Abstract
Klinefelter syndrome (47, XXY) (KS) is a genetic syndrome characterized by the presence of an extra X chromosome and low level of testosterone, resulting in a number of neurocognitive abnormalities, yet little is known about brain function. This study investigated the fMRI-BOLD response from KS relative to a group of Controls to basic motor, perceptual, executive and adaptation tasks. Participants (N: KS = 49; Controls = 49) responded to whether the words “GREEN” or “RED” were displayed in green or red (incongruent versus congruent colors). One of the colors was presented three times as often as the other, making it possible to study both congruency and adaptation effects independently. Auditory stimuli saying “GREEN” or “RED” had the same distribution, making it possible to study effects of perceptual modality as well as Frequency effects across modalities. We found that KS had an increased response to motor output in primary motor cortex and an increased response to auditory stimuli in auditory cortices, but no difference in primary visual cortices. KS displayed a diminished response to written visual stimuli in secondary visual regions near the Visual Word Form Area, consistent with the widespread dyslexia in the group. No neural differences were found in inhibitory control (Stroop) or in adaptation to differences in stimulus frequencies. Across groups we found a strong positive correlation between age and BOLD response in the brain's motor network with no difference between groups. No effects of testosterone level or brain volume were found. In sum, the present findings suggest that auditory and motor systems in KS are selectively affected, perhaps as a compensatory strategy, and that this is not a systemic effect as it is not seen in the visual system.
Keywords: Klinefelter syndrome, Brain activation, Motor cortex, Auditory cortex
Graphical abstract
Highlights
-
•
Klinefelter syndrome (KS) males have an extra X-chromosome.
-
•
KS have increased motor responses in primary motor cortex.
-
•
KS have increased responses to auditory stimuli in auditory cortices.
-
•
KS lack testosterone, but no fMRI effect of testosterone level was observed.
1. Introduction
Klinefelter syndrome (47,XXY) (KS) where the person is phenotypically male but has both two X-chromosomes and a Y-chromosome is the most common sex-chromosome aneuploidy in males, affecting 1 out of 650 men (Bojesen et al., 2003). KS displays a range of anthropometric characteristics, in particular smaller testes and hypogonadism (Høst et al., 2014). Although head size is normal (Chang et al., 2014), both gray matter and white matter volumes have been found to be smaller in KS compared to genetically unaffected male control participants (hereafter called “Controls”) (Bryant et al., 2011, DeLisi et al., 2005, Giedd et al., 2007, Lentini et al., 2013, Skakkebæk et al., 2014b, Warwick et al., 1999). Voxel-based morphometry (VBM) locates these size differences primarily to subcortical areas in combination with insula and medial temporal cortices (Bryant et al., 2011, Hong et al., 2014, Lentini et al., 2013, Skakkebæk et al., 2014b).
KS males also display a number of cognitive deficits and psychiatric morbidity, including learning disabilities (Ratcliffe, 1999), dysfunctional inhibitory control (Kompus et al., 2011, Lee et al., 2011, Temple and Sanfilippo, 2003, van Rijn et al., 2009), working memory impairments (Fales et al., 2003, Skakkebæk et al., 2014b) and below average intelligence, affecting verbal IQ to a larger extent than performance IQ (see Skakkebæk et al., 2015 for a recent review). KS males also often express increased symptoms of depression (Bruining et al., 2009, Turriff et al., 2011), anxiety (Tartaglia et al., 2010), psychotic disorders (Bruining et al., 2009) and autism (Skakkebæk et al., 2014a, van Rijn et al., 2008b).
1.1. Nonspecific brain hemodynamic effects of KS?
Few functional neuroimaging studies have investigated the neurological and brain hemodynamic underpinnings of the KS phenotype (Hong and Reiss, 2014) and most have focused on complex cognitive issues such as language processing (Steinman et al., 2009, van Rijn et al., 2008a) and social cognition (Brandenburg-Goddard et al., 2014, van Rijn et al., 2012). However, KS males also differ from Controls on other factors that are not directly related to cognition, but which may influence the blood-oxygen-level dependent (BOLD) signal detected using fMRI (Ogawa et al., 1990). Vasodilation may be altered by cerebrovascular risk factors, such as hypertension, diabetes and hypercholesterolaemia (D'Esposito et al., 2003). This may affect the transformation of neural activity into changes in blood flow (Handwerker et al., 2012). KS has higher rates of cardiovascular diseases (Bojesen and Gravholt, 2007, Bojesen et al., 2006a) and diabetes (Nielsen et al., 1969), perhaps due to increased obesity (Bojesen et al., 2006b). Blood hematocrit level is also highly variable in KS (Chang et al., 2014) due to its dependency on testosterone levels (Calof et al., 2005). Testosterone levels vary in KS because many receive exogenous testosterone as treatment for their hypogonadotrophic hypogonadism. Hematocrit is thought to influences the BOLD response (Levin et al., 2001).
The adult KS participants in previous experiments have also been highly heterogeneous when it comes to age (van Rijn et al., 2008a, van Rijn et al., 2012), which may contribute to increased variability in the BOLD response due to the above-mentioned factors (Huettel et al., 2001, Richter and Richter, 2003).
Considering these potential confounds, we wished to ask the fundamental question: Do KS males display fMRI-BOLD responses different from Controls at a systemic level? More specifically, do they exhibit differences in BOLD response for simple stimuli across different perceptual modalities with or without behavioral responses? For this purpose, we constructed an experiment where participants experienced both visual and auditory stimuli and had to respond to some of the stimuli while not responding to others (see Section 2.2 for details).
The testable hypothesis for this question was whether KS participants indeed do exhibit greater BOLD responses than Controls, indiscriminately across perceptual and motor cortices. Such a finding would strongly influence the interpretation of previous results related to complex cognitive issues. Secondary to this question was the hypothesis that testosterone level would yield a system level difference in BOLD response. If this were the case, it would in turn invalidate cognitive comparisons that do not control for testosterone level across KS and Controls.
1.2. Specific low-level effects of KS?
If no systems level effects were observed, one could start to investigate the degree to which KS males exhibit systematic localizable effects related to primary perceptual input and/or motor output.
1.2.1. The motor system
KS males suffer from non-specific motor impairments (Ross et al., 2008, Ross et al., 2009) and a larger prevalence of essential tremor (Harlow and Gonzalez-Alegre, 2009) which may cause them to use compensatory motor strategies and also to be less capable of remaining stationary in the scanner. Testing the BOLD signal for motor output and testing whether KS males display increased head movement during scanning is thus an important prerequisite for further neurocognitive investigations of KS as a group.
1.2.1.1. Involuntary movement
Head movement is a primary source of artifacts in fMRI-BOLD experiments (Lund et al., 2005). We hypothesized that involuntary motion during scanning would be increased in KS. In that case many complex cognitive investigations would be invalidated, as movement would lead to differences in data artifacts and signal power loss.
1.2.1.2. Motor cortex effects
Prior to investigation of cognitive effects in KS it remains to be studied whether simple motor output generates comparable fMRI-BOLD responses. In this study we therefore investigated potential behavioral differences in KS and Controls to simple button presses using both auditory and visual stimuli. We hypothesized that motor cortex activation would be higher in KS due to compensatory strategies involved in overcoming motor impairments (Ross et al., 2008, Ross et al., 2009).
1.2.2. Auditory systems
Although data is scarce, KS males have been found to have increased rates of sensorineural hearing impairments (Anderson et al., 1971, Castiglione et al., 2013, Sørensen, 1992), affecting an estimated 20% of the group (Castiglione et al., 2013). Furthermore, an increased proportion of KS males report auditory hallucinations (Boks et al., 2007, Bruining et al., 2009, DeLisi et al., 2005, Sørensen and Nielsen, 1977). Auditory hallucinations have been found to yield an increase in activity in the auditory cortices (Dierks et al., 1999). Verbal IQ in KS is known to be affected to a greater extent than performance IQ (Skakkebæk et al., 2015), perhaps suggesting a role for low-level audition. Together, these findings point towards the possibility of a generally altered, most likely increased activation pattern for auditory input either due to interaction with hallucinations or as coping strategy for hearing impairment and/or hallucinations.
1.2.3. Visual systems
KS males exhibit fewer problems in the primary visual domain. We are at least not aware of reports indicating that vision should be significantly different in the KS population. A subset of the KS group reports visual hallucinations, but to a smaller extent than auditory (Boks et al., 2007, Sørensen and Nielsen, 1977). The visual domain thus remains a possible test-bed for distinguishing between non-specific systemic differences and specific low-level differences between KS and Controls.
1.3. Specific high-level cognitive effects of KS?
As noted above, KS exhibits a number of deficits linked to executive function. For this reason, we added two tasks to investigate these aspects of the neurofunctional profile of KS. We added a simplified Stroop condition to investigate inhibitory executive function and a stimulus frequency manipulation in order to investigate short-term adaptation in both behavior and neural responses (see Materials and methods section for details).
The Stroop task (Stroop, 1935) involves inhibiting a trained response (reading a word) in order to provide an untrained response (naming the color of the letters of the word) and is known to yield increased responses in Broca's region (Novick et al., 2010, Wallentin et al., 2015). Based on this we hypothesized that our KS participants would have greater responses in this region compared to Controls.
Adaptation due to high frequency stimuli have been found to yield decreased responses in parietal areas (Wallentin et al., 2015) and we hypothesized that KS participants would have a smaller decrease than Controls due to a potential deficit in short-term adaptability.
2. Material and methods
2.1. Participants
2.1.1. Klinefelter syndrome participants
Seventy-nine KS participants were recruited through clinics for endocrinology, genetics, and fertility in Denmark. KS males between 18 and 60 years of age were included in a large broad-scale investigation of KS, including psychological testing, physiological measurements and brain scans (see Skakkebæk et al., 2014a, Skakkebæk et al., 2014b for details). Participants with a history of traumatic head injury, neurological disease, color blindness, claustrophobia, extreme obesity and substance abuse were excluded from participating in the fMRI study. Fifty-three KS males completed the fMRI experiment. All participants reported normal hearing and normal or corrected to normal vision. Four participants had to be excluded due to poor performance in the behavioral task. The remaining 49 participants were 18–59 years old (median 35). Forty-five KS participants reported being right-handed, three left-handed and one ambidextrous. Median education length was 13 years (range 7–18 years).
Forty-six KS participants had 47,XXY karyotype and three had mosaicism. Thirty-one KS participants received testosterone treatment (for details about treatment, see Chang et al., 2014). Testosterone level was measured by liquid chromatography tandem mass spectrometry using Perkin Elmer's CHS Steroid MS kit. Mean testosterone level for the current subsample was found to be 16.16 nmol/l (std: 9.29 nmol/l), based on the data obtained in the anthropometrical study on the same KS sample (Chang et al., 2014).
Based on the data obtained in the brain morphometric study of the same KS cohort (Skakkebæk et al., 2014b), mean brain volume (gray + white matter) for the current subsample was estimated to be 1250 ml (std: 88 ml).
2.1.2. Unaffected control participants
Control participants were matched to KS participants on age and level of education (Skakkebæk et al., 2014b). Sixty-five male control participants were included, recruited through advertisements in local hospitals, in local newspapers, at local work services, among volunteer fire fighters, at citizen service offices and at local libraries. Fifty-three participants out of this group also completed the fMRI experiment. Two participants were excluded due to artifacts in the EPI-images and two were excluded due to poor performance in the behavioral task (see Wallentin et al., 2015 for details).
All forty-nine Controls were male (median age: 36 years, range: 19–59 years), Forty-four participants reported being right-handed, five reported left-handedness. Median education length was 13 years (range 8–18 years). Mean testosterone level was 13.68 nmol/l (std: 5.50 nmol/l). Mean brain volume was estimated to be 1302 ml (std: 104 ml).
2.1.3. Ethical approval
All participants received oral and written information about the study before giving their written consent. The study was approved by the Danish Data Protection Agency and local ethics committee (Region Midtjylland, Denmark number M-20080238) and registered at ClinicalTrials.gov (Clinical trial NCT00999310).
2.2. Stimuli
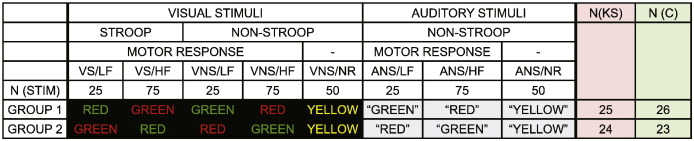
The experiment consisted of 400 trials mixing 250 visual and 150 auditory single word stimuli. Stimuli consisted of three Danish words: “GRØN” (GREEN), “RØD” (RED) and “GUL” (YELLOW). 300 Trials were either GREEN or RED, 100 trials were YELLOW (Fig. 1). Participants responded with button presses using their right hand.
Fig. 1.
Experimental setup. Forty-nine Klinefelter syndrome (KS) males and forty-nine Controls (C) participated in the experiment, see rightmost columns. Participants responded with a button press using their index finger if the color of the letters (for visual stimuli) was green or if a voice said “green” (auditory stimuli) and responded with their middle finger if the color was red or if a voice said “red”. In the case of a yellow visual or “yellow” auditory stimulus, the participants were instructed not to respond. Stimuli could be highly frequent or less frequent. Participants within the contrasted KS and C groups were randomly attributed to two stimulus groups that counterbalanced color and frequency. Participants thus received a total number of 400 stimuli divided into eight types: Visual Stroop/Low Frequency (VS/LF); Visual Stroop/High Frequency (VS/HF); Visual Non-Stroop/Low Frequency (VNS/LF); Visual Non-Stroop/High Frequency (VNS/HF); Visual Non-Stroop/No Response; Auditory Non-Stroop/Low Frequency (ANS/LF); Auditory Non-Stroop/High Frequency (ANS/LF); Auditory Non-Stroop/No Response (ANS/NR).
2.2.1. Visual stimuli
2.2.1.1. Stroop vs Non-Stroop trials
For the visual stimuli, participants were requested to respond with their index finger if the color of the letters was green, and with their middle finger if the color of the letters was red, regardless of the written word. The words GREEN and RED could be written in either green letters or red letters (see Fig. 1). The RED/GREEN part of the visual stimuli thus made up a simplified Stroop task (MacLeod, 1991, Stroop, 1935) where the color of the letters could either be congruent with the written word (Non-Stroop trials) or incongruent (Stroop trials).
2.2.1.2. Frequent vs infrequent trials
Counterbalanced across participants either GREEN or RED was the more frequent stimulus across both auditory and visual conditions (see Fig. 1). The frequent stimulus appeared three times as often (225 trials) as the infrequent stimulus (75 trials). This meant that for the visual stimuli the frequent color would be displayed 75 times in the incongruent condition and 75 times in the congruent condition. The infrequent color was displayed 25 times in the incongruent condition and 25 times in the congruent. All visual stimuli thus had the same within-color probability of a trial being a Stroop trial (50%).
2.2.1.3. No response trials
The word YELLOW was always displayed in yellow letters (50 trials). For YELLOW trials, participants were requested not to respond. These stimuli were included in order to be able to investigate perceptual stimuli in the absence of a motor response.
2.2.2. Auditory stimuli
For the auditory stimuli, the participants responded to the word itself, i.e. index finger for the word “GREEN” (75 or 25 trials) and middle finger for the word “RED” (75 or 25 trials) and no response for “YELLOW” (50 trials). Again, in concordance with the visual stimuli, either “GREEN” or “RED” would be highly frequent and thus presented 75 times or infrequent and presented 25 times (Fig. 1). This allowed an analysis that would indicate whether potential frequency effects were limited to the visual modality or not. Auditory stimuli were recorded in mono in a male neutral voice (sample frequency: 22,050 Hz) using a laptop computer.
2.3. Procedure
Stimuli were presented and responses obtained using Cogent 2000 (http://www.vislab.ucl.ac.uk/cogent_2000.php), executed in MATLAB. Visual stimuli were displayed on a black background and projected onto a screen placed at the foot of the scanner bed and participants viewed the stimuli through a mirror mounted on the head coil. Auditory stimuli were delivered through pneumatic headphones from Avotec (Stuart, FL USA). These also helped to attenuate scanner noise.
The fMRI part of the experiment lasted approximately 20 min. Before entering the scanner, participants were given a short trial run of the task in order to get accustomed to the study design and response procedure. After positioning in the scanner, both KS and Control participants were randomly attributed to one of the two stimulus groups that would get a particular stimulus type more often (Fig. 1). Participants were not informed beforehand that one of the trial types would be more frequent than the other.
2.3.1. fMRI acquisition
A 3T General Electrics Medical Systems (Milwaukee, WI USA) MR system with a standard head coil was used to acquire both T*2-weighted gradient echo, echo-planar images (EPI) with Blood Oxygenation Level-Dependent (BOLD) contrast and T1-weighted structural images. 570 EPI volumes were acquired per participant, not including the first 5 volumes that were discarded to allow for effects of T1 equilibrium. Whole brain coverage was achieved using 36 axial slices of 3.5 mm thickness with an in-plane resolution of 3.33 × 3.33 mm in a 64 × 64 voxel matrix (FOV 213.3 mm). Images were obtained with a TR of 2200 ms, a 30 ms TE and a 90 degrees flip angle. A high-resolution 3D GR T1 anatomical scan was acquired for the additional voxel-based morphometry study (Skakkebæk et al., 2014a, Skakkebæk et al., 2014b). It consisted of 256 × 256 × 134 voxels with a 0.94 × 0.94 × 1.2 mm3 voxel size, obtained with a TR of 6.552 ms, a 2.824 ms TE and a 14 degrees flip angle.
2.4. Data analysis
2.4.1. Pre-processing of fMRI data
All fMRI data were pre-processed and analyzed using Statistical Parametric Mapping software (SPM12; Wellcome Department of Imaging Neuroscience, www.fil.ion.ucl.ac.uk/spm), implemented in MATLAB. Functional images were motion corrected and registered to the first EPI image and normalized to the EPI template in SPM12. Finally, data were spatially smoothed with an isotropic 8 mm full width at half maximum (FWHM) Gaussian kernel to account for differences between participants.
2.4.2. Statistical analyses of fMRI data
Statistical analyses of fMRI data were performed using a two-level general linear model approach (Penny and Holmes, 2007, Worsley and Friston, 1995).
2.4.2.1. Participant level analyses
Eight regressors of interest were included at the first level (Fig. 1): Visual Stroop/Low Frequency (VS/LF); Visual Stroop/High Frequency (VS/HF); Visual Non-Stroop/Low Frequency (VNS/LF); Visual Non-Stroop/High Frequency (VNS/HF); Visual Non-Stroop/No Response (VNS/NR); Auditory Non-Stroop/Low Frequency (ANS/LF); Auditory Non-Stroop/High Frequency (ANS/LF); Auditory Non-Stroop/No Response (ANS/NR). Onset for each trial was defined as the onset of the stimulus. Duration was set to 0.5 s for all trials and conditions. The model was convolved with the standard hemodynamic response function in SPM12 to account for the delay in the BOLD signal. Additional nuisance regressors included error trials and six regressors modeling head motion. In order to conduct analyses relevant for a Group × Stroop × Frequency ANOVA and a Group × Perceptual Modality × Frequency ANOVA, the following contrast measures were sent to 2nd level two-sample analyses: Overall effects of visual input with responses ([1 1 1 1 0 0 0 0]), main effect of Stroop ([1 1 − 1 − 1 0 0 0 0]), main effect of Frequency (visual) ([1 − 1 1 − 1 0 0 0 0]), Stroop × Frequency interaction ([1 − 1 − 1 1 0 0 0 0 ]), effect of Frequency (auditory) ([0 0 0 0 0 1 − 1 0]), overall effects of Non-Stroop input with response across perceptual modality ([0 0 1 1 0 1 1 0]), main effect of perceptual modality for Non-Stroop trials ([0 0 1 1 0 − 1 − 1 0]) and an interaction between modality and frequency ([0 0 1 − 1 0 − 1 1 0]). Beta-estimates for the two No Response conditions were also used in order to look for effects of perceptual modality in the absence of a response.
2.4.2.2. Group level analyses
2nd level analyses were conducted as two-sample t-tests (across participant groups) with additional covariates modeling age, brain volume, stimulus group (Fig. 1) and testosterone level (see Supplementary Fig. S1 for an overview of the 2nd level model). In order to control for false positives, we thresholded results at p < 0.05, family-wise error corrected for multiple comparisons. Putative anatomical labels for peak activation sites were found using the Wake Forest University Pickatlas (Tzourio-Mazoyer et al., 2002). Only peaks > 10 mm apart are reported. Effects that have previously been reported based on the Controls alone (Wallentin et al., 2015) are only briefly summarized (Fig. 5). To further estimate and visualize effects in particular voxels of interest, we extracted beta-estimates from all participants for all eight conditions. The extracted single voxel data within primary perceptual regions were submitted to the same 2 × 2 × 2 ANOVAs as the behavioral data in order to investigate the extent to which lack of significant effects might be due to our stringent multiple comparison correction. This way, we used low-level main effects as functional localizers to investigate orthogonal contrasts, as suggested by Friston and coworkers (Friston et al., 2006).
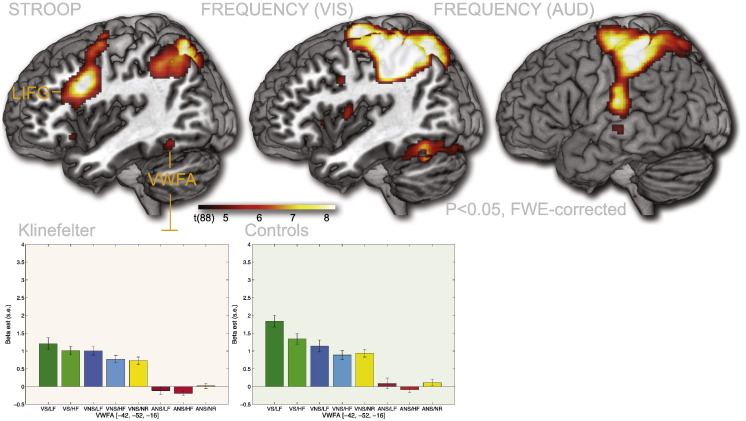
Fig. 5.
Main effects of Stroop (VS/LF & VS/HF > VNS/LF & VNS/HF), Visual Frequency (VS/LF & VNS/LF > VS/HF & VNS/HF) and Auditory Frequency (ANS/LF > ANS/HF), across participant groups. These effects closely mirror those found in a previous analysis of the Controls alone. Left inferior frontal gyrus (LIFG) was seen for the Stroop contrast. Visual Word Form Area (VWFA) was seen both for Stroop and for the Visual Frequency manipulation together with parietal cortex. Frequency in the auditory domain also activated parietal regions as well as primary and secondary auditory regions in the temporal lobe. P < 0.05, FWE-corrected.
3. Results
3.1. Motion parameters
For each participant we calculated the range of movement for all six motion parameters. We found no significant differences between KS and Controls in terms of movement inside the scanner (P > 0.1, uncorrected, for all six t-tests). Descriptive and inferential statistics for each motion parameter can be seen from Supplementary Table S1.
3.2. Behavioral effects
3.2.1. Group × Stroop × Frequency
We conducted a Group (KS vs Controls) × Stroop × Frequency mixed-design ANOVA for both response time and accuracy individually where group was a between-participants effect and Stroop and Frequency were within-participants effects (Fig. 2).
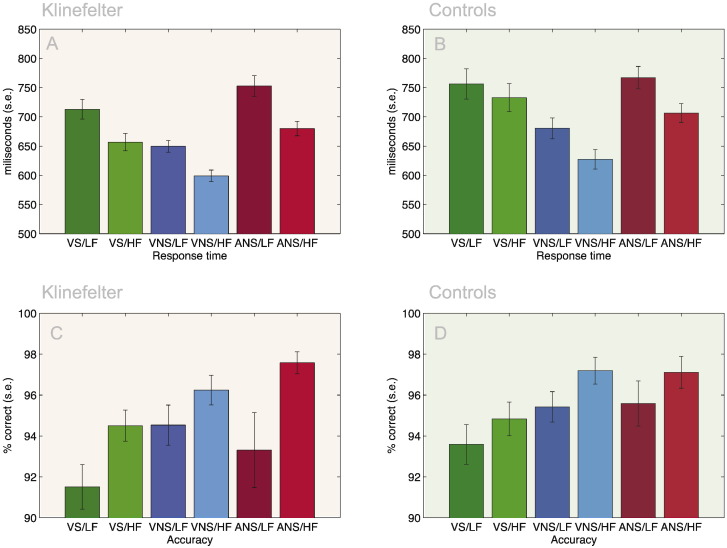
Fig. 2.
Behavioral effects. A Group × Stroop × Frequency ANOVA on the visual data revealed both a response time effect of Stroop (VS > VNS) and Frequency (LF > HF) (see A: four leftmost bars for KS and B: four leftmost bars for Controls) but no overall difference between KS and Controls. An interaction between Stroop and Frequency was found, indicating that the frequency effect is diminished in the presence of a Stroop task, however, this effect was only significant in Controls (A: Compare difference between columns 1–2 and 3–4) and not in KS (B: Compare difference between columns 1–2 and 3–4). Together, this resulted in a significant three-way interaction between Group, Stroop and Frequency. For accuracy (C and D) only main effects of Stroop and Frequency was found. When making another ANOVA contrasting Group, Perceptual Modality and Frequency, we only found main effects of modality and Frequency (A and B - four rightmost bars). See caption for Fig. 1 for abbreviations.
3.2.1.1. Response time
Using log-transformed median response times (RT) for each participant and condition we found no main effect of Group (F(1,96) = 2.40, p = 0.123), but strong effects of Stroop (F(1,96) = 126.73, p < 0.001) and Frequency (F(1,96) = 77.46, p < 0.001). We also found an interaction between Stroop and Frequency (F(1,96) = 5.82, p < 0.05), but no significant interaction between Group and Stroop (F(1,96) = 3.28, p = 0.073) or between Frequency and Group (F(1,96) = 2.23, p = 0.138). Lastly, we found a three-way interaction between Group, Stroop and Frequency (F(1,96) = 5.87, p < 0.05). This effect is due to the fact that the frequency effect is diminished in the presence of Stroop interference in Controls (t(48) = 3.45, p < 0.005), while this interaction is not present in KS (t(48) = 0.01, p = 0.99) (compare columns 1–4 in Fig. 2A and B).
3.2.1.2. Accuracy
For accuracy we found no effect of Group, but main effects of both Stroop (F(1,96) = 22.24, p < 0.001) and Frequency (F(1,96) = 18.91, p < 0.001) (columns 1–4 in Fig. 2C and D). No interactions were significant.
3.2.2. Group × Frequency × Perceptual Modality
To look for effects of Perceptual Modality, we conducted a Group (KS vs Controls) × Frequency × Perceptual Modality mixed-design ANOVA where group was a between-participants effect while Frequency and Modality were within-participants effects (columns 3–6 in Fig. 2).
3.2.2.1. Response time
Again using log-transformed median RTs we found no effect of group F(1,96) = 1.03, p = 0.3, but main effects of both Frequency (F(1,96) = 177.43, p < 0.001) and Perceptual Modality (F(1,96) = 164.97, p < 0.001) (columns 3–6 in Fig. 2A and B). No interactions were significant.
3.2.2.2. Accuracy
When considering accuracy, only Frequency was found to be significant (F(1,96) = 14.62, p < 0.001) (columns 3–6 in Fig. 2C and D). No other effects or interactions were significant.
3.3. fMRI data
3.3.1. Group × Stroop × Frequency ANOVA
3.3.1.1. Main effect of Group (KS vs Control)
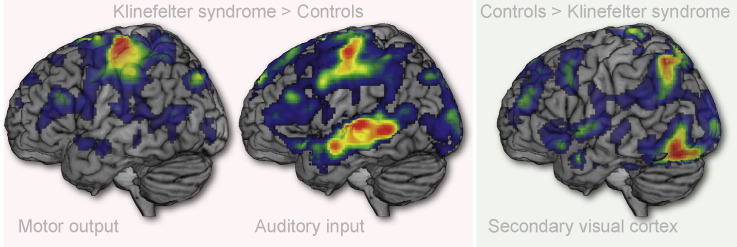
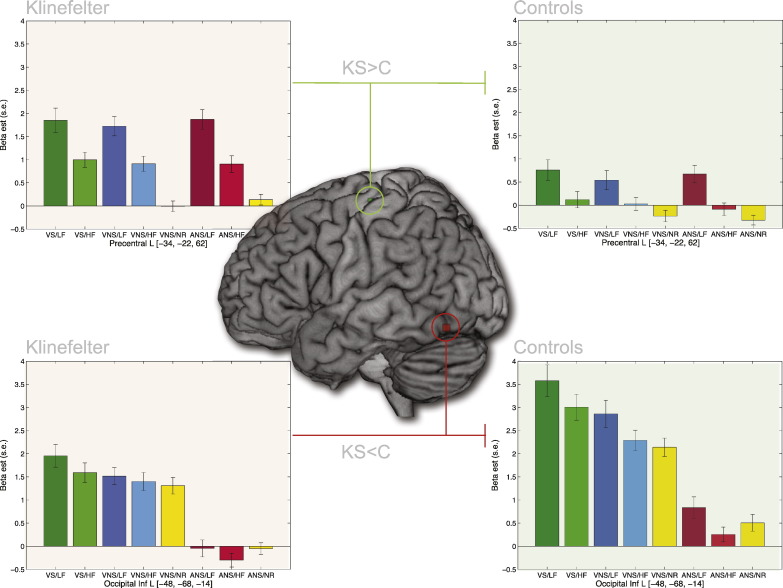
Comparing the two groups across the four visual conditions with motor response (VS/LF, VS/HF, VNS/LF, VNS/HF), one region was found to show increased activation for KS compared to Controls at the whole brain level (Table 1). This region was located in left primary motor cortex (MNI: [− 34, − 22, − 62]; P < 0.05, FWE-corrected). As can be seen from Fig. 3, this region also displays increased activity in KS during auditory trials where there is a motor response (ANS/LF, ANS/HF). Further, it can be seen that this region is sensitive to stimulus frequency in both groups.
Table 1.
Voxel coordinates for whole brain group comparison effects.
| Putative anatomical region | Peak MNI | Z-score |
|---|---|---|
| Group × Stroop × Frequency ANOVA | ||
| Effects of Group (KS > Controls) | ||
| Frontal Precentral L | − 34, − 22, 62 | 4.72 |
| Effects of Group (Controls > KS) | ||
| Occipital Inf L | − 48, − 68, − 14 | 4.67 |
| Group × Modality × Frequency ANOVA | ||
| Effects of Group (KS > Controls) | ||
| Frontal Precentral L | − 34, − 22, 62 | 5.35 |
| Temporal Mid R | 54, − 2, − 18 | 4.64 |
| Cingulum Mid L | − 40, − 14, 52 | 4.83 |
| Effects of Group (Controls > KS) | ||
| Occipital Inf L | − 48, − 68, − 14 | 4.49 |
| Auditory no response condition | ||
| Effects of Group (KS > Controls) | ||
| Temporal Sup R | 62, − 12, − 6 | 4.70 |
| Temporal Mid R | 56, 0, − 18 | 4.70 |
| Visual no response condition | ||
| Effects of Group (KS > Controls) | ||
| Frontal Med R (white matter) | 4, 20, 18 | 4.70 |
P-FWE < 0.05.
Fig. 3.
Main fMRI effects of Group. In the Group × Stroop × Frequency ANOVA two main effects of Group were found. Left primary motor cortex (peak coordinate: [− 34, − 22, 62], Z-score = 4.72) was found to yield greater responses in KS compared to Controls (C) whereas a region in the left inferior occiptotemporal region (peak coordinate: [− 48, − 68, − 14], Z-score = 4.67) displayed greater responses in Controls than in KS. P < 0.05, FWE-corrected. Barplots display mean beta-estimates for each of the eight experimental conditions for both groups and regions. See caption for Fig. 1 for abbreviations.
One region was found to be more active in Controls than in KS (Table 1). This region was located in the left inferior occipital gyrus in the ventral visual pathway (MNI: [− 48, − 68, − 14], see Fig. 3), approximately 10 mm posterior to the spot found to be most sensitive to Frequency and Stroop effects (VWFA – compare Fig. 3, Fig. 5).
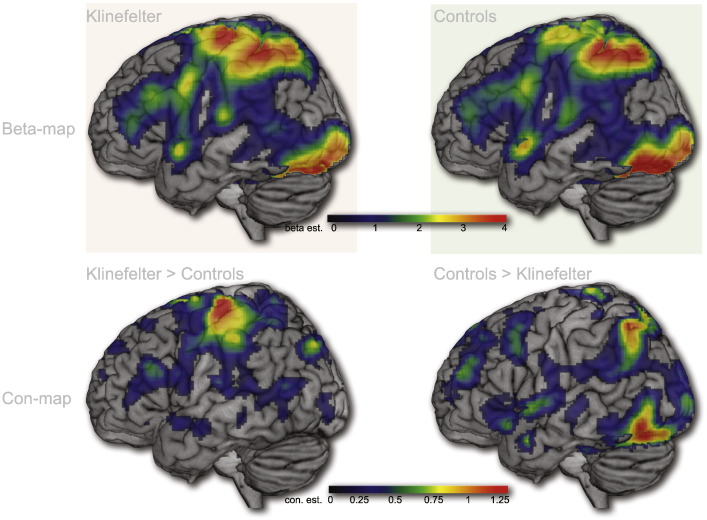
Given the thresholded nature of these effects we wished to qualitatively investigate if the group differences were due to a completely different pattern of activity during the visual tasks with motor responses. We therefore created unthresholded effect maps (mean beta images) including all positive effects as well as contrast maps showing the differences between groups. As can be seen from Fig. 4, the maps are highly similar across groups, suggesting that the observed differences between groups are due to local differences in activation rather than systemic differences hidden under the thresholding veil.
Fig. 4.
Unthresholded effects. Unthresholded average effects across the four conditions included in the first ANOVA (VS/LF, VS/HF, VNS/LF and VNS/HF). Top row displays mean beta estimates across participants for each of the two experimental groups. The bottom row displays unthresholded contrast maps showing regions were the groups' mean responses differ. Qualitatively these images suggest a high degree of similarity in activation patterns across KS and Controls with a few clear areas of difference, roughly corresponding to those that turn up as significant in the statistical tests.
3.3.1.2. Main effect of Stroop
The main effect of Stroop, regardless of participant group, was observed in a number of regions, including inferior frontal gyrus, bilaterally, premotor regions in the left hemisphere, posterior parietal cortex, bilaterally, extending to both the inferior and the superior parietal lobule. Activation in the visual word form area (VWFA) in the inferior part of the left temporal lobe [peak voxel: − 42, − 52, − 16] was also observed. There were no significant decreases in activation as a function of the Stroop task (Fig. 5). These effects mirrored those observed for Controls alone (Wallentin et al., 2015).
3.3.1.3. Main effect of Frequency (Visual)
The main effect of frequency across groups revealed a number of regions that were more active for the visual low frequency trial type, regardless of whether it was a Stroop task or not. These regions included premotor regions, sensorimotor, and parietal regions, including both superior and inferior lobule. Many effects were bilateral, but stronger in the left than in the right hemisphere. Activations were also observed in the visual word form area (VWFA) in the inferior part of the temporal lobe [peak voxel: − 44, − 60, − 16] as well as in the cerebellum. There were no regions where the high frequency stimuli showed greater activation than the low frequency stimuli (Fig. 5). These effects are very similar to those observed for Controls alone (Wallentin et al., 2015).
3.3.1.4. Interaction
No Group × Stroop interaction was found. No Group × Frequency interaction was found either. No Stroop x Frequency interaction was found and we found no regions that mirrored the behavioral interaction between Group, Stroop and Frequency.
3.3.2. Group × Frequency × Perceptual Modality ANOVA
3.3.2.1. Main effect of Group (KS vs Controls)
Comparing the two groups across perceptual modalities using the four Non-Stroop conditions again revealed a significant difference in left primary motor cortex, in right anterior temporal cortex and in the middle cingulate (Table 1).
3.3.2.2. Frequency effect
An analysis of the auditory frequency effects across groups revealed an activation pattern very similar to that observed for the visual data (Fig. 5). Again activations were found as an effect of the low frequency stimuli yielding greater responses than the high frequency stimuli. These effects were observed in the sensorimotor regions and in the parietal cortex, in the left hemisphere as well as in superior/middle temporal cortices, bilaterally (peak left hemisphere: [− 64, − 20, − 4], z-score: 5.05; peak right hemisphere: [64, − 16, 10], z-score: 5.11). No regions displayed greater activation for the high frequency stimuli than for the low frequency. Again, these effects are comparable to those observed for Controls alone (Wallentin et al., 2015).
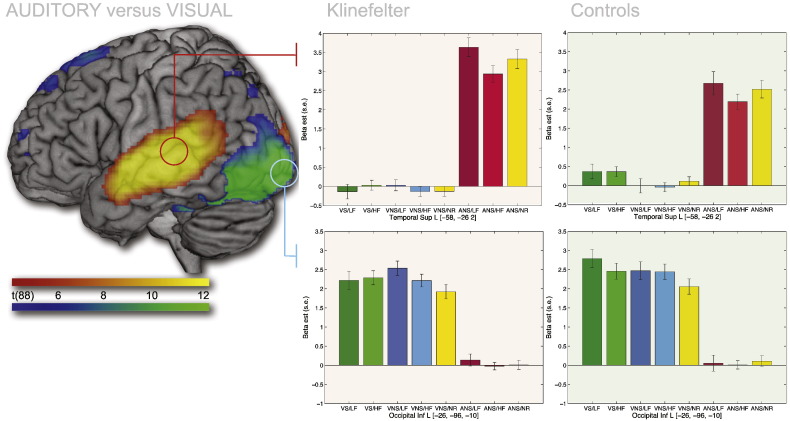
3.3.2.3. Main effect of Perceptual Modality
Regardless of group, the auditory stimuli yielded greater responses in superior and middle temporal gyri, bilaterally (MNI peak voxels left hemisphere: [− 58, − 26, 2], Z-score: Inf; right: [62, − 22, 2], Z-score: Inf), while visual Non-Stroop stimuli yielded greater responses in the occipital lobe, bilaterally (MNI peak voxels left hemisphere: [− 26, − 96, − 10], Z-score: Inf; right: [28, − 96,0], Z-score: Inf). See Fig. 6.
Fig. 6.
Main effects of Perceptual Modality. P < 0.05, FWE-corrected. No interaction between Participant Group and Perceptual Modality was found at the whole-brain corrected level, but inspection of the peak regions activated for each perceptual modality revealed a greater response for KS in auditory cortex (top row) and no difference in visual cortex (bottom row). Note also that auditory cortex is sensitive to frequency. Low Frequency (25 trials) yields greater activity than High Frequency (75 trials) with No Response (50 trials) in the middle. See caption for Fig. 1 for abbreviations. See Supplementary material for a similar display of the right hemisphere.
3.3.2.4. Group × Perceptual Modality interaction
No group differences were found for the perceptual modality contrast at the whole brain level. However, when using the peak voxels for the visual and auditory contrasts (see coordinates above) as regions of interest we found an interaction between modality and group in the auditory regions (left hemisphere: F(1,96) = 8.94, P < 0.005 uncorrected; right hemisphere: F(1,96) = 3.70, P < 0.06, uncorrected), indicating that KS has a greater response in auditory regions. No effect in the visual regions were seen (left hemisphere: F(1,96) = 0.18, P = 0.7; right hemisphere: F(1,96) = 0.13, P = 0.7). See Fig. 6.
3.3.2.5. Group × Perceptual Modality × Frequency interaction
No three-way interactions were observed.
3.4. Perceptual effects in the absence of a motor response
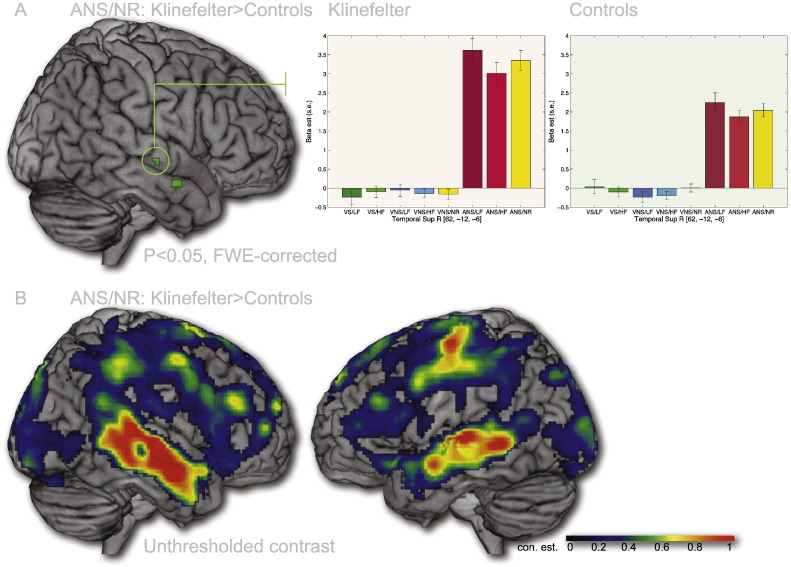
3.4.1. Auditory no response condition (ANS/NR)
To investigate group effects on auditory input in the absence of a response, we conducted a 2nd level analysis using the beta-estimates from the ANS/NR conditions, again including the previously named covariates. We found that KS males had a significantly greater response in two small regions in the right superior temporal [MNI: 62, − 12, − 6, z-score: 4.70] and anterior middle temporal gyri [MNI: 56, 0, − 18; z-score: 4.70]. No regions were found to have higher responses in Controls. The superior temporal region responds selectively to auditory input (Fig. 7A, columns 6–8 in the bar plots). It was also apparent that this region is sensitive to frequency in the auditory domain. Low-frequency stimuli (column 6) yielded greater responses than high frequency stimuli (column 7) whereas the no-response condition (column 8) had a response in between the two auditory response conditions, consistent with its frequency. Using t-tests we found that the difference between high and low frequency auditory input was significant both for KS (t(48) = 3.10, p < 0.005) and Controls (t(48) = 2.50, p < 0.05).
Fig. 7.
Effects of auditory stimuli in the absence of motor response (ANS/NR). A. Significant effects of Participant Group were found in the right temporal lobe in the vicinity of the primary auditory areas (P < 0.05, FWE-corrected). KS participants display a greater response than Controls. Similar to primary auditory regions (Fig. 6 and Supplementary Fig. S2), this area is also sensitive to the frequency of the auditory stimuli. B. Unthresholded contrast estimates indicate that the group differences for auditory stimuli is present below threshold level throughout the auditory region, bilaterally.
We made an unthresholded plot of the mean Group difference (Fig. 7B) in order to investigate if the difference between KS and Controls was as localized as the small blobs suggested or if the size of the significant area was due to thresholding issues. This map clearly shows that KS as a group has an increased response to auditory stimuli relative to Controls across the whole temporal auditory region.
3.4.2. Visual no response condition (VNS/NR)
To look for group effects of visual input in the absence of a response, we conducted a 2nd level analysis using the beta-estimates from the VNS/NR conditions, again including the previously named covariates. We found that KS had a significantly greater response in a region in the medial frontal part of the brain [MNI: 4, 20, 18, z-score: 4.70], however outside gray matter. Furthermore, this effect seems primarily to be driven by a deactivation in Controls (Supplementary Fig. S3). No regions displayed significantly higher responses for Controls.
3.4.3. Effects of covariates
All 2nd level analyses included brain volume, testosterone and age as covariates along with stimulus group (see Methods section). In our previous study (Skakkebæk et al., 2014b) we found that KS males had a smaller total brain volume than Controls. This was also the case for the subgroup of participants who completed the fMRI experiment (t(96) = 2.63, P < 0.01). There was no mean difference between groups in terms of testosterone levels (t(96) = − 1.61), P = 0.11), due to the mixing of both testosterone treated and untreated KS participants (see Methods section). No difference was observed for age (t(96) = 0.08, P = 0.93).
Due to the potential explosion in effects of covariates across the different 2nd level analyzes, we focused on the effects of the visual tasks with a motor response in which we also found significant effects across participant groups at the whole brain level (see Section 3.3.1.1).
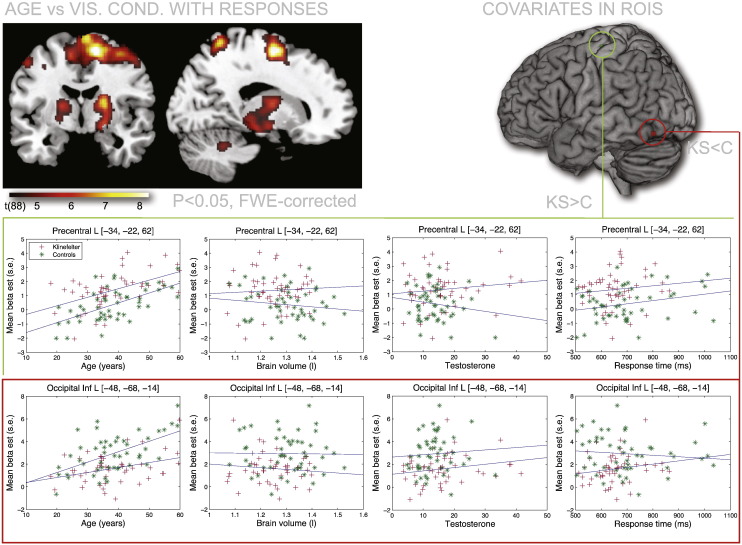
Across participants we found a significant correlation with age in large parts of the motor network, including primary and secondary motor cortices in both hemispheres, but stronger in the right. Effects were also observed in the striatum (putamen) in both hemispheres and in the cerebellum (Fig. 8). No whole brain effects were found for brain volume, testosterone or stimulus group. No significant differences between groups in effects of covariates were found at the whole brain level.
Fig. 8.
Effects of age and other covariates. We found a significant positive effect of age at the corrected whole brain level when contrasted against the visual conditions with a response. This positive correlation was found throughout the motor system, including cortical, striatal and cerebellar regions. No correlations with brain volume, testosterone level or average response time were found, neither at the whole brain level nor when focusing at the two regions where KS and Controls (C) were found to differ.
Focusing on the two regions where main effects of group were observed, we again found a strong positive correlation in the motor region ([MNI: − 34, − 22, 62]) between the visual conditions with responses and age in both the KS group (r = 0.5, P < 0.001, uncorrected) and the Controls (r = 0.61, P < 0.001, uncorrected). No significant correlations were found with any of the other covariates in this region (P > 0.05, uncorrected). The same pattern was seen in the left inferior occipital region (MNI: [− 48, − 68, − 14]). Age was again found to be positively correlated with the BOLD response in both KS (r = 0.36, P < 0.02, uncorrected) and in Controls (r = 0.56, P < 0.001, uncorrected). We also included a correlation analysis with response time. No significant effect of this behavioral measure was seen in either of the two ROIs (Fig. 8).
4. Discussion
The main findings in the present study is that KS participants have an increased fMRI-BOLD response to motor output in primary motor cortex and increased responses to auditory stimuli in areas surrounding the auditory cortices while no difference is found in primary visual areas. These effects seem to reflect specific low-level effects, i.e. the motor cortex effects are present both for visual and auditory stimuli and the effects of auditory stimuli in auditory cortices are present both with and without motor output. The lack of a difference in visual areas suggests that we are not observing a systemic difference.
Conversely, KS males display relatively smaller responses to visual input in a region in the ventral visual stream in the vicinity of the Visual Word Form Area (Dehaene et al., 2002, Wallentin et al., 2015, Wallentin et al., 2014). When looking at the unthresholded effects (Fig. 4, top row), we find that KS and Controls display a highly similar pattern of activation. The areas of difference are areas that to a greater or lesser extent are activated in both groups. Thus, it does thus not seem to be the case that KS or Controls make use of different areas or networks. Rather, we find that different areas are more accentuated and/or expanded in the different groups for these basic tasks. When inspecting the unthresholded contrast images (Fig. 4, bottom), we see that the areas of difference are fairly localized and furthermore that they are localized within regions with a positive response in both groups. We are therefore not, it seems, dealing with a nonspecific, systemic difference between the two groups or differences brought about by low-level hemodynamic differences, artifacts or deactivations. This is an important finding, because it provides grounding both for previous (Brandenburg-Goddard et al., 2014, Steinman et al., 2009, van Rijn et al., 2008a, van Rijn et al., 2012) and for future use of fMRI as a method to investigate the neurofunctional characteristics of KS. Below, we discuss other potential sources for differences in activation between the two groups.
4.1. Possible causes of low-level motor cortex effects
4.1.1. Behavioral effects
The motor cortex activation observed in the KS sample is not likely to have a simple behavioral origin as we did not find any response time differences between the two experimental groups. Further, we did not see any correlation with response time in the motor activation peak (Fig. 8). KS males suffer from unspecific motor impairments (Ross et al., 2008, Ross et al., 2009) and it is possible that these may either cause or be a result of altered neural or hemodynamic responsiveness. The lack of behavioral differences in the present experiment, however, makes such an interpretation less straightforward. One suggestion is that the increased activation reflects a compensatory strategy that allows KS participants to accomplish comparable behavioral results.
4.1.2. Head movement
The observed effects in motor cortex are not likely to be caused by head motion, neither directly nor indirectly. First, we did not observe any group difference in head motion during scanning. This shows that KS participants do not have increased difficulty in remaining still in the scanner. Further, the observed group difference between KS and Controls is located in the vicinity of the hand area of the brain (Yousry et al., 1997), which is at odds with an interpretation of the activation as having a neuronal link to head movement. Again, this is important, because it shows that the KS group can take part in the fMRI experiments without creating signal artifacts.
4.1.3. Tremor
Another possible explanation for the increased motor activation could be essential tremor. KS males are known to suffer from tremor to a larger degree than healthy Controls (Harlow and Gonzalez-Alegre, 2009). In their study Harlow and Gonzalez-Alegre found that 50% of their KS group had experienced uncontrollable tremors compared to 5% of their control participants. Although essential tremor is highly heterogeneous (Benito-León and Louis, 2006), early PET and fMRI studies of essential tremor found increased cerebellar activation but none in primary motor cortices (Bucher et al., 1997, Wills et al., 1994). One paper studied the influence of essential tremor during Stroop using fMRI (Cerasa et al., 2010). Tremor patients displayed increased activation in dorsolateral and parietal regions, close to where we observe Stroop effects, but none in motor cortices. Based on this, tremor seems unlikely as explanation for our results. Unfortunately, we have no tremor measures and thus cannot completely rule out a motor cortex effect of tremor in KS. Further studies are needed.
4.1.4. Testosterone
Very few experiments have used fMRI to study neuro-cognitive effects of testosterone in the normal population, and most look at variation in females (see Celec et al., 2015 for a recent review). Results are mixed and generally related to emotional and/or spatial processing that was not the focus of the current study. No controlled studies exist on the effect of testosterone treatment on cognitive abilities in adult KS, but the effects of testosterone treatment on cognition in the normal population have been limited (Warren et al., 2008) and no effects are seen in elderly hypogonadal patients (Holland et al., 2011). Thus, should there be an effect of testosterone deficiency and testosterone treatment in KS, then we would expect it to be primarily at the physiological level, e.g. through a link to hematocrit levels (Levin et al., 2001). The differences between KS and Controls in this experiment are, however, not likely to be due to testosterone as we found no difference in testosterone levels across the two groups due to the presence of both testosterone treated and untreated KS participants. Also, we did not see any correlation between testosterone level and BOLD response in neither KS nor Controls. Previously we have found no differences in neither global nor local brain volumes between testosterone treated and untreated KS (Skakkebæk et al., 2014b). In general, no one has yet been able to establish a role for testosterone in the cerebral phenotype of KS. There may, however, be several explanations to this, − first, measurement of testosterone only provides a short-term glimpse of the actual androgenisation of any given person (Celec et al., 2015); second, although males with KS are hypogonadal as a group, the hypogonadism develops gradually and becomes manifest at different time points in life (Aksglæde et al., 2006); third, the window of opportunity for testosterone to influence the cerebral phenotype may lie very early in life (Samango-Sprouse et al., 2015), perhaps already in utero; fourth, testosterone can be aromatized to estradiol, which may well be the hormone of importance in the brain due to its large number of estrogen receptors (Toran-Allerand, 2004). Altogether, we find no support for a testosterone effect in motor cortex or elsewhere.
4.1.5. Brain volume
KS and Controls differ in terms of their total gray matter volume (see the Introduction and Section 3.4.3 in the Results section). KS participants might compensate for a smaller motor region by increasing the cortical responsiveness and this might explain why we observe an increased activation in motor cortex. Against this, however, speaks the fact that we did not see any correlation between brain volume and BOLD response within neither the KS group nor the Controls alone (Fig. 8). A putative effect of brain volume would thus have to be limited to explaining the between groups variance and not the within groups variance, which would make the effect difficult to interpret. Further, if it were generally the case that smaller brains compensated by increasing their activation, then we should also expect to see sex differences in motor cortex responses. Although tendencies towards this have been observed, the effect is considered negligible (Pitcher et al., 2004, Sella et al., 2014). Lastly, it is worth noting that local volumetric differences between KS and Controls are not very pronounced in the motor cortices (Bryant et al., 2011, Lentini et al., 2013, Skakkebæk et al., 2014b). We thus consider it unlikely that the observed motor cortex effect should be caused by the difference in brain volume.
4.1.6. Age
We saw a strong correlation between BOLD-response and age throughout the motor system as well as in the inferior occipital cortex (Fig. 8). This effect may be due to changes in the shape and latency of the hemodynamic response function with age (D'Esposito et al., 2003, Huettel et al., 2001, Richter and Richter, 2003) rather than amplitude. No difference in the age/BOLD correlation was seen between KS and Controls and groups were matched on age, indicating that the age correlation cannot be the cause of the observed group differences in BOLD response in motor cortex or in the inferior occipital area. The age dependency of the BOLD signal has not been observed in KS before, and it suggests that the group has a normal aging pattern in their neural responses. It also indicates that the large age spread often seen in studies of KS comes with the cost of decreased power due to age-induced variance in the data. It further highlights the importance of using age-matched control groups and of including age as a covariate in future fMRI studies on KS.
4.2. Possible causes of auditory cortex effects
When removing the threshold, we saw a widespread area in the temporal lobes where KS displayed a greater BOLD response to auditory stimuli than Controls, both with and without a motor response (Fig. 6, Fig. 7). The differences only reached significance at the stringent whole brain corrected level in secondary auditory cortices. Further studies are needed to elucidate the origin of this group effect, but the presence of a difference in BOLD response in low-level auditory cortices in the absence of behavioral differences is puzzling and warrants caution when interpreting findings from more complex auditory fMRI-experiments, such as speech perception.
The effects could potentially be due to compensatory strategies countering sensorineural hearing impairments (Anderson et al., 1971, Castiglione et al., 2013, Sørensen, 1992) or auditory hallucinations (Boks et al., 2007, Bruining et al., 2009, DeLisi et al., 2005, Sørensen and Nielsen, 1977). Auditory hallucinations may increase activity in auditory cortices (Dierks et al., 1999). However, similar to the motor cortex effect, any interpretation involving deficits in the KS group has to be evaluated against the fact that we find no behavioral difference. Furthermore, none of the participants report any hearing problems. Importantly, we also note that auditory cortices in both KS and Controls were found to be sensitive to frequency differences in auditory stimuli (Fig. 7 and Supplementary Fig. S2). This points towards the sensitivity of the BOLD response in this area to pick up even subtle differences in the distribution of stimuli, but it also highlights that the auditory cortex difference between KS and Controls is not related to differences in adaption during the experiment. Our finding is relevant for studies that look into speech lateralization in KS (van Rijn et al., 2008a). Van Rijn et al. found decreased lateralization in KS compared to Controls in superior temporal gyrus. Lateralization indices were found, as it is often done, by comparing the number of activated voxels in a given region of interest across the two hemispheres. However, if KS have greater signal in auditory cortex, then they will tend to have more activated voxels. Activation levels may thus be just above threshold in KS in e.g. the right hemisphere while they are just below threshold in Controls, resulting in a seemingly greater lateralization in the Controls. For this reason, it is advisable not to use relative cluster size as a measure of lateralization. Instead, one may simply compare raw beta coefficient estimates (e.g. see Wallentin et al., 2014). As an example, we subtracted the effect of the auditory No Response condition at peak coordinates for auditory stimuli in auditory cortices (see Section 3.3.2.2. for coordinates) in both hemispheres and compared groups. No significant difference in lateralization could be observed (t(96) = − 0.43, p = 0.67). Further studies are needed in order to investigate if KS exhibit increased auditory responses to all types of sounds or whether the effect is linked to words as studied in the present experiment.
4.3. Possible causes of inferior occipital cortex effect
We found an unpredicted difference between KS and Controls in the left inferior occipital region. This is an area known to be involved in object recognition and to contain a subregion specialized for processing of visual words, an area known as the visual word form area (VWFA) (Dehaene and Cohen, 2011, Dehaene et al., 2002, Wallentin et al., 2014). The peak activations for the Stroop task and the frequency manipulation were located with peak activations with MNI-coordinates Y = − 52 and Y = − 60. The latter is almost identical to the peak location found for the VWFA (Y = − 58) in meta-analyses of word processing studies (Jobard et al., 2003). The VWFA has been found to have a high degree of spatial consistency “within a few millimeters” across studies (Dehaene and Cohen, 2011). The area in which we found a maximal difference between KS and Controls was located somewhat posterior to this (Y = − 68) and given that the area of significant difference was rather small, it is not clear whether the two regions are the same or whether we might be dealing with two separate regions. Previous findings indicate that although the peak area for VWFA has a large consistency, the cortex is organized with gradual changes in sensitivity to specific types of stimuli across the space of the inferior occipital region rather than consisting of small sharply defined functional areas. With regards to reading, the ventral visual regions have been found to exhibit a posterior to anterior gradient with increasing sensitivity to words in anterior parts (Vinckier et al., 2007). Considering the KS phenotype, it may not come as a surprise to find a differential response in regions related to reading. KS is known to be associated with reading impairments (Bender et al., 1986). Previous studies have found that dyslexia is related to a differential pattern of activation along the posterior to anterior gradient, rather than a specific difference in VWFA alone (van der Mark et al., 2009). This is compatible with our results but warrants further studies with different types of orthographical input (e.g. real words, pseudo-words and false fonts) in order to determine the detailed nature of the difference.
4.4. Lack of high-level cognitive effects
Despite the fact that a number of studies have shown high-level cognitive deficits in the KS group, we failed to find any high-level behavioral or neural effects, neither for our Stroop task nor for our adaptation task.
4.4.1. Stroop
The lack of a significant difference in behavior for our simplified Stroop task goes against previous studies that have found KS to exhibit increased Stroop interference (Boone et al., 2001, DeLisi et al., 2005, Temple and Sanfilippo, 2003). We also previously reported a Group difference in Stroop inhibition between KS and Controls (Skakkebæk et al., 2014b Table 2). However, during the work on this manuscript it has come to our attention that the significant finding in that report was based on a calculation error (Corrigendum: Skakkebæk et al., 2016) and that in fact there was no inhibition difference between groups. This lack of a Stroop inhibition difference is thus consistent with the lack of an effect in our simplified fMRI Stroop paradigm. Controversies exist about how to best quantify the inhibition effect investigated using the Stroop paradigm (Chafetz and Matthews, 2004). The effects reported in the articles cited above did not correct for general processing speed. Others have found that when correcting for this, the difference between KS and Controls becomes minimal (Ross et al., 2009). The additional lack of a difference in activation pattern in our fMRI data further speaks against the idea that identical performance is obtained through different strategies. We are left with the conclusion that Stroop may not be ideal paradigm for elucidating the executive deficits otherwise apparent in the KS population (Kompus et al., 2011, Lee et al., 2011, Skakkebæk et al., 2015, Temple and Sanfilippo, 2003, van Rijn et al., 2009).
4.4.2. Frequency adaptation
We also failed to find any group differences for the frequency manipulation. KS are apparently just as able to adapt to stimulus frequency as their age and education matched peers (Wallentin et al., 2015). Investigating adaptation rate in KS has not been attempted before and the lack of a difference in performance in our well-powered experiment is noteworthy and suggests that the many cognitive challenges that KS face are not due to a general lack of behavioral, neuronal and/or hemodynamic adaptability. If anything, the KS group was more sensitive to the Frequency manipulation than Controls as indicated by the behavioral interaction in the visual conditions between Stroop and Frequency found in Controls where Stroop seems to inhibit the Frequency adaptation (Fig. 2). This inhibition was not present in KS.
5. Conclusion
KS males are capable of remaining still in the scanner at a comparable level to Controls. We find that KS participants have increased activity for motor output in motor cortex and to auditory stimuli in auditory cortices, but not to visual stimuli in visual cortex. The lack of visual effects rules out systemic differences in the BOLD response. KS males exhibit diminished fMRI BOLD-responses to visual stimuli in an area slightly posterior to the Visual word form area. This may be causing their reading deficits or be an effect of it. Apart from a positive correlation with age, we fail to find any effects of external covariates such as brain volume, response time and testosterone levels. KS also match Controls on Stroop executive inhibition and adaptation effects, i.e. KS as a group shows no sign of deficits in neural or behavioral short-term adaptation. We suggest that further studies, exploring the exact nature of the basic-level differences observed in this experiment, should be performed before continuing with high-level cognitive fMRI experiments in Klinefelter syndrome.
Funding
This study was supported by grants from the Lundbeck Foundation, the Augustinus Foundation, the Novo Nordisk Foundation and the Aase and Einar Danielsen Foundation. M.W. received funding from the MindLab grant from the Danish Ministry of Science, Technology and Innovation. A.S. received a research fellowship from Aarhus University. C.H.G. was supported by a personal clinical research grant from the Novo Nordisk Foundation.
Acknowledgements
We would like to thank Dora Zeidler and Michael Geneser for their technical assistance.
Footnotes
Supplementary data to this article can be found online at http://dx.doi.org/10.1016/j.nicl.2016.02.002.
Appendix A. Supplementary data
Supplementary material.
References
- Aksglæde L., Wikström A.M., Rajpert-De Meyts E., Dunkel L., Skakkebæk N.E., Juul A. Natural history of seminiferous tubule degeneration in Klinefelter syndrome. Hum. Reprod. Update. 2006;12:39–48. doi: 10.1093/humupd/dmi039. [DOI] [PubMed] [Google Scholar]
- Anderson H., Lindsten J., Wedenberg E. Hearing defects in males with sex chromosome anomalies. Acta Otolaryngol. 1971;72:55–58. doi: 10.3109/00016487109122455. [DOI] [PubMed] [Google Scholar]
- Bender B.G., Puck M.H., Salbenblatt J.A., Robinson A. Dyslexia in 47,XXY boys identified at birth. Behav. Genet. 1986;16:343–354. doi: 10.1007/BF01071315. [DOI] [PubMed] [Google Scholar]
- Benito-León J., Louis E.D. Essential tremor: emerging views of a common disorder. Nat. Clin. Pract. Neurol. 2006;2:666–678. doi: 10.1038/ncpneuro0347. [DOI] [PubMed] [Google Scholar]
- Bojesen A., Gravholt C.H. Klinefelter syndrome in clinical practice. Nat. Clin. Pract. Urol. 2007;4:192–204. doi: 10.1038/ncpuro0775. [DOI] [PubMed] [Google Scholar]
- Bojesen A., Juul S., Gravholt C.H. Prenatal and postnatal prevalence of Klinefelter syndrome: a national registry study. J. Clin. Endocrinol. Metab. 2003;88:622–626. doi: 10.1210/jc.2002-021491. [DOI] [PubMed] [Google Scholar]
- Bojesen A., Juul S., Birkebaek N., Gravholt C. Morbidity in Klinefelter syndrome: a Danish register study based on hospital discharge diagnoses. J. Clin. Endocrinol. Metab. 2006;91:1254–1260. doi: 10.1210/jc.2005-0697. [DOI] [PubMed] [Google Scholar]
- Bojesen A., Kristensen K., Birkebaek N.H., Fedder J., Mosekilde L., Bennett P., Laurberg P., Frystyk J., Flyvbjerg A., Christiansen J.S., Gravholt C.H. The metabolic syndrome is frequent in Klinefelter's syndrome and is associated with abdominal obesity and hypogonadism. Diabetes Care. 2006;29:1591–1598. doi: 10.2337/dc06-0145. [DOI] [PubMed] [Google Scholar]
- Boks M.P.M., de Vette M.H.T., Sommer I.E., van Rijn S., Giltay J.C., Swaab H., Kahn R.S. Psychiatric morbidity and X-chromosomal origin in a Klinefelter sample. Schizophr. Res. 2007;93:399–402. doi: 10.1016/j.schres.2007.03.015. [DOI] [PubMed] [Google Scholar]
- Boone K.B., Swerdloff R.S., Miller B.L., Geschwind D.H., Razani J., Lee A., Gonzalo I.G., Haddal A., Rankin K., Lu P., Paul L. Neuropsychological profiles of adults with Klinefelter syndrome. J. Int. Neuropsychol. Soc. 2001;7:446–456. doi: 10.1017/s1355617701744013. [DOI] [PubMed] [Google Scholar]
- Brandenburg-Goddard M.N., van Rijn S., Rombouts S.A.R.B., Veer I.M., Swaab H. A comparison of neural correlates underlying social cognition in Klinefelter syndrome and autism. Soc. Cogn. Affect. Neurosci. 2014;9:1926–1933. doi: 10.1093/scan/nst190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruining H., Swaab H., Kas M., Van Engeland H. Psychiatric characteristics in a self-selected sample of boys with Klinefelter syndrome. Pediatrics. 2009;123:e865–e870. doi: 10.1542/peds.2008-1954. [DOI] [PubMed] [Google Scholar]
- Bryant D.M., Hoeft F., Lai S., Lackey J., Roeltgen D., Ross J., Reiss A.L. Neuroanatomical phenotype of Klinefelter syndrome in childhood: a voxel-based morphometry study. J. Neurosci. 2011;31:6654–6660. doi: 10.1523/JNEUROSCI.5899-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bucher S.F., Seelos K.C., Dodel R.C., Reiser M., Oertel W.H. Activation mapping in essential tremor with functional magnetic resonance imaging. Ann. Neurol. 1997;41:32–40. doi: 10.1002/ana.410410108. [DOI] [PubMed] [Google Scholar]
- Calof O.M., Singh A.B., Lee M.L., Kenny A.M., Urban R.J., Tenover J.L., Bhasin S. Adverse events associated with testosterone replacement in middle-aged and older men: a meta-analysis of randomized, placebo-controlled trials. J. Gerontol. 2005;60A:1451–1457. doi: 10.1093/gerona/60.11.1451. [DOI] [PubMed] [Google Scholar]
- Castiglione A., Busi M., Martini A. Syndromic hearing loss: an update. Hearing Balance Commun. 2013;11:146–159. [Google Scholar]
- Celec P., Ostatníková D., Hodosy J. On the effects of testosterone on brain behavioral functions. Front. Neurosci. 2015;9:12. doi: 10.3389/fnins.2015.00012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cerasa A., Passamonti L., Novellino F., Salsone M., Gioia M.C., Morelli M., Paglionico S., Giofrè L., Arabia G., Quattrone A. Fronto-parietal overactivation in patients with essential tremor during Stroop task. Neuroreport. 2010;21:148–151. doi: 10.1097/WNR.0b013e328335b42c. [DOI] [PubMed] [Google Scholar]
- Chafetz M.D., Matthews L.H. A new interference score for the Stroop test. Arch. Clin. Neuropsychol. 2004;19:555–567. doi: 10.1016/j.acn.2003.08.004. [DOI] [PubMed] [Google Scholar]
- Chang S., Skakkebæk A., Trolle C., Bojesen A., Hertz J.M., Cohen A., Hougaard D.M., Wallentin M., Pedersen A.D., Østergaard J.R., Gravholt C.H.J. Anthropometry in Klinefelter syndrome – multifactorial influences due to CAG length, testosterone treatment and possibly intrauterine hypogonadism. J. Clin. Endocrinol. Metab. 2014;100:E508–E517. doi: 10.1210/jc.2014-2834. [DOI] [PubMed] [Google Scholar]
- Dehaene S., Cohen L. The unique role of the visual word form area in reading. Trends Cogn. Sci. 2011;15:254–262. doi: 10.1016/j.tics.2011.04.003. [DOI] [PubMed] [Google Scholar]
- Dehaene S., Le Clec'H G., Poline J.-B., Le Bihan D., Cohen L. The visual word form area: a prelexical representation of visual words in the fusiform gyrus. Neuroreport. 2002;13:321–325. doi: 10.1097/00001756-200203040-00015. [DOI] [PubMed] [Google Scholar]
- DeLisi L.E., Maurizio A.M., Svetina C., Ardekani B., Szulc K., Nierenberg J., Leonard J., Harvey P.D. Klinefelter's syndrome (XXY) as a genetic model for psychotic disorders. Am. J. Med. Genet. B Neuropsychiatr. Genet. 2005;135B:15–23. doi: 10.1002/ajmg.b.30163. [DOI] [PubMed] [Google Scholar]
- D'Esposito M., Deouell L.Y., Gazzaley A. Alterations in the BOLD fMRI signal with ageing and disease: a challenge for neuroimaging. Nat. Rev. Neurosci. 2003;4:863–872. doi: 10.1038/nrn1246. [DOI] [PubMed] [Google Scholar]
- Dierks T., Linden D.E.J., Jandl M., Formisano E., Goebel R., Lanfermann H., Singer W. Activation of Heschl's gyrus during auditory hallucinations. Neuron. 1999;22:615–621. doi: 10.1016/s0896-6273(00)80715-1. [DOI] [PubMed] [Google Scholar]
- Fales C.L., Knowlton B.J., Holyoak K.J., Geschwind D.H., Swerdloff R.S., Gonzalo I.G. Working memory and relational reasoning in Klinefelter syndrome. J. Int. Neuropsychol. Soc. 2003;9:839–846. doi: 10.1017/S1355617703960036. [DOI] [PubMed] [Google Scholar]
- Friston K.J., Rotshtein P., Geng J.J., Sterzer P., Henson R.N. A critique of functional localisers. NeuroImage. 2006;30:1077–1087. doi: 10.1016/j.neuroimage.2005.08.012. [DOI] [PubMed] [Google Scholar]
- Giedd J.N., Clasen L.S., Wallace G.L., Lenroot R.K., Lerch J.P., Wells E.M., Blumenthal J.D., Nelson J.E., Tossell J.W., Stayer C., Evans A.C., Samango-Sprouse C.A. XXY (Klinefelter syndrome): a pediatric quantitative brain magnetic resonance imaging case–control study. Pediatrics. 2007;119:e232–e240. doi: 10.1542/peds.2005-2969. [DOI] [PubMed] [Google Scholar]
- Handwerker D.A., Gonzalez-Castillo J., D'Esposito M., Bandettini P.A. The continuing challenge of understanding and modeling hemodynamic variation in fMRI. NeuroImage. 2012;62:1017–1023. doi: 10.1016/j.neuroimage.2012.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harlow T.L., Gonzalez-Alegre P. High prevalence of reported tremor in Klinefelter syndrome. Parkinsonism Relat. Disord. 2009;15:393–395. doi: 10.1016/j.parkreldis.2008.08.009. [DOI] [PubMed] [Google Scholar]
- Holland J., Bandelow S., Hogervorst E. Testosterone levels and cognition in elderly men: a review. Maturitas. 2011;69:322–337. doi: 10.1016/j.maturitas.2011.05.012. [DOI] [PubMed] [Google Scholar]
- Hong D.S., Reiss A.L. Cognitive and neurological aspects of sex chromosome aneuploidies. Lancet Neurol. 2014;13:306–318. doi: 10.1016/S1474-4422(13)70302-8. [DOI] [PubMed] [Google Scholar]
- Hong D.S., Hoeft F., Marzelli M.J., Lepage J.-F., Roeltgen D., Ross J., Reiss A.L. Influence of the X-chromosome on neuroanatomy: evidence from Turner and Klinefelter syndromes. J. Neurosci. 2014;34:3509–3516. doi: 10.1523/JNEUROSCI.2790-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Høst C., Skakkebæk A., Groth K.A., Bojesen A. The role of hypogonadism in Klinefelter syndrome. Asian J. Androl. 2014;16:185–191. doi: 10.4103/1008-682X.122201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huettel S.A., Singerman J.D., McCarthy G. The effects of aging upon the hemodynamic response measured by functional MRI. NeuroImage. 2001;13:161–175. doi: 10.1006/nimg.2000.0675. [DOI] [PubMed] [Google Scholar]
- Jobard G., Crivello F., Tzourio-Mazoyer N. Evaluation of the dual route theory of reading: a metanalysis of 35 neuroimaging studies. NeuroImage. 2003;20:693–712. doi: 10.1016/S1053-8119(03)00343-4. [DOI] [PubMed] [Google Scholar]
- Kompus K., Westerhausen R., Nilsson L.-G., Hugdahl K., Jongstra S., Berglund A., Arver S., Savic I. Deficits in inhibitory executive functions in Klinefelter (47, XXY) syndrome. Psychiatry Res. 2011;189:135–140. doi: 10.1016/j.psychres.2011.02.028. [DOI] [PubMed] [Google Scholar]
- Lee N.R., Wallace G.L., Clasen L.S., Lenroot R.K., Blumenthal J.D., White S.L., Celano M.J., Giedd J.N. Executive function in young males with Klinefelter (XXY) syndrome with and without comorbid attention-deficit/hyperactivity disorder. J. Int. Neuropsychol. Soc. 2011;17:522–530. doi: 10.1017/S1355617711000312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lentini E., Kasahara M., Arver S., Savic I. Sex differences in the human brain and the impact of sex chromosomes and sex hormones. Cereb. Cortex. 2013;23:2322–2336. doi: 10.1093/cercor/bhs222. [DOI] [PubMed] [Google Scholar]
- Levin J.M., Frederick B.d.B., Ross M.H., Fox J.F., von Rosenberg H.L., Kaufman M.J., Lange N., Mendelson J.H., Cohen B.M., Renshaw P.F. Influence of baseline hematocrit and hemodilution on BOLD fMRI activation. Magn. Reson. Imaging. 2001;19:1055–1062. doi: 10.1016/s0730-725x(01)00460-x. [DOI] [PubMed] [Google Scholar]
- Lund T.E., Nørgaard M.D., Rostrup E., Rowe J.B., Paulson O.B. Motion or activity: their role in intra- and inter-subject variation in fMRI. NeuroImage. 2005;26:960–964. doi: 10.1016/j.neuroimage.2005.02.021. [DOI] [PubMed] [Google Scholar]
- MacLeod C.M. Half a century of research on the Stroop effect: an integrative review. Psychol. Bull. 1991;109:163–203. doi: 10.1037/0033-2909.109.2.163. [DOI] [PubMed] [Google Scholar]
- Nielsen J., Johansen K., Yde H. Frequency of diabetes mellitus in patients with Klinefelter's syndrome of different chromosome constitutions and the XYY syndrome. Plasma insulin and growth hormone level after a glucose load. J. Clin. Endocrinol. Metab. 1969;29:1062–1073. doi: 10.1210/jcem-29-8-1062. [DOI] [PubMed] [Google Scholar]
- Novick J.M., Trueswell J.C., Thompson-Schill S.L. Broca's area and language processing: evidence for the cognitive control connection. Lang. Linguist. Compass. 2010;4:906–924. [Google Scholar]
- Ogawa S., Lee T.M., Kay A.R., Tank D.W. Brain magnetic resonance imaging with contrast dependent on blood oxygenation. Proc. Natl. Acad. Sci. U. S. A. 1990;87:9868–9872. doi: 10.1073/pnas.87.24.9868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penny W., Holmes A.P. Random effects analysis. In: Friston K.J., Ashburner J., Kiebel S., Nichols T., Penny W., editors. Statistical Parametric Mapping: The Analysis of Functional Brain Images. Academic Press; London: 2007. pp. 156–165. [Google Scholar]
- Pitcher J.B., Ogston K.M., Miles T.S. Age and sex differences in human motor cortex input–output characteristics. J. Physiol. 2004;546:605–613. doi: 10.1113/jphysiol.2002.029454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ratcliffe S. Long term outcome in children of sex chromosome abnormalities. Arch. Dis. Child. 1999;80:192–195. doi: 10.1136/adc.80.2.192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richter W., Richter M. The shape of the fMRI BOLD response in children and adults changes systematically with age. NeuroImage. 2003;20:1122–1131. doi: 10.1016/S1053-8119(03)00347-1. [DOI] [PubMed] [Google Scholar]
- Ross J.L., Roeltgen D.P., Stefanatos G., Benecke R., Zeger M.P.D., Kushner H., Ramos P., Elder F.F., Zinn A.R. Cognitive and motor development during childhood in boys with Klinefelter syndrome. Am. J. Med. Genet. 2008;146A:708–719. doi: 10.1002/ajmg.a.32232. [DOI] [PubMed] [Google Scholar]
- Ross J.L., Zeger M.P.D., Kushner H., Zinn A.R., Roeltgen D.P. An extra X or Y chromosome: contrasting the cognitive and motor phenotypes in childhood in boys with 47,XYY syndrome or 47,XXY Klinefelter syndrome. Dev. Disabil. Res. Rev. 2009;15:309–317. doi: 10.1002/ddrr.85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samango-Sprouse C., Stapleton E.J., Lawson P., Mitchell F., Sadeghin T., Powell S., Gropman A.L. Positive effects of early androgen therapy on the behavioral phenotype of boys with 47,XXY. Am. J. Med. Genet. C Semin. Med. Genet. 2015;169:150–157. doi: 10.1002/ajmg.c.31437. [DOI] [PubMed] [Google Scholar]
- Sella O., Jones R.D., Huckabee M.-L. Age and gender effects on submental motor-evoked potentials. Age (Dordr.) 2014;36:9735-9711. doi: 10.1007/s11357-014-9735-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skakkebæk A., Bojesen A., Kristensen M.K., Cohen A., Hougaard D.M., Hertz J.M., Fedder J., Laurberg P., Wallentin M., Ostergaard J.R., Pedersen A.D., Gravholt C.H. Neuropsychology and brain morphology in Klinefelter syndrome — the impact of genetics. Andrology. 2014;2:632–640. doi: 10.1111/j.2047-2927.2014.00229.x. [DOI] [PubMed] [Google Scholar]
- Skakkebæk A., Gravholt C.H., Rasmussen P.M., Bojesen A., Jensen J.S., Fedder J., Laurberg P., Hertz J.M., Stergaard J.R., Pedersen A.D., Wallentin M. Neuroanatomical correlates of Klinefelter syndrome studied in relation to the neuropsychological profile. NeuroImage: Clin. 2014;4:1–9. doi: 10.1016/j.nicl.2013.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skakkebæk A., Wallentin M., Gravholt C.H. Neuropsychology and socioeconomic aspects of Klinefelter syndrome: new developments. Curr. Opin. Endocrinol. Diabetes Obes. 2015;22:209–216. doi: 10.1097/MED.0000000000000157. [DOI] [PubMed] [Google Scholar]
- Skakkebæk A., Gravholt C.H., Rasmussen P.M., Bojesen A., Jensen J.S., Fedder J., Laurberg P., Hertz J.M., Østergaard J.R., Pedersen A.D., Wallentin M. Corrigendum to “Neuroanatomical correlates of Klinefelter syndrome studied in relation to the neuropsychological profile” [NeuroImage:Clin 4 (2014) 1–9] NeuroImage: Clin. 2016 doi: 10.1016/j.nicl.2013.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sørensen K. Physical and mental development of adolescent males with Klinefelter syndrome. Horm. Res. Paediatr. 1992;37:55–61. doi: 10.1159/000182402. [DOI] [PubMed] [Google Scholar]
- Sørensen K., Nielsen J. Twenty psychotic males with Klinefelter's syndrome. Acta Psychiatr. Scand. 1977;56:249–255. doi: 10.1111/j.1600-0447.1977.tb00225.x. [DOI] [PubMed] [Google Scholar]
- Steinman K., Ross J., Lai S., Reiss A., Hoeft F. Structural and functional neuroimaging in Klinefelter (47,XXY) syndrome: a review of the literature and preliminary results from a functional magnetic resonance imaging study of language. Dev. Disabil. Res. Rev. 2009;15:295–308. doi: 10.1002/ddrr.84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stroop J.R. Studies of interference in serial verbal reactions. J. Exp. Psychol. 1935;18:643–662. [Google Scholar]
- Tartaglia N., Cordeiro L., Howell S., Wilson R., Janusz J. The spectrum of the behavioral phenotype in boys and adolescents 47,XXY (Klinefelter syndrome) Pediatr. Endocrinol. Rev. 2010;8(Suppl. 1):151–159. [PMC free article] [PubMed] [Google Scholar]
- Temple C.M., Sanfilippo P.M. Executive skills in Klinefelter's syndrome. Neuropsychologia. 2003;41:1547–1559. doi: 10.1016/s0028-3932(03)00061-7. [DOI] [PubMed] [Google Scholar]
- Toran-Allerand C.D. Minireview: a plethora of estrogen receptors in the brain: where will it end? Endocrinology. 2004;145:1069–1074. doi: 10.1210/en.2003-1462. [DOI] [PubMed] [Google Scholar]
- Turriff A., Levy H.P., Biesecker B. Prevalence and psychosocial correlates of depressive symptoms among adolescents and adults with Klinefelter syndrome. Genet. Med. 2011;13:966–972. doi: 10.1097/GIM.0b013e3182227576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tzourio-Mazoyer N., Landeau B., Papathanassiou D., Crivello F., Etard O., Delcroix N., Mazoyer B., Joliot M. Automated anatomical labeling of activations in SPM using a macroscopic anatomical parcellation of the MNI MRI single-subject brain. NeuroImage. 2002;15:273–289. doi: 10.1006/nimg.2001.0978. [DOI] [PubMed] [Google Scholar]
- van der Mark S., Bucher K., Maurer U., Schulz E., Brem S., Buckelmüller J., Kronbichler M., Loenneker T., Klaver P., Martin E., Brandeis D. Children with dyslexia lack multiple specializations along the visual word-form (VWF) system. NeuroImage. 2009;47:1940–1949. doi: 10.1016/j.neuroimage.2009.05.021. [DOI] [PubMed] [Google Scholar]
- van Rijn S., Aleman A., Swaab H., Vink M., Sommer I., Kahn R.S. Effects of an extra X chromosome on language lateralization: an fMRI study with Klinefelter men (47,XXY) Schizophr. Res. 2008;101:17–25. doi: 10.1016/j.schres.2008.02.001. [DOI] [PubMed] [Google Scholar]
- van Rijn S., Swaab H., Aleman A., Kahn R.S. Social behavior and autism traits in a sex chromosomal disorder: Klinefelter (47XXY) syndrome. J. Autism Dev. Disord. 2008;38:1634–1641. doi: 10.1007/s10803-008-0542-1. [DOI] [PubMed] [Google Scholar]
- van Rijn S., Aleman A., De Sonneville L., Swaab H. Cognitive mechanisms underlying disorganization of thought in a genetic syndrome (47,XXY) Schizophr. Res. 2009;112:91–98. doi: 10.1016/j.schres.2009.04.017. [DOI] [PubMed] [Google Scholar]
- van Rijn S., Swaab H., Baas D., de Haan E., Kahn R.S., Aleman A. Neural systems for social cognition in Klinefelter syndrome (47,XXY): evidence from fMRI. Soc. Cogn. Affect. Neurosci. 2012;7:689–697. doi: 10.1093/scan/nsr041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vinckier F., Dehaene S., Jobert A., Dubus J.P., Sigman M., Cohen L. Hierarchical coding of letter strings in the ventral stream: dissecting the inner organization of the visual word-form system. Neuron. 2007;55:143–156. doi: 10.1016/j.neuron.2007.05.031. [DOI] [PubMed] [Google Scholar]
- Wallentin M., Michaelsen J.L.D., Rynne I., Nielsen R.H. Lateralized task shift effects in Broca's and Wernicke's regions and in visual word form area are selective for conceptual content and reflects trial history. NeuroImage. 2014;101:276–288. doi: 10.1016/j.neuroimage.2014.07.012. [DOI] [PubMed] [Google Scholar]
- Wallentin M., Gravholt C.H., Skakkebæk A. Broca's region and visual word form area activation differ during a predictive Stroop task. Cortex. 2015;73:257–270. doi: 10.1016/j.cortex.2015.08.023. [DOI] [PubMed] [Google Scholar]
- Warren M.F., Serby M.J., Roane D.M. The effects of testosterone on cognition in elderly men: a review. CNS Spectrums. 2008;13:887–897. doi: 10.1017/s1092852900016990. [DOI] [PubMed] [Google Scholar]
- Warwick M.M., Doody G.A., Lawrie S.M., Kestelman J.N., Best J.J.K., Johnstone E.C. Volumetric magnetic resonance imaging study of the brain in subjects with sex chromosome aneuploidies. J. Neurol. Neurosurg. Psychiatry. 1999;66:628–632. doi: 10.1136/jnnp.66.5.628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wills A.J., Jenkins I.H., Thompson P.D., Findley L.J., Brooks D.J. Red nuclear and cerebellar but no olivary activation associated with essential tremor: a positron emission tomographic study. Ann. Neurol. 1994;36:636–642. doi: 10.1002/ana.410360413. [DOI] [PubMed] [Google Scholar]
- Worsley K.J., Friston K.J. Analysis of fMRI time-series revisited–again. NeuroImage. 1995;2:173–181. doi: 10.1006/nimg.1995.1023. [DOI] [PubMed] [Google Scholar]
- Yousry T.A., Schmid U.D., Alkadhi H., Schmidt D., Peraud A., Buettner A., Winkler P. Localization of the motor hand area to a knob on the precentral gyrus. A new landmark. Brain J. Neurol. 1997;120(Pt 1):141–157. doi: 10.1093/brain/120.1.141. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material.