Abstract
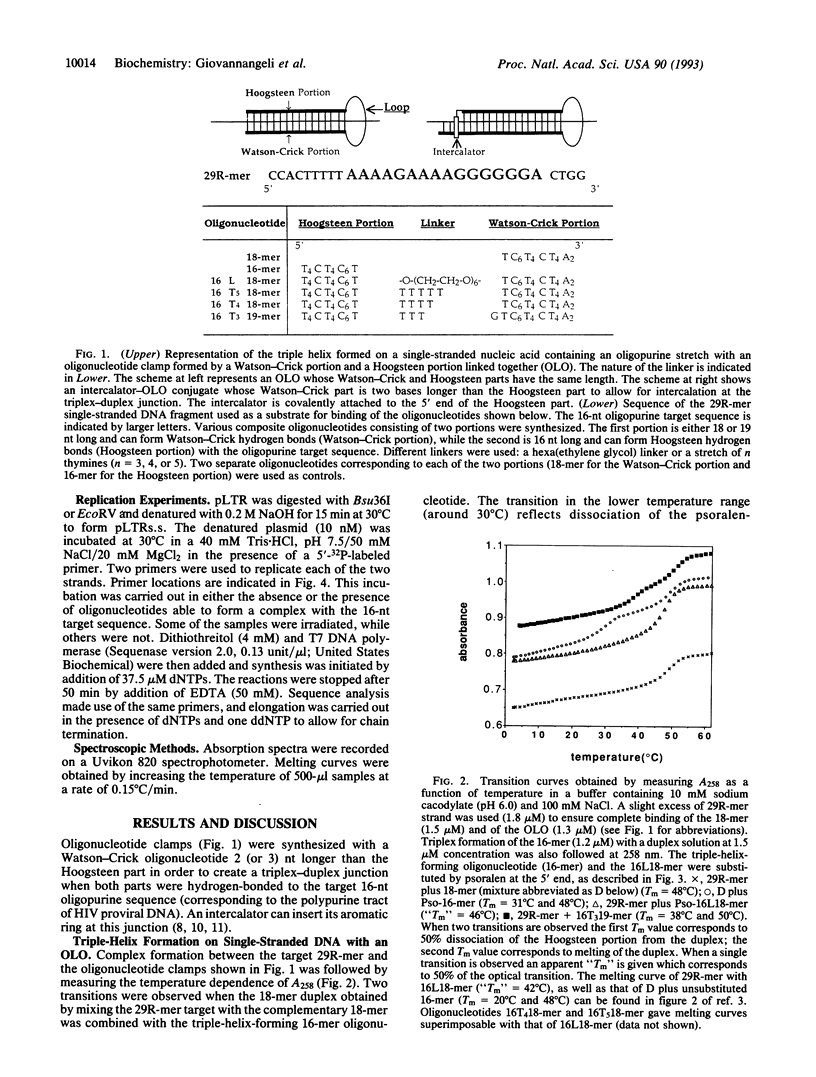
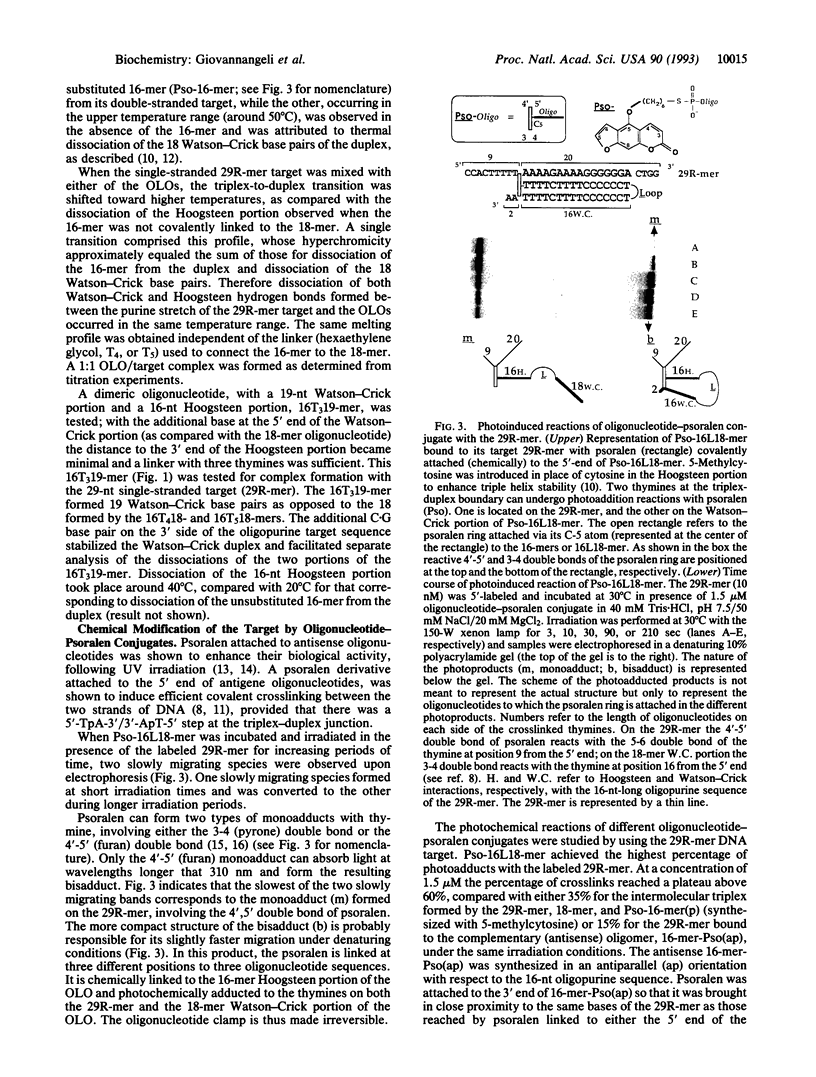
Triple helices can be formed on single-stranded oligopurine target sequences by composite oligonucleotides consisting of two oligonucleotides covalently linked by either a hexaethylene glycol linker or an oligonucleotide sequence. The first oligomer forms Watson-Crick base pairs with the target, while the second oligomer engages in Hoogsteen base pairing, thereby acting as a molecular clamp. The triple-helical complex formed by such an oligonucleotide clamp, or "oligonucleotide-loop-oligonucleotide" (OLO), is more stable than either the corresponding trimolecular triple helix or the double helix formed upon binding of the oligopyrimidine complement to the same oligopurine target. Attaching a psoralen derivative to the 5' end of the OLO allowed us to photoinduce a covalent linkage to the target sequence. The psoralen moiety became covalently linked to all three portions of the triplex, thereby making the oligonucleotide clamp irreversible. These crosslinking reactions introduced strong stop signals during DNA replication, as shown on a plasmid containing a portion of the HIV proviral sequence of human immunodeficiency virus. A 16-mer oligopurine sequence corresponding to the "polypurine tract" of human immunodeficiency virus was chosen as a target for a psoralen-OLO conjugate. Three different stop signals for DNA polymerase were observed, corresponding to different sites of polymerase arrest on its template. Even in the absence of photoinduced crosslinking, the psoralen-OLO conjugate was able to arrest DNA replication. The formation of triple-helical structures on single-stranded targets may provide an alternative to the antisense strategy for the control of gene expression.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Chang E. H., Miller P. S., Cushman C., Devadas K., Pirollo K. F., Ts'o P. O., Yu Z. P. Antisense inhibition of ras p21 expression that is sensitive to a point mutation. Biochemistry. 1991 Aug 27;30(34):8283–8286. doi: 10.1021/bi00098a001. [DOI] [PubMed] [Google Scholar]
- Durand M., Chevrie K., Chassignol M., Thuong N. T., Maurizot J. C. Circular dichroism studies of an oligodeoxyribonucleotide containing a hairpin loop made of a hexaethylene glycol chain: conformation and stability. Nucleic Acids Res. 1990 Nov 11;18(21):6353–6359. doi: 10.1093/nar/18.21.6353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duval-Valentin G., Thuong N. T., Hélène C. Specific inhibition of transcription by triple helix-forming oligonucleotides. Proc Natl Acad Sci U S A. 1992 Jan 15;89(2):504–508. doi: 10.1073/pnas.89.2.504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Escudé C., Sun J. S., Rougée M., Garestier T., Hélène C. Stable triple helices are formed upon binding of RNA oligonucleotides and their 2'-O-methyl derivatives to double-helical DNA. C R Acad Sci III. 1992;315(13):521–525. [PubMed] [Google Scholar]
- Giovannangéli C., Thuong N. T., Hélène C. Oligodeoxynucleotide-directed photo-induced cross-linking of HIV proviral DNA via triple-helix formation. Nucleic Acids Res. 1992 Aug 25;20(16):4275–4281. doi: 10.1093/nar/20.16.4275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hélène C. The anti-gene strategy: control of gene expression by triplex-forming-oligonucleotides. Anticancer Drug Des. 1991 Dec;6(6):569–584. [PubMed] [Google Scholar]
- Hélène C., Toulmé J. J. Specific regulation of gene expression by antisense, sense and antigene nucleic acids. Biochim Biophys Acta. 1990 Jun 21;1049(2):99–125. doi: 10.1016/0167-4781(90)90031-v. [DOI] [PubMed] [Google Scholar]
- Kulka M., Smith C. C., Aurelian L., Fishelevich R., Meade K., Miller P., Ts'o P. O. Site specificity of the inhibitory effects of oligo(nucleoside methylphosphonate)s complementary to the acceptor splice junction of herpes simplex virus type 1 immediate early mRNA 4. Proc Natl Acad Sci U S A. 1989 Sep;86(18):6868–6872. doi: 10.1073/pnas.86.18.6868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ollis D. L., Brick P., Hamlin R., Xuong N. G., Steitz T. A. Structure of large fragment of Escherichia coli DNA polymerase I complexed with dTMP. 1985 Feb 28-Mar 6Nature. 313(6005):762–766. doi: 10.1038/313762a0. [DOI] [PubMed] [Google Scholar]
- Roberts R. W., Crothers D. M. Stability and properties of double and triple helices: dramatic effects of RNA or DNA backbone composition. Science. 1992 Nov 27;258(5087):1463–1466. doi: 10.1126/science.1279808. [DOI] [PubMed] [Google Scholar]
- Sastry S. S., Hearst J. E. Studies on the interaction of T7 RNA polymerase with a DNA template containing a site-specifically placed psoralen cross-link. I. Characterization of elongation complexes. J Mol Biol. 1991 Oct 20;221(4):1091–1110. [PubMed] [Google Scholar]
- Shi Y. B., Gamper H., Hearst J. E. The effects of covalent additions of a psoralen on transcription by E. coli RNA polymerase. Nucleic Acids Res. 1987 Sep 11;15(17):6843–6854. doi: 10.1093/nar/15.17.6843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi Y. B., Gamper H., Van Houten B., Hearst J. E. Interaction of Escherichia coli RNA polymerase with DNA in an elongation complex arrested at a specific psoralen crosslink site. J Mol Biol. 1988 Jan 20;199(2):277–293. doi: 10.1016/0022-2836(88)90314-2. [DOI] [PubMed] [Google Scholar]
- Shimizu M., Konishi A., Shimada Y., Inoue H., Ohtsuka E. Oligo(2'-O-methyl)ribonucleotides. Effective probes for duplex DNA. FEBS Lett. 1992 May 11;302(2):155–158. doi: 10.1016/0014-5793(92)80428-j. [DOI] [PubMed] [Google Scholar]
- Sun J. S., François J. C., Montenay-Garestier T., Saison-Behmoaras T., Roig V., Thuong N. T., Hélène C. Sequence-specific intercalating agents: intercalation at specific sequences on duplex DNA via major groove recognition by oligonucleotide-intercalator conjugates. Proc Natl Acad Sci U S A. 1989 Dec;86(23):9198–9202. doi: 10.1073/pnas.86.23.9198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun J. S., Giovannangeli C., François J. C., Kurfurst R., Montenay-Garestier T., Asseline U., Saison-Behmoaras T., Thuong N. T., Hélène C. Triple-helix formation by alpha oligodeoxynucleotides and alpha oligodeoxynucleotide-intercalator conjugates. Proc Natl Acad Sci U S A. 1991 Jul 15;88(14):6023–6027. doi: 10.1073/pnas.88.14.6023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takasugi M., Guendouz A., Chassignol M., Decout J. L., Lhomme J., Thuong N. T., Hélène C. Sequence-specific photo-induced cross-linking of the two strands of double-helical DNA by a psoralen covalently linked to a triple helix-forming oligonucleotide. Proc Natl Acad Sci U S A. 1991 Jul 1;88(13):5602–5606. doi: 10.1073/pnas.88.13.5602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wain-Hobson S., Sonigo P., Danos O., Cole S., Alizon M. Nucleotide sequence of the AIDS virus, LAV. Cell. 1985 Jan;40(1):9–17. doi: 10.1016/0092-8674(85)90303-4. [DOI] [PubMed] [Google Scholar]
- Xodo L. E., Manzini G., Quadrifoglio F. Spectroscopic and calorimetric investigation on the DNA triplex formed by d(CTCTTCTTTCTTTTCTTTCTTCTC) and d(GAGAAGAAAGA) at acidic pH. Nucleic Acids Res. 1990 Jun 25;18(12):3557–3564. doi: 10.1093/nar/18.12.3557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young S. L., Krawczyk S. H., Matteucci M. D., Toole J. J. Triple helix formation inhibits transcription elongation in vitro. Proc Natl Acad Sci U S A. 1991 Nov 15;88(22):10023–10026. doi: 10.1073/pnas.88.22.10023. [DOI] [PMC free article] [PubMed] [Google Scholar]