Abstract
Varicocele is a common medical condition entangled with many controversies. Though it is highly prevalent in men with infertility, still it marks its presence in males who do have normal fertility. Determining which patients are negatively affected by varicocele would enable clinicians to better select those men who benefitted the most from surgery. Since conventional semen analysis has been limited in its ability to evaluate the negative effects of varicocele on fertility, a multitude of specialized laboratory tests have emerged. In this review, we examine the role and significance of specialized sperm function tests with regards to varicocele. Among the various tests, analysis of sperm DNA fragmentation and measurements of oxidative stress markers provide an independent measure of fertility in men with varicocele. These diagnostic modalities have both diagnostic and prognostic information complementary to, but distinct from conventional sperm parameters. Test results can guide management and aid in monitoring intervention outcomes. Proteomics, metabolomics, and genomics are areas; though still developing, holding promise to revolutionize our understanding of reproductive physiology, including varicocele.
Keywords: male infertility, oxidative stress, semen analysis, sperm DNA fragmentation, sperm function, varicocele, varicocelectomy
INTRODUCTION
Few are the cases in the medical literature that has been extensively researched yet remain coupled with controversies. Varicocele is one good example where its main dispute is choosing the ideal patient who would undoubtedly benefit from surgery as treating a varicocele is not necessarily helpful to all affected patients. Varicocele is a common medical condition that is prevalent in about 15% of the general male population.1 This rate is much higher among patients presenting with infertility, in the range of 40%–50%.2 The high prevalence of varicocele is one reason for the skepticism it carries. Another reason is its occurrence in men with normal fertility.
Numerous studies have linked varicocele to abnormalities in semen parameters and, importantly, demonstrated significant improvement in these parameters after surgery.3,4 These findings, however, may be of limited clinical significance as the main outcome for infertile couples is delivery of a healthy baby rather than improvements in sperm quality. On the contrary, the likely benefit of varicocelectomy on natural pregnancy rates can only be truly evaluated in couples whose female factor infertility has been excluded. As a matter of fact, few prospective studies are available taking into consideration female partners fitting the aforementioned criterion and live birth rate as the primary outcome.5,6,7 While most of them confirmed that sperm quality is generally improved after varicocele repair, the effect of the intervention on pregnancy outcome is more equivocal. The most recent Cochrane meta-analysis on the effects of varicocele repair in subfertility included ten RCTs involving 894 men.8 The combined fixed-effect odds ratio (OR) of the 10 studies for the outcome of pregnancy was 1.47 (95% CI: 1.05–2.05), thus suggesting a benefit of varicocele treatment over expectant management in subfertile couples in whom varicocele was the only abnormal finding. Due to the high heterogeneity of included studies and lack of randomized controlled trials with live birth as the primary outcome measure, it is clear that more research is needed to elucidate the true benefit of repairing varicoceles on pregnancy outcome.
Notwithstanding, semen analysis results, namely, semen volume, sperm count, motility, and morphology are the most frequent measures used in the laboratory investigation of varicocele. This analysis, per se, is not accurate for diagnosis because there is a wide variability in the semen parameters of individuals with varicocele across various germinative cycles.9,10 There is also variation in the methods used by different laboratories when performing semen analyses.11,12 Finally, traditional semen analysis does not evaluate putative varicocele-associated sperm dysfunctions such as immature chromatin or fragmented DNA.13,14 As a consequence, additional tests have been developed to unravel the different aspects of sperm function in relation to the presence of varicocele that cannot be identified by conventional semen analysis. Some of these tests include the hypo-osmotic swelling test, computer-assisted sperm analysis, antisperm antibody test, sperm penetration assay, hemizona assay, reactive oxygen species (ROS) tests, and sperm chromatin integrity tests.15
This review explores the role and clinical significance of these specialized sperm function tests in the context of varicocele. An extensive search of studies examining the relationship between varicocele and sperm function testing was performed using search engines such as MEDLINE and Google Scholar. There was no limit on dates, but only full articles published in English were examined. The overall strategy for study identification and data extraction was based on the following keywords: “varicocele,” “sperm function,” “acrosome reaction,” “antisperm antibodies,” “computer-assisted semen analysis,” “sperm binding to zona pellucida,” “hypo-osmotic swelling test,” “oxidative stress,” “reactive oxygen species,” “sperm DNA fragmentation,” “sperm DNA damage.” Websites and book-chapter citations provide conceptual content only. A list of peer-reviewed articles included is provided in Table 1.
Table 1.
Overview of specialized sperm function tests utilized in varicocele
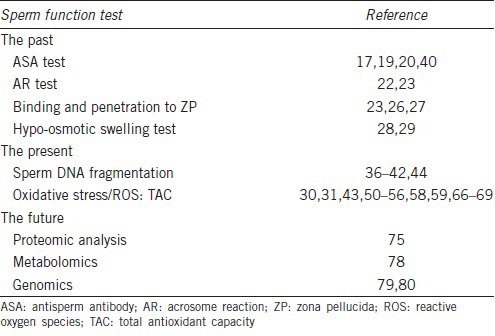
SPECIALIZED SPERM FUNCTIONAL TESTS IN VARICOCELE-ASSOCIATED INFERTILITY
The past
For many years, tests that assessed the presence of antisperm antibodies (ASAs), sperm capacitation, acrosome reaction (AR), and sperm binding to the zona pellucida were utilized to measure the effect of varicocele on sperm function and predict the sperm fertilizing potential (Table 2). Given the complexity to perform some of these tests and the inherent difficulties to interpret their results, most of them have been abandoned or are rarely used nowadays. Nevertheless, we briefly discuss the significance of the most relevant tests in the context of varicocele, as below.
Table 2.
Methods for assessing ASA and functional in vitro tests for measuring sperm fertilization defects
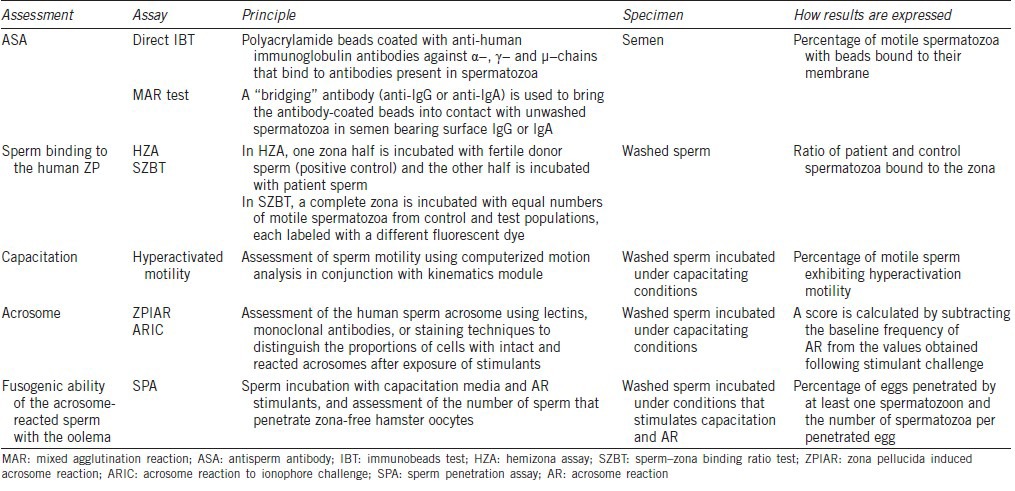
Antisperm antibody (ASA) test
ASA have been implicated as a cause of infertility. They develop when the patient's own immune system identifies the sperm cells as a result of disruption of the blood–testis barrier. As a consequence, sperm lose their ability to perform vital functions such as penetration into the cervical mucus and binding to the zona pellucida.16 Low positivity for ASA has been found in approximately 30% men with clinical varicocele.17
The mechanisms underlying ASA production in men with varicocele is unclear as the blood-testis barrier remained intact after the experimental creation of unilateral varicocele, despite the bilateral deterioration of testicular function.18
The effect of varicocelectomy on ASA titers is equally equivocal. In one study involving 99 patients, ASA titers were reduced in half of the affected subjects after varicocelectomy, and reduction was more pronounced in men with high-grade varicoceles. In this aforementioned study, pregnancy rate within a year after surgery was 2.8 times more frequent in couples with postoperative ASA-negative men (39%) than ASA-positive men (14%; P < 0.05).19 In contrast, Djaladat et al. studied 81 men and found that while ASA titers were reduced in some men, this reduction was not universal and not predictive of improvements in semen parameters.17 Thus, the clinical utility of ASA testing remains questionable as the presence of ASA neither predict semen quality improvement after varicocele repair nor direct a different form of treatment.20
Acrosome reaction (AR)
In vivo, ejaculated human spermatozoa are unable to fertilize until they have undergone capacitation, which allows the AR to take place when they approach or contact the oocyte. Acrosome reaction is accompanied by the release of lytic enzymes and exposure of membrane receptors, which are required for sperm penetration through the zona pellucida (ZP) and for fusion with the oolema.21
Few studies have explored the influence of varicocele on AR. In one study, El Mulla et al. evaluated AR rates (both baseline and after stimulation with ionophore) in spermatozoa obtained from 30 patients with varicocele and 20 controls. The authors found no significant association between varicocele and AR rates.22 In another study, Vigil et al. compared the sperm function, including AR, of fertile men (control), infertility patients (experimental), and men with varicocele.23 The AR rates in response to the stimulation with follicular fluid were significantly lower in infertile patients, but the presence of varicocele did not appear to influence the process. Although acrosome reaction testing has not been used clinically nowadays, the test is still performed for research purposes.
Binding and penetration to zona pellucida (ZP)
Tests assessing sperm fertilizing ability have similarly fallen out of favor. The main reasons are the unavailability of treatments for these functional defects and the widespread practice of intracytoplasmic sperm injection (ICSI). Sperm binding to the ZP is assessed with hemizona assay (HZA) or the sperm–zona binding ratio test,24 while sperm penetration is assessed with the sperm penetration assay (SPA).25
Few studies have evaluated these tests in patients with varicocele. In a small cohort study, Hauser et al. examined sperm binding ability in 12 men with varicocele-related infertility subjected to varicocelectomy. The authors divided the patients into three groups based on the pregnancy outcome and time to achieve pregnancy (i) Group 1 consisted of three couples that achieved pregnancy within 6 months; (ii) Group 2 consisted of four couples who achieved pregnancy between 12 and 18 months; and (iii) Group 3 included five couples in whom pregnancy had not been recorded during the follow-up period. Interestingly, while conventional semen parameters improved in all treated subjects, sperm binding to the ZP, as measured by the hemizona assay (HZA), improved only in the group that achieved pregnancy.26 In another cohort study, SPA was performed on fertile controls, fertile varicocele patients, and infertile varicocele patients.27 SPA results were significantly lower in infertile men with varicocele, but the test alone was unable to predict fertility status of patients with varicocele.27 Vigil et al. also examined the sperm penetration assay by comparing three groups of men (i) Group 1 (control) consisted of fertile men; (ii) Group 2 (experimental) consisted of infertile men without varicocele; and (iii) Group 3 consisted of infertile men with varicocele. The mean number of hamster oocytes penetrated by spermatozoa was significantly lower in infertile patients, particularly those with varicocele: 50% ± 8% in the control, 19% ± 3% in Group 2, and 10% ± 3% in the varicocele group (P < 0.001).23
Although the results of these studies indicate that varicocele negatively affect sperm fusion to the ZP and penetration, they were unable to establish the clinical utility of such tests to predict who might benefit from varicocele repair.
Hypo-osmotic swelling test
The hypo-osmotic swelling test (HOST) is based on the permeability of the intact cell membrane. Spermatozoa “swell” under hypo-osmotic conditions as a result of an influx of water that causes expansion of sperm tail volume. In one study evaluating 35 patients with varicocele, Fuse et al. reported that the percentage of sperm exhibiting tail swelling was significantly lower in men with varicocele compared to men with idiopathic infertility, thus suggesting that varicocele alters sperm membrane function.28 Subsequently, the same group examined HOST in the semen of 60 men before and after varicocele repair. The authors divided the patients into two groups (i) Group 1 consisted of 18 men with varicocele who successfully achieved pregnancy, and (ii) Group 2 consisted of 42 men who did not. The magnitude of postoperative improvement in HOST results was shown to be associated with pregnancy outcomes,29 but preoperative values were not different between the groups.
Collectively, specialized tests, including screening for antisperm antibodies, acrosome reaction, binding and penetration to zona pellucida, and hypo-osmotic swelling are limited in their ability to guide management of infertile men with varicocele. At present, these tests remain mainly reserved for experimental studies.
The present
Recently, oxidative stress has been implicated as an important mediator of varicocele-associated infertility.30 An imbalance between ROS production and decreased total antioxidant capacity (TAC) has been implicated as the result of acidification of spermatozoa cytosol and seminal plasma in men with varicocele. Oxidative stress via ROS, including lipid peroxidation, not only damages membrane function but also leads to DNA damage.31 These altered functional aspects might help to understand the effect of varicocele on male infertility in the presence of a so-called normal semen analysis. Such findings prompted investigators to start utilizing contemporary sperm function tests to the clinical settings.
Sperm DNA fragmentation test
Increased oxidative stress has been associated with sperm DNA damage in men with varicocele. ROS can inflict damage to both nuclear and mitochondrial DNA, and may cause base modification, strand breaks, and chromatin cross-links.32 Moreover, increased oxidative stress seen in varicocele can trigger an apoptosis-like process affecting maturation and nuclear protamination.33 Excessive levels of sperm DNA fragmentation (SDF) have been associated with infertility, poor assisted reproductive technology (ART) outcomes, and miscarriage.15,34,35 Since sperm DNA and chromatin integrity are essential for effective transmission of genetic information to offspring, increased interest has emerged on the clinical value of assessing the quality of sperm DNA.
Several tests have been developed to examine sperm DNA integrity, most of which specifically examine single and/or double breaks occurring at the DNA strands, and are termed “sperm DNA fragmentation tests.” These techniques include sperm chromatin structure assay (SCSA), terminal deoxynucleotide transferase-mediated dUTP nick end labeling (TUNEL) assay, Comet assay, and sperm chromatin dispersion (SCD) test (Figure 1). Despite being out of the scope of this review to discuss each test in detail, a brief description of the most commonly used tests are provided in Table 3. A comprehensive review of about these techniques can be found elsewhere.15,32
Figure 1.
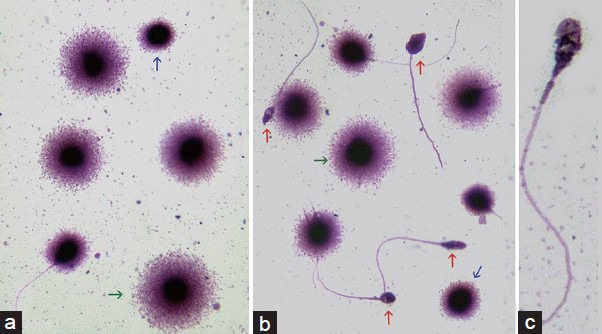
Sperm DNA fragmentation test using the Sperm Chromatin Dispersion (SCD) technique. When sperm classification is performed using the images provided by the SCD-Halosperm® method, normal sperm containing nonfragmented DNA are scored as the sperm population showing large or medium halos of dispersed chromatin surrounding a compact and well-defined core (a, green arrow). Spermatozoa either containing small halos or no halos, i.e., leaving only the chromatin core visible, are considered as those containing fragmented DNA (a and b, blue arrows). Spermatozoa exhibiting highly degraded chromatin are characterized by the presence of small nucleoids presenting nonuniform or faintly stained chromatin core in association with the absence of a halo of dispersed chromatin after direct staining (b, red arrows; c).
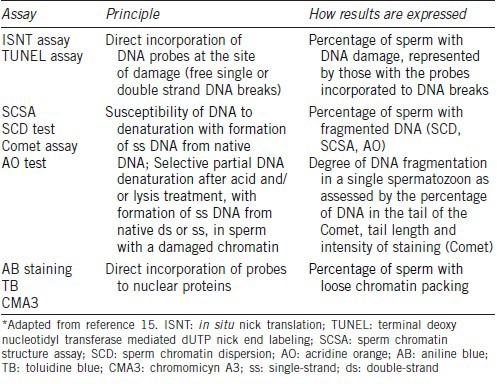
Table 3.
Characteristics of the tests commonly used for assessing sperm DNA damage*
Collectively, sperm DNA fragmentation tests have emerged as important biomarkers for assessing fertility potential in men with varicocele. In one study involving 593 men with various etiology conditions attending infertility clinics, SDF was found to be significantly higher both in men with varicocele (35.7% ± 18.3%) and leukocytospermia (41.7% ± 17.6%) compared to counterparts with testicular cancer and repeated ART failure (P < 0.05). The authors described a specific subpopulation with massive nuclear DNA damage, so-called degraded sperm, to be more prevalent in the group of men with varicocele. Although this class was not exclusive of varicocele patients, it was over-represented in this group (P < 0.001). Using receiver operating characteristics (ROCs) analysis, DDSi, defined as the proportion of degraded sperm in the whole population of spermatozoa with fragmented DNA, was able to identify patients with varicocele with 94% accuracy.14
Many other studies have also linked SDF with varicocele. In an experimental varicocele model, Ozturk et al. demonstrated increased sperm DNA damage with reduction in this damage after varicocelectomy.36 Clinically, this association has also been observed. Talebi et al. compared infertile men with varicocele to infertile men without varicocele and to normal fertile men, and detected a higher proportion of sperm with abnormal DNA and immature chromatin in the varicocele group.37 Blumer et al.38 confirmed previous reports of a negative correlation between sperm morphology and the percentage of sperm with DNA fragmentation (r = −0.450) in men with varicocele. Smith et al.39 found high levels of sperm DNA damage to be associated with varicocele even when semen analysis results were within the reference ranges.
Surgical ligation of varicocele has also been established to reduce the extent of DNA damage, a finding perceived in clinical but not in subclinical varicoceles.40 In one study, Sadek et al.41 assessed the rate of chromatin condensation using aniline blue staining in infertile men with varicocele and showed significant improvement in DNA packing following surgical correction of large varicose veins. Smit et al.42 showed significant improvement in the DNA fragmentation index (DFI) 3 months after varicocelectomy (preoperative 35.2% ± 13.1%; postoperative 30.2% ± 14.7%, P = 0.019). A difference could also be noted between couples that conceived naturally or with ART compared to those who failed (DFI%: 26.6% ± 13.7% vs 37.3% ± 13.9%, P = 0.013). Dada et al.43 reported repair of clinical varicoceles to result in rapid (1 month) decline in free radical levels followed by a slow (3–6 months) decline in DNA damage assessed by the Comet assay. Finally, in a meta-analysis44 exploring the relationship between varicocele and sperm DNA fragmentation, the overall estimate showed that varicocelectomy improved sperm DNA integrity, with a mean difference of −3.37% (95% CI: −4.09–−2.65; P < 0.00001).
The above-mentioned findings have prompted clinicians to test for SDF in varicocele patients and utilize the results for management (Figure 2). Testing is recommended at initial workup to all men with conventional semen analysis within normal ranges. Abnormal test results identify couples at higher risk of remaining childless if an expectant management is taken. In these cases, varicocele repair is recommended to decrease OS and sperm DNA damage. Oral antioxidants and life-style modification (e.g., cessation of smoking, weight loss) as contributory interventions are also recommended and can be combined to varicocele repair. Moreover, testing is useful to monitor the results of varicocele repair.15 Monitoring of treatment outcome is carried out with same tests at 3-month intervals. Persistent abnormal results after interventions could help in the decision of pursuing ART. In this sense, recent evidence has suggested that testicular sperm has lower SDF compared to ejaculated sperm and might result in better reproductive outcome in the cases of intracytoplasmic sperm injection.45
Figure 2.
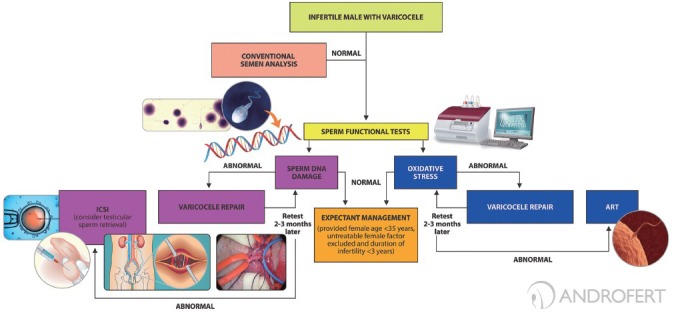
Algorithm proposed for the management of infertile males with varicocele using sperm DNA damage and oxidative stress tests. Testing is recommended at initial workup to all men with conventional semen analysis results within normal ranges. Abnormal test results identify couples at higher risk of remaining childless if an expectant management is taken. Interventions aimed to overcome OS and sperm DNA damage in patients with varicocele include varicocele repair and assisted reproductive techniques. Oral antioxidants and life-style modifications (cessation of smoking, weight loss) can be combined to varicocelectomy. Monitoring is carried out with same tests at 3-month intervals after varicocele treatment. ART are recommended for patients with persistent abnormal sperm function markers after varicocele repair. Testicular spermatozoa may be considered for sperm injections in ART treatment.
Reactive oxygen species
ROS are by-products of oxygen metabolism released from sperm cells and leukocytes. In ideal quantities, ROS serve to optimize sperm function. They control the number of germ cells by either inducing apoptosis or triggering proliferation of spermatogonia.46 They also regulate sperm capacitation, acrosome reaction, mitochondrial sheath stability, and sperm motility.47 ROS are counteracted by antioxidants and are kept at optimal levels. When an imbalance occurs between ROS and antioxidants resulting from either a surplus in the former or a decline in the latter, a state of oxidative stress develops. This has detrimental effects on cells and tissues, with sperm cells being particularly vulnerable. Lipid peroxidation of polyunsaturated fatty acids (PFAs) in the sperm cell membrane occur causing defects of sperm structure and function.31 And as already discussed, DNA damage in the form of point mutations, polymorphisms, deletions, chromosomal rearrangements, frame shifts, and single-stranded or double-stranded breaks can also develop48 accelerating apoptosis.49
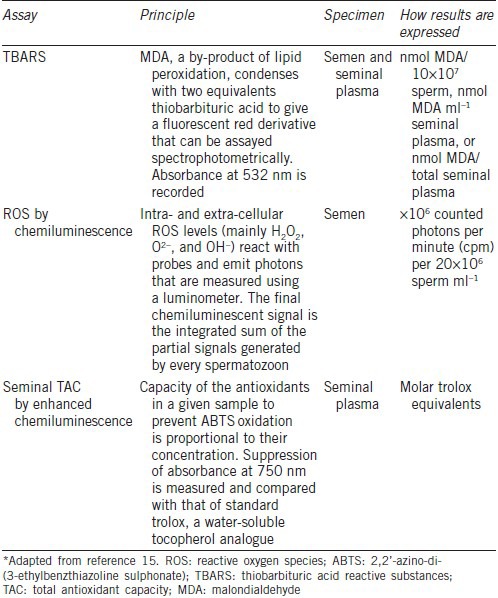
The methods used for ROS detection are broadly divided into two major categories based on their ability to directly or indirectly measure oxidative radicals.15 Indirect measurements involve the assessment of lipid peroxidation products (malondialdehyde), protein oxidation products (carbonyl groups) and oxidized DNA (8-hydroxy-20-deoxyguanosine [8-OHdG]), whereas direct oxidative stress measurements using chemiluminescence can evaluate both intracellular and extracellular ROS levels (Figure 3). Brief descriptions of the most used tests are provided in Table 4. A comprehensive review of the assays principle, methodology, and clinical utility can be found elsewhere.15
Figure 3.
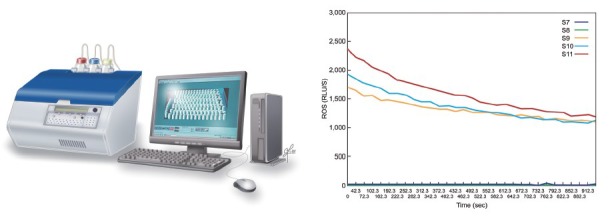
ROS measurement by chemiluminescence assay: AutoLumat 953 Plus Luminometer connected to a computer with a sample result graph.
Table 4.
Characteristics of the test commonly used for assessing oxidative stress*
Many studies have measured seminal markers of oxidative stress in infertile men with varicocele and compared the values with those of fertile men with or without varicocele and infertile men with idiopathic infertility. In a review of studies involving oxidative stress markers in the semen of men with varicocele, we observed that oxidative stress markers were significantly increased in varicocele patients compared to controls.31 Seminal ROS levels measured by chemiluminescence were significantly higher in infertile men with varicocele than fertile controls. In one of the included studies, Allamaneni et al.50 reported that semen ROS levels correlated positively with varicocele grade. The authors showed that men with larger varicoceles had significantly higher semen ROS levels than men with small varicoceles. Mostafa et al. evaluated fertile men with and without varicocele and detected a significant increase in ROS in men with varicocele in comparison to those without.51 Higher seminal levels of specific free radicals, namely nitric oxide (NO) and nitric oxide synthase, have also been detected in infertile men with varicocele compared with fertile men without varicocele.52,53,54,55 Levels of hydrogen peroxide (H2O2) and extracellular seminal superoxide anion have been also shown to be significantly higher in the semen of infertile men with varicocele than in healthy fertile controls.56,57
Among controlled studies that used the malondialdehyde assay, the vast majority demonstrated that seminal malondialdehyde levels were significantly higher in infertile men with varicocele than in fertile healthy controls without varicocele.31
Interestingly, surgical treatment of varicocele has been shown to reduce seminal oxidative stress in varicocele patients.30 In one study, Sakamoto et al.52 found that a time lag of approximately 6 months is required to achieve a marked improvement in seminal ROS markers after varicocele repair. Mostafa et al.58 observed that markers of seminal oxidative stress (NO, H2O2 and malondialdehyde) were significantly reduced whereas antioxidant levels of superoxide dismutase, catalase, glutathione peroxidase and Vitamin C were elevated 3 and 6 months after varicocele repair. Hurtado de Catalfo et al.59 showed that levels of nonenzymatic antioxidants (zinc and selenium) were still abnormal 1 month after varicocele repair, while levels of reduced and oxidized seminal glutathione and antioxidant enzymes were normalized as compared with age-matched fertile controls.
The main clinical value of assessing ROS levels in men with varicocele would be therefore to identify those individuals whose oxidative stress is contributory to their infertility condition. Like SDF, testing results could guide management and follow-up (Figure 2).
Total antioxidant capacity
As stated earlier, an intricate balance between ROS and antioxidants exists maintaining optimal sperm function. Two major antioxidant systems are present, the enzymatic and nonenzymatic antioxidants. Enzymatic antioxidants consist of superoxide dismutase (SOD), catalase, glutathione peroxidase, and glutathione reductase, while the nonenzymatic system consists of scavenger molecules (ascorbate, urate, thiol groups), peroxidation blockers (alpha-tocopherol), and iron-binding molecules (transferrin and lactoferrin).60 Studies have shown an inverse relationship between antioxidant capacity and established oxidative offenses such as lipid peroxidation suggesting an important protective role of antioxidants in infertile men.61
Seminal TAC, enzymatic and nonenzymatic antioxidant measurements have also been used to assess directly oxidative stress (Table 4). Nonenzymatic antioxidants, which account for approximately 65% of the TAC, comprise the main defense system to scavenge excessive ROS.
Several methods are used to assess antioxidant capacity. Total radical-trapping antioxidant parameter (TRAP) monitors the ability of antioxidants to prevent peroxyl radicals from reacting with a probe.62 An antioxidant solution (seminal plasma) is added to 2,2-azobis (2-amidinopropane) dihydrochloride (ABAP), which generates peroxyl radicals, and to R-Phycoerythrin, a red protein pigment capable of harvesting light, in a fluorescence cuvette. Decay of R-Phycoerythrin, which is inhibited by antioxidant activity, is measured every 5 min and the antioxidant potential is identified through determining the lag phase (or delay). Total antioxidant scavenging capacity utilizes gas chromatography to analyze a controlled oxidation reaction, namely “a-keto-g-methiolbutyric acid (KMBA) oxidation to ethylene.”63 Antioxidants ability to prevent ethylene formation relative to a control reaction is quantified.
Chemiluminescence can also be utilized to measure TAC. Radical oxidants react with marker compounds (luminol or lucigenin) producing excited-state species capable of emitting light.64 Antioxidant capacity is assessed through measuring the time of depressed light emission. Finally, a simple, cost effective and reliable method for measuring TAC utilizes a colorometric assay. In this method, H2O2 metamyoglobin interacts with ABAP to produce radical cations that can be detected using spectrophometry.65 Like chemiluminescence assay, the presence of antioxidants induces a lag time in the accumulation of radical cations proportional to the concentration of antioxidant compounds.
Patients with varicocele were found to have lower levels of total seminal antioxidant capacity and specific nonenzymatic antioxidants. Moreover, a positive correlation exists between TAC and the degree of oligo- or asthenozoospermia in patients with varicocele.66,67 In contrast, specific measurements of seminal antioxidant enzyme activity, particularly superoxide dismutase, which scavenges superoxide ions, have yielded conflicting results.
Surgery also seems to improve TAC levels as depicted in few small cohort studies.68,69 Based on the available evidence, TAC seems to be a useful tool in the investigation of men with varicocele; it adds to ROS measurement by providing additional understanding of the interplay between the protective role of antioxidants and oxidative stress in patients with varicocele.
In conclusion, unlike ASA and AR or fertilization defects, sperm DNA fragmentation and oxidative stress are potential targets for therapeutic interventions. Hence, test results could help clinicians not only to identify those individuals more likely to benefit from interventions but also to monitor results of such interventions.
The future
The human seminal plasma is rich in potential biomarkers. It contains numerous proteins and other molecules secreted by virtually all organs of the genital tract from the testis to the meatus of the urethra. For instance, the protein concentration of human seminal plasma is roughly 35–55 mg ml−1, making it an appealing medium for protein identification.70 The proteomic analysis utilizes one- or two-dimensional sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE) in addition to mass spectrometry to confirm the presence of a given protein and measure its quantity. Currently, proteomic analysis is focused on identifying key seminal proteins. Examples of proteins identified so far include fibronectin, lactoferrin, laminin, albumin, and semenogelin.70 Other proteins with specific influence on male fertility include heat shock protein 2 and sperm acrosome membrane-associated protein,71,72 which are found in the sperm acrosome and play a role in sperm–oocyte fusion. The recent publication of a detailed proteomic analysis of the human spermatozoon marked the beginning of the “-omics” revolution.73 Attempts are underway to define the protein structure and function of normal and defective spermatozoa74 and in various disease entities such as varicocele,75 and these issues will be considered elsewhere within this special issue of the journal.
Along these lines, metabolomics, or the study of cellular metabolic products is another area of recent interest in the search of potential male fertility biomarkers. The physiological functions of these small, low molecular weight metabolites are diverse affecting growth, development, and reproduction.76 One example of such markers is those utilized to identify oxidative stress,77 which is increasingly recognized as a major causative factor in the etiology of male infertility.77 We are still understanding the origins of such stress and the central role played by the mitochondria of sperm cells in the generation of reactive oxygen species.78
Finally, genomics is another field of promising outcomes. Genetic abnormalities are estimated to occur in 15%–30% of male factor infertility.79 This number is likely to increase as more genetic causes of infertility are being identified. While genetic abnormalities previously detectable were only large structural chromosomal aberrations, much smaller genomic regions have been found to be responsible for infertility.80 In the near future, identification of point nucleotide changes causing infertility will be possible, allowing for more accurate male infertility biomarkers.80 Microarray technologies, which evaluate men for copy number variations, gene expression levels and single nucleotide polymorphisms hold great promise for identifying highly sensitive and specific genetic biomarkers.
CONCLUSIONS
Varicocele remains one of the most common conditions causing male infertility. Its high prevalence in infertile patients prompts the search for optimal diagnostic modalities capable of accurately selecting patients who would benefit most from surgery. Conventional semen analysis alone is insufficient in the laboratory evaluation of men with varicocele. Andrology has continuously introduced novel advancements to the laboratory evaluation of such patients. The evidence is growing about the clinical importance of utilizing specialized tests to evaluate sperm DNA quality and oxidative stress markers in men with varicocele. Test results can be useful to direct management and to monitor intervention outcomes. However, further research is needed to standardize better protocols, validate test results in larger trials and evaluate the cost-effectiveness of such diagnostic modalities. Proteomics, metabolomics, and genomics are areas, though still developing, with a promise to revolutionize our understanding of reproductive physiology, including varicocele.
AUTHOR CONTRIBUTIONS
AM designed the study, participated in the acquisition of data, summarized the collected evidence, and helped to draft the manuscript. SCE designed the study, participated in the acquisition of data and helped to draft the manuscript. JG helped to draft the manuscript and provided supervision. AA revised the manuscript and helped in coordination. All authors read and approved the final manuscript.
COMPETING INTERESTS
The authors declare no competing interests.
REFERENCES
- 1.Gorelick JI, Goldstein M. Loss of fertility in men with varicocele. Fertil Steril. 1993;59:613–6. [PubMed] [Google Scholar]
- 2.Speroff L, Glass R, Kase NG. Baltimore, USA: Lippincott Williams & Wilkins; 1999. Infertility. Clinical Gynecologic Endocrinology and Infertility; pp. 201–46. [Google Scholar]
- 3.Schlesinger MH, Wilets IF, Nagler HM. Treatment outcome after varicocelectomy. A critical analysis. Urol Clin North Am. 1994;21:517–29. [PubMed] [Google Scholar]
- 4.Pryor JL, Howards SS. Varicocele. Urol Clin North Am. 1987;14:499–513. [PubMed] [Google Scholar]
- 5.Nieschlag E, Hertle L, Fischedick A, Abshagen K, Behre HM. Update on treatment of varicocele: counselling as effective as occlusion of the vena spermatica. Hum Reprod. 1998;13:2147–50. doi: 10.1093/humrep/13.8.2147. [DOI] [PubMed] [Google Scholar]
- 6.Krause W, Muller HH, Schafer H, Weidner W. Does treatment of varicocele improve male fertility?. Results of the ‘Deutsche Varikozelenstudie’, a multicentre study of 14 collaborating centres. Andrologia. 2002;34:164–71. doi: 10.1046/j.1439-0272.2002.00494.x. [DOI] [PubMed] [Google Scholar]
- 7.Madgar I, Weissenberg R, Lunenfeld B, Karasik A, Goldwasser B. Controlled trial of high spermatic vein ligation for varicocele in infertile men. Fertil Steril. 1995;63:120–4. doi: 10.1016/s0015-0282(16)57306-3. [DOI] [PubMed] [Google Scholar]
- 8.Kroese AC, de Lange NM, Collins J, Evers JL. Surgery or embolization for varicoceles in subfertile men. Cochrane Database Syst Rev. 2012;10:CD000479. doi: 10.1002/14651858.CD000479.pub5. [DOI] [PubMed] [Google Scholar]
- 9.Alvarez C, Castilla JA, Martinez L, Ramirez JP, Vergara F, et al. Biological variation of seminal parameters in healthy subjects. Hum Reprod. 2003;18:2082–8. doi: 10.1093/humrep/deg430. [DOI] [PubMed] [Google Scholar]
- 10.Castilla JA, Alvarez C, Aguilar J, Gonzalez-Varea C, Gonzalvo MC, et al. Influence of analytical and biological variation on the clinical interpretation of seminal parameters. Hum Reprod. 2006;21:847–51. doi: 10.1093/humrep/dei423. [DOI] [PubMed] [Google Scholar]
- 11.Keel BA, Stembridge TW, Pineda G, Serafy NT., Sr Lack of standardization in performance of the semen analysis among laboratories in the United States. Fertil Steril. 2002;78:603–8. doi: 10.1016/s0015-0282(02)03296-x. [DOI] [PubMed] [Google Scholar]
- 12.Riddell D, Pacey A, Whittington K. Lack of compliance by UK andrology laboratories with World Health Organization recommendations for sperm morphology assessment. Hum Reprod. 2005;20:3441–5. doi: 10.1093/humrep/dei230. [DOI] [PubMed] [Google Scholar]
- 13.Esteves SC. Clinical relevance of routine semen analysis and controversies surrounding the 2010 World Health Organization criteria for semen examination. Int Braz J Urol. 2014;40:443–53. doi: 10.1590/S1677-5538.IBJU.2014.04.02. [DOI] [PubMed] [Google Scholar]
- 14.Esteves SC, Gosalvez J, Lopez-Fernandez C, Núñez-Calonge R, Caballero P, et al. Diagnostic accuracy of sperm DNA degradation index (DDSi) as a potential noninvasive biomarker to identify men with varicocele-associated infertility. Int Urol Nephrol. 2015;47:1471–7. doi: 10.1007/s11255-015-1053-6. [DOI] [PubMed] [Google Scholar]
- 15.Esteves SC, Sharma RK, Gosalvez J, Agarwal A. A translational medicine appraisal of specialized andrology testing in unexplained male infertility. Int Urol Nephrol. 2014;46:1037–52. doi: 10.1007/s11255-014-0715-0. [DOI] [PubMed] [Google Scholar]
- 16.Esteves SC, Schneider DT, Verza S., Jr Influence of antisperm antibodies in the semen on intracytoplasmic sperm injection outcome. Int Braz J Urol. 2007;33:795–802. doi: 10.1590/s1677-55382007000600007. [DOI] [PubMed] [Google Scholar]
- 17.Djaladat H, Mehrsai A, Rezazade M, Djaladat Y, Pourmand G. Varicocele and antisperm antibody: fact or fiction? South Med J. 2006;99:44–7. doi: 10.1097/01.smj.0000197036.08282.70. [DOI] [PubMed] [Google Scholar]
- 18.Turner TT, Jones CE, Roddy MS. Experimental varicocele does not affect the blood-testis barrier, epididymal electrolyte concentrations, or testicular blood gas concentrations. Biol Reprod. 1987;36:926–32. doi: 10.1095/biolreprod36.4.926. [DOI] [PubMed] [Google Scholar]
- 19.Bozhedomov VA, Lipatova NA, Alexeev RA, Alexandrova LM, Nikolaeva MA, et al. The role of the antisperm antibodies in male infertility assessment after microsurgical varicocelectomy. Andrology. 2014;2:847–55. doi: 10.1111/j.2047-2927.2014.00254.x. [DOI] [PubMed] [Google Scholar]
- 20.Al-Adl A, El-Karamany T, Issa H, Zaazaa M. The influence of antisperm antibodies, intratesticular haemodynamics and the surgical approach to varicocelectomy on seminal variables. Arab J Urol. 2014;12:309–17. doi: 10.1016/j.aju.2014.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Esteves SC, Sharma RK, Thomas AJ, Jr, Agarwal A. Effect of in vitro incubation on spontaneous acrosome reaction in fresh and cryopreserved human spermatozoa. Int J Fertil Womens Med. 1998;43:235–42. [PubMed] [Google Scholar]
- 22.El Mulla KF, Kohn FM, El Beheiry AH, Schill WB. The effect of smoking and varicocele on human sperm acrosin activity and acrosome reaction. Hum Reprod. 1995;10:3190–4. doi: 10.1093/oxfordjournals.humrep.a135885. [DOI] [PubMed] [Google Scholar]
- 23.Vigil P, Wohler C, Bustos-Obregon E, Comhaire F, Morales P. Assessment of sperm function in fertile and infertile men. Andrologia. 1994;26:55–60. doi: 10.1111/j.1439-0272.1994.tb00756.x. [DOI] [PubMed] [Google Scholar]
- 24.Oehninger S, Franken D, Alexander N, Hodgen GD. Hemizona assay and its impact on the identification and treatment of human sperm dysfunctions. Andrologia. 1992;24:307–21. doi: 10.1111/j.1439-0272.1992.tb02660.x. [DOI] [PubMed] [Google Scholar]
- 25.Johnson A, Bassham B, Lipshultz LI, Lamb DJ. A quality control system for the optimized sperm penetration assay. Fertil Steril. 1995;64:832–7. doi: 10.1016/s0015-0282(16)57862-5. [DOI] [PubMed] [Google Scholar]
- 26.Hauser R, Yogev L, Greif M, Hirshenbein A, Botchan A, et al. Sperm binding and ultrasound changes after operative repair of varicocele: correlation with fecundity. Andrologia. 1997;29:145–7. doi: 10.1111/j.1439-0272.1997.tb00309.x. [DOI] [PubMed] [Google Scholar]
- 27.Plymate SR, Nagao RR, Muller CH, Paulsen CA. The use of sperm penetration assay in evaluation of men with varicocele. Fertil Steril. 1987;47:680–3. doi: 10.1016/s0015-0282(16)59121-3. [DOI] [PubMed] [Google Scholar]
- 28.Fuse H, Kazama T, Katayama T. Hypoosmotic swelling test in patients with varicocele. Arch Androl. 1991;27:149–54. doi: 10.3109/01485019108987665. [DOI] [PubMed] [Google Scholar]
- 29.Fuse H, Akashi T, Fujishiro Y, Kazama T, Katayama T. Effect of varicocele on fertility potential: comparison between impregnating and nonimpregnating groups. Arch Androl. 1995;35:143–8. doi: 10.3109/01485019508987865. [DOI] [PubMed] [Google Scholar]
- 30.Hamada A, Esteves SC, Agarwal A. Insight into oxidative stress in varicocele-associated male infertility: part 2. Nat Rev Urol. 2013;10:26–37. doi: 10.1038/nrurol.2012.198. [DOI] [PubMed] [Google Scholar]
- 31.Agarwal A, Hamada A, Esteves SC. Insight into oxidative stress in varicocele-associated male infertility: part 1. Nat Rev Urol. 2012;9:678–90. doi: 10.1038/nrurol.2012.197. [DOI] [PubMed] [Google Scholar]
- 32.Gosálvez J, López-Fernández C, Fernández JL, Esteves SC, Johnston S. Unpacking the mysteries of sperm DNA fragmentation: ten frequently asked questions. J Reprod Biotechnol Fertil. 2015;4:1–16. [Google Scholar]
- 33.Sakkas D, Alvarez JG. Sperm DNA fragmentation: mechanisms of origin, impact on reproductive outcome, and analysis. Fertil Steril. 2010;93:1027–36. doi: 10.1016/j.fertnstert.2009.10.046. [DOI] [PubMed] [Google Scholar]
- 34.Zini A, Boman JM, Belzile E, Ciampi A. Sperm DNA damage is associated with an increased risk of pregnancy loss after IVF and ICSI: systematic review and meta-analysis. Hum Reprod. 2008;23:2663–8. doi: 10.1093/humrep/den321. [DOI] [PubMed] [Google Scholar]
- 35.Robinson L, Gallos ID, Conner SJ, Rajkhowa M, Miller D, et al. The effect of sperm DNA fragmentation on miscarriage rates: a systematic review and meta-analysis. Hum Reprod. 2012;27:2908–17. doi: 10.1093/humrep/des261. [DOI] [PubMed] [Google Scholar]
- 36.Ozturk MI, Koca O, Keles MO, Yilmaz S, Karaman MI. Increased sperm DNA damage in experimental rat varicocele model and the beneficial effect of varicocelectomy. Int J Fertil Steril. 2012;6:95–100. [PMC free article] [PubMed] [Google Scholar]
- 37.Talebi AR, Moein MR, Tabibnejad N, Ghasemzadeh J. Effect of varicocele on chromatin condensation and DNA integrity of ejaculated spermatozoa using cytochemical tests. Andrologia. 2008;40:245–51. doi: 10.1111/j.1439-0272.2008.00852.x. [DOI] [PubMed] [Google Scholar]
- 38.Blumer CG, Restelli AE, Giudice PT, Soler TB, Fraietta R, et al. Effect of varicocele on sperm function and semen oxidative stress. BJU Int. 2012;109:259–65. doi: 10.1111/j.1464-410X.2011.10240.x. [DOI] [PubMed] [Google Scholar]
- 39.Smith R, Kaune H, Parodi D, Madariaga M, Rios R, et al. Increased sperm DNA damage in patients with varicocele: relationship with seminal oxidative stress. Hum Reprod. 2006;21:986–93. doi: 10.1093/humrep/dei429. [DOI] [PubMed] [Google Scholar]
- 40.Garcia-Peiro A, Ribas-Maynou J, Oliver-Bonet M, Navarro J, Checa MA, et al. Multiple determinations of sperm DNA fragmentation show that varicocelectomy is not indicated for infertile patients with subclinical varicocele. Biomed Res Int 2014. 2014:181396. doi: 10.1155/2014/181396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sadek A, Almohamdy AS, Zaki A, Aref M, Ibrahim SM, et al. Sperm chromatin condensation in infertile men with varicocele before and after surgical repair. Fertil Steril. 2011;95:1705–8. doi: 10.1016/j.fertnstert.2011.01.008. [DOI] [PubMed] [Google Scholar]
- 42.Smit M, Romijn JC, Wildhagen MF, Veldhoven JL, Weber RF, et al. Decreased sperm DNA fragmentation after surgical varicocelectomy is associated with increased pregnancy rate. J Urol. 2010;183:270–4. doi: 10.1016/j.juro.2009.08.161. [DOI] [PubMed] [Google Scholar]
- 43.Dada R, Shamsi MB, Venkatesh S, Gupta NP, Kumar R. Attenuation of oxidative stress and DNA damage in varicocelectomy: implications in infertility management. Indian J Med Res. 2010;132:728–30. [PMC free article] [PubMed] [Google Scholar]
- 44.Wang YJ, Zhang RQ, Lin YJ, Zhang RG, Zhang WL. Relationship between varicocele and sperm DNA damage and the effect of varicocele repair: a meta-analysis. Reprod Biomed Online. 2012;25:307–14. doi: 10.1016/j.rbmo.2012.05.002. [DOI] [PubMed] [Google Scholar]
- 45.Esteves SC, Sanchez-Martin F, Sanchez-Martin P, Schneider DT, Gosalvez J. Comparison of reproductive outcome in oligozoospermic men with high sperm DNA fragmentation undergoing intracytoplasmic sperm injection with ejaculated and testicular sperm. Fertil Steril. 2015;104:1398–405. doi: 10.1016/j.fertnstert.2015.08.028. [DOI] [PubMed] [Google Scholar]
- 46.Aitken RJ. The Amoroso lecture. The human spermatozoon – A cell in crisis? J Reprod Fertil. 1999;115:1–7. doi: 10.1530/jrf.0.1150001. [DOI] [PubMed] [Google Scholar]
- 47.Check JH, Graziano V, Cohen R, Krotec J, Check ML. Effect of an abnormal sperm chromatin structural assay (SCSA) on pregnancy outcome following (IVF) with ICSI in previous IVF failures. Arch Androl. 2005;51:121–4. doi: 10.1080/014850190518125. [DOI] [PubMed] [Google Scholar]
- 48.Aitken RJ, Krausz C. Oxidative stress, DNA damage and the Y chromosome. Reproduction. 2001;122:497–506. doi: 10.1530/rep.0.1220497. [DOI] [PubMed] [Google Scholar]
- 49.Turner TT, Lysiak JJ. Oxidative stress: a common factor in testicular dysfunction. J Androl. 2008;29:488–98. doi: 10.2164/jandrol.108.005132. [DOI] [PubMed] [Google Scholar]
- 50.Allamaneni SS, Naughton CK, Sharma RK, Thomas AJ., Jr Agarwal A, editor. Increased seminal reactive oxygen species levels in patients with varicoceles correlate with varicocele grade but not with testis size. Fertil Steril. 2004;82:1684–6. doi: 10.1016/j.fertnstert.2004.04.071. [DOI] [PubMed] [Google Scholar]
- 51.Mostafa T, Anis T, Imam H, El-Nashar AR, Osman IA. Seminal reactive oxygen species-antioxidant relationship in fertile males with and without varicocele. Andrologia. 2009;41:125–9. doi: 10.1111/j.1439-0272.2008.00900.x. [DOI] [PubMed] [Google Scholar]
- 52.Sakamoto Y, Ishikawa T, Kondo Y, Yamaguchi K, Fujisawa M. The assessment of oxidative stress in infertile patients with varicocele. BJU Int. 2008;101:1547–52. doi: 10.1111/j.1464-410X.2008.07517.x. [DOI] [PubMed] [Google Scholar]
- 53.Mehraban D, Ansari M, Keyhan H, Sedighi Gilani M, Naderi G, et al. Comparison of nitric oxide concentration in seminal fluid between infertile patients with and without varicocele and normal fertile men. Urol J. 2005;2:106–10. [PubMed] [Google Scholar]
- 54.Xu Y, Xu QY, Yang BH, Zhu XM, Peng YF. [Relationship of nitric oxide and nitric oxide synthase with varicocele infertility] Zhonghua Nan Ke Xue. 2008;14:414–7. [PubMed] [Google Scholar]
- 55.Abd-Elmoaty MA, Saleh R, Sharma R, Agarwal A. Increased levels of oxidants and reduced antioxidants in semen of infertile men with varicocele. Fertil Steril. 2010;94:1531–4. doi: 10.1016/j.fertnstert.2009.12.039. [DOI] [PubMed] [Google Scholar]
- 56.Mostafa T, Anis T, El Nashar A, Imam H, Osman I. Seminal plasma reactive oxygen species-antioxidants relationship with varicocele grade. Andrologia. 2012;44:66–9. doi: 10.1111/j.1439-0272.2010.01111.x. [DOI] [PubMed] [Google Scholar]
- 57.Mazzilli F, Rossi T, Marchesini M, Ronconi C, Dondero F. Superoxide anion in human semen related to seminal parameters and clinical aspects. Fertil Steril. 1994;62:862–8. doi: 10.1016/s0015-0282(16)57017-4. [DOI] [PubMed] [Google Scholar]
- 58.Mostafa T, Anis TH, El-Nashar A, Imam H, Othman IA. Varicocelectomy reduces reactive oxygen species levels and increases antioxidant activity of seminal plasma from infertile men with varicocele. Int J Androl. 2001;24:261–5. doi: 10.1046/j.1365-2605.2001.00296.x. [DOI] [PubMed] [Google Scholar]
- 59.Hurtado de Catalfo GE, Ranieri-Casilla A, Marra FA, de Alaniz MJ, Marra CA. Oxidative stress biomarkers and hormonal profile in human patients undergoing varicocelectomy. Int J Androl. 2007;30:519–30. doi: 10.1111/j.1365-2605.2007.00753.x. [DOI] [PubMed] [Google Scholar]
- 60.Aitken J, Fisher H. Reactive oxygen species generation and human spermatozoa: the balance of benefit and risk. Bioessays. 1994;16:259–67. doi: 10.1002/bies.950160409. [DOI] [PubMed] [Google Scholar]
- 61.Sharma RK, Pasqualotto FF, Nelson DR, Thomas AJ, Jr, Agarwal A. The reactive oxygen species-total antioxidant capacity score is a new measure of oxidative stress to predict male infertility. Hum Reprod. 1999;14:2801–7. doi: 10.1093/humrep/14.11.2801. [DOI] [PubMed] [Google Scholar]
- 62.Ghiselli A, Serafini M, Maiani G, Azzini E, Ferro-Luzzi A. A fluorescence-based method for measuring total plasma antioxidant capability. Free Radic Biol Med. 1995;18:29–36. doi: 10.1016/0891-5849(94)00102-p. [DOI] [PubMed] [Google Scholar]
- 63.Regoli F, Winston GW. Quantification of total oxidant scavenging capacity of antioxidants for peroxynitrite, peroxyl radicals, and hydroxyl radicals. Toxicol Appl Pharmacol. 1999;156:96–105. doi: 10.1006/taap.1999.8637. [DOI] [PubMed] [Google Scholar]
- 64.Bastos EL, Romoff P, Eckert CR, Baader WJ. Evaluation of antiradical capacity by H2O2-hemin-induced luminol chemiluminescence. J Agric Food Chem. 2003;51:7481–8. doi: 10.1021/jf0345189. [DOI] [PubMed] [Google Scholar]
- 65.Mahfouz R, Sharma R, Sharma D, Sabanegh E, Agarwal A. Diagnostic value of the total antioxidant capacity (TAC) in human seminal plasma. Fertil Steril. 2009;91:805–11. doi: 10.1016/j.fertnstert.2008.01.022. [DOI] [PubMed] [Google Scholar]
- 66.Giulini S, Sblendorio V, Xella S, La Marca A, Palmieri B, et al. Seminal plasma total antioxidant capacity and semen parameters in patients with varicocele. Reprod Biomed Online. 2009;18:617–21. doi: 10.1016/s1472-6483(10)60004-1. [DOI] [PubMed] [Google Scholar]
- 67.Mancini A, Meucci E, Milardi D, Giacchi E, Bianchi A, et al. Seminal antioxidant capacity in pre- and post-operative varicocele. J Androl. 2004;25:44–9. doi: 10.1002/j.1939-4640.2004.tb02757.x. [DOI] [PubMed] [Google Scholar]
- 68.Chen SS, Huang WJ, Chang LS, Wei YH. Attenuation of oxidative stress after varicocelectomy in subfertile patients with varicocele. J Urol. 2008;179:639–42. doi: 10.1016/j.juro.2007.09.039. [DOI] [PubMed] [Google Scholar]
- 69.Ozturk U, Ozdemir E, Buyukkagnici U, Dede O, Sucak A, et al. Effect of spermatic vein ligation on seminal total antioxidant capacity in terms of varicocele grading. Andrologia. 2012;44(Suppl 1):199–204. doi: 10.1111/j.1439-0272.2011.01164.x. [DOI] [PubMed] [Google Scholar]
- 70.Pilch B, Mann M. Large-scale and high-confidence proteomic analysis of human seminal plasma. Genome Biol. 2006;7:R40. doi: 10.1186/gb-2006-7-5-r40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Redgrove KA, Nixon B, Baker MA, Hetherington L, Baker G, et al. The molecular chaperone HSPA2 plays a key role in regulating the expression of sperm surface receptors that mediate sperm-egg recognition. PLoS One. 2012;7:e50851. doi: 10.1371/journal.pone.0050851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Fujihara Y, Satouh Y, Inoue N, Isotani A, Ikawa M, et al. SPACA1-deficient male mice are infertile with abnormally shaped sperm heads reminiscent of globozoospermia. Development. 2012;139:3583–9. doi: 10.1242/dev.081778. [DOI] [PubMed] [Google Scholar]
- 73.Baker MA, Reeves G, Hetherington L, Muller J, Baur I, et al. Identification of gene products present in Triton X-100 soluble and insoluble fractions of human spermatozoa lysates using LC-MS/MS analysis. Proteomics Clin Appl. 2007;1:524–32. doi: 10.1002/prca.200601013. [DOI] [PubMed] [Google Scholar]
- 74.Barazani Y, Agarwal A, Sabanegh ES., Jr Functional sperm testing and the role of proteomics in the evaluation of male infertility. Urology. 2014;84:255–61. doi: 10.1016/j.urology.2014.04.043. [DOI] [PubMed] [Google Scholar]
- 75.Agarwal A, Sharma R, Durairajanayagam D, Ayaz A, Cui Z, et al. Major protein alterations in spermatozoa from infertile men with unilateral varicocele. Reprod Biol Endocrinol. 2015;13:8. doi: 10.1186/s12958-015-0007-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Lindon JC, Nicholson JK. Spectroscopic and statistical techniques for information recovery in metabonomics and metabolomics. Annu Rev Anal Chem (Palo Alto Calif) 2008;1:45–69. doi: 10.1146/annurev.anchem.1.031207.113026. [DOI] [PubMed] [Google Scholar]
- 77.Agarwal A, Said TM. Oxidative stress, DNA damage and apoptosis in male infertility: a clinical approach. BJU Int. 2005;95:503–7. doi: 10.1111/j.1464-410X.2005.05328.x. [DOI] [PubMed] [Google Scholar]
- 78.Koppers AJ, De Iuliis GN, Finnie JM, McLaughlin EA, Aitken RJ. Significance of mitochondrial reactive oxygen species in the generation of oxidative stress in spermatozoa. J Clin Endocrinol Metab. 2008;93:3199–207. doi: 10.1210/jc.2007-2616. [DOI] [PubMed] [Google Scholar]
- 79.Ferlin A, Raicu F, Gatta V, Zuccarello D, Palka G, et al. Male infertility: role of genetic background. Reprod Biomed Online. 2007;14:734–45. doi: 10.1016/s1472-6483(10)60677-3. [DOI] [PubMed] [Google Scholar]
- 80.Lehmann KJ, Kovac JR, Xu J, Fischer MA. Isodicentric Yq mosaicism presenting as infertility and maturation arrest without altered SRY and AZF regions. J Assist Reprod Genet. 2012;29:939–42. doi: 10.1007/s10815-012-9822-y. [DOI] [PMC free article] [PubMed] [Google Scholar]


