Abstract
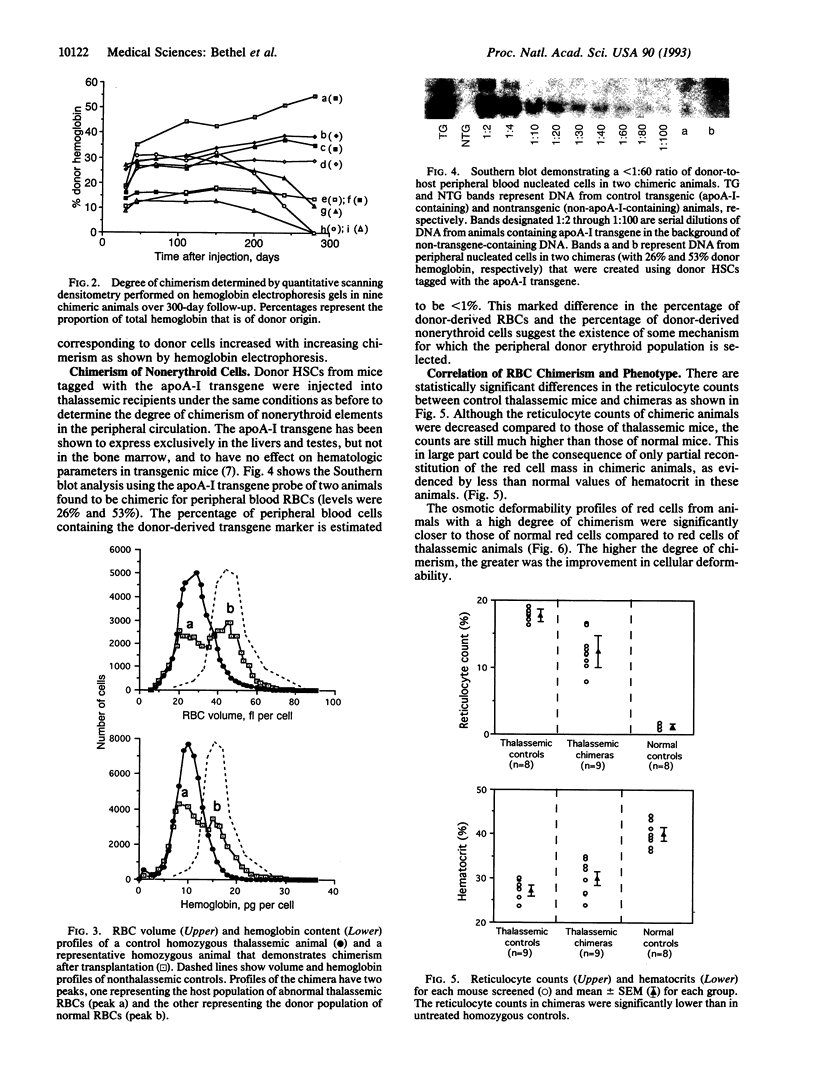
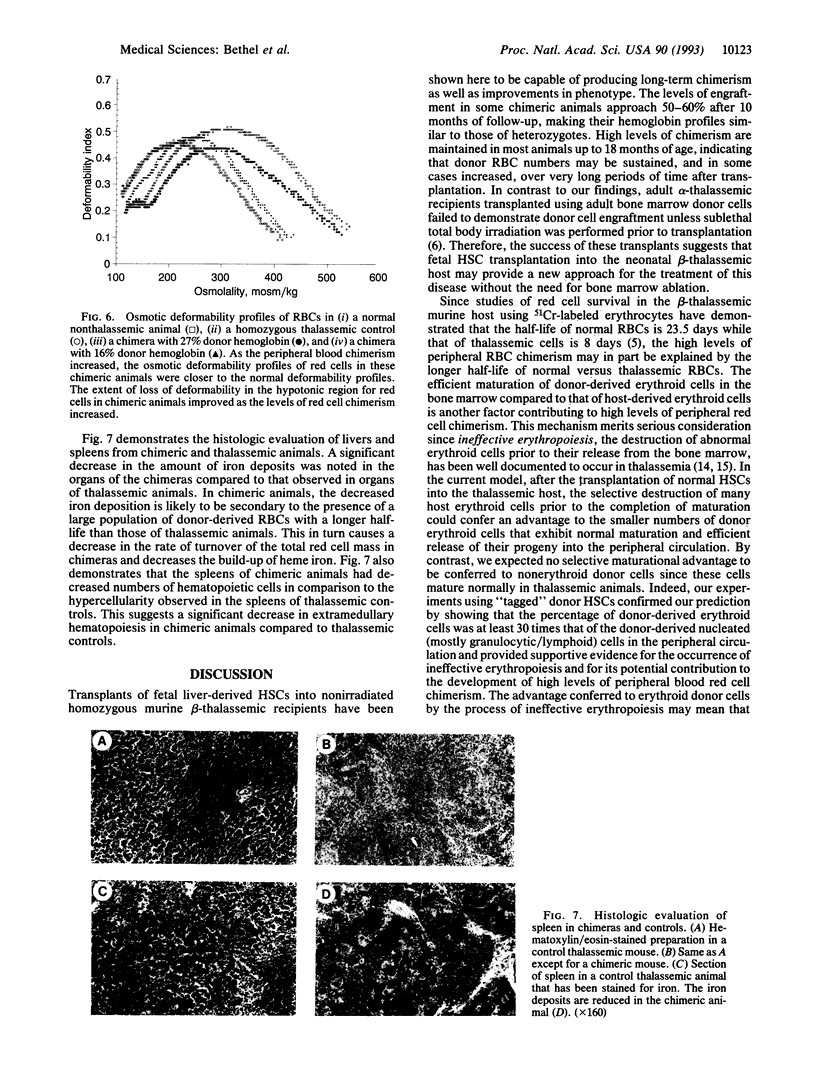
We have explored the application of fetal hematopoietic stem cell (HSC) transplants for cellular replacement in a murine model of beta-thalassemia. Liver-derived HSCs from nonthalassemic syngeneic murine fetal donors were transplanted into nonirradiated neonatal beta-thalassemic recipients. Significant erythrocyte chimerism (9-27%) was demonstrated in the majority of recipients at 1 month and remained stable or increased (up to 55%) during long-term follow-up in almost all cases. Chimeras had improved phenotypes, as evidenced by decreased reticulocyte counts, increased mean erythrocyte deformability, and decreased iron deposits in comparison to controls. To investigate whether the high degree of peripheral blood chimerism was predominantly a feature of erythroid elements or was a general feature of all hematopoietic elements, chimeras were created using donor HSCs "tagged" with a DNA transgene. Whereas donor hemoglobin comprised > 30% of total hemoglobin, nucleated tagged nonerythroid donor cells comprised < 1% of peripheral blood elements. Explanations for the observed selective increase in erythroid chimerism include longer survival of normal donor red cells compared to that of thalassemic red cells and the effective maturation of the donor erythroid elements in the bone marrow in chimeric animals. The latter explanation bears consideration because it is consistent with the process of ineffective erythropoiesis, well documented to occur in thalassemia, in which the majority of thalassemic erythroid cells are destroyed during erythropoiesis prior to release from the bone marrow. Overall, these data demonstrate the potential for significant erythroid chimerism and suggest that fetal HSC transplantation may play a significant role in future treatment.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BRECHER G., SCHNEIDERMAN M. A time-saving device for the counting of reticulocytes. Am J Clin Pathol. 1950 Nov;20(11):1079–1083. doi: 10.1093/ajcp/20.11_ts.1079. [DOI] [PubMed] [Google Scholar]
- Finch C. A., Deubelbeiss K., Cook J. D., Eschbach J. W., Harker L. A., Funk D. D., Marsaglia G., Hillman R. S., Slichter S., Adamson J. W. Ferrokinetics in man. Medicine (Baltimore) 1970 Jan;49(1):17–53. doi: 10.1097/00005792-197001000-00002. [DOI] [PubMed] [Google Scholar]
- Fleischman R. A., Mintz B. Prevention of genetic anemias in mice by microinjection of normal hematopoietic stem cells into the fetal placenta. Proc Natl Acad Sci U S A. 1979 Nov;76(11):5736–5740. doi: 10.1073/pnas.76.11.5736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fliedner T. M., Steinbach K. H. Repopulating potential of hematopoietic precursor cells. Blood Cells. 1988;14(2-3):393–410. [PubMed] [Google Scholar]
- Garrick L. M., Strano-Paul L. A., Hoke J. E., Kirdani-Ryan L. A., Alberico R. A., Everett M. M., Bannerman R. M., Garrick M. D. Tissue iron deposition in untransfused beta-thalassemic mice. Exp Hematol. 1989 Jun;17(5):423–428. [PubMed] [Google Scholar]
- Gōmōri G. Microtechnical Demonstration of Iron: A Criticism of its Methods. Am J Pathol. 1936 Sep;12(5):655–664.1. [PMC free article] [PubMed] [Google Scholar]
- Knyszynski A., Danon D., Kahane I., Rachmilewitz E. A. Phagocytosis of nucleated and mature beta thalassaemic red blood cells by mouse macrophages in vitro. Br J Haematol. 1979 Oct;43(2):251–255. doi: 10.1111/j.1365-2141.1979.tb03748.x. [DOI] [PubMed] [Google Scholar]
- Micklem H. S., Lennon J. E., Ansell J. D., Gray R. A. Numbers and dispersion of repopulating hematopoietic cell clones in radiation chimeras as functions of injected cell dose. Exp Hematol. 1987 Mar;15(3):251–257. [PubMed] [Google Scholar]
- Mohandas N., Clark M. R., Jacobs M. S., Shohet S. B. Analysis of factors regulating erythrocyte deformability. J Clin Invest. 1980 Sep;66(3):563–573. doi: 10.1172/JCI109888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neben S., Redfearn W. J., Parra M., Brecher G., Pallavicini M. G. Short- and long-term repopulation of lethally irradiated mice by bone marrow stem cells enriched on the basis of light scatter and Hoechst 33342 fluorescence. Exp Hematol. 1991 Oct;19(9):958–967. [PubMed] [Google Scholar]
- Pantely G. A., Swenson L. J., Tamblyn C. H., Seaman G. V., Anselone C. G., Johnson W. B., Bristow J. D. Increased vascular resistance due to a reduction in red cell deformability in the isolated hind limb of swine. Microvasc Res. 1988 Jan;35(1):86–100. doi: 10.1016/0026-2862(88)90052-0. [DOI] [PubMed] [Google Scholar]
- Popp R. A., Popp D. M., Johnson F. M., Skow L. C., Lewis S. E. Hematology of a murine beta-thalassemia: a longitudinal study. Ann N Y Acad Sci. 1985;445:432–444. doi: 10.1111/j.1749-6632.1985.tb17213.x. [DOI] [PubMed] [Google Scholar]
- Rubin E. M., Ishida B. Y., Clift S. M., Krauss R. M. Expression of human apolipoprotein A-I in transgenic mice results in reduced plasma levels of murine apolipoprotein A-I and the appearance of two new high density lipoprotein size subclasses. Proc Natl Acad Sci U S A. 1991 Jan 15;88(2):434–438. doi: 10.1073/pnas.88.2.434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Touraine J. L. In-utero transplantation of fetal liver stem cells into human fetuses. Hum Reprod. 1992 Jan;7(1):44–48. doi: 10.1093/oxfordjournals.humrep.a137554. [DOI] [PubMed] [Google Scholar]





