Abstract
Dopamine release during reward-driven behaviors influences synaptic plasticity. However, dopamine innervation and release in the hippocampus and its role during aversive behaviors are controversial. Here we show that in vivo hippocampal synaptic plasticity in the CA3-CA1 circuit underlies contextual learning during inhibitory avoidance (IA) training. Immunohistochemistry and molecular techniques verified sparse dopaminergic innervation of the hippocampus from the midbrain. The long-term synaptic potentiation (LTP) underlying the learning of IA was assessed with a D1-like dopamine receptor agonist or antagonist in ex vivo hippocampal slices and in vivo in freely-moving mice. Inhibition of D1-like dopamine receptors impaired memory of the IA task and prevented the training-induced enhancement of both ex vivo and in vivo LTP induction. The results indicate that dopamine-receptor signaling during an aversive contextual task regulates aversive memory retention and regulates associated synaptic mechanisms in the hippocampus that likely underlie learning.
Keywords: Fear conditioning, Ventral tegmental area, Passive avoidance, Inhibitory avoidance, LTP, In vivo recording, Memory
Introduction
Dopamine (DA) neurons arising from the ventral tegmental area (VTA) and substantia nigra compacta (SNc) contribute during the formation of rewarded behaviors (Bayer and Glimcher, 2005; Schultz, 1986, 1998). DA neurons fire phasic bursts in response to unpredicted rewards, and their phasic firing begins to track neutral stimuli that predict those rewards (Hollerman and Schultz, 1998). This firing characteristic of DA neurons suggests that they are highly effective at pairing neutral stimuli to unconditioned stimuli, and this property provided evidence that DA signals are a neural substrate of reward prediction (Berridge and Robinson, 1998; Dayan and Balleine, 2002; Montague et al., 2004; Schultz et al., 1997).
Recent studies indicate that DA neurons have a more heterogenous response profile (Henny et al., 2012). For example, dorsal VTA neurons are typically inhibited by footshocks, but ventral VTA neurons may be phasically excited by noxious stimuli (Brischoux et al., 2009; Bromberg-Martin et al., 2010; Zweifel et al., 2011). Aversive events have been shown to increase the firing rate of a subset of VTA DA neurons, and as a result, increase DA release in target areas, such as the striatum or medial prefrontal cortex (Budygin et al., 2012; Dong et al., 2010; Lammel et al., 2011). Recent studies indicate that a DA neuron’s response to negative or positive stimuli is largely dependent upon the neuron’s presynaptic inputs, and the dopaminergic signal influences separate brain regions depending on valence (Lammel et al., 2011; Lammel et al., 2014; Lammel et al., 2012). These findings suggest that DA signaling may encode beyond prediction errors and contribute to synaptic plasticity required for updating memory of environmental salience, and that these DA signals act upon specific neural targets.
One such potential target is the hippocampus. Earlier evidence indicated that dopaminergic projections originating primarily from the midbrain (including the VTA, substantia nigra, and retrorubral field) project directly to the hippocampus (Gasbarri et al., 1994a; Gasbarri et al., 1996; Gasbarri et al., 1994b). Quantitative real-time PCR confirmed that D1 and D5 receptors are found in the hippocampus, including in the CA1 (Mu et al., 2011). Physiological evidence demonstrated that D1 and D5 receptors are important for controlling spike timing dependent plasticity within the hippocampus (Yang and Dani, 2014). Also, there is functional evidence that indicates that drugs of abuse, such as nicotine (Tang and Dani, 2009; Zhang et al., 2010) and methylphenidate (Jenson et al., 2015), recruit dopaminergic neurotransmission to influence synaptic plasticity within the hippocampus. Dopaminergic neurotransmission in the hippocampus during specific time points in the retention interval also seem to be important for successful consolidation of long-term memories (Rossato et al., 2009). Despite this evidence, there is currently some controversy about the source of dopaminergic innervation in the hippocampus (Smith and Greene, 2012), suggesting that significant dopaminergic neurotransmission is the result of extra-synaptic volume transmission from other sources (Agnati et al., 1995; Borgkvist et al., 2012), such as the locus coeruleus (Walling et al., 2012).
In this study, we used virally-introduced molecular markers to label dopaminergic synaptic terminals arising from the midbrain to determine whether there is significant innervation from midbrain DA areas. Then, we tested whether neurotransmission at D1-like receptors was important for the acquisition of aversive memories in an inhibitory avoidance (IA) task. After finding a sparse direct projection of midbrain DA neurons to the hippocampus and evidence of dopaminergic influence in the retention of IA learning, we tested the effects of D1-like receptors on measures of synaptic plasticity. We found that dopaminergic activity regulated IA-induced CA1 LTP measured from single pyramidal neurons in ex vivo slices or from in vivo field EPSPs in freely moving mice.
Results
Evidence for innervation of the hippocampus by midbrain dopamine neurons
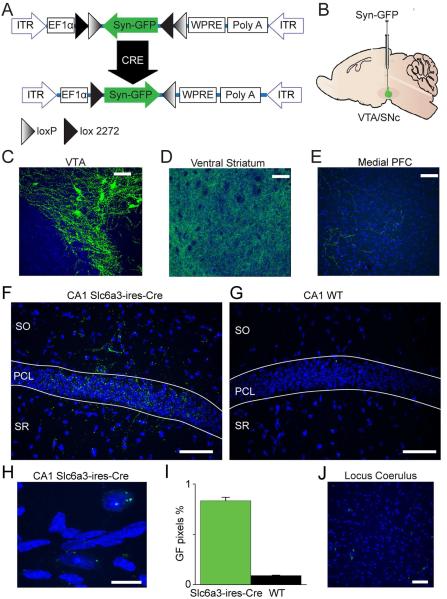
Dopamine transporters (DAT) have been shown to be located on dopamine (DA) fibers and terminals (Nirenberg et al., 1996; Shimada et al., 1991; Shimada et al., 1992). To examine whether there is a direct dopaminergic projection to the hippocampus, we injected a recombinant adeno-associated virus (Grimm et al., 2008; Tsai et al., 2009) (AAV-EF1a-DIO-synaptophysin:GFP) containing a double-floxed inverted open reading frame encoding synaptophysin-GFP into the midbrain DA area of adult Slc6a3ires-cre/+ knock-in and Slc6a3+/+ (WT) mice (Fig. 1A,B). This AAV vector facilitates the expression of GFP specifically in the synaptic terminals of neurons containing DAT, exclusively in Cre-expressing cells. Because synaptophysin is a synaptic vesicle protein, this procedure concentrated the GFP fluorophore into DA terminals of neurons from the ventral tegmental area (VTA) and the substantia nigra (SN). By concentration of the fluorophore into the synaptic terminals, we increased the likelihood of finding DA-positive innervation.
Fig. 1. Midbrain DAT-positive neurons project to the CA1.
(A) Didactic vector map of the AAV-EF1a-DIO-synaptophysin:GFP virus constructed for use in the viral tracing experiments. This DJ8 vector specifically expressed synaptophysin, which was primarily targeted in the fiber terminals of infected neurons. (B) AAV-EF1a-DIO-synaptophysin:GFP was injected into the midbrain dopamine area (VTA/SNc) of Slc6a3ires-cre/+ mice and WT mice served as controls. (C) Confocal image (10x) taken from the VTA of a Slc6a3ires-cre/+ mouse injected with Synaptophysin-GFP virus. The light blue is DAPI (Vector laboratories, Burlingame CA), and green fluorescence indicates the reporter from Synaptophysin-GFP. Cell bodies and processes were labeled in the VTA. (D) As a positive control, a confocal image is shown of the dense innervation of the ventral striatum, which receives innervation from VTA DA neurons. (E) A second positive control showing green DA-terminal puncta in the medial prefrontal cortex (PFC). (F) Image from Slc6a3ires-cre/+ mouse indicating direct dopaminergic projections from the midbrain DA area revealed as green puncta in the CA1. (G) The CA1 of a Slc6a3+/+ (WT littermate) shows practically no green fluorescence. (H) Confocal image (40x) of DAT terminals projecting directly to cell bodies in the PCL of the hippocampal CA1. (I) Quantification of the number of green pixels from Slc6a3ires-cre/+ mice compared to WT littermates: 0.83% ± 0.03, n = 5 for Slc6a3ires-cre/+; 0.09% ± 0.004, n = 3 for WT. (J) Locus coeruleus image illustrating Synaptophysin-GFP (DAT) labeled terminals, but no cell somas, indicating that these are not DAT positive cell bodies. SO: stratum oriens, PCL: pyramidal cell layer, SR: stratum radiatum. Scale bars: 100 μm (C, D, E); 50 μm (F, G, J); 25 μm (H).
As positive controls, two weeks after injection we inspected the brains of injected mice and found prominent labeling of projections and cell bodies in the VTA (Fig. 1C) and labelled terminals in the ventral striatum (Fig. 1D) and medial prefrontal cortex (Fig. 1E), major known targets for innervation by midbrain DA neurons. GFP-positive terminals were also found throughout the CA1 region of Cre-positive mice (Fig. 1F,H). This expression was significantly greater relative to Cre-negative mice (Fig. 1G): 0.83% ± 0.03, n = 5 for Slc6a3ires-cre/+; 0.09% ± 0.004, n = 3 for WT (Fig. 1I). We inspected the locus coeruleus and found punctate terminals, but no cell bodies labeled (Fig. 1J), verifying that the labeling arose from the midbrain not the locus coeruleus. To test the specificity of Slc6a3ires-cre/+ expression, we checked the cerebellum of injected Slc6a3irescre/+ mice and found virtually no green fluorescence in the cerebellum (0.003%, data not shown). Likewise, there was no GFP fluorescence in the hypothalamus, which is near to the midbrain DA areas (Fig. S1A,B). Thus, the injections were restricted to the midbrain DA areas, and there the synaptophysin-GFP was found to co-localize with the catecholamine synthesis enzyme, tyrosine hydroxylase (TH) (Fig. S1C).
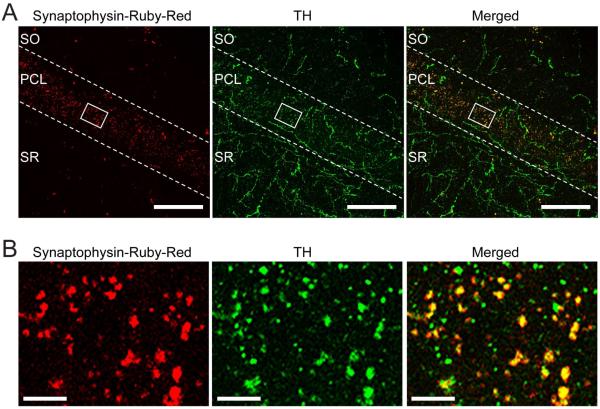
To verify that the synaptophysin signal we observed in the hippocampal CA1 is indeed dopaminergic terminals, we injected the midbrains of a second group of Slc6a3ires-cre/+ mice with Synaptophysin-Ruby-Red (AAV-EF1a-DIO-synaptophysin:RFP) and immunostained with an antibody against TH (Fig. 2A). We observed nearly complete co-localization between the Synaptophysin-Ruby-Red and TH (Fig. 2A,B), confirming that the synaptic terminals are dopaminergic and originate from the midbrain: Ruby-Red/TH double positive terminals, 93 ± 2%, n = 6.
Figure 2. Dopaminergic terminals and axons in the CA1 show high co-localization with tyrosine hydroxylase (TH).
(A) Confocal images of the CA1 field of a Slc6a3ires-cre/+ mouse injected with Synaptophysin-Ruby-Red virus in the VTA/SNc area. Images show punctate Synaptophysin-Ruby-Red signal (in red) mainly along the pyramidal cell layer of CA1 and TH immunoreactivity (in green). A merged overlay of the two signals, shows a high degree of co-localization (yellow-orange) of Synaptophysin-Ruby-Red with TH (Ruby-Red/TH double-positive: 93 ± 2%, n = 6) providing further evidence of the existence of midbrain dopaminergic innervation of the hippocampus. SO: stratum oriens, PCL: pyramidal cell layer, SR: stratum radiatum. Scale bars, 50 μm. (B) High magnification images of the white boxed area in (A), showing individual and composite images of the different labels including the co-localization (Merged). Scale bars, 5 μm.
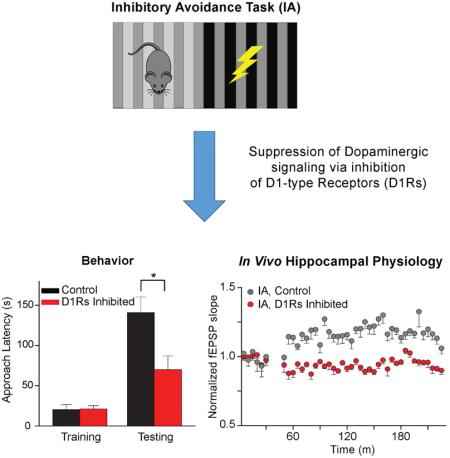
Dopamine regulated acquisition of inhibitory avoidance memories
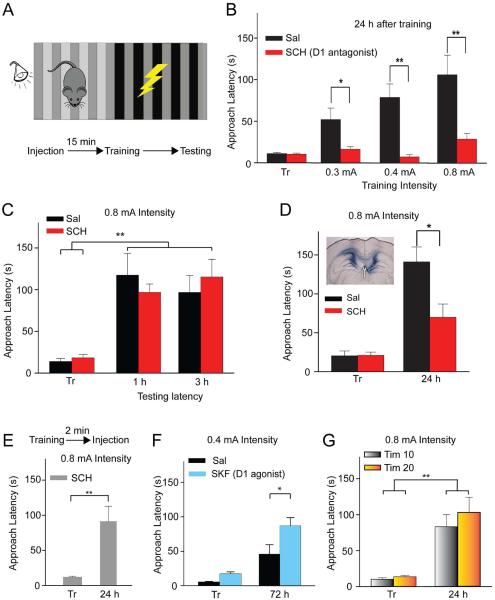
To test the hypothesis that DA regulates acquisition of IA memory, we injected groups of animals with SCH 23390 (SCH), a D1/D5 receptor antagonist, prior to footshock training (Fig 3A). During IA training, the mice received a footshock upon entering the dark side of the training chamber from the lighted side of the chamber. The latency to enter the darkened side was examined at three SCH doses (0.2, 0.1, and 0.05 mg/kg, i.p.). Only the higher doses of SCH slowed the approach latency during training (Fig. S2): p < 0.01, n = 4-8. Therefore, we used the lowest dose of SCH (0.05 mg/kg) that did not slow the initial approach of mice to the dark side of the light/dark chamber (Fig. S2).
Fig. 3. Dopamine regulated acquisition of a IA long-term memory.
(A) Didactic illustration of the mouse in the IA training and testing box. The mouse was placed on the light side, and after a short time a door opened enabling the mouse to move to the dark side where it could be foot shocked. (B) IA training typically elevated the approach latency when tested 24 h later (saline injection, black bars). A low dose of SCH 23390 (0.05 mg/kg, i.p., red bars) just before training blocked this effect at different footshock intensities: n = 9 - 11/group. The results are the following: 0.3 mA, 0.4 mA, and 0.8 mA: Sal 0.3 mA = 52.1 ± 13.80 s, SCH 0.3 mA = 16.44 ± 3.19 s, n = 10, 9; Sal 0.4 mA = 78.5 ± 16.47 s, SCH 0.4 mA = 7.4 ± 2.73 sec, n =10,10; Sal 0.8 mA = 106.17 ± 23.54 s, SCH 0.8mA = 28.78 ± 7.04 sec, n = 12, 9. (C) The same SCH dose (red bars) did not block short-term retention in the IA task: 1 h retention, SCH, n = 32 and Sal, n = 15, p > 0.05; 3 h retention, n = 5, 5, p > 0.05. (D) Local bilateral infusion of 1-μl SCH (1 mg/ml concentration, red bars) into the CA1 prior to training significantly reduced memory retention in the IA paradigm 24 h after training: n = 7, 9 p < 0.05. The insert indicates post hoc staining, indicating the location of the infusion of SCH into the dorsal CA1 region. (E) Systemic injections of a high dose of SCH (0.2 mg/kg) immediately after IA training did not impair retention of the footshock at the 24 h interval: Training = 11.78 ± 1.36 s, Testing = 91.20 ± 21.77 s; p < 0.01. (F) Systemic injection of DA D1-like receptor agonist, SKF 81297 (0.9 mg/kg), enhanced retention of a footshock (0.4 mA) when tested at the 72 h retention interval: Sal = 45.90 ± 13.80 s, n = 10; SKF = 92.5 ± 12.64 s, n = 32; p < 0.05. (G) Two doses of β2-adrenergic antagonist, Tim (i.p.), prior to IA training did not block the retention of a footshock: approach latency was 83.3 ± 16.7 s, p < 0.01, after 10 mg/kg Tim; and was 103.1 ± 21.0 s, p < 0.01 after 20 mg/kg Tim , n = 10, 10.
24 h after training (i.e., footshock), control mice injected with saline significantly delayed entering the dark (previously shocked) side (Fig. 3B, black bars). On the contrary, the latency to approach the dark (shocked) side was not delayed in SCH-treated mice at three different shock intensities (Fig. 3B, red bars). This dose of SCH (0.05 mg/kg, i.p.) also did not impair short-term memory (STM) because approach latency remained similar to controls at 1 h and 3 h after the shock training (Fig. 3C). In summary, the D1/D5 antagonist, at a dose that did not influence approach latency during training and did not impair short-term memory, inhibited long-term memory retention in this IA task.
To test whether the affected dopaminergic receptors resided within the hippocampus, we implanted bilateral cannulas directly above the CA1 and infused SCH (1 μl, 1 mg/ml concentration given at 0.5 μl/min) 15 min prior to IA training. When tested 24 h later, direct hippocampal infusions of the D1-like antagonist significantly reduced approach latency compared to saline controls (Fig. 3D): p < 0.05, unpaired t-test, n = 7,9. Injecting a higher dose (0.2 mg/kg, i.p.) of SCH 23390 after IA training did not influence long-term memory retention 24 h later, as indicated by an approach latency significantly higher after testing (Fig. 3E): training vs. testing p < 0.01, n = 10. Acting opposite to the D1-like antagonist, the D1-like agonist, SKF 81297 (0.9 mg/kg), enhanced IA retention 72 h after a moderate (0.4 mA) footshock (Fig. 3F): p < 0.05, n = 10, 32. This result suggests that elevated DA D1R activity during training enhances retention of a more difficult training task, which was IA memory at a longer retention interval (i.e., 72 h) to a moderate footshock (0.4 mA).
In order to test whether β-adrenergic (norepinephrine, NE) neurotransmission was important for learning the IA task, we injected two doses (10 mg/kg and 20 mg/kg, i.p.) of the β2-adrenergic antagonist, Timolol (Tim), prior to IA training. The approach latency was significantly greater when tested after 24 h retention, indicating that mice still learned to avoid the dark side (shocked side) of the chamber even when β2-adrenergic receptors were inhibited (Fig. 3G): p < 0.01, paired t-tests, n = 10, 10.
Inhibitory avoidance training increased the AMPA/NMDA ratio in the CA1
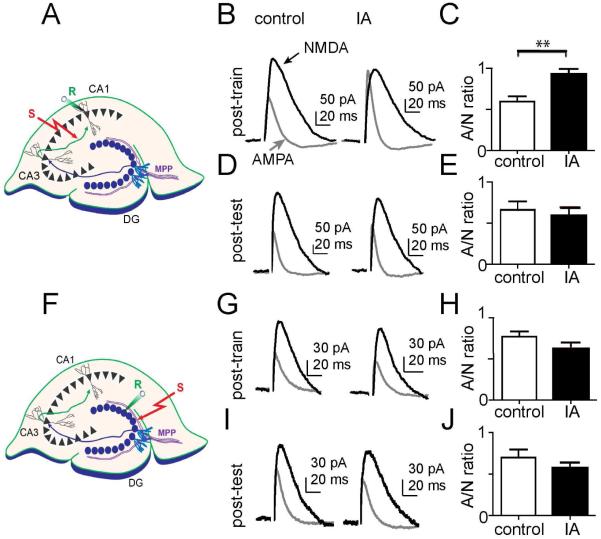
Learning the context of IA is expected to engage synaptic plasticity mechanisms in the CA1 region of the hippocampus (Whitlock et al., 2006). To gain insight into whether LTP occurred in the CA1 region following IA training, we cut ex vivo slices and determined the AMPA/NMDA ratio (Ungless et al., 2001) from CA1 pyramidal neurons while stimulating the Schaffer collateral input (Fig. 4A). IA training increased the AMPA/NMDA ratio at the CA3-CA1 synapses when measured at 1.5 h after the footshock, as compared to walk-through controls that were not shocked (Fig. 4B,C): 0.59 ± 0.06 in control vs. 0.93 ± 0.06 after IA, n = 12, 6, p < 0.01 unpaired t test. The ratio returned to baseline 24 h after the footshock (Fig. 4D,E): 0.66 ± 0.10 in control vs. 0.59 ± 0.10 after IA, n = 6, 6, p > 0.05 unpaired t test. These results demonstrate an association between learned avoidance and an increase in the AMPA/NMDA ratio in the CA1 of the hippocampus.
Fig. 4. IA training increased the AMPA/NMDA current ratio in CA1 pyramidal neurons, but not in dentate gyrus granule cells.
(A) Diagram illustrating the whole-cell recording from CA1 pyramidal neurons in hippocampal slices. The stimulating electrode (S) was placed on the Schaffer collateral path and the recording electrode (R) onto a CA1 pyramidal neuron. (B) and (D) Representative traces of AMPA (grey traces) and NMDA (black traces) receptor mediated whole-cell currents recorded from CA1 pyramidal neurons from control (unshocked, left) and IA (shocked, right) mice, which were decapitated either 1.5 h after training (B), or after testing (D). (C) and (E) The average of AMPA/NMDA ratios from CA1 pyramidal neurons are plotted. IA training significantly increased the AMPA/NMDA ratio in slices prepared from animals decapitated 1.5 h after training: control vs. IA, 0.59 ± 0.06 vs. 0.93 ± 0.06, n = 12, 6, p < 0.01), but not in slices prepared from animals after testing: control vs. IA, 0.66 ± 0.10 vs. 0.59 ± 0.10, n = 6, 6, p > 0.05. (F) Diagram illustrating the whole-cell recording from dentate gyrus granule cells. The stimulating electrode (S) was placed on the medial perforant path (MPP) and the recording electrode (R) onto a DG granule cell. (G) and (I) Representative traces of AMPA (grey) and NMDA (black) receptor mediated whole-cell currents recorded in granule cells from control (unshocked, left) and IA (shocked, right) mice, which were sacrificed either 1.5 h after training (G) or after testing (I). (H) and (J) IA training had no effect on the AMPA/NMDA ratio measured from dentate gyrus granule cells at these time points: 1.5 h, control vs. IA, 0.77 ± 0.06 vs. 0.63 ± 0.07, n = 10, 11, p > 0.05; post-test, control vs. IA, 0.70 ± 0.10 vs. 0.57 ± 0.06, n = 5, 7, p > 0.05.
As a negative control, we tested the effects of IA on the AMPA/NMDA ratio by recording from dentate granule cells while stimulating the medial perforant path (Fig. 4F). We recorded the AMPA/NMDA ratio of granule cells in the dentate gyrus 1.5 h after the footshock and found no difference relative to walk-through controls (Fig. 4G,H): 0.77 ± 0.06 in control vs. 0.63 ± 0.07 at 1.5 h after footshock, n = 10, 11, p > 0.05 unpaired t test. Similarly, 1 d after training and following testing of memory retention, there was no difference between the footshock and the no-footshock groups (Fig. 4I,J): 0.70 ± 0.10 in control vs. 0.57 ± 0.06 at 1 d after training, n = 5, 7, p > 0.05 unpaired t test.
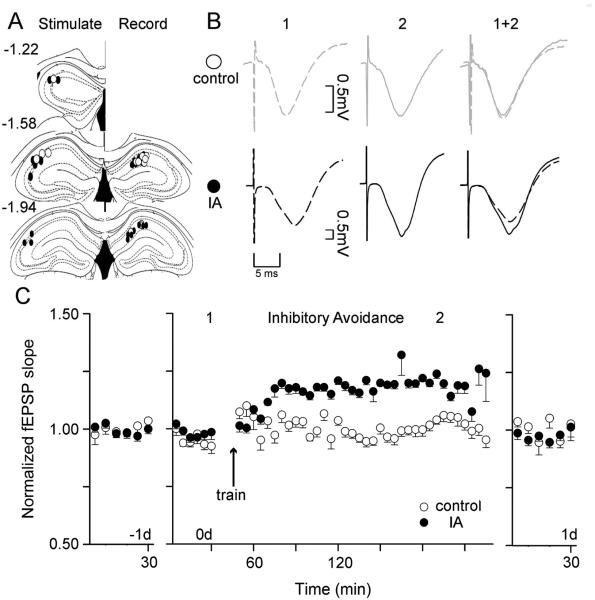
Inhibitory avoidance training increased the slope of CA1 fEPSPs in vivo
The ex vivo slice studies (Fig. 4) suggest that IA-induced long-term synaptic potentiation (LTP) in the CA1 region. Therefore, we measured in vivo synaptic strength along the Schaffer collateral input to CA1 in freely behaving mice to determine whether there was synaptic potentiation associated with the IA learning task in real time. We monitored synaptic transmission from the stratum radiatum of the CA1 by stimulating the contralateral Schaffer collateral axons (indicated in Fig. 5A, also see Fig. S3) before and after IA training (Fig. 5B). The day before training (−1 d) we recorded baseline fEPSP measurements from freely moving mice that were well habituated to the recording box (Fig. 5C). These baseline recordings of synaptic responses were required to remain stable over the 30-min period on the day before (−1 d) and the day of IA training (0 d) to justify continuation of the multi-day in vivo recording paradigm (Fig. 5C). The baseline recordings on 0 d were taken again for 30 min immediately prior to IA training, and we required continued stability from −1 d of the fEPSP slope in both the control and IA groups: p > 0.05, n = 4 for control, n = 9 for IA. Walk-through controls that were exposed to the IA apparatus but not shocked demonstrated no significant change in the slope of the fEPSP of the CA3-CA1 circuit (Fig. 5C, white circles): F (41,123) = 1.165; p > 0.05. IA training significantly increased the slope of the fEPSPs (Fig. 5C, black circles): F (41, 328) = 5.065, p < 0.01. Example fEPSPs are shown (Fig. 5B) at the times indicated in the recording time course (Fig. 5C, 1 and 2). In both groups synaptic transmission returned to baseline levels the following day (1 d): controls, F (17, 51) = 0.50; IA, F(17, 136) = 0.45; both p > 0.05, n = 4, 9.
Fig. 5. IA training enhanced the slope of the in vivo fEPSP of the CA3-CA1 circuit.
(A) Illustration of the post-experimental positioning of stimulating (left) and recording (right) electrodes with white circles for walk-through controls and black circles for IA recordings. All of the illustrated sites indicate successful recordings that produced stable input-output curves on all the recording days and remained within the CA1 (Recording) and Schaffer collateral (Stimulate) pathway. (B) Representative traces from the CA1 taken from mice recorded before (1) and after (2) IA training (black lines) or walk-through controls (unshocked, gray lines). (C) Walk-through control mice that were exposed to the experimental IA chamber but did not receive footshock did not have a significant change in the fEPSP slope (white circles, p > 0.05). Mice trained in the IA paradigm showed a significant increase in the fEPSP slope (p < 0.01). Following testing, the fEPSP slope returned to baseline levels: controls, IA both p > 0.05.
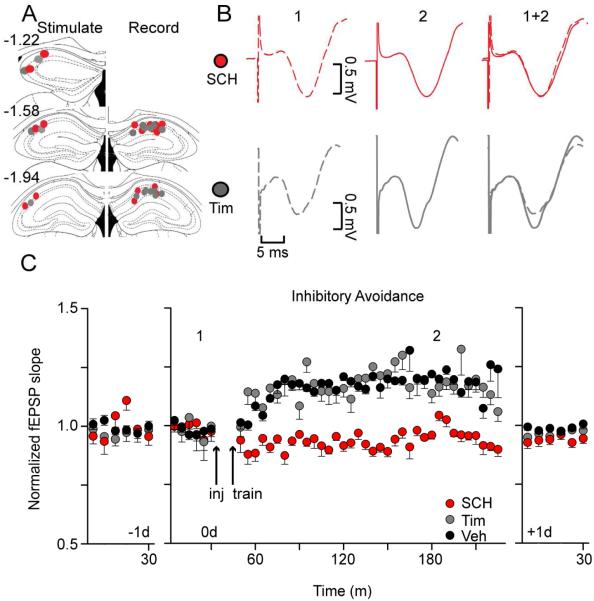
Learning-induced in vivo LTP required activity of D1-like DA receptors
We recorded fEPSPs from the stratum radiatum of the CA1 while the contralateral Schaffer collaterals were stimulated (Fig. 6A). As always, a long-term stable baseline was required on −1 d and 0 d before the injections and before the continuation of recording on 0 d. As in Fig. 5 (without the inhibitors), IA training increased the slope of the fEPSP that persisted after inhibition of β2-adrenergic receptors with Tim (10 mg/kg, i.p., Fig. 6B,C, gray traces and circles): F (41,164) = 1.98; p < 0.01, n = 5. However, following inhibition of D1-like receptors with SCH (0.05 mg/kg, i.p.), IA training did not significantly change the slope of the fEPSP (Fig. 6B,C, red traces and circles): F (41, 246) = 1.35; p > 0.05. As in previous experiments (Fig. 2), SCH significantly impaired footshock memory retention of IA by the implanted mice (Fig. S4, red bar): 61.2 ± 31.0 s approach latency, n = 7, p = 0.12, paired t-test. After Tim injection, implanted mice retained the IA memory (Fig. S4, grey bar): 131.2 ± 20.1 s approach latency, n = 5, p < 0.05, paired t-test. Separate in vivo fEPSP recordings showed that neither drug in the absence of training changed basal synaptic transmission (Fig. S5): F(41, 246) = 1.03, p > 0.05, n = 7 for SCH alone; F (41,164) = 0.94, p > 0.05, n = 5 for Tim alone. These results suggest that DA neurotransmission influences learning and the associated synaptic plasticity along the CA3-CA1 pathway after aversive conditioning.
Fig. 6. D1/D5 receptor antagonist, but not β-adrenergic receptor antagonist, blocked IA training-induced increases in the in vivo fEPSP slope.
(A) Illustration of the post-experimental positioning of stimulating (left) and recording (right) electrodes with grey circles for Tim and red circles for SCH 23390 treated mice. (B) Representative traces from the CA1 taken from mice recorded before (1) and after (2) IA training after injections of either SCH (red traces) or Tim (grey traces). (C) Mice treated with Tim (10 mg/kg, i.p., grey circles) showed a significant increase in the CA1 fEPSP slope: p < 0.01. IA + vehicle treated mice are re-represented from Fig. 5 for comparison (black circles). Mice treated with SCH (0.05 mg/kg, i.p., red circles) showed no change in the fEPSP slope relative to baseline recordings: n = 7, p > 0.05.
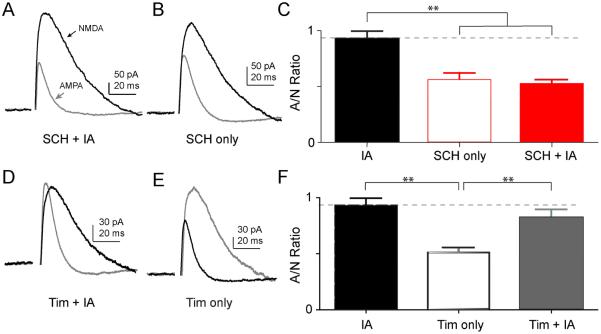
Inhibiting D1-like dopamine receptors prevented IA learning and synaptic plasticity
After demonstrating that the IA learning-induced increase in the AMPA/NMDA ratio were specific in the hippocampus to the CA3-CA1 circuit, we tested the hypothesis that DA D1-like activity is necessary for increases in the AMPA/NMDA ratio. The D1-like receptor inhibitor, SCH, at doses that inhibited learning (0.05 mg/kg, Figs. 3B, Fig S4) also inhibited IA-induced increases in the AMPA/NMDA ratio (Fig. 7A-C). In contrast, the β2-adrenergic receptor antagonist, Tim (10 mg/kg), did not block learning-induced changes in the AMPA/NMDA ratio (Fig. 7D-F). These results are consistent with D1-like receptor activation significantly influencing CA1-CA3 IA-induced synaptic potentiation.
Fig. 7. D1/D5 receptor antagonist, but not β-adrenergic receptor antagonist, blocked the increased AMPA/NMDA current ratio in CA1 pyramidal neurons from mice 1.5 h after training.
Representative traces of AMDA (grey) or NMDA (black) mediated currents after treatment with SCH 23390 + IA (A), SCH only (B), Tim + IA (D), or Tim only (E). (C) Summary of the average data for IA only (black bar), SCH only (open red bar), and SCH + IA (red bar): F (2, 16) = 15.15, p < 0.01, ANOVA; Tukey post hoc multiple comparison test, IA vs. SCH only: 0.93 ± 0.06 vs. 0.55 ± 0.06, p < 0.01; IA vs. SCH + IA: 0.93 ± 0.06 vs. 0.52 ± 0.04, p < 0.01. (F) Summary of the average data for IA only (black bar), Tim only (Tim, open black bar), and Tim + IA (grey bar). TIM treatment did not prevent IA from increasing the AMPA/NMDA ratio: F (2, 18) = 10.62, p < 0.01, ANOVA; Tukey post hoc multiple comparison test, IA vs. TIM only, 0.93 ± 0.06 vs. 0.51 ± 0.04, p < 0.01; TIM + IA vs. TIM only, 0.82 ± 0.07 vs. 0.51 ± 0.04, p < 0.01. The IA data (black bars) were duplicated from Fig. 4C.
Discussion
Fluorescent markers associated with DAT and TH indicated that dopaminergic fibers and terminals project sparsely from the midbrain DA neurons to the dorsal CA1 (Figs. 1, 2). A low dose of a D1-like receptor antagonist did not impair the approach time (latency) for IA training and did not impair short-term memory (Fig. 3C), but did prevent the long-term retention (24 h) of the fear memory (Fig. 3B). Training in the IA task produced significant increases in the AMPA/NMDA ratio of the CA1 synapses (Fig. 4C), and significantly increased the slope of the fEPSP in the CA1 of freely-moving mice (Fig. 5C). D1-like receptor inhibition blocked learning-induced increases in the slope of the fEPSP in the CA3-CA1 circuit (Fig. 6) and blocked the learning-induced enhancement in the AMPA/NMDA ratio in the CA1 (Fig. 7C). Taken together, these results suggest that D1-like receptor activity in the hippocampus contributes to the synaptic plasticity associated with acquisition of contextual memory required for long-term retention of aversive memories.
Anatomical evidence of a direct midbrain dopaminergic projection to the hippocampus
There is known noradrenergic innervation of the hippocampus, and D1-like receptor activity can arise from DA released from noradrenergic innervation. Much of the DA receptor activity may arise from neurons originating in the locus coerulus (Smith and Greene, 2012). Our results also support a sparse direct projection from midbrain DA neurons to the hippocampus as indicated by GFP- and Ruby-Red-synaptophysin staining of DAT-expressing neurons (Figs. 1, 2). Injections were localized to the midbrain and no fluorescence was found in the hypothalamus or from the cell bodies in the locus coeruleus (Fig. 1J), which precludes noradrenergic neurons as the source of the labeling that we observed in the hippocampus. Furthermore, fluorescent labelling of terminals in the CA1 resulting from viral infection of the VTA/SNc region highly co-localized with TH, identifying these terminals as dopaminergic (Fig. 2). These data support previous evidence arising from retrograde tracers injected in the hippocampus that indicated dopaminergic innervation of the hippocampus from the VTA/SNc (Broussard et al., 2012; Gasbarri et al., 1997; Gasbarri et al., 1994b). Our results also confirm recent studies using viral expression of channelrhodopsin in Slc6a3ires-cre/+ mice that labeled relatively sparse dopaminergic axons in the CA1 region (McNamara et al., 2014; Rosen et al., 2015).
Despite the sparse innervation of the hippocampus by midbrain dopaminergic axons, several lines of evidence indicate a significant functional role for this dopaminergic innervation. It was shown that nicotine can cause in vivo hippocampal long-term synaptic plasticity, and this effect required local activation of DA receptors (Tang and Dani, 2009). Inactivation of the VTA with local infusion of TTX inhibited this effect, demonstrating that the required dopamine signal originated, at least in part, from the midbrain (Tang and Dani, 2009). In more recent studies, researchers expressed channelrhodopsin-2 specifically in dopaminergic neurons in the VTA/SNc. Optical stimulation of these dopaminergic fibers in the VTA or locally in the hippocampus in mice exploring novel environments enhanced hippocampal reactivation and improved spatial learning and memory (McNamara et al., 2014). In addition, optogenetic release of dopamine exclusively from the VTA was shown to cause a bidirectional, activity-dependent modulation of Schaffer collateral synapses in hippocampal slices (Rosen et al., 2015). Our data are consistent with those findings, indicating the existence of anatomical connectivity between midbrain dopamine centers and the hippocampus.
Although DA neurons have been traditionally viewed to reinforce rewarding behaviors and/or to signal the expectation of rewards, studies also have indicated that DA can influence the persistence of aversive learning (Moncada et al., 2011; Ortiz et al., 2010; Rossato et al., 2009). A population of ventral VTA neurons located within the paranigral nucleus (PN) has been shown to respond specifically to aversive stimuli within seconds (Brischoux et al., 2009), resulting in increased DA in target areas (Dong et al., 2010; Horvitz, 2000; Kienast et al., 2008). Anatomically, this subpopulation of DA neurons projects to the medial prefrontal cortex and the medial shell of the nucleus accumbens (Lammel et al., 2011; Lammel et al., 2012). Interestingly, in an older retrograde labeling study, it was shown that a population of TH-positive neurons within the PN also connects to the hippocampus (Gasbarri et al., 1994b).
D1-like receptor activity enhanced memory and plasticity in CA3-CA1 synapses
Our data support that the synaptic plasticity associated with aversive learning is dependent upon the temporal coincidence of glutamatergic and dopaminergic neurotransmission. D1-like receptor inhibition after the behavioral training did not prevent remembering the training that occurred just minutes before the D1-like receptor inhibition. The 2-min window after training was sufficient to allow DA to bind to D1 receptors to initiate the mechanisms of LTP and memory retention. Activity initiated by the D1-like receptors continued despite the later arrival of the D1-like antagonist. Thus, synaptic potentiation and learning proceeded owing to the original initiation of these memory-related processes before the antagonist arrived 2-min after the training period.
An interesting concept was that D1-like receptor activity in the CA1 was necessary for retention of long-term, but not short-term IA memory. In the 1-h to 3-h time window, mice still avoided the footshocked side even after D1-like receptors were inhibited during training. Similarly, DA was important for long-term retention at 6 h, but not at 20 min in a different behavioral task (O'Carroll et al., 2006), and a D1-like antagonist applied after training did not affect memory consolidation (Rossato et al., 2009).
Shorter-term memory will likely involve different mechanisms from longer-term memory that is consolidated over time, often requiring periods of sleep. The hippocampal synaptic potentiation indicated in vivo by the fEPSPs or ex vivo by the AMPA/NMDA ratios correlated with the long-term memory tested 24 to 72 h later. In both cases (i.e., the in vivo fEPSP and ex vivo AMPA/NMDA ratio), the indication of LTP was expressed as a relatively global feature of the CA1 area we were studying. That is, different measures that in one case averaged a broad area (in vivo fEPSP) or in the other case picked individual CA1 pyramidal neurons (ex vivo AMPA/NMDA ratio) both indicated the presence of LTP. These results suggest that to retain memory for 24 to 72 h a high percentage of a general area of the CA1 region was contributing: nearly every single CA1 pyramidal neuron we studied had an elevated AMPA/NMDA ratio. Because a large area and high percentage of neurons were contributing, we were able to measure those diverse indications of LTP with our techniques. Even under those successful circumstances, however, the in vivo fEPSP rose slowly over approximately 30 min (see Fig. 5C, black data), suggesting an imperfect match between the learning and presence of LTP as measured by the in vivo fEPSP.
When D1Rs were inhibited, in vivo the global fEPSP did not change during IA training and the ex vivo slices did not show LTP, and the memory was not observed at 24 to 72 h. All of these data to this point are consistent and easily understandable. However, at early times after IA training with D1-like receptors inhibited, short-term memory was observed without global indications of LTP by fEPSPs or AMinPA/NMDA ratios. That result may be because without D1-like activity a high percentage of the dorsal CA1 is not contributing to the memory process needed to retain information for days. However, smaller components of the dorsal hippocampus (or other areas of the hippocampus or brain) could be contributing to the short-term memory seen at 1 to 3 h. Consistent with this speculation, for example, hippocampal dopaminergic activity was shown to influence long-term but not short-term memory retention in appetitive tasks (Bethus et al., 2010). In the present experiments, DA was shown to be important during the encoding phase of a long-term memory in an aversive paradigm. Thus, DA neurotransmission within the CA1 may serve to associate neutral spatial contexts with unexpected unconditioned stimuli. This conclusion is consistent with the hypothesis that DA signals under these circumstances contribute to the updating of the rodent’s internal perception (or map) of environmental saliency (Bethus et al., 2010; McNamara et al., 2014).
Physiological evidence has indicated that dopaminergic neurotransmission within the CA1 influences late phase LTP (L-LTP), a phenomenon that requires protein synthesis and is thought to be a molecular mechanism underlying mnemonic consolidation in CA1 synapses (Frey et al., 1993; Huang et al., 2013; Huang and Kandel, 1995; Luscher and Malenka, 2012). L-LTP is typically produced by stimulating the Schaffer collaterals with high frequency trains that increase the slope of the fEPSP (Frey and Morris, 1997). The D1-like antagonist, SCH, does not block early LTP produced by tetanization, but does block late phase LTP (Swanson-Park et al., 1999). In hippocampal slice recordings it has been shown that weaker stimulation protocols can be enhanced by D1-like receptor activation and blocked by D1-like receptor antagonists or catecholaminergic depletion (Otmakhova and Lisman, 1996; Yang and Dani, 2014). The results support the hypothesis that D1/D5 neurotransmission is important for tagging synapses in the CA3-CA1 circuit for L-LTP (Frey and Morris, 1997; Lisman et al., 2011). In the synaptic tagging and capture hypothesis, learning signals, such as the novel IA context and subsequent association with a footshock, could cause upregulation of plasticity proteins that stabilizes learning-induced synaptic change (Frey and Morris, 1997, 1998). Our data indicate that aversive IA training produces broad synaptic plasticity in the CA3-CA1 synapses that is strongly influenced by D1-like neurotransmission. Thus, DA signaling within the hippocampus serves to consolidate the association of a neutral context with aversive, unconditioned stimuli by contributing to the plasticity of the CA3-CA1 circuitry that likely contributes to memory retention (McHugh et al., 2007; Rumpel et al., 2005).
Conclusions
In summary, DA D1-like receptor activity modulates and regulates hippocampal CA1 synaptic plasticity, associated learning, and long-term memory arising from an aversive contextual task. Although DA signaling from noradrenergic innervation may contribute, dopaminergic innervation of the CA1 arises from the VTA/SNc and β2 adrenergic signaling did not significantly influence memory retention examined in our paradigms. These results support the hypothesis that aversive events recruit subpopulations of DA neurons (Brischoux et al., 2009; Henny et al., 2012; Lammel et al., 2011; Lammel et al., 2014; Lammel et al., 2012) to contribute to learning and memory.
Experimental Procedures
The methodology and reagents for immunohistochemistry, ex vivo electrophysiology, surgery and in vivo electrophysiology, and statistical analysis are described in the Supplemental Experimental Procedures.
Animals
Wild-type C57BL/6J mice 2–4 months old (Jackson Laboratory, Bar Harbor, Maine) had free access to food and water and were housed in accordance with the guidelines specified by the Institutional Animal Care and Use Committee at Baylor College of Medicine or University of Pennsylvania and the Public Health Service Policy on Humane Care and Use of Laboratory Animals. All animals were maintained on a reverse light cycle with the lights off from 6 am to 6pm, and all studies were conducted during the dark phase. For anatomy experiments (Figs. 1, 2), Slc6a3ires-cre/+ mice were obtained from Jackson laboratories (Stock #: 006660, which are commonly referred to as DATires-cre mice).
Inhibitory avoidance behavioral task
Mice were handled in the experimentation room for 3-4 days and habituated to needle injections with saline before the start of the experiments, and all experiments were conducted during the dark cycle. Prior to IA training, all animals were injected i.p. with either saline, SCH 23390, SKF 82197, or Tim. Training consisted of 40 s acclimatizing to the light chamber, and then the trap door to the dark chamber was opened. Once mice walked into the dark chamber, the trap door closed and the mice were given a single footshock (2 s) followed by two minutes within the dark chamber before being returned to their home cages. After IA training, animals were tested for avoidance retention at 1, 3, 24, or 72 h or sacrificed for in vitro neurophysiological assays. IA retention was assayed after training by replacing mice into the light side of the chamber and measuring the latency before the animal returned to the dark context side where the shock had been administered. The foot-shock was not re-administered during the retention assay and measurements were terminated at a ceiling delay interval of 180 s. Statistical analysis was determined using repeated measures ANOVA with the criterion significance set at p < 0.05.
Supplementary Material
HIGHLIGHTS.
Molecular approaches verified dopamine innervation of the hippocampus
Inhibitory avoidance (IA) learning induces ex vivo and in vivo LTP in the CA1
D1-like dopamine receptor inhibition prevents IA induction of LTP
Dopamine activation enhances and inhibition prevents long-term retention of IA
In Brief.
The role of dopamine in signaling rewards is well known, but here Broussard et al. show that dopamine D1-like receptor activity in the hippocampus is necessary for retention of aversive memories. The results indicate dopaminergic innervation and function within the hippocampus underlying long-term synaptic potentiation associated with aversive memory retention.
Acknowledgments
This work was supported by grants from the National Institutes of Health NS21229, DA09411 (JAD); DA021194, MH067119 (FMZ); DA017173 (MDB). We wish to thank Andrea Stout for help with the Metamorph software.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Author Contributions
JIB led the wet lab effort and did the in vivo electrophysiology assisted by DJ. KY did the ex vivo electrophysiology. AL did much of the IA behavior. IG and BA devised and assisted JIB with the vectors and GFP studies. FC did viral injections and aspects of histology and imaging. FZ helped to devised studies on dopamine innervation of the hippocampus. JAD originated, planned, and oversaw the experiments with MDB’s assistance. JAD and JIB did much of the writing. TT performed the TH co-localization studies of Fig. 2 and assisted with the writing.
References
- Agnati LF, Zoli M, Stromberg I, Fuxe K. Intercellular communication in the brain: wiring versus volume transmission. Neuroscience. 1995;69:711–726. doi: 10.1016/0306-4522(95)00308-6. [DOI] [PubMed] [Google Scholar]
- Bayer HM, Glimcher PW. Midbrain dopamine neurons encode a quantitative reward prediction error signal. Neuron. 2005;47:129–141. doi: 10.1016/j.neuron.2005.05.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berridge KC, Robinson TE. What is the role of dopamine in reward: hedonic impact, reward learning, or incentive salience? Brain Res Brain Res Rev. 1998;28:309–369. doi: 10.1016/s0165-0173(98)00019-8. [DOI] [PubMed] [Google Scholar]
- Bethus I, Tse D, Morris RG. Dopamine and memory: modulation of the persistence of memory for novel hippocampal NMDA receptor-dependent paired associates. J Neurosci. 2010;30:1610–1618. doi: 10.1523/JNEUROSCI.2721-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borgkvist A, Malmlof T, Feltmann K, Lindskog M, Schilstrom B. Dopamine in the hippocampus is cleared by the norepinephrine transporter. Int J Neuropsychopharmacol. 2012;15:531–540. doi: 10.1017/S1461145711000812. [DOI] [PubMed] [Google Scholar]
- Brischoux F, Chakraborty S, Brierley DI, Ungless MA. Phasic excitation of dopamine neurons in ventral VTA by noxious stimuli. Proc Natl Acad Sci U S A. 2009;106:4894–4899. doi: 10.1073/pnas.0811507106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bromberg-Martin ES, Matsumoto M, Hikosaka O. Dopamine in motivational control: rewarding, aversive, and alerting. Neuron. 2010;68:815–834. doi: 10.1016/j.neuron.2010.11.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broussard JI, Jenson D, Dani JA. Dopaminergic influence over hippocampal synaptic plasticity and function. Clin Exp Pharmacol. 2012;2:e108. [Google Scholar]
- Budygin EA, Park J, Bass CE, Grinevich VP, Bonin KD, Wightman RM. Aversive stimulus differentially triggers subsecond dopamine release in reward regions. Neuroscience. 2012;201:331–337. doi: 10.1016/j.neuroscience.2011.10.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dayan P, Balleine BW. Reward, motivation, and reinforcement learning. Neuron. 2002;36:285–298. doi: 10.1016/s0896-6273(02)00963-7. [DOI] [PubMed] [Google Scholar]
- Dong Y, Zhang T, Li W, Doyon WM, Dani JA. Route of nicotine administration influences in vivo dopamine neuron activity: habituation, needle injection, and cannula infusion. J Mol Neurosci. 2010;40:164–171. doi: 10.1007/s12031-009-9231-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frey U, Huang YY, Kandel ER. Effects of cAMP simulate a late stage of LTP in hippocampal CA1 neurons. Science. 1993;260:1661–1664. doi: 10.1126/science.8389057. [DOI] [PubMed] [Google Scholar]
- Frey U, Morris RG. Synaptic tagging and long-term potentiation. Nature. 1997;385:533–536. doi: 10.1038/385533a0. [DOI] [PubMed] [Google Scholar]
- Frey U, Morris RG. Synaptic tagging: implications for late maintenance of hippocampal long-term potentiation. Trends Neurosci. 1998;21:181–188. doi: 10.1016/s0166-2236(97)01189-2. [DOI] [PubMed] [Google Scholar]
- Gasbarri A, Packard MG, Campana E, Pacitti C. Anterograde and retrograde tracing of projections from the ventral tegmental area to the hippocampal formation in the rat. Brain Res Bull. 1994a;33:445–452. doi: 10.1016/0361-9230(94)90288-7. [DOI] [PubMed] [Google Scholar]
- Gasbarri A, Packard MG, Sulli A, Pacitti C, Innocenzi R, Perciavalle V. The projections of the retrorubral field A8 to the hippocampal formation in the rat. Exp Brain Res. 1996;112:244–252. doi: 10.1007/BF00227643. [DOI] [PubMed] [Google Scholar]
- Gasbarri A, Sulli A, Packard MG. The dopaminergic mesencephalic projections to the hippocampal formation in the rat. Prog Neuropsychopharmacol Biol Psychiatry. 1997;21:1–22. doi: 10.1016/s0278-5846(96)00157-1. [DOI] [PubMed] [Google Scholar]
- Gasbarri A, Verney C, Innocenzi R, Campana E, Pacitti C. Mesolimbic dopaminergic neurons innervating the hippocampal formation in the rat: a combined retrograde tracing and immunohistochemical study. Brain Res. 1994b;668:71–79. doi: 10.1016/0006-8993(94)90512-6. [DOI] [PubMed] [Google Scholar]
- Grimm D, Lee JS, Wang L, Desai T, Akache B, Storm TA, Kay MA. In vitro and in vivo gene therapy vector evolution via multispecies interbreeding and retargeting of adeno-associated viruses. J Virol. 2008;82:5887–5911. doi: 10.1128/JVI.00254-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henny P, Brown MT, Northrop A, Faunes M, Ungless MA, Magill PJ, Bolam JP. Structural correlates of heterogeneous in vivo activity of midbrain dopaminergic neurons. Nat Neurosci. 2012;15:613–619. doi: 10.1038/nn.3048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hollerman JR, Schultz W. Dopamine neurons report an error in the temporal prediction of reward during learning. Nat Neurosci. 1998;1:304–309. doi: 10.1038/1124. [DOI] [PubMed] [Google Scholar]
- Horvitz JC. Mesolimbocortical and nigrostriatal dopamine responses to salient non-reward events. Neuroscience. 2000;96:651–656. doi: 10.1016/s0306-4522(00)00019-1. [DOI] [PubMed] [Google Scholar]
- Huang W, Zhu PJ, Zhang S, Zhou H, Stoica L, Galiano M, Krnjevic K, Roman G, Costa-Mattioli M. mTORC2 controls actin polymerization required for consolidation of long-term memory. Nat Neurosci. 2013;16:441–448. doi: 10.1038/nn.3351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang YY, Kandel ER. D1/D5 receptor agonists induce a protein synthesis-dependent late potentiation in the CA1 region of the hippocampus. Proc Natl Acad Sci U S A. 1995;92:2446–2450. doi: 10.1073/pnas.92.7.2446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenson D, Yang K, Acevedo-Rodriguez A, Levine A, Broussard JI, Tang J, Dani JA. Dopamine and norepinephrine receptors participate in methylphenidate enhancement of in vivo hippocampal synaptic plasticity. Neuropharmacology. 2015;90:23–32. doi: 10.1016/j.neuropharm.2014.10.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kienast T, Hariri AR, Schlagenhauf F, Wrase J, Sterzer P, Buchholz HG, Smolka MN, Grunder G, Cumming P, Kumakura Y, et al. Dopamine in amygdala gates limbic processing of aversive stimuli in humans. Nat Neurosci. 2008;11:1381–1382. doi: 10.1038/nn.2222. [DOI] [PubMed] [Google Scholar]
- Lammel S, Ion DI, Roeper J, Malenka RC. Projection-specific modulation of dopamine neuron synapses by aversive and rewarding stimuli. Neuron. 2011;70:855–862. doi: 10.1016/j.neuron.2011.03.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lammel S, Lim BK, Malenka RC. Reward and aversion in a heterogeneous midbrain dopamine system. Neuropharmacology. 2014;76(B):351–359. doi: 10.1016/j.neuropharm.2013.03.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lammel S, Lim BK, Ran C, Huang KW, Betley MJ, Tye KM, Deisseroth K, Malenka RC. Input-specific control of reward and aversion in the ventral tegmental area. Nature. 2012;491:212–217. doi: 10.1038/nature11527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lisman J, Grace AA, Duzel E. A neoHebbian framework for episodic memory; role of dopamine-dependent late LTP. Trends Neurosci. 2011;34:536–547. doi: 10.1016/j.tins.2011.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luscher C, Malenka RC. NMDA receptor-dependent long-term potentiation and long-term depression (LTP/LTD) Cold Spring Harb Perspect Biol. 2012;4 doi: 10.1101/cshperspect.a005710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McHugh TJ, Jones MW, Quinn JJ, Balthasar N, Coppari R, Elmquist JK, Lowell BB, Fanselow MS, Wilson MA, Tonegawa S. Dentate gyrus NMDA receptors mediate rapid pattern separation in the hippocampal network. Science. 2007;317:94–99. doi: 10.1126/science.1140263. [DOI] [PubMed] [Google Scholar]
- McNamara CG, Tejero-Cantero A, Trouche S, Campo-Urriza N, Dupret D. Dopaminergic neurons promote hippocampal reactivation and spatial memory persistence. Nat Neurosci. 2014;17:1658–1660. doi: 10.1038/nn.3843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moncada D, Ballarini F, Martinez MC, Frey JU, Viola H. Identification of transmitter systems and learning tag molecules involved in behavioral tagging during memory formation. Proc Natl Acad Sci U S A. 2011;108:12931–12936. doi: 10.1073/pnas.1104495108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montague PR, Hyman SE, Cohen JD. Computational roles for dopamine in behavioural control. Nature. 2004;431:760–767. doi: 10.1038/nature03015. [DOI] [PubMed] [Google Scholar]
- Mu Y, Zhao C, Gage FH. Dopaminergic modulation of cortical inputs during maturation of adult-born dentate granule cells. J Neurosci. 2011;31:4113–4123. doi: 10.1523/JNEUROSCI.4913-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nirenberg MJ, Vaughan RA, Uhl GR, Kuhar MJ, Pickel VM. The dopamine transporter is localized to dendritic and axonal plasma membranes of nigrostriatal dopaminergic neurons. J Neurosci. 1996;16:436–447. doi: 10.1523/JNEUROSCI.16-02-00436.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Carroll CM, Martin SJ, Sandin J, Frenguelli B, Morris RG. Dopaminergic modulation of the persistence of one-trial hippocampus-dependent memory. Learn Mem. 2006;13:760–769. doi: 10.1101/lm.321006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ortiz O, Delgado-Garcia JM, Espadas I, Bahi A, Trullas R, Dreyer JL, Gruart A, Moratalla R. Associative learning and CA3-CA1 synaptic plasticity are impaired in D1R null, Drd1a−/− mice and in hippocampal siRNA silenced Drd1a mice. J Neurosci. 2010;30:12288–12300. doi: 10.1523/JNEUROSCI.2655-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otmakhova NA, Lisman JE. D1/D5 dopamine receptor activation increases the magnitude of early long-term potentiation at CA1 hippocampal synapses. J Neurosci. 1996;16:7478–7486. doi: 10.1523/JNEUROSCI.16-23-07478.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosen ZB, Cheung S, Siegelbaum SA. Midbrain dopamine neurons bidirectionally regulate CA3-CA1 synaptic drive. Nat Neurosci. 2015;18:1763–1771. doi: 10.1038/nn.4152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossato JI, Bevilaqua LR, Izquierdo I, Medina JH, Cammarota M. Dopamine controls persistence of long-term memory storage. Science. 2009;325:1017–1020. doi: 10.1126/science.1172545. [DOI] [PubMed] [Google Scholar]
- Rumpel S, LeDoux J, Zador A, Malinow R. Postsynaptic receptor trafficking underlying a form of associative learning. Science. 2005;308:83–88. doi: 10.1126/science.1103944. [DOI] [PubMed] [Google Scholar]
- Schultz W. Responses of midbrain dopamine neurons to behavioral trigger stimuli in the monkey. J Neurophysiol. 1986;56:1439–1461. doi: 10.1152/jn.1986.56.5.1439. [DOI] [PubMed] [Google Scholar]
- Schultz W. Predictive reward signal of dopamine neurons. J Neurophysiol. 1998;80:1–27. doi: 10.1152/jn.1998.80.1.1. [DOI] [PubMed] [Google Scholar]
- Schultz W, Dayan P, Montague PR. A neural substrate of prediction and reward. Science. 1997;275:1593–1599. doi: 10.1126/science.275.5306.1593. [DOI] [PubMed] [Google Scholar]
- Shimada S, Kitayama S, Lin CL, Patel A, Nanthakumar E, Gregor P, Kuhar M, Uhl G. Cloning and expression of a cocaine-sensitive dopamine transporter complementary DNA. Science. 1991;254:576–578. doi: 10.1126/science.1948034. [DOI] [PubMed] [Google Scholar]
- Shimada S, Kitayama S, Walther D, Uhl G. Dopamine transporter mRNA: dense expression in ventral midbrain neurons. Brain Res Mol Brain Res. 1992;13:359–362. doi: 10.1016/0169-328x(92)90220-6. [DOI] [PubMed] [Google Scholar]
- Smith CC, Greene RW. CNS dopamine transmission mediated by noradrenergic innervation. J Neurosci. 2012;32:6072–6080. doi: 10.1523/JNEUROSCI.6486-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swanson-Park JL, Coussens CM, Mason-Parker SE, Raymond CR, Hargreaves EL, Dragunow M, Cohen AS, Abraham WC. A double dissociation within the hippocampus of dopamine D1/D5 receptor and beta-adrenergic receptor contributions to the persistence of long-term potentiation. Neuroscience. 1999;92:485–497. doi: 10.1016/s0306-4522(99)00010-x. [DOI] [PubMed] [Google Scholar]
- Tang J, Dani JA. Dopamine enables in vivo synaptic plasticity associated with the addictive drug nicotine. Neuron. 2009;63:673–682. doi: 10.1016/j.neuron.2009.07.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsai HC, Zhang F, Adamantidis A, Stuber GD, Bonci A, de Lecea L, Deisseroth K. Phasic firing in dopaminergic neurons is sufficient for behavioral conditioning. Science. 2009;324:1080–1084. doi: 10.1126/science.1168878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ungless MA, Whistler JL, Malenka RC, Bonci A. Single cocaine exposure in vivo induces long-term potentiation in dopamine neurons. Nature. 2001;411:583–587. doi: 10.1038/35079077. [DOI] [PubMed] [Google Scholar]
- Walling SG, Brown RA, Miyasaka N, Yoshihara Y, Harley Selective wheat germ agglutinin (WGA) uptake in the hippocampus from the locus coeruleus of dopamine-beta-hydroxylase-WGA transgenic mice. C.W. Front Behav Neurosci. 2012;6:23. doi: 10.3389/fnbeh.2012.00023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitlock JR, Heynen AJ, Shuler MG, Bear MF. Learning induces long-term potentiation in the hippocampus. Science. 2006;313:1093–1097. doi: 10.1126/science.1128134. [DOI] [PubMed] [Google Scholar]
- Yang K, Dani JA. Dopamine D1 and D5 receptors modulate spike-timing-dependent plasticity at medial perforant path to dentate granule cell synapses. J Neurosci. 2014 doi: 10.1523/JNEUROSCI.2400-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang TA, Tang J, Pidoplichko VI, Dani JA. Addictive nicotine alters local circuit inhibition during the induction of in vivo hippocampal synaptic potentiation. J Neurosci. 2010;30:6443–6453. doi: 10.1523/JNEUROSCI.0458-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zweifel LS, Fadok JP, Argilli E, Garelick MG, Jones GL, Dickerson TM, Allen JM, Mizumori SJ, Bonci A, Palmiter RD. Activation of dopamine neurons is critical for aversive conditioning and prevention of generalized anxiety. Nat Neurosci. 2011;14:620–626. doi: 10.1038/nn.2808. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.