Abstract
Interleukin-33 (IL-33) is a member of the IL-1 cytokine superfamily that potently drives production of a variety of cytokines and contributes to the pathogenesis of inflammatory diseases. IL-33 is a nuclear protein and is released from apoptotic or necrotic cells. Serum IL-33 levels are increased in various diseases, such as atopic dermatitis, chronic hepatitis C infection, and asthma. Here, we show that red blood cells (RBCs) are one of the major sources of plasma IL-33. IL-33 levels are significantly increased in supernatants from lysed RBCs. Plasma IL-33 levels are increased in patients during hemolysis and plasma IL-33 levels show a positive correlation with degree of hemolysis. IL-33 protein and mRNA levels were detected in the late stages of differentiation in ex vivo primary human erythroid progenitor cell cultures, suggesting that IL-33 is expressed during maturation of RBCs. Furthermore, hemoglobin depleted red cell lysates induced IL-8 expression in human epithelial cells. This effect was attenuated in IL-33 decoy receptor expressing cells and was enhanced in IL-33 receptor expressing cells. These results suggest that erythroid progenitor cells produce IL-33 and circulating RBCs represent a major source of IL-33 that is released upon hemolysis.
Introduction
Interluekin-33 (IL-33), a relatively new member of the IL-1 cytokine superfamily has been reported to play a pathogenic role in inflammatory diseases including acute lung injury (ALI) (1, 2), asthma (3, 4), pulmonary fibrosis (5, 6), and rheumatoid arthritis (RA) (7–9). IL-33 binds to ST2, a member of the IL-1 receptor/Toll-like receptor superfamily. ST2 protein consists of two types: a soluble (sST2, a decoy receptor) and a membrane-bound (ST2L) isoform (10–12). IL-33 activates the MAPK signal transduction cascade and increases chemokine release through ligating to ST2L (2, 7, 10, 13). We and others have shown that down-regulation of ST2L attenuated IL-33-induced IL-8 release in human lung epithelial cells (2, 13). ST2 protein expression is regulated at the transcriptional (14, 15) and post-translational level (2). Post-translational ST2L regulatory mechanisms include phosphorylation and ubiquitination (2). The mechanisms that regulate IL-33 expression are incompletely understood. IL-33 is localized to nuclei of fibroblasts (16), endothelial cells (11, 17, 18), and epithelial cells (17, 19, 20). It is released from apoptotic and necrotic cells and is considered to be a “danger signaling” molecule (21–23). Increased serum IL-33 levels have been detected in patients with atopic dermatitis (24), RA (25, 26), asthma (27), and scleroderma (28); however, the source of increased IL-33 in these conditions has not been well studied.
Hemolysis is a general term for excessive breakdown of red blood cells (RBCs). Hemolysis can occur within the circulatory system (intravascular hemolysis) or in the reticuloendothelial system (extravascular hemolysis) (29). Hemolysis also occurs during storage of RBCs and the amount of storage hemolysis increases with the length of time in storage (30). In mammals, circulating mature RBCs maintain a very specialized flexible biconcave discoid shape. Mature RBCs are enucleate and lack organelles providing maximum space for their primary cargo hemoglobin. Circulating RBCs have a limited lifespan (120 days in humans) in the circulation and old RBCs are removed from the circulation by macrophages in the spleen; thus extravascular hemolysis is part of the natural life cycle of a circulating RBC. Intravascular hemolysis can be caused by bacterial infections, toxins, drugs, medications, autoimmune responses and alloimmune responses (31–33). Intravascular hemolysis results in the release of RBC contents into the circulation, which when excessive can cause more hemolysis and vascular dysfunction. Storage hemolysis also causes the release of potentially harmful, vasoactive, RBC-derived components into the RBC unit prior to transfusion into patients (30). These storage related changes may harm patients when older RBC units are transfused into patients and contribute to the RBC “storage lesion” (30). Accumulating evidence indicates that stored RBCs have increased cytokine content. Levels of IL-1 and IL-8 are significantly higher in RBC units that have been stored for 40 days compared to the levels of these cytokines observed in freshly collected RBC units (34). Darbonne, WC et al. demonstrated that 125I-labeled IL-8 rapidly and efficiently bound to RBCs (35). In addition to IL-8, RBCs also bind monocyte chemotactic peptide-1 (MCP-1) (35). A recent study from Lee JS, et. al., showed that longtime storage of RBCs increases the production of IL-8-bound RBC-derived microparticles (36). RBCs also bind insulin and insulin-like growth factors (37, 38). These results support the role of circulating RBCs as carriers of bioactive peptides including cytokines. Diffuse alveolar hemorrhage plays a critical role in the pathogenesis of ALI (39, 40). Recent studies have demonstrated that hemolysis induces inflammatory responses (34, 35, 37, 38, 41, 42); however, the mechanisms have not been well characterized.
Here, we report that RBCs contain IL-33. This is the first study to demonstrate IL-33 expression in differentiating erythroid progenitor cells, and IL-33 is released during hemolysis. IL-33 release during hemolysis may contribute to hemolysis-induced inflammatory responses.
Materials and methods
Cell culture and reagents
Purified human CD34+ progenitor cells were derived from GCSF–treated peripheral blood cells of healthy donors. These cells were grown at 37°C with 5% CO2 in serum-free medium consisting of Iscove’s modified Dulbecco’s medium (IMDM) with 1-thioglycerol, BIT9500 supplement (BITS) (Stem Cell Technologies), BSA (Sigma-Aldrich), and the indicated cytokines (PeproTech). The cells initially underwent 72 h of expansion with 100 ng/ml SCF (PeproTech), 100 ng/ml FMS-like tyrosine kinase 3 ligand (FLT3 ligand) (PeproTech), 100 ng/ml thrombopoietin (TPO) (PeproTech), and 50 ng/ml IL-3 (PeproTech). After expansion cells were then seeded in erythroid differentiation medium, which contains recombinant human erythropoietin at 4.5 U/ml (Procrit; Amgen), 10 ng/ml SCF and BIT9500 supplement. Human bronchial epithelial cells (Beas2B) were grown at 37°C with 5% CO2 in DMEM medium with 10% FBS. Horseradish peroxidase-conjugated goat anti-rabbit and anti-mouse antibodies, reverse transcriptase, and real-time PCR SosoFast reagents were purchased from Bio-Rad Laboratories (Hercules, CA). Amicon Ultra centrifuge filters were purchased from EMD Millipore (Billerica, MA). Anti-IL-33 antibody was from R&D Systems (Minneapolis, MN). Anti-LarminA/C antibody was from Santa Cruz (Dallas, TX). Anti-GAPDH and β-actin antibodies and RBC lysis buffer were from Sigma (St. Louis, MO).
RBC lysates preparation
Human or mouse whole blood was collected in EDTA-treated blood collection tubes, followed by centrifugation at 500 g for 10 min. The RBC fraction was transferred to new tubes and split into two groups: the non-lysed control group was incubated in isoosmotic 0.9% NaCl, the hemolyzed group was incubated with RBC lysis buffer. Both groups were incubated at room temperature for 10 minutes. After a brief centrifugation at 500 g for 10 min, the supernatants of the two groups were subjected to IL-33 immunoblotting.
Hemoglobin free RBC lysate preparation
To remove hemoglobin (64 kDa) from RBC lysates, Amicon ultra (50 kDa) cutoff filter was used. Briefly, RBC lysates were subjected to Amicon ultra (50 kDa) cutoff filter, followed by a centrifugation at 5,000 g for 20 min.
Immunoblotting
Cells were washed with cold PBS and collected in lysis buffer containing 20 mM Tris-HCl (pH 7.4), 150 mM NaCl, 2 mM EGTA, 5 mM β-glycerophosphate, 1 mM MgCl2, 1% Triton X-100, 1 mM sodium orthovanadate, 10 μg/ml protease inhibitors, 1 μg/ml aprotinin, 1 μg/ml leupeptin, and 1 μg/ml pepstatin. Equal amounts of cell lysates (20 μg) or RBC supernatants were subjected to SDS-PAGE, electrotransferred to nitrocellulose membranes and immunoblotted with indicated antibodies.
RNA extraction and Real time RT-PCR
Total RNA were extracted from cells by TRIzol (Sigma) according manufacturer’s instructions. Reverse transcription was performed using a cDNA synthesis kit (Bio-Rad) and Real-time PCR was performed to assess cytokine expression using primers specific to human IL-33 and IL-8 mRNA transcripts. Amplicon expression in each sample was normalized to its 18S mRNA transcript level. The relative abundance of target mRNA in each sample was calculated using the relative crossing time ratio. As 2 raised to the negative of its threshold cycle value times 106 after being normalized to the abundance of its corresponding 18S [e.g., 2 −(IL-33R2 Threshold Cycle)/2 −(18S Threshold Cycle) × 106].
Statistics analysis
All results were subjected to statistical analysis using two-way analysis of variance, and, wherever appropriate, analyzed by Student–Newman-Keuls test. Data are expressed as mean ± S.D.. p<0.05 were considered statistically significant.
Results and Discussion
IL-33 exists in RBC lysates
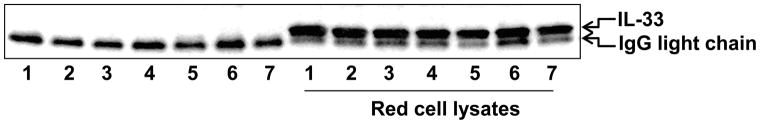
Circulating IL-33 levels are increased in patients with allergic, inflammatory, rheumatologic, and infectious diseases (5, 23, 24, 26–28). However, the source of IL-33 has not been well characterized. RBCs have been shown to bind plasma cytokines, growth factors, and chemokines (35, 37, 38). To investigate whether RBCs are one of the sources of plasma IL-33, we collected mouse whole blood, and compared IL-33 levels in supernatants from lysed RBCs and non-lysed RBC controls by immunoblotting as described in the Materials and Methods. Figure 1 shows that the supernatants from hemolyzed samples contain much higher levels of IL-33 compared to non-lysed controls. These results suggest that IL-33 exists in RBC lysates. This is a novel finding and has important implications in hemolytic diseases and RBC storage in blood banking. RBCs are known to bind IL-8, MCP-1, insulin, and insulin-like growth factors (35, 37, 38), but have not been shown to bind IL-33 until now. IL-8 and MCP-1 bind to the Duffy antigen on circulating RBCs (43). Insulin binds to the insulin receptor on the surface of RBCs (44). Lee JS et al. has also shown that microparticles from RBCs contain IL-8 (36), suggesting that IL-8 is one of internal components in RBC-derived microparticles. The localization of IL-33 in the RBCs remains unclear.
Figure 1. IL-33 is released during hemolysis in vivo.
Isolated mouse RBCs (7 samples/group) were incubated with or without RBC lysis buffer for 10 min. After a brief centrifugation, supernatants were subjected to SDS-PAGE and immunoblotting analysis with an anti-IL-33 antibody.
IL-33 levels are increased in sickle cell patients with higher levels of hemolysis
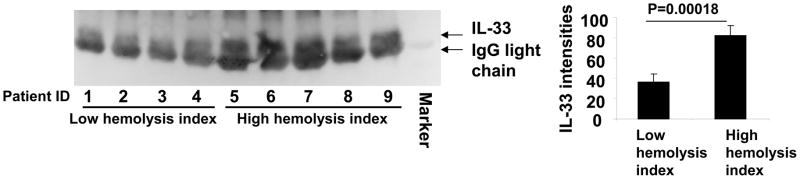
To further investigate the role of the RBC as a storage pool for circulating IL-33, we compared the IL-33 levels in plasma from sickle cell patients known to have undergone low levels or high levels of hemolysis (Table 1). Sickle cell disease patients from our previous Walk-PHASST study were randomly selected within the highest and lowest quartiles of hemolytic component. The hemolytic component is a composed index for hemolysis based on a principle component analysis of factors associated with hemolysis, including lactate dehydrogenase, aspartate, aminotransferase, reticulocyte percentage and total bilirubin in 415 hemoglobin SS patients had a mean of 0 (SD=1.50) (45). IL-33 levels are significantly higher in plasma samples from high hemolyzers compared to the plasma samples from low hemolyzers (Fig. 2). These results support the idea that RBCs are a major source of IL-33 in the circulation. These results also suggest that plasma IL-33 levels may be used as a biomarker for hemolytic disorders, such as sickle cell disease. This finding connects RBC damage during hemolysis directly to a cytokine release.
Table 1.
Plasma samples from subjects with low and high hemolytic component.
| Sample ID | Hemolytic component (relative unit) | Hemolytic group | age |
|---|---|---|---|
| 1 | −4.0508 | Low | 47.4 |
| 2 | −2.529 | Low | 22.3 |
| 3 | −2.4477 | Low | 46.9 |
| 4 | −2.4456 | Low | 20.7 |
| 5 | 2.76036 | High | 42.9 |
| 6 | 2.82356 | High | 48.6 |
| 7 | 2.9443 | High | 47.8 |
| 8 | 3.23965 | High | 37.0 |
| 9 | 3.58382 | High | 41.7 |
Plasma samples of sickle cell disease patient from the Walk-PHASST study were used for Western blot analysis (Fig. 2). Patients were randomly selected within the highest and lowest quartiles of hemolytic component of the Walk-PHASST cohort. The hemolytic component is a composed index for hemolysis based on a principle component analysis of factors associated with hemolysis, including lactate dehydrogenase, aspartate, aminotransferase, reticulocyte percentage and total bilirubin in 415 hemoglobin SS patients had a mean of 0 (SD=1.50) (45).
Figure 2. Plasma IL-33 levels are increased in sickle cell patients with higher hemolysis.
A. IL-33 levels in plasma from patients with low hemolytic rates and high hemolytic rates were analyzed by immunoblotting with an anti-IL-33 antibody. B. Analysis of immunoblot densitometry was performed using Image J software.
Hematopoitic progenitor cells express IL-33
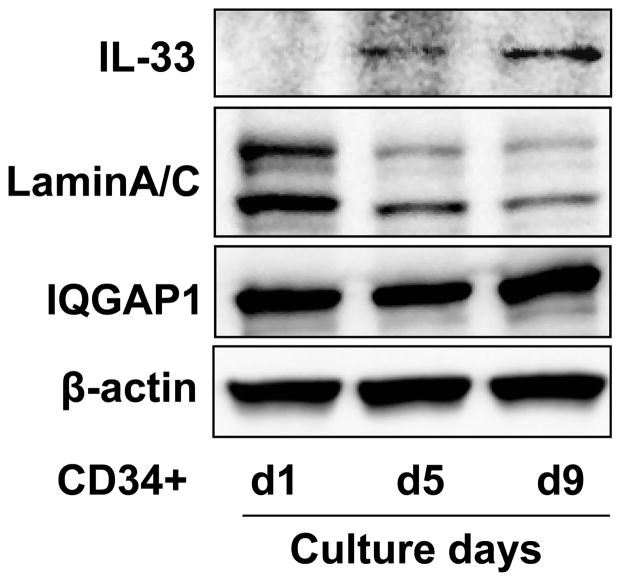
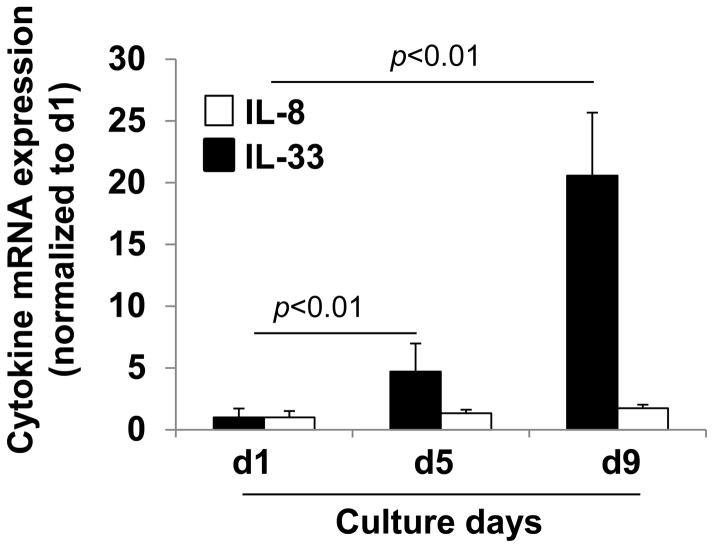
IL-33 is a nuclear protein. It is unclear how IL-33 gets inside of anucleate RBCs. A speculative mechanism is that IL-33 is left behind in the reticulocyte after enucleation of erythroid progenitor cells during the late stages of maturation. To investigate IL-33 synthesis during human erythropoiesis, IL-33 protein expression was examined during different stages of erythroid progenitor cell differentiation using an ex vivo human CD34+ hematopoietic progenitor cell culture system as described in the Materials and Methods. We collected erythroid progenitor cells on days 1, 5, and 9 of erythroid differentiation. As shown in Figure 3, human erythroid progenitor cells show a progressive decrease in expression of the nuclear protein, LaminA/C, while IL-33 expression progressively increases, peaking at day 9. Cytoplasm proteins (IQGAP1 and β-actin) remain no changes. Reduction of LaminA/C indicates the erythroid progenitor cells were differentiated to mature RBC-like cells. IL-33 mRNA, but not IL-8 mRNA levels progressively increase as erythropoiesis proceeds in the human erythroid progenitor cell cultures (Fig. 4). These results suggest that IL-33 is synthesized during RBC differentiation and maturation, and that IL-33 is not stuck on the membrane of RBCs, but is soluble and inside the RBCs. CD34+ progenitors from chronic myeloid leukemia patients have been shown to express ST2L, proliferate and produce cytokine in response to IL-33 (46), while this study is the first to demonstrate CD34+ progenitors also express its ligand, IL-33. Watari K et al. reported that human hematopoietic progenitor cells produce IL-1β (47). These results also support the idea that IL-33 is inside circulating erythrocytes.
Figure 3. IL-33 protein express in erythroid progenitor cells.
Primary human CD34+ hematopoitic progenitor cells were expanded and subjected to unilineage erythroid differentiation culture conditions. Cells were harvested on days 1, 5, and 9 of erythroid differentiation. IL-33, LaminA/C, GAPDH, and β-actin expression were analyzed by immunoblotting.
Figure 4. IL-33 mRNA expression in erythroid progenitor cells.
Primary human CD34+ hematopoitic progenitor cells were expanded and differentiated as described for Figure 3. Erythroid progenitor cells were harvested on days 1, 5, and 9 of erythroid differentiation. Total RNA was extracted and IL-33 and IL-8 mRNA expression were analyzed by real-time PCR.
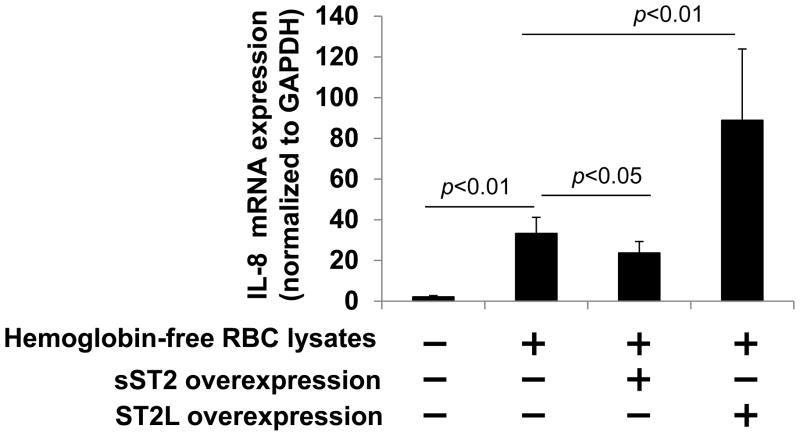
Hemoglobin depleted RBCs lysates increase IL-8 mRNA expression in human lung epithelial cells
We and others have shown that IL-33 treatment increases IL-8 expression in human lung epithelial and endothelial cells (2, 13, 21). To investigate if the IL-33 contained in RBC lysates can increase IL-8 expression, independent of hemoglobin, we removed hemoglobin from lysed RBCs using size exclusion filters, since hemoglobin can affect cytokine release. Beas2B cells were treated with hemoglobin depleted RBC lysates for 3 h and IL-8 mRNA expression was determined by real-time PCR. As shown in Fig. 5, hemoglobin depleted RBC lysates induced IL-8 mRNA expression ~23 fold, this effect was attenuated in IL-33 decoy receptor, sST2-overexpressing cells. Further, hemoglobin depleted RBC lysates-induced IL-8 mRNA expression was enhanced in IL-33 receptor, ST2L over-expressing cells. These results suggest that hemolysis via the release of intra-crythrocyte IL-33 may contribute to IL-8 expression (Fig. 6). Hemolysis can be caused by infection, inflammation, oxidative damage, auto-antibodies, allo-antibodies, toxins, drugs, medications, and inherited genetic lesions (29–32). Hemolysis also contributes to inflammatory responses, endothelial barrier dysfunction, and platelet activation, the effects are similar to IL-33 treatment. This study provides new evidence that IL-33 is synthesized in erythroid progenitor cells and can be released from RBCs undergoing hemolysis. IL-33 may be involved in hemolysis-induced inflammatory responses.
Figure 5. Hemoglobin depleted RBC lysates-induced IL-8 mRNA expression is regulated by IL-33 receptor expression.
RBC lysates were filtered through a 50 kDa cutoff Amicon Ultra column to remove hemoglobin. Beas2B cells were transfected with sST2 or ST2L plasmids for 48 h prior to treatment with the hemoglobin depleted filtrates from hemolyzed RBCs for additional 3 h and total RNA was extracted. IL-8 mRNA levels were determined by real-time PCR.
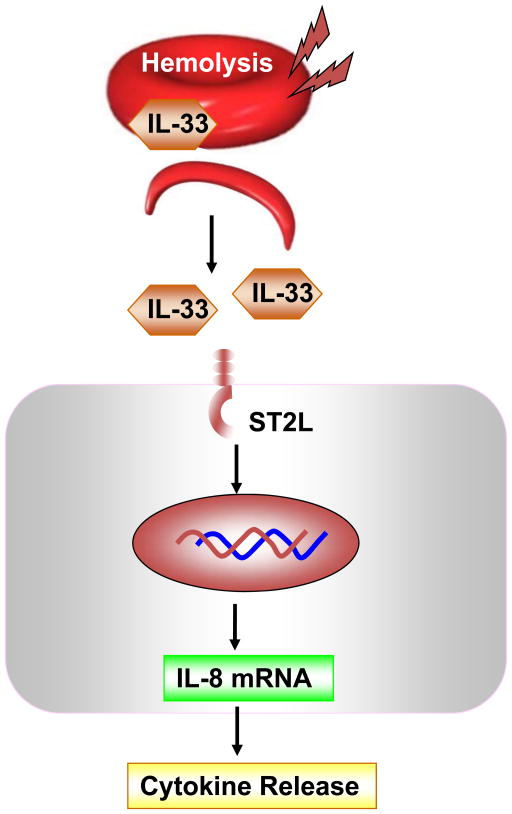
Figure 6. IL-33 releases during hemolysis.
Hemolysis induces release of IL-33 from disrupted RBCs. IL-33 modulates cytokine release through ligation to its receptor, ST2L on surrounding cells.
Acknowledgments
This study was supported by the Vascular Medical Institute seed fund (to Y.Z. and G.B.), US National Institutes of Health RO1 HL112791 (to Y.Z.), and American Heart Association awards 12SDG9050005 (J.Z.).
This study was supported by the US National Institutes of Health (R01 HL01916 and R01HL112791 to Y.Z.), American Heart Association awards 12SDG9050005 (J.Z.), and Vascular Medicine Institute Research Grant (to Y.Z.).
Abbreviations
- RBC
red blood cell
- IL
interleukin
- RA
rheumatoid arthritis
- IMDM
Iscove’s modified Dulbecco’s medium
- ALI
acute lung injury
Footnotes
All authors declare no conflict of interest.
References
- 1.Lefrancais E, Roga S, Gautier V, Gonzalez-de-Peredo A, Monsarrat B, Girard JP, et al. IL-33 is processed into mature bioactive forms by neutrophil elastase and cathepsin G. Proceedings of the National Academy of Sciences of the United States of America. 2012 Jan 31;109(5):1673–8. doi: 10.1073/pnas.1115884109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zhao J, Wei J, Mialki RK, Mallampalli DF, Chen BB, Coon T, et al. F-box protein FBXL19-mediated ubiquitination and degradation of the receptor for IL-33 limits pulmonary inflammation. Nature immunology. 2012 Jul;13(7):651–8. doi: 10.1038/ni.2341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Moffatt MF, Gut IG, Demenais F, Strachan DP, Bouzigon E, Heath S, et al. A large-scale, consortium-based genomewide association study of asthma. The New England journal of medicine. 2010 Sep 23;363(13):1211–21. doi: 10.1056/NEJMoa0906312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Prefontaine D, Lajoie-Kadoch S, Foley S, Audusseau S, Olivenstein R, Halayko AJ, et al. Increased expression of IL-33 in severe asthma: evidence of expression by airway smooth muscle cells. Journal of immunology. 2009 Oct 15;183(8):5094–103. doi: 10.4049/jimmunol.0802387. [DOI] [PubMed] [Google Scholar]
- 5.Luzina IG, Kopach P, Lockatell V, Kang PH, Nagarsekar A, Burke AP, et al. Interleukin-33 potentiates bleomycin-induced lung injury. American journal of respiratory cell and molecular biology. 2013 Dec;49(6):999–1008. doi: 10.1165/rcmb.2013-0093OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Li D, Guabiraba R, Besnard AG, Komai-Koma M, Jabir MS, Zhang L, et al. IL-33 promotes ST2-dependent lung fibrosis by the induction of alternatively activated macrophages and innate lymphoid cells in mice. The Journal of allergy and clinical immunology. 2014 Jun 27; doi: 10.1016/j.jaci.2014.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Palmer G, Talabot-Ayer D, Lamacchia C, Toy D, Seemayer CA, Viatte S, et al. Inhibition of interleukin-33 signaling attenuates the severity of experimental arthritis. Arthritis and rheumatism. 2009 Mar;60(3):738–49. doi: 10.1002/art.24305. [DOI] [PubMed] [Google Scholar]
- 8.Yuan FL, Li X, Lu WG, Li CW, Xu RS, Dong J. IL-33: a promising therapeutic target for rheumatoid arthritis? Expert opinion on therapeutic targets. 2011 May;15(5):529–34. doi: 10.1517/14728222.2011.560838. [DOI] [PubMed] [Google Scholar]
- 9.Xu D, Jiang HR, Kewin P, Li Y, Mu R, Fraser AR, et al. IL-33 exacerbates antigen-induced arthritis by activating mast cells. Proceedings of the National Academy of Sciences of the United States of America. 2008 Aug 5;105(31):10913–8. doi: 10.1073/pnas.0801898105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Schmitz J, Owyang A, Oldham E, Song Y, Murphy E, McClanahan TK, et al. IL-33, an interleukin-1-like cytokine that signals via the IL-1 receptor-related protein ST2 and induces T helper type 2-associated cytokines. Immunity. 2005 Nov;23(5):479–90. doi: 10.1016/j.immuni.2005.09.015. [DOI] [PubMed] [Google Scholar]
- 11.Carriere V, Roussel L, Ortega N, Lacorre DA, Americh L, Aguilar L, et al. IL-33, the IL-1-like cytokine ligand for ST2 receptor, is a chromatin-associated nuclear factor in vivo. Proceedings of the National Academy of Sciences of the United States of America. 2007 Jan 2;104(1):282–7. doi: 10.1073/pnas.0606854104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chackerian AA, Oldham ER, Murphy EE, Schmitz J, Pflanz S, Kastelein RA. IL-1 receptor accessory protein and ST2 comprise the IL-33 receptor complex. Journal of immunology. 2007 Aug 15;179(4):2551–5. doi: 10.4049/jimmunol.179.4.2551. [DOI] [PubMed] [Google Scholar]
- 13.Yagami A, Orihara K, Morita H, Futamura K, Hashimoto N, Matsumoto K, et al. IL-33 mediates inflammatory responses in human lung tissue cells. Journal of immunology. 2010 Nov 15;185(10):5743–50. doi: 10.4049/jimmunol.0903818. [DOI] [PubMed] [Google Scholar]
- 14.Zhao J, Chen Q, Li H, Myerburg M, Spannhake EW, Natarajan V, et al. Lysophosphatidic acid increases soluble ST2 expression in mouse lung and human bronchial epithelial cells. Cellular signalling. 2012 Jan;24(1):77–85. doi: 10.1016/j.cellsig.2011.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Baba Y, Maeda K, Yashiro T, Inage E, Kasakura K, Suzuki R, et al. GATA2 is a critical transactivator for the human IL1RL1/ST2 promoter in mast cells/basophils: opposing roles for GATA2 and GATA1 in human IL1RL1/ST2 gene expression. The Journal of biological chemistry. 2012 Sep 21;287(39):32689–96. doi: 10.1074/jbc.M112.374876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nomura K, Kojima T, Fuchimoto J, Obata K, Keira T, Himi T, et al. Regulation of interleukin-33 and thymic stromal lymphopoietin in human nasal fibroblasts by proinflammatory cytokines. The Laryngoscope. 2012 Jun;122(6):1185–92. doi: 10.1002/lary.23261. [DOI] [PubMed] [Google Scholar]
- 17.Moussion C, Ortega N, Girard JP. The IL-1-like cytokine IL-33 is constitutively expressed in the nucleus of endothelial cells and epithelial cells in vivo: a novel ‘alarmin’? PloS one. 2008;3(10):e3331. doi: 10.1371/journal.pone.0003331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kuchler AM, Pollheimer J, Balogh J, Sponheim J, Manley L, Sorensen DR, et al. Nuclear interleukin-33 is generally expressed in resting endothelium but rapidly lost upon angiogenic or proinflammatory activation. The American journal of pathology. 2008 Oct;173(4):1229–42. doi: 10.2353/ajpath.2008.080014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhang L, Lu R, Zhao G, Pflugfelder SC, Li DQ. TLR-mediated induction of pro-allergic cytokine IL-33 in ocular mucosal epithelium. The international journal of biochemistry & cell biology. 2011 Sep;43(9):1383–91. doi: 10.1016/j.biocel.2011.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shan J, Oshima T, Muto T, Yasuda K, Fukui H, Watari J, et al. Epithelial-derived nuclear IL-33 aggravates inflammation in the pathogenesis of reflux esophagitis. Journal of gastroenterology. 2014 Aug 17; doi: 10.1007/s00535-014-0988-1. [DOI] [PubMed] [Google Scholar]
- 21.Cayrol C, Girard JP. IL-33: an alarmin cytokine with crucial roles in innate immunity, inflammation and allergy. Current opinion in immunology. 2014 Sep 29;31C:31–7. doi: 10.1016/j.coi.2014.09.004. [DOI] [PubMed] [Google Scholar]
- 22.Luthi AU, Cullen SP, McNeela EA, Duriez PJ, Afonina IS, Sheridan C, et al. Suppression of Interleukin-33 Bioactivity through Proteolysis by Apoptotic Caspases. Immunity. 2009 Jul 17;31(1):84–98. doi: 10.1016/j.immuni.2009.05.007. English. [DOI] [PubMed] [Google Scholar]
- 23.Zhao WH, Hu ZQ. The enigmatic processing and secretion of interleukin-33. Cell Mol Immunol. 2010 Jul;7(4):260–2. doi: 10.1038/cmi.2010.3. English. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tamagawa-Mineoka R, Okuzawa Y, Masuda K, Katoh N. Increased serum levels of interleukin 33 in patients with atopic dermatitis. Journal of the American Academy of Dermatology. 2014 May;70(5):882–8. doi: 10.1016/j.jaad.2014.01.867. [DOI] [PubMed] [Google Scholar]
- 25.Kageyama Y, Torikai E, Tsujimura K, Kobayashi M. Involvement of IL-33 in the pathogenesis of rheumatoid arthritis: the effect of etanercept on the serum levels of IL-33. Modern rheumatology/the Japan Rheumatism Association. 2012 Feb;22(1):89–93. doi: 10.1007/s10165-011-0480-1. [DOI] [PubMed] [Google Scholar]
- 26.Hong YS, Moon SJ, Joo YB, Jeon CH, Cho ML, Ju JH, et al. Measurement of interleukin-33 (IL-33) and IL-33 receptors (sST2 and ST2L) in patients with rheumatoid arthritis. Journal of Korean medical science. 2011 Sep;26(9):1132–9. doi: 10.3346/jkms.2011.26.9.1132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Raeiszadeh Jahromi S, Mahesh PA, Jayaraj BS, Madhunapantula SR, Holla AD, Vishweswaraiah S, et al. Serum levels of IL-10, IL-17F and IL-33 in patients with asthma: a case-control study. The Journal of asthma: official journal of the Association for the Care of Asthma. 2014 Dec;51(10):1004–13. doi: 10.3109/02770903.2014.938353. [DOI] [PubMed] [Google Scholar]
- 28.Terras S, Opitz E, Moritz RK, Hoxtermann S, Gambichler T, Kreuter A. Increased serum IL-33 levels may indicate vascular involvement in systemic sclerosis. Annals of the rheumatic diseases. 2013 Jan;72(1):144–5. doi: 10.1136/annrheumdis-2012-201553. [DOI] [PubMed] [Google Scholar]
- 29.Wallace HW, Coburn RF. Relative thresholds for acute intravascular and extravascular mechanical hemolysis. The Journal of thoracic and cardiovascular surgery. 1974 Nov;68(5):792–6. [PubMed] [Google Scholar]
- 30.Lee JS, Gladwin MT. Bad blood: the risks of red cell storage. Nature medicine. 2010 Apr;16(4):381–2. doi: 10.1038/nm0410-381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Thomas SL. Intravascular hemolysis: the sacrifice of few. Blood. 2014 Sep 25;124(13):2011–2. doi: 10.1182/blood-2014-08-595447. [DOI] [PubMed] [Google Scholar]
- 32.Salama A. Drug-induced immune hemolytic anemia. Expert opinion on drug safety. 2009 Jan;8(1):73–9. doi: 10.1517/14740330802577351. [DOI] [PubMed] [Google Scholar]
- 33.Roy-Burman A, Glader BE. Resolution of severe Donath-Landsteiner autoimmune hemolytic anemia temporally associated with institution of plasmapheresis. Critical care medicine. 2002 Apr;30(4):931–4. doi: 10.1097/00003246-200204000-00039. [DOI] [PubMed] [Google Scholar]
- 34.Kristiansson M, Soop M, Saraste L, Sundqvist KG. Cytokines in stored red blood cell concentrates: promoters of systemic inflammation and simulators of acute transfusion reactions? Acta anaesthesiologica Scandinavica. 1996 Apr;40(4):496–501. doi: 10.1111/j.1399-6576.1996.tb04475.x. [DOI] [PubMed] [Google Scholar]
- 35.Darbonne WC, Rice GC, Mohler MA, Apple T, Hebert CA, Valente AJ, et al. Red blood cells are a sink for interleukin 8, a leukocyte chemotaxin. The Journal of clinical investigation. 1991 Oct;88(4):1362–9. doi: 10.1172/JCI115442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Xiong Z, Cavaretta J, Qu L, Stolz DB, Triulzi D, Lee JS. Red blood cell microparticles show altered inflammatory chemokine binding and release ligand upon interaction with platelets. Transfusion. 2011 Mar;51(3):610–21. doi: 10.1111/j.1537-2995.2010.02861.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Polychronakos C, Ruggere MD, Benjamin A, Posner BI, Guyda HJ. The role of cell age in the difference in insulin binding between adult and cord erythrocytes. The Journal of clinical endocrinology and metabolism. 1982 Aug;55(2):290–4. doi: 10.1210/jcem-55-2-290. [DOI] [PubMed] [Google Scholar]
- 38.Morris AH, Joyce JL, Reiter EO. Increased insulin-like growth factor I binding to red blood cells of normal prepubertal children. Pediatric research. 1989 Apr;25(4):409–13. doi: 10.1203/00006450-198904000-00021. [DOI] [PubMed] [Google Scholar]
- 39.Chopra M, Reuben JS, Sharma AC. Acute lung injury:apoptosis and signaling mechanisms. Experimental biology and medicine. 2009 Apr;234(4):361–71. doi: 10.3181/0811-MR-318. [DOI] [PubMed] [Google Scholar]
- 40.Collard HR, Schwarz MI. Diffuse alveolar hemorrhage. Clinics in chest medicine. 2004 Sep;25(3):583–92. vii. doi: 10.1016/j.ccm.2004.04.007. [DOI] [PubMed] [Google Scholar]
- 41.Kiefmann R, Rifkind JM, Nagababu E, Bhattacharya J. Red blood cells induce hypoxic lung inflammation. Blood. 2008 May 15;111(10):5205–14. doi: 10.1182/blood-2007-09-113902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Escobar GA, Cheng AM, Moore EE, Johnson JL, Tannahill C, Baker HV, et al. Stored packed red blood cell transfusion up-regulates inflammatory gene expression in circulating leukocytes. Annals of surgery. 2007 Jul;246(1):129–34. doi: 10.1097/01.sla.0000264507.79859.f9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Neote K, Darbonne W, Ogez J, Horuk R, Schall TJ. Identification of a promiscuous inflammatory peptide receptor on the surface of red blood cells. The Journal of biological chemistry. 1993 Jun 15;268(17):12247–9. [PubMed] [Google Scholar]
- 44.Kappy MS. Insulin binding is a specific marker of fetal erythrocytes in ruminants. Journal of animal science. 1983 May;56(5):1153–60. doi: 10.2527/jas1983.5651153x. [DOI] [PubMed] [Google Scholar]
- 45.Nouraie M, Lee JS, Zhang Y, Kanias T, Zhao X, Xiong Z, et al. The relationship between the severity of hemolysis, clinical manifestations and risk of death in 415 patients with sickle cell anemia in the US and Europe. Haematologica. 2013 Mar;98(3):464–72. doi: 10.3324/haematol.2012.068965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Levescot A, Flamant S, Basbous S, Jacomet F, Feraud O, Anne Bourgeois E, et al. BCR-ABL-induced deregulation of the IL-33/ST2 pathway in CD34+ progenitors from chronic myeloid leukemia patients. Cancer research. 2014 May 15;74(10):2669–76. doi: 10.1158/0008-5472.CAN-13-2797. [DOI] [PubMed] [Google Scholar]
- 47.Watari K, Mayani H, Lee F, Dragowska W, Lansdorp PM, Schrader JW. Production of interleukin 1beta by human hematopoietic progenitor cells. The Journal of clinical investigation. 1996 Apr 1;97(7):1666–74. doi: 10.1172/JCI118593. [DOI] [PMC free article] [PubMed] [Google Scholar]