Abstract
BACKGROUND
Babesia infection is caused by intraerythrocytic tickborne parasites. Cases of transfusion-transmitted babesiosis have been increasingly recognized. To date, no Babesia test has been licensed for screening US blood donors. We conducted a longitudinal study to assess the course and markers of Babesia infection among seropositive donors identified in a seroprevalence study.
STUDY DESIGN AND METHODS
Eligible donors had B. microti indirect fluorescent antibody (IFA) titers ≥1:64. Enrollees were monitored up to 3 years, by IFA and three methods for evidence of parasitemia: B. microti nested PCR analysis (at two laboratories), hamster inoculation, and blood-smear examination.
RESULTS
Among 115 eligible donors, 84 (73%) enrolled. Eighteen enrollees (21%) had evidence of parasitemia for 30 total specimens (17% of 181), which were collected in 9 different months and tested positive by various approaches: PCR (25 specimens/16 persons), hamster inoculation (13 specimens/8 persons), and blood smear (1 specimen positive by all three approaches). Overall, 14 persons had ≥1 specimen with positive PCR results at both laboratories (12 persons) and/or had parasitologically confirmed infection (8 persons). Three of nine persons who had >1 specimen with evidence of parasitemia had nonconsecutive positives. Several enrollees likely had been infected ≥1 year when their last positive specimen was collected. The final three specimens for seven persons tested negative by all study methods, including IFA.
CONCLUSION
Seropositive blood donors can have protracted low-level parasitemia that is variably and intermittently detected by parasitologic and molecular methods. Donor-screening algorithms should include serologic testing and not solely rely on molecular testing.
Keywords: babesiosis, Babesia microti, transfusion, blood donor, donor screening, polymerase chain reaction, hamster inoculation, parasitemia
INTRODUCTION
Human babesiosis is caused by intraerythrocytic protozoan parasites, which are tickborne in nature but also are transmissible via blood transfusion.1–11 Most of the documented US cases of babesiosis have been caused by Babesia microti, which is transmitted by Ixodes scapularis ticks in the Northeast and upper Midwest, primarily during the spring and summer.1–3 B. microti infection can range from asymptomatic to severe. Persons, such as transfusion recipients, who are asplenic, elderly, premature, or immunocompromised are at increased risk for clinically manifest and life-threatening infection.
More than 160 US cases of transfusion-transmitted babesiosis (TTB) have been identified during the 3 decades since the first described TTB case in 1979,12 most (>75%) of which occurred during the last decade.1 To date, no Babesia test has been licensed by the US Food and Drug Administration (FDA) for screening blood donors1,4–6; donor-screening algorithms do not routinely include testing for evidence of Babesia infection.1 Although donors routinely are asked if they have a “history of babesiosis,”6,7 persons with undiagnosed asymptomatic infection can fulfill all criteria for donating blood despite having low levels of potentially transmissible bloodstream parasites, which can suffice to cause infection in transfusion recipients.1
Relatively few B. microti-infected persons have been monitored systematically for extended periods,13–15 most of whom initially had symptomatic acute cases of babesiosis. We assessed the course and laboratory markers of B. microti infection in settings relevant to transfusion medicine by conducting a longitudinal study among seropositive blood donors, who were evaluated up to 3 years, by serologic, parasitologic, and molecular methods as well as structured questionnaires. Although the study was not designed to evaluate the performance of particular methods as diagnostic or donor-screening assays, our findings pertain to the development and implementation of donor-testing and management strategies.
MATERIALS AND METHODS
Study design and enrollment
Seropositive donors whose B. microti indirect fluorescent antibody (IFA) titer was ≥1:64 on initial testing during May 2000 through April 2004 in a previously described seroprevalence study16 were eligible to enroll in the longitudinal study, which began in June 2000; the last study specimen was collected in July 2006. In the seroprevalence study, donors in southeastern Connecticut (Middlesex and New London Counties) were targeted initially; the catchment area gradually expanded within Connecticut, and donors in Massachusetts (Dukes and Nantucket Counties) were added in 2003.
The protocol for the longitudinal study was approved by the institutional review boards of the American Red Cross (ARC) and the Centers for Disease Control and Prevention (CDC). On enrollment, participants provided written informed consent and their first study specimen, referred to as their enrollment specimen. Each study specimen comprised three tubes of blood, which were collected by regional ARC staff and shipped at 4°C on wet ice to the ARC’s Holland Laboratory (one serum-separator tube and one EDTA tube) and to CDC (one EDTA tube). The specimens were tested by IFA (at the ARC) and by three methods for evidence of parasitemia: two parasitologic methods (blood-smear examination and animal inoculation at CDC) and one molecular method (nested PCR analysis at both laboratories). In the data analyses, positive results by any of these three methods, at either laboratory, constituted evidence of parasitemia. Unless otherwise specified, positive and tested positive refer to evidence of parasitemia rather than to seropositivity. Participants who had positive results were encouraged to share them with their physician and were given contact information for a clinical babesiosis expert. Study subjects were asked to provide a specimen every 2–3 months (monthly, if they had evidence of parasitemia) until they had three consecutive specimens with negative results by all methods, including IFA, or 3 years had elapsed.
Laboratory methods
The ARC conducted the serologic testing using a nonautomated IFA assay for immunoglobulin G antibodies to B. microti antigens; IFA slides and reagents were purchased from Focus Technologies, Inc. (Cypress, CA). If seroreactivity was noted at the lowest dilution of serum tested (1:64), the specimen was defined as IFA positive (in accordance with the manufacturer’s instructions and the protocol for the seroprevalence study in which eligible subjects were identified) and was tested to endpoint in serial 2-fold dilutions.17 Of note, the study was not designed to evaluate this particular IFA assay or cut-off (1:64) for donor-testing purposes. Positive and negative controls were used. The same positive control serum specimen was used throughout the study; when B. microti antigen lots changed, the positive control was used to certify the new lot and was observed to perform consistently. Because of the subjectivity inherent to determining the endpoint titer in this nonautomated assay, only highly trained, designated staff conducted the testing. After completion of the study, serial specimens from multiple subjects were retested in parallel, on the same day.
CDC conducted the parasitologic testing: two thick and two thin Giemsa-stained blood smears (10 µL per smear) were examined for Babesia parasites by light microscopy, under oil immersion. In addition, two golden Syrian hamsters (Mesocricetus auratus) were inoculated intraperitoneally with 1-mL aliquots of whole blood and were monitored weekly, by examination of Giemsa-stained thin blood smears, until parasites were noted or 8 weeks had elapsed. Hamsters are competent (amplifying) hosts of B. microti, which is not cultivable in vitro. CDC’s Institutional Animal Care and Use Committee approved animal experiments and procedures.
The ARC and CDC independently analyzed blood specimens by PCR, using primers designed to amplify B. microti DNA from the 18S ribosomal RNA gene18 and a previously described 2-step nested PCR protocol.9,16,17 Total DNA was extracted from 200 µL of whole blood (i.e., a 5-fold lower volume than was inoculated into each hamster), by using the QIAamp DNA Blood Mini Kit (Qiagen, Inc., Valencia, CA). An aliquot of extracted DNA was amplified with primers Bab1 and Bab4, the product was amplified further with internal primers Bab2 and Bab3, and the final product was visualized in a 2% agarose gel stained with ethidium bromide. Positive, negative, and extraction controls were included. DNA extraction, amplification, and electrophoretic analysis were conducted in physically separate work areas; and other standard measures (e.g., irradiation with ultraviolet light) were used to prevent contamination.
Questionnaires
Epidemiologic and clinical data were obtained via structured questionnaires. An extensive “long” questionnaire, which focused on the previous 24 months, was included in the enrollment packet and was completed on site or submitted later. It addressed demographic factors, places of residence and travel, outdoor activities, tick exposures, and clinical data (e.g., flu-like symptoms, anti-Babesia therapy, surgical splenectomy). Persons with tick exposures were asked if the ticks were attached (difficult to pull off) and if they were small versus large, in comparison with unlabeled photographs of I. scapularis versus Dermacentor variabilis ticks, respectively; duration of attachment and tick engorgement were not assessed. During all study visits, participants were asked to complete a “short” questionnaire, which addressed interim activities, exposures, symptoms, and treatment.
Data analysis
Univariate analyses were conducted for descriptive purposes. Proportions were compared by using the chi-square test or, if expected cell counts were <5, the Fisher’s exact test. The Wilcoxon two-sample test was used to compare the ranked distributions of ordinal variables. The serologic results obtained using a nonautomated IFA assay are provided/analyzed for illustrative purposes, even though the absolute magnitude of the titers might not always be reproducible or generalizable to other laboratories. In analyses of the distributions of the serologic data, log2 values were used, from 5 (for an IFA result of <1:64) to 10 (for a titer of 1:1024, the highest documented in the study). Statistical significance was defined as a 2-tailed p value <0.05.
RESULTS
Eighty-four (73%) of 115 eligible B. microti–seropositive donors enrolled in the longitudinal study. Demographic and serologic data for the 84 who enrolled and the 31 who declined to participate were not significantly different (data not shown). The 84 enrollees had a median age of 50 years and 54 (64%) were men. On enrollment, 60 persons (71%) still had an IFA titer ≥1:64, whereas 24 (29%) were seronegative; the median interval between collection of the initial and the enrollment specimens was 51 days (Table 1). In aggregate, the 84 enrollees provided 540 study specimens over a 6-year period.
TABLE 1. Characteristics of the 84 Babesia microti–seropositive study participants, stratified by evidence of parasitemia.
| Ever tested positive | |||||
|---|---|---|---|---|---|
| Variable | All study subjects (n = 84) |
Yes (n = 18) | No (n = 66) | P value | Comments |
| Median age, years (range; IQR) | 50 (19–79; 41–61) | 52 (26–79; 39–62) | 49 (19–79; 41–61) | 0.7 | |
| No. (%) male | 54 (64) | 12 (67) | 42 (64) | 0.8 | |
| No. (%) who donated blood in Connecticut* | 77 (92) | 17 (94) | 60 (91) | 1.0 | |
| No. (%) who completed the "long" questionnaire†,‡ | 80 (95) | 16 (89) | 64 (97) | 0.2 | |
| No./total no. (%) who lived in a rural area† | 49/80 (61) | 11/16 (69) | 38/64 (59) | 0.5 | |
| No./total no. (%) who had ≥1 acre of undeveloped land | 56/79 (71) | 13/16 (81) | 43/63 (68) | 0.4 | |
| No./total no. (%) who saw deer on property or nearby | 73/80 (91) | 14/16 (88) | 59/64 (92) | 0.4 | |
| Median year of initial IFA testing (IQR) | 2001 (2001–2003) | 2000 (2000–2002) | 2002 (2001–2003) | 0.005§ | |
| No. (%) by year | |||||
| 2000‖ | 18 (21) | 10 (56)‖ | 8 (12) | <0.001 | Comparison of 2000 vs. later‖ |
| 2001 | 25 (30) | 2 (11) | 23 (35) | Subjects K and N | |
| 2002 | 14 (17) | 3 (17) | 11 (17) | Subjects H, O, and P | |
| 2003 | 25 (30) | 3 (17) | 22 (33) | Subjects E, Q, and R | |
| 2004 | 2 (2) | 0 | 2 (3) | ||
| Median month of initial IFA testing (range; IQR) | Aug (Mar–Dec; Jul–Oct) | Jul (May–Nov; Jul–Aug) | Aug (Mar–Dec; Jul–Oct) | 0.2 | |
| Median month of enrollment (range; IQR) | Sep (Jan–Dec; Aug–Nov) | Sep (Jan–Nov; Aug–Oct) | Oct (Jan–Dec; Aug–Nov) | 0.2 | |
| Median initial IFA titer (IQR) | 1:64 (1:64–1:128) | 1:256 (1:128–1:512) | 1:128 (1:128–1:128) | <0.001§ | |
| No. (%) by titer | |||||
| 1:64 | 46 (55) | 4 (22) | 42 (64) | 0.002 | Comparison of 1:64 vs. >1:64 |
| 1:128 | 20 (24) | 4 (22) | 16 (24) | ||
| 1:256 | 6 (7) | 3 (17) | 3 (5) | ||
| 1:512 | 8 (10) | 5 (28) | 3 (5) | Subjects D, J, L, O, and R | |
| 1:1024 | 4 (5) | 2 (11) | 2 (3) | Subjects E and P | |
| ≥1:512¶ | 12 (14) | 7 (39) | 5 (8)¶ | 0.003 | Comparison of ≥1:512 vs. <1:512 |
| Median enrollment IFA titer (IQR) | 1:64 (<1:64–1:128) | 1:256 (1:128–1:512) | 1:64 (<1:64–1:128) | <0.001§ | |
| No. (%) by titer | |||||
| <1:64 (IFA negative**) | 24 (29) | 2 (11) | 22 (33) | 0.06 | Comparison of <1:64 vs. ≥1:64 |
| 1:64 | 25 (30) | 1 (6) | 24 (36) | ||
| ≤1:64 | 49 (58) | 3 (17) | 46 (70) | <0.001 | Comparison of ≤1:64 vs. >1:64 |
| 1:128 | 19 (23) | 6 (33) | 13 (20) | ||
| 1:256 | 8 (10) | 2 (11) | 6 (9) | ||
| 1:512 | 5 (6) | 5 (28) | 0 | Subjects A, J, N, P, and R | |
| 1:1024 | 3 (4) | 2 (11) | 1 (2)¶ | Subjects E and O | |
| ≥1:512¶ | 8 (10) | 7 (39) | 1 (2)¶ | <0.001 | Comparison of ≥1:512 vs. <1:512 |
| Subtotal no. who received anti-Babesia treatment | 10 | 6 | 4 | Self-reported data; could be incomplete | |
| No. (%) pretreated (i.e., before enrollment) | 6 (7) | 2 (11) | 4 (6) | 0.4 | Comparison of pretreated vs. not pretreated |
| No. treated after enrollment | 4 | 4 | 0 | ||
| Median no. of study specimens (range; IQR); total†† | 5 (1–17; 3–10); n = 540 | 11 (4–17; 6–14); n = 181 | 5 (1–13; 3–7); n = 359 | <0.001 | |
| Median no. of positive specimens (range); total | – | 2 (1–4); n = 30 | – | ||
| Median interval, days (range; IQR) | |||||
| Initial specimen to enrollment specimen¶ | 51 (9–296; 28–67) | 44 (25–94; 28–55) | 52 (9–296; 28–71)¶ | 0.3 | |
| Initial specimen to last positive specimen | – | 75 (28–770; 54–229) | – | Does not constitute duration of infection | |
| Initial specimen to last study specimen | 409 (26–1,275; 232–757) | 740 (346–1,141; 372–1,078) | 366 (26–1,275; 193–624) | 0.001 | |
| Enrollment specimen to last study specimen | 348 (0–1,087; 184–693) | 677 (295–1,087; 329–1,023) | 318 (0–1,057; 150–566) | <0.001 | |
Unless otherwise specified, no. (%) refers to persons; percentages might not total 100 because of rounding. The word initial refers to the preenrollment specimen/testing in the seroprevalence study. The 18 persons who ever tested positive for evidence of parasitemia are referred to as subjects A through R (Fig. 1). Abbreviations: ARC, American Red Cross; CDC, Centers for Disease Control and Prevention; IFA, indirect fluorescent antibody assay; IQR, interquartile range.
The 77 Connecticut donors include 75 Connecticut residents (16 tested positive) and two Rhode Island residents (one tested positive). The seven Massachusetts donors include six Massachusetts residents (one tested positive) and one Vermont resident.
All 84 enrollees completed the “short” questionnaire at least once. Among the 80 who completed the “long” questionnaire, 76 (95%) described their locale as rural (49 persons) or suburban (31 persons).
All 83 enrollees with available data reported at least one of the following outdoor activities in potential risk areas: doing lawn/yard work, with or without clearing land/brush (79 of 81 persons); gardening (59 of 75); walking (69 of 77); or hiking (48 of 75).
The results were comparable if data for subjects G, M, Q, and R, who had evidence of parasitemia only by PCR, at one laboratory (Fig. 1), were excluded.
Overall, 10 (56%) of the 18 persons with evidence of parasitemia had their initial IFA testing in 2000; the eight (44%) whose initial testing was during 2001–2003 account for five (63%) of the eight total persons who tested positive by hamster inoculation.
Of the 66 without evidence of parasitemia, five (8%) had IFA titers ≥1:512 preenrollment, only one of whom had a titer ≥1:512 on enrollment (see text); three of the other four persons had prolonged intervals between their initial and enrollment specimens (i.e., 71, 120, and 218 days).
Of the 24 who were seronegative on enrollment, 12 (14% of 84) did not have any IFA-positive study specimens, one of whom (subject F) had positive PCR results at both laboratories on enrollment (Fig. 1).
Of the 540 study specimens, seven (7%) were tested by PCR only at CDC (vs. also at the ARC): the final three specimens from each of two subjects (E and R) who previously had had positive results (Fig. 1); and the penultimate specimen from one of the 66 subjects who never had evidence of parasitemia by any method (PCR, hamster inoculation, or blood smear). Overall, six (9%) of the 66 persons without evidence of parasitemia provided only one study specimen.
Eighteen enrollees (21%)—referred to as subjects A though R (Fig. 1)—tested positive for evidence of parasitemia, for a total of 30 specimens (17% of 181) (Table 1). The epidemiologic profiles of these 18 persons with evidence of parasitemia and the 66 enrollees without demonstrable parasitemia were comparable in univariate analyses. However, in aggregate, these 18 persons had higher IFA titers on initial testing and on enrollment (Table 1). The median IFA result for the 30 specimens that had evidence of parasitemia was 1:256 (range, <1:64 to 1:1024).
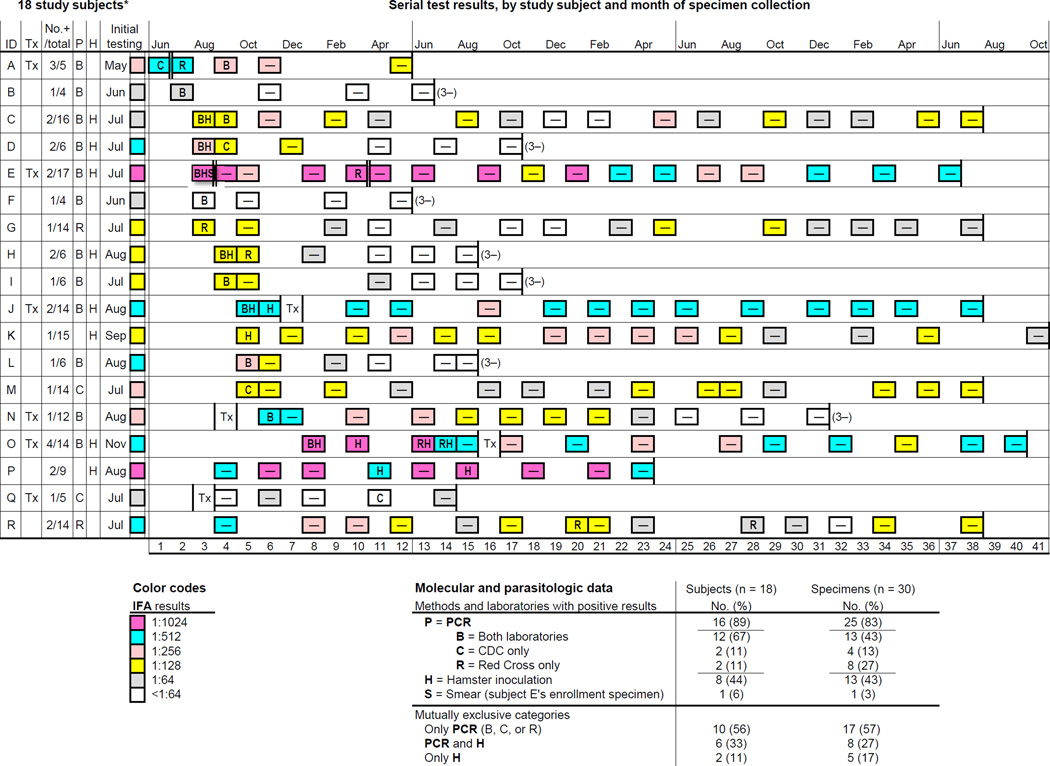
Fig. 1. Characteristics of and serial laboratory data for the 18 Babesia microti–seropositive study subjects with evidence of parasitemia.
These 18 subjects (A through R) provided a total of 181 study specimens, 30 (17%) of which tested positive for evidence of parasitemia by at least one of three methods: PCR analysis (P), at the Centers for Disease Control and Prevention (CDC; C), the Red Cross (R), or both laboratories (B); hamster inoculation (H); and blood-smear examination (S). The specimens were tested by PCR at both laboratories, with the exception of the final three specimens from subjects E and R, which were tested only at CDC. See right bottom for a summary of the molecular and parasitologic results; percentages might not total 100 because of rounding. See left bottom for the color codes used to indicate the indirect fluorescent antibody (IFA) results. The left-hand columns specify each subject’s letter identification (ID), self-reported history of anti-Babesia treatment (Tx), and the numbers of positive and total study specimens (No.+/total). The P and H columns indicate which subjects ever had positive results by PCR and hamster inoculation, respectively. In column P, the summary PCR results (B, C, R, or blank) are mutually exclusive. The initial-testing column specifies the month of collection and the IFA results for the preenrollment specimen tested in a separate study. In the timelines, the 6-year study period was normalized to 3.4 years (41 months); data for the eight subjects whose initial testing was after 2000 were shifted back from 1 to 3 years. The results for the 181 study specimens are provided by month of collection (see column headers); because the IFA results are provided for the preenrollment specimen as well as all 181 study specimens, the data can be reanalyzed ad hoc using different criteria for classifying a specimen as IFA positive (and, therefore, for considering a person eligible to enroll in the study and for releasing the study subject after <3 years of monitoring). For the pertinent subjects, the timing of therapy is shown; for subjects A and E, the timing is indicated by a double-lined border between specimens in consecutive months (E was treated twice). The 1-month data columns are numbered (see footers) to facilitate ad hoc calculations of intervals. The data boxes for the 30 specimens with evidence of parasitemia include the pertinent letters for the positive molecular/parasitologic results and the background color indicative of the IFA results. The data boxes for the other 151 study specimens have a centered negative sign; a white box with a negative sign indicates that the IFA results also were negative (<1:64). For seven subjects, the final three specimens tested negative by all study methods, as indicated by “(3–)”; their last three specimens were collected over a median of 6 months (range, 4–7).
* The 18 subjects are listed in order of the month of their first study specimen with evidence of parasitemia and by the number and clustering of positive specimens; the first 15 subjects tested positive on enrollment. Several subjects found small ticks attached to their skin at various intervals before their preenrollment testing: the interval was ≤1 month for subjects E, L, and Q; and was approximately 3 months for subject J. Subjects C, D, and G also reportedly found small attached ticks; but the timing was unclear. For subjects G, M, and Q—each of whom had only one specimen with evidence of parasitemia, by PCR at one laboratory—CDC repeated the PCR analyses after reextracting DNA from an aliquot of the pertinent specimen. Upon retesting, CDC’s results were positive for subjects G and M and were negative for subject Q. CDC’s results for the first extraction are shown; the Red Cross did not retest the specimens. Of note, subject A’s first two specimens still had discordant PCR results on repeat testing at CDC.
Serial laboratory data for these 18 enrollees are depicted in timelines (Fig. 1; see right bottom for summary data). Their 30 positive specimens had evidence of parasitemia by various permutations and combinations of methods and laboratories—i.e., by PCR analysis at either laboratory (25 specimens), hamster inoculation (13 specimens), and blood-smear examination (one specimen). In the study as a whole, including all 84 participants, the PCR results were concordant for 521 (98%) of the 533 specimens that were tested by both laboratories (Table 1 and Fig. 1). Of the 25 PCR-positive specimens, 12 (48%) had positive results at only one laboratory: these 12 specimens, which account for the overall discordance rate of 2%, were from nine persons, five of whom (A, D, E, H, and O) had other specimens that tested positive by PCR at both laboratories or by hamster inoculation. Overall, 14 (of 18) persons had positive PCR results at both laboratories and/or had parasitologically confirmed infection, four of whom (A, C, D, and P) also had positive lookback investigations—i.e., a B. microti PCR-positive recipient of red blood cells (RBCs) they had donated was identified.17
Overall, nine of the 18 persons had >1 specimen with evidence of parasitemia: six persons had consecutive positive specimens but not necessarily by the same methods or laboratories; and three persons had positive results for nonconsecutive specimens. For example, subject P had two nonconsecutive hamster-positive specimens. Of interest, he did not have demonstrable parasitemia until his fourth study specimen (in April), even though all of his specimens had IFA titers ≥1:512 and a lookback investigation of his blood donation the previous July was positive; >1 year after that July donation, his second hamster-positive specimen (his sixth study specimen) was collected (Fig. 1).
Six of the 18 persons reported receipt of anti-Babesia therapy, four of whom had posttreatment positive PCR results, including subjects N and Q, who were treated preenrollment, and subjects A and E, who were treated after they enrolled (Fig. 1). Subjects N, A, and E are particularly illustrative. Subject N, the only asplenic participant, became acutely ill, was hospitalized, and started a several-week course of anti-Babesia therapy 10 days after his initial IFA testing in August. His enrollment specimen in November, 56 days posttreatment, had positive PCR results. Subjects A and E, who were 32 and 79 years old, respectively, tested positive before and after they were treated. Subject A was treated between the first and second of her three consecutive PCR-positive specimens, which were collected in June, July, and September, 58 days posttreatment (Fig. 1). The fact that a lookback investigation of her blood donation the previous December was positive suggests that she had protracted infection: if she became infected during the preceding spring-or-summer tick season, the interval from acquisition of infection to her last PCR-positive specimen was >1 year. Subject E also likely had protracted infection. His enrollment specimen in August had positive results by all modalities, including blood-smear examination (<1% of the RBCs were infected). He was treated in September and was retreated 7 months later, after PCR positivity was noted again in March.
Subject O, who did not have positive PCR results after treatment, also is noteworthy (Fig. 1): she had four consecutive specimens with evidence of parasitemia; all four tested positive by hamster inoculation, but the PCR results varied; the first two hamster-positive specimens were collected during winter months, in January and March; the other two were from June and July, approximately 1 year after the preceding tick season; and she was seropositive throughout the 3-year period from her preenrollment specimen to her final study specimen (IFA titer, 1:512), which was collected 2 years posttreatment.
Overall, at least three persons (A, O, and P)—two of whom (A and P) had positive lookback investigations—likely had been infected for approximately 1 year or longer when their last specimen with evidence of parasitemia was collected, which tested positive by hamster inoculation or by PCR at both laboratories (i.e., the probability of true-positive results was high). None of the 18 subjects had evidence of parasitemia when last tested; only three (A, P, and Q) withdrew before fulfilling study criteria (Fig. 1). Seven subjects were released after <3 years of monitoring because they had had three consecutive specimens that tested negative by all study methods, including IFA.
Among the remaining eight persons, who were seropositive when last tested but were released because they had been monitored for 3 years, five subjects―C, G, K, M, and R―had a final IFA titer of 1:64 or 1:128. In supplemental testing, serial specimens from subjects C, G, and R were retested by IFA, in parallel, on the same day. Upon retesting, the IFA results typically were the same as those shown in Fig. 1 or differed by only one (2-fold) dilution: subject G’s last four specimens had negative IFA results; subject R’s titers gradually decreased, without fluctuations (final titer, 1:64); and subject C’s titers still fluctuated. The other three subjects (E, J, and O) who were released after having been monitored for 3 years had a final titer of 1:512, despite having been treated and monitored for at least 2 years posttherapy. If subject P (who did not report receipt of treatment) is counted, a total of four persons (22% of 18) had a titer of 1:512 when last tested. Of interest, one of the 66 participants who never had demonstrable parasitemia had prolonged high-level seropositivity: the IFA titer was 1:1024 for all 11 specimens he provided over a 2-year period (data not shown).
DISCUSSION
We assessed the course and laboratory markers of B. microti infection in settings relevant to transfusion medicine by conducting a multi-year longitudinal study among prospectively identified seropositive donors in Babesia-endemic areas. The strengths of the study included the collection of serial specimens, the use of parasitologic as well as molecular amplification techniques, the independent performance of PCR analysis at two laboratories, and the availability of epidemiologic and clinical context. Because of the internal controls inherent to the study design, we were able to identify discordant or inconsistent laboratory results. In the data analyses, we focused on the 18 enrollees who ever tested positive for evidence of parasitemia—particularly, the 14 persons who had positive PCR results at both laboratories and/or had parasitologically confirmed infection (i.e., strong evidence of active infection), four of whom also had positive lookback investigations. None of our conclusions are dependent on data from persons who tested positive only by PCR at one laboratory or who never had evidence of parasitemia, and the study was not designed to determine the proportion of seropositive persons who had demonstrable parasitemia.
The aggregate longitudinal data underscore that persons who fulfill the eligibility criteria for donating blood can have protracted low-level parasitemia that is variably and intermittently detected by parasitologic and molecular amplification techniques. Regardless of the analytic sensitivity of the method used, the results will be negative if the target parasite/DNA is not present in the aliquots tested, which typically are several orders of magnitude smaller than the volumes transfused to adults.1 When this study was initiated, B. microti 2-step nested PCR analysis was considered state of the art. Although using real-time PCR and/or increasing the starting volume (e.g., for extracting/targeting DNA) could improve detection of low parasite densities, the discrepancy between the volumes tested versus transfused would remain a fundamental limitation. In the blood-donor setting, negative PCR results—even for optimally collected, processed, and tested specimens—would not exclude Babesia infection or infectivity. The PCR positivity rates, as determined by nucleic acid testing, for donors infected with viral pathogens such as hepatitis B virus that are associated with relatively high viremias are not applicable to B. microti. In general, with the exception of the window period, which was not addressed by this study, seropositivity is a more sensitive marker than PCR of B. microti infection, although it does not reliably distinguish between active and resolved infection.
Our findings also highlight distinctions between patient and donor settings (i.e., between clinical and transfusion medicine). Patients with acute symptomatic babesiosis typically have patent parasitemia, detectable by careful blood-smear examination.1,3 In contrast, Babesia-infected persons who meet the criteria for donating blood by definition “feel well” and usually have subpatent (smear-negative) parasitemia, which may or may not be detected by methods that amplify parasites/DNA, even though the transfused inoculum may suffice to cause patent parasitemia in a susceptible recipient.1,10 Only one enrollee in our study, an elderly man (subject E), was documented to have a smear-positive specimen, which, as expected, also tested positive by PCR, at both laboratories, and by hamster inoculation. However, PCR and in vivo positivity rates for persons with positive blood smears are not generalizable to donors with subpatent parasitemia.
In a previously described study,19 hamsters reliably developed patent parasitemia if the intraperitoneal inoculum was at least 300 B. microti parasites; approximately one-third of hamsters became infected if the inoculum was as low as 30 parasites. In our study, more persons/specimens tested positive by PCR than by hamster inoculation but not necessarily in both laboratories or for consecutive specimens; and some had positive results only by the in vivo method, either by chance or because a larger volume of blood was tested. The potential role of chance detection is underscored by the higher concordance rate by person than by specimen (e.g., as exemplified by subject A; Fig. 1). Variable detection of parasites/DNA―in aliquots of specimens collected at the same time and in serial specimens―should not be surprising in the context of low-level parasite densities that approximate a Poisson probability distribution. Especially for the 14 persons who were the focus of the analyses, false-positive PCR results were unlikely, although they cannot be excluded for some specimens. Although we did not use quantitative methods, the persons/specimens with positive results by both methods/laboratories likely had higher parasite densities than those with discordant or inconsistent results.
The overall number of persons who still were infected when they enrolled in the longitudinal study is not known, nor is the true duration of infection among the subset of enrollees who tested positive for evidence of parasitemia. However, the aggregate longitudinal data affirm the potential for otherwise healthy persons to have protracted parasitemia.1,9,10,20–23 None of the study subjects had evidence of parasitemia when last tested, but several still had comparatively high-level seroreactivity. Potential explanations include persistent infection, with very low densities of residual or sequestered parasites not detected by the amplification methods we used24; reexposure/reinfection; or other antigenic stimuli.
The increasing recognition of US cases of TTB1 has strengthened the impetus to develop, evaluate, and implement strategies to reduce the risk for transmission.1,4–6,25–27 Our findings support the concept of year-round donor testing: the study specimens that had evidence of parasitemia were collected during 9 different months of the year (even if only hamster-positive specimens are included), which is consistent with the presence of protracted parasitemia in some donors and with the year-round occurrence of cases of TTB.1 Almost all of the identified cases of TTB cases have been linked to RBC transfusions, which have included components that had been leukoreduced, irradiated, or cryopreserved.1 The identified cases linked to whole-blood derived platelets1,9,12,17 presumably were caused by residual RBCs that were infected with B. microti or by the presence of extracellular forms of the parasite.1,28
The FDA's Blood Products Advisory Committee that was convened in July 2010 supported the concept of regional (vs. other selective or universal) donor testing for evidence of Babesia infection26; the details of where to test (in which areas) and how to do so (with what types of approaches and which particular assays/protocols) have not yet been resolved. Our data underscore that donor-screening algorithms should include serologic testing and should not rely solely on molecular testing; indeed, both serologic and molecular testing have been conducted in the donor screening performed to date in selected areas under FDA-approved investigational protocols. To minimize the loss of uninfected donors (while maximizing the detection of infected donors), the definition of a seropositive result for the pertinent assay(s) should be evaluated and candidate reentry algorithms could be investigated; because negative PCR results do not exclude ongoing infection, negative seroconversion, with consistently negative serologic results thereafter (for an as-of-yet unspecified period), also would be needed. Although our study was not designed to evaluate the particulars of potential donor-screening tests or management strategies, multiple participants who had been seropositive and had evidence of parasitemia ultimately had three consecutive specimens that tested negative by all of our study methods/definitions (Fig. 1).
Donor-screening tests targeted at B. microti have the highest near-term priority. However, infection with other species, such as B. duncani, which has caused three documented cases of TTB,22,23,29 is not detected by the available serologic or molecular tests for B. microti.1 For the longer-term future, the ideal screening test would be a high-throughput, highly sensitive and specific marker of active Babesia infection, regardless of the species. Pathogen reduction constitutes an alternative or supplemental mitigation strategy; techniques for cellular components have not yet been approved for use in the United States4,25 but are under investigation.30,31
Acknowledgments
We thank Mark L. Eberhard and Henry Bishop for their insights and technical assistance.
This work was supported in part by the Centers for Disease Control and Prevention (cooperative agreement no. UR9/CCU316878) and by the American Red Cross Biomedical Services.
Footnotes
The authors declare that they have no conflicts of interest relevant to the manuscript submitted to TRANSFUSION.
The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention.
REFERENCES
- 1.Herwaldt BL, Linden JV, Bosserman E, Young C, Olkowska D, Wilson M. Transfusion-associated babesiosis in the United States: a description of cases. Ann Intern Med. 2011;155:509–519. doi: 10.7326/0003-4819-155-8-201110180-00362. [DOI] [PubMed] [Google Scholar]
- 2.Centers for Disease Control and Prevention. Babesiosis surveillance—18 states, 2011. MMWR Morb Mortal Wkly Rep. 2012;61:505–509. [PubMed] [Google Scholar]
- 3.Vannier E, Krause PJ. Human babesiosis. N Engl J Med. 2012;366:2397–2407. doi: 10.1056/NEJMra1202018. [DOI] [PubMed] [Google Scholar]
- 4.Gubernot DM, Nakhasi HL, Mied PA, Asher DM, Epstein JS, Kumar S. Transfusion-transmitted babesiosis in the United States: summary of a workshop. Transfusion. 2009;49:2759–2771. doi: 10.1111/j.1537-2995.2009.02429.x. [DOI] [PubMed] [Google Scholar]
- 5.Leiby DA. Transfusion-associated babesiosis: shouldn't we be ticked off? Ann Intern Med. 2011;155:556–557. doi: 10.7326/0003-4819-155-8-201110180-00363. [DOI] [PubMed] [Google Scholar]
- 6.Leiby DA. Transfusion-transmitted Babesia spp.: bull's-eye on Babesia microti. Clin Microbiol Rev. 2011;24:14–28. doi: 10.1128/CMR.00022-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tonnetti L, Eder AF, Dy B, Kennedy J, Pisciotto P, Benjamin RJ, Leiby DA. Transfusion-transmitted Babesia microti identified through hemovigilance. Transfusion. 2009;49:2557–2563. doi: 10.1111/j.1537-2995.2009.02317.x. [DOI] [PubMed] [Google Scholar]
- 8.Asad S, Sweeney J, Mermel LA. Transfusion-transmitted babesiosis in Rhode Island. Transfusion. 2009;49:2564–2573. doi: 10.1111/j.1537-2995.2009.02380.x. [DOI] [PubMed] [Google Scholar]
- 9.Herwaldt BL, Neitzel DF, Gorlin JB, Jensen KA, Perry EH, Peglow WR, Slemenda SB, Won KY, Nace EK, Pieniazek NJ, Wilson M. Transmission of Babesia microti in Minnesota through four blood donations from the same donor over a 6-month period. Transfusion. 2002;42:1154–1158. doi: 10.1046/j.1537-2995.2002.00189.x. [DOI] [PubMed] [Google Scholar]
- 10.Cangelosi JJ, Sarvat B, Sarria JC, Herwaldt BL, Indrikovs AJ. Transmission of Babesia microti by blood transfusion in Texas. Vox Sang. 2008;95:331–334. doi: 10.1111/j.1423-0410.2008.01094.x. [DOI] [PubMed] [Google Scholar]
- 11.Gubernot DM, Lucey CT, Lee KC, Conley GB, Holness LG, Wise RP. Babesia infection through blood transfusions: reports received by the US Food and Drug Administration, 1997–2007. Clin Infect Dis. 2009;48:25–30. doi: 10.1086/595010. [DOI] [PubMed] [Google Scholar]
- 12.Jacoby GA, Hunt JV, Kosinski KS, Demirjian ZN, Huggins C, Etkind P, Marcus LC, Spielman A. Treatment of transfusion-transmitted babesiosis by exchange transfusion. N Engl J Med. 1980;303:1098–1100. doi: 10.1056/NEJM198011063031906. [DOI] [PubMed] [Google Scholar]
- 13.Krause PJ, Spielman A, Telford SR, 3rd, Sikand VK, McKay K, Christianson D, Pollack RJ, Brassard P, Magera J, Ryan R, Persing DH. Persistent parasitemia after acute babesiosis. N Engl J Med. 1998;339:160–165. doi: 10.1056/NEJM199807163390304. [DOI] [PubMed] [Google Scholar]
- 14.Krause PJ, Gewurz BE, Hill D, Marty FM, Vannier E, Foppa IM, Furman RR, Neuhaus E, Skowron G, Gupta S, McCalla C, Pesanti EL, Young M, Heiman D, Hsue G, Gelfand JA, Wormser GP, Dickason J, Bia FJ, Hartman B, Telford SR, 3rd, Christianson D, Dardick K, Coleman M, Girotto JE, Spielman A. Persistent and relapsing babesiosis in immunocompromised patients. Clin Infect Dis. 2008;46:370–376. doi: 10.1086/525852. [DOI] [PubMed] [Google Scholar]
- 15.Ruebush TK, 2nd, Chisholm ES, Sulzer AJ, Healy GR. Development and persistence of antibody in persons infected with Babesia microti. Am J Trop Med Hyg. 1981;30:291–292. doi: 10.4269/ajtmh.1981.30.291. [DOI] [PubMed] [Google Scholar]
- 16.Johnson ST, Cable RG, Tonnetti L, Spencer B, Rios J, Leiby DA. Seroprevalence of Babesia microti in blood donors from Babesia-endemic areas of the northeastern United States: 2000 through 2007. Transfusion. 2009;49:2574–2582. doi: 10.1111/j.1537-2995.2009.02430.x. [DOI] [PubMed] [Google Scholar]
- 17.Johnson ST, Cable RG, Leiby DA. Lookback investigations of Babesia microti-seropositive blood donors: seven-year experience in a Babesia-endemic area. Transfusion. 2012;52:1509–1516. doi: 10.1111/j.1537-2995.2011.03345.x. [DOI] [PubMed] [Google Scholar]
- 18.Persing DH, Mathiesen D, Marshall WF, Telford SR, Spielman A, Thomford JW, Conrad PA. Detection of Babesia microti by polymerase chain reaction. J Clin Microbiol. 1992;30:2097–2103. doi: 10.1128/jcm.30.8.2097-2103.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Etkind P, Piesman J, Ruebush TK, 2nd, Spielman A, Juranek DD. Methods for detecting Babesia microti infection in wild rodents. J Parasitol. 1980;66:107–110. [PubMed] [Google Scholar]
- 20.Wittner M, Rowin KS, Tanowitz HB, Hobbs JF, Saltzman S, Wenz B, Hirsch R, Chisholm E, Healy GR. Successful chemotherapy of transfusion babesiosis. Ann Intern Med. 1982;96:601–604. doi: 10.7326/0003-4819-96-5-601. [DOI] [PubMed] [Google Scholar]
- 21.Wei Q, Tsuji M, Zamoto A, Kohsaki M, Matsui T, Shiota T, Telford SR, 3rd, Ishihara C. Human babesiosis in Japan: isolation of Babesia microti-like parasites from an asymptomatic transfusion donor and from a rodent from an area where babesiosis is endemic. J Clin Microbiol. 2001;39:2178–2183. doi: 10.1128/JCM.39.6.2178-2183.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Herwaldt BL, Kjemtrup AM, Conrad PA, Barnes RC, Wilson M, McCarthy MG, Sayers MH, Eberhard ML. Transfusion-transmitted babesiosis in Washington State: first reported case caused by a WA1-type parasite. J Infect Dis. 1997;175:1259–1262. doi: 10.1086/593812. [DOI] [PubMed] [Google Scholar]
- 23.Bloch EM, Herwaldt BL, Leiby DA, Shaieb A, Herron RM, Chervenak M, Reed W, Hunter R, Ryals R, Hagar W, Xayavong MV, Slemenda SB, Pieniazek NJ, Wilkins PP, Kjemtrup AM. The third described case of transfusion-transmitted Babesia duncani. Transfusion. 2012;52:1517–1522. doi: 10.1111/j.1537-2995.2011.03467.x. [DOI] [PubMed] [Google Scholar]
- 24.Leiby DA, Chung AP, Gill JE, Houghton RL, Persing DH, Badon S, Cable RG. Demonstrable parasitemia among Connecticut blood donors with antibodies to Babesia microti. Transfusion. 2005;45:1804–1810. doi: 10.1111/j.1537-2995.2005.00609.x. [DOI] [PubMed] [Google Scholar]
- 25.Stramer SL, Hollinger FB, Katz LM, Kleinman S, Metzel PS, Gregory KR, Dodd RY. Emerging infectious disease agents and their potential threat to transfusion safety. Transfusion. 2009;49(Suppl 2):1S–29S. doi: 10.1111/j.1537-2995.2009.02279.x. [DOI] [PubMed] [Google Scholar]
- 26.Food and Drug Administration. July 26, 2010, Meeting of the Blood Products Advisory Committee. [Accessed December 22, 2013];Risk of Babesia infection by blood transfusion and potential strategies for donor testing. Available at: http://www.fda.gov/AdvisoryCommittees/CommitteesMeetingMaterials/BloodVaccinesandOtherBiologics/BloodProductsAdvisoryCommittee/ucm205013.htm.
- 27.Young C, Chawla A, Berardi V, Padbury J, Skowron G, Krause PJ Babesia Testing Investigational Containment Study Group. Preventing transfusion-transmitted babesiosis: preliminary experience of the first laboratory-based blood donor screening program. Transfusion. 2012;52:1523–1529. doi: 10.1111/j.1537-2995.2012.03612.x. [DOI] [PubMed] [Google Scholar]
- 28.Pantanowitz L, Cannon ME. Extracellular Babesia microti parasites. Transfusion. 2001;41:440. doi: 10.1046/j.1537-2995.2001.41040440.x. [DOI] [PubMed] [Google Scholar]
- 29.Kjemtrup AM, Lee B, Fritz CL, Evans C, Chervenak M, Conrad PA. Investigation of transfusion transmission of a WA1-type babesial parasite to a premature infant in California. Transfusion. 2002;42:1482–1487. doi: 10.1046/j.1537-2995.2002.00245.x. [DOI] [PubMed] [Google Scholar]
- 30.Tonnetti L, Proctor MC, Reddy HL, Goodrich R, Leiby DA. Evaluation of the Mirasol platelet reduction technology system against Babesia microti in apheresis platelets and plasma. Transfusion. 2010;50:1019–1027. doi: 10.1111/j.1537-2995.2009.02538.x. [DOI] [PubMed] [Google Scholar]
- 31.Tonnetti L, Thorp AM, Reddy HL, Keil SD, Goodrich RP, Leiby DA. Riboflavin and ultraviolet light reduce the infectivity of Babesia microti in whole blood. Transfusion. 2013;53:860–867. doi: 10.1111/j.1537-2995.2012.03791.x. [DOI] [PubMed] [Google Scholar]