Abstract
Objective
Methanogens colonizing the human gut produce methane and influence host metabolism. We examined metabolic parameters in methane-producing subjects before and after antibiotic treatment.
Methods
11 prediabetic methane-positive subjects (9F, 2M) with obesity (BMI 35.17±7.71 kg/m2) aged 47±9 years were recruited. Subjects underwent breath testing, symptom questionnaire, oral glucose tolerance test (OGTT), lipid profile, stool Methanobrevibacter smithii levels, gastric transit and energy utilization analyses. After a 10 day antibiotic therapy (neomycin 500mg bid/rifaximin 550mg tid), all testing was repeated.
Results
Baseline stool M. smithii levels correlated with breath methane (R=0.7, P=0.05). Eight subjects (73%) eradicated breath methane and showed reduced stool M. smithii (P=0.16). After therapy, methane-eradicated subjects showed significant improvements in low-density lipoprotein (LDL) (P=0.028), total cholesterol (P=0.01) and insulin levels on OGTT (P=0.05 at 120 minutes), lower blood glucose levels on OGTT (P=0.054 at 90 minutes), significant reductions in bloating (P=0.018) and straining (P=0.059), and a trend towards lower stool dry weight. No changes were detected in gastric emptying time or energy harvest.
Conclusions
Breath methane eradication and M. smithii reduction are associated with significant improvements in total cholesterol, LDL and insulin levels, and with lower glucose levels, in prediabetic subjects with obesity. The underlying mechanisms require further elucidation.
Keywords: Obesity, prediabetes, methanogens, cholesterol, insulin, antibiotics
Introduction
Diabetes affects 29.1 million Americans, and is a leading cause of blindness, renal failure, heart disease and stroke 1. In addition, an estimated 86 million Americans have prediabetes 1, which is defined by impaired fasting glucose and/or impaired glucose tolerance. While glycemic dysregulation and insulin resistance are common threads in the progression from pre-diabetes to diabetes, there is also an undeniable relationship with obesity.
The human gastrointestinal (GI) tract is host to a diverse and dynamic community of an estimated 1014 microbes, comprising approximately 1000 species that include archaea, bacteria, and eukaryotes 2. These normally function to establish mutually beneficial symbiotic relationships with their hosts 3, contributing to human health through roles including the breakdown of non-digestible foods for absorption, energy harvest and vitamin synthesis, and modulation of the immune system 4. However, disruptions in the normal balance of gut microbial populations contribute to gut-related conditions including inflammatory bowel disease 5, irritable bowel syndrome (IBS) 6, nonalcoholic fatty liver disease 7, diabetes 8 and obesity 9.
One particular group of gut microbes, the methanogenic archaea (methanogens), has been specifically linked to altered metabolism and weight gain 9. These anaerobic organisms utilize hydrogen, formate and other substrates produced by neighboring microbes to generate methane 10–11. In humans, the predominant methanogen is Methanobrevibacter smithii 12, and there is increasing evidence for a specific role for M. smithii and methane production in weight gain and the development of obesity 13–15. Intestinal methane production is also associated with impaired glucose tolerance 16 and may be linked to altered glycemic control in diabetic subjects 17.
In this study, we sought to determine whether using an antibiotic to eradicate M. smithii (as measured by breath methane) results in improvement in the metabolic profile, by examining metabolic parameters in methane-positive, pre-diabetic subjects with obesity before and after antibiotic treatment.
Methods
Study subjects
Subjects were prospectively recruited from the GI Motility Program of a tertiary care medical center, through advertising, by word of mouth and through the study listing on clinicaltrials.gov. Individuals were eligible to participate if they were 18 to 65 years of age, overweight (BMI >25.0 kg/m2) or obese (BMI >30.0 kg/m2), prediabetic (hemoglobin A1c of 5.7%–6.4%), and had a methane-positive breath test. Methane positivity was defined as ≥3 parts per million (ppm), as in our previous studies 14, 16. Exclusion criteria were: 1) diabetes/diabetes medications; 2) prokinetic medication; 3) pregnancy; 4) history of bariatric or gastrointestinal surgery (other than cholecystectomy or appendectomy); 5) unstable thyroid disease; 6) an active weight loss program; 7) smoking; 8) inability to comply; 9) dietary restrictions (lactose intolerance, vegan, etc.); 10) active celiac disease, Crohn’s disease or ulcerative colitis; 11) active use of laxatives, narcotics, antidiarrheals or antisecretory drugs; or 12) antibiotic use in the past month. The study protocol was approved by the Institutional Review Board at Cedars-Sinai Medical Center, and all subjects provided informed written consent prior to participating in the study.
Study procedures
On an initial screening visit, a hemoglobin A1c test was performed to confirm pre-diabetic status, and a physical exam was performed including measurements of height and weight to determine the subject’s current body mass index (BMI). Body composition measurements were obtained using an InBody 520 Body Composition Analyzer (Biospace Co, Ltd, Seoul, Korea). Due to the possibility of ototoxicity associated with the use of neomycin, a baseline audiogram was performed. This was repeated only if subjects reported a subsequent change in hearing. Subjects were also given a three-day food log to complete.
On the next study visit, subjects returned the food log and completed a demographic/medical questionnaire and a bowel symptom questionnaire which rated their last 7 days of intestinal complaints (bloating, diarrhea, constipation, and abdominal pain) on a visual analog scale (VAS) 18. Subjects also provided a stool sample for determination of methanogen levels. Lastly, subjects’ metabolic rates (MR) were calculated using the Mifflin-St Jeor equations 19.
Lactulose breath test
Subjects underwent a 2-hour lactulose breath test as described previously, and subjects with methane ≥3 ppm were considered methane positive 14, 16. Subjects with hydrogen >20 ppm at or before 90 minutes during the test were considered hydrogen positive 15.
Oral glucose tolerance test (OGTT)
All subjects underwent a standard 75 g OGTT with venous sampling for glucose, insulin and free fatty acid (FFA) levels at −15, −5 and 0 minutes, and 5, 15, 30, 60, 90 and 120 minutes post-ingestion. An additional blood sample was drawn at 0 minutes for measurement of lipid levels.
Gastric emptying time evaluation (Smartpill™)
Gastric emptying time was evaluated using the Smartpill™ (Given Imaging Ltd, Yoqneam, Israel). The SmartPill™ Capsule is a single-use, ingestible capsule that measures pressure, pH and temperature from inside the GI tract and wirelessly transmits that information to a Data Receiver worn on a belt by the subject. Recordings were performed over a 5–6 hour period, during which time subjects recorded activities such as eating, passing gas, nausea and cramping/pain in a diary. While whole gut transit evaluation is possible with this method, due to study visit timing and restrictions, gastric emptying time was the only measurement obtained.
Energy utilization study
Energy intake and losses were measured in all subjects while they followed a weight-maintaining diet for 3 consecutive days. Caloric requirements for each individual subject were calculated by the research dietician based on their metabolic rates, Inbody results and the average caloric intake from the 3-day food records. Three identical sets of customized daily meals were then prepared for each subject (subjects consumed the same breakfast, lunch, dinner and snacks each day), and a fourth set was prepared for determination of caloric content by bomb calorimetry. All meals had the same macronutrient composition ranges, 14.6%–15.6% protein, 29.6%–31.1% fat and 54%–55.7% carbohydrate. Meals were prepared at three basal caloric content levels, 1,400, 1,800 and 2,600 calories respectively. These were augmented as necessary with additional unit foods with same macronutrient composition in order to meet individual subjects’ caloric needs and maintain weight during the 3-day study. The diets were designed in collaboration with the Clinical Translational Research Center (CTRC) staff at Harbor-UCLA and were prepared in their metabolic kitchen. Subjects were asked to consume all of the food given at each meal, and where this was not possible, to freeze and return the leftovers so that they could be used for determination of calories not consumed. A non-absorbable blue food dye (FD&C) was given with breakfast on the first day and with dinner of the 3rd day. Subjects were asked to collect and store all stool from the first appearance of dye and up to and including the second appearance of dye, in accordance with a published study 20. Leftovers and stool samples were collected from the subjects and stored at −20°C for bomb calorimetry. Bomb calorimetry was performed per accepted protocol 21 using an IKA C5000 bomb calorimeter (IKA Wilmington, NC). The individual meal components were freeze-dried, ground to homogeneity, and then a sample of the homogenate was weighed and subjected to calorimetry. The caloric content of the weighed sample was calculated, and multiplied by the weight of the entire homogenate to obtain the caloric content of the entire meal component. The caloric contents of the individual meal components were added together and used to confirm the caloric content of the entire meal. Caloric contents of leftovers and stool were calculated in the same way. The % kcal in stool vs. ingested was then used to calculate the average number of calories harvested daily by each subject.
Antibiotic therapy and follow-up testing
After completion of all of the above tests, subjects were given a course of rifaximin 550 mg three times daily and neomycin 500 mg twice daily, for 10 days. This regimen has been shown to eradicate methane on breath testing in up to 85% of subjects 22. After completing the course of antibiotics, subjects were required to repeat all of the above-described tests within 6 weeks. One subject received an exemption from this requirement and completed testing within 76 days.
Quantitative PCR (q-PCR) of methanogens in stool
Stool specimens were tested for the levels of M. smithii and of total bacteria by q-PCR as previously described, using specific primers for M. smithii and total bacteria 23.
Data and statistical analyses
Demographic data were compared by Student’s t-test when data were normally distributed. When comparing before and after data such as BMI, a paired t-test was used. For comparing microbial counts before and after antibiotics, a Wilcoxon Signed Rank test was used.
Results
Subject demographics and study procedures
A total of 11 pre-diabetic methane-positive subjects (9 female, 2 male) aged 47±9 years of age were recruited for this study and completed all study-related procedures and evaluations. All subjects had obesity, with an average BMI of 35.17±7.71 kg/m2 (Table 1).
Table 1.
Subject characteristics before and after antibiotic treatment
| Total Group (n=11) | Methane Eradicated Only (n=8) | |||||
|---|---|---|---|---|---|---|
| Before Antibiotic Therapy | After Antibiotic Therapy | Before Antibiotic Therapy | After Antibiotic Therapy | |||
| Demographics | Mean±StDev | Mean±StDev | P-value | Mean±StDev | Mean±StDev | P-value |
| Age (years) | 47±9 | - | 47±10 | - | ||
| A1c (%) | 5.72±0.60 | - | 5.77±0.73 | - | ||
| Height (in) | 66.09±3.53 | - | 66.4±4.4 | - | ||
| Weight (lbs) | 219.23±50.56 | 218.66±51.77 | 210.8±57.7 | 209.5±58.6 | 0.64 | |
| BMI (kg/m2) | 35.17±7.71 | 34.98±7.92 | 33.4±8.4 | 33.2±8.7 | 0.54 | |
| Bowel Symptoms (VAS) | ||||||
| Bloating | 55.09±34.18 | 39.91±33.27 | 0.14 | 66.8±32.4 | 38.9±36.1 | 0.018 |
| Abdominal pain | 23.36±27.18 | 18.18±29.91 | 0.43 | 28.6±30.3 | 21.6±34,7 | 0.44 |
| Constipation | 30.18±29.75 | 20.55±33.90 | 0.13 | 31.0±30.5 | 22.3±39.6 | 0.25 |
| Diarrhea | 23.73±30.16 | 14.64±31.01 | 0.29 | 30.5±32.9 | 17.3±36.0 | 0.27 |
| Straining | 31.64±29.25 | 21.55±30.19 | 0.22 | 37.5±31.8 | 18.4±34.5 | 0.059 |
| % stool dry weight | 24.1±5.3 | 22.9±4.4 | 0.33 | 25.1±4.7 | 22.6±4.4 | 0.075 |
| Caloric Harvest | ||||||
| % kcal in stool vs. oral ingestion | 8.1±2.7 | 8.3±3.8 | 0.85 | 8.3±1.8 | 8.3±3.8 | 0.97 |
| Gastric Emptying Time (min) | 182.3±72.8 | 163.5±46.8 | 0.45 | 174.9±71.1 | 164.9±45.8 | 0.61 |
StDev = standard deviation; VAS = visual analogue scale.
Breath methane levels correlate with levels of stool methanogens and are reduced following antibiotic therapy
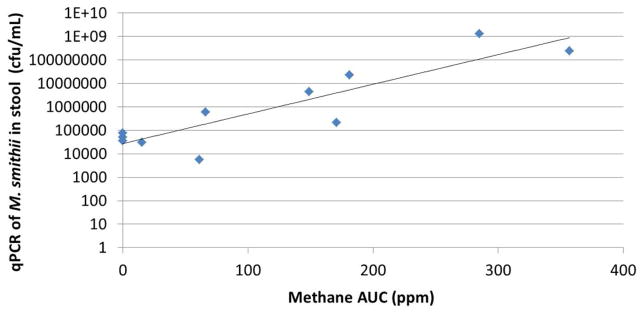
Breath methane levels as determined by lactulose breath test and stool methanogen levels as determined by q-PCR were compared in all subjects before antibiotic therapy. A strong correlation was found between methane area-under-the curve (AUC) and M. smithii levels in stool (R=0.7, P<0.05, Figure 1), confirming breath methane as an acceptable surrogate for intestinal M. smithii levels. Comparisons of breath methane levels before and after antibiotic therapy indicated that breath methane was eradicated in 8 of 11 subjects (73%), which is in accordance with expected eradication rates 22. Eradication of breath methane in these 8 subjects was associated with reductions in stool M. smithii levels, but these did not reach significance (P=0.16) (Table 2).
Figure 1.
Breath methane levels correlate with M. smithii levels determined by stool PCR.
Table 2.
Breath methane and stool methanogen levels before and after antibiotic therapy
| Methane Eradicators (n=8) | Methane Non-Eradicators (n=3) | |||||
|---|---|---|---|---|---|---|
| Pre-Ab | Post-Ab | P-value | Pre-Ab | Post-Ab | P-value | |
| Stool methanogens | 3.6×107 | 1.1×105 | 0.16 | 6.86×108 | 9.4×107 | NS |
| Stool total bacteria | 1.1×109 | 1.2×109 | NS | 3.7×108 | 8.5 ×109 | NS |
Pre-Ab = before antibiotic therapy; Post-Ab = after antibiotic therapy.
P-value is comparing change in methane eradiacators before and after treatment to change in methane non-eradiacators before and after treatment.
NS=not significant.
Effects of methane eradication on bowel symptoms
Subjects completed a bowel symptom questionnaire 18 before and after antibiotic therapy. Of the intestinal complaints assessed (bloating, abdominal pain, constipation, diarrhea, and straining), none were significantly altered in the group as a whole (n=11) (Table 1). However, methane-eradicated subjects (n=8) experienced a significant reduction in bloating (P=0.018), and a reduction in straining that approached significance (P=0.059) (Table 1). There was also a trend to lower stool dry weight (suggesting looser stools) following methane eradication (Table 1).
Eradication of breath methane is associated with improved low-density lipoprotein (LDL) and total cholesterol levels
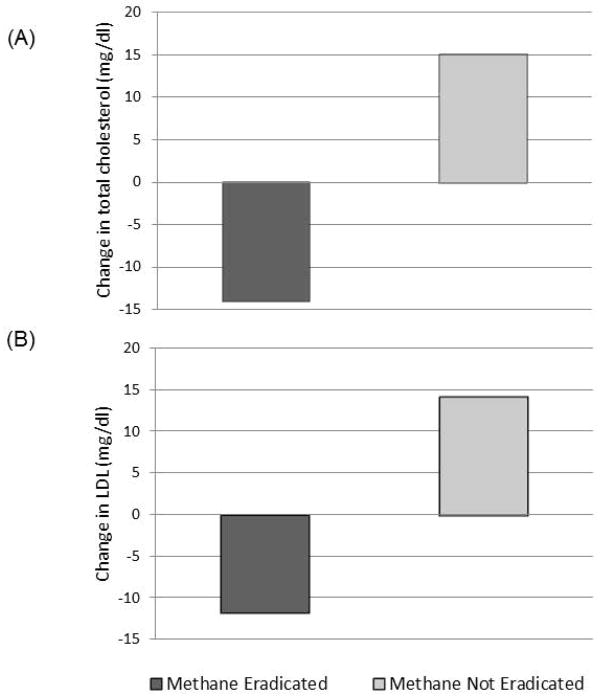
LDL and total cholesterol levels were evaluated in all subjects before and after antibiotic therapy. Methane-eradicated subjects showed significant improvements in both LDL (P=0.028) and total cholesterol (P=0.01) levels compared to non-eradicated subjects (Table 3 and Figure 2).
Table 3.
LDL and total cholesterol levels before and after antibiotic therapy
| Methane Eradicators (n=8) | Methane Non-Eradicators (n=3) | P-value | |||
|---|---|---|---|---|---|
| Pre-Ab | Post-Ab | Pre-Ab | Post-Ab | ||
| Total cholesterol (mg/dl) | 192±51 | 177±52 | 173±41 | 188±49 | 0.01* |
| LDL (mg/dl) | 116±42 | 104±46 | 96±41 | 111±51 | 0.028* |
P-value is comparing change in methane eradiacators before and after treatment to change in methane non-eradiacators before and after treatment.
Figure 2.
Eradication of breath methane is associated with improvements in total cholesterol (A) and LDL (B) levels.
Eradication of breath methane is associated with reduced insulin and glucose levels
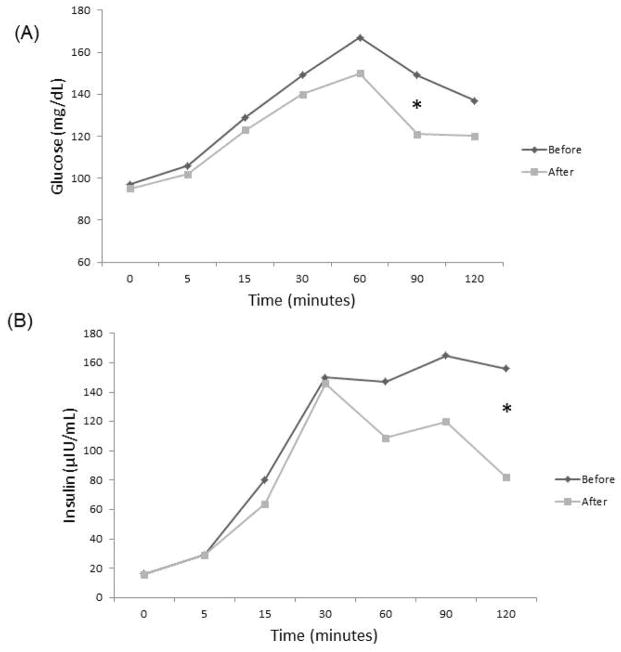
Blood insulin and glucose levels during OGTT were also evaluated in all subjects before and after antibiotic therapy. Following treatment, methane-eradicated subjects exhibited lower blood glucose levels at 60, 90 and 120 minutes (P=0.054 at 90 minutes) (Figure 3a), and also exhibited significantly lower insulin levels at 60, 90 and 120 minutes (P=0.05 at 120 minutes) (Figure 3b).
Figure 3.
Eradication of breath methane is associated with lower blood glucose (A) and insulin (B) levels. * denotes P=0.054 (A) and P=0.05 (B).
Eradication of breath methane did not result in detectable differences in energy utilization
The average number of calories harvested daily by each subject was determined by comparing the number of calories in the meals provided (less those remaining in leftovers) vs. those excreted in stool. This caloric harvest for each subject was then compared before and after antibiotic therapy. There was no apparent change in energy harvest as represented by % kcal loss in stool (Table 1), irrespective of methane eradication status.
Eradication of breath methane does not affect gastric emptying time
Gastric emptying times were determined in all subjects before and after antibiotic therapy. No significant differences in gastric emptying times before and after treatment were identified (182.3±72.8 vs. 163.5±46.8 minutes, respectively) (P=0.45, Table 1). Gastric emptying times in the 8 methane-eradicated subjects also showed no significant reductions (174.9±71.1 vs. 164.9±45.8 minutes, respectively) (P=0.61, Table 1). Intestinal transit times were not measured.
Side Effects of Antibiotic Therapy
There were no significant lasting side effects from the antibiotic therapy. Specifically, no subjects complained of hearing changes, and no cases of Clostridium difficile or antibiotic intolerance requiring discontinuation were reported (data not shown).
Discussion
The results of this study suggest that eradication of methane on breath test in a results in significant improvements in total cholesterol (P=0.01) and LDL (P=0.028) levels, as well as decreased insulin and glucose levels during OGTT, in methane-positive prediabetic subjects with obesity. We found that breath methane is a reliable surrogate for gut colonization with M. smithii, the predominant methanogen in the human gut 12, and that eradication of methane on breath test correlates strongly with reduction of M. smithii levels in stool. Further, methane-eradicated subjects showed significant improvements in gastrointestinal bloating (P=0.018) and reductions in straining and stool % dry weight that approached significance (P=0.059 and P=0.075, respectively). No significant effects on gastric emptying times or energy utilization were detected. This is the first study to suggest that reductions in a specific gut microbial population (methanogens), can have beneficial effects on host metabolism in human subjects, and to indicate that antibiotic therapy can be used to reduce methanogen levels and achieve clinically significant results in pre-diabetic subjects with obesity.
There is a growing body of literature suggesting a role for methanogenic archaea in host metabolism and weight gain (reviewed in 24). In a germ-free mouse model, introduction of a single Bacteroides species (B. thetaiotaomicron) and M. smithii resulted in greater weight gain than the introduction of B. thetaiotaomicron alone 13, and the cecal flora of Ob/Ob mice exhibit increased methanogen levels 9. Our group has previously shown that Sprague-Dawley rats, which are a model of obesity when fed a high-fat diet, exhibit high levels of endogenous colonization with M. smithii 23. We maintained these rats on high-fat vs. regular chow over different time periods, and found that rats fed high-fat chow had higher M. smithii levels in the duodenum, ileum, and cecum than those fed normal chow 23. Further, rats which gained most weight were those with the most bowel segments colonized with M. smithii, irrespective of whether or not they were on high-fat chow 23. An important aspect of this study was that it demonstrated M. smithii colonization throught the small intestine, a finding we later confirmed in human duodenal samples 25. This was significant as M. smithii had previously been thought to only colonize the large intestine, weakening the likelihood that it could play a significant role in caloric harvest and weight gain.
In human studies, we showed that methane-producing individuals with obesity had a 6.8 kg/m2 greater BMI than non-methane producing controls with obesity 14, and subsequently demonstrated that methane and hydrogen on breath test was associated with both greater BMI and higher percent body fat in a general population cohort (N=792 subjects) 15. We have also shown that methane-producing individuals may have impaired glucose tolerance when challenged with a high carbohydrate load, as well as a higher susceptibility to hyperglycemia 16. These data support our current findings that eradication of methanogens results in improved glucose and insulin levels, which are also supported by a recent independent study suggesting a role for intestinal methane production differences in altered glycemic control in diabetic subjects 17. However, that M. smithii may play a role in obesity is not without controversy – Raoult and co-authors found that M. smithii levels were not significantly different in lean subjects vs. subjects with obesity in one study 26, and found negative correlations between M. smithii and BMI in other studies 27. In contrast, Zhang et al. found increased levels of Methanobacteriales, the order which includes M. smithii, in subjects with obesity, and decreased levels of Methanobacteriales in individuals post-bariatric surgery 28. Patil et al. also found significantly increased archaeal density in subjects with obesity, with significant reductions following bariatric surgery 29. These disparities may result from differences between study populations from different geographical regions with different dietary influences, and may also reflect differences in techniques. We note that Patil and co-workers used the same isolation technique used in our laboratory, whereas the Raoult group used a significantly different technique.
Various mechanisms have been proposed to explain how intestinal microbes affect host metabolism and weight gain 30–33 (reviewed in 24). In addition to these, our group has shown that methane gas may also directly affect intestinal transit and gut neuromuscular function 34, findings confirmed by an independent group 35. Reduced gut motility could also result in increased time for nutrient absorption, further enhancing energy harvest. Subjects who eradicated methane in this study reported a significant reduction in gastrointestinal bloating, which is consistent with eradication of methanogens and reduction in methane gas production, although no immediate effects on energy harvest were detected. Methane-eradicated subjects also showed a reduction in straining and a reduction in % stool dry weight which, although they did not reach significance, are consistent with the potential effects of eradication of methanogens, which are associated with constipation 36. No differences were detected in gastric transit, and intestinal transit was not measured in this study. Lastly, alterations in the systemic availability of SCFAs acetate and propionate have been suggested to influence blood lipid levels 37, which may support our finding of improved total cholesterol and LDL levels following eradication of methanogens.
The limitations of this study include the small sample size, the short duration of the study, and the potential confounding effects of the antibiotics on other gut microbial populations. Although small, our population size was sufficient to detect statistically significant differences in total cholesterol and LDL levels pre- vs. post-treatment in methane-eradicated subjects as compared to non-eradicated subjects. These occurred in the absence of any dietary restrictions or weight change during the study period. Although the number of non-eradicated subjects was small, this group represents the non-specific effects of the antibiotics used on other gut microbial populations. Subtherapeutic antibiotic treatment (STAT), which has been used in the agricultural industry since the 1940s, actually promotes weight gain in a number of commercial species 38, and studies in mice have confirmed these effects 38,24. In contrast, treating infant mice fed with higher (therapeutic) doses of the antibiotics ampicillin and neomycin resulted in reductions in inflammatory makers, visceral adipose tissue and high-fat diet-induced adipocyte hypertrophy comapred to untreated controls 31,24. Therefore, antibiotic dose, time and duration of treatment and the specific antibiotic(s) used appear to influence effects on host weight gain and body fat accumulation. A larger study with greater numbers of non-eradicating subjects will be needed to determine the non-specific effects of these antibiotics on other gut microbes, and to determine if this is the reason LDL and total cholesterol levels appear to actually increase in non-eradicated subjects. A longer study duration would be required to fully address whether eradication of methanogens can result in weight loss. We also note that 3 ppm is the limit of detection of breath methane using the Quintron SC gas chromatograph (Quintron Instrument Company), which we found in another study to correlate with stool M. smithii levels of 106. Individuals with lower levels of stool M. smithii undoubtedly produce some breath methane, but this is not currently detectable. This point was recently illustrated by Di Stefano et al, who found that many IBS subjects without detectable methane on the breath had detectable levels of colonic methane 39. Lastly, we did not detect any significant difference in energy utilization during this study, which again may be due in part to the small sample size and study duration.
In summary, this study demonstrates for the first time that eradication of methane on breath test via antibiotic therapy results in significant improvements in total cholesterol, LDL, insulin and glucose levels in methane-positive prediabetic subjects with obesity. These findings suggest that targeting a specific gut microbial population can have clinical benefits in this at-risk population.
What is already known about this subject?
Gut microbes, including methanogens, influence host metabolism and weight gain
Methanobrevibacter smithii is the predominant methanogen in the human gut, and has been linked to weight gain in animal models and human studies
Increased breath methane has been linked to obesity in human subjects, as well as impaired glucose tolerance
What does your study add?
Eradication of breath methane in prediabetic subjects with obesity results in significant improvements in total cholesterol andd LDL levels, as well as decreased insulin and glucose during OGTT
Subjects who eradicated breath methane also showed significant improvements in gastrointestinal bloating as well as reductions in straining and stool % dry weight
Our results suggest that reductions in a specific gut microbial population can have beneficial effects on host metabolism in human subjects
Acknowledgments
Funding: This study was supported by an Innovation Award from the American Diabetes Association to Dr Ruchi Mathur (1-12-IN-28). The Cedars-Sinai CTRC is supported by the National Center for Research Resources, Grant UL1RR033176, and is now at the National Center for Advancing Translational Sciences, Grant UL1TR000124. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
We thank the staff of the Wildlife Habitat/Nutrition Laboratory at Washington State University (Pullman, WA) for performing the bomb calorimetry. We also thank the staff of the CTRC at Cedars-Sinai for their support and assistance in the performance of this study. The CTRC is supported by the National Center for Research Resources, Grant UL1RR033176, and is now at the National Center for Advancing Translational Sciences, Grant UL1TR000124.
Footnotes
Disclosure: Cedars-Sinai has a licensing agreement with Salix Pharmaceuticals. Dr. Mark Pimentel is a consultant for Salix Pharmaceuticals, and is on the advisory board for Salix Pharmaceuticals. The remaining authors have no financial conflicts of interest to disclose.
Author Contributions: RM designed the study, performed clinical studies, analyzed the data and wrote the manuscript. KSC recruited subjects, assisted with clinical studies and compiled data. WM performed laboratory tests and did the initial data analysis. MM calculated the metabolic rates, designed the diets, monitored subject compliance and contributed to the manuscript. RT and ZM performed additional laboratory testing. DS and RB performed additional data analysis and contributed to and edited the manuscript. GB assisted with troubleshooting experiments and wrote and reviewed the manuscript. SW supervised the laboratory testing. MP analyzed the data and wrote and edited the manuscript.
References
- 1.Centers for Disease Control and Prevention. National Diabetes Statistics Report: Estimates of Diabetes and Its Burden in the United States, 2014. Atlanta, GA: 2014. [Google Scholar]
- 2.The Gut Microbiota. Science. 2012:336. special issue. [Google Scholar]
- 3.Backhed F, Ley RE, Sonnenburg JL, Peterson DA, Gordon JI. Host-bacterial mutualism in the human intestine. Science. 2005;307:1915–20. doi: 10.1126/science.1104816. [DOI] [PubMed] [Google Scholar]
- 4.Littman DR, Pamer EG. Role of the commensal microbiota in normal and pathogenic host immune responses. Cell Host Microbe. 2011;10:311–23. doi: 10.1016/j.chom.2011.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sokol H, Pigneur B, Watterlot L, et al. Faecalibacterium prausnitzii is an anti-inflammatory commensal bacterium identified by gut microbiota analysis of Crohn disease patients. Proc Natl Acad Sci U S A. 2008;105:16731–6. doi: 10.1073/pnas.0804812105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kerckhoffs AP, Samsom M, van der Rest ME, et al. Lower Bifidobacteria counts in both duodenal mucosa-associated and fecal microbiota in irritable bowel syndrome patients. World J Gastroenterol. 2009;15:2887–92. doi: 10.3748/wjg.15.2887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mouzaki M, Comelli EM, Arendt BM, et al. Intestinal microbiota in patients with nonalcoholic fatty liver disease. Hepatology. 2013;58:120–7. doi: 10.1002/hep.26319. [DOI] [PubMed] [Google Scholar]
- 8.Musso G, Gambino R, Cassader M. Interactions between gut microbiota and host metabolism predisposing to obesity and diabetes. Annu Rev Med. 2011;62:361–80. doi: 10.1146/annurev-med-012510-175505. [DOI] [PubMed] [Google Scholar]
- 9.Turnbaugh PJ, Ley RE, Mahowald MA, Magrini V, Mardis ER, Gordon JI. An obesity-associated gut microbiome with increased capacity for energy harvest. Nature. 2006;444:1027–31. doi: 10.1038/nature05414. [DOI] [PubMed] [Google Scholar]
- 10.McKay LF, Holbrook WP, Eastwood MA. Methane and hydrogen production by human intestinal anaerobic bacteria. Acta Pathol Microbiol Immunol Scand B. 1982;90:257–60. doi: 10.1111/j.1699-0463.1982.tb00114.x. [DOI] [PubMed] [Google Scholar]
- 11.Gibson GR, Cummings JH, Macfarlane GT, et al. Alternative pathways for hydrogen disposal during fermentation in the human colon. Gut. 1990;31:679–83. doi: 10.1136/gut.31.6.679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Weaver GA, Krause JA, Miller TL, Wolin MJ. Incidence of methanogenic bacteria in a sigmoidoscopy population: an association of methanogenic bacteria and diverticulosis. Gut. 1986;27:698–704. doi: 10.1136/gut.27.6.698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Samuel BS, Gordon JI. A humanized gnotobiotic mouse model of host-archaeal-bacterial mutualism. Proc Natl Acad Sci U S A. 2006;103:10011–6. doi: 10.1073/pnas.0602187103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Basseri RJ, Basseri B, Pimentel M, et al. Intestinal methane production in obese individuals is associated with a higher body mass index. Gastroenterol Hepatol. 2012;8:22–8. [PMC free article] [PubMed] [Google Scholar]
- 15.Mathur R, Amichai M, Chua KS, Mirocha J, Barlow GM, Pimentel M. Methane and hydrogen positivity on breath test is associated with greater body mass index and body fat. J Clin Endocrinol Metab. 2013;98:E698–702. doi: 10.1210/jc.2012-3144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mathur R, Goyal D, Kim G, Barlow GM, Chua KS, Pimentel M. Methane-producing human subjects have higher serum glucose levels during oral glucose challenge than non-methane producers: a pilot study of the effects of enteric methanogens on glycemic regulation. Res J Endocrinol Metab. 2014;2 [Google Scholar]
- 17.Cesario V, Di Rienzo TA, Pitocco D, Campanale M, Barbaro F, D’Angelo G, et al. Methane intestinal production and poor metabolic control in type I diabetes complicated by autonomic neuropathy. Minerva Endocrinol. 2014;39(3):201–7. [PubMed] [Google Scholar]
- 18.Wewers ME, Lowe NK. A critical review of visual analogue scales in the measurement of clinical phenomena. Res Nurs Health. 1990;13:227–36. doi: 10.1002/nur.4770130405. [DOI] [PubMed] [Google Scholar]
- 19.Mifflin MD, St Jeor ST, Hill LA, Scott BJ, Daugherty SA, Koh YO. A new predictive equation for resting energy expenditure in healthy individuals. Am J Clin Nutr. 1990;51:241–7. doi: 10.1093/ajcn/51.2.241. [DOI] [PubMed] [Google Scholar]
- 20.Jumpertz R, Le DS, Turnbaugh PJ, et al. Energy-balance studies reveal associations between gut microbes, caloric load, and nutrient absorption in humans. Am J Clin Nutr. 2011;94:58–65. doi: 10.3945/ajcn.110.010132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wu GD, Lewis JD, Hoffmann C, et al. Sampling and pyrosequencing methods for characterizing bacterial communities in the human gut using 16S sequence tags. BMC Microbiol. 2010;10:206. doi: 10.1186/1471-2180-10-206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Low K, Hwang L, Hua J, Zhu A, Morales W, Pimentel M. A combination of rifaximin and neomycin is most effective in treating irritable bowel syndrome patients with methane on lactulose breath test. J Clin Gastroenterol. 2010;44:547–50. doi: 10.1097/MCG.0b013e3181c64c90. [DOI] [PubMed] [Google Scholar]
- 23.Mathur R, Kim G, Morales W, et al. Intestinal Methanobrevibacter smithii but not total bacteria is related to diet-induced weight gain in rats. Obesity (Silver Spring) 2013;21:748–54. doi: 10.1002/oby.20277. [DOI] [PubMed] [Google Scholar]
- 24.Mathur R, Barlow GM. Obesity and the microbiome. Expert Rev Gastroenterol Hepatol. 2015;9:1087–99. doi: 10.1586/17474124.2015.1051029. [DOI] [PubMed] [Google Scholar]
- 25.Kim G, Giamarellos-Bourboulis EJ, Chang C, Pyleris E, Pistiki K, Pimentel M. Quantitation of Bacteria in Duodenal Aspirates by qPCR Appears to Identify Viable Organisms in IBS. Gastroenterology. 2013;144:S-908. [Google Scholar]
- 26.Armougom F, Henry M, Vialettes B, Raccah D, Raoult D. Monitoring bacterial community of human gut microbiota reveals an increase in Lactobacillus in obese patients and Methanogens in anorexic patients. PLoS One. 2009;4:e7125. doi: 10.1371/journal.pone.0007125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Million M, Angelakis E, Maraninchi M, et al. Correlation between body mass index and gut concentrations of Lactobacillus reuteri, Bifidobacterium animalis, Methanobrevibacter smithii and Escherichia coli. Int J Obes (Lond) 2013;37:1460–6. doi: 10.1038/ijo.2013.20. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 28.Zhang H, DiBaise JK, Zuccolo A, et al. Human gut microbiota in obesity and after gastric bypass. Proc Natl Acad Sci U S A. 2009;106:2365–70. doi: 10.1073/pnas.0812600106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Patil DP, Dhotre DP, Chavan SG, et al. Molecular analysis of gut microbiota in obesity among Indian individuals. J Biosci. 2012;37:647–57. doi: 10.1007/s12038-012-9244-0. [DOI] [PubMed] [Google Scholar]
- 30.DiBaise JK, Zhang H, Crowell MD, Krajmalnik-Brown R, Decker GA, Rittmann BE. Gut microbiota and its possible relationship with obesity. Mayo Clin Proc. 2008;83:460–9. doi: 10.4065/83.4.460. [DOI] [PubMed] [Google Scholar]
- 31.Cani PD, Bibiloni R, Knauf C, et al. Changes in gut microbiota control metabolic endotoxemia-induced inflammation in high-fat diet-induced obesity and diabetes in mice. Diabetes. 2008;57:1470–81. doi: 10.2337/db07-1403. [DOI] [PubMed] [Google Scholar]
- 32.Samuel BS, Shaito A, Motoike T, et al. Effects of the gut microbiota on host adiposity are modulated by the short-chain fatty-acid binding G protein-coupled receptor, Gpr41. Proc Natl Acad Sci USA. 2008;105:16767–72. doi: 10.1073/pnas.0808567105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Samuel BS, Hansen EE, Manchester JK, et al. Genomic and metabolic adaptations of Methanobrevibacter smithii to the human gut. Proc Natl Acad Sci USA. 2007;104:10643–8. doi: 10.1073/pnas.0704189104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pimentel M, Lin HC, Enayati P, et al. Methane, a gas produced by enteric bacteria, slows intestinal transit and augments small intestinal contractile activity. Am J Physiol Gastrointest Liver Physiol. 2006;290:G1089–95. doi: 10.1152/ajpgi.00574.2004. [DOI] [PubMed] [Google Scholar]
- 35.Jahng J, Jung IS, Choi EJ, Conklin JL, Park H. The effects of methane and hydrogen gases produced by enteric bacteria on ileal motility and colonic transit time. Neurogastroenterol Motil. 2012;24:185–90. e92. doi: 10.1111/j.1365-2982.2011.01819.x. [DOI] [PubMed] [Google Scholar]
- 36.Kunkel D, Basseri RJ, Makhani MD, Chong K, Chang C, Pimentel M. Methane on breath testing is associated with constipation: a systematic review and meta-analysis. Dig Dis Sci. 2011;56:1612–8. doi: 10.1007/s10620-011-1590-5. [DOI] [PubMed] [Google Scholar]
- 37.Wolever TM, Fernandes J, Rao VA, Chiasson JL, Josse RG, Leiter LA. Positive methane-producing status associated with increased serum cholesterol in subjects with impaired glucose tolerance. Diabetes Care. 1995;18:1010–2. doi: 10.2337/diacare.18.7.1010. [DOI] [PubMed] [Google Scholar]
- 38.Blaser MJ. Disappearing microbiota and human metabolic health. 2011. In: Finlay B, editor. Microbiota: Agents for Health and Disease. Henry Stewart Talks Ltd; London: 2011. [Google Scholar]
- 39.Di Stefano M, Mengoli C, Bergonzi M, et al. Breath Methane Excretion Is not An Accurate Marker of Colonic Methane Production in Irritable Bowel Syndrome. The Am J Gastroenterol. 2015;110:891–8. doi: 10.1038/ajg.2015.47. [DOI] [PubMed] [Google Scholar]