Abstract
The idea that our cognitive abilities change with age has support from empirical research as well as from anecdotal reports. Cognition has many component processes, some of which are impaired by normal aging like attention and memory as a result of changes in perceptual systems or speed of processing. Other cognitive domains improve in functioning as aging continues such as wisdom and some kinds of decision making. Many years of research in the psychology of cognitive aging has described patterns of age-related changes in cognitive processes with older adults performing worse than younger adults on tests of attention, working memory and episodic memory and better on tests of general knowledge. More recent work in task-related functional neuroimaging has further elucidated the effects of aging on brain circuitry related to these cognitive processes. Generally, studies show that older adults activate regions of the frontal cortex more than younger adults while younger adults activate more posterior cortical areas. This paper describes normal patterns of cognitive change in healthy aging, describes how some of these processes can be explored with functional neuroimaging, and briefly describes the work attempting to describe differences between normal and pathological cognitive aging.
Keywords: geropsychiatry, cognitive disorders, late life, cognitive abilities, pathological cognitive aging, cognitive aging, fMRI, episodic memory, attention, working memory
Introduction
As people age, there are declines in cognition that fall short of dementia but still impact functional abilities and independence1. The goal of successful aging is to maintain intact cognitive functioning all the way until death. Normal cognitive aging is not dementia and does not result in the loss of neurons. Rather there are changes in brain functioning that may have a financial impact on society. Older adults with normal cognitive changes are more susceptible to financial scams and may have difficulty with financial decisions1. Moreover, behaviors which affect health and safety (driving skills, health care decisions, medication adherence) also are impacted by normal variations in cognitive aging. First, it is necessary to understand how cognition changes in healthy older adults and that is the focus of this review. A second step would be to develop interventions that are likely to slow, stop, or reverse normal cognitive aging.
Cognitive Aging
The field of cognitive psychology is concerned with discovering the form of mental representation and the processes that access them. It is the study of how people perceive, learn, remember, and think about information. The study of cognitive aging seeks to examine how these processes change over time and between people. Normal aging has been defined as aging changes that occur in individuals free of overt diseases of the nervous system. Neurological disorders like Alzheimer’s disease (AD) and Parkinson’s disease (PD) are not normal manifestations of aging fated for everyone who lives long enough to have them. But these diseases do appear in an age-dependent manner and can share some cognitive features with normal aging making it difficult to completely distinguish between signs and symptoms of overt disease and normal aging. As there are decreases in biological functions and mental abilities that are normal and happen with the passage of time, it is helpful to understand these changes in order to understand what happens when there is disease in addition to the normal changes.
Specifically, studies in healthy older adults show declines in some cognitive domains while showing improvements in others. Most commonly, healthy older adults show impairments on tasks of attention, working memory, and episodic memory relative to younger adults (i.e., 2–4. However, older adults also show improvements on cognitive tasks where they can rely on experience to perform well such as tests assessing wisdom and general knowledge4. One way to conceptualize how aging affects cognition is through the model described below.
Model of Information Processing and Aging
Cowan5,6 proposed a model of information processing that describes the relationships between attention, working memory, and long term memory storage. In this model, working memory is conceptualized as the activated portion of long-term memory. Working memory contains information both inside and outside the focus of attention which has a very restricted capacity, limited in some cases to as little as one item (e.g.,7). In the context of the Cowan5,6 information processing model, aging may result in difficulties in controlling the focus of attention. Attentional control impairments imply that older adults allow both relevant and irrelevant information to enter into the focus of attention which will impair performance on any kind of task requiring the ability to differentiate relevant from irrelevant information. Older adults may also fail to suppress attention to irrelevant information that has already entered working memory8. Thus, the effect of aging on the focus of attention may be to blur the boundaries or widen the focus of attention such that older adults have less control over the content of what is currently within the focus of attention (see Figure 1).
Figure 1. Model of Cognition and Aging.
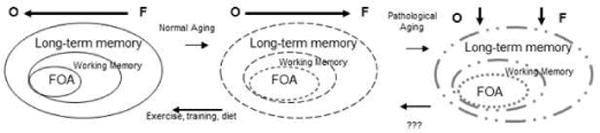
Figure 1 diagrams a model of age-related changes in cognition based on the model of Cowan5,6 and includes patterns of brain activation as measured by fMRI. Dotted and grayed lines indicate impairments. When younger adults (left panel) perform attention and memory tests they show brain activation patterns that are balanced between occipital and frontal regions or even have a shift towards greater posterior activation. Normal cognitive aging (middle panel) may degrade the control processes of the focus of attention (FOA) thereby affecting working memory and long-term memory. The functional activation patterns show increased in frontal activation relative to posterior regions. Lifestyle modifications may be effective in slowing or reversing some of the aspects of cognitive aging. Cognitive dysfunction seen in pathological cognitive aging may affect all aspects of cognition. The activation patterns will show decreases in frontal and occipital regions.
In the past two decades with the increased access to and availability of MRI scanners, studies have focused on the connection between brain functioing and cognitive processing. The relationship between brain functioining and cognition across the life span is a dynamic one. Understanding how age-related changes in brain functioning affects cognition is important for deliniating differences in normal and pathological aging.
Functional Neuroimaging Studies of Attention, Working Memory, and Episodic Memory in Healthy Older Adults
Older adults show impairments on tasks of attention, working memory and episodic memory relative to younger adults (i.e., 2–4). Age differences in brain activation in functional imaging studies during performance of attention, working memory and episodic memory tasks have been found in many studies (i.e. 9–11). These studies show similarities in activation patterns for older adults relative to younger adults across tasks. Generally, older adults show increases in frontal activation (e.g., 9) and decreases in occipitotemporal activity (e.g., 12) relative to younger adults. While this activation pattern was first described by Grady et al.12, Davis and colleagues 13 labeled the pattern the posterior-anterior shift in aging (PASA). PASA patterns have been seen in a number of different cognitive domains including tests of attention, working memory and episodic memory. Importantly, successful completion of each of these three tasks requires the control of attention. A summary of the prior imaging studies of older and younger adults in these cognitive domains is presented below.
Functional imaging studies of age differences in tasks requiring control of attentional resources show that older adults activate more regions of frontal cortex than younger adults (e.g., 10,14–16). In a study of age differences in Stroop interference, older and younger adults activated similar brain regions during the interference task, but older adults were slower to perform the task and showed more activation in regions of the frontal cortex relative to younger adults10. Older adults have also shown more frontal activation in tests of sustained, selective, and cross modal shifting attention (from auditory to visual;16). Thus, across a broad range of attention tasks, age differences in attentional control resulted in differences in brain activation such that older adults required increased frontal cortical activation.
Functional neuroimaging studies of working memory have shown that the age difference in working memory performance may be related to the ability to recruit frontal brain regions to compensate for poor performance. Increases in prefrontal activation for older adults relative to younger adults appear to depend upon successful task performance and suggest a compensatory process is involved in increased activation for older adults 17,18. Three examples across different tasks involving working memory processes support this proposal. First, Rypma and D’Esposito11 found that faster performing younger adults showed less dorsolateral prefrontal cortex activation relative to slower younger adults. However, faster performing older adults showed increased activation relative to slower older adults. Second, Mattay et al. 19 found that older adults who performed as well as younger adults on the 1-back condition of an N-back working memory task activated more prefrontal cortex bilaterally. However, on the higher working memory load conditions of 2-back and 3-back, older adults performed worse and had less frontal activation than younger adults. Finally, Grossman et al.20 found that on a sentence comprehension task with a high working memory component, older adults with equivalent comprehension scores as younger adults activated more premotor and inferior frontal cortices. Thus, although older adults performed similarly to younger adults in some working memory tasks, they required more frontal activation to do so.
Research on episodic memory shows that during encoding older adults activated more bilateral frontal areas compared to younger adults. Cabeza and colleagues9 have proposed the hemispheric asymmetry reduction for older adults (HAROLD) model of age differences in frontal cortex activation during memory tasks. This is an age-related modification of the hemispheric encoding/retrieval asymmetry (HERA; 21) pattern of brain activation often seen in younger adults where encoding processes activate left prefrontal cortex while retrieval processes activate right prefrontal cortex. Older adults showed a reduction in this asymmetry such that there was more bilateral activation in both encoding and retrieval tasks. Morcom et al.22 found encoding-related activity was left lateralized in younger adults as predicted by HERA and bilateral in older adults as predicted by HAROLD. Dennis et al.23 showed that older adults had increased left prefrontal cortex activity compared to younger adults for words that were successfully encoded relative to words that were forgotten. However, conflicting data have been found by Daselaar et al.24. They found that poor performing older adults had increased overall brain activity during episodic memory encoding relative to younger adults and a group of older adults who performed at the level of younger adults on an episodic memory task. Thus, the relationship between increased activation during episodic encoding and age remains to be further elucidated.
Work by Cabeza and colleagues 25 emphasized the similarities between age differences in brain activation seen across tests of visual attention, working memory, and episodic memory tasks in one study with the same group of subjects. Older adults showed increased activation in the prefrontal cortex and decreased occipital activations during all three task types. Cabeza et al. interpreted these findings as evidence that there are task-independent age-related changes in brain activity representing sensory decline often seen in older adults, in addition to functional compensation for these sensory changes with recruitment of additional frontal cortical areas during these three tasks. Davis et al.13 further explored the PASA effect to examine whether this pattern resulted as an effect of task difficulty, whether it was related to compensation, and whether it generalized to activations on visual perception and episodic retrieval tasks. To investigate the difficulty explanation, Davis et al. 13 matched older and younger adults on task performance and the PASA pattern was still seen. Frontal increases were positively correlated with improved performance while occipital decreases were negatively correlated with performance thus providing evidence that the additional frontal activation is the result of neural compensation26. Finally, the deactivation pattern mirrored the activation pattern showing more deactivation in frontal regions and less deactivation in posterior cortex. Thus overall, these data patterns support the proposal that the PASA effect reflects age-related neural compensation to maintain adequate task performance.
To summarize the data from functional imaging studies of attention, working memory and episodic memory, age differences in brain activation were found across all tasks such that older adults recruited more frontal cortical areas to perform at the same accuracy level as younger adults, supporting the proposal that the additional activation is the result of neural compensation17. Additionally, decreases in posterior cortical areas were seen, potentially indicating a shift toward a frontal cortex-dominated pattern of brain activation. In terms of the Cowan model, older adults may have difficulty controlling the focus of attention8,27. This difficulty may be reflected by an increased need to recruit frontal brain regions25 as well as lessen the ability to suppress activation of irrelevant information28.
Normal versus Pathological Aging: Functional Imaging Evidence
The studies reviewed above detailed differences in brain activation between younger adults and healthy older adults from cross sectional studies. However, understanding how normal aging develops into pathological aging requires additional examination of longitudinal studies as well as cross sectional studies comparing younger adults, healthy older adults, adults with mild cognitive impairment (MCI), and patients with Alzheimer’s disease (AD). Sperling and colleagues have conducted a number of studies examining normal aging in cross sectional studies29,30, longitudinal studies31, and in comparison to patients with age-related pathologies30,32. These studies examined associative encoding during a face-name encoding task in healthy younger and older adults as well as in adults with MCI and AD to examine the age and disease effects on episodic encoding and related brain functioning during the this task30,33,34. The task activates an episodic encoding network that includes the hippocampus, dorsolateral prefrontal cortex, fusiform cortex, and the pulvinar nucleus of the thalamus; decreased activation was found in the posterior cingulate cortex35. One study directly comparing healthy younger and older adults found similar hippocampal activation but different activation patterns in the frontal and parietal cortices34. Both older and younger adults showed increased hippocampal activation during successful encoding of face-name pairs that were successfully remembered33. When healthy older adults were followed longitudinally and performed the fMRI face-name encoding task two years after a baseline assessment, decreased hippocampal activation was associated with clinical decline31, implying that decreased hippocampal activation in healthy older adults may be a biomarker for pathological aging.
To describe differences between normal and pathological cognitive aging, it is necessary to examine differences between those with normal cognition and those with MCI and AD. Cross sectional studies of healthy older adults and those with MCI showed mixed results with some studies showing hyperactivation for the MCI group32,36 while other showed hypoactivation for the MCI group37,38 compared to the healthy older adults on tests of episodic memory. When task-related activation of those with AD was compared to healthy older adults, generally decreases in task-related activation have been seen in medial temporal regions (i.e., 30,36). However, hypoactivation has also been observed in frontal regions and is interpreted as a compensation response to the inability to engage medial temporal regions30.
Overall, task-based fMRI data showed mixed results for discriminating normal aging from MCI and AD. It appears to depend on the progression of MCI or AD30. Additional information is needed to interpret the fMRI task-based differences between normal and pathological aging. More recent studies are examining how genetic risk for AD, APOE genotype in particular, and amyloid load contribute to differentiating between normal and pathological cognitive aging39–42. These studies generally show that between 20 – 30% of older adults who show no clinical evidence of cognitive decline have significant amyloid burden. In addition, studies have shown that amyloid plaques have been seen approximately 20–30 years prior to the development of cognitive decline43. As the access to amyloid imaging becomes more widely available, studies are following adults at younger ages before disease begins to fully understand how amyloid and APOE genotype affect the trajectory of the development of pathological cognitive aging. How these processes relate to cognitive performance and affect brain functioning continues to be examined.
Summary
Research on cognitive aging has flourished in recent years detailing how cognitive processes change with increasing age. In addition neuroimaging studies examining the effects of aging on brain circuitry show consistent patterns across a number of cognitive tasks. Recent work is being done to examine biomarkers that differentiate normal and pathological cognitive processes and studies in the near future will be able to describe how these biomarkers relate to patterns of normal cognitive aging shown during fMRI. At this time the data appear to be mixed in terms of the consistent patterns for discriminating normal and pathological aging using taskrelated fMRI alone. However, the examination of amyloid load and APOE genotype is providing more context with which to interpret the task-based fMRI data. Continued efforts in identifying and defining normal aging and the development of methods to slow or reverse it will be useful in continuing to differentiate normal from pathological cognitive processes.
Footnotes
Conflict of Interest
Julie A Dumas has no relevant conflicts to disclose.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by the author.
References
- 1.Blazer DG, Yaffe K, Karlawish J. Cognitive aging: a report from the Institute of Medicine. Jama. 2015;313:2121–2. doi: 10.1001/jama.2015.4380. [DOI] [PubMed] [Google Scholar]
- 2.Verhaeghen P, Marcoen A, Goossens L. Facts and fiction about memory aging: a quantitative integration of research findings. Journal of Gerontology. 1993;48:157–71. doi: 10.1093/geronj/48.4.p157. [DOI] [PubMed] [Google Scholar]
- 3.Verhaeghen P, Cerella J. Aging, executive control, and attention: a review of meta-analyses. Neuroscience Biobehavioral Reviews. 2002;26:849–57. doi: 10.1016/s0149-7634(02)00071-4. [DOI] [PubMed] [Google Scholar]
- 4.Craik FI, Salthouse T. Handbook of Aging and Cogntion II. Mahwah, NJ: Erlbaum; 2000. [Google Scholar]
- 5.Cowan N. Evolving conceptions of memory storage, selective attention, and their mutual constraints within the human information processing system. Psychological Bulletin. 1988;104:163–91. doi: 10.1037/0033-2909.104.2.163. [DOI] [PubMed] [Google Scholar]
- 6.Cowan N. An embedded-process model of working memory. In: M A, Shah P, editors. Models of working memory: Mechanisms of active maintenance and executive control. New York, NY: Cambridge University Press; 1999. pp. 62–101. [Google Scholar]
- 7.McElree B. Working memory and focal attention. Journal of Experimental Psychology: Learning, Memory, and Cognition. 2001;27:817–35. [PMC free article] [PubMed] [Google Scholar]
- 8.Dumas JA, Hartman M. Adult age differneces in access and deletion functions of inhibition. Neuropsychology, Development and Cognition Section B, Aging, Neuropsychology, and Cognition. 2008;15:330–57. doi: 10.1080/13825580701534601. [DOI] [PubMed] [Google Scholar]
- 9.Cabeza R, Anderson ND, Locantore JK, McIntosh AR. Aging Gracefully: Compensatory Brain Activity in High-Performing Older Adults. NeuroImage. 2002;17:1394–402. doi: 10.1006/nimg.2002.1280. [DOI] [PubMed] [Google Scholar]
- 10.Langenecker SA, Nielson KA, Rao SM. fMRI of healthy older adults during Stroop interference. Neuroimage. 2004;21:192–200. doi: 10.1016/j.neuroimage.2003.08.027. [DOI] [PubMed] [Google Scholar]
- 11.Rypma B, D’Esposito M. Isolating the neural mechanisms of age-related changes in human working memory. Nature Neuroscience. 2000;3:509–15. doi: 10.1038/74889. [DOI] [PubMed] [Google Scholar]
- 12.Grady CL, Maisog JM, Horowitz B, Ungerleider LG, Mentis MJ, Salerno JA, Piertrini P, Wagner E, Haxby JV. Age-related changes in cortical blood flow activation during visual processing of faces and location. Journal of Neuroscience. 1994;14:1450–62. doi: 10.1523/JNEUROSCI.14-03-01450.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Davis SW, Dennis NA, Daselaar SM, Fleck MS, Cabeza R. Que PASA? The Posterior Anterior Shift in Aging. Cerebral Cortex. 2008;18:1201–9. doi: 10.1093/cercor/bhm155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Madden DJ, Spaniol J, Whiting WL, Bucur B, Provenzale JM, Cabeza R, White LE, Huettel SA. Adult age differences in the functional neuroanatomy of visual attention: a combined fMRI and DTI study. Neurobiology of Aging. 2007;28:459–76. doi: 10.1016/j.neurobiolaging.2006.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Thomsen T, Specht K, Rimol LM, Hammar A, Nyttinges J, Ersland L, Hugdahl K. Brain localization of attentional control in different age groups by combining functional and structural MRI. Neuroimage. 2004;22:912–9. doi: 10.1016/j.neuroimage.2004.02.015. [DOI] [PubMed] [Google Scholar]
- 16.Townsend J, Adamo M, Haist F. Changing channels: an fMRI study of aging and crossmodal attention shifts. Neuroimage. 2006;31:1682–92. doi: 10.1016/j.neuroimage.2006.01.045. [DOI] [PubMed] [Google Scholar]
- 17.Stern Y. Critical Review: What is cognitive reserve? Theory and research application on the reserve concept. Journal of International Neurpsychological Society. 2002;8:448–60. [PubMed] [Google Scholar]
- 18.Han SD, Bangen KJ, Bondi MW. Functional magnetic resonance imaging of compensatory neural recruitment in aging and risk for Alzheimer’s disease: review and recommendation. Dementia and Geriatric Cognitive Disorders. 2008;27:1–10. doi: 10.1159/000182420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mattay VS, Fera F, Tessitore A, Hariri AR, Berman KF, Das S, Meyer-Lindenberg A, Goldberg TE, Callicot JH, Weinberger DR. Neuropsychological corelates of age-related changes in working memory capacity. Neuroscience Letters. 2006;392:32–7. doi: 10.1016/j.neulet.2005.09.025. [DOI] [PubMed] [Google Scholar]
- 20.Grossman M, Cooke A, DeVita C, Chen W, Moore P, Detre J, Alsop D, Gee J. Sentence processing strategies in healthy seniors with poor sentence comprehension: an fMRI study. Brain and Language. 2002;80:296–313. doi: 10.1006/brln.2001.2581. [DOI] [PubMed] [Google Scholar]
- 21.Tulving E, Kapur S, Craik FI, Moscovitch M, Houle S. Hemispheric encoding/retrieval asymmetry in episodic memory: positron emission tomography findings. Proceedings of the National Academy of Science. 1994;91:2016–20. doi: 10.1073/pnas.91.6.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Morcom AM, Good CD, Frackowiak SJ, Rugg MD. Age effects on the neural correlates of successful memory encoding. Brain. 2003;126:213–29. doi: 10.1093/brain/awg020. [DOI] [PubMed] [Google Scholar]
- 23.Dennis NA, Daselaar S, Cabeza R. Effects of aging on transient and sustained successful memory encoding activity. Neurobiol Aging. 2007;28:1749–58. doi: 10.1016/j.neurobiolaging.2006.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Daselaar SM, Veltman DJ, Rombouts S, Raaijmakers J, Jonker C. Neuroanatomical correlates of episodic encoding and retrieval in young and elderly subjects. Brain. 2003;126:43–56. doi: 10.1093/brain/awg005. [DOI] [PubMed] [Google Scholar]
- 25.Cabeza R, Daselaar SM, Dolcos F, Prince SE, Budde M, Nyberg L. Task-independent and task-specific age effects on brain activity during working memory, visual attention and episodic memory retrieval. Cerebral Cortex. 2004;14:364–75. doi: 10.1093/cercor/bhg133. [DOI] [PubMed] [Google Scholar]
- 26.Stern YS. What is cognitive researve? Theory and research application of the reserve concept. Journal of the International Neuropsychological Society. 2002;8:448–60. [PubMed] [Google Scholar]
- 27.Gazzaley A, Clapp W, Kelley J, McEvoy K, Knight RT, D’Esposito M. Age-related top-down suppression deficit in the early stages or cortical visual memory processing. Proceedes of the National Academy of Science. 2008;105:13122–6. doi: 10.1073/pnas.0806074105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gazzaley A, Cooney JW, Rissman J, D’Esposito M. Top-down suppression deficit underlies working memory impairment in normal aging. Nature Neuroscience. 2005;8:1298–300. doi: 10.1038/nn1543. [DOI] [PubMed] [Google Scholar]
- 29.Rand-Giovannetti E, Chua EF, Driscoll AE, Schacter DL, Albert M, Sperling RA. Hippocampal and neocortical activation during repetitive encoding in older persons. Neurobiology of Aging. 2006;27:173–82. doi: 10.1016/j.neurobiolaging.2004.12.013. [DOI] [PubMed] [Google Scholar]
- 30.Sperling R. Functional MRI studies of associative encoding in normal aging, mild cognitive impairment, and Alzheimer’s disease. Ann N Y Acad Sci. 2007;1097:146–55. doi: 10.1196/annals.1379.009. [DOI] [PubMed] [Google Scholar]
- 31.O’Brien JL, O’Keefe KM, LaViolette PS, DeLuca AN, Blacker D, Dickerson BC, Sperling RA. Longitudinal fMRI in elderly reveals loss of hippocampal activation with clinical decline. Neurology. 2010;74:1969–76. doi: 10.1212/WNL.0b013e3181e3966e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Dickerson BC, Salat DH, Greve DN, Chua EF, Rand-Giovannetti E, Rentz DM, Bertram L, Mullin K, Tanzi RE, Blacker D, Albert MS, Sperling RA. Increased hippocampal activation in mild cognitive impairment compared to normal aging and AD. Neurology. 2005;65:404–11. doi: 10.1212/01.wnl.0000171450.97464.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Miller SL, Celone K, DePeau K, Diamond E, Dickerson BC, Rentz D, Pihlajamaki M, Sperling RA. Age-related memory impairment associated with loss of parietal deactivation but preserved hippocampal activation. Proc Natl Acad Sci U S A. 2008;105:2181–6. doi: 10.1073/pnas.0706818105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sperling R, Chua E, Cocchiarella A, Rand-Giovannetti E, Poldrack R, Schacter DL, Albert M. Putting names to faces: successful encoding of associative memories activates the anterior hippocampal formation. Neuroimage. 2003;20:1400–10. doi: 10.1016/S1053-8119(03)00391-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sperling RA, Bates JF, Cocchiarella AJ, Schacter DL, Rosen BR, Albert MS. Encoding novel face-name associations: a functional MRI study. Hum Brain Mapp. 2001;14:129–39. doi: 10.1002/hbm.1047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Golby A, Silverberg G, Race E, Gabrieli S, O’Shea J, Knierim K, Stebbins G, Gabrieli J. Memory encoding in Alzheimer’s disease: an fMRI study of explicit and implicit memory. Brain. 2005;128:773–87. doi: 10.1093/brain/awh400. [DOI] [PubMed] [Google Scholar]
- 37.Machulda MM, Ward HA, Borowski B, Gunter JL, Cha RH, O’Brien PC, Petersen RC, Boeve BF, Knopman D, Tang-Wai DF, Ivnik RJ, Smith GE, Tangalos EG, Jack CR., Jr Comparison of memory fMRI response among normal, MCI, and Alzheimer’s patients. Neurology. 2003;61:500–6. doi: 10.1212/01.wnl.0000079052.01016.78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Small SA, Perera GM, DeLaPaz R, Mayeux R, Stern Y. Differential regional dysfunction of the hippocampal formation among elderly with memory decline and Alzheimer’s disease. Ann Neurol. 1999;45:466–72. doi: 10.1002/1531-8249(199904)45:4<466::aid-ana8>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- 39.Kennedy KM, Rodrigue KM, Devous MD, Sr, Hebrank AC, Bischof GN, Park DC. Effects of beta-amyloid accumulation on neural function during encoding across the adult lifespan. Neuroimage. 2012;62:1–8. doi: 10.1016/j.neuroimage.2012.03.077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hedden T, Mormino EC, Amariglio RE, Younger AP, Schultz AP, Becker JA, Buckner RL, Johnson KA, Sperling RA, Rentz DM. Cognitive profile of amyloid burden and white matter hyperintensities in cognitively normal older adults. J Neurosci. 2012;32:16233–42. doi: 10.1523/JNEUROSCI.2462-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hedden T, Schultz AP, Rieckmann A, Mormino EC, Johnson KA, Sperling RA, Buckner RL. Multiple Brain Markers are Linked to Age-Related Variation in Cognition. Cereb Cortex. 2014 doi: 10.1093/cercor/bhu238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Resnick SM, Bilgel M, Moghekar A, An Y, Cai Q, Wang MC, Thambisetty M, Prince JL, Zhou Y, Soldan A, Wong DF, O’Brien RJ, Ferrucci L, Albert MS. Changes in Abeta biomarkers and associations with APOE genotype in 2 longitudinal cohorts. Neurobiol Aging. 2015;36:2333–9. doi: 10.1016/j.neurobiolaging.2015.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Jansen WJ, Ossenkoppele R, Knol DL, Tijms BM, Scheltens P, Verhey FR, Visser PJ, Aalten P, Aarsland D, Alcolea D, Alexander M, Almdahl IS, Arnold SE, Baldeiras I, Barthel H, van Berckel BN, Bibeau K, Blennow K, Brooks DJ, van Buchem MA, Camus V, Cavedo E, Chen K, Chetelat G, Cohen AD, Drzezga A, Engelborghs S, Fagan AM, Fladby T, Fleisher AS, van der Flier WM, Ford L, Forster S, Fortea J, Foskett N, Frederiksen KS, Freund-Levi Y, Frisoni GB, Froelich L, Gabryelewicz T, Gill KD, Gkatzima O, Gomez-Tortosa E, Gordon MF, Grimmer T, Hampel H, Hausner L, Hellwig S, Herukka SK, Hildebrandt H, Ishihara L, Ivanoiu A, Jagust WJ, Johannsen P, Kandimalla R, Kapaki E, Klimkowicz-Mrowiec A, Klunk WE, Kohler S, Koglin N, Kornhuber J, Kramberger MG, Van Laere K, Landau SM, Lee DY, de Leon M, Lisetti V, Lleo A, Madsen K, Maier W, Marcusson J, Mattsson N, de Mendonca A, Meulenbroek O, Meyer PT, Mintun MA, Mok V, Molinuevo JL, Mollergard HM, Morris JC, Mroczko B, Van der Mussele S, Na DL, Newberg A, Nordberg A, Nordlund A, Novak GP, Paraskevas GP, Parnetti L, Perera G, Peters O, Popp J, Prabhakar S, Rabinovici GD, Ramakers IH, Rami L, Resende de Oliveira C, Rinne JO, Rodrigue KM, Rodriguez-Rodriguez E, Roe CM, Rot U, Rowe CC, Ruther E, Sabri O, Sanchez-Juan P, Santana I, Sarazin M, Schroder J, Schutte C, Seo SW, Soetewey F, Soininen H, Spiru L, Struyfs H, Teunissen CE, Tsolaki M, Vandenberghe R, Verbeek MM, Villemagne VL, Vos SJ, van Waalwijk van Doorn LJ, Waldemar G, Wallin A, Wallin AK, Wiltfang J, Wolk DA, Zboch M, Zetterberg H. Prevalence of cerebral amyloid pathology in persons without dementia: a meta-analysis. Jama. 2015;313:1924–38. doi: 10.1001/jama.2015.4668. [DOI] [PMC free article] [PubMed] [Google Scholar]


