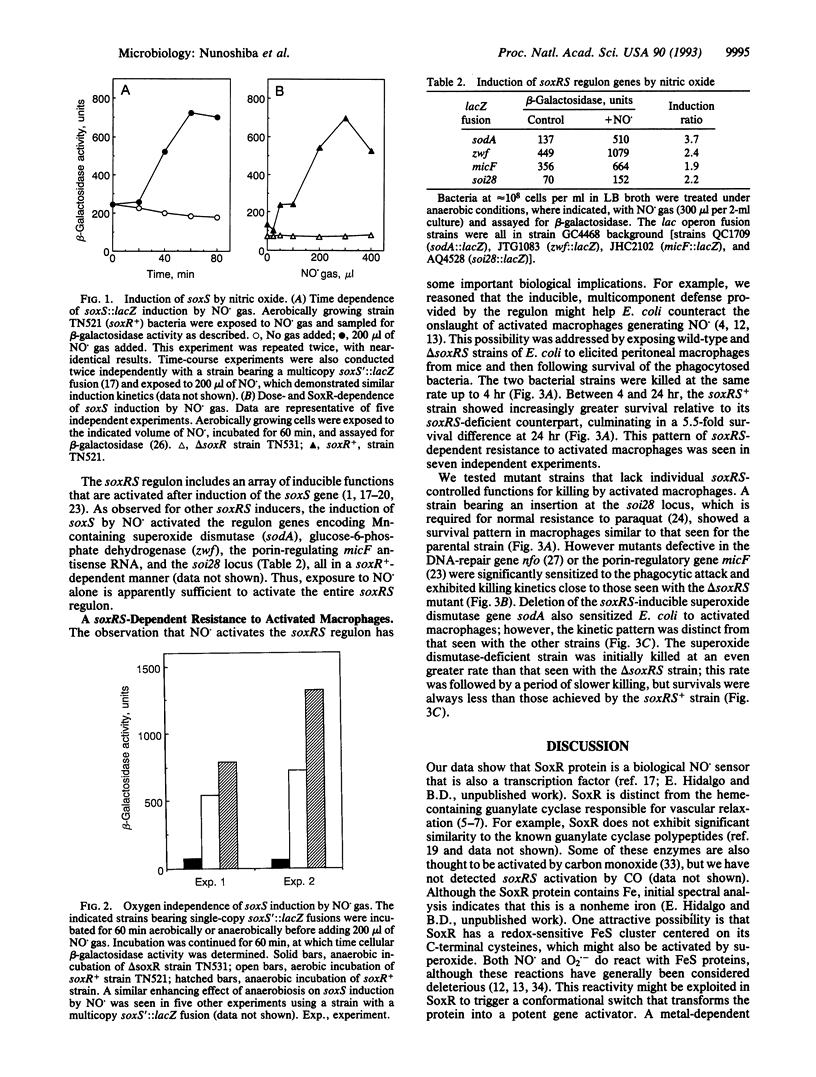
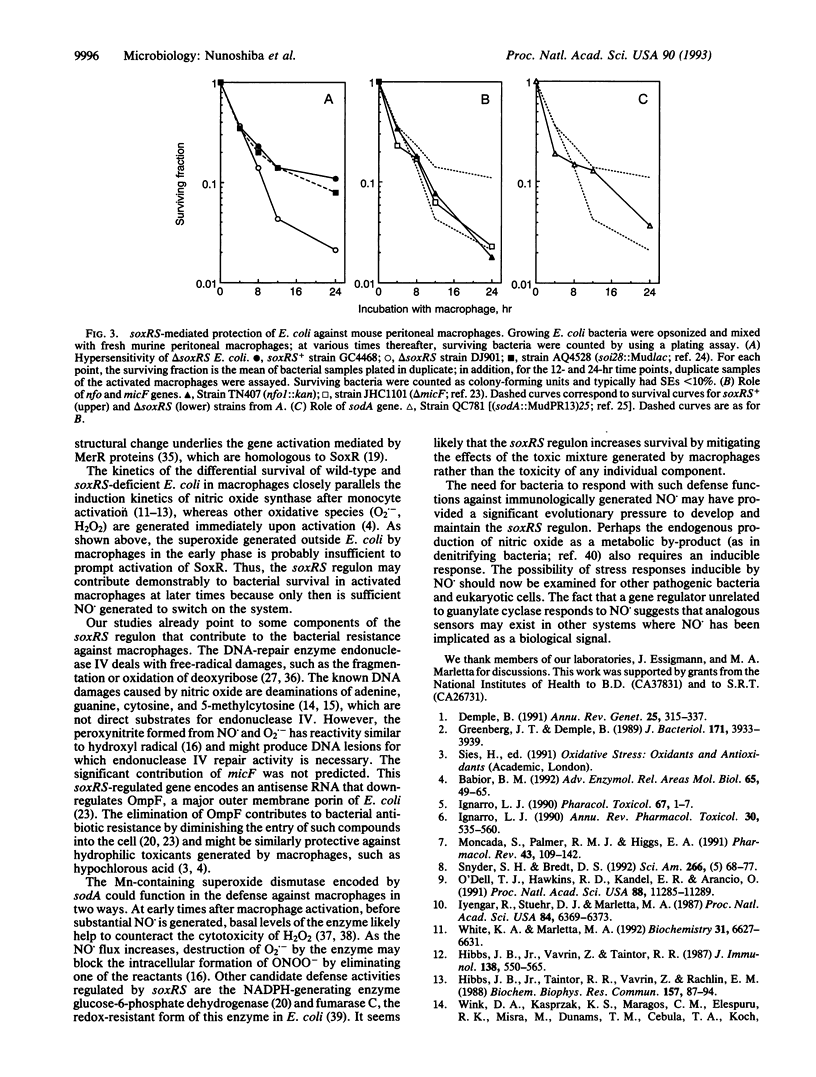
Abstract
Nitric oxide is a free radical (NO) formed biologically through the oxidation of L-arginine by nitric oxide synthases. NO is produced transiently in mammalian cells for intercellular signaling and in copious quantities to cause cytostasis and cytotoxicity. In the latter situation, NO is a deliberate cytotoxic product of activated macrophages, along with other reactive oxygen species such as hydrogen peroxide (H2O2) and superoxide (O2-). Escherichia coli has a complex set of responses to H2O2 and O2- that involves approximately 80 inducible proteins; we wondered whether these bacteria might induce analogous defenses against nitric oxide. We show here that a multigene system controlled by the redox-sensitive transcriptional regulator SoxR is activated by NO in vivo. This induction confers bacterial resistance to activated murine macrophages with kinetics that parallel the production of NO by these cells. Elimination of specific SoxR-regulated genes diminishes the resistance of these bacteria to the cytotoxic macrophages. The required functions include manganese-containing superoxide dismutase, endonuclease IV (a DNA-repair enzyme for oxidative damage), and micF, an antisense regulator of the outer membrane porin OmpF. These results demonstrate that SoxR is a sensor for cellular exposure to NO, and that the soxRS response system may contribute to bacterial virulence.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Amábile-Cuevas C. F., Demple B. Molecular characterization of the soxRS genes of Escherichia coli: two genes control a superoxide stress regulon. Nucleic Acids Res. 1991 Aug 25;19(16):4479–4484. doi: 10.1093/nar/19.16.4479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Babior B. M. The respiratory burst oxidase. Adv Enzymol Relat Areas Mol Biol. 1992;65:49–95. doi: 10.1002/9780470123119.ch2. [DOI] [PubMed] [Google Scholar]
- Buchmeier N. A., Heffron F. Intracellular survival of wild-type Salmonella typhimurium and macrophage-sensitive mutants in diverse populations of macrophages. Infect Immun. 1989 Jan;57(1):1–7. doi: 10.1128/iai.57.1.1-7.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlioz A., Touati D. Isolation of superoxide dismutase mutants in Escherichia coli: is superoxide dismutase necessary for aerobic life? EMBO J. 1986 Mar;5(3):623–630. doi: 10.1002/j.1460-2075.1986.tb04256.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chou J. H., Greenberg J. T., Demple B. Posttranscriptional repression of Escherichia coli OmpF protein in response to redox stress: positive control of the micF antisense RNA by the soxRS locus. J Bacteriol. 1993 Feb;175(4):1026–1031. doi: 10.1128/jb.175.4.1026-1031.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Compan I., Touati D. Interaction of six global transcription regulators in expression of manganese superoxide dismutase in Escherichia coli K-12. J Bacteriol. 1993 Mar;175(6):1687–1696. doi: 10.1128/jb.175.6.1687-1696.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cunningham R. P., Saporito S. M., Spitzer S. G., Weiss B. Endonuclease IV (nfo) mutant of Escherichia coli. J Bacteriol. 1986 Dec;168(3):1120–1127. doi: 10.1128/jb.168.3.1120-1127.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demple B. Regulation of bacterial oxidative stress genes. Annu Rev Genet. 1991;25:315–337. doi: 10.1146/annurev.ge.25.120191.001531. [DOI] [PubMed] [Google Scholar]
- Gardner P. R., Fridovich I. Superoxide sensitivity of the Escherichia coli aconitase. J Biol Chem. 1991 Oct 15;266(29):19328–19333. [PubMed] [Google Scholar]
- Goretski J., Zafiriou O. C., Hollocher T. C. Steady-state nitric oxide concentrations during denitrification. J Biol Chem. 1990 Jul 15;265(20):11535–11538. [PubMed] [Google Scholar]
- Greenberg J. T., Demple B. A global response induced in Escherichia coli by redox-cycling agents overlaps with that induced by peroxide stress. J Bacteriol. 1989 Jul;171(7):3933–3939. doi: 10.1128/jb.171.7.3933-3939.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenberg J. T., Monach P., Chou J. H., Josephy P. D., Demple B. Positive control of a global antioxidant defense regulon activated by superoxide-generating agents in Escherichia coli. Proc Natl Acad Sci U S A. 1990 Aug;87(16):6181–6185. doi: 10.1073/pnas.87.16.6181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hibbs J. B., Jr, Taintor R. R., Vavrin Z., Rachlin E. M. Nitric oxide: a cytotoxic activated macrophage effector molecule. Biochem Biophys Res Commun. 1988 Nov 30;157(1):87–94. doi: 10.1016/s0006-291x(88)80015-9. [DOI] [PubMed] [Google Scholar]
- Hibbs J. B., Jr, Vavrin Z., Taintor R. R. L-arginine is required for expression of the activated macrophage effector mechanism causing selective metabolic inhibition in target cells. J Immunol. 1987 Jan 15;138(2):550–565. [PubMed] [Google Scholar]
- Ignarro L. J. Biosynthesis and metabolism of endothelium-derived nitric oxide. Annu Rev Pharmacol Toxicol. 1990;30:535–560. doi: 10.1146/annurev.pa.30.040190.002535. [DOI] [PubMed] [Google Scholar]
- Ignarro L. J. Haem-dependent activation of guanylate cyclase and cyclic GMP formation by endogenous nitric oxide: a unique transduction mechanism for transcellular signaling. Pharmacol Toxicol. 1990 Jul;67(1):1–7. doi: 10.1111/j.1600-0773.1990.tb00772.x. [DOI] [PubMed] [Google Scholar]
- Imlay J. A., Linn S. DNA damage and oxygen radical toxicity. Science. 1988 Jun 3;240(4857):1302–1309. doi: 10.1126/science.3287616. [DOI] [PubMed] [Google Scholar]
- Iyengar R., Stuehr D. J., Marletta M. A. Macrophage synthesis of nitrite, nitrate, and N-nitrosamines: precursors and role of the respiratory burst. Proc Natl Acad Sci U S A. 1987 Sep;84(18):6369–6373. doi: 10.1073/pnas.84.18.6369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kogoma T., Farr S. B., Joyce K. M., Natvig D. O. Isolation of gene fusions (soi::lacZ) inducible by oxidative stress in Escherichia coli. Proc Natl Acad Sci U S A. 1988 Jul;85(13):4799–4803. doi: 10.1073/pnas.85.13.4799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koppenol W. H., Moreno J. J., Pryor W. A., Ischiropoulos H., Beckman J. S. Peroxynitrite, a cloaked oxidant formed by nitric oxide and superoxide. Chem Res Toxicol. 1992 Nov-Dec;5(6):834–842. doi: 10.1021/tx00030a017. [DOI] [PubMed] [Google Scholar]
- Levin J. D., Johnson A. W., Demple B. Homogeneous Escherichia coli endonuclease IV. Characterization of an enzyme that recognizes oxidative damage in DNA. J Biol Chem. 1988 Jun 15;263(17):8066–8071. [PubMed] [Google Scholar]
- Lewis R. S., Deen W. M., Tannenbaum S. R., Wishnok J. S. Membrane mass spectrometer inlet for quantitation of nitric oxide. Biol Mass Spectrom. 1993 Jan;22(1):45–52. doi: 10.1002/bms.1200220106. [DOI] [PubMed] [Google Scholar]
- Liochev S. I., Fridovich I. Fumarase C, the stable fumarase of Escherichia coli, is controlled by the soxRS regulon. Proc Natl Acad Sci U S A. 1992 Jul 1;89(13):5892–5896. doi: 10.1073/pnas.89.13.5892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lissner C. R., Swanson R. N., O'Brien A. D. Genetic control of the innate resistance of mice to Salmonella typhimurium: expression of the Ity gene in peritoneal and splenic macrophages isolated in vitro. J Immunol. 1983 Dec;131(6):3006–3013. [PubMed] [Google Scholar]
- Marks G. S., Brien J. F., Nakatsu K., McLaughlin B. E. Does carbon monoxide have a physiological function? Trends Pharmacol Sci. 1991 May;12(5):185–188. doi: 10.1016/0165-6147(91)90544-3. [DOI] [PubMed] [Google Scholar]
- Moncada S., Palmer R. M., Higgs E. A. Nitric oxide: physiology, pathophysiology, and pharmacology. Pharmacol Rev. 1991 Jun;43(2):109–142. [PubMed] [Google Scholar]
- Nguyen T., Brunson D., Crespi C. L., Penman B. W., Wishnok J. S., Tannenbaum S. R. DNA damage and mutation in human cells exposed to nitric oxide in vitro. Proc Natl Acad Sci U S A. 1992 Apr 1;89(7):3030–3034. doi: 10.1073/pnas.89.7.3030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nunoshiba T., Hidalgo E., Amábile Cuevas C. F., Demple B. Two-stage control of an oxidative stress regulon: the Escherichia coli SoxR protein triggers redox-inducible expression of the soxS regulatory gene. J Bacteriol. 1992 Oct;174(19):6054–6060. doi: 10.1128/jb.174.19.6054-6060.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Dell T. J., Hawkins R. D., Kandel E. R., Arancio O. Tests of the roles of two diffusible substances in long-term potentiation: evidence for nitric oxide as a possible early retrograde messenger. Proc Natl Acad Sci U S A. 1991 Dec 15;88(24):11285–11289. doi: 10.1073/pnas.88.24.11285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Privalle C. T., Fridovich I. Anaerobic inductions of active forms of superoxide dismutases in Escherichia coli. Free Radic Res Commun. 1991;12-13 Pt 1:419–428. doi: 10.3109/10715769109145812. [DOI] [PubMed] [Google Scholar]
- Snyder S. H., Bredt D. S. Biological roles of nitric oxide. Sci Am. 1992 May;266(5):68-71, 74-7. doi: 10.1038/scientificamerican0592-68. [DOI] [PubMed] [Google Scholar]
- Summers A. O. Untwist and shout: a heavy metal-responsive transcriptional regulator. J Bacteriol. 1992 May;174(10):3097–3101. doi: 10.1128/jb.174.10.3097-3101.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White K. A., Marletta M. A. Nitric oxide synthase is a cytochrome P-450 type hemoprotein. Biochemistry. 1992 Jul 28;31(29):6627–6631. doi: 10.1021/bi00144a001. [DOI] [PubMed] [Google Scholar]
- Wink D. A., Kasprzak K. S., Maragos C. M., Elespuru R. K., Misra M., Dunams T. M., Cebula T. A., Koch W. H., Andrews A. W., Allen J. S. DNA deaminating ability and genotoxicity of nitric oxide and its progenitors. Science. 1991 Nov 15;254(5034):1001–1003. doi: 10.1126/science.1948068. [DOI] [PubMed] [Google Scholar]
- Wu J., Weiss B. Two-stage induction of the soxRS (superoxide response) regulon of Escherichia coli. J Bacteriol. 1992 Jun;174(12):3915–3920. doi: 10.1128/jb.174.12.3915-3920.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]


