Abstract
Objective
The effectiveness of annual diabetic eye exams in children is unclear. We sought to determine the prevalence and onset of ocular pathology in children with diabetes mellitus (DM), identify risk factors for ocular disease, and recommend a screening regimen for asymptomatic children.
Design
Retrospective consecutive cohort study.
Subjects
Children less than age 18 years with type 1 or 2 DM examined over a 4 year period.
Methods
All children underwent a complete eye exam, including dilated fundoscopy and cycloplegic refraction. A literature review was performed, identifying the youngest reported age and shortest reported duration of DM prior to the diagnosis of diabetic retinopathy.
Main outcome measures
Prevalence of diabetic retinopathy, cataract, high refractive error, and strabismus.
Results
370 children (mean age 11.2 years, range 1–17.5) had 693 examinations, with mean DM duration 5.2 years (range 0.1–16.2), mean HbA1c 8.6 (range 5 to ≥14). No children had diabetic retinopathy. 12 had cataract; 5 required extraction but were identified by decreased vision, not diabetic screening. 19 had strabismus; only one was microvascular paralytic strabismus. 41 had high refractive error. There were no associations between these conditions and duration or control of DM. In the literature, the youngest age at diagnosis of severe diabetic retinopathy was 15 years and the shortest duration of disease was 5 years.
Conclusion
Diabetic retinopathy is rare in children regardless of duration and control of DM. Based upon our study and literature review, screening examinations for type 1 diabetics could begin at age 15 years or at 5 years after the diagnosis of DM, whichever occurs later, unless the child is judged by the endocrinologist as being at unusally high risk. Other ocular complications are identifiable through existing amblyopia screening methods.
Diabetes mellitus (DM) is a well known cause of multiple ophthalmic problems in adults, including diabetic retinopathy (DR), macular edema, cataract, refractive change, and microvascular paralytic strabismus. Diabetic retinopathy and macular edema progress to the ultimate ocular complication of blindness in 12,000 to 24,000 new patients each year in the United States, making DM the leading cause of blindness in American adults aged 20 to 74 years.1 The Early Treatment of Diabetic Retinopathy Study (ETDRS) and the Diabetic Retinopathy Study (DRS), demonstrated that early recognition and treatment of diabetic macular edema and proliferative diabetic retinopathy in patients with DM reduced the risk of moderate and severe vision loss.2, 3 Therefore, there has been a fervent public health effort to establish ophthalmic screening regimens for those with DM, beginning at an early age. For a screening program to be worthwhile, it must identify a disease that is asymptomatic and has a cost-effective treatment, conditions that generally are met by diabetic retinopathy.
Current guidelines by the American Academy of Ophthalmology (AAO) encourage annual screening examinations for all patients with Type 1 DM to begin 5 years after diagnosis of DM.4 However, the age at diagnosis and prevalence of DR in children is not well established, with varied reports in the literature, and there is a paucity of information about the onset and prevalence of other diabetic ocular complications in children, as the majority of studies have focused on DR. Some data are available with regards to modifiable risk factors to prevent the development of ophthalmic complications of DM, but not particularly in the very young. Findings from the Diabetes Control and Complications Trial (DCCT) demonstrated that intensive glucose control in children aged 13 to 17 years of age with type 1 DM reduced the risk of development of DR by 53%.5, 6 The risk of DR appears to increase with increased duration of DM 7–9, but one study of DM in young children suggested that development of DM type 1 at a very young age (i.e., < 5 years) might protect against the development of DR.10 Even less is known about DR risk and incidence in children with type 2 DM, which is an increasingly important population to study given the growing prevalence of children with this disorder.
In light of our limited knowledge of the age at onset and prevalence of these ocular complications, the clinical effectiveness of annual diabetic eye exams in children is unclear. We sought to determine the prevalence and onset of ocular pathology in children with DM, including DR, cataract, high refractive error and strabismus. We also sought to identify potential risk factors for ocular disease and to recommend an updated ophthalmic screening regimen for asymptomatic children with DM based upon our study results and a review of the literature.
METHODS
We conducted a retrospective consecutive cohort study of children aged less than 18 years with type 1 or 2 DM, who underwent one or more complete dilated eye examinations at our institution over a 4 year period between 2009 and 2013. The study protocol was approved by the Institutional Review Board of The Children’s Hospital of Philadelphia, conformed to the requirements of the United States Health Insurance Portability and Privacy Act, and adhered to the tenets of the Declaration of Helsinki.
A search of the electronic medical record was performed to identify all children examined in the outpatient ophthalmology clinic meeting the inclusion criteria. Data collected included gender, race, ethnicity, the child’s age at each eye examination, age at diagnosis of DM, the presence and severity of DR, the presence and severity of cataract, refractive error, the presence and type of strabismus, and serum hemoglobin A1c (HbA1c) levels. Duration of DM was calculated based upon the age at diagnosis of DM and age at time of the eye examination. The HbA1c recorded was the most recent HbA1c measurement obtained prior to each particular visit date. If multiple ophthalmology examinations were available for one patient, an average (± standard deviation) of each prior HbA1c measurement was calculated.
Retinopathy screening was conducted with dilated fundoscopic examinations performed by pediatric ophthalmologists. Refractive errors were obtained by cycloplegic refractions and were classified by the amount of spherical equivalence (in diopters, D) into the following categories: high myopia (4.0 D of myopia or greater), myopia (0.25 D to 3.75 D of myopia), hyperopia (plano to 2.75 D of hyperopia), and high hyperopia (3.0 D of hyperopia or greater). High astigmatism was defined as 1.5 D or greater cylindrical refractive error. “High refractive error” was a composite variable defined as the presence of one or more of high myopia, high hyperopia, and high astigmatism.
Statistical analysis
Descriptive statistics (mean, standard deviation, median, minimum, and maximum) were calculated for baseline characteristics of the subjects. The primary outcomes were the prevalence with 95% confidence interval of diabetic retinopathy, cataract, strabismus, and high refractive error. Potential risk factors were categorized for analysis according to threshold values suggested in the literature. These factors included age at DM diagnosis (≤5 yrs, >5 to <10 yrs, ≥10 yrs) 11–13, DM duration (≤5 yrs, >5 to <10 yrs, ≥10 yrs), and mean HbA1c (≤7.5, >7.5 to <10, ≥10).14 The associations between ocular complications and age at DM diagnosis, duration of DM, and HbA1c, were analyzed in univariate analyses. Fisher’s exact test was used to assess for statistical significance, which was defined as a P value less than 0.05. Kaplan-Meier survival curves were plotted to demonstrate the time to development of each ocular complication from the diagnosis of DM. All statistical analyses were performed using SAS for Windows v9.4 (SAS, Inc., Cary, NC).
Literature search
Studies reporting the occurrence of diabetic retinopathy and other ocular complications in children with DM were identified through the following methods. A Pubmed search was performed for various combinations of the following terms: diabetes mellitus, diabetic retinopathy, proliferative diabetic retinopathy, children, pediatric, complications, cataract, strabismus, cranial neuropathy, cranial nerve palsy, screening, and guidelines. The American Academy of Pediatrics and American Academy of Ophthalmology guidelines were reviewed as well. The reference sections of relevant papers and guidelines were searched to identify additional potential studies.
RESULTS
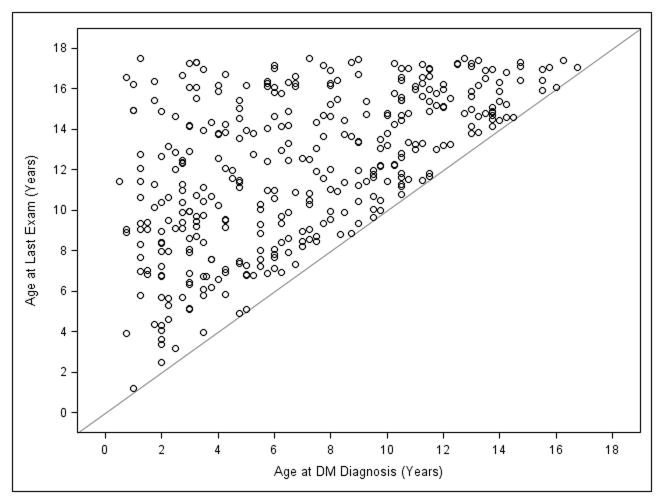
Three hundred seventy children underwent 693 diabetic screening examinations during the study period. Baseline characteristics of these subjects appear in Table 1. Three hundred thirty eight children had Type 1 DM, and 32 children had Type 2 DM. Mean age at first eye exam was 11.2 (SD 3.7) years, ranging from 1 to 17.5 years. Mean age at DM diagnosis was 7.0 (SD 4.1) years, ranging from 0.5 to 16.8 years. Mean DM duration at the time of examination was 5.2 (SD 3.7) years, ranging from 0.1 to 16.2 years, with mean HbA1c of 8.6 (SD 1.9), ranging from 5 to ≥14 (values greater than 14 were reported by the lab as “≥14” only). The ages at diagnosis of DM and the ages at last examination for each child appear in Figure 1.
Table 1.
Baseline characteristics of study population
| Diabetes | |||
|---|---|---|---|
|
|
|||
| Characteristic | Type 1 (n=338) | Type 2 (n=32) | Overall (n=370) |
| Gender – n (%) | |||
| Male | 183 (54) | 12 (38) | 195 (53) |
| Female | 155 (46) | 20 (62) | 175 (47) |
|
| |||
| Ethnicity – n (%) | |||
| Hispanic | 24 (07) | 0 (00) | 24 (07) |
| Non-Hispanic | 297 (88) | 29 (91) | 326 (88) |
| Unknown | 17 (05) | 3 (09) | 20 (05) |
|
| |||
| Race – n (%) | |||
| Asian | 14 (04) | 1 (03) | 15 (04) |
| African American | 75 (22) | 27 (84) | 102 (28) |
| White | 225 (67) | 4 (13) | 229 (62) |
| More than One Reported | 1 (00) | 0 (00) | 1 (00) |
| Unknown | 23 (07) | 0 (00) | 23 (06) |
|
| |||
| Age at Diabetes Onset1 | |||
| Mean ± SD | 6.6 ± 4.0 | 11.8 ± 2.7 | 7.0 ± 4.1 |
| Median (min, max) | 6.0 (0.5, 16.8) | 11.9 (5.8, 16.0) | 6.5 (0.5, 16.8) |
| ≤5 | 144 (43) | 0 (00) | 144 (39) |
| >5 to <10 | 108 (32) | 5 (16) | 113 (31) |
| ≥10 | 77 (23) | 27 (84) | 104 (28) |
| Missing | 9 (03) | 0 (00) | 9 (02) |
|
| |||
| Duration of Diabetes at Last Visit2 | |||
| Mean ± SD | 5.4 ± 3.7 | 2.8 ± 2.3 | 5.2 ± 3.7 |
| Median (min, max) | 4.9 (0.1, 16.2) | 2.0 (0.1, 10.1) | 4.5 (0.1, 16.2) |
| ≤5 | 168 (50) | 27 (84) | 195 (53) |
| >5 to <10 | 111 (33) | 2 (06) | 113 (31) |
| ≥10 | 41 (12) | 1 (03) | 42 (11) |
| Missing | 18 (05) | 2 (06) | 20 (05) |
|
| |||
| Age at First Exam (yrs) | |||
| Mean ± SD | 10.9 ± 3.7 | 14.1 ± 2.2 | 11.2 ± 3.7 |
| Median (min, max) | 11.0 (0.66, 17.5) | 14.4 (7.7, 17.2) | 11.4 (0.66, 17.5) |
| ≤ 10 | 143 (42) | 2 (06) | 145 (39) |
| >10 to <15 | 133 (39) | 17 (53) | 150 (41) |
| ≥15 | 53 (16) | 11 (34) | 64 (17) |
| Missing | 9 (03) | 2 (06) | 11 (03) |
|
| |||
| Age at Last Exam (yrs) | |||
| Mean ± SD | 11.9 ± 3.8 | 14.5 ± 2.1 | 12.1 ± 3.7 |
| Median (min, max) | 12.0 (1.2, 17.5) | 14.9 (7.7, 17.2) | 12.5 (1.2, 17.5) |
| ≤ 10 | 112 (33) | 1 (03) | 113 (31) |
| >10 to <15 | 131 (39) | 15 (47) | 146 (39) |
| ≥15 | 86 (25) | 14 (44) | 100 (27) |
| Missing | 9 (03) | 2 (06) | 11 (03) |
|
| |||
| Average HgbA1c During Study Period | |||
| Mean ± SD | 8.6 ± 1.8 | 8.9 ±2.9 | 8.6 ± 1.9 |
| Median (min, max) | 8.2 (5.0, 14.0) | 8.2 (5.5, 14.0) | 8.2 (5.0, 14.0) |
| ≤7.5 | 98 (29) | 14 (44) | 112 (30) |
| >7.5 to <10 | 164 (49) | 3 (09) | 167 (45) |
| ≥10 | 62 (18) | 14 (44) | 76 (21) |
| Missing | 14 (04) | 1 (03) | 15 (04) |
n=361: 9 Subjects Missing Age of Diabetes Onset.
n=350: 11 Subjects Missing With No Eye Exam Information + 9 Subjects Missing Age of Onset.
Figure 1.
Age at last examination of all 370 children with diabetes mellitus in reference to age at diagnosis of diabetes mellitus. DM, diabetes mellitus.
The prevalence of ocular complications appear in table 2. No children were found to have diabetic retinopathy in any of the 693 examinations (0%, 95% CI 0–1%; Table 2). Eighteen eyes of 12 children (3.3%, 95% CI 1.5 to 5.1) were found to have cataracts (Table 3). The average age at cataract diagnosis was 13.6 years, at a mean 5.3 years after DM diagnosis. The youngest age at cataract diagnosis in our study was 7.5 years, 4.5 years after DM diagnosis. No associations were found between cataract formation and age at diagnosis, duration, or control (as indicated by HbA1c) of DM (Table 4). Not all of these cataracts were thought to be attributable to complications of DM (Table 3). Specifically, one child with a unilateral cataract had ipsilateral CMV retinitis, which was the presumed cataract etiology. Another child with bilateral cataracts had received whole body irradiation for metastatic neuroblastoma several years prior to his cataract diagnosis, and the radiation may have been the more causative element. Nine eyes of five children required cataract extraction. In all five cases, the diagnosis of cataract was made after the children presented with symptoms of decreased vision; they were not diagnosed at the time of routine diabetic eye screening.
Table 2.
Prevalence of ocular complications by type of diabetes mellitus
| Diabetes | |||
|---|---|---|---|
|
|
|||
| Ocular Complication | Type 1 (n=338) | Type 2 (n=32) | Overall (N=370) |
| Retinopathy – n (%, (95% CI))1 | |||
| 0 (0.0, (0.0,1.1)) | 0 (0.0, (0.0,10.7)) | 0 (0.0, (0.0,1.0)) | |
|
| |||
| Cataract – n (%, (95% CI))1 | |||
| 10 (3.0, (1.1, 4.8)) | 2 (6.3, (0.0, 14.6)) | 12 (3.3, (1.5, 5.1)) | |
|
| |||
| Strabismus – n (%, (95% CI))1 | |||
| 16 (4.8, (2.5, 7.0)) | 3 (9.4, (0.0, 19.5)) | 19 (5.2, (2.9, 7.4)) | |
|
| |||
| Refractive Error at Last Visit2 | |||
| (Min, max) | (−8.00, 7.00) | (−4.00, 4.25) | (−8.00, 7.00) |
| Refractive Error Categories – n (%, (95% CI))2 | |||
| High myopia: ≤ −4.0 D | 8 (02.7, (01.0, 05.0)) | 1 (03.7, (00.0, 10.8)) | 9 (02.8, (01.0, 05.0)) |
| Myopia: > − 4.0 D to ≤ −0.25 D | 110 (37.4, (31.8, 43.0)) | 14 (51.9, (33.0, 70.7)) | 124 (38.6, (33.3, 44.0)) |
| Hyperopia: >−0.25 to < 3.0 D | 169 (57.5, (51.8, 63.1)) | 10 (37.0, (18.8, 55.3)) | 179 (55.8, (50.3, 61.2)) |
| High hyperopia: ≥ 3.0 D | 7 (02.4, (01.0, 04.0)) | 2 (07.4, (00.0, 17.3)) | 9 (02.8, (01.0, 05.0)) |
|
| |||
| Astigmatism: ≥ 1.5 D in Either Eye 2,3 | |||
| n (%, (95% CI)) | 19 (06.5, (03.7, 09.3)) | 4 (14.8, (01.4, 28.2)) | 23 (07.2, (04.3, 10.0)) |
n=368: 2 subjects missing outcome information.
n=321: 49 subjects with no refractive error measurements.
Absolute value of cylinder ≥ 1.5 D in either eye.
Table 3.
Demographics of 12 pediatric cataracts associated with diabetes mellitus
| Subject | Age at cataract dx (years) | DM duration at dx (years) | Cataract morphology | Surgery | Age at surgery (years) | Mean HgbA1c | Highest HgbA1c |
|---|---|---|---|---|---|---|---|
| 1 | 9 | 3.25 | peripheral cortical wedge (unilateral) | N | N/A | 7.3 | 7.7 |
| 21 | 7.5 | 3.75 | 1+ PSC (unilateral) | N | N/A | 7.4 | 8.4 |
| 3 | 14 | 14 | dense, intumescent OU | Y; OU | 14 | “High” | 14 |
| 4 | 17 | 2.25 | 2+ PSC OU | N | N/A | N/A | N/A |
| 5 | 13.5 | 0 | mature white OU | Y; OU | 13.5 | “High” | 14 |
| 6 | 15 | 0 | 1+ PSC OD, 3+NSC OS | Y; OU | 15 | 9 | 13.6 |
| 7 | 14 | 0 | trace PSC (unilateral) | N | N/A | 10.2 | 14 |
| 8 | 14 | 2.5 | trace PSC (unilateral) | N | N/A | 9.9 | 14 |
| 9 | 11.5 | 6.5 | peripheral deposits OU, trace PSC (unilateral) | N | N/A | 11 | 11.4 |
| 10 | 16 | 2 | trace PSC (unilateral) | N | N/A | 11 | 14 |
| 112 | 18 | 15 | dense PSC OU | Y; OU | 18 | 13 | 14 |
| 12 | 13.8 | 14 | dense PSC OU | Y; OD | 14 | 8.3 | 9.7 |
| Mean (SD) | 13.6 (3.0) | 5.3 (5.8) | 14.9 (1.8) | 9.7 (1.9) | 12.3 (2.5) |
SD, standard deviation; PSC, posterior subcapsular cataract.
Patient had ipsilateral CMV retinitis
Patient received whole body irradiation for metastatic neuroblastoma
Table 4.
Ocular complications and potential risk factors
| Characteristics | n1 (n=368) | Cataract (n=12) n (%) |
Strabismus (n=19) n (%) |
Any Ocular Complications2 (n=30) n (%) |
|||
|---|---|---|---|---|---|---|---|
| Type of Diabetes | P=0.283 | P=0.22 | P=0.30 | ||||
|
| |||||||
| Type 1 | 336 | 10 (03.0) | 16 (04.8) | 25 (07.4) | |||
| Type 2 | 32 | 2 (06.3) | 3 (09.4) | 5 (15.6) | |||
|
| |||||||
| Age at Diabetes Onset (Yrs) | P=0.01 | P=0.43 | P=0.04 | ||||
|
| |||||||
| ≤5 | 143 | 4 (02.8) | 10 (7.0) | 13 (09.0) | |||
| >5 to <10 | 112 | 0 (00.0) | 3 (02.7) | 3 (02.7) | |||
| ≥10 | 104 | 8 (07.7) | 6 (05.8) | 14 (46.7) | |||
| Missing | 9 | 0 (00.0) | 0 (00.0) | 0 (00.0) | |||
|
| |||||||
| Duration of Diabetes at Last Visit (Yrs) | P=0.56 | P=0.19 | 0.33 | ||||
|
| |||||||
| ≤5 | 195 | 8 (04.1) | 10 (05.1) | 18 (09.2) | |||
| >5 to <10 | 111 | 2 (01.8) | 4 (03.6) | 6 (05.3) | |||
| ≥10 | 42 | 1 (02.4) | 5 (11.9) | 5 (11.9) | |||
| Missing | 20 | 1 (05.0) | 0 (00.0) | 1 (05.0) | |||
|
| |||||||
| Average HgbA1c During Study Period | P=0.37 | P=0.06 | P=0.09 | ||||
|
| |||||||
| ≤7.5 | 111 | 5 (04.5) | 7 (06.3) | 11 (09.8) | |||
| >7.5 to <10 | 166 | 3 (01.8) | 4 (02.4) | 7 (04.2) | |||
| ≥10 | 76 | 4 (05.3) | 6 (07.9) | 10 (13.1) | |||
| Missing | 15 | 0 (0.00) | 2 (13.3) | 2 (13.3) | |||
2 subjects missing outcome information.
One subject with cataract and strabismus.
P-values are from Fisher exact tests.
Nineteen (5.2%) patients were found to have strabismus. The average age at strabismus diagnosis was 11 years, 3.7 years after DM diagnosis. The youngest age at strabismus diagnosis was 2.7 years. Of these 19 children, only one patient was noted to have a paralytic strabismus from an abducens nerve palsy, which resolved spontaneously. This transient abducens nerve palsy was thought to be a microvascular neuropathy from DM after a thorough neurologic workup, which included brain CT, brain MRI, myasthenia gravis antibody panel, and lumbar puncture with CSF studies and opening pressure, yielded normal results. This abducens neuropathy occurred at 12 years of age, which was 1.5 years after diagnosis of DM; information on her HbA1c, however, was not available. The child with sixth nerve palsy was diagnosed after presenting with diplopia and not during a routine diabetic screening exam. No associations were found between strabismus and type, age at diagnosis, duration or control of DM (Table 4).
Forty-one patients (11%) were found to have high refractive error, with 2.8% having high hyperopia, 2.8% having high myopia and 7.2% having high astigmatism in at least one eye. The majority of children (55.8%) were found to have mild hyperopia (Table 2). There was no significant relationship found between refractive errors and type, age at diagnosis, duration or control of DM.
DISCUSSION
We found no cases of diabetic retinopathy among a large cohort of children with type 1 and 2 DM of duration up to 16 years and blood glucose control ranging from good to poor. Our cohort included children diagnosed at a very young age and followed through adolescence (Figure 1). While other investigators have reported similar results15, we also reviewed the literature to identify studies reporting the occurrence of diabetic retinopathy during childhood (Table 5). Among these studies, the prevalence of DR in children was reported to be between 9% and 28%.7–9, 16–23 One possible reason for this wide range of prevalence estimates (0% to 28%) might be differing baseline characteristics of the study samples, including different ages at examination or blood glucose control. Another reason might be different screening modalities. The use of fundus photography has been found to be more sensitive than fundoscopy for detecting mild retinopathy, particularly in children, who are more difficult to examine than adults.8, 21 Finally, improvements in the effectiveness of diabetes diagnosis and management over time may be resulting in a decrease in the incidence of DR.23, 24 Some of these studies present clinical data from as far back as 30 to 40 years ago, prior to publication of the major diabetic retinopathy studies, such as the DCCT in 1993, which identified modifiable risk factors for DR and established glucose control goals.5
Table 5.
Studies reporting occurrence of diabetic retinopathy in children.
| Article | Youngest AGE at onset of any DR (years) | Shortest DURATION of DM at onset of any DR (years) | Youngest AGE at onset of pre-proliferative* or proliferative DR (years) | Shortest DURATION of DM at onset of pre-proliferative* or proliferative DR (years) |
|---|---|---|---|---|
| Kubin et al. 2011 | 8 | 1.7 | None | None |
| Kernell et al. 1997 | 9.5 | 1.5 | PDR at 21.5 | PDR at 9.5 |
| Palmberg et al. 1981 | > 20 | < 1 yr (in a 29 year old patient) | 1 case of DR requiring laser after age 20 | 13 |
| Donaghue et al. 1997 | 8 | 1.2 | None | None |
| Holl et al. 1998 | 5.5 | 2.2 | 1 case of DR requiring laser, age not reported | Not reported |
| Kullberg et al. 2002 | > 9 | Not reported; ranged 6 to 13 in study | > 18 yrs | Not reported; ranged 6 to 13 |
| Minuto et al. 2012 | Not reported | Not reported | 1 case of DR requiring laser, age not reported | Not reported |
| Maguire et al. 2005 | < 11 | Not reported | None | None |
| WESDR II (Klein et al. 1984) | ≤ 9 | 1 | PDR 15–19 | PDR at 5 to 6 |
| WDRS (1990–2002) (Lecaire et al. 2006) | ≤ 9 | ≤ 4 | Not reported | PDR at ≤ 7 yrs |
| Falck et al. 1996 | 11.5 | 1.3 | Severe NPDR 18.8; no PDR | Severe NPDR 5.5; no PDR |
| Mayer-Davis et al. 2012 | Not reported | Not reported | One case of PDR in child with type 2 DM, age not reported | Not reported |
Pre-proliferative retinopathy defined as severe non-proliferative diabetic retinopathy
The majority of children with DR in prior studies had mild non-proliferative DR, many of whom were found to have only a single microaneurysm or retinal hemorrhage unilaterally.7, 8, 16, 18, 19 The youngest age at which a child was diagnosed with DR was 5.5 years, and this child had a single microaneurysm in one eye.7 We feel that the value of screening for such mild disease is questionable, considering the large number of normal examinations being performed. A more reasonable screening target may be identification of sight-threatening DR, which might be close to requiring treatment (Table 5). In our review of the literature, we could identify only five possible cases of children less than 18 years of age with sight-threatening DR.7, 9, 22 Holl et al. reported one child who had received laser prior to their study, but did not report the age of the patient at treatment or duration of DM.7 Minuto et al. reported one patient requiring laser for “sight threatening DR”, but does not provide the age at treatment; the authors report that among all patients with DR (n=26), the median age was 26.5 years and the first quartile age was 19.8 years. Therefore, it is not clear that this patient was less than 18 years old when treated for DR.22 A minimum age for the group is not provided, and no duration of DM prior to treatment was reported.22 Similarly, a more recent study of Type 1 and Type 2 DM in youth, reported a single case of proliferative diabetic retinopathy (PDR) in a child with Type 2 DM, though the age of this child at diagnosis of PDR was not provided and could be in the third decade of life, based upon the study methods described.25 Three additional cases of PDR possibly diagnosed prior to age 18 years were reported in 1984 from the Wisconsin Epidemiologic Study of Diabetic Retinopathy, preceding the DCCT.9 The ages of these patients are reported only as a range from 15 years to 19 years.9 The shortest duration of DM prior to the development of PDR was provided as 5 to 6 years.9 Based upon this review, the earliest documented age of severe DR is 15 years, conservatively assuming the lower end of the range noted above, and the shortest duration of DM prior to severe DR is 5 years. Of note, none of the studies discussed above reported a case of clinically significant macular edema in a child.
The mean incidence of diabetic cataracts in children has been reported to be 0.7 to 3.4%.26, 27. In our study, we found a similar cataract incidence of 3.3% (12 children, 19 eyes). The youngest age at cataract diagnosis in our study was 7.5 years, 4.5 years after DM diagnosis with an average age of 13.2 years, 4.4 years after DM diagnosis. These findings are similar to a recently published series of cataracts in patients with type 1 DM in which the mean age at cataract diagnosis was 11.4 years, 2.3 years after DM diagnosis, with the youngest cataract diagnosed at age 5 years.28 Notably, two of their patients presented with cataracts prior to diagnosis of DM, and five cataracts were diagnosed at the time of DM diagnosis. In this report, Wilson et al. describe pediatric diabetic cataracts of varied morphology from posterior subcapsular, lamellar, flake-like, and dense milky-white cataracts. They propose the variation in cataract morphology in these children may be due to differences in age of DM diagnosis and severity of DM.28 In our study, we also found varied cataract morphologies, including posterior subcapsular, posterior flecks, mature white, and intumescent cataracts. Nine eyes of 5 children in our cohort required cataract surgery. All of these children with visually significant cataracts presented symptomatically or were discovered during vision screening examinations.
The prevalence of high refractive errors and strabismus in our cohort were found to be similar to a non-diabetic pediatric population.29, 30 However, the case of a possible microvascular abducens neuropathy due to DM was an interesting finding and has not been reported in the literature in a child to our knowledge. However, subclinical peripheral neuropathy has been detected by nerve conduction studies in half of children with a Type 1 DM duration of greater than 5 years31, therefore a microvascular insult causing strabismus in children is biologically plausible. This particular patient had the majority of her DM care at another institution, therefore very little information regarding control of her DM or other comorbid conditions, including other microvascular insults, was available. She presented symptomatically with diplopia, rather than being diagnosed with esotropia on routine screening examination.
The American Academy of Ophthalmology guidelines recommend annual screenings for DR to begin 5 years after the diagnosis of DM, and the American Academy of Pediatrics guidelines suggest initiating annual examinations 3 to 5 years after DM diagnosis or after the age of 9 years, whichever occurs later.4, 32 Based upon our study results and review of the literature, screening for ocular complications of DM could begin later than suggeted by these guidelines. No children in our study were diagnosed with retinopathy. The earliest documented age of severe DR in the literature was 15 years, and the shortest duration of DM prior to the development of severe DR was 5 years. In our study, 121 children had DM for at least 5 years and an examination prior to age 15 years, and there were 213 examinations prior to age 15 that involved children who had DM for at least 5 years. The children with visually significant cataracts were diagnosed when they presented for vision loss. Similarly, the one child with a diabetic sixth nerve palsy presented with diplopia. Finally, there are already established amblyopia and amblyopia risk factor screening programs in place in schools and pediatricians’ offices that are effectively identifying strabismus and high refractive errors in school-aged children. The United States Preventive Services Task Force currently recommends vision screening for all children at least once between the ages of 3 and 5 years to detect the presence of amblyopia or its risk factors.30
We suggest the collaborative consensus groups that publish recommendations for screening consider updating the current guidelines. Based upon the available evidence, we believe that screening for diabetic retinopathy may commence at 15 years of age or at 5 years following the diagnosis of DM, whichever occurs later. In addition, examinations could begin earlier in children considered to be at unusually high risk for systemic diabetic complications, as judged by the treating endocrinologist (e.g., children with chronically poorly controlled blood glucose levels or in the case of pregnancy). In addition, as only a small percentage of our sample had type 2 DM, and there is a paucity of information in the literature on the prevalence and incidence of DR in children with type 2 DM, this growing population may need to be considered separately. Until additional data are available, children with type 2 DM could be considered within the high risk category and begin DR screening upon DM diagnosis, as do adults with type 2 DM.
Our study has limitations worth considering. We did not include fluorescein angiographic analysis to identify occult DR, however fluorescein angiography is not routinely performed in children, not widely available in pediatric ophthalmology offices, nor is it recommended for routine DR screening in adults or children.4 Furthermore, we did not perform ocular color fundus photography to screen for DR. Fundus photography has been found to be more sensitive that ophthalmoscopy in the diagnosis of mild DR.8, 21 However, we believe our methodology is more clinically relevant to the screening techniques employed by most pediatric ophthalmologists, and the utility of identifying very mild background DR is questionable. The generalizability of our results to children in other geographic regions and of different ethnic backgrounds may be limited by the racial and ethnic profile of our study sample. However, the children in our study were referred by their endocrinologists or primary care pediatricians for diabetic retinopathy screening, and our cohort is therefore representative of a typical population of children presenting for such screening. Results relating to blood glucose control in this study may be limited by the possibility that the collected HbA1c values were not always representative of the patient’s overall glucose control. We did not collect all available HbA1c values (only the value preceding each examination) nor information on HbA1c variability, which has been found to be an independent risk factor for the development of diabetic retinopathy in patients with type 1 DM.10 However, each HbA1c collected is a random spot sampling of each patient’s glucose control and free from any potential bias of more frequent testing in an uncontrolled patient. Furthermore, our study does not include information on concurrent hypertension, maternal history of gestational diabetes or other prenatal insults that have been suggested as potential risk factors for DR in children.33 Finally, the successful implementation of a clinical guideline depends upon its acceptance by clinicians, and some physicians may feel uncomfortable with our screening recommendations. However, the recommendation to not examine children with diabetes mellitus until age 15 years is based upon the current evidence available in the scientific literature.
Vision threatening diabetic retinopathy is extremely rare in children, regardless of the duration or control of DM. Current screening guidelines appear to create an unnecessary financial and logistical burden for families and unnecessary appropriation of resources of pediatric ophthalmologists and the healthcare system. Based upon the available evidence, we believe that screening examinations for DR could begin at age 15 years or after 5 years of DM duration, whichever occurs later, with exception made for high-risk children and type 2 diabetics. Annual examinations could then continue into adulthood, when the risk of developing sight-threatening diabetic retinopathy increases. A collaborative consensus group should consider revising the current diabetic retinopathy screening guidelines.
Acknowledgments
This research was supported by National Institutes of Health grants P30 EY01583-26, 5T35DK060441-10, UL1RR02499, and L30 EY018451-03. The sponsor or funding organization had no role in the design or conduct of this research.
Footnotes
None of the authors has a conflict of interest to report.
Presented in part at the 2015 Annual Meeting of the American Association for Pediatric Ophthalmology and Strabismus in New Orleans, LA, and the 2015 Eastern Society for Pediatric Research in Philadelphia, PA
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Prevention Cfdca. National Diabetes Fact Sheet: National Estimates and General Information on Diabetes and Prediabetes in the United States, 2011. Atlanta, GA: US Department of Health & Human Services, Centers for Disease Control and Prevention; 2011. [Google Scholar]
- 2.Photocoagulation treatment of proliferative diabetic retinopathy. Clinical application of Diabetic Retinopathy Study (DRS) findings, DRS Report Number 8. The Diabetic Retinopathy Study Research Group. Ophthalmology. 1981;88(7):583–600. [PubMed] [Google Scholar]
- 3.Photocoagulation therapy for diabetic eye disease. Early Treatment Diabetic Retinopathy Study Research Group. JAMA. 1985;254(21):3086. [PubMed] [Google Scholar]
- 4.Panel Aaoor. Preferred Practice Pattern 2014: Diabetic Retinopathy. San Francisco: American Academy of Ophthalmology; 2003. [Google Scholar]
- 5.The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. The Diabetes Control and Complications Trial Research Group. N Engl J Med. 1993;329(14):977–86. doi: 10.1056/NEJM199309303291401. [DOI] [PubMed] [Google Scholar]
- 6.Retinopathy and nephropathy in patients with type 1 diabetes four years after a trial of intensive therapy. The Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications Research Group. N Engl J Med. 2000;342(6):381–9. doi: 10.1056/NEJM200002103420603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Holl Rw, Lang Ge, Grabert M, Heinze E, Lang Gk, Debatin Km. Diabetic retinopathy in pediatric patients with type-1 diabetes: effect of diabetes duration, prepubertal and pubertal onset of diabetes, and metabolic control. J Pediatr. 1998;132(5):790–4. doi: 10.1016/s0022-3476(98)70305-1. [DOI] [PubMed] [Google Scholar]
- 8.Palmberg P, Smith M, Waltman S, Krupin T, Singer P, Burgess D, Wendtlant T, Achtenberg J, Cryer P, Santiago J, White N, Kilo C, Daughaday W. The natural history of retinopathy in insulin-dependent juvenile-onset diabetes. Ophthalmology. 1981;88(7):613–8. doi: 10.1016/s0161-6420(81)34975-6. [DOI] [PubMed] [Google Scholar]
- 9.Klein R, Klein Be, Moss Se, Davis Md, Demets Dl. The Wisconsin epidemiologic study of diabetic retinopathy. II. Prevalence and risk of diabetic retinopathy when age at diagnosis is less than 30 years. Arch Ophthalmol. 1984;102(4):520–6. doi: 10.1001/archopht.1984.01040030398010. [DOI] [PubMed] [Google Scholar]
- 10.Hermann Jm, Hammes Hp, Rami-Merhar B, Rosenbauer J, Schutt M, Siegel E, Holl Rw. HbA1c variability as an independent risk factor for diabetic retinopathy in type 1 diabetes: a German/Austrian multicenter analysis on 35,891 patients. PLoS One. 2014;9(3):e91137. doi: 10.1371/journal.pone.0091137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Harvey Jn, Allagoa B. The long-term renal and retinal outcome of childhood-onset Type 1 diabetes. Diabet Med. 2004;21(1):26–31. doi: 10.1046/j.1464-5491.2003.01062.x. [DOI] [PubMed] [Google Scholar]
- 12.Monti Mc, Lonsdale Jt, Montomoli C, Montross R, Schlag E, Greenberg Da. Familial risk factors for microvascular complications and differential male-female risk in a large cohort of American families with type 1 diabetes. J Clin Endocrinol Metab. 2007;92(12):4650–5. doi: 10.1210/jc.2007-1185. [DOI] [PubMed] [Google Scholar]
- 13.Svensson M, Eriksson Jw, Dahlquist G. Early glycemic control, age at onset, and development of microvascular complications in childhood-onset type 1 diabetes: a population-based study in northern Sweden. Diabetes Care. 2004;27(4):955–62. doi: 10.2337/diacare.27.4.955. [DOI] [PubMed] [Google Scholar]
- 14.Donaghue Kc, Chiarelli F, Trotta D, Allgrove J, Dahl-Jorgensen K. ISPAD Clinical Practice Consensus Guidelines 2006–2007. Microvascular and macrovascular complications. Pediatr Diabetes. 2007;8(3):163–70. doi: 10.1111/j.1399-5448.2007.00250.x. [DOI] [PubMed] [Google Scholar]
- 15.Lueder Gt, Pradhan S, White Nh. Risk of retinopathy in children with type 1 diabetes mellitus before 2 years of age. Am J Ophthalmol. 2005;140(5):930–1. doi: 10.1016/j.ajo.2005.05.023. [DOI] [PubMed] [Google Scholar]
- 16.Donaghue Kc, Fairchild Jm, Chan A, Hing Sj, King J, Howard Nj, Silink M. Diabetes microvascular complications in prepubertal children. J Pediatr Endocrinol Metab. 1997;10(6):579–85. doi: 10.1515/jpem.1997.10.6.579. [DOI] [PubMed] [Google Scholar]
- 17.Flack A, Kaar Ml, Laatikainen L. A prospective, longitudinal study examining the development of retinopathy in children with diabetes. Acta Paediatr. 1996;85(3):313–9. doi: 10.1111/j.1651-2227.1996.tb14023.x. [DOI] [PubMed] [Google Scholar]
- 18.Kernell A, Dedorsson I, Johansson B, Wickstrom Cp, Ludvigsson J, Tuvemo T, Neiderud J, Sjostrom K, Malmgren K, Kanulf P, Mellvig L, Gjotterberg M, Sule J, Persson La, Larsson Li, Aman J, Dahlquist G. Prevalence of diabetic retinopathy in children and adolescents with IDDM. A population-based multicentre study. Diabetologia. 1997;40(3):307–10. doi: 10.1007/s001250050679. [DOI] [PubMed] [Google Scholar]
- 19.Kubin M, Tossavainen P, Hannula V, Lahti S, Hautala N, Falck A. Prevalence of retinopathy in Finnish children and adolescents with type 1 diabetes: a cross-sectional population-based retrospective study. Arch Dis Child. 2011;96(10):963–8. doi: 10.1136/adc.2011.210807. [DOI] [PubMed] [Google Scholar]
- 20.Kullberg Ce, Abrahamsson M, Arnqvist Hj, Finnstrom K, Ludvigsson J. Prevalence of retinopathy differs with age at onset of diabetes in a population of patients with Type 1 diabetes. Diabet Med. 2002;19(11):924–31. doi: 10.1046/j.1464-5491.2002.00839.x. [DOI] [PubMed] [Google Scholar]
- 21.Maguire A, Chan A, Cusumano J, Hing S, Craig M, Silink M, Howard N, Donaghue K. The case for biennial retinopathy screening in children and adolescents. Diabetes Care. 2005;28(3):509–13. doi: 10.2337/diacare.28.3.509. [DOI] [PubMed] [Google Scholar]
- 22.Minuto N, Emmanuele V, Vannati M, Russo C, Rebora C, Panarello S, Pistorio A, Lorini R, D’annunzio G. Retinopathy screening in patients with type 1 diabetes diagnosed in young age using a non-mydriatic digital stereoscopic retinal imaging. J Endocrinol Invest. 2012;35(4):389–94. doi: 10.3275/8016. [DOI] [PubMed] [Google Scholar]
- 23.Lecaire T, Palta M, Zhang H, Allen C, Klein R, D’alessio D. Lower-than-expected prevalence and severity of retinopathy in an incident cohort followed during the first 4–14 years of type 1 diabetes: the Wisconsin Diabetes Registry Study. Am J Epidemiol. 2006;164(2):143–50. doi: 10.1093/aje/kwj166. [DOI] [PubMed] [Google Scholar]
- 24.Henricsson M, Nystrom L, Blohme G, Ostman J, Kullberg C, Svensson M, Scholin A, Arnqvist Hj, Bjork E, Bolinder J, Eriksson Jw, Sundkvist G. The incidence of retinopathy 10 years after diagnosis in young adult people with diabetes: results from the nationwide population-based Diabetes Incidence Study in Sweden (DISS) Diabetes Care. 2003;26(2):349–54. doi: 10.2337/diacare.26.2.349. [DOI] [PubMed] [Google Scholar]
- 25.Mayer-Davis Ej, Davis C, Saadine J, D’agostino Rb, Jr, Dabelea D, Dolan L, Garg S, Lawrence Jm, Pihoker C, Rodriguez Bl, Klein Be, Klein R. Diabetic retinopathy in the SEARCH for Diabetes in Youth Cohort: a pilot study. Diabet Med. 2012;29(9):1148–52. doi: 10.1111/j.1464-5491.2012.03591.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Klein Be, Klein R, Moss Se. Prevalence of cataracts in a population-based study of persons with diabetes mellitus. Ophthalmology. 1985;92(9):1191–6. doi: 10.1016/s0161-6420(85)33877-0. [DOI] [PubMed] [Google Scholar]
- 27.Montgomery El, Batch Ja. Cataracts in insulin-dependent diabetes mellitus: sixteen years’ experience in children and adolescents. J Paediatr Child Health. 1998;34(2):179–82. doi: 10.1046/j.1440-1754.1998.00195.x. [DOI] [PubMed] [Google Scholar]
- 28.Wilson Me, Jr, Levin Av, Trivedi Rh, Kruger Sj, Elliott La, Ainsworth, Awner S, Cruz Oa, Kivlin J, Vroman Dt, Young Wo. Cataract associated with type-1 diabetes mellitus in the pediatric population. J AAPOS. 2007;11(2):162–5. doi: 10.1016/j.jaapos.2005.10.005. [DOI] [PubMed] [Google Scholar]
- 29.Eye examination in infants, children, and young adults by pediatricians. Pediatrics. 2003;111(4 Pt 1):902–7. [PubMed] [Google Scholar]
- 30.Vision screening for children 1 to 5 years of age: US Preventive Services Task Force Recommendation statement. Pediatrics. 2011;127(2):340–6. doi: 10.1542/peds.2010-3177. [DOI] [PubMed] [Google Scholar]
- 31.Eng Gd, Hung W, August Gp, Smokvina Md. Nerve conduction velocity determinations in juvenile diabetes: continuing study of 190 patients. Arch Phys Med Rehabil. 1976;57(1):1–5. [PubMed] [Google Scholar]
- 32.Lueder Gt, Silverstein J. Screening for retinopathy in the pediatric patient with type 1 diabetes mellitus. Pediatrics. 2005;116(1):270–3. doi: 10.1542/peds.2005-0875. [DOI] [PubMed] [Google Scholar]
- 33.Snow V, Weiss Kb, Mottur-Pilson C. The evidence base for tight blood pressure control in the management of type 2 diabetes mellitus. Ann Intern Med. 2003;138(7):587–92. doi: 10.7326/0003-4819-138-7-200304010-00017. [DOI] [PubMed] [Google Scholar]