Abstract
Even though the inevitable process of aging by itself cannot be considered a disease, it is directly linked to life span and is the driving force behind all age-related diseases. It is an undisputable fact that age-associated diseases are among the leading causes of death in the world, primarily in industrialized countries. During the last several years, an intensive search of antiaging treatments has led to the discovery of a variety of drugs that promote health span and/or life extension. The biguanide compound metformin is widely used for treating people with type 2 diabetes and appears to show protection against cancer, inflammation, and age-related pathologies. Here, we summarize the recent developments about metformin use in translational aging research and discuss its role as a potential geroprotector.
Metformin, which is widely used to treat type 2 diabetes, may provide some protection against age-related pathologies. But more work is needed to understand its molecular mechanisms and translational potential.
Over the past two decades, metformin has emerged as the first-line treatment for people with type 2 diabetes (T2DM) and is the most widely prescribed antidiabetic drug in the world (American Diabetes Association 2014). In addition to its use in T2DM, metformin is being prescribed for the treatment of polycystic ovary syndrome, diabetic nephropathy, and gestational diabetes, and has shown early promise as a treatment for cancer. Historically, despite its well-accepted antidiabetic properties in the 1950s, and use for hyperglycemia treatment in England in 1958, metformin remained contraindicated largely because of concerns about lactic acidosis and it was not approved by the U.S. Food and Drug Administration until 1994 (Bailey and Turner 1996; Mahmood et al. 2013). We now know that the rare event of lactic acidosis occurs in >0.01 to 0.08 cases (average, 0.03) per 1000 patient-years caused by an insufficient metformin clearance by the kidneys (Bailey and Turner 1996). Therefore, the risk of side effects is relatively low in comparison to the multiple benefits of metformin.
The exact molecular mechanisms of metformin’s therapeutic action still remain unknown. Metformin is a biguanide compound originally derived from a guanidine derivative found in the plant Galega officinalis. It acts as an insulin sensitizer and exerts its principal metabolic action on the liver. In addition to its glucoregulatory action, metformin has gained attention for its pleiotropic effects and activity in a variety of tissues, such as muscles, adipose tissue, ovary, endothelium, and brain (Diamanti-Kandarakis et al. 2010; Foretz et al. 2014). Food intake (Adeyemo et al. 2014; Pernicova and Korbonits 2014) and body weight (Glueck et al. 2001) are decreased as a result of a direct action of metformin on the hypothalamic centers regulating satiety and feeding (Stevanovic et al. 2012); it may also influence metabolic and cellular processes associated with the development of chronic conditions of aging, including inflammation, fatty liver, oxidative damage, protein glycation, cellular senescence, diminished autophagy, apoptosis, and development of several types of cancer (Isoda et al. 2006; Kita et al. 2012; Hirsch et al. 2013; Woo et al. 2014). A number of recent studies support the role of metformin in improving health span and life span in different animal models (Anisimov et al. 2011; Cabreiro et al. 2013; Martin-Montalvo et al. 2013; Anisimov 2014; De Haes et al. 2014). The possibility exists, therefore, for similar beneficial actions of metformin in human health and longevity.
The purpose of this article is to review the role of metformin as a possible geroprotector drug. We will try to summarize recent evidence for the antiaging properties of metformin, the molecular mechanisms implicated in this role, and, finally, discuss new (research opportunities) directions to better understand the translational potential of metformin.
HOW DOES METFORMIN WORK?
Metformin is excreted intact in the urine, without being metabolized by the liver or kidney. About 50%–60% of an oral dose is absorbed into the systemic circulation and distributed in most tissues at similar concentrations, although higher concentrations are found in gastrointestinal tract, liver, and kidney (Bailey and Turner 1996; Gong et al. 2012; Pawlyk et al. 2014). Age, gender, nutritional status, lifestyle, and genetic variations represent some of the factors that influence metformin’s susceptibility and distribution to target tissues. For instance, membrane transporter polymorphism is a key determinant in the pharmacokinetic properties of this drug (Chen et al. 2013; Pawlyk et al. 2014). Metformin exerts its therapeutic effects, through a number of mechanisms and physiological pathways that resemble those generated by caloric restriction (CR), an experimental model known to extend life span and health span in various organisms. Indeed, microarray analyses have shown that metformin induces the same gene expression profile as CR (Dhahbi et al. 2005; Spindler 2006; Martin-Montalvo et al. 2013), despite no reduction in food intake (Mercken et al. 2012; de Cabo et al. 2014).
The inhibition of hepatic gluconeogenesis and lipogenesis by metformin occurs via alterations in cellular energetics. The decrease in cellular respiration that results from metformin’s inhibition of mitochondrial complex I activity (El-Mir et al. 2000; Owen et al. 2000) yields lower ATP levels. Although the interaction with mitochondrial copper ion appears essential for the metabolic effects of metformin (Logie et al. 2012), more still needs to be learned about whether the drug inhibits respiration through direct or indirect action (Fontaine 2014). The nonclassical effects of metformin on the expression of glucose transporters and glycolytic enzymes (up-regulation) result indirectly from mitochondrial respiratory chain inhibition (Owen et al. 2000). This inhibition of the electron transport chain (Batandier et al. 2006; Guarente 2008) combined with the induction of antioxidant gene expression by the SKN-1/Nrf2 transcription pathway (Onken and Driscoll 2010) provides mechanistic insights into metformin’s role in lowering the production of reactive oxygen species (ROS). AMP-activated protein kinase (AMPK) is a key sensor of energy status that regulates metabolic energy balance at whole-body level (Hardie et al. 2012). The increase in AMP/ATP and ADP/ATP ratios stimulates AMPK (Stephenne et al. 2011); however, metformin can activate AMPK without eliciting detectable changes in AMP, ADP, and ATP levels (Hawley et al. 2002). It was later determined that the tumor suppressor protein LKB1 (alternatively termed SK11) was responsible for the activating phosphorylation of AMPK in response to metformin (Shaw et al. 2005). Indeed, the LKB1–AMPK pathway controls the expression of key hepatic gluconeogenic genes by regulating the transcriptional coactivator cAMP-response element-binding protein (CREB)-regulated transcription coactivator 2 (CRTC2, also known as TORC2) (Shaw et al. 2005), a key regulator of fasting glucose metabolism (Koo et al. 2005). The role of LKB1–AMPK as mediator of metformin’s action on hepatic gluconeogenesis and lipogenesis (Zhou et al. 2001; Zou et al. 2004; Shaw et al. 2005) was put to test in studies using conditional Ampk knockout mice (Foretz et al. 2010). The observed inhibition of glucononeogenesis, independent of LKB1–AMPK signaling, was accompanied by a decrease in hepatic energy state in response to concentrations of metformin that were far higher than those reached in hepatic portal vein after standard treatment (Foretz et al. 2010). When therapeutic concentrations of metformin were tested, hepatic gluconeogenesis was suppressed via AMPK activation (Cao et al. 2014) and formation of AMPK αβγ complexes (Meng et al. 2014). The ability of AMPK to improve lipid metabolism helps explain the reduction in hepatic steatosis by metformin (Woo et al. 2014), which requires the inhibitory phosphorylation of acetyl-CoA carboxylase (ACC) by AMPK, an essential step toward the lipid-lowering and insulin-sensitizing effects of metformin (Fullerton et al. 2013). Moreover, metformin treatment decreases the levels of sterol regulatory element-binding protein 1 (SREBP-1), a key lipogenic transcription factor, via direct phosphorylation by AMPK (Zhou et al. 2001; Li et al. 2011). The regulation of lipid metabolism by metformin also takes place by enhancing the fatty acid β-oxidation pathway (Collier et al. 2006). New molecular mechanisms by which metformin inhibits hepatic gluconeogenesis have been proposed and include the ability of the drug to inhibit adenylate cyclase through AMP accumulation, thereby blocking the glucagon-signaling pathway (Miller et al. 2013), and direct inhibition of mitochondrial glycerophosphate dehydrogenase (mGPD) (Madiraju et al. 2014). In the latter study, metformin-mediated mGPD inhibition was accompanied by lower mitochondrial NADH/NAD+ ratios, a result inconsistent with prior reports showing that complex I inhibition by metformin increased this ratio (Owen et al. 2000). The different doses and route of administration of metformin between the two studies might explain these discrepancies (Baur and Birnbaum 2014).
Another potential mechanism through which metformin inhibits hepatic gluconeogenesis is the down-modulated expression of genes encoding for the gluconeogenic enzymes, phosphoenolpyruvate carboxykinase (PEPCK), and glucose-6-phosphatase (G6Pase), a molecular mechanism that requires AMPK-mediated up-regulation of orphan nuclear receptor short heterodimer partner (SHP) expression (Kim et al. 2008). Additionally, metformin improves glucose homeostasis by promoting an increase in insulin-independent phosphorylation of insulin receptor and insulin receptor substrates (IRS)-1 and (IRS)-2, and subsequent translocation of glucose transporters GLUT4 to the plasma membrane (Gunton et al. 2003; Yuan et al. 2003). The regulation of the incretin hormone (e.g., glucagon-like peptide 1) and insulin secretory responses with metformin treatment has been reported (Cho and Kieffer 2011; Maida et al. 2011; Kim et al. 2014).
Metformin also acts as an inhibitor of mechanistic target of rapamycin complex 1 (mTORC1) through AMPK-dependent and -independent mechanisms. AMPK activation by metformin inhibits the protein kinase mTOR, thus preventing the phosphorylation of downstream targets, including S6K, rpS6, and 4E-BP1 (Dowling et al. 2007). Inhibition of the Ras-related GTP binding (Rag) GTPases (Kalender et al. 2010) and up-regulation of REDD1, a hypoxia-inducible factor 1 (HIF-1) target (Shoshani et al. 2002; Ben Sahra et al. 2011), are among the AMPK-independent mechanisms by which metformin inhibits mTORC1 signaling. Because of the many faces of mTOR in life span and metabolism, it is intriguing that metformin may act as a potential therapeutic drug for the treatment of aging and age-related diseases, such as cancer and metabolic syndrome (Johnson et al. 2013).
METFORMIN AS AN ANTI-AGING DRUG
Recent reviews have reported the geroprotective effects of biguanides, mainly metformin, because of its superior safety profile (Bulterijs 2011; Berstein 2012; Miles et al. 2014). As indicated earlier, metformin treatment enhances insulin sensitivity, induces glycolysis, and suppresses hepatic gluconeogenesis. There is some evidence that metformin may also have cardioprotective effects (Eurich et al. 2013; Hong et al. 2013) and contribute to the prevention of some forms of human cancer (Cazzaniga et al. 2013; Anisimov 2014; Laskov et al. 2014). This therapeutic profile of metformin supports its use for age-related diseases and longevity. Of significance, many studies have confirmed the positive effect of metformin on life span of worms, flies, mice, and rats. Moreover, diabetic and cardiovascular disease patients who are prescribed metformin have increased rates of survival (Scarpello 2003; Yin et al. 2013), and it was recently proposed that metformin might promote longevity by preventing frailty in older adults with T2DM (Wang et al. 2014). Chronic treatment with metformin among patients with diabetes might reduce the risk of cognitive decline and dementia (Ng et al. 2014; Patrone et al. 2014) and improve survival in several types of cancer (Greenhill 2015; Ko et al. 2015; Lin et al. 2015; Rego et al. 2015).
STUDIES IN INVERTEBRATE MODELS
Many molecular mechanisms implicated in aging and age-related diseases have been elucidated in Caenorhabditis elegans, an experimental model widely used for the identification of new pharmacological agents capable of delaying the aging process (Olsen et al. 2006; Lapierre and Hansen 2012). Metformin supplementation (50 mM dose) was found to increase the mean life span of C. elegans by about 40% without maximum life span extension. This increase in health span had CR-like features that involved activation of the LKB1–AMPK–SKN1 pathway both in wild-type worms and in mutant animals with disrupted insulin pathway (Onken and Driscoll 2010). The increase in carbohydrate levels in metformin-treated worms provides a good source of ATP to better survive 2 to 3 d of anoxia exposure through a mechanism that depends on specific AMPK subunits (LaRue and Padilla 2011). Active bacterial metabolism is a critical nutritional requirement for C. elegans life span (Lenaerts et al. 2008; Cabreiro and Gems 2013). Biguanide-treated worms lived longer (∼30% increase compared with their normal life span) only when cultured with a Escherichia coli strain sensitive to the drug, which contrasts with the pathogenic effects of drug-resistant bacteria on nematode health and aging. An alteration in microbial folate and methionine metabolism helps explain the extended longevity, which is consistent with the notion that metformin is a CR-mimetic drug. Metformin is primarily used for the management of hyperglycemia in T2DM, which led Cabreiro and colleagues to test whether high glucose adversely affected bacterial growth inhibition by metformin and, consequently, C. elegans longevity. The reduction in metformin-induced life span extension in response to glucose supplementation led the investigators to suggest that altering gut microbiota might represent a new therapeutic approach for delaying aging and the treatment of age-related diseases (Cabreiro et al. 2013). Conversely, glucose restriction extends C. elegans life span by inducing mitohormesis, a physiological process based on mitochondrial oxidative stress (Schulz et al. 2007; Zarse et al. 2012). Of significance, metformin-treated nematodes showed increased respiration and higher ROS production, consistent with the generation of a mitohormetic signal (De Haes et al. 2014). These investigators established that the mitohormetic signal was propagated by the hydrogen peroxide scavenger peroxiredoxin PRDX-2, whose expression was up-regulated after metformin treatment, and deletion of the prdx2 gene led to decreased overall life expectancy. C. elegans treated with metformin also had a youthful morphology for a longer time, which contributed to their improved health span (De Haes et al. 2014).
The beneficial effects of metformin on the life span of nematodes do not appear to be evolutionarily conserved in Drosophila. AMPK activation increases life span in Drosophila (Tohyama and Yamaguchi 2010; Stenesen et al. 2013), and metformin treatment reduces lipid storage via robust activation of AMPK without promoting longevity in either male or female flies. Perturbations in intestinal homeostasis may be responsible for metformin toxicity in flies when taken in high enough doses (Slack et al. 2012) and when different antidiabetic compounds were tested for their potential antiaging properties (Jafari et al. 2007). In the latter study, metformin given in doses of 0.4, 0.8, and 1.6 mg/ml did not decrease the mortality rate in flies (Jafari et al. 2007). Even though the prolongevity effects of metformin have yet to be found, this drug can inhibit age- and oxidative-stress-induced DNA damage and delay stem cell aging in Drosphilia (Na et al. 2013).
STUDIES IN RODENT MODELS
C57BL/6J mice and Fisher 344 (F344) rats are the preferred strains of rodents for use in gerontological studies (Anisimov et al. 2012). The physiology of these animals, mainly at the cellular level, is very similar to humans, which allows the study of various compounds for their life extending properties and the extrapolation of these findings to human aging. The sole publication on the impact of metformin in rat longevity indicated a lack of effect of the biguanine on mean life span and mean of the last surviving 10% male F344 rats, compelling the investigators to question the claims about metformin acting as a CR-mimetic drug (Smith et al. 2010). However, this strain of rats is resistant to the health benefits of CR and, thus, may provide a partial explanation for the lack of prolongevity effects of metformin in F344 rats (Smith et al. 2010). This study was rather inconclusive and new approaches will be required to determine whether metformin can prolong life span in rats.
More studies were performed in different mouse strains using male and female animals. In general, female mice responded better to metformin vis-à-vis mean life span extension, as compared with male mice and rats. Metformin treatment (100 mg/kg in drinking water for 5 consecutive days every month) significantly increased mean (+8%) and maximal life span (+9%) of short-lived, cancer-prone female HER-2/neu transgenic mice (strain FVB/N carrying a HER-2/neu oncogene) with significant reduction in the mean size and accumulation of mammary adenocarcinoma (Anisimov et al. 2005a,b). When combined with melatonin, metformin inhibited the growth of a HER2 mammary tumor and Ehrlich tumor growth in mice, whereas metformin treatment alone was shown to slow down the development of spontaneous mammary tumors and increase mean life span in female HER-2/neu transgenic mice (Anisimov et al. 2010a).
The geroprotective effects of metformin and its ability to suppress spontaneous tumorigenesis were also observed in other mouse strains. Long-term treatment with metformin significantly increased mean (+37.9%) and maximum life span (+10.3%) of female outbred SHR mice, and slowed down the age-associated disturbances in the estrous function without impacting on body weight or food intake (Anisimov et al. 2008). However, metformin treatment did not alter the incidence or mean latency of tumors, an unexpected finding that was attributed to the inherent genetic makeup of the SHR mouse strain. Nevertheless, this result emphasizes the fact that metformin can prolong life independently of its ability to suppress cancer (Blagosklonny and Campisi 2008). It is interesting to note that the responsiveness of female SHR mice to the prolongevity effects of metformin was dependent of the age of the animals at the onset of treatment. An increase in the mean life span was observed when metformin treatment was started at the age of 3 or 9 mo (+14.1% and +6.1%, respectively), but not at 15 mo of age (Anisimov et al. 2011). Focusing the analysis on tumor-free mice only, there was a significant increase (20.7% and 7.1%) but significant reduction (−12.8%) in mean life span when metformin administration was initiated in 3-, 9-, and 15-mo-old animals, respectively.
The possibility that metformin can improve the outcomes of two neurological disorders was investigated both in male and female mice. In the first study, different metformin doses (0, 2, or 5 mg/ml) were given in the drinking water of 5-wk-old transgenic mice with Huntington’s disease (the R6/2 line with ∼150 glutamine repeats) (Ma et al. 2007). The investigators observed that metformin, only at 2 mg/ml, significantly increased mean life span (+20.1%) and decreased the duration of hind limb clasping, a phenotypic marker of motor defect, in male but not female animals. In the second study, three doses of metformin (0.5, 2, and 5 mg/ml) were given in the drinking water of male and female SOD1G93A mice (transgenic model of amyotrophic lateral sclerosis [ALS]) from 35 d of age (Kaneb et al. 2011). Metformin treatment had no effect on disease onset, progression or survival in male SOD1G93A mice at any dose while eliciting a dose-dependent negative neurological response in females owing to metformin’s ability to inhibit estrogen production (Rice et al. 2009). Inhibition of estrogen can accelerate ALS progression and reduce life span in female SOD1G93A mice (Choi et al. 2008). All treatment groups appeared to weigh less and displayed no significant differences in their life span, as compared with control mice. However, a tendency toward increased survival was observed with reduction in the dose of metformin (Kaneb et al. 2011).
The notion that the prolongevity effects of metformin depend on the developmental stage of the animal at the onset of treatment was further explored in inbred male and female 129/Sv mice (Anisimov et al. 2010b). Addition of metformin (100 mg/kg) in drinking water of 3-mo-old male animals elicited a significant decrease in mean life span (−13.4%) without affecting maximum life span. A higher incidence of chromosome aberrations was also noted in metformin-treated male mice. In females, metformin did not influence maximum life span, but it slightly increased mean and median life span by 4.4% and 7.8%, respectively, with a significant reduction in the total incidence of malignant tumors. However, an increase of benign angiogenic tumors was observed in metformin-treated female mice (Anisimov et al. 2010b). The reasons for these gender-specific differences on metformin responses are still under study and may be attributed to the fact that males and females have different mechanisms of aging. Potential gender-related variability in outcomes are exemplified by the next series of reports. Deletion of ribosomal S6 protein kinase 1 (S6K1), a component of the mTOR pathway, significantly increased the life span of female C57BL/6 mice (+20.4%) without changes in that of male animals (Selman et al. 2009). Subcutaneous administration of metformin (100 mg/kg body weight) in 3-, 5-, and 7-d-old 129/Sv mouse pups caused an inversion of the gender response to the prolongevity effects of the drug (Anisimov et al. 2015). These investigators reported an increase in mean life span (+20%) and a slight maximum life span extension (+3.5%) in males who received metformin neonatally, while a decrease in mean and median life span (−9.1% and −13.8%, respectively) without significant differences in maximum life span was observed when female mouse pups were treated with metformin, as compared with control animals. The neonatal period is critical for the development of the hypothalamic circuits that control energy homeostasis (Contreras et al. 2013) and it has been suggested that reprogramming of these circuits, especially the mTOR-signaling pathway, may be part of the aging process (Blagosklonny 2013). Many aspects of aging are controlled by the hypothalamus, and alteration of hypothalamic pathways might allow the manifestations of aging to be modified (Zhang et al. 2013).
The long-term effects of metformin supplementation (0.1% and 1% w/w) in the food was performed in male C57BL/6 mice, starting from the age of 54 wk for the remainder of their lives (Martin-Montalvo et al. 2013). The mean life span of mice supplemented with 0.1% metformin increased by 5.83%, while that of mice on 1% metformin was significantly reduced (−14.4%), likely caused by renal failure. Diet supplementation with 0.1% metformin tended to preserve body weight with advancing age, a condition known to increase longevity in mice (Pearson et al. 2008). There were no significant differences in the number of pathologies in mice on 0.1% metformin; however, liver cancer incidence was significantly reduced with 1% metformin supplementation (3.3% vs. 26.5% in metformin- and vehicle-treated mice, respectively) (Martin-Montalvo et al. 2013). An improvement in physical performance and glucose homeostasis combined with increased insulin sensitivity, and a reduction in low-density lipoprotein and cholesterol levels occurred in 0.1% metformin-fed mice without a decrease in caloric intake. By preserving overall health span in mice, metformin may prevent the development of metabolic syndrome through significant reduction in oxidative stress and chronic inflammation (Martin-Montalvo et al. 2013). These investigators reported similar gene expression patterns in the liver (and skeletal muscle) of mice fed 40% CR and 0.1% metformin, reinforcing the role of metformin as a CR mimetic (Mercken et al. 2012; de Cabo et al. 2014). Of significance, the prolongevity effect of metformin was observed also in a second strain of male mice (hybrid B6C3F1), with a 4.15% increase in mean life span in response to 0.1% metformin supplementation in the diet (Martin-Montalvo et al. 2013).
CONCLUSIONS AND PERSPECTIVES
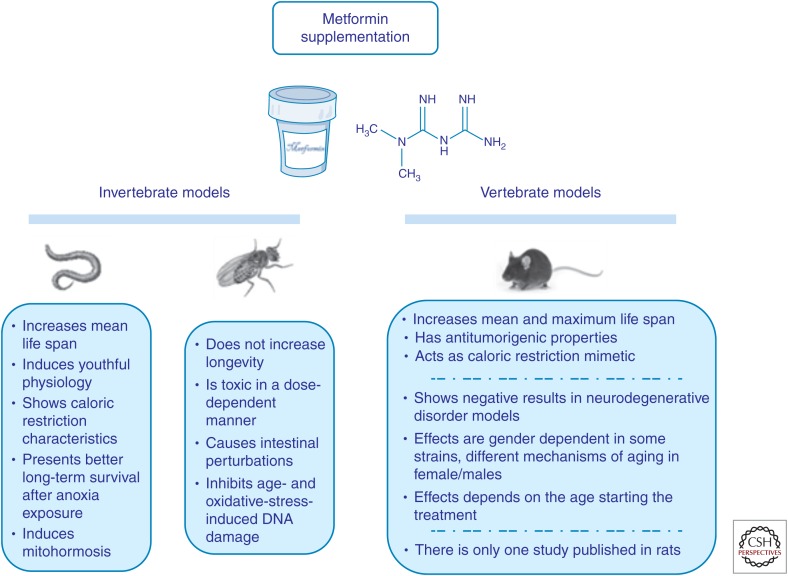
According to recent published data in different animal models, metformin appears to be a promising candidate as a life-extending drug (Fig. 1). This compound is generally well tolerated and its long history of clinical use makes it an even more attractive candidate. Besides, metformin is more beneficial than any other anti-diabetic drug in reducing age-related diseases and improving survival in diabetic patients. Although the initial results are very hopeful, more work is needed to elucidate several aspects that still remain unclear. Many of these positive results have been obtained using doses of metformin that exceed therapeutic levels in humans (Martin-Castillo et al. 2010; Aldea et al. 2014). Moreover, the modes of administration varied among research teams, with the addition of metformin either in drinking water or to the diet. Although female mice were initially found to show a better response to metformin supplementation, recent results from our laboratory indicated no gender or stain differences in the actions of metformin (Martin-Montalvo et al. 2013). Therefore, to establish the molecular mechanisms and pathways of aging, it is imperative to investigate potential hormone-metformin interactions in male and female animals of varying ages, as the age of starting metformin treatment determines whether an increase in mean and maximum life span occurs (Menendez et al. 2011; Anisimov et al. 2015). There are not enough studies to conclude whether there are epigenetic/genetic differences in metformin effect on aging, life span, and tumorigenesis. Because not all organisms studied seem to respond positively to metformin supplementation (e.g., flies and rats), new approaches with different protocols and experimental designs would be crucial to understanding how metformin might be a good geroprotector throughout phylogeny, including in humans.
Figure 1.
Summary of the effects of metformin supplementation in invertebrate (Caenorhabditis elegans and Drosophila melanogaster) and vertebrate models (rodents, mainly mice).
A new interesting functional interplay has emerged during the last years that might explain some of the molecular mechanisms through which metformin could improve health and life span. There is some evidence that the anticancer protection conferred by metformin treatment may involve the modulation of miRNAs (Pulito et al. 2014). These small noncoding RNAs regulate gene expression at the posttranscriptional level and metformin modulates miRNAs that regulate apoptosis and inhibit proliferation (Li et al. 2012).
Despite these advances, it is the hope that better coordination among basic and clinical researchers and use of more sophisticated approaches will facilitate the development of new interventions aimed at improving human health and life span.
ACKNOWLEDGMENTS
This work is supported by the Intramural Research Program of the National Institute on Aging, National Institutes of Health. CIBER de Fisiopatología de la Obesidad y Nutrición is an initiative of ISCIII. The funding agency had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Footnotes
Editors: S. Jay Olshansky, George M. Martin, and James L. Kirkland
Additional Perspectives on Aging available at www.perspectivesinmedicine.org
REFERENCES
- Adeyemo MA, McDuffie JR, Kozlosky M, Krakoff J, Calis KA, Brady SM, Yanovski JA. 2014. Effects of metformin on energy intake and satiety in obese children. Diabetes Obes Metab 17: 363–370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aldea M, Craciun L, Tomuleasa C, Berindan-Neagoe I, Kacso G, Florian IS, Crivii C. 2014. Repositioning metformin in cancer: Genetics, drug targets, and new ways of delivery. Tumour Biol 35: 5101–5110. [DOI] [PubMed] [Google Scholar]
- American Diabetes Association. 2014. Standards of medical care in diabetes—2014. Diabetes Care 37: S14–S80. [DOI] [PubMed] [Google Scholar]
- Anisimov VN. 2014. Do metformin a real anticarcinogen? A critical reappraisal of experimental data. Ann Transl Med 2: 60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anisimov VN, Berstein LM, Egormin PA, Piskunova TS, Popovich IG, Zabezhinski MA, Kovalenko IG, Poroshina TE, Semenchenko AV, Provinciali M, et al. 2005a. Effect of metformin on life span and on the development of spontaneous mammary tumors in HER-2/neu transgenic mice. Exp Gerontol 40: 685–693. [DOI] [PubMed] [Google Scholar]
- Anisimov VN, Egormin PA, Bershtein LM, Zabezhinskii MA, Piskunova TS, Popovich IG, Semenchenko AV. 2005b. Metformin decelerates aging and development of mammary tumors in HER-2/neu transgenic mice. Bull Exp Biol Med 139: 721–723. [DOI] [PubMed] [Google Scholar]
- Anisimov VN, Berstein LM, Egormin PA, Piskunova TS, Popovich IG, Zabezhinski MA, Tyndyk ML, Yurova MV, Kovalenko IG, Poroshina TE, et al. 2008. Metformin slows down aging and extends life span of female SHR mice. Cell Cycle 7: 2769–2773. [DOI] [PubMed] [Google Scholar]
- Anisimov VN, Egormin PA, Piskunova TS, Popovich IG, Tyndyk ML, Yurova MN, Zabezhinski MA, Anikin IV, Karkach AS, Romanyukha AA. 2010a. Metformin extends life span of HER-2/neu transgenic mice and in combination with melatonin inhibits growth of transplantable tumors in vivo. Cell Cycle 9: 188–197. [DOI] [PubMed] [Google Scholar]
- Anisimov VN, Piskunova TS, Popovich IG, Zabezhinski MA, Tyndyk ML, Egormin PA, Yurova MV, Rosenfeld SV, Semenchenko AV, Kovalenko IG, et al. 2010b. Gender differences in metformin effect on aging, life span and spontaneous tumorigenesis in 129/Sv mice. Aging 2: 945–958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anisimov VN, Berstein LM, Popovich IG, Zabezhinski MA, Egormin PA, Piskunova TS, Semenchenko AV, Tyndyk ML, Yurova MN, Kovalenko IG, et al. 2011. If started early in life, metformin treatment increases life span and postpones tumors in female SHR mice. Aging 3: 148–157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anisimov VN, Zabezhinski MA, Popovich IG, Pliss GB, Bespalov VG, Alexandrov VA, Stukov AN, Anikin IV, Alimova IN, Egormin Pcapital A C, et al. 2012. Rodent models for the preclinical evaluation of drugs suitable for pharmacological intervention in aging. Expert Opin Drug Discov 7: 85–95. [DOI] [PubMed] [Google Scholar]
- Anisimov VN, Popovich IG, Zabezhinski MA, Egormin PA, Yurova MN, Semenchenko AV, Tyndyk ML, Panchenko AV, Trashkov AP, Vasiliev AG, et al. 2015. Sex differences in aging, life span and spontaneous tumorigenesis in 129/Sv mice neonatally exposed to metformin. Cell Cycle 14: 46–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bailey CJ, Turner RC. 1996. Metformin. N Engl J Med 334: 574–579. [DOI] [PubMed] [Google Scholar]
- Batandier C, Guigas B, Detaille D, El-Mir MY, Fontaine E, Rigoulet M, Leverve XM. 2006. The ROS production induced by a reverse-electron flux at respiratory-chain complex 1 is hampered by metformin. J Bioenerg Biomembr 38: 33–42. [DOI] [PubMed] [Google Scholar]
- Baur JA, Birnbaum MJ. 2014. Control of gluconeogenesis by metformin: Does redox trump energy charge? Cell Metab 20: 197–199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ben Sahra I, Regazzetti C, Robert G, Laurent K, Le Marchand-Brustel Y, Auberger P, Tanti JF, Giorgetti-Peraldi S, Bost F. 2011. Metformin, independent of AMPK, induces mTOR inhibition and cell-cycle arrest through REDD1. Cancer Res 71: 4366–4372. [DOI] [PubMed] [Google Scholar]
- Berstein LM. 2012. Metformin in obesity, cancer and aging: Addressing controversies. Aging 4: 320–329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blagosklonny MV. 2013. Big mice die young but large animals live longer. Aging 5: 227–233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blagosklonny MV, Campisi J. 2008. Cancer and aging: More puzzles, more promises? Cell Cycle 7: 2615–2618. [DOI] [PubMed] [Google Scholar]
- Bulterijs S. 2011. Metformin as a geroprotector. Rejuvenation Res 14: 469–482. [DOI] [PubMed] [Google Scholar]
- Cabreiro F, Gems D. 2013. Worms need microbes too: Microbiota, health and aging in Caenorhabditis elegans. EMBO Mol Med 5: 1300–1310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cabreiro F, Au C, Leung KY, Vergara-Irigaray N, Cocheme HM, Noori T, Weinkove D, Schuster E, Greene ND, Gems D. 2013. Metformin retards aging in C. elegans by altering microbial folate and methionine metabolism. Cell 153: 228–239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao J, Meng S, Chang E, Beckwith-Fickas K, Xiong L, Cole RN, Radovick S, Wondisford FE, He L. 2014. Low concentrations of metformin suppress glucose production in hepatocytes through AMP-activated protein kinase (AMPK). J Biol Chem 289: 20435–20446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cazzaniga M, DeCensi A, Pruneri G, Puntoni M, Bottiglieri L, Varricchio C, Guerrieri-Gonzaga A, Gentilini OD, Pagani G, Dell’Orto P, et al. 2013. The effect of metformin on apoptosis in a breast cancer presurgical trial. Br J Cancer 109: 2792–2797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen S, Zhou J, Xi M, Jia Y, Wong Y, Zhao J, Ding L, Zhang J, Wen A. 2013. Pharmacogenetic variation and metformin response. Curr Drug Metab 14: 1070–1082. [DOI] [PubMed] [Google Scholar]
- Cho YM, Kieffer TJ. 2011. New aspects of an old drug: Metformin as a glucagon-like peptide 1 (GLP-1) enhancer and sensitizer. Diabetologia 54: 219–222. [DOI] [PubMed] [Google Scholar]
- Choi CI, Lee YD, Gwag BJ, Cho SI, Kim SS, Suh-Kim H. 2008. Effects of estrogen on lifespan and motor functions in female hSOD1 G93A transgenic mice. J Neurol Sci 268: 40–47. [DOI] [PubMed] [Google Scholar]
- Collier CA, Bruce CR, Smith AC, Lopaschuk G, Dyck DJ. 2006. Metformin counters the insulin-induced suppression of fatty acid oxidation and stimulation of triacylglycerol storage in rodent skeletal muscle. Am J Physiol Endocrinol Metab 291: E182–E189. [DOI] [PubMed] [Google Scholar]
- Contreras C, Novelle MG, Leis R, Dieguez C, Skrede S, Lopez M. 2013. Effects of neonatal programming on hypothalamic mechanisms controlling energy balance. Horm Metab Res 45: 935–944. [DOI] [PubMed] [Google Scholar]
- de Cabo R, Carmona-Gutierrez D, Bernier M, Hall MN, Madeo F. 2014. The search for antiaging interventions: From elixirs to fasting regimens. Cell 157: 1515–1526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Haes W, Frooninckx L, Van Assche R, Smolders A, Depuydt G, Billen J, Braeckman BP, Schoofs L, Temmerman L. 2014. Metformin promotes lifespan through mitohormesis via the peroxiredoxin PRDX-2. Proc Natl Acad Sci 111: E2501–E2509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhahbi JM, Mote PL, Fahy GM, Spindler SR. 2005. Identification of potential caloric restriction mimetics by microarray profiling. Physiol Genomics 23: 343–350. [DOI] [PubMed] [Google Scholar]
- Diamanti-Kandarakis E, Christakou CD, Kandaraki E, Economou FN. 2010. Metformin: An old medication of new fashion: Evolving new molecular mechanisms and clinical implications in polycystic ovary syndrome. Eur J Endocrinol 162: 193–212. [DOI] [PubMed] [Google Scholar]
- Dowling RJ, Zakikhani M, Fantus IG, Pollak M, Sonenberg N. 2007. Metformin inhibits mammalian target of rapamycin-dependent translation initiation in breast cancer cells. Cancer Res 67: 10804–10812. [DOI] [PubMed] [Google Scholar]
- El-Mir MY, Nogueira V, Fontaine E, Averet N, Rigoulet M, Leverve X. 2000. Dimethylbiguanide inhibits cell respiration via an indirect effect targeted on the respiratory chain complex I. J Biol Chem 275: 223–228. [DOI] [PubMed] [Google Scholar]
- Eurich DT, Weir DL, Majumdar SR, Tsuyuki RT, Johnson JA, Tjosvold L, Vanderloo SE, McAlister FA. 2013. Comparative safety and effectiveness of metformin in patients with diabetes mellitus and heart failure: Systematic review of observational studies involving 34,000 patients. Circ Heart Fail 6: 395–402. [DOI] [PubMed] [Google Scholar]
- Fontaine E. 2014. Metformin and respiratory chain complex. I: The last piece of the puzzle? Biochem J 463: e3–e5. [DOI] [PubMed] [Google Scholar]
- Foretz M, Hebrard S, Leclerc J, Zarrinpashneh E, Soty M, Mithieux G, Sakamoto K, Andreelli F, Viollet B. 2010. Metformin inhibits hepatic gluconeogenesis in mice independently of the LKB1/AMPK pathway via a decrease in hepatic energy state. J Clin Invest 120: 2355–2369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foretz M, Guigas B, Bertrand L, Pollak M, Viollet B. 2014. Metformin: From mechanisms of action to therapies. Cell Metab 20: 953–966. [DOI] [PubMed] [Google Scholar]
- Fullerton MD, Galic S, Marcinko K, Sikkema S, Pulinilkunnil T, Chen ZP, O’Neill HM, Ford RJ, Palanivel R, O’Brien M, et al. 2013. Single phosphorylation sites in Acc1 and Acc2 regulate lipid homeostasis and the insulin-sensitizing effects of metformin. Nat Med 19: 1649–1654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glueck CJ, Fontaine RN, Wang P, Subbiah MT, Weber K, Illig E, Streicher P, Sieve-Smith L, Tracy TM, Lang JE, et al. 2001. Metformin reduces weight, centripetal obesity, insulin, leptin, and low-density lipoprotein cholesterol in nondiabetic, morbidly obese subjects with body mass index greater than 30. Metabolism 50: 856–861. [DOI] [PubMed] [Google Scholar]
- Gong L, Goswami S, Giacomini KM, Altman RB, Klein TE. 2012. Metformin pathways: Pharmacokinetics and pharmacodynamics. Pharmacogenet Genomics 22: 820–827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenhill C. 2015. Gastric cancer: Metformin improves survival and recurrence rate in patients with diabetes and gastric cancer. Nat Rev Gastroenterol Hepatol 12: 124. [DOI] [PubMed] [Google Scholar]
- Guarente L. 2008. Mitochondria—A nexus for aging, calorie restriction, and sirtuins? Cell 132: 171–176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunton JE, Delhanty PJ, Takahashi S, Baxter RC. 2003. Metformin rapidly increases insulin receptor activation in human liver and signals preferentially through insulin-receptor substrate-2. J Clin Endocrinol Metab 88: 1323–1332. [DOI] [PubMed] [Google Scholar]
- Hardie DG, Ross FA, Hawley SA. 2012. AMPK: A nutrient and energy sensor that maintains energy homeostasis. Nat Rev Mol Cell Biol 13: 251–262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hawley SA, Gadalla AE, Olsen GS, Hardie DG. 2002. The antidiabetic drug metformin activates the AMP-activated protein kinase cascade via an adenine nucleotide-independent mechanism. Diabetes 51: 2420–2425. [DOI] [PubMed] [Google Scholar]
- Hirsch HA, Iliopoulos D, Struhl K. 2013. Metformin inhibits the inflammatory response associated with cellular transformation and cancer stem cell growth. Proc Natl Acad Sci 110: 972–977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong J, Zhang Y, Lai S, Lv A, Su Q, Dong Y, Zhou Z, Tang W, Zhao J, Cui L, et al. 2013. Effects of metformin versus glipizide on cardiovascular outcomes in patients with type 2 diabetes and coronary artery disease. Diabetes Care 36: 1304–1311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isoda K, Young JL, Zirlik A, MacFarlane LA, Tsuboi N, Gerdes N, Schonbeck U, Libby P. 2006. Metformin inhibits proinflammatory responses and nuclear factor-κB in human vascular wall cells. Arterioscler Thromb Vasc Biol 26: 611–617. [DOI] [PubMed] [Google Scholar]
- Jafari M, Khodayari B, Felgner J, Bussel II, Rose MR, Mueller LD. 2007. Pioglitazone: An anti-diabetic compound with anti-aging properties. Biogerontology 8: 639–651. [DOI] [PubMed] [Google Scholar]
- Johnson SC, Rabinovitch PS, Kaeberlein M. 2013. mTOR is a key modulator of ageing and age-related disease. Nature 493: 338–345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalender A, Selvaraj A, Kim SY, Gulati P, Brule S, Viollet B, Kemp BE, Bardeesy N, Dennis P, Schlager JJ, et al. 2010. Metformin, independent of AMPK, inhibits mTORC1 in a rag GTPase-dependent manner. Cell Metab 11: 390–401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaneb HM, Sharp PS, Rahmani-Kondori N, Wells DJ. 2011. Metformin treatment has no beneficial effect in a dose-response survival study in the SOD1G93A mouse model of ALS and is harmful in female mice. PLoS ONE 6: e24189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim YD, Park KG, Lee YS, Park YY, Kim DK, Nedumaran B, Jang WG, Cho WJ, Ha J, Lee IK, et al. 2008. Metformin inhibits hepatic gluconeogenesis through AMP-activated protein kinase-dependent regulation of the orphan nuclear receptor SHP. Diabetes 57: 306–314. [DOI] [PubMed] [Google Scholar]
- Kim MH, Jee JH, Park S, Lee MS, Kim KW, Lee MK. 2014. Metformin enhances glucagon-like peptide 1 via cooperation between insulin and Wnt signaling. J Endocrinol 220: 117–128. [DOI] [PubMed] [Google Scholar]
- Kita Y, Takamura T, Misu H, Ota T, Kurita S, Takeshita Y, Uno M, Matsuzawa-Nagata N, Kato K, Ando H, et al. 2012. Metformin prevents and reverses inflammation in a non-diabetic mouse model of nonalcoholic steatohepatitis. PLoS ONE 7: e43056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ko EM, Sturmer T, Hong JL, Castillo WC, Bae-Jump V, Funk MJ. 2015. Metformin and the risk of endometrial cancer: A population-based cohort study. Gynecol Oncol 136: 341–347. [DOI] [PubMed] [Google Scholar]
- Koo SH, Flechner L, Qi L, Zhang X, Screaton RA, Jeffries S, Hedrick S, Xu W, Boussouar F, Brindle P, et al. 2005. The CREB coactivator TORC2 is a key regulator of fasting glucose metabolism. Nature 437: 1109–1111. [DOI] [PubMed] [Google Scholar]
- Lapierre LR, Hansen M. 2012. Lessons from C. elegans: Signaling pathways for longevity. Trends Endocrinol Metab 23: 637–644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LaRue BL, Padilla PA. 2011. Environmental and genetic preconditioning for long-term anoxia responses requires AMPK in Caenorhabditis elegans. PLoS ONE 6: e16790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laskov I, Drudi L, Beauchamp MC, Yasmeen A, Ferenczy A, Pollak M, Gotlieb WH. 2014. Anti-diabetic doses of metformin decrease proliferation markers in tumors of patients with endometrial cancer. Gynecol Oncol 134: 607–614. [DOI] [PubMed] [Google Scholar]
- Lenaerts I, Walker GA, Van Hoorebeke L, Gems D, Vanfleteren JR. 2008. Dietary restriction of Caenorhabditis elegans by axenic culture reflects nutritional requirement for constituents provided by metabolically active microbes. J Gerontol A Biol Sci Med Sci 63: 242–252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Xu S, Mihaylova MM, Zheng B, Hou X, Jiang B, Park O, Luo Z, Lefai E, Shyy JY, et al. 2011. AMPK phosphorylates and inhibits SREBP activity to attenuate hepatic steatosis and atherosclerosis in diet-induced insulin-resistant mice. Cell Metab 13: 376–388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li W, Yuan Y, Huang L, Qiao M, Zhang Y. 2012. Metformin alters the expression profiles of microRNAs in human pancreatic cancer cells. Diabetes Res Clin Pract 96: 187–195. [DOI] [PubMed] [Google Scholar]
- Lin JJ, Gallagher EJ, Sigel K, Mhango G, Galsky MD, Smith CB, LeRoith D, Wisnivesky JP. 2015. Survival of patients with stage IV lung cancer with diabetes treated with metformin. Am J Respir Crit Care Med 191: 448–454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Logie L, Harthill J, Patel K, Bacon S, Hamilton DL, Macrae K, McDougall G, Wang HH, Xue L, Jiang H, et al. 2012. Cellular responses to the metal-binding properties of metformin. Diabetes 61: 1423–1433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma TC, Buescher JL, Oatis B, Funk JA, Nash AJ, Carrier RL, Hoyt KR. 2007. Metformin therapy in a transgenic mouse model of Huntington’s disease. Neurosci Lett 411: 98–103. [DOI] [PubMed] [Google Scholar]
- Madiraju AK, Erion DM, Rahimi Y, Zhang XM, Braddock DT, Albright RA, Prigaro BJ, Wood JL, Bhanot S, MacDonald MJ, et al. 2014. Metformin suppresses gluconeogenesis by inhibiting mitochondrial glycerophosphate dehydrogenase. Nature 510: 542–546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahmood K, Naeem M, Rahimnajjad NA. 2013. Metformin: The hidden chronicles of a magic drug. Eur J Intern Med 24: 20–26. [DOI] [PubMed] [Google Scholar]
- Maida A, Lamont BJ, Cao X, Drucker DJ. 2011. Metformin regulates the incretin receptor axis via a pathway dependent on peroxisome proliferator-activated receptor-α in mice. Diabetologia 54: 339–349. [DOI] [PubMed] [Google Scholar]
- Martin-Castillo B, Vazquez-Martin A, Oliveras-Ferraros C, Menendez JA. 2010. Metformin and cancer: Doses, mechanisms and the dandelion and hormetic phenomena. Cell Cycle 9: 1057–1064. [DOI] [PubMed] [Google Scholar]
- Martin-Montalvo A, Mercken EM, Mitchell SJ, Palacios HH, Mote PL, Scheibye-Knudsen M, Gomes AP, Ward TM, Minor RK, Blouin MJ, et al. 2013. Metformin improves healthspan and lifespan in mice. Nat Commun 4: 2192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menendez JA, Cufi S, Oliveras-Ferraros C, Vellon L, Joven J, Vazquez-Martin A. 2011. Gerosuppressant metformin: Less is more. Aging 3: 348–362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meng S, Cao J, He Q, Xiong L, Chang E, Radovick S, Wondisford FE, He L. 2014. Metformin activates AMP-activated protein kinase by promoting formation of the αβγ heterotrimeric complex. J Biol Chem 290: 3793–3802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mercken EM, Carboneau BA, Krzysik-Walker SM, de Cabo R. 2012. Of mice and men: The benefits of caloric restriction, exercise, and mimetics. Ageing Res Rev 11: 390–398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miles JM, Rule AD, Borlaug BA. 2014. Use of metformin in diseases of aging. Curr Diab Rep 14: 490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller RA, Chu Q, Xie J, Foretz M, Viollet B, Birnbaum MJ. 2013. Biguanides suppress hepatic glucagon signalling by decreasing production of cyclic AMP. Nature 494: 256–260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Na HJ, Park JS, Pyo JH, Lee SH, Jeon HJ, Kim YS, Yoo MA. 2013. Mechanism of metformin: Inhibition of DNA damage and proliferative activity in Drosophila midgut stem cell. Mech Ageing Dev 134: 381–390. [DOI] [PubMed] [Google Scholar]
- Ng TP, Feng L, Yap KB, Lee TS, Tan CH, Winblad B. 2014. Long-term metformin usage and cognitive function among older adults with diabetes. J Alzheimers Dis 41: 61–68. [DOI] [PubMed] [Google Scholar]
- Olsen A, Vantipalli MC, Lithgow GJ. 2006. Using Caenorhabditis elegans as a model for aging and age-related diseases. Ann NY Acad Sci 1067: 120–128. [DOI] [PubMed] [Google Scholar]
- Onken B, Driscoll M. 2010. Metformin induces a dietary restriction-like state and the oxidative stress response to extend C. elegans healthspan via AMPK, LKB1, and SKN-1. PLoS ONE 5: e8758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owen MR, Doran E, Halestrap AP. 2000. Evidence that metformin exerts its anti-diabetic effects through inhibition of complex 1 of the mitochondrial respiratory chain. Biochem J 348: 607–614. [PMC free article] [PubMed] [Google Scholar]
- Patrone C, Eriksson O, Lindholm D. 2014. Diabetes drugs and neurological disorders: New views and therapeutic possibilities. Lancet Diabetes Endocrinol 2: 256–262. [DOI] [PubMed] [Google Scholar]
- Pawlyk AC, Giacomini KM, McKeon C, Shuldiner AR, Florez JC. 2014. Metformin pharmacogenomics: Current status and future directions. Diabetes 63: 2590–2599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearson KJ, Baur JA, Lewis KN, Peshkin L, Price NL, Labinskyy N, Swindell WR, Kamara D, Minor RK, Perez E, et al. 2008. Resveratrol delays age-related deterioration and mimics transcriptional aspects of dietary restriction without extending life span. Cell Metab 8: 157–168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pernicova I, Korbonits M. 2014. Metformin—Mode of action and clinical implications for diabetes and cancer. Nat Rev Endocrinol 10: 143–156. [DOI] [PubMed] [Google Scholar]
- Pulito C, Donzelli S, Muti P, Puzzo L, Strano S, Blandino G. 2014. microRNAs and cancer metabolism reprogramming: The paradigm of metformin. Ann Transl Med 2: 58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rego DF, Pavan LM, Elias ST, De Luca Canto G, Guerra EN. 2015. Effects of metformin on head and neck cancer: A systematic review. Oral Oncol 51: 416–422. [DOI] [PubMed] [Google Scholar]
- Rice S, Pellatt L, Ramanathan K, Whitehead SA, Mason HD. 2009. Metformin inhibits aromatase via an extracellular signal-regulated kinase-mediated pathway. Endocrinology 150: 4794–4801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scarpello JH. 2003. Improving survival with metformin: The evidence base today. Diabetes Metab 29: 6S36–6S43. [DOI] [PubMed] [Google Scholar]
- Schulz TJ, Zarse K, Voigt A, Urban N, Birringer M, Ristow M. 2007. Glucose restriction extends Caenorhabditis elegans life span by inducing mitochondrial respiration and increasing oxidative stress. Cell Metab 6: 280–293. [DOI] [PubMed] [Google Scholar]
- Selman C, Tullet JM, Wieser D, Irvine E, Lingard SJ, Choudhury AI, Claret M, Al-Qassab H, Carmignac D, Ramadani F, et al. 2009. Ribosomal protein S6 kinase 1 signaling regulates mammalian life span. Science 326: 140–144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw RJ, Lamia KA, Vasquez D, Koo SH, Bardeesy N, Depinho RA, Montminy M, Cantley LC. 2005. The kinase LKB1 mediates glucose homeostasis in liver and therapeutic effects of metformin. Science 310: 1642–1646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shoshani T, Faerman A, Mett I, Zelin E, Tenne T, Gorodin S, Moshel Y, Elbaz S, Budanov A, Chajut A, et al. 2002. Identification of a novel hypoxia-inducible factor 1-responsive gene, RTP801, involved in apoptosis. Mol Cell Biol 22: 2283–2293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slack C, Foley A, Partridge L. 2012. Activation of AMPK by the putative dietary restriction mimetic metformin is insufficient to extend lifespan in Drosophila. PLoS ONE 7: e47699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith DL Jr, Elam CF Jr, Mattison JA, Lane MA, Roth GS, Ingram DK, Allison DB. 2010. Metformin supplementation and life span in Fischer-344 rats. J Gerontol A Biol Sci Med Sci 65: 468–474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spindler SR. 2006. Use of microarray biomarkers to identify longevity therapeutics. Aging Cell 5: 39–50. [DOI] [PubMed] [Google Scholar]
- Stenesen D, Suh JM, Seo J, Yu K, Lee KS, Kim JS, Min KJ, Graff JM. 2013. Adenosine nucleotide biosynthesis and AMPK regulate adult life span and mediate the longevity benefit of caloric restriction in flies. Cell Metab 17: 101–112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stephenne X, Foretz M, Taleux N, van der Zon GC, Sokal E, Hue L, Viollet B, Guigas B. 2011. Metformin activates AMP-activated protein kinase in primary human hepatocytes by decreasing cellular energy status. Diabetologia 54: 3101–3110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevanovic D, Janjetovic K, Misirkic M, Vucicevic L, Sumarac-Dumanovic M, Micic D, Starcevic V, Trajkovic V. 2012. Intracerebroventricular administration of metformin inhibits ghrelin-induced hypothalamic AMP-kinase signalling and food intake. Neuroendocrinology 96: 24–31. [DOI] [PubMed] [Google Scholar]
- Tohyama D, Yamaguchi A. 2010. A critical role of SNF1A/dAMPKα (Drosophila AMP-activated protein kinase α) in muscle on longevity and stress resistance in Drosophila melanogaster. Biochem Biophys Res Commun 394: 112–118. [DOI] [PubMed] [Google Scholar]
- Wang CP, Lorenzo C, Espinoza SE. 2014. Frailty attenuates the impact of metformin on reducing mortality in older adults with type 2 diabetes. J Endocrinol Diabetes Obes 2: 1030. [PMC free article] [PubMed] [Google Scholar]
- Woo SL, Xu H, Li H, Zhao Y, Hu X, Zhao J, Guo X, Guo T, Botchlett R, Qi T, et al. 2014. Metformin ameliorates hepatic steatosis and inflammation without altering adipose phenotype in diet-induced obesity. PLoS ONE 9: e91111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin M, Zhou J, Gorak EJ, Quddus F. 2013. Metformin is associated with survival benefit in cancer patients with concurrent type 2 diabetes: A systematic review and meta-analysis. Oncologist 18: 1248–1255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan L, Ziegler R, Hamann A. 2003. Metformin modulates insulin post-receptor signaling transduction in chronically insulin-treated Hep G2 cells. Acta Pharmacol Sin 24: 55–60. [PubMed] [Google Scholar]
- Zarse K, Schmeisser S, Groth M, Priebe S, Beuster G, Kuhlow D, Guthke R, Platzer M, Kahn CR, Ristow M. 2012. Impaired insulin/IGF1 signaling extends life span by promoting mitochondrial l-proline catabolism to induce a transient ROS signal. Cell Metab 15: 451–465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang G, Li J, Purkayastha S, Tang Y, Zhang H, Yin Y, Li B, Liu G, Cai D. 2013. Hypothalamic programming of systemic ageing involving IKK-β, NF-κB and GnRH. Nature 497: 211–216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou G, Myers R, Li Y, Chen Y, Shen X, Fenyk-Melody J, Wu M, Ventre J, Doebber T, Fujii N, et al. 2001. Role of AMP-activated protein kinase in mechanism of metformin action. J Clin Invest 108: 1167–1174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zou MH, Kirkpatrick SS, Davis BJ, Nelson JS, Wiles WGt, Schlattner U, Neumann D, Brownlee M, Freeman MB, Goldman MH. 2004. Activation of the AMP-activated protein kinase by the anti-diabetic drug metformin in vivo. Role of mitochondrial reactive nitrogen species. J Biol Chem 279: 43940–43951. [DOI] [PubMed] [Google Scholar]