Abstract
Cisplatin is currently one of the most effective chemotherapeutic drugs used for treating ovarian cancer; however, resistance to cisplatin is common. In this study, we explored an experimental strategy for overcoming cisplatin resistance of human ovarian cancer from the new perspective of cancer cell metabolism. By using two pairs of genetically matched cisplatin-sensitive and cisplatin-resistant ovarian cancer cell lines, we tested the hypothesis that downregulating hypoxia-inducible factor-1 (HIF-1), which regulates metabolic enzymes involved in glycolysis, is a promising strategy for overcoming cisplatin resistance of human ovarian cancer cells. We found that cisplatin downregulated the level of the regulatable α subunit of HIF-1, HIF-1α, in cisplatin-sensitive ovarian cancer cells through enhancing HIF-1α degradation but did not downregulate HIF-1α in their cisplatin-resistant counterparts. Overexpression of a degradation-resistant HIF-1α (HIF-1α ΔODD) reduced cisplatin-induced apoptosis in cisplatin-sensitive cells, whereas genetic knockdown of HIF-1α or pharmacological promotion of HIF-1α degradation enhanced response to cisplatin in both cisplatin-sensitive and cisplatin-resistant ovarian cancer cells. We further demonstrated that knockdown of HIF-1α improved the response of cisplatin-resistant ovarian cancer cells to cisplatin by redirecting the aerobic glycolysis in the resistant cancer cells towards mitochondrial oxidative phosphorylation, leading to cell death through overproduction of reactive oxygen species. Our findings suggest that the HIF-1α-regulated cancer metabolism pathway could be a novel target for overcoming cisplatin resistance in ovarian cancer.
Keywords: Cisplatin, ovarian cancer, resistance, HIF-1, cancer metabolism
1. Introduction
Approximately 80% of patients with ovarian cancer respond to initial cytoreductive surgery followed by adjuvant chemotherapy with carboplatin and paclitaxel or cisplatin and paclitaxel; however, approximately 70% of patients treated this way develop recurrence, and the percentage is even higher among patients with stage III and IV disease [1–3]. Recurrent and metastatic ovarian cancer is often resistant to standard platinum-based chemotherapy [4]. Strategies tried to overcome such chemoresistance include combining platinum-based chemotherapy with new molecularly targeted drugs—e.g., bevacizumab, a vascular endothelial cell growth factor A (VEGF-A)-neutralizing antibody, or olaparib, a poly (ADP-ribose) polymerase (PARP) inhibitor [1,4–6]. Bevacizumab plus chemotherapy has been shown to shrink or slow the growth of advanced epithelial ovarian cancers, but this combination does not seem to help women live longer [5]. Olaparib is used only in patients with tumors that have mutations in the BRCA genes because the drug works only against cells with a blocked BRCA pathway; however, only a small proportion of women with ovarian cancer have mutated BRCA genes [6]. Therefore, there is a strong need to develop new strategies to overcome platinum resistance in recurrent and metastatic ovarian cancer [7].
Among many strategies for treating recurrent and metastatic ovarian cancer, targeting of cancer cell metabolism is particularly appealing because most ovarian cancer cells are highly proliferative and thus highly dependent on metabolism of glucose, a major source for the socalled biomass, which includes lipids, nucleotides, and amino acids that are building blocks required for cell growth and proliferation [8,9]. It was discovered nearly a century ago that in highly proliferative cancer cells, glucose is mainly converted into lactate instead of entering into the mitochondria for generation of ATP through the Krebs cycle and subsequent NADH oxidative phosphorylation, even when O2 level is sufficient [10–12]. This phenomenon is called aerobic glycolysis or the Warburg effect [13]. By adopting such a low energy efficient cell metabolism, highly proliferative cancer cells consume a large number of glucose molecules and generate ample amounts of intermediate metabolites and NADPH reducing equivalent for structural needs of cell growth and proliferation [10–12].
The enzymes that regulate the flow of glycolysis are transcriptionally regulated mainly by three major transcription factors: hypoxia-inducible factor-1 (HIF-1), p53, and Myc [14]. Among them, HIF-1, a heterodimer consisting of a constitutively expressed HIF-1β submit and a regulatory HIF-1α subunit, appears to be the key player [15,16]. HIF-1 regulates almost all the enzymes leading to glucose breakdown during glycolysis and regulates lactate production and lactate removal from cells by activating lactate dehydrogenase A (LDH-A) and the lactate transporter MCT4 [15]. Besides stimulating glycolysis, HIF-1 inhibits mitochondrial respiratory chain function in several ways [17–20]. For example, HIF-1 activates pyruvate dehydrogenase (PDH) kinase-1 (PDK1), which in turn inhibits the activity of PDH, leading to inhibition of the conversion of pyruvate into acetyl-CoA for metabolizing in the Krebs cycle [19]. Inhibition of HIF-1 in cancer cells may redirect aerobic glycolysis towards mitochondrial oxidative phosphorylation. The resulting entry of a large amount of pyruvate into the mitochondria, followed by production of acetyl-CoA and subsequent metabolism through the Krebs cycle, could result in cytotoxic levels of reactive oxygen species (ROS), a byproduct of oxidative phosphorylation during the formation of ATP, leading to cell death via apoptosis [21].
In this study, we tested our hypothesis that downregulating HIF-1α is a promising strategy for overcoming cisplatin resistance in ovarian cancer from a perspective of regulation of cancer metabolism. We found a novel HIF-1α-related mechanism by which ovarian cancer cells develop resistance to cisplatin. Our results show that cisplatin in combination with genetic knockdown of HIF-1α expression or pharmacological promotion of HIF-1α degradation can induce apoptosis in cisplatin-resistant ovarian cancer cells through overproduction of ROS. Targeting HIF-1α may therefore be an attractive approach for improving clinical outcomes of ovarian cancer treatment.
2. Materials and methods
2.1. Cell lines and cell culture
Human ovarian cancer lines A2780, A2780/CP, PEO1, and PEO4 were kindly provided by Dr. Yinhua Yu (The University of Texas MD Anderson Cancer Center). The cells were maintained in DMEM/F12 complete medium supplemented with 10% fetal bovine serum at 37°C in a humidified atmosphere containing 5% CO2.
2.2. Reagents
Cisplatin was obtained from the outpatient pharmacy at MD Anderson. 1-methyl-1, 9-pyrazoloanthrone (1-methyl-1, 9 PA), N-acetyl cysteine (NAC), and Z-Leu-Leu-Leu-al (MG132) were purchased from Sigma-Aldrich.
2.3. siRNA duplexes, cDNA construct, and transfection
HIF-1α–targeted siRNA (target DNA sequence #1, CAAAGTTCACCTGAGCCTA; #2, GATTAACTCAGTTTGAACT) and control siRNA were purchased from Sigma-Aldrich and were previously described [22]. The siRNA (200 pmol) and Lipofectamine 2000 (Life Technologies) (5 µL) were mixed in 100 µL of minimal essential medium (Opti-MEM, Life Technologies) for 15 minutes, and the siRNA/Lipofectamine 2000 mixture was added into the culture medium. Six hours later, the medium was replaced with regular medium, and then the cells were cultured for an additional 48 h prior to the detection of HIF-1α expression knockdown by Western blotting.
Flag-tagged LDH-A cDNA was obtained by RT-PCR using the RNA extracted from MCF7 breast cancer cells as the template and using following pair of primers: forward primer, GCCGACTAGTATGGATTACAAGGATGACGACGATAAGGCAACTCTAAAGGATCAGCTG; reverse primer, GCGCCTCGAGTTAAAATTGCAGCTCCTTTTGGATCCCCCAAAG. The LDH-A cDNA was subcloned into pLEX-MCS lentiviral construct (Thermo Fisher Scientific) via the SpeI and XhoI sites. The recombinant lentivirus was produced in HEK293T cells following co-transfection with psPAX2 and pMD2.G using Lipofectamine 2000 according to the manufacturer’s instructions. The lentivirus-containing medium from the HEK293T cells was harvested, aliquoted, and stored at −80°C prior to use.
HIF-1α ΔODD construct was described previously [23,24] and was transfected into the targeted cells using Lipofectamine 2000.
2.4. Cell proliferation and clonogenic assays
Cell proliferation assay was performed as we previously described [25,26]. In brief, at the end of treatment, 20 µL of 10 mg/mL methylthiazolyldiphenyltetrazolium bromide (MTT) solution in phosphate-buffered saline was added to each well of a 48-well tissue culture plate containing 200 µL of medium. Three hours later, cells were lysed with a lysis buffer (200 µL/well) containing 20% SDS in dimethyl formamide/H2O (1:1, v/v; pH 4.7) at 37°C for at least 6 h. The relative number of surviving cells in each group was determined by measuring the optical density (OD) of the cell lysates at an absorbance wavelength of 570 nm. The OD value in each treatment group was then expressed as a percentage of the OD value in the untreated control cells, and OD values were plotted against treatments.
For clonogenic assay, cells were seeded in triplicate into 6-cm dishes at 1000 cells/dish after appropriate treatment. The cells were cultured in 10% FBS medium in a 37°C, 5% CO2 incubator for 10 days. Surviving clones in the dishes were fixed and stained with a solution containing 0.2% crystal violet in 10% ethanol for 30 minutes and then photographed [27,28].
2.5. Western blotting analysis
After appropriate treatment, cells were lysed in a lysis buffer containing 50 mM TrisHCl (pH 7.4), 150 mM NaCl, 0.5% NP-40, 50 mM NaF, 1 mM Na3VO4, 1 mM phenylmethylsulfonyl fluoride, 25 µg/mL aprotinin, and 25 µg/mL leupeptin and kept on ice for 15 minutes [22]. The lysates were cleared by centrifugation, and the supernatants were collected. Equal amounts of protein lysate, as determined using the Pierce Coomassie Plus colorimetric protein assay (Thermo Fisher Scientific), were separated by SDS–polyacrylamide gel electrophoresis (PAGE), blotted onto nitrocellulose, blocked with 5% milk in TrisHCl-buffered saline for 30 minutes, and then probed at 4°C overnight with various primary antibodies as follows: HIF-1α (BD Biosciences); PARP, LDH-A, PKM2, and HK2 (Cell Signaling Technology); PDK1 (Enzo Life Sciences); Alexa Fluor 555-conjugated anti-phospho-H2A.X antibody (Ser139) (clone JBW30, EMD Millipore); and β-actin (Sigma-Aldrich). The signals were visualized using the enhanced chemiluminescence detection kit (GE Healthcare).
2.6. ROS detection
Intracellular ROS were detected by using the total ROS detection kit (Enzo Life Sciences) according to the manufacturer’s instructions [29]. Briefly, cells were seeded in a 12-well plate at approximately 40% to 60% confluence. After indicated treatment, cells were washed with a buffer provided in the kit and were either (i) stained directly with ROS detection solution at 37°C for 1 h and then analyzed by using a fluorescence microscope or (ii) trypsinized and resuspended in Eppendorf tubes, then stained with ROS detection solution at 37°C for 1 h, and then analyzed with an LSRFortessa cell analyzer (BD Biosciences).
2.7. Live/dead cell viability assay
The LIVE/DEAD cell viability assay kit (Life Technologies) was used to detect cell death as we recently described [30]. In brief, following treatment, cells were incubated with 4 µM calcein acetoxymethyl ester and 2 µM ethidium homodimer-1 together in a 37°C, 5% CO2 incubator for 45 minutes. The cells were then rinsed gently with phosphate-buffered saline and then observed for cell viability under a fluorescence microscope. Live cells were identified by green fluorescence when excited at 485 nm, and dead cells were identified by bright red fluorescence when excited at 544 nm.
2.8. Apoptosis assays
Apoptosis was measured by detection of PARP cleavage using Western blotting with an antibody that recognizes both cleaved and uncleaved PARP (Cell Signaling Technology); by quantitative measurement of the levels of cytoplasmic histone-associated DNA fragments (mononucleosomes and oligonucleosomes) using a colorimetric Cell Death Detection ELISA kit (Roche Diagnostics Corp.); or by quantitative measurement of apoptotic cells using a flow cytometer after staining of cells with FITC-conjugated annexin V and propidium iodide (Life Technologies), according to the vendor’s protocols [24,31].
3. Results
3.1. Cisplatin downregulates HIF-1α protein level in cisplatin-sensitive ovarian cancer cells but not in cisplatin-resistant ovarian cancer cells
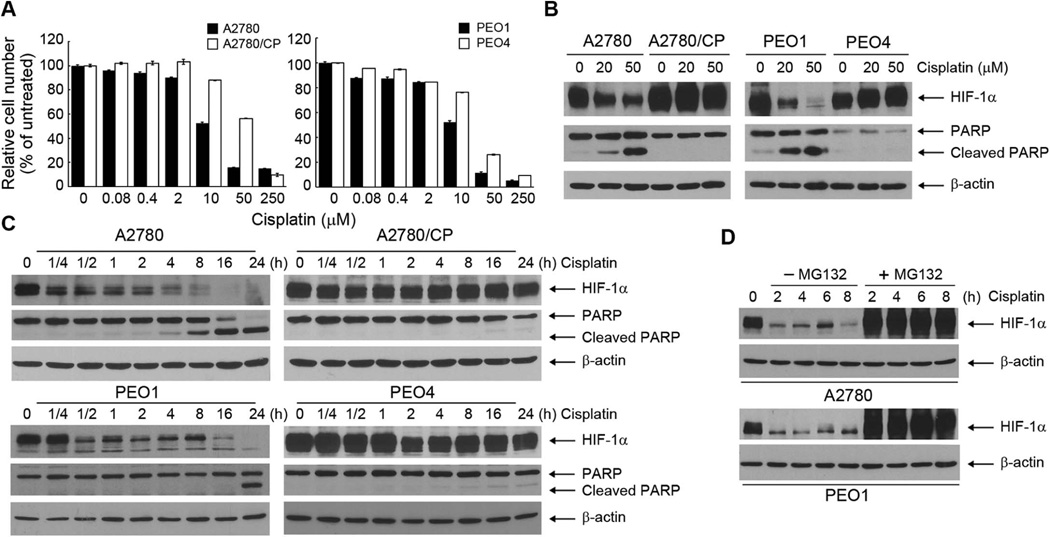
To test our hypothesis that downregulating HIF-1α is a promising strategy for overcoming cisplatin resistance in ovarian cancer, we used two pairs of isogeneic cisplatin-sensitive and cisplatin-resistant ovarian cancer cell lines. A2780 ovarian cancer cells are cisplatin sensitive; A2780/CP is a subline of A2780 that acquired cisplatin resistance in vitro [32]. PEO1 was derived from a patient with primary ovarian cancer sensitive to cisplatin; PEO4 was derived from the same patient after recurrence and metastasis developed following cisplatin treatment [33,34]. As expected, A2780/CP and PEO4 cells exhibited considerable resistance to cisplatin, whereas their respective counterparts exhibited sensitivity to cisplatin, as assessed by an MTT assay following treatment of the cells with various concentrations of cisplatin in culture for 72 h (Figure 1A). Consistent with the MTT assay results, Western blotting analysis showed dose-dependent and treatment time–dependent induction of PARP cleavage, a marker of apoptosis, in A2780 and PEO1 cells but not in A2780/CP and PEO4 cells after treatment with cisplatin (Figure 1B and 1C). Interestingly, the level of HIF-1α was markedly downregulated in the cisplatin-sensitive cells but not in the cisplatin-resistant cells (Figure 1B and 1C). The downregulation of HIF-1α was detected as early as 15 minutes after cisplatin treatment in A2780 cells and as early as 30 minutes after cisplatin treatment in PEO1 cells. These time points were much earlier than the time points when PARP cleavage was first detected (~8 h in A2780 cells and ~24 h in PEO1 cells), which corresponded with the time points when downregulation of HIF-1α was strongest. Inhibition of proteasomal degradation by co-treatment of the cells with MG132, a specific and cell-permeable proteasome inhibitor, completely abolished cisplatin-induced degradation of HIF-1α (Figure 1D), suggesting that cisplatin induced downregulation of HIF-1α through promoting HIF-1α degradation.
Figure 1. Cisplatin downregulates HIF-1α protein level and induces apoptosis in cisplatin-sensitive ovarian cancer cells but not in cisplatin-resistant ovarian cancer cells.
(A) A2780, A2780/CP, PEO1, and PEO4 ovarian cancer cells were untreated or treated with the indicated concentrations of cisplatin for 72 h before being subjected to an MTT assay. The data shown are the OD values of treated cell groups at the end of treatment, expressed as a percentage of the OD value of the corresponding untreated cells. (B) A2780, A2780/CP, PEO1, and PEO4 ovarian cancer cells were treated with indicated concentrations of cisplatin for 24 h. Cell lysates were then prepared and subjected to Western blotting analysis using the indicated antibodies. (C) A2780, A2780/CP, PEO1, and PEO4 ovarian cancer cells were treated with 50 µM cisplatin for the indicated times. Cell lysates were then prepared and subjected to Western blotting analysis using the indicated antibodies. (D) A2780 and PEO1 ovarian cancer cells were treated with 50 µM cisplatin with or without 5 µM MG132 for the indicated times. Cell lysates were then prepared and subjected to Western blotting analysis using the indicated antibodies.
3.2. Overexpression of a degradation-resistant HIF-1α reduces cisplatin-induced apoptosis in cisplatin-sensitive ovarian cancer cells
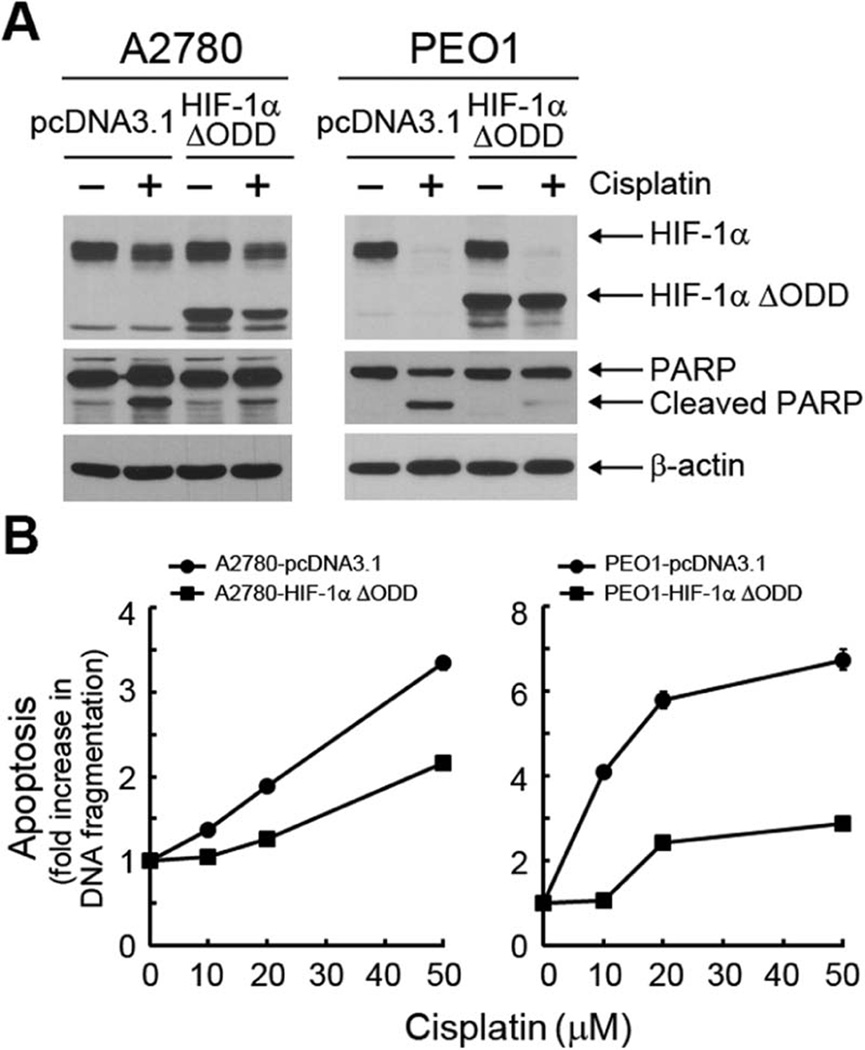
To determine whether there was a causal link between cisplatin-induced downregulation of HIF-1α and cisplatin-induced induction of apoptosis, we examined whether overexpression of a degradation-resistant HIF-1α, in which the oxygen-dependent degradation (ODD) domain of HIF-1α was deleted [24,35], could prevent cisplatin-induced apoptosis. Figure 2A shows that overexpression of HIF-1α ΔODD in A2780 and PEO1 cells strongly protected the cells against cisplatin-induced apoptosis, as shown by a marked reduction in the amount of PARP cleavage, particularly in PEO1 cells, compared to the results for the control vector-transfected cells. Following treatment with cisplatin at doses ranging from 10 µM to 50 µM, a quantitative apoptosis ELISA detected considerably less histone-associated DNA fragmentation in A2780 and PEO1 cells transfected with HIF-1α-ΔODD than in A2780 and PEO1 cells transfected with the control vector (Figure 2B). The protective effect of HIF-1α ΔODD overexpression was greater in PEO1 cells than in A2780 cells. These findings suggest that cisplatin-induced downregulation of HIF-1α played a critical role in inducing apoptosis in the parental cisplatinsensitive ovarian cancer cells.
Figure 2. Overexpression of a degradation-resistant HIF-1α reduces cisplatin-induced apoptosis in cisplatin-sensitive ovarian cancer cells.
(A) and (B) A2780 and PEO1 ovarian cancer cells were transiently transfected with HIF-1α ΔODD plasmid or pcDNA3.1 control vector for 24 h. In (A), the cells were then either untreated or treated with 20 µM cisplatin for another 24 h. Cell lysates were then prepared and subjected to Western blotting analysis using the indicated antibodies. In (B), the cells were then treated with the indicated concentrations of cisplatin for 24 h and then subjected to an apoptosis ELISA. The bars indicating the standard deviations for apoptosis are not seen because they are smaller than the symbols on the lines, except for the 50 µM cisplatin dose in PEO1-pcDNA3.1 cells.
3.3. Knockdown of HIF-1α expression restores sensitivity to cisplatin in cisplatin-resistant ovarian cancer cells
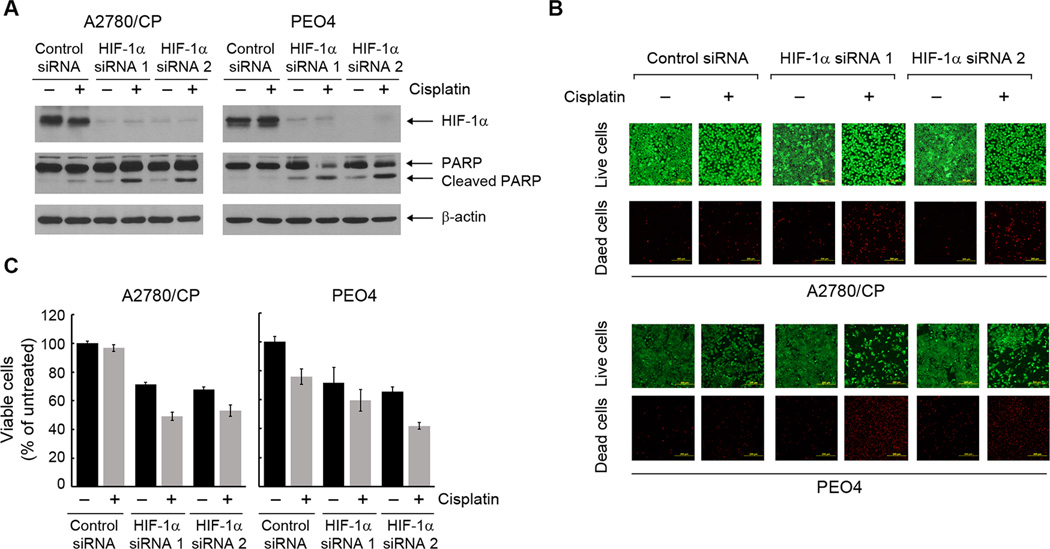
We next examined whether knockdown of HIF-1α by siRNA in the cisplatin-resistant cells could restore their sensitivity to cisplatin. Figure 3A shows that successful knockdown of HIF-1α by each of two independent siRNAs led to strong induction of PARP cleavage in A2780/CP and PEO4 cells, whereas cisplatin alone or HIF-1α siRNA alone induced either no PARP cleavage or only weak PARP cleavage. To further confirm cell death in the cisplatin-resistant cells following treatment with cisplatin plus HIF-1α siRNA, we used a fluorescence-based live/dead cell viability assay (Figure 3B). The number of dead cells, identified by bright red fluorescence, was markedly higher following treatment with cisplatin plus HIF-1α siRNA than following treatment with either cisplatin alone or HIF-1α siRNA alone. We confirmed this important finding by an MTT assay (Figure 3C). Of note, because the experiment was limited by the transfection efficiency of siRNA, HIF-1α knockdown was not expected to occur in 100% of the cells following transient treatment with siRNA; nevertheless, HIF-1α knockdown by siRNA clearly improved the response of cisplatin-resistant cells to cisplatin treatment.
Figure 3. Genetic knockdown of HIF-1α expression restores sensitivity to cisplatin in cisplatin-resistant ovarian cancer cells.
(A) and (B) A2780/CP and PEO4 ovarian cancer cells were transiently transfected with one of two different HIF-1α-specific siRNAs or control siRNA using Lipofectamine 2000 for 72 h. The cells were then either untreated or treated with 20 µM cisplatin for 24 h. In (A), cell lysates were then prepared and subjected to Western blotting analysis using the indicated antibodies. In (B), the cells were subjected to a live/dead cell viability assay and observed under a fluorescence microscope (scale bars, 200 µm). (C) A2780/CP and PEO4 cells were subjected to HIF-1α knockdown as described in (A). The cells were then either untreated or treated with 20 µM cisplatin for 72 h before being subjected to an MTT assay. The data shown are the OD values of treated cell groups at the end of treatment, expressed as a percentage of the OD value of the corresponding untreated cells.
Taken together with the finding in Figures 1 and 2, these results strongly indicate that lack of downregulation of HIF-1α in response to cisplatin treatment is a novel mechanism by which these ovarian cancer cells resist cisplatin-induced apoptosis. Further, these results suggest that HIF-1α downregulation is a promising strategy for restoring sensitivity to cisplatin in cisplatin-resistant ovarian cancer cells.
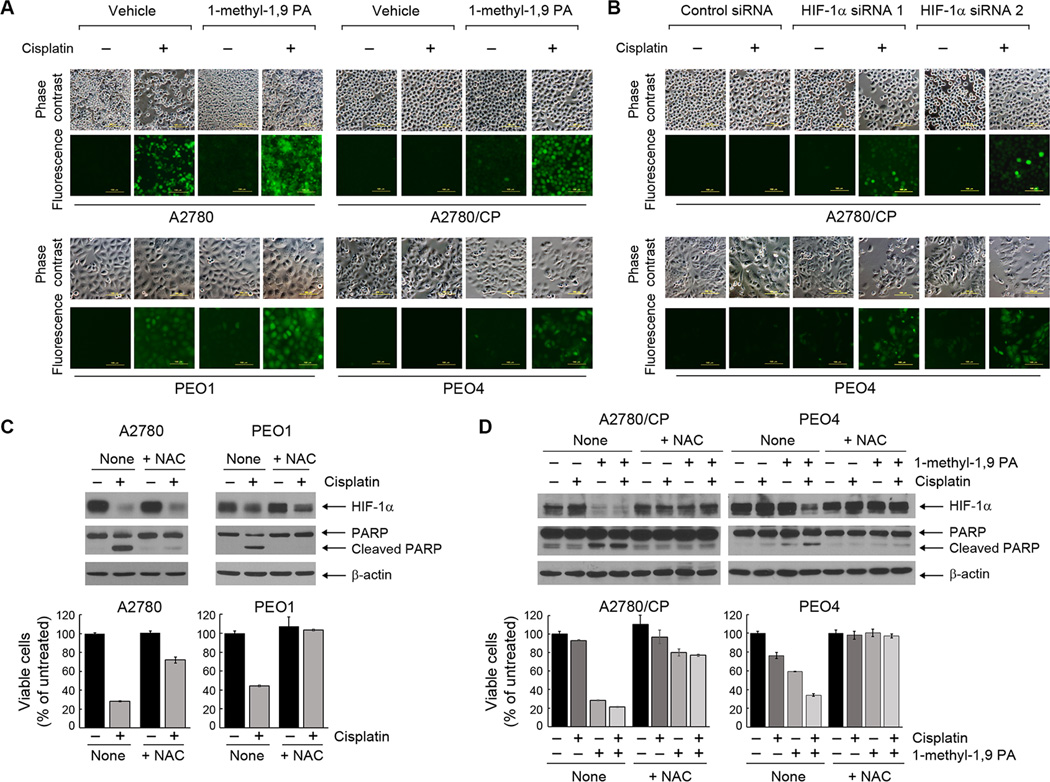
3.4. Pharmacological promotion of HIF-1α degradation enhances sensitivity to cisplatin in both cisplatin-sensitive and cisplatin-resistant ovarian cancer cells
We previously reported that a derivative of 1, 9-pyrazoloanthrone (1, 9 PA), 1-methyl-1, 9 PA, can induce strong downregulation of HIF-1α in multiple types of cancer cells by promoting HIF-1α protein degradation via the ubiquitination pathway [31]. To determine whether 1-methyl-1, 9 PA could mimic the effect of HIF-1α silencing on sensitizing cisplatin-resistant ovarian cancer cells to cisplatin, we first confirmed that 1-methyl-1, 9 PA could downregulate HIF-1α in A2780, A2780/CP, PEO1, and PEO4 cells, as we previously reported it did in many other cancer cell lines [31]. We then determined whether 1-methyl-1, 9 PA could improve the response of the cells to cisplatin treatment in an HIF-1α downregulation-dependent manner.
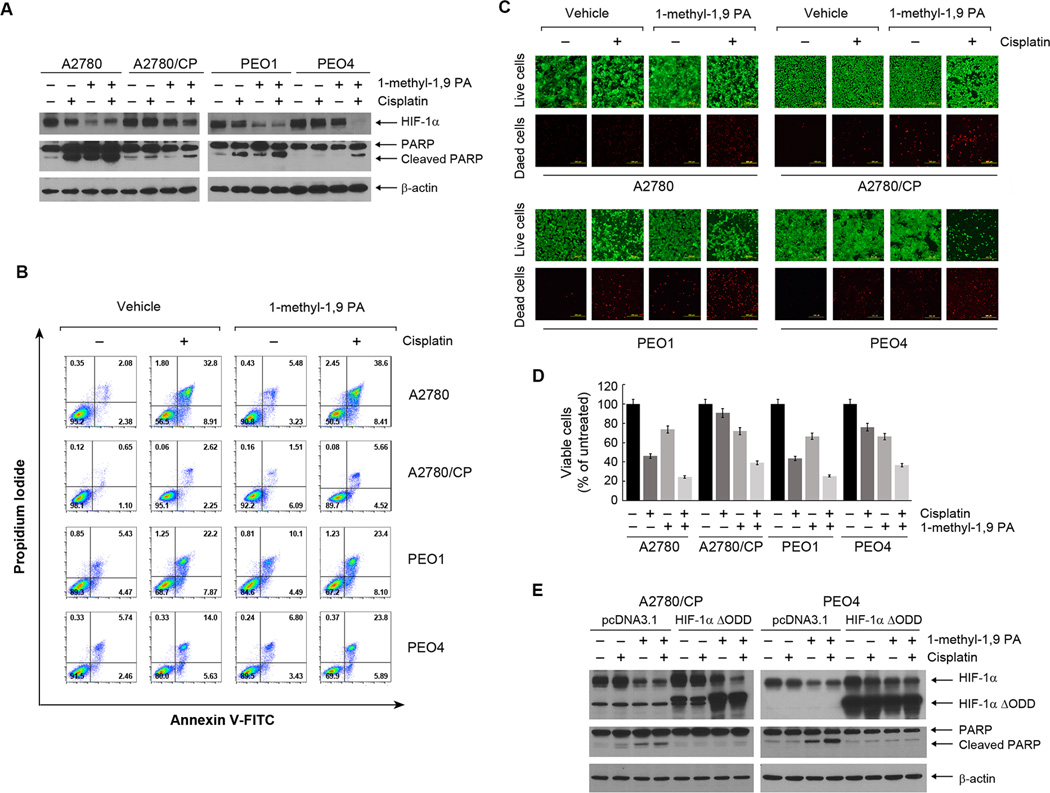
Figure 4A show that treatment of the cells with 1-methyl-1, 9 PA downregulated HIF-1α, and the effect was more evident in the cisplatin-sensitive A2780 and PEO1 cells than in the cisplatin-resistant A2780/CP and PEO4 cells. Importantly, HIF-1α downregulation was substantially greater with the combination of cisplatin and 1-methyl-1, 9 PA than with cisplatin alone, particularly in the cisplatin-resistant A2780/CP and PEO4 cells. The combination of cisplatin and 1-methyl-1, 9 PA produced a greater level of apoptosis than was observed with either treatment alone, as shown by the results for PARP cleavage. The conclusion was further shown by the results for annexin V staining by flow cytometric analysis (Figure 4B). 1-methyl-1, 9 PA in combination with cisplatin did not have significant effect on cisplatin-induced accumulation of cells in the S phase of cell cycle in cisplatin-sensitive or -resistant cells; although it appears that 1-methyl-1, 9 PA alone lowered the percentage of cells in the G1 phase in cisplatin-resistant cells but not in cisplatin-sensitive cells (Supplementary Figure 1).
Figure 4. Pharmacological promotion of HIF-1α degradation enhances sensitivity to cisplatin in both cisplatin-sensitive and cisplatin-resistant ovarian cancer cells.
(A) The indicated ovarian cancer cells were treated with 20 µM cisplatin, 10 µM 1-methyl-1, 9 PA, or both for 24 h. Cell lysates were then prepared and subjected to Western blotting analysis using the indicated antibodies. (B) The indicated cells were treated as described in (A) for 36 h, and the cells were then harvested and subjected to flow cytometry analysis after staining with FITC-conjugated annexin V and propidium iodide. (C) The indicated cells were treated as described in (A) for 24 h and then subjected to a live/dead cell viability assay and observed under a fluorescence microscope (scale bars, 200 µm). (D) The indicated cells were treated as described in (A) for 72 h before being subjected to an MTT assay. The data shown are the OD values of treated cell groups at the end of treatment, expressed as a percentage of the OD value of the corresponding untreated cells. (E) A2780/CP and PEO4 ovarian cancer cells were transiently transfected with HIF-1α ΔODD plasmid or pcDNA3.1 control vector for 24 h. After the transfection, the cells were treated with 20 µM cisplatin, 10 µM 1-methyl-1, 9 PA, or both for 24 h as described in (A). Cell lysates were then prepared and subjected to Western blotting analysis using the indicated antibodies.
The effect of the combination treatment on inducing apoptotic cell death in cisplatin-resistance cells was confirmed independently by fluorescence-based live/dead cell viability assay (Figure 4C), MTT cell proliferation and survival assay (Figure 4D), and clonogenic assay (Supplementary Figure 2).
To confirm that this effect of 1-methyl-1, 9 PA was mediated through downregulation of HIF-1α, we transfected A2780/CP and PEO4 cells with the degradation-resistant HIF-1α ΔODD construct. We found that overexpression of HIF-1α ΔODD largely abolished the effect of 1-methyl-1, 9 PA alone and in combination with cisplatin on inducing PARP cleavage (Figure 4E). This result strongly indicates that 1-methyl-1, 9 PA sensitized cisplatin-resistant ovarian cancer cells to cisplatin by downregulating HIF-1α.
3.5. Cisplatin plus HIF-1α downregulation induces apoptosis of cisplatin-resistant ovarian cancer cells through inducing ROS overproduction
As mentioned in the introduction, HIF-1α downregulation may lead to cell death via apoptosis in cancer cells that are highly dependent on the Warburg effect. To elucidate the biochemical mechanism by which targeting HIF-1α sensitized cisplatin-resistant cells to cisplatin treatment, we tested our hypothesis that cisplatin treatment leads to overproduction of ROS in cisplatin-sensitive cells in part through downregulating HIF-1α. By using a green fluorescence-based ROS detection kit, we found that cisplatin induced ROS overproduction in cisplatin-sensitive A2780 and PEO1 cells but not in cisplatin-resistant A2780/CP and PEO4 cells (Figure 5A). In contrast, the combination of cisplatin and 1-methyl-1, 9 PA induced marked ROS overproduction not only in cisplatin-sensitive A2780 and PEO1 cells but also in cisplatin-resistant A2780/CP and PEO4 cells. We further confirmed that result by treating A2780/CP and PEO4 cells with the combination of cisplatin and HIF-1α siRNA (Figure 5B). Of note, the 1-methyl-1, 9-PA-induced ROS enhanced cisplatin-induced DNA damage, as shown by an increase in γ-H2AX-positive cells after the combination treatment (Supplementary Figure 3). Together, these findings indicate that cisplatin treatment led to overproduction of ROS in cisplatin-sensitive ovarian cancer cells and that targeting HIF-1α can enhance cisplatin-induced ROS production in cisplatin-resistant ovarian cancer cells.
Figure 5. Cisplatin plus HIF-1α downregulation induces apoptosis of cisplatin-resistant ovarian cancer cells through inducing ROS overproduction.
(A) The indicated ovarian cancer cells were treated with 20 µM cisplatin, 10 µM 1-methyl-1, 9 PA, or both for 24 h. The cells were then stained with Enzo’s ROS detection kit and observed under a fluorescence microscope (scale bars, 100 µm). (B) A2780/CP and PEO4 ovarian cancer cells were transiently transfected with one of two different HIF-1α-specific siRNAs or control siRNA using Lipofectamine 2000 for 72 h. The cells were then either untreated or treated with 20 µM cisplatin for 24 h. The cells were then stained with Enzo’s ROS detection kit and observed under a fluorescence microscope (scale bars, 100 µm). (C) Top, A2780 and PEO1 ovarian cancer cells were treated with 20 µM cisplatin with or without 5 mM NAC for 24 h. Cell lysates were then prepared and subjected to Western blotting analysis using the indicated antibodies. Bottom, the cells were treated with 20 µM cisplatin with or without 5 mM NAC for 72 h before being subjected to MTT assay. The data shown are the OD values of treated cell groups at the end of treatment, expressed as a percentage of the OD value of the corresponding untreated cells. (D) Top, A2780/CP and PEO4 cells were treated with 20 µM cisplatin, 10 µM 1-methyl-1, 9 PA, or both, in the presence or absence of 5 mM NAC for 24 h. Cell lysates were then prepared and subjected to Western blotting analysis using the indicated antibodies. Bottom, the cells were treated as described above for 72 h before being subjected to MTT assay as in (C).
We next examined whether the overproduction of ROS as a result of HIF-1α downregulation played a causal role in the induction of apoptosis following treatment with cisplatin in cisplatin-sensitive cells and following treatment with the combination of cisplatin and 1-methyl-1, 9 PA in cisplatin-resistant cells. As shown in Figure 5C, cisplatin-induced apoptosis in A2780 and PEO1 cells, as measured by detection of PARP cleavage and quantitation of histone-associated DNA fragmentation, was markedly reduced when the cells were co-treated with N-acetyl cysteine (NAC), a potent and cell-permeable antioxidant. Similarly, apoptosis induced by the combination of cisplatin and 1-methyl-1, 9 PA in A2780/CP and PEO4 cells was markedly reduced in the presence of NAC (Figure 5D). Together, these results strongly indicate that HIF-1α downregulation sensitizes cisplatin-resistant ovarian cancer cells by inducing overproduction of ROS following cisplatin treatment.
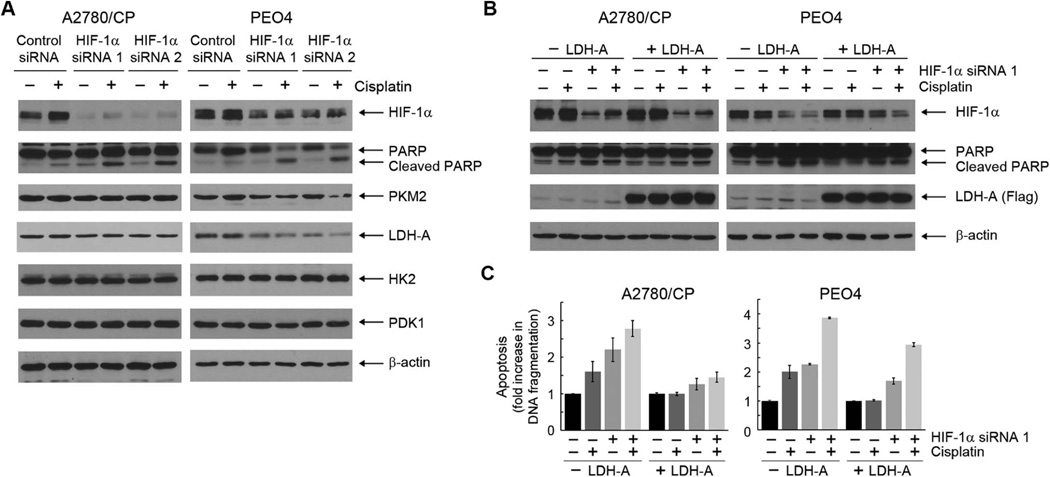
3.6. Apoptosis induced by cisplatin plus HIF-1α downregulation can be partially reduced by overexpression of LDH-A
To further confirm the role of overproduction of ROS, as a result of redirection of aerobic glycolysis to mitochondrial oxidative phosphorylation through HIF-1α downregulation, in restoring sensitivity to cisplatin in cisplatin-resistant cells, we first examined changes in the expression of a few glycolytic enzymes known to be regulated by HIF-1 transcription factor. Figure 6A shows that among several glycolytic enzymes examined, including PKM2, LDH-A, HK2, and PDK1, LDH-A was the enzyme exhibiting the greatest decrease in expression level following knockdown of HIF-1α. We then examined whether experimental overexpression of LDH-A, which was expected to drive the flow of glucose metabolism to glycolysis from mitochondrial oxidative phosphorylation, could reduce cisplatin-induced apoptosis. As shown by detection of PARP cleavage using Western blotting (Figure 6B) and measurement of histone-associated DNA fragmentation by quantitative apoptosis ELISA (Figure 6C), overexpression of a flag-tagged LDH-A by lentiviral infection clearly reduced, albeit not completely, the level of apoptosis following treatment with the combination of cisplatin and HIF-1α siRNA in A2780/CP and PEO4 cells. As a technical note, because of the cellular stress compounded by lentiviral infection and the following siRNA Lipofectamine transfection, both A2780/CP and PEO4 cells exhibited cisplatin-induced apoptosis, whereas such cisplatin-induced apoptosis was not observed in these cells under the same conditions in experiments described earlier in this paper; however, the combination treatment clearly produced a higher level of apoptosis than did cisplatin or HIF-1α siRNA alone.
Figure 6. Apoptosis induced by cisplatin plus HIF-1α downregulation can be partially reduced by overexpression of LDH-A.
(A) A2780/CP and PEO4 ovarian cancer cells were transiently transfected with one of two different HIF-1α-specific siRNAs or control siRNA using Lipofectamine 2000 for 72 h. The cells were untreated or treated with 20 µM cisplatin for 24 h. Cell lysates were then prepared and subjected to Western blotting analysis using the indicated antibodies. (B) and (C) A2780 and PEO1 ovarian cancer cells were infected with a pLEX-based recombinant lentivirus containing human LDH-A cDNA or not for 24 h. The cells were then transiently transfected with each of two different HIF-1α-specific siRNAs or control siRNA using Lipofectamine 2000 for 72 h. In the last 24 h of siRNA treatment, the cells were exposed to 20 µM cisplatin or not. After the treatment, cell lysates were prepared and subjected to Western blotting analysis using the indicated antibodies (B) or subjected to apoptosis ELISA (C).
Together, our findings support our hypothesis that HIF-1α downregulation overcomes resistance of ovarian cancer cells to cisplatin through redirecting aerobic glycolysis, which is dominant in cisplatin-resistant cancer cells, towards mitochondrial oxidative phosphorylation, leading to cell death through overproduction of ROS.
4. Discussion
Here, we report findings from our study testing our hypothesis that cisplatin resistance in ovarian cancer cells can be overcome by targeting HIF-1-regulated cancer metabolism. First, we demonstrated that cisplatin induced apoptosis in cisplatin-sensitive ovarian cancer cells in a HIF-1α-downregulation-dependent manner and that resistance to cisplatin-induced apoptosis could be overcome by downregulating HIF-1α through RNA interference or pharmacological promotion of HIF-1α degradation. Next, we studied the mechanism by which downregulating HIF-1α improves response of cisplatin-resistant ovarian cancer cells and found that redirection of HIF-1-regulated aerobic glycolysis towards mitochondrial oxidative phosphorylation can sensitize the cells to cisplatin through overproduction of ROS that leads to apoptosis.
The cytotoxic mode of action of cisplatin is well established and is mediated by interaction of cisplatin with DNA to form DNA adducts, primarily intrastrand crosslink adducts [36]. Resistance mechanisms that limit the extent of DNA damage by cisplatin include reduced drug uptake, increased drug inactivation, and increased DNA adduct repair [36]. However, strategies developed on the basis of these known resistance mechanisms did not fully reverse cisplatin resistance. It is also well documented that cisplatin treatment can activate multiple signal transduction pathways, including those involving ATR, p53, p73, and MAPK [36]. New treatment strategies based on a departure from the classic views of cisplatin’s mechanism of action and mechanisms of resistance may offer new hope for improving the rate of response to cisplatin in ovarian cancer patients.
HIF-1, a master regulator of oxygen homeostasis in almost all nucleated mammalian cells, activates over 100 genes involved in a variety of cellular functions that have therapeutic implications, including angiogenesis (via activating the gene coding for VEGF-A [37]) and cancer metabolism. Cancer cells have a higher demand than other cells for metabolic inputs to aid growth and proliferation, and this could be an Achilles heel of cancer cells that could be exploited therapeutically. In our current study, we explored an experimental strategy for overcoming cisplatin resistance of human ovarian cancer from the new perspective of targeting HIF-1-regulated cancer cell metabolism. By using two pairs of genetically matched, cisplatin-sensitive and cisplatin-resistant ovarian cancer cell lines, we showed that cisplatin can induce downregulation of HIF-1α in cisplatin-sensitive ovarian cancer cells but not in the counterpart cisplatin-resistant ovarian cancer cells. Although the exact biochemical mechanism underlying cisplatin-induced downregulation of HIF-1α is still not known and further exploration of the detailed mechanism in a separate study is clearly needed, we found that cisplatin-induced downregulation of HIF-1α appeared to occur through promotion of HIF-1α degradation.
We used a small molecule compound, 1-methyl-1, 9 PA, that we previously reported can promote HIF-1α degradation [31]. 1-methyl-1, 9 PA downregulated HIF-1α level as did cisplatin but apparently via a different mechanism. We demonstrated that 1-methyl-1, 9 PA improved response of the cisplatin-resistant A2780/CP and PEO4 cells to cisplatin treatment in a HIF-1α-downregulation-dependent manner, and we demonstrated that the response to cisplatin was mitigated by co-treatment with the antioxidant NAC, indicating that ROS contributed to the response. It would be ideal to carry out a xenograft study to evaluate this strategy in vivo; however, we were unable to do so because of poor solubility of 1-methyl-1, 9 PA in saline (data not shown). HIF-1α is currently considered a hot target for drug development; however, as is typical for transcription factors, direct targeting of HIF-1α is technically challenging [38,39]. As of this writing, there are no Food and Drug Administration–approved drugs targeting HIF-1α.
In addition to regulating cancer metabolism, HIF-1 regulates angiogenesis, cell survival and apoptosis, cell migration, and other cellular processes [37,40]. The potential clinical significance of our current findings can be further explored in two ways. First, it is worth exploring whether a HIF-1α inhibitor that has been tested or is currently being tested in clinical trials, such as EZN-2968, an antisense oligonucleotide inhibitor of HIF-1α [41], can improve response to cisplatin in patients with cisplatin-resistant ovarian cancer. Second, it will be interesting to determine whether results similar to the ones achieved in our current study can be achieved by therapeutic targeting of cancer metabolism–related gene products transcriptionally regulated by HIF-1. One such target candidate is PDK1, inhibition of which could lead to activation of PDH, redirecting cancer cell metabolism from aerobic glycolysis to mitochondrial oxidative phosphorylation [29].
HIF-1 has been implicated in chemoresistance in several preclinical and clinical studies [37]. It is not known whether resistance to drugs other than cisplatin can be overcome through targeting HIF-1-regulated cancer metabolism, as we reported in the current study. Addressing this question would require development of isogeneic drug-sensitive and drug-resistant cell line models similar to the ones that we used in our current study. In addition, cisplatin is used in several other types of solid tumors; it would therefore be interesting to test whether the strategy of combining cisplatin with HIF-1α downregulation works in other types of cancers with acquired or inherent resistance to cisplatin.
In summary, in a departure from conventional strategies for overcoming cisplatin resistance, we explored an alternative therapeutic strategy linking cisplatin resistance to HIF-1-regulated metabolism in ovarian cancer cells. Our findings suggest that HIF-1α and possibly HIF-1-regulated cancer metabolism genes could be novel targets for overcoming cisplatin resistance in ovarian cancer. Further exploration of this untested area may open a new avenue toward overcoming cisplatin resistance, which could lead to a major advance in our efforts to improve clinical outcomes of ovarian cancer treatment.
Supplementary Material
Highlights.
Cisplatin downregulates HIF-1α in cisplatin-sensitive ovarian cancer cells
Inhibition of HIF-1α resensitizes cisplatin-resistant ovarian cancer cells to cisplatin
Inhibition of HIF-1α induces ROS overexpression in cisplatin-resistant cells
LDH-A confers resistance to HIF-1α inhibition-induced resensitization to cisplatin
Acknowledgments
This work was supported in part by a challenge grants program of Shanghai Municipal Health Bureau, China (grant number 2013ZYJB0201), and by US National Institutes of Health (NIH) R01 awards (CA179015 and CA129036). The work was also supported in part by the NIH through MD Anderson’s Cancer Center Support Grant, CA016672. We thank Stephanie Deming of the Department of Scientific Publications at The University of Texas MD Anderson Cancer Center for editorial assistance.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of interest
The authors declare no conflict of interest.
References
- 1.Jayson GC, Kohn EC, Kitchener HC, Ledermann JA. Ovarian cancer. Lancet. 2014;384:1376–1388. doi: 10.1016/S0140-6736(13)62146-7. [DOI] [PubMed] [Google Scholar]
- 2.Tew WP, Fleming GF. Treatment of ovarian cancer in the older woman. Gynecol. Oncol. 2015;136:136–142. doi: 10.1016/j.ygyno.2014.10.028. [DOI] [PubMed] [Google Scholar]
- 3.Liu J, Matulonis UA. New strategies in ovarian cancer: translating the molecular complexity of ovarian cancer into treatment advances. Clin. Cancer Res. 2014;20:5150–5156. doi: 10.1158/1078-0432.CCR-14-1312. [DOI] [PubMed] [Google Scholar]
- 4.Davis A, Tinker AV, Friedlander M. "Platinum resistant" ovarian cancer: what is it, who to treat and how to measure benefit? Gynecol. Oncol. 2014;133:624–631. doi: 10.1016/j.ygyno.2014.02.038. [DOI] [PubMed] [Google Scholar]
- 5.Della PC, Banerjee S. Bevacizumab in combination with chemotherapy in platinum-sensitive ovarian cancer. Onco. Targets. Ther. 2014;7:1025–1032. doi: 10.2147/OTT.S40527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Eskander RN, Tewari KS. PARP inhibition and synthetic lethality in ovarian cancer. Expert. Rev. Clin. Pharmacol. 2014;7:613–622. doi: 10.1586/17512433.2014.930662. [DOI] [PubMed] [Google Scholar]
- 7.Damia G, Sessa C. Successes and limitations of targeted cancer therapy in ovarian cancer. Prog. Tumor Res. 2014;41:89–97. doi: 10.1159/000355905. [DOI] [PubMed] [Google Scholar]
- 8.Vander Heiden MG, Locasale JW, Swanson KD, Sharfi H, Heffron GJ, Amador-Noguez D, Christofk HR, Wagner G, Rabinowitz JD, Asara JM, Cantley LC. Evidence for an alternative glycolytic pathway in rapidly proliferating cells. Science. 2010;329:1492–1499. doi: 10.1126/science.1188015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Vander Heiden MG. Targeting cancer metabolism: a therapeutic window opens. Nat. Rev. Drug Discov. 2011;10:671–684. doi: 10.1038/nrd3504. [DOI] [PubMed] [Google Scholar]
- 10.Vander Heiden MG, Cantley LC, Thompson CB. Understanding the Warburg effect: the metabolic requirements of cell proliferation. Science. 2009;324:1029–1033. doi: 10.1126/science.1160809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kroemer G, Pouyssegur J. Tumor cell metabolism: cancer's Achilles' heel. Cancer Cell. 2008;13:472–482. doi: 10.1016/j.ccr.2008.05.005. [DOI] [PubMed] [Google Scholar]
- 12.Hsu PP, Sabatini DM. Cancer cell metabolism: Warburg and beyond. Cell. 2008;134:703–707. doi: 10.1016/j.cell.2008.08.021. [DOI] [PubMed] [Google Scholar]
- 13.Warburg O. On the origin of cancer cells. Science. 1956;123:309–314. doi: 10.1126/science.123.3191.309. [DOI] [PubMed] [Google Scholar]
- 14.Yeung SJ, Pan J, Lee MH. Roles of p53, MYC and HIF-1 in regulating glycolysis - the seventh hallmark of cancer. Cell Mol. Life Sci. 2008;65:3981–3999. doi: 10.1007/s00018-008-8224-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Semenza GL. HIF-1: upstream and downstream of cancer metabolism. Curr. Opin. Genet. Dev. 2010;20:51–56. doi: 10.1016/j.gde.2009.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Aragones J, Fraisl P, Baes M, Carmeliet P. Oxygen sensors at the crossroad of metabolism. Cell Metab. 2009;9:11–22. doi: 10.1016/j.cmet.2008.10.001. [DOI] [PubMed] [Google Scholar]
- 17.Selak MA, Armour SM, MacKenzie ED, Boulahbel H, Watson DG, Mansfield KD, Pan Y, Simon MC, Thompson CB, Gottlieb E. Succinate links TCA cycle dysfunction to oncogenesis by inhibiting HIF-alpha prolyl hydroxylase. Cancer Cell. 2005;7:77–85. doi: 10.1016/j.ccr.2004.11.022. [DOI] [PubMed] [Google Scholar]
- 18.Zhang H, Gao P, Fukuda R, Kumar G, Krishnamachary B, Zeller KI, Dang CV, Semenza GL. HIF-1 inhibits mitochondrial biogenesis and cellular respiration in VHL-deficient renal cell carcinoma by repression of C-MYC activity. Cancer Cell. 2007;11:407–420. doi: 10.1016/j.ccr.2007.04.001. [DOI] [PubMed] [Google Scholar]
- 19.Kim JW, Tchernyshyov I, Semenza GL, Dang CV. HIF-1-mediated expression of pyruvate dehydrogenase kinase: a metabolic switch required for cellular adaptation to hypoxia. Cell Metab. 2006;3:177–185. doi: 10.1016/j.cmet.2006.02.002. [DOI] [PubMed] [Google Scholar]
- 20.Papandreou I, Cairns RA, Fontana L, Lim AL, Denko NC. HIF-1 mediates adaptation to hypoxia by actively downregulating mitochondrial oxygen consumption. Cell Metab. 2006;3:187–197. doi: 10.1016/j.cmet.2006.01.012. [DOI] [PubMed] [Google Scholar]
- 21.Pathania D, Millard M, Neamati N. Opportunities in discovery and delivery of anticancer drugs targeting mitochondria and cancer cell metabolism. Adv. Drug Deliv. Rev. 2009;61:1250–1275. doi: 10.1016/j.addr.2009.05.010. [DOI] [PubMed] [Google Scholar]
- 22.Lu H, Li X, Luo Z, Liu J, Fan Z. Cetuximab reverses the Warburg effect by inhibiting HIF-1-regulated LDH-A. Mol. Cancer Ther. 2013;12:2187–2199. doi: 10.1158/1535-7163.MCT-12-1245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Luwor RB, Lu Y, Li X, Mendelsohn J, Fan Z. The antiepidermal growth factor receptor monoclonal antibody cetuximab/C225 reduces hypoxia-inducible factor-1 alpha, leading to transcriptional inhibition of vascular endothelial growth factor expression. Oncogene. 2005;24:4433–4441. doi: 10.1038/sj.onc.1208625. [DOI] [PubMed] [Google Scholar]
- 24.Li X, Lu Y, Liang K, Pan T, Mendelsohn J, Fan Z. Requirement of hypoxia-inducible factor-1alpha down-regulation in mediating the antitumor activity of the anti-epidermal growth factor receptor monoclonal antibody cetuximab. Mol. Cancer Ther. 2008;7:1207–1217. doi: 10.1158/1535-7163.MCT-07-2187. [DOI] [PubMed] [Google Scholar]
- 25.Liang K, Qiu S, Lu Y, Fan Z. Autocrine/paracrine erythropoietin regulates migration and invasion potential and the stemness of human breast cancer cells. Cancer Biol. Ther. 2014;15:89–98. doi: 10.4161/cbt.26717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Liang K, Esteva FJ, Albarracin C, Stemke-Hale K, Lu Y, Bianchini G, Yang CY, Li Y, Li X, Chen CT, Mills GB, Hortobagyi GN, Mendelsohn J, Hung MC, Fan Z. Recombinant human erythropoietin antagonizes trastuzumab treatment of breast cancer cells via Jak2-mediated Src activation and PTEN inactivation. Cancer Cell. 2010;18:423–435. doi: 10.1016/j.ccr.2010.10.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Franken NA, Rodermond HM, Stap J, Haveman J, van Bree C. Clonogenic assay of cells in vitro. Nat. Protoc. 2006;1:2315–2319. doi: 10.1038/nprot.2006.339. [DOI] [PubMed] [Google Scholar]
- 28.Lu H, Liang K, Lu Y, Fan Z. The anti-EGFR antibody cetuximab sensitizes human head and neck squamous cell carcinoma cells to radiation in part through inhibiting radiation-induced upregulation of HIF-1alpha. Cancer Lett. 2012;322:78–85. doi: 10.1016/j.canlet.2012.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bonnet S, Archer SL, Allalunis-Turner J, Haromy A, Beaulieu C, Thompson R, Lee CT, Lopaschuk GD, Puttagunta L, Bonnet S, Harry G, Hashimoto K, Porter CJ, Andrade MA, Thebaud B, Michelakis ED. A mitochondria-K+ channel axis is suppressed in cancer and its normalization promotes apoptosis and inhibits cancer growth. Cancer Cell. 2007;11:37–51. doi: 10.1016/j.ccr.2006.10.020. [DOI] [PubMed] [Google Scholar]
- 30.Li X, Lu Y, Lu H, Luo J, Hong Y, Fan Z. AMPK-mediated energy homeostasis and associated metabolic effects on cancer cell response and resistance to cetuximab. Oncotarget. 2015;6:11507–11518. doi: 10.18632/oncotarget.3432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lu Y, Li X, Lu H, Fan Z. 1, 9-Pyrazoloanthrones downregulate HIF-1alpha and sensitize cancer cells to cetuximab-mediated anti-EGFR therapy. PLoS One. 2010;5:e15823. doi: 10.1371/journal.pone.0015823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Behrens BC, Hamilton TC, Masuda H, Grotzinger KR, Whang-Peng J, Louie KG, Knutsen T, McKoy WM, Young RC, Ozols RF. Characterization of a cis-diamminedichloroplatinum(II)-resistant human ovarian cancer cell line and its use in evaluation of platinum analogues. Cancer Res. 1987;47:414–418. [PubMed] [Google Scholar]
- 33.Langdon SP, Lawrie SS, Hay FG, Hawkes MM, McDonald A, Hayward IP, Schol DJ, Hilgers J, Leonard RC, Smyth JF. Characterization and properties of nine human ovarian adenocarcinoma cell lines. Cancer Res. 1988;48:6166–6172. [PubMed] [Google Scholar]
- 34.Chu E, Lai GM, Zinn S, Allegra CJ. Resistance of a human ovarian cancer line to 5-fluorouracil associated with decreased levels of 5-fluorouracil in DNA. Mol. Pharmacol. 1990;38:410–417. [PubMed] [Google Scholar]
- 35.Huang LE, Gu J, Schau M, Bunn HF. Regulation of hypoxia-inducible factor 1alpha is mediated by an O2-dependent degradation domain via the ubiquitin-proteasome pathway. Proc. Natl. Acad. Sci. U. S. A. 1998;95:7987–7992. doi: 10.1073/pnas.95.14.7987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Siddik ZH. Cisplatin: mode of cytotoxic action and molecular basis of resistance. Oncogene. 2003;22:7265–7279. doi: 10.1038/sj.onc.1206933. [DOI] [PubMed] [Google Scholar]
- 37.Semenza GL. Defining the role of hypoxia-inducible factor 1 in cancer biology and therapeutics. Oncogene. 2010;29:625–634. doi: 10.1038/onc.2009.441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lin X, David CA, Donnelly JB, Michaelides M, Chandel NS, Huang X, Warrior U, Weinberg F, Tormos KV, Fesik SW, Shen Y. A chemical genomics screen highlights the essential role of mitochondria in HIF-1 regulation. Proc. Natl. Acad. Sci. U. S. A. 2008;105:174–179. doi: 10.1073/pnas.0706585104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Nagle DG, Zhou YD. Natural product-based inhibitors of hypoxia-inducible factor-1 (HIF-1) Curr. Drug Targets. 2006;7:355–369. doi: 10.2174/138945006776054979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Semenza GL. HIF-1 inhibitors for cancer therapy: from gene expression to drug discovery. Curr. Pharm. Des. 2009;15:3839–3843. doi: 10.2174/138161209789649402. [DOI] [PubMed] [Google Scholar]
- 41.Jeong W, Rapisarda A, Park SR, Kinders RJ, Chen A, Melillo G, Turkbey B, Steinberg SM, Choyke P, Doroshow JH, Kummar S. Pilot trial of EZN-2968, an antisense oligonucleotide inhibitor of hypoxia-inducible factor-1 alpha (HIF-1alpha), in patients with refractory solid tumors. Cancer Chemother. Pharmacol. 2014;73:343–348. doi: 10.1007/s00280-013-2362-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.