Abstract
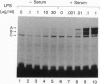
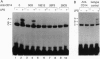
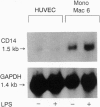
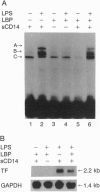
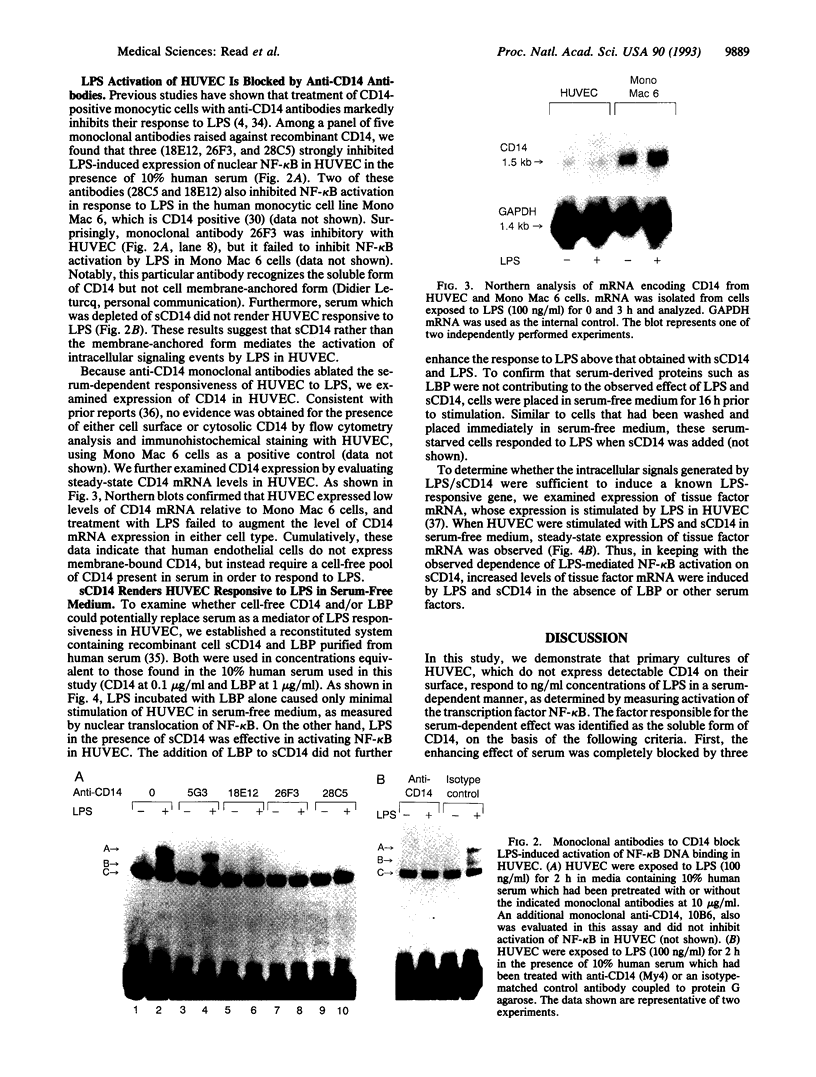
Lipopolysaccharide (LPS), a major envelope component of Gram-negative bacteria, is the most frequent causative agent of septic shock and disseminated intravascular coagulation. LPS activates both CD14-positive (monocytes, macrophages, polymorphonuclear leukocytes) and CD14-negative (B-cell lines, endothelial cells) cells. CD14, a 55-kDa glycosyl-phosphatidylinositol-anchored membrane protein present on mature myeloid cells, serves as a receptor for LPS in complex with a soluble (serum-derived) LPS-binding protein (LBP). In this report, we show that human umbilical vein endothelial cells (HUVEC), which do not express measurable CD14 protein, become 3000-fold more sensitive to LPS-induced activation in the presence of serum, as measured by activation of the transcription factor NF-kappa B and expression of mRNA encoding tissue factor, a procoagulant molecule. This enhanced responsiveness of HUVEC is specifically mediated by the cell-free pool of CD14 (soluble CD14, sCD14) found in serum. The role of sCD14 in HUVEC activation by LPS was established by (i) the blocking effect of monoclonal anti-CD14 antibodies which discriminate between cell-bound and sCD14, (ii) the lack of the serum-enhancing effect after immunodepletion of sCD14, and (iii) establishing a reconstituted system in which recombinant sCD14 was sufficient to enhance the effects of LPS in the absence of serum and without a requirement for LBP. Thus, this mechanism of endothelial cell activation by LPS involves a cell-free pool of sCD14 most likely shed from CD14-positive cells of the monocytic lineage.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bagasra O., Wright S. D., Seshamma T., Oakes J. W., Pomerantz R. J. CD14 is involved in control of human immunodeficiency virus type 1 expression in latently infected cells by lipopolysaccharide. Proc Natl Acad Sci U S A. 1992 Jul 15;89(14):6285–6289. doi: 10.1073/pnas.89.14.6285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bazil V., Baudys M., Hilgert I., Stefanová I., Low M. G., Zbrozek J., Horejsí V. Structural relationship between the soluble and membrane-bound forms of human monocyte surface glycoprotein CD14. Mol Immunol. 1989 Jul;26(7):657–662. doi: 10.1016/0161-5890(89)90048-5. [DOI] [PubMed] [Google Scholar]
- Bazil V., Strominger J. L. Shedding as a mechanism of down-modulation of CD14 on stimulated human monocytes. J Immunol. 1991 Sep 1;147(5):1567–1574. [PubMed] [Google Scholar]
- Beekhuizen H., Blokland I., Corsèl-van Tilburg A. J., Koning F., van Furth R. CD14 contributes to the adherence of human monocytes to cytokine-stimulated endothelial cells. J Immunol. 1991 Dec 1;147(11):3761–3767. [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- Brand K., Fowler B. J., Edgington T. S., Mackman N. Tissue factor mRNA in THP-1 monocytic cells is regulated at both transcriptional and posttranscriptional levels in response to lipopolysaccharide. Mol Cell Biol. 1991 Sep;11(9):4732–4738. doi: 10.1128/mcb.11.9.4732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen C. S., Hawiger J. Reactivity of synthetic peptide analogs of adhesive proteins in regard to the interaction of human endothelial cells with extracellular matrix. Blood. 1991 May 15;77(10):2200–2206. [PubMed] [Google Scholar]
- Clark B. D., Collins K. L., Gandy M. S., Webb A. C., Auron P. E. Genomic sequence for human prointerleukin 1 beta: possible evolution from a reverse transcribed prointerleukin 1 alpha gene. Nucleic Acids Res. 1986 Oct 24;14(20):7897–7914. doi: 10.1093/nar/14.20.7897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins T., Williams A., Johnston G. I., Kim J., Eddy R., Shows T., Gimbrone M. A., Jr, Bevilacqua M. P. Structure and chromosomal location of the gene for endothelial-leukocyte adhesion molecule 1. J Biol Chem. 1991 Feb 5;266(4):2466–2473. [PubMed] [Google Scholar]
- Cordle S. R., Donald R., Read M. A., Hawiger J. Lipopolysaccharide induces phosphorylation of MAD3 and activation of c-Rel and related NF-kappa B proteins in human monocytic THP-1 cells. J Biol Chem. 1993 Jun 5;268(16):11803–11810. [PubMed] [Google Scholar]
- Crossman D. C., Carr D. P., Tuddenham E. G., Pearson J. D., McVey J. H. The regulation of tissue factor mRNA in human endothelial cells in response to endotoxin or phorbol ester. J Biol Chem. 1990 Jun 15;265(17):9782–9787. [PubMed] [Google Scholar]
- Cybulsky M. I., Chan M. K., Movat H. Z. Acute inflammation and microthrombosis induced by endotoxin, interleukin-1, and tumor necrosis factor and their implication in gram-negative infection. Lab Invest. 1988 Apr;58(4):365–378. [PubMed] [Google Scholar]
- Cybulsky M. I., Fries J. W., Williams A. J., Sultan P., Eddy R., Byers M., Shows T., Gimbrone M. A., Jr, Collins T. Gene structure, chromosomal location, and basis for alternative mRNA splicing of the human VCAM1 gene. Proc Natl Acad Sci U S A. 1991 Sep 1;88(17):7859–7863. doi: 10.1073/pnas.88.17.7859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danner R. L., Van Dervort A. L., Doerfler M. E., Stuetz P., Parrillo J. E. Antiendotoxin activity of lipid A analogues: requirements of the chemical structure. Pharm Res. 1990 Mar;7(3):260–263. doi: 10.1023/a:1015874012484. [DOI] [PubMed] [Google Scholar]
- Frey E. A., Miller D. S., Jahr T. G., Sundan A., Bazil V., Espevik T., Finlay B. B., Wright S. D. Soluble CD14 participates in the response of cells to lipopolysaccharide. J Exp Med. 1992 Dec 1;176(6):1665–1671. doi: 10.1084/jem.176.6.1665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golenbock D. T., Hampton R. Y., Qureshi N., Takayama K., Raetz C. R. Lipid A-like molecules that antagonize the effects of endotoxins on human monocytes. J Biol Chem. 1991 Oct 15;266(29):19490–19498. [PubMed] [Google Scholar]
- Harlan J. M., Harker L. A., Reidy M. A., Gajdusek C. M., Schwartz S. M., Striker G. E. Lipopolysaccharide-mediated bovine endothelial cell injury in vitro. Lab Invest. 1983 Mar;48(3):269–274. [PubMed] [Google Scholar]
- Haskill S., Beg A. A., Tompkins S. M., Morris J. S., Yurochko A. D., Sampson-Johannes A., Mondal K., Ralph P., Baldwin A. S., Jr Characterization of an immediate-early gene induced in adherent monocytes that encodes I kappa B-like activity. Cell. 1991 Jun 28;65(7):1281–1289. doi: 10.1016/0092-8674(91)90022-q. [DOI] [PubMed] [Google Scholar]
- Iademarco M. F., McQuillan J. J., Rosen G. D., Dean D. C. Characterization of the promoter for vascular cell adhesion molecule-1 (VCAM-1). J Biol Chem. 1992 Aug 15;267(23):16323–16329. [PubMed] [Google Scholar]
- Kirkland T. N., Qureshi N., Takayama K. Diphosphoryl lipid A derived from lipopolysaccharide (LPS) of Rhodopseudomonas sphaeroides inhibits activation of 70Z/3 cells by LPS. Infect Immun. 1991 Jan;59(1):131–136. doi: 10.1128/iai.59.1.131-136.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirkland T. N., Virca G. D., Kuus-Reichel T., Multer F. K., Kim S. Y., Ulevitch R. J., Tobias P. S. Identification of lipopolysaccharide-binding proteins in 70Z/3 cells by photoaffinity cross-linking. J Biol Chem. 1990 Jun 5;265(16):9520–9525. [PubMed] [Google Scholar]
- Kitchens R. L., Ulevitch R. J., Munford R. S. Lipopolysaccharide (LPS) partial structures inhibit responses to LPS in a human macrophage cell line without inhibiting LPS uptake by a CD14-mediated pathway. J Exp Med. 1992 Aug 1;176(2):485–494. doi: 10.1084/jem.176.2.485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krüger C., Schütt C., Obertacke U., Joka T., Müller F. E., Knöller J., Köller M., König W., Schönfeld W. Serum CD14 levels in polytraumatized and severely burned patients. Clin Exp Immunol. 1991 Aug;85(2):297–301. doi: 10.1111/j.1365-2249.1991.tb05722.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee J. D., Kato K., Tobias P. S., Kirkland T. N., Ulevitch R. J. Transfection of CD14 into 70Z/3 cells dramatically enhances the sensitivity to complexes of lipopolysaccharide (LPS) and LPS binding protein. J Exp Med. 1992 Jun 1;175(6):1697–1705. doi: 10.1084/jem.175.6.1697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levin J., Poore T. E., Young N. S., Margolis S., Zauber N. P., Townes A. S., Bell W. R. Gram-negative sepsis: detection of endotoxemia with the limulus test. With studies of associated changes in blood coagulation, serum lipids, and complement. Ann Intern Med. 1972 Jan;76(1):1–7. doi: 10.7326/0003-4819-76-1-1. [DOI] [PubMed] [Google Scholar]
- Libermann T. A., Baltimore D. Activation of interleukin-6 gene expression through the NF-kappa B transcription factor. Mol Cell Biol. 1990 May;10(5):2327–2334. doi: 10.1128/mcb.10.5.2327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackman N., Brand K., Edgington T. S. Lipopolysaccharide-mediated transcriptional activation of the human tissue factor gene in THP-1 monocytic cells requires both activator protein 1 and nuclear factor kappa B binding sites. J Exp Med. 1991 Dec 1;174(6):1517–1526. doi: 10.1084/jem.174.6.1517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin T. R., Mathison J. C., Tobias P. S., Letúrcq D. J., Moriarty A. M., Maunder R. J., Ulevitch R. J. Lipopolysaccharide binding protein enhances the responsiveness of alveolar macrophages to bacterial lipopolysaccharide. Implications for cytokine production in normal and injured lungs. J Clin Invest. 1992 Dec;90(6):2209–2219. doi: 10.1172/JCI116106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyrick B. O., Ryan U. S., Brigham K. L. Direct effects of E coli endotoxin on structure and permeability of pulmonary endothelial monolayers and the endothelial layer of intimal explants. Am J Pathol. 1986 Jan;122(1):140–151. [PMC free article] [PubMed] [Google Scholar]
- Molitor J. A., Walker W. H., Doerre S., Ballard D. W., Greene W. C. NF-kappa B: a family of inducible and differentially expressed enhancer-binding proteins in human T cells. Proc Natl Acad Sci U S A. 1990 Dec;87(24):10028–10032. doi: 10.1073/pnas.87.24.10028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montgomery K. F., Osborn L., Hession C., Tizard R., Goff D., Vassallo C., Tarr P. I., Bomsztyk K., Lobb R., Harlan J. M. Activation of endothelial-leukocyte adhesion molecule 1 (ELAM-1) gene transcription. Proc Natl Acad Sci U S A. 1991 Aug 1;88(15):6523–6527. doi: 10.1073/pnas.88.15.6523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mukaida N., Shiroo M., Matsushima K. Genomic structure of the human monocyte-derived neutrophil chemotactic factor IL-8. J Immunol. 1989 Aug 15;143(4):1366–1371. [PubMed] [Google Scholar]
- Neish A. S., Williams A. J., Palmer H. J., Whitley M. Z., Collins T. Functional analysis of the human vascular cell adhesion molecule 1 promoter. J Exp Med. 1992 Dec 1;176(6):1583–1593. doi: 10.1084/jem.176.6.1583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishizawa M., Nagata S. Regulatory elements responsible for inducible expression of the granulocyte colony-stimulating factor gene in macrophages. Mol Cell Biol. 1990 May;10(5):2002–2011. doi: 10.1128/mcb.10.5.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patrick D., Betts J., Frey E. A., Prameya R., Dorovini-Zis K., Finlay B. B. Haemophilus influenzae lipopolysaccharide disrupts confluent monolayers of bovine brain endothelial cells via a serum-dependent cytotoxic pathway. J Infect Dis. 1992 May;165(5):865–872. doi: 10.1093/infdis/165.5.865. [DOI] [PubMed] [Google Scholar]
- Pober J. S., Cotran R. S. Cytokines and endothelial cell biology. Physiol Rev. 1990 Apr;70(2):427–451. doi: 10.1152/physrev.1990.70.2.427. [DOI] [PubMed] [Google Scholar]
- Pohlman T. H., Munford R. S., Harlan J. M. Deacylated lipopolysaccharide inhibits neutrophil adherence to endothelium induced by lipopolysaccharide in vitro. J Exp Med. 1987 May 1;165(5):1393–1402. doi: 10.1084/jem.165.5.1393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pohlman T. H., Winn R. K., Callahan K. S., Maier R. V., Harlan J. M. A glycolipid precursor of bacterial lipopolysaccharide (lipid X) lacks activity against endothelial cells in vitro and is not toxic in vivo. J Surg Res. 1988 Aug;45(2):228–237. doi: 10.1016/0022-4804(88)90069-8. [DOI] [PubMed] [Google Scholar]
- Pomerantz R. J., Feinberg M. B., Trono D., Baltimore D. Lipopolysaccharide is a potent monocyte/macrophage-specific stimulator of human immunodeficiency virus type 1 expression. J Exp Med. 1990 Jul 1;172(1):253–261. doi: 10.1084/jem.172.1.253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pugin J., Schürer-Maly C. C., Leturcq D., Moriarty A., Ulevitch R. J., Tobias P. S. Lipopolysaccharide activation of human endothelial and epithelial cells is mediated by lipopolysaccharide-binding protein and soluble CD14. Proc Natl Acad Sci U S A. 1993 Apr 1;90(7):2744–2748. doi: 10.1073/pnas.90.7.2744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riedo F. X., Munford R. S., Campbell W. B., Reisch J. S., Chien K. R., Gerard R. D. Deacylated lipopolysaccharide inhibits plasminogen activator inhibitor-1, prostacyclin, and prostaglandin E2 induction by lipopolysaccharide but not by tumor necrosis factor-alpha. J Immunol. 1990 May 1;144(9):3506–3512. [PubMed] [Google Scholar]
- Schumann R. R., Leong S. R., Flaggs G. W., Gray P. W., Wright S. D., Mathison J. C., Tobias P. S., Ulevitch R. J. Structure and function of lipopolysaccharide binding protein. Science. 1990 Sep 21;249(4975):1429–1431. doi: 10.1126/science.2402637. [DOI] [PubMed] [Google Scholar]
- Schütt C., Schilling T., Grunwald U., Schönfeld W., Krüger C. Endotoxin-neutralizing capacity of soluble CD14. Res Immunol. 1992 Jan;143(1):71–78. doi: 10.1016/0923-2494(92)80082-v. [DOI] [PubMed] [Google Scholar]
- Sen R., Baltimore D. Inducibility of kappa immunoglobulin enhancer-binding protein Nf-kappa B by a posttranslational mechanism. Cell. 1986 Dec 26;47(6):921–928. doi: 10.1016/0092-8674(86)90807-x. [DOI] [PubMed] [Google Scholar]
- Simmons D. L., Tan S., Tenen D. G., Nicholson-Weller A., Seed B. Monocyte antigen CD14 is a phospholipid anchored membrane protein. Blood. 1989 Jan;73(1):284–289. [PubMed] [Google Scholar]
- Steffan A. M., Lafon M. E., Gendrault J. L., Schweitzer C., Royer C., Jaeck D., Arnaud J. P., Schmitt M. P., Aubertin A. M., Kirn A. Primary cultures of endothelial cells from the human liver sinusoid are permissive for human immunodeficiency virus type 1. Proc Natl Acad Sci U S A. 1992 Mar 1;89(5):1582–1586. doi: 10.1073/pnas.89.5.1582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tobias P. S., Soldau K., Ulevitch R. J. Isolation of a lipopolysaccharide-binding acute phase reactant from rabbit serum. J Exp Med. 1986 Sep 1;164(3):777–793. doi: 10.1084/jem.164.3.777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whelan J., Ghersa P., Hooft van Huijsduijnen R., Gray J., Chandra G., Talabot F., DeLamarter J. F. An NF kappa B-like factor is essential but not sufficient for cytokine induction of endothelial leukocyte adhesion molecule 1 (ELAM-1) gene transcription. Nucleic Acids Res. 1991 May 25;19(10):2645–2653. doi: 10.1093/nar/19.10.2645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright S. D., Ramos R. A., Hermanowski-Vosatka A., Rockwell P., Detmers P. A. Activation of the adhesive capacity of CR3 on neutrophils by endotoxin: dependence on lipopolysaccharide binding protein and CD14. J Exp Med. 1991 May 1;173(5):1281–1286. doi: 10.1084/jem.173.5.1281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright S. D., Ramos R. A., Tobias P. S., Ulevitch R. J., Mathison J. C. CD14, a receptor for complexes of lipopolysaccharide (LPS) and LPS binding protein. Science. 1990 Sep 21;249(4975):1431–1433. doi: 10.1126/science.1698311. [DOI] [PubMed] [Google Scholar]
- Ziegler-Heitbrock H. W., Thiel E., Fütterer A., Herzog V., Wirtz A., Riethmüller G. Establishment of a human cell line (Mono Mac 6) with characteristics of mature monocytes. Int J Cancer. 1988 Mar 15;41(3):456–461. doi: 10.1002/ijc.2910410324. [DOI] [PubMed] [Google Scholar]
- van Deventer S. J., Buller H. R., ten Cate J. W., Sturk A., Pauw W. Endotoxaemia: an early predictor of septicaemia in febrile patients. Lancet. 1988 Mar 19;1(8586):605–609. doi: 10.1016/s0140-6736(88)91412-2. [DOI] [PubMed] [Google Scholar]
- von Asmuth E. J., Leeuwenberg J. F., Ceska M., Buurman W. A. LPS and cytokine-induced endothelial cell IL-6 release and ELAM-1 expression; involvement of serum. Eur Cytokine Netw. 1991 Aug-Sep;2(4):291–297. [PubMed] [Google Scholar]