Abstract
The purpose of this study was to explore potential mediators of the relationship between depression and obesity in a sample of low-income, minority women. Data were extracted from a sample of 535 women enrolled in a weight loss intervention for the prevention of type 2 diabetes. Using a non-parametric bootstrapping procedure, the potential mediation effects of stress eating and sleep disturbance on the relationship between depression and obesity were tested. Results of a single mediation model indicated that depressive symptomatology was significantly associated with obesity (β = 0.800, SE = 0.290, p = 0.006), and that stress eating (β = 0.166, 95% CI [0.046, 0.328]) and sleep disturbance (β = 1.032, 95% CI [0.612, 1.427]) were significant independent mediators of this relationship. Sleep disturbance remained a significant mediator in a combined mediation model (β = 1.009, 95% CI [0.653, 1.399]). Findings add to the growing literature on the psychosocial factors implicated in the link between depression and obesity, particularly among disadvantaged populations. Future longitudinal research should aim to establish causal pathways between obesity, stress eating, sleep disturbance, and depression.
Keywords: mediation, obesity, depression, stress eating, sleep disturbance
Overweight and obesity are problems of global proportion, with nearly 40% of adults worldwide considered overweight (body mass index [BMI] ≥ 25 kg/m2) and 13% considered obese (BMI ≥ 30 kg/m2) [1]. Over the past three decades, these prevalence rates have risen in high-, middle-, and low-income countries alike [1], with some of the highest rates in North America, Western Europe, Oceania, and parts of Africa and Latin America [2]. This increase in prevalence is alarming given that overweight and in particular obesity increase risk for other health problems, including cardiovascular disease, stroke, metabolic syndrome, cancer, osteoarthritis, sleep apnea, and reproductive problems [3].
Depression is another problem of global proportion, affecting approximately 4% of adults worldwide [4]. Similar to obesity, it is increasingly prevalent across high-, middle-, and low-income countries [4] – and is considered the number one cause of disability in the world [5]. It is associated with increased prevalence of other mental health problems, including anxiety, substance use, impulse control disorders, and suicide [6]. It is also considered a risk factor for a wide range of medical problems including cardiovascular disease, diabetes, and noncompliance with medical treatment [7].
In developed countries such as the United States, significant racial and ethnic differences in the prevalence and treatment of overweight, obesity, and depression have gained attention as health disparities. The prevalence of overweight and obesity are 1.2 times greater among Hispanics and Latinos than among Non-Hispanic Whites [8], and low-income, minority populations are less likely to seek treatment for depression [9]. Postulated reasons for these differences include lower education and income, lack of affordable healthy food and venues for physical activity, increased exposure to fast food, lack of social norms that value healthy eating and exercise, and lack of awareness or stigma about mental health and available services among minority populations [9,10,11].
It is thus clear, from the research to date, that overweight, obesity, and depression are all problems within low-income, minority populations. It may also be that these problems are functionally related to one another within such populations. Studies in the U.S. and abroad have found associations between obesity and depressive symptoms, history of depression, and psychological distress [12,13] – and one important and relatively unexplored element in the link between depression and obesity, particularly in low-income, minority populations, is stress. In neighborhoods of lower income, stressors such as financial strain, work stress, marital and family conflict, and community-wide strain are common [14] and potentially increase stress-related behaviors such as stress-induced eating (otherwise known as “stress eating” [14]) and problems such as sleep apnea [15]. Dysregulated food intake and sleep problems are indeed shared symptoms of depression and obesity [16] – and it may be that these symptoms mediate the relationship between depression and obesity.
A prime example of how obesity, depression, stress eating, and sleep problems may be interrelated can be found in the community of East Harlem, New York. Due to the high rates of poverty [17] and disease, particularly obesity and obesity-related conditions such as diabetes [18], found in East Harlem, academic and community partners in the neighborhood came together in 2005 to develop Project Help Educate to Eliminate Diabetes (HEED), a weight loss intervention for individuals at risk for developing type 2 diabetes. Salient to the current study was the community partners’ assertion that eating and sleep problems were prevalent phenomena in a community characterized by chronic life stress associated with poverty and could be potential causes of the high rates of obesity witnessed in the community.
Building upon previous research on the relationship between depression and obesity, the current study utilized data from Project HEED to examine how stress-related symptoms, specifically stress eating and sleep disturbance, mediate the relationship between depression and obesity. It was hypothesized that there would be a significant direct relationship between depression and obesity, and that this relationship would be mediated by stress eating and sleep disturbance.
METHOD
Participants
Between May and July 2007, participants were recruited via advertisements, announcements, and events at community agencies in East Harlem, NY to participate in Project HEED, a randomized controlled trial aimed at measuring the effectiveness of a peer-led lifestyle intervention in promoting weight loss among overweight adults. Participants were eligible for the trial if they: 1) at least 18 years of age; 2) resided in East Harlem, NY; 3) spoke English or Spanish; 4) had a BMI of at least 25 kg/m2; and 5) were available to participate in a group intervention. Participants were excluded if they: 1) were diagnosed with diabetes; 2) were prescribed glucose-altering medications; or 3) were currently pregnant.
All participants provided written informed consent. Participants were not compensated for completing the baseline measures described below and used for the current analysis. A total of 641 adults were recruited for the study; however, because men constituted only 16% of the sample and have been shown to exhibit lower obesity-depression co-morbidity [20], they were excluded from the current analysis. The study was approved by the Institutional Review Board at the Icahn School of Medicine at Mount Sinai.
Measures
All measures were developed and approved by the Project HEED Evaluation Subcommittee, which identified domains of interest and asked study staff to supply validated scales to explore these domains [19]. The committee piloted scales and items in the community, revised the measures accordingly, and wrote supplemental questions as needed. The measures were compiled into a survey designed for a fourth grade reading level and translated into Spanish. Trained community members piloted the initial survey with 183 East Harlem adults at multiple community locations. After this initial pilot phase, existing measures and scales were adjusted in accordance with the community's input, including the inclusion of a specific question on eating in response to stress [21]. The survey items relevant to this analysis are outlined below.
Obese
Participants’ height and weight were measured by study staff at study entry and used to calculate BMI. In the current study, obesity was entered into the hypothesized mediation model as a dichotomous outcome variable, with a BMI greater than or equal to 30 considered obese and a BMI less than 29 kg/m2 considered non-obese.
Patient Health Questionnaire-8 Item Version (PHQ-8)
Depression was measured using 8 of the 9 items of the PHQ-9 [22]. The question regarding suicidal ideation and intent was excluded, as suicidality was a specific exclusion criterion for the study. On the PHQ-9, participants are asked how often, over the past two weeks, they have experienced symptoms such as apathy, depression, difficulty concentrating, and low self-esteem. Responses are coded as 0, “not at all”; 1, “several days”; 2, “more than half the days”; or 3, “nearly every day.” These responses are summed for a total score, with a PHQ-8 score of 10 or more considered clinically significant depression. Therefore, in the current study, depression was entered into the hypothesized mediation model as a dichotomous variable, with participants categorized as depressed if their PHQ-8 score was greater or equal to 10. The internal consistency of the PHQ-8, measured by Cronbach's alpha, was 0.78.
Stress Eating
Stress eating was assessed using a single item. Participants were asked, “How often do you eat as a way to manage your stress?” Responses were captured via a 5-point Likert scale, with 1 indicating “never” and 5 indicating “always”. Stress eating was entered into the hypothesized mediation model as a dichotomous variable, with participants categorized as stress eaters if they responded “always”, “often”, or “sometimes”.
Sleep Disturbance
Sleep disturbance was operationalized as having high sleep apnea risk and assessed using the Berlin Questionnaire [23]. On the Berlin Questionnaire, participants were asked to respond to nine questions regarding their sleep habits and quality, with response options varying by question. Sample items include: “Do you snore?” (with potential responses of “yes” or “no”); “How often do you snore?” (with potential responses ranging from “never or nearly never” to “nearly every day”); and “How often do you feel tired or fatigued after your sleep?” (with potential responses ranging form “never or nearly never” to “nearly every day”). An individual's sleep apnea risk is classified as “high” or “low” based on his or her responses to these nine items as well as his or her hypertension and obesity status. Sleep disturbance was entered into the hypothesized mediation model as a dichotomous variable, with participants categorized as having sleep disturbance if they met criteria for high sleep apnea risk. The Berlin Questionnaire has been shown to have adequate test-retest reliability [24].
Demographic Variables
A self-report questionnaire was used to assess demographic items including age, ethnicity, race, marital status, and household income.
Procedure
The current study was a secondary, cross-sectional, analysis of baseline data from Project HEED. During Project HEED, study staff met with participants for a baseline assessment after obtaining their written informed consent. During this assessment, study staff first obtained various physical data, including participants’ height and weight. Weight was taken without shoes and using a Siltec PS500L scale (Precision Weighing Balances, Bradford, MA). Study staff then administered the baseline survey to participants. The survey lasted approximately 30 minutes and included the measures described above. After completing the survey, participants were randomized to either an intervention or control group. Additional details concerning the parent study's methods can be found elsewhere [19].
Statistical Analysis
We first described participant characteristics using summary statistics including frequencies, means, and standard deviations. We compared participant characteristics by obesity status (obese v. non-obese) in order to describe any differences among overweight and obese individuals in our sample, using χ2 tests for categorical variables and t-tests for continuous variables. We then used the procedure described by Cuevas and colleagues [25] to examine the total effect of depressive symptomatology on obesity and the indirect effect of depressive symptomatology on obesity through stress eating and sleep disturbance. We controlled for possible confounders and covariates significantly related to main outcome, including age, race, marital status, and income. Each model was tested using the INDIRECT macro in SPSS 20.0 [26,27]. This macro produced information on the direct association between depression and obesity, and multivariate logistic regressions were used to confirm the direct effect measured by the mediation model. The indirect effects were tested using a non-parametric, bias-corrected bootstrapping procedure that provided an empirical approximation of the sampling distribution of the product of the estimated coefficients in the indirect paths using 1,000 resamples from the data set. Parameter estimates were generated using logistic regression. Unstandardized estimates were presented and are reported. All other analyses were conducted in SAS 9.3. Statistical significance was set at the p = 0.05 level.
RESULTS
Participant Characteristics
Table 1 summarizes participant characteristics. Our sample of 535 females had a mean age of 43.7 (SD = 15.0). Participants were predominantly of minority racial status, under-educated, and living in poverty. All were overweight and 60% were obese, with a mean BMI of 32.2 (SD = 5.6). In comparing obese and non-obese participants, African Americans were more likely than other racial or ethnic groups to be obese (74% vs. 57%, respectively, p = 0.001) and obese participants were less likely than non-obese participants to have an annual income less than $15,000 (45% vs. 60%, p = 0.001).
TABLE 1.
Participant characteristics at baseline
| Characteristic | Overall (N=535) | Obese BMI ≥ 30 (N=324; 61%) | Non-Obese BMI 25-29 (N=211; 39%) | p |
|---|---|---|---|---|
| Age, mean ± SD | 43.7 ± 15.0 | 44.6 ± 14.4 | 42.3 ± 15.9 | 0.087 |
| BMI, mean ± SD | 32.2 ± 5.6 | 35.3 ± 5.0 | 27.4 ± 1.4 | <.0001 |
| Race, N (%) | 0.006 | |||
| Black | 108 (20%) | 80 (25%) | 28 (13%) | |
| Hispanic | 408 (76%) | 235 (73%) | 173 (82%) | |
| White | 4 (1%) | 3 (1%) | 5 (2%) | |
| Other | 14 (3%) | 6 (2 %) | 5 (2%) | |
| Income < $15K/year, N (%) | 225 (51%) | 120 (45%) | 105 (60%) | 0.001 |
| Insurance | 0.001 | |||
| No Insurance | 221 (42%) | 109 (34%) | 112 (54%) | |
| Medicaid | 122 (23%) | 84 (26%) | 38 (18%) | |
| Medicare | 45 (8%) | 29 (9%) | 16 (8%) | |
| Commercial | 143 (27%) | 100 (31%) | 43 (21%) | |
| < High school education | 249 (46%) | 142 (44%) | 107 (51%) | 0.102 |
| Married/living with partner | 323 (60%) | 183 (56%) | 140 (66%) | 0.023 |
| Employed | 0.050 | |||
| Working F/P time | 164 (31%) | 109 (34%) | 55 (26%) | |
| Looking for work/laid off | 35 (7%) | 25 (8%) | 10 (5%) | |
| Keeping house/Retired/Unable work | 276 (52%) | 152 (47%) | 124 (59%) | |
| School/Other | 60 (11%) | 38 (12%) | 22 (10%) | |
| Excellent/Very good health | 68 (13%) | 35 (11%) | 33 (16%) | 0.111 |
| Stressful life | 221 (41%) | 143 (44%) | 78 (37%) | 0.099 |
| Extremely/Very stressful | 97 (18%) | 62 (19%) | 35 (17%) | 0.435 |
| Depressed (PHQ8 ≥ 10) | 88 (16%) | 63 (19%) | 25 (12%) | 0.021 |
| Stress eating | 235 (44%) | 162 (50%) | 73 (35%) | 0.001 |
| High sleep apnea risk | 181 (34%) | 164 (51%) | 17 (8%) | <.0001 |
| Current smoker | 43 (8%) | 32 (10%) | 11 (5%) | 0.0723 |
| Drink about once/week (Reference: Never drink) | 154 (29%) | 104 (32%) | 50 (24%) | 0.0402 |
Overall, 16% of participants endorsed a PHQ-8 ≥ 10, indicating clinically significant major depression. Over 40% of participants reported having a stressful life, with 44% reporting stress eating and 34% presenting with high sleep apnea risk. Obese participants were more likely than non-obese participants to be depressed, stress eat, and meet criteria for high sleep apnea risk.
Main Analyses
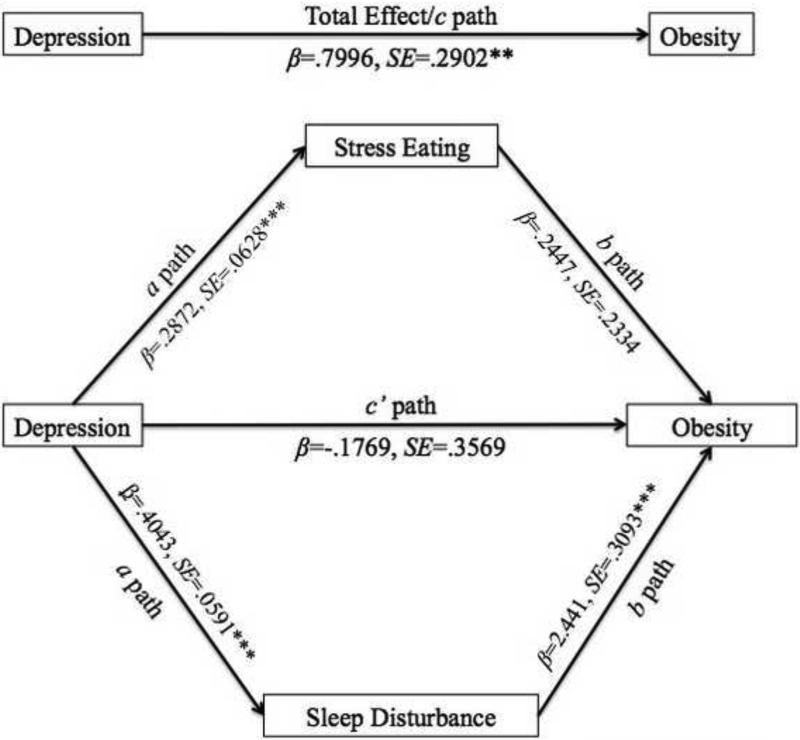
Figure 1 illustrates the hypothesized conceptual model of the relationship between depression and obesity, mediated by stress eating and sleep disturbance. Depressive symptomatology was significantly associated with obesity after adjusting for age, race/ethnicity, income, and marital status (β = 0.800, SE = 0.290, p = 0.006). Depressive symptomatology was significantly associated with greater likelihood of stress eating (β = 0.287, SE = 0.063, p < 0.001), and stress eating was significantly associated with greater likelihood of obesity (β = 0.568, SE = 0.210, p = 0.007). A single mediation model indicated that stress eating was a significant mediator between depression and obesity (indirect effect β = 0.166, 95% CI [0.046, 0.328]). Depressive symptomatology was also significantly associated with greater likelihood of sleep disturbance (β = 0.404, SE = 0.059, p < 0.001), and sleep disturbance was significantly associated with greater likelihood of obesity (β = 2.487, SE = 0.307, p < 0.001). A single mediation model indicated that sleep disturbance, too, was a significant mediator between depression and obesity (indirect effect β = 1.033, 95% CI [0.612, 1.427]).
Figure 1.
Hypothesized conceptual model of the relationship between depression and obesity (c path), mediated by stress eating and sleep disturbance (ab paths). Note: β = Unstandardized parameter estimate from the regression model; SE = Standard error for the parameter estimate; p = probability for testing the null hypothesis that the parameter estimate is equal to 0; *p<0.05, **p<0.001, ***p<0.0001.
Since stress eating and sleep disturbance were significantly related in the sample (p = 0.001), a multiple mediator analysis was carried out to account for the shared variance. This combined mediation analysis showed that sleep disturbance continued to significantly mediate the relationship between depression and obesity (indirect effect β = 1.009, 95% CI [0.653, 1.399]). However, stress eating was no longer a significant mediator in this combined analysis (indirect effect β = 0.684, 95% CI [−0.053, 0.225]). The results of both the single and multiple mediator analyses are displayed in Table 2.
TABLE 2.
Results of single and multiple mediator models for relationship between depression and obesity
| Results of Single and Multiple Mediator Models | |||||
|---|---|---|---|---|---|
| Single Mediator Models | |||||
| Estimate of Indirect Effect | BCa 95% CI | ||||
| Proposed Mediator | 1 | 2 | SE | Lower | Upper |
| Stress Eating | .1631 | .1662 | 0717 | .0464 | .3278 |
| Sleep Disturbance | 1.0053 | 1.0325 | .2097 | .6116 | 1.4268 |
| Multiple Mediator Model | |||||
|---|---|---|---|---|---|
| Estimate of Indirect Effect | BCa 95% CI | ||||
| Proposed Mediators | 1 | 2 | SE | Lower | Upper |
| Stress Eating | .0703 | .0684 | .0708 | −.0527 | .2251 |
| Sleep Disturbance | .9866 | 1.0087 | .1938 | .6533 | 1.399 |
| Combined | 1.0569 | 1.0770 | .2008 | .6985 | 1.4670 |
Notes. Results were calculated using the SPSS INDIRECT macro syntax for estimating models with binary (or continuous) outcomes and continuous mediators. Logistic regression is used for binary outcomes.
1= The indirect effect calculated in the original sample
2= The mean of the indirect effect estimates calculated across 1000 bootstrap samples
SE = The standard deviation of the 1000 bootstrap estimates of the indirect effect
BCa 95% CI = Bias corrected and accelerated 95% confidence interval
Depression = PHQ-8 score ≥ 10
Stress Eating = Endorsed eating as a way to control stress
Sleep Disturbance = High risk of sleep apnea
Covariates = age, race, marital status, income
DISCUSSION
The aim of the current study was to examine the relationships between depression, obesity, and stress-related responses in a large sample of Black and Latino, low-income adults from East Harlem, New York. Specifically, the aim was to understand whether the relationship between depression and obesity was mediated by two specific behavioral correlates of stress – stress eating and sleep disturbance. To meet this aim, we undertook single and multiple mediation analyses. Results of the single mediation analysis found that depressive symptomatology was significantly associated with obesity, and that stress eating and sleep disturbance were indeed significant independent mediators of this relationship. Results of the multiple mediation analysis found that sleep disturbance alone was a significant mediator of this relationship, indicating that it may be a stronger mediator.
These findings add to the growing literature on the psychosocial factors implicated in the link between depression and obesity, particularly among disadvantaged populations. Consistent with the findings presented here, previous studies have found that depressed individuals are likely to report experiencing stress-related symptoms associated with increased weight. For example, Konttinen et al. [14] found that depressive symptomatology is positively associated with emotional (i.e., stress) eating, characterized by the consumption of sugary, sweet foods in particular. Other studies have found that depressed individuals report sleep difficulties [28], and that sleep difficulties increase risk for obesity [29]. The current study extends previous research by examining how stress eating and sleep disturbance link depression and obesity.
Our findings, particularly the full mediation of sleep disturbance in the relationship between depression and obesity, have broad clinical implications. Sleep disturbance is prevalent among low-income, minority populations such as the one in this study. This is attributable to numerous issues, including obesity-related sleep apnea [30,31], but it may also be related to chronic stress [32]. One potential way to improve quality of life among adults similar to those in our sample is to help them to find ways to tackle the chronic and daily stress that may exacerbate the eating and sleep problems they experience. In the current study, participants were not asked to identify specific stressors or reasons for endorsing stress; however, the stressors that low-income, minority individuals face in their daily lives have been well documented and include financial hardship, racial discrimination and prejudice, and lack of access to medical care, proper nutrition, and other resources [33]. This stress is not easily extinguished. It may be important to address the daily and chronic stress these adults face using approaches such as mindfulness-based stress reduction (MBSR), which has been effective in Black and Latino populations [34,35].
An additional way to improve quality of life among adults like those in our sample may be to educate and encourage low-income, minority adults to seek treatment for depression. Although this may seem like anything but a novel suggestion, the fact is that adults from low-income and minority backgrounds often fail to seek mental health treatment due to myriad factors, including cost, stigma, and language and structural barriers [36]. Innovative strategies to improve access to quality mental health care include universal clinical depression screening, expanding care teams to include navigators and community health workers, and embedding care in “everyday” locations, including barbershops and beauty parlors [37].
Limitations of the current study mainly include assessment issues. We assessed for the presence of stress eating using single question rather than a more comprehensive scale and further dichotomized the responses; this decreases the variability of the measure and could cause bias in the results. In addition, the current study was based upon baseline data only; thus, causality between depression, stress eating, sleep disturbance, and obesity cannot be established. However, the current study had several strengths, including a large sample size, measured height and weight, and efforts by the research team to collaborate with community stakeholders in designing a culturally sensitive assessment.
The current study is one of the first to conceptualize stress eating and sleep disturbance as specific mediators of the relationship between depression and obesity in a low-income, minority population. Thus, it serves as a catalyst for future research examining the ways in which stress-related symptoms can affect the relationship between depression and obesity in underserved populations. Such research will help identify modifiable factors that can be targeted to reduce the burden of depression and obesity across the globe.
ACKNOWLEDGEMENTS
This study was supported by the National Center on Minority Health and Health Disparities (1R24 MD001691-03) and the New York State Department of Health Diabetes Prevention and Control Program (C020123 and C021751).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Ng M, Fleming T, Robinson M, et al. Global, regional, and national prevalence of overweight and obesity in children and adults during 1980-2013: a systematic analysis for the Global Burden of Disease Study 2013. Lancet. 2014 Aug;384(9945):766–781. doi: 10.1016/S0140-6736(14)60460-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.World Health Organization . Obesity and overweight [Internet] World Health Organization; Geneva (Switzerland): Jan, 2015. [2015 Apr 1]. Available from: http://www.who.int/mediacentre/factsheets/fs311/en/ [Google Scholar]
- 3.National Heart, Lung, and Blood Institute . What are the health risks of overweight and obesity? [Internet] National Institutes of Health; Bethesda (MD): Jul 13, 2012. [2015 Apr 1]. Available from: http://www.nhlbi.nih.gov/health/health-topics/topics/obe/risks. [Google Scholar]
- 4.World Health Organization . Depression [Internet] World Health Organization; Geneva (Switzerland): Oct, 2012. [2015 Apr 1]. Available from: http://www.who.int/mediacentre/factsheets/fs369/en/ [Google Scholar]
- 5.Smith K. Mental health: A world of depression. Nature. 2014 Nov;515:180–181. doi: 10.1038/515180a. [DOI] [PubMed] [Google Scholar]
- 6.Kessler RC, Berglund P, Demler O, et al. The epidemiology of major depressive disorder: results from the National Comorbidity Survey Replication (NCS-R). JAMA. 2003 Jun;289(23):3095–3105. doi: 10.1001/jama.289.23.3095. [DOI] [PubMed] [Google Scholar]
- 7.Carney RM, Freedland KE, Miller GE, Jaffe AS. Depression as a risk factor for cardiac mortality and morbidity: A review of potential mechanisms. J Psychosom Res. 2002 Oct;53(4):897–902. doi: 10.1016/s0022-3999(02)00311-2. [DOI] [PubMed] [Google Scholar]
- 8.Ogden CL, Lamb MM, Carroll MD, Flegal KM. Obesity and socioeconomic status in Adults: United States 1988-1994. NCHS Data Brief No 50. National Center for Health Statistics; Hyattsville, MD: 2010. [PubMed] [Google Scholar]
- 9.Bahls C. Health policy brief: Achieving equity in health. Robert Wood Johnson Foundation; Princeton, NJ: 2011. [Google Scholar]
- 10.Morland K, Diez Roux AV, Wing S. Supermarkets, other food stores, and obesity: the atherosclerosis risk in communities study. Am J Prev Med. 2006 Apr;30(4):333–339. doi: 10.1016/j.amepre.2005.11.003. [DOI] [PubMed] [Google Scholar]
- 11.Wang Y, Beydoun MA. The obesity epidemic in the United States—gender, age, socioeconomic, racial/ethnic, and geographic characteristics: a systematic review and meta-regression analysis. Epidemiol Rev. 2007 May;29:6–28. doi: 10.1093/epirev/mxm007. [DOI] [PubMed] [Google Scholar]
- 12.Simon GE, Von Korff M, Saunders K, Miglioretti DL, Crane PK, van Belle G, Kessler RC. Association between obesity and psychiatric disorders in the US adult population. Arch Gen Psychiatry. 2006 Jul;63(7):824–830. doi: 10.1001/archpsyc.63.7.824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Markowitz S, Friedman MA, Arent SM. Understanding the relation between depression and obesity: causal mechanisms and implications for treatment. Clin Psychol Sci Pr. 2008 Mar;15(1):1–20. [Google Scholar]
- 14.Konttinen H, Manniesto S, Sarlio-Lahteenkorva S, Silventoinen K, Haukkala A. Emotional eating, depressive symptoms and self-reported food consumption: a population-based study. Appetite. 2010 Jun;54:473–479. doi: 10.1016/j.appet.2010.01.014. [DOI] [PubMed] [Google Scholar]
- 15.Mouchacca J, Abbott GR, Ball K. Associations between psychological stress, eating, physical activity, sedentary behaviours and body weight among women: a longitudinal study. BMC Public Health. 2013 Sep;13:828. doi: 10.1186/1471-2458-13-828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Reeves GM, Postolache TT, Snitker S. Childhood depression and obesity: Connection between these growing problems in growing children. Int J Child Health Hum Dev. 2008 Aug;1(2):103–114. [PMC free article] [PubMed] [Google Scholar]
- 17.Olson EC, Van Wye G, Kerber B, Thorpe L, Frieden TR. Take Care East Harlem: NYC Community Health Profiles, Second Edition. NYC Department of Health and Mental Hygiene; New York, NY: 2006. [Google Scholar]
- 18.Black JL, Macinko J. The changing distribution and determinants of obesity in the neighborhoods of New York City, 2003-2007. Am J Epidemiol. 2010 Apr;171(7):765–775. doi: 10.1093/aje/kwp458. [DOI] [PubMed] [Google Scholar]
- 19.Parikh P, Simon EP, Fei K, Looker H, Goytia C, Horowitz CR. Results of a pilot diabetes prevention intervention in East Harlem, New York City: Project HEED. Am J Public Health. 2010 Apr;100(Suppl):S232–S239. doi: 10.2105/AJPH.2009.170910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dutton GR, Needham BL. Obesity. In: Richards CS, O'Hara MW, editors. The Oxford Handbook of Depression and Comorbidity. Oxford University Press; New York, NY: 2014. pp. 335–348. [Google Scholar]
- 21.Horowitz CR, Eckhardt S, Talavera S, Goytia C, Lorig K. Effectively translating diabetes prevention: a successful model in a historically underserved community. Transl Behav Med. 2011 Sep;1(3):443–452. doi: 10.1007/s13142-011-0067-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kroenke K, Spitzer RL. The PHQ-9: a new depression diagnostic and severity measure. Psychiatr Ann. 2002 Sep;32(9):509–521. [Google Scholar]
- 23.Netzer NC, Stoohs RA, Netzer CM, Clark K, Strohl KP. Using the Berlin Questionnaire to identify patients at risk for the sleep apnea syndrome. Ann Intern Med. 1999 Oct;131(7):485–491. doi: 10.7326/0003-4819-131-7-199910050-00002. [DOI] [PubMed] [Google Scholar]
- 24.Sforza E, Chouchou F, Pichot V, Hermann F, Barthelemy JC, Roche F. Is the Berlin Questionnaire a useful tool to diagnose obstructive sleep apnea in the elderly? Sleep Med. 2011 Feb;12(2):142–146. doi: 10.1016/j.sleep.2010.09.004. [DOI] [PubMed] [Google Scholar]
- 25.Cuevas AG, Reitzel LR, Cao Y, et al. Mediators of discrimination and self-rated health among African Americans. Am J Health Behav. 2013 Nov;37(6):745–754. doi: 10.5993/AJHB.37.6.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Preacher KJ, Hayes AF. SPSS and SAS procedures for estimating indirect effects in simple mediation models. Behav Res Methods Instrum Comput. 2004 Nov;36(4):717–731. doi: 10.3758/bf03206553. [DOI] [PubMed] [Google Scholar]
- 27.Preacher KJ, Hayes AF. Asymptotic and resampling strategies for assessing and comparing indirect effects in multiple mediator models. Behav Res Methods. 2008 Aug;40(3):879–891. doi: 10.3758/brm.40.3.879. [DOI] [PubMed] [Google Scholar]
- 28.Pigeon WR, Pinquart M, Connor K. Meta-analysis of sleep disturbance and suicidal thoughts and behaviors. J Clin Psychiatry. 2012 Sep;73(9):e1160–1167. doi: 10.4088/JCP.11r07586. [DOI] [PubMed] [Google Scholar]
- 29.Spiegel K, Tasali E, Leproult R, Van Cauter E. Effects of poor and short sleep on glucose metabolism and obesity risk. Nat Rev Endocrinol. 2009 May;5(5):253–261. doi: 10.1038/nrendo.2009.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Punjabi NM. The epidemiology of adult obstructive sleep apnea. Proc Am Thorac Soc. 2008 Feb;5(2):136–143. doi: 10.1513/pats.200709-155MG. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Piccolo RS, Yang M, Bilwise DL, Yaggi HK, Araujo AB. Racial and socioeconomic disparities in sleep and chronic disease. Results of a longitudinal investigation. Ethn Dis. 2013;23(4):499–507. Autumn. [PMC free article] [PubMed] [Google Scholar]
- 32.Han KS, Kim L, Shim I. Stress and sleep disorder. Exp Neurobiol. 2012 Dec;21(4):141–150. doi: 10.5607/en.2012.21.4.141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Baum A, Garofalo JP, Yali AM. Socioeconomic status and chronic stress. Does stress account for SES effects on health? Ann N Y Acad Sci. 1999;895:131–144. doi: 10.1111/j.1749-6632.1999.tb08111.x. [DOI] [PubMed] [Google Scholar]
- 34.Szanton SL, Wenzel J, Connolly AB, Piferi RL. Examining mindfulness-based stress reduction: perceptions from minority older adults residing in a low-income housing facility. BMC Complement Altern Med. 2011 May;11:44. doi: 10.1186/1472-6882-11-44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Roth B, Robbins D. Mindfulness-based stress reduction and health-related quality of life: findings from a bilingual inner-city patient population. Psychosom Med. 2004 Jan-Feb;66(1):113–123. doi: 10.1097/01.psy.0000097337.00754.09. [DOI] [PubMed] [Google Scholar]
- 36.Hansen MC, Cabassa LJ. Pathways to depression care: help-seeking experiences of low-income Latinos with diabetes and depression. J Immigr Minor Health. 2012 Dec;14(6):1097–1106. doi: 10.1007/s10903-012-9590-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kazdin AE, Blase SL. Rebooting psychotherapy research and practice to reduce the burden of mental illness. Perspect Psychol Sci. 2011 Jan;6(1):21–37. doi: 10.1177/1745691610393527. [DOI] [PubMed] [Google Scholar]