Abstract
Purpose
Atherosclerotic renovascular disease remains highly prevalent and presents an array of clinical syndromes. Recent prospective trials have dampened enthusiasm for revascularization generally, but clinicians recognize the need to identify patients likely to benefit from vascular intervention.
Recent findings
highlight the inflammatory nature of vascular occlusive disease and the limits of the kidney to adapt to reduced blood flow. Although moderate reductions can be tolerated, severe impairment of renal perfusion leads to tissue hypoxia and activates inflammatory injury within the kidney. Hence, assessment of kidney viability and potential tools to modify mitochondrial and inflammatory damage may be important to identify patients for whom clinical intervention should be undertaken.
Implications for practice
Clinicians must recognize clinical syndromes that identify “high-risk” groups and apply revascularization in those likely to benefit. Future efforts to protect the kidney (e.g. mitochondrial protection) and/or cell-based therapy may amplify the clinical recovery when combined with restoring renal blood flow.
Keywords: renal artery stenosis, hypertension, renovascular hypertension, stent, ischemic nephropathy, flash pulmonary edema
Introduction
Management of atherosclerotic renovascular disease (ARVD) continues to be plagued with confusion. While many clinicians favor attempting balloon angioplasty for younger hypertensive patients with fibromuscular dysplasias, an array of prospective, randomized clinical trials (RCTs) for atherosclerotic disease up to now fail to demonstrate additional benefits from restoring renal artery patency when added to optimal medical therapy. Hence, endovascular revascularization procedures for ARVD have fallen substantially over the last decade. These trial data are at odds with clinical experience and adverse outcomes after renal artery occlusion reported for some patients (1, 2). Additional reports identify specific high-risk patient subgroups with major clinical benefits with revascularization (3–5). Some authors and specialty groups have weighed in with opinions regarding “appropriate use” of renal artery stenting (6, 7). In this review, we will summarize recent publications in this field including emerging perspectives on the status of the post-stenotic kidney, evaluation of the RCTs to date, and specific clinical instances where intervening to restore renal circulation is of clear benefit.
Vascular Occlusive disease affecting the kidney
Unquestionably, renovascular disease can activate pressor pathways leading to accelerated hypertension. One must recognize, however, that atherosclerosis is a systemic disorder characterized by activation of multiple inflammatory pathways. Studies of vessels in patients with early ARVD indicate disturbances in both tissue and circulating inflammatory cell types, even before hypertension or parenchymal kidney injury are apparent (8). Recent studies also implicate immune mechanisms in driving experimental hypertension and its target manifestations (9). While the prevalence of ARVD continues to rise with age and associated atherosclerotic disease, occasional reports appear in adolescent subjects with severe risk factors, including genetic dyslipidemias (10). While most ARVD lesions are of minor hemodynamic significance, some progress to produce a spectrum of clinical manifestations that eventually includes renovascular hypertension, accelerated cardiovascular morbidity, circulatory congestion and parenchymal renal functional deterioration (FIGURE 1). Multiple observational and registry studies in the past suggest that renal revascularization can lead to improved blood pressure control, although it rarely reverses hypertension completely in ARVD. A recent report from the HERCULES investigators reconfirms this point, indicating that 202 patients experienced systolic BP reduction from 162±18 to 146 ± 16 mmHg (p<.001) after 36 months with no changes in medications (11). These authors argue that selection of patients with treatment resistant hypertension (more than 70% were uncontrolled on three or more medications) led to sustained, clinically important blood pressure reduction. Few features specifically related to the degree of vascular occlusion actually predict the blood pressure response to revascularization. Studies of temporary balloon occlusion indicate that a translesional gradient of at least 10–20% between aortic pressures and post-lesional renal artery pressure is required for measurable activation of the renin-angiotensin system (12), long an established major component underlying renovascular hypertension (13). The degree of “hyperemic systolic gradient” (HSG) induced by dopamine or other agents has been proposed as a predictor of pressure response after revascularization (14). These observations are particularly important as results from prospective RCTs in patients with ARVD indicate that the degree of stenosis is frequently overestimated, even in experienced catheterization laboratories (15). Review of ARVD lesions by the angiographic core laboratory for CORAL for 239 “roll-in” patients downgraded estimates of stenosis from 73% lesion occlusion to 66% on average, leaving fewer than 20% of subjects in this trial with occlusive lesions above 80%. In this trial, the correlation between severity of vascular stenosis and translesional pressure gradients was low (r=0.21, p=.06) (16). As many authors have observed, the hemodynamic properties of the renal vessels are not the sole determinant of clinical manifestations related to ARVD (17).
Figure 1.
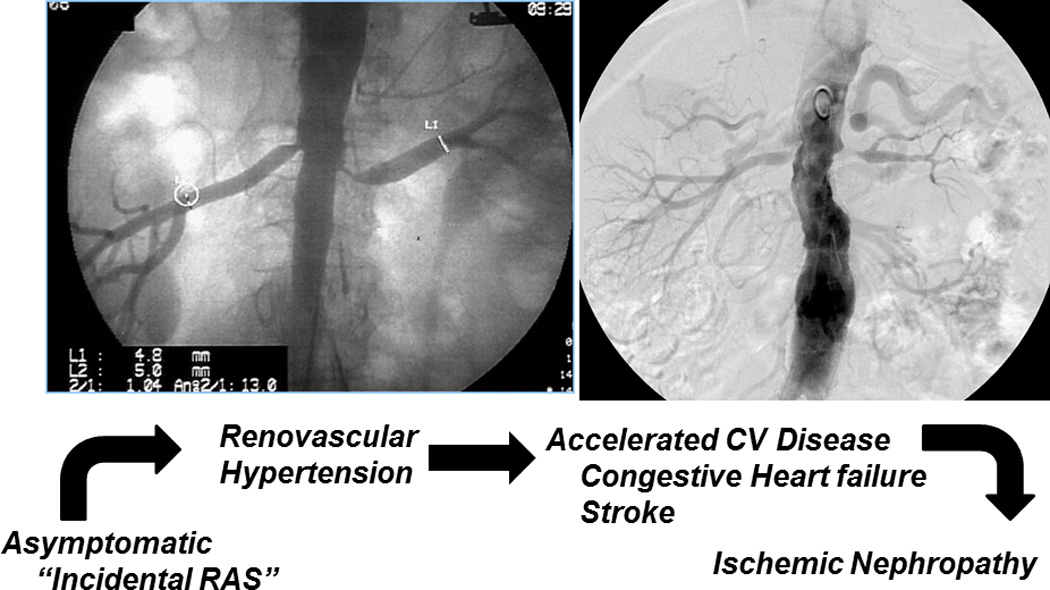
Spectrum of atherosclerotic renovascular disease: Clinical manifestations vary considerably depending in part on the severity of vascular occlusion, but also the state of the underlying kidney (see text). While many lesions are of minor hemodynamic importance, prolonged and severe vascular occlusion accelerates hypertension, circulatory congestion and ultimately threatens viability of the kidney (figure from (3) with permission)
Medical therapy for renovascular hypertension has become more standardized and effective. Although activation of the renin-angiotensin system has long been recognized as central to the initiation of renovascular hypertension, application of agents to block this system, specifically angiotensin converting enzyme (ACE) inhibitors and angiotensin receptor blockers (ARB’s) has been inconsistent, likely due in part to concerns about the potential fall in glomerular filtration pressures due to removal of efferent vascular effects of angiotensin within the kidney. Investigators from the CORAL trial examined the prevalent use of renin-angiotensin blockade prior to randomization in 853 of 931 trial participants (18). Overall, 49% of subjects had been treated with these agents before entering CORAL. A lower number than average was observed in those with pre-existing CKD (eGFR<60 ml/min/1.73m2) and a higher proportion in those with pre-existing diabetes. Importantly, patients treated with renin-angiotensin blockade had lower systolic BP (148 ± 23 vs 152 ± 23, p=.003) and were more likely to have reached goal BP (30% vs 22% p=.01). After enrollment in CORAL, all participants were administered renin-angiotensin blockade, specifically to address whether renal revascularization provides additional benefits beyond removing the effects of the renin-angiotensin system. In both CORAL and ASTRAL, overall goal pressures were achieved in remarkably high proportions of the groups treated with or without revascularization (15). Aggressive use of statins and glycemic control were benchmarks of the CORAL population as well. While some patients cannot tolerate renin-angiotensin blockade without deteriorating renal function, this was infrequent in the prospective trials and previously reported registry data (19, 20). A major conclusion from the prospective RCT’s must be that current medical therapy can be remarkably effective for many patients with ARVD.
Wider application of endovascular aortic stent grafts sometimes is associated with a clinical syndrome of iatrogenic renal artery stenosis, as these grafts can migrate or be placed across the renal arteries. A prospective RCT reporting results of a fenestrated endovascular graft in 67 patients indicated that 129 renal vessels were targeted by fenestration at the time of placement. Four developed renal artery occlusion and 12 developed significant stenosis. A total of 15 patients (22%) required secondary interventions for renal artery stenosis/occlusion (21).
Condition of the Post-Stenotic Kidney
An important corollary of improved medical management of renovascular hypertension is the fact that most ARVD remains in place for long periods of time before being identified and considered for revascularization. The kidney can tolerate moderate reductions in blood flow without developing either tissue hypoxia or identifiable tissue injury. In part this reflects the balance between blood flow (and therefore oxygen supply) and reduced oxygen consumption that follows reduced filtration (22). These observations reinforce the conclusion that antihypertensive drug therapy to lower systemic pressures can be tolerated without progressive loss of renal function over prolonged periods of time, sometimes for years. As a result, many patients in the treatment trials had stable kidney function despite substantially reduced renal perfusion pressures. Experimental and clinical studies indicate that beyond certain limits, however, vascular occlusive disease does reduce renal cortical oxygenation, activating renal inflammatory pathways, oxidative stress and tissue fibrosis (23). Measurement of renal vein effluent levels of neutrophil gelatinase-associated lipocalin (NGAL) suggest that pro-inflammatory signaling pathways persist even after restoring renal blood flow in human subjects (24) and may affect both post-stenotic and non-stenotic contralateral kidneys with a shift toward metabolomics profiles associated with chronic kidney disease (25). Transjugular biopsy specimens from the post-stenotic kidney demonstrate appearance of both CD-3+ T-cells and CD-68+ macrophages (26). These changes are associated with interstitial inflammation, loss of viable tubular structures and glomerular obsolescence. Experimental studies in acute ischemic injury suggest that the polarity of macrophages within the post-ischemic kidney is an important determinant of recovery after restoring blood flow and that failure to transition to the reparative (M-2) phenotype is associated with failure to regenerate tubules and progression to chronic kidney disease (27).
Human kidneys are unusually tolerant to some forms of ischemic injury. Unlike experimental models in which severe tubular necrosis develops after 30–45 minutes, a study of human kidney tissue obtained during total isolated renal artery occlusion for 30–60 minutes demonstrated few histologic changes or biomarker evidence of injury biomarker release or clinical acute kidney injury (AKI)(28). An early sign of ischemic injury in this study was distortion of mitochondrial structures. Application of mitochondrial protection peptides in ischemic models accelerates ATP recovery and limits ischemic injury in rodents (29, 30). These studies provide evidence that targeted stabilization of cardiolipin within mitochondrial structures stabilizes the electron transport chain, allowing preservation of ATP production and minimizing release of toxic oxygen species in a variety of organs, including kidney, muscle, heart and brain. Importantly, studies of renovascular swine models treated with mitochondrial protection agents indicate that weekly injections reduce oxidative stress and fibrosis, improve vascular density and tissue oxygenation, and prevent the loss of GFR (31). Previous studies indicated that administration of mitochondrial protection agents at the time of restoring renal blood flow with endovascular procedures improves renal functional outcomes (32).
Prolonged hemodynamic compromise does activate inflammatory pathways within the kidneys . Measurement of renal vein cytokines indicates ongoing net release of pro-inflammatory markers including IL-10 and TNF-alpha both in humans and experimental models (24, 33). Restoring blood flow with endovascular procedures fails to reverse this process, but administration of adipose-derived mesenchymal stem cells (MSC) in experimental ARVD can reduce cytokine release and reduced tissue markers of inflammatory injury (33). Whether such maneuvers can modify human renal tissue inflammatory injury remains to be seen.
Taken together, these studies indicate that ARVD undergoes a transition from purely hemodynamic compromise limiting kidney function to a condition of ongoing parenchymal injury that includes oxidative injury, inflammation and ultimately fibrosis. (FIGURE 2). Clinical studies directed toward both mitochondrial protection and adjunctive therapy with mesenchymal stromal/stem cells are in progress (Clinicaltrials.gov). How best to define the state of the kidney both to restore blood flow and recover kidney function before irreversible injury ensues remains a major challenge for clinicians.
Figure 2.
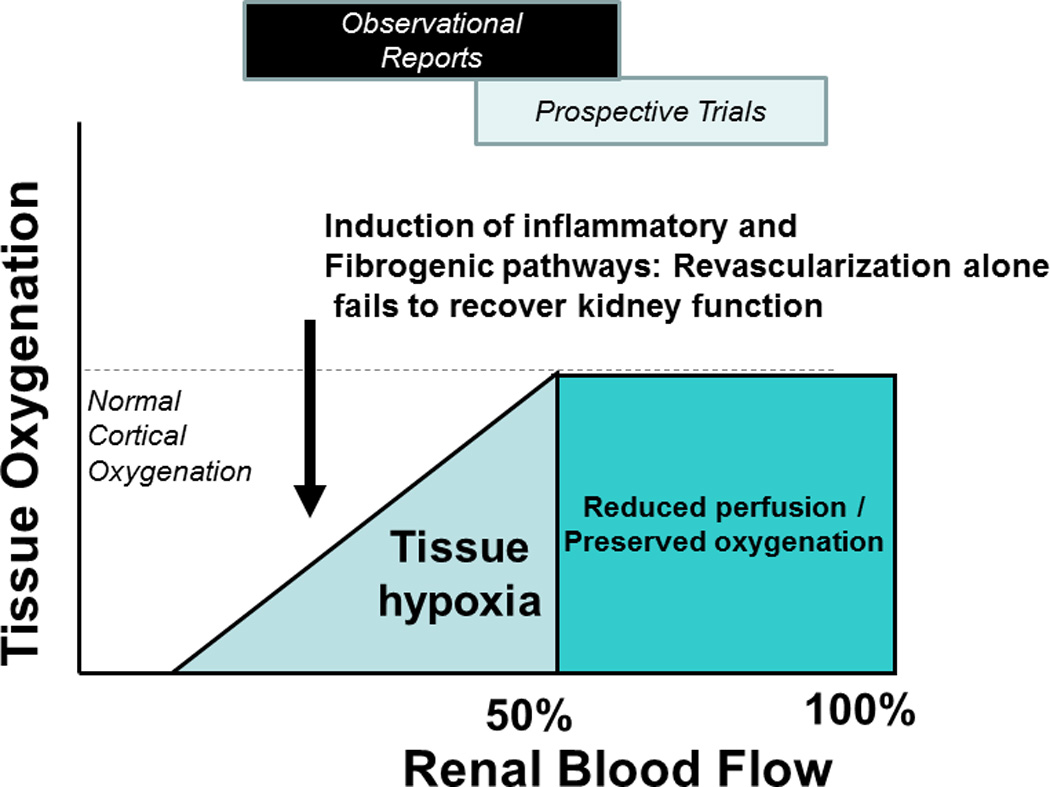
Tissue oxygenation and renal blood flow: Because the kidney is abundantly perfused in its function as a filtering organ, it can tolerate moderate reductions in blood flow without developing overt tissue hypoxia. Beyond a critical threshold, however, further reductions lead to tissue hypoxia and activation of oxidative stress and tissue inflammatory injury within the kidney. The clinical effects following renal artery revascularization depend upon the underlying state of the kidney. Many of the prospective RCTs have been hampered by recruitment of relatively minor renovascular disease as compared to observational studies of more severe clinical syndromes. (Figure from reference 26, with permission)
When to pursue renal revascularization
Selection of patients for vascular intervention obviously depends on the likelihood of obtaining some clinical benefit. Considering the complexity of factors regulating blood pressure and kidney function outlined above, this is rarely straightforward. Paradoxically, the likelihood of clinical benefit regarding blood pressure control (and possibly renal function as well) is highly related to the duration of manifest ARVD. Hence, those with shorter duration of overt renovascular hypertension are most likely to lower arterial pressure after revascularization. Multiple predictive clinical features were examined for unilateral atherosclerotic disease in a publication from Italy (34) based on a single-center experience over the years between 1990–2008. They report that baseline levels of kidney function (eGFR by MDRD) in 158 patients analyzed were lower (eGFR 45 vs 63 ml/min/1.73m2, p<.05) in those without renal functional recovery. Ultrasound derived measures of resistive index (RI) defined as ((peak systolic velocity – end-diastolic velocity)/peak systolic velocity) were independent predictors of actual recovery of eGFR (defined as an increase more than 20% of baseline eGFR), even after adjusting for age, gender, obesity, and baseline serum creatinine above 1.8 mg/dL. In general agreement with previous reports, RI within the contralateral kidney (using a cutpoint of 0.73, somewhat lower than 0.8 as others have proposed) was the best single predictor of functional outcome, possibly as it defined the state of the parenchymal small vessels within the kidney not directly subject to large vessel disease. Thirty-six of 158 (22.8%) treated patients had identifiable increases in eGFR over 12 months. Importantly, no ultrasound parameters predicted the response of blood pressure, reinforcing the separation of blood pressure regulation from kidney function in ARVD.
Technical aspects of endovascular revascularization continue to improve. In the series from Italy for 158 patients, major complications affecting the kidney (3 leading to nephrectomy, 1 arterial thrombosis, and 1 restenosis) were infrequent. Retroperitoneal bleeding, and partial renal ischemia from branch occlusion developed in 5 additional patients for a complication rate of 6.3%. Reported complications in the CORAL trial identified and corroborated by the angiographic core laboratory were infrequent, totalling 2.2% in the main arm of the trial. This was in contradistinction to the Roll-In phase of the trial where 239 patients were evaluated by the angiographic core. In this group, 13% (n=28) experienced “major” angiographic complications including dissection (n=11; 4.5%), embolus (n=9; 3.7%), occlusion (n=9; 3.7%), thrombus (n=3; 1.2%), vessel rupture (n=2; 0.8%), wire perforation of a branch artery (n=2; 0.8%), and pseudoaneurysm (n=1; 0.4%) (16). Many of the initial complications were attributed to concurrent use of a large distal protection device (Angiogard) initially required for the trial. Development of this device was discontinued and not utilized for the main portion of CORAL. Results three years after enrollment for 202 patients treated with the HERCULES cobalt/chromium stent specifically designed for the renal artery confirmed “procedural success” (stent deployment) in more than 99% of arteries (11). Restenosis after 9 months was 22/209 (10.5%). Long-term blood pressure levels were maintained well-below enrollment levels (74% had meaningful step-down in pressure levels) without change in medications. The complication rate for 30 days was 1.5%. Taken together, endovascular stent procedures in experienced laboratories is reasonably safe.
“Evidence-biased Medicine?”
In the current milieu, RCT data are considered by some to represent a form of “gold-standard” evidence. Multiple proposed RCTs with atherosclerotic renal artery stenosis have been limited by poor recruitment, in part the result of previous clinical experience and lack of consensus. As a result, even carefully performed trials have been subject to selection bias for relatively low risk participants (3). While prospective RCTs in fact provide evidence for “average participants” of large groups, such data may not apply to specific individuals with atypical features. Concluding that a “negative trial” (e.g. one that fails to identify overall population benefit from renal revascularization) means that such therapy should be abandoned likely deprives specific, high-risk patients from major benefit. Reports of successful renal revascularization regularly provide examples of individuals with pivotal recovery of renal function or relief from pulmonary edema, most often in patients that were underrepresented in the relatively small, prospective trials conducted up to now. (1, 2, 5). An example of such a patient is illustrated in FIGURE 3, depicting creatinine values and BP information for over several years when the role of ARVD was overlooked for several years. This individual progressed to advanced renal failure, was listed for kidney transplant, and later developed episodes of “flash pulmonary edema”. Remarkably, the solitary post-stenotic kidney remained viable and both kidney function and refractory hypertension regressed after successful endovascular stenting. Table 1 illustrates a recent “consensus” document published by the Society for Cardiovascular Angiography and Intervention (SCAI) that identifies clinical syndromes for which renal artery interventions may be considered “appropriate use”.
Figure 3.
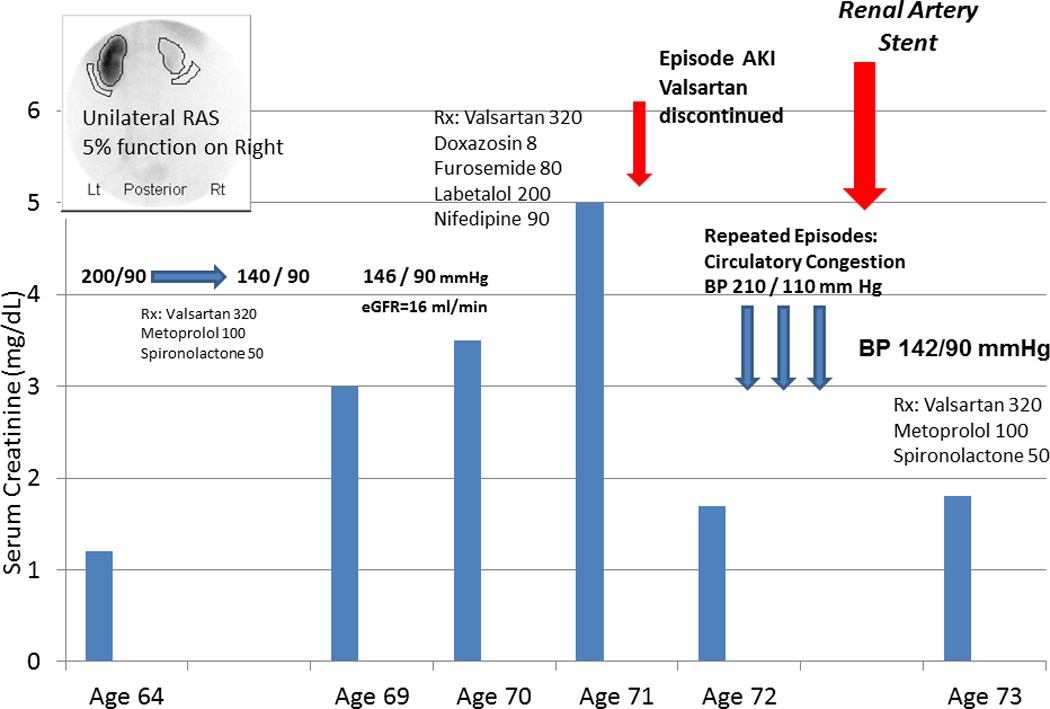
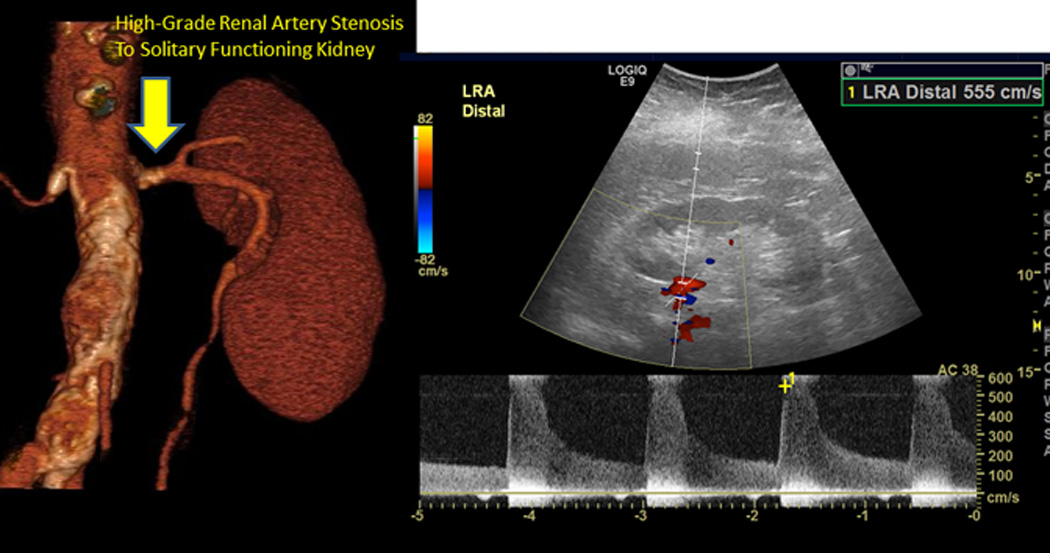
(A) Serum creatinine, blood pressure and medications over an eight year period in a patient with unilateral atherosclerotic renovascular disease associated with a nonfunctioning kidney (less than 5% by renal scan). This patient developed severe hypertension that responded well to a regiment based upon an angiotensin receptor blocker (valsartan). Several years later, however, she developed worsening renal failure with an eGFR = 16 ml/min/1.73m2, leading to evaluation for kidney transplantation. Her disease was managed conservatively, but she developed severe hypertension and worsening renal function (creatinine above 5.0 mg/dL), leading her physician to withhold the ARB. Serum creatinine fell, although blood pressure was difficult to control and she developed episodes of acute pulmonary edema. (B) Doppler ultrasound identified a stenosis to her remaining functional kidney (peak systolic velocity above 500 cm/s). This was treated with endovascular stenting, with marked improvement in blood pressure levels, stable kidney function and tolerance to restarting the ARB (valsartan).
TABLE 1. Clinical Scenarios in Which Treatment of Significant RASα May be Considered.
A summary table from a consensus statement issued by the Society for Cardiovascular Angiography and Interventions (SCAI) identifies the reasonably appropriate indications for renal artery stenting. The document attempts to synthesize both levels of evidence and experience into an overall scheme for which renal revascularization offers true clinical benefits. (reproduced from Reference 6, with permission)
| Appropriate Care |
|
| May Be Appropriate Care |
|
| Rarely Appropriate Care |
|
Significant RAS is an angiographically moderate lesion (50–70%) with physiologic confirmation of severity or a > 70% stenosis
Conclusion
Atherosclerotic renal artery stenosis presents a wide variety of clinical syndromes, many of which are managed initially with current medical therapy. Recent data highlight the transition from primarily a hemodynamic condition limiting renal blood flow to a complex inflammatory process in the kidney characterized by oxidative stress, inflammation and macrophage activation. Restoring vessel patency can interrupt this transition and may provide major benefits to high-risk patients, particularly when combined with investigational procedures to modify injury pathways. Identifying such patients in whom medical therapy is failing and for whom renal revascularization can stabilize circulatory control and renal function is the central challenge for clinicians.
Key Points.
Prospective randomized clinical trials enrolling relatively low risk patients fail to demonstrate major additional benefits from renal revascularization. Most patients are now treated initially primarily with medical therapy.
Studies of vascular stenosis and injury within the post-stenotic kidney indicate a progression from hemodynamic compromise to inflammatory and fibrotic injury.
Numerous clinical observations reinforce the benefit of renal revascularization in specific “high-risk” subsets that develop clinical manifestations despite medical management.
Acknowledgments
The authors would like to thank Beverly Tietje for her coordination of the clinical studies described in this report.
Financial Support : The work described was supported by Award Number R01 DK100081 from the National Institute for Diabetes, Digestive and Kidney Diseases (NIDDK) and NIH/NCRR CTSA Grant Number UL1 RR024150. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIDDK or the National Institutes of Health.
Footnotes
Conflicts of Interest: Neither author has conflicts of interest to report.
Contributor Information
Stephen C. Textor, Division of Nephrology and Hypertension, Mayo Clinic
Michael M. McKusick, Division of Interventional Radiology, Department of Radiology, Mayo Clinic
References
- 1.Mark PB, Schiffrin EL, Jennings GL, et al. Renovascular hypertension: to stent or not to stent? Hypertension. 2014;64:1165–1168. doi: 10.1161/HYPERTENSIONAHA.114.04497. [DOI] [PubMed] [Google Scholar]
- 2.Textor SC. Attending rounds: a patient with accelerated hypertension and an atrophic kidney. Clin J Am Soc Nephrol. 2014;9:1117–1123. doi: 10.2215/CJN.09030813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Herrmann SM, Saad A, Textor SC. Management of atherosclerotic renovascular disease after Cardiovascular Outcomes in Renal Atherosclerotic Lesions (CORAL) Nephrology, dialysis, transplantation : official publication of the European Dialysis and Transplant Association - European Renal Association. 2015;30:366–375. doi: 10.1093/ndt/gfu067. Review of trials with exploration of limited entry criteria and selection bias that limits generalizability in clinical practice.
- 4.Textor SC. Renovascular hypertension: is there still a role for stent revascularization? Current Opinion in Nephrology & Hypertension. 2013;22:525–530. doi: 10.1097/MNH.0b013e328363ffe0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Ritchie J, Green D, Chrysochou C, et al. High-risk clinical presentations in atherosclerotic renovascular disease: prognosis and response to renal artery revascularization. American journal of kidney diseases : the official journal of the National Kidney Foundation. 2014;63:186–197. doi: 10.1053/j.ajkd.2013.07.020. Important identification of “high-risk” subsets from an regional database for atherosclerotic renovascular disease in the United Kingdom. These authors identified clinical syndromes of episodic pulmonary edema and rapidly progressive renal failure with severe hypertension for which renal revascularization confered important survival benefits.
- 6. Parikh SA, Shishehbor MH, Gray BH, et al. SCAI expert consensus statement for renal artery stenting appropriate use. Catheterization & Cardiovascular Interventions. 2014;84:1163–1171. doi: 10.1002/ccd.25559. This is a practical and important view of those involved in renal revascularization regarding most “appropriate use” of endovascular renal revascularization based on currently available evidence.
- 7.White CJ. The "chicken little" of renal stent trials: the CORAL trial in perspective. Jacc: Cardiovascular Interventions. 2014;7:111–113. doi: 10.1016/j.jcin.2013.12.002. [DOI] [PubMed] [Google Scholar]
- 8.Kotliar C, Juncos L, Inserra F, et al. Local and systemic cellular immunity in early renal artery atherosclerosis. ClinJAmSocNephrol. 2012;7:224–230. doi: 10.2215/CJN.06270611. [DOI] [PubMed] [Google Scholar]
- 9.De Ciuceis C, Rossini C, La Boria E, et al. Immune mechanisms in hypertension. High Blood Pressure & Cardiovascular Prevention. 2014;21:227–234. doi: 10.1007/s40292-014-0040-9. [DOI] [PubMed] [Google Scholar]
- 10.Webb TN, Ramratnam M, Evans RW, et al. Atherosclerotic renal artery stenosis as a cause for hypertension in an adolescent patient. Pediatric Nephrology. 2014;29:1457–1460. doi: 10.1007/s00467-014-2774-y. [DOI] [PubMed] [Google Scholar]
- 11. Chrysant GS, Bates MC, Sullivan TM, et al. Proper patient selection yields significant and sustained reduction in systolic blood pressure following renal artery stenting in patients with uncontrolled hypertension: long-term results from the HERCULES trial. Journal of Clinical Hypertension. 2014;16:497–503. doi: 10.1111/jch.12341. Observational report of recently completed trial of endovascular renal artery stent for patients with clinically refractory renovascular hypertension. These data likely represent current state of restenosis, technical success and clinical impact of renal artery stent procedures.
- 12.De Bruyne B, Manoharan G, Pijls NHJ, et al. Assessment of renal artery stenosis severity by pressure gradient measurements. Journal of the American College of Cardiology. 2006;48:1851–1855. doi: 10.1016/j.jacc.2006.05.074. [DOI] [PubMed] [Google Scholar]
- 13.Vaughan ED., Jr Curable renal hypertension: renin, marker or cause? Question answered. American Journal of Hypertension. 2014;27:1000–1003. doi: 10.1093/ajh/hpu111. [DOI] [PubMed] [Google Scholar]
- 14.Mangiacapra F, Trana C, Sarno G, et al. Translesional pressure gradients to predict blood pressure response after renal artery stenting in patients with renovascular hypertension. CircCardiovascInterven. 2010;3:537–542. doi: 10.1161/CIRCINTERVENTIONS.110.957704. [DOI] [PubMed] [Google Scholar]
- 15. Cooper CJ, Murphy TP, Cutlip DE, et al. Stenting and medical therapy for atherosclerotic renal-artery stenosis. The New England journal of medicine. 2014;370:13–22. doi: 10.1056/NEJMoa1310753. This is the largest prospective, randomized trial evaluating the effects of renal artery stenting when added to “optimal” medical therapy including angiotensin receptor blockade, statins, glucose control and protocolized blood pressure control. For this population with relatively minor hypertension and preserved renal function, no improvements in major combined outcomes were observed with renal artery stenting.
- 16.Murphy TP, Cooper CJ, Cutlip DE, et al. Roll-in experience from the Cardiovascular Outcomes with Renal Atherosclerotic Lesions (CORAL) study. Journal of Vascular & Interventional Radiology. 2014;25:511–520. doi: 10.1016/j.jvir.2013.09.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ritchie J, Alderson HV, Kalra PA. Where now in the management of renal artery stenosis? Implications of the ASTRAL and CORAL trials. Current Opinion in Nephrology & Hypertension. 2014;23:525–532. doi: 10.1097/MNH.0000000000000059. [DOI] [PubMed] [Google Scholar]
- 18. Evans KL, Tuttle KR, Folt DA, et al. Use of renin-angiotensin inhibitors in people with renal artery stenosis. Clinical Journal of The American Society of Nephrology: CJASN. 2014;9:1199–1206. doi: 10.2215/CJN.11611113. This report examines the pre-enrollment use of renin-angiotensin system blockade in CORAL enrollees, suggesting persistent clinical bias against using these agents in patients with reduced GFR (below 60 ml/min/1.73m2). All patients were subsequently treated with angiotensin receptor blockade in the CORAL trial.
- 19.Hackam DG, Duong-Hua ML, Mamdani M, et al. Angiotensin inhibition in renovascular disease: a population-based cohort study. AmHeartJ. 2008;156:549–555. doi: 10.1016/j.ahj.2008.05.013. [DOI] [PubMed] [Google Scholar]
- 20.Chrysochou C, Foley RN, Young JF, et al. Dispelling the myth: the use of renin-angiotensin blockade in atheromatous renovascular disease. Nephrol Dial Transplant. 2012;27:1403–1409. doi: 10.1093/ndt/gfr496. [DOI] [PubMed] [Google Scholar]
- 21. Oderich GS, Greenberg RK, Farber M, et al. Results of the United States multicenter prospective study evaluating the Zenith fenestrated endovascular graft for treatment of juxtarenal abdominal aortic aneurysms. Journal of Vascular Surgery. 2014;60:1420–1428. e1421–e1425. doi: 10.1016/j.jvs.2014.08.061. This is an important paper describing the emerging role of endovascular aortic stent grafts in producing renal artery occlusion requiring renal revascularization.
- 22.Evans RG, Goddard D, Eppel GA, et al. Factors that render the kidney susceptible to tissue hypoxia in hypoxemia. AmJPhysiol RegulIntegrComp Physiol. 2011;300:R931–R940. doi: 10.1152/ajpregu.00552.2010. [DOI] [PubMed] [Google Scholar]
- 23.Lerman LO, Textor SC. Gained in translation: protective paradigms for the poststenotic kidney. Hypertension. 2015;65:976–982. doi: 10.1161/HYPERTENSIONAHA.114.04364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Saad A, Herrmann SMS, Crane J, et al. Stent revascularization restores cortical blood flow and reverses tissue hypoxia in atherosclerotic renal artery stenosis but fails to reverse inflammatory pathways or glomerular filtration rate. Circulation: Cardiovascular Interventions. 2013;6:428–435. doi: 10.1161/CIRCINTERVENTIONS.113.000219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rhee EP, Clish CB, Pierce KA, et al. Metabolomics of renal venous plasma from individuals with unilateral renal artery stenosis and essential hypertension. Journal of Hypertension. 2015;33:836–842. doi: 10.1097/HJH.0000000000000470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Textor SC, Lerman LO. Paradigm Shifts in Atherosclerotic Renovascular Disease: Where Are We Now? J Am Soc Nephrol. 2015;26:2074–2080. doi: 10.1681/ASN.2014121274. Summary of recent developments related to understanding the transition from hemodynamic to inflammatory and profibrotic changes within the post-stenotic kidney
- 27. Lech M, Grobmayr R, Ryu M, et al. Macrophage phenotype controls long-term AKI outcomes--kidney regeneration versus atrophy. J Am Soc Nephrol. 2014;25:292–304. doi: 10.1681/ASN.2013020152. Important experimental study indicating that recovery from ischemic injury depends upon intact transitions to the reparative “M2” macrophage for tubular regeneration. These data are part of a series of reports indicating that polarity shifts in macrophage functional differentiation are central to determining whether long-term kidney damage results from acute insults, such as ischemic vascular disease.
- 28.Parekh DJ, Weinberg JM, Ercole B, et al. Tolerance of the human kidney to isolated controlled ischemia. J Am Soc Nephrol. 2013;24:506–517. doi: 10.1681/ASN.2012080786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Szeto HH, Liu S, Soong Y, et al. Improving mitochondrial bioenergetics under ischemic conditions increases warm ischemia tolerance in the kidney. American Journal of Physiology - Renal Physiology. 2015;308:F11–F21. doi: 10.1152/ajprenal.00366.2014. Summary of the potential for mitochondrial protection to prevent irreversible kidney injury in ischemic kidney injury.
- 30.Birk AV, Liu S, Soong Y, et al. The mitochondrial-targeted compound SS-31 re-energizes ischemic mitochondria by interacting with cardiolipin. Journal of the American Society of Nephrology. 2013;24:1250–1261. doi: 10.1681/ASN.2012121216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Eirin A, Ebrahimi B, Zhang X, et al. Mitochondrial protection restores renal function in swine atherosclerotic renovascular disease. Cardiovascular Research. 2014;103:461–472. doi: 10.1093/cvr/cvu157. Experimental study in swine renal artery stenosis demonstrating the role of mitochondrial protection in preserving the renal microvasculature and glomerular filtration rate.
- 32.Eirin A, Li Z, Zhang X, et al. A mitochondrial permeability transition pore inhibitor improves renal outcomes after revascularization in experimental atherosclerotic renal artery stenosis. Hypertension. 2012;60:1242–1249. doi: 10.1161/HYPERTENSIONAHA.112.199919. [DOI] [PubMed] [Google Scholar]
- 33.Eirin A, Zhang X, Zhu X-Y, et al. Renal vein cytokine release as an index of renal parenchymal inflammation in chronic experimental renal artery stenosis. Nephrology Dialysis Transplantation. 2014;29:274–282. doi: 10.1093/ndt/gft305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Bruno RM, Daghini E, Versari D, et al. Predictive role of renal resistive index for clinical outcome after revascularization in hypertensive patients with atherosclerotic renal artery stenosis: a monocentric observational study. Cardiovascular Ultrasound. 2014;12:9. doi: 10.1186/1476-7120-12-9. Careful analysis of multiple clinical factors and doppler ultrasound measurements of renal resistive index in predicting outcomes of renal revascularization in unilateral renal artery stenosis. Identifies differences in the role of resistive index for renal functional as compared to blood pressure responses.


