Abstract
Despite considerable evidence for deleterious effects of aging on place learning and memory, less is known about the trajectory and the putative neural mechanisms of these decrements. The virtual Morris Water Task (vMWT) is a human analog of a non-human spatial navigation task. The present study investigated longitudinal changes in place learning in 51 healthy, non-demented adults (age 30–83) who completed the vMWT and a neuropsychological battery at two time-points (interval= ~8 years). We also assessed cross-sectional associations between vMWT and brain structure, biochemical integrity, and standardized neuropsychological measures in a subset of 22 individuals who underwent MR imaging at follow-up. Despite no longitudinal decrement in vMWT performance, there were cross-sectional age differences on the vMWT favoring younger adults. Negative associations were observed between vMWT latency and gray matter volumes in the right hippocampus, bilateral thalamus, and right medial orbitofrontal cortex, and between vMWT latency and white matter fractional anisotropy in the bilateral uncinate fasciculus. Collectively, these results suggest a pattern of differences in the structural integrity of regions supporting successful navigation even in the absence of longitudinal performance decrements.
Keywords: normal aging, place learning, memory, virtual navigation, hippocampus, DTI
1. INTRODUCTION
Intact spatial learning and memory are important for older adults to independently and successfully navigate complex environments. In survey research, older adults endorse self-perceived difficulties with navigation and report using behavioral coping strategies, such as avoidance of unfamiliar places and reliance on maps, to alleviate navigation problems (Bryden, Charlton, Oxley, & Lowndes, 2013; Burns, 1999). Spatial navigation deficits are also seen in individuals with mild cognitive impairment (MCI) and may be an early indicator of Alzheimer’s disease (AD) (Gazova et al., 2012; Laczó et al., 2009; Hort et al., 2007; deIpolyi et al., 2007; see Lithfous, Dufour, & Després, 2013, for review).
Spatial learning and memory has been extensively studied in rodents using the Morris water task (MWT) (Morris, 1981, 1984), a classic paradigm in which a rodent is placed in a large pool of water and must navigate the environment to find a hidden escape platform. Successful navigation in the MWT requires allocentric processing, in which the platform location is situated in a framework external to the observer (e.g., coordinate space) (Wolbers & Wiener, 2014). This is in contrast to egocentric representations, which provide information relative to the particular perspective of the observer. Effective performance on the MWT depends on the hippocampus (Morris, Garrud, Rawlins, & O’Keefe, 1982; West, Coleman, Flood, & Troncoso, 1994; Redish & Touretzky, 1998), and age-related alterations in hippocampal integrity have been linked to learning and memory deficits on the MWT (Gage, Dunnett, & Björklund, 1984; Gage, Chen, Buzsaki, & Armstrong, 1988; Geinisman et al., 1995; Geinisman, de Toledo-Morrell, & Morrell, 1986; Gallagher & Nicolle, 1993). Thus, this task may provide a robust basis for cross-species comparisons of age-related changes in spatial memory.
Although previous research using real-world analogues of the MWT have shown similar navigation deficits in aging humans (Newman & Kaszniak, 2000; Laczó et al., 2009), life-sized maze learning paradigms are challenging to implement because of space considerations and older adults’ limited mobility. The use of virtual environments has proven a fruitful alternative for exploring spatial learning and memory in humans. A computerized version of the MWT (vMWT) allows individuals to take a first-person perspective while exploring a virtual pool to find the hidden platform (Moffat & Resnick, 2002; Hamilton et al., 2002). Compared to younger adults, older individuals travel longer distances to reach the platform, spend a smaller proportion of their total distance in the goal quadrant, take paths with fewer platform intersections, and are impaired in constructing a cognitive map of the environment (Moffat & Resnick, 2002; Driscoll et al., 2003; Driscoll et al., 2005; Moffat et al., 2007).
Numerous cross-sectional studies have demonstrated age deficits in spatial learning and memory, but only one known study has used a longitudinal design to assess changes in spatial cognition. Lövdén et al. (2012) implemented a four-month spatial navigation training program and found that this training was associated with improved navigation performance and maintenance of hippocampal volumes four months after the termination of training. Meanwhile, a control group showed decreased hippocampal volume over the follow-up interval. Collectively, these findings suggest that spatial navigation training may be protective against age-related changes to the hippocampus. While this study provides important information about experience-related changes in hippocampal morphology, albeit in a sample of older individuals not screened for cognitive impairment, no longer-term longitudinal investigations of spatial navigation have been performed. Thus, to the best of our knowledge, this is the first study to characterize the trajectory of changes in spatial cognition within the context of healthy aging.
Subcortical structures including the hippocampus and striatum appear to be critical in supporting spatial navigation abilities (Morris et al., 1982; Maguire et al., 1998; Ekstrom et al., 2003; Devan, Goad, & Petri, 1996; Voermans et al., 2004; Moffat et al., 2007; Burgess, 2008). In particular, the hippocampus supports allocentric processing required for effective navigation on the vMWT (Astur et al., 2002; Goodrich-Hunsaker et al., 2010; Bartsch et al., 2010). Age-related structural and functional changes in hippocampal integrity may contribute to poorer spatial navigation performance in older adults (Driscoll et al., 2003; Moffat, Elkins, & Resnick, 2006; Antonova et al., 2009). In addition, increased age is associated with lower use of allocentric spatial memory, as older adults tend to prefer response strategies such as learning a series of movements relative to one’s starting position (Bohbot et al., 2012; Rodgers, Sindone, & Moffat, 2012; Wiener et al., 2013); this strategy does not require knowledge of the relationships between landmarks. In younger adults, use of a response strategy is associated with striatal structures, particularly the caudate nucleus (Bohbot et al., 2007; Iaria et al., 2003; Bohbot, Iaria, & Petrides, 2004). Consistent with an age-related shift to use of a response strategy, structural and functional imaging studies suggest that older adults may rely more heavily on the prefrontal cortex and other extrahippocampal regions to successfully navigate an environment (Konishi et al., 2013; Moffat et al., 2007).
The preponderance of studies exploring brain-behavior relationships using spatial navigation paradigms has investigated gray matter structures (see Moffat, 2009, for review). However, the integrity of white matter (WM) pathways is increasingly recognized as essential for healthy aging (Gunning-Dixon & Raz, 2000; Gunning-Dixon, Brickman, Cheng, & Alexopoulos, 2009; Guttmann et al., 1998; Bennett et al., 2010; Bennet & Madden, 2014). Diffusion tensor imaging (DTI) allows for assessments of the integrity of white matter connectivity in the brain by estimating diffusion along the major axis and two minor axes of an ellipsoid. Normal aging is associated with lower fractional anisotropy (FA), a measure of the degree of anisotropic diffusion, and higher mean diffusivity (MD), the average amount of water diffusion across the three principle axes of the diffusion ellipsoid (Abe et al., 2002; Gunning-Dixon, Brickman, Cheng, & Alexopoulos, 2009; Yoon, Shim, Lee, Shon, & Yang, 2008). In healthy aging, lower FA is observed particularly in the genu of the corpus callosum and bilateral frontal regions (Barrick, Charlton, Clark, & Markus, 2010; Sullivan & Pfefferbaum, 2003). AD patients, on the other hand, show more widespread alterations in FA and MD, including in the parahippocampal gyrus, posterior cingulum, splenium of the corpus callosum, and temporal WM (Barrick et al., 2010; Chua, Wen, Slavin, & Sachdev, 2008; Sullivan & Pfefferbaum, 2003; Naggara et al., 2006). The extent to which age-related changes in WM microstructural properties affect spatial cognition is largely unknown.
In addition to yielding structural assessments of brain integrity, MR imaging permits evaluation of brain metabolite levels in vivo via proton magnetic resonance spectroscopy (1H MRS). In particular, both healthy aging (Driscoll et al., 2003) and AD (Dixon, Bradley, Budge, Styles, & Smith, 2002; Schuff et al., 1997; Schuff et al., 1999; Angelie et al., 2001) are associated with lower hippocampal concentrations of N-acetylaspartate (NAA), a compound thought to reflect neuronal functioning and viability (Moffett, Ross, Arun, Madhavarao, & Namboodiri, 2007). A previous study using the vMWT (Driscoll et al., 2003) found that impaired place learning was associated with lower hippocampal NAA/Creatine (Cr) and lower hippocampal volume, suggesting that age-related neuronal loss or changes in neuronal morphology may contribute to spatial learning and memory deficits.
The overall aim of the present study was to create a comprehensive description of neuropsychological, morphometric, and neurochemical features associated with spatial learning and memory within the context of normal aging. We quantified age-related changes in learning and memory on the vMWT over an average interval of eight years in adults ranging in age from 30 to 83 years at baseline and compared performance on the vMWT to performance on standardized neuropsychological tests commonly employed to assess cognition in humans. We hypothesized that vMWT performance would be associated with measures of episodic memory, visuospatial ability, and executive functioning but not with tests of attention or language. In addition, we performed assessments of the underlying structural (volume and white matter microstructural organization) and biochemical (metabolite concentrations) brain integrity at the follow-up visit in a subset of the returning participants. We hypothesized that impaired spatial navigation would be associated with: 1) lower gray matter volume in the hippocampal formation and prefrontal regions previously implicated in successful spatial navigation; 2) reduced FA in the uncinate fasciculus, which connects the anterior temporal lobe to prefrontal cortical areas; and 3) lower hippocampal NAA/Cr, indicating reduced neuronal viability.
2. METHODS
2.1. Participants
The final sample included 51 adults from the Baltimore Longitudinal Study of Aging (BLSA) who participated in a previous study investigating place learning in older adults (Moffat & Resnick, 2002); none had participated in the BLSA Neuroimaging substudy. Participants ranged in age from 30 to 83 years at baseline (M = 59.7) and were recalled for follow-up testing an average of 7.8 years later (see Table 1). BLSA participants are generally healthy, community- dwelling volunteers who periodically (age <60 every 4 years; age 60–79 biennially, and age 80+ annually) return to the National Institute on Aging (NIA) for thorough medical, physiological, and neuropsychological evaluations (Shock et al., 1984). The BLSA visit involves a comprehensive assessment of physical and behavioral health over a two to three day period; given the complexity of scheduling a variety of procedures, the order of assessments (vMWT, neuropsychological testing, MRI) was not fixed for participants in the current sample. Attempt were made to minimize potential impact of fatigue on the vMWT or neuropsychological testing. Participants stayed in the clinical research unit, where the testing is also performed, for the duration of the visit, allowing them to rest as needed between examinations. All participants were screened for dementia based on results of physical, neurologic, and neuropsychological examinations (Kawas et al., 2000); diagnoses of dementia and Alzheimer’s disease (AD) followed DSM-III-R and National Institute of Neurological and Communicative Disorders and Stroke and the Alzheimer’s Disease and Related Disorders (NINCDS-ADRDA) criteria, respectively. Participants who met criteria for probable (n = 1) or possible (n = 3) AD at follow-up were excluded from analyses.
Table 1.
Demographic Characteristics and Performance on Standardized Neuropsychological Tests
| Baseline | Follow-Up | t score | |
|---|---|---|---|
| Age (years) | 59.38 (11.73) | 67.01 (11.29) | −24.65*** |
| Sex (M:F) | 20:26 | 20:26 | 0 |
| Years Education | 16.53 (2.36) | 16.53 (2.36) | 0 |
| MMSE | 28.72 (1.41) | 29.03 (1.07) | 0.88 |
| CESD | 6.82 (8.17) | 5.46 (5.95) | 1.24 |
| CVLT (total correct) | 56.88 (10.77) | 52.78 (9.80) | 3.12** |
| Card Rotations (correct-incorrect) | 98.89 (33.95) | 94.04 (36.67) | 1.13 |
| Digit Span Fwd (total correct) | 8.53 (2.26) | 8.53 (2.18) | 0 |
| Digit Span Bkwd (total correct) | 7.65 (1.95) | 7.38 (1.89) | .98 |
| BVRT (total errors) | 4.33 (2.93) | 6.24 (3.39) | −3.87** |
| Category Fluency (mean correct) | 16.81 (2.83) | 16.65 (3.45) | .19 |
| Letter Fluency (mean correct) | 16.42 (4.64) | 15.90 (4.74) | .65 |
| Trails A (time in seconds) | 30.53 (8.34) | 34.58 (12.32) | −1.75 |
| Trails B (time in seconds) | 70.00 (26.37) | 75.48 (28.66) | −.91 |
Note. MMSE: Mini-Mental Status Examination; CES-D: Center for Epidemiologic Studies Depression Scale; CVLT: California Verbal Learning Test, Immediate Recall Trials 1–5; BVRT: Benton Visual Retention Test
p < .001;
p < .0001
Participants of the original place-learning study, the results of which have been previously published (Moffat and Resnick, 2002), were asked to partake in the present study when returning for their regularly scheduled BLSA visit and recruited over a period of four years. Of the original 86 participants who completed the place-learning task, 52 were recruited. One participant was excluded on the basis of computer malfunction and no viable place learning data obtained at the second time point. Three original participants withdrew from the BLSA in the interval between baseline and follow-up. Two refused to participate as they were no longer traveling to the NIA for BLSA visits due to advanced age (90+ years of age) and frailty. There were no significant differences in sex, age at baseline, education, depression scores or Mini-Mental Status Exam (MMSE, Folstein et al., 1975; although MMSE is obtained in the BLSA only for participants age 60 and older) between the group that returned for a follow-up visit compared to those we were unable to recruit or schedule for follow-up. However, there were significant differences between these two groups on four of the 10 neuropsychological assessments performed (Card Rotations Test, p = .05; Benton Visual Retention Test, p = .04; Category verbal fluency test, p = .007; Trail-Making Test A, p = .005), with participants who did not return for follow-up having poorer performance than those who returned. Despite this, only performance on Trail-Making Test A (Reitan, 1992), a measure of attention and processing speed, survived Bonferroni correction for multiple comparisons (p = .04, corrected). Of the 52 recruited at follow-up, 22 agreed to undergo MR imaging and the sample was divided into middle-aged (< 60 years; age range 40 – 59 years (M = 50.8); N = 9; 3 males) and older (≥ 60 years; age range 60 – 78 years (M = 67.9); N = 13; 6 males) adults for analyses purposes.
2.2. Procedures
2.2.1. Neuropsychological Testing
At each visit, participants completed a thorough neuropsychological battery in a single testing session lasting 2 hours. The neuropsychological battery was designed to assess performance across five cognitive domains: 1) Memory. The California Verbal Learning Test (CVLT) (Delis et al., 1987) is a list learning task that assesses verbal episodic memory. List A immediate (sum of five immediate recall trials) and delayed recall scores were used. The Benton Visual Retention Test (BVRT) (Benton, 1968) requires participants to reproduce designs after a brief viewing period. This is a test of non-verbal episodic memory. 2) Attention. Digits Forward (Wechsler, 1981) involves presentation of a string of numbers that a participant must repeat back to the examiner. Trail-Making Test A (Reitan, 1992) requires participants to connect a set of dots in sequential order as quickly as possible without making mistakes. 3) Executive Functioning: Digits Backward (Wechsler, 1981) requires participants to listen to a string of numbers and repeat them backward. Effective performance requires working memory abilities. In Trail-Making Test B (Reitan, 1992) participants must connect dots alternating between numbers and letters. This assesses ability to flexibly switch between mental sets, a component of executive functioning. 4). Language. Letter verbal fluency test (VFT-L) (Benton, 1968) requires participants to name all of the words they can think of that begin with a letter. Category verbal fluency test (VFT-C) (Newcombe, 1969) assesses a participant’s ability to name words within a given semantic category. 5) Visuospatial Abilities. The Card Rotations Test (Wilson et al., 1975) requires participants to mentally rotate objects in two-dimensional space.
2.2.2. vMWT
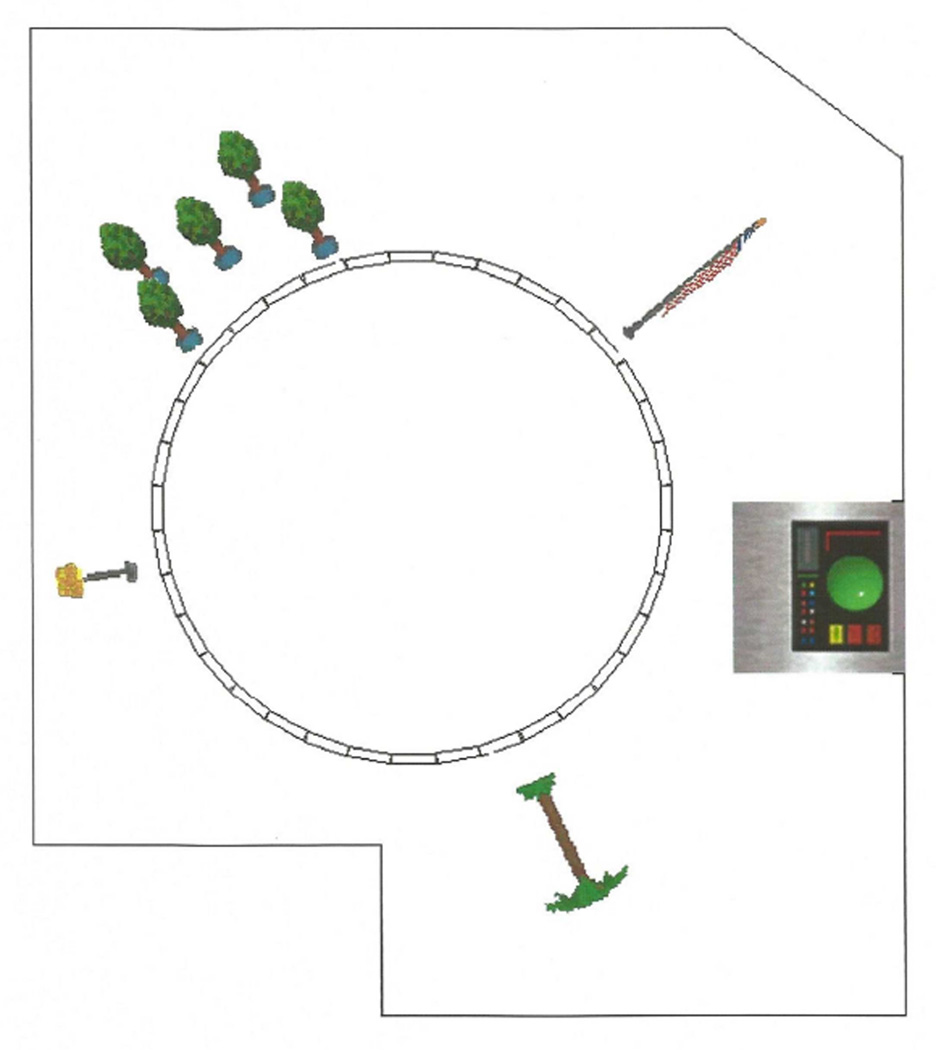
The vMWT was designed with a modified version of the Game Creation System (Pie in the Sky Software, Fairport, NY). The task was administered on a Dell IBM-compatible Pentium III computer with a 17-in (43.2 cm) monitor. Participants completed the vMWT in a separate session from the neuropsychological testing but during the same BLSA visit. The entire administration took approximately 30 minutes, including joystick training and practice trials. The participant was seated in a chair so that the head was approximately 50 cm from the monitor. The vMWT was presented from a first-person perspective. The room consisted of a circular pool of water placed within an irregularly shaped room with several distal objects that could be used as visual cues to guide navigation (Figure 1). All scoring of the vMWT task was fully automated. A software program recorded the participant’s coordinate position and heading direction in degrees every 20 ms. This information was used to calculate total distance traveled and total time to reach the platform on the learning trials, distance traveled in the goal quadrant and percentage of time spent in the goal quadrant on the probe trial, and speed of movement.
Figure 1.
Overhead view of the vMWT environment (Moffat & Resnick, 2002). Object cues represent (clockwise from top left) a group of trees, a flag, a picture, a palm tree, and a lamp.
2.2.3. vMWT Pretest Training
Participants completed pretraining in a virtual environment consisting of two rooms with objects situated on either end of the interconnected hallways to familiarize them with the computerized environment and the use of a joystick. After experimenter instruction, participants were allowed to freely explore the virtual environment. After this initial practice session allowing participants to become familiar navigating a virtual environment using a joystick, a practice vMWT was presented. The practice MWT was smaller than the test vMWT but otherwise similar in design and configuration, including visual objects that could serve as distal cues. The location of the platform and the types of visual objects in the practice vMWT were different than in the test vMWT. Participants completed five practice trials of the vMWT, with the platform alternating between being visible and hidden. This pretest training was performed at both the baseline and follow-up visits.
2.2.4. vMWT Learning Trials
A square platform was hidden beneath the surface of the water, and participants were instructed to locate the platform as quickly and accurately as possible. Upon crossing over the platform, the platform became visible and elevated above the pool. The platform remained elevated for a 12-second interval during which time the participants could freely scan the environment, although they were not explicitly instructed to do so. After the 12-second interval, a new trial began with the participant starting from a different area of the pool. Participants were informed that the platform remained in the same location on each trial and were instructed to try to remember its location. Each trial began with the participant facing a different direction and being located in one of the three quadrants of the pool that did not contain the platform. Participants completed six learning trials.
2.2.5. vMWT Probe Trial
Following the completion of six learning trials, participants completed a probe trial in the same environment. In this trial, the platform was removed but participants were not told that it was missing. Participants were given 60 seconds of search time. The proportion of time spent and distance traveled in the vicinity of the platform is a measure of participants’ memory for the platform location. Performance at or below chance on the probe trial (i.e., 25% of time or path distance in the goal quadrant) is an indication that a participant did not learn the platform location.
2.2.6. vMWT Visible Platform Trial
After completing the probe trial, participants were placed in the same room but with the platform visible above the surface of the water. They were instructed to navigate to the platform as quickly and accurately as possible. This trial required focused attention, visuomotor integration, joystick control, and other abilities that may be impaired during aging. Thus, performance on the visible platform trial was used to control for perceptual or motor capabilities that may have affected vMWT performance independently of place learning.
2.2.7. Magnetic Resonance Imaging (MRI)
All imaging was performed on a 3T Philips MRI system at the Kennedy Krieger Institute. Of the 52 participants recalled at follow-up, 22 agreed to undergo MR imaging as a part of their follow-up assessment. Data for one participant was excluded from analysis due to low quality of images.
2.2.8. Volumetric Analysis
T1-weighted images were acquired with a 3D magnetization prepared rapid acquisition gradient echo (MPRAGE) sequence (sagittal acquisition: SENSE factor 2, 1 mm isotropic voxels, 256 × 256 mm field of view, TR = 2300 ms, TE = 3 ms, flip angle = 8°) obtained at the beginning of each imaging session. Cortical surface reconstruction and volumetric segmentation was performed using FreeSurfer (version 5.1; Laboratory for Computational Neuroimaging, Martinos Center for Biomedical Imaging, Boston, MA; http://surfer.nmr.mgh.harvard.edu). Technical details of these procedures are described in prior publications (Fischl et al., 2002; Fischl et al., 2004). Briefly, this processing includes segmentation of subcortical white matter and deep gray matter volumetric structures as well as transformation of each participant’s reconstructed brain to an average spherical surface representation to permit automatic parcellation of the cerebral cortex. Regional labels were automatically assigned on the cortical surface model based on probabilistic information using the Desikan-Killiany Atlas (Desikan et al., 2006). Regional brain volumes were quantified and entered into SPSS statistical software for analysis.
2.2.9. 1H MRS
Axial T1-weighted images were acquired for voxel placement. Spectra were acquired from two 8cc (2 × 2 x 2 cm) voxels placed in the posterior left and right hippocampus respectively. Spectra were acquired with a point resolved pulse sequence (PRESS TR = 2000 ms, TE=35 ms, 1024 points, 2000 Hz spectral width, 256 averages, scan time 8 min 34 sec). Sixteen averages of unsuppressed water spectra were also collected with the same parameters. Water suppression was achieved using three chemical-shift selected (CHESS) presaturation pulses. All sequences were within FDA guideline SAR limits of 3.0 Watts/kg average power deposition for head imaging. Water suppressed single voxel spectra were analyzed using fully automated, standard curve fitting software, LCModel (Provencher, 2001), to determine values of NAA (neuron-axonal compound), Glx (glutamine + glutamate), Cho (choline, related to inflammation and demyelination, amongst other processes), Cr (measure of cellular energy currency) and mI (myo-inositol, present in glial cells predominantly). The average Cramer Rao lower bounds (CRLBs) were calculated by LCModel for each fitted metabolite, and all of the metabolite concentrations were estimated with CRLBs lower than 20% of the calculated concentrations. Spectra were analyzed blinded to the subject status. Values were expressed as ratios with creatine (e.g., NAA/Cr, Glx/Cr) for statistical analysis.
2.2.10. DTI
A 3-minute 30 seconds diffusion tensor imaging (DTI) sequence was acquired at the end of the MRS scanning procedure, involving a spin-echo dataset with 60 interleaved slices, each 2.2 mm thick, with a field of view of 212 mm x 212 mm with a 96 × 96 matrix, providing continuous whole brain coverage (TE 69 ms; TR 6.1 s). Following an acquisition without diffusion sensitization (b = 0 s mm−2), images were acquired with diffusion gradients acquired (b = 700 s mm−2) in 32 directions. Although this is not as sensitive as many currently used DTI pulse sequences, 32 directions was a standard DTI protocol at the time the data were acquired. DTI data were analyzed using the Tract-Based Spatial Statistics (TBSS) pipeline from the FMRIB Software Library (FSL) (Smith et al., 2006). Briefly, images were realigned to remove distortions. FA images were created by fitting a tensor model to the raw diffusion data using FDT (Behrens et al., 2003a; Behrens et al., 2003b) and then brain-extracted using BET (Smith, 2002). All participants’ FA data were aligned into a common space using nonlinear registration and were visually inspected for errors in normalization. The individual FA maps were averaged to create a group-averaged FA map, which was thinned to create a skeleton of white matter tracts. Each participant’s aligned FA data were then projected onto this skeleton. The resulting data were used for cross-subject statistical analysis. Voxelwise statistics were performed using a permutation-based inference tool for nonparametric statistical thresholding (FSL’s Randomise) (Winkler, Ridgway, Webster, Smith, & Nichols, 2014). Voxelwise correlations between vMWT performance and FA were performed, entering age and vMWT speed of movement as covariates. The statistical threshold for significant clusters was set to p < .001, uncorrected. This uncorrected threshold was selected because we had a priori predictions about associations between uncinate fasciculus microstructural organization and spatial learning and memory. Controlling for family-wise error prevents spurious discoveries but is very conservative and sacrifices sensitivity for increased specificity (Schwartz et al., 2014). As this is one of the first studies to investigate associations between WM microstructural integrity and vMWT performance, and our imaging sub-sample is modest, we used an uncorrected threshold and consider our findings exploratory. Significant clusters were identified using a DTI-based atlas of human white matter (Mori, Wakana, Van Zijl, & Nagae-Poetscher, 2005).
2.2.11. Statistical Analyses
Analyses were performed in IBM SPSS Statistics Version 20 (IBM Corp., released 2011). Inspection of vMWT summary variables (e.g., total latency and distance over learning trials, latency and distance in probe trial goal quadrant) showed that they were positively skewed. A logarithmic transformation was applied to meet the statistical assumptions of normality (Manikandan, 2010; Moffat et al., 2007).
3. RESULTS
3.1. Age-Related Changes in Performance on the vMWT and Neuropsychological Battery
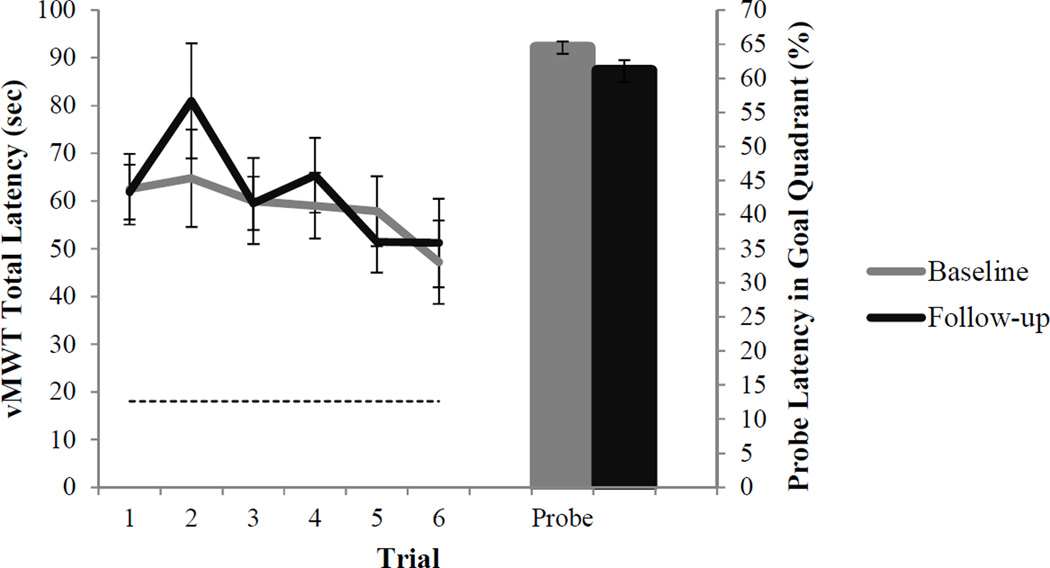
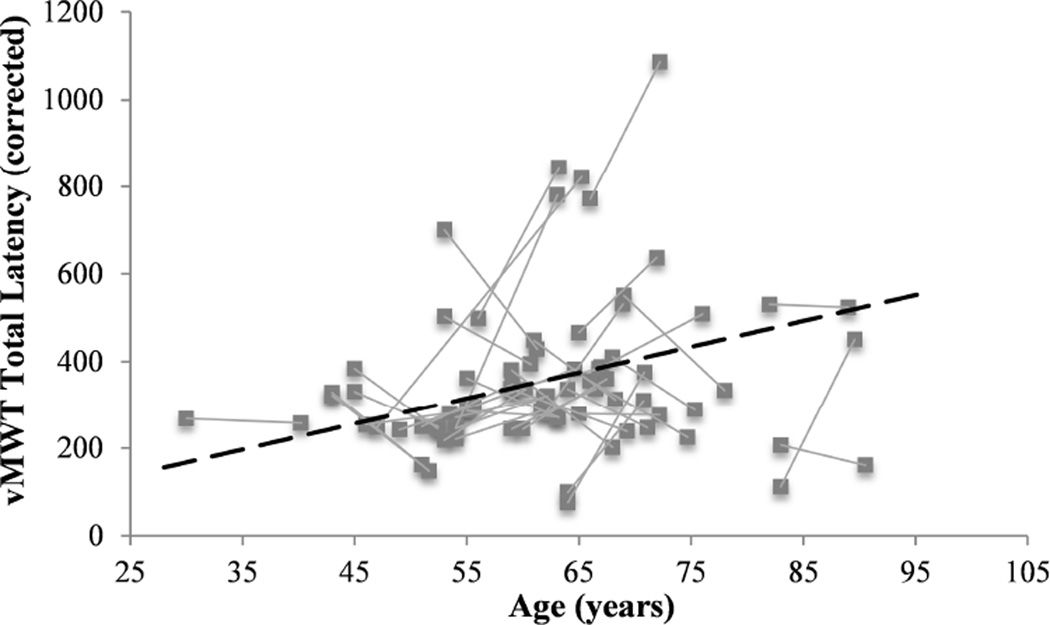
The effects of age-related changes in spatial navigation performance were assessed using a general linear model with sex, baseline age, and duration of follow-up interval as between-subjects variables, time (baseline or follow-up) as a within-subjects variable, speed of movement as a covariate, and total latency to complete the vMWT (sum of latencies for the six learning trials) as a dependent variable. There was no significant main effect of time (baseline to follow-up) on latency to complete the vMWT, F(1,34) = 2.23, p = .15, 95% CI [−.20, .07]. Main effects of age (p = .14) and sex (p = .23) were not significant, nor were the interactions between time and age and between time and sex (p’s > .05). Additionally, there was no change between baseline and follow-up in total distance on vMWT learning trials nor percentage of time or distance spent searching in the goal quadrant on the probe trial (p’s > .05) (Figure 2). Three participants at baseline and two participants at follow-up performed at or below chance on the probe trial (i.e., less than 25% of time or distance in the goal quadrant); however, excluding these individuals from the sample did not alter the overall pattern of results. Participants showed considerable variability in the change in total latency (M = 36.2, SD = 205.3) and distance (M = 25.2, SD = 191.8) to complete learning trials. Intra-individual trajectories of change in vMWT total latency (after regressing out effects of swim speed using separate linear regression models for baseline and follow-up) are depicted in Figure 3.
Figure 2.
Longitudinal change in vMWT performance (Mean ± SEM). Dotted line represents perfect performance.
Figure 3.
Variability of intra-individual change in vMWT performance from baseline to follow-up. Dotted trendline represents average trajectory of change. Corrected total latency reflects residual values after regressing out the effects of swim speed in a linear regression model.
To assess the effects of age and sex on vMWT performance cross-sectionally, we performed general linear models for baseline and follow-up separately, using vMWT summary variables (e.g., total latency, total distance, probe trial latency or distance) as the dependent measures, age and sex as independent variables, and vMWT speed of movement as a covariate. At baseline, there was a significant main effect of age, F(1, 37) = 7.8, p = .008, while the main effect of sex was not significant. There was also a significant main effect of age on total vMWT distance, F(1, 37) = 5.5, p = .03. No effects of age and sex on probe trial performance were significant (p’s > .1). At follow-up, there was a significant effect of age on total distance to complete the vMWT learning trials, F(1, 40) = 8.4, p = .006, but there were no significant effects of age or sex on total latency or probe trial performance (p’s > .1).
Pairwise comparisons were performed between neuropsychological tests administered at baseline and follow-up in order to identify age-related changes in cognition (Table 1). Participants showed a significant decline in performance on the CVLT, t(31) = 3.12, p = .04 (Bonferroni corrected), and the BVRT, t(32) = −3.87, p = .009 (Bonferroni corrected). There were no significant age-related changes in performance on other neuropsychological tests, and associations between vMWT performance (e.g., total latency, total distance, probe latency, probe distance) and neuropsychological tests were not significant at baseline or follow-up after controlling for age.
3.2. vMWT Performance and Regional Brain Volumes
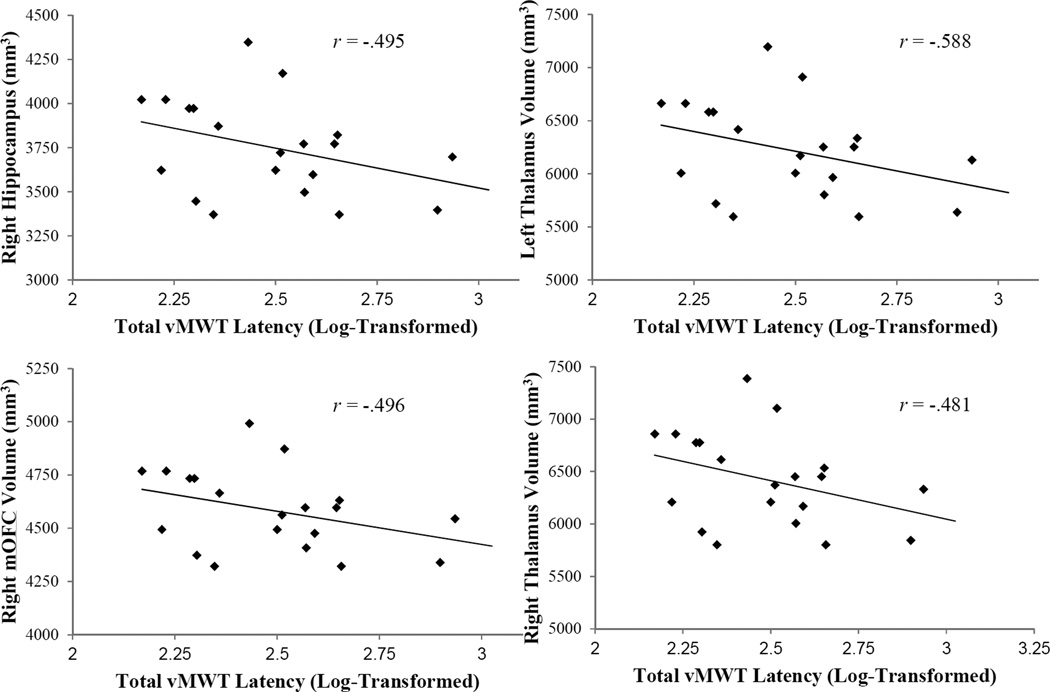
As MR imaging was performed at the follow-up visit, only follow-up vMWT data were used in the following analyses. Brain volumes previously found to be associated with spatial navigation abilities were selected for analyses based on a priori hypotheses. These regions included the hippocampus, parahippocampal gyrus, retrosplenial cortex, caudate nucleus, thalamus, posterior cingulate gyrus, lateral orbitofrontal cortex, medial orbitofrontal cortex, and middle frontal cortex (see Moffat et al., 2009 for review). The association between vMWT performance and regional brain volumes was evaluated using partial correlations controlling for age, intracranial volume, sex, and speed of movement. There was a significant negative correlation between total vMWT latency and gray matter volume in the right hippocampus, r(14) = −.55, p = .03, left thalamus, r(14) = −.55, p = .03, right thalamus, r(14) = −.54, p = .03, and right medial orbitofrontal volume, r(15) = −.56, p = .02 (Figure 4). There was also a significant negative association between total vMWT distance and gray matter volume in the right hippocampus, r = −.58, p = .02. Associations between regional gray matter volumes and total distance, probe time, and probe distance were not significant.
Figure 4.
Correlation between vMWT total latency and volume of the right hippocampus (A), right medial orbitofrontal cortex (B), left thalamus (C), and right thalamus (D). Effects were adjusted for age, and all correlations are significant at p < .05.
To explore age differences in the associations between spatial navigation ability and gray matter volumes, regression models were created with vMWT summary variables as dependent variables, speed of movement and ICV as covariates (entered into the model before the independent variables), and age, regional brain volume, and an age by brain volume interaction term as independent variables. The age by volume interaction term was not significant in any of these analyses (p’s > .05). Given that many studies of age differences in spatial learning and memory assess effects in different age groups, we split the sample into middle-aged (< 60 years; N = 9; 3 males) and older (≥ 60 years; N = 13; 6 males) adults. In middle-aged adults, there was a significant negative correlation between total vMWT latency and gray matter volume in the right hippocampus, r(4) = −.80, p = .03; left thalamus, r(4) = −.82, p = .02; and right thalamus, r(4) = −.77, p = .04. In contrast, older adults showed no significant relationship between vMWT performance and right hippocampal volume or bilateral thalamus volumes. However, there was a significant negative correlation between total vMWT latency and right medial orbitofrontal volume, r(8) = −.77, p = .005. There were no significant age differences in associations between vMWT total distance or probe trial performance and regional brain volumes.
3.3. vMWT Performance and Brain Metabolites
We performed correlations between vMWT summary variables and metabolite concentrations (expressed as ratios with Cr), controlling for age and vMWT speed of movement. After correcting for multiple comparisons, there were no significant correlations between vMWT variables and brain metabolite concentrations (p’s > .1).
3.4. vMWT Performance and White Matter Microstructure
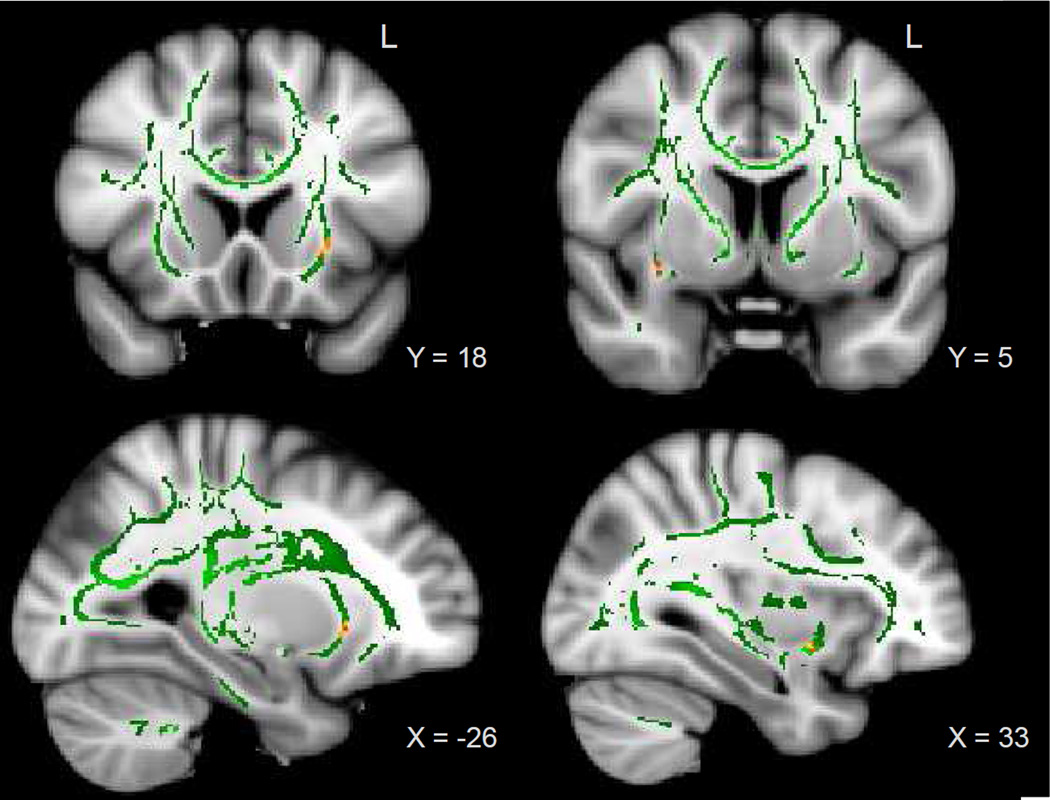
Voxelwise correlations between vMWT performance (total latency, total distance, and probe trial percent latency and distance in goal quadrant) and measures of WM microstructural organization (FA, MD, axial diffusivity [AD], and radial diffusivity [RD])) were performed, entering age and vMWT speed of movement as covariates. No clusters showing a correlation between age and FA were significant at the p < .001 level (uncorrected), but there was a significant positive association between age and MD in the splenium of the corpus callosum (p < .001, uncorrected). This should be interpreted cautiously, as MD tends to have lower reliability than other diffusion measures (Duan, Zhao, He, & Shu, 2015; Heiervang, Behrens, Mackay, Robson, & Johansen-Berg, 2006). There was a significant negative association between FA and vMWT latency in the left (p < .001) and right (p < .001) uncinate fasciculus (Figure 5) after adjusting for age and speed of movement. No other associations between measures of WM microstructural organization (including AD or RD) and vMWT summary variables were significant.
Figure 5.
TBSS results: Mean FA skeleton (green) overlaid on MNI152 template brain. Voxels with a significant negative correlation between total vMWT latency and FA (red-yellow), controlling for age and speed of movement, p < .001. Significant clusters can be observed in the left (left panel) and right (right panel) uncinate fasciculus.
4. DISCUSSION
The present study is the first investigation of virtual navigation assessing age-related changes in performance over a period of several years. While a prior investigation by Lövdén et al. (2012) used a longitudinal study design to investigate spatial navigation, the study involved four months of active vMWT training and had a much shorter (four month) follow-up interval. In the present study, despite cross-sectional effects of age on vMWT performance at baseline and follow-up, there was no significant longitudinal decrement in spatial learning and memory over an average interval of eight years. Nonetheless, neuroimaging data acquired at follow-up demonstrated that smaller gray matter volume in the right hippocampus, right medial orbitofrontal cortex, and bilateral thalamus and decreased WM fractional anisotropy in the uncinate fasciculus were associated with poorer spatial learning and memory.
Previous behavioral studies have demonstrated significant age deficits in spatial navigation abilities in both rodents (Gage et al., 1984; Gage, Chen, Buzsaki, & Armstrong, 1988; Geinisman et al., 1995; Geinisman, de Toledo-Morrell, & Morrell, 1986; Gallagher & Nicolle, 1993) and humans (Driscoll et al., 2005; Moffat & Resnick, 2002; Moffat, Zonderman, & Resnick, 2001; Lithfous, Dufour, & Després, 2013.). Many of these studies compare only two age groups (young and old) often leaving out the middle-aged, further limiting ability to draw inferences about within-individual trajectories of age-related decline in spatial navigation performance. One of the few studies to use middle-aged individuals as a separate comparison group assessed age differences in vMWT performance between young (20–39 years), middle-aged (40–59 years), and older adults (60+ years) (Driscoll et al., 2005). There were significant differences in spatial cognition between the three groups, with older age associated with poorer navigation performance. Although middle-aged individuals performed intermediately between the other two groups, their performance more closely resembled older adults than younger adults. Another cross-sectional study with participants ranging from 25 to 93 years of age found a quadratic relationship between age and total distance traveled on vMWT learning trials, with sharper age differences in older individuals (Moffat & Resnick, 2002). Probe trial performance showed that young adults traveled the greatest distance in the goal quadrant, followed by middle-aged and then older adults. Again, performance in the middle-aged group (45–65 years) more closely resembled that of older than younger participants. Collectively, these findings suggest that spatial learning and memory may begin to decline as early as the 30s and 40s, continuing a downward trajectory into older adulthood.
The current investigation found no evidence of longitudinal age-related decline in spatial navigation performance nor decline in 8 of the 10 neuropsychological tests administered, whereas two of the neuropsychological tests assessing verbal and visual memory did show longitudinal change. There are several potential explanations for these null findings. Although total latency to complete the vMWT learning trials showed a non-significant increase over the eight-year follow-up interval, there was significant inter-individual variability in performance change over time. This heterogeneity reduced statistical power to detect age-related changes in vMWT performance. Interestingly, visual inspection of the data indicates that the participants showing the greatest increases in vMWT latency over the follow-up interval were middle-aged at baseline, although no statistical effect of age on change in navigation performance was observed. The high degree of intra-individual variability in the current sample could be a consequence of the aging process. Alternatively, it may reflect poor test-retest reliability of the vMWT. Despite being proposed as a potential biomarker of age-related cognitive decline (e.g., Lithfous, Doufour, & Després, 2013), there is little available evidence about the psychometric properties of the vMWT. In the current sample, test-retest reliability is impossible to disentangle from age-related effects over the 8-year follow-up interval. Thus, additional investigation into the psychometric properties of the vMWT is needed for this measure to have future clinical utility.
Sample characteristics may also have contributed to a lack of detectable longitudinal change in vMWT performance. Although the age of the sample ranged from 30 to 83 at baseline, 53% were under age 60 but only 19% were under the age of 50. This may have reduced our power to detect changes in spatial cognition that may occur in the 30s or 40s. While the larger effects on tests of episodic memory may be detectable despite this selection bias, smaller effects may be missed. It is also possible that practice effects may have led to an underestimation of age-related changes, but this is not a fully satisfactory explanation for the lack of longitudinal change on the vMWT. Participants in the present study completed the vMWT at two time points an average of eight years apart. It is unlikely that practice effects contributed significantly to performance over this relatively lengthy follow-up interval. Another factor that may have limited power to detect age-related changes in vMWT performance is non-random attrition. Of the original 86 participants, we were able to recruit 52 at follow-up. Although there were no significant differences in sex, age at baseline, education, depression scores, or MMSE scores between participants who returned for follow-up versus those who did not, there were differences on several neuropsychological tests, which suggests that attrition may have been non-random. Additionally, the BLSA sample is not population-based. The majority of the sample is Caucasian, highly educated, has good access to medical care, and has remained relatively healthy over the follow-up interval, which may not be representative of the general population of normally aging adults. Finally, the BLSA provides prospective physical, neurologic, and neuropsychological data as well as informant reports that allowed us to exclude any participants who went on to develop MCI or possible or probable AD during the follow-up interval. Hence, this sample is likely more reflective of true healthy aging compared to other cross-sectional studies that may include individuals who are in pre-clinical or early stages of impairment. These factors combined may have decreased sensitivity for detection of age-related decline on the vMWT.
Consistent with translational evidence from rodents and other studies of human spatial learning (Morris et al., 1982; Driscoll et al., 2003; Head & Isom, 2010), the present study demonstrated an association between more effective spatial navigation and larger right hippocampal volume. The right hippocampus is thought to be particularly important for use of allocentric spatial representations, which are needed for effective navigation in the vMWT (Iglói et al., 2010). For example, Bohbot et al. (2007) found that people who used a spontaneous spatial memory strategy had higher right hippocampal volume compared to those using a response strategy. Lower FA in the right hippocampus, an indication of microstructural disorganization of tissue, has been associated with slower formation of a cognitive map and less efficient use of cognitive maps during navigation (Iaria et al., 2008). Additionally, navigation impairment in patients with MCI and AD has been associated with lower right hippocampal volume in several studies (Nedelska et al., 2012; deIpolyi et al., 2007).
In the present study, after splitting the sample into separate age groups, the association between vMWT total latency and right hippocampal volume was seen only in middle-aged individuals but was not present in older adults. This age difference in brain-behavior relationship is consistent with research showing that older adults are less likely to use spatial strategies, which are hippocampal-dependent (Rodgers, Sidone, & Moffat, 2012; Wiener et al., 2013). A cross-sectional study of spatial strategy use across the lifespan found that the proportion of individual who use allocentric strategies steadily declines from childhood to older adulthood (Bohbot et al., 2012). Instead, older adults shift toward using response strategies, such as learning a specific pattern of movements from a given start point. Whereas allocentric, or spatial, strategies depend on the hippocampus (Moffat et al., 2006; Antonova et al., 2009; Rodgers, Sidone, & Moffat, 2012), response strategies involve the striatum (Packard & McGaugh, 1992; Iaria et al., 2003; Konishi et al., 2013). However, as this study was not designed to assess age-related strategy differences in spatial navigation, there was no control task involving egocentric processing or use of a response strategy. Although our findings broadly support the research indicating that older adults show a decrement in allocentric processing that is related to hippocampal impairment, in the absence of a relationship in older adults between vMWT performance and volume of the caudate nucleus or other extrahippocampal structures, these findings should be interpreted with caution.
In addition to quantifying gray matter volume, we assessed metabolite concentrations in the hippocampus. A previous investigation (Driscoll et al., 2003) found that NAA/Cr, a putative neuronal marker, was lower in older adults and was negatively correlated with percentage of time spent in the goal quadrant on the probe trial of the vMWT. In the present sample, there were no significant associations between hippocampal metabolite concentrations and vMWT performance. However, the previous study differed from the current investigation in several important ways. The prior study compared young and older adults, which may have magnified age effects compared to our current sample that included middle-aged and older adults. The difference in results between these two studies may reflect methodological differences in general and more specifically, differences in participant sampling. Most cross-sectional studies of normal older individuals may be contaminated with participants in preclinical stages of impairment that goes unnoticed due to inherent lack of prospective information and diagnosis available for the present sample. Loss of neuropil or glial cell bodies may contribute to gray matter atrophy, while NAA/Cr concentrations remain within the range expected during healthy aging (Driscoll et al., 2006).
This study also points to the role of prefrontal regions in facilitating effective spatial navigation. There was a significant association between right medial orbitofrontal cortex volume and vMWT performance. Human studies have found that the medial orbitofrontal cortex is involved in flexibly updating associations between stimuli and rewarded responses (Elliott, Dolan, & Frith, 2000; Plassmann, O’Doherty, & Rangel, 2010). In rodents, the orbitofrontal cortex encodes goal locations and actions required to achieve the goal, such as choosing a heading direction to reach an escape platform (Feierstein, Quirk, Uchida, Sosulski, & Mainen, 2006). One study demonstrated that inducing a reversible lesion of the rat’s orbitofrontal cortex interfered with both acquisition and consolidation of spatial memory in the MWT (Vafaei & Rashidy-Pour, 2004). In humans, more effective navigation performance has been associated with larger volumes of prefrontal cortex more generally (Moffat et al., 2007) and the medial orbitofrontal cortex specifically (Bohbot et al., 2007). Additionally, a recent investigation by Dahmani and Bohbot (2015) showed that in younger adults, use of a spatial strategy was associated with increased BOLD activity and gray matter density in the medial prefrontal cortex, particularly in the orbitofrontal cortex. The current study extends these findings into older age, suggesting that medial orbitofrontal cortex may support navigation throughout the lifespan. One possibility is that these prefrontal regions may be involved in selection and initiation of navigation strategies, although additional research is needed to test this hypothesis.
Importantly, the DTI findings relating decreased FA in the left and right uncinate fasciculus to poorer vMWT performance are congruent with the observed pattern of gray matter volumetric associations. Fibers of the uncinate fasciculus originate from the temporal lobe, connecting the anterior temporal regions with the orbitofrontal cortex (Catani & de Schotten, 2012). Although the function of the uncinate fasciculus is not well understood, it is thought to play a role in episodic memory formation and retrieval (Diehl et al., 2008; Levine et al., 1998). Differences in microstructural integrity of the uncinate fasciculus have been observed in Alzheimer’s disease (Kiuchi et al., 2009; Yasmin et al., 2008; Larroza et al., 2014; Serra et al., 2012), with FA in the bilateral uncinate fasciculus negatively associated with severity of cognitive impairment (Morikawa et al., 2010). While the findings in the present study did not survive correction for family-wise error, they provide suggestive evidence that microstructural differences in the uncinate fasciculus may contribute to age-related spatial navigation impairment. As navigation has been proposed as a potential biomarker of risk of age-related cognitive impairment (Lithfous, Dufour, & Després, 2013), further characterizing white matter pathways that subserve navigation abilities may highlight potential targets for early diagnosis or intervention. Additionally, more research is needed to determine how prefrontal cortical areas such as the medial orbitofrontal cortex interact with subcortical regions to coordinate goal-directed behavior, select and employ search strategies, and flexibly update spatial representations due to changing task demands.
In conclusion, the present study identified cross-sectional age differences in performance on a place learning task, while longitudinal performance was characterized by considerable intra-and inter-individual variability. Navigation ability was associated with volumetric differences in cortical and subcortical gray matter areas involved in spatial memory and goal-directed behavior as well as white matter pathways implicated in episodic memory. Additional investigation is needed to better characterize the behavioral and neuroanatomical features of spatial navigation deficits that may begin as early as middle age.
HIGHLIGHTS.
We assessed longitudinal change in vMWT performance over a period of 8 years.
No longitudinal decline in spatial learning and memory was observed at follow-up.
Cross-sectionally, older adults showed impaired vMWT performance.
Place learning correlates with indices of gray and white matter integrity.
ACKNOWLEDGMENTS
This research was supported by the Intramural Research Program of the National Institute on Aging, National Institutes of Health.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abe O, Aoki S, Hayashi N, Yamada H, Kunimatsu A, Mori H, Yoshikawa T, Okubo T, Ohtomo K. Normal aging in the central nervous system: quantitative MR diffusion-tensor analysis. Neurobiol Aging. 2002;23:433–441. doi: 10.1016/s0197-4580(01)00318-9. [DOI] [PubMed] [Google Scholar]
- Angelie E, Bonmartin A, Boudraa A, Gonnaud P-M, Mallet J-J, Sappey-Marinier D. Regional differences and metabolic changes in normal aging of the human brain: proton MR spectroscopic imaging study. AJNR Am J Neuroradiol. 2001;22:119–127. [PMC free article] [PubMed] [Google Scholar]
- Antonova E, Parslow D, Brammer M, Dawson GR, Jackson SHD, Morris RG. Age-related neural activity during allocentric spatial memory. Memory. 2009;17:125–143. doi: 10.1080/09658210802077348. [DOI] [PubMed] [Google Scholar]
- Astur RS, Taylor LB, Mamelak AN, Philpot L, Sutherland RJ. Humans with hippocampus damage display severe memory impairments in a virtual Morris water task. Behav Brain Res. 2002;132:77–84. doi: 10.1016/s0166-4328(01)00399-0. [DOI] [PubMed] [Google Scholar]
- Barrick TR, Charlton RA, Clark CA, Markus HS. White matter structural decline in normal ageing: a prospective longitudinal study using tract-based spatial statistics. Neuroimage. 2010;51:565–577. doi: 10.1016/j.neuroimage.2010.02.033. [DOI] [PubMed] [Google Scholar]
- Bartsch T, Schönfeld R, Müller FJ, Alfke K, Leplow B, Aldenhoff J, Deuschl G, Koch JM. Focal lesions of human hippocampal CA1 neurons in transient global amnesia impair place memory. Science. 2020;328:1412–1415. doi: 10.1126/science.1188160. [DOI] [PubMed] [Google Scholar]
- Behrens TEJ, Woolrich MW, Jenkinson M, Johansen-Berg H, Nunes RG, Clare S, Matthews PM, Brady JM, Smith SM. Characterization and propagation of uncertainty in diffusion-weighted MR imaging. Magn Reson Med. 2003a;50:1077–1088. doi: 10.1002/mrm.10609. [DOI] [PubMed] [Google Scholar]
- Behrens TEJ, Johansen-Berg H, Woolrich MW, Smith SM, Wheeler-Kingshott CAM, Boulby PA, Barker GJ, Sillery EL, Sheehan K, Ciccarelli O, Thompson AJ, Brady JM, Matthews PM. Non-invasive mapping of connections between human thalamus and cortex using diffusion imaging. Nat Neurosci. 2003b;6:750–757. doi: 10.1038/nn1075. [DOI] [PubMed] [Google Scholar]
- Bennett IJ, Madden DJ, Vaidya CJ, Howard DV, Howard JH., Jr Age-related differences in multiple measures of white matter integrity: a diffusion tensor imaging study of healthy aging. Human Brain Mapp. 2010;31:378–390. doi: 10.1002/hbm.20872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett IJ, Madden DJ. Disconnected aging: cerebral white matter integrity and age-related differences in cognition. Neurosci. 2014;276:187–205. doi: 10.1016/j.neuroscience.2013.11.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benton AL. Differential behavioral effects in frontal lobe disease. Neuropsychologia. 1968;6:53–60. [Google Scholar]
- Bohbot VD, Lerch J, Thorndycraft B, Iaria G, Zijdenbos AP. Gray matter differences correlate with spontaneous strategies in a human virtual navigation task. J Neurosci. 2007;27:10078–10083. doi: 10.1523/JNEUROSCI.1763-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bohbot VD, McKenzie S, Konishi K, Fouquet C, Kurdi V, Schachar R, et al. Virtual navigation strategies from childhood to senescense: evdience for changes across the lifespan. Front Aging Neurosci. 2012;4:28. doi: 10.3389/fnagi.2012.00028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bryden KJ, Charlton JL, Oxley JA, Lowndes GJ. Self-reported wayfinding ability of older drivers. Accid Anal Prev. 2013;59:277–282. doi: 10.1016/j.aap.2013.06.017. [DOI] [PubMed] [Google Scholar]
- Burgess N. Spatial cognition and the brain. Ann N Y Acad Sci. 2008;1124:77–97. doi: 10.1196/annals.1440.002. [DOI] [PubMed] [Google Scholar]
- Burns PC. Navigation and the mobility of older drivers. J Gerontol B Psychol Sci Soc Sci. 1999;54:S49–S55. doi: 10.1093/geronb/54b.1.s49. [DOI] [PubMed] [Google Scholar]
- Catani M, de Schotten MT. Atlas of Human Brain Connections. Oxford: Oxford University Press; 2012. [Google Scholar]
- Chua TC, Wen W, Slavin MJ, Sachdev PS. Diffusion tensor imaging in mild cognitive impairment and Alzheimer’s disease: a review. Curr Opin Neurol. 2008;21:83–92. doi: 10.1097/WCO.0b013e3282f4594b. [DOI] [PubMed] [Google Scholar]
- Dahmani L, Bohbot VD. Dissociable contributions of the prefrontal cortex to hippocampus- and caudate nucleus-dependent virtual navigation strategies. Neurobiol Learn Mem. 2015;117:42–50. doi: 10.1016/j.nlm.2014.07.002. [DOI] [PubMed] [Google Scholar]
- deIpoly AR, Rankin KP, Mucke L, Miller BL, Gorno-Tempini ML. Spatial cognition and the human navigation network in AD and MCI. Neurology. 2007;69:986–997. doi: 10.1212/01.wnl.0000271376.19515.c6. [DOI] [PubMed] [Google Scholar]
- Delis DC, Kramer JH, Kaplan E, Ober BA. California Verbal Learning Test – research edition. New York: The Psychological Corporation; 1987. [Google Scholar]
- Desikan RS, Ségonne F, Fischl B, Quinn BT, Dickerson BC, Blacker D, Buckner RL, Dale AM, Maguire RP, Hyman BT, Albert MS, Killiany RJ. An automated labeling system for subdividing the human cerebral cortex on MRI scans into gyral based regions of interest. NeuroImage. 2006;31:968–980. doi: 10.1016/j.neuroimage.2006.01.021. [DOI] [PubMed] [Google Scholar]
- Devan BD, Goad EH, Petri HL. Dissociation of hippocampal and striatal contributions to spatial navigation in the water maze. Neurobiol Learn Mem. 1996;66:305–323. doi: 10.1006/nlme.1996.0072. [DOI] [PubMed] [Google Scholar]
- Diehl B, Busch RM, Duncan JS, Piao Z, Tkach J, Lüders HO. Abnormalities in diffusion tensor imaging of the uncinate fasciculus relate to reduced memory in temporal lobe epilepsy. Epilepsia. 2008;49:1409–1418. doi: 10.1111/j.1528-1167.2008.01596.x. [DOI] [PubMed] [Google Scholar]
- Dixon RM, Bradley KM, Budge MM, Styles P, Smith AD. Longitudinal quantitative proton magnetic resonance spectroscopy of the hippocampus in Alzheimer’s disease. Brain. 2002;125:2332–2341. doi: 10.1093/brain/awf226. [DOI] [PubMed] [Google Scholar]
- Driscoll I, Hamilton DA, Petropoulos H, Yeo RA, Brooks WM, Baumgartner RN, Sutherland RJ. The aging hippocampus: cognitive, biochemical and structural findings. Cereb Cortex. 2003;13:1344–1351. doi: 10.1093/cercor/bhg081. [DOI] [PubMed] [Google Scholar]
- Driscoll I, Hamilton DA, Yeo RA, Brooks WM, Sutherland RJ. Virtual navigation in humans: the impact of age, sex, and hormones on place learning. Horm Behav. 2005;47:326–335. doi: 10.1016/j.yhbeh.2004.11.013. [DOI] [PubMed] [Google Scholar]
- Driscoll I, Howard SR, Stone JC, Monfils MH, Tomanek B, Brooks WM, Sutherland RJ. Neurosci. 2006;139:1173–1185. doi: 10.1016/j.neuroscience.2006.01.040. [DOI] [PubMed] [Google Scholar]
- Duan F, Zhao T, He Y, Shu N. Test-retest reliability of diffusion measures in cerebral white matter: a multiband diffusion MRI study. J Magn Reson Imaging. 2015 doi: 10.1002/jmri.24859. epub ahead of print. [DOI] [PubMed] [Google Scholar]
- Ekstrom AD, Kahan MJ, Caplan JB, Fields TA, Isham EA, Newman EL, Fried I. Cellular networks underlying human spatial navigation. Nature. 2003;425:184–188. doi: 10.1038/nature01964. [DOI] [PubMed] [Google Scholar]
- Elliott R, Dolan RJ, Frith CD. Dissociable functions in the medial and lateral orbitofrontal cortex: evidence from human neuroimaging studies. Cereb Cortex. 2000;10:308–317. doi: 10.1093/cercor/10.3.308. [DOI] [PubMed] [Google Scholar]
- Feierstein CE, Quirk MC, Uchida N, Sosulski DL, Mainen ZF. Representation of spatial goals in rat orbitofrontal cortex. Neuron. 2006;51:495–507. doi: 10.1016/j.neuron.2006.06.032. [DOI] [PubMed] [Google Scholar]
- Fischl B, Salat DH, Busa E, Albert M, Dieterich M, Haselgrove C, et al. Whole brain segmentation: automated labeling of neuroanatomical structures in the human brain. Neuron. 2002;33:341–355. doi: 10.1016/s0896-6273(02)00569-x. [DOI] [PubMed] [Google Scholar]
- Fischl B, van der Kouwe A, Destrieux C, Halgren E, Ségonne F, Salat DH, et al. Automatically parcellating the human cerebral cortex. Cereb Cortex. 2004;14:11–22. doi: 10.1093/cercor/bhg087. [DOI] [PubMed] [Google Scholar]
- Folstein MF, Folstein SE, McHugh PR. Mini-mental state: a practical method for grading the cognitive status of patients for the clinician. J Psychiatr Res. 1975;12:189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- Gage FH, Chen KS, Buzsaki G, Armstrong D. Experimental approaches to age-related cognitive impariments. Neurobiol aing. 1988;9:645–655. doi: 10.1016/s0197-4580(88)80129-5. [DOI] [PubMed] [Google Scholar]
- Gage FH, Dunnett SB, Björklund A. Spatial learning and motor deficits in aged rats. Neurobiol Aging. 1984;5:43–48. doi: 10.1016/0197-4580(84)90084-8. [DOI] [PubMed] [Google Scholar]
- Gallagher M, Nicolle MM. Animal models of normal aging: relationship between cognitive decline and markers in hippocampal circuitry. Behav Brain Res. 1993;57:155–162. doi: 10.1016/0166-4328(93)90131-9. [DOI] [PubMed] [Google Scholar]
- Gazova I, Vlcek K, Laczó J, Nedelska Z, Hyncicova E, Mokrisova I, et al. Spatial navigation: a unique window into physiological and pathological aging. Front Aging Neurosci. 2012;4:16. doi: 10.3389/fnagi.2012.00016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geinisman Y, de Toledo-Morrell L, Morrell F. Aged rats need a preserved complement of perforated axospinous synapses per hippocampal neuron to maintain good spatial memory. Brain Res. 1986;398:266–275. doi: 10.1016/0006-8993(86)91486-1. [DOI] [PubMed] [Google Scholar]
- Geinisman Y, de Toledo-Morrell L, Morrell F, Heller RE. Hippocampal markers of age-related memory dysfunction: behavioral, electrophysiological and morphological perspectives. Prog Neurobiol. 1995;45:223–252. doi: 10.1016/0301-0082(94)00047-l. [DOI] [PubMed] [Google Scholar]
- Goodrich-Hunsaker NJ, Livingstone SA, Skelton RW, Hopskins RO. Spatial deficits in a virtual water maze in amnesic participants with hippocampal damage. Hippocampus. 2010;20:481–491. doi: 10.1002/hipo.20651. [DOI] [PubMed] [Google Scholar]
- Gunning-Dixon FM, Raz N. The cognitive correlates of white matter abnormalities in normal aging: a quantitative review. Neuropsychol. 2000;14:224–232. doi: 10.1037//0894-4105.14.2.224. [DOI] [PubMed] [Google Scholar]
- Gunning-Dixon FM, Brickman AM, Cheng JC, Alexopoulos GS. Aging of cerebral white matter: a review of MRI findings. Int J Geriatr Psychiatry. 2009;24:109–117. doi: 10.1002/gps.2087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guttmann CRG, Jolesz FA, Kikinis R, Killiany RJ, Moss MB, Sandor T, Albert MS. White matter changes with normal aging. Neurol. 1998;50:972–978. doi: 10.1212/wnl.50.4.972. [DOI] [PubMed] [Google Scholar]
- Hamilton DA, Driscoll I, Sutherland RJ. Human place learning in a virtual Morris water task: some important constraints on the flexibility of place navigation. Behav Brain Res. 2002;129:159–170. doi: 10.1016/s0166-4328(01)00343-6. [DOI] [PubMed] [Google Scholar]
- Head D, Isom M. Age effects on wayfinding and route learning skills. Behav Brain Res. 2010;209:49–58. doi: 10.1016/j.bbr.2010.01.012. [DOI] [PubMed] [Google Scholar]
- Heiervang E, Behrens TEJ, Mackay CE, Robson MD, Johansen-Berg H. Between session reproducibility and between subject variability of diffusion MR and tractography measures. Neuroimage. 2006;33:867–877. doi: 10.1016/j.neuroimage.2006.07.037. [DOI] [PubMed] [Google Scholar]
- Hort J, Laczó J, Vyhnálek M, Bojar M, Bureš J, Vlček K. Spatial navigation deficit in amnestic mild cognitive impairment. Proc Natl Acad Sci U S A. 2007;104:4042–4047. doi: 10.1073/pnas.0611314104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iaria G, Petrides M, Dagher A, Pike B, Bohbot VD. Cognitive strategies dependent on the hippocampus and caudate nucleus in human navigation: variability and change with practice. J Neurosci. 2003;23:5945–5952. doi: 10.1523/JNEUROSCI.23-13-05945.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iglói K, Doeller CF, Berthoz A, Rondi-Reig L, Burgess N. Lateralized human hippocampal activity predicts navigation based on sequence or place memory. Proc Natl Acad Sci U S A. 2010;107:14466–14471. doi: 10.1073/pnas.1004243107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawas C, Gray S, Brookmeyer R, Fozard J, Zonderman A. Age-specific incidence rates of Alzheimer’s disease: the Baltimore Longitudinal Study of Aging. Neurol. 2000;54:2072–2077. doi: 10.1212/wnl.54.11.2072. [DOI] [PubMed] [Google Scholar]
- Kiuchi K, Morikawa M, Taoka T, Nagashima T, Yamauchi T, Makinodan M, et al. Abnormalities of the uncinate fasciculus and posterior cingulate fasciculus in mild cognitive impairment and early Alzheimer’s disease: a diffusion tensor tractography study. Brain Res. 2009;1287:184–191. doi: 10.1016/j.brainres.2009.06.052. [DOI] [PubMed] [Google Scholar]
- Konishi K, Etchamendy N, Roy S, Marighetto A, Rajah N, Bohbot VD. Decreased fMRI activity in the hippocampus in favor of the caudate nucleus in older adults tested in a virtual navigation task. Hippocampus. 2013;23:1005–1014. doi: 10.1002/hipo.22181. [DOI] [PubMed] [Google Scholar]
- Laczó J, Vlcek K, Vyhnálek M, Vajnerová O, Ort M, Holmerová I, et al. Spatial navigation testing discriminates two types of amnestic mild cognitive impairment. Behav Brain Res. 2009;202:252–259. doi: 10.1016/j.bbr.2009.03.041. [DOI] [PubMed] [Google Scholar]
- Larroza A, moratal D, D’ocón Alcañiz V, Arana E Alzheimer’s Disease Neuroimaging Initiative. Tractography of the uncinate fasciculus and the posterior cingulate fasciculus in patients with mild cognitive impairment and Alzheimer disease. Neurologia. 2014;29:11–20. doi: 10.1016/j.nrl.2013.02.002. [DOI] [PubMed] [Google Scholar]
- Ledoux AA, Boyer P, Phillips JL, Labelle A, Smith A, Bohbot VD. Structural hippocampal anomalies in a schizophrenia population correlate with navigation performance on a wayfinding task. Front Behav Neruosci. 2014;8:88. doi: 10.3389/fnbeh.2014.00088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levin B, Black SE, Cabeza R, Sinden M, Mcintosh AR, Toth JP, et al. Episodic memory and the self in a case of isolated retrograde amnesia. Brain. 1998;121:1951–1973. doi: 10.1093/brain/121.10.1951. [DOI] [PubMed] [Google Scholar]
- Lithfous S, Dufour A, Després O. Spatial navigation in normal aging and the prodromal stage of Alzheimer’s disease: insights from imaging and behavioral studies. Ageing Res Rev. 2013;12:201–213. doi: 10.1016/j.arr.2012.04.007. [DOI] [PubMed] [Google Scholar]
- Lövdén M, Schaefer S, Noack H, Bodammer NC, Kühn S, Heinze HJ, Düzel E, Bäckman L, Lindenberger U. Spatial navigation training protects the hippocampus against age-related changes during early and late adulthood. Neurobiol Aging. 2012;33:620.e9–620.e22. doi: 10.1016/j.neurobiolaging.2011.02.013. [DOI] [PubMed] [Google Scholar]
- Maguire EA, Burgess N, Donnett JG, Frackowiak RSJ, Frith SD, O’Keefe J. Knowing where and getting there: a human navigation network. Science. 1998;280:921–924. doi: 10.1126/science.280.5365.921. [DOI] [PubMed] [Google Scholar]
- Manikandan S. Data transformation. J Pharmacol Pharmacother. 2010;1:126–127. doi: 10.4103/0976-500X.72373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moffat SD. Aging and spatial navigation: what do we know and where do we go? Neuropsychol Rev. 2009;19:478–489. doi: 10.1007/s11065-009-9120-3. [DOI] [PubMed] [Google Scholar]
- Moffat SD, Elkins W, Resnick SM. Age differences in the neural systems supporting human allocentric spatial navigation. Neurobiol Aging. 2006;27:965–972. doi: 10.1016/j.neurobiolaging.2005.05.011. [DOI] [PubMed] [Google Scholar]
- Moffat SD, Kennedy KM, Rodriguez KM, Raz N. Extrahippocampal contributions to age differences in human spatial navigation. Cereb Cortex. 2007;17:1274–1282. doi: 10.1093/cercor/bhl036. [DOI] [PubMed] [Google Scholar]
- Moffat SD, Resnick SM. Effects of age on virtual environment place navigation and allocentric cognitive mapping. Behav Neurosci. 2002;116:851–859. doi: 10.1037//0735-7044.116.5.851. [DOI] [PubMed] [Google Scholar]
- Moffat SD, Zonderman AB, Resnick SM. Age differences in spatial memory in a virtual environment navigation task. Neurobiol Aging. 2001;22:787–796. doi: 10.1016/s0197-4580(01)00251-2. [DOI] [PubMed] [Google Scholar]
- Moffett JR, Ross B, Arun P, Madhavarao CN, Namboodiri AM. N-Acetylaspartate in the CNS: from neurodiagnostics to neurobiology. Prog Neurobiol. 2007;81:89–131. doi: 10.1016/j.pneurobio.2006.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mori S, Wakana S, Van Zijl PC, Nagae-Poetscher L. MRI atlas of human white matter. Waltham, MA: Academic Press; 2005. [DOI] [PubMed] [Google Scholar]
- Morikawa M, Kiuchi K, Taoka T, Nagauchi K, Kichikawa K, Kishimoto T. Uncinate fasciculus-correlated cognition in Alzheimer’s disease: a diffusion tensor imaging study by tractography. Psychogeriatrics. 2010;10:15–20. doi: 10.1111/j.1479-8301.2010.00312.x. [DOI] [PubMed] [Google Scholar]
- Morris R. Spatial localization does not require the presence of local cues. Learn Motiv. 1981;12:239–260. [Google Scholar]
- Morris R. Developments of a water-maze procedure for studying spatial learning in the rat. J Neurosci Methods. 1984;11:47–60. doi: 10.1016/0165-0270(84)90007-4. [DOI] [PubMed] [Google Scholar]
- Morris R, Garrud P, Rawlins JNP, O’Keefe J. Place navigation impaired in rats with hippocampal lesions. Nature. 1982;297:681–683. doi: 10.1038/297681a0. [DOI] [PubMed] [Google Scholar]
- Naggara O, Oppenheim C, Rieu D, Raoux N, Rodrigo S, Dalla Barba G, Meder J-F. Diffusion tensor imaging in early Alzheimer’s disease. Psychiatry Res. 2006;146:243–249. doi: 10.1016/j.pscychresns.2006.01.005. [DOI] [PubMed] [Google Scholar]
- Nedelska Z, Andel R, Laczó J, Vlcek K, Horinek D, Lisy J, et al. Spatial navigation impairment is proportional to right hippocampal volume. Proc Natl Acad Sci U S A. 2012;109:2590–2594. doi: 10.1073/pnas.1121588109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newcombe F. A study of psychological deficits. London: Oxford University Press; 1969. Missile wounds of the brain. [Google Scholar]
- Newman MC, Kaszniak AW. Spatial memory and aging: performance on a human analog of the Morris water maze. Neuropsychol Dev Cogn B Aging Neuropsychol Cogn. 2000;7:86–93. [Google Scholar]
- Packard MG, McGaugh JL. Double dissociation of fornix and caudate nucleus lesions on acquisition of two water maze tasks: further evidence for multiple memory systems. Behav Neurosci. 1992;10:439–446. doi: 10.1037//0735-7044.106.3.439. [DOI] [PubMed] [Google Scholar]
- Plassman H, O’Doherty JP, Rangel A. Appetitive and aversive goal values are encoded in the medial orbitofrontal cortex at the time of decision making. J Neurosci. 2010;30:10799–10808. doi: 10.1523/JNEUROSCI.0788-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Provencher SW. Automatic quantitation of localized in vivo 1H spectra with LCModel. NMR Biomed. 2001;14:260–264. doi: 10.1002/nbm.698. [DOI] [PubMed] [Google Scholar]
- Redish A, Touretzky DS. The role of the hippocampus in solving the Morris Water Maze. Neral Comput. 1998;10:73–111. doi: 10.1162/089976698300017908. [DOI] [PubMed] [Google Scholar]
- Reitan RM. Trail Making Test: Manual for Administration and Scoring. Reitan Neuropsychology Laboratory. 1992 [Google Scholar]
- Rodgers MK, III, Sindone JA, Moffat SD. Effects of age on navigation strategy. Neurobiol Aging. 2012;33:202.e15–202.22. doi: 10.1016/j.neurobiolaging.2010.07.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuff N, Amend D, Ezekiel F, Steinman SK, Tanabe J, Norman D, et al. Changes of hippocampal N-acetyl aspartate and volume in Alzheimer’s disease. A proton MR spectroscopic imaging and MRI study. Neurol. 1997;49:1513–1521. doi: 10.1212/wnl.49.6.1513. [DOI] [PubMed] [Google Scholar]
- Schuff N, Amend DL, Knowlton R, Norman D, Fein G, Weiner MW. Age-related metabolite changes and volume loss in the hippocampus by magnetic resonance spectroscopy and imaging. Neurobiol Aging. 1999;20:279–285. doi: 10.1016/s0197-4580(99)00022-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz CG, Reid RI, Gunter JL, Senjem ML, Przybelski SA, Zuk SM, Whitwell JL, Vemuri P, Josephs KA, Kantarci K, Thompson PM, Petersen RC, Jack CR., Jr Improved DTI registration allows voxel-based analysis that outperforms Tract-Based Spatial Statistics. NeuroImage. 2014;94:65–78. doi: 10.1016/j.neuroimage.2014.03.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serra L, Cercignani M, Basile B, Spano B, Perri R, Fadda L, Marra C, Giubilei F, Caltagirone C, Bozzali M. White matter damage along the uncinate fasciculus contributes to cognitive decline in AD and DLB. Curr Alzheimer Res. 2012;9:326–333. doi: 10.2174/156720512800107555. [DOI] [PubMed] [Google Scholar]
- Shock NW, Greulich RC, Andres R, Arenberg D, Costa PT, Jr, Lakatta E, Tobin JD. Normal human aging: the Baltimore Longitudinal Study of Aging. Washington, D.C.: U.S. Government Printing Office; 1984. [Google Scholar]
- Smith SM. Fast robust automated brain extraction. Human Brain Mapping. 2002;17:143–155. doi: 10.1002/hbm.10062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith SM, Jenkinson M, Johansen-Berg H, Rueckert D, Nichols TE, Mackay CE, et al. Tract-based spatial statistics: voxelwise analysis of multi-subject diffusion data. Neuroimage. 2006;31:1487–1505. doi: 10.1016/j.neuroimage.2006.02.024. [DOI] [PubMed] [Google Scholar]
- Sullivan EV, Pfefferbaum A. Diffusion tensor imaging in normal aging and neuropsychiatric disorders. Eur J Radiol. 2003;45:244–255. doi: 10.1016/s0720-048x(02)00313-3. [DOI] [PubMed] [Google Scholar]
- Vafaei AA, Rashidy-Pour A. Reversible lesion of the rat’s orbitofrontal cortex interferes with hippocampus-dependent spatial memory. Behav Brain Res. 2004;149:61–68. doi: 10.1016/s0166-4328(03)00209-2. [DOI] [PubMed] [Google Scholar]
- Voermans NC, Magnus Petersson K, Daudey L, Weber B, vn Spaendonck KP, Kremer HPH, Fernández G. Interaction between human hippocampus and the caudate nucleus during route recognition. Neuron. 2004;43:427–435. doi: 10.1016/j.neuron.2004.07.009. [DOI] [PubMed] [Google Scholar]
- Wechsler D. Wechsler Adult Intelligence Scale–Revised (WAIS–R) New York: Psychological Corporation; 1981. [Google Scholar]
- West MJ, Coleman PD, Flood DG, Troncoso JC. Differences in the pattern of hippocampal neuronal loss in normal ageing and Alzheimer’s disease. Lancet. 1994;344:769–772. doi: 10.1016/s0140-6736(94)92338-8. [DOI] [PubMed] [Google Scholar]
- Wiener JM, de Condappa O, Harris MA, Wolbers T. Maladaptive bias for extrahippocampal navigation strategies in aging humans. J Neurosci. 2013;33:6012–6017. doi: 10.1523/JNEUROSCI.0717-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson JR, DeFries JC, McClearn GE, Vandenberg SG, Johnson RC, Rashad MN. Cognitive abilities: use of family data as a control to assess sex and age differences in two ethnic groups. Int J Aging Hum Dev. 1975;6:261–276. doi: 10.2190/BBJP-XKUG-C6EW-KYB7. [DOI] [PubMed] [Google Scholar]
- Winkler AM, Ridgway GR, Webster MA, Smith SM, Nichols TE. Permutation inference for the general linear model. Neuroimage. 2014;92:381–397. doi: 10.1016/j.neuroimage.2014.01.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolbers T, Wiener JM. Challenges for identifying the neural mechanisms that support spatial navigation: the impact of spatial scale. Front Hum Nuerosci. 8:571. doi: 10.3389/fnhum.2014.00571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yasmin H, Nakata Y, Aoki S, Abe O, Sato N, Nemoto K, et al. Diffusion abnormalities of the uncinate fasciculus in Alzheimer’s disease: diffusion tensor tract-specific analysis using a new method to measure the core of the tract. Neuroradiol. 2008;50:293–299. doi: 10.1007/s00234-007-0353-7. [DOI] [PubMed] [Google Scholar]
- Yoon B, Shim YS, Lee KS, Shon YM, Yang DW. Region-specific changes of cerebral white matter during normal aging: a diffusion-tensor analysis. Arch Gerontol Geriatr. 2008;47:129–138. doi: 10.1016/j.archger.2007.07.004. [DOI] [PubMed] [Google Scholar]