Abstract
Blindness is a unique model for understanding the role of experience in the development of the brain's functional and anatomical architecture. Documenting changes in the structure of anatomical networks for this population would substantiate the notion that the brain's core network-level organization may undergo neuroplasticity as a result of life-long experience. To examine this issue, we compared whole-brain networks of regional cortical-thickness covariance in early blind and matched sighted individuals. This covariance is thought to reflect signatures of integration between systems involved in similar perceptual/cognitive functions. Using graph-theoretic metrics, we identified a unique mode of anatomical reorganization in the blind that differed from that found for sighted. This was seen in that network partition structures derived from subgroups of blind were more similar to each other than they were to partitions derived from sighted. Notably, after deriving network partitions, we found that language and visual regions tended to reside within separate modules in sighted but showed a pattern of merging into shared modules in the blind. Our study demonstrates that early visual deprivation triggers a systematic large-scale reorganization of whole-brain cortical-thickness networks, suggesting changes in how occipital regions interface with other functional networks in the congenitally blind.
Highlights
-
•
We examined cortical-thickness covariance networks in blind and sighted.
-
•
The two groups showed different partition structures.
-
•
In blind, occipital and lateral–temporal regions merged within modules.
1. Introduction
Blindness is associated with changes to both the functional and anatomical organization of the brain (e.g., Noppeney, 2007, Voss and Zatorre, 2012b). Most strikingly, occipital regions are involved in and have been causally linked to different non-visual processes in the blind (Amedi et al., 2004, Cohen et al., 1997, Collignon et al., 2007), and there are some suggestions that their recruitment is linked to enhanced abilities in the remaining senses (Amedi et al., 2003, Gougoux et al., 2005). Anatomically, early blindness is accompanied by atrophy of gray matter volume and increased cortical thickness in occipital cortex (Bridge et al., 2009, Jiang et al., 2009, Park et al., 2009, Qin et al., 2013, Voss and Zatorre, 2012a) that may also be related to non-visual behaviors in blind individuals (Voss et al., 2014, Voss and Zatorre, 2012a). In addition, blindness also impacts thalamic subregions involved in visual processing (Cecchetti et al., 2015, Ptito et al., 2008), the shape and volume of corpus callosum (Tomaiuolo et al., 2014), and hippocampal volume (Fortin et al., 2008).
It is still poorly understood whether the specialization for non-visual information in the blind's occipital cortex reflects mostly localized changes, or whether blindness induces a larger-scale reorganization associated with a different mode of global information exchange at the whole-brain level. More specifically, is the occipital cortex re-programmed without affecting the large-scale organization of brain networks, or does re-programming occur at the level of whole-brain networks inducing occipital regions to cluster differently with other regions? An influential view on brain organization suggests that the development of domain selectivity in occipital regions, as well as superior parietal, parahippocampal, and several other brain areas, is independent of visual experience (Dormal and Collignon, 2011, Mahon and Caramazza, 2011, Reich et al., 2012; see Ricciardi et al., 2014 for recent review). On this view, the maintained functional selectivity in occipital regions in early blind would arise from a pre-existing (possibly innate) set of neural connections, which are similar for blind and sighted individuals (e.g., Hannagan et al., 2015, Mahon and Caramazza, 2011, Reich et al., 2011). For example, several studies have documented a maintained pattern of resting-state functional connectivity between functionally specific occipital regions (e.g. the visual word form area, the numerical form area, the parahippocampal place area) across blind and sighted individuals (Abboud et al., 2015, He et al., 2013, Reich et al., 2011).
In addition, several functional neuroimaging studies have pointed to a large-scale reorganization of extended brain networks. Schepers et al. (2012) showed that processing auditory inputs in blind produces stronger neural synchronization between auditory and visual cortices in the gamma band and suggested that “the deprived visual cortex is integrated into a larger network related to its new function” (for similar conclusions, see Collignon et al., 2011, Collignon et al., 2013, Klinge et al., 2010, Wittenberg et al., 2004). Also, resting-state neuroimaging studies in the blind have revealed stronger connectivity between occipital and frontal or parietal regions (e.g., Deen et al., 2015; see Bock and Fine, 2014 for a recent review). In summary, the extent to which early-acquired blindness may induce large-scale reorganization of brain networks remains controversial.
Our goal here was to determine whether there is large-scale, network-level reorganization of anatomical features in the blind. Voss and Zatorre (2015) showed that anatomical covariance between a specific seed region in occipital cortex and a region in the superior frontal gyrus differs for blind and sighted, but no network-level study has examined the two populations. When evaluated from a graph-theoretic perspective, regional covariation patterns in cortical thickness show a modular network organization (e.g., Chen et al., 2008), and areas within these modules tend to be associated with similar behavioral or cognitive function. Moreover, correlations between distant cortical regions are thought to be signatures of functional integration between different systems (Alexander-Bloch et al., 2013a, Alexander-Bloch et al., 2013b). Therefore, features of these structural networks are taken to reflect the brain's core capacity for information transmission across cortical regions, and the structure of these networks has been shown to differ between clinical and non-clinical populations (for review, see Alexander-Bloch et al., 2013b).
Interestingly, prior work (Chen et al., 2008) has shown that in structural networks of sighted, frontal, lateral–temporal, and occipital regions tend to cluster within separate cliques (“modules” in graph-theoretic parlance). This makes structural networks an interesting target for study in congenitally blind since auditory and language functions have notably been mapped inside occipital structures in this population (e.g., Bedny et al., 2011, Collignon et al., 2011). This raises the general possibility that blindness would be associated with some sort of “multisensory merging” between sensory systems that would be manifested in a weakening of the structural separation between occipital and lateral–temporal regions.
Importantly, blindness has a unique status as a model system for examining the role of experience in anatomical development and functional activity. For this reason, documenting changes in network structure in this population would convincingly substantiate the notion that the brain's core network-level structural organization may undergo neuroplasticity as result of life-long experience.
2. Methods
2.1. Participants
The blind participant group consisted of 18 congenitally blind (7 female, mean age: 44.1 ± 13.7; 11 male, mean age: 42.45 ± 12.44) and the sighted control group (N = 18) matched the CB group on age and gender distribution (7 female controls, mean age: 45.6 ± 14.55; 11 male controls, mean age: 40.0 ± 6.9). Additional characteristics of the blind group are provided in Supplementary Table 1. All procedures involving human participants were approved by the research ethic and scientific boards of the Centre for Interdisciplinary Research in Rehabilitation of Greater Montreal and the Quebec Bio-Imaging Network.
2.2. Acquisition and preprocessing pipeline for structural images
Structural data used in this study were collected in the functional neuroimaging unit (UNF) of the University of Montreal, Canada. Images were obtained using a 3T TRIO TIM system (Siemens, Erlangen, Germany) equipped with a 12-channel head coil. Data were acquired using a T1-weighted 3D magnetization prepared rapid acquisition gradient echo sequence (MPRAGE) with the parameters: voxel size 1 × 1 × 1.2 mm3; matrix size 240 × 256; TR 2300 ms; TE 2.91 ms; TI 900 ms; FoV 256; 160 slices.
Processing of structural data was performed using FreeSurfer version v5.3.0 (Massachusetts General Hospital, Harvard Medical School). The preprocessing pipeline (Dale et al., 1999, Fischl et al., 1999) consisted of non-brain tissue removal, Talairach transformation, white matter and gray matter segmentation, intensity normalization, topology correction, surface inflation, atlas registration, and parcellation of the cerebral cortex according to the Destriux atlas (74 regions per hemisphere, Destrieux et al., 2010). Each of these automatically executed steps was followed by quality control assessments implemented jointly by H. A. and U. H. Interventions in this quality control step consisted of 1) replacing low-quality structural scans of 4 participants with higher quality alternate scans for the same participants; 2) manual Talairach alignment (n = 2); 3) manual adjustment of the skull stripping procedure to assure that dura matter or meninges were not falsely recognized as grey matter or white matter (n = 4); 4) correction for missed labeling of white matter (n = 15); and 5) use of control points to correct the intensity normalization of white matter (n = 5).
2.3. Analysis of cortical thickness at regional level
To evaluate our data against prior results, we conducted a region-based univariate analysis of cortical thickness. For each of the 148 regions automatically parcelated by FreeSurfer, we contrasted the mean cortical thickness of the blind and sighted groups using unpaired T-tests, and controlling for multiple comparisons using FDR correction.
2.4. Partial least squares analysis for group-linked CT covariance
To evaluate whether there is a network-level covariance pattern that discriminates the two groups, we used a partial least squares regression (PLSR) approach (Krishnan et al., 2011, McIntosh and Lobaugh, 2004). In the current implementation, the to-be-predicted variable was the [36 participants × 1] vector coding the participant's group identifier (0.5 for sighted, − 0.5 for blind), and the explanatory data were the [36 participants × 148 regions] matrix. We implemented PLSR using the PLS package in the statistical software R (Mevik and Wehrens, 2007), with 2 components analyzed based on an initial evaluation of prediction accuracy profiles as estimated by the RMSEP parameter in a Leave One Out classification scheme. The workflow for evaluating statistical significance followed the one detailed in Krishnan et al. (2011). Specifically, as in previous studies, we used permutations to evaluate whether the fit between participant's scores and the predicted variable exceeded what would be expected by chance. For this purpose, a sampling distribution was constructed from 500 permutations, with each permutation randomly assigning the group labels to participants. To identify regions whose brain scores were systematic across implementations of the PLSR algorithm, we used a bootstrapping procedure that was run 100 times (see McIntosh et al., 1996). In each instance, we bootstrapped, with replacement, rows from the original [36 × 148] CT-value matrix, to populate a proxy [36 × 148] matrix. For each proxy matrix, PLSR was run, and the loadings for the estimated 2 components were retrieved. This loading matrix was rotated to match the direction of the loadings in the original data via a Procrustes Rotation, and the Y-loadings (Brain loadings) for the first component saved. Finally, we calculated the standard deviation of the 100 bootstrap loadings, per region, and then obtained a Z-score per region [region loading/sd(loading)]. Only regions that passed a Z-score of ± 2.5 were considered significantly “salient.”
2.5. Regression approach to assess bivariate correlations between region-pairs
Our regression approach followed that described by Lerch et al. (2006), and included partialling out the effect of age, followed by FDR correction for multiple comparisons (Benjamini & Hochberg, 1995). For each pair of brain regions (of the 148 in our parcellation), we determined whether the relationship between cortical thickness (CT) in any two given regions (CT1, CT2) differed for blind or sighted. This was evaluated via the following regression model applied to each region-pair:
This approach is based on identifying brain regions for which the regression slope differs between groups, and therefore the parameter of interest was the [CT2 × Group] interaction term (see Lerch et al., 2006). Because regression is non-symmetric, the number of regressions conducted was (1482 − 148 = 21,756). We used robust regression to minimize the impact of outliers and corrected the family-wise false-positive error rate for multiple comparisons using FDR correction applied to the p-values computed for this interaction term.
2.6. Structural networks from cortical-thickness values: construction and validation against random networks
Each participant's cortical surface was automatically segmented into 148 anatomical regions of interest (74 per hemisphere) using FreeSurfer's automated methods. From the mean CT in each region, we constructed pair-wise correlation matrices at the group level by correlating across participants the CT value in each region against those in all other regions using Pearsons's product–moment correlation coefficient (R).
We treated these as adjacency matrices and constructed binarized matrices at six density thresholds: 10%, 20%, 30%, 40%, 50%, and 60%, as it may be that certain network-level differences maintain only at certain density levels (e.g., Garrison et al., 2015). Density here refers to the percentage of strongest connections maintained out of the complete connectivity matrix (e.g., a 10% density threshold would maintain 1088 connections in a 148-node network [(1482 − 148)/2 * 10%]. We analyzed the binarized connectivity matrices using a community detection algorithm based on maximizing the modularity of the resulting partition. Modularity is a graph-theoretic measure that can be used to characterize the quality of a network partition. Its value increases as network elements partition into clusters (“modules,” or “communities”) that are more densely connected within rather than between them. Here, we used a fast-unfolding community detection algorithm (Blondel et al., 2008) based on modularity optimization to determine partitions of the blind and sighted group-level network. As we describe below, some of our analyses were conducted on network partitions at all six densities, whereas, in further analyses, we focus on the blind and sighted networks at the 10% density alone, as this was the threshold that demonstrated the strongest discrimination between the groups.
The partitioning algorithm returns an index reflecting the modularity of the network (Blondel et al., 2008). The resulting values were compared to ones obtained for random networks matched for node degree distributions. Since the modularity optimization algorithm is non-deterministic in that it can produce different partitions for the same binarized matrix, we performed 100 modularity solutions for each group (both real and random) and kept the maximum Q value for each. This test was performed for 6 different edge-density thresholds (10%–60% in 10% intervals) to evaluate if the modularity of the structural networks exceeded that of matched-degree random ones.
2.7. Determining within- and between-group partition similarity
To determine whether the structure of community partitions statistically differed between blind and sighted, we conducted a permutation-based procedure. The principle of the analysis was to determine the similarity of network partitions found for equal subgroups (N = 9) within each population and compare this value to the similarity of partitions found for equal-sized subgroups between the two populations.
Each permutation was conducted as follows. Within each participant group (sighted, blind), we i) split participants into two randomly selected subgroups, ii) constructed the 148-region covariance matrix, iii) thresholded the matrix at an edge density of 10%, and iv) derived the optimal partitioning structure using the methods described above (specifically, we found the partition with max-Q from the binarized matrix out of a set of 100 solutions of the partitioning algorithm for the current binarized matrix).
The within-population partition similarity (e.g., 9 sighted vs. 9 sighted; 9 blind vs. 9 blind) and the between-population partition similarity (9 sighted vs. 9 blind) were assessed using Normalized Mutual Information (NMI), a measure with values between 0 and 1, where higher values indicate more similar partition structures (Danon et al., 2005). One hundred permutations were conducted, establishing three distributions: NMI values for within-population partitions in sighted, NMI values for within-population partitions in blind, and NMI values for between-populations comparisons. This entire procedure was also conducted for the other edge densities (20, 30, 40, 50, and 60%).
2.8. Operationalizing language-related and visual-related regions
After obtaining whole-brain partitions of regions by their cortical-thickness covariance, we more closely examined the organization of brain areas typically implicated in language- and vision-related functions (see rational in introduction). To use an objective rather than subjective criterion, we used a meta-analysis method of quantitative reverse inference, implemented via the online tool Neurosynth (Yarkoni et al., 2011). This tool enables probabilistic conclusions, based on a large scale of neuroimaging studies (more than 10,000 at time of use). We used it to obtain “reverse inference” maps through a judicious method for establishing which regions tend to be associated with a particular function. Rather than showing which regions are disproportionally reported by studies where a certain term is dominant (forward inference; P(activation | term)), this method identifies regions whose report in a neuroimaging study is diagnostic of a certain term being dominant in the study (reverse inference; P(term | activation)). This specific implementation of reverse inference, rather than one based on subjective impression, indicates which regions are selectively involved in a particular process, and reflects a principled use of prior data for arriving at labels. As such, the two functional sets of regions we define reflect voxels whose activation in neuroimaging studies was highly diagnostic of the study reporting results related to language comprehension (“language comprehension” query) or visual processing (“visual” query). This method has been used to draw interesting functional conclusions on language (e.g., Skipper, 2014). We also note that in the implementation, we considered only voxels where the probability of reverse inference was p < .01 (FDR corrected for entire brain) and we used these maps to categorize FreeSurfer regions within these two sets of functional maps.
3. Results
3.1. Replication and extension of prior univariate and bivariate findings
3.1.1. Regional results
Prior to conducting the network analysis, we conducted several analyses on the regional level to evaluate whether the current cohort replicated prior findings reported for blind. Our data replicated essential findings (Park et al., 2009) of increased CT in blind occipital regions (lingual gyrus) and decreased CT in motor regions (right post-central gyrus and central sulcus bilaterally). Apart from these, few other regions showed significant differences (p < .05, uncorrected for multiple comparisons). Increased CT for blind was found in the pericallosal sulcus bilaterally, the right orbital sulcus, and right subparietal sulcus, whereas reduced CT was found in the right planum polare. (Supplementary Fig. 1 shows all statistically significant regions presented on the cortical surface.) We also conducted a partial least squares analysis, which in some cases offers a more sensitive multivariate test of brain structure differences across populations. This analysis revealed a set of regions (identified by a statistically significant latent factor, p = .038, based on 500 permutations, see Methods) that subsumed the regions identified by the univariate analysis but also contained more extensive sections of the ACC (see Supplementary Fig. 2). In conclusion, our analyses replicated an extended prior work on cortical-thickness differences between sighted and blind.
As another indicant of data quality, we evaluated whether we could identify negative correlations between cortical thickness and age, as classically observed in the literature (e.g., Thambisetty et al., 2010). Twenty-five of the 148 regions showed statistically significant negative correlations with age, another 107 showed non-significant negative correlations, and 11 showed non-significant positive correlations.
3.1.2. Results of bivariate regression
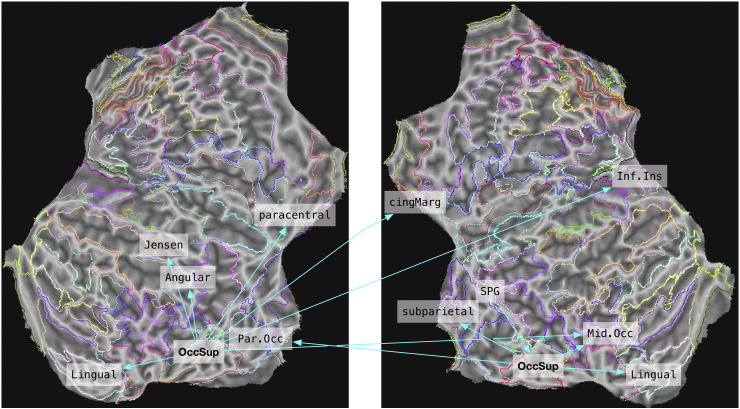
Here we evaluated differences in covariance among all pairs of brain regions (1482 − 148 = 21,756). After FDR correction, there were 90 cases where correlation between regional covariance was moderated by Group (i.e., different slopes for blind and sighted, see Methods). Within these 90-region pairs, most of the regions appeared once, but several appeared repeatedly. The regions most frequently implicated were the right superior parietal lobule (12 connections), left superior occipital gyrus (8 connections), right superior occipital gyrus (7 connections), right circular sulcus of insula (7 connections), and right posterior lateral fissure (7 connections). Fig. 1 shows the regions whose connectivity with the left and right superior occipital gyri differed for blind and sighted. As shown in the figure, these involved almost exclusively occipital and parietal regions. In a follow-up analysis, we compared the regression coefficients between these regions after deriving them separately for each group (and after partialling out the effect of age). In all but one case, regression slopes were higher for sighted than blind. The exception was the inferior insula for which sighted showed a negative slope and blind a positive one. An analysis of correlations, after partialling out age, revealed a similar pattern: correlations tended to be positive and significant for sighted, but not statistically significant for blind (we report regression coefficients and correlation values in Supplementary Table 2).
Fig. 1.
Structural correlations of superior occipital cortex. The figure shows regions whose structural correlations with the superior occipital gyri differed significantly between groups (family-wise error corrected using FDR). Differences were identified by a [Group × CT] interaction term within a multiple regression model (see Text). “Jensen”—intermediate sulcus of Jensen; “Par.Occ”—parieto occipital sulcus. In all cases apart from the connection to the inferior insula, correlations were stronger for sighted than for blind. Arrow directions indicate the predicting (origin) and predicted variable (target) in the regression model.
3.2. Different network arrangements in blind and sighted
We first evaluated whether there is a difference in network partition structure between the blind and sighted cohorts. Here a partition refers to an optimized way that regions cluster together as subsets, or “modules” (Blondel et al., 2008). We found that within-group partition similarity (for both sighted and blind) was substantially higher than between-group partition similarity. The NMI means were as follows: blind within population [M = .35 (± 0.06)], sighted within population [M = .33 (± 0.07)], between-populations similarity [M = .23 (± 0.04)]. Thus, for both groups, partitions showed greater similarity when constructed from participants within the same group.
The resulting distributions of partition similarity for the 10% density threshold are shown in Fig. 2. The differences in distributions were tested using Kolmogorov–Smirnov tests and were statistically significant (NMI blind vs. between: D = 0.7796, p < 2.2 × 10–16; sighted vs. between: D = 0.6832, p < 2.2 × 10–16).
Fig. 2.
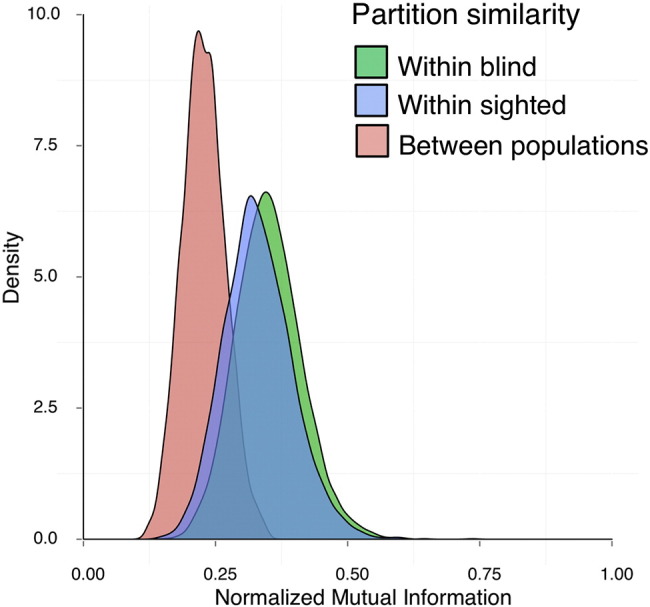
Distributions of partition similarity. Distributions of partition similarity when the partitions were generated from equally sized subgroups within the sighted population (blue), blind population (green), or between the two populations (pink). Partition structure was more similar within the sighted and blind populations than between the two populations.
Note that the separation of both within-group distributions from the between-group distribution is not a mathematical necessity (in Fig. 2: separation of pink distribution from both green and blue distributions). Sighted participants' partitions could have shown more similarity to those of other sighted, but blind participants' partitions could have been equally similar to those of blind or sighted, indicative of a loss of coherent arrangement in blindness. Instead, the current data pattern indicates a different partitioning arrangement within blind and sighted.
Network partitions can differ substantially depending on the edge density (percentage of strongest connections) chosen to construct the networks (e.g., Garrison et al., 2015). The NMI results reported above (Fig. 2) were found for 10% density (at this threshold, the minimum correlation value for blind was 0.76, and for sighted, 0.73). To verify that this finding is not an artifact of the threshold chosen, we repeated the permutation procedure for other densities. (For these thresholds, the minimum correlation values for blind and sighted were 20% {0.71, 0.67}, 30% {0.67, 0.63}, 40% {0.62, 0.59}, 50% {0.60, 0.55}, 60% {0.56, 0.51}). In all cases, we found the same pattern, with stronger within-group than between-group similarity. In addition, the within-group NMI distributions for the 10% density were associated with the highest means (for both blind and sighted), suggesting that this density level best captured partition features common to participants within each group. For this reason, in the subsequent analyses we focused on this edge density level.
3.3. Spatial features of network partitions in blind and sighted
3.3.1. Spatial structure of networks partitions
The community detection algorithm (Blondel et al., 2008) identifies an optimal partition of a covariance matrix into separate modules. We applied this algorithm to the networks derived from each group's (N = 18) full covariance matrix (see Supplementary Fig. 3 for raw correlation matrices). Both groups' network partitions were more modular than randomly wired networks matched for node degree distribution (at all edge densities, Kolmogorov–Smirnov tests, p < .001).
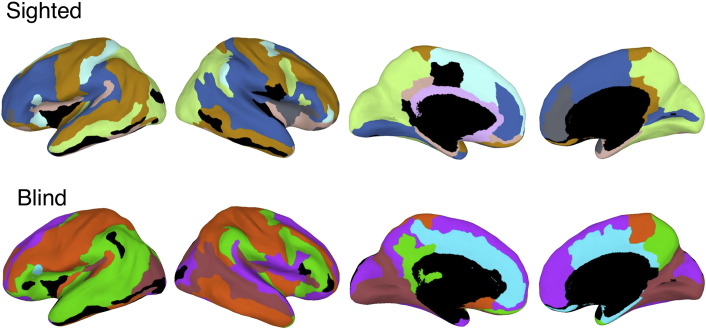
Fig. 3 shows the modular organization of blind and sighted network partitions, with each module marked by a separate color. For sighted, our results matched the partition structure reported by Chen et al. (2008). Specifically, Chen et al. reported a “language-related” module consisting of the lateral–temporal cortices, inferior frontal gyri (IFG), and supramarginal gyri (SMG), all bilaterally, as well as the right lingual gyrus and left lateral-occipitotemporal gyrus. Our partitioning revealed a highly similar arrangement (blue module in Fig. 3). This replication is notable given that our current procedure used a more detailed parcellation than Chen et al.'s (74 vs. 26 regions per hemisphere), which can strongly impact the structure of modules. For instance, in Chen et al., the cingulate gyrus was a single region, but in our parcellation, it was partitioned into 4 subregions.
Fig. 3.
Modular organization of structural networks in sighted and blind. Partition structure was obtained using a community detection algorithm that maximizes the modularity of the partition (see Methods). The algorithm assigns regions to modules, and in the figure, regions within each module are color coded via a shared unique color. Regions in black are regions not assigned to any module (singletons). The median wall was not included in the analysis.
For the sighted, of the 24 perisylvian anatomical regions broadly associated with language functions (SMG, PT, PP, TTS, TTG, STG, STS, MTG, IFGop, IFGtr, IFGor, posterior Sylvian fissure, bilaterally), only six were outside this module. Similarly, we found a strong homogenous organization for vision-related regions. All 15 anatomical regions corresponding to the dorsal and ventral visual streams, bilaterally, resided within a single module. The other modules consisted of combinations of fronto-parietal regions, and one module was made of orbitofrontal and adjacent insular regions.
For the blind, we found a different network organization, most evident in the module-membership of regions linked to language and visual processing. The first difference was that the left and right lateral–temporal cortices did not reside within the same module. In addition, in both hemispheres, these temporal regions were within modules that also contained regions from dorsal or ventral visual streams (green, purple, and brown clusters). Examining the organization of visual regions, we documented a fragmentation of the dorsal and ventral visual streams evident in that subregions within those were associated with several different modules. Finally, the purple module that enclosed visual regions (roughly area V2) also included frontal, parietal, and temporal regions. Another module (brown) covered regions corresponding to V3 and V4 in the blind and was connected to right lateral–temporal regions (STS).
3.3.2. Within- and cross-sensory connections in blind and sighted
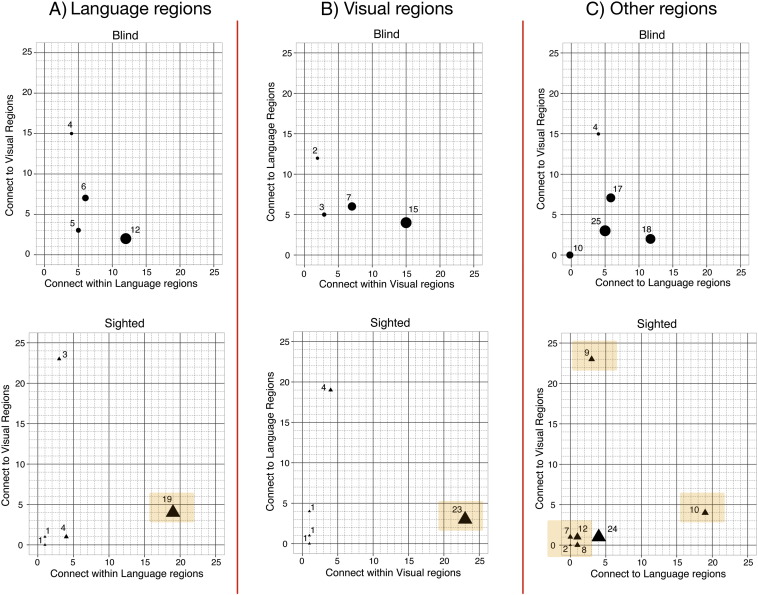
The assignment of regions to modules suggests that in the blind, there is a stronger integration, in terms of clustering, between language and visual regions. To better understand this issue, after obtaining the optimal partitions for sighted and blind networks (i.e., the most optimal assignment of regions to modules), we quantified i) the number of within-module connections that each language region had to other language or visual regions, and similarly, ii) the number of within-module connections that each visual region had to other language or visual regions. (These regions were operationalized via a meta-analysis tool, Neurosynth, that uses quantitative reverse inference to link regional activity to cognitive function; see Methods and Supplementary Fig. 4 for statistical brain maps). This produced multivariate distributions reflecting, for each region, its number of within-module connections to language or visual regions. The relative frequency of these combinations, across regions, was the parameter of interest.
As shown in Fig. 4A, language regions were more strongly interconnected in sighted than in blind, i.e., each was connected more extensively to other language regions. Similarly, visual regions (Fig. 4B) were more strongly interconnected in sighted than in blind. Notably, in the sighted, there was one module in which 19 language regions were interconnected and another module in which 23 visual regions were interconnected. In the blind, the respective values were 12 and 15. These data point to the existence of modules, for sighted, where visual or language regions tended to strongly cluster with other regions related to the same modality. In contrast, these modules tend to merge in the blind group. Interestingly, for the sighted, we also identified 3 “language” regions whose within-module connections tended to be visual regions (each was connected to 24 visual regions) and we also identified 4 “visual” regions that were mainly connected to language regions. The former consisted of the left TTG, left MTG, and the right inferior precentral sulcus. The latter consisted of the lingual gyrus bilaterally, right calcarine sulcus, and right post-central sulcus.
Fig. 4.
Within-module connectivity of language regions, visual regions, and all other regions in sighted and blind. The three panels plot multivariate distributions showing within-module connectivity of language and visual regions. Panel A: connectivity of language regions to language and visual regions in blind (upper row) and sighted (lower row). Panel B: connectivity of visual regions to language and visual regions in blind and sighted. Panel C: connectivity of non-language and non-visual regions (“other”) to language and visual regions in blind and sighted. Orange highlights: in sighted, there were larger cluster of interconnected language regions (panel A), as well as interconnected visual regions (panel B). In addition, in sighted, among the “other” regions (panel C), there were large clusters of regions predominantly connected to either language or visual regions, as well as a large cluster of regions (next to the origin) that were not connected to either language or visual regions. All these were reduced in blind.
For completeness, we repeated the same analysis, but for all remaining anatomical regions—that is, for those not included in the language and visual sets (Fig. 4C). As in the prior analysis, we counted for each region the number of its connections to language- and visual-related regions within its module. Here, too, we found signatures of different arrangement for blind and sighted. For sighted, there were many entries toward the lower-right and upper-left quadrants of the plot (Ns = 10, 9) reflecting those 19 regions that were connected within their modules to either a large number of language regions, or visual regions, but not both. In contrast, for the blind, there were no entries in these quadrants. A complementary finding evident in Fig. 4C for this set of regions is that for the sighted there were ~ 30 regions with very little connectivity to either language or visual regions (these are entries in the lower left quadrant with values ≤ 1 on both the x and y axes). For the blind, however, there were only 10 such regions. In short, for these “other” regions, in the sighted, we documented more instances of regions that were strongly connected only within a sensory modality, as well as more instances of regions that did not track covariance of either modality.
4. Discussion
Understanding whether blindness-related neuroplasticity is manifested in large-scale, network-level brain reorganization is an important but unresolved issue. With few exceptions (Voss and Zatorre, 2015), extant studies of anatomical changes in the blind, using cortical-thickness or voxel-based morphometry, have used univariate approaches. These have documented strong atrophy in occipital cortex volume, which is accompanied by increased thickness (Bridge et al., 2009, Jiang et al., 2009, Park et al., 2009, Qin et al., 2013, Voss and Zatorre, 2012a, Voss and Zatorre, 2012b), as well as changes in specific subcortical regions and corpus callosum (Tomaiuolo et al., 2014, Ptito et al., 2008, Fortin et al., 2008, Cecchetti et al., 2015). Our findings, which were based on network-level analyses, are the first to show that blindness induces a systematic large-scale reorganization of whole-brain cortical-thickness networks, producing a unique structural architecture. This was most evident in our analysis of similarity of network partitions within vs. between groups, which showed that network partition structures derived from subgroups of blind were more similar to each other than they were to partitions derived from sighted, and the converse was also the case (Fig. 2).
Beyond simply documenting topological differences in the form of partition structure, we also examined the within-module connectivity structure of regions associated with language and visual functions (as defined by a meta-analysis tool). These regions mostly segregate in sighted individuals (Chen et al., 2008) but showed a tendency to “merge” within structural partitions in blind individuals. Furthermore, using a pair-wise regional covariance analysis, we documented numerous instances of brain regions whose covariance differed for blind and sighted, including the superior occipital gyrus (bilaterally) and the right superior parietal lobule. In what follows, we first address the implications of the results for current understanding on the determinants of the topology of structural networks, and then we discuss implications for theories of neuroplasticity in the blind.
4.1. Evidence for experience-dependent network-level neuroplasticity
A review of cortical-thickness covariance networks (Alexander-Bloch et al., 2013b) underlines two main findings pertinent to understanding the current results. First, it has been shown that areas with strong cortical-thickness covariance tend to be associated with similar behavioral or cognitive function. Second, though nearby brain areas tend to be more strongly correlated, long-distance correlations also exist, and these latter connections are thought to be signatures of integration between different systems (Chen et al., 2008).
One issue that has remained unclear in interpreting CT covariance networks is to what extent does this structural covariance reflect coordinated neurodevelopment due (in part) to functional co-activation, or a shared genetic influence that impacts diverse phenotypic traits (Alexander-Bloch et al., 2013b, Evans, 2013a, Evans, 2013b). There have been numerous demonstrations that life-long practice can affect morphometric features of specific brain regions (for review, see May, 2011) and that particular skills may be associated with changes in covariance between particular region-pairs (Evans, 2013a, Evans, 2013b). Yet, the impact of experience on the network-level organization of structural networks is poorly understood. Some studies documented a relation between brain disorders including schizophrenia and autism and structural covariance, which is consistent with the impact of experience, but may also be related to genetic factors (for review, see Alexander-Bloch et al., 2013b). Given that there is no reason to suppose that our blind population had a fundamentally different genetic organization that impacts the brain's structural network, and given our findings that speak to a “merging” of the sensory systems from a between- to within-module organization, our results suggest that these changes result from a life-long alteration in sensory experience. As such, they strongly suggest that life-long experience can affect the cortical-thickness covariance architecture at the whole-brain level (see Kim et al., 2014 for similar conclusions in deafness). Our results thus support the notion raised in prior works that structural network organization can be an index of functional organization.
4.2. Implications for current network-level interpretations of neuroplasticity in blind
To our knowledge, our study is the first to document whole-brain level reorganization of cortical-thickness networks in the blind. Voss and Zatorre (2015) found that pair-wise CT correlations between a single occipital seed region of interest and superior frontal cortex differed for blind and sighted, but they did not examine whole-brain network-level properties. The bulk of network-related work in blind is based on studies of resting state correlations (RSC), as well as few studies of network features of white-matter connectivity. Much of this work has been concisely reviewed by Bock and Fine (2014) and we therefore focus, in this section, only on those studies that bear directly on the current findings.
One clear pattern that emerges from studies of RSC in blind is that blind and sighted broadly show similar patterns of RSC within visual cortices. Butt et al. (2013) documented this pattern and found that the RSC patterns of any given blind participant were as similar to those of the sighted group as they were to the blind group, suggesting no unique reorganization in the blind. However, between-hemisphere RSC was reduced in blind. In addition, this study, like others (e.g., Deen et al., 2015), revealed stronger RSC between left IFG and striate cortex in the blind. Striem-Amit et al. (2015) similarly reported that RSC of visual cortex in blind manifests typical “retinotopic” organization principles found in sighted (differentiation by eccentricity, laterality, and elevation), with no evidence for differences between the two populations. Yet, even within the visual system, there are some differences in RSC patterns. First, RSC between V1, V2, and V3 shows different hierarchical and homotopic RSC in blind vs. sighted (Butt et al., 2015). Second, subtle features of RSC, such as the regional homogeneity of the correlation magnitude (a measure of spatial autocorrelation) within visual cortex do differ between blind and sighted (Jiang et al., 2015). To summarize, there appears to be high similarity in the organization of RSC within occipital regions of blind and sighted, which is consistent with the notion that central aspects of its organization are independent of visual experience. However, there are also some organizational differences, particularly in RSC between hemispheres, or between different levels of the visual hierarchy. Our findings for occipital regions and, more generally, for the visual processing streams are consistent with prior work indicating changes in network-level organization of these regions. First, the modularity analysis indicated that in the sighted, occipital and parietal regions within the dorsal stream were in the same module, whereas in the blind, they were distributed across three separate modules. In addition, the bivariate analyses of functional connectivity strength, evaluated via regression and follow-up correlation-based analyses, indicated that the blind show significantly weaker correlations between the superior occipital cortex and a set of mainly occipital and parietal regions. While (bivariate) functional connectivity measures and modularity analyses target different organizational features, the weaker occipito-parietal correlations we find for the blind are consistent with the modularity findings.
Other studies have reported whole-brain examinations of RSC in blind. Burton et al. (2014) carried out pair-wise analyses of RSC between 62 manually defined regions of interest covering main nodes in well-known resting state networks. They found that, if anything, RSC connectivity patterns between visual and auditory regions were stronger in sighted (a repeatedly demonstrated pattern, see Bock and Fine, 2014 for review). In addition, RSC between visual and somatosensory regions tended to be positive for sighted but negative for blind. Finally, RSC between visual cortex and frontal and parietal regions associated with cognitive control was higher in blind (but this pattern was complicated as in almost all cases the blind showed negative correlations, and the sighted showed stronger negative correlations). Striem-Amit et al. (2015) also reported reduced RSC between V1 and somatosensory and auditory regions, but stronger connectivity with the left IFG (for similar results, see Deen et al., 2015). Wang et al. (2014) partitioned whole-brain RSC patterns to networks via independent component analysis and showed reduced connectivity between visual and sensorimotor networks in blind.
The aforementioned demonstrations of reduced RSC between visual and auditory systems in the blind appear, prima facie, to contrast with the large amount of studies reporting robust task-dependent activations of occipital regions during the processing of non-visual inputs as well as with the possibility that these sensory systems are more tightly coupled in blind (e.g., Collignon et al., 2013, Klinge et al., 2010, Schepers et al., 2012). They may also appear incompatible with our specific finding showing that visual and language regions tend to more frequently share structural modules in blind. We suggest that this is an apparent inconsistency that originates from the presupposition that resting state networks are good proxies for networks instantiated during cognitive/perceptual processing. Recent work, however, has shown that whole-brain functional connectivity networks can fundamentally change in different task contexts and are not constrained by resting-state topologies. This holds particularly true for connectivity structures of sensory systems (e.g., Andric and Hasson, 2015, Di et al., 2013, Mennes et al., 2013, Moussa et al., 2011, Wen et al., 2015). Furthermore, as we mentioned in the Introduction, the early blind show stronger neural synchronization between auditory and visual cortices during auditory processing (Schepers et al., 2012), and during an auditory spatial task, they show stronger connectivity between dorsal visual regions and areas typically involved in spatial attention (Collignon et al., 2011). Finally, during tactile motion discrimination, the blind show stronger connectivity between area hMT + and somatosensory regions (Sani et al., 2010), a pattern that is opposite to that found for visual and sensorimotor regions in RSC of blind and sighted (Burton et al., 2014).
To summarize, the findings from task-invoked connectivity analyses show that the blind often show stronger connectivity between sensory regions (for review see Ricciardi et al., 2014), which does not coincide with the resting-state data. Instead, these patterns are consistent with our findings of a less strict separation between language- and vision-related regions in the blind. Finally, some studies suggest that there may only be moderate similarity between the structure of RSC networks and CT-covariance networks. Using a between-network similarity measure, Hosseini and Kesler (2013) documented a moderate degree of convergence in structure, independent of the density at which the networks were thresholded. The same work also found weak agreement in the location of “hub” regions in these networks. In other work (Alexander-Bloch, Raznahan, Bullmore, & Giedd, 2013), the correlation between structural and RSC connectivity matrices was moderate (around 0.3).
Apart from RSC studies, there has also been some related work on white matter (WM) connectivity in the blind. In general, blindness is thought to be associated with reduced WM integrity, including a reduction in integrity of occipitotemporal connections (Bock and Fine, 2014, but see Shimony et al., 2006). However, little is known of WM connectivity in the blind. Shu et al. (2009) examined network-level features of WM connectivity using a 90-node network. The authors reported differences in several network features: the blind showed increased path length, reduced global efficiency, and decreased node degree. They also found that most changes to network features were found in occipital regions of the blind, which was interpreted in terms of reduced occipital connectivity with other brain regions. A main difference between WM and CT connectivity analyses is that a WM connectivity network can be calculated for each participant (with a connection defined between any two regions that share a certain number of fiber connections), so the information-carrying variance pertains to differences in connections between regions. In contrast, for CT networks, the variance is based on inter-individual variance. These two sources of variance can load on different latent factors, and there is some work (Gong et al., 2012) showing that WM connections do not account for the majority of cross-regional CT covariance.
4.3. Relation to cross-modal plasticity in blind
Cross-modal plasticity has been repeatedly documented in the blind, in that visual cortices are demonstrably involved in a wide range of non-visual tasks (see, e.g., Frasnelli et al., 2011, for review). Our finding of reduced segregation of auditory and visual regions in the blind is consistent with prior work showing signatures of auditory, lexical, and semantic processing in the blind's visual cortex (e.g., Amedi et al., 2003, Bedny et al., 2011, Roder et al., 1999, Weeks et al., 2000). As we highlighted in the Introduction, there is no debate that cross-modal plasticity is not an epiphenomenal reorganization subsequent to sensory deprivation but instead reflects functional recruitment of occipital networks by non-visual perceptual/cognitive operations (Amedi et al., 2004, Cohen et al., 1997, Collignon et al., 2007). Yet, one of the main current debates concerns to what extent this reorganization reflects, i) a limited cross-modal re-programming of visual cortices in the blind (perhaps relying on “hardwired” domain-general capacities of the region) without affecting the large-scale organization of brain networks, or (perhaps additionally) ii) a re-programming that occurs at the scale of whole-brain networks so that occipital cortex changes its clustering with other regions. Speaking to these issues, three of our findings speak more strongly to the second possibility. First, overall topological organization (partition structure) differed between blind and sighted, such that CT covariance networks were more similar within a population than between populations (Fig. 2). Second, sighted individuals showed a more pronounced demonstration of connections that were contained within a sensory modality (Fig. 4A, B). Third, perhaps the strongest support was seen in the connectivity patterns of regions not typically implicated in either visual or language processing (“other regions,” Fig. 4C): for sighted, these showed a stronger tendency to connect to either language- or visual-related regions. Conjointly, for sighted, a larger subset of these regions was not connected to either language or visual regions.
These findings point to a general pattern of network-level structural reorganization that we term “multisensory merging” which reflects a relative weakening of sensory-systems separation in the blind (we emphasize this is relative, since even in sighted there is evidence for direct cortico-cortical connections, including between primary sensory cortices, e.g., Falchier et al., 2002, Klinge et al., 2010, Rockland and Ojima, 2003). Because it has been shown that auditory competence in the blind is related to structural features of occipital cortex (Voss and Zatorre, 2012a), such changes support a functional reorganization interpretation rather than one based simply on lack of sensory innervation. It is notable that for the blind, we found changes in connection profiles of areas associated with language processing, even though these regions do not appear to differ in CT between blind and sighted. In fact, anatomical changes in the preserved auditory cortex are seldom reported (for null results, see, Anurova et al., 2015, Jiang et al., 2009, Modi et al., 2012, Voss and Zatorre, 2012a, Voss and Zatorre, 2012b, but see Park et al., 2009, for an exception). Thus, the impact of deprivation on auditory/language regions may be in their connectivity profile rather than local morphometry per se.
One prominent view in the field of cross-modal plasticity in the blind suggests “functional constancy” – the idea that, in the blind, occipital regions maintain their functional preference, but apply a preserved computation to different modalities (for reviews see Dormal and Collignon, 2011, Ricciardi et al., 2014). Indeed, the study of neural processing in the blind has been instrumental in showing that the brain contains numerous regions that process abstract stimulus features, independent of the modality via which input is presented (see Ricciardi et al., 2014 for extensive review). Our findings elaborate on this position: on the local (regional) scale, certain functionalities might be maintained across blind and sighted, completely consistent with meso-scale supramodal organization. However, at the macro-scale (where functions may map onto network organizations; Anderson, 2010, Pessoa, 2014, Poldrack, 2006), there might be organizational changes. Our data are therefore also consistent with the view that occipital areas can take on new inputs and/or functions as a consequence of blindness (e.g., Bedny et al., 2015). Language processing in the occipital cortex of blind individuals has been treated as a paradigmatic example indicating that regions typically considered “visual” can dramatically switch function to process linguistic inputs (e.g., Bedny et al., 2011). Our observation that language and visual regions tend to arrange in separate modules in sighted but show a pattern of merging into shared modules in the blind seems to support this idea. We note, however, that several studies have shown that, even in sighted individuals, certain occipital regions are linked in sophisticated ways to auditory and speech processing. For instance, during spoken sentence comprehension, the lingual gyrus shows repetition suppression to syntactically simple sentences but not more complex ones (Hasson et al., 2006), it tracks statistical regularity of auditory series (Tobia et al., 2012), and tracks lexical frequency and other speech properties during auditory naturalistic language comprehension (Boldt et al., 2013, Brennan et al., 2012). It is therefore an interesting and open question whether sophisticated language functions in occipital cortex that are found in the blind (Lane et al., 2015) reflect completely novel functional developments or an enhancement of existing capacities already observable, even if to a much lower extent, in the sighted.
4.4. Limitations and potential future developments
We obtained our findings with group sizes that are typical for study of the blind but relatively small for between-group studies. It may be that including more individuals would increase the precision and improve the power of such connectivity analyses. Relatedly, we tracked the within-module organization of relatively granular sets of regions (related to language comprehension and visual processing as operationalized via a meta-analysis), as this was the first study of organization of cortical-thickness networks in the blind. This, admittedly, sacrificed some level of resolution, and it would be interesting in future work to examine the organization of specific sensory subsystems, perhaps based on functional localizers. Future studies could also determine whether this large-scale reorganization of brain networks relates to perceptual functions in the blind and whether the age of blindness onset impacts such structural covariance networks. Finally, our main interest here was in assessing similarity in partition structures and the organization of different regions within modules. Related measures for examining networks (which are less concerned with spatial organization per se) focus on abstract topological features of these networks, which are related to their capacity to transfer information. Given our findings, it may be that such features change as well in meaningful ways, but we note that interpreting such topological metrics in relation to functional reorganization is quite complex (e.g., see Supplementary analysis, Nodal features, in Supplementary Materials).
4.5. Summary
In conclusion, we demonstrate for the first time that early visual deprivation triggers a unique structural organization of cortical-thickness networks at the whole-brain level. Since cortical-thickness covariance is thought to reflect signatures of integration between different systems involved in similar perceptual/cognitive functions (Alexander-Bloch et al., 2013b, Chen et al., 2008, Evans, 2013a, Evans, 2013b), our results suggest that congenital blindness strongly affects how occipital regions interact with other functional networks. This study therefore impacts on those current theories of brain organization suggesting that the preserved domain specialization observed in the blind's occipital regions is established on a basis of similar connectomic architectures in blind and sighted (e.g., Hannagan et al., 2015, Mahon and Caramazza, 2011). Our observations rather suggest that early blindness is accompanied by a reorganization of the partition structure of anatomical networks, which is one of the core global features of such networks, notably inducing some occipital regions to more strongly covary with regions typically involved in language processing (consistent with prior functional imaging work, e.g., Bedny et al., 2011).
Acknowledgment
This project was supported by European Research Council Starting Grants: ERC-STG #263318 NeuroInt to U.H., and ERC-STG #337573 MADVIS to O.C, the Fond de Recherches en Santé du Québec, and the Canadian Institutes of Health Research. The data were collected while O.C was a post-doctoral fellow with Dr. Franco Lepore, and we thank Dr. Lepore for allowing us to use these data. We also thank Giulia Dormal, Latifa Lazzouni, Maxime Pelland, and the UNF (Unité de Neuroimagerie Fonctionelle) team of the Research Center at the Geriatric Institute of the University of Montreal for their invaluable help in collecting these data, and the Institut Nazareth et Louis Braille of Montreal for their help in recruiting the blind participants.
Footnotes
Supplementary data to this article can be found online at http://dx.doi.org/10.1016/j.neuroimage.2015.12.048.
Appendix A. Supplementary data
Supplementary data
References
- Abboud S., Maidenbaum S., Dehaene S., Amedi A. A number-form area in the blind. Nat. Commun. 2015;6:6026. doi: 10.1038/ncomms7026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander-Bloch A., Raznahan A., Bullmore E., Giedd J. The convergence of maturational change and structural covariance in human cortical networks. J. Neurosci. 2013;33(7):2889–2899. doi: 10.1523/JNEUROSCI.3554-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander-Bloch A., Giedd J.N., Bullmore E. Imaging structural co-variance between human brain regions. Nat. Rev. Neurosci. 2013;14(5):322–336. doi: 10.1038/nrn3465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amedi A., Raz N., Pianka P., Malach R., Zohary E. Early ‘visual’ cortex activation correlates with superior verbal memory performance in the blind. Nat. Neurosci. 2003;6(7):758–766. doi: 10.1038/nn1072. [DOI] [PubMed] [Google Scholar]
- Amedi A., Floel A., Knecht S., Zohary E., Cohen L.G. Transcranial magnetic stimulation of the occipital pole interferes with verbal processing in blind subjects. Nat. Neurosci. 2004;7(11):1266–1270. doi: 10.1038/nn1328. [DOI] [PubMed] [Google Scholar]
- Anderson M.L. Neural reuse: a fundamental organizational principle of the brain. Behav. Brain Sci. 2010;33(4):245–266. doi: 10.1017/S0140525X10000853. (discussion 266-313) [DOI] [PubMed] [Google Scholar]
- Andric M., Hasson U. Global features of functional brain networks change with contextual disorder. NeuroImage. 2015;117:103–113. doi: 10.1016/j.neuroimage.2015.05.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anurova I., Renier L.A., De Volder A.G., Carlson S., Rauschecker J.P. Relationship Between Cortical Thickness and Functional Activation in the Early Blind. Cereb Cortex. 2015;25(8):2035–2048. doi: 10.1093/cercor/bhu009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bedny M., Pascual-Leone A., Dodell-Feder D., Fedorenko E., Saxe R. Language processing in the occipital cortex of congenitally blind adults. Proc. Natl. Acad. Sci. U. S. A. 2011;108(11):4429–4434. doi: 10.1073/pnas.1014818108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bedny M., Richardson H., Saxe R. “Visual” cortex responds to spoken language in blind children. J. Neurosci. 2015;35(33):11674–11681. doi: 10.1523/JNEUROSCI.0634-15.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benjamini Y., Hochberg Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing (PDF) J. R. Stat. Soc. Series B. 1995;57(1):289–300. [Google Scholar]
- Blondel V.D., Guillaume J.-L., Lambiotte R., Lefebvre E. Fast unfolding of communities in large networks. J. Stat. Mech: Theory Exp. 2008;10:10008. [Google Scholar]
- Bock A.S., Fine I. Anatomical and functional plasticity in early blind individuals and the mixture of experts architecture. Front. Hum. Neurosci. 2014;8:971. doi: 10.3389/fnhum.2014.00971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boldt R., Malinen S., Seppä M., Tikka P., Savolainen P., Hari R., Carlson S. Listening to an audio drama activates two processing networks, one for all sounds, another exclusively for speech. PLoS One. 2013;8(5):e64489. doi: 10.1371/journal.pone.0064489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brennan J., Nir Y., Hasson U., Malach R., Heeger D.J., Pylkkanen L. Syntactic structure building in the anterior temporal lobe during natural story listening. Brain Lang. 2012;120(2):163–173. doi: 10.1016/j.bandl.2010.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bridge H., Cowey A., Ragge N., Watkins K. Imaging studies in congenital anophthalmia reveal preservation of brain architecture in 'visual' cortex. Brain. 2009;132(Pt 12):3467–3480. doi: 10.1093/brain/awp279. [DOI] [PubMed] [Google Scholar]
- Burton H., Snyder A.Z., Raichle M.E. Resting state functional connectivity in early blind humans. Front. Syst. Neurosci. 2014;8:51. doi: 10.3389/fnsys.2014.00051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butt O.H., Benson N.C., Datta R., Aguirre G.K. The fine-scale functional correlation of striate cortex in sighted and blind people. J. Neurosci. 2013;33(41):16209–16219. doi: 10.1523/JNEUROSCI.0363-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butt O.H., Benson N.C., Datta R., Aguirre G.K. Hierarchical and homotopic correlations of spontaneous neural activity within the visual cortex of the sighted and blind. Front. Hum. Neurosci. 2015;9:25. doi: 10.3389/fnhum.2015.00025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cecchetti L., Ricciardi E., Handjaras G., Kupers R., Ptito M., Pietrini P. Congenital blindness affects diencephalic but not mesencephalic structures in the human brain. Brain Struct. Funct. 2015 doi: 10.1007/s00429-014-0984-5. [DOI] [PubMed] [Google Scholar]
- Chen Z.J., He Y., Rosa-Neto P., Germann J., Evans A.C. Revealing modular architecture of human brain structural networks by using cortical thickness from MRI. Cereb. Cortex. 2008;18(10):2374–2381. doi: 10.1093/cercor/bhn003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen L.G., Celnik P., Pascual-Leone A., Corwell B., Falz L., Dambrosia J.…Hallett M. Functional relevance of cross-modal plasticity in blind humans. Nature. 1997;389(6647):180–183. doi: 10.1038/38278. [DOI] [PubMed] [Google Scholar]
- Collignon O., Lassonde M., Lepore F., Bastien D., Veraart C. Functional cerebral reorganization for auditory spatial processing and auditory substitution of vision in early blind subjects. Cereb. Cortex. 2007;17(2):457–465. doi: 10.1093/cercor/bhj162. [DOI] [PubMed] [Google Scholar]
- Collignon O., Vandewalle G., Voss P., Albouy G., Charbonneau G., Lassonde M., Lepore F. Functional specialization for auditory-spatial processing in the occipital cortex of congenitally blind humans. Proc. Natl. Acad. Sci. U. S. A. 2011;108(11):4435–4440. doi: 10.1073/pnas.1013928108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collignon O., Dormal G., Albouy G., Vandewalle G., Voss P., Phillips C., Lepore F. Impact of blindness onset on the functional organization and the connectivity of the occipital cortex. Brain. 2013;136(Pt 9):2769–2783. doi: 10.1093/brain/awt176. [DOI] [PubMed] [Google Scholar]
- Dale A.M., Fischl B., Sereno M.I. Cortical surface-based analysis. I. Segmentation and surface reconstruction. NeuroImage. 1999;9(2):179–194. doi: 10.1006/nimg.1998.0395. [DOI] [PubMed] [Google Scholar]
- Danon L., Díaz-Guilera A., Duch J., Arenas A. Comparing community structure identification. J. Stat. Mech: Theory Exp. 2005;2005(09):P09008. [Google Scholar]
- Deen B., Saxe R., Bedny M. Occipital cortex of blind individuals is functionally coupled with executive control areas of frontal cortex. J. Cogn. Neurosci. 2015;27(8):1633–1647. doi: 10.1162/jocn_a_00807. [DOI] [PubMed] [Google Scholar]
- Destrieux C., Fischl B., Dale A., Halgren E. Automatic parcellation of human cortical gyri and sulci using standard anatomical nomenclature. NeuroImage. 2010;53(1):1–15. doi: 10.1016/j.neuroimage.2010.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di X., Gohel S., Kim E.H., Biswal B.B. Task vs. rest-different network configurations between the coactivation and the resting-state brain networks. Front. Hum. Neurosci. 2013;7:493. doi: 10.3389/fnhum.2013.00493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dormal G., Collignon O. Functional selectivity in sensory-deprived cortices. J. Neurophysiol. 2011;105(6):2627–2630. doi: 10.1152/jn.00109.2011. [DOI] [PubMed] [Google Scholar]
- Evans A.C. Networks of anatomical covariance. NeuroImage. 2013;80:489–504. doi: 10.1016/j.neuroimage.2013.05.054. [DOI] [PubMed] [Google Scholar]
- Evans A.C. Networks of anatomical covariance. NeuroImage. 2013;80:489–504. doi: 10.1016/j.neuroimage.2013.05.054. [DOI] [PubMed] [Google Scholar]
- Falchier A., Clavagnier S., Barone P., Kennedy H. Anatomical evidence of multimodal integration in primate striate cortex. J. Neurosci. 2002;22(13):5749–5759. doi: 10.1523/JNEUROSCI.22-13-05749.2002. (doi: 20026562) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischl B., Sereno M.I., Dale A.M. Cortical surface-based analysis. II: inflation, flattening, and a surface-based coordinate system. NeuroImage. 1999;9(2):195–207. doi: 10.1006/nimg.1998.0396. [DOI] [PubMed] [Google Scholar]
- Fortin M., Voss P., Lord C., Lassonde M., Pruessner J., Saint-Amour D.…Lepore F. Wayfinding in the blind: larger hippocampal volume and supranormal spatial navigation. Brain. 2008;131(Pt 11):2995–3005. doi: 10.1093/brain/awn250. [DOI] [PubMed] [Google Scholar]
- Frasnelli J., Collignon O., Voss P., Lepore F. Crossmodal plasticity in sensory loss. Prog. Brain Res. 2011;191:233–249. doi: 10.1016/B978-0-444-53752-2.00002-3. [DOI] [PubMed] [Google Scholar]
- Garrison K.A., Scheinost D., Finn E.S., Shen X., Constable R.T. The (in)stability of functional brain network measures across thresholds. NeuroImage. 2015;118:651–661. doi: 10.1016/j.neuroimage.2015.05.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gong G., He Y., Chen Z.J., Evans A.C. Convergence and divergence of thickness correlations with diffusion connections across the human cerebral cortex. NeuroImage. 2012;59(2):1239–1248. doi: 10.1016/j.neuroimage.2011.08.017. [DOI] [PubMed] [Google Scholar]
- Gougoux F., Zatorre R.J., Lassonde M., Voss P., Lepore F. A functional neuroimaging study of sound localization: visual cortex activity predicts performance in early-blind individuals. PLoS Biol. 2005;3(2) doi: 10.1371/journal.pbio.0030027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hannagan T., Amedi A., Cohen L., Dehaene-Lambertz G., Dehaene S. Origins of the specialization for letters and numbers in ventral occipitotemporal cortex. Trends Cogn. Sci. 2015;19(7):374–382. doi: 10.1016/j.tics.2015.05.006. [DOI] [PubMed] [Google Scholar]
- Hasson U., Nusbaum H.C., Small S.L. Repetition suppression for spoken sentences and the effect of task demands. J. Cogn. Neurosci. 2006;18(12):2013–2029. doi: 10.1162/jocn.2006.18.12.2013. [DOI] [PubMed] [Google Scholar]
- He C., Peelen M.V., Han Z., Lin N., Caramazza A., Bi Y. Selectivity for large nonmanipulable objects in scene-selective visual cortex does not require visual experience. NeuroImage. 2013;79:1–9. doi: 10.1016/j.neuroimage.2013.04.051. [DOI] [PubMed] [Google Scholar]
- Hosseini S.M., Kesler S.R. Comparing connectivity pattern and small-world organization between structural correlation and resting-state networks in healthy adults. NeuroImage. 2013;78:402–414. doi: 10.1016/j.neuroimage.2013.04.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang J., Zhu W., Shi F., Liu Y., Li J., Qin W.…Jiang T. Thick visual cortex in the early blind. J. Neurosci. 2009;29(7):2205–2211. doi: 10.1523/JNEUROSCI.5451-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang A., Tian J., Li R., Liu Y., Jiang T., Qin W., Yu C. Alterations of regional spontaneous brain activity and gray matter volume in the Blind. Neural Plast. 2015 doi: 10.1155/2015/141950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim E., Kang H., Lee H. Morphological brain network assessed using graph theory and network filtration in deaf adults. Hear. Res. 2014;315:88–98. doi: 10.1016/j.heares.2014.06.007. [DOI] [PubMed] [Google Scholar]
- Klinge C., Eippert F., Roder B., Buchel C. Corticocortical connections mediate primary visual cortex responses to auditory stimulation in the blind. J. Neurosci. 2010;30(38):12798–12805. doi: 10.1523/JNEUROSCI.2384-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krishnan A., Williams L.J., McIntosh A.R., Abdi H. Partial least squares (PLS) methods for neuroimaging: a tutorial and review. NeuroImage. 2011;56(2):455–475. doi: 10.1016/j.neuroimage.2010.07.034. [DOI] [PubMed] [Google Scholar]
- Lane C., Kanjlia S., Omaki A., Bedny M. “Visual” cortex of congenitally blind adults responds to syntactic movement. J. Neurosci. 2015;35(37):12859–12868. doi: 10.1523/JNEUROSCI.1256-15.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lerch J.P., Worsley K., Shaw W.P., Greenstein D.K., Lenroot R.K., Giedd J., Evans A.C. Mapping anatomical correlations across cerebral cortex (MACACC) using cortical thickness from MRI. NeuroImage. 2006;31(3):993–1003. doi: 10.1016/j.neuroimage.2006.01.042. [DOI] [PubMed] [Google Scholar]
- Mahon B.Z., Caramazza A. What drives the organization of object knowledge in the brain? Trends Cogn. Sci. 2011;15(3):97–103. doi: 10.1016/j.tics.2011.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- May A. Experience-dependent structural plasticity in the adult human brain. Trends Cogn. Sci. 2011;15(10):475–482. doi: 10.1016/j.tics.2011.08.002. [DOI] [PubMed] [Google Scholar]
- McIntosh A.R., Lobaugh N.J. Partial least squares analysis of neuroimaging data: applications and advances. NeuroImage. 2004;23(Suppl. 1):S250–S263. doi: 10.1016/j.neuroimage.2004.07.020. [DOI] [PubMed] [Google Scholar]
- McIntosh A.R., Bookstein F.L., Haxby J.V., Grady C.L. Spatial pattern analysis of functional brain images using partial least squares. NeuroImage. 1996;3(3 Pt 1):143–157. doi: 10.1006/nimg.1996.0016. [DOI] [PubMed] [Google Scholar]
- Mennes M., Kelly C., Colcombe S., Castellanos F.X., Milham M.P. The extrinsic and intrinsic functional architectures of the human brain are not equivalent. Cereb. Cortex. 2013;23(1):223–229. doi: 10.1093/cercor/bhs010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mevik B.-H., Wehrens R. The PLS package: principal component and partial least squares regression in R. J. Stat. Softw. 2007;18(2) [Google Scholar]
- Modi S., Bhattacharya M., Singh N., Tripathi R.P., Khushu S. Effect of visual experience on structural organization of the human brain: a voxel based morphometric study using DARTEL. Eur. J. Radiol. 2012;81(10):2811–2819. doi: 10.1016/j.ejrad.2011.10.022. [DOI] [PubMed] [Google Scholar]
- Moussa M.N., Vechlekar C.D., Burdette J.H., Steen M.R., Hugenschmidt C.E., Laurienti P.J. Changes in cognitive state alter human functional brain networks. Front. Hum. Neurosci. 2011;5:83. doi: 10.3389/fnhum.2011.00083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noppeney U. The effects of visual deprivation on functional and structural organization of the human brain. Neurosci. Biobehav. Rev. 2007;31(8):1169–1180. doi: 10.1016/j.neubiorev.2007.04.012. [DOI] [PubMed] [Google Scholar]
- Park H.J., Lee J.D., Kim E.Y., Park B., Oh M.K., Lee S., Kim J.J. Morphological alterations in the congenital blind based on the analysis of cortical thickness and surface area. NeuroImage. 2009;47(1):98–106. doi: 10.1016/j.neuroimage.2009.03.076. [DOI] [PubMed] [Google Scholar]
- Pessoa L. Understanding brain networks and brain organization. Phys. Life Rev. 2014;11(3):400–435. doi: 10.1016/j.plrev.2014.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poldrack R.A. Can cognitive processes be inferred from neuroimaging data? Trends Cogn. Sci. 2006;10(2):59–63. doi: 10.1016/j.tics.2005.12.004. [DOI] [PubMed] [Google Scholar]
- Ptito M., Schneider F.C., Paulson O.B., Kupers R. Alterations of the visual pathways in congenital blindness. Exp. Brain Res. 2008;187(1):41–49. doi: 10.1007/s00221-008-1273-4. [DOI] [PubMed] [Google Scholar]
- Qin W., Liu Y., Jiang T., Yu C. The development of visual areas depends differently on visual experience. PLoS One. 2013;8(1):e53784. doi: 10.1371/journal.pone.0053784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reich L., Szwed M., Cohen L., Amedi A. A ventral visual stream reading center independent of visual experience. Curr. Biol. 2011;21(5):363–368. doi: 10.1016/j.cub.2011.01.040. [DOI] [PubMed] [Google Scholar]
- Reich L., Maidenbaum S., Amedi A. The brain as a flexible task machine: implications for visual rehabilitation using noninvasive vs. invasive approaches. Curr. Opin. Neurol. 2012;25(1):86–95. doi: 10.1097/WCO.0b013e32834ed723. [DOI] [PubMed] [Google Scholar]
- Ricciardi E., Bonino D., Pellegrini S., Pietrini P. Mind the blind brain to understand the sighted one! Is there a supramodal cortical functional architecture? Neurosci. Biobehav. Rev. 2014;41:64–77. doi: 10.1016/j.neubiorev.2013.10.006. [DOI] [PubMed] [Google Scholar]
- Rockland K.S., Ojima H. Multisensory convergence in calcarine visual areas in macaque monkey. Int. J. Psychophysiol. 2003;50(1–2):19–26. doi: 10.1016/s0167-8760(03)00121-1. [DOI] [PubMed] [Google Scholar]
- Roder B., Teder-Salejarvi W., Sterr A., Rosler F., Hillyard S.A., Neville H.J. Improved auditory spatial tuning in blind humans. Nature. 1999;400(6740):162–166. doi: 10.1038/22106. [DOI] [PubMed] [Google Scholar]
- Sani L., Ricciardi E., Gentili C., Vanello N., Haxby J.V., Pietrini P. Effects of visual experience on the human MT + functional connectivity networks: An fMRI study of motion perception in sighted and congenitally blind individuals. Front. Syst. Neurosci. 2010;4:159. doi: 10.3389/fnsys.2010.00159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schepers I.M., Hipp J.F., Schneider T.R., Roder B., Engel A.K. Functionally specific oscillatory activity correlates between visual and auditory cortex in the blind. Brain. 2012;135(Pt 3):922–934. doi: 10.1093/brain/aws014. [DOI] [PubMed] [Google Scholar]
- Shimony J.S., Burton H., Epstein A.A., McLaren D.G., Sun S.W., Snyder A.Z. Diffusion tensor imaging reveals white matter reorganization in early blind humans. Cereb. Cortex. 2006;16(11):1653–1661. doi: 10.1093/cercor/bhj102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shu N., Liu Y., Li J., Li Y., Yu C., Jiang T. Altered anatomical network in early blindness revealed by diffusion tensor tractography. PLoS One. 2009;4(9) doi: 10.1371/journal.pone.0007228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skipper J.I. Echoes of the spoken past: how auditory cortex hears context during speech perception. Philos. Trans. R. Soc. Lond. Ser. B Biol. Sci. 2014;369(1651):20130297. doi: 10.1098/rstb.2013.0297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Striem-Amit E., Ovadia-Caro S., Caramazza A., Margulies D.S., Villringer A., Amedi A. Functional connectivity of visual cortex in the blind follows retinotopic organization principles. Brain. 2015;138(Pt 6):1679–1695. doi: 10.1093/brain/awv083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thambisetty M., Wan J., Carass A., An Y., Prince J.L., Resnick S.M. Longitudinal changes in cortical thickness associated with normal aging. NeuroImage. 2010;52(4):1215–1223. doi: 10.1016/j.neuroimage.2010.04.258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tobia M.J., Iacovella V., Davis B., Hasson U. Neural systems mediating recognition of changes in statistical regularities. NeuroImage. 2012;63(3):1730–1742. doi: 10.1016/j.neuroimage.2012.08.017. [DOI] [PubMed] [Google Scholar]
- Tomaiuolo F., Campana S., Collins D.L., Fonov V.S., Ricciardi E., Sartori G.…Ptito M. Morphometric changes of the corpus callosum in congenital blindness. PLoS One. 2014;9(9):e107871. doi: 10.1371/journal.pone.0107871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voss P., Zatorre R.J. Occipital cortical thickness predicts performance on pitch and musical tasks in blind individuals. Cereb. Cortex. 2012;22(11):2455–2465. doi: 10.1093/cercor/bhr311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voss P., Zatorre R.J. Organization and reorganization of sensory-deprived cortex. Curr. Biol. 2012;22(5):R168–R173. doi: 10.1016/j.cub.2012.01.030. [DOI] [PubMed] [Google Scholar]
- Voss P., Zatorre R.J. Early visual deprivation changes cortical anatomical covariance in dorsal-stream structures. NeuroImage. 2015 doi: 10.1016/j.neuroimage.2014.12.063. [DOI] [PubMed] [Google Scholar]
- Voss P., Pike B.G., Zatorre R.J. Evidence for both compensatory plastic and disuse atrophy-related neuroanatomical changes in the blind. Brain. 2014;137(Pt 4):1224–1240. doi: 10.1093/brain/awu030. [DOI] [PubMed] [Google Scholar]
- Wang D., Qin W., Liu Y., Zhang Y., Jiang T., Yu C. Altered resting-state network connectivity in congenital blind. Hum. Brain Mapp. 2014;35(6):2573–2581. doi: 10.1002/hbm.22350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weeks R., Horwitz B., Aziz-Sultan A., Tian B., Wessinger C.M., Cohen L.G.…Rauschecker J.P. A positron emission tomographic study of auditory localization in the congenitally blind. J. Neurosci. 2000;20(7):2664–2672. doi: 10.1523/JNEUROSCI.20-07-02664.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wen X., Zhang D., Liang B., Zhang R., Wang Z., Wang J.…Huang R. Reconfiguration of the brain functional network associated with visual task demands. PLoS One. 2015;10(7):e0132518. doi: 10.1371/journal.pone.0132518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wittenberg G.F., Werhahn K.J., Wassermann E.M., Herscovitch P., Cohen L.G. Functional connectivity between somatosensory and visual cortex in early blind humans. Eur. J. Neurosci. 2004;20(7):1923–1927. doi: 10.1111/j.1460-9568.2004.03630.x. [DOI] [PubMed] [Google Scholar]
- Yarkoni T., Poldrack R.A., Nichols T.E., Van Essen D.C., Wager T.D. Large-scale automated synthesis of human functional neuroimaging data. Nat. Methods. 2011;8(8):665–670. doi: 10.1038/nmeth.1635. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary data