Abstract
Objectives To examine the association between dipeptidyl peptidase-4 (DPP-4) inhibitors and the risk of heart failure or hospital admission for heart failure in patients with type 2 diabetes.
Design Systematic review and meta-analysis of randomised and observational studies.
Data sources Medline, Embase, Cochrane Central Register of Controlled Trials, and ClinicalTrials.gov searched up to 25 June 2015, and communication with experts.
Eligibility criteria Randomised controlled trials, non-randomised controlled trials, cohort studies, and case-control studies that compared DPP-4 inhibitors against placebo, lifestyle modification, or active antidiabetic drugs in adults with type 2 diabetes, and explicitly reported the outcome of heart failure or hospital admission for heart failure.
Data collection and analysis Teams of paired reviewers independently screened for eligible studies, assessed risk of bias, and extracted data using standardised, pilot tested forms. Data from trials and observational studies were pooled separately; quality of evidence was assessed by the GRADE approach.
Results Eligible studies included 43 trials (n=68 775) and 12 observational studies (nine cohort studies, three nested case-control studies; n=1 777 358). Pooling of 38 trials reporting heart failure provided low quality evidence for a possible similar risk of heart failure between DPP-4 inhibitor use versus control (42/15 701 v 33/12 591; odds ratio 0.97 (95% confidence interval 0.61 to 1.56); risk difference 2 fewer (19 fewer to 28 more) events per 1000 patients with type 2 diabetes over five years). The observational studies provided effect estimates generally consistent with trial findings, but with very low quality evidence. Pooling of the five trials reporting admission for heart failure provided moderate quality evidence for an increased risk in patients treated with DPP-4 inhibitors versus control (622/18 554 v 552/18 474; 1.13 (1.00 to 1.26); 8 more (0 more to 16 more)). The pooling of adjusted estimates from observational studies similarly suggested (with very low quality evidence) a possible increased risk of admission for heart failure (adjusted odds ratio 1.41, 95% confidence interval 0.95 to 2.09) in patients treated with DPP-4 inhibitors (exclusively sitagliptin) versus no use.
Conclusions The relative effect of DPP-4 inhibitors on the risk of heart failure in patients with type 2 diabetes is uncertain, given the relatively short follow-up and low quality of evidence. Both randomised controlled trials and observational studies, however, suggest that these drugs may increase the risk of hospital admission for heart failure in those patients with existing cardiovascular diseases or multiple risk factors for vascular diseases, compared with no use.
Introduction
Of over 380 million people with diabetes worldwide, most (85-95%) have type 2 diabetes.1 Dipeptidyl peptidase-4 (DPP-4) inhibitors are a relatively new class of incretin based agents for treating type 2 diabetes. Evidence from randomised controlled trials has established that DPP-4 inhibitors reduce levels of glycated haemoglobin (HbA1c),2 3 do not affect body weight,2 pose a low risk of hypoglycaemia,4 and do not increase the risk of cardiovascular events.5 6 7 The American Diabetes Association and European Association for the Study of Diabetes have recommended this drug class as second line agents for type 2 diabetes management.8
A recent major trial9 (SAVOR-TIMI 53) reported an increased risk of admission to hospital for heart failure (hazard ratio 1.27, 95% confidence interval 1.07 to 1.51) with the DPP-4 inhibitor saxagliptin. Although unexpected, the finding raised concern among professionals and health authorities. In 2014, the US Food and Drug Administration (FDA) requested the clinical trial data from the manufacturer to investigate the potential association between use of saxagliptin and heart failure. The FDA then recommended that “Patients should not stop taking saxagliptin and should speak with their health care professionals about any questions or concerns. Health care professionals should continue to follow the prescribing recommendations in the drug labels.”10
Subsequently, the EXAMINE trial11 testing alogliptin, and the TECOS trial12 testing sitagliptin, reported no significant effect on hospital admission for heart failure. Evidence from observational studies has been inconsistent,13 14 15 16 17 and the effect of DPP-4 inhibitors on heart failure remains controversial.
A systematic review of trials and observational studies offers an opportunity to consider the total body of evidence and potentially resolve the concern. We therefore undertook a systematic review to assess the extent to which DPP-4 inhibitors affect the risk of heart failure or hospital admission for heart failure in patients with type 2 diabetes.
Methods
We followed the standards set by the meta-analysis of observational studies in epidemiology (MOOSE)18 and preferred reporting items for systematic reviews and meta-analyses (PRISMA)19 for the reporting of our study.
Eligibility criteria
We included randomised controlled trials, non-randomised controlled trials, cohort studies, and case-control studies that compared DPP-4 inhibitors against placebo, lifestyle modification, or active antidiabetic drugs in adults with type 2 diabetes. We required follow-up for at least 12 weeks (not applicable to case-control studies), and explicit reporting of the outcome of heart failure or hospital admission for heart failure (either as raw data or adjusted effect estimates with 95% confidence intervals). We classified study designs according to recommendations by the Cochrane Non-Randomised Studies Methods Group. Trials, particularly phase III studies, reported heart failure either as a normal adverse event or a serious adverse event. For serious adverse events, admission for heart failure may have been included. We defined heart failure reported in such trials as an unspecified outcome.
Literature search
We searched Medline, Embase, and the Cochrane Central Register of Controlled Trials (CENTRAL) from inception to 25 June 2015. We combined both MeSH and free text terms for identifying relevant articles. An information expert (DP) developed our search strategies (web appendix 1).
We also searched ClinicalTrials.gov to identify additional eligible studies. Section 801 of the US Food and Drug Administration Amendments Act (FDAAA 801) requires responsible parties to submit summary results of clinical trials, including serious adverse events and adverse events with frequency over 5%, to this trial registry.20 21 In doing so, important information regarding heart failure can be collected. We used generic names of each drug to identify relevant studies, and limited our search to studies labelled as “completed” or “terminated,” in which summary results were available.
We also contacted content experts and industry representatives, and searched for conference abstracts on the American Diabetes Association and European Association for the Study of Diabetes, for additional information.
Study process
Teams of two paired reviewers, trained in health research methods, independently screened titles, abstracts, and full texts for eligibility; assessed risk of bias; and collected data from each eligible study, using standardised, pilot tested forms, together with detailed instructions. Reviewers resolved disagreement through discussion or, if required, by adjudication by a third reviewer (XS).
Risk of bias assessment
We used the Cochrane Collaboration’s tool22 to assess the risk of bias of randomised controlled trials. The items included random sequence generation, allocation concealment, blinding of participants, caregivers, and assessors of outcomes (that is, heart failure or hospital admission for heart failure), and adjudication of the outcomes. By assessing the risk of bias associated with blinding of patients, caregivers, and outcome assessors, we modified the instrument by removing the “unclear” option, an approach that we have previously shown to be reliable and valid.23
We used the Newcastle-Ottawa quality assessment scale24 to assess the risk of bias of cohort studies and case-control studies. We removed the items “representativeness of the exposed cohort” and “was follow-up long enough for outcomes to occur” for cohort studies and the item “representativeness of the cases” for case-control studies because these items relate to applicability of results. We, however, added two items: the ascertainment of type 2 diabetes and the ascertainment of potential confounding factors for these both types of studies, because misclassification could result from suboptimal approaches to these issues. We planned to assess publication bias but were unable to do so owing to very low event rates.
Data collection
We collected the following information from each eligible randomised controlled trial:
• General study characteristics: author name, year of publication, total number of patients randomised, number of treatment groups, length of follow-up, study phase, funding source, trial registry number, countries involved, and number of study sites
• Patient characteristics: sex, age, diabetes duration, body mass index, baseline HbA1c level, and fasting plasma glucose values
• Interventions: medications common to all groups (baseline treatment), details of DPP-4 inhibitors treatment and control group (eg, drug generic name, and duration of treatment)
• Outcomes: the definition of heart failure, number of events, and patients included for analyses in each group, as well as adjusted data if available.
For each trial, if the initial treatment assignment was switched (eg, patients in placebo group started receiving DPP-4 inhibitors after 24 weeks), we collected the outcome data before that point. If a trial had multiple reports, we collated all data into one study.25 If a trial had both reports from ClincialTrials.gov and journal publications, we carefully checked data from these two sources for consistency. If outcome data were reported at multiple follow-up points, we used data from the longest follow-up.
For observational studies, we collected data similar to randomised controlled trials (eg, total number of patients, sex, age, diabetes duration, body mass index, baseline HbA1c). We documented, for each observational study, the definition of outcomes and sources of data for the outcomes. In addition, we documented information on:
• Study design (eg, retrospective or prospective cohort study)
• Data source (eg, claims data, electronic medical records)
• Methods used to ascertain type 2 diabetes status (eg, International Classification of Diseases (ICD) code)
• Exposures (eg, DPP-4 inhibitors, and other exposure variables)
• Methods used to control confounding (eg, logistic or cox regression, and control variables).
We collected adjusted estimates and their associated 95% confidence intervals, as well as adjustment factors, in addition to raw event data and exposure time.
Data analysis
We conducted separate analyses for randomised controlled trials and observational studies. We also separately analysed the data on heart failure and hospital admission for heart failure, because those two outcomes, although sharing the same clinical and pathophysiological features, represent differential seriousness of the effect of DPP-4 inhibitors treatment on patients and society. Heart failure could be subclinical and might not be diagnosed; admission for heart failure is, however, always a clinical event and a condition important to patients and clinicians. We considered admission for heart failure as the more important outcome for patients.
For the analysis of trials, we pooled outcome data using Peto’s methods because of very low event rates,26 27 and reported pooled Peto odds ratios and associated 95% confidence intervals. We examined heterogeneity among studies with the Cochrane χ2 test and the I2 statistic. We explored sources of heterogeneity with four prespecified subgroup hypotheses:
• Type of control (placebo v active treatment; larger effect in trials with placebo control)
• Length of follow-up (≤52 v >52 weeks; larger effect in those with longer follow-up)
• Mode of treatment (monotherapy v add-on or combination therapy; larger effect in those with add on or combination therapy)
• Individual DPP-4 inhibitors (different DPP-4 inhibitors v control).
We carried out sensitivity analyses by using alternative effect measures (odds ratios v risk ratios), pooling methods (Peto v Mantel-Hanszel method), and statistical models regarding heterogeneity (random v fixed effects).
In the analysis of observational studies, we qualitatively summarised the data for heart failure, because of the substantial variations in the comparison (that is, type of control) and patient populations in those studies. We pooled adjusted estimates of hospital admission for heart failure from cohort and nested case-control studies using a random effects model. Although the effect measures differ for those two designs (hazard ratios for cohort studies and odds ratios for nested case-control studies), they are relative measures and the effect estimates are close when the event rate is low (<5%).
Quality of evidence
We used the grading of recommendations assessment, development, and evaluation (GRADE) methodology to rate quality of the evidence for heart failure and hospital admission for heart failure as high, moderate, low, or very low.28 Randomised controlled trials begin as high quality evidence, but can be rated down because of risk of bias,29 imprecision,30 inconsistency,31 indirectness,32 and publication bias.33 Observational studies begin as low quality evidence, but can be rated up for a large magnitude of effect, a dose-response gradient, or presence of plausible confounders or other biases that increase confidence in the estimated effect.34
Patient involvement
No patients were involved in setting the research question or the outcome measures, nor were they involved in developing plans for design or implementation of the study. No patients were asked to advise on interpretation or writing up of results. There are no plans to disseminate the results of the research to study participants or the relevant patient community.
Results
Of 11 440 potentially relevant reports identified, after title and abstract screening, 820 reports proved potentially eligible. On full text screening, 55 studies proved eligible, including 43 randomised controlled trials, representing 68 775 patients, reported in 77 reports9 11 12 35 36 37 38 39 40 41 42 43 44 45 46 47 48 49 50 51 52 53 54 55 56 57 58 59 60 61 62 63 64 65 66 67 68 69 70 71 72 73 74 75 76 77 78 79 80 81 82 83 84 85 86 87 88 89 90 91 92 93 94 95 96 97 98 99 100 101 102 103 104 105 106 107 108 (45 from journal reports, 31 from the ClinicalTrials.gov website, and one conference abstract) and 12 observational studies,13 14 15 16 17 109 110 111 112 113 114 115 involving 1 777 358 patients, reported in nine cohort studies and three nested case-control studies (nine from journal reports, one from a trial registry, and two conference abstracts; fig 1). Two cohort studies15 116 analysed patient data from the same claims database, one presenting a subpopulation of the other; we included only the larger cohort study.15
Fig 1 Flowchart of article selection
Evidence from randomised controlled trials
Trials reporting heart failure
Of the 43 randomised controlled trials, 38 reported heart failure, of which 33 (87%) were international studies, and 35 (92%) were clearly labelled as phase III trials. These 38 trials enrolled 109-2789 patients (total n=31 680; mean age range 49.7-72.6 years, mean body mass index 24.0-32.8, mean baseline HbA1c 7.1-9.9%, mean fasting plasma glucose 7.7-11.1 mmol/L, and mean duration of diabetes 1.7-17.5 years; table 1). Nine trials used DPP-4 inhibitors as monotherapy, 27 as add-on or combination therapy, and two as both monotherapy and combination therapy. Length of follow-up ranged from 12 to 206 weeks (median 52; table 2).
Table 1.
Characteristics of included randomised controlled trials
| Author (year) | International study | No of countries involved | No of study sites | Study phase | Total No of patients randomised | Length of follow-up (weeks) | Male patients (No, %) | Mean age (years) | Mean body mass index | Mean HbA1c (%) | Mean FPG (mmol/L) | Mean diabetes duration (years) |
| Trials reporting heart failure | ||||||||||||
| Arjona Ferreira (2013)a35, 36 | Yes | NR | NR | III | 426 | 54 | 158 (57) | 64.5 | 26.8 | 7.8 | 8.1 | 10.4 |
| Arjona Ferreira (2013)b37, 38 | Yes | 12 | 31 | III | 129 | 54 | 77 (59.7) | 59.5 | 26.8 | 7.8 | 9.0 | 17.5* |
| Bosi (2011)39 | Yes | NR | NR | III | 803 | 52 | 414 (51.6) | 55.1 | 31.5 | 8.2 | 9.0 | 7.2 |
| Ferrannini (2009)40 | Yes | 24 | 402 | III | 2789 | 52 | 1490 (53.4) | 57.5 | 31.8 | 7.3 | 9.2 | 5.7 |
| Fonseca (2013)41 | Yes | 12 | 58 | III | 313 | 26 | 195 (62.3) | 56.0 | 29.9 | 9.8 | 9.8 | NR |
| Garber (2007)42 | Yes | 2 | 123 | III | 463 | 24 | 199 (50) | 54.0 | 32.4 | 8.7 | 10.1 | 4.7 |
| Henry (2014)43, 44 | NR | NR | NR | III | 1615 | 54 | 912 (56.5) | NR | 30.9 | 8.8 | 10.0 | 7.9 |
| Iwamoto (2010)45, 46 | Yes | 1 | 97 | II | 363 | 12 | 224 (61.7) | 59.8 | 24.5 | 7.6 | 8.2 | 5.4 |
| NCT00094770 (2009)47, 48, 49 | Yes | NR | 173 | III | 1172 | 104 | 694 (59.2) | 56.7 | 31.2 | 7.7 | 9.2 | 6.4 |
| NCT00103857 (2009)50, 51 | Yes | NR | 140 | III | 1091 | 104 | 539 (49.4) | 53.5 | NR | 8.8 | 11.1 | NR |
| NCT00121641 (2011)52, 53 | NR | 6 | 135 | III | 403 | 206 | 204 (50.9) | 53.5 | 31.7 | 7.9 | 9.7 | 2.6 |
| NCT00121667 (2011)54, 55 | Yes | 9 | 154 | III | 745 | 206 | 377 (50.7) | 54.6 | 31.4 | NR | NR | NR |
| NCT00286442 (2011)56, 57 | Yes | 15 | 115 | III | 527 | 26 | 265 (50.3) | 54.8 | 32.0 | 7.9 | 9.5 | 6.0 |
| NCT00286468 (2011)58, 59 | Yes | 15 | 125 | III | 585 | 26 | 261 (52.2) | 56.6 | 30.1 | NR | NR | 7.7 |
| NCT00295633 (2009)60, 61, 62 | Yes | 8 | 133 | III | 565 | 76 | 643 (49.2) | 52.0 | 30.2 | 9.5 | 11.1 | 1.7 |
| NCT00327015 (2009)63, 64, 65 | Yes | 13 | 211 | III | 1309 | 24 | 643 (49.2) | 52.0 | 30.2 | 9.5 | 11.1 | 1.7 |
| NCT00395343 (2009)66, 67 | Yes | 24 | 100 | III | 641 | 24 | 326 (50.9) | 57.8 | 31.0 | 8.7 | 9.8 | 12.5 |
| NCT00482729 (2009)68, 69, 70 | Yes | 2 | 229 | III | 1250 | 44 | 708 (56.8) | 49.7 | NR | 9.9 | NR | NR |
| NCT00575588 (2010)71, 72, 73 | Yes | 11 | 130 | III | 858 | 104 | 444 (51.7) | 57.5 | 31.4 | 7.7 | 9.0 | 5.4 |
| NCT00614939 (2010)74, 75, 76 | Yes | 13 | 75 | III | 170 | 52 | 73 (42.9) | 66.5 | 30.7 | 8.3 | 9.9 | 16.7 |
| NCT00622284 (2011)77, 78 | Yes | 16 | 209 | III | 1560 | 104 | 933 (60.2) | 56.6 | 30.2 | 7.7 | 9.1 | 715 (47.1)† |
| NCT00642278 (2013)79, 80 | Yes | 13 | 85 | II | 451 | 12 | 236 (52.3) | 52.9 | 31.5 | 7.8 | 9.0 | NR |
| NCT00707993 (2013)81, 82 | Yes | 15 | 110 | III | 441 | 54 | 198 (44.9) | 69.9 | 29.8 | 7.5 | 8.1 | 6.1 |
| NCT00757588 (2011)83, 84 | Yes | 10 | 72 | III | 457 | 24 | 188 (41.3) | 57.2 | 32.3 | 8.7 | 9.6 | 11.9 |
| NCT00798161 (2011)85, 86 | Yes | 14 | 133 | III | 791 | 24 | 426 (53.9) | 55.3 | 29.1 | 8.7 | 10.9 | 562 (74.3)† |
| NCT00838903 (2014)87, 88 | Yes | 10 | 289 | III | 1049 | 164 | 482 (47.6) | 54.5 | 32.6 | 8.1 | 9.2 | 6.0 |
| NCT00856284 (2013)89, 90 | Yes | 32 | 310 | III | 2639 | 104 | 1312 (49.7) | 55.4 | 31.2 | 7.6 | NR | 5.5 |
| NCT00954447 (2012)91 | Yes | 19 | 167 | III | 1263 | 52 | 658 (52.2) | 60.0 | 31.0 | 8.3 | 8.3 | NR |
| NCT01006603 (2013)92 | Yes | 13 | 152 | IV | 720 | 52 | 445 (61.8) | 72.6 | NR | NR | NR | NR |
| NCT01189890 (2013)93 | Yes | NR | NR | III | 480 | 30 | 202 (42.1) | 70.7 | NR | 7.8 | 9.4 | NR |
| NCT01263483 (2011)94, 95 | No | 1 | 31 | II and III | 230 | 12 | 142 (61.7) | 62.1 | 24.0 | 8.0 | NR | 7.8 |
| NCT01289990 (2014)96 | Yes | 19 | 243 | III | 2700 | 76 | 1492 (55.3) | 55.6 | NR | NR | NR | NR |
| Pratley (2009)97, 98 | Yes | 14 | 125 | III | 493 | 26 | 287 (58.2) | 55.4 | 32.8 | 8.0 | NR | 7.6 |
| Pratley (2014)99 | Yes | 13 | 198 | III | 784 | 26 | 374 (47.7) | 53.5 | 30.7 | NR | NR | 4.0 |
| Rosenstock (2006)100 | Yes | 17 | NR | III | 353 | 24 | 196 (55.5) | 56.3 | 31.5 | 8.0 | 9.2 | NR |
| Rosenstock (2010)101 | Yes | 23 | 268 | III | 655 | 26 | 320 (48.9) | 52.6 | 31.1 | 8.8 | 10.6 | 3.2 |
| Seino (2012)102 | No | 1 | 30 | III | 288 | 12 | 198 (68.8) | 52.6 | 25.9 | 8.0 | NR | 6.3 |
| Yang (2015)103 | No | 1 | 25 | III | 109 | 24 | 57 (54.3) | 56.2 | 25.0 | 7.1 | 7.7 | 3.6 |
| Trials reporting hospital admission for heart failure | ||||||||||||
| Green (2015) (TECOS)12 | Yes | 38 | 673 | III | 14 735 | 156‡ | 10 374 (70.7) | 65.5 | 30.2 | 7.2 | NR | 11.6 |
| Krum (2014) (VIVIDD)104 | NR | NR | NR | NR | 253 | 52 | NR | 63 | NR | 7.8 | NR | NR |
| Laakso (2015)105 | Yes | 9 | 52 | III | 235 | 52 | 149 (63.4) | 66.6 | NR | 8.1 | NR | NR |
| Scirica (2013) (SAVOR-TIMI 53)9, 106 | Yes | 26 | 788 | IV | 16 492 | 109‡ | 11 037 (66.9) | 65.0 | 31.1 | NR | 8.7 | 10.3* |
| Zannad (2015) (EXAMINE)11, 107, 108 | Yes | 49 | 898 | III | 5380 | 76‡ | 3651 (67.9) | 60.9 | 29.5 | NR | NR | 9.2 |
FPG=fasting plasma glucose; NR=not reported.
*Median diabetes duration (years).
†No (%) of patients with no more than five years’ duration.
‡Median follow-up time (weeks).
Table 2.
Interventions tested and event rates in randomised controlled trials
| Author (year) | Drug treatments used across groups | DPP-4 inhibitors | Control | Duration of treatment (weeks) | |||
| Type | Events/analysed patients (No) | Type | Events/analysed patients (No) | ||||
| Trials reporting heart failure | |||||||
| Arjona Ferreira (2013)a35, 36 | None | Sitagliptin | 0/210 | Glipizide | 4/212 | 54 | |
| Arjona Ferreira (2013)b37, 38 | None | Sitagliptin | 2/64 | Glipizide | 2/65 | 54 | |
| Bosi (2011)39 | Metformin, and pioglitazone 30 mg | Alogliptin | 2/404 | Add-on pioglitazone 15 mg | 1/399 | 52 | |
| Ferrannini (2009)40 | Metformin | Vildagliptin | 2/1389 | Glimepiride | 2/1383 | 52 | |
| Fonseca (2013)41 | Metformin and pioglitazone | Sitagliptin | 0/157 | Placebo | 0/156 | 26 | |
| Garber (2007)42 | Pioglitazone | Vildagliptin | 1/304 | Placebo | 1/158 | 24 | |
| Henry (2014)43, 44 | Pioglitazone | Sitagliptin | 2/691 | No additional drugs | 0/693 | 54 | |
| Iwamoto (2010)45, 46 | None | Sitagliptin | 1/290 | Placebo | 0/73 | 12 | |
| NCT00094770 (2009)47, 48, 49 | Metformin | Sitagliptin | 2/588 | Glipizide | 1/584 | 104 | |
| NCT00103857 (2009)50, 51 | Metformin | Sitagliptin | 1/372 | No additional drugs | 0/364 | 104 | |
| NCT00121641 (2011)52, 53 | None | Saxagliptin | 1/306 | Placebo | 0/95 | 206 | |
| NCT00121667 (2011)54, 55 | Metformin | Saxagliptin | 3/564 | Placebo | 2/179 | 206 | |
| NCT00286442 (2011)56, 57 | Metformin | Alogliptin | 1/423 | Placebo | 0/104 | 26 | |
| NCT00286468 (2011)58, 59 | Glyburide | Alogliptin | 1/401 | Placebo | 0/99 | 26 | |
| NCT00295633 (2009)60, 61, 62 | TZD | Saxagliptin | 0/381 | Placebo | 1/184 | 76 | |
| NCT00327015 (2009)63, 64, 65 | Metformin | Saxagliptin | 0/643 | No additional drugs | 2/328 | 24 | |
| NCT00395343 (2009)66, 67 | Insulin with or without metformin | Sitagliptin | 0/322 | Placebo | 2/319 | 24 | |
| NCT00482729 (2009)68, 69, 70 | Metformin | Sitagliptin | 1/625 | No additional drugs | 0/621 | 44 | |
| NCT00575588 (2010)71, 72, 73 | Metformin | Saxagliptin | 1/428 | Glipizide | 1/430 | 104 | |
| NCT00614939 (2010)74, 75, 76 | OADs and/or insulin | Saxagliptin | 1/85 | Placebo | 0/85 | 52 | |
| NCT00622284 (2011)77, 78 | Metformin | Linagliptin | 3/776 | Glimepiride | 2/775 | 104 | |
| NCT00642278 (2013)79, 80 | Metformin | Sitagliptin | 0/65 | Placebo | 0/65 | 12 | |
| Sitagliptin | 0/65 | Canagliflozin | 1/321 | ||||
| NCT00707993 (2013)81, 82 | None | Alogliptin | 1/222 | Glipizide | 1/219 | 52 | |
| NCT00757588 (2011)83, 84 | Insulin with or without metformin | Saxagliptin | 2/304 | Placebo | 0/151 | 24 | |
| NCT00798161 (2011)85, 86 | None | Linagliptin | 0/142 | Placebo | 0/72 | 24 | |
| Metformin | Linagliptin | 1/286 | No additional drugs | 0/291 | |||
| NCT00838903 (2014)87, 88 | Metformin | Sitagliptin | 1/302 | Glimepiride | 1/307 | 156 | |
| Sitagliptin | 1/302 | Placebo | 0/101 | ||||
| NCT00856284 (2013)89, 90 | Metformin | Alogliptin | 3/1751 | Glipizide | 1/878 | 104 | |
| NCT00954447 (2012)91 | Basal insulin and/or OADs | Linagliptin | 3/631 | Placebo | 2/630 | 52 | |
| NCT01006603 (2013)92 | None | Saxagliptin | 1/359 | Glimepiride | 3/359 | 52 | |
| NCT01189890 (2013)93 | None | Sitagliptin | 0/241 | Glimepiride | 1/236 | 30 | |
| NCT01263483 (2011)94, 95 | Voglibose | Alogliptin | 0/155 | Placebo | 1/75 | 12 | |
| NCT01289990 (2014)96 | None | Sitagliptin | 1/223 | Placebo | 0/223 | 76 | |
| Sitagliptin | 1/223 | Empagliflozin | 0/453 | ||||
| Pratley (2009)97, 98 | Pioglitazone or pioglitazone, plus metformin or SU | Alogliptin | 3/397 | Placebo | 0/97 | 26 | |
| Pratley (2014)99 | None | Alogliptin | 0/222 | Placebo | 0/106 | 26 | |
| Metformin | Alogliptin | 0/220 | No additional drugs | 0/220 | |||
| Rosenstock (2006)100 | Pioglitazone | Sitagliptin | 0/175 | Placebo | 0/178 | 24 | |
| Rosenstock (2010)101 | Pioglitazone | Alogliptin | 0/327 | No additional drugs | 0/163 | 26 | |
| Seino (2012)102 | Metformin | Alogliptin | 1/188 | Placebo | 0/100 | 12 | |
| Yang (2015)103 | None | Anagliptin | 0/68 | Placebo | 1/40 | 24 | |
| Trials reporting hospital admission for heart failure | |||||||
| Green (2015) (TECOS)12 | One or two OADs (metformin, pioglitazone, or SU) or insulin with or without metformin | Sitagliptin | 228/7332 | Placebo | 229/7339 | 156* | |
| Krum (2014) (VIVIDD)104 | Standard diabetes treatment | Vildagliptin | 13/128 | Placebo | 10/124 | 52 | |
| Laakso (2015)105 | None | Linagliptin | 7/113 | Placebo or glimepiride | 6/120 | 52 | |
| Scirica (2013) (SAVOR-TIMI 53)9, 106 | Antihyperglycaemic drugs | Saxagliptin | 289/8280 | Placebo | 228/8212 | 109* | |
| Zannad (2015) (EXAMINE)11, 107, 108 | Standard of care treatment for type 2 diabetes mellitus | Alogliptin | 85/2701 | Placebo | 79/2679 | 78* | |
BG=biguanide; TZD=thiazolidinedione; OADs=oral antidiabetic drugs; SU=sulfonylurea.
*Median treatment time (weeks).
All 38 trials were industry funded. Most (n=24) were identified from ClinicalTrials.gov, of which four91 92 93 96 have not been published in a peer reviewed journal. Because of the limited information in the trial registry, we were unable to adequately assess the risk of bias for these four trials. On the basis of the information we collected, 16 (42%) trials adequately generated their randomisation sequence; 11 (29%) adequately concealed allocation; all trials blinded patients, caregivers, and outcome assessors; eight (21%) adjudicated heart failure events; and four (11%) used blinded assessors to adjudicate heart failure (web appendix 2). The treatment groups of each included trial were generally balanced with respect to demographic and clinical characteristics.
Effects on heart failure
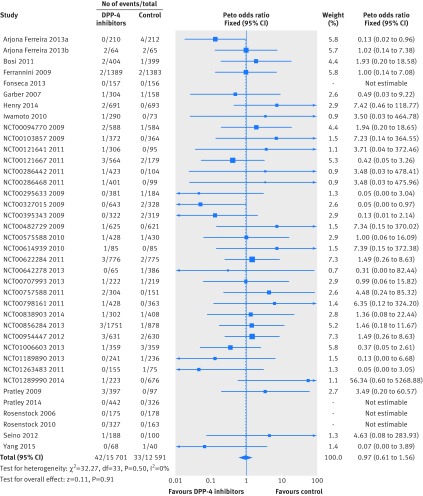
The 38 trials reported 75 heart failure events occurring in 28 292 patients who were treated with at least one drug (raw event rate 0.27%). The definition of heart failure was available in only one trial37; 33 (87%) trials reported heart failure as serious adverse events. The pooling of data from these trials showed no significant difference in the risk of heart failure between DPP-4 inhibitors treatment and control. Event rates were 0.27% for DPP-4 inhibitors versus 0.26% for controls (odds ratio 0.97 (95% confidence interval 0.61 to 1.56), I2=0%; risk difference 2 fewer (19 fewer to 28 more) events per 1000 patients with type 2 diabetes over five years; fig 2 and table 3). We rated the quality of evidence as low because of risk of bias and imprecision (table 3).
Fig 2 Risk of heart failure in patients with type 2 diabetes who received DPP-4 inhibitors versus control from randomised controlled trials
Table 3.
GRADE evidence profile of DPP-4 inhibitors and risk of heart failure in type 2 diabetes
| Quality assessment | Summary of findings | Quality of evidence | ||||||||||
| No of participants (studies), follow-up period | Risk of bias | Inconsistency | Indirectness | Imprecision | Publication bias | Study event rates | Relative risk (95% CI) | Anticipated absolute effects (5- year time frame)§ | ||||
| With control | With DPP-4 inhibitors | Risk with control | Risk difference with DPP-4 inhibitors (95% CI) | |||||||||
| Heart failure | ||||||||||||
| 28 292 (38), 12-206 weeks | Serious limitation, due to risk of bias* | No serious limitations | No serious limitations | Serious limitation, confidence interval includes important benefit and harm | Undetected | 33/12 591 (0.26%) | 42/15 701 (0.27%) | Odds ratio 0.97 (0.61 to 1.56) | 50 per 1000† | 2 fewer (19 fewer to 28 more) | ⊕⊕ΟΟ Low due to risk of bias and imprecision |
|
| Hospital admission for heart failure | ||||||||||||
| 37 028 (5), 1-3 years | No serious limitations | No serious limitations | No serious limitations | Serious limitation, confidence interval includes no harm and important harm | Undetected | 552/18 474 (3%) | 622/18 554 (3.4%) | Odds ratio 1.13 (1.00 to 1.26) | 60 per 1000‡ | 8 more (0 more to 16 more) | ⊕⊕⊕Ο Moderate due to imprecision |
|
*Most trials had unclear risk of bias on random sequence generation and allocation concealment (web appendix 2), and the follow-up (median of 52 weeks) was not long enough for heart failure to occur in patients at low risk of cardiovascular disease.
†Baseline risk estimate for heart failure in a five year time frame comes from the control arm of the cohort study we identified to best represent our target population (Kannan 2015111), with 528 events of heart failure in 13 185 participants (4.0%) at four year follow-up across the control and intervention arms.
‡Baseline risk estimate for hospital admission for heart failure in a five year time frame comes from control arms of the five trials we identified to best represent our target population (fig 3) with 552 events in 18 474 participants (30 per 1000) over a 2.5 year follow-up period, in the absence of observational studies providing more credible baseline risk estimates.
§Units are no of events per 1000 patients with type 2 diabetes mellitus over a five year time frame.
The subgroup analysis by type of control (placebo v active drugs) showed no difference in treatment effects (interaction P=0.57; comparison with placebo, odds ratio 1.17 (95% confidence interval 0.58 to 2.33); comparison with active drugs, 0.89 (0.47 to 1.66); fig A in web appendix 3). The subgroup analyses of the other three prespecified hypotheses showed no difference in treatment effects (figs B-D in appendix 3). Sensitivity analysis using alternative effect measures, statistical methods, and analysis models did not show important changes in pooled effects (figs E-G in web appendix 3).
Trials reporting hospital admission for heart failure
We included three large trials9 11 12 (SAVOR-TIMI 53, EXAMINE, and TECOS) and two small trials104 105 reporting hospital admission for heart failure; all were designed to assess the cardiovascular safety of DPP-4 inhibitors compared with placebo (table 1). The SAVOR-TIMI 53 trial investigated saxagliptin in patients with diabetes who had a renal impairment and cardiovascular disease or multiple risk factors for vascular disease. The EXAMINE trial recruited patients receiving alogliptin with type 2 diabetes and a recent acute coronary syndrome. The TECOS trial examined sitagliptin in patients with type 2 diabetes and cardiovascular disease. In addition, one small trial104 assessed vildagliptin in patients with type 2 diabetes as well as heart failure and a left ventricular ejection fraction less than 40%; the other small trial105 assessed linagliptin in patients with type 2 diabetes with moderate to severe renal impairment.
All three large trials were international studies. The median length of follow-up ranged from 76 to 156 weeks (table 1). Those trials enrolled 5380-16 492 patients (total n=36 607; mean age range 60.9-65.5 years, mean body mass index 29.5-31.1, and duration of diabetes 9.2-11.6 years). The two small trials followed up patients for 52 weeks; mean age ranged from 63 to 66.6 years and mean HbA1c levels ranged from 7.8% to 8.1%.
All trials, but one104 (which had unclear details because it was presented as an abstract), adequately generated their randomisation sequence and adequately concealed allocation; all trials blinded patients, caregivers, outcome assessors, and centrally adjudicated hospital admission for heart failure outcome through a clinical events classification committee who were blinded to treatment allocation. All trials were funded by industry (web appendix 2).
Effects on hospital admission for heart failure
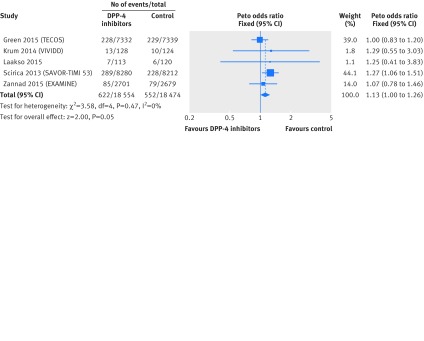
All five trials9 11 12 104 105 reported unadjusted rates of hospital admission for heart failure. Overall, 1174 events of admission for heart failure occurred in 37 028 patients (raw event rate 3.4% for DPP-4 inhibitors v 3.0% for controls; table 3). Pooling across trials showed a borderline increase in the risk of hospital admission for heart failure in patients with type 2 diabetes using DPP-4 inhibitors versus control (odds ratio 1.13 (95% confidence interval 1.00 to 1.26), I2=0%; risk difference 8 more (0 more to 16 more) per 1000 patients with type 2 diabetes over five years; fig 3 and table 3). We rated the quality of evidence as moderate due to imprecision (table 3). Sensitivity analysis by use of alternative effect measures, statistical methods, and analysis models did not show important changes in the pooled effects (figs H-J in web appendix 3).
Fig 3 Risk of hospital admission for heart failure in patients with type 2 diabetes who received DPP-4 inhibitors versus control from randomised controlled trials
Evidence from observational studies
Of 12 observational studies, four109 110 111 112 reported heart failure, and eight13 14 15 16 17 113 114 115 reported hospital admission for heart failure; nine13 14 15 109 110 111 113 114 115 were cohort studies and the other three16 17 112 were nested case-control studies (fig 1).
Observational studies reporting heart failure
Of the four studies reporting heart failure, two prospective cohort studies109 110 compared DPP-4 inhibitors versus sulfonylureas and sitagliptin versus sulfonylureas. One retrospective cohort study111 assessed DPP-4 inhibitors versus sulfonylureas and reported the findings from the subgroup of DPP-4 inhibitors. Finally, one nested case-control study112 using claims data investigated use of sitagliptin versus no use in patients admitted to hospital for acute coronary syndrome (table 4 and table 5). Sample sizes ranged from 616 to 13 185, and the mean or median length of follow-up ranged from one to four years. Enrolled patients had a mean or median age ranging from 55 to 65.8 years. None of the studies explicitly defined provided diagnostic criteria for heart failure.
Table 4.
Characteristics of included observational studies
| Author (year) | Study design | Data source | Countries | Funding | Total No of patients | Follow-up (years) | Male patients (No (%)) | Mean age (years) | Mean body mass index | Mean HbA1c (%) | Mean FPG (mmol/L) | Mean diabetes duration (years) | CVD at baseline |
| Studies reporting heart failure | |||||||||||||
| Gitt (2013)109 | Prospective cohort study | Registry data | Germany | Private for-profit funding | 616 | 1 | 3097 (49.8) | 65.8† | NR | 7.3† | 7.7† | 4.9† | Included patients had CVD or had no CVD at baseline |
| NCT01357135 (2014)110 | Prospective cohort study | Electronic medical records | France | Private for-profit funding | 3453 | 3 | 2004 (58.0) | 63.5 | NR | NR | NR | NR | NR |
| Kannan (2015)*, 111 | Retrospective cohort study | Electronic health records | USA | No funding | 13 185 | 4† | 7827 (54.6) | 60.6 | 32.6† | NR | NR | NR | Included patients had no history of CVD or congestive heart failure at baseline |
| Eurich (2014)112 | Nested case-control study | Claims data | USA | NR | 5027 | NA | 3268 (65) | 55 | NR | NR | NR | NR | Included patients had no history of heart failure in the 3 years before admission to hospital for an acute coronary syndrome event |
| Studies reporting hospital admission for heart failure | |||||||||||||
| Fadini (2015)13 | Retrospective cohort study | Registry data | Italy | Public funding | 127 555 | 2.6 | 66 201 (51.9) | 67.0 | NR | NR | NR | NR | Included patients had CVD or no CVD at baseline |
| Fu (2015)14 | Retrospective cohort study | Claims data | USA | NR | 218 556 | 0.5 | NR | NR | NR | NR | NR | NR | Included patients had CVD or no CVD at baseline |
| Seong (2015)113 | Retrospective cohort study | Claims data | South Korea | No funding | 349 476 | 0.6 | 191 167 (54.7) | 58.3 | NR | NR | NR | NR | Included patients had no history of CVD within 2.5 years before cohort entry |
| Suh (2015)114 | Retrospective cohort study | Claims data | South Korea | NR | 935 519 | 0.9 | 518 614 (55.4) | 59.4 | NR | NR | NR | NR | NR |
| Velez (2015)*, 115 |
Retrospective cohort study | Electronic medical records | USA | Public funding | 4224 | 2.0† | 2265 (53.6) | 60.8 | NR | 8.0 | NR | 2.5 | Included patients had CVD or no CVD at baseline |
| Wang (2014)15 | Retrospective cohort study | Claims data | Taiwan | Public funding | 16 576 | 1.5† | 8615 (52.0) | 64.3 | NR | NR | NR | 8.6 | Included patients had CVD or no CVD at baseline |
| Weir (2014)16 | Nested case-control study | Claims data | USA | NR | 45 434 | NA | 27 013 (59.5) | 54.6 | NR | 7.5 | NR | NR | Included patients were recently diagnosed with heart failure |
| Yu (2015)*, 17 | Nested case-control study | Electronic medical records | UK | Public funding | 57 737 | NA | 32 795 (56.8) | 61.6 | NR | NR | NR | 2.3 | Included patients had CVD or no CVD at baseline |
FPG=fasting plasma glucose; CVD=cardiovascular disease; NR=not reported; NA=not applicable.
*Three studies accessed incretin agents (both glucagon-like peptide 1 receptor agonists and DPP-4 inhibitors) and the risk of heart failure, so the data above were the characteristics of total patients included.
†Median value.
Table 5.
Exposures, outcomes, and results of included observational studies
| Author (year) | Exposure of interest | Control group | No of events or cases | Total no of analysed patients | Adjusted estimates (95% CI) | Adjusted covariate |
| Studies reporting heart failure | ||||||
| Eurich (2014)112 | Sitagliptin use | No use | 457 | 5027 | OR 0.75 (0.38 to 1.46) | Demographics, clinical and laboratory data, pharmacy claims, healthcare use and propensity scores (conditional probability of being treated with metformin or sulfonylurea or insulin or sitagliptin) |
| Kannan (2015)111 | DPP-4 inhibitors (combined with metformin) | Sulfonylureas (combined with metformin) | 528* | 13 185 (55 110 person years)* | HR 1.10 (1.04 to 1.17) | Age, sex, race, body mass index, number of encounters, median household income, smoking status, systolic and diastolic blood pressure, hypertension, dyslipidaemia, cerebral vascular event, presence of neuropathy, retinopathy, dementia, chronic obstructive pulmonary disease, cancer, atrial fibrillation, antihypertensive drugs, lipid lowering agents, antiplatelet agents, and propensity for being on metformin and sulfonylureas at baseline, lipid profile, estimated glomerular filtration rate |
| Gitt (2013)109 | DPP-4 inhibitors | Sulfonylureas | 11 | 616 | NR | NR |
| NCT01357135 (2014)110 | Sitagliptin (combined with metformin) | Sulfonylureas (combined with metformin) | 2 | 2607 | NR | NR |
| Studies reporting hospital admission for heart failure | ||||||
| Fadini (2015)13 | DPP-4 inhibitors | Sulfonylureas | 1181 | 110 757 | HR 0.78 (0.62 to 0.97) | Age, sex, use of certain medications (drugs for hypertension, dyslipidaemia, chronic obstructive pulmonary disease, non-steroidal anti-inflammatory drugs, and antiplatelet drugs), presence of previous hospital admissions, Charlson index level grouped into three categories, previous use of oral glucose lowering drugs, cotreatment with metformin, and adherence level categorised on the basis of the medication possession ratio (MPR (%); <80% v ≥80%) |
| Fu (2015)14 | DPP-4 inhibitors | Sulfonylureas | 495 | 218 556 | No CVD at baseline: HR 0.59 (0.38 to 0.89); CVD at baseline: 0.95 (0.78 to 1.15) |
Adjusted covariates of Cox proportional hazard models were not stated explicitly; each comparison consisted of patients matched 1:1 on a propensity score based on demographics, general clinical characteristics, and hospital admission for heart failure risk factors from one year before baseline; analyses were stratified by presence of CVD |
| Seong (2015)113 | DPP-4 inhibitors | Sulfonylureas and pioglitazone | 212 | 349 476 (211 959 person years) | DPP-4 inhibitors v sulfonylureas: adjusted HR 0.93 (0.62 to 1.41); DPP-4 inhibitors v pioglitazone: 0.21 (0.15 to 0.28) | Adjusted factors included age, sex, duration of diabetes at baseline; comorbidities in year before the index date (microvascular complications of diabetes (retinopathy, neuropathy, or nephropathy), peripheral vascular disease, hypertension, and dyslipidemia), and associated Charlson score; diabetes related hospital admission and total number of hypoglycaemic drug classes used in year before the index date; and use of the following drug classes in year before the index date: hypoglycaemic, lipid lowering, antihypertensive, antiplatelet (drug names not listed here) |
| Suh (2015)114 | DPP-4 inhibitors | Pioglitazone | 998 | 935 519 | Sitagliptin v pioglitazone: adjusted HR 0.97 (0.80 to 1.16); vildagliptin v pioglitazone: 1.22 (0.99 to 1.50) | Age and sex |
| Velez (2015)115 | DPP-4 inhibitors | Control (no details) | 127 | 3987 | HR 0.58 (0.38, 0.88) | Propensity score, number of antidiabetic drugs, duration of diabetes, baseline beta blocker use, and use of angiotensin converting enzyme inhibitor or angiotensin receptor blocker |
| Wang (2014)15 | Sitagliptin use | No use | 614 | 16 576 | HR 1.21 (1.04 to 1.42) | Adjusted covariates of Cox proportional hazard models were not stated explicitly; potential confounding were mitigated by the propensity score matching approach, and covariates included age, sex, duration of diabetes, antidiabetic drugs used, comorbidities, and outpatient visit |
| Weir (2014)16 | Sitagliptin use | No use | 824 | 9062 | OR 1.84 (1.16 to 2.92) | Demographics (age, sex, and socioeconomic status), most recent clinical laboratory data (HbA1c, low and high density lipoprotein cholesterol, triglycerides, estimated glomerular filtration rate, albuminuria, and haemoglobin concentrations), history of CVD (ischaemic heart disease, myocardial infarction, dyslipidaemia, hypertension, arrhythmia, and valve disease), and prescription drug use (antiplatelet drugs, anticoagulants, statins, calcium channel blockers, β blockers, angiotensin converting enzyme inhibitors, renin inhibitors, diuretics, and nitrates) |
| Yu (2015)17 | DPP-4 inhibitors (sitagliptin, vildagliptin, and saxagliptin, alone or in combination with other antidiabetic drugs) | Other oral antidiabetic drugs | 1118* | 18 744* | OR 0.88 (0.63 to 1.22) | Sex, body mass index, excessive alcohol use, smoking status, HbA1c level, comorbidities (neuropathy, renal disease, retinopathy, atrial fibrillation, cancer (other than non-melanoma skin cancer), chronic obstructive pulmonary disease, coronary artery disease, dyslipidaemia, hypertension, previous myocardial infarction, peripheral arteriopathy, previous coronary revascularisation, peripheral vascular disease, and previous stroke), number of prescriptions, number of physician visits, and use of the following drugs in the year before cohort entry: angiotensin converting enzyme inhibitors, angiotensin receptor blockers, beta blockers, calcium channel blockers, diuretics, fibrates, statins, aspirin, and other non-steroidal anti-inflammatory drugs |
NR=not reported; HR=hazard ratio; OR=odds ratio; CVD=cardiovascular disease.
*These two studies accessed incretin drugs and the risk of heart failure, and data of events/cases and total number of analysed patients regarding glucagon-like peptide 1 receptor agonists and DPP-4 inhibitors were not reported separately, so the data above were of total study patients.
Four studies used registry data, electronic health or medical records, or claims data for their analyses. Patients with type 2 diabetes were ascertained by physicians in one prospective cohort study109 or by ICD-9 Clinical Modification (CM) codes in one nested case-control study112; the other two cohort studies110 111 did not explicitly report the ascertainment of type 2 diabetes. None of these studies mentioned the ascertainment of exposure to DPP-4 inhibitors agents and other confounding variables; the accuracy of ascertaining exposure and confounding factors was unclear. Of these three cohort studies, only one111 demonstrated that the outcome of interest was not present at start of study, and mentioned the method used to assess the outcome of interest. Of these four studies, two111 112 controlled for the effect of confounding factors (web appendices 4 and 5).
Effects on heart failure
All three cohort studies109 110 111 reported unadjusted rates of heart failure, involving 541 events among 16 408 patients (raw event rate 3.3%). Because of the heterogeneous and indirect nature of the identified evidence—with substantial variations in comparisons and types of patients—we did not pool data across studies. The outcome information is presented in table 5.
One retrospective cohort study111 and one nested case-control study112 reported adjusted data. The retrospective cohort study, including 13 185 patients and with a median follow-up of four years, suggested that, compared with sulfonylureas, DPP-4 inhibitors was statistically associated with an increased risk of congestive heart failure (adjusted hazard ratio 1.10, 95% confidence interval 1.04 to 1.17). The nested case-control study, selecting 457 heart failure cases and 4570 controls, showed no statistical difference in the risk of heart failure between use and no use of sitagliptinin the 90 days before acute coronary syndrome (adjusted odds ratio 0.75, 95% confidence interval 0.38 to 1.46, table 5). Using GRADE, we rated the quality of evidence in the identified studies as very low, owing to risk of bias, indirectness, and imprecision in addition to the inherent risk for confounding given the observational design.
Observational studies reporting hospital admission for heart failure
Of the eight studies reporting hospital admission for heart failure, six retrospective cohort studies,13 14 15 113 114 115 using registry data, claims data, or electronic medical records, assessed DPP-4 inhibitors versus active drugs (eg, sulfonylureas, pioglitazone), and the use of sitagliptin versus no use of sitagliptin. The other two nested case-control studies16 17 assessed use of sitagliptin versus no use of sitagliptin, and incretin based drugs (including the DPP-4 inhibitors subgroup) versus other oral antidiabetic drugs (tables 4 and 5). The sample sizes of these eight studies ranged from 4224 to 935 519, and the mean or median length of follow-up ranged from 0.5 to 2.6 years. Enrolled patients had a mean age ranging from 54.6 to 67 years, mean baseline HbA1c level ranging from 7.5% to 8.0%, and a mean duration of diabetes ranging from 2.3 to 8.6 years.
The eight studies used registry data, claims data, or electronic medical records for analyses. Only two studies15 113 explicitly reported use of ICD codes to ascertain patients with diabetes; one study13 ascertained exposure to DPP-4 inhibitors by using anatomical therapeutic chemical classes; three studies13 15 113 explicitly stated use of ICD codes to ascertain other confounding variables. Four studies13 15 113 114 used ICD-9 or ICD-10 codes to assess outcomes. Three cohort studies13 113 114 clarified that the outcome of interest was not present at enrolment. All eight studies controlled for potential confounding factors, but failed to specify the extent to which the data were complete in the database (web appendices 4 and 5).
Effects on hospital admission for heart failure
All but one retrospective cohort study115 reported unadjusted rates of hospital admission for heart failure. The five cohort studies13 14 15 113 114 included 3500 events among 1 630 884 patients (raw event rate 0.2%; 1466 events (0.2%) in 912 309 patients from the DPP-4 inhibitors group, and 2034 events (0.3%) in 718 575 patients from the control group). The two nested case-control studies16 17 involved 1942 cases among 27 806 patients. Because of the variety of confounding factors investigated in the studies, we did not pool the unadjusted data.
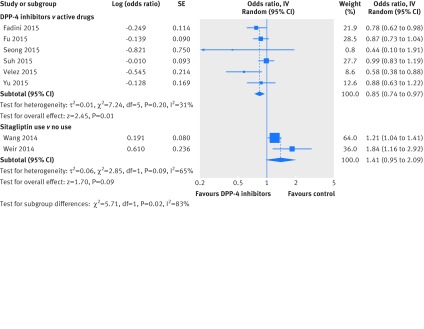
All eight studies reported adjusted estimates of hospital admission for heart failure. Of these, six studies—five cohort studies and one nested case-control study—compared DPP-4 inhibitors with active drugs (sulfonylureas, pioglitazone, other oral antidiabetic drugs). Pooling of adjusted estimates from these six studies showed that DPP-4 inhibitors were associated with reduced risk of hospital admission for heart failure (adjusted odds ratio 0.85, 95% confidence interval 0.74 to 0.97; I2=31%). However, pooling of the cohort study15 (16 576 patients and 614 events), and the nested case-control study16 (824 cases and 8238 controls) suggested a non-significant trend for increased risk of admission for heart failure compared with no use of sitagliptin (adjusted odds ratio 1.41, 0.95 to 2.09; I2=65%). There was significant subgroup effect by type of control (interaction P=0.02, fig 4). Using GRADE, we rated the quality of evidence as very low, due to risk of bias, heterogeneity, and imprecision in addition to the inherent risk for confounding given the observational design.
Fig 4 Risk of hospital admission for heart failure in patients with type 2 diabetes who received DPP-4 inhibitors versus control based on adjusted data from observational studies. SE=standard error; IV=inverse variance
Table 6 summarises the evidence regarding the effects of DPP-4 inhibitors on heart failure or hospital admission for heart failure.
Table 6.
Risk of heart failure or hospital admission for heart failure among patients with type 2 diabetes receiving DPP-4 inhibitor treatment
| Comparison | No of studies (events or cases, patients) | DPP-4 inhibitors (events/patients) | Control (events/patients) | Effect estimate (95%CI) | Cardiovascular morbidities at baseline |
| Heart failure | |||||
| Randomised controlled trials | |||||
| DPP-4 inhibitors v control | 38 (75, 28 292) | 42/15 701 | 33/12 591 | Pooled OR 0.97 (0.61 to 1.56) | Typically without CVD |
| Observational studies | |||||
| DPP-4 inhibitors v SU | 1 (11, 616) | 8/436 | 3/153 | Unadjusted OR 0.88 (0.22 to 3.48) | With or without CVD |
| DPP-4 inhibitors v SU | 1 (528, 13 185) | NR | NR | Adjusted HR 1.10 (1.04 to 1.17) | No history of CVD or congestive heart failure |
| Sitagliptin v SU | 1 (2, 2607) | 1/1874 | 1/733 | Unadjusted OR 0.39 (0.02 to 6.26) | NR |
| Sitagliptin use v no use | 1 (457, 5027) | — | — | Adjusted OR 0.75 (0.38 to 1.46) | Admission to hospital for an acute coronary syndrome event |
| Hospital admission for heart failure | |||||
| Randomised controlled trials | |||||
| DPP-4 inhibitors v control | 5 (1174, 37 028) | 622/18 554 | 522/18 474 | Pooled OR 1.13 (1.00 to 1.26) | CVD or multiple risk factors for vascular disease |
| Observational studies | |||||
| DPP-4 inhibitors v active control (pooled estimates) | 6 (4341, 1 618 295) | — | — | Pooled adjusted OR 0.85 (0.74 to 0.97) | With or without CVD |
| DPP-4 inhibitors v SU | 3 (1875, 657 596) | 380/202 292 | 1495/455 304 | Adjusted HR 0.84 (0.74 to 0.96) | With or without CVD |
| DPP-4 inhibitors v pioglitazone | 2 (1060, 1 031 432) | 796/776 449 | 264/254 983 | Adjusted HR 0.67 (0.57 to 0.78) | With or without CVD |
| DPP-4 inhibitors v other OADs | 1 (1118, 18 744)* | Adjusted OR 0.88 (0.63 to 1.22) | With or without CVD | ||
| DPP-4 inhibitors v control | 1 (127, 3987) | NR | NR | Adjusted HR 0.58 (0.38, 0.88) | With or without CVD |
| Sitagliptin use v no use (pooled estimates) | 2 (1438, 25 638) | — | — | Pooled adjusted OR 1.41 (0.95 to 2.09) | — |
| Sitagliptin use v no use | 1 (614, 16 576) | 339/8288 | 275/8288 | Adjusted HR 1.21 (1.04 to 1.42) | With or without CVD |
| Sitagliptin use v no use | 1 (824, 9062)* | Adjusted OR 1.84 (1.16 to 2.92) | Heart failure at baseline | ||
CVD=cardiovascular disease; SU=sulfonylurea; OR=odds ratio; HR=hazard ratio; NR=not reported; OADs=oral antidiabetic drugs.
*Nested case-control study.
Discussion
Main findings
The only evidence of moderate quality from our results is from randomised controlled trials that examined the effect of DPP-4 inhibitors on hospital admission for heart failure. These studies suggested a small increase, in both relative and absolute terms, in heart failure admissions in patients using DPP-4 inhibitors than those not. The results, however, are of borderline significance. Evidence from observational studies is of very low quality, and thus has little bearing on any inferences about DPP-4 inhibitor effects on heart failure admission.
With respect to the incidence of heart failure, trial evidence leaves uncertainty regarding the relative effect of DPP-4 inhibitors. Because the follow-up was relatively short and the baseline risk of patients was very low in those trials, the incidence of heart failure was very low (well under 1% per year), and with the small number of events, the confidence intervals around relative effects are wide. In addition, heart failure was unspecified in all but one of the phase III trials. Many (87%) reported heart failure as serious adverse events, in which admission for heart failure might have been included according to the definition of serious adverse events. The pooled estimate could thus represent a composite of heart failure with or without admission for heart failure. The observational studies again provide very low quality evidence and have little effect on inferences, although results are consistent. Overall, the current evidence provides no support for the hypothesis that DPP-4 inhibitors increase the incidence of heart failure.
Strengths and limitations
Our study has several strengths. Firstly, we used rigorous methods to systematically identify and include data from both randomised and non-randomised studies to examine the effect of DPP-4 inhibitors on risk of heart failure and hospital admission for heart failure. Secondly, in addition to published reports, we have identified additional data from ClinicalTrials.gov. Our study included four randomised controlled trials and three observational studies that were not published in journals. Thirdly, we instituted a rigorous approach to ensure the data were accurate. In particular, we carefully checked the data reported in ClinicalTrials.gov and journal publications for consistency. Fourthly, we addressed several prespecified subgroup analyses to explore sources of heterogeneity. Finally, we used GRADE to assess the quality of the body of evidence.
Our study also had some limitations. Firstly, for various reasons, some trials are likely not to report outcome data in their full publications. However, we have obtained additional data through the search of the ClinicalTrials.gov and conference abstracts, which minimised the risk of outcome reporting bias. Secondly, given the limitations of reported data, we were unable to confirm whether the increased risk of hospital admission for heart failure was a class effect or a specific effect of saxagliptin. Other limitations included those of the primary studies, such as the risk of bias of observational studies, the potentially variable specification of outcomes (heart failure and hospital admission for heart failure), and the likelihood of variable and incomplete ascertainment of heart failure in the clinical trials.
Comparison with other studies
Four previous meta-analyses7 117 118 119 have explored the effect of DPP-4 inhibitors on the risk of heart failure. Of those studies, one7 found that treatment with DPP-4 inhibitors for 29 weeks or longer was associated with an increased risk of new onset of heart failure (risk ratio 1.16, 95% confidence interval 1.01 to 1.33), but not with treatment for less than 29 weeks (0.67, 0.32 to 1.40). The second117 included 24 randomised controlled trials that enrolled no less than 100 patients and followed up patients for 24 weeks; the third118 exclusively included 37 trials for analysis; the fourth119 included trials and observational studies. All the last three studies found that DPP-4 inhibitors were statistically associated with an increased risk of heart failure (risk ratio 1.16 (1.01 to 1.33), odds ratio 1.19 (1.03 to 1.37), odds ratio 1.15 (1.02 to 1.29), respectively).
Compared with these studies, our review has added substantial information. Firstly, we separately addressed heart failure and hospital admission as a result of heart failure. Secondly, we included both observational studies and randomised controlled trials. With respect to the trials, two important large trials11 12 were published subsequent to the previous reviews and allowed us to analyse the effect of DPP-4 inhibitors on hospital admission for heart failure. We also included additional large observational studies that carry important information regarding the risk of heart failure or admission for heart failure.
Our findings regarding the effect of DPP-4 inhibitors on heart failure were not consistent with previous meta-analyses. This difference is probably due to the fact that the previous studies were dominated by large trials reporting positive association with hospital admission for heart failure (eg, SAVOR TIMI-53), and more recent trials that have failed to find an effect were not considered.
We also found all four meta-analyses in our study7 117 118 119 to have several methodological issues. Firstly, these reviews have pooled data for heart failure and hospital admissions for heart failure. We believe that a more appropriate analysis should consider the two outcomes separately. We identified varying results when analysing the two outcomes separately. More importantly, the pooling of the two outcomes together would probably result in misleading effect estimates, when the authors aimed to assess the effect of DPP-4 inhibitors on the risk of heart failure. Another meta-analysis7 investigated DPP-4 inhibitors on the risk of new onset of heart failure, but this study included trials, such as SAVOR TIMI-53 and EXAMINE that already included patients with heart failure at baseline. The third meta-analysis117 failed to include outcome data published in ClinicalTrials.gov. The final meta-analysis119 combined randomised controlled trials and observational studies to generate grand effect estimates. Because of the substantial differences in the design and analysis of the type of studies, and the considerable variation in observational studies, the grand pooling will introduce misleading findings.
Implications for practice
The current evidence suggests a possible increased risk of hospital admission for heart failure in those patients with type 2 diabetes treated with DPP-4 inhibitors and with cardiovascular diseases or multiple risk factors for vascular diseases at baseline. Although the effect is small if it exists, and the associated confidence interval includes no effect, our results suggest the advisability of caution in the use of DPP-4 inhibitors for patients with type 2 diabetes who are at high risk for heart failure.
Conclusions
The relative effect of DPP-4 inhibitors on heart failure remains uncertain in patients with type 2 diabetes, given the relatively short follow-up and low quality of evidence. The current evidence suggests a small increase in the risk of hospital admission for heart failure in patients with existing cardiovascular diseases or multiple risk factors for vascular diseases. Additional randomised controlled trials enrolling patients with existing cardiovascular diseases or multiple risk factors for vascular diseases will be required to definitively assess the effect of DPP-4 inhibitors on such patients. Such trials, if enrolling patients at high risk of exacerbation and admission, may be feasible. In the meantime, the possible increase in hospital admission for heart failure could be one issue that patients and clinicians consider in choosing antidiabetic drug treatment for patients with existing cardiovascular diseases.
What is already known on this topic
Several occurrences of heart failure or hospital admission for heart failure have been reported in patients with type 2 diabetes taking DPP-4 inhibitors
Systematic reviews of randomised controlled trials and observational studies have suggested an increased risk of heart failure or admission for heart failure associated with the agents
What this study adds
The relative effect of DPP-4 inhibitors on the risk of heart failure is uncertain
Current evidence from trials and observational studies suggests a small increase in risk of admission for heart failure in patients with type 2 diabetes who have existing cardiovascular diseases or multiple risk factors for vascular diseases, relative to no use
Web Extra.
Extra material supplied by the author
Web appendix 1: Search strategies
Web appendix 2: Risk of bias of included randomised controlled trials
Web appendix 3: Supplementary forest plots
Web appendix 4: Risk of bias of included cohort studies
Web appendix 5: Risk of bias of included case-control studies
We thank Daphne Plaut for developing the search strategy and conducting the initial literature search.
Contributors: XS and SL conceived the study. XS acquired the funding. XS and LL had full access to all of the data in the study, and take responsibility for the integrity of the data and the accuracy of the data analysis. XS and LL designed the study. XS and LL developed and tested the data collection forms. LL, KD, JL, PZ, LZ, JS, MMB, ZNS, EW, JWB, SE, GM, LPR, POV, YW, QC, and XS acquired the data. LL and XS conducted the analysis, interpreted the data, and drafted the manuscript. LL, XS, GHG, POV, SL, MMB, ZNS, EW, JWB, SE, GM, LPR, KD, JL, PZ, LZ, JS, YW, and QC critically revised the manuscript. XS is the guarantor.
Funding: This study was supported by the National Natural Science Foundation of China (grant no 71573183), “Thousand Youth Talents Plan” of China (grant no D1024002) and Sichuan Province, and Young Investigator Award of Sichuan University (grant no. 2013SCU04A37). These funders had no role in the study design, writing of the manuscript, or decision to submit this or future manuscripts for publication. SL is funded by the National Natural Science Foundation of China (grant No 81400811 and 21534008). ZNS is funded by the Canadian Diabetes Association. JWB is funded by a New Investigator Award from the Canadian Institutes of Health Research and Canadian Chiropractic Research Foundation. SE is funded by MITACS Elevate and Restracomp Postdoctoral Awards.
Competing interests: All authors have completed the ICMJE uniform disclosure form at www.icmje.org/coi_disclosure.pdf and declare: support from the National Natural Science Foundation of China, “Thousand Youth Talents Plan” of China and Sichuan Province, and Young Investigator Award of Sichuan University for the submitted work; no financial relationships with any organisations that might have an interest in the submitted work in the previous three years, and no other relationships or activities that could appear to have influenced the submitted work.
Ethical approval: Not required.
Data sharing: No additional data available.
The lead author (the manuscript’s guarantor) affirms that the manuscript is an honest, accurate, and transparent account of the study being reported; that no important aspects of the study have been omitted; and that any discrepancies from the study as planned have been explained.
References
- 1.International Diabetes Federation. IDF DIABETES ATLAS (Sixth edition). http://www.idf.org/files/idf_publications/idf_diabetes_atlas_EN/idf_diabetes_atlas_EN/assets/common/downloads/publication.pdf (accessed May 8 2015).
- 2.Karagiannis T, Paschos P, Paletas K, Matthews DR, Tsapas A. Dipeptidyl peptidase-4 inhibitors for treatment of type 2 diabetes mellitus in the clinical setting: systematic review and meta-analysis. BMJ 2012;344:e1369. 10.1136/bmj.e1369. 22411919. [DOI] [PubMed] [Google Scholar]
- 3.Esposito K, Chiodini P, Maiorino MI, Bellastella G, Capuano A, Giugliano D. Glycaemic durability with dipeptidyl peptidase-4 inhibitors in type 2 diabetes: a systematic review and meta-analysis of long-term randomised controlled trials. BMJ Open 2014;4:e005442. 10.1136/bmjopen-2014-005442. 24916090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kawalec P, Mikrut A, Łopuch S. The safety of dipeptidyl peptidase-4 (DPP-4) inhibitors or sodium-glucose cotransporter 2 (SGLT-2) inhibitors added to metformin background therapy in patients with type 2 diabetes mellitus: a systematic review and meta-analysis. Diabetes Metab Res Rev 2014;30:269-83. 10.1002/dmrr.2494. 24829965. [DOI] [PubMed] [Google Scholar]
- 5.Monami M, Ahrén B, Dicembrini I, Mannucci E. Dipeptidyl peptidase-4 inhibitors and cardiovascular risk: a meta-analysis of randomized clinical trials. Diabetes Obes Metab 2013;15:112-20. 10.1111/dom.12000. 22925682. [DOI] [PubMed] [Google Scholar]
- 6.Patil HR, Al Badarin FJ, Al Shami HA, et al. Meta-analysis of effect of dipeptidyl peptidase-4 inhibitors on cardiovascular risk in type 2 diabetes mellitus. Am J Cardiol 2012;110:826-33. 10.1016/j.amjcard.2012.04.061. 22703861. [DOI] [PubMed] [Google Scholar]
- 7.Savarese G, Perrone-Filardi P, D’Amore C, et al. Cardiovascular effects of dipeptidyl peptidase-4 inhibitors in diabetic patients: A meta-analysis. Int J Cardiol 2015;181:239-44. 10.1016/j.ijcard.2014.12.017. 25528528. [DOI] [PubMed] [Google Scholar]
- 8.Inzucchi SE, Bergenstal RM, Buse JB, et al. Management of hyperglycemia in type 2 diabetes, 2015: a patient-centered approach: update to a position statement of the American Diabetes Association and the European Association for the Study of Diabetes. Diabetes Care 2015;38:140-9. 10.2337/dc14-2441. 25538310. [DOI] [PubMed] [Google Scholar]
- 9.Scirica BM, Bhatt DL, Braunwald E, et al. SAVOR-TIMI 53 Steering Committee and Investigators. Saxagliptin and cardiovascular outcomes in patients with type 2 diabetes mellitus. N Engl J Med 2013;369:1317-26. 10.1056/NEJMoa1307684. 23992601. [DOI] [PubMed] [Google Scholar]
- 10.U.S. Food and Drug Administration. Saxagliptin (marketed as Onglyza and Kombiglyze XR): drug safety communication - FDA to review heart failure risk. http://www.fda.gov/Safety/MedWatch/SafetyInformation/SafetyAlertsforHumanMedicalProducts/ucm385471.htm (accessed August 27 2015).
- 11.Zannad F, Cannon CP, Cushman WC, et al. EXAMINE Investigators. Heart failure and mortality outcomes in patients with type 2 diabetes taking alogliptin versus placebo in EXAMINE: a multicentre, randomised, double-blind trial. Lancet 2015;385:2067-76. 10.1016/S0140-6736(14)62225-X. 25765696. [DOI] [PubMed] [Google Scholar]
- 12.Green JB, Bethel MA, Armstrong PW, et al. TECOS Study Group. Effect of Sitagliptin on Cardiovascular Outcomes in Type 2 Diabetes. N Engl J Med 2015;373:232-42. 10.1056/NEJMoa1501352. 26052984. [DOI] [PubMed] [Google Scholar]
- 13.Fadini GP, Avogaro A, Degli Esposti L, et al. OsMed Health-DB Network. Risk of hospitalization for heart failure in patients with type 2 diabetes newly treated with DPP-4 inhibitors or other oral glucose-lowering medications: a retrospective registry study on 127,555 patients from the Nationwide OsMed Health-DB Database. Eur Heart J 2015;36:2454-62. 10.1093/eurheartj/ehv301. 26112890. [DOI] [PubMed] [Google Scholar]
- 14.Fu AZ, Johnston S, Sheehan J, et al. Risk of hospitalization for heart failure with dipeptidyl peptidase-4 inhibitors vs. sulfonylureas and with saxagliptin vs. sitagliptin in a U.S. claims database. American Diabetes Association 75th Scientific Sessions (2015): 164-LB-2015 [Board 164]. Presented on June 7, 2015.
- 15.Wang KL, Liu CJ, Chao TF, et al. Sitagliptin and the risk of hospitalization for heart failure: a population-based study. Int J Cardiol 2014;177:86-90. 10.1016/j.ijcard.2014.09.038. 25499347. [DOI] [PubMed] [Google Scholar]
- 16.Weir DL, McAlister FA, Senthilselvan A, Minhas-Sandhu JK, Eurich DT. Sitagliptin use in patients with diabetes and heart failure: a population-based retrospective cohort study. JACC Heart Fail 2014;2:573-82. 10.1016/j.jchf.2014.04.005. 24998080. [DOI] [PubMed] [Google Scholar]
- 17.Yu OH, Filion KB, Azoulay L, Patenaude V, Majdan A, Suissa S. Incretin-based drugs and the risk of congestive heart failure. Diabetes Care 2015;38:277-84. 10.2337/dc14-1459. 25205143. [DOI] [PubMed] [Google Scholar]
- 18.Stroup DF, Berlin JA, Morton SC, et al. Meta-analysis of observational studies in epidemiology: a proposal for reporting. Meta-analysis Of Observational Studies in Epidemiology (MOOSE) group. JAMA 2000;283:2008-12. 10.1001/jama.283.15.2008. 10789670. [DOI] [PubMed] [Google Scholar]
- 19.Moher D, Liberati A, Tetzlaff J, Altman DG. PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. BMJ 2009;339:b2535. 10.1136/bmj.b2535. 19622551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.ClinicalTrials.gov. Why should i register and submit results? https://www.clinicaltrials.gov/ct2/manage-recs/background (accessed August 25 2015).
- 21.U.S. Food and Drug Administration. Food and Drug Administration Amendments Act (FDAAA) of 2007. US Public Law 110-85 section 801. https://www.gpo.gov/fdsys/pkg/PLAW-110publ85/pdf/PLAW-110publ85.pdf (accessed August 25 2015).
- 22.Higgins JPTAD, Sterne JAC. Assessing risk of bias in included studies. In: Higgins JPT, Green S, eds. Cochrane handbook for systematic reviews of interventions. Version 5.1.0.Cochrane Collaboration, 2011.
- 23.Akl EA, Sun X, Busse JW, et al. Specific instructions for estimating unclearly reported blinding status in randomized trials were reliable and valid. J Clin Epidemiol 2012;65:262-7. 10.1016/j.jclinepi.2011.04.015. 22200346. [DOI] [PubMed] [Google Scholar]
- 24.Wells GASB, O’Connell D, Peterson J, et al. The Newcastle-Ottawa scale (NOS) for assessing the quality of non-randomized studies in meta-analysis. http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp (accessed August 25 2015).
- 25.Higgins JPT, Green S, eds. Cochrane handbook for systematic reviews of interventions. Version 5.1.0.Cochrane Collaboration, 2011.
- 26.Bradburn MJ, Deeks JJ, Berlin JA, Russell Localio A. Much ado about nothing: a comparison of the performance of meta-analytical methods with rare events. Stat Med 2007;26:53-77. 10.1002/sim.2528. 16596572. [DOI] [PubMed] [Google Scholar]
- 27.Higgins JPTDJ, Altman DG. Special topics in statistics. In: Higgins JPT, Green S, eds. Cochrane handbook for systematic reviews of interventions. Version 5.1.0.Cochrane Collaboration, 2011.
- 28.Guyatt GH, Oxman AD, Vist GE, et al. GRADE Working Group. GRADE: an emerging consensus on rating quality of evidence and strength of recommendations. BMJ 2008;336:924-6. 10.1136/bmj.39489.470347.AD. 18436948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Guyatt GH, Oxman AD, Vist G, et al. GRADE guidelines: 4. Rating the quality of evidence--study limitations (risk of bias). J Clin Epidemiol 2011;64:407-15. 10.1016/j.jclinepi.2010.07.017. 21247734. [DOI] [PubMed] [Google Scholar]
- 30.Guyatt GH, Oxman AD, Kunz R, et al. GRADE guidelines 6. Rating the quality of evidence--imprecision. J Clin Epidemiol 2011;64:1283-93. 10.1016/j.jclinepi.2011.01.012. 21839614. [DOI] [PubMed] [Google Scholar]
- 31.Guyatt GH, Oxman AD, Kunz R, et al. GRADE Working Group. GRADE guidelines: 7. Rating the quality of evidence--inconsistency. J Clin Epidemiol 2011;64:1294-302. 10.1016/j.jclinepi.2011.03.017. 21803546. [DOI] [PubMed] [Google Scholar]
- 32.Guyatt GH, Oxman AD, Kunz R, et al. GRADE Working Group. GRADE guidelines: 8. Rating the quality of evidence--indirectness. J Clin Epidemiol 2011;64:1303-10. 10.1016/j.jclinepi.2011.04.014. 21802903. [DOI] [PubMed] [Google Scholar]
- 33.Guyatt GH, Oxman AD, Montori V, et al. GRADE guidelines: 5. Rating the quality of evidence--publication bias. J Clin Epidemiol 2011;64:1277-82. 10.1016/j.jclinepi.2011.01.011. 21802904. [DOI] [PubMed] [Google Scholar]
- 34.Guyatt GH, Oxman AD, Sultan S, et al. GRADE Working Group. GRADE guidelines: 9. Rating up the quality of evidence. J Clin Epidemiol 2011;64:1311-6. 10.1016/j.jclinepi.2011.06.004. 21802902. [DOI] [PubMed] [Google Scholar]
- 35.Arjona Ferreira JC, Marre M, Barzilai N, et al. Efficacy and safety of sitagliptin versus glipizide in patients with type 2 diabetes and moderate-to-severe chronic renal insufficiency. Diabetes Care 2013;36:1067-73. 10.2337/dc12-1365. 23248197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Merck. Sitagliptin versus glipizide in participants with type 2 diabetes mellitus and chronic renal insufficiency (MK-0431-063 AM1). National Library of Medicine (US), 2000. https://clinicaltrials.gov/show/NCT00509262.
- 37.Arjona Ferreira JC, Corry D, Mogensen CE, et al. Efficacy and safety of sitagliptin in patients with type 2 diabetes and ESRD receiving dialysis: a 54-week randomized trial. Am J Kidney Dis 2013;61:579-87. 10.1053/j.ajkd.2012.11.043. 23352379. [DOI] [PubMed] [Google Scholar]
- 38.Merck. Sitagliptin versus glipizide in participants with type 2 diabetes mellitus and end-stage renal disease (MK-0431-073 AM1). National Library of Medicine (US), 2000. https://clinicaltrials.gov/show/NCT00509236.
- 39.Bosi E, Ellis GC, Wilson CA, Fleck PR. Alogliptin as a third oral antidiabetic drug in patients with type 2 diabetes and inadequate glycaemic control on metformin and pioglitazone: a 52-week, randomized, double-blind, active-controlled, parallel-group study. Diabetes Obes Metab 2011;13:1088-96. 10.1111/j.1463-1326.2011.01463.x. 21733058. [DOI] [PubMed] [Google Scholar]
- 40.Ferrannini E, Fonseca V, Zinman B, et al. Fifty-two-week efficacy and safety of vildagliptin vs. glimepiride in patients with type 2 diabetes mellitus inadequately controlled on metformin monotherapy. Diabetes Obes Metab 2009;11:157-66. 10.1111/j.1463-1326.2008.00994.x. 19125777. [DOI] [PubMed] [Google Scholar]
- 41.Fonseca V, Staels B, Morgan JD 2nd, et al. Efficacy and safety of sitagliptin added to ongoing metformin and pioglitazone combination therapy in a randomized, placebo-controlled, 26-week trial in patients with type 2 diabetes. J Diabetes Complications 2013;27:177-83. 10.1016/j.jdiacomp.2012.09.007. 23116881. [DOI] [PubMed] [Google Scholar]
- 42.Garber AJ, Schweizer A, Baron MA, Rochotte E, Dejager S. Vildagliptin in combination with pioglitazone improves glycaemic control in patients with type 2 diabetes failing thiazolidinedione monotherapy: a randomized, placebo-controlled study. Diabetes Obes Metab 2007;9:166-74. 10.1111/j.1463-1326.2006.00684.x. 17300592. [DOI] [PubMed] [Google Scholar]
- 43.Henry RR, Staels B, Fonseca VA, et al. Efficacy and safety of initial combination treatment with sitagliptin and pioglitazone--a factorial study. Diabetes Obes Metab 2014;16:223-30. 10.1111/dom.12194. 23909985. [DOI] [PubMed] [Google Scholar]
- 44.Merck. MK0431 and pioglitazone co-administration factorial study in patients with type 2 diabetes mellitus (0431-102 AM2). National Library of Medicine (US), 2000. https://clinicaltrials.gov/show/NCT00722371.
- 45.Iwamoto Y, Taniguchi T, Nonaka K, et al. Dose-ranging efficacy of sitagliptin, a dipeptidyl peptidase-4 inhibitor, in Japanese patients with type 2 diabetes mellitus. Endocr J 2010;57:383-94. 10.1507/endocrj.K09E-272. 20332588. [DOI] [PubMed] [Google Scholar]
- 46.Merck. A Study of an investigational drug sitagliptin for type 2 diabetes mellitus. National Library of Medicine (US), 2000. https://clinicaltrials.gov/show/NCT00127192.
- 47.Merck. An investigational drug study in patients with type 2 diabetes mellitus. National Library of Medicine (US), 2000. https://clinicaltrials.gov/show/NCT00094770.
- 48.Seck T, Nauck M, Sheng D, et al. Sitagliptin Study 024 Group. Safety and efficacy of treatment with sitagliptin or glipizide in patients with type 2 diabetes inadequately controlled on metformin: a 2-year study. Int J Clin Pract 2010;64:562-76. 10.1111/j.1742-1241.2010.02353.x. 20456211. [DOI] [PubMed] [Google Scholar]
- 49.Nauck MA, Meininger G, Sheng D, Terranella L, Stein PP. Sitagliptin Study 024 Group. Efficacy and safety of the dipeptidyl peptidase-4 inhibitor, sitagliptin, compared with the sulfonylurea, glipizide, in patients with type 2 diabetes inadequately controlled on metformin alone: a randomized, double-blind, non-inferiority trial. Diabetes Obes Metab 2007;9:194-205. 10.1111/j.1463-1326.2006.00704.x. 17300595. [DOI] [PubMed] [Google Scholar]
- 50.Merck. MK0431 (sitagliptin) and metformin co-administration factorial study in patients with type 2 diabetes mellitus. National Library of Medicine (US), 2000. https://clinicaltrials.gov/show/NCT00103857.
- 51.Goldstein BJ, Feinglos MN, Lunceford JK, Johnson J, Williams-Herman DE. Sitagliptin 036 Study Group. Effect of initial combination therapy with sitagliptin, a dipeptidyl peptidase-4 inhibitor, and metformin on glycemic control in patients with type 2 diabetes. Diabetes Care 2007;30:1979-87. 10.2337/dc07-0627. 17485570. [DOI] [PubMed] [Google Scholar]
- 52.Bristol-Myers Squibb. Saxagliptin treatment in subjects with type 2 diabetes who are not controlled with diet and exercise. National Library of Medicine (US), 2000. https://clinicaltrials.gov/show/NCT00121641.
- 53.Rosenstock J, Aguilar-Salinas C, Klein E, Nepal S, List J, Chen R. CV181-011 Study Investigators. Effect of saxagliptin monotherapy in treatment-naïve patients with type 2 diabetes. Curr Med Res Opin 2009;25:2401-11. 10.1185/03007990903178735. 19650754. [DOI] [PubMed] [Google Scholar]
- 54.Bristol-Myers Squibb. Study assessing saxagliptin treatment in type 2 diabetic subjects who are not controlled with metformin alone. National Library of Medicine (US), 2000. https://clinicaltrials.gov/show/NCT00121667.
- 55.DeFronzo RA, Hissa MN, Garber AJ, et al. Saxagliptin 014 Study Group. The efficacy and safety of saxagliptin when added to metformin therapy in patients with inadequately controlled type 2 diabetes with metformin alone. Diabetes Care 2009;32:1649-55. 10.2337/dc08-1984. 19478198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Takeda. Efficacy and safety of alogliptin combined with metformin in participants with type 2 diabetes mellitus. National Library of Medicine (US), 2000. https://clinicaltrials.gov/show/NCT00286442.
- 57.Nauck MA, Ellis GC, Fleck PR, Wilson CA, Mekki Q. Alogliptin Study 008 Group. Efficacy and safety of adding the dipeptidyl peptidase-4 inhibitor alogliptin to metformin therapy in patients with type 2 diabetes inadequately controlled with metformin monotherapy: a multicentre, randomised, double-blind, placebo-controlled study. Int J Clin Pract 2009;63:46-55. 10.1111/j.1742-1241.2008.01933.x. 19125992. [DOI] [PubMed] [Google Scholar]
- 58.Takeda. Study of alogliptin combined with sulfonylurea in subjects with type 2 diabetes mellitus. National Library of Medicine (US), 2000. https://clinicaltrials.gov/show/NCT00286468.
- 59.Pratley RE, Kipnes MS, Fleck PR, Wilson C, Mekki Q. Alogliptin Study 007 Group. Efficacy and safety of the dipeptidyl peptidase-4 inhibitor alogliptin in patients with type 2 diabetes inadequately controlled by glyburide monotherapy. Diabetes Obes Metab 2009;11:167-76. 10.1111/j.1463-1326.2008.01016.x. 19125778. [DOI] [PubMed] [Google Scholar]
- 60.Bristol-Myers Squibb. A study assessing saxagliptin treatment in type 2 diabetic subjects who are not controlled with TZD therapy alone. National Library of Medicine (US), 2000. https://clinicaltrials.gov/show/NCT00295633.
- 61.Hollander PL, Li J, Frederich R, Allen E, Chen R. CV181013 Investigators. Safety and efficacy of saxagliptin added to thiazolidinedione over 76 weeks in patients with type 2 diabetes mellitus. Diab Vasc Dis Res 2011;8:125-35. 10.1177/1479164111404575. 21562064. [DOI] [PubMed] [Google Scholar]
- 62.Hollander P, Li J, Allen E, Chen R. CV181-013 Investigators. Saxagliptin added to a thiazolidinedione improves glycemic control in patients with type 2 diabetes and inadequate control on thiazolidinedione alone. J Clin Endocrinol Metab 2009;94:4810-9. 10.1210/jc.2009-0550. 19864452. [DOI] [PubMed] [Google Scholar]
- 63.Bristol-Myers Squibb. A phase 3 study of BMS-477118 in combination with metformin in subjects with type 2 diabetes who are not controlled with diet and exercise. National Library of Medicine (US), 2000. https://clinicaltrials.gov/show/NCT00327015.
- 64.Pfützner A, Paz-Pacheco E, Allen E, Frederich R, Chen R. CV181039 Investigators. Initial combination therapy with saxagliptin and metformin provides sustained glycaemic control and is well tolerated for up to 76 weeks. Diabetes Obes Metab 2011;13:567-76. 10.1111/j.1463-1326.2011.01385.x. 21342412. [DOI] [PubMed] [Google Scholar]
- 65.Jadzinsky M, Pfützner A, Paz-Pacheco E, Xu Z, Allen E, Chen R. CV181-039 Investigators. Saxagliptin given in combination with metformin as initial therapy improves glycaemic control in patients with type 2 diabetes compared with either monotherapy: a randomized controlled trial. Diabetes Obes Metab 2009;11:611-22. 10.1111/j.1463-1326.2009.01056.x. 19515181. [DOI] [PubMed] [Google Scholar]
- 66.Merck. Sitagliptin added-on to insulin study. National Library of Medicine (US), 2000. https://clinicaltrials.gov/show/NCT00395343.
- 67.Vilsbøll T, Rosenstock J, Yki-Järvinen H, et al. Efficacy and safety of sitagliptin when added to insulin therapy in patients with type 2 diabetes. Diabetes Obes Metab 2010;12:167-77.20092585. [DOI] [PubMed] [Google Scholar]
- 68.Merck. MK0431A comparative study in patients with type 2 diabetes. National Library of Medicine (US), 2000. https://clinicaltrials.gov/show/NCT00482729.
- 69.Reasner C, Olansky L, Seck TL, et al. The effect of initial therapy with the fixed-dose combination of sitagliptin and metformin compared with metformin monotherapy in patients with type 2 diabetes mellitus. Diabetes Obes Metab 2011;13:644-52. 10.1111/j.1463-1326.2011.01390.x. 21410627. [DOI] [PubMed] [Google Scholar]
- 70.Olansky L, Reasner C, Seck TL, et al. A treatment strategy implementing combination therapy with sitagliptin and metformin results in superior glycaemic control versus metformin monotherapy due to a low rate of addition of antihyperglycaemic agents. Diabetes Obes Metab 2011;13:841-9. 10.1111/j.1463-1326.2011.01416.x. 21535346. [DOI] [PubMed] [Google Scholar]
- 71.AstraZeneca. Bristol-Myers Squibb. 52-week add-on to metformin comparison of saxagliptin and sulphonylurea, with a 52-week extension period. National Library of Medicine (US), 2000. https://clinicaltrials.gov/show/NCT00575588.
- 72.Göke B, Gallwitz B, Eriksson JG, Hellqvist Å, Gause-Nilsson I. Saxagliptin vs. glipizide as add-on therapy in patients with type 2 diabetes mellitus inadequately controlled on metformin alone: long-term (52-week) extension of a 52-week randomised controlled trial. Int J Clin Pract 2013;67:307-16. 10.1111/ijcp.12119. 23638466. [DOI] [PubMed] [Google Scholar]
- 73.Göke B, Gallwitz B, Eriksson J, Hellqvist A, Gause-Nilsson I. D1680C00001 Investigators. Saxagliptin is non-inferior to glipizide in patients with type 2 diabetes mellitus inadequately controlled on metformin alone: a 52-week randomised controlled trial. Int J Clin Pract 2010;64:1619-31. 10.1111/j.1742-1241.2010.02510.x. 20846286. [DOI] [PubMed] [Google Scholar]
- 74.AstraZeneca. Treatment effect of saxagliptin compared with placebo in patients with type 2 diabetes and renal impairment. National Library of Medicine (US), 2000. https://clinicaltrials.gov/show/NCT00614939.
- 75.Nowicki M, Rychlik I, Haller H, et al. Long-term treatment with the dipeptidyl peptidase-4 inhibitor saxagliptin in patients with type 2 diabetes mellitus and renal impairment: a randomised controlled 52-week efficacy and safety study. Int J Clin Pract 2011;65:1230-9. 10.1111/j.1742-1241.2011.02812.x. 21977965. [DOI] [PubMed] [Google Scholar]
- 76.Nowicki M, Rychlik I, Haller H, Warren ML, Suchower L, Gause-Nilsson I. D1680C00007 Investigators. Saxagliptin improves glycaemic control and is well tolerated in patients with type 2 diabetes mellitus and renal impairment. Diabetes Obes Metab 2011;13:523-32. 10.1111/j.1463-1326.2011.01382.x. 21332627. [DOI] [PubMed] [Google Scholar]
- 77.Boehringer Ingelheim Pharmaceuticals. Efficacy and safety of BI 1356 in combination with metformin in patients with type 2 diabetes. National Library of Medicine (US), 2000. https://clinicaltrials.gov/show/NCT00622284.
- 78.Gallwitz B, Rosenstock J, Rauch T, et al. 2-year efficacy and safety of linagliptin compared with glimepiride in patients with type 2 diabetes inadequately controlled on metformin: a randomised, double-blind, non-inferiority trial. Lancet 2012;380:475-83. 10.1016/S0140-6736(12)60691-6. 22748821. [DOI] [PubMed] [Google Scholar]
- 79.Johnson & Johnson Pharmaceutical Research & Development. L.L.C. An efficacy, safety, and tolerability study of canagliflozin (JNJ-28431754) in patients with type 2 diabetes. National Library of Medicine (US), 2000. https://clinicaltrials.gov/show/NCT00642278.
- 80.Rosenstock J, Aggarwal N, Polidori D, et al. Canagliflozin DIA 2001 Study Group. Dose-ranging effects of canagliflozin, a sodium-glucose cotransporter 2 inhibitor, as add-on to metformin in subjects with type 2 diabetes. Diabetes Care 2012;35:1232-8. 10.2337/dc11-1926. 22492586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Takeda. Efficacy and safety of alogliptin compared to glipizide in elderly diabetics. National Library of Medicine (US), 2000. https://clinicaltrials.gov/show/NCT00707993.
- 82.Rosenstock J, Wilson C, Fleck P. Alogliptin versus glipizide monotherapy in elderly type 2 diabetes mellitus patients with mild hyperglycaemia: a prospective, double-blind, randomized, 1-year study. Diabetes Obes Metab 2013;15:906-14. 10.1111/dom.12102. 23531118. [DOI] [PubMed] [Google Scholar]
- 83.Bristol-Myers Squibb. AstraZeneca. Safety and efficacy of saxagliptin plus insulin with or without metformin. National Library of Medicine (US), 2000. https://clinicaltrials.gov/show/NCT00757588.
- 84.Barnett AH, Charbonnel B, Donovan M, Fleming D, Chen R. Effect of saxagliptin as add-on therapy in patients with poorly controlled type 2 diabetes on insulin alone or insulin combined with metformin. Curr Med Res Opin 2012;28:513-23. 10.1185/03007995.2012.665046. 22313154. [DOI] [PubMed] [Google Scholar]
- 85.Boehringer Ingelheim Pharmaceuticals. Safety and efficacy of linagliptin (BI 1356) plus metformin in type 2 diabetes, factorial design. National Library of Medicine (US), 2000. https://clinicaltrials.gov/show/NCT00798161.
- 86.Haak T, Meinicke T, Jones R, Weber S, von Eynatten M, Woerle HJ. Initial combination of linagliptin and metformin improves glycaemic control in type 2 diabetes: a randomized, double-blind, placebo-controlled study. Diabetes Obes Metab 2012;14:565-74. 10.1111/j.1463-1326.2012.01590.x. 22356132. [DOI] [PubMed] [Google Scholar]
- 87.GlaxoSmithKline. Efficacy and safety of albiglutide in treatment of type 2 diabetes. National Library of Medicine (US), 2000. https://clinicaltrials.gov/show/NCT00838903.
- 88.Ahrén B, Johnson SL, Stewart M, et al. HARMONY 3 Study Group. HARMONY 3: 104-week randomized, double-blind, placebo- and active-controlled trial assessing the efficacy and safety of albiglutide compared with placebo, sitagliptin, and glimepiride in patients with type 2 diabetes taking metformin. Diabetes Care 2014;37:2141-8. 10.2337/dc14-0024. 24898304. [DOI] [PubMed] [Google Scholar]
- 89.Takeda. Efficacy and safety of alogliptin plus metformin compared to glipizide plus metformin in patients with type 2 diabetes mellitus (ENDURE). National Library of Medicine (US), 2000. https://clinicaltrials.gov/show/NCT00856284.
- 90.Del Prato S, Camisasca R, Wilson C, Fleck P. Durability of the efficacy and safety of alogliptin compared with glipizide in type 2 diabetes mellitus: a 2-year study. Diabetes Obes Metab 2014;16:1239-46. 10.1111/dom.12377. 25132212. [DOI] [PubMed] [Google Scholar]
- 91.Boehringer Ingelheim Pharmaceuticals; Eli Lilly and Company. Efficacy and safety of linagliptin in combination with insulin in patients with type 2 diabetes. National Library of Medicine (US), 2000. https://clinicaltrials.gov/show/NCT00954447.
- 92.AstraZeneca. Bristol-Myers Squibb. Saxagliptin compared to glimepiride in elderly type 2 diabetes patients, with inadequate glycemic control on metformin (GENERATION). National Library of Medicine (US), 2000. https://clinicaltrials.gov/show/NCT01006603.
- 93.Merck. Safety and efficacy of sitagliptin compared with glimepiride in elderly participants with type 2 diabetes mellitus (MK-0431-251 AM2). National Library of Medicine (US), 2000. https://clinicaltrials.gov/show/NCT01189890.
- 94.Takeda Pharmaceutical Company Limited. Efficacy and safety of alogliptin used in combination with α-glucosidase inhibitor in participants with type 2 diabetes in Japan. National Library of Medicine (US), 2000. https://clinicaltrials.gov/show/NCT01263483.
- 95.Seino Y, Fujita T, Hiroi S, Hirayama M, Kaku K. Alogliptin plus voglibose in Japanese patients with type 2 diabetes: a randomized, double-blind, placebo-controlled trial with an open-label, long-term extension. Curr Med Res Opin 2011;27(Suppl 3):21-9. 10.1185/03007995.2011.614936. 22106975. [DOI] [PubMed] [Google Scholar]
- 96.Ingelheim B; Eli Lilly and Company. Safety and efficacy of empagliflozin (BI 10773) and sitagliptin versus placebo over 76 weeks in patients with type 2 diabetes. National Library of Medicine (US), 2000. https://clinicaltrials.gov/show/NCT01289990.
- 97.Pratley RE, Reusch JEB, Fleck PR, Wilson CA, Mekki Q. Alogliptin Study 009 Group. Efficacy and safety of the dipeptidyl peptidase-4 inhibitor alogliptin added to pioglitazone in patients with type 2 diabetes: a randomized, double-blind, placebo-controlled study. Curr Med Res Opin 2009;25:2361-71. 10.1185/03007990903156111. 19650752. [DOI] [PubMed] [Google Scholar]
- 98.Takeda. Study of alogliptin combined with pioglitazone in subjects with type 2 diabetes mellitus. National Library of Medicine (US), 2000. https://clinicaltrials.gov/show/NCT00286494.
- 99.Pratley RE, Fleck P, Wilson C. Efficacy and safety of initial combination therapy with alogliptin plus metformin versus either as monotherapy in drug-naïve patients with type 2 diabetes: a randomized, double-blind, 6-month study. Diabetes Obes Metab 2014;16:613-21. 10.1111/dom.12258. 24400655. [DOI] [PubMed] [Google Scholar]
- 100.Rosenstock J, Brazg R, Andryuk PJ, Lu K, Stein P. Sitagliptin Study 019 Group. Efficacy and safety of the dipeptidyl peptidase-4 inhibitor sitagliptin added to ongoing pioglitazone therapy in patients with type 2 diabetes: a 24-week, multicenter, randomized, double-blind, placebo-controlled, parallel-group study. Clin Ther 2006;28:1556-68. 10.1016/j.clinthera.2006.10.007. 17157112. [DOI] [PubMed] [Google Scholar]
- 101.Rosenstock J, Inzucchi SE, Seufert J, Fleck PR, Wilson CA, Mekki Q. Initial combination therapy with alogliptin and pioglitazone in drug-naïve patients with type 2 diabetes. Diabetes Care 2010;33:2406-8. 10.2337/dc10-0159. 20724648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Seino Y, Miyata Y, Hiroi S, Hirayama M, Kaku K. Efficacy and safety of alogliptin added to metformin in Japanese patients with type 2 diabetes: a randomized, double-blind, placebo-controlled trial with an open-label, long-term extension study. Diabetes Obes Metab 2012;14:927-36. 10.1111/j.1463-1326.2012.01620.x. 22583697. [DOI] [PubMed] [Google Scholar]
- 103.Yang HK, Min KW, Park SW, et al. A randomized, placebo-controlled, double-blind, phase 3 trial to evaluate the efficacy and safety of anagliptin in drug-naïve patients with type 2 diabetes. Endocr J 2015;62:449-62. 10.1507/endocrj.EJ14-0544. 25819061. [DOI] [PubMed] [Google Scholar]
- 104.Krum K, Lukashevich V, Bolli GB, Kozlovski P, Kothny W, Ponikowski P. No significant difference in risk of heart failure hospitalization with vildagliptin in diabetic patients with systolic chronic heart failure: VIVIDD Study. American Diabetes Association 74th Scientific Sessions (2014): 1028-P-2014. Presented on June 15, 2014.
- 105.Laakso M, Rosenstock J, Groop PH, et al. Treatment with the dipeptidyl peptidase-4 inhibitor linagliptin or placebo followed by glimepiride in patients with type 2 diabetes with moderate to severe renal impairment: a 52-week, randomized, double-blind clinical trial. Diabetes Care 2015;38:e15-7. 10.2337/dc14-1684. 25614693. [DOI] [PubMed] [Google Scholar]
- 106.AstraZeneca. Bristol-Myers Squibb. Does saxagliptin reduce the risk of cardiovascular events when used alone or added to other diabetes medications (SAVOR- TIMI 53). National Library of Medicine (US), 2000. https://clinicaltrials.gov/show/NCT01107886.
- 107.Takeda. Cardiovascular outcomes study of alogliptin in patients with type 2 diabetes and acute coronary syndrome(EXAMINE). National Library of Medicine (US), 2000. https://clinicaltrials.gov/show/NCT00968708.
- 108.White WB, Cannon CP, Heller SR, et al. EXAMINE Investigators. Alogliptin after acute coronary syndrome in patients with type 2 diabetes. N Engl J Med 2013;369:1327-35. 10.1056/NEJMoa1305889. 23992602. [DOI] [PubMed] [Google Scholar]
- 109.Gitt AK, Bramlage P, Binz C, Krekler M, Deeg E, Tschöpe D. Prognostic implications of DPP-4 inhibitor vs. sulfonylurea use on top of metformin in a real world setting - results of the 1 year follow-up of the prospective DiaRegis registry. Int J Clin Pract 2013;67:1005-14. 10.1111/ijcp.12179. 23981060. [DOI] [PubMed] [Google Scholar]
- 110.Sharp M, Corp D. An Observational Study of Type II Diabetics Treated With Dual Therapy With or Without Sitagliptin (Januvia®/Xelevia®, MK-0431-201). National Library of Medicine (US), 2000. https://clinicaltrials.gov/show/NCT01357135.
- 111.Kannan S, Pantalone KM, Matsuda S, Wells BJ, Karafa M, Zimmerman RS. Risk of overall mortality and cardiovascular events in patients with type 2 diabetes on dual drug therapy including metformin: A large database study from the Cleveland Clinic. J Diabetes 2015; [Epub ahead of print]. 10.1111/1753-0407.12301. .25929426. [DOI] [PubMed]
- 112.Eurich DT, Weir DL, Simpson SH, Senthilselvan A, McAlister FA. Risk of new onset heart failure in patients using sitagliptin. American Diabetes Association 74th Scientific Sessions (2014): 135-LB-2014. Presented on 15 June, 2014.
- 113.Seong JM, Choi NK, Shin JY, et al. Differential cardiovascular outcomes after dipeptidyl peptidase-4 inhibitor, sulfonylurea, and pioglitazone therapy, all in combination with metformin, for type 2 diabetes: a population-based cohort study. PLoS One 2015;10:e0124287. 10.1371/journal.pone.0124287. 25992614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Suh S, Seo GH, Jung CH, et al. Increased risk of hospitalization for heart failure with newly prescribed dipeptidyl peptidase-4 inhibitors and pioglitazone using the Korean health insurance claims database. Diabetes Metab J 2015;39:247-52. 10.4093/dmj.2015.39.3.247. 26124995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Velez M, Peterson EL, Wells K, et al. Association of antidiabetic medications targeting the glucagon-like peptide 1 pathway and heart failure events in patients with diabetes. J Card Fail 2015;21:2-8. 10.1016/j.cardfail.2014.10.012. 25451709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Chen DY, Wang SH, Mao CT, et al. Sitagliptin and cardiovascular outcomes in diabetic patients with chronic kidney disease and acute myocardial infarction: A nationwide cohort study. Int J Cardiol 2015;181:200-6. 10.1016/j.ijcard.2014.12.029. 25528312. [DOI] [PubMed] [Google Scholar]
- 117.Wu S, Hopper I, Skiba M, Krum H. Dipeptidyl peptidase-4 inhibitors and cardiovascular outcomes: meta-analysis of randomized clinical trials with 55,141 participants. Cardiovasc Ther 2014;32:147-58. 10.1111/1755-5922.12075. 24750644. [DOI] [PubMed] [Google Scholar]
- 118.Monami M, Dicembrini I, Mannucci E. Dipeptidyl peptidase-4 inhibitors and heart failure: a meta-analysis of randomized clinical trials. Nutr Metab Cardiovasc Dis 2014;24:689-97. 10.1016/j.numecd.2014.01.017. 24793580. [DOI] [PubMed] [Google Scholar]
- 119.Clifton P. Do dipeptidyl peptidase IV (DPP-IV) inhibitors cause heart failure?Clin Ther 2014;36:2072-9. 10.1016/j.clinthera.2014.10.009. 25453730. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Web appendix 1: Search strategies
Web appendix 2: Risk of bias of included randomised controlled trials
Web appendix 3: Supplementary forest plots
Web appendix 4: Risk of bias of included cohort studies
Web appendix 5: Risk of bias of included case-control studies