Abstract
Inflammatory cells such as microglia need energy to exert their functions and to maintain their cellular integrity and membrane potential. Subsequent to cerebral ischemia, inflammatory cells infiltrate tissue with limited blood flow where neurons and astrocytes died due to insufficient supply with oxygen and glucose. Using dual tracer positron emission tomography (PET), we found that concomitant with the presence of inflammatory cells, transport and consumption of glucose increased up to normal levels but returned to pathological levels as soon as inflammatory cells disappeared. Thus, inflammatory cells established sufficient glucose supply to satisfy their energy demands even in regions with insufficient supply for neurons and astrocytes to survive. Our data suggest that neurons and astrocytes died from oxygen deficiency and inflammatory cells metabolized glucose non-oxidatively in regions with residual availability. As a consequence, glucose metabolism of inflammatory cells can mask metabolic deficits in neurodegenerative diseases. We further found that the PET tracer did not bind to inflammatory cells in severely hypoperfused regions and thus only a part of the inflammation was detected. We conclude that glucose consumption of inflammatory cells should be taken into account when analyzing disease-related alterations of local cerebral metabolism.
Keywords: Glucose metabolism, Neuroinflammation, Positron emission tomography, [11C]PK11195, FDG, Cerebral ischemia
Highlights
-
•
Inflammatory cells consume high amounts of glucose in supply-limited brain regions.
-
•
Glucose metabolism of inflammatory cells masks metabolic deficits in the brain.
-
•
In vivo markers only reach inflammatory cells in regions with residual blood supply.
-
•
Measuring inflammation and metabolism provide complementary information.
Introduction
Harmful or beneficial — disorders of the brain may induce neuroinflammation affecting the course of disease (Graeber and Streit, 2010, Graeber, 2010). After an ischemic injury, the peak of the inflammatory response is typically observed one week after the onset of ischemia (Hallenbeck et al., 1986). In ischemic regions, neurons and astrocytes die because of insufficient supply of nutrients and oxygen. Inflammatory cells, however, also rely on energy supply to exert cellular functions and to maintain their membrane potential. In a double tracer long-term follow-up positron emission tomography (PET) study in rats we analyzed the development of inflammation in relation to local glucose metabolism following permanent occlusion of the middle cerebral artery (MCAo). Inflammation was localized and quantified using [11C]PK11195, a PET tracer that binds to the translocator protein expressed by activated microglia and macrophages (Banati, 2002, Politis et al., 2012, Rojas et al., 2007, Thiel and Heiss, 2011). Since [11C]PK11195 does not allow for differentiation, we summarize here activated microglia and microglia- or monocyte-derived macrophages as inflammatory cells, keeping in mind that other types of inflammatory cells could be involved. Glucose metabolism was measured using [18F]-2-fluoro-2-deoxy-D-glucose ([18F]FDG) as PET tracer. Additionally, we measured blood flow 1 h after MCAo using [15O]H2O-PET for localization of the primarily affected ischemic territory and performed structural magnetic resonance imaging (MRI) before each PET scan. In vivo data were compared with ex vivo immunostaining.
Materials and methods
Animals and Surgery
All animal procedures were performed in accordance with the German Laws for Animal Protection and were approved by the local animal care committee and local governmental authorities (LANUV NRW). Male Wistar rats (n = 5, Janvier, France; weight: 320 to 363 g; age ~ 10 weeks; pairwise housed in type-4 cages filled with Lignocel in an inverse 12 h day–night cycle with lights on at 8:30 pm in a temperature (22 ± 11 °C) and humidity (55 ± 5%) controlled room; experiments performed between 9 am and 4 pm) were anesthetized with 5% isoflurane and maintained with 2.5% isoflurane in 65%/35% nitrous oxide/oxygen. Throughout the surgical procedure and the MRI and PET imaging procedures, body temperature was maintained at 37.0 ± 0.5 °C using a thermostatically controlled heating pad (MEDRES, Cologne, Germany). Before acute PET imaging, animals were prepared for induction of ischemia using macrospheres (Gerriets et al., 2004). Briefly, the left common carotid artery, internal carotid artery, and external carotid artery were exposed through a midline incision of the neck and the external carotid artery and the pterygopalatine branch of the internal carotid artery were ligated. A PE-50 catheter was filled with saline and two TiO2 macrospheres (diameter of 0.315–0.355 mm; BRACE, Alzenau, Germany). This catheter was inserted into the common carotid artery, advanced to the origin of the pterygopalatine artery, and fixed in place. After placing the rats in the micro-PET scanner and running baseline regional cerebral blood flow (rCBF) measurements using [15O]H2O as tracer, macrospheres were injected through a saline-filled catheter placed in the internal carotid artery to occlude the middle cerebral artery. Following PET imaging, the catheter was removed and the animals were allowed to recover. Each animal was additionally imaged by T2-weighted MRI and PET using [11C]PK11195 and [18F]FDG at baseline (7 days before MCAo) as well as at days 2, 7, 14, 21 and 42 after MCAo. During the experiment, all animals received an intensified care with moistening of food pellets, subcutaneous saline injections (5 mL 0.9%NaCl/day for 8 days) and an intra- and postoperative analgesia with Carprofen (Rimadyl®; 5 mg/kg/day s.c. for 3 days).
PET
PET imaging was performed using a microPET Focus 220 scanner (Concorde Microsystems, Inc, Knoxville, TN; 63 image planes; 1.5-mm full width at half maximum). For each animal and each imaging session transmission data from a Co57 point source were acquired for attenuation correction. During induction of ischemia rats received an intravenous bolus injection of [15O]H2O (59–89 MBq/rat in 0.5 mL) before as well as 5, 30, and 60 min after MCAo without changing the position of the animals in the PET scanner. Emission data were acquired for 2 min. Thereafter, an intravenous bolus of [18F]-2-fluoro-2-deoxy-D-glucose ([18F]FDG) (52–74 MBq/rat in 0.5 mL) was injected into the tail vein at 75 min after embolization of macrospheres, and emission data were acquired for 60 min.
At baseline (7 days before MCAo) as well as 2, 7, 14, 21, and 42 days, rats received an intravenous bolus injection of [11C]PK11195 (59–89 MBq/rat, specific activity 18.5–81.4 GBq/μmol) in the tail vein. Emission data were acquired for 30 min and the animals remained in the μPET-scanner without changing their position. 100 min after injection of [11C]PK11195 (~ 5 half-lives of the [11C]-labeled tracer) a bolus of [18F]FDG (52–74 MBq/rat in 0.5 mL) was injected in the tail vein and emission data were acquired for 60 min.
[18F]FDG and [11C]PK11195 data were histogrammed in time frames of 6 × 30 s, 3 × 60 s, 3 × 120 s, and either 12 × 240 s for [18F]FDG or 4 × 240 s for [11C]PK11195, Fourier rebinned, and images (voxel size: 0.4 mm × 0.4 mm × 0.8 mm) were reconstructed using 2-dimensional filtered back projection. Binding potential of the radiotracer [11C]PK11195 was calculated using the simplified reference tissue model (SRTM) using the contralateral hemisphere as reference region (Lammertsma and Hume, 1996). [15O]H2O data were Fourier rebinned and images were reconstructed using 2-dimensional filtered back projection. CBF was assessed as percentage injected dose of [15O]H2O averaged over 2 minute acquisition time. A 1.5 mm Gaussian Filter was applied to the CBF images.
An image derived input function was extracted from the [18F]FDG-PET data and parametric images of the kinetic constants were determined by a voxel-by-voxel application of a two-tissue-compartment kinetic model (Backes et al., 2011). K1 is the rate constant for [18F]FDG transport from blood into the brain tissue and is related to regional cerebral blood flow (Walberer et al., 2012). The metabolic rate constant or net influx rate constant for [18F]FDG is given by Ki = K1 k3 / (k2 + k3). The cerebral metabolic rate of glucose CMRglc is given by: CMRglc = Kglc Cp, where Kglc is the net influx rate constant of glucose (Kglc = Ki / [0.38 + 1.22 Ki/K1]) and Cp the plasma glucose level. Note, that Kglc takes into account changes of the lumped constant that result from changes in the relative contributions of glucose transport and hexokinase activity (Backes et al., 2011). The plasma glucose level was determined after each [18F]FDG PET measurement from a tail vein blood sample (measured values: 75–233 mg/dL, mean = 128.5 ± 28.8 mg/dL).
Magnetic resonance imaging
At baseline (7 days before MCAo) as well as 2, 7, 14, 21 and 42 days after induction of ischemia, rats were (re-)anesthetized with isoflurane, and experiments were conducted on a 4.7 T BioSpec system (Bruker BioSpin, Ettlingen, Germany) with a 30 cm bore horizontal magnet, equipped with a self-shielded gradient system (max gradient: 100 mT/m; rise time: < 250 μs). Radio frequency transmission was achieved with a Helmholtz coil (12 cm diameter), and the signal was detected with a 22 mm diameter surface coil. The animals were positioned prone in a dedicated cradle using a stereotactic head holder with the surface coil placed directly over the head. A multislice multiecho Carr–Purcell–Meiboom–Gill sequence was used to acquire multi-echo trains in twenty contiguous T2-weighted coronal slices with a thickness of 1 mm (relaxation time/echo time = 4279/12.5 msecs, 16 echoes, field of view = 4.6 cm, matrix 128 × 128) for the detection of infarct and edema. During scanning, body temperature and respiration rate were monitored and maintained using a combination of in-house equipment and DASYLab 9.0 (DasyLab, Moenchengladbach, Germany).
Radiochemistry
The radiolabeled tracers [15O]H2O, [11C]PK11195 and [18F]FDG were produced as described previously in more detail (Schroeter et al., 2009, Walberer et al., 2012).
Histology and Immunohistochemistry
After PET-measurements at day 42, animals were decapitated under deep anesthesia with isoflurane. The brains were rapidly removed, frozen in 2-methylbutane, and stored at -80°C prior to further histological and immunohistochemical processing. Adjacent serial coronal brain sections were cut at 500 μm intervals (slice thickness 10 μm) and stained with hematoxylin and eosin (H&E) according to standard protocols. The mAb against the complement receptor 3/CD11b identified microglia/macrophages (clone OX42, dilution 1:1000, AbD Serotec, Oxford, UK, cat-# MCA275R). Microglia activation was assessed by staining for MHC class II (clone Ox6, dilution 1:400, AbD Serotec, Oxford, UK, cat-#MCA46G). Phagocytic cells were identified with mAb ED1 (clone ED1, dilution 1:1000, AbD Serotec, Oxford, UK, cat-# MCA341). The neuronal marker NeuN (clone A60, dilution 1:1000, Millipore, Billerica, USA, cat-# MAB377) was used to assess neuronal integrity in the peri-infarct zone, in order to exactly distinguish infarcted from intact tissue. For visualization, we used the ABC Elite kit (Vector Laboratories, Burlingame, CA, USA) with diaminobenzidine (Sigma, Munich, Germany) or Vector SG substrate kit for peroxidase (Vector laboratories, Burlingame, CA, USA.) for anti-NeuN staining as the final reaction product.
Image analysis
For co-registration of PET-, MRI- and histological images, the image analysis software VINCI was used (Cízek et al., 2004). PET and MR images were first manually co-registered to a 3D brain atlas constructed from brain slices presented by Swanson (Swanson, 2003). Slices that corresponded to the histological slices were then identified in the 3D brain atlas and saved as 2D images together with the co-registered PET and MR images. Compositions of the 2D PET, MRI and histological images were performed using GIMP an open source software for image processing (http://gimp.org).
For analysis of the time course of inflammation in relation to metabolism, in each individual animal all voxels in the 3D PET datasets with a binding potential of [11C]PK11195 BPND > 1 seven days after MCA occlusion were marked. For each animal the average binding potential, K1, and Kglc in this region were determined for baseline, days 2, 7, 14, 21, and 42.
Statistics
One way repeated measures ANOVA was performed for K1, Kglc and BPND for the factor “time”. Differences to baseline were tested using pairwise multiple comparison with Holm–Sidak p-value adjustment. Correlation of K1 and Kglc with BPND after MCAo was tested using a Pearson product-moment correlation test. Calculations were performed using the software package R (http://www.R-project.org/). All values are given as mean ± standard error.
PK11195 binding and histology at day seven after MCAo
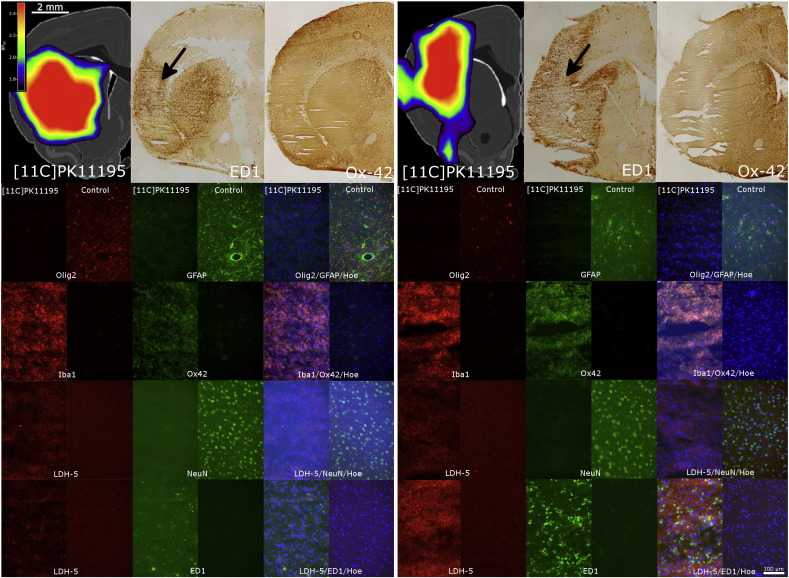
In order to support our findings from this study with histologic data acquired at day 7 after MCAo we re-analyzed histologic data in relation to PK11195 uptake from a study performed by our group that had already been published (Schroeter et al., 2009). In brief, [11C]PK11195-PET and subsequent histological staining with H&E, Ox42, and ED1 was performed in 5 male Wistar rats seven days after MCAo following the experimental procedures described above. The MCA was occluded using the macrosphere model in the same way as in this study. Here we re-analyzed the data and performed additional stainings of brain sections from two rats in order to determine the status of brain tissue in the region of maximum [11C]PK11195 binding at day seven. To verify the absence of neurons, astrocytes and oligodendrocytes within the [11C]PK11195-positive region we used the neuronal marker NeuN (clone A60, dilution 1:500 for fluorescence, Millipore, Billerica, USA, cat-# MAB377) to assess neuronal integrity, an antibody against glial fibrillary acidic protein (GFAP, clone GA5, dilution 1:500, Millipore, #AB5320, Darmstadt, Germany) as marker for astrocytes, and an antibody against oligodendrocyte transcription factor 2 (Olig2, dilution 1:300, AB9610, Millipore, Darmstadt, Germany) for oligodendrocytes. The monoclonal antibody (mAb) against the complement receptor 3/CD11b identified microglia/macrophages (clone OX42, dilution 1:300 for fluorescence, AbD Serotec, Oxford, UK, cat-# MCA275R). Microglia activation was assessed by staining for Ionized calcium binding adapter molecule 1 (Iba1, dilution 1:500, Wako Pure Chemical Industries, Osaka, Japan, cat.#019-19741). Phagocytic cells were identified with mAb ED1 (clone ED1, 1:500 for fluorescence, AbD Serotec, Oxford, UK, cat-# MCA341). To detect areas with predominantly glycolytic metabolism we stained for Lactate Dehydrogenase Isoenzyme 5 (LDH-5), an enzyme that is transcriptionally regulated by the hypoxia inducible factors (HIF) 1a and 2a (dilution 1:250, #ab101562, Abcam, Cambridge, UK). Secondary antibodies for fluorescence microscopy were goat-anti-rabbit 568 and goat-anti-mouse 488 (Alexa Fluor, Invitrogen/Life Technologies, Carlsbad, CA, USA). For nuclear co-staining, Hoechst 33342 (Life Technologies, Darmstadt, Germany) was applied after the secondary antibody.
Representative images of the [11C]PK11195-positive and the homologous region in the healthy hemisphere were taken using an inverted fluorescence phase-contrast microscope (Keyence BZ-9000E, Osaka, Japan) with 4 ×, 20 ×, and 40 × objectives.
Results
Acute changes of CBF and metabolism after permanent occlusion of the MCA
We analyzed development of CBF during the first hour after MCAo with the help of serial [15O]H2O PET imaging (Fig. 1). The results were already published and extensively discussed in (Walberer et al., 2012). In short, we found that CBF in the MCA territory decreased substantially within 30 min after occlusion with no relevant changes between 30 and 60 min. We conclude that after permanent occlusion regional flow at 60 min is representative for future damage. CBF at 60 min correlated well with the unidirectional transport parameter of FDG K1. A combination of the kinetic parameters of FDG K1 and Ki allowed for the discrimination of infarct core (low K1, low Ki) from early viable tissue (low K1, high Ki).
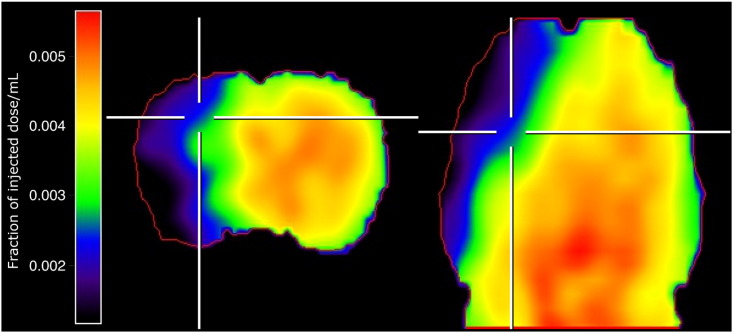
Fig. 1.
Cerebral blood flow 1 h after permanent occlusion of the middle cerebral artery. The region supplied by the MCA was characterized by a severe reduction of CBF measured by [15O]H2O PET. Data is from the same rat and spatially co-registered to the data shown in Fig. 2. Left: coronal section, right: axial section. Locations of the sections are indicated by white markers. The red line indicates the boundary of the brain.
Development of inflammation and metabolism during six weeks after MCAo
The maximum of [11C]PK11195-BPND as measure for inflammation was observed seven days after stroke in the border zone of the ischemic core (Fig. 2, Fig. 3) and in the thalamus of the affected hemisphere. Inflammation in the thalamus persisted until the end of the six weeks observation period. Note, the thalamus is not part of the supply territory of the MCA, but since its neurons project to severely injured cortical regions, it is subject to secondary chronic inflammation (Schroeter et al., 2009, Schroeter et al., 2006). In the following we focus on primary inflammation in directly affected brain regions.
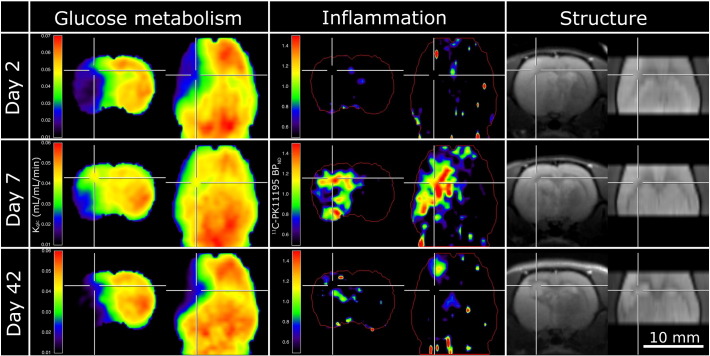
Fig. 2.
Inflammation and glucose metabolism after occlusion of the middle cerebral artery (MCAo). Left column: glucose consumption rate constant (Kglc) determined from [18F]FDG-PET; middle column: binding potential (BPND) of [11C]PK11195 binding for localization of inflammation; right column: structural T2 weighted MRI image. Top row: two days after MCAo, middle row: 7 days after MCAo, bottom row: 42 days after MCAo. In the region marked by the crosshairs, the presence of inflammation at day seven was accompanied by a relative increase of glucose metabolism from day 2 to day 7. Data are taken from the same rat and spatially co-registered to the data shown in Fig. 1. In each column left: coronal section, right: axial section. Locations of the sections are indicated by white markers.
Fig. 3.
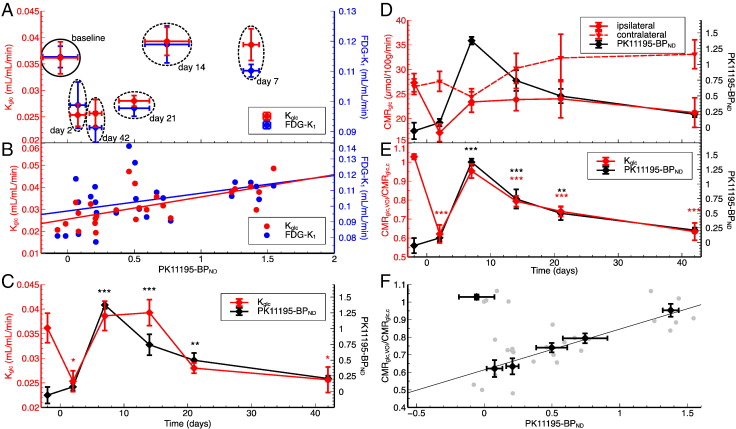
Inflammation and glucose metabolism in the region of primary inflammation ([11C]PK11195-BPND > 1 at day seven after MCAo) (n = 5). (A) Glucose consumption rate constant (Kglc, red) and the rate constant for unidirectional transport of glucose from blood to brain tissue (K1, blue) over inflammation level (BPND) before and at different time points after MCAo. (B) Data from individual rats for time points after MCAo. The lines are linear fits. BPND was significantly correlated with Kglc (r = 0.65, p = 0.0005) and close to significantly correlated with K1 (r = 0.39, p = 0.0508) (C) Time course of glucose consumption rate constant (red) and inflammation level (BPND, black). (D) Time course of CMRglc in the region of primary inflammation (VOI) and the healthy contralateral hemisphere and their ratio (E). (F) CMRglc,VOI/CMRglc,c data from individual rats (grey), averages for each time point, and linear fit for time points after MCAo (r = 0.81, p < 0.0001). Asterisks indicate significant differences from baseline (*p < 0.05, **p < 0.01, ***p < 0.001).
In each animal, we identified primary inflammation by a binding potential (BPND) of [11C]PK11195-BPND > 1 seven days after stroke (excluding the thalamus). The threshold of BPND > 1 is well above the BPND noise level on a voxel basis, which is in the order of 0.3. BPND is a measure for the number of activated microglia and macrophages per volume and as such a surrogate measure of inflammation. The anatomic location of primary inflammation was restricted to the affected hemisphere in each animal but differed individually due to inter-individual variability of the ischemic territory. We therefore analyzed the time course of glucose transport and metabolism as well as the level of inflammation in the respective region of each animal (Table 1, Fig. 2, Fig. 3). At 1 h after MCA occlusion CBF was reduced by 39% (28%–55%) in this region (Table 1). MCAo had an overall effect on K1 (F(5,20) = 4.86, p = 0.005), Kglc (F(5,20) = 7.01, p < 0.001), and BPND (F(5,20) = 28.82, p < 0.001). Before MCAo, metabolism was normal (K1 = 0.11 ± 0.004 mL/mL/min, Kglc = 0.036 ± 0.003 mL/mL/min) and, as expected, no inflammation was detected (BPND = − 0.06 ± 0.13). Two days after stroke, glucose transport and consumption were reduced in the region of primary inflammation (K1 = 0.099 ± 0.008 mL/mL/min, p = 0.095; Kglc = 0.025 ± 0.002 mL/mL/min, p = 0.026), indicating severely reduced glucose metabolism. No significant level of inflammation could yet be observed (BPND = 0.08 ± 0.06, p = 0.38). Seven days after the onset of ischemia, the level of inflammation reached its maximum (BPND = 1.38 ± 0.06, p < 0.001) and remained high for more than one week (day 14: BPND = 0.74 ± 0.16, p < 0.001; day 21: BPND = 0.50 ± 0.11, p = 0.003). Glucose transport and metabolism returned to normal (i.e., baseline) levels in this particular region during that time (day 7: K1 = 0.11 ± 0.002 mL/mL/min, p = 1.0; Kglc = 0.039 ± 0.003 mL/mL/min, p = 0.77; day 14: K1 = 0.12 ± 0.006 mL/mL/min, p = 1.0; Kglc = 0.039 ± 0.003 mL/mL/min, p = 0.77; day 21: K1 = 0.098 ± 0.003 mL/mL/min, p = 0.092; Kglc = 0.028 ± 0.001 mL/mL/min, p = 0.088). The simultaneous increase of K1 and Kglc indicate that the increase of Kglc is a consequence not only of increased hexokinase activity but also of increased unidirectional transport of glucose into tissue. Six weeks after stroke, inflammation resolved (BPND = 0.21 ± 0.05, p = 0.18) and metabolism returned to the same level as two days after the onset of ischemia but before the onset of inflammation (K1 = 0.091 ± 0.006 mL/mL/min, p = 0.013; Kglc = 0.026 ± 0.003 mL/mL/min, p = 0.026). Immunohistochemistry showed that brain tissue was infarcted in this region (Fig. 4).
Table 1.
Region of primary inflammation (PK11195-BPND > 1) at day 7 after MCAo. Volume of the region, mean BPND, CMRglc as fraction of the contralateral hemisphere, and CBF reduction in the region at 1 h after MCAo as fraction of injected dose of [15O]H2O in VOI relative to the contralateral hemisphere are listed for each individual animal.
| Rat # | VOI vol. (μL) | PK11195-BPND(d7) | CMRglc,VOI/CMRglc,ctra(d7) | [15O]H2OVOI/[15O]H2Octra(1h) |
|---|---|---|---|---|
| 1 | 248.4 | 1.41 | 0.88 | 0.72 |
| 2 | 154.5 | 1.26 | 0.89 | 0.71 |
| 3 | 60.6 | 1.23 | 1.06 | 0.56 |
| 4 | 175.6 | 1.44 | 1.02 | 0.45 |
| 5 | 86.6 | 1.54 | 0.91 | 0.62 |
| Mean ± SE | 145.2 ± 33.6 | 1.38 ± 0.06 | 0.95 ± 0.04 | 0.61 ± 0.05 |
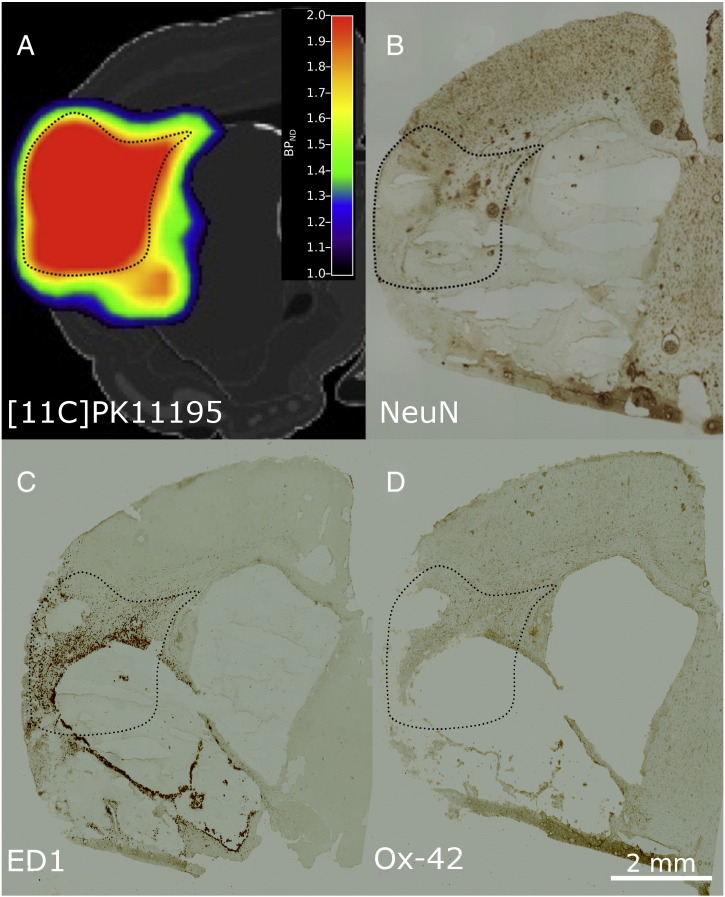
Fig. 4.
(A) [11C]PK1195 binding seven days after occlusion of the left MCA. Post-mortem NeuN (B), ED1 (C), and Ox-42 (D) staining of the same brain region and the same animal five weeks later. At day 42, the predominant part of the region with high [11C]PK1195 binding is necrotic.
The region of primary inflammation was always part of the region that appeared as infarcted in the T2 weighted MR images (Fig. 2). However, this region could not be identified based on the MR images.
Development of CMRglc
We also analyzed the development of CMRglc in the region of primary inflammation (Figs. 3D–F). The time course of the CMRglc is not completely following the PK11195-BPND time course. In a detailed analysis (not shown here) we found that CMRglc relative to the blood glucose level was deregulated after MCAo in two rats (rat 1 and 2 in Table 1) causing an average increase of CMRglc in the healthy contralateral hemisphere. However, the time course of CMRglc in the region of primary inflammation relative to CMRglc in contralateral hemisphere (CMRglc,VOI/CMRglc,c) follows the development of inflammation. Statistics for CMRglc,VOI/CMRglc,c: overall effect (F(5,20) = 20.78, p < 0.001), base line (1.02 ± 0.01), day 2 (0.62 ± 0.05, p < 0.001), day 7 (0.95 ± 0.04, p = 0.14), day 14 (0.79 ± 0.03, p < 0.001), day 21 (0.74 ± 0.03, p < 0.001), day 42 (0.63 ± 0.05, p < 0.001).
After stroke, glucose consumption correlated significantly with the average binding potential of [11C]PK11195 in the region of primary inflammation (Fig. 3F; Pearson correlation of Kglc vs. [11C]PK11195-BPND at day 2,7,14,21,42 after MCAo: r = 0.65, p = 0.0005, Pearson correlation of CMRglc,VOI/CMRglc,c vs. [11C]PK11195-BPND at days 2, 7, 14, 21, and 42 after MCAo: r = 0.81, p < 0.0001) indicating that the transient increase in glucose metabolism reflects glucose consumption by inflammatory cells.
Cellular population of the primary inflammation region at day 7
In order to verify that the rise of glucose consumption in the inflamed region at day 7 did not reflect a transient recovery of neurons or astrocytes, we reanalyzed data of rats that had been treated in the same way as rats in the present study, but had been sacrificed at day 7 after MCAo (Schroeter et al., 2009). Immunohistochemistry confirmed that the inflamed region identified by [11C]PK11195 was void of neurons, astrocytes, and oligodendrocytes (negative staining for NeuN, GFAP, Olig2), exclusively populated by immune cells (all cells Ox-42 and Iba1 positive), which were co-located with the staining for LDH-5, the enzyme that drives lactate production from pyruvate (Bittar et al., 1996) (Fig. 5). The upregulation of LDH-5 indicates that these cells metabolize glucose non-oxidatively via glycolysis.
Fig. 5.
[11C]PK11195 binding and immunostaining at day seven after MCAo in two representative animals. The top row gives an overview of the location of [11C]PK11195 binding relative to ED1 and Ox-42 staining. The images below show a detailed analysis of the cellular composition in a representative [11C]PK11195-postive region (column labeled by ‘[11C]PK11195’) in comparison to the homologous region in the healthy contralateral hemisphere (‘Control’). The approximate location is indicated on the ED1 slices by a black arrow. Staining was performed for neurons (NeuN), astrocytes (GFAP), oligodendrocytes (Olig2), microglia/macrophages (Iba1, Ox42), and phagocytizing macrophages (ED1). In the fused images cell nuclei are stained in blue by Hoechst 33342 (Hoe). Additional staining for lactate-dehydrogenase-5 (LDH-5) indicates regions with predominant transformation of pyruvate into lactate characteristic for non-oxidative glucose metabolism.
[11C]PK11195-postive and [11C]PK11195-negative inflammation region
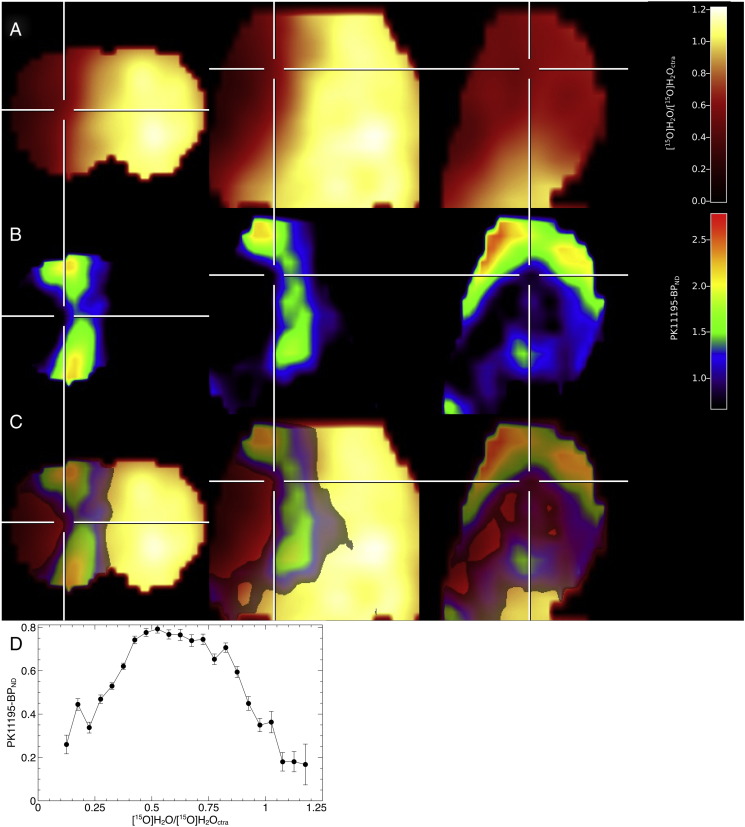
Only a fraction of the region infiltrated by macrophages was labeled by [11C]PK11195 (Fig. 5). Staining for phagocytic cells, there was no obvious difference between [11C]PK11195-positive and [11C]PK11195-negative cells, neither in cell density nor in morphological structure. Regional comparison of CBF (measured 60 min after MCAo) and [11C]PK11195-BPND at day 7 shows that in regions where CBF is below ~ 30% of the normal CBF, [11C]PK11195-BPND is linearly correlated to CBF (Fig. 6D), indicating that tracer delivery to tissue is limited in these regions and [11C]PK11195-BPND underestimates the true number of macrophages in low perfusion regions. The major part of [11C]PK11195 binding is located in regions with a residual blood flow of 30%–80%.
Fig. 6.
[11C]PK11195 binding and cerebral blood flow (CBF). (A) Coronal, axial, and sagittal section of CBF measured by [15O]H2O-PET 60 min after MCAo shown as fraction of CBF in the healthy hemisphere. (B) [11C]PK11195 binding at day 7 after MCAo in the same animal. (C) Overlay of [11C]PK11195 binding and CBF. (D) Regional analysis of [11C]PK11195-BPND (mean ± SE) and CBF in n = 5 rats. There is a systematic decrease in [11C]PK11195 binding in regions with low CBF.
The observed rise of Kglc during inflammation was confined to the [11C]PK11195-positive fraction of the inflamed region (Fig. 2). Note that the absence of [18F]FDG accumulation implied absent glucose metabolism: tissue not reached by the PET tracer was neither reached by glucose as the energy substrate. Thus, inflammatory cells were only able to satisfy their energy needs by consumption of glucose where glucose was available.
Discussion
Although it is not surprising that inflammatory cells consume glucose to satisfy their energy needs (Wise et al., 1983), it is remarkable that in regions with insufficient blood flow for neurons and astrocytes to survive, inflammatory cells managed to extract and consume the same amounts of glucose per time and volume tissue as neurons and astrocytes consumed under healthy conditions.
Our results indicate that (1) Inflammatory cells seem to rely upon a very efficient glucose transport system that is capable of extracting high amounts of glucose even in ischemic tissue. (2) Neurons and astrocytes in the region of primary inflammation died of oxygen and not of glucose deficiency. (3) High glucose consumption in presumably oxygen deficient tissue indicates that glucose taken-up by inflammatory cells is non-oxidatively metabolized. (4) [11C]PK11195 labels activated microglia and macrophages in tissue with sufficient residual blood flow to deliver the tracer, but underestimates inflammation in regions with CBF <~30% of normal.
[11C]PK11195 binds to the translocator protein (TSPO), which is primarily located on the outer mitochondrial membrane of activated microglia and macrophages. Changes in TSPO expression are related to alterations in the functional state of mitochondria (Banati et al., 2004). Unlike microglia and unlike adaptive immune cells (e.g. T cells), macrophages can switch to non-oxidative metabolism in hypoxic conditions (Fumagalli et al., 2015, Riboldi et al., 2013, Sica et al., 2011). In accordance with the results from immunostaining (Fig. 5), it is therefore most likely that macrophages are responsible for the transient increase in glucose consumption that we observe in the ischemic area. However, the origin of these macrophages (from CNS or hematogenous) is less clear. It was shown in rat brain slices that within 2 h microglia can transform into amoeboid macrophages (Stence et al., 2001). Although hypoxia induces autophagic cell death in microglia cultures (Yang et al., 2014), in vivo hypoxia could trigger the transformation of microglia into macrophages. Microglia also have the capacity to become polarized into M1-like and M2-like phenotypes (Prinz and Priller, 2014). Thus, the functional status of these cell populations is not determined by their origin.
[15O]H2O measurements were only performed immediately before and up to 1 h after the permanent occlusion of the MCA. The analysis of the early development of regional CBF after occlusion showed that major changes in CBF occurred within the first 30 min while there were only minor changes from 30 to 60 min (Walberer et al., 2012). After permanent MCA occlusion in cats it was shown that CBF changes between 1 and 24 h were minimal (Heiss et al., 1994). In our study, we identified the location of the occluding macrosphere post mortem and confirmed that the MCA was still blocked at six weeks after occlusion and reperfusion due to flushing away of the occluding obstacle could be excluded. We therefore assume here that the individual CBF map of each animal at 60 min after permanent occlusion more or less reflects the future situation.
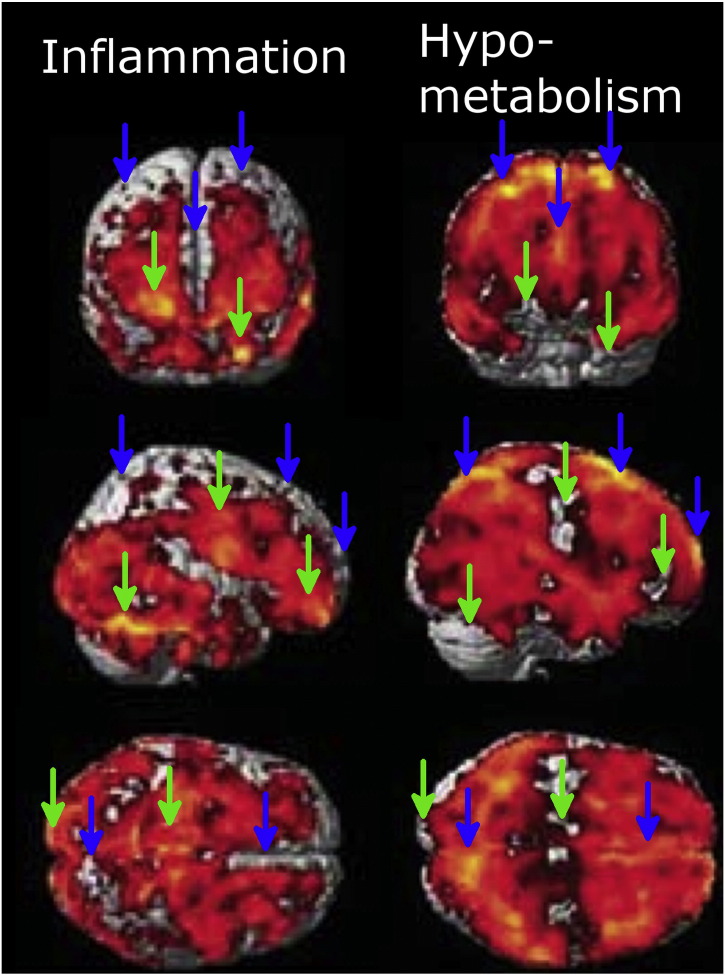
Our findings could influence the detection of neuroinflammation and hypo-metabolism in other pathologies. Neurodegenerative diseases such as Alzheimer’s disease or Parkinson’s disease affect cerebral glucose metabolism and induce inflammation. Our observations indicate that (1) energy metabolism of inflammatory cells can mask metabolic deficits in regions with neuronal damage, and (2) the in vivo detection of inflammation using tracer methods is impaired in severely hypo-perfused tissue. A recent study on glucose metabolism and inflammation in patients suffering from Parkinson’s disease showed – although not explicitly discussed by the authors – a mismatch of regions with reduced glucose metabolism and regions with inflammation (Edison et al., 2013). The comparison of Fig. 1, Fig. 3 in their publication indicates that on the one hand, regions with the highest level of inflammation did not show a reduction in glucose metabolism while on the other hand, those regions with the most prominent reductions of glucose consumption did not show [11C]PK11195 binding (Fig. 7). Our own data demonstrate that intact glucose metabolism in a [11C]PK11195 positive region can be caused by glucose consumption of inflammatory cells, while a lack of [11C]PK11195 binding regions with impaired glucose consumption does not necessarily exclude inflammation. In summary, previous data and our current findings suggest that [18F]FDG and [11C]PK11195 PET provide complementary information regarding glucose consumption and neuroinflammation. Glucose consumption of inflammatory cells needs to be taken into account when diagnosing neurodegenerative diseases by measuring local metabolism.
Fig. 7.
Inflammation and hypometabolism in patients with Parkinson's disease and dementia. The left column shows inflammation as revealed by a significant binding of [11C]PK11195. The right column shows regions with significant reduction of CMRglc as identified by using [18F]FDG-PET (note that brighter color indicates reduced metabolism). Blue arrows indicate regions with severe hypometabolism and no [11C]PK11195 binding, green arrows indicate those regions with a high [11C]PK11195 binding which do not show reductions in metabolism. [This figure is composed from images taken from Fig. 1, Fig. 3 in Edison et al. (2013). Reprinted by permission from Macmillan Publishers Ltd: Neuropsychopharmacology (Edison et al. (2013)), copyright 2013.]
Acknowledgments
This work was financially supported by grants from the EU-FP7 programs TargetBraIn (HEALTH-F2-2012-279017) and BrainPath (FP7-PEOPLE-2013-IAPP -612360).
References
- Backes H., Walberer M., Endepols H., Neumaier B., Graf R., Wienhard K., Mies G. Whiskers area as extracerebral reference tissue for quantification of rat brain metabolism using (18)F-FDG PET: application to focal cerebral ischemia. J. Nucl. Med. 2011;52:1252–1260. doi: 10.2967/jnumed.110.085266. [DOI] [PubMed] [Google Scholar]
- Banati R.B. Visualising microglial activation in vivo. Glia. 2002;40:206–217. doi: 10.1002/glia.10144. [DOI] [PubMed] [Google Scholar]
- Banati R.B., Egensperger R., Maassen A., Hager G., Kreutzberg G.W., Graeber M.B. Mitochondria in activated microglia in vitro. J. Neurocytol. 2004;33:535–541. doi: 10.1007/s11068-004-0515-7. [DOI] [PubMed] [Google Scholar]
- Bittar P.G., Charnay Y., Pellerin L., Bouras C., Magistretti P.J. Selective distribution of lactate dehydrogenase isoenzymes in neurons and astrocytes of human brain. J. Cereb. Blood Flow Metab. 1996;16:1079–1089. doi: 10.1097/00004647-199611000-00001. [DOI] [PubMed] [Google Scholar]
- Cízek J., Herholz K., Vollmar S., Schrader R., Klein J., Heiss W.-D. Fast and robust registration of PET and MR images of human brain. NeuroImage. 2004;22:434–442. doi: 10.1016/j.neuroimage.2004.01.016. [DOI] [PubMed] [Google Scholar]
- Edison P., Ahmed I., Fan Z., Hinz R., Gelosa G., Ray Chaudhuri K., Walker Z., Turkheimer F.E., Brooks D.J. Microglia, amyloid, and glucose metabolism in Parkinson's disease with and without dementia. Neuropsychopharmacology. 2013;38:938–949. doi: 10.1038/npp.2012.255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fumagalli S., Perego C., Pischiutta F., Zanier E.R., De Simoni M.-G. The ischemic environment drives microglia and macrophage function. Front. Neurol. 2015;6:81. doi: 10.3389/fneur.2015.00081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerriets T., Stolz E., Walberer M., Müller C., Kluge A., Bachmann A., Fisher M., Kaps M., Bachmann G. Noninvasive quantification of brain edema and the space-occupying effect in rat stroke models using magnetic resonance imaging. Stroke. 2004;35:566–571. doi: 10.1161/01.STR.0000113692.38574.57. [DOI] [PubMed] [Google Scholar]
- Graeber, M.B., 2010. Changing face of microglia. Sci. (New York, NY) 330, 783–788. [DOI] [PubMed]
- Graeber M.B., Streit W.J. Microglia: biology and pathology. Acta Neuropathol. 2010;119:89–105. doi: 10.1007/s00401-009-0622-0. [DOI] [PubMed] [Google Scholar]
- Hallenbeck J.M., Dutka A.J., Tanishima T., Kochanek P.M., Kumaroo K.K., Thompson C.B., Obrenovitch T.P., Contreras T.J. Polymorphonuclear leukocyte accumulation in brain regions with low blood flow during the early postischemic period. Stroke. 1986;17:246–253. doi: 10.1161/01.str.17.2.246. [DOI] [PubMed] [Google Scholar]
- Heiss W.D., Graf R., Wienhard K., Lottgen J., Saito R., Fujita T., Rosner G., Wagner R. Dynamic penumbra demonstrated by sequential multitracer PET after middle cerebral artery occlusion in cats. J. Cereb. Blood Flow Metab. 1994;14:892–902. doi: 10.1038/jcbfm.1994.120. [DOI] [PubMed] [Google Scholar]
- Lammertsma A.A., Hume S.P. Simplified reference tissue model for PET receptor studies. NeuroImage. 1996;4:153–158. doi: 10.1006/nimg.1996.0066. [DOI] [PubMed] [Google Scholar]
- Politis M., Giannetti P., Su P., Turkheimer F., Keihaninejad S., Wu K., Waldman A., Malik O., Matthews P.M., Reynolds R., Nicholas R., Piccini P. Increased PK11195 PET binding in the cortex of patients with MS correlates with disability. Neurology. 2012;79:523–530. doi: 10.1212/WNL.0b013e3182635645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prinz M., Priller J. Microglia and brain macrophages in the molecular age: from origin to neuropsychiatric disease. Nat. Rev. Neurosci. 2014;15:300–312. doi: 10.1038/nrn3722. [DOI] [PubMed] [Google Scholar]
- Riboldi E., Porta C., Morlacchi S., Viola A., Mantovani A., Sica A. Hypoxia-mediated regulation of macrophage functions in pathophysiology. Int. Immunol. 2013;25:67–75. doi: 10.1093/intimm/dxs110. [DOI] [PubMed] [Google Scholar]
- Rojas S., Martin A., Arranz M.J., Pareto D., Purroy J., Verdaguer E., Llop J., Gomez V., Gispert J.D., Millan O., Chamorro A., Planas A.M. Imaging brain inflammation with [(11)C]PK11195 by PET and induction of the peripheral-type benzodiazepine receptor after transient focal ischemia in rats. J. Cereb. Blood Flow Metab. 2007;27:1975–1986. doi: 10.1038/sj.jcbfm.9600500. [DOI] [PubMed] [Google Scholar]
- Schroeter M., Zickler P., Denhardt D.T., Hartung H.P., Jander S. Increased thalamic neurodegeneration following ischaemic cortical stroke in osteopontin-deficient mice. Brain. 2006;129:1426–1437. doi: 10.1093/brain/awl094. [DOI] [PubMed] [Google Scholar]
- Schroeter M., Dennin M.A., Walberer M., Backes H., Neumaier B., Fink G.R., Graf R. Neuroinflammation extends brain tissue at risk to vital peri-infarct tissue: a double tracer [11C]PK11195- and [18F]FDG-PET study. J. Cereb. Blood Flow Metab. 2009;29:1216–1225. doi: 10.1038/jcbfm.2009.36. [DOI] [PubMed] [Google Scholar]
- Sica A., Melillo G., Varesio L. Hypoxia: a double-edged sword of immunity. J. Mol. Med. (Berl) 2011;89:657–665. doi: 10.1007/s00109-011-0724-8. [DOI] [PubMed] [Google Scholar]
- Stence N., Waite M., Dailey M.E. Dynamics of microglial activation: a confocal time-lapse analysis in hippocampal slices. Glia. 2001;33:256–266. [PubMed] [Google Scholar]
- Swanson L.W. Acad. Press; 2003. [BOOK] Brain maps III: structure of the rat brain. [Google Scholar]
- Thiel A., Heiss W.-D. Imaging of microglia activation in stroke. Stroke. 2011;42:507–512. doi: 10.1161/STROKEAHA.110.598821. [DOI] [PubMed] [Google Scholar]
- Walberer M., Backes H., Rueger M.A., Neumaier B., Endepols H., Hoehn M., Fink G.R., Schroeter M., Graf R. Potential of early [(18)F]-2-fluoro-2-deoxy-D-glucose positron emission tomography for identifying hypoperfusion and predicting fate of tissue in a rat embolic stroke model. Stroke. 2012;43:193–198. doi: 10.1161/STROKEAHA.111.624551. [DOI] [PubMed] [Google Scholar]
- Wise R.J., Rhodes C.G., Gibbs J.M., Hatazawa J., Palmer T., Frackowiak R.S., Jones T. Disturbance of oxidative metabolism of glucose in recent human cerebral infarcts. Ann. Neurol. 1983;14:627–637. doi: 10.1002/ana.410140605. [DOI] [PubMed] [Google Scholar]
- Yang Z., Zhao T.-Z., Zou Y.-J., Zhang J.H., Feng H. Hypoxia induces autophagic cell death through hypoxia-inducible factor 1α in microglia. PLoS One. 2014;9 doi: 10.1371/journal.pone.0096509. [DOI] [PMC free article] [PubMed] [Google Scholar]