Abstract
Epilepsy is a common brain disorder characterized by the occurrence of spontaneous seizures. These bursts of synchronous firing arise from abnormalities of neuronal networks. Excitability of individual neurons and neuronal networks is largely governed by ion channels and, indeed, abnormalities of a number of ion channels resulting from mutations or aberrant expression and trafficking underlie several types of epilepsy. Here, we focus on the hyperpolarization-activated cyclic nucleotide-gated ion (HCN) channels that conduct Ih current. This conductance plays complex and diverse roles in the regulation of neuronal and network excitability. We describe the normal function of HCN channels and discuss how aberrant expression, assembly, trafficking, and posttranslational modifications contribute to experimental and human epilepsy.
HCN channels help regulate neuronal and network excitability. Their aberrant expression, trafficking, and posttranslational modifications contribute to epilepsy.
HCN CHANNELS AND Ih IN THE BRAIN
Expression, Assembly, and Subcellular Distribution
Hyperpolarization-activated cyclic nucleotide-gated ion (HCN) channels conduct the Ih current and are encoded by four genes (HCN1, HCN2, HCN3, and HCN4) (Santoro et al. 1997, 1998; Gauss et al. 1998; Ludwig et al. 1998; Ishii et al. 1999). Structurally similar to voltage-gated K+ channels, HCN channels are formed as a tetramer of subunits, each with a six-transmembrane domain topology, including a pore region that conducts ion flow, and intracellular amino and carboxyl termini. Four HCN molecules of the same isotype can assemble as homomeric channels. However, the four isoforms can also functionally assemble in different combinations to form heteromeric channels with different properties (Robinson and Siegelbaum 2003; Santoro and Baram 2003; Brewster et al. 2005; Biel et al. 2009; Zolles et al. 2009). The variation in Ih properties among cell types and developmental stages is likely a result of differential expression of various HCN isoforms in both homomeric and heteromeric forms (Kuisle et al. 2006; Brewster et al. 2007; Bender and Baram 2008; Kanyshkova et al. 2009). For example, the isoform predominantly expressed in cortex and hippocampus, HCN1, endows the current with more rapid activation kinetics and a minimal sensitivity to cyclic adenosine 3′, 5′-monophosphate (cAMP), whereas Ih in cells from thalamus and cardiac sinoatrial node where the primary isoform expressed is HCN2 or HCN4 shows slower activation and substantial sensitivity to cAMP (Biel et al. 2009; Noam et al. 2011). In addition, the profile of HCN subunit expression in a specific neuron may vary during development (Brewster et al. 2007).
Interactions with associated proteins greatly influence the function of HCN channels, also in an isoform-specific manner (Santoro et al. 2004; Lewis et al. 2009; Zolles et al. 2009; Lewis and Chetkovich 2011; Noam et al. 2014). The conductive properties of HCN channels are further regulated by interactions with molecules such as cAMP. cAMP binds a sequence on the carboxyl terminus of the channel and influences HCN function by accelerating its kinetics and shifting its voltage-activation curve to more depolarized values (Robinson and Siegelbaum 2003; Biel et al. 2009). The sensitivity of HCN channels to cAMP is isoform specific with HCN4 > HCN2 >> HCN1. The number of HCN channels expressed on a cell’s surface influences the magnitude and properties of Ih (see below). HCN expression is regulated by neuronal and network activity in an isoform-dependent manner (Brewster et al. 2002; Noam et al. 2011). Other factors that influence HCN membrane expression are posttranscriptional modifications, particularly glycosylation and phosphorylation (Jung et al. 2010; Williams et al. 2015). HCN channels undergo extensive glycosylation and the extent of glycosylation influences the number of channels expressed on the membrane (Much et al. 2003; Zha et al. 2008) and their homomeric or heteromeric assembly (Zha et al. 2008; Hegle et al. 2010).
The function of HCN channels depends also on their subcellular distribution (Magee 1998; Poolos et al. 2002; Berger et al. 2003; Santoro and Baram 2003; Aponte et al. 2006; Bender et al. 2007; Ying et al. 2007; Noam et al. 2010; Wilkars et al. 2012). Subcellular localization can vary dramatically among different neuronal subtypes and includes somatic, dendritic, and axonal. In cortical and hippocampal pyramidal cells, for example, HCN channels localize primarily to dendrites where they regulate the integration and summation of synaptic input (Stuart and Spruston 1998; Magee 1999; Lorincz et al. 2002; Poolos et al. 2002; Berger et al. 2003; Wang et al. 2003; Brewster et al. 2007). Furthermore, in cortical and hippocampal interneuronal populations, HCN channels localize preferentially to the somatic region of the cell where they coregulate cellular properties like the resting membrane potential and spontaneous firing frequency (Maccaferri and McBain 1996; Lupica et al. 2001). Axonal HCN channels have been identified in a subgroup of interneuron populations as well as in certain excitatory neurons (Notomi and Shigemoto 2004; Lujan et al. 2005; Aponte et al. 2006; Brewster et al. 2007). HCN channels are also found in axonal terminals of a subset of entorhinal neurons where they act to inhibit presynaptic glutamate release (Bender et al. 2007; Huang et al. 2011).
The subunit composition, channel phosphorylation, and interaction with accessory proteins and other ion channels contribute significantly to the different biophysical properties of HCN channels on diverse neuronal populations in different brain regions (Chen et al. 2002; George et al. 2009; Jung et al. 2010; Hammelmann et al. 2011). In addition, the contribution of HCN channels to neuronal excitability depends on their subcellular localization, the type and levels of coexpressed ion channels, the nature and strength of synaptic inputs, and likely other as-yet less-well-defined properties (Noam et al. 2011). In fact, as was first described in 1998, HCN channels in pyramidal neurons are arrayed in a gradient density pattern along the somatodendritic axis, reaching a density in the distal dendrites that is seven- to 10-fold that of the soma (Magee 1998; Lorincz et al. 2002). This nonuniform somatodendritic distribution of ion channel subunits turns out to be a property of a number of other voltage-gated ion channels as well, including the K+ channel Kv4.2 and the Ca2+ channel Cav2.3 (Beck and Yaari 2008; Nusser 2009). The dendritic predominance of HCN channel distribution in pyramidal neurons appears to be mediated in part by interaction with an accessory protein, TPR-containing Rab8b-interacting protein (TRIP8b). This protein binds to the carboxyl terminus of the HCN protein and increases its surface membrane expression in pyramidal dendrites (Lewis et al. 2009; Santoro et al. 2009; Lewis and Chetkovich 2011). Interestingly, HCN channel trafficking to the axons of entorhinal cortical neurons is independent of TRIP8b (Huang et al. 2012). Conversely, filamin A binding with HCN channel subunits decreases their surface membrane expression via dynamin-dependent endocytosis (Noam et al. 2014). Thus, accessory protein interactions underlie HCN channel trafficking, and may contribute to activity-dependent regulation of their expression.
PROPERTIES OF Ih AND ITS ROLE IN REGULATION OF NEURONAL AND NETWORK EXCITABILITY
HCN channels generate Ih, a current with unusual biophysical properties (Accili et al. 2002; Robinson and Siegelbaum 2003; Biel et al. 2009). As mentioned above, they are structurally similar to K+ channels but lack their high selectivity for K+ ions and, in fact, conduct mostly Na+ ions under physiological conditions. Thus, Ih is an inward, depolarizing current. The voltage-dependent activation of HCN channels is also atypical, increasing with hyperpolarization from rest rather than with depolarization as is common with other channels. Thus, neuronal depolarization tends to inactivate HCN channels, whereas hyperpolarization tends to activate them. HCN channels do not display inactivation, being fractionally open and active around resting potential. Indeed, the channels contribute to the resting potential, such that neurons expressing HCN channels typically have a more depolarized resting membrane potential than those that do not express the channel (Lupica et al. 2001; Mesirca et al. 2014). This biophysical feature of Ih constitutes a critical contribution to the regulation of neuronal excitability. Because HCN channels are one of the few voltage-gated channels open at resting potential, Ih comprises about half of the resting conductance of many neuron types. This allows HCN channels to exert a strong influence on neuronal resting potential and input resistance. Finally, HCN channel activation is modulated by cyclic nucleotides such as cAMP. Numerous signaling pathways that alter intracellular cAMP levels, therefore, modulate HCN channel activity.
Ih produces an inward current yet is deactivated (with a slow time course on the order of tens of msec) by depolarization; this yields an inherent negative-feedback property that imparts a stabilizing effect on neuronal excitability. For example, neuronal depolarization deactivates the steady-state inward current produced by HCN channels, hyperpolarizing the membrane potential back toward rest. Conversely, a hyperpolarizing input will turn on Ih, depolarizing the neuron back toward rest, an action that might also lead to rebound depolarization and action potential firing (Chen et al. 2001). Paired with an inactivating inward conductance, such as a transient “T-type” Ca2+ current, Ih contributes to rhythmic “pacemaker” activity, such as is seen in thalamocortical neurons, cardiac sinoatrial nodal cells, and neurons in the basal ganglia (McCormick and Bal 1997; Huguenard 2001; Chan et al. 2004).
The pacemaking function of HCN channels is prominent when they are localized in the cell soma near the sites of action potential generation. In neocortical and hippocampal pyramidal neurons, HCN channels are localized at low density in the soma and at higher density in the apical dendrites. This dendritic localization produces an additional inhibitory effect. Within the dendrites, resting Ih conductance constitutes a “leak” that diminishes the input resistance of the dendrites to incoming synaptic currents, decreasing the voltage change produced by an excitatory postsynaptic potential (EPSP) and decreasing the ability of synaptic input to drive action potential firing. Conversely, when Ih is inactivated, input resistance is higher and EPSPs are increased in magnitude. The effect of Ih on dendritic input resistance is augmented by an interaction with M-type K+ channels present in the dendrites (George et al. 2009). Thus, Ih causes both resting potential depolarization that promotes hyperexcitability and an inhibitory effect on action potential firing. Several important studies found that pharmacological inhibition of Ih or genetic deletion of HCN1 channels increases excitability in neocortical and hippocampal pyramidal neurons. For example, increased summation of excitatory synaptic inputs in hippocampal and neocortical pyramidal neurons was found on pharmacological inhibition of Ih (Berger et al. 2001; Poolos et al. 2002). Genetic deletion of HCN1 channels or pharmacological blockade in vivo of HCN channels enhanced synaptic plasticity and spatial learning (Nolan et al. 2004; Wang et al. 2007) and increased dendritic Ca2+ spiking (Tsay et al. 2007). However, in neurons, including interneurons, where Ih does not have a prominent dendritic localization, its depolarizing action on resting potential yields a predominantly excitatory influence, for example, on the spontaneous activity of interneurons (Lupica et al. 2001).
HCN CHANNELS IN EPILEPSY: ANIMAL STUDIES
HCN Expression and Function in Experimental Epilepsy Studies
Rodent models of seizures and epileptogenesis have provided direct evidence for activity-dependent effects on HCN channel expression and function. The first studies to implicate HCN channel expression changes in epileptogenesis involved the hyperthermia-induced seizures in infant (p10) rats. These seizures resulted in chronic enhancement of Ih in CA1 pyramidal neurons and were associated with a modest depolarizing shift of the voltage dependence of Ih, and significant slowing in the activation and deactivation kinetics (Chen et al. 2001). These changes persisted for weeks to months following the initial epilepsy-inducing insult. Follow-up studies examined the molecular basis of the changes in HCN function and suggested that altered channel properties resulted from a change in the relative abundance of HCN1 and HCN2 channels in favor of the slower HCN2. Specifically, studies of the expression pattern of HCN channels following developmental prolonged febrile seizures and also kainate-induced seizures found persistent loss of HCN1 messenger RNA (mRNA) and protein expression, transient down-regulation of HCN2 expression (Brewster et al. 2002, 2005), and increased formation of heteromeric HCN1/HCN2 channels (Brewster et al. 2005).
Subsequent to the initial description of reduced HCN1 expression and increased Ih availability in the experimental febrile status epilepticus (SE) model, this loss of HCN1 channel expression coupled with reduction of Ih has been the most common finding in rodent models of acquired epilepsy (Table 1). The changes in Ih biophysical parameters, HCN1 channel protein expression, and HCN1 transcription suggest that multiple mechanisms underlie the acute and chronic loss of HCN1 channel expression and function following an epileptogenic brain insult. In post-SE models, Ih in the dendrites of pyramidal neurons is reduced as rapidly as 1 h post-SE, and remains so at least for a month, at a point when the animal is chronically epileptic (Shah et al. 2004; Marcelin et al. 2009; Jung et al. 2011). Some model-dependent differences in the time course of this process have been observed, that may be a function of the severity of the resulting epilepsy phenotype, with the pilocarpine model tending to produce a more rapidly developing epilepsy than kainate or experimental febrile SE (Dube et al. 2006; Jung et al. 2007; Williams et al. 2009). In addition, loss of HCN channel expression has been observed following other brain insults, including perinatal hypoxia (Zhang et al. 2006), cortical dysplasia (Hablitz and Yang 2010; Li et al. 2012), and subarachnoid hemorrhage. Indeed, a common regulatory mechanism consisting of an increase in the expression of the repressor neuron-restrictive silencer factor (NRSF/repressor element-1 silencing transcription factor [REST]), that potently represses HCN1 and other key neuronal genes, might exist in these scenarios (McClelland et al. 2011, 2014).
Table 1.
Listing of studies in various epilepsy models that detect decreased HCN1 expression within the CA1 region of the hippocampus
| Study | Model | Hippocampal region |
|---|---|---|
| (Brewster et al. 2002) | Febrile seizures | CA1 |
| (Shah et al. 2004) | Systemic KA | EC layer III |
| (Jung et al. 2007) | Pilocarpine | CA1 |
| (Powell et al. 2008) | Systemic KA/kindling | CA1, CA3, DG, EC |
| (Marcelin et al. 2009) | Pilocarpine | CA1 |
| (Jung et al. 2010) | Pilocarpine | CA1 |
| (Jung et al. 2011) | Pilocarpine | CA1 |
| (McClelland et al. 2011) | Systemic KA | CA1 |
HCN, Hyperpolarization-activated cyclic nucleotide-gated ion; KA, kainic acid; DG, dentate gyrus; EC, entorhinal cortex.
Several mechanisms underlying loss of Ih following a brain insult have been elucidated. In pyramidal neuronal dendrites, loss of Ih amplitude within the first hour post-SE is accompanied by a loss of surface membrane expression of HCN1 channel subunits, whereas total channel protein expression is unchanged suggesting that HCN1 channel subunits are rapidly internalized following SE (Jung et al. 2011). In several models, reduction in HCN1 mRNA expression can be detected starting at 8, 16, or 48, to 72 h following the insult. This is mediated by increased expression and activity of NRSF. NRSF binds to a cognate binding site (neuron-restrictive silencer element [NRSE]) situated within the first intron of the HCN1 gene (McClelland et al. 2011). Indeed, when the function of NRSF was inhibited following kainic acid (KA)-induced seizures by using NRSE-containing oligodeoxynucleotides, the expression of HCN1 was rescued and Ih was restored (McClelland et al. 2011). Importantly, inhibition of NRSF function following KA-induced seizures significantly ameliorated the subsequent epileptic phenotype. Although restitution of HCN1 expression and Ih is unlikely to be responsible for this amelioration (because NRSF regulates the transcription of numerous genes) (McClelland et al. 2014), it implicates HCN channel dysregulation and dysfunction as a contributing factor to the epileptogenic process.
Another factor contributing to the loss of Ih in epilepsy is a down-regulation of voltage-dependent activation or “gating.” Although Ih amplitude and HCN1 channel expression are reduced by ∼50% following SE, the remaining channels also show a hyperpolarizing shift in their gating, reducing the fraction of channels that are activated at typical neuronal resting potential, thus promoting hyperexcitability (Chen et al. 2001; Jung et al. 2007). HCN channel gating is strongly modulated by phosphorylation-dependent mechanisms, with down-regulation correlating with decreased phosphorylation mediated by either the protein kinase p38 mitogen-activated protein kinase (MAPK) or increased activity of the protein phosphatase 2B (PP2B or calcineurin). Hippocampal tissue from chronically epileptic animals shows a loss of p38 MAPK activity with a concomitant increase in PP2B activity; pharmacological reversal of these phosphorylation signaling changes leads to normalized HCN channel gating and reduced neuronal hyperexcitability (Jung et al. 2010). This suggests that posttranslational signaling mechanisms, in addition to transcriptional mechanisms, contribute to HCN channelopathy in epilepsy models.
Other lines of evidence indicating a role for HCN channels in neuronal excitability and epilepsy have come from studies demonstrating the effects of clinically used antiepileptic drugs on Ih. The first such demonstration showed that acetazolamide up-regulated Ih via a depolarizing shift in gating of Ih recorded from thalamic neurons (Munsch and Pape 1999). Subsequently, lamotrigine was found to up-regulate Ih in hippocampal pyramidal neurons, also by an action on gating (Poolos et al. 2002), whereas gabapentin increased the maximal current amplitude (Surges et al. 2003). Interestingly, up-regulation of Ih by lamotrigine increased the spontaneous action potential output of hippocampal interneurons, enhancing the inhibition of pyramidal neurons (Peng et al. 2010).
Knockout and Genetic Model Studies
The advent of genome manipulation in mice has significantly aided our understanding of the role of HCN channels in pathological conditions such as epilepsy. Deletion of the HCN2 gene revealed that HCN2−/– mice had generalized, spike-wave electroencephalographic (EEG) discharges, consistent with generalized epilepsy. Whole-cell current-clamp recordings from thalamocortical neurons from HCN2−/– mice revealed stereotyped high-frequency burst discharges, suggesting that HCN2 channel expression is required for normal thalamocortical oscillations, and its absence promotes epilepsy (Ludwig et al. 2003). These findings show that loss of HCN2 channel function, most likely in thalamic nuclei, are sufficient to produce an epilepsy phenotype.
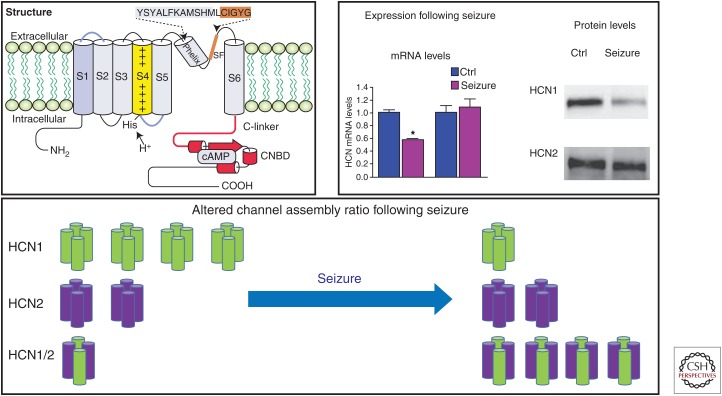
Analysis of HCN channels in thalamocortical neurons in inbred rat strains with generalized epilepsy, such as Genetic Absence Epilepsy Rats from Strasbourg (GAERS), and Wistar Albino Glaxo/Rij (WAG/Rij) rats show abnormal HCN channel expression (Fig 1). In the GAERS rats, a reduced Ih voltage-dependent activation resulting from a subunit shift in the expression of HCN channels (reduced HCN2 and increased HCN1) and, hence, decreased sensitivity to cAMP in thalamocortical neurons, was found in the WAG/Rij strain (Budde et al. 2005; Kuisle et al. 2006); loss of neocortical Ih and HCN1 expression paralleled the onset of spontaneous absence seizures (Kole et al. 2007). In both models, the genetic etiology of the epilepsy phenotype is probably multifactorial, yet involves dysregulation of HCN channel expression.
Figure 1.
Abnormal hyperpolarization-activated cyclic nucleotide-gated ion (HCN) channel regulation and assembly following seizures. HCN channel subunits consist of six transmembrane segments (top left). The cytoplasmic carboxyl terminus contains a 120-amino-acid cyclic nucleotide-binding domain (CNBD). HCN1 messenger RNA (mRNA) and protein levels are significantly reduced in the hippocampus following seizures in many animal models of epilepsy; HCN2 levels do not alter appreciably (top right). Altered HCN channel assembly following seizures may result from a reduction of HCN1 subunits (bottom). SF, Silencer factor; cAMP, cyclic adenosine 3′, 5′-monophosphate.
Deletion of the HCN1 gene does not produce an epileptic phenotype; however, HCN1 knockout (KO) mice had reduced threshold to seizures induced by electrical stimulation (amygdala kindling) and also by the chemoconvulsants kainate or pilocarpine (Huang et al. 2009; Santoro et al. 2010). HCN1 KO mice were more susceptible to seizure-related death (irrespective of model used) as compared with wild-type controls. These studies support an anti-excitatory or seizure-protective role of HCN1. However, whereas knockout studies are powerful, their interpretation should consider potential confounders. In view of the complex mechanisms regulating HCN channel expression, heteromeric assembly and trafficking, the potential effects of deletion of a specific HCN channel isoform on the others requires consideration. For example, careful analysis should be performed to detect compensatory expression of other HCN channels, which may result from loss of one channel. Although global analyses of hippocampus or cortex failed to uncover compensatory changes in HCN2 (Santoro et al. 2010), Brewster et al. (2006) found a significant increase in HCN2 expression at both the mRNA and protein level within pyramidal neurons of hippocampal area CA1 in HCN1−/− mice. Intriguingly, immunohistochemistry suggested enhanced trafficking of HCN2 channels to dendrites of CA1 pyramidal cells. This compensatory increase in HCN2 expression in HCN1 null mice may generate dendritic Ih and restore the role of Ih in reducing excitability.
HCN CHANNELS AND EPILEPSY: HUMAN STUDIES
HCN Expression and Function in Hippocampus from Resected Patient Tissue
In resected hippocampal tissue from patients with temporal lobe epilepsy, there were enhanced levels of HCN1 expression and dendritic localization in the granule cells of the dentate gyrus (DG) in patients with mesial temporal sclerosis (MTS) (Bender et al. 2003). Separately, Surges et al. (2012) found enhanced Ih in these DG neurons from patients with MTS compared with those without MTS, and concordant increased HCN1 mRNA expression. Similar changes were found in DG neurons from epileptic rats induced with the pilocarpine model, with augmented HCN1 expression and Ih that mirrored the human phenotype. Given the importance of Ih in attenuating postsynaptic potentials at dendrites and limiting their spread in pyramidal neurons of CA1 region, this up-regulation of HCN1 and Ih in granule cells of the dentate gyrus may be a protective mechanism resulting in controlled dentate gyrus gating. Further evidence of altered Ih in human epilepsy was shown in studies of tissue from neocortical resections. In pyramidal neurons from patients with a high presurgical seizure frequency, Ih was reduced compared with those with relatively lower seizure frequency (Wierschke et al. 2010). Because altered expression of HCN channels in human hippocampal tissue can only be analyzed in people with existing epilepsy, it is difficult to say whether altered HCN channel expression is a cause or consequence of epilepsy.
Human Mutations in HCN Channels and Their Role in Epilepsy
Altered HCN channel expression and function, and the demonstration of a causal role of these alterations in experimental models of epileptogenesis promote the question of a potential role of mutations in HCN channels in human epilepsy. Thus far, Mendelian-inherited epilepsy has not been shown to arise from HCN channel mutation. However, several studies have shown the association between HCN channel mutation and human genetic epilepsy. Tang et al. (2008) undertook the first study mapping mutations in HCN channels in patients with idiopathic generalized epilepsy (IGE), and this analysis uncovered several functional variants within HCN1 and HCN2; however, the impact of these variants on channel function were not fully understood. A study that screened patients with febrile seizures and the defined syndrome generalized epilepsy with febrile seizures plus (GEFS+) uncovered a triple proline deletion in HCN2; when recombinant channels were exogenously expressed, the magnitude of the resulting Ih was 35% larger than with wild-type channels (Dibbens et al. 2010). However, because this gene variant did not strictly cosegregate with an epilepsy phenotype (being found in unaffected individuals as well), it is not clearly causative of disease. Another study that screened families with epilepsy for mutations in HCN1 and HCN2 found a recessive loss-of-function point mutation in HCN2 in a patient with sporadic IGE (DiFrancesco et al. 2011). The mutation (E515K) was located within a region of the channel known to affect gating, and subsequent functional analysis revealed that the homomeric mutant channel inhibited function by causing a large hyperpolarizing shift of activation along with slowed activation kinetics. This was the first evidence in humans of a loss-of-function point mutation in HCN2, which may contribute to generalized epilepsy with recessive inheritance.
An intriguing recent association between HCN channel mutation and catastrophic early epilepsy emerged from a comprehensive study on a European population. This study employed whole exome sequencing on parent–child trios that included probands with early infantile epileptic encephalopathy (Nava et al. 2014), uncovering six different heterozygous mutations in HCN1. The mutations identified in this study affected highly conserved amino acids and were all located on intracellular portions of the channel. Affected individuals presented with similar phenotypes; however, functional analysis of these mutations revealed divergent effects on HCN channel function. The investigators explored this further by coexpressing heterozygous mutant channels with wild-type channels, and found that most mutated channels had a dominant negative effect on wild-type channels. The study also found a high level of sequence conservation and low frequency of copy number variation among control population, suggesting that the conservation of HCN channels is essential for their appropriate function. Recently, Nakamura et al. (2013) uncovered a mutation in HCN2 that may contribute to hyperexcitability and specifically to febrile seizures in children. The relevant mutation was predicted to replace a residue in the intracellular amino terminus. Together, these human studies provide support for the role of abnormal HCN channel function in seizures and epilepsy.
SUMMARY
The HCN-regulated ion channels that conduct Ih have crucial and diverse roles in normal physiology. Specifically, they contribute to regulation of neuronal and network excitability. These roles position HCN channels, and specifically their aberrant expression, trafficking, posttranslational modification, and interactions with other proteins as key targets in understanding the hyperexcitability found in epilepsy. To date, some of the molecular mechanisms of HCN channelopathy in both genetic and acquired epilepsy have been dissected, yet there is much to learn about this ion channel regulation in epilepsy. Importantly, the study of HCN channels has broader implications, because these channels represent an ideal model system in which to investigate the interplay of diverse ion channels and signaling cascades and their potential roles in a variety of epilepsies. It is hoped that these multiple signaling pathways may represent potential novel therapeutic targets for reversing abnormal channel expression and function (“channelopathy”) not only of the HCN channels, but of others. The novel mechanisms underlying ion channelopathies in epilepsy offer promise for treating epilepsy that is refractory to conventional therapy.
Footnotes
Editors: Gregory L. Holmes and Jeffrey L. Noebels
Additional Perspectives on Epilepsy: The Biology of a Spectrum Disorder available at www.perspectivesinmedicine.org
REFERENCES
- Accili EA, Proenza C, Baruscotti M, DiFrancesco D. 2002. From funny current to HCN channels: 20 years of excitation. News Physiol Sci 17: 32–37. [DOI] [PubMed] [Google Scholar]
- Aponte Y, Lien CC, Reisinger E, Jonas P. 2006. Hyperpolarization-activated cation channels in fast-spiking interneurons of rat hippocampus. J Physiol 574: 229–243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beck H, Yaari Y. 2008. Plasticity of intrinsic neuronal properties in CNS disorders. Nat Rev Neurosci 9: 357–369. [DOI] [PubMed] [Google Scholar]
- Bender RA, Baram TZ. 2008. Hyperpolarization activated cyclic-nucleotide gated (HCN) channels in developing neuronal networks. Prog Neurobiol 86: 129–140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bender RA, Soleymani SV, Brewster AL, Nguyen ST, Beck H, Mathern GW, Baram TZ. 2003. Enhanced expression of a specific hyperpolarization-activated cyclic nucleotide-gated cation channel (HCN) in surviving dentate gyrus granule cells of human and experimental epileptic hippocampus. J Neurosci 23: 6826–6836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bender RA, Kirschstein T, Kretz O, Brewster AL, Richichi C, Ruschenschmidt C, Shigemoto R, Beck H, Frotscher M, Baram TZ. 2007. Localization of HCN1 channels to presynaptic compartments: Novel plasticity that may contribute to hippocampal maturation. J Neurosci 27: 4697–4706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berger T, Larkum ME, Luscher HR. 2001. High Ih channel density in the distal apical dendrite of layer V pyramidal cells increases bidirectional attenuation of EPSPs. J Neurophysiol 85: 855–868. [DOI] [PubMed] [Google Scholar]
- Berger T, Senn W, Luscher HR. 2003. Hyperpolarization-activated current Ih disconnects somatic and dendritic spike initiation zones in layer V pyramidal neurons. J Neurophysiol 90: 2428–2437. [DOI] [PubMed] [Google Scholar]
- Biel M, Wahl-Schott C, Michalakis S, Zong X. 2009. Hyperpolarization-activated cation channels: From genes to function. Physiol Rev 89: 847–885. [DOI] [PubMed] [Google Scholar]
- Brewster A, Bender RA, Chen Y, Dube C, Eghbal-Ahmadi M, Baram TZ. 2002. Developmental febrile seizures modulate hippocampal gene expression of hyperpolarization-activated channels in an isoform- and cell-specific manner. J Neurosci 22: 4591–4599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brewster AL, Bernard JA, Gall CM, Baram TZ. 2005. Formation of heteromeric hyperpolarization-activated cyclic nucleotide-gated (HCN) channels in the hippocampus is regulated by developmental seizures. Neurobiol Dis 19: 200–207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brewster AL, Dube CM, Bender RA, Patel N, Lee M, Ring A, Baram TZ. 2006. HCN1-deficient mice: Functional and molecular characterization provides insight into cell-specific roles of HCN channels in hippocampal network excitability. In Annual Meeting of the Society for Neuroscience, Program 334.6, Atlanta. [Google Scholar]
- Brewster AL, Chen Y, Bender RA, Yeh A, Shigemoto R, Baram TZ. 2007. Quantitative analysis and subcellular distribution of mRNA and protein expression of the hyperpolarization-activated cyclic nucleotide-gated channels throughout development in rat hippocampus. Cereb Cortex 17: 702–712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Budde T, Caputi L, Kanyshkova T, Staak R, Abrahamczik C, Munsch T, Pape HC. 2005. Impaired regulation of thalamic pacemaker channels through an imbalance of subunit expression in absence epilepsy. J Neurosci 25: 9871–9882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan CS, Shigemoto R, Mercer JN, Surmeier DJ. 2004. HCN2 and HCN1 channels govern the regularity of autonomous pacemaking and synaptic resetting in globus pallidus neurons. J Neurosci 24: 9921–9932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen K, Aradi I, Thon N, Eghbal-Ahmadi M, Baram TZ, Soltesz I. 2001. Persistently modified h-channels after complex febrile seizures convert the seizure-induced enhancement of inhibition to hyperexcitability. Nat Med 7: 331–337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen K, Aradi I, Santhakumar V, Soltesz I. 2002. h-Channels in epilepsy: New targets for seizure control? Trends Pharmacol Sci 23: 552–557. [DOI] [PubMed] [Google Scholar]
- Dibbens LM, Reid CA, Hodgson B, Thomas EA, Phillips AM, Gazina E, Cromer BA, Clarke AL, Baram TZ, Scheffer IE, et al. 2010. Augmented currents of an HCN2 variant in patients with febrile seizure syndromes. Ann Neurol 67: 542–546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiFrancesco JC, Barbuti A, Milanesi R, Coco S, Bucchi A, Bottelli G, Ferrarese C, Franceschetti S, Terragni B, Baruscotti M, et al. 2011. Recessive loss-of-function mutation in the pacemaker HCN2 channel causing increased neuronal excitability in a patient with idiopathic generalized epilepsy. J Neurosci 31: 17327–17337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dube C, Richichi C, Bender RA, Chung G, Litt B, Baram TZ. 2006. Temporal lobe epilepsy after experimental prolonged febrile seizures: Prospective analysis. Brain 129: 911–922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gauss R, Seifert R, Kaupp UB. 1998. Molecular identification of a hyperpolarization-activated channel in sea urchin sperm. Nature 393: 583–587. [DOI] [PubMed] [Google Scholar]
- George MS, Abbott LF, Siegelbaum SA. 2009. HCN hyperpolarization-activated cation channels inhibit EPSPs by interactions with M-type K+ channels. Nat Neurosci 12: 577–584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hablitz JJ, Yang J. 2010. Abnormal pyramidal cell morphology and HCN channel expression in cortical dysplasia. Epilepsia 51: 52–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammelmann V, Zong X, Hofmann F, Michalakis S, Biel M. 2011. The cGMP-dependent protein kinase II is an inhibitory modulator of the hyperpolarization-activated HCN2 channel. PLoS ONE 6: e17078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hegle AP, Nazzari H, Roth A, Angoli D, Accili EA. 2010. Evolutionary emergence of N-glycosylation as a variable promoter of HCN channel surface expression. Am J Physiol Cell Physiol 298: C1066–1076. [DOI] [PubMed] [Google Scholar]
- Huang Z, Walker MC, Shah MM. 2009. Loss of dendritic HCN1 subunits enhances cortical excitability and epileptogenesis. J Neurosci 29: 10979–10988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang Z, Lujan R, Kadurin I, Uebele VN, Renger JJ, Dolphin AC, Shah MM. 2011. Presynaptic HCN1 channels regulate Cav3.2 activity and neurotransmission at select cortical synapses. Nat Neurosci 14: 478–486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang Z, Lujan R, Martinez-Hernandez J, Lewis AS, Chetkovich DM, Shah MM. 2012. TRIP8b-independent trafficking and plasticity of adult cortical presynaptic HCN1 channels. J Neurosci 32: 14835–14848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huguenard J. 2001. Thalamocortical circuits and excitability. Epilepsy Curr 1: 13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishii TM, Takano M, Xie LH, Noma A, Ohmori H. 1999. Molecular characterization of the hyperpolarization-activated cation channel in rabbit heart sinoatrial node. J Biol Chem 274: 12835–12839. [DOI] [PubMed] [Google Scholar]
- Jung S, Jones TD, Lugo JN Jr, Sheerin AH, Miller JW, D’Ambrosio R, Anderson AE, Poolos NP. 2007. Progressive dendritic HCN channelopathy during epileptogenesis in the rat pilocarpine model of epilepsy. J Neurosci 27: 13012–13021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung S, Bullis JB, Lau IH, Jones TD, Warner LN, Poolos NP. 2010. Downregulation of dendritic HCN channel gating in epilepsy is mediated by altered phosphorylation signaling. J Neurosci 30: 6678–6688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung S, Warner LN, Pitsch J, Becker AJ, Poolos NP. 2011. Rapid loss of dendritic HCN channel expression in hippocampal pyramidal neurons following status epilepticus. J Neurosci 31: 14291–14295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanyshkova T, Pawlowski M, Meuth P, Dube C, Bender RA, Brewster AL, Baumann A, Baram TZ, Pape HC, Budde T. 2009. Postnatal expression pattern of HCN channel isoforms in thalamic neurons: Relationship to maturation of thalamocortical oscillations. J Neurosci 29: 8847–8857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kole MH, Brauer AU, Stuart GJ. 2007. Inherited cortical HCN1 channel loss amplifies dendritic calcium electrogenesis and burst firing in a rat absence epilepsy model. J Physiol 578: 507–525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuisle M, Wanaverbecq N, Brewster AL, Frere SG, Pinault D, Baram TZ, Luthi A. 2006. Functional stabilization of weakened thalamic pacemaker channel regulation in rat absence epilepsy. J Physiol 575: 83–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis AS, Chetkovich DM. 2011. HCN channels in behavior and neurological disease: Too hyper or not active enough? Mol Cell Neurosci 46: 357–367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis AS, Schwartz E, Chan CS, Noam Y, Shin M, Wadman WJ, Surmeier DJ, Baram TZ, Macdonald RL, Chetkovich DM. 2009. Alternatively spliced isoforms of TRIP8b differentially control h channel trafficking and function. J Neurosci 29: 6250–6265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li B, Luo C, Tang W, Chen Z, Li Q, Hu B, Lin J, Zhu G, Zhang JH, Feng H. 2012. Role of HCN channels in neuronal hyperexcitability after subarachnoid hemorrhage in rats. J Neurosci 32: 3164–3175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lorincz A, Notomi T, Tamas G, Shigemoto R, Nusser Z. 2002. Polarized and compartment-dependent distribution of HCN1 in pyramidal cell dendrites. Nat Neurosci 5: 1185–1193. [DOI] [PubMed] [Google Scholar]
- Ludwig A, Zong X, Jeglitsch M, Hofmann F, Biel M. 1998. A family of hyperpolarization-activated mammalian cation channels. Nature 393: 587–591. [DOI] [PubMed] [Google Scholar]
- Ludwig A, Budde T, Stieber J, Moosmang S, Wahl C, Holthoff K, Langebartels A, Wotjak C, Munsch T, Zong X, et al. 2003. Absence epilepsy and sinus dysrhythmia in mice lacking the pacemaker channel HCN2. EMBO J 22: 216–224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lujan R, Albasanz JL, Shigemoto R, Juiz JM. 2005. Preferential localization of the hyperpolarization-activated cyclic nucleotide-gated cation channel subunit HCN1 in basket cell terminals of the rat cerebellum. Eur J Neurosci 21: 2073–2082. [DOI] [PubMed] [Google Scholar]
- Lupica CR, Bell JA, Hoffman AF, Watson PL. 2001. Contribution of the hyperpolarization-activated current (Ih) to membrane potential and GABA release in hippocampal interneurons. J Neurophysiol 86: 261–268. [DOI] [PubMed] [Google Scholar]
- Maccaferri G, McBain CJ. 1996. The hyperpolarization-activated current (Ih) and its contribution to pacemaker activity in rat CA1 hippocampal stratum oriens-alveus interneurones. J Physiol 497: 119–130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magee JC. 1998. Dendritic hyperpolarization-activated currents modify the integrative properties of hippocampal CA1 pyramidal neurons. J Neurosci 18: 7613–7624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magee JC. 1999. Dendritic Ih normalizes temporal summation in hippocampal CA1 neurons. Nat Neurosci 2: 848. [DOI] [PubMed] [Google Scholar]
- Marcelin B, Chauviere L, Becker A, Migliore M, Esclapez M, Bernard C. 2009. h Channel-dependent deficit of theta oscillation resonance and phase shift in temporal lobe epilepsy. Neurobiol Dis 33: 436–447. [DOI] [PubMed] [Google Scholar]
- McClelland S, Flynn C, Dube C, Richichi C, Zha Q, Ghestem A, Esclapez M, Bernard C, Baram TZ. 2011. Neuron-restrictive silencer factor-mediated hyperpolarization-activated cyclic nucleotide gated channelopathy in experimental temporal lobe epilepsy. Ann Neurol 70: 454–464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McClelland S, Brennan GP, Dube C, Rajpara S, Iyer S, Richichi C, Bernard C, Baram TZ. 2014. The transcription factor NRSF contributes to epileptogenesis by selective repression of a subset of target genes. eLife 3: e01267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCormick DA, Bal T. 1997. Sleep and arousal: Thalamocortical mechanisms. Annu Rev Neurosci 20: 185–215. [DOI] [PubMed] [Google Scholar]
- Mesirca P, Alig J, Torrente AG, Muller JC, Marger L, Rollin A, Marquilly C, Vincent A, Dubel S, Bidaud I, et al. 2014. Cardiac arrhythmia induced by genetic silencing of “funny” (f) channels is rescued by GIRK4 inactivation. Nat Commun 5: 4664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Much B, Wahl-Schott C, Zong X, Schneider A, Baumann L, Moosmang S, Ludwig A, Biel M. 2003. Role of subunit heteromerization and N-linked glycosylation in the formation of functional hyperpolarization-activated cyclic nucleotide-gated channels. J Biol Chem 278: 43781–43786. [DOI] [PubMed] [Google Scholar]
- Munsch T, Pape HC. 1999. Upregulation of the hyperpolarization-activated cation current in rat thalamic relay neurones by acetazolamide. J Physiol 519: 505–514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura Y, Shi X, Numata T, Mori Y, Inoue R, Lossin C, Baram TZ, Hirose S. 2013. Novel HCN2 mutation contributes to febrile seizures by shifting the channel’s kinetics in a temperature-dependent manner. PLoS ONE 8: e80376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nava C, Dalle C, Rastetter A, Striano P, de Kovel CG, Nabbout R, Cances C, Ville D, Brilstra EH, Gobbi G, et al. 2014. De novo mutations in HCN1 cause early infantile epileptic encephalopathy. Nat Genet 46: 640–645. [DOI] [PubMed] [Google Scholar]
- Noam Y, Zha Q, Phan L, Wu RL, Chetkovich DM, Wadman WJ, Baram TZ. 2010. Trafficking and surface expression of hyperpolarization-activated cyclic nucleotide-gated channels in hippocampal neurons. J Biol Chem 285: 14724–14736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noam Y, Bernard C, Baram TZ. 2011. Towards an integrated view of HCN channel role in epilepsy. Curr Opin Neurobiol 21: 873–879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noam Y, Ehrengruber MU, Koh A, Feyen P, Manders EM, Abbott GW, Wadman WJ, Baram TZ. 2014. Filamin A promotes dynamin-dependent internalization of hyperpolarization-activated cyclic nucleotide-gated type 1 (HCN1) channels and restricts Ih in hippocampal neurons. J Biol Chem 289: 5889–5903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nolan MF, Malleret G, Dudman JT, Buhl DL, Santoro B, Gibbs E, Vronskaya S, Buzsaki G, Siegelbaum SA, Kandel ER, et al. 2004. A behavioral role for dendritic integration: HCN1 channels constrain spatial memory and plasticity at inputs to distal dendrites of CA1 pyramidal neurons. Cell 119: 719–732. [DOI] [PubMed] [Google Scholar]
- Notomi T, Shigemoto R. 2004. Immunohistochemical localization of Ih channel subunits, HCN1–4, in the rat brain. J Comp Neurol 471: 241–276. [DOI] [PubMed] [Google Scholar]
- Nusser Z. 2009. Variability in the subcellular distribution of ion channels increases neuronal diversity. Trends Neurosci 32: 267–274. [DOI] [PubMed] [Google Scholar]
- Peng BW, Justice JA, Zhang K, He XH, Sanchez RM. 2010. Increased basal synaptic inhibition of hippocampal area CA1 pyramidal neurons by an antiepileptic drug that enhances Ih. Neuropsychopharmacology 35: 464–472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poolos NP, Migliore M, Johnston D. 2002. Pharmacological upregulation of h-channels reduces the excitability of pyramidal neuron dendrites. Nat Neurosci 5: 767–774. [DOI] [PubMed] [Google Scholar]
- Powell KL, Ng C, O'Brien TJ, Xu SH, Williams DA, Foote SJ, Reid CA. 2008. Decreases in HCN mRNA expression in the hippocampus after kindling and status epilepticus in adult rats. Epilepsia 49: 1686–1695. [DOI] [PubMed] [Google Scholar]
- Robinson RB, Siegelbaum SA. 2003. Hyperpolarization-activated cation currents: From molecules to physiological function. Annu Rev Physiol 65: 453–480. [DOI] [PubMed] [Google Scholar]
- Santoro B, Baram TZ. 2003. The multiple personalities of h-channels. Trends Neurosci 26: 550–554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santoro B, Grant SG, Bartsch D, Kandel ER. 1997. Interactive cloning with the SH3 domain of N-src identifies a new brain specific ion channel protein, with homology to Eag and cyclic nucleotide-gated channels. Proc Natl Acad Sci 94: 14815–14820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santoro B, Liu DT, Yao H, Bartsch D, Kandel ER, Siegelbaum SA, Tibbs GR. 1998. Identification of a gene encoding a hyperpolarization-activated pacemaker channel of brain. Cell 93: 717–729. [DOI] [PubMed] [Google Scholar]
- Santoro B, Wainger BJ, Siegelbaum SA. 2004. Regulation of HCN channel surface expression by a novel C-terminal protein-protein interaction. J Neurosci 24: 10750–10762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santoro B, Piskorowski RA, Pian P, Hu L, Liu H, Siegelbaum SA. 2009. TRIP8b splice variants form a family of auxiliary subunits that regulate gating and trafficking of HCN channels in the brain. Neuron 62: 802–813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santoro B, Lee JY, Englot DJ, Gildersleeve S, Piskorowski RA, Siegelbaum SA, Winawer MR, Blumenfeld H. 2010. Increased seizure severity and seizure-related death in mice lacking HCN1 channels. Epilepsia 51: 1624–1627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shah MM, Anderson AE, Leung V, Lin X, Johnston D. 2004. Seizure-induced plasticity of h channels in entorhinal cortical layer III pyramidal neurons. Neuron 44: 495–508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stuart G, Spruston N. 1998. Determinants of voltage attenuation in neocortical pyramidal neuron dendrites. J Neurosci 18: 3501–3510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Surges R, Freiman TM, Feuerstein TJ. 2003. Gabapentin increases the hyperpolarization-activated cation current Ih in rat CA1 pyramidal cells. Epilepsia 44: 150–156. [DOI] [PubMed] [Google Scholar]
- Surges R, Kukley M, Brewster A, Ruschenschmidt C, Schramm J, Baram TZ, Beck H, Dietrich D. 2012. Hyperpolarization-activated cation current Ih of dentate gyrus granule cells is upregulated in human and rat temporal lobe epilepsy. Biochem Biophys Res Commun 420: 156–160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang B, Sander T, Craven KB, Hempelmann A, Escayg A. 2008. Mutation analysis of the hyperpolarization-activated cyclic nucleotide-gated channels HCN1 and HCN2 in idiopathic generalized epilepsy. Neurobiol Dis 29: 59–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsay D, Dudman JT, Siegelbaum SA. 2007. HCN1 channels constrain synaptically evoked Ca2+ spikes in distal dendrites of CA1 pyramidal neurons. Neuron 56: 1076–1089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Z, Xu NL, Wu CP, Duan S, Poo MM. 2003. Bidirectional changes in spatial dendritic integration accompanying long-term synaptic modifications. Neuron 37: 463–472. [DOI] [PubMed] [Google Scholar]
- Wang M, Ramos BP, Paspalas CD, Shu Y, Simen A, Duque A, Vijayraghavan S, Brennan A, Dudley A, Nou E, et al. 2007. α2A-adrenoceptors strengthen working memory networks by inhibiting cAMP-HCN channel signaling in prefrontal cortex. Cell 129: 397–410. [DOI] [PubMed] [Google Scholar]
- Wierschke S, Lehmann TN, Dehnicke C, Horn P, Nitsch R, Deisz RA. 2010. Hyperpolarization-activated cation currents in human epileptogenic neocortex. Epilepsia 51: 404–414. [DOI] [PubMed] [Google Scholar]
- Wilkars W, Liu Z, Lewis AS, Stoub TR, Ramos EM, Brandt N, Nicholson DA, Chetkovich DM, Bender RA. 2012. Regulation of axonal HCN1 trafficking in perforant path involves expression of specific TRIP8b isoforms. PLoS ONE 7: e32181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams PA, White AM, Clark S, Ferraro DJ, Swiercz W, Staley KJ, Dudek FE. 2009. Development of spontaneous recurrent seizures after kainate-induced status epilepticus. J Neurosci 29: 2103–2112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams AD, Jung S, Poolos NP. 2015. Protein kinase C bidirectionally modulates Ih and hyperpolarization-activated cyclic nucleotide-gated (HCN) channel surface expression in hippocampal pyramidal neurons. J Physiol 593: 2779–2792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ying SW, Jia F, Abbas SY, Hofmann F, Ludwig A, Goldstein PA. 2007. Dendritic HCN2 channels constrain glutamate-driven excitability in reticular thalamic neurons. J Neurosci 27: 8719–8732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zha Q, Brewster AL, Richichi C, Bender RA, Baram TZ. 2008. Activity-dependent heteromerization of the hyperpolarization-activated, cyclic-nucleotide gated (HCN) channels: Role of N-linked glycosylation. J Neurochem 105: 68–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang K, Peng BW, Sanchez RM. 2006. Decreased Ih in hippocampal area CA1 pyramidal neurons after perinatal seizure-inducing hypoxia. Epilepsia 47: 1023–1028. [DOI] [PubMed] [Google Scholar]
- Zolles G, Wenzel D, Bildl W, Schulte U, Hofmann A, Muller CS, Thumfart JO, Vlachos A, Deller T, Pfeifer A, et al. 2009. Association with the auxiliary subunit PEX5R/Trip8b controls responsiveness of HCN channels to cAMP and adrenergic stimulation. Neuron 62: 814–825. [DOI] [PubMed] [Google Scholar]