Abstract
The extracellular matrix (ECM) is a complex and dynamic scaffold that maintains tissue structure and dynamics. However, the view of the ECM as an inert architectural support has been increasingly challenged. The ECM is a vibrant meshwork, a crucial organizer of cellular microenvironments. It plays a direct role in cellular interactions regulating cell growth, survival, spreading, proliferation, differentiation and migration through the intricate relationship among cellular and acellular tissue components. This complex interrelationship preserves cardiac function during homeostasis; however it is also responsible for pathologic remodeling following myocardial injury. Therefore, enhancing our understanding of this cross-talk may provide mechanistic insights into the pathogenesis of heart failure and suggest new approaches to novel, targeted pharmacologic therapies. This review explores the implications of ECM-cell interactions in myocardial cell behavior and cardiac function at baseline and following myocardial injury.
Introduction
The mammalian heart is composed of diverse cell populations that collaborate to maintain cardiac function under homeostatic and pathological conditions. At the cellular level, the main permanent components of the heart are myocytes, fibroblasts, endothelial cells, pericytes and smooth muscle cells [1–3]. These cell types lie on a three-dimensional collagen network and maintain the electrical, chemical and biomechanical responsiveness of the organ, cooperating to maintain cardiac tissue stability required for both diastolic and systolic cardiac function. The arrangement of cardiac fibroblasts (CF) on this scaffold allows them to contract the collagen fibers that surround cardiomyocytes, exerting mechanical force on tissue and myocytes [4, 5]. Extracellular matrix (ECM) integrity is maintained via cell-cell and cell-ECM interactions, as well as through fibroblast-mediated ECM degradation and synthesis [2, 6–8]. In doing so, fibroblasts are considered “first responders” to a number of stimuli including chemical, mechanical and electrical signals [6]. CF are involved in many aspects of cardiac function, including heterotypic communication with cardiomyocytes to maintain the electrical activity [9–13], production of growth factors and cytokines [14], intercellular signaling with other CF [2, 10, 15, 16], and endothelial [17–21] cells that can impact cellular events such as angiogenesis [21–25], cell proliferation [26], cardiomyocyte hypertrophy [27] or apoptosis [28]. However, a key primary function of CF is the synthesis, homeostatic maintenance and degradation of the ECM, which acts as framework that integrates the activity of individual cells to coordinate the contractile function of the myocardium [29–32]. Cardiac ECM is a complex meshwork of fibers comprised of matrix proteins in which cardiac myocytes, fibroblasts, immune cells, cardiac progenitors, vascular cells and many other cell types reside. ECM has been classically thought to provide a structural framework for an organ. However, it is now well recognized that the ECM serves a number of additional functions. Hormones, growth factors, drugs, and other molecules travel in the extracellular space where they come in contact with ECM components. The versatility of cell-ECM interactions permits generation of tightly regulated adaptive and reparative responses through intracellular signals across the cell membrane.
Cardiac extracellular matrix
The cardiac ECM is a highly organized network that provides the architectural scaffold surrounding and connecting various cardiac cell populations. In addition to its function in tissue support, the myocardial ECM acts as a signal transducer for cell-cell communication modulating cell motility, survival and cell proliferation (Figure 1). Further, the ECM regulates other molecules in the interstitial space [33, 34] and distributes mechanical forces throughout the organ [3]. The ECM is also essential for efficient cardiac function via myocyte alignment, regulation of blood flow during contraction, compliance and maintenance of appropriate tissue tensile modulus. Therefore, the ECM is crucial to maintain appropriate cardiac integrity and pump function [35]. Conversely, disruption of ECM homeostasis is a central factor for cardiac dysfunction, pathologic remodeling and fibrosis following cardiac injury [3]. ECM homeostasis relies on a tight balance between matrix metalloproteinases (MMPs) and tissue inhibitors of metalloproteinases (TIMPs), which collectively regulate ECM components in the process of cardiac remodeling [36–38]. CF can also increase or decrease the rate of synthesis and degradation of the ECM depending on myocardial demands.
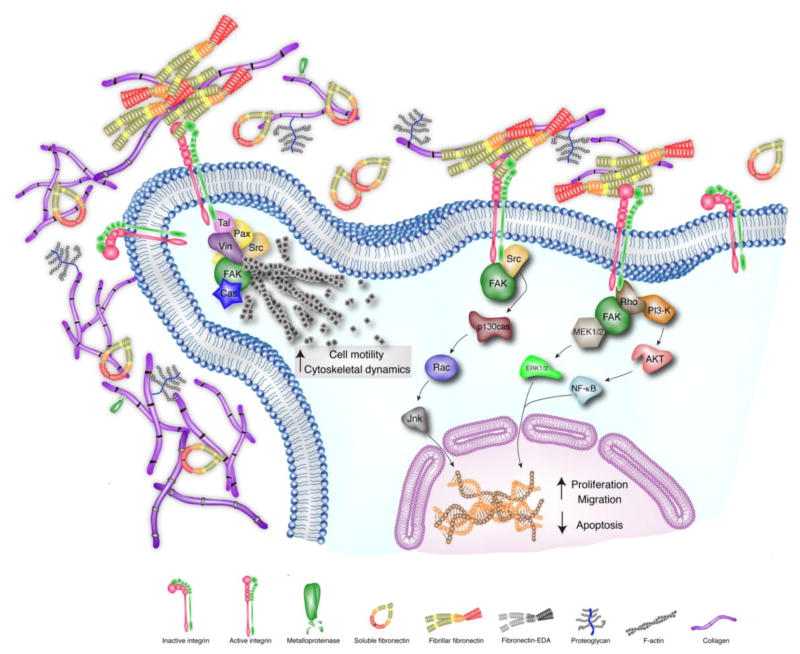
Figure 1. ECM-Cell Interactions in homeostatic myocardium.
Extracellular matrix proteins bind to integrin-cell surface receptors to trigger signaling pathways involved in cell spreading, cytoskeletal rearrangement, cell proliferation, survival and migration. As a result of the interaction between ECM proteins and the cell surface, integrins cluster and the cytoplasmic integrin domains synergize with focal-activated kinase proteins (FAK) to regulate the actin cytoskeleton reorganization and cell migration. Fibronectin and collagen promote cell proliferation, migration and cell survival primarily via FAK activation and downstream signaling pathways as well as the Rho cascade. Further detail of the downstream pathways represented in Figure 1 is described elsewhere [104, 105, 223–225].
The cardiac ECM is a dynamic and intricate network composed essentially of structural and non-structural proteins and sugars that are further subdivided into glycoproteins, proteoglycans and glycosaminoglycans. Some proteins serve a structural function, such as collagen (largely collagen I, ≈ 80%, and collagen III, ≈ 10%) [39, 40], whereas others have nonstructural roles, such as matricellular proteins. Glycoproteins such as fibronectin or laminin can play both structural and non-structural roles [41–43]. Moreover, the ECM is filled with a diverse assortment of growth factors, cytokines, matrikines and proteases such as MMPs and TIMPs [44–48].
ECM-Cell Interactions in homeostatic myocardium
Receptors for ECM-cell interaction
Cell adhesion is crucial for tissue formation, structure and integrity. The connection between the ECM and the cells that comprise the organ is critical for its optimal function. In this context, the cell surface possesses two types of ECM receptors: non-integrin and integrin receptors; their role in homeostasis and fibrosis are only partially understood.
Non integrin receptors
These include CD36, proteoglycans, and some laminin-binding proteins. The binding of collagen type I and IV to the proteoglycan CD44 plays an essential role in cell adhesion and movement [49].
Integrin receptors
The main mediators of ECM-cell interactions are integrins. Integrins are non-covalently associated, heterodimeric transmembrane receptors with more than 18 α and 8 β subunits identified in mammals; these subunits can combine to form at least 24 distinct receptors. The binding of integrins to ECM components (collagen, laminin, fibronectin, thrombospondin, tenascin-c, osteopontin and periostin [50]) transmits intracellular signaling events. Because the integrins do not possess enzymatic activity, they must trigger downstream molecules to transmit their signal(s) [50–52] (Figure 1). The integrin cytoplasmic domain is essential in this process and has been shown to bind numerous molecules such as calreticulin [53], focal adhesion kinase (FAK) [54], melusin [55] and muscle integrin-binding protein (MIBP) [56], the latter two being preferentially expressed in muscle [53, 55–57]. Integrin receptors also bind components of the cytoskeleton such as talin [58] and α-actinin [59]. Ultimately, signaling from the integrins may influence pathways through which other cellular effectors (such as growth factors) may also signal, including those requiring Akt, Raf, phosphoinositide 3-kinase (PI3-K), or mitogen-activated protein kinases (MAPKs) and extracellular signal–regulated kinases (ERKs) [60]. As a consequence, the integrins can influence a wide range of cellular functions, including cell spreading, proliferation, apoptosis, migration and differentiation [61–65] (Figure 1).
Intercellular communication via structural ECM proteins
Collagen
Collagens are present in the majority of the organs and constitute 2% to 4% of the human body [66]. Fibrillar collagen (which includes type I and III) is synthesized as a triple α-helix precursor polypeptide with the representative Gly-X-Y repeat sequence [37]; it is then proteolytically processed by removal of amino and carboxy terminal propeptides before insertion into nascent fibrils in the extracellular space. Collagen is the major structural protein of the cardiac interstitium and serves several key functions. First, collagen provides a scaffold on which muscle cells and blood vessels reside [67]. It also provides lateral connections between cells and muscle bundles to govern architecture [67, 68]; its tensile strength and resilience are important determinants of diastolic and systolic myocardial stiffness [69]. Collagen also serves to resist myocardial deformation, maintains shape, tensile modulus and wall thickness and prevents ventricular aneurysm and rupture [70, 71]. Collagen types I and III are the major components of the myocardial ECM and provide the myocardium with tensile strength (collagen I) and distensibility (collagen III) [39]. Myocytes are surrounded by a basement membrane of which the principal structural component is collagen type IV while collagen I and III are arranged in successive layers of organization. Apart from its architectural role, collagen is also involved in intracellular signal transduction. It has been reported that collagen can promote cell survival in vitro by inhibition of apoptosis, via a β1 integrin-dependent mechanism [72]. In addition, collagen participates in cell spreading through p130Cas phosphorylation via FAK-dependent and FAK-independent integrin receptor pathways [73]. Collagen is also implicated in the induction of proliferation via FAK activation and downstream signaling pathways (Src, MEK, PI3-kinase, and p38 MAPK) [70, 74] (Figure 1). Finally, collagen plays a key role in cell migration through the activation of FAK and PI3-K, leading to elevated Rac1 activity as a downstream consequence in activated cell migration [75, 76] (Figure 1).
Fibronectin
Fibronectin (FN) is a ubiquitous, large structural glycoprotein composed of two subunits linked by a pair of disulfide bridges at the C-termini. FN is a multidomain protein composed of a number of repeated modular structures organized into functional domains. The specific domains of FN can interact with multiple binding partners, including collagen, fibrin, fibulin, heparin, TGF-β and FN itself [77–82]. FN polymerization into the ECM is required for the deposition of collagen-I and thrombospondin-1 [81]. FN is present in a soluble form secreted by hepatocytes and in the plasma; it exists in an insoluble form synthesized by fibroblasts, epithelial cells and other differentiated cell types. Plasma FN can diffuse into tissues and be incorporated in the fibrillar matrix [83]. FN mRNA has three alternative splicing sites (termed EDA, EDB and IIICS [84]) generating up to 20 different variants in humans [85]. The levels of expression of the spliced variants and their relative proportions vary during embryonic development and in pathological processes [86–90]. EDA and EDB exons tend to be excluded in most adult tissues, whereas they are generally included during events comprising tissue rearrangements, such as wound healing [91]. In addition to the structural role of FN, this protein also plays a pivotal role in cell behavior through interaction with integrin receptors [92]. Association of the α5β1 integrin with FN results in local accumulation of signaling molecules and cytoskeletal components at sites of focal adhesions as well as stimulation of specific proteins associated with focal adhesion, including FAK [93–95], paxilin [96, 97], tensin [98] and p130cas [99, 100]. As a result of the interaction between FN and the cell surface, integrins cluster and the interaction of their cytoplasmic integrin domains with FAK [101, 102] leads to the recruitment of Rho GTPases, PKC, MAP and Src kinases that subsequently regulate key steps in actin cytoskeleton reorganization and a specific global patterning of gene expression with implications in cell migration [103] (Figure 1). Not only does FN play a relevant role in cell spreading and migration, but it is also crucial for cell growth, survival and proliferation through different α5β1 downstream signaling pathways involving NF-kappaB, which in turn, increases c-Myc and cyclin D1 expression, and decreased p21 and PTEN expression via PI3-K/Akt pathways [104] (Figure 1). In addition, FN stimulates caveolin-1 signaling to the RhoA-PI3-K/Akt-Erk 1/2 pathway, which appears to contribute to cell proliferation [105] (Figure 1). Inhibiting FN polymerization may provide a novel therapeutic approach. Inhibitory peptides of FN polymerization delivered in models of experimental liver and flow-induced vascular remodeling and fibrosis models attenuated excess collagen deposition as well as early leukocyte infiltration and cell proliferation. Excess deposition of FN and collagen characteristic of tissue remodeling were also attenuated by these inhibitory peptides [106, 107].
ECM-cell interactions in the injured myocardium
Under normal conditions, the ECM provides structural support for the heart, acts as a reservoir for cytokines and growth factors and provides a connection with surrounding cells that is important for transmission of extracellular cues (Figure 1). Following pathologic stimulation, injury or stress, the ECM undergoes remodeling of its structural components and matricellular protein levels [47, 108, 109]. CF are largely responsible for secretion and regulation of the ECM. CF are influenced by autocrine and paracrine signals via extracellular proteins from a variety of cell types in the heart and beyond (i.e. cytokines and growth factors). Upon cardiac injury, CF respond to these signals by transforming to smooth muscle actin-expressing “myofibroblasts,” leading to changes in proliferation and migration as well as secretion of ECM proteins to promote wound healing (Figure 2). Myofibroblasts secrete large amounts of ECM proteins including collagens, fibronectin, periostin, MMPs and their inhibitors, TIMPs [110, 111]. Specifically, CF have been shown to secrete MMP-1,-2,-3,-9,-13,-14 and TIMP-1,-2,-3 and -4 after injury or pathologic stimulation [14, 112, 113]. The transition of fibroblasts to myofibroblasts appears to be necessary for cardiac healing after injury. However, persistent myofibroblast activity leads to excessive accumulation of these ECM proteins and, ultimately, fibrosis. Importantly, the ECM proteins secreted from myofibroblasts serve as an intermediary network for intercellular communication by transducing intracellular signals via various cell surface receptors, often leading to the development of cardiac fibrosis, ventricular stiffening and dysfunction [3, 27, 110, 114–116] (Figure 2). In addition, ECM proteins secreted by CF are actively involved in inflammatory-mediated response following cardiac insult. There are several known proteins that are important in ECM-cell communication that play a role in cardiac pathophysiology.
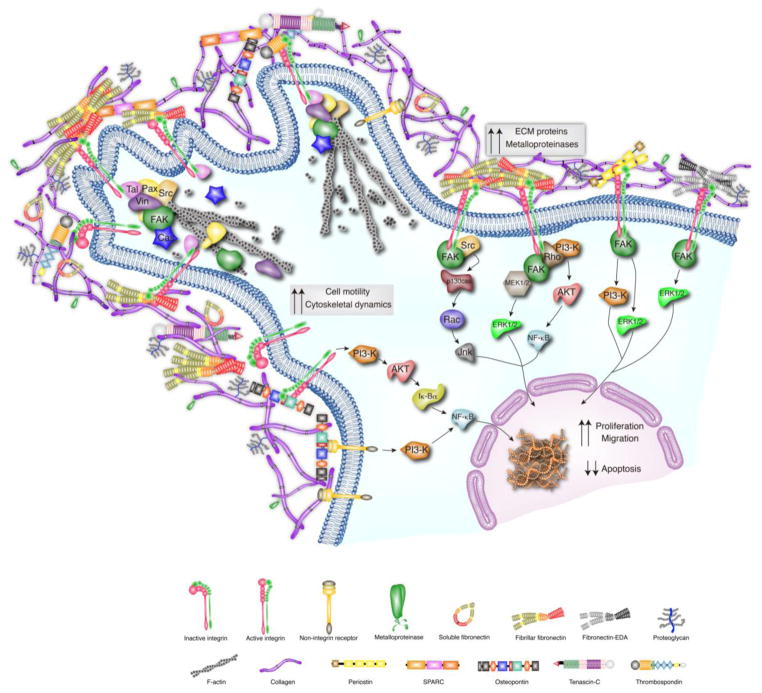
Figure 2. ECM-Cell Interactions in the injured myocardium.
Following cardiac injury, the ECM undergoes remodeling that involves increased expression of collagens, MMPs, EDA fibronectin and matricellular proteins (thrombospondins (TSP), periostin, SPARC, osteopontin (OPN), and tenascin-C (TNC)). Many of the increased ECM proteins induce intracellular signaling. In the injured myocardium, fibronectin, periostin, OPN, TNC and TSP interact with integrins and stimulate changes in FAK, PI-3K and RhoA activation. Matricellular proteins can also interact with non-integrin receptors to activate downstream signaling cascades. In addition, SPARC is known to bind to structural components of the ECM and promotes de-adhesion through disassembly of focal adhesions. This ECM-mediated intracellular signaling and de-adhesion effects lead to changes in cell motility, proliferation, migration and apoptosis. Further detail of the downstream pathways represented in Figure 2 is described elsewhere [127, 128, 149, 150, 153, 161, 173, 183, 197, 226].
Intercellular communication through Integrins
Integrin signaling has been found to play a role in cardiomyocyte hypertrophy. Specifically, hemodynamic overload induces changes in the heart such as release of cytokines and growth factors, myocardial stretch and remodeling of the ECM. These changes in the ECM often induce signaling through integrin receptors leading to changes in protein expression, growth and survival of myocytes. In vitro studies have indicated that integrin β1 mediates the phosphorylation of MAP kinase signaling pathways that are important in hypertrophy, such as ERK, p38 and JNK, in neonatal rat ventricular myocytes [117]. Likewise, stretching of CF, such as that which occurs in cardiac hypertrophy and dysfunction, induces signaling through ERK1/2 and JNK pathways that is integrin and matrix dependent [118]. Importantly, integrin inhibitors have shown promising results in Phase II and III in clinical trials in cancer patients [119]. In addition, pharmacological inhibition of integrins has shown attenuated effects in pathologic liver and lung fibrosis. These data suggest that blocking specific integrins may have a clinical benefit in the treatment of pathologic and adverse remodeling in patients with fibrotic diseases [120]
Intercellular communication via Matricellular proteins
Matricellular proteins are non-structural, secreted macromolecules that are nominally expressed in the normal myocardium, but are re-expressed following cardiac injury. These proteins interact with cell surface receptors, growth factors and other ECM proteins and act as a link between matrix proteins and cells in order to modulate cell behavior. The role of matricellular proteins as novel regulators of inflammation is also discussed further in this issue [121]). Matricellular proteins include thrombospondins (TSP), osteopontin (OPN), tenascin-C (TNC), periostin and SPARC (secreted protein acid and rich in cysteine)[122].
Thrombospondins are a matricellular family of multi-domain, multimeric and multifunctional proteins involved in ECM synthesis and deposition, cell-ECM interactions and tissue remodeling. TSP play an important role in stabilizing the ECM since these proteins bind to multiple components of the ECM, such as collagen, fibronectin, laminin, and heparin sulfate. TSP bind to cell surface receptors, such as integrins, CD36, and CD47 [123–126], modulating cell-ECM interactions, including focal adhesions [127, 128] (Figure 2). Although these matricellular proteins are not detectable in normal adult ECM, their expression increases greatly in response to cardiac injury [129–131] and participate in heart failure progression [132]. The TSP family consists of five members subdivided based on their structural organization and oligomerization status; TSP-1,-2 form trimers and -3, -4 and -5 form pentamers. TSP-1, -2, -3, -4 expression levels are significantly increased during hypertensive or pressure overload cardiomyopathy, contributing to cardiac remodeling and fibrosis. [133–141]. TSP-1 null mice display increased hypertrophy and LV dilation, impaired myofibroblast differentiation, reduced collagen expression, as well as increased MMP expression and activation in a pressure overload model of HF [141]. In addition, TSP-1 binds to the scavenger receptor CD36 and mediates apoptotic effects via the CD47-dependent pathway, leading to a resolution of inflammation [142, 143]. It has also been described that TSP-1-CD47 interaction attenuates inflammation due to a delicate balance between T cell activation and apoptosis [144].
TSP-2 activates the pro-survival AKT signaling pathway through inhibiting MMP activity [140]. TSP-2 null mice experience cardiac rupture and increased MMP activity after Angiotensin II infusion [135]. TSP-2 also shows a protective role against cardiac inflammation in a model of acute viral myocarditis [145]. Conversely, TSP-4 appears to inhibit the fibrotic response. TSP-4 null mice experience increased hypertrophy and fibrosis, LV dilatation, decreased LV function, and pronounced fibrotic response with less mature collagen structure in a pressure overload model, transverse aortic constriction (TAC) [137, 146, 147]. However, TSP-4 deficient mice show an attenuated vascular inflammatory response through multiple mechanisms, including reduced monocyte/macrophage recruitment and migration into the lesion [148].
Osteopontin is a multifunctional protein that can act as a cytokine when secreted as a soluble protein or as an ECM bound matricellular protein. OPN affects gene expression, cell adhesion, spreading and survival by signaling through integrins and CD44 pathways [149–152] (Figure 2). Both secreted and ECM bound OPN act as anti-apoptotic signals through integrin signaling and NF-kappaB activation [153] (Figure 2). OPN also serves as a chemoattractant for multiple cell types such as monocytes, endothelial cells, smooth muscle cells and epithelial cells in vitro [150]. OPN is re-expressed in experimental models of MI and likely plays a role in cardiac repair and remodeling. OPN-null mice subjected to MI display augmented cardiac dilation and decreased collagen deposition in the infarct area compared to WT mice [154]. The role of OPN in the fibrotic response may be partly due to increased macrophage chemotaxis or effects on fibroblast adhesion and proliferation [155]. In addition, OPN has been described to play a role during inflammation through macrophage recruitment and cell retention into the sites of injury (reviewed in [156]).
Tenascins are a group of large, oligomeric ECM glycoproteins comprised of four members (-C, -R, -Z and –W). Tenascin-C (TNC) expression is generally restricted to development, and it is prominently repressed in adult tissues. However, an increase in TNC levels after myocardial infarction (MI) [157], myocarditis [158] or pressure overload [159] has been reported in the setting of cardiac remodeling. TNC can detach cardiomyocytes from the ECM after MI, possibly leading to cardiomyocyte apoptosis and invasion of inflammatory cells [160]. CF stimulated with TNC in vitro show increased migration, α-smooth muscle actin synthesis, collagen gel contraction, myofibroblast transition and participates in cytoskeletal rearrangement [161] (Figure 2). Additionally, ablation of TNC in mice leads to delayed myofibroblast recruitment to the site of injury [162]. Following cardiac insult, TNC is released into the bloodstream, leading to its development as a reliable biomarker that can predict the degree of cardiac remodeling and subsequent mortality in humans [163–166]. The increase in TNC following cardiac injury is exacerbated by the action of several factors released in pathologic cardiac remodeling, such as TGF-β and FGF-2, therefore suggesting a role of this glycoprotein in regulating inflammation and fibrosis. Finally, loss of TNC has been reported to be protective against the maladaptive responses exhibited during myocardial repair. Thus, TNC is emerging as a target to attenuate adverse pathological ventricular remodeling following cardiac injury [167]. In addition, loss of TNC attenuates inflammation following cardiac fibrosis. TNC interacts with integrins localized on the surface of the macrophage, upregulating IL-6, and FAK-Src through NF-κB and augmenting the inflammatory response [168].
Periostin (Osteoblast specific factor 2) is a secreted matricellular protein, originally identified in osteoblast lineages [169] that contains 4 repetitive fasciclin domains [170]. These domains contain sequences that allow binding to glycosaminoglycans, collagen I/V, FN, TNC, heparin and integrins [171, 172] and play a role in cell adhesion. Specifically, periostin can signal through αvβ3 and αvβ5 integrins to induce migration of smooth muscle cells in vitro [173] (Figure 2). Periostin binding to integrins leads to activation of PI3-K, Rho-kinase, and FAK signaling pathways affecting cell migration [173, 174] (Figure 2). Periostin expression is detectable in the developing heart but is largely undetectable in the adult myocardium under homeostatic conditions [172, 175–179]. However, periostin is rapidly re-expressed by myofibroblast cells in response to myocardial injury or pressure overload stimulation [176, 180–185] to prevent cardiac rupture by stimulating fibroblast recruitment, myofibroblast transdifferentiation and collagen deposition, orchestrating cardiac remodeling and fibrosis [175, 178]. Periostin-null mice show an improvement in their cardiac morbidity although they are prone to cardiac rupture following MI compared to WT mice [175]. Loss of periostin leads to preserved cardiac function, decreased fibrosis and attenuated cardiac hypertrophy in a pressure overload model of HF as well as a genetically induced model of hypertrophy [175, 176]. In addition, periostin null mice exhibit less inflammatory cell recruitment (less macrophages in the injury site) consistent with a reduction in fibrotic area [175]. Future study of inducible, cell-type restricted periostin null mice will provide invaluable insights regarding cell-specific effects of periostin in myocardial remodeling.
SPARC is another classic matricellular protein that regulates cell function and tissue remodeling by inhibiting cell cycle, mediating growth factor signaling and through adhesion effects including cytoskeletal rearrangement [161] (Figure 2). Like other matricellular proteins, SPARC expression levels are increased in the heart after infarction as well as in hypertrophy and fibrosis [155]. In animal models of MI, SPARC is mainly expressed in myofibroblast and macrophage [186, 187] compartments. Further discussion on the implications for cardiac repair and fibrosis of SPARC expression in macrophages is reviewed by Dr. Bradshaw [188]. Mice lacking SPARC that underwent MI injury experienced increased mortality as a result of cardiac rupture and HF [187]. These mice also had disorganized ECM with immature collagen fibers. Conversely, adenoviral overexpression of SPARC in mice reduced cardiac dilation and dysfunction [187]. After TAC, SPARC null mice display reduced collagen deposition associated with decreased diastolic stiffness [189]. In vitro, SPARC has been shown to affect cell adhesion and growth factor signaling that is involved in fibrosis, angiogenesis and tissue repair. Specifically, SPARC can bind platelet derived growth factor (PDGF), inhibiting its action at the PDGF receptor [190], and can inhibit PDGF-mediated smooth muscle cell proliferation [191]. In fibroblasts, SPARC ablation decreases mature collagen formation in the matrix and affects FN matrix assembly. SPARC also appears to regulate TGF-β signaling in CF; knockdown of SPARC in primary CF leads to a decreased ratio of p-Smad2/Smad2 after TGF-β stimulation [187].
Intercellular communication via structural ECM proteins
Fibronectin EDA is a FN splice variant of the type III repeat extra domain A (EDA) that is upregulated after cardiac injury [192, 193]. Fibronectin EDA affects signaling in multiple cardiac cell types. EDA fibronectin acts as a ligand of toll like receptors on immune cells and activates mast cells [194, 195]. EDA also regulates fibroblast proliferation and migration as well as their transition to myofibroblasts through FAK/ERK1/2 signaling pathways [196, 197]. After MI, mice lacking fibronectin EDA display preserved cardiac function and decreased remodeling. The fibronectin EDA-null mice have normal scar formation after MI, but experience less fibrosis in the remote myocardium and reduced myofibroblast transdifferentiation in the ventricular wall compared to WT mice [192]. In addition, EDA null mice display a reduction in macrophage infiltration, both in infarct and remote areas and in the production of detrimental cytokines that affect cardiomyocyte survival (such as TNFα or RANTES) [192].
Intercellular communication via metalloproteinases
The MMP family contains over 25 zinc-dependent proteases that have been classified based on their preferential substrate [198, 199]. The major types of MMPs found in the heart include collagenases (MMP-1,-8 and -13), gelatinases (MMP-2 and -9), stromelysins (MMP-3, -10, and -11) and membrane-type MMPs (MMP-14) [46, 198, 200, 201]. Endogenous control of MMP activity occurs through a group of specific MMP inhibitors, TIMPs. There are four known TIMPs that complex with active MMPs in a 1:1 ratio [202–204]. MMPs and TIMPs are known to play important roles in ECM maintenance and degradation. Several MMPs and TIMPs have been shown to contribute to the development and progression of heart disease [46, 108, 205–208]. Following MI in human patients, there is a significant increase in MMPs immediately after infarction. MMP levels then decrease as healing progresses, but this is followed by a second increase in MMP levels that is associated with ventricular dilation and dysfunction [209, 210]. MMPs and TIMPs are secreted by cardiomyocytes, cardiac fibroblasts, leukocytes, vascular smooth muscle cells and endothelial cells [46, 211–213] and have been shown to play direct and indirect roles in cardiac remodeling and intercellular communication. For example, MMPs are able to cleave and mobilize growth factors and cytokines, which can elicit multiple effects in the heart such as cell proliferation, migration, inflammation and angiogenesis [214]. Overexpression of TIMPs in fibroblasts leads to changes in collagen synthesis and apoptosis. These effects were shown to be independent of MMP activity, as inhibition of MMPs did not recapitulate the observed effects [215]. Further, MMP-7 is expressed in the heart by cardiomyocytes and macrophages and plays a role in Cx43 cleavage, which is important in gap junction cell communication [216]. Additionally, we have shown a direct role for MMP-13 in heart failure, in which MMP-13 is capable of cleaving the protease-activated receptor-1 (PAR-1) leading to downstream ERK1/2 phosphorylation. Inhibition of this MMP-13-mediated PAR-1 signaling was shown to be protective in a mouse model of acute cardiac hypertrophy [217]. MMPs are also capable of cleaving ECM proteins (collagens, proteoglycans, fibronectin, etc.) revealing cryptic biologically active (matricryptic) sites that can elicit signaling within the site of cardiac injury [218, 219]. For example, Lindsey et al. recently identified a previously unrecognized MMP-2 and -9 cleavage site of collagen I resulting in release of an 18-kD peptide fragment (C-1158/59). Expression of this matricryptin negatively correlates with E/e′ ratios (a marker of LV filling pressure) in human patients. It is also elevated in mice 7 days post MI when MMP-9 returns to baseline expression level, suggesting a role for MMP-9 in the formation as well as degradation of C-1158/59 in mice. This fragment also contributes to increased mouse CF migration and capillary formation of endothelial cells in vitro. Interestingly, treatment of mice with a synthetic peptide mimicking the endogenous matricryptin (p1159/59) after MI attenuated LV dilation and preserved LV structure [220]. Further discussion on how MMPs act as an input and output signals for post-myocardial remodeling is reviewed by Dr. Lindsay and colleagues [221].
Conclusions and Future Directions
In summary, the ECM plays a critical role in the maintenance of the functional myocardium as well as the regulation of the heart’s response to stress or injury. The ECM is relatively stable in the healthy adult heart, but this changes following cardiac injury. Specific ECM proteins interact with cells and play an active role in intercellular signaling to control cell behavior that is critical to the repair process. Current HF therapeutics do not target ECM molecules known to facilitate the development of HF, such as the myofibroblast transition and excess collagen deposition. Ongoing studies targeting receptors for the ECM components as well as targeting of cytokines, enzymes and signaling molecules have shown potential for new, targeted therapeutics, including several in various stages of clinical trials (largely in areas other than heart failure) [222]. Effective antifibrotic therapies would be a significant contribution in the treatment of HF, as well as a myriad other fibrotic diseases. However, more information regarding specific ECM components and their roles in cardiac remodeling is needed to advance this field of therapeutic development. Many experiments have studied individual components of the ECM, however, further insights are needed regarding the interaction of ECM proteins and how they synergistically regulate cardiac remodeling after injury. Interestingly, the development of synthetic ECM has recently emerged as a way to elucidate the interaction of native ECM molecules with living cells, to further understand how the ECM regulates their environment. Tissue engineering will open new avenues to create intelligent scaffolds to support regeneration of diseased or damaged tissue. We believe that an increased understanding of the mechanisms underlying pathologic cardiac fibroblast activation and cardiac ECM-cell communication will yield novel therapeutic strategies. Unlike the current therapeutic paradigm, these new approaches will directly target cardiac remodeling and will further contribute to the reduction in mortality and morbidity resulting from this devastating disease.
Highlights.
ECM acts as a dynamic scaffold for cells and is a reservoir for signaling molecules
ECM-cell communication is critical for cell behavior before and after cardiac injury
Better understanding of ECM-mediated signaling may lead to new HF targets
Acknowledgments
This work was funded in part by R01HL129722, R01HL091475, P01HL069779 (BCB) and T32HL125204 (AES).
Footnotes
Disclosures
None.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Banerjee I, Fuseler JW, Price RL, Borg TK, Baudino TA. Determination of cell types and numbers during cardiac development in the neonatal and adult rat and mouse. American journal of physiology Heart and circulatory physiology. 2007;293:H1883–91. doi: 10.1152/ajpheart.00514.2007. [DOI] [PubMed] [Google Scholar]
- 2.Camelliti P, Borg TK, Kohl P. Structural and functional characterisation of cardiac fibroblasts. Cardiovascular research. 2005;65:40–51. doi: 10.1016/j.cardiores.2004.08.020. [DOI] [PubMed] [Google Scholar]
- 3.Baudino TA, Carver W, Giles W, Borg TK. Cardiac fibroblasts: friend or foe? American journal of physiology Heart and circulatory physiology. 2006;291:H1015–26. doi: 10.1152/ajpheart.00023.2006. [DOI] [PubMed] [Google Scholar]
- 4.Catalucci D, Latronico MV, Ellingsen O, Condorelli G. Physiological myocardial hypertrophy: how and why? Frontiers in bioscience : a journal and virtual library. 2008;13:312–24. doi: 10.2741/2681. [DOI] [PubMed] [Google Scholar]
- 5.Holmes JW, Borg TK, Covell JW. Structure and mechanics of healing myocardial infarcts. Annual review of biomedical engineering. 2005;7:223–53. doi: 10.1146/annurev.bioeng.7.060804.100453. [DOI] [PubMed] [Google Scholar]
- 6.Brown RD, Ambler SK, Mitchell MD, Long CS. The cardiac fibroblast: therapeutic target in myocardial remodeling and failure. Annual review of pharmacology and toxicology. 2005;45:657–87. doi: 10.1146/annurev.pharmtox.45.120403.095802. [DOI] [PubMed] [Google Scholar]
- 7.Weber KT. Fibrosis in hypertensive heart disease: focus on cardiac fibroblasts. Journal of hypertension. 2004;22:47–50. doi: 10.1097/00004872-200401000-00011. [DOI] [PubMed] [Google Scholar]
- 8.Baxter SC, Morales MO, Goldsmith EC. Adaptive changes in cardiac fibroblast morphology and collagen organization as a result of mechanical environment. Cell biochemistry and biophysics. 2008;51:33–44. doi: 10.1007/s12013-008-9013-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kamkin A, Kiseleva I, Isenberg G, Wagner KD, Gunther J, Theres H, et al. Cardiac fibroblasts and the mechano-electric feedback mechanism in healthy and diseased hearts. Progress in biophysics and molecular biology. 2003;82:111–20. doi: 10.1016/s0079-6107(03)00009-9. [DOI] [PubMed] [Google Scholar]
- 10.Banerjee I, Yekkala K, Borg TK, Baudino TA. Dynamic interactions between myocytes, fibroblasts, and extracellular matrix. Annals of the New York Academy of Sciences. 2006;1080:76–84. doi: 10.1196/annals.1380.007. [DOI] [PubMed] [Google Scholar]
- 11.Chilton L, Giles WR, Smith GL. Evidence of intercellular coupling between co-cultured adult rabbit ventricular myocytes and myofibroblasts. The Journal of physiology. 2007;583:225–36. doi: 10.1113/jphysiol.2007.135038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kohl P. Heterogeneous cell coupling in the heart: an electrophysiological role for fibroblasts. Circulation research. 2003;93:381–3. doi: 10.1161/01.RES.0000091364.90121.0C. [DOI] [PubMed] [Google Scholar]
- 13.Louault C, Benamer N, Faivre JF, Potreau D, Bescond J. Implication of connexins 40 and 43 in functional coupling between mouse cardiac fibroblasts in primary culture. Biochimica et biophysica acta. 2008;1778:2097–104. doi: 10.1016/j.bbamem.2008.04.005. [DOI] [PubMed] [Google Scholar]
- 14.Porter KE, Turner NA. Cardiac fibroblasts: at the heart of myocardial remodeling. Pharmacology & therapeutics. 2009;123:255–78. doi: 10.1016/j.pharmthera.2009.05.002. [DOI] [PubMed] [Google Scholar]
- 15.Camelliti P, Devlin GP, Matthews KG, Kohl P, Green CR. Spatially and temporally distinct expression of fibroblast connexins after sheep ventricular infarction. Cardiovascular research. 2004;62:415–25. doi: 10.1016/j.cardiores.2004.01.027. [DOI] [PubMed] [Google Scholar]
- 16.Camelliti P, Green CR, LeGrice I, Kohl P. Fibroblast network in rabbit sinoatrial node: structural and functional identification of homogeneous and heterogeneous cell coupling. Circulation research. 2004;94:828–35. doi: 10.1161/01.RES.0000122382.19400.14. [DOI] [PubMed] [Google Scholar]
- 17.Bowers SL, Borg TK, Baudino TA. The dynamics of fibroblast-myocyte-capillary interactions in the heart. Annals of the New York Academy of Sciences. 2010;1188:143–52. doi: 10.1111/j.1749-6632.2009.05094.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Johnson TL, Nerem RM. Endothelial connexin 37, connexin 40, and connexin 43 respond uniquely to substrate and shear stress. Endothelium : journal of endothelial cell research. 2007;14:215–26. doi: 10.1080/10623320701617233. [DOI] [PubMed] [Google Scholar]
- 19.Mather S, Dora KA, Sandow SL, Winter P, Garland CJ. Rapid endothelial cell-selective loading of connexin 40 antibody blocks endothelium-derived hyperpolarizing factor dilation in rat small mesenteric arteries. Circulation research. 2005;97:399–407. doi: 10.1161/01.RES.0000178008.46759.d0. [DOI] [PubMed] [Google Scholar]
- 20.Pepper MS, Montesano R, el Aoumari A, Gros D, Orci L, Meda P. Coupling and connexin 43 expression in microvascular and large vessel endothelial cells. The American journal of physiology. 1992;262:C1246–57. doi: 10.1152/ajpcell.1992.262.5.C1246. [DOI] [PubMed] [Google Scholar]
- 21.Villaschi S, Nicosia RF. Paracrine interactions between fibroblasts and endothelial cells in a serum-free coculture model. Modulation of angiogenesis and collagen gel contraction. Laboratory investigation; a journal of technical methods and pathology. 1994;71:291–9. [PubMed] [Google Scholar]
- 22.Armulik A, Abramsson A, Betsholtz C. Endothelial/pericyte interactions. Circulation research. 2005;97:512–23. doi: 10.1161/01.RES.0000182903.16652.d7. [DOI] [PubMed] [Google Scholar]
- 23.Hinz B. Formation and function of the myofibroblast during tissue repair. The Journal of investigative dermatology. 2007;127:526–37. doi: 10.1038/sj.jid.5700613. [DOI] [PubMed] [Google Scholar]
- 24.Kalluri R, Zeisberg M. Fibroblasts in cancer. Nature reviews Cancer. 2006;6:392–401. doi: 10.1038/nrc1877. [DOI] [PubMed] [Google Scholar]
- 25.Montesano R, Pepper MS, Orci L. Paracrine induction of angiogenesis in vitro by Swiss 3T3 fibroblasts. Journal of cell science. 1993;105(Pt 4):1013–24. doi: 10.1242/jcs.105.4.1013. [DOI] [PubMed] [Google Scholar]
- 26.Ieda M, Tsuchihashi T, Ivey KN, Ross RS, Hong TT, Shaw RM, et al. Cardiac fibroblasts regulate myocardial proliferation through beta1 integrin signaling. Developmental cell. 2009;16:233–44. doi: 10.1016/j.devcel.2008.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fujiu K, Nagai R. Fibroblast-mediated pathways in cardiac hypertrophy. Journal of molecular and cellular cardiology. 2014;70:64–73. doi: 10.1016/j.yjmcc.2014.01.013. [DOI] [PubMed] [Google Scholar]
- 28.Heineke J, Molkentin JD. Regulation of cardiac hypertrophy by intracellular signalling pathways. Nature reviews Molecular cell biology. 2006;7:589–600. doi: 10.1038/nrm1983. [DOI] [PubMed] [Google Scholar]
- 29.Gaudesius G, Miragoli M, Thomas SP, Rohr S. Coupling of cardiac electrical activity over extended distances by fibroblasts of cardiac origin. Circulation research. 2003;93:421–8. doi: 10.1161/01.RES.0000089258.40661.0C. [DOI] [PubMed] [Google Scholar]
- 30.Eghbali M. Cardiac fibroblasts: function, regulation of gene expression, and phenotypic modulation. Basic research in cardiology. 1992;87(Suppl 2):183–9. doi: 10.1007/978-3-642-72477-0_16. [DOI] [PubMed] [Google Scholar]
- 31.Zeisberg EM, Tarnavski O, Zeisberg M, Dorfman AL, McMullen JR, Gustafsson E, et al. Endothelial-to-mesenchymal transition contributes to cardiac fibrosis. Nature medicine. 2007;13:952–61. doi: 10.1038/nm1613. [DOI] [PubMed] [Google Scholar]
- 32.Ng CP, Hinz B, Swartz MA. Interstitial fluid flow induces myofibroblast differentiation and collagen alignment in vitro. Journal of cell science. 2005;118:4731–9. doi: 10.1242/jcs.02605. [DOI] [PubMed] [Google Scholar]
- 33.Berk BC, Fujiwara K, Lehoux S. ECM remodeling in hypertensive heart disease. The Journal of clinical investigation. 2007;117:568–75. doi: 10.1172/JCI31044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Schellings MW, Pinto YM, Heymans S. Matricellular proteins in the heart: possible role during stress and remodeling. Cardiovascular research. 2004;64:24–31. doi: 10.1016/j.cardiores.2004.06.006. [DOI] [PubMed] [Google Scholar]
- 35.Curtis MW, Russell B. Micromechanical regulation in cardiac myocytes and fibroblasts: implications for tissue remodeling. Pflugers Archiv: European journal of physiology. 2011;462:105–17. doi: 10.1007/s00424-011-0931-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ahmed SH, Clark LL, Pennington WR, Webb CS, Bonnema DD, Leonardi AH, et al. Matrix metalloproteinases/tissue inhibitors of metalloproteinases: relationship between changes in proteolytic determinants of matrix composition and structural, functional, and clinical manifestations of hypertensive heart disease. Circulation. 2006;113:2089–96. doi: 10.1161/CIRCULATIONAHA.105.573865. [DOI] [PubMed] [Google Scholar]
- 37.Jugdutt BI. Remodeling of the myocardium and potential targets in the collagen degradation and synthesis pathways. Current drug targets Cardiovascular & haematological disorders. 2003;3:1–30. doi: 10.2174/1568006033337276. [DOI] [PubMed] [Google Scholar]
- 38.Benjamin MM, Khalil RA. Matrix metalloproteinase inhibitors as investigative tools in the pathogenesis and management of vascular disease. Exs. 2012;103:209–79. doi: 10.1007/978-3-0348-0364-9_7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Segura AM, Frazier OH, Buja LM. Fibrosis and heart failure. Heart failure reviews. 2014;19:173–85. doi: 10.1007/s10741-012-9365-4. [DOI] [PubMed] [Google Scholar]
- 40.Bishop JE, Laurent GJ. Collagen turnover and its regulation in the normal and hypertrophying heart. European heart journal. 1995;16(Suppl C):38–44. doi: 10.1093/eurheartj/16.suppl_c.38. [DOI] [PubMed] [Google Scholar]
- 41.Halper J, Kjaer M. Basic components of connective tissues and extracellular matrix: elastin, fibrillin, fibulins, fibrinogen, fibronectin, laminin, tenascins and thrombospondins. Advances in experimental medicine and biology. 2014;802:31–47. doi: 10.1007/978-94-007-7893-1_3. [DOI] [PubMed] [Google Scholar]
- 42.Magnusson MK, Mosher DF. Fibronectin: structure, assembly, and cardiovascular implications. Arteriosclerosis, thrombosis, and vascular biology. 1998;18:1363–70. doi: 10.1161/01.atv.18.9.1363. [DOI] [PubMed] [Google Scholar]
- 43.Ramirez F, Sakai LY, Dietz HC, Rifkin DB. Fibrillin microfibrils: multipurpose extracellular networks in organismal physiology. Physiological genomics. 2004;19:151–4. doi: 10.1152/physiolgenomics.00092.2004. [DOI] [PubMed] [Google Scholar]
- 44.Taipale J, Keski-Oja J. Growth factors in the extracellular matrix. FASEB journal : official publication of the Federation of American Societies for Experimental Biology. 1997;11:51–9. doi: 10.1096/fasebj.11.1.9034166. [DOI] [PubMed] [Google Scholar]
- 45.Corda S, Samuel JL, Rappaport L. Extracellular matrix and growth factors during heart growth. Heart failure reviews. 2000;5:119–30. doi: 10.1023/A:1009806403194. [DOI] [PubMed] [Google Scholar]
- 46.Fan D, Takawale A, Lee J, Kassiri Z. Cardiac fibroblasts, fibrosis and extracellular matrix remodeling in heart disease. Fibrogenesis & tissue repair. 2012;5:15. doi: 10.1186/1755-1536-5-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bowers SL, Banerjee I, Baudino TA. The extracellular matrix: at the center of it all. Journal of molecular and cellular cardiology. 2010;48:474–82. doi: 10.1016/j.yjmcc.2009.08.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lockhart M, Wirrig E, Phelps A, Wessels A. Extracellular matrix and heart development. Birth defects research Part A, Clinical and molecular teratology. 2011;91:535–50. doi: 10.1002/bdra.20810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Cichy J, Pure E. The liberation of CD44. The Journal of cell biology. 2003;161:839–43. doi: 10.1083/jcb.200302098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hynes RO. Integrins: versatility, modulation, and signaling in cell adhesion. Cell. 1992;69:11–25. doi: 10.1016/0092-8674(92)90115-s. [DOI] [PubMed] [Google Scholar]
- 51.Clark EA, Brugge JS. Integrins and signal transduction pathways: the road taken. Science. 1995;268:233–9. doi: 10.1126/science.7716514. [DOI] [PubMed] [Google Scholar]
- 52.Schoenwaelder SM, Burridge K. Bidirectional signaling between the cytoskeleton and integrins. Current opinion in cell biology. 1999;11:274–86. doi: 10.1016/s0955-0674(99)80037-4. [DOI] [PubMed] [Google Scholar]
- 53.Coppolino MG, Woodside MJ, Demaurex N, Grinstein S, St-Arnaud R, Dedhar S. Calreticulin is essential for integrin-mediated calcium signalling and cell adhesion. Nature. 1997;386:843–7. doi: 10.1038/386843a0. [DOI] [PubMed] [Google Scholar]
- 54.Guan JL. Role of focal adhesion kinase in integrin signaling. The international journal of biochemistry & cell biology. 1997;29:1085–96. doi: 10.1016/s1357-2725(97)00051-4. [DOI] [PubMed] [Google Scholar]
- 55.Brancaccio M, Guazzone S, Menini N, Sibona E, Hirsch E, De Andrea M, et al. Melusin is a new muscle-specific interactor for beta(1) integrin cytoplasmic domain. The Journal of biological chemistry. 1999;274:29282–8. doi: 10.1074/jbc.274.41.29282. [DOI] [PubMed] [Google Scholar]
- 56.Li J, Mayne R, Wu C. A novel muscle-specific beta 1 integrin binding protein (MIBP) that modulates myogenic differentiation. The Journal of cell biology. 1999;147:1391–8. doi: 10.1083/jcb.147.7.1391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Schaller MD, Otey CA, Hildebrand JD, Parsons JT. Focal adhesion kinase and paxillin bind to peptides mimicking beta integrin cytoplasmic domains. The Journal of cell biology. 1995;130:1181–7. doi: 10.1083/jcb.130.5.1181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Tadokoro S, Shattil SJ, Eto K, Tai V, Liddington RC, de Pereda JM, et al. Talin binding to integrin beta tails: a final common step in integrin activation. Science. 2003;302:103–6. doi: 10.1126/science.1086652. [DOI] [PubMed] [Google Scholar]
- 59.Otey CA, Pavalko FM, Burridge K. An interaction between alpha-actinin and the beta 1 integrin subunit in vitro. The Journal of cell biology. 1990;111:721–9. doi: 10.1083/jcb.111.2.721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Cary LA, Guan JL. Focal adhesion kinase in integrin-mediated signaling. Frontiers in bioscience : a journal and virtual library. 1999;4:D102–13. doi: 10.2741/cary. [DOI] [PubMed] [Google Scholar]
- 61.Ruoslahti E, Reed JC. Anchorage dependence, integrins, and apoptosis. Cell. 1994;77:477–8. doi: 10.1016/0092-8674(94)90209-7. [DOI] [PubMed] [Google Scholar]
- 62.Lauffenburger DA, Horwitz AF. Cell migration: a physically integrated molecular process. Cell. 1996;84:359–69. doi: 10.1016/s0092-8674(00)81280-5. [DOI] [PubMed] [Google Scholar]
- 63.Assoian RK. Anchorage-dependent cell cycle progression. The Journal of cell biology. 1997;136:1–4. doi: 10.1083/jcb.136.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Bottazzi ME, Assoian RK. The extracellular matrix and mitogenic growth factors control G1 phase cyclins and cyclin-dependent kinase inhibitors. Trends in cell biology. 1997;7:348–52. doi: 10.1016/S0962-8924(97)01114-8. [DOI] [PubMed] [Google Scholar]
- 65.Fassler R, Rohwedel J, Maltsev V, Bloch W, Lentini S, Guan K, et al. Differentiation and integrity of cardiac muscle cells are impaired in the absence of beta 1 integrin. Journal of cell science. 1996;109(Pt 13):2989–99. doi: 10.1242/jcs.109.13.2989. [DOI] [PubMed] [Google Scholar]
- 66.Weber KT. Cardiac interstitium in health and disease: the fibrillar collagen network. Journal of the American College of Cardiology. 1989;13:1637–52. doi: 10.1016/0735-1097(89)90360-4. [DOI] [PubMed] [Google Scholar]
- 67.Borg TK, Caulfield JB. The collagen matrix of the heart. Federation proceedings. 1981;40:2037–41. [PubMed] [Google Scholar]
- 68.Weber KT, Janicki JS, Pick R, Abrahams C, Shroff SG, Bashey RI, et al. Collagen in the hypertrophied, pressure-overloaded myocardium. Circulation. 1987;75:I40–7. [PubMed] [Google Scholar]
- 69.Weber KT, Clark WA, Janicki JS, Shroff SG. Physiologic versus pathologic hypertrophy and the pressure-overloaded myocardium. Journal of cardiovascular pharmacology. 1987;10(Suppl 6):S37–50. [PubMed] [Google Scholar]
- 70.Dawson R, Milne G, Williams RB. Changes in the collagen of rat heart in copper-deficiency-induced cardiac hypertrophy. Cardiovascular research. 1982;16:559–65. doi: 10.1093/cvr/16.10.559. [DOI] [PubMed] [Google Scholar]
- 71.Factor SM, Robinson TF, Dominitz R, Cho SH. Alterations of the myocardial skeletal framework in acute myocardial infarction with and without ventricular rupture. A preliminary report. The American journal of cardiovascular pathology. 1987;1:91–7. [PubMed] [Google Scholar]
- 72.Mooney A, Jackson K, Bacon R, Streuli C, Edwards G, Bassuk J, et al. Type IV collagen and laminin regulate glomerular mesangial cell susceptibility to apoptosis via beta(1) integrin-mediated survival signals. The American journal of pathology. 1999;155:599–606. doi: 10.1016/s0002-9440(10)65155-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Sanders MA, Basson MD. Collagen IV regulates Caco-2 cell spreading and p130Cas phosphorylation by FAK-dependent and FAK-independent pathways. Biological chemistry. 2008;389:47–55. doi: 10.1515/BC.2008.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Koohestani F, Braundmeier AG, Mahdian A, Seo J, Bi J, Nowak RA. Extracellular matrix collagen alters cell proliferation and cell cycle progression of human uterine leiomyoma smooth muscle cells. PloS one. 2013;8:e75844. doi: 10.1371/journal.pone.0075844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Jean L, Yang L, Majumdar D, Gao Y, Shi M, Brewer BM, et al. The Rho family GEF Asef2 regulates cell migration in three dimensional (3D) collagen matrices through myosin II. Cell adhesion & migration. 2014;8:460–7. doi: 10.4161/19336918.2014.983778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Loffek S, Hurskainen T, Jackow J, Sigloch FC, Schilling O, Tasanen K, et al. Transmembrane collagen XVII modulates integrin dependent keratinocyte migration via PI3K/Rac1 signaling. PloS one. 2014;9:e87263. doi: 10.1371/journal.pone.0087263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Chung CY, Erickson HP. Glycosaminoglycans modulate fibronectin matrix assembly and are essential for matrix incorporation of tenascin-C. Journal of cell science. 1997;110(Pt 12):1413–9. doi: 10.1242/jcs.110.12.1413. [DOI] [PubMed] [Google Scholar]
- 78.Dallas SL, Sivakumar P, Jones CJ, Chen Q, Peters DM, Mosher DF, et al. Fibronectin regulates latent transforming growth factor-beta (TGF beta) by controlling matrix assembly of latent TGF beta-binding protein-1. The Journal of biological chemistry. 2005;280:18871–80. doi: 10.1074/jbc.M410762200. [DOI] [PubMed] [Google Scholar]
- 79.Kadler KE, Hill A, Canty-Laird EG. Collagen fibrillogenesis: fibronectin, integrins, and minor collagens as organizers and nucleators. Current opinion in cell biology. 2008;20:495–501. doi: 10.1016/j.ceb.2008.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Sabatier L, Chen D, Fagotto-Kaufmann C, Hubmacher D, McKee MD, Annis DS, et al. Fibrillin assembly requires fibronectin. Molecular biology of the cell. 2009;20:846–58. doi: 10.1091/mbc.E08-08-0830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Sottile J, Hocking DC. Fibronectin polymerization regulates the composition and stability of extracellular matrix fibrils and cell-matrix adhesions. Molecular biology of the cell. 2002;13:3546–59. doi: 10.1091/mbc.E02-01-0048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Twal WO, Czirok A, Hegedus B, Knaak C, Chintalapudi MR, Okagawa H, et al. Fibulin-1 suppression of fibronectin-regulated cell adhesion and motility. Journal of cell science. 2001;114:4587–98. doi: 10.1242/jcs.114.24.4587. [DOI] [PubMed] [Google Scholar]
- 83.Moretti FA, Chauhan AK, Iaconcig A, Porro F, Baralle FE, Muro AF. A major fraction of fibronectin present in the extracellular matrix of tissues is plasma-derived. The Journal of biological chemistry. 2007;282:28057–62. doi: 10.1074/jbc.M611315200. [DOI] [PubMed] [Google Scholar]
- 84.Kumazaki T, Mitsui Y, Hamada K, Sumida H, Nishiyama M. Detection of alternative splicing of fibronectin mRNA in a single cell. Journal of cell science. 1999;112(Pt 10):1449–53. doi: 10.1242/jcs.112.10.1449. [DOI] [PubMed] [Google Scholar]
- 85.Muro AF, Chauhan AK, Gajovic S, Iaconcig A, Porro F, Stanta G, et al. Regulated splicing of the fibronectin EDA exon is essential for proper skin wound healing and normal lifespan. The Journal of cell biology. 2003;162:149–60. doi: 10.1083/jcb.200212079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Carnemolla B, Balza E, Siri A, Zardi L, Nicotra MR, Bigotti A, et al. A tumor-associated fibronectin isoform generated by alternative splicing of messenger RNA precursors. The Journal of cell biology. 1989;108:1139–48. doi: 10.1083/jcb.108.3.1139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Ffrench-Constant C, Hynes RO. Alternative splicing of fibronectin is temporally and spatially regulated in the chicken embryo. Development. 1989;106:375–88. doi: 10.1242/dev.106.2.375. [DOI] [PubMed] [Google Scholar]
- 88.Ffrench-Constant C, Van de Water L, Dvorak HF, Hynes RO. Reappearance of an embryonic pattern of fibronectin splicing during wound healing in the adult rat. The Journal of cell biology. 1989;109:903–14. doi: 10.1083/jcb.109.2.903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Oyama F, Hirohashi S, Shimosato Y, Titani K, Sekiguchi K. Deregulation of alternative splicing of fibronectin pre-mRNA in malignant human liver tumors. The Journal of biological chemistry. 1989;264:10331–4. [PubMed] [Google Scholar]
- 90.Jarnagin WR, Rockey DC, Koteliansky VE, Wang SS, Bissell DM. Expression of variant fibronectins in wound healing: cellular source and biological activity of the EIIIA segment in rat hepatic fibrogenesis. The Journal of cell biology. 1994;127:2037–48. doi: 10.1083/jcb.127.6.2037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Caputi M, Baralle FE, Melo CA. Analysis of the linkage between fibronectin alternative spliced sites during ageing in rat tissues. Biochimica et biophysica acta. 1995;1263:53–9. doi: 10.1016/0167-4781(95)00067-q. [DOI] [PubMed] [Google Scholar]
- 92.Takagi J. Structural basis for ligand recognition by RGD (Arg-Gly-Asp)-dependent integrins. Biochemical Society transactions. 2004;32:403–6. doi: 10.1042/BST0320403. [DOI] [PubMed] [Google Scholar]
- 93.Guan JL, Trevithick JE, Hynes RO. Fibronectin/integrin interaction induces tyrosine phosphorylation of a 120-kDa protein. Cell regulation. 1991;2:951–64. doi: 10.1091/mbc.2.11.951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Kornberg L, Earp HS, Parsons JT, Schaller M, Juliano RL. Cell adhesion or integrin clustering increases phosphorylation of a focal adhesion-associated tyrosine kinase. The Journal of biological chemistry. 1992;267:23439–42. [PubMed] [Google Scholar]
- 95.Schaller MD, Borgman CA, Cobb BS, Vines RR, Reynolds AB, Parsons JT. pp125FAK a structurally distinctive protein-tyrosine kinase associated with focal adhesions. Proceedings of the National Academy of Sciences of the United States of America. 1992;89:5192–6. doi: 10.1073/pnas.89.11.5192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Burridge K, Turner CE, Romer LH. Tyrosine phosphorylation of paxillin and pp125FAK accompanies cell adhesion to extracellular matrix: a role in cytoskeletal assembly. The Journal of cell biology. 1992;119:893–903. doi: 10.1083/jcb.119.4.893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Turner CE. Paxillin is a major phosphotyrosine-containing protein during embryonic development. The Journal of cell biology. 1991;115:201–7. doi: 10.1083/jcb.115.1.201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Bockholt SM, Burridge K. Cell spreading on extracellular matrix proteins induces tyrosine phosphorylation of tensin. The Journal of biological chemistry. 1993;268:14565–7. [PubMed] [Google Scholar]
- 99.Nojima Y, Morino N, Mimura T, Hamasaki K, Furuya H, Sakai R, et al. Integrin-mediated cell adhesion promotes tyrosine phosphorylation of p130Cas, a Src homology 3-containing molecule having multiple Src homology 2-binding motifs. The Journal of biological chemistry. 1995;270:15398–402. doi: 10.1074/jbc.270.25.15398. [DOI] [PubMed] [Google Scholar]
- 100.Vuori K, Ruoslahti E. Tyrosine phosphorylation of p130Cas and cortactin accompanies integrin-mediated cell adhesion to extracellular matrix. The Journal of biological chemistry. 1995;270:22259–62. doi: 10.1074/jbc.270.38.22259. [DOI] [PubMed] [Google Scholar]
- 101.Zamir E, Geiger B. Molecular complexity and dynamics of cell-matrix adhesions. Journal of cell science. 2001;114:3583–90. doi: 10.1242/jcs.114.20.3583. [DOI] [PubMed] [Google Scholar]
- 102.Yamada KM, Pankov R, Cukierman E. Dimensions and dynamics in integrin function. Brazilian journal of medical and biological research = Revista brasileira de pesquisas medicas e biologicas/Sociedade Brasileira de Biofisica [et al] 2003;36:959–66. doi: 10.1590/s0100-879x2003000800001. [DOI] [PubMed] [Google Scholar]
- 103.Hocking DC, Chang CH. Fibronectin matrix polymerization regulates small airway epithelial cell migration. American journal of physiology Lung cellular and molecular physiology. 2003;285:L169–79. doi: 10.1152/ajplung.00371.2002. [DOI] [PubMed] [Google Scholar]
- 104.Han SW, Roman J. Fibronectin induces cell proliferation and inhibits apoptosis in human bronchial epithelial cells: pro-oncogenic effects mediated by PI3-kinase and NF-kappa B. Oncogene. 2006;25:4341–9. doi: 10.1038/sj.onc.1209460. [DOI] [PubMed] [Google Scholar]
- 105.Park JH, Ryu JM, Han HJ. Involvement of caveolin-1 in fibronectin-induced mouse embryonic stem cell proliferation: role of FAK, RhoA, PI3K/Akt, and ERK 1/2 pathways. Journal of cellular physiology. 2011;226:267–75. doi: 10.1002/jcp.22338. [DOI] [PubMed] [Google Scholar]
- 106.Altrock E, Sens C, Wuerfel C, Vasel M, Kawelke N, Dooley S, et al. Inhibition of fibronectin deposition improves experimental liver fibrosis. Journal of hepatology. 2015;62:625–33. doi: 10.1016/j.jhep.2014.06.010. [DOI] [PubMed] [Google Scholar]
- 107.Chiang HY, Korshunov VA, Serour A, Shi F, Sottile J. Fibronectin is an important regulator of flow-induced vascular remodeling. Arteriosclerosis, thrombosis, and vascular biology. 2009;29:1074–9. doi: 10.1161/ATVBAHA.108.181081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Cleutjens JP, Creemers EE. Integration of concepts: cardiac extracellular matrix remodeling after myocardial infarction. Journal of cardiac failure. 2002;8:S344–8. doi: 10.1054/jcaf.2002.129261. [DOI] [PubMed] [Google Scholar]
- 109.Kim HE, Dalal SS, Young E, Legato MJ, Weisfeldt ML, D’Armiento J. Disruption of the myocardial extracellular matrix leads to cardiac dysfunction. J Clin Invest. 2000;106:857–66. doi: 10.1172/JCI8040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Davis J, Molkentin JD. Myofibroblasts: Trust your heart and let fate decide. J Mol Cell Cardiol. 2013 doi: 10.1016/j.yjmcc.2013.10.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Dostal D, Glaser S, Baudino TA. Cardiac fibroblast physiology and pathology. Compr Physiol. 2015;5:887–909. doi: 10.1002/cphy.c140053. [DOI] [PubMed] [Google Scholar]
- 112.Vanhoutte D, Schellings M, Pinto Y, Heymans S. Relevance of matrix metalloproteinases and their inhibitors after myocardial infarction: a temporal and spatial window. Cardiovascular research. 2006;69:604–13. doi: 10.1016/j.cardiores.2005.10.002. [DOI] [PubMed] [Google Scholar]
- 113.Turner NA, Porter KE. Regulation of myocardial matrix metalloproteinase expression and activity by cardiac fibroblasts. IUBMB life. 2012;64:143–50. doi: 10.1002/iub.594. [DOI] [PubMed] [Google Scholar]
- 114.Howard CM, Baudino TA. Dynamic cell-cell and cell-ECM interactions in the heart. J Mol Cell Cardiol. 2014;70:19–26. doi: 10.1016/j.yjmcc.2013.10.006. [DOI] [PubMed] [Google Scholar]
- 115.Krenning G, Zeisberg EM, Kalluri R. The origin of fibroblasts and mechanism of cardiac fibrosis. J Cell Physiol. 2010;225:631–7. doi: 10.1002/jcp.22322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Harston RK, Kuppuswamy D. Integrins are the necessary links to hypertrophic growth in cardiomyocytes. J Signal Transduct. 2011;2011:521742. doi: 10.1155/2011/521742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Lal H, Verma SK, Smith M, Guleria RS, Lu G, Foster DM, et al. Stretch-induced MAP kinase activation in cardiac myocytes: differential regulation through beta1-integrin and focal adhesion kinase. J Mol Cell Cardiol. 2007;43:137–47. doi: 10.1016/j.yjmcc.2007.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.MacKenna DA, Dolfi F, Vuori K, Ruoslahti E. Extracellular signal-regulated kinase and c-Jun NH2-terminal kinase activation by mechanical stretch is integrin-dependent and matrix-specific in rat cardiac fibroblasts. J Clin Invest. 1998;101:301–10. doi: 10.1172/JCI1026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Desgrosellier JS, Cheresh DA. Integrins in cancer: biological implications and therapeutic opportunities. Nature reviews Cancer. 2010;10:9–22. doi: 10.1038/nrc2748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Henderson NC, Arnold TD, Katamura Y, Giacomini MM, Rodriguez JD, McCarty JH, et al. Targeting of alphav integrin identifies a core molecular pathway that regulates fibrosis in several organs. Nature medicine. 2013;19:1617–24. doi: 10.1038/nm.3282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Papageorgiou A, Rikens M. Novel regulators of cardiac inflammation: matricellular proteins expand their repertoire. JMCC, Journal of Molecular and Cellular Cardiology. 2016:JMC9609. doi: 10.1016/j.yjmcc.2016.01.008. in press. [DOI] [PubMed] [Google Scholar]
- 122.Matsui Y, Morimoto J, Uede T. Role of matricellular proteins in cardiac tissue remodeling after myocardial infarction. World J Biol Chem. 2010;1:69–80. doi: 10.4331/wjbc.v1.i5.69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Qabar AN, Lin Z, Wolf FW, O’Shea KS, Lawler J, Dixit VM. Thrombospondin 3 is a developmentally regulated heparin binding protein. The Journal of biological chemistry. 1994;269:1262–9. [PubMed] [Google Scholar]
- 124.Narouz-Ott L, Maurer P, Nitsche DP, Smyth N, Paulsson M. Thrombospondin-4 binds specifically to both collagenous and non-collagenous extracellular matrix proteins via its C-terminal domains. The Journal of biological chemistry. 2000;275:37110–7. doi: 10.1074/jbc.M007223200. [DOI] [PubMed] [Google Scholar]
- 125.Chen H, Herndon ME, Lawler J. The cell biology of thrombospondin-1. Matrix biology : journal of the International Society for Matrix Biology. 2000;19:597–614. doi: 10.1016/s0945-053x(00)00107-4. [DOI] [PubMed] [Google Scholar]
- 126.Chen FH, Herndon ME, Patel N, Hecht JT, Tuan RS, Lawler J. Interaction of cartilage oligomeric matrix protein/thrombospondin 5 with aggrecan. The Journal of biological chemistry. 2007;282:24591–8. doi: 10.1074/jbc.M611390200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Gao AG, Lindberg FP, Dimitry JM, Brown EJ, Frazier WA. Thrombospondin modulates alpha v beta 3 function through integrin-associated protein. The Journal of cell biology. 1996;135:533–44. doi: 10.1083/jcb.135.2.533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Murphy-Ullrich JE, Hook M. Thrombospondin modulates focal adhesions in endothelial cells. The Journal of cell biology. 1989;109:1309–19. doi: 10.1083/jcb.109.3.1309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Adams JC. Thrombospondins: multifunctional regulators of cell interactions. Annual review of cell and developmental biology. 2001;17:25–51. doi: 10.1146/annurev.cellbio.17.1.25. [DOI] [PubMed] [Google Scholar]
- 130.Kirk J, Cingolani OH. Thrombospondins in the Transition from Myocardial Infarction to Heart Failure. Journal of Molecullar and Cellular Cardiology. 2015;10(90):102–110. doi: 10.1016/j.yjmcc.2015.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Tan K, Lawler J. The interaction of Thrombospondins with extracellular matrix proteins. Journal of cell communication and signaling. 2009;3:177–87. doi: 10.1007/s12079-009-0074-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.How CB, Ming KE, Chin CY. Does religious affiliation influence glycaemic control in primary care patients with type 2 diabetes mellitus? Mental health in family medicine. 2011;8:21–8. [PMC free article] [PubMed] [Google Scholar]
- 133.Schellings MW, van Almen GC, Sage EH, Heymans S. Thrombospondins in the heart: potential functions in cardiac remodeling. Journal of cell communication and signaling. 2009;3:201–13. doi: 10.1007/s12079-009-0070-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Mustonen E, Ruskoaho H, Rysa J. Thrombospondins, potential drug targets for cardiovascular diseases. Basic & clinical pharmacology & toxicology. 2013;112:4–12. doi: 10.1111/bcpt.12026. [DOI] [PubMed] [Google Scholar]
- 135.Schroen B, Heymans S, Sharma U, Blankesteijn WM, Pokharel S, Cleutjens JP, et al. Thrombospondin-2 is essential for myocardial matrix integrity: increased expression identifies failure-prone cardiac hypertrophy. Circulation research. 2004;95:515–22. doi: 10.1161/01.RES.0000141019.20332.3e. [DOI] [PubMed] [Google Scholar]
- 136.Mustonen E, Aro J, Puhakka J, Ilves M, Soini Y, Leskinen H, et al. Thrombospondin-4 expression is rapidly upregulated by cardiac overload. Biochemical and biophysical research communications. 2008;373:186–91. doi: 10.1016/j.bbrc.2008.05.164. [DOI] [PubMed] [Google Scholar]
- 137.Frolova EG, Sopko N, Blech L, Popovic ZB, Li J, Vasanji A, et al. Thrombospondin-4 regulates fibrosis and remodeling of the myocardium in response to pressure overload. FASEB journal : official publication of the Federation of American Societies for Experimental Biology. 2012;26:2363–73. doi: 10.1096/fj.11-190728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Cingolani OH, Kirk JA, Seo K, Koitabashi N, Lee DI, Ramirez-Correa G, et al. Thrombospondin-4 is required for stretch-mediated contractility augmentation in cardiac muscle. Circulation research. 2011;109:1410–4. doi: 10.1161/CIRCRESAHA.111.256743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Lynch JM, Maillet M, Vanhoutte D, Schloemer A, Sargent MA, Blair NS, et al. A thrombospondin-dependent pathway for a protective ER stress response. Cell. 2012;149:1257–68. doi: 10.1016/j.cell.2012.03.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Swinnen M, Vanhoutte D, Van Almen GC, Hamdani N, Schellings MW, D’Hooge J, et al. Absence of thrombospondin-2 causes age-related dilated cardiomyopathy. Circulation. 2009;120:1585–97. doi: 10.1161/CIRCULATIONAHA.109.863266. [DOI] [PubMed] [Google Scholar]
- 141.Xia Y, Dobaczewski M, Gonzalez-Quesada C, Chen W, Biernacka A, Li N, et al. Endogenous thrombospondin 1 protects the pressure-overloaded myocardium by modulating fibroblast phenotype and matrix metabolism. Hypertension. 2011;58:902–11. doi: 10.1161/HYPERTENSIONAHA.111.175323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Lopez-Dee Z, Pidcock K, Gutierrez LS. Thrombospondin-1: multiple paths to inflammation. Mediators of inflammation. 2011;2011:296069. doi: 10.1155/2011/296069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Febbraio M, Silverstein RL. CD36: implications in cardiovascular disease. The international journal of biochemistry & cell biology. 2007;39:2012–30. doi: 10.1016/j.biocel.2007.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Lamy L, Foussat A, Brown EJ, Bornstein P, Ticchioni M, Bernard A. Interactions between CD47 and thrombospondin reduce inflammation. Journal of immunology. 2007;178:5930–9. doi: 10.4049/jimmunol.178.9.5930. [DOI] [PubMed] [Google Scholar]
- 145.Papageorgiou AP, Swinnen M, Vanhoutte D, VandenDriessche T, Chuah M, Lindner D, et al. Thrombospondin-2 prevents cardiac injury and dysfunction in viral myocarditis through the activation of regulatory T-cells. Cardiovascular research. 2012;94:115–24. doi: 10.1093/cvr/cvs077. [DOI] [PubMed] [Google Scholar]
- 146.Cingolani OH, Kirk JA, Seo K, Koitahashi N, Lee DI, Ramirez-Correa G, et al. Thrombospondin-4 Is Required for Stretch-Mediated Contractility Augmentation in Cardiac Muscle. Circulation Research. 2011;109:1410–4. doi: 10.1161/CIRCRESAHA.111.256743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Moens AL, Cingolani O, Spinale FS, Kass DA. Exacerbated cardiac remodeling to pressure-overload in mice lacking thrombospondin-4. Hypertension. 2008;52:749. [Google Scholar]
- 148.Frolova EG, Pluskota E, Krukovets I, Burke T, Drumm C, Smith JD, et al. Thrombospondin-4 regulates vascular inflammation and atherogenesis. Circulation research. 2010;107:1313–25. doi: 10.1161/CIRCRESAHA.110.232371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Collins AR, Schnee J, Wang W, Kim S, Fishbein MC, Bruemmer D, et al. Osteopontin modulates angiotensin II-induced fibrosis in the intact murine heart. Journal of the American College of Cardiology. 2004;43:1698–705. doi: 10.1016/j.jacc.2003.11.058. [DOI] [PubMed] [Google Scholar]
- 150.Frangogiannis NG, Youker KA, Rossen RD, Gwechenberger M, Lindsey MH, Mendoza LH, et al. Cytokines and the microcirculation in ischemia and reperfusion. Journal of molecular and cellular cardiology. 1998;30:2567–76. doi: 10.1006/jmcc.1998.0829. [DOI] [PubMed] [Google Scholar]
- 151.Standal T, Borset M, Sundan A. Role of osteopontin in adhesion, migration, cell survival and bone remodeling. Experimental oncology. 2004;26:179–84. [PubMed] [Google Scholar]
- 152.Wang KX, Denhardt DT. Osteopontin: role in immune regulation and stress responses. Cytokine & growth factor reviews. 2008;19:333–45. doi: 10.1016/j.cytogfr.2008.08.001. [DOI] [PubMed] [Google Scholar]
- 153.Scatena M, Almeida M, Chaisson ML, Fausto N, Nicosia RF, Giachelli CM. NF-kappaB mediates alphavbeta3 integrin-induced endothelial cell survival. The Journal of cell biology. 1998;141:1083–93. doi: 10.1083/jcb.141.4.1083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Trueblood NA, Xie Z, Communal C, Sam F, Ngoy S, Liaw L, et al. Exaggerated left ventricular dilation and reduced collagen deposition after myocardial infarction in mice lacking osteopontin. Circ Res. 2001;88:1080–7. doi: 10.1161/hh1001.090842. [DOI] [PubMed] [Google Scholar]
- 155.Frangogiannis NG. Matricellular proteins in cardiac adaptation and disease. Physiol Rev. 2012;92:635–88. doi: 10.1152/physrev.00008.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Singh M, Ananthula S, Milhorn DM, Krishnaswamy G, Singh K. Osteopontin: a novel inflammatory mediator of cardiovascular disease. Frontiers in bioscience : a journal and virtual library. 2007;12:214–21. doi: 10.2741/2059. [DOI] [PubMed] [Google Scholar]
- 157.Imanaka-Yoshida K, Hiroe M, Nishikawa T, Ishiyama S, Shimojo T, Ohta Y, et al. Tenascin-C modulates adhesion of cardiomyocytes to extracellular matrix during tissue remodeling after myocardial infarction. Laboratory investigation; a journal of technical methods and pathology. 2001;81:1015–24. doi: 10.1038/labinvest.3780313. [DOI] [PubMed] [Google Scholar]
- 158.Imanaka-Yoshida K, Hiroe M, Yasutomi Y, Toyozaki T, Tsuchiya T, Noda N, et al. Tenascin-C is a useful marker for disease activity in myocarditis. The Journal of pathology. 2002;197:388–94. doi: 10.1002/path.1131. [DOI] [PubMed] [Google Scholar]
- 159.Xia Y, Lee K, Li N, Corbett D, Mendoza L, Frangogiannis NG. Characterization of the inflammatory and fibrotic response in a mouse model of cardiac pressure overload. Histochemistry and cell biology. 2009;131:471–81. doi: 10.1007/s00418-008-0541-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Murphyullrich JE, Lightner VA, Aukhil I, Yan YZ, Erickson HP, Hook M. Focal Adhesion Integrity Is down-Regulated by the Alternatively Spliced Domain of Human Tenascin. Journal of Cell Biology. 1991;115:1127–36. doi: 10.1083/jcb.115.4.1127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Murphy-Ullrich JE. The de-adhesive activity of matricellular proteins: is intermediate cell adhesion an adaptive state? The Journal of clinical investigation. 2001;107:785–90. doi: 10.1172/JCI12609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Tamaoki M, Imanaka-Yoshida K, Yokoyama K, Nishioka T, Inada H, Hiroe M, et al. Tenascin-C regulates recruitment of myofibroblasts during tissue repair after myocardial injury. Am J Pathol. 2005;167:71–80. doi: 10.1016/S0002-9440(10)62954-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Liabeuf S, Barreto DV, Kretschmer A, Barreto FC, Renard C, Andrejak M, et al. High circulating levels of large splice variants of tenascin-C is associated with mortality and cardiovascular disease in chronic kidney disease patients. Atherosclerosis. 2011;215:116–24. doi: 10.1016/j.atherosclerosis.2010.11.038. [DOI] [PubMed] [Google Scholar]
- 164.Yao HC, Han QF, Zhao AP, Yao DK, Wang LX. Prognostic values of serum tenascin-C in patients with ischaemic heart disease and heart failure. Heart, lung & circulation. 2013;22:184–7. doi: 10.1016/j.hlc.2012.10.005. [DOI] [PubMed] [Google Scholar]
- 165.Sarli B, Topsakal R, Kaya EG, Akpek M, Lam YY, Kaya MG. Tenascin-C as predictor of left ventricular remodeling and mortality in patients with dilated cardiomyopathy. Journal of investigative medicine : the official publication of the American Federation for Clinical Research. 2013;61:728–32. doi: 10.2310/JIM.0b013e3182880c11. [DOI] [PubMed] [Google Scholar]
- 166.Sato A, Hiroe M, Akiyama D, Hikita H, Nozato T, Hoshi T, et al. Prognostic value of serum tenascin-C levels on long-term outcome after acute myocardial infarction. Journal of cardiac failure. 2012;18:480–6. doi: 10.1016/j.cardfail.2012.02.009. [DOI] [PubMed] [Google Scholar]
- 167.Nishioka T, Onishi K, Shimojo N, Nagano Y, Matsusaka H, Ikeuchi M, et al. Tenascin-C may aggravate left ventricular remodeling and function after myocardial infarction in mice. American journal of physiology Heart and circulatory physiology. 2010;298:H1072–8. doi: 10.1152/ajpheart.00255.2009. [DOI] [PubMed] [Google Scholar]
- 168.Shimojo N, Hashizume R, Kanayama K, Hara M, Suzuki Y, Nishioka T, et al. Tenascin-C may accelerate cardiac fibrosis by activating macrophages via the integrin alphaVbeta3/nuclear factor-kappaB/interleukin-6 axis. Hypertension. 2015;66:757–66. doi: 10.1161/HYPERTENSIONAHA.115.06004. [DOI] [PubMed] [Google Scholar]
- 169.Horiuchi K, Amizuka N, Takeshita S, Takamatsu H, Katsuura M, Ozawa H, et al. Identification and characterization of a novel protein, periostin, with restricted expression to periosteum and periodontal ligament and increased expression by transforming growth factor beta. Journal of bone and mineral research : the official journal of the American Society for Bone and Mineral Research. 1999;14:1239–49. doi: 10.1359/jbmr.1999.14.7.1239. [DOI] [PubMed] [Google Scholar]
- 170.Bastiani MJ, Harrelson AL, Snow PM, Goodman CS. Expression of fasciclin I and II glycoproteins on subsets of axon pathways during neuronal development in the grasshopper. Cell. 1987;48:745–55. doi: 10.1016/0092-8674(87)90072-9. [DOI] [PubMed] [Google Scholar]
- 171.Elkins T, Hortsch M, Bieber AJ, Snow PM, Goodman CS. Drosophila fasciclin I is a novel homophilic adhesion molecule that along with fasciclin III can mediate cell sorting. The Journal of cell biology. 1990;110:1825–32. doi: 10.1083/jcb.110.5.1825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Snider P, Hinton RB, Moreno-Rodriguez RA, Wang J, Rogers R, Lindsley A, et al. Periostin is required for maturation and extracellular matrix stabilization of noncardiomyocyte lineages of the heart. Circulation research. 2008;102:752–60. doi: 10.1161/CIRCRESAHA.107.159517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Li G, Jin R, Norris RA, Zhang L, Yu S, Wu F, et al. Periostin mediates vascular smooth muscle cell migration through the integrins alphavbeta3 and alphavbeta5 and focal adhesion kinase (FAK) pathway. Atherosclerosis. 2010;208:358–65. doi: 10.1016/j.atherosclerosis.2009.07.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Norris RA, Moreno-Rodriguez R, Hoffman S, Markwald RR. The many facets of the matricelluar protein periostin during cardiac development, remodeling, and pathophysiology. Journal of cell communication and signaling. 2009;3:275–86. doi: 10.1007/s12079-009-0063-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Oka T, Xu J, Kaiser RA, Melendez J, Hambleton M, Sargent MA, et al. Genetic manipulation of periostin expression reveals a role in cardiac hypertrophy and ventricular remodeling. Circulation research. 2007;101:313–21. doi: 10.1161/CIRCRESAHA.107.149047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Shimazaki M, Nakamura K, Kii I, Kashima T, Amizuka N, Li M, et al. Periostin is essential for cardiac healing after acute myocardial infarction. The Journal of experimental medicine. 2008;205:295–303. doi: 10.1084/jem.20071297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Johnatty SE, Dyck JR, Michael LH, Olson EN, Abdellatif M. Identification of genes regulated during mechanical load-induced cardiac hypertrophy. Journal of molecular and cellular cardiology. 2000;32:805–15. doi: 10.1006/jmcc.2000.1122. [DOI] [PubMed] [Google Scholar]
- 178.Stanton LW, Garrard LJ, Damm D, Garrick BL, Lam A, Kapoun AM, et al. Altered patterns of gene expression in response to myocardial infarction. Circulation research. 2000;86:939–45. doi: 10.1161/01.res.86.9.939. [DOI] [PubMed] [Google Scholar]
- 179.Wang D, Oparil S, Feng JA, Li P, Perry G, Chen LB, et al. Effects of pressure overload on extracellular matrix expression in the heart of the atrial natriuretic peptide-null mouse. Hypertension. 2003;42:88–95. doi: 10.1161/01.HYP.0000074905.22908.A6. [DOI] [PubMed] [Google Scholar]
- 180.Kruzynska-Frejtag A, Machnicki M, Rogers R, Markwald RR, Conway SJ. Periostin (an osteoblast-specific factor) is expressed within the embryonic mouse heart during valve formation. Mechanisms of development. 2001;103:183–8. doi: 10.1016/s0925-4773(01)00356-2. [DOI] [PubMed] [Google Scholar]
- 181.Wilde J, Yokozeki M, Terai K, Kudo A, Moriyama K. The divergent expression of periostin mRNA in the periodontal ligament during experimental tooth movement. Cell and tissue research. 2003;312:345–51. doi: 10.1007/s00441-002-0664-2. [DOI] [PubMed] [Google Scholar]
- 182.Katsuragi N, Morishita R, Nakamura N, Ochiai T, Taniyama Y, Hasegawa Y, et al. Periostin as a novel factor responsible for ventricular dilation. Circulation. 2004;110:1806–13. doi: 10.1161/01.CIR.0000142607.33398.54. [DOI] [PubMed] [Google Scholar]
- 183.Norris RA, Damon B, Mironov V, Kasyanov V, Ramamurthi A, Moreno-Rodriguez R, et al. Periostin regulates collagen fibrillogenesis and the biomechanical properties of connective tissues. Journal of cellular biochemistry. 2007;101:695–711. doi: 10.1002/jcb.21224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Stansfield WE, Andersen NM, Tang RH, Selzman CH. Periostin is a novel factor in cardiac remodeling after experimental and clinical unloading of the failing heart. The Annals of thoracic surgery. 2009;88:1916–21. doi: 10.1016/j.athoracsur.2009.07.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Hakuno D, Kimura N, Yoshioka M, Mukai M, Kimura T, Okada Y, et al. Periostin advances atherosclerotic and rheumatic cardiac valve degeneration by inducing angiogenesis and MMP production in humans and rodents. The Journal of clinical investigation. 2010;120:2292–306. doi: 10.1172/JCI40973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Chlenski A, Cohn SL. Modulation of matrix remodeling by SPARC in neoplastic progression. Semin Cell Dev Biol. 2010;21:55–65. doi: 10.1016/j.semcdb.2009.11.018. [DOI] [PubMed] [Google Scholar]
- 187.Schellings MW, Vanhoutte D, Swinnen M, Cleutjens JP, Debets J, van Leeuwen RE, et al. Absence of SPARC results in increased cardiac rupture and dysfunction after acute myocardial infarction. J Exp Med. 2009;206:113–23. doi: 10.1084/jem.20081244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Bradshaw AD. The role of secreted protein acidic and rich in cysteine (SPARC) in cardiac repair and fibrosis: Does expression of SPARC by macrophages influence outcomes? Journal of molecular and cellular cardiology. 2015 doi: 10.1016/j.yjmcc.2015.11.014. [DOI] [PubMed] [Google Scholar]
- 189.Bradshaw AD, Baicu CF, Rentz TJ, Van Laer AO, Boggs J, Lacy JM, et al. Pressure overload-induced alterations in fibrillar collagen content and myocardial diastolic function: role of secreted protein acidic and rich in cysteine (SPARC) in post-synthetic procollagen processing. Circulation. 2009;119:269–80. doi: 10.1161/CIRCULATIONAHA.108.773424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Raines EW, Lane TF, Iruela-Arispe ML, Ross R, Sage EH. The extracellular glycoprotein SPARC interacts with platelet-derived growth factor (PDGF)-AB and - BB and inhibits the binding of PDGF to its receptors. Proc Natl Acad Sci U S A. 1992;89:1281–5. doi: 10.1073/pnas.89.4.1281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Motamed K, Funk SE, Koyama H, Ross R, Raines EW, Sage EH. Inhibition of PDGF-stimulated and matrix-mediated proliferation of human vascular smooth muscle cells by SPARC is independent of changes in cell shape or cyclin-dependent kinase inhibitors. J Cell Biochem. 2002;84:759–71. doi: 10.1002/jcb.10095. [DOI] [PubMed] [Google Scholar]
- 192.Arslan F, Smeets MB, Riem Vis PW, Karper JC, Quax PH, Bongartz LG, et al. Lack of fibronectin-EDA promotes survival and prevents adverse remodeling and heart function deterioration after myocardial infarction. Circ Res. 2011;108:582–92. doi: 10.1161/CIRCRESAHA.110.224428. [DOI] [PubMed] [Google Scholar]
- 193.Knowlton AA, Connelly CM, Romo GM, Mamuya W, Apstein CS, Brecher P. Rapid expression of fibronectin in the rabbit heart after myocardial infarction with and without reperfusion. The Journal of clinical investigation. 1992;89:1060–8. doi: 10.1172/JCI115685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Schoneveld AH, Hoefer I, Sluijter JP, Laman JD, de Kleijn DP, Pasterkamp G. Atherosclerotic lesion development and Toll like receptor 2 and 4 responsiveness. Atherosclerosis. 2008;197:95–104. doi: 10.1016/j.atherosclerosis.2007.08.004. [DOI] [PubMed] [Google Scholar]
- 195.Gondokaryono SP, Ushio H, Niyonsaba F, Hara M, Takenaka H, Jayawardana ST, et al. The extra domain A of fibronectin stimulates murine mast cells via toll-like receptor 4. J Leukoc Biol. 2007;82:657–65. doi: 10.1189/jlb.1206730. [DOI] [PubMed] [Google Scholar]
- 196.Serini G, Bochaton-Piallat ML, Ropraz P, Geinoz A, Borsi L, Zardi L, et al. The fibronectin domain ED-A is crucial for myofibroblastic phenotype induction by transforming growth factor-beta1. The Journal of cell biology. 1998;142:873–81. doi: 10.1083/jcb.142.3.873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Kohan M, Muro AF, White ES, Berkman N. EDA-containing cellular fibronectin induces fibroblast differentiation through binding to alpha4beta7 integrin receptor and MAPK/Erk 1/2-dependent signaling. FASEB journal : official publication of the Federation of American Societies for Experimental Biology. 2010;24:4503–12. doi: 10.1096/fj.10-154435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Spinale FG. Myocardial matrix remodeling and the matrix metalloproteinases: influence on cardiac form and function. Physiological reviews. 2007;87:1285–342. doi: 10.1152/physrev.00012.2007. [DOI] [PubMed] [Google Scholar]
- 199.Vu TH, Werb Z. Matrix metalloproteinases: effectors of development and normal physiology. Genes & development. 2000;14:2123–33. doi: 10.1101/gad.815400. [DOI] [PubMed] [Google Scholar]
- 200.Moshal KS, Tyagi N, Moss V, Henderson B, Steed M, Ovechkin A, et al. Early induction of matrix metalloproteinase-9 transduces signaling in human heart end stage failure. Journal of cellular and molecular medicine. 2005;9:704–13. doi: 10.1111/j.1582-4934.2005.tb00501.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Wilson EM, Spinale FG. Myocardial remodelling and matrix metalloproteinases in heart failure: turmoil within the interstitium. Annals of medicine. 2001;33:623–34. doi: 10.3109/07853890109002108. [DOI] [PubMed] [Google Scholar]
- 202.Vanhoutte D, Heymans S. TIMPs and cardiac remodeling: ‘Embracing the MMP-independent-side of the family’. Journal of molecular and cellular cardiology. 2010;48:445–53. doi: 10.1016/j.yjmcc.2009.09.013. [DOI] [PubMed] [Google Scholar]
- 203.Brew K, Dinakarpandian D, Nagase H. Tissue inhibitors of metalloproteinases: evolution, structure and function. Biochim Biophys Acta. 2000;1477:267–83. doi: 10.1016/s0167-4838(99)00279-4. [DOI] [PubMed] [Google Scholar]
- 204.Gomez DE, Alonso DF, Yoshiji H, Thorgeirsson UP. Tissue inhibitors of metalloproteinases: structure, regulation and biological functions. Eur J Cell Biol. 1997;74:111–22. [PubMed] [Google Scholar]
- 205.Altieri P, Brunelli C, Garibaldi S, Nicolino A, Ubaldi S, Spallarossa P, et al. Metalloproteinases 2 and 9 are increased in plasma of patients with heart failure. European journal of clinical investigation. 2003;33:648–56. doi: 10.1046/j.1365-2362.2003.01187.x. [DOI] [PubMed] [Google Scholar]
- 206.Barton PJ, Birks EJ, Felkin LE, Cullen ME, Koban MU, Yacoub MH. Increased expression of extracellular matrix regulators TIMP1 and MMP1 in deteriorating heart failure. The Journal of heart and lung transplantation : the official publication of the International Society for Heart Transplantation. 2003;22:738–44. doi: 10.1016/s1053-2498(02)00557-0. [DOI] [PubMed] [Google Scholar]
- 207.Bhalla V, Georgiopoulou VV, Azeem AA, Marti CN, Cole RT, Laskar SR, et al. Matrix metalloproteinases, tissue inhibitors of metalloproteinases, and heart failure outcomes. International journal of cardiology. 2011;151:237–9. doi: 10.1016/j.ijcard.2011.06.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Lopez B, Gonzalez A, Diez J. Role of matrix metalloproteinases in hypertension-associated cardiac fibrosis. Current opinion in nephrology and hypertension. 2004;13:197–204. doi: 10.1097/00041552-200403000-00008. [DOI] [PubMed] [Google Scholar]
- 209.Phatharajaree W, Phrommintikul A, Chattipakorn N. Matrix metalloproteinases and myocardial infarction. Can J Cardiol. 2007;23:727–33. doi: 10.1016/s0828-282x(07)70818-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Creemers EE, Cleutjens JP, Smits JF, Daemen MJ. Matrix metalloproteinase inhibition after myocardial infarction: a new approach to prevent heart failure? Circulation research. 2001;89:201–10. doi: 10.1161/hh1501.094396. [DOI] [PubMed] [Google Scholar]
- 211.Lagente V, Boichot E. Matrix metalloproteinases in tissue remodelling and inflammation. Basel; Boston: Birkhäuser; 2008. [Google Scholar]
- 212.Spinale FG. Matrix metalloproteinases: regulation and dysregulation in the failing heart. Circulation research. 2002;90:520–30. doi: 10.1161/01.res.0000013290.12884.a3. [DOI] [PubMed] [Google Scholar]
- 213.Yonggang Ma RPI, de Castro Bras Lisandra E, Toba Hiroe, Yabluchanskiy Andrey, Deleon-Pennel Kristine Y, Hall Michael E, Lange Richard A, Lindsey Merry L. Cross Talk Between Inflammation and Extracellular Matrix Following Myocardial Infarction. In: Matthijs W, Blankesteijn RA, editors. Inflammation in Heart Failure. 2014. pp. 67–76. [Google Scholar]
- 214.Nagase H, Visse R, Murphy G. Structure and function of matrix metalloproteinases and TIMPs. Cardiovascular research. 2006;69:562–73. doi: 10.1016/j.cardiores.2005.12.002. [DOI] [PubMed] [Google Scholar]
- 215.Lovelock JD, Baker AH, Gao F, Dong JF, Bergeron AL, McPheat W, et al. Heterogeneous effects of tissue inhibitors of matrix metalloproteinases on cardiac fibroblasts. Am J Physiol Heart Circ Physiol. 2005;288:H461–8. doi: 10.1152/ajpheart.00402.2004. [DOI] [PubMed] [Google Scholar]
- 216.Lindsey ML, Escobar GP, Mukherjee R, Goshorn DK, Sheats NJ, Bruce JA, et al. Matrix metalloproteinase-7 affects connexin-43 levels, electrical conduction, and survival after myocardial infarction. Circulation. 2006;113:2919–28. doi: 10.1161/CIRCULATIONAHA.106.612960. [DOI] [PubMed] [Google Scholar]
- 217.Jaffré F, Friedman AE, Hu Z, Mackman N, Blaxall BC. β-adrenergic receptor stimulation transactivates protease-activated receptor 1 via matrix metalloproteinase 13 in cardiac cells. Circulation. 2012;125:2993–3003. doi: 10.1161/CIRCULATIONAHA.111.066787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Ricard-Blum S, Salza R. Matricryptins and matrikines: biologically active fragments of the extracellular matrix. Experimental dermatology. 2014;23:457–63. doi: 10.1111/exd.12435. [DOI] [PubMed] [Google Scholar]
- 219.Davis GE, Bayless KJ, Davis MJ, Meininger GA. Regulation of tissue injury responses by the exposure of matricryptic sites within extracellular matrix molecules. The American journal of pathology. 2000;156:1489–98. doi: 10.1016/S0002-9440(10)65020-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Lindsey ML, Iyer RP, Zamilpa R, Yabluchanskiy A, DeLeon-Pennell KY, Hall ME, et al. A Novel Collagen Matricryptin Reduces Left Ventricular Dilation Post-Myocardial Infarction by Promoting Scar Formation and Angiogenesis. Journal of the American College of Cardiology. 2015;66:1364–74. doi: 10.1016/j.jacc.2015.07.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Lindsey ML, Iyer RP, Jung M, DeLeon-Pennell KY, Ma Y. Matrix metalloproteinases as input and output signals for post-myocardial infarction remodeling. Journal of molecular and cellular cardiology. 2015 doi: 10.1016/j.yjmcc.2015.12.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Martin ML, Blaxall BC. Cardiac intercellular communication: are myocytes and fibroblasts fair-weather friends? Journal of cardiovascular translational research. 2012;5:768–82. doi: 10.1007/s12265-012-9404-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Hsia DA, Mitra SK, Hauck CR, Streblow DN, Nelson JA, Ilic D, et al. Differential regulation of cell motility and invasion by FAK. The Journal of cell biology. 2003;160:753–67. doi: 10.1083/jcb.200212114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Matsuo M, Sakurai H, Ueno Y, Ohtani O, Saiki I. Activation of MEK/ERK and PI3K/Akt pathways by fibronectin requires integrin alphav-mediated ADAM activity in hepatocellular carcinoma: a novel functional target for gefitinib. Cancer science. 2006;97:155–62. doi: 10.1111/j.1349-7006.2006.00152.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Vicente-Manzanares M, Choi CK, Horwitz AR. Integrins in cell migration--the actin connection. Journal of cell science. 2009;122:199–206. doi: 10.1242/jcs.018564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Chong HC, Tan CK, Huang RL, Tan NS. Matricellular proteins: a sticky affair with cancers. Journal of oncology. 2012;2012:351089. doi: 10.1155/2012/351089. [DOI] [PMC free article] [PubMed] [Google Scholar]