Abstract
Previous research has found that adolescent ethanol (EtOH) exposure alters drug seeking behaviors, cognition and neuroplasticity. Using male Sprague Dawley rats, differences in spatial working memory, non-spatial discrimination learning and behavioral flexibility were explored as a function of age at the onset (mid-adolescent vs. adult) of chronic EtOH exposure (CET). Concentrations of mature brain-derived neurotrophic factor (mBDNF) and beta-nerve growth factor (β-NGF) in the prefrontal cortex and hippocampus were also assessed at different time-points: during CET, following acute abstinence (48-hrs), and after protracted abstinence (6–8 wks). Our results revealed that an adolescent onset of CET leads to increased EtOH consumption that persisted into adulthood. In both adult and adolescent onset CET groups, there were significant long-term reductions in prefrontal cortical mBDNF and β-NGF levels. However, only adult onset CET rats displayed decreased hippocampal BDNF levels. Spatial memory, assessed by spontaneous alternation and delayed alternation, was not significantly affected by CET as a function of age of drinking onset, but higher blood–EtOH levels were correlated with lower spontaneous alternation scores. Regardless of the age of onset, EtOH exposed rats were impaired on non-spatial discrimination learning and displayed inflexible behavioral patterns upon reversal learning. Our results indicate that adolescent EtOH exposure changes long-term consumption patterns producing behavioral and neural dysfunctions that persist across the lifespan.
Introduction
Approximately 17 million adults suffer from an alcohol use disorder (AUD) and the majority of adults diagnosed with an AUD began consuming alcohol during adolescence [1]. Adolescence is identified as a vulnerable developmental time period during which exposure to drugs, including alcohol, can have long lasting effects on memory, cognition, anxiety and social interaction [2–4]. Early ethanol (EtOH) exposure appears to solidify adolescent-typical behaviors in rodents, such as increased impulsivity, decreased behavioral inhibition, and behavioral inflexibility, well into adulthood [5,6]. These unique behavioral effects are sustained through long-lasting neural changes in critical brain regions such as the frontal cortex and the hippocampus [7,8]. Thus, early EtOH exposure can produce a state wherein the adult brain is primed for later chronic alcohol abuse and associated behavioral impairment [9].
The hippocampus is vulnerable to chronic EtOH exposure and these alterations contribute to memory deficits associated with alcoholism [10–13]. In rodents, chronic exposure to EtOH results in reduced interneuron, pyramidal and granule cell numbers [14–16], as well as reduced long-term potentiation within in the hippocampus [17,18]. Hippocampal neurogenesis is also very sensitive to chronic EtOH exposure. Several chronic EtOH delivery paradigms, both intermittent and continuous, decrease the number of newly born surviving neurons (by 40–60%) within the dentate gyrus [12,19]. High blood ethanol concentrations (BECs) are needed to observe reductions in neurogenesis [20]. Because newborn hippocampal neurons in adolescent rats are less likely to incorporate into a functional neural network following excessive EtOH treatment, the adolescent hippocampus displays a distinct vulnerabililty to chronic EtOH [21]. In human and non-human primates, hippocampal volume loss is greater following adolescent-onset alcohol consumption, compared with adult-onset [22,23]. These EtOH induced changes in hippocampal structure are believed to contribute to the initial spatial processing, episodic learning, and memory impairments observed in alcoholics [24].
The frontal cortex is also adversely affected by EtOH exposure, perhaps to a greater degree than the hippocampus [25–27]; a region that is developmentally sensitive to EtOH toxicity [28]. The extent of reduction in frontal cortical volume is largely dependent upon the extent of alcohol exposure [28–31]. Shrinkage of the frontal cortex in alcoholics is related to reduced neuronal size, branching of basal dendrites [32] and glial cell density [33]. Markers of cell death are increased in the prefrontal cortex of alcoholics, suggesting alcohol-related neural degeneration within this region [34]. Chronic EtOH exposure in adolescence leads to the induction of a persistent neural immune response within the frontal cortex that contributes to alcohol-related brain damage [35]. In both humans and nonhuman mammals, increased EtOH consumption correlates with decreases in behavioral flexibility and response inhibition, behaviors that are modulated by the frontal cortex [36–39].
EtOH is known to disrupt several signaling cascades and one target effector is BDNF [40]. Abnormalities in neurotrophins are associated with cognitive deterioration [41,42] and neuronal atrophy [43]. In particular, brain-derived neurotrophic factor (BDNF) and nerve growth factor (NGF) are of interest due to their role in homeostatic neural function and recovery during and after EtOH exposure [44–47]. Previous research indicates a biphasic temporal effect of EtOH on BDNF and NGF levels: short exposures appear to increase levels, while prolonged exposures seem to reduce levels [44]. In addition, BDNF levels are not changed during excessive adolescent binge EtOH exposure [21]. Altered levels of neurotrophins appear to contribute to alcohol-related brain damage and affiliated cognitive impairment [48].
Given the potential interactions between the timing of EtOH exposure, brain regional sensitivity, and neurotrophin levels, the current project investigated whether age differences (mid-adolescence versus adulthood) at onset of CET would modulate the degree of neural and behavioral dysfunction typically associated with AUDs. Chronic EtOH exposure in drinking water has been used as a model to evaluate the effects of long-term alcohol abuse observed in middle-aged to advanced-aged populations [49,50]. Specifically, this model was instrumental in revealing that the forebrain cholinergic population and cholinergic innervation of the hippocampus and cortex are very sensitive to long-term EtOH toxicity. CET models have also demonstrated that exogenous NGF application improved neural and behavioral outcomes after CET [51–55].
We monitored total EtOH consumption throughout a 6-month forced EtOH exposure paradigm, followed by a battery of behavioral tests that included spatial and non-spatial discrimination learning, as well as reversal learning as a measure of behavioral flexibility. Visuospatial function has been reported to be initially impaired in abstinent alcoholics and is associated with reduced hippocampal volume [24]. Behavioral or cognitive flexibility, which is dependent on the frontal cortex, is also impaired in abstinent alcoholics [56]. Thus, tasks were chosen that are dependent on hippocampal and frontal cortical functioning. Neurotrophic factors (mBDNF, β-NGF) were measured within the frontal cortex and hippocampus at three time-points to examine neural adaptions as a function of intoxication, withdrawal and protracted abstinence. It was hypothesized that initiation of chronic EtOH exposure during adolescence would produce persistent disruptions in drinking patterns, neurotrophin levels and cognitive function.
Materials and Methods
Ethics Statement, Minimization of Potential Pain, Distress and Treatment of Animal Subjects
All experimental procedures were in compliance with the National Institutes of Health (NIH) Guide for Care and Use of Laboratory Animals and approved by the Institutional Animal Care and Use Committee (IACUC) at the State University of New York at Binghamton. During treatment, behavioral testing, and tissue collection procedures were devised to minimize the potential pain and distress of the animals used in this study. All rats were frequently monitored, at least three times a week, for health status.
Adult (postnatal day [PD] 72–75; n = 40) and adolescent (PD 35; n = 40) male, Sprague-Dawley rats were obtained from litters bred at Binghamton University. Rats were doubly housed in a temperature (20°C) and humidity controlled room under a 12-hour light/dark cycle, 7:00 a.m.-7:00 p.m. No more than one rat from each litter was included in a given treatment condition. Rats were provided with ad libitum access to rat chow. Rats were randomly assigned to 1 of 3 tissue collection time-points. At time-point 1 (T1; intoxication), brain tissue was collected during the 28th week of CET while rats were still consuming 20% EtOH. At time-point 2 (T2; acute abstinence or withdrawal), brain tissue was collected 48-hrs after CET ended, in an EtOH-free state. At time-point 3 (T3; protracted abstinence or recovered), brain tissue was collected after behavioral testing, approximately 6–8 weeks following the cessation of CET. Rats were randomly assigned to the following treatment onset conditions: Adolescent control (T1 = 4*, T2 = 4* [*note: T1 and T2 groups were combined into a common control group]; T3 = 8); Adult control (T1 = 4*, T2 = 4*; T3 = 8); Adolescent Onset CET (T1 = 8; T2 = 8; T3 = 8); Adult Onset CET (T1 = 8; T2 = 8; T3 = 8). Fig 1 illustrates the treatment and behavioral timeline for rats undergoing CET and the treatment conditions are defined in detail below.
Fig 1. Chronic Ethanol Treatment (CET) Protocol Outline.
Schematic illustrating the age range, ethanol (EtOH) exposure procedures, brain collection time points, and behavioral testing paradigms and for both adolescent onset and adult onset CET animals.
Chronic EtOH treatment (CET)
CET began on PD 35 for mid-adolescents and PD 72–75 for adults. Rats exposed to CET were provided with an aqueous solution of EtOH (95% EtOH v/v) diluted with tap water to the appropriate v/v as the sole source of liquid for the duration of CET. Bottles were weighed and refilled every Monday, Wednesday and Friday. The grams of EtOH per kilogram of body weight was determined by the following formula: (milliliters of EtOH consumed x the percent concentration of EtOH x the density of EtOH/ weight of the animal). Average daily EtOH consumption was determined by dividing the average weekly consumption by seven days across the EtOH exposure phase. CET rats initiated their treatment using a”fading on” procedure [52,57,58]: EtOH exposure started at 6% (v/v) for 4 days and incrementally increased by 3% every 5 days until reaching 12% (v/v), at which point the concentration was increased to 20% (v/v) and maintained for 28 weeks. At the beginning of 20% (v/v) exposure, adolescent onset CET rats were PD 50 and adult onset CET rats were approximately PD 90. After 28 weeks, animals in the T3 groups were gradually “faded off” CET in a series of deescalating EtOH concentrations: 12% (v/v) for 5 days, 9% (v/v) for 5 days, 6% (v/v) for 5 days before given ad libitum access to water. Control rats were provided with unlimited access to tap water. Water consumption data were collected from age-matched, male, Sprague Dawley rats (S1 Fig).
After 1 month of exposure to 20% EtOH v/v, tail blood was collected from all rats at approximately 12:00 AM. This procedure was repeated at months 2, 4, and 6 of CET. Plasma was separated using a centrifuge and blood EtOH concentrations (BEC) were analyzed via Analox (AM1, Analox Instruments, London, United Kingdom). Tail bloods were collected and analyzed from both CET and control groups in order to maintain equal treatment parameters across all animals and in order to validate the BEC assay.
Brain Collection
Upon rapid decapitation at their given time-points (T1, T2, T3; Fig 1), each rat’s brain was immediately extracted and fresh tissue was extracted from a hemi- dissection. The prefrontal cortex was blocked and collected to include all subregions, and the entire hippocampus was dissected from the hemisphere. Tissue was stored at -80°C to be used for neurotrophin enzyme-linked immunosorbent (ELISA) assays.
Enzyme-linked immunosorbent assay (ELISA)
The concentration levels of mBDNF (Emax® Immunassay system, Promega, Madison, WI, USA) and β-NGF (Duoset ELISA β-NGF kit, R&D Systems, Minneapolis, MN) were assessed using the respective vendor procedures and protocols. Each region was homogenized, as described by Gearhart and colleagues [59], and returned to the -80°C freezer until ELISA analysis. Total protein concentrations for each region of interest were determined with BCA assay (Pierce TM BCA Protein Assay Kit, Thermo Scientific, Rockford, IL). The total protein concentration for each neurotrophin and of each sample was calculated using regression analysis based on the standard curve optical densities.
Mature BDNF ELISA.
Corning Costar® 96-well flat bottom plates (Corning Life Sciences, Corning, NY) were coated and incubated overnight at 4°C with monoclonal anti-BDNF carbonate buffer (supplied by Promega). Plates were washed in Tris-buffered saline with Tween 20 (TBST) following all incubation periods. The next day, plates were incubated with block and sample buffer for 1-hr at room temperature. Standards (supplied by Promega) were diluted in blocking buffer to create working concentrations of 500, 250, 125, 62.5, 31.5, 15.625, 7.8125, and 0 pg/mL. All samples were diluted at a ratio of 1:4 for the frontal cortex and 1:5 for the hippocampus to allow for an appropriate detection range of the standard curve. Following block, samples were plated and incubated for 2-hrs at room temperature. Samples were then incubated with the supplied BDNF polyclonal antibody for 2-hrs at room temperature. BDNF plates were washed and treated with anti-IgY horseradish peroxidase (HRP) conjugate for 1-hr. After the final wash, a color development reaction was initiated by adding TMB One solution for 10 min at room temperature. The color development reactions were stopped by adding 1 N HCl. Optical densities were measured using a Tecan plate reader (Tecan Infinite M200 Pro, Mannëdorf, Switzerland) and plates were read at a 450 nm wavelength.
β-NGF ELISA
Corning Costar® 96-well flat bottom plates were coated and incubated overnight with goat anti-rat β-NGF (R&D systems) at 20°C. Plates were washed with 0.05% Tween-20 in phosphate buffered saline following each incubation. The following day, plates were blocked with BSA reagent diluent for 1-hr at room temperature. Standards (supplied by R&D systems) were diluted in blocking buffer to create working concentrations of: 1000, 500, 250, 125, 62.5, 31.5, 15.625, and 0 pg/mL. Samples were diluted at a ratio of 1:4 for the frontal cortex and 1:5 for the hippocampus to allow for an appropriate detection range of the standard curve. Samples were plated and incubated for 2-hrs at room temperature. Plates were then incubated with a detection antibody for 2-hrs at room temperature, followed by an incubation with streptavidin-HRP for 20 minutes. A color development reaction was initiated by adding vendor supplied H2O2 and tetramethylbenzidine for 20 minutes at room temperature. The color development reactions were stopped by adding 2 N H2SO4. Optical densities were measured using a Tecan plate reader at a 450 nm wavelength.
Behavioral Testing
Following the cessation of CET and the fade-off EtOH procedure, T3 rats began a 3-week EtOH free recovery period. During this time rats were weighed and handled daily. Prior to the start of behavioral testing, rats were food restricted to 90% of their free feed weight over the course of 5 days. Rats were behaviorally tested in the following sequence: spontaneous alternation, delayed alternation, non-spatial discrimination learning, and behavioral flexibility.
Spontaneous Alternation
Previous findings indicate that a rat performs optimally on a spontaneous alternation maze task when the animal is slightly food restricted, presumably by increasing their motivation to explore a new environment [60]. Once a rat reached 90% of their free weight, they were tested on a single session of spontaneous alternation. Spontaneous alteration was conducted in a plus maze (105.5 x 14.4 x 15 cm) with clear plastic walls and black painted wooden floors. The testing room was rich in visual cues. To habituate the animal to the testing room, rats were placed in the testing room for 20-min before initiating testing. The rat was then placed into the center of the apparatus, and allowed to explore the maze for 18-min of testing, during which arm entries (all four paws within an arm) were recorded. An alternation was defined as entry into four different arms in overlapping successive sequences of 4 arm entries (e.g., the successive arm entries of A, D, C, B, D, A, C, D, B, D, A, C, D, A, C; the first sequence of ADCB was an alternation, but the following sequence of 4 arm entries DCBD was not). The percent alternation score is equal to the ratio of (actual alternations/possible alternations [trial number-3]) X 100. (For the above data set: 5/(15–3) = .416 X 100 = 41.6%.) This criterion is similar to that used in previous experiments [61–63]. Spontaneous alternation scores were corrected for differences in activity between CET and control groups: Arm entries were only recorded up to the average number of entries made by the adult control group (25 total entries), which had the lowest activity level.
Delayed Alternation
After spontaneous alternation testing, rats continued food restriction until they reached 85% of their free feed weight. For the two sessions of delayed alternation testing, a T-maze was used that had clear Plexiglas sidewalls (12 cm high), with two goal arms (55 cm), and a start arm (66 cm). The arms were made of wood, painted black, and the maze was elevated 80 cm from the floor. Transparent Plexiglas guillotine doors separated the start box and the two goal boxes from the choice area. The T-maze was located in the same testing room as the spontaneous alternation maze with the same visual cues. The mazes were placed in the same location so that exposure to spontaneous alternation would also serve as pre-exposure for delayed alternation.
After a 20-min habituation period, the rat was placed in the start box of the maze. On the first trial of each testing day, each rat was allowed a free choice to enter either the left or right arm of the maze, and either response was rewarded with a ½ Frosted Cheerio (General Mills, Minneapolis, MN). After consuming the reward, the rat was manually removed from the goal box and placed back into the start box. There was a 30-sec delay interval between the end of the previous trial and the beginning of the next trial. A correct choice (entering the previously non-visited goal arm) was rewarded with ½ Cheerio. An incorrect response (repeat visit to the previous arm) was not rewarded and the rat was confined to the goal box for a 10-sec time-out period before being returned to the start box. The correct response after an error trial was to alternate to the opposing arm. Percent alternation scores were determined by the number of correct alternations divided by the total number of possible alternations.
Non-spatial discrimination learning and Reversal Learning
Following delayed alternation testing, rats began dig training in their home cage for 3 days. Small ceramic bowls (diameter = 9 cm; depth = 4 cm) were filled with wood shavings and baited with ¼ frosted Cheerio. Training began by placing a Cheerio portion on top of the shavings; Cheerios were incrementally placed lower in the shavings until the animal learned to dig the reward out from the bottom of the bowl.
Non-spatial discrimination learning and reversal testing took place in a white opaque plastic box (70.3 x 40 x 36.4 cm) with a black floor. A white, opaque, removable divider sectioned the apparatus into the start box (16.5 x 40) and the testing area (53.8 x 40 cm). Two ceramic bowls, filled with digging substrate termed medium (see Table 1), were located near the back wall separated by another removable divider (19.8 x 25.9 cm). The first phase of training consisted of habituation to the chamber. After 5 min, the divider was lifted allowing the rat to access the baited bowls. When a rat approached the bowls reliably within 30 sec and ate the reward on 6-consecutive trials within 2 min, the rat would advance to discrimination training. This benchmark (a rat approaching and eating from the correct baited bowl on 6 consecutive trials) was used to determine the total number of trials required to reach criterion for the simple, compound and reversal discrimination tasks.
Table 1. Non-Spatial Discrimination Reversal Learning Examples.
| Discrimination Task | Medium-based Cues | Scent-base Cue | ||
|---|---|---|---|---|
| Bowl 1 | Bowl 2 | Bowl 1 | Bowl 2 | |
| Simple | easter grass | shredded paper | clove/bedding | nutmeg/bedding |
| Compound 1 | thyme/rocks | citronella/tubes | piña colada/confetti | lavender/colored beads |
| Reversal 2 | thyme/rocks | citronella/tubes | piña colada/confetti | lavender/colored beads |
| Compound 2 | rosemary/sand | cinnamon/gravel | sweet pea/sand | vanilla/wood chips |
| Reversal 2 | rosemary/sand | cinnamon/gravel | sweet pea/sand | vanilla/wood chips |
Bolded exemplar indicates the rewarded cue. Half of the animals underwent training with medium-based cues, and the other half underwent training with scent-based cues. The type of exemplar used did not interact with treatment conditions (all p’s>0.10).
The second phase of training consisted of each rat learning two simple discriminations: a scent-based and medium-based discrimination. The final phase of testing required each rat to perform a series of five discriminations (see Table 1): (1) Simple discrimination consisted of a single dimension (scent or medium); (2) Compound discrimination included both a unique smell and digging medium that were distinctive from those used in previous discriminations; (3) After reaching criteria on the first compound discrimination, the rule was changed (reversed) such that the previous incorrect combination of stimuli were now correct; (4) A second compound discrimination was trained in which each rat learned a novel complex discrimination with new stimuli (both scent and medium); (5) A final reversal was conducted that switched the rewarded stimuli from the second compound discrimination.
Experimental Design and Statistics
Analyses were performed in SPSS (IBM Corporation, version 22, Armonk, New York). The neurotrophin data were analyzed using a 2 (Age: adult vs. adolescent) x 2 (Treatment: CET vs. control [water]) x 3 (Time-point of tissue collection: intoxicated [during CET], withdrawal [48-hrs post CET], or protracted recovery [6-8-wks post CET]) factorial analysis of variance. All behavioral data, including both drinking and cognitive assessment, were analyzed with a 2 (Age: adult vs. adolescent) x 2 (Treatment: CET vs. control) factorial analysis of variance. Bivariate correlations were analyzed using Pearson’s correlation coefficient between BECs, neurotrophin level and behavior.
Results
Treatment Parameters
All rats gained weight across treatment, but CET and Control rats differed at specific time-points
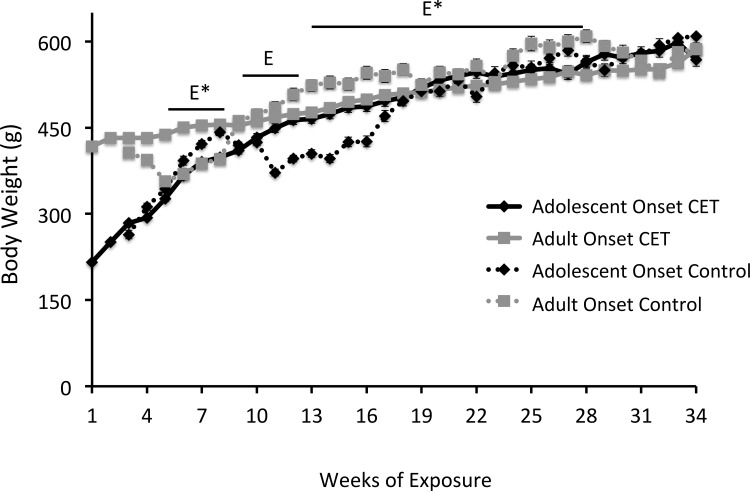
All rats gained weight (Fig 2) during the course of the experiment (F[27,1431] = 853.44, p<0.001). Adolescent onset CET rats weighed significantly more (3.5%) than their age-matched controls (F[1, 38] = 30.63, p<0.0001] during weeks 8–11, but no difference was found between these two groups during the remainder of the experiment. Adult onset CET rats weighed significantly more than age-matched controls during weeks 4–7, but adult control rats were significantly heavier (8.4%) than adult onset CET rats from weeks 12–27 (F[1,38] = 6.13, p<0.02).
Fig 2. All rats gained weight across treatment, but there were time-points when group weight differences were evident.
A significant difference was observed between adolescent onset CET and their age-matched controls during weeks 8–11 [E, p <0.05], but not for the remainder of the experiment. During weeks 4–7 and 12–27 [E*, p <0.05], a significant difference was also observed between adult onset CET and their age- matched controls.
Adolescent onset CET rats consumed more EtOH solution
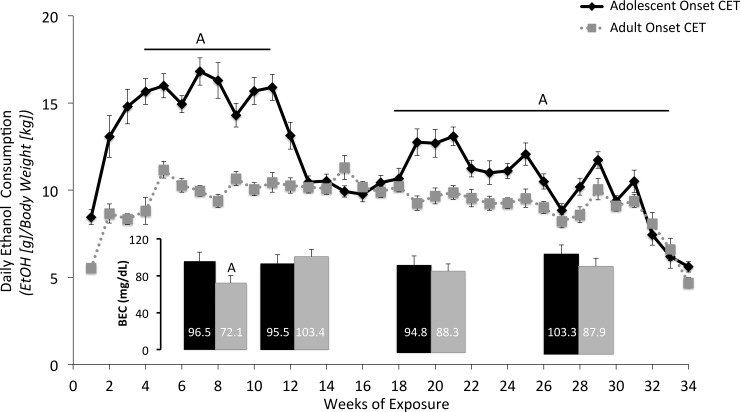
As shown in Fig 3, there were age differences in the amount of EtOH consumed during treatment. The average daily EtOH consumption for adult onset rats averaged 9.28 ± 0.61 (SEM) g/kg, whereas adolescent onset rats consumed 11.80 ± 1.11 g/kg. There was a significant interaction between Age and Time (analyzed as weekly intake) on EtOH consumption (F[30,1380] = 11.02, p<0.001). Follow-up analyses were conducted by dividing the 34-week treatment phase into 3 (fading-on or fading-off periods) or 4-week bins. Adolescent onset rats drank more than adult onset rats during weeks 4–7 (F[3,138] = 8.83, p<0.05), 8-11(F[3,138] = 4.12, p<0.05), 16–19 (F[3,138] = 11.63, p<0.05), 20–23 (F[3,138] = 6.32, p<0.05), 24–27 (F[3,138] = 3.5, p<0.05), 28–31 (F[3,138] = 9.62, p<0.05). No significant differences were found between weeks 12–15 and 32–34.
Fig 3. Adolescent onset of drinking, relative to adult onset, leads to higher EtOH consumption levels and initial higher blood ethanol concentrations.
A difference in EtOH consumption was observed between the adult and adolescent CET onset rats on weeks 5–11 and 16–31, [A, p <0.05], where adolescent onset rats consumed significantly more EtOH solution compared to adult onset rats. Despite significant differences in consumption, a significant difference in BEC was only observed after 1 month of CET [A, p <0.05], where adolescent onset rats had significantly higher BEC levels compared to adult onset rats.
As shown in S1 Fig, there was a significant difference in average daily water consumption between adolescent and adult rats during weeks 1–4 (F[3,45] = 3.97, p<0.2). Adolescent animals consumed more water compared to adult animals during week 1 (F[1,18] = 87.66, p<0.001), week 2 (F[1,15] = 28.80, p<0.001), and week 3 (F[1,15] = 12.82, p<0.01). After week 4, there was no significant effect of Age on average daily intake (all F’s<2.2, p’s>0.05). These results indicate that age-related differences in normal liquid consumption equalize at about P55, the age at which adolescent onset CET animals began exposure to 20% (v/v) CET.
Rats exposed to chronic EtOH had BECs in the binge range
CET rats had significantly higher BECs than control groups at each analysis time-point (month 2, 3, 4 and 6; F [1,76] = 239.56, p<0.001), indicating that control rats were not exposed to EtOH, whereas CET rats were intoxicated. The average BECs across the months were above the binge range of 80 mg/dL (Reilly et al, 2014). A significant Age effect was only found in the month 1 BEC analysis, where adult onset CET rats had lower BECs compared to adolescent onset animals (Fig 3; F[1,47] = 5.13, p<0.05).
Chronic EtOH drinking reduced neurotrophin levels in the prefrontal cortex
Brain derived neurotrophin factor
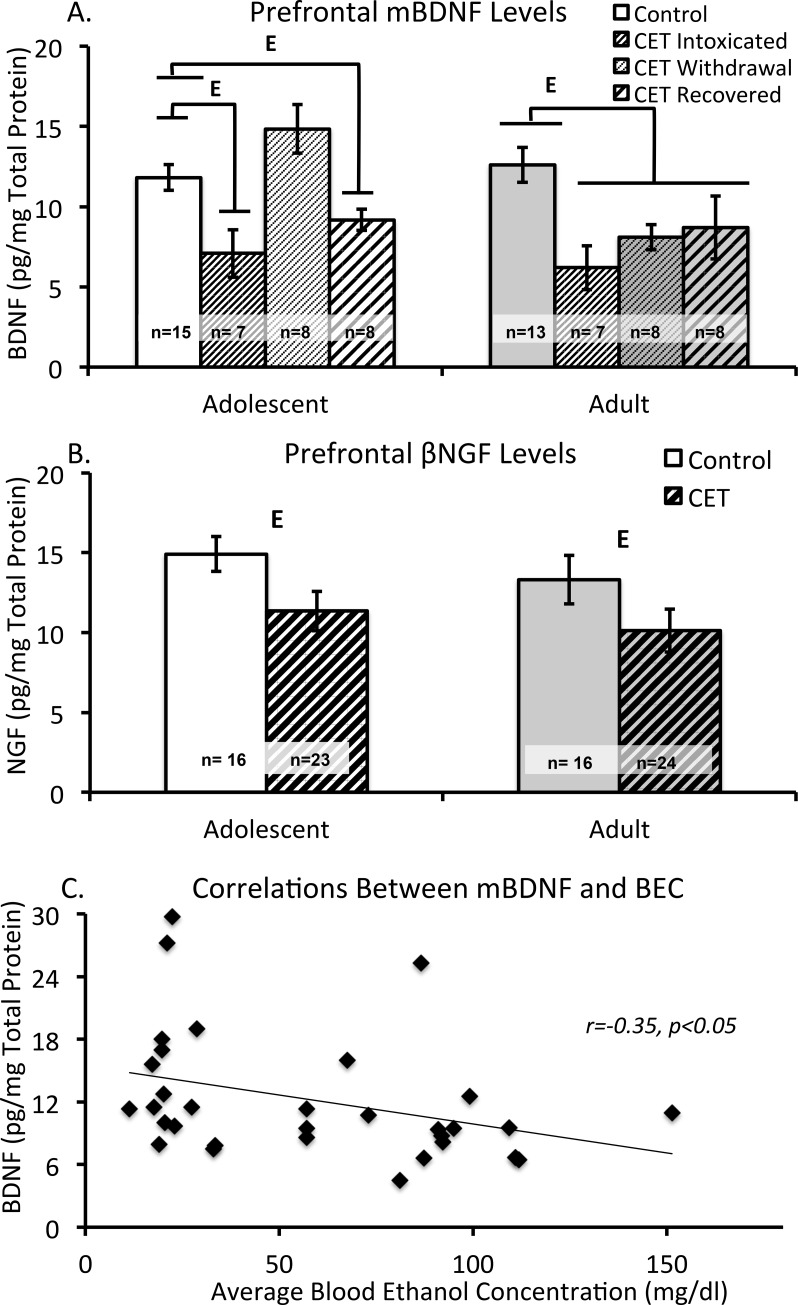
The cortices of 5 rats (1- Adolescent CET/T1; 1- Adolescent control/T3; 1-Adult CET/T1; 2-Adult Control T1) were not processed due to limited tissue. We hypothesized that there would be no differences across time-points for neurotrophin content in the control groups. This hypothesis was supported by the ANOVA (both F’s <1.21, p>0.30), allowing us to collapse our data across time-points and use a single control group at each age. There was a main effect of Treatment (CET vs. Control) on prefrontal cortex BDNF levels (F[1,66] = 21.29, p<0.0001), where CET rats had lower BDNF levels than controls rats. In the CET groups, there was significant Age x Time interaction (F[2,66] = 3.55, p<0.05). As shown in Fig 4A, adolescent onset CET rats at T1 (intoxicated) and T3 (protracted abstinence/recovered) had significantly decreased BDNF levels relative to control rats (T1: F[1,21] = 9.27, p<0.05; T3: F[1,22] = 4.76, p<0.05). At T2 (acute abstinence/withdrawal) there were was a trend (F[1,21] = 3.75, p = 0.07) for adolescent onset CET rats to have higher BDNF levels than control rats, but this difference failed to reach significance. In contrast, the adult onset CET rats had suppressed BDNF levels, relative to their control group, at all time points (F[1,32] = 11.66, p<0.01). Unlike the adolescent CET rats, adult CET rats did not differ across the three time points (F<1).
Fig 4. Chronic EtOH exposure alters prefrontal neurotrophin levels.
(A) Average concentration of mature BDNF levels (pg/mg) in the prefrontal cortex according to Age, Treatment group and Time-point for tissue collection (control groups were collapsed across time-points). In the adolescent group, time-point 1 [E, p<0.05, intoxicated; thin stripe] and time-point 3 [E, p<0.05, recovered; thick stripe] rats had significantly less BDNF content compared to controls. There was also a significant main effect of Treatment in the adult consumption groups [E, p<0.05], with CET rats having lower BDNF content compared to controls. (B) Mean concentration levels (pg/mg) for β-NGF in the prefrontal cortex by Age and Treatment group, collapsed across time-points. There was a main effect of Treatment [E, p<0.05], with CET rats displaying significantly decreased levels of β-NGF in the prefrontal cortex. (C) Correlation between average BEC levels during CET and BDNF content in the prefrontal cortex. There was a significant negative correlation between average BEC and BDNF content: Rats that had higher average BECs had reduced BDNF levels within the prefrontal cortex.
β-Nerve growth factor
The cortex of 1 rat (1-Adolescent CET/T2) was not processed due to limited tissue availability. There were no age related differences in prefrontal cortex β-NGF levels in control (both F’s<1.76, p’s>0.20) or CET groups (both F’s<1.13, p’s>0.30) across the 3 time-points. Therefore, the data were collapsed across time points for both treatment groups. A main effect of Treatment was found (F[1,71] = 6.01, p<0.05), with CET rats having lower levels of β-NGF (Fig 4B). However, there was no main effect of Age (F’s< 0.58, p’s >0.451, or Time point (F’s< 1.31, p’s >0.28) and the interaction between those variables was non-significant (F’s< 1.3, p’s> 0.28).
Higher BECs correlated with lower prefrontal cortical BDNF levels
Regardless of the age at which EtOH exposure initiated, rats with higher BECs had decreased BDNF content within the prefrontal cortex (Fig 4C). Average BEC levels had a significant, negative correlation with prefrontal BDNF content, r = -0.35, p<0.05. This was the only regional neurotrophin level that correlated with BEC.
Chronic EtOH drinking reduces hippocampal BDNF levels in adult onset rats
Brain derived neurotrophin factor
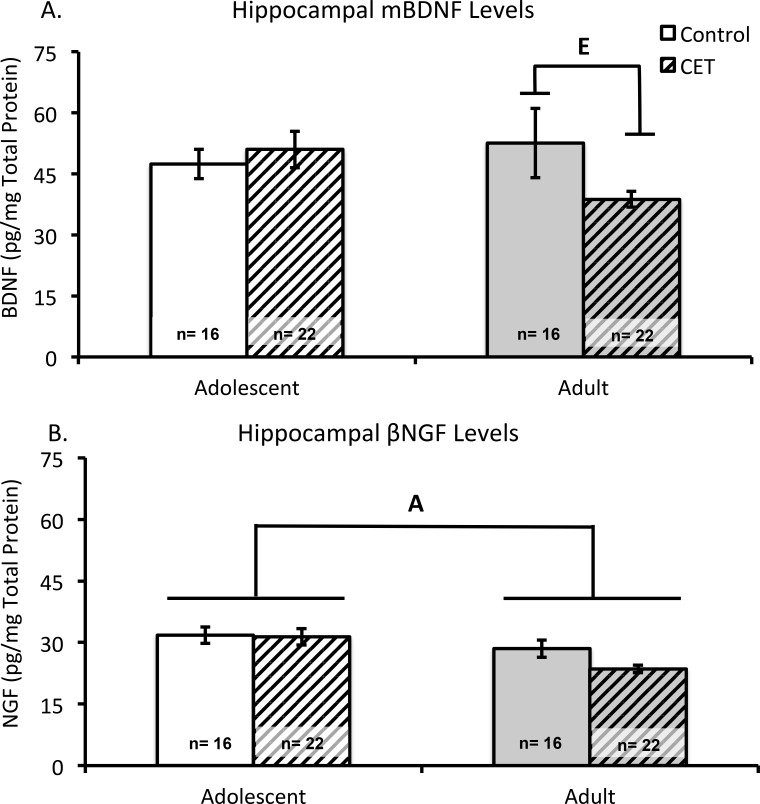
The hippocampi of 4 subjects (1-Adolescent CET/T1; 1-Adolescent CET/T2; 2 Adult CET/T2) were not processed due to dissection issues or tissue availability. Neither adolescent nor adult rats, CET nor control, significantly differed in hippocampal BDNF levels as a function of Time point (F’s<1, p’s> 0.60); thus, data were collapsed across time-points (Fig 5A). Analysis of variance revealed a trend towards significance in the interaction between Age and Treatment (F(1,68) = 3.79, p = 0.056). Post-hoc analyses revealed that adult onset CET rats had significantly lower hippocampal BDNF levels than adult control rats (F[1,36] = 4.16, p<0.05). This treatment effect was not observed between the adolescent onset rats (F<1).
Fig 5. Chronic EtOH drinking decreases hippocampal neurotrophin levels only in adult onset EtOH drinking rats.
(A) Mean concentration of mature BDNF levels (pg/mg) in the hippocampus according to Age and Treatment conditions, collapsed across time-points for tissue collection. Adult CET rats have less BDNF neurotrophin content compared to adult controls [E, p <0.05]. (B) Mean concentration levels of β-NGF (pg/mg) in the hippocampus according to Age and Treatment groups, collapsed across time-points for tissue collection. There was a main effect of Age [A, p<0.05] on β-NGF levels, where adult rats have significantly less β-NGF detected in the hippocampus compared to adolescent rats.
β-Nerve Growth Factor
Both control and CET groups were collapsed across time-points as levels of β-NGF in the hippocampus did not differ as a function of time-points (F’s<1, p’s>0.90). Regardless of treatment condition, animals that began the exposure protocol as adolescents had significantly higher concentrations of β-NGF in the hippocampus than their adult counterparts (F[1,68] = 9.48, p<0.01; Fig 5B). There was no significant interaction between Age and Treatment (F[1,72] = 1.65, p>0.20).
Behavioral Testing
All T-3 groups (Adolescent onset CET, Adolescent water control, Adult onset CET, Adult water control) had 8 subjects that completed all phases of behavioral testing.
Higher BECs correlated with lower spontaneous alternation scores
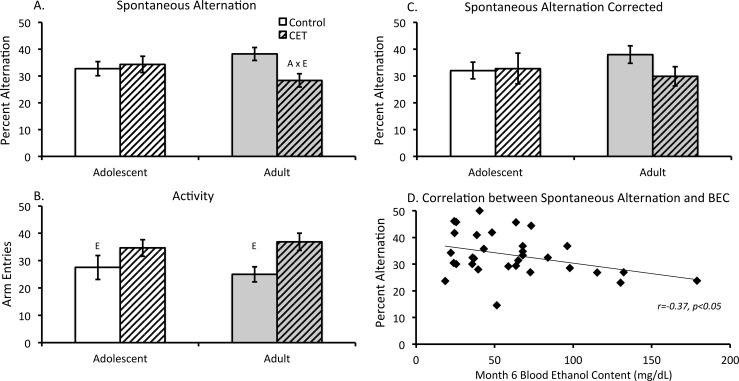
Analysis of the percent alternation scores revealed a significant Age x Treatment interaction (F [1,28] = 4.66, p<0.05; Fig 6A). Adult onset CET rats displayed lower alternation scores compared to their age-matched controls. In contrast, adolescent onset CET rats did not display significantly different alternation scores relative to age-matched controls. However, CET rats, regardless of age, made significantly more arm entries compared to control rats (F [1, 28] = 7.77, p<0.01; Fig 6B). Thus, alternation scores were corrected for overall activity, with percent alternation scores only including a maximum of 25 arm entries (the lowest group activity level). Subsequent analysis failed to yield a significant difference between adult onset CET and the adult control group (F[1,14) = 2.95, p = 0.11; (Fig 6C).
Fig 6. Lower spontaneous alternation behavior correlated with higher blood EtOH concentrations.
(A) Percent alternation analysis reveals an Age by Treatment interaction [A x E, p<0.05], with adult onset CET rats alternating significantly less than age-matched controls. (B) Mean number of arm entries according to Age and Treatment groups. Regardless of age, CET rats made significantly more arm entries compared to controls. (C) Analysis of percent alteration scores corrected for a maximum of 25 arm entries revealed no difference between adult control and CET rats. (D) Final (month 6) BECs were significantly negatively correlated with spontaneous alternation behavior: As BECs increased, spontaneous alternation performance decreased.
However, the correlation analysis revealed a negative relation (r = -0.37, p<0.05) between high BECs and low spontaneous alternation scores (Fig 6D). Regardless of the age at which EtOH exposure initiated, rats with higher BECs at the end of the CET paradigm had significantly fewer spontaneous alternations.
Delayed Alternation is not affected by chronic EtOH drinking
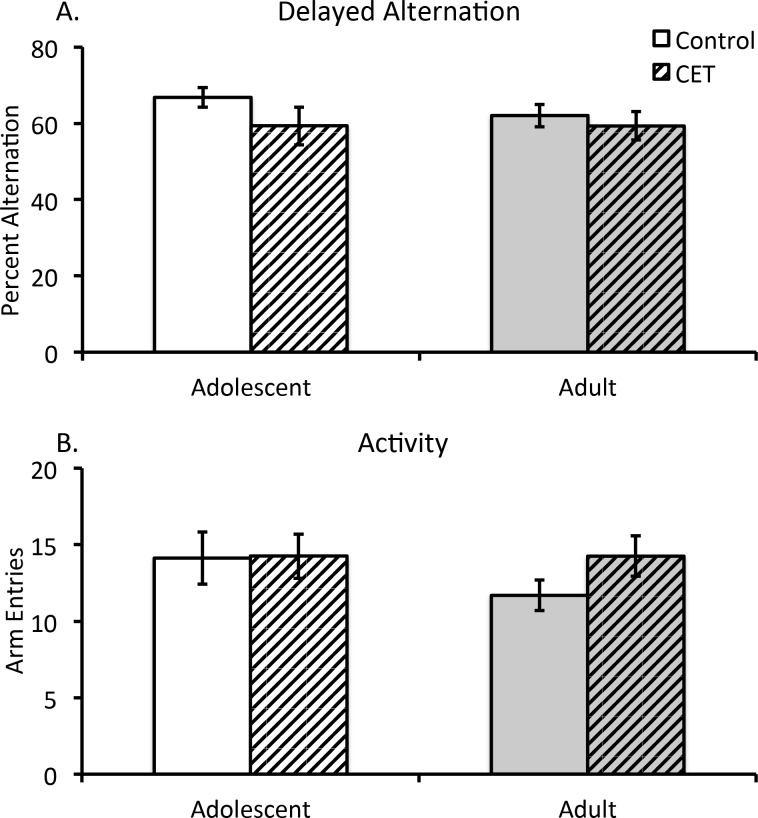
There were no significant differences in delayed alternation scores or arm entries as a function of Age or Treatment (F’s[1,28) <1.73; p>0.10). Furthermore, there was no interaction between Age and Treatment on delayed alternation scores (All F’s <0.25, p’s>0.05; Fig 7).
Fig 7. Chronic EtOH drinking did not impair delayed alternation behavior.
(A) Average percent delayed alternation according to Age and Treatment conditions. There was no effect of Age or CET on delayed alternation performance. (B) Average number of arm entries according to Age and Treatment groups. No group differences were found.
Chronic EtOH drinking impairs non-spatial discrimination learning and behavioral flexibility
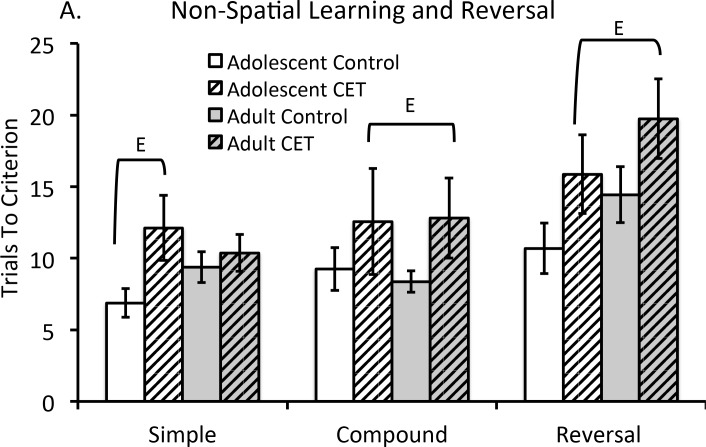
Fig 8A demonstrates the average number of trials required to reach criterion as a function of the type of non-spatial discrimination task for each group. The number of trials to criterion varied as a function of test (F[4,112] = 12.96, p<0.001). There was a main effect of CET that demonstrated impaired non-spatial discrimination learning, (F[1,28] = 8.82, p<0.01), but EtOH exposure interacted with the type of task (F[4,112] = 2.99, p<0.025). We therefore analyzed separate ANOVAs for each type of task. This analysis revealed that CET rats required more trials to learn the simple discrimination (F [1, 28] = 4.33, p<0.05). However, the effect was driven by the CET adolescent group requiring more trials to master the simple discrimination rule (F[1,28] = 4.65, p<0.05), whereas adult CET rats did not differ from adult controls (F<1). Since there was no significant difference across both compound (F[1,28] = 3.36, p>0.05) and reversal tasks (F[1,28] = 1.55, p>0.05), each test was collapsed. Both CET age groups required more trials to learn the complex discriminations (F[1, 28] = 4.93, p<0.05). Furthermore, the greatest deficit (Cohen’s d = 0.79) was seen in reversal learning, where CET rats were impaired on reversal learning regardless of the age of onset (F[1, 28] = 7.27, p<0.025).
Fig 8. Chronic EtOH drinking, regardless of age of drinking onset, impaired complex non-spatial discrimination learning and reduced behavioral flexibility.
(A) The average number of trials required to reach criterion during non-spatial discrimination learning and reversal tasks. Criterion was defined as making a correct discrimination on 6 consecutive trials. Significant CET effects [E] were found on the simple discrimination task, compound discrimination and reversal phases. In these three tasks, CET rats all required more trials to reach criterion compared to controls (E, p’s<0.05).
We did not find a significant correlation between BEC levels at the end of CET and the number of trials to reach criterion for non-spatial discrimination learning or reversal learning (all p’s>0.05).
Discussion
Four main findings emerged from the present study: first, adolescent onset of chronic EtOH drinking led to increased EtOH consumption that persisted beyond the adolescent period into adulthood. Second, chronic EtOH drinking altered both brain BDNF and NGF levels during intoxication, withdrawal and protracted abstinence. In the prefrontal cortex, CET produced long-term decreases in both BDNF and NGF, regardless of the age at which EtOH consumption was initiated. However, adolescent onset CET rats displayed a unique increase in BDNF levels during the acute abstinence (withdrawal) phase. In the hippocampus, only rats that started consuming EtOH as adults displayed reductions in BDNF. Third, higher BECs were correlated with lower hippocampal-dependent spontaneous alternation behavior. Fourth, regardless of BECs or age of drinking onset, non-spatial discrimination learning and behavioral flexibility, which is dependent on the frontal cortex, was impaired by chronic EtOH drinking.
Epidemiological work has consistently demonstrated that adolescent alcohol abuse increases the lifetime risk for developing a later AUD [9,64,65]. This has been mirrored in animal models demonstrating that adolescent EtOH exposure predisposes rats to consume elevated levels of EtOH in adulthood, compared to an EtOH naïve rat [66–71]. Rats with an adolescent onset of CET had comparable BEC levels to adult onset CET rats after month 1, despite the fact that adult onset CET rats consumed, on average, 10% less EtOH. The data suggest a greater metabolic tolerance in the adolescent onset CET group relative to the adult onset ETOH group. This finding complements data showing that adolescent rats exposed to a chronic, intermittent binge EtOH treatment display an increased metabolic rate, relative to adult rats [72]. The comparable BEC levels across the adolescent and adult onset CET groups likely contributed to the similar profile of behavioral impairment seen in both exposure groups.
Our CET animals exhibited impairments in non- spatial discrimination learning, as well as overall reductions in neurotrophin levels. It is hypothesized that alcohol-related brain damage, leading to behavioral dysfunction, is a result of alterations in the balance between neurotrophins, which are reduced, and neuroimmune signaling, which is typically increased, after chronic EtOH exposure [48,73]. At high doses, EtOH decreases cyclic AMP-responsive element binding protein (CREB)-DNA binding while increasing nuclear factor kappa-light-chain enhancer of activated B cells (NF-kB; [74]). EtOH-induced disruptions of CREB and NF-kB levels may result in decreased neurotrophin levels and increased chemokine and cytokine activation, which exacerbate EtOH-induced glutamate excitoxicity, which could manifest in behavioral deficits [48].
Neuroimaging studies of the alcoholic brain have demonstrated that the frontal lobes, relative to other brain regions, have the most pronounced abnormalities [75,76]. These findings are paralleled by an increase in markers of cell death and neural neuroinflammation within the frontal cortex in both rodent models of adolescent binge EtOH exposure and in post mortem brain tissue of alcoholics with an early age of drinking onset [26]. Abstinent alcoholics display decreased gray matter volume in the dorsolateral, dorsomedial and ventromedial prefrontal cortex that are correlated with increased impulsivity [77]. Orbital frontal cortical shrinkage is also observed in abstinent alcoholics [78] and has been associated with impaired behavioral flexibility [37], including reversal learning [79]. Similarly, rodents exposed to chronic intermittent EtOH exposure as adults [80] or adolescents [26] display an initial impairment in reversal learning, but in adult mice the reversal impairment was no longer evident with an extended recovery period (10-days post EtOH). Our data demonstrate that long-term, continuous EtOH exposure leads to a persistent and long-lasting impairment in reversal learning, regardless of age of drinking onset or BEC.
However, brain and behavioral recovery following abstinence from alcohol consumption has been observed in humans, but there are considerable individual differences [81]. Following excessive binge EtOH exposure in rodents, in the acute abstinence phase (48-hrs post EtOH), there is a burst of cell genesis and an increase in phosphorylated CREB in multiple brain regions, including the cortex and hippocampus [82]. However, the increase in these markers of plasticity eventually subsides. Alcoholic patients going through withdrawal show an acute transient increase in plasma concentrations of BDNF levels, but not NGF levels [83,84]. Furthermore, the intensity of acute alcohol withdrawal symptoms have been correlated with an increase in BDNF serum levels [46], which was taken as evidence that BDNF may be involved in neuroadaptation during the early alcohol withdrawal period. The transient increase in frontal cortical mBDNF observed in the current study (during the acute abstinence phase in adolescent onset CET rats) may represent an attempt to compensate for neurodegeneration. This effect could be indicative of an intrinsic mechanism for the repair of alcohol-induced structural damage, similar to what has been observed after adolescent binge EtOH exposure [20]. Age at the time of EtOH exposure maybe a key factor in both the expression and extent of the recovery of function observed in abstinent alcoholics. The manner in which age and alcohol interact to modulate both neural adaption and plasticity markers needs further examination.
In the adult nervous system, the mature forms of BDNF and NGF support neuronal survival and are critical for synaptic plasticity that is altered by addiction [85]. However, both pro-NGF and pro-BDNF activate the p75 receptor that leads to cell death [86,87]. Chronic, continuous EtOH exposure decreases the mRNA for BDNF within the adult cortex during CET [88] and within the hippocampus 48-hrs after EtOH cessation [89]. The down-regulation of BDNF transcription, induced by CET, could lead to the reduction of BDNF synthesis, thereby resulting in decreased detection of mBDNF levels after prolonged EtOH consumption. Chronic EtOH intake in rats also decreases the immunoreactivity of NGF and choline acetyltransferase, an enzyme regulated by NGF, within the hippocampus and medial septum [51]. This suggests that NGF synthesis and/or biological activity is also affected by chronic EtOH drinking. This is supported by studies demonstrating that exogenous NGF delivery recovers both behavioral impairment and the loss of forebrain cholinergic neurons that project to the hippocampus and cortex in the CET model [53,54].
We observed a significant loss of NGF in the frontal cortex, regardless of age of drinking onset, an effect not observed in the hippocampus. This regional sensitivity may be attributed to the fact that most ELISA kits measure both the pro- and mature forms of NGF [90]. Considering the opposing effects of pro and mature forms of NGF and BDNF on cell survival, it is critical in future studies to determine the ratio between pro and mature neurotrophins. The altered balance across pro- and mature neurotrophin levels could be a biomarker of neurodegeneration or repair in several diseases, including chronic alcoholism.
Behavioral deficits on tasks that rely on the hippocampus and/or frontal cortex are seen in abstinent alcoholics [91,92] and rodent models of chronic continuous or intermittent EtOH exposure [93]. Factors that contribute to the extent of both hippocampal and cortical impairment are drinking history duration and degree of intoxication, which can be reflected in BECs. It has been stated that a high volume of alcohol drinking (35 drinks per week for men; 28 drinks per week for women) for an extended period of time (more than 5 years) are key risk factors in the development of alcohol-related brain damage [94]. The forced EtOH consumption models (EtOH in the drinking fluid or liquid diet) are analogs of the later stages of sustained alcohol addiction [50]. Such forced EtOH consumption models are commonly used to achieve high EtOH intake for extended periods of time [49], which appear to modulate alcohol-induced behavioral dysfunction.
Spatial memory impairments have been observed after adult onset CET with a long duration (12 months) of EtOH exposure [95] or with shorter durations (6–8 months) when BEC are over 100 mg/dl [96]. Sex also appears to modulate the behavioral effects of chronic EtOH: we found that adult female rats exposed to CET were impaired on delayed matching to position, whereas adult male CET rats only displayed impairment when the rule was reversed to non-matching-to-position [57]. In the current study, we found that higher BECs at the end of treatment were correlated with lower spontaneous alternation performance, a hippocampal dependent task [97]. However, our data reveal that the same correlation between BEC and reversal learning, an orbital frontal cortical dependent task [98], was not significant. Regardless, there was a main effect of CET on reversal learning, irrespective of age of drinking onset. This suggests that EtOH-induced abnormalities in frontocortical-dependent behaviors can be found across a range of metabolic EtOH concentrations. Thus, frontocortical behaviors, like neural plasticity measures, appear to be more sensitive to EtOH, compared to hippocampal-dependent behaviors.
Conclusions
Recent data establishes adolescence as a vulnerable time period for EtOH related toxicity that leads to neuropathological, behavioral and motivational changes [99]. Initiating alcohol drinking at a young age leads to a greater propensity for the development of AUDs. Individuals who begin drinking by the age of 13 are over 3 times more likely to engage binge drinking (more than 5 drinks per episode) and extreme drinking (greater than 10 drinks per occasion; [100]. Exposure to EtOH during adolescence in rodents can produce greater EtOH intake during adulthood [101,102] and we replicated those finding in a continuous access model.
However, adolescence is a protracted period of developmental neural adaption that appears to have epochs of vulnerability to alcohol exposure, which influences certain long-term behavioral outcomes, including abuse propensity [103]. In rodents, researchers have identified three adolescent epochs: early- (PD 21–34), mid- (PD 34–46), and late- (PD 46–59) adolescence [103,104]. In a summary of recent data, Spear [103] suggests that early adolescent EtOH exposure has been associated with affective and hippocampal abnormalities, whereas late adolescent EtOH exposure disrupts behaviors dependent on the developing pre-frontal cortical systems.
The lack of persistent hippocampal dysfunction after mid-adolescent onset CET may be related to the unique, time- dependent, developmental windows that occur during adolescence. One caveat of our model is that the EtOH “fade on” period takes the adolescent rats through “emerging adulthood” [99]. Thus, the impact of the moderate to high EtOH doses did not occur until emerging adulthood. Exposure to binge EtOH levels during early adolescence have been linked to deficits in spatial memory acquisition and reversal learning [105,106]. These EtOH related deficits are either not elicited or less pronounced when exposure occurs during mid to late adolescence [103]. Therefore, we hypothesize that the lack of age- dependent, EtOH related behavioral deficits was possibly due to the fact that we missed a critical window of EtOH exposure. If EtOH exposure was initiated at an earlier age, it is possible that we would have detected pronounced behavioral and neural deficits compared to those seen with adult onset exposure.
However, even moderate drinking during mid-adolescence contributed to heavy EtOH consumption in adulthood. It has been shown, and we replicated, that by PD 55, water consumption stabilizes across adulthood [107]. Thus, differences in EtOH consumption from day 55 onward are not due to adolescent-typical hyperdipsia.
The current data extend our understanding of the different patterns of neuroadaptation, specifically of BDNF and NGF, within two key brain regions (hippocampus and frontal cortex) in an animal model of late-stage sustained alcohol addiction. By collecting brain samples at different phases of the addiction cycle (intoxication, acute and protracted abstinence), we revealed that chronic EtOH exposure beginning in mid-adolescence or early adulthood leads to reductions in frontocortical plasticity during intoxication and following protracted abstinence. The neurotrophin reductions that occur during active drinking and protracted abstinence may modulate several disease process associated with alcoholism, including both functional (decision making) and structural (neuropathological) changes that likely contribute to the cycle of addictive behaviors [108].
Supporting Information
Data is presented for each Treatment condition as a function of age (Age of Onset) at which drinking started (postnatal day 35 = Adolescent; postnatal day 73–75 = Adult), and time (Timepoint) when brains were collected (1 = during ethanol consumption; 2 = 48-hrs after ethanol removal; 3 = 6–8 weeks after ethanol fading-off and behavioral testing). Data is present for each behavioral task (DA = Delayed Alternation [Day 1; Day 2]; SA = Spontaneous Alternation; % = Percent alternation; # = Number of arm entries). Discrimination data is summarized as the number of trials to reach criterion. There is also neurotrophin data (Brain-Derived Neurotropic Factor [BDNF] and Nerve Growth Factor [NGF], both presented as pg/mg of protein, as a function of brain region (PFC = prefrontal cortex; HPC = hippocampus).
(PDF)
Estimated daily EtOH consumption (g/kg) as a function of ethanol concentration (6%, 9%, 12%, 20%) for 28 weeks for rats that started drinking at either postnatal day 35 (see Age at Onset; Adolescent) or postnatal days 73–75 (see Age at Onset; Adult). Brains were collected at 3 time points (Timepoint): 1 = During ethanol consumption; 2 = 48-hours after ethanol removal; 3 = 6 to 8 weeks post ethanol exposure and after behavioral testing.
(PDF)
A difference in water consumption was observed between adult and adolescent age- matched animals on weeks 1–4 [A, p <0.05], where adolescent animals consumed significantly more liquid compared to adult age- matched rats. No further age dependent differences were found on consumption levels after week 4.
(TIF)
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
This research was funded by a pilot project grant from the Developmental Exposure Alcohol Research Center (P50AA01782306) and an R01 grant to LMS (RO1AA021775), both funded by the National Institute on Alcohol Abuse and Alcoholism. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Substance Abuse and Mental Health Services Administration. Results from the 2013 National Survey on Drug Use and Health: Summary of National Findings, NSDUH Series H-48, HHS Publication No. (SMA) 14–4863.
- 2.Swartzwelder HS, Hogan A, Risher M-L, Swartzwelder RA, Wilson WA, Acheson SK. Effect of sub-chronic intermittent ethanol exposure on spatial learning and ethanol sensitivity in adolescent and adult rats. Alcohol Fayettev N. 2014. June;48(4):353–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gass JT, Glen WB, McGonigal JT, Trantham-Davidson H, Lopez MF, Randall PK, et al. Adolescent alcohol exposure reduces behavioral flexibility, promotes disinhibition, and increases resistance to extinction of ethanol self-administration in adulthood. Neuropsychopharmacol Off Publ Am Coll Neuropsychopharmacol. 2014. October;39(11):2570–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Varlinskaya EI, Truxell E, Spear LP. Chronic intermittent ethanol exposure during adolescence: effects on social behavior and ethanol sensitivity in adulthood. Alcohol Fayettev N. 2014. August;48(5):433–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Spear LP, Varlinskaya EI. Sensitivity to ethanol and other hedonic stimuli in an animal model of adolescence: implications for prevention science? Dev Psychobiol. 2010. April;52(3):236–43. 10.1002/dev.20457 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mejia-Toiber J, Boutros N, Markou A, Semenova S. Impulsive choice and anxiety-like behavior in adult rats exposed to chronic intermittent ethanol during adolescence and adulthood. Behav Brain Res. 2014. February 22;266C:19–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hamilton KR, Felton JW, Risco CM, Lejuez CW, MacPherson L. Brief report: The interaction of impulsivity with risk-taking is associated with early alcohol use initiation. J Adolesc. 2014. September 29;37(8):1253–6. 10.1016/j.adolescence.2014.08.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Broadwater MA, Liu W, Crews FT, Spear LP. Persistent loss of hippocampal neurogenesis and increased cell death following adolescent, but not adult, chronic ethanol exposure. Dev Neurosci. 2014;36(3–4):297–305. 10.1159/000362874 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hanson KL, Medina KL, Padula CB, Tapert SF, Brown SA. Impact of Adolescent Alcohol and Drug Use on Neuropsychological Functioning in Young Adulthood: 10-Year Outcomes. J Child Adolesc Subst Abuse. 2011. January 1;20(2):135–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Vetreno RP, Broadwater M, Liu W, Spear LP, Crews FT. Adolescent, but not adult, binge ethanol exposure leads to persistent global reductions of choline acetyltransferase expressing neurons in brain. PLOS One. 2014;9(11):e113421 10.1371/journal.pone.0113421 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Spear LP. Adolescent neurobehavioral characteristics, alcohol sensitivities, and intake: Setting the stage for alcohol use disorders? Child Dev Perspect. 2011. December 1;5(4):231–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nixon K, McClain JA. Adolescence as a critical window for developing an alcohol use disorder: current findings in neuroscience. Curr Opin Psychiatry. 2010. May;23(3):227–32. 10.1097/YCO.0b013e32833864fe [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Crews F, He J, Hodge C. Adolescent cortical development: a critical period of vulnerability for addiction. Pharmacol Biochem Behav. 2007. February;86(2):189–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Miki T, Harris SJ, Wilce PA, Takeuchi Y, Bedi KS. Effects of alcohol exposure during early life on neuron numbers in the rat hippocampus. I. Hilus neurons and granule cells. Hippocampus. 2003;13(3):388–98. [DOI] [PubMed] [Google Scholar]
- 15.Miki T, Harris SJ, Wilce PA, Takeuchi Y, Bedi KS. Effects of age and alcohol exposure during early life on pyramidal cell numbers in the CA1-CA3 region of the rat hippocampus. Hippocampus. 2004;14(1):124–34. [DOI] [PubMed] [Google Scholar]
- 16.Lundqvist C, Alling C, Knoth R, Volk B. Intermittent ethanol exposure of adult rats: hippocampal cell loss after one month of treatment. Alcohol Alcohol Oxf Oxfs. 1995. November;30(6):737–48. [PubMed] [Google Scholar]
- 17.Thinschmidt JS, Walker DW, King MA. Chronic ethanol treatment reduces the magnitude of hippocampal LTD in the adult rat. Synap N Y N. 2003. June 15;48(4):189–97. [DOI] [PubMed] [Google Scholar]
- 18.Fujii S, Yamazaki Y, Sugihara T, Wakabayashi I. Acute and chronic ethanol exposure differentially affect induction of hippocampal LTP. Brain Res. 2008. May 23;1211:13–21. 10.1016/j.brainres.2008.02.052 [DOI] [PubMed] [Google Scholar]
- 19.Richardson HN, Chan SH, Crawford EF, Lee YK, Funk CK, Koob GF, et al. Permanent impairment of birth and survival of cortical and hippocampal proliferating cells following excessive drinking during alcohol dependence. Neurobiol Dis. 2009. October;36(1):1–10. 10.1016/j.nbd.2009.05.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Geil CR, Hayes DM, McClain JA, Liput DJ, Marshall SA, Chen KY, et al. Alcohol and adult hippocampal neurogenesis: promiscuous drug, wanton effects. Prog Neuropsychopharmacol Biol Psychiatry. 2014. October 3;54:103–13. 10.1016/j.pnpbp.2014.05.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.McClain JA, Morris SA, Marshall SA, Nixon K. Ectopic hippocampal neurogenesis in adolescent male rats following alcohol dependence. Addict Biol. 2014. July;19(4):687–99. 10.1111/adb.12075 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ozsoy S, Durak AC, Esel E. Hippocampal volumes and cognitive functions in adult alcoholic patients with adolescent-onset. Alcohol Fayettev N. 2013. February;47(1):9–14. [DOI] [PubMed] [Google Scholar]
- 23.Taffe MA, Kotzebue RW, Crean RD, Crawford EF, Edwards S, Mandyam CD. Long-lasting reduction in hippocampal neurogenesis by alcohol consumption in adolescent nonhuman primates. Proc Natl Acad Sci U S A. 2010. June 15;107(24):11104–9. 10.1073/pnas.0912810107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hoefer ME, Pennington DL, Durazzo TC, Mon A, Abé C, Truran D, et al. Genetic and behavioral determinants of hippocampal volume recovery during abstinence from alcohol. Alcohol Fayettev N. 2014. November;48(7):631–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pfefferbaum A, Rosenbloom M, Deshmukh A, Sullivan E. Sex differences in the effects of alcohol on brain structure. Am J Psychiatry. 2001. February;158(2):188–97. [DOI] [PubMed] [Google Scholar]
- 26.Vetreno RP, Qin L, Crews FT. Increased receptor for advanced glycation end product expression in the human alcoholic prefrontal cortex is linked to adolescent drinking. Neurobiol Dis. 2013. November;59:52–62. 10.1016/j.nbd.2013.07.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Volkow ND, Baler RD. Brain imaging biomarkers to predict relapse in alcohol addiction. JAMA Psychiatry. 2013. July;70(7):661–3. 10.1001/jamapsychiatry.2013.1141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Squeglia LM, Tapert SF, Sullivan EV, Jacobus J, Meloy MJ, Rohlfing T, et al. Brain development in heavy-drinking adolescents. Am J Psychiatry. 2015. June;172(6):531–42. 10.1176/appi.ajp.2015.14101249 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Harper C. The neuropathology of alcohol-related brain damage. Alcohol Alcohol Oxf Oxfs. 2009. April;44(2):136–40. [DOI] [PubMed] [Google Scholar]
- 30.Kril JJ, Macdonald V, Patel S, Png F, Halliday GM. Distribution of brain atrophy in behavioral variant frontotemporal dementia. J Neurol Sci. 2005. May 15;232(1–2):83–90. [DOI] [PubMed] [Google Scholar]
- 31.De Bellis MD, Narasimhan A, Thatcher DL, Keshavan MS, Soloff P, Clark DB. Prefrontal cortex, thalamus, and cerebellar volumes in adolescents and young adults with adolescent-onset alcohol use disorders and comorbid mental disorders. Alcohol Clin Exp Res. 2005. September;29(9):1590–600. [DOI] [PubMed] [Google Scholar]
- 32.Harper C. The neuropathology of alcohol-specific brain damage, or does alcohol damage the brain? J Neuropathol Exp Neurol. 1998. February;57(2):101–10. [DOI] [PubMed] [Google Scholar]
- 33.Miguel-Hidalgo JJ. Withdrawal from free-choice ethanol consumption results in increased packing density of glutamine synthetase-immunoreactive astrocytes in the prelimbic cortex of alcohol-preferring rats. Alcohol Alcohol Oxf Oxfs. 2006. August;41(4):379–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Whittom A, Villarreal A, Soni M, Owusu-Duku B, Meshram A, Rajkowska G, et al. Markers of apoptosis induction and proliferation in the orbitofrontal cortex in alcohol dependence. Alcohol Clin Exp Res. 2014. November;38(11):2790–9. 10.1111/acer.12559 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Crews FT, Qin L, Sheedy D, Vetreno RP, Zou J. HMGB1/TLR Receptor Danger Signaling Increases Brain Neuroimmune Activation in Alcohol Dependence. Biol Psychiatry. 2013. April 1;73(7):602–12. 10.1016/j.biopsych.2012.09.030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Houston RJ, Derrick JL, Leonard KE, Testa M, Quigley BM, Kubiak A. Effects of heavy drinking on executive cognitive functioning in a community sample. Addict Behav. 2014. January;39(1):345–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Oscar-Berman M, Kirkley SM, Gansler DA, Couture A. Comparisons of Korsakoff and non-Korsakoff alcoholics on neuropsychological tests of prefrontal brain functioning. Alcohol Clin Exp Res. 2004. April;28(4):667–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Coleman LG, He J, Lee J, Styner M, Crews FT. Adolescent binge drinking alters adult brain neurotransmitter gene expression, behavior, brain regional volumes, and neurochemistry in mice. Alcohol Clin Exp Res. 2011. April;35(4):671–88. 10.1111/j.1530-0277.2010.01385.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Coleman LG, Liu W, Oguz I, Styner M, Crews FT. Adolescent binge ethanol treatment alters adult brain regional volumes, cortical extracellular matrix protein and behavioral flexibility. Pharmacol Biochem Behav. 2014. January;116:142–51. 10.1016/j.pbb.2013.11.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Logrip ML, Janak PH, Ron D. Escalating ethanol intake is associated with altered corticostriatal BDNF expression. J Neurochem. 2009. June;109(5):1459–68. 10.1111/j.1471-4159.2009.06073.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Carlino D, De Vanna M, Tongiorgi E. Is altered BDNF biosynthesis a general feature in patients with cognitive dysfunctions? Neurosci Rev J Bringing Neurobiol Neurol Psychiatry. 2013. August;19(4):345–53. [DOI] [PubMed] [Google Scholar]
- 42.Forlenza OV, Diniz BS, Teixeira AL, Radanovic M, Talib LL, Rocha NP, et al. Lower Cerebrospinal Fluid Concentration of Brain-Derived Neurotrophic Factor Predicts Progression from Mild Cognitive Impairment to Alzheimer’s Disease. Neuromolecular Med. 2015. September;17(3):326–32. 10.1007/s12017-015-8361-y [DOI] [PubMed] [Google Scholar]
- 43.Murer MG, Yan Q, Raisman-Vozari R. Brain-derived neurotrophic factor in the control human brain, and in Alzheimer’s disease and Parkinson’s disease. Prog Neurobiol. 2001. January;63(1):71–124. [DOI] [PubMed] [Google Scholar]
- 44.Miller R, King MA, Heaton MB, Walker DW. The effects of chronic ethanol consumption on neurotrophins and their receptors in the rat hippocampus and basal forebrain. Brain Res. 2002. September 20;950(1–2):137–47. [DOI] [PubMed] [Google Scholar]
- 45.Joe K-H, Kim Y-K, Kim T-S, Roh S-W, Choi S-W, Kim Y-B, et al. Decreased plasma brain-derived neurotrophic factor levels in patients with alcohol dependence. Alcohol Clin Exp Res. 2007. November;31(11):1833–8. [DOI] [PubMed] [Google Scholar]
- 46.Huang M-C, Chen C-H, Chen C-H, Liu S-C, Ho C-J, Shen WW, et al. Alterations of serum brain-derived neurotrophic factor levels in early alcohol withdrawal. Alcohol Alcohol Oxf Oxfs. 2008. June;43(3):241–5. [DOI] [PubMed] [Google Scholar]
- 47.Jockers-Scherübl MC, Zubraegel D, Baer T, Linden M, Danker-Hopfe H, Schulte-Herbrüggen O, et al. Nerve growth factor serum concentrations rise after successful cognitive-behavioural therapy of generalized anxiety disorder. Prog Neuropsychopharmacol Biol Psychiatry. 2007. January 30;31(1):200–4. [DOI] [PubMed] [Google Scholar]
- 48.Vetreno RP, Crews FT. Binge ethanol exposure during adolescence leads to a persistent loss of neurogenesis in the dorsal and ventral hippocampus that is associated with impaired adult cognitive functioning. Front Neurosci. 2015;9:35 10.3389/fnins.2015.00035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Coleman RA, Young BM, Turner LE, Cook RT. A practical method of chronic ethanol administration in mice. Methods Mol Biol Clifton NJ. 2008;447:49–59. [DOI] [PubMed] [Google Scholar]
- 50.D’Souza El-Guindy NB, Kovacs EJ, De Witte P, Spies C, Littleton JM, de Villiers WJS, et al. Laboratory models available to study alcohol-induced organ damage and immune variations: choosing the appropriate model. Alcohol Clin Exp Res. 2010. September 1;34(9):1489–511. 10.1111/j.1530-0277.2010.01234.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Aloe L, Tirassa P. The effect of long-term alcohol intake on brain NGF-target cells of aged rats. Alcohol Fayettev N. 1992. August;9(4):299–304. [DOI] [PubMed] [Google Scholar]
- 52.Arendt T. Impairment in memory function and neurodegenerative changes in the cholinergic basal forebrain system induced by chronic intake of ethanol. J Neural Transm Suppl. 1994;44:173–87. [DOI] [PubMed] [Google Scholar]
- 53.Arendt T, Allen Y, Marchbanks RM, Schugens MM, Sinden J, Lantos PL, et al. Cholinergic system and memory in the rat: effects of chronic ethanol, embryonic basal forebrain brain transplants and excitotoxic lesions of cholinergic basal forebrain projection system. Neuroscience. 1989;33(3):435–62. [DOI] [PubMed] [Google Scholar]
- 54.Lukoyanov NV, Pereira PA, Paula-Barbosa MM, Cadete-Leite A. Nerve growth factor improves spatial learning and restores hippocampal cholinergic fibers in rats withdrawn from chronic treatment with ethanol. Exp Brain Res. 2003. January;148(1):88–94. [DOI] [PubMed] [Google Scholar]
- 55.Ehrlich D, Pirchl M, Humpel C. Effects of long-term moderate ethanol and cholesterol on cognition, cholinergic neurons, inflammation, and vascular impairment in rats. Neuroscience. 2012. March 15;205:154–66. 10.1016/j.neuroscience.2011.12.054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Trick L, Kempton MJ, Williams SCR, Duka T. Impaired fear recognition and attentional set-shifting is associated with brain structural changes in alcoholic patients. Addict Biol. 2014. November;19(6):1041–54. 10.1111/adb.12175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Savage LM, Candon PM, Hohmann HL. Alcohol-induced brain pathology and behavioral dysfunction: using an animal model to examine sex differences. Alcohol Clin Exp Res. 2000. April;24(4):465–75. [PubMed] [Google Scholar]
- 58.Cadete-Leite A, Pereira PA, Madeira MD, Paula-Barbosa MM. Nerve growth factor prevents cell death and induces hypertrophy of basal forebrain cholinergic neurons in rats withdrawn from prolonged ethanol intake. Neuroscience. 2003;119(4):1055–69. [DOI] [PubMed] [Google Scholar]
- 59.Gearhart DA, Middlemore M-L, Terry AV. ELISA methods to measure cholinergic markers and nerve growth factor receptors in cortex, hippocampus, prefrontal cortex, and basal forebrain from rat brain. J Neurosci Methods. 2006. January 30;150(2):159–73. [DOI] [PubMed] [Google Scholar]
- 60.Foreman N, Toates F, Donohoe T. Spontaneous and learned turning behaviour in food- or water-restricted hooded rats. Q J Exp Psychol B. 1990. May;42(2):153–73. [PubMed] [Google Scholar]
- 61.Anzalone S, Vetreno RP, Ramos RL, Savage LM. Cortical cholingeric abnormalities contribute to the amnesic state induced by pyrithiamine-induced thiamine deficiency in the rat. Eur J Neurosci. 2010. September;32(5):847–58. 10.1111/j.1460-9568.2010.07358.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Hall JM, Vetreno RP, Savage LM. Differential cortical neurotrophin and cytogenetic adaptation after voluntary exercise in normal and amnestic rats. Neuroscience. 2014. January 31;258:131–46. 10.1016/j.neuroscience.2013.10.075 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Ragozzino ME, Artis S, Singh A, Twose TM, Beck JE, Messer WS. The Selective M1 Muscarinic Cholinergic Agonist CDD-0102A Enhances Working Memory and Cognitive Flexibility. J Pharmacol Exp Ther. 2012. March;340(3):588–94. 10.1124/jpet.111.187625 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.DeWit DJ, Adlaf EM, Offord DR, Ogborne AC. Age at first alcohol use: a risk factor for the development of alcohol disorders. Am J Psychiatry. 2000. May;157(5):745–50. [DOI] [PubMed] [Google Scholar]
- 65.Dawson DA, Goldstein RB, Chou SP, Ruan WJ, Grant BF. Age at first drink and the first incidence of adult-onset DSM-IV alcohol use disorders. Alcohol Clin Exp Res. 2008. December;32(12):2149–60. 10.1111/j.1530-0277.2008.00806.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Fabio MC, Nizhnikov ME, Spear NE, Pautassi RM. Binge ethanol intoxication heightens subsequent ethanol intake in adolescent, but not adult, rats. Dev Psychobiol. 2014. April;56(3):574–83. 10.1002/dev.21101 [DOI] [PubMed] [Google Scholar]
- 67.Alaux-Cantin S, Warnault V, Legastelois R, Botia B, Pierrefiche O, Vilpoux C, et al. Alcohol intoxications during adolescence increase motivation for alcohol in adult rats and induce neuroadaptations in the nucleus accumbens. Neuropharmacology. 2013. April;67:521–31. 10.1016/j.neuropharm.2012.12.007 [DOI] [PubMed] [Google Scholar]
- 68.Broadwater M, Spear LP. Consequences of ethanol exposure on cued and contextual fear conditioning and extinction differ depending on timing of exposure during adolescence or adulthood. Behav Brain Res. 2013. November 1;256:10–9. 10.1016/j.bbr.2013.08.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Criado JR, Ehlers CL. Effects of adolescent onset voluntary drinking followed by ethanol vapor exposure on subsequent ethanol consumption during protracted withdrawal in adult Wistar rats. Pharmacol Biochem Behav. 2013. January;103(3):622–30. 10.1016/j.pbb.2012.10.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Gilpin NW, Karanikas CA, Richardson HN. Adolescent binge drinking leads to changes in alcohol drinking, anxiety, and amygdalar corticotropin releasing factor cells in adulthood in male rats. PLOS One. 2012;7(2):e31466 10.1371/journal.pone.0031466 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Maldonado-Devincci AM, Badanich KA, Kirstein CL. Alcohol during adolescence selectively alters immediate and long-term behavior and neurochemistry. Alcohol Fayettev N. 2010. February;44(1):57–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Silvers JM, Tokunaga S, Mittleman G, Matthews DB. Chronic intermittent injections of high-dose ethanol during adolescence produce metabolic, hypnotic, and cognitive tolerance in rats. Alcohol Clin Exp Res. 2003. October;27(10):1606–12. [DOI] [PubMed] [Google Scholar]
- 73.Mayfield J, Ferguson L, Harris RA. Neuroimmune signaling: a key component of alcohol abuse. Curr Opin Neurobiol. 2013. August;23(4):513–20. 10.1016/j.conb.2013.01.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Zou J, Crews F. CREB and NF-kappaB transcription factors regulate sensitivity to excitotoxic and oxidative stress induced neuronal cell death. Cell Mol Neurobiol. 2006. August;26(4–6):385–405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.O’Neill J, Cardenas VA, Meyerhoff DJ. Effects of abstinence on the brain: quantitative magnetic resonance imaging and magnetic resonance spectroscopic imaging in chronic alcohol abuse. Alcohol Clin Exp Res. 2001. November;25(11):1673–82. [PubMed] [Google Scholar]
- 76.Rando K, Hong K-I, Bhagwagar Z, Li C-SR, Bergquist K, Guarnaccia J, et al. Association of Frontal and Posterior Cortical Gray Matter V olume With Time to Alcohol Relapse: A Prospective Study. Am J Psychiatry. 2011. February;168(2):183–92. 10.1176/appi.ajp.2010.10020233 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Le Berre A-P, Pitel A-L, Chanraud S, Beaunieux H, Eustache F, Martinot J-L, et al. Chronic alcohol consumption and its effect on nodes of frontocerebellar and limbic circuitry: comparison of effects in France and the United States. Hum Brain Mapp. 2014. September;35(9):4635–53. 10.1002/hbm.22500 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Tanabe J, Tregellas JR, Dalwani M, Thompson L, Owens E, Crowley T, et al. Medial orbitofrontal cortex gray matter is reduced in abstinent substance-dependent individuals. Biol Psychiatry. 2009. January 15;65(2):160–4. 10.1016/j.biopsych.2008.07.030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Fortier CB, Steffen EM, Lafleche G, Venne JR, Disterhoft JF, McGlinchey RE. Delay discrimination and reversal eyeblink classical conditioning in abstinent chronic alcoholics. Neuropsychology. 2008. March;22(2):196–208. 10.1037/0894-4105.22.2.196 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Badanich KA, Becker HC, Woodward JJ. Effects of chronic intermittent ethanol exposure on orbitofrontal and medial prefrontal cortex-dependent behaviors in mice. Behav Neurosci. 2011. December;125(6):879–91. 10.1037/a0025922 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Pfefferbaum A, Rosenbloom MJ, Chu W, Sassoon SA, Rohlfing T, Pohl KM, et al. White matter microstructural recovery with abstinence and decline with relapse in alcohol dependence interacts with normal ageing: a controlled longitudinal DTI study. Lancet Psychiatry. 2014. August;1(3):202–12. 10.1016/S2215-0366(14)70301-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Crews FT, Nixon K. Mechanisms of neurodegeneration and regeneration in alcoholism. Alcohol Alcohol Oxf Oxfs. 2009. April;44(2):115–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Köhler S, Klimke S, Hellweg R, Lang UE. Serum brain-derived neurotrophic factor and nerve growth factor concentrations change after alcohol withdrawal: preliminary data of a case-control comparison. Eur Addict Res. 2013;19(2):98–104. 10.1159/000342334 [DOI] [PubMed] [Google Scholar]
- 84.Reynolds PM, Mueller SW, MacLaren R. A comparison of dexmedetomidine and placebo on the plasma concentrations of NGF, BDNF, GDNF, and epinephrine during severe alcohol withdrawal. Alcohol Fayettev N. 2015. February;49(1):15–9. [DOI] [PubMed] [Google Scholar]
- 85.Davis MI. Ethanol-BDNF interactions: still more questions than answers. Pharmacol Ther. 2008. April;118(1):36–57. 10.1016/j.pharmthera.2008.01.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Hempstead BL. Deciphering proneurotrophin actions. Handb Exp Pharmacol. 2014;220:17–32. 10.1007/978-3-642-45106-5_2 [DOI] [PubMed] [Google Scholar]
- 87.Lu B, Pang PT, Woo NH. The yin and yang of neurotrophin action. Nat Rev Neurosci. 2005. August;6(8):603–14. [DOI] [PubMed] [Google Scholar]
- 88.Baek JK, Heaton MB, Walker DW. Up-regulation of high-affinity neurotrophin receptor, trk B-like protein on western blots of rat cortex after chronic ethanol treatment. Brain Res Mol Brain Res. 1996. August;40(1):161–4. [DOI] [PubMed] [Google Scholar]
- 89.MacLennan AJ, Lee N, Walker DW. Chronic ethanol administration decreases brain-derived neurotrophic factor gene expression in the rat hippocampus. Neurosci Lett. 1995. September 8;197(2):105–8. [DOI] [PubMed] [Google Scholar]
- 90.Soligo M, Protto V, Florenzano F, Bracci-Laudiero L, De Benedetti F, Chiaretti A, et al. The mature/pro nerve growth factor ratio is decreased in the brain of diabetic rats: Analysis by ELISA methods. Brain Res. 2015. August 15; [DOI] [PubMed] [Google Scholar]
- 91.Pitel AL, Segobin SH, Ritz L, Eustache F, Beaunieux H. Thalamic abnormalities are a cardinal feature of alcohol-related brain dysfunction. Neurosci Biobehav Rev. 2014. August 6; [DOI] [PubMed] [Google Scholar]
- 92.Stavro K, Pelletier J, Potvin S. Widespread and sustained cognitive deficits in alcoholism: a meta-analysis. Addict Biol. 2013. March;18(2):203–13. 10.1111/j.1369-1600.2011.00418.x [DOI] [PubMed] [Google Scholar]
- 93.Vetreno RP, Hall JM, Savage LM. Alcohol-related amnesia and dementia: animal models have revealed the contributions of different etiological factors on neuropathology, neurochemical dysfunction and cognitive impairment. Neurobiol Learn Mem. 2011. November;96(4):596–608. 10.1016/j.nlm.2011.01.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Ridley NJ, Draper B, Withall A. Alcohol-related dementia: an update of the evidence. Alzheimers Res Ther. 2013;5(1):3 10.1186/alzrt157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Beracochea D, Durkin TP, Jaffard R. On the involvement of the central cholinergic system in memory deficits induced by long term ethanol consumption in mice. Pharmacol Biochem Behav. 1986. March;24(3):519–24. [DOI] [PubMed] [Google Scholar]
- 96.Hodges H, Allen Y, Sinden J, Mitchell SN, Arendt T, Lantos PL, et al. The effects of cholinergic drugs and cholinergic-rich foetal neural transplants on alcohol-induced deficits in radial maze performance in rats. Behav Brain Res. 1991. April 18;43(1):7–28. [DOI] [PubMed] [Google Scholar]
- 97.Deacon RMJ, Rawlins JNP. T-maze alternation in the rodent. Nat Protoc. 2006;1(1):7–12. [DOI] [PubMed] [Google Scholar]
- 98.Hamilton DA, Brigman JL. Behavioral flexibility in rats and mice: contributions of distinct frontocortical regions. Genes Brain Behav. 2015. January;14(1):4–21. 10.1111/gbb.12191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Spear LP, Swartzwelder HS. Adolescent alcohol exposure and persistence of adolescent-typical phenotypes into adulthood: a mini-review. Neurosci Biobehav Rev. 2014. September;45:1–8. 10.1016/j.neubiorev.2014.04.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Hingson RW, Zha W. Age of drinking onset, alcohol use disorders, frequent heavy drinking, and unintentionally injuring oneself and others after drinking. Pediatrics. 2009. June;123(6):1477–84. 10.1542/peds.2008-2176 [DOI] [PubMed] [Google Scholar]
- 101.Pascual MM, Pastor V, Bernabeu RO. Nicotine-conditioned place preference induced CREB phosphorylation and Fos expression in the adult rat brain. Psychopharmacology (Berl). 2009. November;207(1):57–71. [DOI] [PubMed] [Google Scholar]
- 102.Acevedo MB, Molina JC, Nizhnikov ME, Spear NE, Pautassi RM. High ethanol dose during early adolescence induces locomotor activation and increases subsequent ethanol intake during late adolescence. Dev Psychobiol. 2010. July 1;52(5):424–40. 10.1002/dev.20444 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Spear LP. Adolescent alcohol exposure: Are there separable vulnerable periods within adolescence? Physiol Behav. 2015. September 1;148:122–30. 10.1016/j.physbeh.2015.01.027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Tirelli E, Laviola G, Adriani W. Ontogenesis of behavioral sensitization and conditioned place preference induced by psychostimulants in laboratory rodents. Neurosci Biobehav Rev. 2003. March;27(1–2):163–78. [DOI] [PubMed] [Google Scholar]
- 105.Markwiese BJ, Acheson SK, Levin ED, Wilson WA, Swartzwelder HS. Differential effects of ethanol on memory in adolescent and adult rats. Alcohol Clin Exp Res. 1998. April;22(2):416–21. [PubMed] [Google Scholar]
- 106.Vetreno RP, Crews FT. Adolescent binge drinking increases expression of the danger signal receptor agonist HMGB1 and Toll-like receptors in the adult prefrontal cortex. Neuroscience. 2012. December 13;226:475–88. 10.1016/j.neuroscience.2012.08.046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Vetter CS, Doremus-Fitzwater TL, Spear LP. Time course of elevated ethanol intake in adolescent relative to adult rats under continuous, voluntary-access conditions. Alcohol Clin Exp Res. 2007. July;31(7):1159–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Koob GF, Volkow ND. Neurocircuitry of addiction. Neuropsychopharmacol Off Publ Am Coll Neuropsychopharmacol. 2010. January;35(1):217–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data is presented for each Treatment condition as a function of age (Age of Onset) at which drinking started (postnatal day 35 = Adolescent; postnatal day 73–75 = Adult), and time (Timepoint) when brains were collected (1 = during ethanol consumption; 2 = 48-hrs after ethanol removal; 3 = 6–8 weeks after ethanol fading-off and behavioral testing). Data is present for each behavioral task (DA = Delayed Alternation [Day 1; Day 2]; SA = Spontaneous Alternation; % = Percent alternation; # = Number of arm entries). Discrimination data is summarized as the number of trials to reach criterion. There is also neurotrophin data (Brain-Derived Neurotropic Factor [BDNF] and Nerve Growth Factor [NGF], both presented as pg/mg of protein, as a function of brain region (PFC = prefrontal cortex; HPC = hippocampus).
(PDF)
Estimated daily EtOH consumption (g/kg) as a function of ethanol concentration (6%, 9%, 12%, 20%) for 28 weeks for rats that started drinking at either postnatal day 35 (see Age at Onset; Adolescent) or postnatal days 73–75 (see Age at Onset; Adult). Brains were collected at 3 time points (Timepoint): 1 = During ethanol consumption; 2 = 48-hours after ethanol removal; 3 = 6 to 8 weeks post ethanol exposure and after behavioral testing.
(PDF)
A difference in water consumption was observed between adult and adolescent age- matched animals on weeks 1–4 [A, p <0.05], where adolescent animals consumed significantly more liquid compared to adult age- matched rats. No further age dependent differences were found on consumption levels after week 4.
(TIF)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.