Abstract
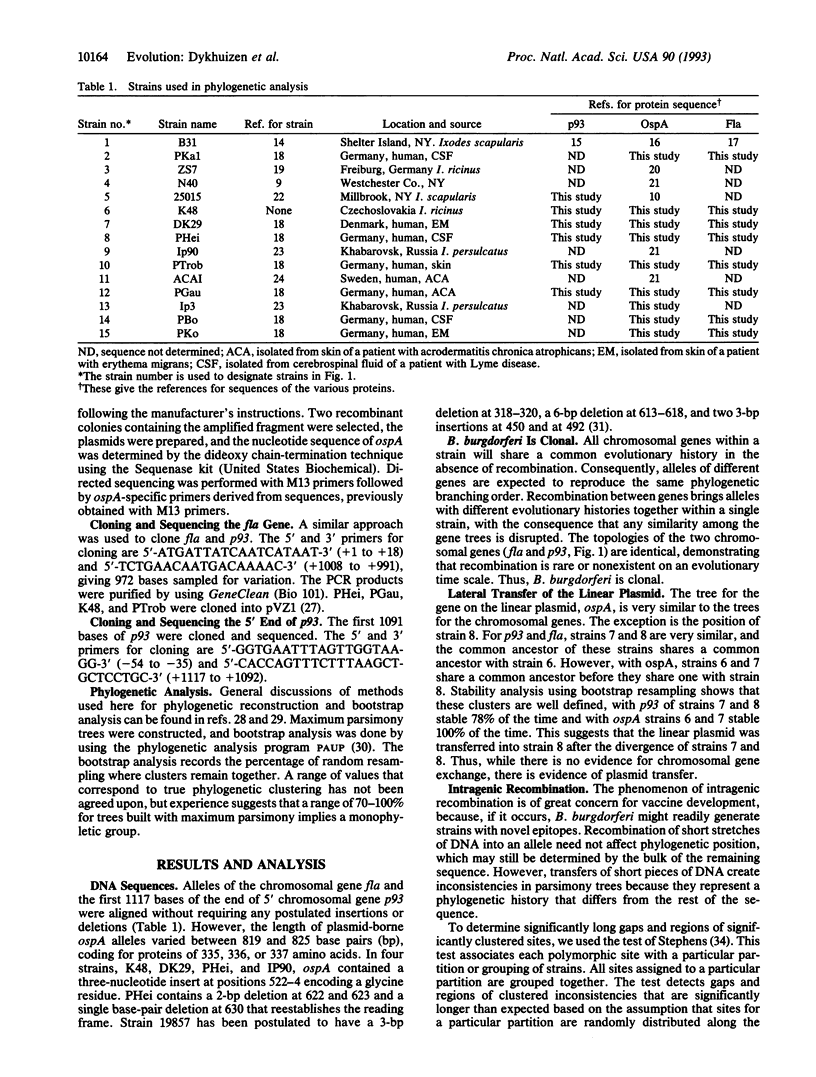
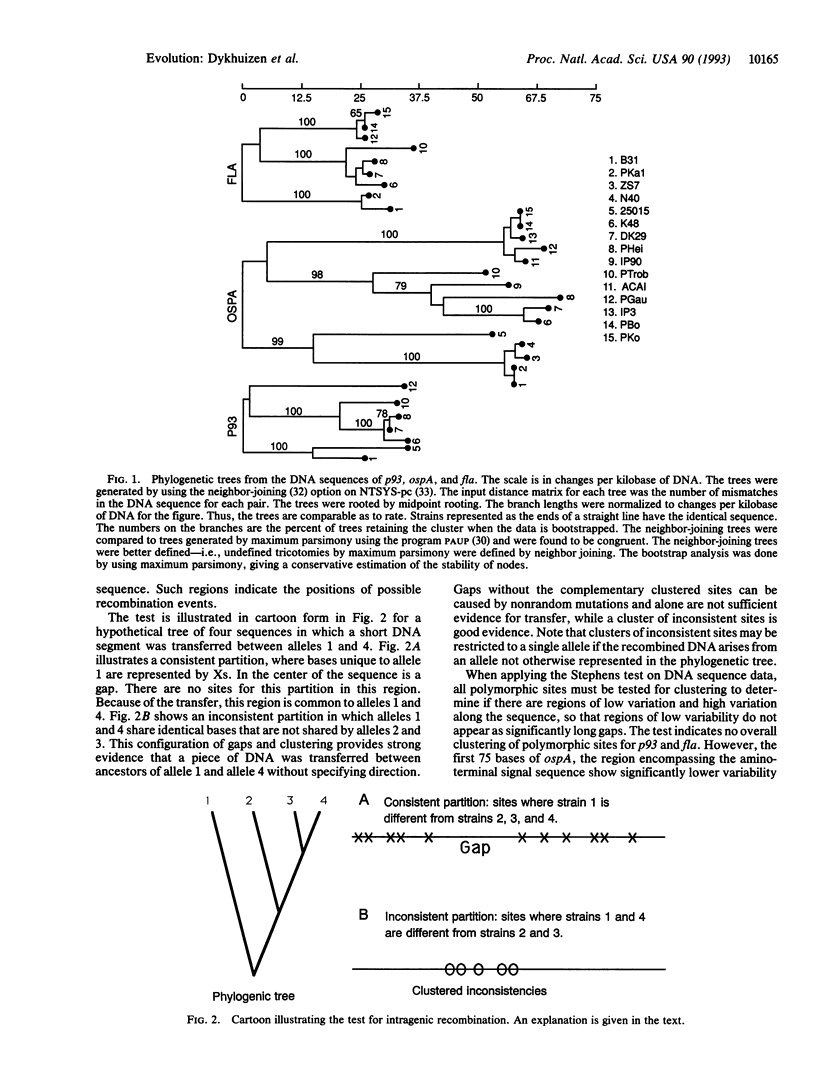
The chromosomal genes fla and p93 and the ospA gene from a linear plasmid were sequenced from up to 15 isolates of Borrelia burgdorferi, which causes Lyme borreliosis in man. Comparison of the gene trees provides no evidence for genetic exchange between chromosomal genes, suggesting B. burgdorferi is strictly clonal. Comparison of the chromosomal gene trees with that of the plasmid-encoded ospA reveals that plasmid transfer between clones is rare. Evidence for intragenic recombination was found in only a single ospA allele. The analysis reveals three common clones and a number of rare clones that are so highly divergent that vaccines developed against one are unlikely to provide immunity to organisms from others. Consequently, an understanding of the geographic and genetic variability of B. burgdorferi will prove essential for the development of effective vaccines and programs for control. While the major clones might be regarded as different species, the clonal population structure, the geographic localization, and the widespread incidence of Lyme disease suggest that B. burgdorferi should remain the name for the entire array of organisms.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson J. F., Magnarelli L. A., McAninch J. B. New Borrelia burgdorferi antigenic variant isolated from Ixodes dammini from upstate New York. J Clin Microbiol. 1988 Oct;26(10):2209–2212. doi: 10.1128/jcm.26.10.2209-2212.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asbrink E., Hovmark A., Hederstedt B. The spirochetal etiology of acrodermatitis chronica atrophicans Herxheimer. Acta Derm Venereol. 1984;64(6):506–512. [PubMed] [Google Scholar]
- Baranton G., Postic D., Saint Girons I., Boerlin P., Piffaretti J. C., Assous M., Grimont P. A. Delineation of Borrelia burgdorferi sensu stricto, Borrelia garinii sp. nov., and group VS461 associated with Lyme borreliosis. Int J Syst Bacteriol. 1992 Jul;42(3):378–383. doi: 10.1099/00207713-42-3-378. [DOI] [PubMed] [Google Scholar]
- Barbour A. G., Garon C. F. Linear plasmids of the bacterium Borrelia burgdorferi have covalently closed ends. Science. 1987 Jul 24;237(4813):409–411. doi: 10.1126/science.3603026. [DOI] [PubMed] [Google Scholar]
- Barbour A. G., Hayes S. F. Biology of Borrelia species. Microbiol Rev. 1986 Dec;50(4):381–400. doi: 10.1128/mr.50.4.381-400.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergström S., Bundoc V. G., Barbour A. G. Molecular analysis of linear plasmid-encoded major surface proteins, OspA and OspB, of the Lyme disease spirochaete Borrelia burgdorferi. Mol Microbiol. 1989 Apr;3(4):479–486. doi: 10.1111/j.1365-2958.1989.tb00194.x. [DOI] [PubMed] [Google Scholar]
- Boerlin P., Peter O., Bretz A. G., Postic D., Baranton G., Piffaretti J. C. Population genetic analysis of Borrelia burgdorferi isolates by multilocus enzyme electrophoresis. Infect Immun. 1992 Apr;60(4):1677–1683. doi: 10.1128/iai.60.4.1677-1683.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burgdorfer W., Barbour A. G., Hayes S. F., Benach J. L., Grunwaldt E., Davis J. P. Lyme disease-a tick-borne spirochetosis? Science. 1982 Jun 18;216(4552):1317–1319. doi: 10.1126/science.7043737. [DOI] [PubMed] [Google Scholar]
- Craft J. E., Fischer D. K., Shimamoto G. T., Steere A. C. Antigens of Borrelia burgdorferi recognized during Lyme disease. Appearance of a new immunoglobulin M response and expansion of the immunoglobulin G response late in the illness. J Clin Invest. 1986 Oct;78(4):934–939. doi: 10.1172/JCI112683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dattwyler R. J., Luft B. J. Immunodiagnosis of Lyme borreliosis. Rheum Dis Clin North Am. 1989 Nov;15(4):727–734. [PubMed] [Google Scholar]
- Dykhuizen D. E., Green L. Recombination in Escherichia coli and the definition of biological species. J Bacteriol. 1991 Nov;173(22):7257–7268. doi: 10.1128/jb.173.22.7257-7268.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Felsenstein J. Phylogenies from molecular sequences: inference and reliability. Annu Rev Genet. 1988;22:521–565. doi: 10.1146/annurev.ge.22.120188.002513. [DOI] [PubMed] [Google Scholar]
- Fikrig E., Barthold S. W., Kantor F. S., Flavell R. A. Protection of mice against the Lyme disease agent by immunizing with recombinant OspA. Science. 1990 Oct 26;250(4980):553–556. doi: 10.1126/science.2237407. [DOI] [PubMed] [Google Scholar]
- Fikrig E., Barthold S. W., Persing D. H., Sun X., Kantor F. S., Flavell R. A. Borrelia burgdorferi strain 25015: characterization of outer surface protein A and vaccination against infection. J Immunol. 1992 Apr 1;148(7):2256–2260. [PubMed] [Google Scholar]
- Gassmann G. S., Kramer M., Göbel U. B., Wallich R. Nucleotide sequence of a gene encoding the Borrelia burgdorferi flagellin. Nucleic Acids Res. 1989 May 11;17(9):3590–3590. doi: 10.1093/nar/17.9.3590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henikoff S., Eghtedarzadeh M. K. Conserved arrangement of nested genes at the Drosophila Gart locus. Genetics. 1987 Dec;117(4):711–725. doi: 10.1093/genetics/117.4.711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jonsson M., Noppa L., Barbour A. G., Bergström S. Heterogeneity of outer membrane proteins in Borrelia burgdorferi: comparison of osp operons of three isolates of different geographic origins. Infect Immun. 1992 May;60(5):1845–1853. doi: 10.1128/iai.60.5.1845-1853.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kriuchechnikov V. N., Korenberg E. I., Shcherbakov S. V., Kovalevskii Iu V., Levin M. L. Identifikatsiia borrelii, izolirovannykh v SSSR ot kleshchei Ixodes persulcatus Schulze. Zh Mikrobiol Epidemiol Immunobiol. 1988 Dec;(12):41–44. [PubMed] [Google Scholar]
- Luft B. J., Mudri S., Jiang W., Dattwyler R. J., Gorevic P. D., Fischer T., Munoz P., Dunn J. J., Schubach W. H. The 93-kilodalton protein of Borrelia burgdorferi: an immunodominant protoplasmic cylinder antigen. Infect Immun. 1992 Oct;60(10):4309–4321. doi: 10.1128/iai.60.10.4309-4321.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luft B. J., Pawagi S., Jiang W., Fiseene S., Gorevic P. D., Dunn J. Analysis and expression of the Borrelia burgdorferi P/Gau fla gene: identification of heterogeneity with the B31 strain. FEMS Microbiol Lett. 1992 May 15;72(1):63–67. doi: 10.1016/0378-1097(92)90490-f. [DOI] [PubMed] [Google Scholar]
- Saitou N., Nei M. The neighbor-joining method: a new method for reconstructing phylogenetic trees. Mol Biol Evol. 1987 Jul;4(4):406–425. doi: 10.1093/oxfordjournals.molbev.a040454. [DOI] [PubMed] [Google Scholar]
- Schaible U. E., Kramer M. D., Museteanu C., Zimmer G., Mossmann H., Simon M. M. The severe combined immunodeficiency (scid) mouse. A laboratory model for the analysis of Lyme arthritis and carditis. J Exp Med. 1989 Oct 1;170(4):1427–1432. doi: 10.1084/jem.170.4.1427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schubach W. H., Mudri S., Dattwyler R. J., Luft B. J. Mapping antibody-binding domains of the major outer surface membrane protein (OspA) of Borrelia burgdorferi. Infect Immun. 1991 Jun;59(6):1911–1915. doi: 10.1128/iai.59.6.1911-1915.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steere A. C., Grodzicki R. L., Kornblatt A. N., Craft J. E., Barbour A. G., Burgdorfer W., Schmid G. P., Johnson E., Malawista S. E. The spirochetal etiology of Lyme disease. N Engl J Med. 1983 Mar 31;308(13):733–740. doi: 10.1056/NEJM198303313081301. [DOI] [PubMed] [Google Scholar]
- Stephens J. C. Statistical methods of DNA sequence analysis: detection of intragenic recombination or gene conversion. Mol Biol Evol. 1985 Nov;2(6):539–556. doi: 10.1093/oxfordjournals.molbev.a040371. [DOI] [PubMed] [Google Scholar]
- Wallich R., Helmes C., Schaible U. E., Lobet Y., Moter S. E., Kramer M. D., Simon M. M. Evaluation of genetic divergence among Borrelia burgdorferi isolates by use of OspA, fla, HSP60, and HSP70 gene probes. Infect Immun. 1992 Nov;60(11):4856–4866. doi: 10.1128/iai.60.11.4856-4866.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallich R., Schaible U. E., Simon M. M., Heiberger A., Kramer M. D. Cloning and sequencing of the gene encoding the outer surface protein A (OspA) of a European Borrelia burgdorferi isolate. Nucleic Acids Res. 1989 Nov 11;17(21):8864–8864. doi: 10.1093/nar/17.21.8864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilske B., Preac-Mursic V., Göbel U. B., Graf B., Jauris S., Soutschek E., Schwab E., Zumstein G. An OspA serotyping system for Borrelia burgdorferi based on reactivity with monoclonal antibodies and OspA sequence analysis. J Clin Microbiol. 1993 Feb;31(2):340–350. doi: 10.1128/jcm.31.2.340-350.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilske B., Preac-Mursic V., Schierz G., Kühbeck R., Barbour A. G., Kramer M. Antigenic variability of Borrelia burgdorferi. Ann N Y Acad Sci. 1988;539:126–143. doi: 10.1111/j.1749-6632.1988.tb31846.x. [DOI] [PubMed] [Google Scholar]
- Zumstein G., Fuchs R., Hofmann A., Preac-Mursic V., Soutschek E., Wilske B. Genetic polymorphism of the gene encoding the outer surface protein A (OspA) of Borrelia burgdorferi. Med Microbiol Immunol. 1992;181(2):57–70. doi: 10.1007/BF00189424. [DOI] [PubMed] [Google Scholar]


