Abstract
Purpose
Fat suppression (FS) via chemically selective saturation (CHESS) eliminates fat-water oscillations in multi-echo gradient echo (mGRE) R2*-MRI. However, for increasing R2* values as seen with increasing liver iron content (LIC), the water signal spectrally overlaps with the CHESS band, which may alter R2*. Here, we investigate the effect of CHESS on R2* and describe a heuristic correction for the observed CHESS-induced R2* changes.
Methods
Eighty patients (49/31 female/male, mean age: 18.3±11.7 years) with iron overload were scanned with a non-FS and a CHESS-FS mGRE sequence at 1.5T and 3T. Mean liver R2* values were evaluated using 3 published fitting approaches. Measured and model-corrected R2* values were compared and statistically analyzed.
Results
At 1.5T, CHESS led to a systematic R2* reduction (P<0.001 for all fitting algorithms) especially toward higher R2*. Our model described the observed changes well and reduced the CHESS-induced R2* bias after correction (linear regression slopes: 1.032/0.927/0.981). No CHESS-induced R2* reductions were found at 3T.
Conclusion
The CHESS-induced R2* bias at 1.5T needs to be considered when applying R2*-LIC biopsy calibrations for clinical LIC assessment which were established without FS at 1.5T. The proposed model corrects the R2* bias and could therefore improve clinical iron overload assessment based on linear R2*-LIC calibrations.
Keywords: hepatic iron overload, R2* quantification, fat suppression (CHESS), liver MRI, quantitative MRI
Introduction
Hematologic disorders such as β-thalassemia and sickle cell disease require repeated blood transfusions for disease management. A common side effect of frequent blood transfusions is the accumulation of extrinsic iron, especially in the liver (1-5). Because extrinsic iron accumulation eventually leads to organ damage, early monitoring and treatment of iron burden is essential.
Traditionally, iron overload has been monitored by measuring hepatic iron content from needle biopsy samples (6). In recent years, MRI has evolved as a non-invasive alternative to biopsies to assess iron overload by quantifying the transverse relaxation rates R2 (= 1/T2) or R2* (= 1/T2*). Both parameters correlate well with liver iron content (LIC) in biopsy calibration studies (7-11). A major advantage of R2*-based approaches is that R2* can be measured rapidly by multi-echo gradient recalled echo (mGRE) imaging within a single breath hold (10,12,13), whereas R2 measurements take up to several minutes (8,14).
R2* is typically obtained by analyzing the exponential signal decay seen in the mGRE sequence. A major confounder of such R2* measurements is the presence of fat (e.g., in patients with hepatic steatosis) (15), because fat introduces characteristic sinusoidal modulations in the mGRE signal evolution (16). These modulations stem from the different resonance frequencies for water and lipid protons (17) and eventually affect the R2* evaluation. Post-processing techniques such as iterative decomposition of water and fat with echo asymmetry and least-squares estimation (IDEAL) (18-20) or other advanced data modeling algorithms (21,22) have been presented to simultaneously quantify R2* and the actual fat-water content. Another method to address fat-water modulations without the need of post-processing is in-phase echo time (TE) sampling which uses only TEs at which fat and water components are approximately in-phase (TE increments of approximately 2×2.3 ms at 1.5 T). However, the use of this approach is hampered due to fat-fat signal interferences (23) and in patients with high LIC because the main portion of the detectable MR signal has already decayed at the first TE.
To avoid fat-water modulations, and thereby to improve the R2* evaluation, fat suppression (FS) techniques have been applied in recent iron assessment studies (24,25). An easy method for achieving FS is to include chemically selective saturation (CHESS) (26) radio-frequency (RF) pulses into the mGRE sequence. While it has been shown that CHESS pulses have almost no effect on hepatic R2 measurements (27), it is not clear how they affect R2* measurements and consequently R2*-based LIC quantification as existing R2*-LIC biopsy calibrations have been mainly derived in the absence of fat suppression. Moreover, it has been shown very recently (28) that spectral presaturation with inversion recovery (SPIR, (29)) in mGRE imaging of transfusional iron overload leads to lowered R2* values by up to 7%. As SPIR also applies chemically selective RF pulses similarly to CHESS, such R2* alterations could occur with CHESS as well.
Increasing R2* values, as observed in the case of increasing LIC, are associated with broadened spectral signal profiles that might overlap with the saturation band of the applied CHESS pulse. Therefore, we hypothesize that the partial saturation effect due the application of CHESS could affect the observed water R2* and thus impact clinical R2*-based LIC estimation. We further hypothesize that potential water R2* alterations are a direct result of the employed CHESS pulse and are observable regardless of the presence or absence of fat.
In this study, we systematically investigated the effect of CHESS FS on R2* at 1.5 T and 3 T in a group of 80 subjects with transfusional iron overload. We quantified R2* by using 3 previously published R2* fitting approaches (9,10,24), and propose a heuristic model for a phenomenological correction if CHESS-induced R2* changes are present.
Methods
Patient population
Eighty subjects [49/31 female/male, mean age ± standard deviation (SD): 18.3±11.7 years (range: 20 months – 53 years); 44/36 pediatric/adult patients, mean age ± SD pediatric cases: 10.6±4.6 years, mean age ± SD adult cases: 29.4±8.8 years] enrolled in an institutional review board approved study to assess iron overload (www.clinicaltrials.gov #NCT01572922) were investigated. All participants have transfusional iron overload due to multiple blood transfusions received as part of their therapy (cumulative number of blood transfusion in lifetime ≥ 12). Diagnoses were: sickle cell disease (n = 48), β-thalassemia major (n = 12), Diamond-Blackfan anemia (n = 5), acute myeloid leukemia (n = 4), severe aplastic anemia (n = 2), acute lymphocytic leukemia (n = 2), dyskeratosis congenita, rhabdomyosarcoma, chronic lymphocytic leukemia, Wilms' tumor, Langerhans's cell histiocytosis, myelodysplastic syndrome, and severe combined immunodeficiency (n = 1 each). Participants underwent MRI scanning at clinical field strengths of 1.5 and 3 T. Written informed consent was obtained from the adult participants or parents or legal guardians of minors. If general sedation was required during the MRI exam, the 3 T scan was omitted to avoid the risks associated with an additional sedation (n = 23). 3 T MRI was also not performed for subjects with 3 T incompatible implants (n = 1) or due to other clinically motivated contraindications to 3 T MRI (n = 3). Overall, 80 and 53 data sets were obtained at 1.5 and 3 T, respectively.
Heuristic Description of the Effect of CHESS on R2*
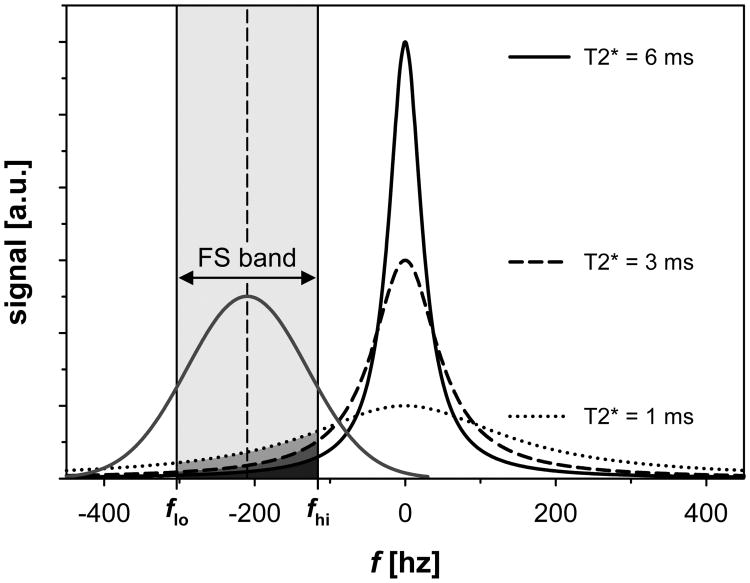
The ideal noise-free signal evolution S(t) for an mGRE sequence in the scenario of iron overload can be approximated as a mono-exponential decay (16). Although inhomogeneous T2* line broadening is usually associated with a Gaussian spectral profile, the generally used, simple description of the mGRE signal as a mono-exponential decay translates via Fourier transformation (FT) into a Lorentzian profile in the frequency domain f with a full width at half maximum (FWHM) that is directly related to R2*:
| [1] |
Here, S0 represents the mGRE signal at t = 0. Figure 1 shows different Lorentzian T2* line profiles together with a conventional Gaussian CHESS FS pulse. The FS pulse partially overlaps with the spectral profile of the water signal and consequently affects spectral components within its frequency band. Under the assumption that the spectral components within the frequency band of the CHESS pulse are fully saturated and thus removed from the observed mGRE signal, one may explain the effect of the CHESS pulse on the T2* broadened water line as spectral “hole burning” (30). In this simplified model, the total number of contributing signal components is reduced and the spectral profile distorted which can be approximated with a narrower effective spectral line width. Consequently a lower apparent R2* value would be measured. Heuristically, this effect could be described by calculating the change in the total area of the spectral R2* profile. Therefore, we approximate the frequency profile of the CHESS pulse as a uniform saturation range (FS band) defined by the center frequency (f0) of the CHESS pulses relative to the water center resonance frequency (i.e., at 0 Hz) and the pulse bandwidth (BW). Then, FS R2*, the apparent R2* value affected by the CHESS FS pulse, can be obtained from the non-FS R2* value by (see Fig. 1):
Figure 1.
Lorentzian spectral profiles for different T2* values as expected from a mono-exponential mGRE signal decay (Eq. 1). The saturation band (‘FS band’) of a conventional Gaussian CHESS pulse is indicated in light grey (respective net areas ΔAFS that are removed from the total spectral areas are indicated in darker grey tones). The CHESS pulse is illustrated by using the parameters at 1.5 T: center frequency f0 relative to water resonance frequency = -210 Hz and pulse bandwidth BW = 187.5 Hz. The water resonance frequency was assumed at a reference location of 0 Hz.
| [2] |
Here, ΔAFS represents the net area of the Lorentzian profile within the FS band, and fhi and flo the upper and lower boundary values of the FS band, respectively (i.e., fhi = f0+BW/2 and flo = f0-BW/2). S0 was set to 1 as it represents a scaling factor only. No further normalization of ΔAFS was required because the total integral of the Lorentzian equals 1.
Equation 2 cannot be analytically solved for R2* to calculate non-FS R2* values from the FS R2* values found under the effect of the CHESS pulse. However, a numerical correction factor can be derived and applied to any measured FS R2* value to obtain the corresponding non-FS R2* value.
MRI Protocol
MRI scans were performed as described previously (10) using identical imaging parameters at both magnetic field strengths (1.5 T Magnetom Avanto and 3 T Magnetom Trio, Siemens Healthcare, Erlangen, Germany). Each exam included an axial 2D multi-slice non-FS and FS mGRE image series that was centered at the location of the main portal vein and acquired with the following otherwise equivalent pulse sequence parameters: TR = 200 ms, TE1 = 1.1 ms, ΔTE = 0.8 ms, 20 echoes, bipolar readout gradient, matrix size: 128×104 (readout × phase-encoding direction), slice thickness: 10 mm, pixel bandwidth: 1950 Hz/px, and flip angle: 35°. Ten interleaved slices were acquired in the non-FS protocol, and five interleaved slices in the FS protocol. A slice gap of 10 mm (i.e., 100% of the slice thickness) was used for both protocols to minimize potential slice cross-talk. The field of view was identical for both protocols and ranged from 210 to 450 mm according to the subjects' size. For the FS mGRE protocol, the vendor's standard clinical Gaussian CHESS pulse (flip angle: 110°, duration Δt = 10240/5120 μs, center frequency f0 = -210/-407 Hz, and BW = 187.5/375 Hz at 1.5/3 T) was applied before each TR interval. Each sequence was acquired in a single breath hold maneuver (total acquisition time TA ≈ 21 s for each protocol). In case breath holding was not possible (e.g., because subjects were sedated or could not hold their breath sufficiently long) images were acquired with 3 to 5 averages to blur out artifacts from breathing motion (number of data sets acquired with signal averaging: 31/7 at 1.5/3 T).
Presence of Hepatic Fat, R2* Mapping, and Statistical Analysis
Our study investigates the effect of CHESS on the transverse relaxation rate R2* of the water signal, i.e. the water peak. However, hepatic fat presents a major confounder in R2* quantification (16), so that potential R2* differences without and with CHESS could arise from both the application of the CHESS pulse and the presence of fat. To evaluate the effect of CHESS on R2* in a controlled group, each enrolled subject was retrospectively tested for potential hepatic fat infiltration prior to further analysis using an approach described in (21,31). The evaluation of the hepatic fraction (FF) is described in detail in Section 1 of the Supporting Information. Subjects with FF ≥ 5% were excluded from further statistical R2* analysis to ensure that the final cohort exhibited no hepatic steatosis (grade 0: FF < 5% (32)).
For each included mGRE magnitude data set, pixel-wise R2* mapping was done in MATLAB (The MathWorks, Natick, MA) using customized non-linear least square (NLSQ) fitting routines. Several fitting approaches have been presented for R2* analysis in iron overload (9,10,24,33). To test whether our hypothesis applies for different fitting techniques, R2* maps were calculated for 3 fitting approaches: The first fit model (fit (i)) describes the mGRE signal evolution as a mono-exponential decay and considers image noise N as a constant offset:
| [3] |
This model has been employed in the R2*-LIC calibration study presented by Wood et al (9). All 3 parameters (S0, R2*, N) were used as free parameters during NLSQ fitting. The second fitting approach (fit (ii)) models the signal decay as
| [4] |
according to (34). The fitting routine estimates N from the image background, which is then subtracted from each mGRE image according to Eq. 4 before actual data fitting, so that only 2 parameters, S0 and R2*, are fitted. This technique has been used in the R2*-LIC calibration study by Hankins et al. (10). The third fitting routine (fit (iii)) employs a quadratic signal model
| [5] |
that has been presented recently (24) as an improved fitting approach for accurate and precise R2* quantification in the presence of noise. Again, S0, R2*, and N are set as free fit parameters.
For both imaging protocols and each fitting algorithm, representative R2* maps were calculated from the central slice of the multi-slice stack. Subsequently, whole liver regions of interest (ROIs) were manually drawn and any unwanted structures (e.g., blood vessels or artifacts) were excluded based on a histogram analysis (35). For each acquisition and each fitting approach, the mean liver R2* value was obtained from the pixel-wise mean value of the remaining pixels inside the ROI (see Fig. 2). If the fraction of remaining pixels dropped below 40% of the initial total number of pixels within the whole liver ROI, the respective data set was considered as a failed fit and not included in the comparison.
Figure 2.
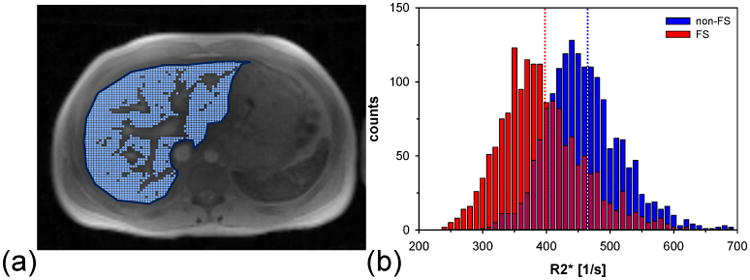
Example of whole liver R2* measurement. (a) Manually selected whole liver ROI (dark blue) and included pixels (overlaid as light blue dots) based on histogram evaluation. Blood vessel were excluded from the R2* analysis. (b) Histogram of remaining R2* values for non-FS and FS mGRE acquisition. In this example R2* data obtained via fit (iii) are shown. The dotted lines indicate the respective mean R2* values. Note the clear shift in the FS R2* results to lower values.
Statistical analysis was done in SigmaPlot 12.0 (Systat Software, San Jose, CA). R2* data sets for each fitting algorithm and each field strength were compared based on the Wilcoxon signed rank test. Linear regression and Bland-Altman plotting were used to quantitatively evaluate differences in the measured R2* values. For all statistical tests, a significance level of P = 0.05 was employed.
Results
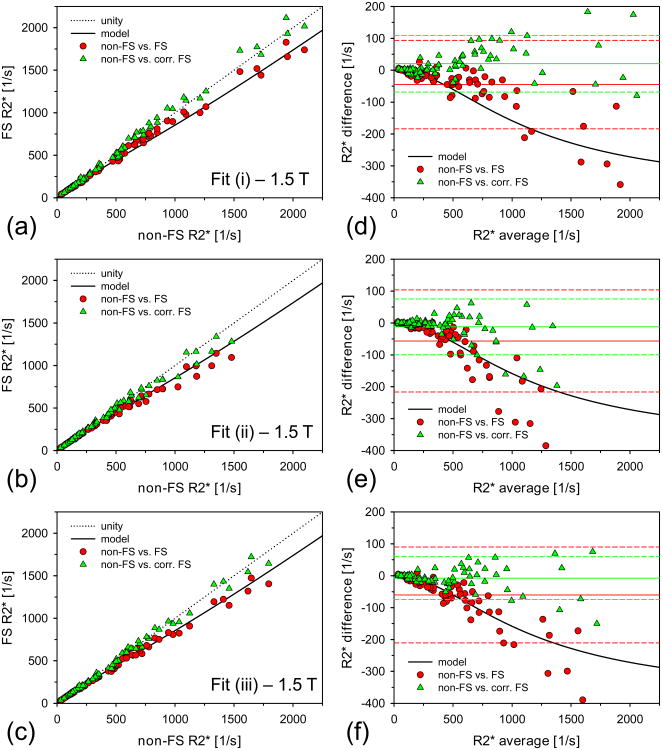
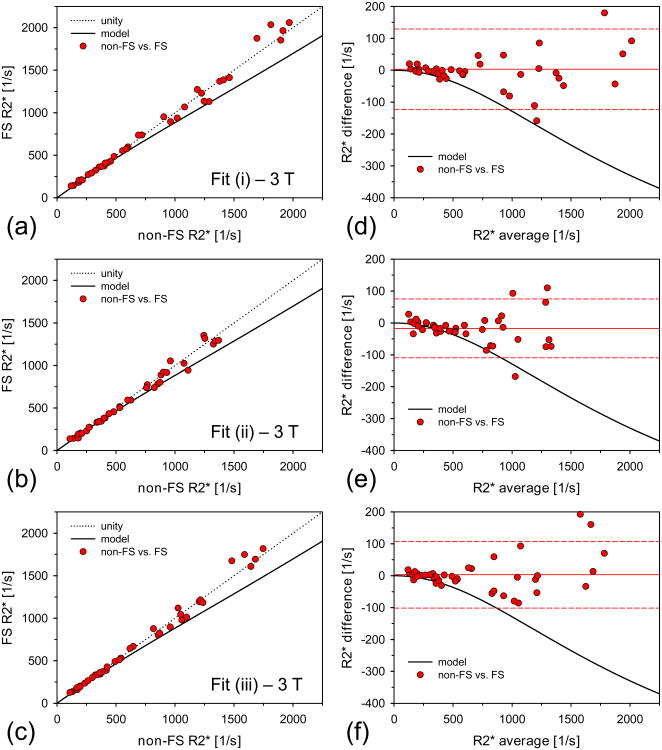
Five subjects of our entire study cohort exhibited hepatic steatosis grade 1 (32) with elevated FFs in the range of 5.9-30.0% (associated R2* range: 114-199 1/s at 1.5 T). These patients were excluded from the statistical analysis. Further, for all three fit models, 1 additional data set from the 1.5 T measurements and 10 additional data sets from the 3 T measurements were excluded because they were classified as failed fit based on our criterion of the percentage of included pixels. The final cohort consisted of n =74 subjects at 1.5 T and n = 39 cases at 3 T. For these subjects, overall mean liver R2* values ranged from 31 to 2097 1/s at 1.5 T and 109 to 2060 1/s at 3 T. Table 1 summarizes the range of mean liver R2* values as well as the overall mean R2* values across the patient population for each measurement protocol and each fitting method. Figures 3 and 4 show the data of the R2* measurements and the associated Bland-Altman plots for 1.5 T and 3 T, respectively. Table 2 shows the corresponding results of the linear regressions as well as the bias and 95% confidence intervals of the Bland-Altman analysis.
Table 1.
Range of R2* values and mean ± SD R2* values across patients (74/39 available data sets at 1.5 T/3 T) for the employed imaging protocols and the 3 different fitting approaches.
| Fit model | (i) | (ii) | (iii) | ||||
|---|---|---|---|---|---|---|---|
|
| |||||||
| R2* range (min.-max.) [1/s] | Mean R2*± SD [1/s] | R2* range (min.-max.) [1/s] | Mean R2*± SD [1/s] | R2* range (min.-max.) [1/s] | Mean R2*± SD [1/s] | ||
| 1.5 T | non-FS | 36-2097 | 530±495 | 31-1479 | 426±347 | 32-1794 | 473±418 |
| FS | 39-1828 | 485§±435 | 33-1143 | 370§±274 | 36-1473 | 413§±348 | |
| corr. FS | 39-2116 | 551§±512 | 33-1340 | 414±323 | 36-1721 | 466§±411 | |
|
| |||||||
| 3 T | non-FS | 120-1968 | 801±573 | 110-1369 | 626±385 | 109-1747 | 704±494 |
| FS | 140-2060 | 804±592 | 137-1354 | 609§±380 | 127-1818 | 707±511 | |
Note: Mean R2* values were compared for each fitting algorithm and each field strength by the Wilcoxon signed rank test. Significant differences in the comparison of non-FS and FS as well as non-FS and corrected FS values are indicated by the § symbol. Abbreviations: min., minimum; max., maximum; SD, standard deviation; non-FS, without fat saturation; FS, with fat saturation; corr. FS, corrected fat saturation.
Figure 3.
Non-FS and FS R2* results at 1.5 T. (a)/(b)/(c) 1.5 T R2* results calculated via fit (i)/(ii)/(iii) for non-FS and FS acquisitions together with the corrected FS R2* values. The solid black line indicates the predicted R2* values due to the 1.5 T CHESS FS pulse according to our heuristic model (Eq. 2). (d)/(e)/(f) Corresponding Bland-Altman plots for data shown in (a)/(b)/(c). The mean difference values are indicated as solid lines. The dashed lines represent respective limits of agreement (±1.96×SD of differences). Mean difference values and limits of agreement are given in Table 2.
Figure 4.
Non-FS and FS R2* results at 3 T. (a)/(b)/(c) 3 T R2* results calculated via fit (i)/(ii)/(iii) for non-FS and FS acquisitions. The solid black line indicates the predicted R2* values due to the 3 T CHESS FS pulse according to our heuristic model (Eq. 2). The predicted R2* changes are not represented by the measured data. No correction of the 3 T FS R2* values was necessary. (d)/(e)/(f) Corresponding Bland-Altman plots for data shown in (a)/(b)/(c). The mean difference values are indicated as solid lines. The dashed lines represent respective limits of agreement (±1.96×SD of differences). Mean difference values and limits of agreement are given in Table 2.
Table 2.
Results of linear regression and Bland-Altman analyses for non-FS, FS, and corrected FS data sets.
| Linear regression | Bland Altman | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
|
| ||||||||||
| Fit model | (i) | (ii) | (iii) | (i) | (ii) | (iii) | ||||
|
| ||||||||||
| slope ± error |
y-int. ± error [1/s] |
slope ± error |
y-int. ± error [1/s] |
slope ± error |
y-int. ± error [1/s] |
+1.96×S D mean -1.96×SD [1/s] |
+1.96×S D mean -1.96×SD [1/s] |
+1.96×S D mean -1.96×SD [1/s] |
||
| 1.5 T | non-FS vs. FS | 0.876§ ± 0.009 | 21§ ± 6 | 0.783§ ± 0.011 | 36§ ± 6 | 0.829§ ± 0.008 | 21§ ± 5 | 94-45-184 | 104-56-216 | 90-60-211 |
| non-FS vs. corr. FS | 1.032§ ± 0.010 | 4 ± 7 | 0.927§ ± 0.013 | 19§ ± 7 | 0.981§ ± 0.010 | 1 ± 6 | 110 21-68 | 75-12-99 | 60-7-75 | |
|
| ||||||||||
| 3 T | non-FS vs. FS | 1.027 ± 0.018 | -19 ± 18 | 0.980 ± 0.020 | -4 ± 15 | 1.028 ± 0.017 | -17 ± 15 | 129 3-123 | 75-17-109 | 108 3-101 |
Note: Slope and y-intercept of each linear regression were tested by the two-sided t-test for being statistically different from 1 and 0, respectively, at a significance level of P = 0.05. Values for which statistical significance was found are indicated by the § symbol. Abbreviations: y-int., y-intercept; SD, standard deviation; non-FS, without fat saturation; FS, with fat saturation; corr. FS, corrected fat saturation.
At 1.5 T, mean FS R2* values differed significantly from the non-FS R2* values for all 3 fitting algorithms (see Table 1). As expected from our heuristic model, the FS R2* values were systematically lower than the non-FS R2* values, with deviations of up to approximately 15%. The slopes of the linear regressions were all below 1 and the y-intercepts were systematically higher than 0 (see Table 2). The Bland-Altman plots revealed that absolute R2* differences became larger for increasing R2* values (Fig. 3). The CHESS-induced R2* alterations as predicted by our model (Eq. 2) appeared consistent with the measured data (see Fig. 3). By using Eq. 2 to generate a look-up table, a correction factor was applied to each measured FS R2* value, yielding sets of “corrected” FS R2* values. The corrected FS R2* values were not statistically different from the non-FS R2* values for fit (ii), but remained significantly different from the non-FS R2* values for fits (i) and (iii). Still, the model-based correction substantially reduced the FS-induced bias for all 3 fitting approaches so that minor residual differences are likely to be clinically negligible. The slopes of the linear regressions between corrected FS and non-FS values were much closer to unity (0.927-1.032) than the slopes for the FS vs. non-FS regressions (0.783-0.876). For fits (i) and (iii), the correction even removed the systematic shifts of the y-intercepts.
In contrast to the 1.5 T findings, no CHESS-induced R2* alterations were observed at 3 T. Although the mean FS R2* values were significantly different from the non-FS values for fit (ii), linear regression and Bland-Altman analyses showed that the differences were very small. For all 3 fitting algorithms, neither the slopes nor the y-intercepts of the linear regressions between non-FS and FS data were significantly different from 1 and 0, respectively. However, our model would predict a systematic reduction of up to approximately 10% for the FS R2* values at 3 T too, which is not represented by the measured data (see Fig. 4 and Discussion). Therefore, FS correction as applied at 1.5 T was not necessary at 3 T.
Discussion
In this study, we investigated the effect of FS via CHESS pulses on R2* measurements for iron overload assessment in a controlled cohort that exhibits no hepatic fat (steatosis grade 0). To demonstrate general applicability, R2* mapping was performed with 3 different fitting approaches that have been previously employed in R2*-LIC calibration studies (9,10) or presented as improved fitting techniques in the scenario of iron overload (24).
Overall, we measured systematically lower R2* values at 1.5 T for the mGRE imaging protocol that included the vendor's CHESS FS pulse. Especially for high R2* values (> 900 1/s), R2* reductions of up to 15% were seen. Without further consideration or correction, these changes would directly translate into an equivalent LIC underestimation based on existing linear R2*-LIC calibrations derived without CHESS (7,9,10). At 3 T, such R2* changes were not observed for the FS mGRE protocol. The results were consistent among the 3 fitting approaches. However, depending on the fit algorithm, there were differences in the actual R2* values even for the same imaging protocol, as seen from the respective R2* ranges and overall mean R2* values (Table 1): Fit (i) (Eq. 3) employed by Wood et. al (9) systematically produced the largest values, which is consistent with findings reported in a recent study (24) and is mainly explained by the different ways by which image noise is incorporated into the fit model. Feng et al. (24) demonstrated that the noise problem in R2* mapping can be addressed by fitting either the first or the second moment of the mGRE signal. The mathematical description of the second moment of the mGRE signal exhibits a much simpler form (see Eq. 5) (24) than the first moment model and was therefore implemented in our study (fit (iii)). Feng et al. further reported an increased R2* overestimation in case of R2* fitting via Eq. 3 (fit (i)) for decreasing signal to noise ratio (SNR), whereas R2* is gradually underestimated for lower SNRs in the so-called truncation R2* fitting models (36-39). As CHESS pulses also lead to an overall reduction of the SNR, usually in the range of 10-20%, such SNR-dependent R2* errors might be the reason why our proposed correction leads to slightly over-corrected FS R2* values for fit (i) and slightly under-corrected R2* values for fit (ii) at 1.5 T. For fit (iii) (i.e., fitting of the second moment of the mGRE signal) the linear regression between non-FS and corrected R2* values is closest to unity (see Table 2). However, the R2* differences arising because of the use of different fitting approaches do not necessarily translate into an erroneous or biased LIC estimate. The LIC can be correctly assessed as long as the employed R2* fitting method matches the method used to generate the R2*-LIC calibration curve (33).
For all fitting approaches, linear regression analysis between 1.5 T non-FS and FS R2* values shows y-intercepts systematically higher than 0. This could be an indicator that the CHESS-induced R2* changes at 1.5 T cannot be satisfactorily reproduced by a simple scaling effect of the non-FS R2* values and exhibits a more complex behavior. The detected R2* changes can be explained by a partial saturation of water signal components that are spectrally located within the FS band of the CHESS pulse. These signal components are also saturated, which ultimately alters the R2* value. To quantitatively account for this partial saturation effect, we presented a descriptive model (Eq. 2) that assumes a Lorentzian spectral profile as implied by a mono-exponential signal evolution and a uniform (square) saturation range (FS band). The model is based on the physical properties of the CHESS pulse only (i.e., center frequency f0 and spectral bandwidth BW) and does not require any additional input such as pulse sequence parameters.
It is important to note that our derived model (Eq. 2) is a heuristic approach that well describes the observed behavior. There are several limitations which are not considered in our presented model and which would need to be incorporated into an accurate and fully valid description (discussed below). As a simple but mathematically more stringent alternative, the spectral hole burning of the CHESS pulse could be formally described via a spectral band pass filter. The signal after application of CHESS could then be retrieved via inverse FT of the band pass filtered signal. In Section 2 of the Supporting Information, we describe and evaluate such a formally derived model for the spectral hole burning of the CHESS pulse. However, as shown in the Supporting Information, this model does not provide an improved description of the observed effects. The band pass description would lead to an oscillatory pattern in the signal after application of CHESS which would translate into a model mismatch in the R2* extraction of the FS mGRE data via mono-exponential fitting. A direct comparison of band pass modeled and measured signals revealed that the measured FS data do not show an oscillatory behavior and satisfactorily follow an exponential decay thereby justifying the application of fit equations (i)-(iii) for the analysis of the FS data (see Section 2 of Supporting Information).
At 3 T, the measurements did not show a R2* bias due to CHESS. Consequently, no correction is required for the FS R2* values. However from our model, we would expect systematically smaller FS R2* compared to the non-FS R2* values also at 3 T. Therefore, phantom measurements were made to further study the behavior of R2* under the influence of CHESS in a reproducible and controllable setting (minimal magnetic field inhomogeneity etc.). Agarose phantoms doped with iron nanoparticles as well as MnCl2 solutions were used. These measurements and results are summarized in detail in Section 3 of the Supporting Information. For none of the phantoms, systematic R2* changes due to CHESS could be observed neither at 1.5 T nor at 3 T. Additional R2 measurements showed that in these phantoms R2* and R2 are very similar. In the light of these results, it is crucial to note that T2* and T2 broadening reflect two physically different scenarios: inhomogeneous and homogeneous line broadening respectively. In case of homogeneous T2 line broadening, the CHESS pulse does not lead to spectral hole burning in a sense that water signal components within the frequency band of the CHESS pulse are saturated and thus removed from the observed mGRE signal. In fact, as soon as the CHESS pulse spectrally overlaps with the T2 broadened signal peak, the CHESS pulse essentially excites the entire peak which leads to an overall signal reduction due to (partial) saturation but does not introduce a systematic R2 bias. This overall signal reduction results from direct saturation of the entire homogeneously broadened peak and has been described in Mulkern et al. (40) based on simulations of the Bloch equations: such a saturation effect leads to a reduction of the overall detectable signal (40,41) without altering the underlying T2 line profile so that R2 remains unchanged. In the scenario of transfusional iron overload, however, the ratio of R2* and R2 is larger compared to the values found in the employed phantoms. The phantoms showed a maximal R2* vs. R2 ratio of about 2 whereas in iron overload the R2* vs. R2 ratio is about 3.5 and more for R2* values > 500 1/s at 1.5 T (extracted from (9)). In (42), the authors report a linear R2-to-R2* conversion with a slope of 13.617 and a y-intercept of -169.781. Consequently, in iron overload T2* broadening is more pronounced than the underlying T2 broadening so that the CHESS pulse can affect the T2* line profile which could lead to the observed R2* changes. As in iron overload, R2* and also R2 increase with the magnetic field strength (B0) (8,9,16,43-46), T2 broadening might not be negligible for an accurate theoretical description of the saturation effect of the water peak due to CHESS. Our simple approach to model the effect of CHESS on R2* via hole burning assumes spectral broadening as a purely inhomogeneous line broadening effect (i.e. line broadening due to T2′ contributions from field inhomogeneity effects alone) does not consider underlying homogeneous T2 line broadening. This could be one reason why our heuristic description (as well as the formal band pass model, see Section 2 of Supporting Information) fails to correctly describe the effect of CHESS on R2* at 3 T. In this context, it has be noted however, that R2* increases proportionally with B0 (44,46) whereas R2 only increases by a factor of 1.4 from 1.5 T to 3 T (46). Therefore, less overlap of the CHESS pulse and the underlying T2 line would be expected at 3 T. Consequently, other effects need to be considered for a fundamental theoretical description of the effect of CHESS on R2* in iron overload in addition to underlying homogeneous line broadening.
One important effect that was not included into our model could arise from insufficient or non-uniform saturation due to B1 inhomogeneities. Such B1 inhomogeneities could lead to spatially varying saturation efficiency throughout the liver. To address this issue, we also compared non-FS and FS R2* values in small ROIs which were located in the center part of the right liver lobe where B1 inhomogeneities are usually small. The results found within these small ROIs (not shown) were very consistent to the whole liver results – R2* bias at 1.5 T, no R2* bias at 3 T – so that B1 inhomogeneities appear not to be one of the primary reasons for the observed R2* behavior. Another important issue that our model does not account for is signal noise. This might be important because slightly different reductions of SNR due to the CHESS pulse were seen at 1.5 T and 3 T respectively (SNR reduction due to CHESS: ∼25% at 1.5 T vs. ∼10% at 3 T; detailed data not shown). This observation appears consistent to the aforementioned fact that less overlap between the CHESS pulse and the underlying T2 broadened line (46), and thus, less SNR reduction due to CHESS is to be expected at 3 T.
Also, our model assumes a uniform saturation profile rather than a more realistic Gaussian FS band. The effect of a Gaussian FS band is also addressed in Section 2 of the Supporting Information but does not improve the model and only leads to minor alterations. Our model does also not consider potential relaxation effects during the application of the CHESS pulse as described in (41,47): In case of very short transverse relaxation times, relaxation during application of an RF pulse cannot be neglected. For signal components with short transverse relaxation times, the magnetization actually rotated into the transverse plane may be a lot less than that expected from the nominal pulse flip angle (41). Therefore, the CHESS pulse might only partly saturate signals with short transverse relaxation times so that these signal components would not be completely removed from the total signal as assumed by our model. Finally, the model neglects effects from local off-resonance effects due to the presence of iron (48,49).
We would like to emphasize that our model is not intended as a fundamental theoretical explanation of the effect of CHESS on R2* in iron overload. Besides the various effects discussed above which are not included in our heuristic approach, we believe that an accurate and fundamental description has to account for the unique microscopic make-up of liver tissue in the context of iron storage (e.g. effects from iron cluster size and density) and would necessitate a more complex description of the underlying physics (50), e.g. via Bloch equations, which was, however, beyond the scope of our study but could be the aim of further work.
Nevertheless, despite the fact that our heuristic description involves several simplifications, it is consistent with the measured data over the entire R2* range at 1.5 T where CHESS causes systematic R2* alterations in our group of patients with transfusional iron overload. Our model can be used for phenomenological correction of the FS-induced R2* bias at 1.5 T as can be seen from the linear regression between non-FS and corrected FS data. Our model does not follow the measured R2* data at 3 T. However within the range of R2* values of our study cohort, our measurements did not show a CHESS-induced R2* bias at 3 T so that CHESS could be applied without requiring a method for R2* correction in that case.
Our findings of a CHESS-induced R2* bias at 1.5 T are important for clinical R2*-based LIC assessment, as they are in contrast to a previous study at 1.5 T by Sanches-Rocha et al. (51) that reported no changes in R2* due to FS via CHESS. However, in that study, measured R2* values were confined to a range below approximately 330 1/s, wherein our results showed that changes in R2* for non-FS and FS acquisitions are small (< 5%; see Fig. 3). It should be noted as well that Sanches-Rocha et al. evaluated only T2* rather than R2*, where potential differences might manifest more subtle because of the inverse relationship between T2* and R2*. Furthermore, in a very recent biopsy-calibrated R2*-LIC study by Henninger et al. mGRE imaging in combination with CHESS was done (11). In this study, the authors did not find a significant difference in the slopes or y-intercepts of the R2*-LIC calibrations compared to other biopsy-calibrated studies e.g. by Wood et al. (9) and Hankins et al. (10), which were established via mGRE sequences without any FS technique. However, R2* values reported by Henninger et al. are also confined to a range below 500 1/s where CHESS-induced changes are quite small. The actual value of the slope of their R2*-LIC conversion found by Henninger et al. is slightly smaller compared to the slopes found by Wood et al. and Hankins et al. This could be consistently explained by minor R2* reductions due to the applied CHESS pulses.
Because we investigated the effects on R2* due to FS via CHESS pulses, our findings might not be directly transferrable if alternative saturation techniques are used. CHESS pulses exhibit certain limitations, such as sensitivity to off-resonance effects (e.g. as present in scenarios of elevated LIC (48,49)), that can be overcome by using inversion recovery for magnetization nulling such as short TI inversion recovery (STIR) for a more efficient FS. However, these techniques rely on clearly distinct T1 times of water and fat. This is not necessarily the case in the presence of iron in tissue, as iron can also shorten T1 (25,52,53). Consequently, inversion-based fat suppression techniques or combined approaches such SPIR may also partially affect the water signal and introduce uncertainty into the R2* measurement. SPIR impacts R2* quantitation in transfusional siderosis at 1.5 T as shown in a recent study by Meloni et al. (28). The authors independently report a R2* reduction due to FS of up to about 7%. These results are remarkably concordant to our findings. Meloni et al. also attribute these observations to SPIR-induced suppression of water signal components from iron-broadened line profiles. Unfortunately, this study does not investigate potential SPIR-induced R2* changes at 3 T. One advantage of the employed CHESS pulses over inversion-based methods might be the shorter time duration which facilitated multi-slice coverage within a single breath hold without the need for segmentation and thereby maintained FS efficiency.
As an alternative to applying any of the FS techniques, confounding effects on R2* from fat could be considered by means of appropriate fat-water modeling (18-22). Such techniques might be limited in patients with severe iron overload, as the accessible temporal range to evaluate fat-water modulations is restricted due to the fast signal decay (54). However, the presence of fat and iron is more common for non-transfusional iron overload in which LIC is usually not that high (16). In scenarios of mild iron overload, fat-water modeling has been successfully demonstrated (19,31,55) so that no potentially R2*-confounding FS technique would be required for a correct R2* quantification.
In summary, based on the mGRE techniques and CHESS pulses employed, our results demonstrate that the use of FS via CHESS in R2*-based iron overload measurements can lead to a biased R2* evaluation at 1.5 T especially for high R2* values. We propose a heuristic model via spectral hole burning that consistently explains the observed R2* changes due to the applied CHESS pulse and successfully reduces the FS-induced R2* bias at 1.5 T. The model allows for a simple correction and only requires the actual parameters of the CHESS module as input. We further found that CHESS FS does not affect the R2* measurements for transfusional iron overload assessment at 3 T. Therefore no correction is required. Compared with complex fat-water signal modeling, the use of FS appeals as a simpler tool to avoid confounding effects from lipid signals in R2*-LIC measurements (24,25). To date, published clinical biopsy R2*-LIC calibration curves have been mainly established using mGRE acquisitions without FS and at 1.5 T only (7,9,10). Hence, our findings are of high relevance for clinical R2*-based iron overload assessment in scenarios wherein non-FS mGRE sequences are substituted with CHESS FS mGRE acquisitions, such as in patients with suspected steatosis and hemochromatosis (56). Without correction or further consideration of the effect of the FS technique on R2*, any mGRE technique including FS would necessitate a new biopsy calibration study for accurate LIC estimation.
Supplementary Material
Supporting Figure S1. (a), (c) Selection of ROIs in liver parenchyma and (b), (d) associated averaged magnitude mGRE signals (blue crosses) together with results from non-linear least square fit (solid lines) described in (3) for two cases (case 1: mean hepatic FF = 1.9%, case 2: mean hepatic FF: 12.6%). Subjects with a mean hepatic FF of ≥ 5% were excluded from further analysis.
Supporting Figure S2. Numerically simulated mGRE signal without and with CHESS for 1.5 T (top) and 3 T (bottom). In the left panels, the gray lines indicate non-FS mGRE signal for 100, 300, 600, and 1200 1/s. The red and blue lines indicate the simulated signal evolution assuming boxcar and Gaussian CHESS profiles respectively. As a second step, the simulated signals were discretized according to the TEs of the employed mGRE sequence and fitted to a mono-exponential function to calculate pairs of non-FS and FS R2* values (right panels).
Supporting Figure S3. Left panel: Plots of non-FS vs. FS R2* values as retrieved from a formal mathematical description of the CHESS-induced spectral hole burning. Solid lines indicate 1.5 T results, dashed lines indicate 3 T data. Red color represents values assuming a boxcar CHESS profile, blue represents the values in case of a Gaussian CHESS profile. Right panel: In addition to the data shown on the left, non-FS and FS R2* according to our heuristic model (green) are included together with the measured data.
Supporting Figure S4. Measured non-FS (circles) and FS (diamonds) together with simulated signal data at 1.5 T (top row) and 3 T (bottom row). No oscillations as expected from the formal band pass model are visible in the FS data. However, background noise needs to be considered during R2* fitting as can be seen from a constant signal offset in the measured data for longer TEs.
Supporting Figure S5. (a) Non-FS R2*, FS R2*, and R2 measurements in 3 different phantom types (top row: BNF particles, circles; mid row: DSPIO particles, rectangles; bottom row: MnCl2, triangles) at 1.5 T. For R2* quantification, the same 3 fitting models were applied as in patients. R2 quantification was done using fit (ii). (b) Corresponding 3 T results.
Acknowledgments
The authors would like to acknowledge Vani Shanker, PhD, for help with editing of the manuscript, Gail Fortner, BSN, for patient recruitment, and Kamisha Flower, BS, for data management.
Funding: This study was supported by NIH grant 5 R01 DK088988 from the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) and the American Lebanese Syrian Associated Charities (ALSAC – the fund-raising organization of St. Jude Children's Research Hospital).
Footnotes
Parts of this study were presented (program number 1671) at the 2014 Annual Meeting of the International Society of Magnetic Resonance in Medicine in Milan, Italy.
References
- 1.Angelucci E, Brittenham GM, McLaren CE, Ripalti M, Baronciani D, Giardini C, Galimberti M, Polchi P, Lucarelli G. Hepatic iron concentration and total body iron stores in thalassemia major. The New England journal of medicine. 2000;343(5):327–331. doi: 10.1056/NEJM200008033430503. [DOI] [PubMed] [Google Scholar]
- 2.Brittenham GM. Iron-chelating therapy for transfusional iron overload. The New England journal of medicine. 2011;364(2):146–156. doi: 10.1056/NEJMct1004810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Olivieri NF. Progression of iron overload in sickle cell disease. Seminars in hematology. 2001;38(1 Suppl 1):57–62. doi: 10.1016/s0037-1963(01)90060-5. [DOI] [PubMed] [Google Scholar]
- 4.Prati D, Maggioni M, Milani S, Cerino M, Cianciulli P, Coggi G, Forni GL, Magnano C, Meo A, Gramignoli R, Rebulla P, Fiorelli G, Cappellini MD Cooleycare Cooperative G. Clinical and histological characterization of liver disease in patients with transfusion-dependent beta-thalassemia. A multicenter study of 117 cases. Haematologica. 2004;89(10):1179–1186. [PubMed] [Google Scholar]
- 5.Taher AT, Musallam KM, Inati A. Iron overload: consequences, assessment, and monitoring. Hemoglobin. 2009;33(Suppl 1):S46–57. doi: 10.3109/03630260903346676. [DOI] [PubMed] [Google Scholar]
- 6.Angelucci E, Baronciani D, Lucarelli G, Baldassarri M, Galimberti M, Giardini C, Martinelli F, Polchi P, Polizzi V, Ripalti M, et al. Needle liver biopsy in thalassaemia: analyses of diagnostic accuracy and safety in 1184 consecutive biopsies. British journal of haematology. 1995;89(4):757–761. doi: 10.1111/j.1365-2141.1995.tb08412.x. [DOI] [PubMed] [Google Scholar]
- 7.Anderson LJ, Holden S, Davis B, Prescott E, Charrier CC, Bunce NH, Firmin DN, Wonke B, Porter J, Walker JM, Pennell DJ. Cardiovascular T2-star (T2*) magnetic resonance for the early diagnosis of myocardial iron overload. European heart journal. 2001;22(23):2171–2179. doi: 10.1053/euhj.2001.2822. [DOI] [PubMed] [Google Scholar]
- 8.St Pierre TG, Clark PR, Chua-anusorn W, Fleming AJ, Jeffrey GP, Olynyk JK, Pootrakul P, Robins E, Lindeman R. Noninvasive measurement and imaging of liver iron concentrations using proton magnetic resonance. Blood. 2005;105(2):855–861. doi: 10.1182/blood-2004-01-0177. [DOI] [PubMed] [Google Scholar]
- 9.Wood JC, Enriquez C, Ghugre N, Tyzka JM, Carson S, Nelson MD, Coates TD. MRI R2 and R2* mapping accurately estimates hepatic iron concentration in transfusion-dependent thalassemia and sickle cell disease patients. Blood. 2005;106(4):1460–1465. doi: 10.1182/blood-2004-10-3982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hankins JS, McCarville MB, Loeffler RB, Smeltzer MP, Onciu M, Hoffer FA, Li CS, Wang WC, Ware RE, Hillenbrand CM. R2* magnetic resonance imaging of the liver in patients with iron overload. Blood. 2009;113(20):4853–4855. doi: 10.1182/blood-2008-12-191643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Henninger B, Zoller H, Rauch S, Finkenstedt A, Schocke M, Jaschke W, Kremser C. R2* Relaxometry for the Quantification of Hepatic Iron Overload: Biopsy-Based Calibration and Comparison with the Literature. RoFo : Fortschritte auf dem Gebiete der Rontgenstrahlen und der Nuklearmedizin. 2015;187(6):472–479. doi: 10.1055/s-0034-1399318. [DOI] [PubMed] [Google Scholar]
- 12.Chandarana H, Lim RP, Jensen JH, Hajdu CH, Losada M, Babb JS, Huffman S, Taouli B. Hepatic iron deposition in patients with liver disease: preliminary experience with breath-hold multiecho T2*-weighted sequence. AJR American journal of roentgenology. 2009;193(5):1261–1267. doi: 10.2214/AJR.08.1996. [DOI] [PubMed] [Google Scholar]
- 13.Westwood M, Anderson LJ, Firmin DN, Gatehouse PD, Charrier CC, Wonke B, Pennell DJ. A single breath-hold multiecho T2* cardiovascular magnetic resonance technique for diagnosis of myocardial iron overload. Journal of magnetic resonance imaging. 2003;18(1):33–39. doi: 10.1002/jmri.10332. [DOI] [PubMed] [Google Scholar]
- 14.Wood JC, Ghugre N. Magnetic resonance imaging assessment of excess iron in thalassemia, sickle cell disease and other iron overload diseases. Hemoglobin. 2008;32(1-2):85–96. doi: 10.1080/03630260701699912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.George DK, Goldwurm S, MacDonald GA, Cowley LL, Walker NI, Ward PJ, Jazwinska EC, Powell LW. Increased hepatic iron concentration in nonalcoholic steatohepatitis is associated with increased fibrosis. Gastroenterology. 1998;114(2):311–318. doi: 10.1016/s0016-5085(98)70482-2. [DOI] [PubMed] [Google Scholar]
- 16.Sirlin CB, Reeder SB. Magnetic resonance imaging quantification of liver iron. Magnetic resonance imaging clinics of North America. 2010;18(3):359–381. ix. doi: 10.1016/j.mric.2010.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hamilton G, Yokoo T, Bydder M, Cruite I, Schroeder ME, Sirlin CB, Middleton MS. In vivo characterization of the liver fat (1)H MR spectrum. NMR in biomedicine. 2011;24(7):784–790. doi: 10.1002/nbm.1622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Reeder SB, Pineda AR, Wen Z, Shimakawa A, Yu H, Brittain JH, Gold GE, Beaulieu CH, Pelc NJ. Iterative decomposition of water and fat with echo asymmetry and least-squares estimation (IDEAL): application with fast spin-echo imaging. Magnetic resonance in medicine. 2005;54(3):636–644. doi: 10.1002/mrm.20624. [DOI] [PubMed] [Google Scholar]
- 19.Yu H, Shimakawa A, McKenzie CA, Brodsky E, Brittain JH, Reeder SB. Multiecho water-fat separation and simultaneous R2* estimation with multifrequency fat spectrum modeling. Magnetic resonance in medicine. 2008;60(5):1122–1134. doi: 10.1002/mrm.21737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Vasanawala SS, Yu H, Shimakawa A, Jeng M, Brittain JH. Estimation of liver T(2) in transfusion-related iron overload in patients with weighted least squares T(2) IDEAL. Magnetic resonance in medicine. 2012;67(1):183–190. doi: 10.1002/mrm.22986. [DOI] [PubMed] [Google Scholar]
- 21.Bydder M, Yokoo T, Hamilton G, Middleton MS, Chavez AD, Schwimmer JB, Lavine JE, Sirlin CB. Relaxation effects in the quantification of fat using gradient echo imaging. Magnetic resonance imaging. 2008;26(3):347–359. doi: 10.1016/j.mri.2007.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.O'Regan DP, Callaghan MF, Wylezinska-Arridge M, Fitzpatrick J, Naoumova RP, Hajnal JV, Schmitz SA. Liver fat content and T2*: simultaneous measurement by using breath-hold multiecho MR imaging at 3.0 T--feasibility. Radiology. 2008;247(2):550–557. doi: 10.1148/radiol.2472070880. [DOI] [PubMed] [Google Scholar]
- 23.Hernando D, Kuhn JP, Mensel B, Volzke H, Puls R, Hosten N, Reeder SB. R2* estimation using “in-phase” echoes in the presence of fat: the effects of complex spectrum of fat. Journal of magnetic resonance imaging. 2013;37(3):717–726. doi: 10.1002/jmri.23851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Feng Y, He T, Gatehouse PD, Li X, Harith Alam M, Pennell DJ, Chen W, Firmin DN. Improved MRI R2 * relaxometry of iron-loaded liver with noise correction. Magnetic resonance in medicine. 2013;70(6):1765–1774. doi: 10.1002/mrm.24607. [DOI] [PubMed] [Google Scholar]
- 25.Henninger B, Kremser C, Rauch S, Eder R, Zoller H, Finkenstedt A, Michaely HJ, Schocke M. Evaluation of MR imaging with T1 and T2* mapping for the determination of hepatic iron overload. European radiology. 2012;22(11):2478–2486. doi: 10.1007/s00330-012-2506-2. [DOI] [PubMed] [Google Scholar]
- 26.Haase A, Frahm J, Hanicke W, Matthaei D. 1H NMR chemical shift selective (CHESS) imaging. Physics in medicine and biology. 1985;30(4):341–344. doi: 10.1088/0031-9155/30/4/008. [DOI] [PubMed] [Google Scholar]
- 27.Papakonstantinou O, Foufa K, Benekos O, Alexopoulou E, Mademli M, Balanika A, Economopoulos N, Kelekis NL. Use of fat suppression in R(2) relaxometry with MRI for the quantification of tissue iron overload in beta-thalassemic patients. Magnetic resonance imaging. 2012;30(7):926–933. doi: 10.1016/j.mri.2012.03.002. [DOI] [PubMed] [Google Scholar]
- 28.Meloni A, Tyszka JM, Pepe A, Wood JC. Effect of inversion recovery fat suppression on hepatic R2* quantitation in transfusional siderosis. AJR American journal of roentgenology. 2015;204(3):625–629. doi: 10.2214/AJR.14.12786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kaldoudi E, Williams SC, Barker GJ, Tofts PS. A chemical shift selective inversion recovery sequence for fat-suppressed MRI: theory and experimental validation. Magnetic resonance imaging. 1993;11(3):341–355. doi: 10.1016/0730-725x(93)90067-n. [DOI] [PubMed] [Google Scholar]
- 30.Hodgson N, Weber H. Optical Resonators: Fundamentals, Advanced Concepts and Applications. London: Springer; 1997. p. 659. [Google Scholar]
- 31.Bydder M, Shiehmorteza M, Yokoo T, Sugay S, Middleton MS, Girard O, Schroeder ME, Wolfson T, Gamst A, Sirlin C. Assessment of liver fat quantification in the presence of iron. Magnetic resonance imaging. 2010;28(6):767–776. doi: 10.1016/j.mri.2010.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tang A, Tan J, Sun M, Hamilton G, Bydder M, Wolfson T, Gamst AC, Middleton M, Brunt EM, Loomba R, Lavine JE, Schwimmer JB, Sirlin CB. Nonalcoholic fatty liver disease: MR imaging of liver proton density fat fraction to assess hepatic steatosis. Radiology. 2013;267(2):422–431. doi: 10.1148/radiol.12120896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Meloni A, Rienhoff HY, Jr, Jones A, Pepe A, Lombardi M, Wood JC. The use of appropriate calibration curves corrects for systematic differences in liver R2* values measured using different software packages. British journal of haematology. 2013;161(6):888–891. doi: 10.1111/bjh.12296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gudbjartsson H, Patz S. The Rician distribution of noisy MRI data. Magnetic resonance in medicine. 1995;34(6):910–914. doi: 10.1002/mrm.1910340618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.McCarville MB, Hillenbrand CM, Loeffler RB, Smeltzer MP, Song R, Li CS, Hankins JS. Comparison of whole liver and small region-of-interest measurements of MRI liver R2* in children with iron overload. Pediatric radiology. 2010;40(8):1360–1367. doi: 10.1007/s00247-010-1596-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Beaumont M, Odame I, Babyn PS, Vidarsson L, Kirby-Allen M, Cheng HL. Accurate liver T2 measurement of iron overload: a simulations investigation and in vivo study. Journal of magnetic resonance imaging. 2009;30(2):313–320. doi: 10.1002/jmri.21835. [DOI] [PubMed] [Google Scholar]
- 37.He T, Gatehouse PD, Smith GC, Mohiaddin RH, Pennell DJ, Firmin DN. Myocardial T2* measurements in iron-overloaded thalassemia: An in vivo study to investigate optimal methods of quantification. Magnetic resonance in medicine. 2008;60(5):1082–1089. doi: 10.1002/mrm.21744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.He T, Zhang J, Carpenter JP, Feng Y, Smith GC, Pennell DJ, Firmin DN. Automated truncation method for myocardial T2* measurement in thalassemia. Journal of magnetic resonance imaging. 2013;37(2):479–483. doi: 10.1002/jmri.23780. [DOI] [PubMed] [Google Scholar]
- 39.Yin X, Shah S, Katsaggelos AK, Larson AC. Improved R2* measurement accuracy with absolute SNR truncation and optimal coil combination. NMR in biomedicine. 2010;23(10):1127–1136. doi: 10.1002/nbm.1539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mulkern RV, Williams ML. The general solution to the Bloch equation with constant rf and relaxation terms: application to saturation and slice selection. Medical physics. 1993;20(1):5–13. doi: 10.1118/1.597063. [DOI] [PubMed] [Google Scholar]
- 41.Robson MD, Gatehouse PD, Bydder M, Bydder GM. Magnetic resonance: an introduction to ultrashort TE (UTE) imaging. Journal of computer assisted tomography. 2003;27(6):825–846. doi: 10.1097/00004728-200311000-00001. [DOI] [PubMed] [Google Scholar]
- 42.Christoforidis A, Perifanis V, Spanos G, Vlachaki E, Economou M, Tsatra I, Athanassiou-Metaxa M. MRI assessment of liver iron content in thalassamic patients with three different protocols: comparisons and correlations. European journal of haematology. 2009;82(5):388–392. doi: 10.1111/j.1600-0609.2009.01223.x. [DOI] [PubMed] [Google Scholar]
- 43.Bulte JW, Miller GF, Vymazal J, Brooks RA, Frank JA. Hepatic hemosiderosis in non-human primates: quantification of liver iron using different field strengths. Magnetic resonance in medicine. 1997;37(4):530–536. doi: 10.1002/mrm.1910370409. [DOI] [PubMed] [Google Scholar]
- 44.Storey P, Thompson AA, Carqueville CL, Wood JC, de Freitas RA, Rigsby CK. R2* imaging of transfusional iron burden at 3T and comparison with 1.5T. Journal of magnetic resonance imaging. 2007;25(3):540–547. doi: 10.1002/jmri.20816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Anwar M, Wood J, Manwani D, Taragin B, Oyeku SO, Peng Q. Hepatic Iron Quantification on 3 Tesla (3 T) Magnetic Resonance (MR): Technical Challenges and Solutions. Radiology research and practice. 2013;2013:628150. doi: 10.1155/2013/628150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ghugre NR, Doyle EK, Storey P, Wood JC. Relaxivity-iron calibration in hepatic iron overload: Predictions of a Monte Carlo model. Magnetic resonance in medicine. 2014 doi: 10.1002/mrm.25459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Robson MD, Gatehouse PD. Consequences of T2 relaxation during half-pulse slice selection for ultrashort TE imaging. Magnetic resonance in medicine. 2010;64(2):610–615. doi: 10.1002/mrm.22323. [DOI] [PubMed] [Google Scholar]
- 48.Hernando D, Cook RJ, Diamond C, Reeder SB. Magnetic susceptibility as a B0 field strength independent MRI biomarker of liver iron overload. Magnetic Resonance in Medicine. 2013;70(3):648–656. doi: 10.1002/mrm.24848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Taylor BA, Loeffler RB, Song R, McCarville MB, Hankins JS, Hillenbrand CM. Simultaneous field and R2 mapping to quantify liver iron content using autoregressive moving average modeling. Journal of magnetic resonance imaging. 2012;35(5):1125–1132. doi: 10.1002/jmri.23545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ghugre NR, Wood JC. Relaxivity-iron calibration in hepatic iron overload: probing underlying biophysical mechanisms using a Monte Carlo model. Magnetic resonance in medicine. 2011;65(3):837–847. doi: 10.1002/mrm.22657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Sanches-Rocha L, Serpa B, Figueiredo E, Hamerschlak N, Baroni R. Comparison between multi-echo T2* with and without fat saturation pulse for quantification of liver iron overload. Magnetic resonance imaging. 2013;31(10):1704–1708. doi: 10.1016/j.mri.2013.07.013. [DOI] [PubMed] [Google Scholar]
- 52.Feng Y, He T, Carpenter JP, Jabbour A, Alam MH, Gatehouse PD, Greiser A, Messroghli D, Firmin DN, Pennell DJ. In vivo comparison of myocardial T1 with T2 and T2* in thalassaemia major. Journal of magnetic resonance imaging. 2013;38(3):588–593. doi: 10.1002/jmri.24010. [DOI] [PubMed] [Google Scholar]
- 53.Stark DD, Moseley ME, Bacon BR, Moss AA, Goldberg HI, Bass NM, James TL. Magnetic resonance imaging and spectroscopy of hepatic iron overload. Radiology. 1985;154(1):137–142. doi: 10.1148/radiology.154.1.3964933. [DOI] [PubMed] [Google Scholar]
- 54.Hernando D, Sharma SD, Reeder SB. Limits of liver fat quantification in the presence of severe iron overload; Proceedings of the 22nd Annual Meeting of ISMRM; Milan, Italy. 2014; abstract: 612. [Google Scholar]
- 55.Kuhn JP, Hernando D, Munoz del Rio A, Evert M, Kannengiesser S, Volzke H, Mensel B, Puls R, Hosten N, Reeder SB. Effect of multipeak spectral modeling of fat for liver iron and fat quantification: correlation of biopsy with MR imaging results. Radiology. 2012;265(1):133–142. doi: 10.1148/radiol.12112520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Powell EE, Ali A, Clouston AD, Dixon JL, Lincoln DJ, Purdie DM, Fletcher LM, Powell LW, Jonsson JR. Steatosis is a cofactor in liver injury in hemochromatosis. Gastroenterology. 2005;129(6):1937–1943. doi: 10.1053/j.gastro.2005.09.015. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting Figure S1. (a), (c) Selection of ROIs in liver parenchyma and (b), (d) associated averaged magnitude mGRE signals (blue crosses) together with results from non-linear least square fit (solid lines) described in (3) for two cases (case 1: mean hepatic FF = 1.9%, case 2: mean hepatic FF: 12.6%). Subjects with a mean hepatic FF of ≥ 5% were excluded from further analysis.
Supporting Figure S2. Numerically simulated mGRE signal without and with CHESS for 1.5 T (top) and 3 T (bottom). In the left panels, the gray lines indicate non-FS mGRE signal for 100, 300, 600, and 1200 1/s. The red and blue lines indicate the simulated signal evolution assuming boxcar and Gaussian CHESS profiles respectively. As a second step, the simulated signals were discretized according to the TEs of the employed mGRE sequence and fitted to a mono-exponential function to calculate pairs of non-FS and FS R2* values (right panels).
Supporting Figure S3. Left panel: Plots of non-FS vs. FS R2* values as retrieved from a formal mathematical description of the CHESS-induced spectral hole burning. Solid lines indicate 1.5 T results, dashed lines indicate 3 T data. Red color represents values assuming a boxcar CHESS profile, blue represents the values in case of a Gaussian CHESS profile. Right panel: In addition to the data shown on the left, non-FS and FS R2* according to our heuristic model (green) are included together with the measured data.
Supporting Figure S4. Measured non-FS (circles) and FS (diamonds) together with simulated signal data at 1.5 T (top row) and 3 T (bottom row). No oscillations as expected from the formal band pass model are visible in the FS data. However, background noise needs to be considered during R2* fitting as can be seen from a constant signal offset in the measured data for longer TEs.
Supporting Figure S5. (a) Non-FS R2*, FS R2*, and R2 measurements in 3 different phantom types (top row: BNF particles, circles; mid row: DSPIO particles, rectangles; bottom row: MnCl2, triangles) at 1.5 T. For R2* quantification, the same 3 fitting models were applied as in patients. R2 quantification was done using fit (ii). (b) Corresponding 3 T results.