Abstract
This study evaluated the feasibility of using the Ribulose Bisphosphate Carboxylase Large subunit gene (rbcL) and the Internal Transcribed Spacers 1 and 2 of the nuclear rDNA (nuITS1 and nuITS2) markers for identifying a very diverse, albeit poorly known group, of green microalgae from neotropical inland waters. Fifty-one freshwater green microalgae strains isolated from Brazil, the largest biodiversity reservoir in the neotropics, were submitted to DNA barcoding. Currently available universal primers for ITS1-5.8S-ITS2 region amplification were sufficient to successfully amplify and sequence 47 (92%) of the samples. On the other hand, new sets of primers had to be designed for rbcL, which allowed 96% of the samples to be sequenced. Thirty-five percent of the strains could be unambiguously identified to the species level based either on nuITS1 or nuITS2 sequences’ using barcode gap calculations. nuITS2 Compensatory Base Change (CBC) and ITS1-5.8S-ITS2 region phylogenetic analysis, together with morphological inspection, confirmed the identification accuracy. In contrast, only 6% of the strains could be assigned to the correct species based solely on rbcL sequences. In conclusion, the data presented here indicates that either nuITS1 or nuITS2 are useful markers for DNA barcoding of freshwater green microalgae, with advantage for nuITS2 due to the larger availability of analytical tools and reference barcodes deposited at databases for this marker.
Introduction
DNA barcoding is a method used for species identification, which identifies specimens based on DNA sequence similarity against a sequence database of a priori defined species[1]. This powerful technique has brought significant improvements to applications such as taxonomy [2–4], ecology [5, 6], biosecurity [7–9] and food product regulation [10–12]. DNA-based identification is particularly useful for unveiling cryptic diversity at various taxonomic levels and identifying species where there are few or difficult to observe structural characters [13–17].
The green algae, Chlorophyta, are an ancient and taxonomically diverse lineage with approximately 8,000 described species [18, 19]. It is estimated that at least 5,000 species still remain undescribed, notably in tropical and subtropical areas [19]. Chlorophytes are important producers in aquatic and humid terrestrial ecosystems, which are often used as bioindicators in water monitoring and ecological studies [20, 21]. In addition, there is a growing interest in using green microalgae for biotechnological applications such as the production of fuels, chemicals, food and animal feed [22, 23]. The identification of green microalgae can be a difficult task and often requires careful microscopic examination of live cultured cells by a trained specialist [14, 24, 25]. Even so, the presence of cryptic species and phenotypic plasticity found in some species may hamper conclusive morphologic species diagnosis [26, 27]. DNA barcodes could provide the means to identify green microalgae consistently and rapidly, regardless of life stage [13, 28, 29].
Targets for potential Chlorophyta DNA barcodes have included chloroplast (rbcL, tufA and Cp23S), mitochondrial (COI) and nuclear genes (18S rDNA, nuITS1 and nuITS2) [13, 28–30]. However, none of these markers were considered ideal for use across all lineages tested [13, 29, 31, 32]. Given the complexity and heterogeneity of chlorophytes, the protist working group of the Consortium for the Barcode of Life (CBOL) recommended the use of a two-step barcoding pipeline in which a universal pre-barcode marker should be used first, followed by the use of a group-specific second barcode [29]. A dual marker barcode based on matK and rbcL genes has been formally proposed for use in DNA barcoding embryophytes [4]. However, the matK gene is absent in chlorophytes precluding its use in this group [33]. Despite the unavailability of a universal PCR toolkit for rbcL amplification, this marker is considered a promising barcode for green algae [13]. Indeed, there are currently 4,449 rbcL sequences from chlorophyte species deposited at the Barcode of Life Data Systems (BOLD), a taxonomically curated database [3]. Apart from rbcL, the most promising candidates for green microalgae barcoding are the nuITS1 and nuITS2 markers [13, 14, 26, 28, 30, 34]. The ITS1-5.8S-ITS2 region from virtually all Viridiplantae can be amplified with a single set of universal primers [35], despite these being markers of high variability [13]. Furthermore, it is possible to analyze not only the nuITS1 and nuITS2 primary sequence, but also their secondary structures [36]. Although there are reports indicating that nuITS1 and nuITS2 might be insufficiently conserved or confounded by introgression or biparental inheritance patterns, a growing body of evidence has shown that simultaneous analysis of nucleotide data and compensatory base changes (CBCs) with secondary structure information can overcome most of the limitations of this potential barcode [14, 28, 30]. In addition, nuITS1 and nuITS2 have been the molecular markers of choice in several recent taxonomic revisions of freshwater chlorophytes species that were based on integrated morphological, physiological and molecular approaches [14, 26, 27, 34, 37–42] The use of nuITS1- and nuITS2-based phylogenies promoted considerable changes in green microalgae taxonomy, especially in taxa with simple morphology and few ultrastructural characteristics such as coccoid chlorophytes [26, 27].
This study aimed to identify neotropic green microalgae specimens isolated from Brazilian inland waters through the use of rbcL, nuITS1 and nuITS2 molecular markers as DNA barcodes. Brazilian continental waters comprise a biodiversity reservoir of enormous global significance and might contain up to 25% of the world’s algae species [43]. Novel primers for neotropic specimens’ rbcL gene amplification and sequencing are presented, as well as comparisons between rbcL, nuITS1 and nuITS2 markers variability, primers universality and databases accuracy and comprehensiveness.
Materials and Methods
Isolation and culturing
All the sample collections were made under the authorization SISBIO #39146 (09/26/2013) conceded by the Instituto Chico Mendes de Conservação da Biodiversidade (ICMBio) of the Brazillian Ministry of the Environment (MMA). The collections made on private land were also authorized by the owner of the land. This study did not involve endangered or protected species. Water samples were collected from the sites shown in S1 Fig. The collection environments included natural freshwater bodies within the Amazon rainforest, the Cerrado savanna and the Pantanal flooded grasslands, as well as anthropogenic wastewater deposits from the sugarcane industry (vinasse), pisciculture ponds and wastewater from swine farming. Sampling areas were delimited as being a 1 km radius centered in the geographic coordinates shown in S1 Fig. The collected environmental samples were submitted to an enrichment step through suspension in modified Bold's Basal Medium–BBM [44] and subsequent culturing at 28°C, light intensity of 50 μEm-2 s-1 and 16/8h light/dark regime. After 15 days of culture, the microalgae strains were isolated by two subsequent rounds of subculturing on BBM agar plates supplemented with ampicillin (100 μg/ml), chloramphenicol (25 μg/ml) and amphotericin B (2,5 μg/ml) under the same conditions described above. Individualized macroscopic colonies on agar plates were collected and inoculated into liquid BBM media to derive axenic cultures. The absence of contaminants was confirmed through microscopic inspection. The isolated strains were deposited in the Collection of Microorganisms and Microalgae Applied to Agroenergy and Biorefineries at Embrapa (Brasília/DF–Brazil).
DNA extraction, amplification and sequencing
Total genomic DNA was isolated from 30 mg of fresh algal biomass using the Cetyl Trimethylammonium Bromide (CTAB) DNA extraction protocol adapted by [45]. The rbcL and ITS1-5.8S-ITS2 DNA regions were submitted to PCR amplification using the primers described in Table 1. The 25 μL PCR reaction mix was composed of 14.5 μL of ultrapure water, 5 μL of GoTaq 5X PCR buffer, 1.5 μL MgCl2 25 mM, 0.75 μL BSA 10 mg/mL, 0.5 μL dNTPs 10 mM, 0.25 μL of GoTaq DNA polymerase (5 U/μL) (Promega, USA), 0.25 μL of each primer (10 μM) and 2.0 μl of DNA template (50–100 ng/μL). The PCR amplification protocol used for both markers was: 96°C for 5 min, 40 cycles of 96° C for 1 min, (primer annealing temperature—see Table 1) for 1 min and 72°C for 1 min, with a final extension at 72° C for 5 min. The PCR products (5 μL) were visualized on agarose gels and selected for direct sequencing. Sequences were determined bi-directionally for at least two different amplicons using the BigDye Terminator v.3.1 Cycle Sequencing Kit on the ABI 3130 automated DNA sequencer (both from Life Technologies, USA), in accordance with the manufacturer’s instructions. The forward and reverse sequences were aligned and edited using Geneious 6.1 software [46], generating consensus nucleotide positions with QV ≥ 20. Sequences were deposited in GenBank under the accession numbers: rbcL sequences (KT307991 to KT308039); ITS1-5.8S-ITS2 sequences (KT308040 to KT308042; KT308046 to KT308076; KT308078 to KT308086; KT445859 to KT445863).
Table 1. List of primers used in this study, including the primer sequences, amplicon length, annealing temperature and the sequencing success rate for a total of 51 strains tested.
| Primer pair | Molecular marker | Sequence | Amplicon length (Nucleotides span) | Annealing temperature | Sequencing success rate | Reference |
|---|---|---|---|---|---|---|
| Fw_ITS1/Rv_ITS4 | ITS1-5.8S-ITS2 | Fw_ITS1: 5’–AGGAGAAGTCGTAACAAGGT– 3’ Rv_ITS4: 5’–TCCTCCGCTTATTGATATGC– 3’ | ≈ 650 pb | 52°C | 92,15% | [35] |
| Fw_rbcL_192/Rv_rbcL_657 | rbcL | Fw_rbcL_192: 5’–GGTACTTGGACAACWGTWTGGAC– 3’ Rv_rbcL_657: 5’–GAAACGGTCTCKCCARCGCAT– 3’ | 465 pb (position 192 to 657) | 52°C | 82,35% | This study |
| Fw_rbcL_375/Rv_rbcL_1089 | rbcL | Fw_rbcL_375: 5’–TTTGGTTTCAAAGCIYTWCGTGC– 3’ Rv_rbcL_1089: 5’–ATACCACGRCTACGRTCTTT– 3’ | 714 pb (position 375 to 1089) | 52°C | 50,98% | This study |
| Fw_rbcL_192/Rv_rbcL_1089 | rbcL | Fw_rbcL_192: 5’–GGTACTTGGACAACWGTWTGGAC– 3’ Rv_rbcL_1089: 5’–ATACCACGRCTACGRTCTTT– 3’ | 897 pb (position 192 to 1089) | 52°C | 37,25% | This study |
| Fw_rbcLa_f/Rv_rbcL_ajf634R | rbcL | Fw_rbcLa_f 5’–ATGTCACCACAAACAGAAACTAAAGC– 3’ Rv_rbcL_ajf634R: 5’–GAAACGGTCTCTCCAACGCAT– 3’ | 654 pb (position 1 to 654) | 54°C | 15,69% | [4] |
| Fw_rbcL_109/Rv_rbcL_657 | rbcL | Fw_rbcL_109: 5’–TTCTTGCTGCITTYCGTATG– 3’ Rv_rbcL_657: 5’–GAAACGGTCTCKCCARCGCAT– 3’ | 548 pb (position 109 to 657) | 52°C | 13,75% | This study |
| Fw_rbcLa_f/rbcLA_rev | rbcL | Fw_rbcLa_f: 5’–ATGTCACCACAAACAGAGACTAAAGC– 3’ rbcLA_rev: 5’–GTAAAATCAAGTCCACCRCG– 3’ | 599 pb (position 1 to 599) | 54°C | 7,84% | [4] |
| Fw_rbcL_109/Rv_rbcL_1089 | rbcL | Fw_rbcL_109: 5’–TTCTTGCTGCITTYCGTATG– 3’ Rv_rbcL_1089: 5’–ATACCACGRCTACGRTCTTT– 3’ | 980 pb (position 109 to 1089) | 52°C | 1,96% | This study |
| Fw_rbcL_RH1/rbcL_724R | rbcL | Fw_rbcL_RH1: 5’–ATGTCACCACAAACAGAAACTAAAGC– 3’ rbcL_724R: 5’–TCGCATGTACCTGCAGTAGC– 3’ | 743 pb (position 1 to 743) | 54°C | 1,96% | [4] |
| Fw_rbcL_RH1/rbcL_1385R | rbcL | Fw_rbcL_RH1: 5’–ATGTCACCACAAACAGAAACTAAAGC– 3’ rbcL_1385R: 5’–AATTCAAATTTAATTTCTTTCC– 3’ | 1406 pb (position 1 to 1406) | 48°C | 0% | [13] |
Molecular data analysis
Sequences were aligned automatically using ClustalW [47] under default parameters using MEGA5 software [48]. The nuITS1, 5.8S and nuITS2 sequences were annotated using ITSx v. 1.0.11 [49]. For similarity searches, the rbcL sequences were submitted to the Barcode of Life Data Systems (BOLD systems) using the Plant identification tool, while nuITS2 sequences were submitted to the Basic Local Alignment Search Tool (BLASTN) for comparisons against nucleotide sequences deposited at the Genbank. The nuITS2 secondary structures were predicted by either direct fold (energy minimization) or homology modelling [50]. Subsequently, in order to locate hemi-compensatory base changes (hemi-CBCs) and compensatory base changes (CBCs), each sequence-structure along with its top match on ITS2 Blast tool were aligned and analyzed with 4SALE v. 1.7 [51, 52].
The barcode gap was inferred based on uncorrected pair–wise (p) distance matrices. MEGA5 software was used for calculation. The taxon samplings used were reference nuITS1, nuITS2 and rbcL sequences derived from recent taxonomic revisions of the Chlorella and Desmodesmus genera [14, 53, 54] (S1–S3 Tables). The maximum intraspecific distances and minimum interspecific distances obtained were computed.
For phylogenetic tree analysis, the ITS1-5.8S-ITS2 sequences from Embrapa|LBA#2–3, #22–23, #26–27, #30, #32–36, #39, #42–44 and #50 strains were included in the dataset together with their respectively closest sequences at GenBank. Desmodesmus sp., Chlorella sp. and Micractinium sp. ITS1-5.8S-ITS2 reference sequences [14, 39, 53–55]. The dendrograms were constructed through the maximum likelihood (ML) method using MEGA5 software. The GTR model with invariable sites (I) and gamma distribution shape parameter (G) was chosen. The neighbor-joining (NJ) algorithm was used to generate the initial tree for ML computation. A phylogenetic test using the Bootstrap method (1,000 replicates) was used.
Morphologic Identification
Microscopic morphologic identification at the genus level was performed according to Bellinger & Sigee, 2015 [56]. Further identification to species levels was accomplished by comparison with the species original descriptions that are available at the AlgaeBase [57]. In the case of the as of yet undescribed species, the morphological comparisons were made with the closest strains obtained in the molecular identification step: Desmodesmus sp. MAT2008c [58]; Micractinium sp. CCAP 211/92 [39]; Desmodesmus sp. GM4a [59]. A Carl Zeiss Axio Imager A2 microscope (Zeiss.co, Brazil) equipped with Differential Interference Contrast (DIC) was used for morphological analysis.
Results
Barcode markers primer universality
A total of 51 unialgal strains (named Embrapa|LBA#1 to #51) were isolated from natural water bodies within the Cerrado savanna, the Pantanal wetlands and the Amazon rainforest, as well as anthropogenic wastewater deposits (S1 Fig). Coccoid morphotypes were the most abundant among the isolated strains (51%), followed by monadoids/palmelloids morphotypes (41%) (data not shown).
The ITS1-5.8S-ITS2 region could be successfully sequenced from DNA samples extracted from 47 strains (92,15% sequencing success rate) by using the universal primers described by White and coworkers (1990) [35] (Table 1). Even though all the 51 samples could be amplified with this set of primers, the presence of multiple PCR products impaired direct sequencing of four samples. On the other hand, the sequencing success rate obtained using the rbcL gene universal primer sets described by Hall and coworkers (2010) [13] or the sets proposed for embryophytes by the CBOL Plant working group [4], ranged from 0% to 15,69% (Table 1). In order to circumvent this problem, new sets of primers targeting rbcL gene partial amplification (Table 1) were designed based on 175 rbcL reference sequences from distinct Chlorophyta taxa mined from BOLD Systems. The newly designed primer pairs Fw_rbcL_192/Rv_rbcL_657 and Fw_rbcL_357/Rv_rbcL-1089 could successfully amplify and sequence 82,35% and 50,98% of the dataset, respectively (Table 1). The combination of the sequencing results from both these rbcL primer pairs allowed the construction of quality consensus sequences (QV≥20) for 49 samples (96,08% sequencing success rate). A total of 18 distinct 5.8S genotypes, 23 distinct nuITS1 genotypes, 23 nuITS2 distinct genotypes and 26 distinct rbcL genotypes were obtained.
Similarity search based on nuITS1, nuITS2 and rbcL markers
In order to perform the molecular identification of Embrapa|LBA strains, the rbcL sequences obtained were submitted to similarity searches against the DNA barcoding dedicated database, BOLD systems. The closest matches retrieved for rbcL sequences ranged from 90% to 99% of similarity (Table 2). Currently, there are very few nuITS1 and nuITS2 sequences from chlorophytes deposited at taxonomically curated databases such as BOLD, therefore similarity searches were performed against the GenBank. The closest matches retrieved for nuITS1 sequences ranged from 70% to 100% of similarity and for nuITS2 sequences ranged from 81% to 100% of similarity (Table 2). Embrapa|LBA strains retrieved matches from species that belong to the Chlorophyceae and Trebouxiophyceae classes, especially to the orders Chlamydomonadales, Chlorococcales, Sphaeropleales and Chlorellales (Table 2). Ten nuITS1 sequences, 14 nuITS2 sequences and 0 rbcL sequences retrieved matches with a 100% similarity (Table 2).
Table 2. Molecular identification of the strains used in this study, including the percentual of identity, accession number and the name of the identified species on the Barcode of Life Database (based on rbcL marker sequence) and GenBank (based on nuITS2 marker sequence).
| Strain | ITS1 (GenBank) | ITS2 (GenBank) | rbcL (BOLD) | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Closest match species | Identity | GenBank access | Closest match species | Identity | Number of CBCs / hCBCs | GenBank access | Closest match species | Identity | GenBank access | |
| LBA#1 | Desmodesmus armatus | 95% | KP281288.1 | Desmodesmus bicellularis | 91% | 1 / 7 | AB917134.1 | Scenedesmus quadricauda | 90% | AB084332.1 |
| LBA#2 | Desmodesmus sp. MAT-2008c | 100% | EU502836.1 | Desmodesmus sp. MAT-2008c | 100% | 0 / 0 | EU502836.1 | Acutodesmus obliquus | 93% | DQ396875.1 |
| LBA#3 | Desmodesmus sp. MAT-2008c | 100% | EU502836.1 | Desmodesmus sp. MAT-2008c | 100% | 0 / 0 | EU502836.1 | Acutodesmus obliquus | 90% | DQ396875.1 |
| LBA#4 | Chlamydopodium starrii | 70% | AB983644.1 | Chlorococcum oleofaciens | 91% | 1 / 2 | AB983633.1 | Chlorococcum ellipsoideum | 91% | EF113431.1 |
| LBA#5 | Desmodesmus sp. Tow 10/11 T-12W | 79% | DQ417556.1 | Desmodesmus regularis | 84% | 4 / 2 | AM228924.1 | Desmodesmus santosii | 93% | GU192417.1 |
| LBA#6 | Chlamydopodium starrii | 70% | AB983644.1 | Chlorococcum oleofaciens | 94% | - | AB983633.1 | Chlorococcum ellipsoideum | 91% | EF113431.1 |
| LBA#7 | Desmodesmus sp. Tow 10/11 T-12W | 79% | DQ417556.1 | Desmodesmus regularis | 84% | 4 / 2 | AM228924.1 | Desmodesmus santosii | 93% | GU192417.1 |
| LBA#8 | Chlamydomonas sp. KU107 | 94% | KM061447.1 | Chlamydomonas sp. KU107 | 87% | 0 / 1 | KM061447.1 | Chlamydomonas oblonga | 95% | EF113424.1 |
| LBA#9 | Chlamydopodium starrii | 90% | AB983644.1 | Chlamydopodium starrii | 93% | 0 / 1 | AB983644.1 | Chlorococcum ellipsoideum | 92% | KC810301.1 |
| LBA#10 | Chlamydopodium starrii | 90% | AB983644.1 | Chlamydopodium starrii | 93% | 0 / 1 | AB983644.1 | Chlorococcum ellipsoideum | 92% | KC810301.1 |
| LBA#11 | Chlamydopodium starrii | 90% | AB983644.1 | Chlamydopodium starrii | 93% | 0 / 1 | AB983644.1 | Chlorococcum ellipsoideum | 92% | KC810301.1 |
| LBA#12 | Chlamydopodium starrii | 90% | AB983644.1 | Chlamydopodium starrii | 93% | 0 / 1 | AB983644.1 | - | - | - |
| LBA#13 | Coelastrella sp. shy-188 | 96% | KP702302.1 | Scenedesmus rubescens | 95% | 0 / 2 | JX513884.1 | Scenedesmus quadricauda | 90% | AB084332.1 |
| LBA#14 | Chlamydopodium starrii | 90% | AB983644.1 | Chlamydopodium starrii | 93% | 0 / 1 | AB983644.1 | Chlorococcum ellipsoideum | 92% | KC810301.1 |
| LBA#15 | Chlamydopodium starrii | 90% | AB983644.1 | Chlamydopodium starrii | 93% | 0 / 1 | AB983644.1 | Chlorococcum ellipsoideum | 92% | KC810301.1 |
| LBA#16 | - | - | - | - | - | - | - | Ecballocystopsis dichotomus | 90% | JX018187.1 |
| LBA#17 | Chlamydopodium starrii | 90% | AB983644.1 | Chlamydopodium starrii | 93% | 0 / 1 | AB983644.1 | Chlorococcum ellipsoideum | 92% | KC810301.1 |
| LBA#18 | Chlamydopodium starrii | 90% | AB983644.1 | Chlamydopodium starrii | 93% | 0 / 1 | AB983644.1 | Chlorococcum ellipsoideum | 92% | KC810301.1 |
| LBA#19 | - | - | - | - | - | - | - | Ecballocystopsis dichotomus | 90% | JX018187.1 |
| LBA#20 | Coelastrum astroideum | 76% | GQ375093.1 | Scenedesmus arcuatus | 81% | 0 / 6 | AY170855.1 | Hariotina reticulata | 93% | JQ394815.1 |
| LBA#21 | Coelastrella sp. shy-188 | 96% | KP702302.1 | Scenedesmus rubescens | 95% | 0 / 2 | JX513884.1 | Desmodesmus costato-granulatus | 94% | GU192427.1 |
| LBA#22 | Desmodesmus ultrasquamatus | 100% | GU192392.1 | Desmodesmus ultrasquamatus | 99% | 0 / 0 | GU192392.1 | Desmodesmus costato-granulatus | 93% | GU192427.1 |
| LBA#23 | Desmodesmus ultrasquamatus | 100% | GU192392.1 | Desmodesmus ultrasquamatus | 99% | 0 / 0 | GU192392.1 | Desmodesmus costato-granulatus | 94% | GU192427.1 |
| LBA#24 | Desmodesmus ultrasquamatus | 94% | GU192392.1 | Desmodesmus ultrasquamatus | 94% | 0 / 3 | AM228926.1 | Desmodesmus costato-granulatus | 94% | GU192427.1 |
| LBA#25 | Desmodesmus ultrasquamatus | 94% | GU192392.1 | Desmodesmus ultrasquamatus | 94% | 0 / 3 | AM228926.1 | Desmodesmus costato-granulatus | 94% | GU192427.1 |
| LBA#26 | Desmodesmus sp. MAT-2008c | 100% | EU502836.1 | Desmodesmus sp. MAT-2008c | 100% | 0 / 0 | EU502836.1 | Acutodesmus obliquus | 92% | DQ396875.1 |
| LBA#27 | Chlorella sorokiniana | 100% | KM061456.1 | Chlorella sorokiniana | 100% | 0 / 0 | KJ676113.1 | Chlorella sorokiniana | 99% | HM101339.1 |
| LBA#28 | - | - | - | - | - | - | - | Selenastrum sp. KMMCC 1456 | 94% | JQ315488.1 |
| LBA#29 | Chlorella sp. MAT-2008a | 92% | EU502833.1 | Chlorella sp. MAT-2008a | 91% | 0 / 2 | EU502833.1 | Chlorella sp. IFRPD 1018 | 93% | AB260911.1 |
| LBA#30 | Desmodesmus sp. MAT-2008c | 100% | EU502836.1 | Desmodesmus sp. MAT-2008c | 100% | 0 / 0 | EU502836.1 | Acutodesmus obliquus | 93% | DQ396875.1 |
| LBA#31 | Chlorella sp. MAT-2008a | 92% | EU502833.1 | Chlorella sp. MAT-2008ª | 91% | 0 / 2 | EU502833.1 | Chlorella sp. IFRPD 1018 | 93% | AB260911.1 |
| LBA#32 | Micractinium sp. CCAP 211/92 | 99% | FM205863.1 | Micractinium sp. CCAP 211/92 | 100% | 0 / 0 | FM205863.1 | Chlorella pyrenoidosa | 99% | FM205863.1 |
| LBA#33 | Micractinium sp. CCAP 211/92 | 99% | FM205863.1 | Micractinium sp. CCAP 211/92 | 100% | 0 / 0 | FM205863.1 | Chlorella pyrenoidosa | 99% | FM205863.1 |
| LBA#34 | Micractinium sp. CCAP 211/92 | 99% | FM205863.1 | Micractinium sp. CCAP 211/92 | 100% | 0 / 0 | FM205863.1 | Chlorella pyrenoidosa | 99% | FM205863.1 |
| LBA#35 | Desmodesmus sp. GM4a | 100% | AB917128.1 | Desmodesmus sp. GM4a | 99% | 0 / 1 | AB917128.1 | Desmodesmus baconii | 93% | KC315289.1 |
| LBA#36 | Desmodesmus sp. MAT-2008c | 100% | EU502836.1 | Desmodesmus sp. MAT-2008c | 100% | 0 / 0 | EU502836.1 | Acutodesmus obliquus | 93% | DQ396875.1 |
| LBA#37 | Chlamydomonas sp. YB3-2 | 90% | JN862852.1 | Chlamydomonas applanata | 92% | 1 / 2 | FR865616.1 | Ascochloris multinucleata | 94% | EF113411.1 |
| LBA#38 | Chlamydomonas sp. YB3-2 | 90% | JN862852.1 | Chlamydomonas applanata | 92% | 1 / 2 | FR865616.1 | Ascochloris multinucleata | 94% | EF113411.1 |
| LBA#39 | Chlorella sorokiniana KU207 | 100% | KM061456.1 | Chlorella sorokiniana | 100% | 0 / 0 | KJ676113.1 | Chlorella sorokiniana | 99% | HM101339.1 |
| LBA#40 | Chlamydomonas zebra | 79% | AF033294.1 | Chlamydomonas sp. XJU-36 | 95% | 2 / 0 | FJ572059.1 | Chlamydomonas orbicularis | 96% | AB511849.1 |
| LBA#41 | Chlamydomonas sp. KU107 | 94% | KM061447.1 | Chlamydomonas sp. KU107 | 87% | 0 / 3 | KM061447.1 | Chlamydomonas oblonga | 95% | EF113424.1 |
| LBA#42 | Micractinium sp. CCAP 211/92 | 99% | FM205863.1 | Micractinium sp. CCAP 211/92 | 100% | 0 / 0 | FM205863.1 | Chlorella pyrenoidosa | 99% | FM205863.1 |
| LBA#43 | Micractinium sp. CCAP 211/92 | 99% | FM205863.1 | Micractinium sp. CCAP 211/92 | 100% | 0 / 0 | FM205863.1 | Chlorella pyrenoidosa | 99% | FM205863.1 |
| LBA#44 | Micractinium sp. CCAP 211/92 | 99% | FM205863.1 | Micractinium sp. CCAP 211/92 | 100% | 0 / 0 | FM205863.1 | Chlorella pyrenoidosa | 99% | FM205863.1 |
| LBA#45 | Chlorococcum oleofaciens | 82% | AB983633.1 | Spongiochloris spongiosa | 86% | - | U34776.1 | Protosiphon botryoides | 92% | EF113465.1 |
| LBA#46 | Uronema sp. AF-2012 | 98% | JX092263.1 | Uronema trentonense | 100% | 0 / 0 | HF920659.1 | - | - | - |
| LBA#47 | Tetracystis tetraspora | 95% | KM020024.1 | Dunaliella sp. SPMO 300–4 | 85% | 2 / 0 | DQ377118.1 | Nautococcus solutus | 91% | AB360758.1 |
| LBA#48 | - | - | - | - | - | - | - | Gungnir sp. NIES-1851 | 93% | AB603749.1 |
| LBA#49 | Lobochlamys segnis | 83% | FR865604.1 | Chlamydomonas sp. CCAP 11/150 | 90% | 0 / 1 | FR865545.1 | Asterococcus korschikoffii | 90% | AB175944.1 |
| LBA#50 | Chlorella sp. KMMCC 1468 | 99% | JQ315774.1 | Chlorella sorokiniana | 96% | 0 / 0 | LK021940.1 | Chlorella sp. IFRPD 1014 | 99% | AB260910.1 |
| LBA#51 | Chlorococcum oleofaciens | 74% | AB983630.1 | Chlorococcum sp. CCAP 11/52 | 84% | 2 / 1 | FR865591.1 | Chlamydopodium vacuolatum | 95% | EF113426.1 |
The compensatory and hemi-compensatory base changes (CBCs/hemi-CBCs) between the indicated sequence and its closest match in the ITS2 Database are shown. An hyphen (-) is indicated for samples that could not be amplified and/or sequenced, and for the nuITS2 sequences for which secondary structure predictions and CBCs/Hemi-CBCs analysis were not possible.
Barcode gap analysis
Similarity searches only configure the first step for DNA barcoding since they provide information about the closest matches present in reference databases, but not necessarily species-level identification. In order to establish a genetic distance threshold for species-level identification that is applicable to chlorophytes, barcode gap analyses were conducted based on reference sequences from two species-dense green microalgae genera, Chlorella and Desmodesmus (S2–S4 Figs; S1–S3 Tables).
Chlorella genus nuITS1 intraspecific distances ranged from 0 to 0,014, while nuITS1 interspecific distances ranged from 0,058 to 0,199 (S2A Fig). Desmodesmus genus nuITS1 intraspecific distances ranged from 0 to 0,018, while nuITS1 interspecific distances ranged from 0,029 to 0,193 (S2B Fig). The presence of a barcode gap (gap between maximum intraspecific and minimum interspecific distances) was observed for all species analyzed (S2 Fig). Chlorella genus nuITS2 intraspecific distances ranged from 0 to 0,071, while nuITS2 interspecific distances ranged from 0,076 to 0,204 (S3A Fig). Desmodesmus genus nuITS2 intraspecific distances ranged from 0 to 0,02, while nuITS2 interspecific distances ranged from 0,032 to 0,167 (S3B Fig). The presence of a barcode gap was also observed for all species analyzed (S3 Fig). Desmodesmus rbcL genus intraspecific distances ranged from 0 to 0,108, while rbcL interspecific distances ranged from 0,015 to 0,086 (S4 Fig). The presence of a barcode gap is observed for all species based on rbcL sequences, except for Desmodesmus serratus species (S4 Fig).
Distance thresholds for species-level identification were inferred for each marker based on the minimum interspecific distances observed for each marker (S2–S4 Figs), as follows: i) nuITS1 sequences (< 0,029); ii) nuITS2 sequences (< 0,032); ii) rbcL sequences (< 0,015). The application of these distance thresholds to the data presented in Table 2 suggests that species-level identification has been achieved for: i) 35% of the nuITS1 sequences, namely Embrapa|LBA#2–3, #22–23, #26–27, #30, #32–36, #39, #42–44, #46 and #50; ii) 33% of the nuITS2 sequences, namely Embrapa|LBA#2–3, #22–23, #26–27, #30, #32–36, #39, #42–44 and #46. iii) 18% of the rbcL sequences, namely Embrapa|LBA#27, #32–34, #39, #42–44 and #50.
Additionally, even though nuITS2 Embrapa|LBA#50 sequence presents only 96% of identity to its GenBank closest match, it can also be considered that species-level identification has been achieved, since the lowest interspecific distance calculated specifically for the Chlorella genus nuITS2 sequences is 0,076 (S3A Fig). On the other hand, rbcL based identification assigned Embrapa|LBA #32–34 and #42–44 strains to Chlorella pyrenoidosa species, which is not currently a taxonomically accepted name [57]. Therefore, Embrapa|LBA #32–34 and #42–44 strains were excluded from the subset of strains identified to the species-level based on rbcL sequences.
In conclusion, the results presented so far indicate that 18, 18 and 3 Embrapa|LBA strains were identified to the species-level based on nuITS1, nuITS2 or rbcL sequences, respectively.
Morphologic, Phylogenetic and Compensatory Base Changes (CBCs) analyses
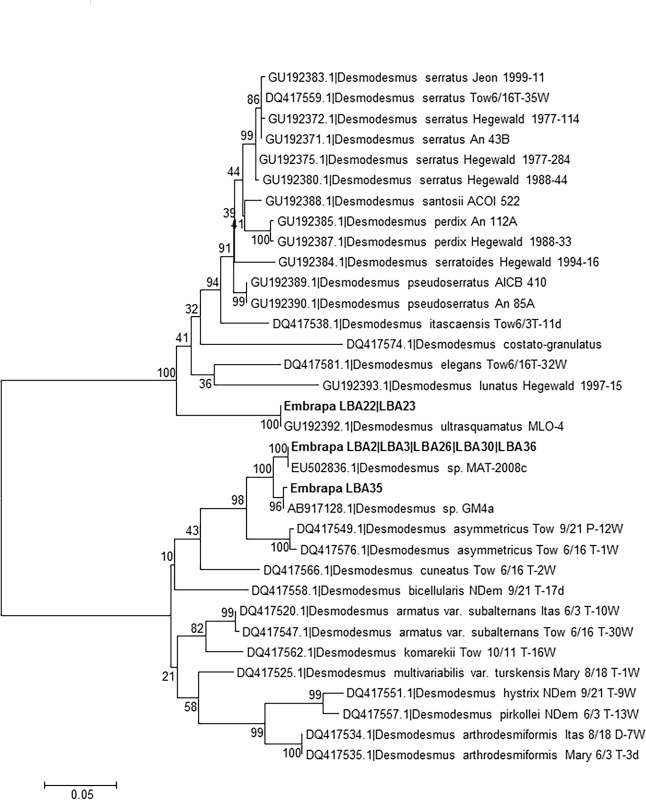
In order to confirm the species-level identification based on barcode gap calculations, the strains Embrapa|LBA#2–3, #22–23, #26–27, #30, #32–36, #39, #42–44,#46 and #50 were identified based on morphology. The strains Embrapa|LBA#22–23 were identified as Desmodesmus ultrasquamatus, Embrapa|LBA#27, 39 and 50 were identified as Chlorella sorokiniana and Embrapa|LBA#46 was identified as Uronema trentonense, according to these species original descriptions [57]. The molecular identification of strains Embrapa|LBA#2–3, #26, #30, #32–36 and #42–44 (Table 2) suggest that they correspond to species still not formally described. Indeed, strains Embrapa|LBA#2–3, #26, #30 and #36 correspond to unicellular spineless coccoid Desmodemus species with sizes ranging from 4–6 μm (Fig 1A and 1D), similar to the description of its closest GenBank match (Table 2) the strain Desmodesmus sp. MAT-2008c isolated in Australia [58]. Strains Embrapa|LBA#32–34 and #42–44 correspond to coccoid bristleless Micractinium species with sizes ranging from 3–5 μm (Fig 1B and 1E), which is congruent with the description reported for its closest GenBank match (Table 2) the strain Micractinium sp. CCAP 211/92 isolated from a soil sample collected from Mahe Island, Seychelles [39]. Strain Embrapa|LBA#35 corresponds to a two-, four- or eight-celled coenobia forming Desmodemus species that present few spines and dimensions of 3–6 x 8–13 μm (Fig 1C and 1F), similar to the description of its closest GenBank match (Table 2) the strain Desmodesmus sp. GM4a isolated from German inland waters [59].
Fig 1. Representative DIC microscopic images of Embrapa|LBA strains assigned to not formally described species.
(A and D) Embrapa|LBA#36. (B and E) Embrapa|LBA#32. (C and F) Embrapa|LBA#35. Scale bars = 5 μm.
Furthermore, the species-level identification obtained for strains Embrapa|LBA#2–3, #22–23, #26–27, #30, #32–36, #39, #42–44,#46 and #50 is corroborated by the absence of Compensatory Base Changes (CBCs) between nuITS2 sequences of these strains and their closest matches at GenBank (Table 2). Additionally, phylogenetic analyses using reference ITS1-5.8S-ITS2 sequences from currently accepted Chlorella, Micractinium and Desmodesmus species also corroborate species-level identification of strains Embrapa|LBA#2–3, #22–23, #26–27, #30, #32–36, #39, #42–44 and #50 (Figs 2 and 3). Figs 2 and 3 clearly demonstrate that sequences from these strains group together with their closest matches from GenBank (Table 2) in monophyletic clades.
Fig 2. Phylogenetic tree for Chlorella and Micractinium genera inferred based on ITS1-5.8S-ITS2 sequences.
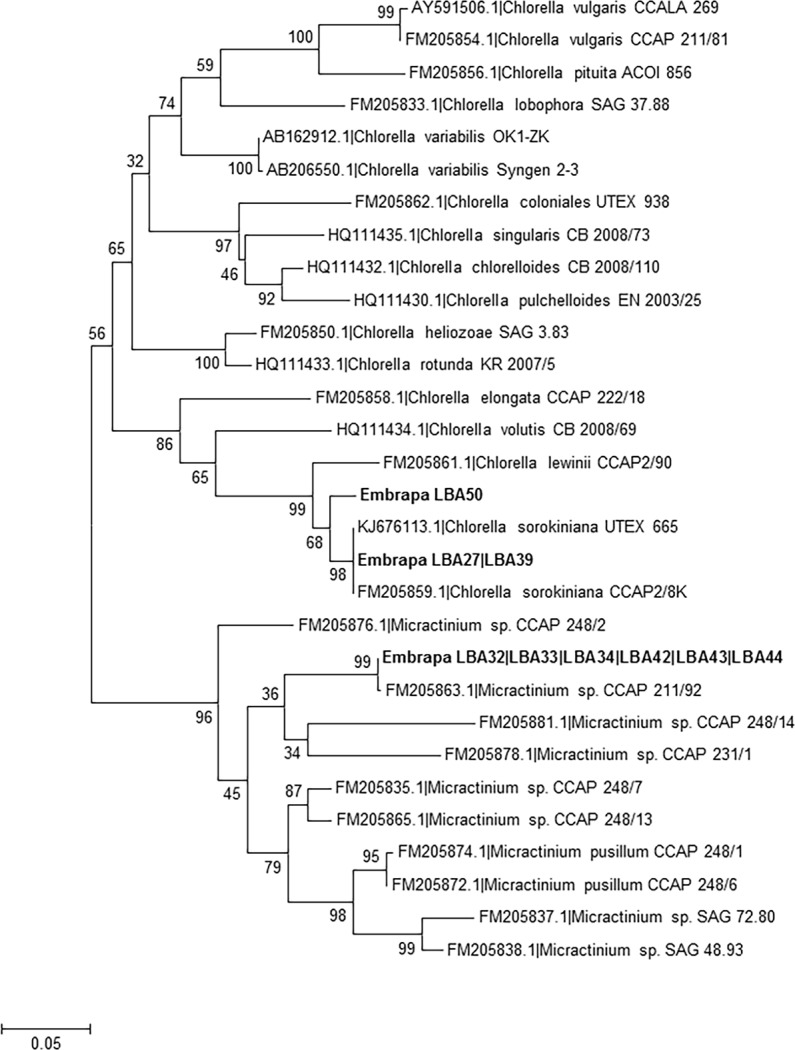
Chlorella sp. and Micractinium sp. ITS1-5.8S-ITS2 reference barcode sequences reported by Luo et al. (2010) [39] and Bock et al. (2011) [14] were included in the analysis together with Embrapa|LBA#27, #32–34, #39, #42–44 and #50 strains sequences and their respectively closest sequences at GenBank. Identical sequences were omitted for simplification. The phylogenetic tree was inferred using the Maximum Composite Likelihood method based on dataset of 472 aligned positions of 31 nucleotide sequences. For the analysis, the GTR+G+I model was chosen. For the analysis, the GTR model with invariable sites (I) and gamma distribution shape parameter (G) was chosen. The bootstrap values (1000 replicates) are shown next to the branches.
Fig 3. Phylogenetic tree for Desmodesmus genus inferred based on ITS1-5.8S-ITS2 sequences.
Demodesmus sp. ITS1-5.8S-ITS2 reference barcode sequences reported by Fawley et al. (2011) [53] and Gorelova et al. (2014) [54] were included in the analysis together with Embrapa|LBA#2–3, #22–23, #26, #30 and #35–36 strains sequences and their respectively closest sequences at GenBank. Identical sequences were omitted for simplification. The phylogenetic tree was inferred using the Maximum Composite Likelihood method based on a dataset of 470 aligned positions of 34 nucleotide sequences. For the analysis, the GTR+G+I model was chosen. The bootstrap values (1000 replicates) are shown next to the branches.
Discussion
A dual marker DNA barcode system has been proposed as a potential solution to cope with the great diversity of protists, however there is no current consensus about which marker should be used [29, 32]. Ideally the two chosen markers should be easily amplified/sequenced using a single set of primers and sufficiently variable to permit clear species delimitations without loss of the phylogenetic signal [29, 32]. Even though tufA has been reported to be a promising barcode for chlorophytes [13, 31, 32], the number of green algae tufA sequences deposited at GenBank is three times lower than the number of deposits for the protein-coding plastid gene rbcL or the non-coding regions of nuclear rDNA ITS1 and ITS2 (over 6,000 sequences deposited for rbcL and nuITS1 and over 7,000 sequences deposited for nuITS2 markers up to December/2015). Furthermore, recent taxonomic revisions of green algae have been based mainly on rbcL, nuITS1 or nuITS2 sequences [14, 26, 27, 32, 34, 37–42, 60]. In addition, there are thousands of rbcL sequences from chlorophytes deposited at BOLD systems, which is the most complete taxonomically curated DNA database available [3]. Therefore, although a formal proposal for Chlorophyta DNA barcodes has not been made, a preference for rbcL, nuITS1 and nuITS2 markers by several research groups involved in green algae taxonomy can be observed.
Brazil holds the largest reservoir of algal genetic resources in the neotropical region [43, 61]. In order to evaluate the applicability of nuITS1, nuITS2 and rbcL markers as DNA barcodes for neotropic freshwater chlorophytes, a subset of green microalgae strains was isolated from Brazilian inland water bodies (S1 Fig). This study, however, did not intend to perform an exhaustive sampling of all the Chlorophyta taxa present in the neotropics. Instead, it used specimens from this largely unexplored biodiversity hotspot as test case. DNA from all 51 Embrapa|LBA strains could be amplified and sequenced for at least one of the markers tested. The higher primer universality obtained for ITS1-5.8S-ITS2 region compared to the rbcL marker (Table 1) is in agreement with previous studies [13, 28, 62]. This can be explained by the presence of highly conserved neighbor regions flanking nuITS (1 and 2) markers, such as the 18S and 28S rDNA genes that function as annealing sites for the primers, described by White and coworkers (1990) [35], which are not available for the rbcL gene.
The levels of nucleotide diversity observed among the 5.8S, nuITS1, nuITS2 and rbcL sequences were of 0,046, 0,537, 0,321 and 0,250, respectively. Indeed, although nuITS1, nuITS2 and rbcL markers may fluctuate depending on the taxa analyzed, these markers rank among the most diverse barcode candidates for chlorophytes [13, 28, 31]. On the other hand, the 5.8S marker might not present sufficient resolution for species discrimination. Therefore, although other studies used the nuclear rDNA region ITS1-5.8S-ITS2 as a barcode for Chlorophyta (14, 34, 39), in this study the nuITS1 and nuITS2 regions were used separately to avoid genetic distance calculation bias eventually introduced by the simultaneous analysis of DNA regions with distinct evolutionary rates.
It is noteworthy that 53% of the nuITS1 and 42% of the nuITS2 matches retrieved from GenBank lacked the Latin binomial that characterizes the complete species name, compared to 10% of the rbcL matches retrieved from BOLD (Table 2). This might be due to the combination of two factors: i) CBOL’s effort to preserve traditional taxonomic nomenclature; ii) The overall tendency in phycology to gradually move away from species identifiers based on Latin binomials pushed by the faster rate of genetic information discovery compared with the traditional taxonomic descriptions [24]. Importantly, species names that are not currently taxonomically accepted were found at both the BOLD and GenBank databases. That is the case, for example, of the strains Embrapa|LBA#32–34 and #42–44, which were assigned as Chlorella pyrenoidosa (Table 2), currently Pseudochlorella pyrenoidosa [26, 38], at BOLD systems. Although this finding is not unexpected within GenBank, it is especially relevant in a taxonomically curated database such as BOLD. A possible explanation is that these are, actually, non-validated reference sequences mined directly from GenBank that are currently under taxonomic revision by BOLD collaborators. Indeed, it can be observed that the Acutodesmus obliquus rbcL reference sequence DQ396875.1 retrieved from BOLD (Table 2) is deposited with the old species name, Scenedesmus obliquus, at GenBank (data not shown).
Only few sequences retrieved matches with 100% of identity from GenBank and BOLD (Table2), suggesting incomplete taxa coverage within the reference databases analyzed. This is corroborated by the fact that there are less than 500 hundred rbcL records from the neotropical region (only 21 from Brazil) deposited at BOLD up to July/2015. Thus, it seems that the incongruences observed between species names retrieved from nuITS1, nuITS2 and rbcL similarity searches (Table 2) are mainly due to reference databases incompleteness rather than to real conflicts derived from distinct species identification by each marker. This is important information to be considered since the possibility of biased performance, eventually leading to sample misidentification, when using search algorithms such as BLAST is increased when analyzing poorly sampled groups [63].
Barcode gap analyses can provide the means to improve the accuracy for species level identification [1, 17]. A barcode gap is present when the maximum intraspecific distance is lower than the minimum interspecific distance for a certain taxon, thereby revealing a corresponding distance threshold that can be applied to delimit species [17]. However, the same distance threshold may not be applicable to every species and should be determined for each taxon analyzed [32, 63, 64]. Due to the unavailability of a complete set of reference sequences for most of the taxa listed in Table 2, the analyses were based on sequences Chlorella and Desmodesmus genera for nuITS1 and nuITS2, and for Desmodesmus genus for rbcL. These reliable reference barcode sequences are originated from recent revisions of these genera based on integrative taxonomy approaches (S2–S4 Figs; S1–S3 Tables). As expect, the barcode gap analyses based on nuITS1, nuITS2 and rbcL makers (S2–S4 Figs) indicate that it is not possible to establish a single universal distance threshold that would avoid incorrect identifications and, at the same time, include all specimens into the correct species. However, assuming that incorrect specimen identification is more problematic than simply not assigning a specimen to any species, distance thresholds were inferred for each marker based on the minimum interspecific distances observed (S2–S4 Figs) allowing species-level identification.
There are several reports suggesting that the presence of compensatory base changes (CBCs) in nuITS2 secondary structures correlate with reproductive isolation [65–67]. A large-scale testing with ~300.000 nuITS2 secondary structures revealed that if a CBC is present then there are two different species with a probability of ~93% [65, 67]. Therefore, the detection of CBCs between the Embrapa|LBA strains nuITS2 sequences and their closest matches at GenBank seems to be a reasonable predictor that species-level identification has not been achieved. In accordance, the CBCs analyses shown in Table 2 corroborate the species-level identification achieved based on barcode gap calculations. Additionally, the morphological (Fig 1) and phylogenetic analyses (Figs 2 and 3) also corroborate the species-level identification based on barcode gap calculations.
The DNA barcoding results presented here using a subset of neotropic freshwater green microalgae as a test case suggest that nuITS1 and nuITS2 are the most useful markers, while rbcL presented lower primer universality and species-level identification power. Although, both nuITS1 and nuITS2 precisely identified the same 18 strains to the species-level based on barcode gap calculations, nuITS2 accounts with a more complete set of reference sequences deposited at databases and an automated and well developed pipeline for secondary structure analysis [50]. The S5 Fig depicts the tentative DNA barcoding workflow for green microalgae specimens based on the results presented.
Conclusions
DNA barcoding can make specimens identification to species level faster, more reliable and accessible to non-specialists. Defining of the appropriate DNA barcodes for Chlorophyta identification and the availability of taxonomically curated DNA databases are pivotal to this task. The results presented here indicate that a DNA barcoding pipeline based on nuITS2 should be useful for green microalgae species identification. It is clear, however, that there is an urgent need for the deposition of more taxonomically accurate reference barcodes in curated databases (e.g.: BOLD Systems). Therefore, extensive efforts on integrative taxonomy are crucial, ideally encompassing the use of both DNA markers. These studies are especially relevant for poorly studied taxa such as tropical chlorophytes.
Supporting Information
Map of Brazilian biomes, including the Amazon tropical rainforest (1), the Caatinga xeric shrublands (2), the Cerrado tropical Savanna (3), the Pantanal flooded grassland (4), the Mata Atlântica tropical rainforest (5) and the Pampa subtropical grassland (6). The geographic coordinates of the six distinct locations sampled and the respective isolated strains in each site are shown. The strains isolated were deposited in the Collection of Microorganisms and Microalgae Applied to Agroenergy and Biorefineries at Embrapa (Brasília/DF–Brazil). The Brazilian territory is highlighted in black in the map of the neotropical region (inset).
(TIF)
The maximum intraspecific distances (◆) and minimum interspecific distances (□) based on nuITS1 marker between Chlorella (A) and Desmodesmus (B) genera species are shown. The dataset was composed of reference barcode sequences reported for each genera (S1 and S2 Tables).
(TIF)
The maximum intraspecific distances (◆) and minimum interspecific distances (□) based on nuITS2 marker between Chlorella (A) and Desmodesmus (B) genera species are shown. The dataset was composed of reference barcode sequences reported for each genera (S1 and S2 Tables).
(TIF)
The maximum intraspecific distances (◆) and minimum interspecific distances (□) based on rbcL marker between Desmodesmus genus species are shown. The dataset was composed of reference barcode sequences reported this genus (S3 Table).
(TIF)
nuITS2 should be primarily sequenced and submitted to similarity searches against GenBank. Similarity values obtained must be compatible with the barcode gap thresholds calculated using reference sequences for the taxon indicated (a). The absence of CBCs between the query nuITS2 sequence and its closest match retrieved from similarity search is necessary to confirm species diagnosis (b). Finally, the current status of the assigned species name must be checked using a reference database (e.g.: AlgaeBase) (c). If nuITS2 is not sufficient for a species diagnosis, other markers/methods should be tried (d).
(TIF)
(DOCX)
(DOCX)
(DOCX)
Acknowledgments
The authors are grateful to the Empresa Brasileira de Pesquisa Agropecuária (EMBRAPA), Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES), Financiadora de Estudos e Projetos (FINEP) and Conselho Nacional de Pesquisa (CNPq) for supporting this work. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
The authors are grateful to the Empresa Brasileira de Pesquisa Agropecuária (EMBRAPA), Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES), Financiadora de Estudos e Projetos (FINEP) and Conselho Nacional de Pesquisa (CNPq) for supporting this work. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Hebert PD, Cywinska A, Ball SL, deWaard JR. Biological identifications through DNA barcodes. Proceedings Biological sciences / The Royal Society. 2003;270(1512):313–21. 10.1098/rspb.2002.2218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hebert PD, Ratnasingham S, deWaard JR. Barcoding animal life: cytochrome c oxidase subunit 1 divergences among closely related species. Proceedings Biological sciences / The Royal Society. 2003;270 Suppl 1:S96–9. 10.1098/rsbl.2003.0025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ratnasingham S, Hebert PD. bold: The Barcode of Life Data System (http://www.barcodinglife.org). Molecular ecology notes. 2007;7(3):355–64. 10.1111/j.1471-8286.2007.01678.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hollingsworth PM, Forrest LL, Spouge JL, Hajibabaei M, Ratnasingham S, van der Bank M, et al. A DNA barcode for land plants. Proceedings of the National Academy of Sciences of the United States of America. 2009;106(31):12794–7. 10.1073/pnas.0905845106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Leray M, Knowlton N. DNA barcoding and metabarcoding of standardized samples reveal patterns of marine benthic diversity. Proceedings of the National Academy of Sciences of the United States of America. 2015;112(7):2076–81. 10.1073/pnas.1424997112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Francis RA. Wall ecology: A frontier for urban biodiversity and ecological engineering. Prog Phys Geogr. 2011;35(1):43–63. [Google Scholar]
- 7.Collins RA, Armstrong KF, Meier R, Yi Y, Brown SDJ, Cruickshank RH, et al. Barcoding and border biosecurity: identifying cyprinid fishes in the aquarium trade. PloS one. 2012;7(1):e28381 10.1371/journal.pone.0028381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Palhares RM, Drummond MG, Brasil BDAF, Cosenza GP, Brandao MDL, Oliveira G. Medicinal plants recommended by the World Health Organization: DNA Barcode identification associated with chemical analyses guarantees their quality. PloS one. 2015;10(5). doi: UNSP e0127866 10.1371/journal.pone.0127866 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Palhares RM, Drummond MG, Brasil BS, Krettli AU, Oliveira GC, Brandão MG. The use of an integrated molecular-, chemical-and biological-based approach for promoting the better use and conservation of medicinal species: A case study of Brazilian quinas. J Ethnopharmacol. 2014;155(1):815–22. 10.1016/j.jep.2014.06.040 [DOI] [PubMed] [Google Scholar]
- 10.Carvalho DC, Neto DA, Brasil BS, Oliveira DA. DNA barcoding unveils a high rate of mislabeling in a commercial freshwater catfish from Brazil. Mitochondrial DNA. 2011;22(sup1):97–105. [DOI] [PubMed] [Google Scholar]
- 11.Carvalho DC, Palhares RM, Drummond MG, Frigo TB. DNA Barcoding identification of commercialized seafood in South Brazil: A governmental regulatory forensic program. Food Control. 2015;50:784–8. [Google Scholar]
- 12.Drummond MG, Brasil BSAF, Dalsecco LS, Brasil RSAF, Teixeira LV, Oliveira DAA. A versatile real-time PCR method to quantify bovine contamination in buffalo products. Food Control. 2013;29(1):131–7. [Google Scholar]
- 13.Hall JD, Fucikova K, Lo C, Lewis LA, Karol KG. An assessment of proposed DNA barcodes in freshwater green algae. Cryptogam, Algol. 2010;31(4):529–55. [Google Scholar]
- 14.Bock C, Krienitz L, Proeschold T. Taxonomic reassessment of the genus Chlorella (Trebouxiophyceae) using molecular signatures (barcodes), including description of seven new species. Fottea. 2011;11(2):293–312. [Google Scholar]
- 15.Costa ES, Plastino EM, Petti R, Oliveira EC, Oliveira MC. The Gracilariaceae Germplasm Bank of the University of São Paulo, Brazil—a DNA barcoding approach. J Appl Phycol. 2012;24(6):1643–53. [Google Scholar]
- 16.Pawlowski J, Holzmann M. A plea for DNA barcoding of Foraminifera. J Foraminiferal Res. 2014;44(1):62–7. [Google Scholar]
- 17.Hebert PDN, Stoeckle MY, Zemlak TS, Francis CM. Identification of birds through DNA barcodes. PLoS Biol. 2004;2:1657–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Becker B, Marin B. Streptophyte algae and the origin of embryophytes. Ann Bot. 2009;103(7):999–1004. 10.1093/aob/mcp044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Guiry MD. How many species of algae are there? J Phycol. 2012;48(5):1057–63. [DOI] [PubMed] [Google Scholar]
- 20.Torres MA, Barros MP, Campos SCG, Pinto E, Rajamani S, Sayre RT, et al. Biochemical biomarkers in algae and marine pollution: a review. Ecotoxicol Environ Saf. 2008;71(1):1–15. 10.1016/j.ecoenv.2008.05.009 [DOI] [PubMed] [Google Scholar]
- 21.Amengual-Morro C, Niell GM, Martínez-Taberner A. Phytoplankton as bioindicator for waste stabilization ponds. J Environ Manage. 2012;95:S71–S6. 10.1016/j.jenvman.2011.07.008 [DOI] [PubMed] [Google Scholar]
- 22.Subhadra B, Grinson-George. Algal biorefinery‐based industry: an approach to address fuel and food insecurity for a carbon‐smart world. J Sci Food Agric. 2011;91(1):2–13. 10.1002/jsfa.4207 [DOI] [PubMed] [Google Scholar]
- 23.Nascimento IA, Marques SSI, Cabanelas ITD, Pereira SA, Druzian JI, de Souza CO, et al. Screening microalgae strains for biodiesel production: lipid productivity and estimation of fuel quality based on fatty acids profiles as selective criteria. Bioenergy Res. 2013;6(1):1–13. [Google Scholar]
- 24.Clerck O, Guiry MD, Leliaert F, Samyn Y, Verbruggen H. Algal taxonomy: a road to nowhere? J Phycol. 2013;49(2):215–25. [DOI] [PubMed] [Google Scholar]
- 25.Škaloud P. Variation and taxonomic significance of some morphological features in European strains of Klebsormidium (Klebsormidiophyceae, Streptophyta). Nova Hedwigia. 2006;83(3–4):533–50. [Google Scholar]
- 26.Krienitz L, Bock C. Present state of the systematics of planktonic coccoid green algae of inland waters. Phytoplankton responses to human impacts at different scales: Springer; 2012. p. 295–326. [Google Scholar]
- 27.Krienitz L, Huss VA, Bock C. Chlorella: 125 years of the green survivalist. Trends in plant science. 2015;20(2):67–9. 10.1016/j.tplants.2014.11.005 . [DOI] [PubMed] [Google Scholar]
- 28.Buchheim MA, Keller A, Koetschan C, Forster F, Merget B, Wolf M. Internal transcribed spacer 2 (nu ITS2 rRNA) sequence-structure phylogenetics: towards an automated reconstruction of the green algal tree of life. PloS one. 2011;6(2):e16931 10.1371/journal.pone.0016931 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pawlowski J, Audic S, Adl S, Bass D, Belbahri L, Berney C, et al. CBOL protist working group: barcoding eukaryotic richness beyond the animal, plant, and fungal kingdoms. PLoS biology. 2012;10(11):e1001419 10.1371/journal.pbio.1001419 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Caisová L, Marin B, Melkonian M. A close-up view on ITS2 evolution and speciation—a case study in the Ulvophyceae (Chlorophyta, Viridiplantae). BMC evolutionary biology. 2011;11:262 10.1186/1471-2148-11-262 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Du G, Wu F, Mao Y, Guo S, Xue H, Bi G. DNA barcoding assessment of green macroalgae in coastal zone around Qingdao, China. J Ocean Univ China. 2014;13(1):97–103. [Google Scholar]
- 32.Leliaert F, Verbruggen H, Vanormelingen P, Steen F, López-Bautista JM, Zuccarello GC, et al. DNA-based species delimitation in algae. Eur J Phycol. 2014;49(2):179–96. [Google Scholar]
- 33.Pombert J-F, Otis C, Lemieux C, Turmel M. The chloroplast genome sequence of the green alga Pseudendoclonium akinetum (Ulvophyceae) reveals unusual structural features and new insights into the branching order of chlorophyte lineages. Mol Biol Evol. 2005;22(9):1903–18. [DOI] [PubMed] [Google Scholar]
- 34.Hegewald E, Bock C, Krienitz L. A phylogenetic study on Scenedesmaceae with the description of a new species of Pectinodesmus and the new genera Verrucodesmus and Chodatodesmus (Chlorophyta, Chlorophyceae). Fottea. 2013;13(2):14. [Google Scholar]
- 35.White TJ, Bruns T, Lee SJWT, Taylor JW. Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics: Academic Press; 1990. 315–22 p. [Google Scholar]
- 36.Koetschan C, Förster F, Keller A, Schleicher T, Ruderisch B, Schwarz R, et al. The ITS2 Database III—sequences and structures for phylogeny. Nucleic acids research. 2010;38(suppl 1):D275–D9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pröschold T, Bock C, Luo W, Krienitz L. Polyphyletic distribution of bristle formation in Chlorellaceae: Micractinium, Diacanthos, Didymogenes and Hegewaldia gen. nov.(Trebouxiophyceae, Chlorophyta). Phycological Res. 2010;58(1):1–8. [Google Scholar]
- 38.Darienko T, Gustavs L, Mudimu O, Menendez CR, Schumann R, Karsten U, et al. Chloroidium, a common terrestrial coccoid green alga previously assigned to Chlorella (Trebouxiophyceae, Chlorophyta). Eur J Phycol. 2010;45(1):79–95. [Google Scholar]
- 39.Luo W, Proschold T, Bock C, Krienitz L. Generic concept in Chlorella-related coccoid green algae (Chlorophyta, Trebouxiophyceae). Plant biology. 2010;12(3):545–53. 10.1111/j.1438-8677.2009.00221.x . [DOI] [PubMed] [Google Scholar]
- 40.Pröschold T, Darienko T, Silva PC, Reisser W, Krienitz L. The systematics of Zoochlorella revisited employing an integrative approach. Environ Microbiol. 2011;13(2):350–64. 10.1111/j.1462-2920.2010.02333.x [DOI] [PubMed] [Google Scholar]
- 41.Krienitz L, Hegewald EH, Hepperle D, Huss VAR, Rohr T, Wolf M. Phylogenetic relationship of Chlorella and Parachlorella gen. nov.(Chlorophyta, Trebouxiophyceae). Phycologia. 2004;43(5):529–42. [Google Scholar]
- 42.Pöschold T, Marin B, Schlosser UG, Melkonian M. Molecular phylogeny and taxonomic revision of Chlamydomonas (Chlorophyta). I. Emendation of Chlamydomonas Ehrenberg and Chloromonas Gobi, and description of Oogamochlamys gen. nov. and Lobochlamys gen. nov. Protist. 2001;152(4):265–300. 10.1078/1434-4610-00068 . [DOI] [PubMed] [Google Scholar]
- 43.Agostinho AA, Thomaz SM, Gomes LC. Conservation of the biodiversity of Brazil's inland waters. Conserv Biol. 2005;19(3):646–52. [Google Scholar]
- 44.Schlösser U. Additions to the culture collection of algae since 1994. Bot Acta. 1997;110(5):424–9. [Google Scholar]
- 45.Bonato ALV, do Valle CB, Jank L, Resende RMS, Leguizamon GOdC. Extração de DNA genômico de Brachiaria e Panicum maximum. Embrapa Gado de Corte Comunicado técnico. 2002.
- 46.Kearse M, Moir R, Wilson A, Stones-Havas S, Cheung M, Sturrock S, et al. Geneious Basic: an integrated and extendable desktop software platform for the organization and analysis of sequence data. Bioinformatics. 2012;28(12):1647–9. 10.1093/bioinformatics/bts199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Thompson JD, Higgins DG, Gibson TJ. CLUSTAL W: improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position-specific gap penalties and weight matrix choice. Nucleic acids research. 1994;22(22):4673–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Tamura K, Peterson D, Peterson N, Stecher G, Nei M, Kumar S. MEGA5: molecular evolutionary genetics analysis using maximum likelihood, evolutionary distance, and maximum parsimony methods. Mol Biol Evol. 2011;28(10):2731–9. 10.1093/molbev/msr121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Bengtsson-Palme J, Ryberg M, Hartmann M, Branco S, Wang Z, Godhe A, et al. Improved software detection and extraction of ITS1 and ITS2 from ribosomal ITS sequences of fungi and other eukaryotes for analysis of environmental sequencing data. Methods Ecol Evol. 2013;4(10):914–9. 10.1111/2041-210X.12073 [DOI] [Google Scholar]
- 50.Koetschan C, Hackl T, Müller T, Wolf M, Förster F, Schultz J. ITS2 database IV: interactive taxon sampling for internal transcribed spacer 2 based phylogenies. Mol Phylogenet Evol. 2012;63(3):585–8. 10.1016/j.ympev.2012.01.026 [DOI] [PubMed] [Google Scholar]
- 51.Seibel PN, Müller T, Dandekar T, Schultz J, Wolf M. 4SALE–a tool for synchronous RNA sequence and secondary structure alignment and editing. BMC bioinformatics. 2006;7(1):498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Seibel PN, Müller T, Dandekar T, Wolf M. Synchronous visual analysis and editing of RNA sequence and secondary structure alignments using 4SALE. BMC research notes. 2008;1(1):91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Fawley MW, Fawley KP, Hegewald E. Taxonomy of Desmodesmus serratus (Chlorophyceae, Chlorophyta) and related taxa on the basis of morphological and DNA sequence data. Phycologia. 2011;50(1):23–56. 10.2216/10-16.1 [DOI] [Google Scholar]
- 54.Gorelova OA, Baulina OI, Solovchenko AE, Chekanov KA, Chivkunova OB, Fedorenko TA, et al. Similarity and diversity of the Desmodesmus spp. microalgae isolated from associations with White Sea invertebrates. Protoplasma. 2015;252(2):489–503. 10.1007/s00709-014-0694-0 . [DOI] [PubMed] [Google Scholar]
- 55.Bock C, Pröschold T, Krienitz L. Two new Dictyosphaerium-morphotype lineages of the Chlorellaceae (Trebouxiophyceae): Heynigia gen. nov. and Hindakia gen. nov. European Journal of Phycology. 2010;45(3):267–77. [Google Scholar]
- 56.Bellinger EG, Sigee DC. Freshwater Algae: Identification and Use as Bioindicators: Wiley; 2015. [Google Scholar]
- 57.AlgaeBase [Internet]. Galway: National University of Ireland. 2015 [cited 16 december 2015]. Available from: http://www.algaebase.org.
- 58.Timmins M, Thomas-Hall SR, Darling A, Zhang E, Hankamer B, Marx UC, et al. Phylogenetic and molecular analysis of hydrogen-producing green algae. Journal of experimental botany. 2009;60(6):1691–702. 10.1093/jxb/erp052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Hoshina R. DNA analyses of a private collection of microbial green algae contribute to a better understanding of microbial diversity. BMC research notes. 2014;7:592 10.1186/1756-0500-7-592 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Loughnane CJ, McIvor LM, Rindi F, Stengel DB, Guiry MD. Morphology, rbc L phylogeny and distribution of distromatic Ulva (Ulvophyceae, Chlorophyta) in Ireland and southern Britain. Phycologia. 2008;47(4):416–29. [Google Scholar]
- 61.Freitas LC, Loverde-Oliveira SM. Checklist of green algae (Chlorophyta) for the state of Mato Grosso, Central Brazil. Check List. 2013;9:1471–83. [Google Scholar]
- 62.Nozaki H, Krienitz L. Morphology and phylogeny of Eudorina minodii (Chodat) Nozaki et Krienitz, comb. nov.(Volvocales, Chlorophyta) from Germany. Eur J Phycol. 2001;36(1):23–8. [Google Scholar]
- 63.Hoef-Emden K. Pitfalls of establishing DNA barcoding systems in protists: the cryptophyceae as a test case. PloS one. 2012;7(8):e43652 10.1371/journal.pone.0043652 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Collins RA, Cruickshank RH. The seven deadly sins of DNA barcoding. Molecular ecology resources. 2013;13(6):969–75. 10.1111/1755-0998.12046 . [DOI] [PubMed] [Google Scholar]
- 65.Wolf M, Chen S, Song J, Ankenbrand M, Muller T. Compensatory base changes in ITS2 secondary structures correlate with the biological species concept despite intragenomic variability in ITS2 sequences—a proof of concept. PloS one. 2013;8(6):e66726 10.1371/journal.pone.0066726 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Coleman AW. Is there a molecular key to the level of “biological species” in eukaryotes? A DNA guide. Mol Phylogenet Evol. 2009;50(1):197–203. 10.1016/j.ympev.2008.10.008 [DOI] [PubMed] [Google Scholar]
- 67.Müller T, Philippi N, Dandekar T, Schultz J, Wolf M. Distinguishing species. RNA. 2007;13(9):1469–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Map of Brazilian biomes, including the Amazon tropical rainforest (1), the Caatinga xeric shrublands (2), the Cerrado tropical Savanna (3), the Pantanal flooded grassland (4), the Mata Atlântica tropical rainforest (5) and the Pampa subtropical grassland (6). The geographic coordinates of the six distinct locations sampled and the respective isolated strains in each site are shown. The strains isolated were deposited in the Collection of Microorganisms and Microalgae Applied to Agroenergy and Biorefineries at Embrapa (Brasília/DF–Brazil). The Brazilian territory is highlighted in black in the map of the neotropical region (inset).
(TIF)
The maximum intraspecific distances (◆) and minimum interspecific distances (□) based on nuITS1 marker between Chlorella (A) and Desmodesmus (B) genera species are shown. The dataset was composed of reference barcode sequences reported for each genera (S1 and S2 Tables).
(TIF)
The maximum intraspecific distances (◆) and minimum interspecific distances (□) based on nuITS2 marker between Chlorella (A) and Desmodesmus (B) genera species are shown. The dataset was composed of reference barcode sequences reported for each genera (S1 and S2 Tables).
(TIF)
The maximum intraspecific distances (◆) and minimum interspecific distances (□) based on rbcL marker between Desmodesmus genus species are shown. The dataset was composed of reference barcode sequences reported this genus (S3 Table).
(TIF)
nuITS2 should be primarily sequenced and submitted to similarity searches against GenBank. Similarity values obtained must be compatible with the barcode gap thresholds calculated using reference sequences for the taxon indicated (a). The absence of CBCs between the query nuITS2 sequence and its closest match retrieved from similarity search is necessary to confirm species diagnosis (b). Finally, the current status of the assigned species name must be checked using a reference database (e.g.: AlgaeBase) (c). If nuITS2 is not sufficient for a species diagnosis, other markers/methods should be tried (d).
(TIF)
(DOCX)
(DOCX)
(DOCX)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.