Abstract
Purpose:
The aim of this study was to evaluate a new surgical technique for implant site preparation that could allow to enhance bone density, ridge width, and implant secondary stability.
Materials and Methods:
The edges of the iliac crests of 2 sheep were exposed and ten 3.8 × 10-mm Dynamix implants (Cortex) were inserted in the left sides using the conventional drilling method (control group). Ten 5 × 10-mm Dynamix implants (Cortex) were inserted in the right sides (test group) using the osseodensification procedure (Versah). After 2 months of healing, the sheep were killed, and biomechanical and histological examinations were performed.
Results:
No implant failures were observed after 2 months of healing. A significant increase of ridge width and bone volume percentage (%BV) (approximately 30% higher) was detected in the test group. Significantly better removal torque values and micromotion under lateral forces (value of actual micromotion) were recorded for the test group in respect with the control group.
Conclusion:
Osseodensification technique used in the present in vivo study was demonstrated to be able to increase the %BV around dental implants inserted in low-density bone in respect to conventional implant drilling techniques, which may play a role in enhancing implant stability and reduce micromotion.
Key Words: bone volume, osseodensification, osseointegration, bone expansion, poor bone density
The implant mechanical stability at the time of surgery, known as primary stability, is a crucial factor to achieve implant osseointegration.1 High primary implant stability is even more necessary in immediate loading protocols, and it was reported that an implant micromotion above 50 to 100 μm could induce periimplant bone resorption or implant failures.2–4
The factors that mainly involved in enhancing implant primary stability are bone density,5,6 surgical protocol,7 and implant thread type, and geometry.8 The insertion torque peak was demonstrated to be directly related to implant primary stability and host bone density9; high insertion torque could significantly increase the initial bone-to-implant contact percentage (%BIC) with respect to implant inserted with low insertion torque values.10 Ottoni et al11 demonstrated a failure reduction rate of 20% in single tooth implant restoration for every 9.8 N cm of torque added.
The mechanical friction between implant surface and bone walls of the osteotomic site gives primary implant stability. The osseointegration process leads to new bone apposition on the implant surface and allows reaching the implant secondary stability that is the functional contact between alive bone and titanium dental implant.
In case of poor bone density, such as upper human jaw, the insufficient bone amount around the implants could negatively influence the histomorphometric parameters (such as %BIC and bone volume percentage [%BV]) and, consequently, both primary and secondary implant stabilities.
Undersized implant site preparation12,13 and the use of osteotomes to condense bone14,15 are surgical techniques proposed to increase primary implant stability and %BIC in poor density bone. Different healing patterns and periimplant bone remodeling models were also observed16,17 between standard site preparation and undersized implant site preparation. Specifically designed implants for low-density bone were also developed18 testifying the hardness of the challenge to reach a sufficient implant stability in poor bone density.
The use of the osteotomes in poor density bone allows fracturing and condensing of bone trabeculae,19,20 but this technique does not improve periimplant bone density (%BV) or implant stability. It is demonstrated that fractured trabeculae in periimplant bone, caused using the osteotome technique, induce a delayed secondary stability with respect to conventional drilling procedures during healing.21
Besides, tooth loss, old age, and removable or unsuitable removable dentures inevitably lead to alveolar bone resorption both in height and width.22 It was reported that bone reduction in a width of approximately 25% after 1 year of tooth extraction and the mandible showed a bone loss rate 4 times higher than the upper maxilla.23 Narrow alveolar bone ridges are common in edentulous patients needing dental implant restoration, and many surgical techniques have been developed, over the years, to perform bone expansion or augmentation. The alveolar ridge splitting/expansion technique in 1 stage was proposed as a valid alternative to the 2-stage Guided Bone Regeneration (GBR).24 The predictability of horizontal and vertical augmentation techniques by bone regeneration, using bone substitutes or autogenous bone, is still not clear, and surgical complications are common.25 However, osteodistraction osteogenesis and ridge splitting technique26 are considered efficient to increase bone width27 with lesser complication incidence.
Osseodensification (OD), a nonextraction technique, was developed by Huwais in 201328 and made possible with specially designed burs to increase bone density as they expand an osteotomy.29 These burs combine advantages of osteotomes with the speed and tactile control of the drilling procedures. Standard drills remove and excavate bone during implant site preparation; while osteotomes preserve bone, they tend to induce fractures of the trabeculae that require long remodeling time and delayed secondary implant stability. The new burs allow bone preservation and condensation through compaction autografting during osteotomy preparation, increasing the periimplant bone density (%BV), and the implant mechanical stability was reported by in vitro testing.30
According to the manufacturer, these special burs demonstrated the ability to expand narrow bone ridges similarly to split crest techniques. The bur geometry, rotating in reverse mode at a rotating speed of 800 to 1500 rpm with profuse saline solution irrigation to prevent bone overheating, allows to compact the bone along the inner surface of the implant osteotomic site without cutting. The bouncing motion (in and out movement) is helpful to create a rate-dependent stress to produce a rate-dependent strain, and allows saline solution pumping to gently pressurize the bone walls. This combination facilitates an increased bone plasticity and bone expansion.
The aim of the present in vivo study is to evaluate the efficacy of this new OD technique of implant site preparation to enhance implant secondary stability, periimplant bone density (%BV) and to increase ridge width in poor density bone.
Materials and Methods
Surgery
The Ethics Committee for Animal Research of the Veterinary School of the University of Teramo (Teramo, Italy) approved the study protocol, which followed guidelines established by the European Union Council Directive of February 2013 (R.D.53/2013).
Two female sheep, 4 to 5 years old, were included in the study. Clinical examination determined that all animals were in good general health. Exclusion criteria included general contraindications (pregnancy, systemic disease) to implant surgery and active infection, or severe inflammation in the area intended for implant placement.
The animals were given thiopental (Thiopental, Höchst, Austria) for induction of anesthesia as needed. After orotracheal intubation and ventilation, anesthesia was sustained with nitrous oxide–oxygen with 0.5% halothane. Physiologic saline solution was administered during surgery for fluid replacement.
The edges of the iliac crests were exposed through a skin incision of 15 cm in length. The skin and fascial layers were opened and closed separately. After dissection of the soft tissues, the bone was exposed and 5 osteotomic sites were prepared in each (left and right) side of the iliac crest for a total of 20 osteotomic implant sites.
On the left side of both animals, the implant conventional drilling procedure was performed after the drill sequence recommended by the manufacturer under profuse saline irrigation (1000 rpm). Ten 3.8 × 10-mm Dynamix implants (Cortex, Shlomi, Israel) were inserted in the left iliac crest side of each sheep (control group) because the bone ridge width did not allow inserting wider diameter implants. The initial bone ridge width measured between 4 and 6 mm and did not allow to insert implants with a diameter wider than 3.8 mm using conventional drilling procedures. At first, we tried to have an osteotomic site of 5 mm with conventional burs but it inevitably involved a bone defect generation (dehiscence). We decided to use 3.8-mm diameter implants in the control group to compare between them implants surrounded by bone and not implants with bone dehiscence to others with healthy bone.
On the right side of both animals, the implant site was prepared using Densah Burs (Versah, LLC, Jackson, MI) (www.versah.com) following the OD protocol for implants used: 2-mm pilot drill (1200 rpm), Densah Bur VT1828 in reverse rotation at 1200 rpm, Densah Bur VT2838 in reverse rotation at 1200 rpm, and Densah Bur VT3848 in reverse rotation at 1200 rpm. Ten 5 × 10-mm Dynamix implants (Cortex) were inserted in the right iliac crest side of each sheep (test group). The burs were used in a bouncing motion under profuse saline sterile solution irrigation. In the right iliac crest, the 5.0-mm diameter implant was used to test whether the ridge expansion could be obtained with this new technique (Figs. 1–2).
Fig. 1.
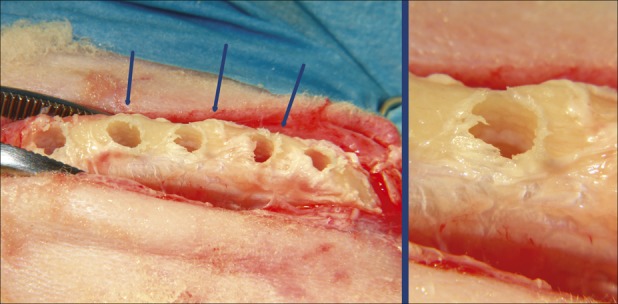
Left side: Clinical photograph of osteotomic site preparation in test group. The bone ridge width was 4 to 6 mm but no dehiscence or fenestration occurred after the 5-mm diameter site preparation. The blue arrows indicate the areas in which the bone ridge expansion is more evident. Right side: A particular osteotomic site in test group. This typical bone hole margins testified that OD burs did not cut the bone but expand it.
Fig. 2.
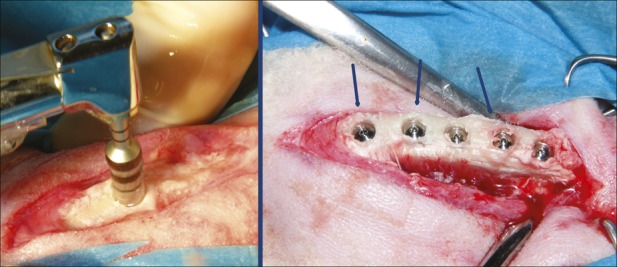
Left side: Clinical photograph of OD burs in action under profuse saline solution irrigation. No bone dehiscence occurred despite the great bur diameter. Right side: Implant positioning in test group. The blue arrows indicate the areas in which bone ridge expansion is more evident.
Cover screws were secured and the surgical wounds were closed by a resorbable periosteal muscular inner suture, followed by an external cutaneous 2-0 silk suture.
Each animal underwent systemic antibiotic therapy for 5 days with 8-mL long acting Clamoxil (Pfizer Limited, Sandwich, MA). After the surgery, animals received appropriate veterinary care and were allowed free access to water and standard laboratory nutritional support throughout the trial period. The sheep were killed 2 months after implantation by an overdose of sodium thiopental (Thiopental, Höchst, Austria).
Value of Actual Micromotion Analysis
Bone blocks containing the implants were retrieved from each side of the iliac crest. Each implant was fitted with a 1-piece 11-mm straight abutment.
The bone blocks were, then, fixed on a customized loading device to measure implant secondary stability according to a previously described technique. Briefly, a digital force gauge (AccuForce Cadet; Ametek, Largo, FL) and, on the opposite side, a digital micrometer (Mitutoyo Digimatic Micrometer, Kawasaki, Japan) was used to measure implant micromotion during load application. Horizontal forces of 25 N/mm were applied onto the abutment of each implant perpendicularly to the major axis, and the lateral displacement was measured by the digital micrometer 10 mm above the crest. This parameter represents the value of the actual micromotion (VAM) and it was previously published.31
RTV Testing
Removal torque value (RTV) was measured at the time of animal sacrifice (2 months after implantation). The RTV was evaluated and recorded for each implant and in every group. It was measured with a digital hand-operated torque wrench (Tonichi STC400CN) by unscrewing the implants until interfacial failure occurred. The digital torque wrench automatically registered the peak removal torque value on the digital display. After the initial interface detachment, the implants were screwed back into their initial position as accurately as possible and retrieved for histological analysis. Although the interfacial detachment created an artifact at the interface, its analysis would still be reliable according to Sennerby et al,32 who used a similar procedure to study the morphology of the bone-metal rupture.
Histological Analysis
After biomechanical measurements, the specimen were immediately fixed in 10% neutral-buffered formalin and processed for histological analysis. After dehydration, the specimens were infiltrated with a methyl-methacrylate resin from a starting solution 50% ethanol/resin and subsequently 100% resin, with each step lasting 24 hours. After polymerization, the blocks were sectioned and then ground down to approximately 40 microns. Toluidine blue staining was used to analyze the different ages and remodeling pattern of the bone. The histomorphometric analysis was performed by digitizing the images from the microscope through a JVC TK-C1380 Color Video Camera (JVC Victor Company, Yokohama, Japan) and a frame grabber. The images were acquired with a ×10 objective over the entire implant surface. Subsequently, the digitized images were analyzed by the image analysis software IAS 2000 (Delta Sistemi, Roma, Italy).
For each section, the 2 most central sections were analyzed and morphometrically measured. The histomorphometric parameters calculated were %BIC and %BV.
Statistical Analysis
Statistical differences of %BIC, %BV, RTV, and VAM between the 2 groups were checked using the statistical software GraphPad Prism 5 (www.graphpad.com). An unpaired t test with Welch correction was used to verify the statistical significance (P < 0.05) of the differences between the average values of each parameter evaluated.
Results
No implant failure was observed after 2 months of healing. The clinical examination, performed immediately after the bone block retrieval, showed no crestal bone resorption and no implants had bone fenestration or dehiscence (Fig. 3). A considerable bone expansion (ridge width) was clinically observed in the test group; 5-mm diameter implants were easily inserted in the narrow ridge (about 4- to 6-mm width) using the OD method without any bone dehiscence around implants.
Fig. 3.
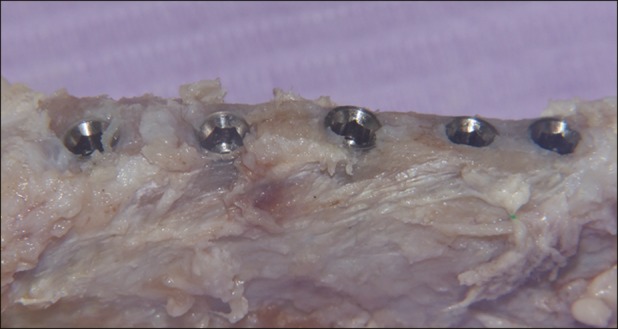
Sample after animal sacrifice in test group. Implants appear osteointegrated and showed no bone resorption.
Histological Results
Control group
A thin layer of newly formed bone covered implant threads. Some bone fractured trabeculae were observed. Newly formed bone connected the fractured bone trabeculae to bone fragments and/or to the implant surface (Fig. 4). The newly woven bone contained fractured bone trabeculae and some bone chips or bone powder. Some bone chips were present at the bone-implant interface. A bone remodeling process, involving bands of osteoid tissue and bone resorption areas, was evident, in a lesser quantity with respect to the test side.
Fig. 4.
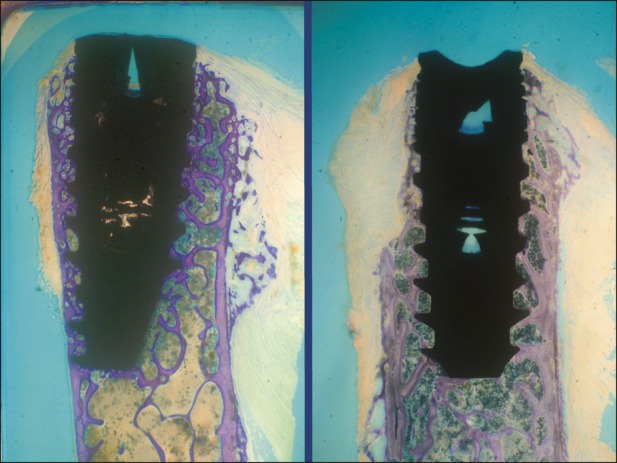
Implants in the control group. It is evident that bone ridge width did not allow inserting the wider diameter implant without creating bone defects. Newly formed bone connected bone trabeculae to titanium implant surface. Bone resorption of approximately 0.3 to 0.5 mm in implant neck area is visible in both samples (toluidine blue, ×10 magnification).
Test group
The thickening of the bone ridge at the coronal zone was observed. The bone, in this area, presented many small circular or ellipsoidal medullary cavities lined by osteoid tissue layers with active osteoblasts (Fig. 5). In the central implant region, bone trabeculae repaired by composite bone in direct contact with the titanium implant surface were observed and ridge expansion was evident (Fig. 6). Some bone chips were involved in the bone remodeling process, mostly in the apical implant zone.
Fig. 5.
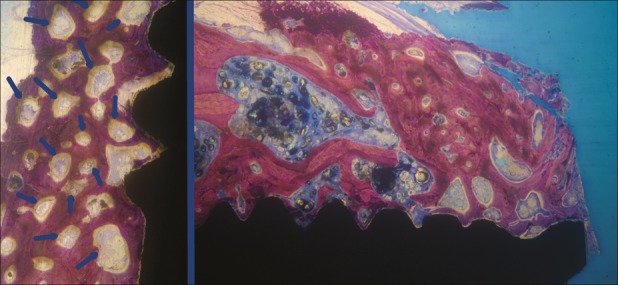
Left side: Test group. The most peculiar feature of the healing pattern was the unusual granular aspect. The granules observed in the trabeculae, seemed like mineralization nuclei (highlighted by blue arrows). These granules were surrounded by active osteoblasts, osteoid tissue, and osteons (toluidine blue, ×30 magnification) Right side: Implant coronal area in test group. No bone resorption was observed. Many mineralization nuclei were present in the most coronal implant area (toluidine blue, ×25 magnification).
Fig. 6.
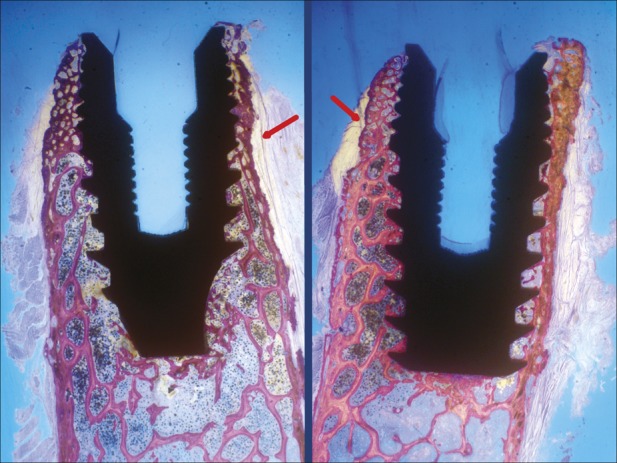
Implants in the test group. The granular aspect was more visible in the coronal portion of the implants. The bone density increase is also evident in this area. The cortical wall changed its direction denoting a bone ridge expansion (red arrows) (toluidine blue, ×10 magnification).
The most peculiar feature of the healing pattern was at the level of the more coronal cortical walls where the bone presented an unusual granular aspect. In these areas, osteoid tissue bands, osteons, and newly formed bone were also visible (Fig. 5). In these zones, the bone trabeculae showed the specific granular aspect also in the inner part, whereas the outer side showed lamellar bone layers. These bone trabeculae were thickened because of incorporation of autogenous bone fragments during the healing process. The granules observed in the trabeculae seemed like mineralization nuclei. Close to these granules, woven bone areas mixed with lamellar bone were also observed.
The percentage of bone surface lined by osteoid bands in the coronal area was much higher than that found in other areas of the implants and higher than that usually observed after 2 months of healing in this animal model and in the control group. The increase of bone density was particularly evident in the most coronal implant region.
Bone chips and resorption of newly formed trabeculae were also observed. Active bone remodeling was found to be directed more toward bone apposition and bone density increase than toward bone resorption.
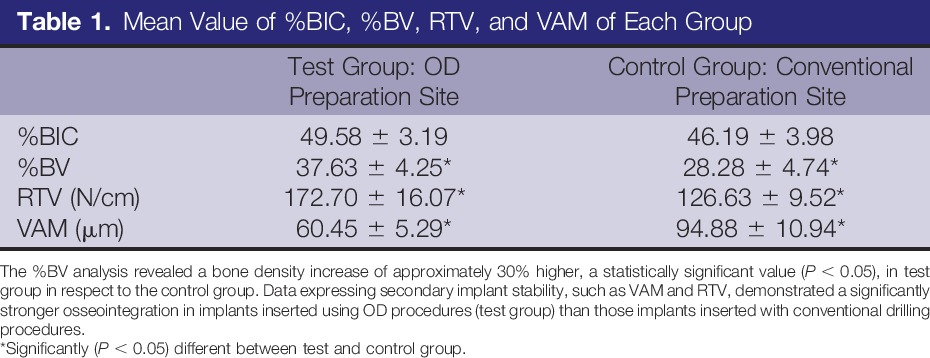
Histomorphometric Results
The mean values of %BIC, %BV, RTV, and secondary stability (VAM) for conventional site preparation (control group) and in implants inserted in sites prepared using OD burs (test group) are summarized in the Table 1. No significant difference in %BIC was detected between the 2 groups. The %BV analysis revealed a bone density increase of approximately 30% higher, a statistically significant value (P < 0.05), in test group in respect to control group.
Table 1.
Mean Value of %BIC, %BV, RTV, and VAM of Each Group
Biomechanical Results
The test group showed statistically significant (P < 0.05) better biomechanical performances (approximately 30%–40% higher) than control group in the parameters evaluated as the RTV and the VAM. Mean values of each parameter evaluated are expressed in Table 1.
Discussion
Poor bone density is common in human upper jaw, especially in elderly patients needing a fixed implant supported rehabilitation. In D3 or D4 bone type, it is difficult to achieve a good implant primary stability because of the poor %BV around the titanium implant surface.5 Bone density classification according to Lekholm and Zarb (1985),33 based on the morphology and distribution of cortical and trabecular bones, individuated 4 bone quality types. Poor bone density (D3-D4) is common in the upper jaw region,34 and in this bone type, it is difficult to achieve a high implant primary stability.
If the primary implant stability is inadequate, the early implant failure rate could rise beyond critical levels.35 Immediate loading protocols are also discouraged in case of poor bone quality or low primary implant stability and longer healing time is needed in these cases, with some disadvantages for the patient.
In case of poor bone density, such as in the human upper jaw, the insufficient bone amount around the implants could negatively influence the histomorphometric parameters (such as %BIC and %BV) and, consequently, both primary and secondary implant stabilities.
Several surgical techniques36 have been proposed to avoid or reduce bone sacrifice during implant placement procedures in low-density bone and to enhance primary implant stability and bone quality. Some authors suggested to undersize the osteotomic implant site in respect to the implant diameter of approximately 10% to reduce bone cutting and enhance primary implant stability.7,13 An undersized osteotomic site greater than 10% did not add mechanical benefit and, however, this expedient allows to increase implant initial stability but it is not able to modify the %BV around the implant as compared with nonundersized sites.37
The alternative to implant drilling procedures is the osteotome technique14 that aimed to compact the bone with the mechanical action of cylindrical steel instruments along the osteotomic walls. This procedure created trabecular fractures with debris, which caused an obstruction of the osseointegration process.21,38 The healing process, in case of osteotome preparation method, involve 2 phases: fractured trabeculae and bone chips resorption followed by new bone formation onto the implant surface.
The OD technique, using special burs in noncutting rotation, tested in this study demonstrated the ability to increase in a significant way (approximately 30% higher) the %BV around the implants and to improve secondary implant stability (expressed as removal torque values and micromotion under lateral forces). The histological analysis showed that the healing process is not obstructed by this bone condensation and, moreover, that bone density increase is evident around implant surface (especially in the upper portion of the implant). Besides, the high presence of mineralization nuclei lined by osteoid tissue and osteoblast observed in test group strongly suggests that, in the long run, the bone could still increase its density. The special geometry design of the burs tested allows pulverizing the bone, creating higher mineralization nuclei number than that of the control group.
Implants inserted using this new OD method showed statistically higher biomechanical values of 30% to 40% than implants inserted with conventional drills. This osseodensification technique allows enhancing the primary implant stability, as it was demonstrated by lower VAM recorded in test group in this study. The VAM of the implant is a parameter already published39 to successfully evaluate implant stability that is directly correlated to stability parameters as %BIC, RTV, and ISQ. The removal torque values (RTVs), which is a parameter directly correlated with the periimplant %BV and %BIC, was positively influenced by 30% of BV rise in test group and reached values of approximately 40% more in OD sites than in the conventional sites.
The differences in RTVs and micromotion under lateral forces (VAM) are very remarkable between the 2 groups (high statistical meaning) and only a little amount of these differences could be attributed to greater implant surfaces in the test group (in the control group, implant diameters are 1 mm smaller than those of the test group). In fact, the surface area of the 5 × 10-mm implant is only 26% more extended than the 3.8 × 10-mm implant (official data from the manufacturer).
In the test group, wider diameter implants were used with respect to the control group (5 vs 3.8 mm), whereas the bone ridge width was 4 to 6 mm: a high risk of bone dehiscence existed with conventional drilling procedures. It was decided to place smaller diameter implants in the control group because implants associated to bone dehiscence should not be compared with implant surrounded by healthy bone. No bone resorption, dehiscence, or fenestration were demonstrated during the clinical and histological examinations; after 2 months of healing, using the OD technique, testifying that this surgical method is able to expand the bone taking advantage of viscoelastic bone properties and compacting bone chips (autografting) along the osteotomy without useless bone sacrifice. The ridge expansion obtained with OD tecnique is particularly evident in samples showed in Figure 6, in which the cortical wall changes its original direction testifying the bone width increase.
Moreover, the great increase of the biomechanical strength in the test group did not negatively affect %BIC or %BV (was not related to a similar increase of the %BIC or %BV), but nevertheless enhanced healing. This could be explained by the change in the bone strength observed under the microscope induced by the OD procedure. This new implant site preparation technique significantly improved the secondary implant stability and the histological analysis revealed new a healing pattern of the surgical bone with a fast and big amount of newly formed bone along osteotomy walls.
Conclusion
The OD technique, used in the present in vivo study, was demonstrated to be able to increase implant primary stability and maintained implant secondary stability, and to increase the %BV around dental implants inserted in low-density bone in respect with conventional implant drilling procedures. OD allowed to avoid bone sacrifice, that appears unavoidable with conventional drilling procedures, and to prevent fractured trabeculae causing a delayed bone growth, as happened with the osteotome technique.
Additionally, this study validated the bone expansion attitude of this OD technique showing that wider implant diameter could be inserted in narrow ridge without creating bone dehiscence or fenestration. Future in vivo human studies are needed to confirm the results showed in the present article.
Disclosure
P. Trisi is a consultant for Cortex Dental Implants. The other authors claim to have no financial interest, either directly or indirectly, in the products or information listed in the article.
Approval
The Ethics Committee for Animal Research of the Veterinary School of the University of Teramo (Teramo, Italy) approved the study protocol with the protocol number 8110.
Acknowledgments
The authors wish to thank Cortex Dental Implants and Versah, LLC for providing the burs and implants used in this study.
References
- 1.Albrektsson T, Brånemark PI, Hansson HA, et al. Osseointegrated titanium implants. Requirements for ensuring a long-lasting, direct bone-to-implant anchorage in man. Acta Orthop Scand. 1981;52:155–170. [DOI] [PubMed] [Google Scholar]
- 2.Søballe K, Brockstedt-Rasmussen H, Hansen ES, et al. Hydroxyapatite coating modifies implant membrane formation. Controlled micromotion in dogs. Acta Orthop Scand. 1992;63:128–140. [DOI] [PubMed] [Google Scholar]
- 3.Søballe K, Hansen ES, Brocksted-Rasmussen H, et al. Hydroxyapatite coating converts fibrous tissue to bone around loaded implants. J Bone Joint Surg Br. 1993;75:270–278. [DOI] [PubMed] [Google Scholar]
- 4.Szmukler-Moncler S, Salama H, Reingewirtz J, et al. Timing of loading and effect of micromotion on bone-dental implant interface: Review of experimental literature. J Biomed Mater Res. 1998;43:192–203. [DOI] [PubMed] [Google Scholar]
- 5.Marquezan M, Osório A, Sant'Anna E, et al. Does bone mineral density influence the primary stability of dental implants? A systematic review. Clin Oral Implants Res. 2012;23:767–774. [DOI] [PubMed] [Google Scholar]
- 6.Trisi P, De Benedittis S, Perfetti G, et al. Primary stability, insertion torque and bone density of cylindric implant ad modum Brånemark: Is there a relationship? An in vitro study. Clin Oral Implants Res. 2011;22:567–570. [DOI] [PubMed] [Google Scholar]
- 7.Turkyilmaz I, Aksoy U, McGlumphy EA. Two alternative surgical techniques for enhancing primary implant stability in the posterior maxilla: A clinical study including bone density, insertion torque, and resonance frequency analysis data. Clin Implant Dent Relat Res. 2008;10:231–237. [DOI] [PubMed] [Google Scholar]
- 8.Dos Santos MV, Elias CN, Cavalcanti Lima JH. The effects of superficial roughness and design on the primary stability of dental implants. Clin Implant Dent Relat Res. 2011;13:215–223. [DOI] [PubMed] [Google Scholar]
- 9.Trisi P, Perfetti G, Baldoni E, et al. Implant micromotion is related to peak insertion torque and bone density. Clin Oral Implants Res. 2009;20:467–471. [DOI] [PubMed] [Google Scholar]
- 10.Capparé P, Vinci R, Di Stefano DA, et al. Correlation between initial BIC and the Insertion Torque/Depth Integral Recorded with an Instantaneous Torque-Measuring Implant Motor: An in vivo Study. Clin implant Dent Relat Res. 2015;17(suppl 2):e613–e620. [DOI] [PubMed] [Google Scholar]
- 11.Ottoni JM, Oliveira ZF, Mansini R, et al. Correlation between placement torque and survival of single-tooth implants. Int J Oral Maxillofac Implants. 2005;20:769–776. [PubMed] [Google Scholar]
- 12.Alghamdi H, Anand PS, Anil S. Undersized implant site preparation to enhance primary implant stability in poor bone density: A prospective clinical study. J Oral Maxillofac Surg. 2011;69:506–512. [DOI] [PubMed] [Google Scholar]
- 13.Degidi M, Daprile G, Piattelli A. Influence of underpreparation on primary stability of implants inserted in poor quality bone sites: An in vitro study. J Oral Maxillofac Surg. 2015;73:1084–1088. [DOI] [PubMed] [Google Scholar]
- 14.Summers RB. A new concept in maxillary implant surgery: The osteotome technique. Compendium. 1994;15:152, 154–156, 158 passim; quiz 162. [PubMed] [Google Scholar]
- 15.Boustany CM, Reed H, Cunningham G, et al. Effect of a modified stepped osteotomy on the primary stability of dental implants in low-density bone: A cadaver study. Int J Oral Maxillofac Implants. 2015;30:48–55. [DOI] [PubMed] [Google Scholar]
- 16.Campos FE, Gomes JB, Marin C, et al. Effect of drilling dimension on implant placement torque and early osseointegration stages: An experimental study in dogs. J Oral Maxillofac Surg. 2012;70:e43–e50. [DOI] [PubMed] [Google Scholar]
- 17.Coelho PG, Marin C, Teixeira HS, et al. Biomechanical evaluation of undersized drilling on implant biomechanical stability at early implantation times. J Oral Maxillofac Surg. 2013;71:e69–e75. [DOI] [PubMed] [Google Scholar]
- 18.Munjal S, Munjal S, Hazari P, et al. Evaluation of specifically designed implants placed in the low-density jaw bones: A clinico-radiographical study. Contemp Clin Dent. 2015;6:40–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shalabi MM, Wolke JG, de Ruijter AJ, et al. A mechanical evaluation of implants placed with different surgical techniques into trabecular bone of goats. J Oral Implantol. 2007;33:51–58. [DOI] [PubMed] [Google Scholar]
- 20.Shalabi MM, Wolke JG, de Ruijter AJ, et al. Histological evaluation of oral implants inserted with different surgical techniques into trabecular bone of goats. Clin Oral Implants Res. 2007;18:489–495. [DOI] [PubMed] [Google Scholar]
- 21.Büchter A, Kleinheinz J, Wiesmann HP, et al. Biological and biomechanical evaluation of bone remodelling and implant stability after using an osteotome technique. Clin Oral Implants Res. 2005;16:1–8. [DOI] [PubMed] [Google Scholar]
- 22.Hansson S, Halldin A. Alveolar ridge resorption after tooth extraction: A consequence of a fundamental principle of bone physiology. J Dent Biomech. 2012;3:1758736012456543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Carlsson G, Persson G. Morphologic changes of the mandible after extraction and wearing dentures: A longitudinal clinical and x-ray cephalometric study covering 5 years. Odontol Revy. 1967;18:27–54. [PubMed] [Google Scholar]
- 24.Bassetti MA, Bassetti RG, Bosshardt DD. The alveolar ridge splitting/expansion technique: A systematic review. Clin Oral Implants Res. 2015 Jan 14. [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 25.Esposito M, Grusovin MG, Felice P, et al. The efficacy of horizontal and vertical bone augmentation procedures for dental implants: A Cochrane systematic review. Eur J Oral Implantol. 2009;2:167–184. [PubMed] [Google Scholar]
- 26.Stricker A, Fleiner J, Stübinger S, et al. Bone loss after ridge expansion with or without reflection of the periosteum. Clin Oral Implants Res. 2015;26:529–536. [DOI] [PubMed] [Google Scholar]
- 27.Tang YL, Yuan J, Song YL, et al. Ridge expansion alone or in combination with guided bone regeneration to facilitate implant placement in narrow alveolar ridges: A retrospective study. Clin Oral Implants Res. 2015;26:204–211. [DOI] [PubMed] [Google Scholar]
- 28.Huwais S, inventor; Fluted osteotome and surgical method for use. US Patent Application US2013/0004918. January 3, 2013.
- 29.Huwais S. Autografting Osteotome. Geneva, Switzerland: World Intellectual Property Organization Publication; 2014. WO2014/077920. [Google Scholar]
- 30.Huwais S, Meyer E. Osseodensification: A novel approach in implant o preparation to increase primary stability, bone mineral density and bone to implant contact. Int J Oral Maxillofac Implants. 2015. [DOI] [PubMed] [Google Scholar]
- 31.Trisi P, Berardini M, Falco A, et al. Effect of implant thread geometry on secondary stability, bone density, and bone-to-implant contact: A biomechanical and histological analysis. Implant Dent. 2015;24:384–391. [DOI] [PubMed] [Google Scholar]
- 32.Sennerby L, Thomsen P, Ericson LE. A morphometric and biomechanic comparison of titanium implants inserted in rabbit cortical and cancellous bone. Int J Oral Maxillofac Implants. 1992;7:62–71. [PubMed] [Google Scholar]
- 33.Lekholm U, Zarb GA. Patient selection and preparation. In: Brånemark PI, Zarb GA, Alberktsson T, eds. Tissue Integrated Prostheses: Osseointegration in Clinical Dentistry. Chicago, IL: Quintessence; 1985:199–209. [Google Scholar]
- 34.Hao Y, Zhao W, Wang Y, et al. Assessments of jaw bone density at implant sites using 3D cone-beam computed tomography. Eur Rev Med Pharmacol Sci. 2014;18:1398–1403. [PubMed] [Google Scholar]
- 35.Meredith N. Assessment of implant stability as a prognostic determinant. Int J Prosthodont. 1998;11:491–501. [PubMed] [Google Scholar]
- 36.Tabassum A, Walboomers XF, Wolke JG, et al. Bone particles and the undersized surgical technique. J Dent Res. 2010;89:581–586. [DOI] [PubMed] [Google Scholar]
- 37.Tabassum A, Meijer GJ, Walboomers XF, et al. Evaluation of primary and secondary stability of titanium implants using different surgical techniques. Clin Oral Implants Res. 2014;25:487–492. [DOI] [PubMed] [Google Scholar]
- 38.Stavropoulos A, Nyengaard JR, Lang NP, et al. Immediate loading of single SLA implants: Drilling vs. osteotomes for the preparation of the implant site. Clin Oral Implants Res. 2008;19:55–65. [DOI] [PubMed] [Google Scholar]
- 39.Trisi P, Berardini M, Falco A, et al. Validation of Value of Actual Micromotion (VAM) as a direct measure of implant micromobility after healing (secondary implant stability): An in vivo histologic and biomechanical study. Clin Oral Implants Res. 2015. [DOI] [PubMed] [Google Scholar]


