Abstract
The genus Cladosporium (Cladosporiaceae, Dothideomycetes), which represents one of the largest genera of dematiaceous hyphomycetes, has been intensively investigated during the past decade. In the process, three major species complexes (C. cladosporioides, C. herbarum and C. sphaerospermum) were resolved based on morphology and DNA phylogeny, and a monographic revision of the genus (s. lat.) published reflecting the current taxonomic status quo. In the present study a further 19 new species are described based on phylogenetic characters (nuclear ribosomal RNA gene operon, including the internal transcribed spacer regions ITS1 and ITS2, as well as partial actin and translation elongation factor 1-α gene sequences) and morphological differences. For a selection of the species with ornamented conidia, scanning electron microscopic photos were prepared to illustrate the different types of surface ornamentation. Surprisingly, during this study Cladosporium ramotenellum was found to be a quite common saprobic species, being widely distributed and occurring on various substrates. Therefore, an emended species description is provided. Furthermore, the host range and distribution data for several previously described species are also expanded.
Key words: Cladosporiaceae, Emendation, Phylogeny, Taxonomic novelties, Taxonomy
Taxonomic novelties: New species: Cladosporium aciculare Bensch, Crous & U. Braun; C. aggregatocicatricatum Bensch, Crous & U. Braun; C. angustiherbarum Bensch, Crous & U. Braun; C. angustiterminale Bensch, Crous & U. Braun; C. austroafricanum Bensch, Crous & U. Braun; C. austrohemisphaericum Bensch, Crous & U. Braun; C. ipereniae Bensch, Crous & U. Braun; C. limoniforme Bensch, Crous & U. Braun; C. longicatenatum Bensch, Crous & U. Braun; C. longissimum Bensch, Crous & U. Braun; C. montecillanum Bensch, Crous & U. Braun; C. parapenidielloides Bensch, Crous & U. Braun; C. penidielloides Bensch, Crous & U. Braun; C. pseudochalastosporoides Bensch, Crous & U. Braun; C. puyae Bensch, Crous & U. Braun; C. rhusicola Bensch, Crous & U. Braun; C. ruguloflabelliforme Bensch, Crous & U. Braun; C. rugulovarians Bensch, Crous & U. Braun; C. versiforme Bensch, Crous & U. Braun
Introduction
Members of the genus Cladosporium are dematiaceous hyphomycetes characterised by a unique coronate structure of the conidiogenous loci and conidial hila, consisting of a central convex dome surrounded by a raised periclinal rim (David 1997). The genus belongs in a separate family, the Cladosporiaceae, which is a sister family to Mycosphaerellaceae and Teratosphaeriaceae, residing in the Capnodiales (Dothideomycetes) (Schoch et al., 2006, Schoch et al., 2009a, Schoch et al., 2009b, Crous et al., 2009b, Quaedvlieg et al., 2014). Based on comprehensive revisions of numerous cladosporioid genera, Cladosporium is now both phylogenetically and morphologically defined and delimited against numerous morphologically similar but distinct genera to which various excluded former Cladosporium species have been reallocated (Crous et al., 2006, Crous et al., 2007a, Schubert et al., 2007a, Braun et al., 2008). Furthermore, a polyphasic approach was undertaken to establish species entities in the three major species complexes within Cladosporium, viz. C. herbarum s. lat., C. sphaerospermum s. lat. and C. cladosporioides s. lat. (Zalar et al., 2007, Schubert et al., 2007b, Bensch et al., 2010). A surprising result of these studies was the high diversity of species and genotypes that exist in nature in formerly accepted, morphologically defined species. Most recently, a monographic revision of Cladosporium s. lat. has been published, comprising about 170 true Cladosporium species and reflecting the current status quo (Bensch et al. 2012).
However, examination of the diversity, phylogeny and taxonomy of Cladosporium on various host substrates from different geographical regions is still ongoing, and the present paper represents an additional contribution highlighting the huge biodiversity in Cladosporium. A multilocus DNA sequence typing approach, employing three loci (the internal transcribed spacers of the rDNA genes (ITS), and partial actin and translation elongation factor 1-α gene sequences), as well as morphological examinations and cultural characteristics were used for the identification and delimitation of several new species.
Material and methods
Isolates
Isolates included in this study were obtained from the culture collection of the Centraalbureau voor Schimmelcultures (CBS-KNAW Fungal Biodiversity Centre), Utrecht, Netherlands, and from several other collections (Table 1), or were freshly isolated from a range of different substrates and placed in the working collection of Pedro Crous (CPC), housed at CBS. Single-conidial isolates were obtained using techniques outlined in Crous et al. (1991). Isolates were inoculated onto 2 % potato-dextrose agar (PDA), synthetic nutrient-poor agar (SNA), 2 % malt extract agar (MEA) and oatmeal agar (OA) (Crous et al. 2009c), and incubated under continuous near-ultraviolet light at 25 °C to promote sporulation. All cultures in this study are maintained at the CBS (Table 1). Nomenclatural novelties and descriptions were deposited in MycoBank (www.MycoBank.org; Crous et al. 2004).
Table 1.
Cladosporium isolates included for sequence and morphological analyses.
| Species | Culture accession number(s)1 | Substrate | Country | Collector | GenBank accession numbers2 |
||
|---|---|---|---|---|---|---|---|
| ITS | tef1 | act | |||||
| Cercospora beticola (outgroup) | CBS 116456 | Beta vulgaris | Italy | — | AY840527 | AY840494 | AY840458 |
| Cladosporium acalyphae | CBS 125982*; CPC 11625 | Acalypha australis | South Korea | H.D. Shin | HM147994 | HM148235 | HM148481 |
| C. aciculare | CBS 140488*; CPC 16547 | Syzygium corynanthum | Australia | P.W. Crous | KT600411 | KT600509 | KT600607 |
| C. aggregatocicatricatum | CBS 140493*; CPC 14709; ICMP 170869 | Culture contaminant | New Zealand | C.F. Hill | KT600448 | KT600547 | KT600645 |
| CBS 113751 | Grape berry | USA: Washington | F.M. Dugan lab | KT600449 | KT600548 | KT600646 | |
| CBS 284.84 | Tempeh | The Netherlands | — | KT600450 | KT600549 | KT600647 | |
| CPC 12055; EXF-2288 | Hypersaline water from precrystalisation pond | Slovenia | P. Zalar | KT600451 | KT600550 | KT600648 | |
| CPC 13365 | Asteriscus sericeus | Germany | N. Ale-Agha | KT600452 | KT600551 | KT600649 | |
| C. allicinum | CBS 121624*; CPC 12211 | Hordeum vulgare | Belgium | J.Z. Groenewald | EF679350 | EF679425 | EF679502 |
| CBS 121.47; VTT D-76045 | Food, frozen Phaseolus vulgaris | The Netherlands | — | KT600364 | KT600461 | KT600560 | |
| CBS 155.60 | Metal | The Netherlands | — | KT600365 | KT600462 | KT600561 | |
| CBS 160.59 | Man, sputum | The Netherlands | — | KT600366 | KT600463 | KT600562 | |
| CBS 188.53; IFO 5267 | — | Japan | — | KT600367 | KT600464 | KT600563 | |
| CBS 374.53; IMI 163999 | Centaurea rhapontica = Rhaponticum scariosum subsp. rhaponticum | Switzerland | — | KT600368 | KT600465 | KT600564 | |
| CBS 420.92 | Acer campestre, leaf spot | Germany | H.A. van der Aa | KT600369 | KT600466 | KT600565 | |
| CPC 12212 | Hordeum vulgare | Belgium | J.Z. Groenewald | EF679351 | EF679426 | EF679503 | |
| CPC 13146 | Puccinia bromina subsp. symphyti-bromarum var. paucispora, aecia | Germany | K. Schubert | KT600370 | KT600467 | KT600566 | |
| CPC 14194 | Outside air | The Netherlands | Applied and Industrial Mycology group, CBS | KT600371 | KT600468 | KT600567 | |
| CPC 14268 | Unidentified tree | France | P.W. Crous | KT600372 | KT600469 | KT600568 | |
| CPC 14303; BA 1702 | Food, bean | Bulgaria | B. Andersen | KT600373 | KT600470 | KT600569 | |
| CPC 16759 | Alnus glutinosa | The Netherlands | W. Quaedvlieg | KT600374 | KT600471 | KT600570 | |
| CPC 18260 | Nivenia stokoei | South Africa | P.W. Crous | KT600375 | KT600472 | KT600571 | |
| CPC 21646 | Arachis hypogaea | Senegal | M.P. Sarr | KT600376 | KT600473 | KT600572 | |
| CPC 21906 | Robinia pseudoacacia, leaf on ground | Germany | R. Jarling & R. Schumacher | KT600377 | KT600474 | KT600573 | |
| C. angustiherbarum | CBS 140479*; CPC 17814 | Pinus ponderosa | USA: Utah | W. Quaedvlieg | KT600378 | KT600475 | KT600574 |
| C. angustiterminale | CBS 140480*; CPC 15564 | Banksia grandis | Australia | A.R. Wood | KT600379 | KT600476 | KT600575 |
| C. angustisporum | CBS 125983*; CPC 12437 | Alloxylon wickhamii | Australia | B.A. Summerell | HM147995 | HM148236 | HM148482 |
| C. aphidis | CBS 132182**; CPC 13204 | On aphids | Germany | N. Ale-Agha | JN906978 | JN906985 | JN906998 |
| C. arthropodii | CBS 124043**; CPC 16160 | Arthropodium cirratum | New Zealand | C.F. Hill | JN906979 | JN906985 | JN906998 |
| C. asperulatum | CBS 126340*; CPC 14040 | Protea susannae | Portugal | P.W. Crous | HM147998 | HM148239 | HM148485 |
| CBS 126339; CPC 11158 | Eucalyptus leaf litter | India | W. Gams | HM147997 | HM148238 | HM148484 | |
| CPC 15614 | Glycine max, seeds | Mexico | M. de Jesús Yáñez-Morales | KT600380 | KT600477 | KT600576 | |
| C. australiense | CBS 125984*; CPC 13226 | Eucalyptus moluccana | Australia | B.A. Summerell | HM147999 | HM148240 | HM148486 |
| C. austroafricanum | CBS 140481*; CPC 16763 | Leaf litter | South Africa | M. Gryzenhout | KT600381 | KT600478 | KT600577 |
| C. austrohemisphaericum | CBS 140482*; CPC 12068 | Lagunaria patersonia, black mould on fruit surface | New Zealand | C.F. Hill | KT600382 | KT600479 | KT600578 |
| CPC 16250 | Cussonia thyrsiflora | South Africa | P.W. Crous | KT600383 | KT600480 | — | |
| CPC 17029 | Musa sp. | Australia | P.W. Crous | KT600384 | KT600481 | KT600579 | |
| C. basiinflatum | CBS 822.84* | Hordeum vulgare | Germany | — | HM148000 | HM148241 | HM148487 |
| C. chalastosporoides | CBS 125985*; CPC 13864 | Fruiting bodies of Teratosphaeria proteae-arboreae on leaves of Protea nitida | South Africa | P.W. Crous | HM148001 | HM148242 | HM148488 |
| C. chubutense | CBS 124457*; CPC 13979; CIEFAP 321 | Pinus ponderosa | Argentina | A. Greslebin | FJ936158 | FJ936161 | FJ936165 |
| C. cladosporioides | CBS 101367 | Soil | Brazil | — | HM148002 | HM148243 | HM148489 |
| CBS 112388* | Indoor air | Germany | Ch. Trautmann | HM148003 | HM148244 | HM148490 | |
| CBS 113738 | Grape bud | USA: Washington | F.M. Dugan lab | HM148004 | HM148245 | HM148491 | |
| CBS 113740 | Grape berry | USA: Washington | F.M. Dugan lab | HM148006 | HM148247 | HM148493 | |
| CPC 11120 | Viola mandshurica | South Korea | H.D. Shin | HM148017 | HM148258 | HM148504 | |
| CPC 11121 | Celosia cristata | South Korea | H.D. Shin | HM148018 | HM148259 | HM148505 | |
| CPC 11161 | Eucalyptus sp. | India | W. Gams | HM148022 | HM148263 | HM148509 | |
| CPC 11404 | Rubus coreanus | South Korea | H.D. Shin | HM148025 | HM148266 | HM148512 | |
| CPC 12187 | Stellaria aquatica leaves | South Korea | H.D. Shin | HM148027 | HM148268 | HM148514 | |
| CPC 12214 | Morus rubra leaves | Germany | N. Ale-Agha | HM148028 | HM148269 | HM148515 | |
| CPC 12760 | Spinach seed, Spinacia oleracea | USA: Washington | L. du Toit | HM148029 | HM148270 | HM148516 | |
| CPC 13669 | Eucalyptus robertsonii subsp. hemisphaerica | Australia | B.A. Summerell | HM148035 | HM148276 | HM148522 | |
| CPC 14021 | Wheat | South Africa | — | HM148042 | HM148283 | HM148529 | |
| CPC 14024 | Pawpaw | South Africa | — | HM148043 | HM148284 | HM148530 | |
| CPC 14244 | Magnolia sp. | USA: Louisiana | P.W. Crous | HM148044 | HM148285 | HM148531 | |
| CPC 14292; BA1691 | Soil, pea field | Denmark | B. Andersen | HM148046 | HM148287 | HM148533 | |
| CPC 14293; BA1692 | Cellulose powder, paint manufacturer | Denmark | B. Andersen | HM148047 | HM148288 | HM148534 | |
| CPC 14355; BA1676 | Food, mouldy pea | USA: Laramie | B. Andersen | HM148048 | HM148289 | HM148535 | |
| CPC 15610 | Rumex sp. | Mexico | M. de Jesús Yáñez-Morales | KT600385 | KT600482 | KT600580 | |
| CPC 15615 | Wild tree | Mexico | M. de Jesús Yáñez-Morales | KT600386 | KT600483 | KT600581 | |
| CPC 15626 | Wild plant | Mexico | M. de Jesús Yáñez-Morales | KT600387 | KT600484 | KT600582 | |
| CPC 18138 | Pine needles plus insects | Mexico | M. de Jesús Yáñez-Morales | KT600388 | KT600485 | KT600583 | |
| C. colombiae | CBS 274.80B* | Cortaderia sp. | Colombia | W. Gams | FJ936159 | FJ936163 | FJ936166 |
| C. cucumerinum | CBS 108.23 | Cucumis sativus | — | W.W. Gilbert | HM148068 | HM148312 | HM148557 |
| CBS 171.52**; MUCL 10092 | Cucumis sativus | The Netherlands | — | HM148072 | HM148316 | HM148561 | |
| CBS 172.54 | Cucumis sativus | The Netherlands | G.W. van der Helm | HM148073 | HM148317 | HM148562 | |
| CBS 174.62 | Cucumis sativus | The Netherlands | G.W. van der Helm | HM148075 | HM148319 | HM148564 | |
| CBS 175.54 | Cucumis sativus | The Netherlands | G.W. van der Helm | HM148077 | HM148321 | HM148566 | |
| C. delicatulum | CBS 126342; CPC 14287; BA 1681 | Indoor air | Denmark | B. Andersen | HM148079 | HM148323 | HM148568 |
| CBS 126344; CPC 11389; reference | Tilia cordata | Germany | K. Schubert | HM148081 | HM148325 | HM148570 | |
| CPC 13148 | Puccinia bromina subsp. symphyti-bromarum | Germany | K. Schubert | HM148082 | HM148326 | HM148571 | |
| CPC 14307; BA 1706 | Sea weed | Denmark | B. Andersen | HM148086 | HM148330 | HM148575 | |
| CPC 15612 | Juglans regia | Mexico | M. de Jesús Yáñez-Morales | KT600389 | KT600486 | KT600584 | |
| C. dominicanum | CBS 119415*; EXF-732; dH 16386 | Hypersaline water | Dominican Republic | N. Gunde-Cimerman | DQ780353 | JN906986 | EF101368 |
| CPC 15932 | Dracaena fragrans | Philippines | C.J.R. Cumagun | KT600390 | KT600487 | KT600585 | |
| CPC 20109 | Unknown vine | Taiwan | P.W. Crous | KT600391 | KT600488 | KT600586 | |
| C. echinulatum | CBS 123191; CPC 15386; reference | Dianthus barbatus | New Zealand | C.F. Hill | JN906980 | JN906987 | JN906999 |
| C. exasperatum | CBS 125986*; CPC 14638 | Eucalyptus tintinnans | Australia | B.A. Summerell | HM148090 | HM148334 | HM148579 |
| C. exile | CBS 125987*; CPC 11828 | Chasmothecia of Phyllactinia guttata on leaves of Corylus avellana | USA: Washington | D. Glawe | HM148091 | HM148335 | HM148580 |
| C. flabelliforme | CBS 126345*; CPC 14523 | Melaleuca cajuputi | Australia | B.A. Summerell | HM148092 | HM148336 | HM148581 |
| C. funiculosum | CBS 122128; ATCC 16160; IFO 6536; JCM 10682 | Ficus carica | Japan | — | HM148093 | HM148337 | HM148582 |
| CBS 122129*; ATCC 38010; IFO 6537; JCM 10683 | Vigna umbellata | Japan | — | HM148094 | HM148338 | HM148583 | |
| C. fusiforme | CBS 119414*; EXF-449 | Hypersaline water | Slovenia | L. Butinar | DQ780388 | JN906988 | EF101372 |
| C. gamsianum | CBS 125989*; CPC 11807 | Strelitzia sp. | South Africa | W. Gams | HM148095 | HM148339 | HM148584 |
| CPC 15617 | Glycine max, seeds | Mexico | M. de Jesús Yáñez-Morales | KT600392 | KT600489 | KT600587 | |
| C. globisporum | CBS 812.96* | Meat stamp | Sweden | M. Olsen | HM148096 | HM148340 | HM148585 |
| C. halotolerans | CBS 119416*; EXF-572 | Hypersaline water of salterns | Namibia | N. Gunde-Cimerman | DQ780364 | JN906989 | EF101397 |
| FMR 13493 | Man | Spain | — | LN834365 | LN834461 | LN834549 | |
| UTHSC DI-13-164 | Man, bone marrow | USA | — | LN834366 | LN834462 | LN834550 | |
| UTHSC DI-13-206 | Man, BAL fluid | USA | — | LN834369 | LN834465 | LN834553 | |
| UTHSC DI-13-249 | Man, nasal | USA | — | LN834373 | LN834469 | LN834557 | |
| C. herbaroides | CBS 121626*; CPC 12052; EXF-1733 | Hypersaline water from salterns | Israel | P. Zalar | EF679357 | EF679432 | EF679509 |
| C. herbarum | CBS 121621**; CPC 12177 | Hordeum vulgare | The Netherlands | J.Z. Groenewald | EF679363 | EF679440 | EF679516 |
| CPC 12178 | Hordeum vulgare | The Netherlands | P.W. Crous | EF679364 | EF679441 | EF679517 | |
| CPC 12179 | Hordeum vulgare | The Netherlands | P.W. Crous | EF679365 | EF679442 | EF679518 | |
| CPC 12180 | Hordeum vulgare | The Netherlands | P.W. Crous | EF679366 | EF679443 | EF679519 | |
| CPC 12181 | Hordeum vulgare | The Netherlands | P.W. Crous | EF679367 | EF679444 | EF679520 | |
| CPC 12183 | Hordeum vulgare | The Netherlands | P.W. Crous | EF679368 | EF679445 | EF679521 | |
| C. hillianum | CBS 125988*; CPC 15459; C92 | Leaf mould of Typha orientalis | New Zealand | R. Beever | HM148097 | HM148341 | HM148586 |
| CPC 15458 | Leaf mould of Typha orientalis | New Zealand | R. Beever | HM148098 | HM148342 | HM148587 | |
| C. inversicolor | CBS 143.65 | Tilia sp. leaf | The Netherlands | — | HM148100 | HM148344 | HM148589 |
| CBS 401.80*; ATCC 200941 | Triticum aestivum leaf | The Netherlands | — | HM148101 | HM148345 | HM148590 | |
| CBS 464.82; ATCC 200945 | Alnus sp. seeds | The Netherlands | G.S. de Hoog | HM148102 | HM148346 | HM148591 | |
| CPC 14190 | Outside air | The Netherlands | M. Meijer | HM148106 | HM148350 | HM148595 | |
| CPC 18238 | Freylinia lanceolata | South Africa | P.W. Crous | KT600393 | KT600490 | KT600588 | |
| C. ipereniae | CBS 140483*; CPC 16238 | Puya sp. | Chile | A. van Iperen | KT600394 | KT600491 | KT600589 |
| CPC 16855 | Arctostaphylos pallida | USA: California | P.W. Crous | KT600395 | KT600492 | KT600590 | |
| C. iranicum | CBS 126346*; CPC 11554 | Citrus sinensis leaf | Iran | W. Gams | HM148110 | HM148354 | HM148599 |
| C. iridis | CBS 138.40** | Iris sp. | The Netherlands | — | EF679370 | EF679447 | EF679523 |
| C. langeronii | CBS 189.54* | Man, ulcero-nodular mycosis of hand and arm | Brazil | da Fonseca | DQ780379 | JN906990 | EF101357 |
| C. licheniphilum | CBS 125990*; CPC 13224 | Phaeophyscia orbicularis and Physcia sp. | Germany | W. von Brackel | HM148111 | HM148355 | HM148600 |
| C. limoniforme | CBS 113737 | Grape berry | USA: Washington | F.M. Dugan lab | KT600396 | KT600493 | KT600591 |
| CBS 140484*; CPC 12039 | Musa acuminata | Egypt | R.S. Summerbell | KT600397 | KT600494 | KT600592 | |
| CPC 12048; EXF-1060 | Hypersaline water | Israel | P. Zalar | KT600398 | KT600495 | KT600593 | |
| CPC 12049; EXF-1062 | Hypersaline water | Israel | P. Zalar | KT600399 | KT600496 | KT600594 | |
| CPC 12050; EXF-1081 | Hypersaline water | Israel | P. Zalar | KT600400 | KT600497 | KT600595 | |
| CPC 13923 | Eucalyptus sp. | Cyprus | A. van Iperen | KT600401 | KT600498 | KT600596 | |
| CPC 18086; KSU C1 | Tomato | — | — | KT600402 | KT600499 | KT600597 | |
| C. longicatenatum | CBS 140485*; CPC 17189 | Unknown plant | Australia | P.W. Crous | KT600403 | KT600500 | KT600598 |
| C. longissimum | CBS 300.96* | Soil along coral reef coast | Papua New Guinea | A. Aptroot | DQ780352 | EU570259 | EF101385 |
| C. macrocarpum | CBS 121623*; CPC 12755 | Spinacia oleracea | USA: Washington | L. du Toit | EF679375 | EF679453 | EF679529 |
| CBS 108.85 | Diospyros kaki | Morocco | — | KT600404 | KT600501 | KT600599 | |
| CBS 175.62 | Hordeum vulgare | The Netherlands | Bierbrouwerij Amstel | AJ244229 | KT600502 | KT600600 | |
| CPC 19063 | Hordeum sp. | Iran | — | KT600405 | KT600503 | KT600601 | |
| C. montecillanum | CBS 140486*; CPC 17953 | Pine needles | Mexico | M. de Jesús Yáñez-Morales | KT600406 | KT600504 | KT600602 |
| CPC 15605 | Taraxacum sp. | Mexico | M. de Jesús Yáñez-Morales | KT600407 | KT600505 | KT600603 | |
| CPC 17804 | Pine needles | Mexico | M. de Jesús Yáñez-Morales | KT600408 | KT600506 | KT600604 | |
| C. myrtacearum | CBS 126350**; CPC 14567 | Corymbia foelscheana | Australia | B.A. Summerell | HM148117 | HM148361 | HM148606 |
| CBS 126349; CPC 13689 | Eucalyptus placita | Australia | B.A. Summerell | HM148116 | HM148360 | HM148605 | |
| CPC 16319 | Indigofera sp. | South Africa | A.R. Wood | KT600409 | KT600507 | KT600605 | |
| C. paracladosporioides | CBS 171.54*; ATCC 11278, 200943; IFO 6369; IMI 049626; MUCL 917; NCTC 4097 | — | — | — | HM148120 | HM148364 | HM148609 |
| C. parapenidielloides | CBS 140487*; CPC 17193 | Eucalyptus sp. | Australia | P.W. Crous | KT600410 | KT600508 | KT600606 |
| C. penidielloides | CBS 140489*; CPC 17674 | Acacia verticillata | Australia | P.W. Crous | KT600412 | KT600510 | KT600608 |
| C. perangustum | CBS 125996*; CPC 13815 | Cussonia sp. | South Africa | P.W. Crous | HM148121 | HM148365 | HM148610 |
| CBS 126364; CPC 14532 | Erythrophleum chlorostachys | Australia | B.A. Summerell | HM148122 | HM148366 | HM148611 | |
| CPC 13686 | Eucalyptus placita | Australia | B.A. Summerell | HM148138 | HM148382 | HM148627 | |
| CPC 13730 | Protea caffra | South Africa | P.W. Crous | HM148140 | HM148384 | HM148629 | |
| CPC 14566 | Corymbia foelscheana | Australia | B.A. Summerell | HM148147 | HM148391 | HM148636 | |
| CPC 15192 | Protea cynaroides | South Africa | L. Mostert | HM148149 | HM148393 | HM148638 | |
| CPC 18494 | Ananas comosus | Panama | — | KT600413 | KT600511 | KT600609 | |
| CPC 18496 | Ananas comosus | Panama | — | KT600414 | KT600512 | KT600610 | |
| C. phaenocomae | CBS 128769*; CPC 18223 | Phaenocoma prolifera | South Africa | K.L. Crous & P.W. Crous | JF499837 | JF499875 | JF499881 |
| C. phlei | CBS 358.69** | Phleum pratense | Germany | — | JN906981 | JN906991 | JN907000 |
| C. phyllactiniicola | CBS 126352; CPC 11836* | Chasmothecia of Phyllactinia guttata on leaves of Corylus avellana | USA: Washington | D. Glawe | HM148150 | HM148394 | HM148639 |
| CBS 126355; CPC 11830 | Chasmothecia of Phyllactinia guttata on leaves of Corylus avellana | USA: Washington | D. Glawe | HM148153 | HM148397 | HM148642 | |
| C. phyllophilum | CBS 125992**; CPC 11333 | Taphrina sp. on Prunus cerasus | Germany | K. Schubert | HM148154 | HM148398 | HM148643 |
| CPC 13873 | On Teratosphaeria proteae-arboreae on Protea arborea | South Africa | P.W. Crous | HM148155 | HM148399 | HM148644 | |
| C. pini-ponderosae | CBS 124456*; CPC 13980; CIEFAP 322 | Pinus ponderosa | Argentina | A. Greslebin | FJ936160 | FJ936164 | FJ936167 |
| C. pseudochalastosporoides | CBS 140490*; CPC 17823 | Pine needles | Mexico | M. de Jesús Yáñez-Morales | KT600415 | KT600513 | KT600611 |
| C. pseudocladosporioides | CBS 125993*; CPC 14189 | Outside air | The Netherlands | M. Meijer | HM148158 | HM148402 | HM148647 |
| CPC 11605 | Agrimonia pilosa | South Korea | H.D. Shin | HM148167 | HM148411 | HM148656 | |
| CPC 13339 | Eucalyptus molucana | Australia | P.W. Crous | HM148170 | HM148414 | HM148659 | |
| CPC 13529 | Sagittaria graminea | Italy | W. Gams & K.A. Seifert | HM148172 | HM148416 | HM148661 | |
| CPC 13683; NSW 734672 | Eucalyptus placita | Australia | B.A. Summerell | HM148173 | HM148417 | HM148662 | |
| CPC 14295; BA 1694 | Soil | Chile: Easter Island | B. Andersen | HM148188 | HM148432 | HM148677 | |
| CPC 14382 | Acer macrophyllum | Canada | B. Callan | HM148190 | HM148434 | HM148679 | |
| CPC 18014 | Aspalathus linearis | South Africa | — | KT600416 | KT600514 | KT600612 | |
| C. psychrotolerans | CBS 119412*; EXF-391; dH 16390 | Hypersaline water | Slovenia | S. Sonjak | DQ780386 | JN906992 | EF101365 |
| C. puyae | CBS 274.80A* | Puya goudotiana | Colombia | W. Gams | KT600418 | KT600516 | KT600614 |
| C. ramotenellum | CBS 109031; JBT 13731 | Cheese | Denmark | J. Frisvad | KT600419 | KT600517 | KT600615 |
| CBS 109501; dH 12343 | Man, deep mycosis | Turkey | — | KT600420 | KT600518 | KT600616 | |
| CBS 121627; CPC 12047; EXF-967 | Air conditioning system (bathroom) | Slovenia | M. Butala | EF679385 | EF679463 | EF679539 | |
| CBS 121628*; CPC 12043; EXF-454 | Hypersaline water from reverse ponds | Slovenia | P. Zalar | EF679384 | EF679462 | EF679538 | |
| CBS 118.24; ATCC 36972; MUCL 10098 | Paeonia sp. | Italy | — | KT600421 | KT600519 | KT600617 | |
| CBS 133.29; ATCC 36970 | Populus tremuloides, leaf spot | — | — | KT600422 | KT600520 | KT600618 | |
| CBS 169.54; CBS 170.54; IMI 025324; NCTC 6740; dH 15462 | Arundo sp., leaf | UK | — | AJ300335 | KT600521 | KT600619 | |
| CBS 261.80 | Margarine | Spain | — | KT600423 | KT600522 | KT600620 | |
| CPC 11395 | Dioscorea tenuipes | South Korea | H.D. Shin | KT600424 | KT600523 | KT600621 | |
| CPC 11401 | Weigela subsessilis | South Korea | H.D. Shin | KT600425 | KT600524 | KT600622 | |
| CPC 11826 | Chasmothecia of Phyllactinia guttata on leaves of Corylus sp. | USA: Washington | D. Glawe | KT600426 | KT600525 | KT600623 | |
| CPC 11832 | Chasmothecia of Phyllactinia guttata on leaves of Corylus sp. | USA: Washington | D. Glawe | KT600427 | KT600526 | KT600624 | |
| CPC 12126; Hill 1192 | Yucca elephantipes, leaf spot | New Zealand | C.F. Hill | KT600428 | KT600527 | KT600625 | |
| CPC 12313 | Rosa sp. | Germany | N. Ale-Agha | KT600429 | KT600528 | KT600626 | |
| CPC 12385 | Eucalyptus sp. | Australia | P.W. Crous | KT600430 | KT600529 | KT600627 | |
| CPC 13407 | Ginkgo biloba | Portugal | P.W. Crous | KT600431 | KT600530 | KT600628 | |
| CPC 13789 | Protea sp. | Spain: Tenerife | P.W. Crous | KT600432 | KT600531 | KT600629 | |
| CPC 13792 | Unknown plant | Spain: Tenerife | P.W. Crous | KT600433 | KT600532 | KT600630 | |
| CPC 13795 | Leucospermum sp. | Spain: Tenerife | P.W. Crous | KT600434 | KT600533 | KT600631 | |
| CPC 13798 | Leucadendron sp. | Spain: Tenerife | P.W. Crous | KT600435 | KT600534 | KT600632 | |
| CPC 13801 | Leucospermum sp. | Spain: Tenerife | P.W. Crous | KT600436 | KT600535 | KT600633 | |
| CPC 13943 | Quercus infectoria | Cyprus | A. van Iperen | KT600437 | KT600536 | KT600634 | |
| CPC 14300; BA 1699 | Indoor building material | Denmark | B. Andersen | KT600438 | KT600537 | KT600635 | |
| CPC 14306; BA1705 | Food, garfish gill | Denmark | B. Andersen | KT600439 | KT600538 | KT600636 | |
| C. rectoides | CBS 125994*; CPC 11624 | Vitis flexuosa | South Korea | H.D. Shin | HM148193 | HM148438 | HM148683 |
| CBS 126357; CPC 11405 | Plectranthus sp. | South Korea | H.D. Shin | HM148194 | HM148439 | HM148684 | |
| C. rhusicola | CBS 140492*; CPC 15219 | Rhus sp. | South Africa | F. Roets | KT600440 | KT600539 | KT600637 |
| C. ruguloflabelliforme | CBS 140494*; CPC 19707 | Diatrapaceae sp. on Aloe sp. | South Africa | P.W. Crous | KT600458 | KT600557 | KT600655 |
| C. rugulovarians | CBS 140495*; CPC 18444 | Leaf sheaths of unidentified Poaceae | Brazil | P.W. Crous | KT600459 | KT600558 | KT600656 |
| C. salinae | CBS 119413*; EXF-335 | Hypersaline water | Slovenia | S. Sonjak | DQ780374 | JN906993 | EF101390 |
| C. sinuosum | CBS 121629*; CPC 11839; ICMP 15819 | Fuchsia excorticata | New Zealand | A. Blouin | EF679386 | EF679464 | EF679540 |
| CBS 164.48; ATCC 11285 | Unidentified moss | France | — | KT600441 | KT600540 | KT600638 | |
| CBS 393.68 | Air | The Netherlands | — | KT600442 | KT600541 | KT600639 | |
| CPC 14000; MRC 02998 | Wheat | South Africa | — | KT600443 | KT600542 | KT600640 | |
| CPC 15454 | Crocus sativus | New Zealand | J. Rennie | KT600444 | KT600543 | KT600641 | |
| CPC 17632 | Eryngium maritimum | Germany | U. Damm | KT600445 | KT600544 | KT600642 | |
| CPC 18365 | Iris pseudacorus | The Netherlands | P.W. Crous | KT600446 | KT600545 | KT600643 | |
| C. sphaerospermum | CBS 102045; EXF-2524; MZKI B-1066 | Hypersaline water | Spain | P. Zalar | DQ780351 | EU570262 | EF101378 |
| CBS 193.54*; ATCC 11289; IMI 49637 | Man, nails | The Netherlands | G.A. de Vries | DQ780343 | EU570261 | EF101380 | |
| CPC 11822 | Phyllactinia guttata on Corylus avellana | USA | D. Glawe | EU570254 | EU570263 | EU570270 | |
| CPC 12476 | Ambrosia artemisiifolia | Germany | J. Nitzsche | EU570255 | EU570264 | EU570271 | |
| CPC 13368 | Phaseolus lunatus | Germany | N. Ale-Agha | EU570256 | EU570265 | EU570272 | |
| CPC 13995; CAMS 000750 | Thatch | South Africa | G. Marais | EU570257 | EU570266 | EU570273 | |
| CPC 14016; MRC 10263 | Triticum aestivum | South Africa | — | EU570258 | EU570267 | EU570274 | |
| C. subinflatum | CBS 121630*; CPC 12041; EXF-343 | Hypersaline water from salterns | Slovenia | S. Sonjak | EF679389 | EF679467 | EF679543 |
| CPC 15565 | Iris sp. | Ukraine | A. Akulov | KT600447 | KT600546 | KT600644 | |
| C. subuliforme | CBS 126500*; CPC 13735 | Chamaedorea metallica | Thailand | I. Hidayat & J. Meeboon | HM148196 | HM148441 | HM148686 |
| CPC 15833 | Citrus sp. | Mexico | M. de Jesús Yáñez-Morales | KT600453 | KT600552 | KT600650 | |
| CPC 15838 | Agave tequilana var. azul | Mexico | M. de Jesús Yáñez-Morales | KT600454 | KT600553 | KT600651 | |
| CPC 16318 | Eucalyptus sp. | South Africa | A.R. Wood | KT600455 | KT600554 | KT600652 | |
| CPC 18243 | Cotton (Gossypium sp.), leaves | Brazil | D.B. da Silva | KT600456 | KT600555 | KT600653 | |
| C. tenuissimum | CBS 125995**; CPC 14253 | Lagerstroemia sp. | USA: Louisiana | P.W. Crous | HM148197 | HM148442 | HM148687 |
| CBS 117.79 | Fruit | Burundi | J. Rammelo | HM148200 | HM148445 | HM148690 | |
| CPC 10882 | Gnaphalium affine | South Korea | H.D. Shin | HM148204 | HM148449 | HM148694 | |
| CPC 11555 | Citrus sinensis | Iran | W. Gams | HM148205 | HM148450 | HM148695 | |
| CPC 11805 | Strelitzia sp. | South Africa | W. Gams | HM148207 | HM148452 | HM148697 | |
| CPC 12795 | Musa sp. | Polynesia | I. Budenhagen | HM148209 | HM148454 | HM148699 | |
| CPC 13222 | Callistemon viminalis | Australia | P.W. Crous | HM148210 | HM148455 | HM148700 | |
| CPC 14250 | Magnolia sp. | USA: Louisiana | P.W. Crous | HM148211 | HM148456 | HM148701 | |
| C. uredinicola | ATCC 46649; CPC 5390 | Hyperparasite on Cronartium fusiforme f. sp. quercum on Quercus nigra leaves | USA: Alabama | — | AY251071 | HM148467 | HM148712 |
| C. variabile | CBS 121635**; CPC 12751 | Spinacia oleracea | USA | — | EF679402 | EF679480 | EF679556 |
| C. varians | CBS 126360; CPC 11327 | Ulmus sp. | Germany | K. Schubert | HM148222 | HM148468 | HM148713 |
| CBS 126362*; CPC 13658 | Catalpa bungei | Russia | V.A. Melnik | HM148224 | HM148470 | HM148715 | |
| C. velox | CBS 119417*; CPC 11224 | Bambusa sp. | India | W. Gams | DQ780361 | JN906995 | EF101388 |
| CPC 18450 | Zea mays | Brazil | P.W. Crous | KT600457 | KT600556 | KT600654 | |
| C. verrucocladosporioides | CBS 126363*; CPC 12300 | Rhus chinensis | South Korea | H.D. Shin | HM148226 | HM148472 | HM148717 |
| C. versiforme | CBS 140491*; CPC 19053 | Hordeum sp. | Iran | — | KT600417 | KT600515 | KT600613 |
| C. xylophilum | CBS 113749 | Bing cherry fruits | USA | F.M. Dugan lab | HM148228 | HM148474 | HM148719 |
| CBS 125997*; CPC 12403 | Dead wood of Picea abies | Russia | D.A. Shabunin | HM148230 | HM148476 | HM148721 | |
| CBS 126588; CPC 13512 | Salix viminalis twigs | Italy | W. Gams | HM148231 | HM148477 | HM148722 | |
| CPC 12101 | Galls of Apiosporina morbosa | Canada | K.A. Seifert | HM148232 | HM148478 | HM148723 | |
| CPC 16356 | Musa sp. | Mexico | M. de Jesús Yáñez-Morales | KT600460 | KT600559 | KT600657 | |
* Ex-type culture.
** Ex-epitype culture.
ATCC: American Type Culture Collection, Virginia, USA; BA: Personal culture collection of Birgitte Andersen, Denmark; CBS: CBS-KNAW Fungal Biodiversity Centre, Utrecht, The Netherlands; CIEFAP: Centro de Investigación y Extensión Forestal Andino Patagónico, Argentina; CPC: Culture collection of Pedro Crous, housed at CBS; dH: de Hoog Culture Collection, housed at CBS; EXF: Fungal strains in the Culture collection Ex: Culture collection of extremophilic microorganisms, Department of Biology, Biotechnical Faculty, University of Ljubljana, Slovenia (Infrastructural Centre Mycosmo, MRIC UL); FMR: Facultat de Medicina, Universitat Rovira i Virgili, Reus, Spain; Hill: Personal culture collection of Frank Hill, New Zealand; ICMP: International Collection of Micro-organisms from Plants, Landcare Research, Private Bag 92170, Auckland, New Zealand; IFO: Institute for Fermentation, Osaka, Japan; IMI: International Mycological Institute, CABI-Bioscience, Egham, Bakeham Lane, UK; MRC: Medical Research Council, Cape Town, South Africa; MUCL: Mycotheque de l'Universite catholique de Louvain, Laboratoire de Mycologie Systematique et Appliquee, Universite catholique de Louvain, Louvain-la-Neuve, Belgium; MZKI: Microbiological Culture Collection of the National Institute of Chemistry, Ljubljana, Slovenia; NCTC: National Collection of Type Cultures, PHLS Central Public Health Laboratory, London, UK; UTHSC: Fungus Testing Laboratory at the University of Texas Health Science Center, San Antonio, TX, USA; VTT: VTT Culture Collection, VTT Technical Research Centre of Finland, Finland.
act: partial actin gene, tef1: partial translation elongation factor 1-alpha gene, ITS: internal transcribed spacer region including intervening 5.8S rRNA gene.
DNA isolation, amplification and sequence analysis
Fungal colonies were established on agar plates, and genomic DNA was isolated as described in Groenewald et al. (2013). DNA amplification of the internal transcribed spacer regions and intervening 5.8S rRNA gene (ITS) of the nrDNA cistron, partial actin (act) and translation elongation factor 1-alpha (tef1) followed Groenewald et al., 2005, Groenewald et al., 2013. Representative isolates and / or species were selected from the NCBI nucleotide database mainly based on degree of nucleotide similarity and / or morphological similarity to the strains examined in this study. Phylogenetic analyses consisted of parsimony analyses of an alignment representing the C. cladosporioides complex and a separate alignment for the combined C. herbarum / sphaerospermum complexes. The analyses were performed as described by Lombard et al. (2014). Novel sequences were deposited in NCBI's GenBank nucleotide database (Table 1) and the alignments and trees in TreeBASE (study accession number S18262).
Morphology
Light microscopy (LM). Microscopic observations of isolates were made from colonies cultivated for 7 d under continuous nearultraviolet light at 25 °C on SNA. Preparations were mounted in Shear's solution (Crous et al. 2009c). To study conidial development and branching patterns of conidial chains, squares of transparent adhesive tape (Titan Ultra Clear Tape, Conglom Inc., Toronto, Canada) were placed on conidiophores growing in the zone between the colony margin and 2 cm inwards, and mounted between two drops of Shear's solution under a glass cover slip. Conidial terminology follows Schubert et al. (2007b) and Bensch et al. (2012) where the different types of conidia are shown and discussed. Wherever possible, 50 measurements (× 1 000 magnification, differential interference contrast microscopy, Zeiss Axioscope 2 PLUS) were made of conidia with outliers given in parentheses. Average values and standard deviations are also listed. Photographic images were captured with a Zeiss Axio Imager 2 light microscope using differential interference contrast (DIC) illumination and an AxioCam MRc5 camera and ZEN software. For cultural characteristics, colonies were cultivated on PDA, SNA, OA and MEA for 14 d at 25 °C in the dark, after which the surface and reverse colours were rated using the charts of Rayner (1970).
Low-temperature scanning electron microscopy (SEM)
Isolates of Cladosporium spp. were grown on SNA with 30 g agar/L for 3–4 d at room temperature under black light. Relevant parts of the small colonies with conidiophores and conidia were selected under a binocular, excised with a surgical blade as small agar (3 × 3 mm) blocks, and transferred to a copper cup for snap-freezing in nitrogen slush. To prevent disruption of the intricate structure of the conidiophores by liquid nitrogen, a piece of Scotch tape was placed lightly over the opening of the copper cup. Agar blocks were glued to the copper surface with frozen tissue medium (KP-Cryoblock, Klinipath, Duiven, Netherlands) mixed with 1 part colloidal graphite (Agar Scientific, Stansted, UK). Samples were examined in a JEOL 5600LV scanning electron microscope (JEOL, Tokyo, Japan) equipped with an Oxford CT1500 Cryostation for cryo-electron microscopy (cryoSEM). Electron micrographs were acquired from uncoated frozen samples, or after sputter-coating by means of a gold target for three times during 30 s. Micrographs of uncoated samples were taken at an acceleration voltage of 3 kV, and consisted out of 30 averaged fast scans (SCAN 2 mode), and at 5 kV in case of the coated samples (PHOTO mode).
Results
DNA phylogeny
To simplify layout of the trees and to maximise the quality of the alignment, two separate alignments were created: one alignment representing the C. cladosporioides complex and the other the combined C. herbarum / sphaerospermum complexes. Novel sequences generated in this study were added to sequences deposited in the NCBI's GenBank nucleotide database (mainly representing the data from Schubert et al., 2007b, Zalar et al., 2007, and Bensch et al., 2010, Bensch et al., 2012).
The manually adjusted alignment of the C. cladosporioides complex contained 120 sequences (including the outgroup sequence) and the three loci were represented by a total of 957 characters (ITS: 493, tef1: 261, act: 203) including alignment gaps, which were used in the analysis. Of the 957 characters, 292 were parsimony-informative (ITS: 11, tef1: 179, act: 102), 188 were variable and parsimony-uninformative (ITS: 122, tef1: 40, act: 26), and 477 were constant (ITS: 360, tef1: 42, act: 75). Ninety-six equally most parsimonious trees (TL = 2 202 steps; CI = 0.415; RI = 0.784; RC = 0.325), the first of which is shown in Fig. 1, were obtained from the parsimony analysis of the combined genes. The ITS sequences were the least successful in resolving species with only three out of the 44 species resolved, followed by tef1 with 38 out of 44 species and act being slightly more suitable with 39 out of 44 species (data not shown, single gene trees available in TreeBASE).
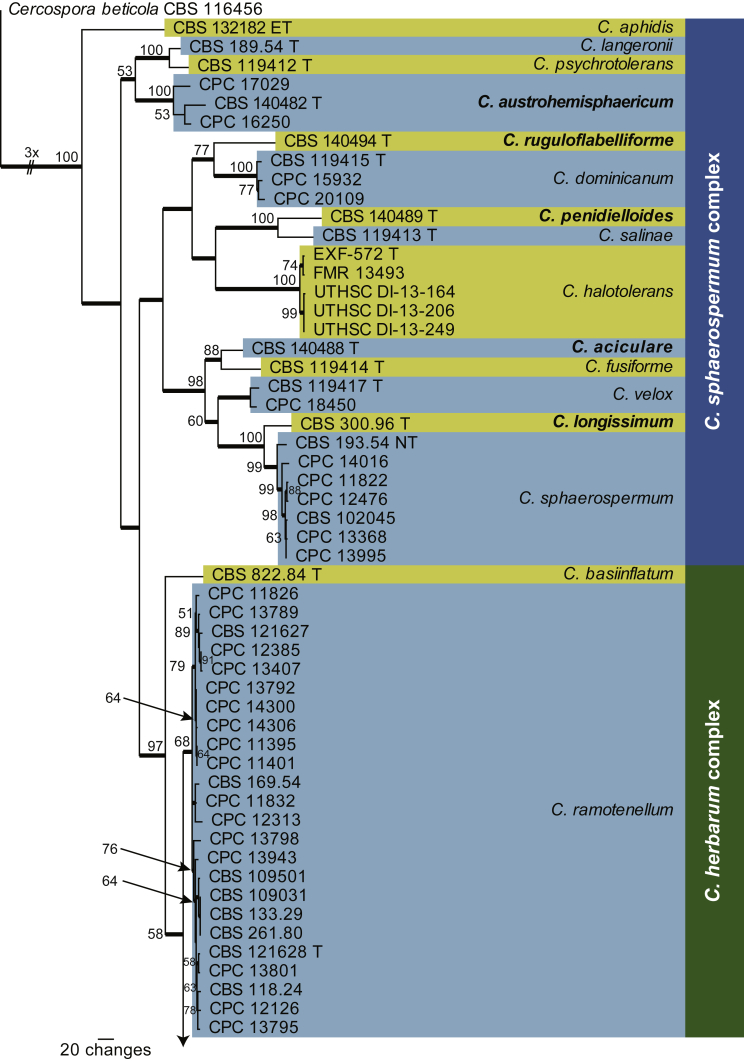
Fig. 1.
The first of 96 equally most parsimonious trees obtained from a heuristic search with 100 random taxon additions of the combined ITS, tef1 and act sequence alignment of the cladosporioides complex using PAUP v. 4.0b10. The scale bar shows 20 changes, and bootstrap support values from 1 000 replicates are shown at the nodes. Thickened lines indicate the strict consensus branches and species names are indicated to the right of the tree. Species boundaries are indicated with coloured blocks. Names of novel species are printed in bold face and the type status of strains are indicated next to the culture collection number (T: ex-type; ET: ex-epitype; NT: ex-neotype; R: reference). The tree was rooted to Cercospora beticola (CBS 116456).
The manually adjusted alignment of the C. herbarum / sphaerospermum complexes contained 112 sequences (including the outgroup sequence) and the three loci were represented by a total of 965 characters (ITS: 496, tef1: 270, act: 199) including alignment gaps which were used in the analysis. Of the 965 characters, 406 were parsimony-informative (ITS: 79, tef1: 206, act: 121), 146 were variable and parsimony-uninformative (ITS: 89, tef1: 28, act: 29), and 413 were constant (ITS: 328, tef1: 36, act: 49). One thousand equally most parsimonious trees (TL = 2 535 steps; CI = 0.453; RI = 0.825; RC = 0.374), the first of which is shown in Fig. 2, were obtained from the parsimony analysis of the combined genes. In the sphaerospermum complex (as delimited in Fig. 2), the ITS sequences were the least successful in resolving species with only 10 out of the 14 species resolved, followed by act with 13 out of 14 species and tef1 being slightly more suitable in resolving all species (data not shown, single gene trees available in TreeBASE). In the herbarum complex (as delimited in Fig. 2), the ITS sequences were the least successful in resolving species with only three out of the 19 species resolved, followed by tef1 with 18 out of 19 species and act being slightly more suitable in resolving all species (data not shown, single gene trees available in TreeBASE).
Fig. 2.
The first of 1 000 equally most parsimonious trees obtained from a heuristic search with 100 random taxon additions of the combined ITS, tef1 and act sequence alignment of the herbarum / sphaerospermum complexes using PAUP v. 4.0b10. The scale bar shows 20 changes, and bootstrap support values from 1 000 replicates are shown at the nodes. Thickened lines indicate the strict consensus branches and species names are indicated to the right of the tree. Species boundaries are indicated with coloured blocks. Names of novel species are printed in bold face and the type status of strains are indicated next to the culture collection number (T: ex-type; ET: ex-epitype; NT: ex-neotype; R: reference). The tree was rooted to Cercospora beticola (CBS 116456).
Taxonomy
The status of numerous unidentified isolates included in this study was resolved, which revealed several novel species. The circumscriptions and delimitations of these species are mainly based on quantitative as well as qualitative morphological features and on molecular data. Features that proved to be diagnostic at species rank were discussed in Bensch et al. (2012) and have been also applied here. The new taxa are treated in alphabetical order below. Detailed descriptions, illustrations and comments as well as the species complex they are belonging to, are given. Additional comments on species complexes are further provided in the Discussion. Some previously described species, for which updated information on species concept, host range and / or geographic distribution are available, are also included.
Cladosporium aciculare Bensch, Crous & U. Braun, sp. nov. MycoBank MB814621. Fig. 3.
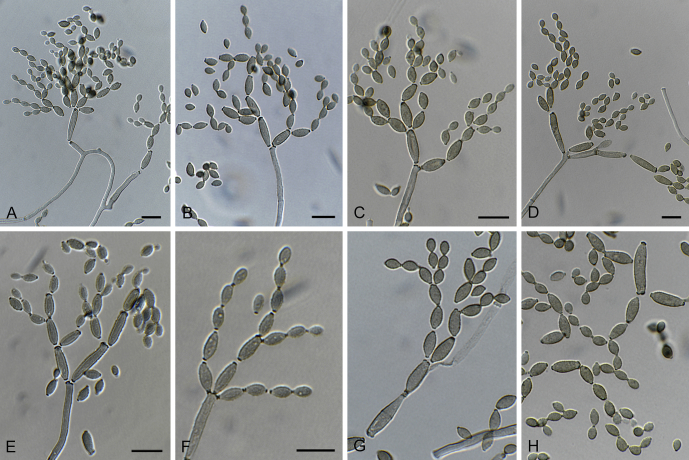
Fig. 3.
Cladosporium aciculare (CBS 140488). A–C. Conidiophores and conidial chains. D–F. Tip of conidiophores and numerous conidia. G. Ramoconidium and conidia. Scale bars = 10 μm.
Etymology: Named after the typical form of the conidiophores being acicular, needle-shaped, with a broader base and pointed towards the apex.
Mycelium sparingly formed, short cylindrical, usually unbranched, 2–4.5 μm wide, sometimes swollen up to 6 μm, with swellings and constrictions, pale to often medium olivaceous-brown, sometimes subhyaline, smooth or asperulate, sometimes verruculose towards the base of conidiophores, walls slightly thickened. Conidiophores macro- or semimacronematous, solitary or in loose groups of up to three arising terminally or laterally from hyphae, sometimes as short and narrow lateral prolongations, about 1.5 μm wide, straight or somewhat flexuous, neither nodulose nor geniculate, but usually subuliforme, awl-like with a wider base, 3–4 μm, and slightly to distinctly attenuated towards the apex, (1.5–)2–2.5(–3) μm, 28–250 μm long, usually unbranched, multiseptate, not constricted, pale to medium olivaceous-brown, paler towards the apex, micronematous ones subhyaline, smooth or almost so, sometimes asperulate, walls slightly thickened. Conidiogenous cells integrated, mainly terminal, often seceding as ramoconidia, cylindrical, 15–40 μm long, with 2–4(–5) distal conidiogenous loci crowded at the outermost apex, loci subdenticulate, (0.8–)1–1.5 μm diam, thickened and darkened-refractive. Ramoconidia commonly formed, narrowly cylindrical, 22–40 × 2–2.5 μm, 0(–1)-septate, pale olivaceous, walls unthickened, base about 2(–3) μm wide, not attenuated towards the base, sometimes hardly distinguishable from secondary ramoconidia. Conidia numerous, catenate, branching in all directions, 1–3(–5) conidia in the terminal unbranched part of the chain; small terminal conidia obovoid, ellipsoid, 3–4 × (1.5–)1.8–2 μm [av. (± SD) 3.6 (± 0.4) × 1.9 (± 0.2)], aseptate, distinctly attenuated towards the base; intercalary conidia ellipsoid to fusiform, 4–10 × 2–2.5 μm [av. (± SD) 6.5 (± 2.0) × 2.2 (± 0.2)], aseptate, with 1–3 distal hila, attenuated towards apex and base; secondary ramoconidia ellipsoid or cylindrical, 5–18(–23) × 2–2.5(–3) μm [av. (± SD) 12.8 (± 5.0) × 2.3 (± 0.3)], 0–1-septate, with 2–4(–5) distal hila, subhyaline or pale olivaceous-brown, smooth or almost so, walls unthickened, hila protuberant, conspicuous, subdenticulate, 0.5–1.5 μm diam, thickened and darkened-refractive; microcyclic conidiogenesis not occurring.
Culture characteristics: Colonies on PDA attaining 38–57 mm after 14 d, olivaceous-grey to iron-grey, grey-olivaceous towards margins, reverse olivaceous-black, velvety to fluffy; margins white, somewhat feathery, narrow, regular, aerial mycelium loose, diffuse to more dense in some spots, fluffy, growth flat, without prominent exudates, sporulation profuse. Colonies on MEA reaching 34–47 mm, glaucous-grey to pale greenish grey, grey-olivaceous due to profuse sporulation, olivaceous-black towards margin, reverse iron-grey to greenish black, powdery to fluffy, margins white, narrow, somewhat feathery, regular, aerial mycelium fluffy, dense, glaucous-grey, high, covering large parts of the colony, growth low convex. Colonies on OA attaining 40–53 mm, pale greenish grey, iron-grey at margins, olivaceous due to abundant sporulation, reverse leaden-grey to leaden-black, powdery to fluffy, margins regular, glabrous, aerial mycelium loose, diffuse to loosely fluffy, high, growth flat.
Specimen examined: Australia, New South Wales, North Washpool State Forest, isol. from Syzygium corynanthum (Myrtaceae), 1 Mar. 2009, P.W. Crous (CBS H-22359, holotype; ex-type culture CBS 140488 = CPC 16547).
Substrate and distribution: On Syzygium; Australia.
Notes: Cladosporium aciculare clusters with species belonging to the sphaerospermum species complex, even in the clade of the eponymous species, but the conidial shape departs from the globose to subglobose shape typical for members of the sphaerospermum species complex. Phylogenetically it is allied to C. fusiforme (Fig. 2) but the shape and length of conidiophores and width of conidia are quite different from that species (Zalar et al., 2007, Bensch et al., 2012).
Its morphology reminds one of C. subuliforme, a species isolated from an Arecaceae in Thailand and belonging to the cladosporioides species complex (Bensch et al. 2010). The conidia of the latter species are somewhat longer and wider [[small terminal conidia 2.5–4.5(–5.5) × 2–2.5 μm, intercalary conidia 5.5–12(–13) × 2–3(–3.5) μm, secondary ramoconidia (6–)8–25(–28) × 2–3(–3.5) μm] and the conidiophores are longer (up to 330 μm long) having a wider swollen base up to 8(–10) μm wide].
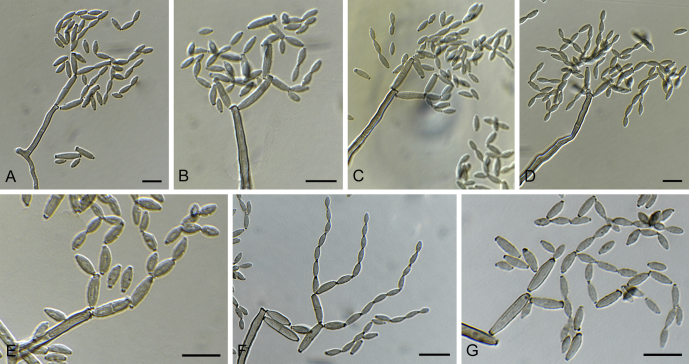
Cladosporium aggregatocicatricatum Bensch, Crous & U. Braun, sp. nov. MycoBank MB814622. Fig. 4, Fig. 5.
Fig. 4.
Cladosporium aggregatocicatricatum (CBS 140493). A–B, D, F. Conidiophores and conidia. C, E. Macronematous conidiophores with conidiogenous loci situated at about the same level on lateral prolongations or round about the stalk at about the same height. G. Tip of a conidiophore with several conidiogenous loci forming conidia. H. Conidia. Scale bars = 10 μm.
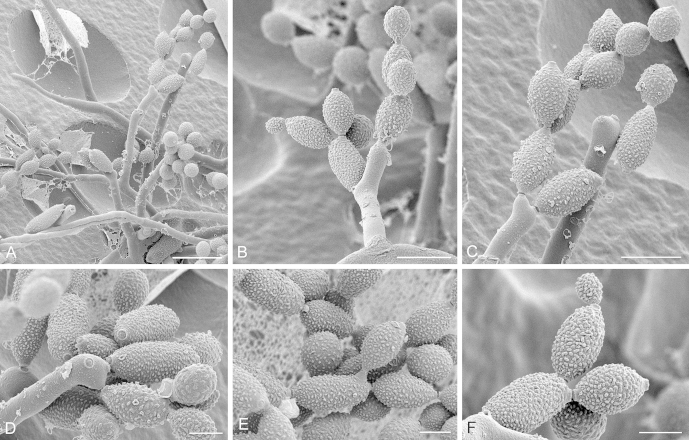
Fig. 5.
Cladosporium aggregatocicatricatum (CBS 140493). A. Part of a colony showing substrate hyphae, aerial hyphae and sparse elongated conidiophores. B–D. Conidiophores with conidial chains. Note the highly elongated secondary ramoconidia in (B) with lack of ornamentation at the connection of conidia. Note also the shape of the conidiophore in (C). E. Hila on a secondary ramoconidium and conidiogenous loci on geniculate conidiophores. These areas appear very smooth. F. Two different stages of conidia formation on a secondary ramoconidium. The height of the individual ornamentations is markedly visible here. In (B) and (F) wart-like structures are visible on relatively broad aerial hyphae. Scale bars = 5 (E–F), 10 (B–D), 20 (A) μm.
Etymology: Name refers to the conidiogenous zone of the conidi-ophores with conidiogenous loci often being crowded and situated at about the same level on lateral prolongations or round about the stalk at the same height (see Fig. 4C, E, G).
Mycelium unbranched or sparingly branched forming long ropes, infertile hyphae 0.5–1.5 μm wide, subhyaline to pale olivaceous-brown, septate, smooth or asperulate, fertile hyphae 2–3 μm wide, pale to medium olivaceous-brown, multiseptate, verruculose or irregularly rough-walled, granulate, walls unthickened or almost so. Conidiophores solitary, macronematous, occasionally micronematous, arising from plagiotropous or ascending hyphae, narrowly cylindrical-oblong, once or several times slightly to distinctly, loosely to densely geniculate-sinuous or subnodulose with unilateral swellings or lateral prolongations, after Conidi-ogenous growth sometimes continuing in a 30–45° angle, unbranched, occasionally branched, 30–550 μm long or even longer, 2–3.5(–4) μm wide, multiseptate, pale to medium olivaceous-brown, often somewhat paler towards the apex, smooth, asperulate or verruculose, walls unthickened or slightly thickened. Micronematous conidiophores short, narrow, pale olivaceous, with a single terminal conidiogenous locus. Conidiogenous cells integrated, terminal and intercalary, intercalary cells often separated by non-conidiogenous cells, cylindrical, up to 70 μm long, slightly to distinctly geniculate, sometimes several geniculations in short succession, loci often situated at about the same level on lateral prolongations or round about the stalk at the same height or subnodulose with loci formed on lateral shoulders, with 1–6 loci per cell, loci crowded, sometimes forming sympodial clusters of pronounced scars, subdenticulate, 1–2 μm diam, thickened and darkened-refractive. Ramoconidia occasionally formed. Conidia catenate, in short branched chains with 1–2 conidia in the terminal unbranched part of the chain, small terminal conidia subglobose, obovoid to ellipsoid, apex rounded, often with an additional hilum near the base, (3–)4–8 × 3–3.5(–4.5) μm [av. (± SD) 5.3 (± 1.4) × 3.0 (± 0.6)], aseptate, intercalary conidia ovoid, ellipsoid, 6.5–15 × (3–)3.5–4 μm [av. (± SD) 10.0 (± 2.8) × 3.3 (± 0.4)], 0–1-septate, septa often not very conspicuous, with 1–3(–4) distal scars, secondary ramoconidia ellipsoid to subcylindrical, 11–27 × (3–)3.5–4.5 μm [av. (± SD) 17.1 (± 5.1) × 3.5 (± 0.5)], 0–2(–3)-septate, with (1–)2–4(–5) distal scars, attenuated towards apex and base, pale olivaceous-brown, verruculose to short spinulose, spines up to 0.5 μm high, walls more or less unthickened, hila conspicuous, subdenticulate, 0.5–2 μm diam, thickened and darkened-refractive; microcyclic conidiogenesis occurring forming secondary conidiophores.
In vivo (on Asteriscus sericeus): Conidiophores cylindrical-oblong, subnodulose with small lateral shoulders, or slightly geniculate-sinuous, sometimes once branched towards the apex, very long, up to 425 μm or even longer, at the base about 7–8 μm wide, slightly attenuated towards the apex and somewhat paler, 4–6.5 μm wide, medium to dark brown at the base, almost smooth to often asperulate or minutely verruculose, walls thickened, two-layered, 1–1.5 μm thick. Ramoconidia up to 54 μm long, aseptate, asperulate or slightly verruculose. Conidial chains somewhat longer than in culture, small terminal conidia globose, subglobose or obovoid, 3–5 × (2–)2.5–4 μm, almost smooth to irregularly rough-walled, intercalary conidia 4.5–15 × 3–4.5(–5) μm, secondary ramoconidia up to 25 μm long, 4–6 μm wide, occasionally swollen up to 8 μm, 0–2(–3)-septate, septa becoming sinuous with age, pale to medium brown, almost smooth to asperulate or densely minutely verruculose; microcyclic conidiogenesis occurring.
Culture characteristics: Colonies on PDA reaching 53–63 mm after 14 d, pale olivaceous-grey to olivaceous-grey, reverse iron-grey to olivaceous-black, floccose to fluffy; margins grey-olivaceous, feathery, narrow; aerial mycelium abundant, pale olivaceous-grey, fluffy to floccose; growth effuse with somewhat elevated colony centre. Colonies on MEA attaining 41–45 mm, grey-olivaceous, pale olivaceous-grey towards margins, reverse iron-grey, velvety to floccose; margins white, feathery, narrow; aerial mycelium sparse, smoke-grey, floccose; growth effuse. Colonies on OA reaching up to 59 mm, grey-olivaceous, olivaceous-grey towards margins, reverse olivaceous-grey, with pale greenish grey margins, velvety to floccose; margins narrow, glabrous; aerial mycelium floccose, loose; growth effuse, flat; without prominent exudates, sporulation profuse.
Specimens examined: Germany, Nordrhein-Westfalen, Essen, isol. from Asteriscus sericeus (Asteraceae), 10 Sep. 2006, coll. N. Ale-Agha, isol. P.W. Crous, CPC 13365–13367. Netherlands, isol. from tempeh, isol. by J.P.A. Stevense, CBS 284.84. New Zealand, contaminant on culture plate, 1 Aug. 2007, C.F. Hill (CBS H-22364, holotype; ex-type culture CBS 140493 = CPC 14709 = ICMP 170869). Slovenia, Sečovlje, 45.4767, 13.623, saltern, hypersaline water from precrystalisation pond, 2004, P. Zalar, CPC 12055 = EXF-2288. USA, isolated from grape berry, F.M. Dugan lab, CBS 113751.
Substrate and distribution: On plant material, tempeh, fruits and hypersaline water; Australasia (New Zealand), Europe (Germany, Netherlands, Slovenia), North America (USA).
Notes: With its ornamented conidia and the geniculate subnodulose conidiophores this species belongs to the herbarum species complex. It resembles C. stanhopeae, a species described by Allescher (1895) from faded leaves of Stanhopea (Orchidaceae) from the botanical garden in Munich. The latter species also possesses quite long conidiophores with the conidiogenous loci often arranged at about the same level (like a garland around the stalk) and very similar conidial measurements (Bensch et al. 2012). However, we hesitate in using this name for the isolates cited above since C. stanhopeae is only known from the type specimen and none of the strains listed above were isolated from a host belonging to the Orchidaceae. Therefore, we prefer to introduce a new name. The conidiophores of C. aggregatocicatricatum are longer (both in vivo and in vitro) and somewhat wider than in C. stanhopeae.
Cladosporium allicinum (Fr.: Fr.) Bensch et al., Stud. Mycol. 72: 50. 2012.
Specimens examined: Bulgaria, Hubavene, isol. from bean, food, Jan. 2007, B.A. Andersen, CPC 14303 = BA 1702. France, Larnas, isol. from an unidentified tree, 21 Aug. 2007, P.W. Crous, CPC 14268. Germany, isol. from Robinia pseudoacacia (Fabaceae), leaf on ground, 11 Jan. 2015, coll. R. Jarling & R. Schumacher, isol. P.W. Crous, CPC 21906; Gerolstein, Roter Hecke, isol. from leaf spot of Acer campestre (Sapindaceae), 1 Jul. 1992, H.A. van der Aa, CBS 420.92; Bavaria, Munich, park of Nymphenburg palace, isol. from aecia of Puccinia bromina subsp. symphyti-bromarum var. paucispora, 2006, K. Schubert, CPC 13146. Japan, isol. from unknown substrate by Kurata, CBS 188.53 = IFO 5267. Netherlands, isol. from sputum of man, CBS 160.59; Delft, isol. from metal, CBS 155.60; Nijmegen, isol. from Alnus glutinosa (Betulaceae), 2 Jun. 2009, W. Quadvlieg, CPC 16759; Rotterdam, isol. from frozen Phaseolus vulgaris (Fabaceae), isol. by J.C. Mooi, CBS 121.47 = VTT D-76045; Zwolle, isol. from outside air, 1 Jul. 2007, Applied and Industrial Mycology group at CBS, CPC 14194. Senegal, Louga, from Arachis hypogaea (Fabaceae), 2011, M.P. Sarr, CPC 21646. South Africa, Western Cape Province, Fernkloof, isol. from Nivenia stokoei (Iridaceae), 4 May 2010, P.W. Crous, CPC 18260. Switzerland, Kt. Graubünden, Val Tuors, isol. from Centaurea rhapontica = Rhaponticum scariosum subsp. rhaponticum (Asteraceae), isol. by E. Müller on 21 Jul. 1953, CBS 374.53 = IMI 163999.
Cladosporium angustiherbarum Bensch, Crous & U. Braun, sp. nov. MycoBank MB814623. Fig. 6, Fig. 7.
Fig. 6.
Cladosporium angustiherbarum (CBS 140479). A–G, I. Conidiophores and conidial chains. H. Tip of a conidiophore and ornamented conidia. Scale bars = 10 μm.
Fig. 7.
Cladosporium angustiherbarum (CBS 140479). A. View on the agar surface showing conidiophores sprouting from structures beneath the agar surface or arising from aerial hyphae with conidial chains. B. Running hyphae with a number of conidia. Note the visibility of septa in these hyphae. Behind the septa, initials of conidiophores can be observed as bulges. C. A bundle of aerial hyphae bearing a number of conidiophores that stick out in different directions. D. Netting of aerial hyphae with conidiophores. E, H. Conidiophores with conidial chains. F–G. Conidial chains showing details of surface ornamentation, especially in (G). Ornamentation is present early during the formation of the conidia (F) I. Tip of a capitate conidiophore with conidial chains. I. Secondary ramoconidia with details of conidial hila (including one conidium initial). Scale bars = 2 (G), 5 (E–F, I–J), 10 (B–D, H), 20 (A) μm.
Etymology: Name refers to its morphological similarity with C. herbarum but also to its narrower conidiophores and conidia.
Mycelium loosely branched, 1–3(–5) μm wide, septate, subhyaline or pale olivaceous-brown, smooth or verruculose, with constrictions and swellings, walls unthickened. Conidiophores macro-, semimacro- and micronematous, arising terminally or laterally from hyphae, solitary, erect. Macronematous conidiophores mostly arising laterally from hyphae, with a cylindrical stipe, towards the apex once or several times subnodulose, sometimes in short succession giving the upper part a knotty / gnarled appearance, or with lateral prolongations or swollen shoulders, unbranched, occasionally once branched, branchlets also with swellings, 5–60 × (2–)2.5–3.5(–4) μm, swellings 3–6.5 μm diam, septate, septa neither constricted nor darkened, pale or medium olivaceous-brown, smooth, walls somewhat thickened. Conidiogenous cells integrated, mainly terminal, occasionally intercalary, nodulose, mostly with a single swelling at the apex or per cell in intercalary ones, or laterally prolongating and swollen at the apex or with few swellings and lateral shoulders and geniculations in short succession with up to seven loci crowded towards the apex, somewhat constricted at nodules, cells 5–19 μm long, loci protuberant, subdenticulate, 1–1.5 μm diam. Micro- and semimacronematous conidiophores commonly formed either as short lateral outgrowth of hyphae or filiform and longer, maximum length ambiguous, often arising terminally from hyphae, 3–100 μm long or even longer, 1–2 μm wide, mostly without distinct swellings, multiseptate, some septa distinctly thickened and darkened, smooth or minutely verruculose or verruculose, subhyaline or pale olivaceous, walls unthickened. Conidiogenous cells integrated, terminal or intercalary, narrowly cylindrical or subnodulose, up to 22 μm long, with up to four loci per cell. Ramoconidia with a truncate, non-cladosporioid base (sensu Bensch et al. 2012) not observed. Conidia catenate, in branched or short unbranched chains with up to 4(–5) conidia in the terminal unbranched part of the chain, small terminal conidia subglobose, obovoid or ellipsoid, occasionally globose, (3.5–)4–9 × 3.5–4.5(–5) μm [av. (± SD) 6.1 (± 1.5) × 4.1 (± 0.5)], aseptate, intercalary conidia limoniform, ellipsoid, 5–9 × (3.5–)4–4.5(–5) μm [av. (± SD) 7.5 (± 1.3) × 4.3 (± 0.4)], 0(–1)-septate, with 1(–2) distal hila, secondary ramoconidia ellipsoid or subcylindrical, sometimes obclavate often formed by semimacronematous conidiophores, (7–)8–21 × (3–)4–6(–6.5) μm [av. (± SD) 14.1 (± 4.8) × 5.1 (± 0.9)], obclavate ones up to 25 μm long and 4 μm wide, 0–1(–2)-septate, with 1–2(–3) distal hila, pale or medium olivaceous-brown, minutely verruculose to verrucose, walls slightly thick-walled, hila protuberant, 1–1.5 μm diam, thickened and darkened-refractive; microcyclic conidiogenesis occurring, secondary conidiophores up to 30 μm long.
Culture characteristics: Colonies on PDA attaining 57–70 mm after 14 d, iron-grey, olivaceous-grey towards margins, grey-olivaceous, reverse greyish blue to iron-grey, fluffy; margin feathery; aerial mycelium loose, diffuse, sometimes high and fluffy; growth flat, few prominent exudates formed, sporulation profuse. Colonies on MEA reaching 58–63 mm, olivaceous, iron-grey towards margins, reverse iron-grey, velvety; margin white, feathery; aerial mycelium loose diffuse, rarely forming small white fluffy patches; colony centre radially furrowed, wrinkled, without prominent exudates, sporulation profuse. Colonies on OA attaining up to 65 mm, iron-grey, olivaceous due to sporulation, reverse leaden-grey to iron-grey, velvety or felty; aerial mycelium loose diffuse to denser and felty, mainly in colony centre, growth flat, without exudates.
Specimen examined: USA, Utah, Escalante National Monument, Grand Staircase, isol. from Pinus ponderosa (Pinaceae), Oct. 2009, coll. W. Quaedvlieg, isol. P.W. Crous (CBS H-22351, holotype; ex-type culture CBS 140479 = CPC 17814).
Notes: The conidia and conidiophores of this new species, which belongs to the herbarum species complex, remind one of C. herbarum but it differs in having shorter and narrower conidiophores as well as narrower conidia. Phylogenetically it is allied to C. phlei, albeit with low support, but morphologically C. phlei differs in having longer and wider conidiophores and conidia. Furthermore, the conidia in C. phlei are formed singly in vivo (in vitro solitary or in short chains).
There are three other species that have been reported from Argentina from the same host, Pinus ponderosa, namely C. pini-ponderosae, C. chubutense and C. cladosporioides s. lat. (Schubert et al., 2009, Bensch et al., 2010, Bensch et al., 2012). Cladosporium pini-ponderosae can be readily distinguished by having wider, non-nodulose conidiophores, longer and somewhat wider intercalary conidia and secondary ramoconidia; C. chubutense forms subcylindrical ramoconidia, longer conidiophores and longer and somewhat narrower secondary ramoconidia; and C. cladosporioides has non-nodulose conidiophores as well as longer and smooth conidia.
Cladosporium angustiterminale Bensch, Crous & U. Braun, sp. nov. MycoBank MB814624. Fig. 8.
Fig. 8.
Cladosporium angustiterminale (CBS 140480). A–F. Conidiophores and conidial chains. G. Peculiar conidiogenesis characterised by forming several conidiogenous loci at about the same level, followed by continuing growth with narrower conidiophores and additional loci at a higher level. Scale bars = 10 μm.
Etymology: Name refers to the peculiar conidiogenesis characterised by forming several conidiogenous loci, followed by proliferated, narrower conidiophores (Fig. 8G).
Mycelium sparse, branched, 1.5–3 μm wide, septate, subhyaline or pale olivaceous, smooth or often verruculose. Conidiophores macronematous or semi-macronematous, cylindrical-oblong, non-nodulose, geniculate-sinuous, occasionally continuing growth with up to an 90° angle, unbranched or once branched, up to 175 μm long, 3–4.5(–5) μm wide, semimacronematous conidiophores narrower, 2–2.5 μm wide, multiseptate, usually 1–5-septate, pale olivaceous to medium olivaceous-brown, smooth or almost so, occasionally asperulate or minutely verruculose, especially towards the base, walls unthickened or slightly thickened. Conidiogenous cells terminal and intercalary, cylindrical, non-nodulose but often geniculate-sinuous at or towards the apex, loci crowded at the apex and also at a lower level forming clusters of pronounced scars, 2–5 conidiogenous loci formed at about the same level, after conidiogenesis conidiophores can start growing again with stalks often being narrower and at a higher level additional loci may be formed, loci often situated at lateral shoulders due to sympodial proliferation or displaced to the side of stalks, with up to seven loci per cell, 12–25(–45) μm long, loci protuberant, 1–2 μm diam, thickened and darkened-refractive. Ramoconidia commonly formed, cylindrical-oblong, 19–35 × 2.5–4 μm, 0–1(–2)-septate, smooth, with (2–)3–5 distal hila, base unthickened, 2.5–3 μm wide, somewhat refractive, differentiation between ramoconidia and secondary ramoconidia under light microscopy sometimes not evident. Conidia catenate, in branched chains, branching in all directions, with 1–3 conidia in the terminal unbranched part of the chain, small terminal conidia subglobose, obovoid or ellipsoid, 2.5–5 × 2–2.5 μm [av. (± SD) 3.7 (± 0.7) × 2.2 (± 0.2)], aseptate, apex rounded, intercalary conidia ellipsoid or limoniform, 4–9.5(–13.5) × (2–)2.5–3(–3.5) μm [av. (± SD) 7.4 (± 2.7) × 2.9 (± 0.4)], aseptate, rarely 1-septate, with 1–3(–5) distal hila, crowded at the distal end, (0.5–)0.8–1.2 μm diam, secondary ramoconidia ellipsoid, subcylindrical or cylindrical, (7–)8–25 × (2.5–)3–3.5(–4) μm [av. (± SD) 15.5 (± 6.1) × 3.4 (± 0.8)], occasionally swollen up to 6.5 μm, 0–1-septate, septum median, pale olivaceous or pale olivaceous-brown, smooth or almost so, walls unthickened or only slightly thickened, with (2–)3–5(–6) hila at the apex forming clusters of pronounced scars, 1–2(–2.5) μm diam, subdenticulate, thickened and darkened-refractive.
Culture characteristics: Colonies on PDA attaining 46–64 mm after 14 d, olivaceous-grey to pale olivaceous-grey, grey-olivaceous towards margins, reverse olivaceous-black, fluffy, margins white, somewhat feathery, broad, aerial mycelium loose diffuse to denser and fluffy, growth flat. Colonies on MEA reaching 37–47 mm, grey-olivaceous to olivaceous-grey, sometimes pale olivaceous-grey at margins, reverse olivaceous-grey to iron-grey, fluffy, margins white, somewhat feathery, aerial mycelium fluffy, growth low convex, radially furrowed and folded. Colonies on OA 50–57 mm, olivaceous-grey to pale olivaceous-grey, reverse leaden-grey to iron-grey, powdery to fluffy, margins crenate, glabrous, aerial mycelium loose diffuse to fluffy, abundant, growth flat. Without prominent exudates; sporulation profuse on all media.
Specimen examined: Australia, Western Australia, Augusta, isol. from Banksia grandis (Proteaceae), 2 Aug. 2008, coll. A.R. Wood, isol. P.W. Crous (CBS H-22352, holotype; ex-type culture CBS 140480 = CPC 15564).
Notes: This species, which belongs to the cladosporioides species complex (Fig. 1), has an interesting conidiogenesis with conidiophores proliferating after giving rise to conidia in being distinctly narrower or somewhat constricted above the conidiogenous zone. In C. rectoides the conidiophores sometimes also proceed to grow at an angle of 45–90° but its ramoconidia, intercalary conidia and secondary ramoconidia are longer than in C. angustiterminale.
Cladosporium asperulatum Bensch et al., Stud. Mycol. 67: 21. 2010.
Specimen examined: Mexico, Tlacotepec, isol. from seeds of Glycine max (Fabaceae), 16 Sep. 2008, coll. M. de Jesús Yáñez-Morales, isol. P.W. Crous, CPC 15614.
Notes: This Mexican isolate fits the species concept of Cladosporium asperulatum (Bensch et al. 2010), but the conidia are mostly smooth or almost so, rarely asperulate, and small terminal and intercalary conidia are somewhat narrower than described in the type in being 1.5–2.5(–3) μm wide. Cladosporium asperulatum is thus far known from India and Portugal, and was isolated from hosts belonging to Myrtaceae and Proteaceae.
Cladosporium austroafricanum Bensch, Crous & U. Braun, sp. nov. MycoBank MB814625. Fig. 9.
Fig. 9.
Cladosporium austroafricanum (CBS 140481). A–F. Conidiophores and conidial chains. G. Conidia. Scale bars = 10 μm.
Etymology: Name refers to the country of origin, South Africa.
Mycelium loosely branched, (1–)2–5 μm wide, multiseptate, sometimes slightly swollen, subhyaline, pale olivaceous to medium olivaceous-brown, densely verruculose, sometimes almost smooth, walls unthickened or slightly thick-walled, sometimes aggregated and forming loose hyphal aggregations. Conidiophores macro- or semimacronematous, erect, arising solitarily from hyphae or in loose groups from loose hyphal aggregations, more or less straight, cylindrical-oblong, neither nodulose nor geniculate, up to 210 μm long, 3–5 μm wide, mostly unbranched, sometimes branched, when branched then branchlets often quite long, multiseptate, sometimes slightly constricted at septa and attenuated towards the base, pale olivaceous to medium olivaceous-brown, smooth, walls unthickened or thickened; semimacronematous conidiophores paler and narrower, about 2–2.5 μm wide. Conidiogenous cells integrated, mainly terminal, cylindrical or cylindrical-oblong, mostly neither nodulose nor geniculate, rarely geniculate-sinuous, 11–30(–45) μm long, usually with (1–)2–3 conspicuous loci at the outermost apex, subdenticulate, 1–2.5 μm diam, thickened and darkened-refractive. Ramoconidia occasionally formed, base 3–3.5 μm wide, unthickened or slightly thickened, somewhat refractive. Conidia catenate, in branched chains, branching in all directions, with 1–4 conidia in the terminal unbranched part of the chain, small terminal conidia obovoid or ellipsoid, 2.5–5 × (1.5–)2–2.5 μm [av. (± SD) 3.9 (± 0.7) × 2.1 (± 0.3)], intercalary conidia limoniform, ovoid or ellipsoid, 4–12 × 2–3(–3.5) μm [av. (± SD) 7.3 (± 2.7) × 2.7 (± 0.5)], aseptate, with 1–4 distal hila, secondary ramoconidia ellipsoid, subcylindrical or cylindrical-oblong, (8–)11–40 × (2.5–)3–4 μm [av. (± SD) 21.8 (± 9.9) × 3.4 (± 0.5)], 0–1(–2)-septate, septum median or often in the upper half, somewhat darkened, with (2–)3–4(–6) distal hila, pale olivaceous or olivaceous-brown, smooth, walls unthickened or almost so, hila conspicuous, subdenticulate, 0.5–2.5 μm diam, thickened and darkened-refractive; microcyclic conidiogenesis not occurring.
Culture characteristics: Colonies on PDA attaining 70–83 mm after 14 d, smoke-grey to pale olivaceous-grey, grey-olivaceous towards margins, reverse olivaceous-black, fluffy to wooly-felty; margins feathery; aerial mycelium abundant, covering almost the whole colony; growth flat; several very small exudates formed. Colonies on MEA reaching 67–72 mm, pale olivaceous-grey to smoke-grey, reverse olivaceous-grey, wooly-felty or fluffy; margins white, glabrous and narrow; aerial mycelium abundantly formed, covering large parts of the colony, dense; growth flat, radially furrowed and somewhat folded or wrinkled, without exudates. Colonies on OA attaining 60–68 mm, smoke-grey or white, grey-olivaceous at margins, reverse leaden-grey to olivaceous-grey, fluffy due to abundant, dense and high aerial mycelium; margins regular, glabrous; without prominent exudates. Sporulation profuse on all media.
Specimen examined: South Africa, Western Cape Province, Cape Town, next to M3 road, leaf litter, 2 Jun. 2009, coll. M. Gryzenhout, isol. P.W. Crous (CBS H-22349, holotype; ex-type culture CBS 140481 = CPC 16763).
Notes: Conidiophores and conidia resemble those of C. cladosporioides but the two species are phylogenetically distinct and in C. austroafricanum the unbranched upper part of the conidial chain is much shorter with only 1–4 conidia, its secondary ramoconidia are more frequently 1-septate and somewhat longer, and ramoconidia are only occasionally formed.
Cladosporium austrohemisphaericum Bensch, Crous & U. Braun, sp. nov. MycoBank MB814626. Fig. 10.
Fig. 10.
Cladosporium austrohemisphaericum (CBS 140482). A–F. Micro-, semimacro- and macronematous conidiophores and conidial chains. G. Ramoconidium and conidia. H. Conidia. Scale bars = 10 μm.
Etymology: From the Latin “auster” (= south) and “hemisphaerium”, referring to the Southern Hemisphere, the origin of this species.
Mycelium immersed, sparingly branched, 1–4 μm wide, septate, subhyaline to very pale olivaceous-brown, asperulate, minutely verruculose, verruculose or even verrucose, walls unthickened, without any swellings and constrictions. Conidiophores micro- to semimacronematous or macronematous, arising terminally and laterally from erect or ascending hyphae, erect, solitary, straight to flexuous, filiform to narrowly cylindrical-oblong, sometimes once geniculate at or towards the apex, unbranched or once branched, branches often only as short lateral peg-like prolongations just below a septum, 20–135(–180) × (2–)2.5–3.5 μm, at the base up to 4.5 μm wide, septate, often only with up to four not very conspicuous septa, sometimes disarticulating at septa and forming ramoconidia and fragments, subhyaline to pale or medium olivaceous-brown, minutely verruculose, asperulate, sometimes verrucose or irregularly rough-walled especially towards the base and almost smooth at or towards the apex, walls unthickened or slightly thick-walled, slightly attenuating towards the apex, sometimes conidiophores reduced to conidiogenous cells. Conidiogenous cells integrated, mostly terminal, sometimes intercalary, filiform to narrowly cylindrical-oblong, sometimes once geniculate, non-nodulose, (6–)13–45(–60) μm long, with 1–3(–4) apical loci, conspicuous, subdenticulate to denticulate, 1–2 μm diam, thickened and darkened-refractive. Ramoconidia cylindrical-oblong, 12–36 × 2–3(–3.5) μm, 0–1(–2)-septate, subhyaline to pale olivaceous-brown, almost smooth to asperulate or minutely verruculose, base broadly truncate, 2–3 μm wide, neither thickened nor darkened. Conidia numerous, catenate, formed in branched chains, branching in all directions, in younger chains often dichotomously branched, 1–3 conidia in the terminal unbranched part of the chain, small terminal conidia globose, subglobose to obovoid or ovoid, 2–5(–7) × (1–)1.5–3 μm (av. ± SD: 3.3 ± 1.0 × 2.1 ± 0.5), aseptate, subhyaline to pale or medium olivaceous-brown, minutely verruculose to verruculose or verrucose, hila 0.5–0.8 μm diam or narrower, intercalary conidia ovoid to ellipsoid-ovoid, 4–11 × 2–3.5 μm (av. ± SD: 7.1 ± 2.1 × 2.6 ± 0.4), 0(–1)-septate, septa sometimes not very conspicuous, surface ornamentation as in small terminal conidia, rounded or only very slightly attenuated towards the ends, with 2–4 distal hila, 0.5–1 μm diam, secondary ramoconidia ellipsoid to subcylindrical, (8–)10–27(–30) × 2–3.5(–4) μm (av. ± SD: 18.5 ± 6.2 × 2.9 ± 0.4), 0–1(–2)-septate, with age constricted at septa, septum median or in the upper half, 1–3(–4) distal hila, subhyaline to pale olivaceous-brown, almost smooth to loosely verruculose or irregularly rough-walled, not or only slightly attenuated towards apex and base, hila conspicuous, subdenticulate, 1–2 μm diam, thickened and darkened-refractive; microcyclic conidiogenesis not occurring.
Culture characteristics: Colonies on PDA attaining 35–45 mm after 14 d, grey-olivaceous to dull green or iron-grey, reverse greyish blue to olivaceous-black, velvety to powdery, margin white, narrow, glabrous to feathery, regular, aerial mycelium absent or sparse, loose, diffuse, growth flat or low convex, without prominent exudates, sporulation profuse. Colonies on MEA reaching 26–44 mm, grey-olivaceous to greenish grey or glaucous-grey at margins, paler in the centre, reverse olivaceous to olivaceous-grey or iron-grey, velvety to powdery, margin white, very narrow, feathery, radially furrowed, growth flat to low convex with slightly elevated colony centre, wrinkled and folded, few prominent exudates formed, sporulation profuse. Colonies on OA attaining 26–34 mm, grey-olivaceous or iron-grey, smoke-grey due to abundant sporulation, reverse leaden-grey to leaden-black, powdery, margin white, very narrow, glabrous, slightly undulate, aerial mycelium absent or diffuse, without prominent exudates.
Specimens examined: Australia, Queensland, Brisbane, Brisbane Botanical Garden, isol. from Musa sp. (Musaceae), 14 Jul. 2009, P.W. Crous, CPC 17029. South Africa, Western Cape Province, Betty's bay, isol. from Cussonia thyrsiflora (Araliaceae), 14 Jan. 2009, P.W. Crous, CPC 16250. New Zealand, Auckland, Morrin Reserve, −37.00, 175.00, isolated from black mould on the surface of a fruit of Lagunaria patersonia (Malvaceae), 18 Apr. 2005, C.F. Hill, Hill 1163 (CBS H-22350, holotype; ex-type culture CBS 140482 = CPC 12068).
Substrate and distribution: On plant material and fruits of different hosts; Australasia (Australia, New Zealand), South Africa.
Notes: With its ornamented globose, subglobose or ovoid terminal conidia and the non-nodulose conidiophores C. austrohemisphaericum belongs to the sphaerospermum species complex but doesn't cluster with C. sphaerospermum, the eponymous species. Both morphologically and phylogenetically it is allied to C. langeronii and C. psychrotolerans which form a separate clade distant from C. sphaerospermum. However, C. langeronii differs in having usually shorter and wider ramoconidia, (10–)11–22(–42) × (3–)3.5–4.5(–5) μm, wider and darker, often medium or dark brown conidia; and C. psychrotolerans deviates in having longer and wider ramoconidia, 19–43(–47) × (2–)3–4(–4.5) μm, and smooth or minutely verruculose conidia. Conidial measurements of C. dominicanum are also similar but ramoconidia are rarely formed in that species and its conidia are smooth or almost so (Zalar et al., 2007, Bensch et al., 2012).
Cladosporium cladosporioides (Fresen.) G.A. de Vries, Contr. Knowl. Genus Cladosporium: 57. 1952.
Specimens examined: Mexico, Mexico State, forest garden, isol. from pine needles plus insects, 22 Mar. 2010, coll. M. de Jesús Yáñez-Morales, isol. P.W. Crous, CPC 18138; Montecillo, isol. from a wild plant, 1 Oct. 2008, coll. M. de Jesús Yáñez-Morales, isol. P.W. Crous, CPC 15626; Tlacotepec, isol. from Rumex sp. (Polygonaceae), 22 Sep. 2008, coll. M. de Jesús Yáñez-Morales, isol. P.W. Crous, CPC 15610; isol. from a wild tree, 16 Sep. 2008, coll. M. de Jesús Yáñez-Morales, isol. P.W. Crous, CPC 15615.
Cladosporium delicatulum Cooke, Grevillea 5: 17. 1876.
Specimen examined: Mexico, Tlacotepec, isol. from Juglans regia (Juglandaceae), 16 Sep. 2008, coll. M. de Jesús Yáñez-Morales, isol. P.W. Crous, CPC 15612.
Notes: Conidiophores, ramoconidia and conidia of the Mexican isolate fit the species concept of C. delicatulum very well (Bensch et al. 2010). It is the first record of this species for Mexico.
Cladosporium dominicanum Zalar et al., Stud. Mycol. 58: 169. 2007.
Specimens examined: Philippines, isol. from Dracaena fragrans (Asparagaceae), 2008, coll. C.J.R. Cumagun, isol. P.W. Crous, CPC 15932. Taiwan, FIRDI campus, isol. from unknown vine, 17 Dec. 2011, P.W. Crous, CPC 20109.
Notes: Until now Cladosporium dominicanum has been isolated from fruit surfaces and hypersaline waters in (sub)tropical climates of Asia (Iran) and Central America (Dominican Republic) (Zalar et al., 2007, Bensch et al., 2012). With these two isolates it is now also reported from the Philippines and Taiwan.
Cladosporium gamsianum Bensch et al., Stud. Mycol. 67: 49. 2010.
Specimen examined: Mexico, South region, Tamanlipas, isol. from soybean seeds (Glycine max, Fabaceae), 1 Dec. 2007, coll. M. de Jesús Yáñez-Morales, isol. P.W. Crous, CPC 15617.
Notes: The morphology of this Mexican isolate fits the species concept of C. gamsianum. The conidiophores with the typical annellations at the apex are formed solitarily or fasciculate with up to four conidiophores in small fascicles. They are smooth to asperulate. The conidia, especially secondary ramoconidia, are somewhat longer [10–20 × 2.5–3(–3.5) μm, 0–1-septate], with up to five conidia in the terminal unbranched part of the conidial chains.
Until now C. gamsianum was only known from the type locality in South Africa. This is the first record from Mexico, where it was also isolated from a new host, which implies that C. gamsianum may probably be more widely distributed.
Cladosporium inversicolor Bensch et al., Stud. Mycol. 67: 55. 2010.
Specimen examined: South Africa, Cape, Kirstenbosch Botanical Garden, isol. from Freylinia lanceolata (Scrophulariaceae), 8 May 2010, P.W. Crous, CPC 18238.
Note: Previously reported from Europe, North and South America, the species is now also recorded from South Africa.
Cladosporium ipereniae Bensch, Crous & U. Braun, sp. nov. MycoBank MB814627. Fig. 11, Fig. 12.
Fig. 11.
Cladosporium ipereniae (CBS 140483). A–D. Unbranched or branched macronematous conidiophores and conidial chains. E–G. Tip of conidiophores and conidia with variable surface ornamentation. Scale bars = 10 μm.
Fig. 12.
Cladosporium ipereniae (CBS 140483). A. Overview of conidiophores and hyphae. B, D. Conidiophores arising from plagiotropous hyphae or sprouting from structures beneath the agar surface with conidial chains. C. Branched conidiophore arising from a plagiotropous hypha with conidial chains. E–F. Tip of conidiophores with conidial chains. Note the constricted septum of the conidiophore in (E). G. Branchlet of a conidiophore with conidia. Note the round shape of the conidia and the elevated scar region. Note also the very short conidiophorous structure on the aerial hyphae. H–I. Conidial chains showing details of the characteristic surface ornamentation that appears to run parallel on the cell wall of larger spores. Note also the scar on the ramoconidium. Scale bars = 2 (H–I), 5 (F–G), 10 (B–C, E), 20 (D), 100 (A) μm.
Etymology: Named after Arien van Iperen, technician at the Centraalbureau voor Schimmelcultures, who collected the type specimen, and for her valuable work on maintaining the numerous Cladosporium isolates.
Mycelium loosely branched, 1.5–5 μm wide, multiseptate, subhyaline to medium olivaceous-brown, smooth, verruculose or irregularly rough-walled, unthickened or somewhat thickened, sometimes aggregated forming ropes of several hyphae or loose stromatic hyphal aggregations of swollen cells, swollen cells 6–9 μm diam, sometimes only a single cell distinctly swollen at the base of conidiophore. Conidiophores macro- and micronematous; macronematous ones solitary, in pairs of two or loosely fasciculate with 3–6 conidiophores in a fascicle, arising terminally or laterally from hyphae or from swollen hyphal cells or from small stromatic hyphal aggregations, subcylindrical or cylindrical, slightly attenuated towards the apex, unbranched, occasionally once or twice branched, non-nodulose, occasionally once geniculate-sinuous at the apex, (10–)35–85 μm long, 2.5–3.5 μm wide at the apex, 3.5–4.5(–5) μm towards or at the base, 1–3-septate, slightly constricted at septa, pale or medium olivaceous or olivaceous-brown, smooth or almost so, sometimes verruculose or irregularly rough-walled, especially towards the base, walls slightly thickened. Micronematous conidiophores subhyaline or pale olivaceous, narrower, 2–3 μm wide. Conidi-ogenous cells integrated, terminal, occasionally intercalary, non-nodulose, sometimes once geniculate at the apex, 11–35 μm long, with (1–)2–4 distal scars, mostly crowded at the outermost apex, loci conspicuous, subdenticulate, 1–1.5 μm diam, thickened and darkened-refractive. Ramoconidia rarely formed. Conidia catenate, branching in all directions or dichotomously branched, 1–4(–5) conidia in the terminal unbranched part of the chain; small terminal conidia subglobose, obovoid, (2.5–)3–4(–5) × (2–)2.5–3(–3.5) μm [av. (± SD) 3.5 (± 0.7) × 2.6 (± 0.4)], apex broadly rounded, distinctly attenuated towards the base, intercalary conidia ovoid, limoniform or ellipsoid, (4–)4.5–8(–10) × (2–)2.5–3.5 μm [av. (± SD) 6.1 (± 1.7) × 3.0 (± 0.4)], aseptate, with 1–2(–4) distal hila, attenuated towards apex and base, secondary ramoconidia ellipsoid or subcylindrical, (5–)6.5–18(–22) × 2.5–3.5(–4.5) μm [av. (± SD) 11.7 (± 4.1) × 3.1 (± 0.4)], aseptate, rarely 1-septate, pale olivaceous-brown or subhyaline, surface ornamentation variable, lightmicroscopically smooth or almost so or often loosely verruculose or sometimes verrucose or irregularly rough-walled, under SEM delicately ornamented showing a somewhat irregularly reticulate surface or slightly to distinctly embossed stripes probably caused by diminishing turgor and shriveling of tender young conidia, walls unthickened or slightly thickened, usually with 3 distal hila, hila subdenticulate, 0.5–1.5(–1.8) μm diam, thickened and darkened-refractive. Microcyclic conidiogenesis usually not occurring, but intercalary and small terminal conidia especially those formed by micronematous conidiophores germinating or rostrate.
Culture characteristics: Colonies on PDA reaching 10–44 mm after 14 d, iron-grey to olivaceous-black, reverse olivaceous-black, velvety or powdery; margins narrow, white, glabrous or feathery, regular, aerial mycelium pale olivaceous-grey, loose to dense, wooly-felty, covering larger parts, mainly in colony centre, growth flat, forming numerous small to large exudates. Colonies on MEA reaching 28–35 mm, greenish grey, glaucous-grey at margins, pale olivaceous-grey due to aerial mycelium, reverse olivaceous-grey, velvety to wooly, margins white, narrow, regular, glabrous, aerial mycelium forming some dense patches in colony centre, growth flat but radially furrowed or wrinkled and folded, without exudates. Colonies on OA attaining 24–33 mm, olivaceous-grey to iron-grey, with patches of white due to dense wooly aerial mycelium, reverse leaden-grey to iron-grey, margins glabrous, regular, growth flat, without exudates; sporulation profuse on all media.
Specimens examined: Chile, La Serrana, isol. from Puya sp. (Bromeliaceae), 7 Dec. 2008, coll. A. van Iperen, isol. P.W. Crous (CBS H-22353, holotype; ex-type culture CBS 140483 = CPC 16238; CPC 16239). USA, California, Oakland, Huckleberry Botanic Regional Preserve, isol. from Arctostaphylos pallida (Ericaceae), 6 Mar. 2009, P.W. Crous, CPC 16855.
Substrate and distribution: On plant material; Chile, USA.
Notes: Cladosporium ipereniae belongs to the cladosporioides species complex and is morphologically and phylogenetically close to C. phyllophilum, C. phyllactiniicola and C. licheniphilum. However, Cladosporium phyllophilum, a species occurring on woody host plants usually associated with Taphrina species, possesses longer and smooth conidia. In C. phyllactiniicola, a mycophilic species occurring on chasmothecia of Phyllactinia guttata, and C. licheniphilum, a species growing on thalli and apothecia of lichens, the conidiogenous loci and conidial hila are wider, 1–2 μm diam, and the conidia are smooth or almost so or sometimes finely asperulate, but never loosely verruculose, verrucose or irregularly rough-walled as sometimes occurring in C. ipereniae. Cladosporium puyae, a new species introduced below, is described from the same host genus from Colombia, but differs in having longer conidia and wider conidiogenous loci and hila.
Cladosporium limoniforme Bensch, Crous & U. Braun, sp. nov. MycoBank MB814628. Fig. 13, Fig. 14.
Fig. 13.
Cladosporium limoniforme (CBS 140484). A–E. Micronematous conidiophores forming large amounts of conidia. F–H. Conidial chains. Scale bars = 10 μm.
Fig. 14.
Cladosporium limoniforme (CBS 140484). A. Overview of a cluster of conidiophores that seem to originate from one base, illustrating the density of conidial chains. B–C, E. Conidiophores and conidial chains either sprouting from structures beneath the agar surface or arising from running hyphae with conidial chains. Note the smooth surface of the conidiophores in contrast to the ornamented conidia (C). Note also the branching conidiophore in (E). D, F. Tip of conidiophores and conidia with details of scars. G–H. Details of conidia, ornamentation and scars. Scale bars = 1 (F), 2 (D, G, H), 10 (B, C, E), 20 (A) μm.
Etymology: Named after the shape of the limoniform intercalary conidia.
Mycelium sparingly formed, usually unbranched, 1.5–3 μm wide, pale olivaceous-brown or subhyaline, asperulate to minutely verruculose, walls unthickened, sometimes forming small ropes of a few hyphae. Conidiophores micronematous to semimacronematous, sometimes macronematous, short, sometimes only as very short lateral branches of hyphae, not very prominent, sometimes hardly distinguishable from hyphae, usually reduced to conidiogenous cells or 1(–2)-septate, terminally arising from hyphae, occasionally laterally arising from plagiotropous hyphae, unbranched, usually neither geniculate nor nodulose, rarely once geniculate, 5–90(–130) × (1–)2–3(–4) μm, mostly only up to 60 μm long, subhyaline, pale brown to pale olivaceous-brown, concolourous with hyphae, smooth or almost so to asperulate or somewhat irregularly rough-walled. Conidiogenous cells integrated, terminal, occasionally intercalary, narrowly cylindrical, neither geniculate nor nodulose, 15–34(–50) μm long, with 1–3 pronounced scars at the apex or situated on short lateral outgrowths at the apex in terminal cells, in intercalary cells a single or two loci situated on small lateral prolongations just below a septum, conidiogenous loci 1–1.5 μm diam, somewhat thickened and darkened-refractive. Ramoconidia 15–34 μm long, 0(–1)-septate, base 2–2.5 μm wide, somewhat refractive. Conidia catenate, very numerous, usually 3–7(–8) conidia in the terminal unbranched part of the chain, relatively short, pale olivaceous-brown or pale brown, ornamentation variable, loosely verruculose, sometimes somewhat spiny or irregularly rough-walled, walls unthickened, small terminal conidia obovoid to subglobose, apex rounded, attenuated towards the base, 3–4 × 2–2.5 μm [av. (± SD) 4.0 (± 0.7) × 2.4 (± 0.4)], aseptate, intercalary conidia limoniform, ovoid to ellipsoid, sometimes fusiform, sometimes rostrate, 4–10(–12) × 2.5–3(–3.5) μm [av. (± SD) 7.6 (± 2.2) × 2.9 (± 0.4)], aseptate, rarely 1-septate, attenuated towards apex and base, with 1–3 distal hila, secondary ramoconidia ellipsoid, fusiform to subcylindrical, (8–)9.5–23(–30) × 3–3.5 μm [av. (± SD) 15.7 (± 4.8) × 3.2 (± 0.3)], 0–1-septate, pale olivaceous-brown or pale brown, surface ornamentation variable, loosely verruculose, sometimes somewhat spiny or irregularly rough-walled, walls unthickened, with 2–3(–4) distal hila, hila protuberant, 0.5–1(–1.5) μm diam, slightly thickened and somewhat darkened-refractive; microcyclic conidiogenesis occasionally occurring.
Culture characteristics: Colonies on PDA attaining 34–65 mm after 14 d, smoke-grey, iron-grey to dark grey-olivaceous, sometimes dull green due to abundant sporulation, reverse iron-grey to olivaceous-black, velvety to granular or floccose; margins regular, broad, white, glabrous to feathery; aerial mycelium sparse, diffuse, sometimes more abundantly formed in colony centre and then villose to densely tufted; growth flat, regular, sometimes with numerous small to large prominent exudates. Colonies on MEA reaching 39–57 mm, grey-olivaceous, greenish olivaceous to smoke-grey or glaucous-grey towards margins, sometimes large parts smoke-grey to glaucous-grey or whitish due to aerial mycelium, reverse olivaceous-grey, iron-grey to black, granular, velvety to floccose; margins regular, narrow to broad, white, feathery to glabrous; aerial mycelium sparse or covering large parts of the colony; growth flat with somewhat elevated colony centre, radially furrowed, sporulation profuse. Colonies on OA attaining up to 69 mm, grey-olivaceous to olivaceous due to abundant sporulation forming concentric zones, reverse pale olivaceous-grey to olivaceous-grey or leaden-grey, velvety, floccose to felty; margins regular, narrow to broad, glabrous to feathery, greenish olivaceous; aerial mycelium absent, sparse or more abundantly formed covering large parts of the colony, smoke-grey; growth flat, without prominent exudates, sporulation profuse.
Specimens examined: Cyprus, Polis, isol. from Eucalyptus sp. (Myrtaceae), 18 Mar. 2007, coll. A. van Iperen, isol. P.W. Crous, CPC 13923. Egypt, isolated from Musa acuminata (Musaceae), 2005, coll. R.S. Summerbell, isol. P.W. Crous (CBS H-22354, holotype; ex-type culture CBS 140484 = CPC 12039). Israel, Dead Sea, Ein Bokek, isol. from hypersaline water, 2004, P. Zalar, EXF-1062 = CPC 12049; Ein Gedi, 31.45, 35.3833, isol. from hypersaline water, 2004, P. Zalar, EXF-1060 = CPC 12048, EXF-1081 = CPC 12050. USA, isolated from grape berry, F.M. Dugan lab, CBS 113737. Unknown, from tomato, CPC 18086 = KSU C1.
Substrate and distribution: Isolated from plant material and hypersaline water; Africa (Egypt), Asia (Israel), Europe (Cyprus) and North America (USA).
Notes: Cladosporium limoniforme is well characterised by its few micronematous conidiophores forming large amounts of conidia and its limoniform intercalary conidia. Conidial surface ornamentation is typical for species belonging to the herbarum complex. It is phylogenetically but not morphologically allied to C. aggregatocicatricatum. The latter species clearly differs in having much longer macronematous conidiophores being once or several times slightly to distinctly geniculate-sinuous or subnodulose with clusters of pronounced scars at apices or intercalary. Morphologically C. limoniforme resembles C. subtilissimum and C. salinae. However, Cladosporium subtilissimum possesses slightly wider conidiophores, longer and wider conidia as well as wider conidiogenous loci and hila. Cladosporium salinae also forms micronematous conidiophores with similar measurements, but the conidiophores in C. salinae are usually slightly or distinctly geniculate-sinuous at or towards the apex forming sympodial clusters of pronounced denticulate Conidi-ogenous loci. Furthermore, the conidia are usually smooth (Zalar et al., 2007, Bensch et al., 2012).
Cladosporium longicatenatum Bensch, Crous & U. Braun, sp. nov. MycoBank MB814629. Fig. 15, Fig. 16.
Fig. 15.
Cladosporium longicatenatum (CBS 140485). A, C–E. Conidiophores with long, dichotomously branched conidial chains. B. Ramoconidium with conidial chains. F–G. Tip of conidiophores with conidia attached. Scale bars = 10 μm.
Fig. 16.
Cladosporium longicatenatum (CBS 140485). A. Overview of hyphae and clusters of rounded cells visible on the agar surface that give rise to slender elongated conidiophores and conidia. B. Very elongated smooth secondary ramoconidia give rise to intercalary and small terminal conidia. Note the pattern of secondary ramoconidia on the conidiophore. C. Detail of conidial hila. D–F. Conidiophores, secondary ramoconidia and intercalary conidia from different angles. G–H. Secondary ramoconidia, intercalary and small terminal conidia. Note an aerial hypha in (G) with septa. Scale bars = 2 (C), 5 (E–H), 10 (B, D), 20 (A) μm.
Etymology: Name refers to the very long conidial chains, with up to 20 or even more conidia in a branched chain.
Mycelium abundant, loosely branched, filiform, 1–3 μm wide, multiseptate, without swellings and constrictions, subhyaline, pale or medium olivaceous or olivaceous-brown, surface ornamentation variable, smooth, verruculose or irregularly rough-walled, walls unthickened or slightly thick-walled, sometimes forming small stromatic hyphal aggregations of few swollen cells. Conidiophores micro- or semimacronematous, sometimes hardly distinguishable from hyphae, erect, straight or somewhat flexuous, filiform or narrowly cylindrical, neither nodulose nor geniculate, usually unbranched, rarely once branched, 13–250 × 2.5–3(–4) μm, sometimes up to 650 μm long, multiseptate, pale olivaceous or medium olivaceous-brown, smooth, verruculose, verrucose or irregularly rough-walled, especially towards the base of conidiophores, walls somewhat thickened, about 0.5 μm thick. Conidiogenous cells integrated, usually terminal, cylindrical, 12–53 μm long, neither geniculate nor nodulose, usually with 2–3 loci at the outermost apex, sometimes up to five loci situated at the laterally proliferated apex, loci protuberant, subdenticulate, 1–1.5(–2) μm diam, somewhat thickened and darkened-refractive. Ramoconidia cylindrical, 22–42 × 2.5–3(–4) μm, 0(–1)-septate, base broadly truncate, 2.5–3(–4) μm wide, not thickened, slightly refractive. Conidia catenate, in very long, usually loosely dichotomously branched chains, up to 12 conidia in the terminal unbranched part, up to 20 conidia or more in a chain, small terminal conidia narrowly ellipsoid, sometimes fusiform, (3.5–)5.5–7 × 2–2.5 μm [av. (± SD) 6.0 (± 1.0) × 2.2 (± 0.2)], subhyaline or pale olivaceous, attenuated towards apex and base, intercalary conidia narrowly ellipsoid or subcylindrical, 7–17 × 2–2.5(–3) μm [av. (± SD) 11.0 (± 3.4) × 2.4 (± 0.3)], 0(–1)-septate, with 1–2(–3) distal hila, 0.5–1 μm diam, secondary ramoconidia subcylindrical or cylindrical, 10–30(–38) × 2.5–3.5 μm [av. (± SD) 18.8 (± 7.4) × 2.9 (± 0.4)], 0(–2)-septate, with (1–)2–3 distal hila, pale olivaceous or pale olivaceous-brown, lightmicroscopically smooth or almost so, verruculose or loosely irregularly rough-walled, outer wall seemingly detached, with SEM smooth or almost so or delicately irregularly reticulate, walls unthickened or almost so, hila 1–2 μm diam, thickened and darkened-refractive; microcyclic Conidi-ogenous not observed.
Culture characteristics: Colonies on PDA attaining 45–59 mm after 14 d, iron-grey to olivaceous-black, reverse leaden-black to olivaceous-black, felty, margins white, narrow, glabrous to somewhat feathery, regular, aerial mycelium loose, diffuse to fluffy, growth flat, few prominent exudates formed. Colonies on MEA reaching 29–37 mm, olivaceous-grey to iron-grey with patches of smoke-grey, reverse olivaceous-grey to iron-grey, velvety to fluffy, margins white, narrow, glabrous, aerial mycelium loose diffuse to more densely and fluffy, growth low convex with somewhat elevated colony centre, radially furrowed, wrinkled, without exudates. Colonies on OA attaining 43–54 mm, grey-olivaceous to olivaceous-grey with patches of white or smoke-grey, reverse leaden-grey to iron-grey, velvety to fluffy, margins hyaline, glabrous, narrow, aerial mycelium fluffy, white to smoke-grey, low to high, growth flat, without exudates; sporulation profuse on all media.
Specimen examined: Australia, Queensland, Noosa Bay, isol. from an unknown host, 27 Jul. 2009, P.W. Crous (CBS H-22355, holotype; ex-type culture CBS 140485 = CPC 17189).
Substrate and distribution: On plant material; Australia.
Notes: Cladosporium longicatenatum, an element of the cladosporioides species complex, clusters with C. exasperatum, which also possesses a reticulate conidial surface ornamentation which is, however, much more prominent with often distinctly embossed stripes. Furthermore, the latter species deviates in having shorter conidiophores, 15–100 μm long, shorter conidial chains with only up to six conidia in the terminal unbranched part of the chain and wider conidia, 3–4.5(–5) μm (Bensch et al. 2010).
Cladosporium parapenidielloides (CBS 140487), which is also phylogenetically closely allied, differs in having shorter conidi-ophores, sparse mycelium and a different surface ornamentation. The conidial chains are shorter, ramoconidia are absent, conidial measurements are similar but intercalary and secondary ramoconidia somewhat shorter, but more commonly septate.
Cladosporium longissimum Bensch, Crous & U. Braun, sp. nov. MycoBank MB814630. Fig. 17.
Fig. 17.
Cladosporium longissimum (CBS 300.96). A–C. Macronematous conidiophores and conidial chains. D. Branchlet of a conidiophore and conidia. E. Tip of a conidiophore and conidia. Scale bars = 10 μm.
Etymology: Name refers to the very long conidiophores, up to 512 μm long.
Mycelium immersed and superficial; hyphae loosely to densely branched, 1–4(–5) μm wide, septate, sometimes with swellings and constrictions, smooth to minutely verruculose, walls unthickened or almost so, sometimes forming ropes, subhyaline to pale olivaceous-brown, at the base of conidiophores darker and somewhat swollen, especially the longer ones, sometimes forming stromatic hyphal aggregations. Conidiophores macronematous to micronematous, solitary, arising terminally and laterally from hyphae, erect, flexuous, filiform to narrowly cylindrical-oblong, neither nodulose nor geniculate. Macronematous conidiophores unbranched or branched, branches mostly only as short denticle-like lateral prolongations, often very long, up to 512 μm, 2.5–3.5(–4) μm wide, multiseptate, 4–19 septa, not constricted at septa, regularly septate, pale to medium or even dark olivaceous-brown, smooth, walls unthickened or slightly thickened, somewhat wider at the base, up to 4 μm wide. Conidiogenous cells integrated, terminal or intercalary, neither geniculate nor nodulose, 13–39 μm long, with up to four loci crowded at the apex, closely aggregated, subdenticulate, 1–1.5 μm diam, thickened and darkened-refractive. Micronematous conidiophores numerous, as short peg-like lateral prolongations or longer, filiform, narrower, shorter and paler, sometimes geniculate, less septate, with up to nine septa, not constricted, subhyaline to pale olivaceous-brown, smooth, walls unthickened. Conidiogenous cells filiform to narrowly cylindrical-oblong, occasionally geniculate, 9–16 μm long, often with a single apical locus, sometimes with up to three loci, subdenticulate, 1–1.2 μm diam. Ramoconidia frequently formed both from macro- and micronematous conidiophores, 15–52 × 2–3 μm, 0–1(–3)-septate, narrowly cylindrical-oblong, with up to four apical hila, not attenuated towards the base. Conidia catenate, in branched chains, up to four conidia in the unbranched part, branching in all directions, sometimes irregular in outline due to lateral denticulate hila, small terminal conidia numerous, globose to subglobose, 2–4 × 2–3 μm (av. ± SD: 3.1 ± 0.8 × 2.5 ± 0.4), aseptate, intercalary conidia subglobose, ovoid to narrowly ellipsoid, 4–7(–8) × 2.5–3(–3.5) μm (av. ± SD: 5.4 ± 1.2 × 2.8 ± 0.3), 0(–1)-septate, secondary ramoconidia ovoid, narrowly ellipsoid to subcylindrical, (6–)8–25(–28) × 2–3(–4) μm (av. ± SD: 15.4 ± 6.3 × 2.7 ± 0.5), 0–1(–2)-septate, not constricted at septa, subhyaline to pale or medium olivaceous-brown, smooth to asperulate or irregularly rough-walled, walls unthickened or only slightly thickened, attenuated towards apex and base, with up to five distal hila, subdenticulate to denticulate, 0.5–1.5 μm diam, thickened and darkened-refractive; microcyclic conidiogenesis occasionally occurring. Conidia formed by micronematous conidiophores often paler and slightly narrower, 2–25 × 1.5–2 μm, 0–1(–2)-septate.
Culture characteristics: Colonies on PDA iron-grey, olivaceous-grey to grey-olivaceous or olivaceous due to sporulation and mycelium, reverse leaden-grey to iron-grey or olivaceous-black, velvety to fluffy, margin whitish, feathery, narrow, aerial mycelium abundant, loose to dense, high, fluffy, growth convex to raised, few very small, not prominent exudates formed, sporulating. Colonies on MEA olivaceous-grey to grey-olivaceous, greenish olivaceous towards margins, reverse olivaceous-grey to iron-grey, velvety to wooly-fluffy, margin white, narrow, glabrous to feathery, aerial mycelium high, fluffy, without prominent exudates, sporulation profuse. Colonies on OA smoke-grey to olivaceous, reverse pale mouse-grey to mouse-grey or olivaceous-grey, wooly-felty, margin hyaline to white, glabrous, aerial mycelium high, fluffy to felty, without exudates, sporulating.
Specimen examined: Papua New Guinea, Madang, Jais Aben, isol. from soil along coral reef coast, Nov. 1995, coll. A. Aptroot, isol. A. van Iperen (CBS H-22356, holotype; ex-type culture CBS 300.96).
Substrate and distribution: Isolated from soil; Papua New Guinea.
Notes: The new species clusters close to C. sphaerospermum but the latter species differs in having shorter and wider conidi-ophores with darkened septa in dense succession, minutely verruculose or verrucose, wider small terminal conidia, 3–5 × 3–3.5 μm, and wider more frequently septate conidia, 3–4(–5) μm wide, 0–3-septate (Zalar et al. 2007). Zalar et al. (2007) hesitated to introduce this isolate as a new species since it is only known from a single isolate, but morphological and phylogenetic differences are sufficient enough to justify its recognition as a distinct species.
Cladosporium cycadicola, described from Australia on Cycas and also belonging to the sphaerospermum species complex (Crous et al. 2014), is morphologically similar in having macro- and micronematous conidiophores and similar conidial measurements. The conidiophores in vitro can be also quite long, up to 600 μm, but the transition between conidiophores and conidia is often not very evident. However, it deviates from C. longissimum in having shorter ramoconidia, shorter conidial chains and somewhat longer intercalary conidia, 4–11(–13) × 2–2.5(–2.8) μm.
Cladosporium macrocarpum Preuss, in Sturm, Deutsch. Fl. 3(26): 27. 1848.
Specimens examined: Iran, isol. from Hordeum sp. (Poaceae), 12 May 2009, isol. by P.W. Crous, CPC 19063. Morokko, Rabat, isol. from Diospyros kaki (Ebenaceae), isol. by L. Najim, CBS H-10355, CBS 108.85. Netherlands, isol. from Hordeum vulgare (Poaceae), Oct. 1962, Bierbrouwerij Amstel, isol. by A.C. Stolk, CBS 175.62.
Notes: Cladosporium versiforme (CBS 140491), introduced below, was also isolated from Hordeum sp. in Iran as was CPC 19063, which proved to be C. macrocarpum. Cladosporium versiforme differs, however, in having narrower macronematous conidiophores, 3–4 μm, with narrower swellings, 5–7 μm. Its conidia are very variable in shape, size and colour, and two different types are formed. The intercalary and secondary ramoconidia of the herbarum-like type formed by macronematous conidiophores are narrower than in C. macrocarpum.
Cladosporium montecillanum Bensch, Crous & U. Braun, sp. nov. MycoBank MB814631. Fig. 18.
Fig. 18.
Cladosporium montecillanum (CBS 140486). A–G. Conidiophores and conidial chains. Scale bars = 10 μm.
Etymology: Named after the place where it was collected, Montecillo, Mexico.
Mycelium sparingly formed and branched, 1–4 μm wide, septate, sometimes with swellings and constriction especially at septa, subhyaline or pale olivaceous, medium olivaceous-brown at the base of conidiophores and sometimes swollen up to 7 μm, verruculose, walls unthickened. Conidiophores macro- or semimacronematous, erect, straight or somewhat flexuous, arising from hyphae or swollen hyphal cells, cylindrical-oblong, often subnodulose at the apex, occasionally slightly geniculate, unbranched, 25–130 × 2.5–4 μm, sometimes up to 5.5 μm at the base and attenuated towards the apex, semimacronematous ones 2–2.5 μm wide, 0–6-septate, septa often not very conspicuous, pale to medium olivaceous-brown, smooth, sometimes verruculose towards the base, walls unthickened or somewhat thickened, about 0.5 μm thick. Conidiogenous cells integrated, mainly terminal, occasionally intercalary, cylindrical, sometimes subnodulose at the apex or intercalary and slightly geniculate due to sympodial proliferation, 11–40 μm long, with mainly 1–4 loci per cell, loci conspicuous, 1–1.5(–2) μm diam, thickened and darkened-refractive. Ramoconidia with a truncate, non-cladosporioid base not observed. Conidia numerous, catenate, in branched chains with 1–4(–6) conidia in the terminal unbranched part, branching in all direction, small terminal conidia subglobose, obovoid or ellipsoid, 3–5(–6) × (1.5–)2–2.5 μm [av. (± SD) 4.1 (± 0.7) × 2.3 (± 0.3)], apex broadly rounded, intercalary conidia ellipsoid, more or less attenuated towards apex and base, 4–9(–11) × (2–)2.5–3(–3.5) μm [av. (± SD) 6.7 (± 2.0) × 2.7 (± 0.4)], aseptate, with 1–3(–4) distal hila, some of them seem to have a halo since the wall being paler than the lumen, secondary ramoconidia ellipsoid or subcylindrical, 6.5–22(–27) × 2.5–3.5(–4) μm [av. (± SD) 14.1 (± 5.5) × 3.2 (± 0.4)], 0(–1)-septate, with 2–4 distal hila, pale olivaceous or pale olivaceous-brown, smooth, walls unthickened, hila conspicuous, 0.5–1.5(–2) μm diam, thickened and darkened-refractive; microcyclic conidiogenesis occasionally observed.
Culture characteristics: Colonies on PDA reaching 65–77 mm after 14 d, grey-olivaceous, olivaceous to olivaceous-black, grey-olivaceous at margins, reverse olivaceous-black, powdery to fluffy, margins white, feathery, regular, aerial mycelium loose diffuse to fluffy, growth flat. Colonies on MEA attaining 70 mm, greenish grey, grey-olivaceous, iron-grey at margins, reverse olivaceous-grey to iron-grey, velvety to fluffy, margins white, glabrous to somewhat feathery, aerial mycelium sparse, diffuse or more commonly formed, white, fluffy, growth flat to low convex, radially furrowed or folded. Colonies on OA reaching 55–70 mm, olivaceous to olivaceous-grey or smoke-grey, reverse leaden-grey to iron-grey, powdery to fluffy, margins glabrous, narrow, aerial mycelium loose diffuse, in a few patches denser, fluffy, smoke-grey, growth flat. On all media sporulation profuse but without prominent exudates.
Specimens examined: Mexico, Montecillo, Texcoco, isol. from pine needles (Pinus sp., Pinaceae), 12 Oct. 2009, coll. M. de Jesús Yáñez-Morales, isol. P.W. Crous, CPC 17804; from same substrate and locality, 26 Nov. 2009, coll. M. de Jesús Yáñez-Morales, isol. P.W. Crous (CBS H-22357, holotype; ex-type culture CBS 140486 = CPC 17953); Montecillo, isol. from Taraxacum sp. (Asteraceae), 1 Oct. 2008, coll. M. de Jesús Yáñez-Morales, isol. P.W. Crous, CPC 15605.
Substrate and distribution: On plant material; Mexico.
Notes: This species reminds one of C. cladosporioides, C. tenuissimum and C. pseudocladosporioides but these three species possess somewhat longer intercalary conidia and secondary ramoconidia, form ramoconidia and in C. cladosporioides and C. tenuissimum the conidiophores are distinctly longer (Bensch et al. 2012).
Cladosporium myrtacearum K. Schub. et al., Australas. Pl. Pathol. 34: 513. 2005. Fig. 19, Fig. 20.
Fig. 19.
Cladosporium myrtacearum (CPC 16319). A–C. Conidiophores and conidial chains. D–E. Tips of conidiophores and conidia. Scale bars = 10 μm.
Fig. 20.
Cladosporium myrtacearum (CPC 16319). A. Overview on agar surface with conidiophores arising from the surface. B. Running hyphae giving rise to several conidiophores. C–F. Tips of conidiophores with conidial chains. Note that the reticulate surface ornamentation differs among the secondary ramo-, intercalary and terminal conidia. Mostly secondary ramoconidia have less surface ornamentation. G. Details of intercalary and small terminal conidia and hila on conidia. Note the smoother surface of these terminal conidia as if the ornamentation is still being formed. H. Secondary ramoconidium with a remarkable expanded non-ornamented hilus region. I. Very early conidia formed on secondary ramoconidia. J–K. Details of terminal chains, hila and branching of secondary ramoconidia on the conidiophore. Scale bars = 5 (F–K), 10 (C–E), 20 (B), 50 (A) μm.
Mycelium abundantly formed, superficial hyphae branched, 1.5–5 μm wide, multiseptate, sometimes slightly constricted at septa or intercalarily swollen, subhyaline, pale or medium olivaceous-brown, minutely verruculose, verruculose or verrucose, sometimes irregularly rough-walled or appearing to be covered by a halo, walls somewhat thickened in wider, fertile hyphae, sometimes forming ropes of a few hyphae. conidi-ophores macronematous, arising solitarily or in small groups of 2–4, arising terminally or laterally from hyphae, cylindrical-oblong, often geniculate towards the apex or apex swollen, capitate, unbranched or once branched, branchlets often quite long, (35–)55–220(–320) × 3.5–4.5 μm, multiseptate (with 3–9 usually not very conspicuous septa), pale to medium olivaceous-brown, smooth, minutely verruculose or irregularly rough-walled towards the base, walls somewhat thickened. Conidiogenous cells integrated, mainly terminal but also intercalary, in terminal cells apex often slightly swollen, capitate, in intercalary ones geniculate due to sympodial proliferation, 9–35 μm long, with 2–4 loci per cell, loci conspicuous, (1–)1.5–2 μm diam, thickened and darkened-refractive. Ramoconidia cylindrical-oblong, 20–55 × 3.5–4.5 μm, 0–3-septate, base 2–3 μm wide, somewhat refractive. Conidia catenate with 1–4 conidia in the terminal unbranched part of the chain, straight, small terminal conidia obovoid or ellipsoid, 4.5–8.5 × (2.5–)3–3.5(–4.5) μm [av. ± SD, 6.0 (± 1.3) × 3.1 (± 0.6) μm], aseptate, intercalary conidia ellipsoid, fusiform, 6–13(–15) × 3–4(–4.5) μm [av. ± SD, 9.4 (± 2.5) × 3.6 (± 0.5) μm], 0(–1)-septate, with 1–3(–4) distal hila, secondary ramoconidia ellipsoid, subcylindrical or fusiform, 10–21(–23.5) × 3.5–4.5(–5) μm [av. ± SD, 15.9 (± 3.5) × 4.0 (± 0.5) μm], 0–1(–2)-septate, septum median, not very conspicuous, becoming sinuous with age, with (1–)2–3(–5) distal hila, hila 1–2 μm diam, pale olivaceous-green or olivaceous-brown, surface ornamentation variable, usually irregularly rough-walled due to reticulate surface or embossed stripes, coarsely verruculose, rugose or verrucose (LM), sometimes outer wall seemingly detaching, in intercalary and small terminal conidia surface ornamentation more prominent, secondary ramoconidia sometimes appearing to be almost smooth or minutely verruculose, hila conspicuous, 0.5–2 μm diam, thickened and darkened-refractive; microcyclic conidiogenesis occurring.
Specimen examined: South Africa, North West Province, Magaliesberg, south of Mooinooi, isol. from Indigofera sp. (Fabaceae), 25 Jan. 2009, coll. A.R. Wood, isol. P.W. Crous, CPC 16319.
Substrate and distribution: On Myrtaceae (Corymbia, Eucalyptus) and Fabaceae (Indigofera); Australia, South Africa.
Notes: The description given above is based on CPC 16319 from South Africa. This isolate clusters with C. myrtacearum which is known from two isolates from Australia, the ex-type isolated from Corymbia and an additional isolate from Eucalyptus, with both hosts belonging to the Myrtaceae (Braun et al., 2005, Bensch et al., 2010). These three isolates form a highly supported subclade (see Fig. 1). Morphologically, CPC 16319 deviates from the isolates from Australia in forming longer conidiophores and ramoconidia and a more prominent surface ornamentation. Whether these morphological differences represent intraspecific variation can only be clarified with additional isolates. Therefore, the biology, host range and distribution of C. myrtacearum still remain unclear as already stated in Bensch et al. (2010).
Cladosporium parapenidielloides Bensch, Crous & U. Braun, sp. nov. MycoBank MB814632. Fig. 21, Fig. 22.
Fig. 21.
Cladosporium parapenidielloides (CBS 140487). A–D. Conidiophores and conidial chains. E–G. Tip of conidiophores and conidia. Scale bars = 10 μm.
Fig. 22.
Cladosporium parapenidielloides (CBS 140487). A, D. Overview of a part of a colony showing a dense field of conidiophores that arise from cells at the level of the agar surface and forming conidial chains. B. Dislodged conidial chains on the agar surface. C, E. Conidial chains with branching at different secondary ramoconidia and intercalary conidia. F–G. Conidiogenous loci on a conidiophore and conidial hila on secondary ramoconidia. Note the very broad contact area (diameter > 2 μm) between secondary ramoconidium and conidiophore in (G). Ornamentation is absent on these conidia, although some pattern formation on the secondary ramoconidium is visible in this figure. Scale bars = 2 (E–F), 5 (C, G), 10 (A–B, D) μm.
Etymology: Name refers to the morphological similarity to Cladosporium penidielloides, but the conidia are shorter and narrower in C. penidielloides.
Mycelium sparingly formed and branched, 1.5–3(–4) μm wide, septate, subhyaline, pale olivaceous, walls unthickened or almost so, smooth, verruculose or irregularly rough-walled, sometimes slightly swollen at the base of conidiophores. Conidiophores macro- and semimacronematous, solitary, rarely in pairs of two, arising terminally and laterally from hyphae, erect, straight or somewhat flexuous, non-nodulose, sometimes once geniculate towards the apex, unbranched, 13–67 × 2.5–3.5(–4) μm, subcylindrical or cylindrical, 1–2(–3)-septate, septa often not very conspicuous, pale to medium olivaceous-brown, smooth or partly verruculose, sometimes loosely verrucose, walls unthickened or almost so, slightly attenuated towards the apex. Conidiogenous cells integrated, terminal, 12–32 μm long, with (1–)2–3 distal loci or once geniculate towards the apex with a single locus on a small lateral shoulder, loci conspicuous but small, often subdenticulate, 0.8–1.5 μm diam, thickened and darkened-refractive. Ramoconidia (sensu Bensch et al. 2012) not observed. Conidia catenate, in branched chains, usually dichotomously branched, with 1–6 conidia in the terminal unbranched part, small terminal conidia narrowly ellipsoid, 4–6 × 2(–2.2) μm [av. (± SD) 5.3 (± 0.8) × 2.0 (± 0.1)], intercalary conidia fusiform or narrowly ellipsoid, 5.5–12(–14) × (1.5–)2–2.5(–3) μm [av. (± SD) 8.4 (± 2.6) × 2.3 (± 0.4)], 0–1-septate, attenuated towards apex and base, with 1–2 distal hila, secondary ramoconidia subcylindrical, narrowly ellipsoid, sometimes fusiform, 10–19 × (2–)2.5–3 μm [av. (± SD) 13.8 (± 2.5) × 2.6 (± 0.3)], 0–1-septate, septum median, subhyaline or pale olivaceous, smooth or almost so, loosely verruculose or slightly irregularly rough-walled, walls unthickened, with 2–3 distal hila, hila conspicuous, 0.5–1.5(–1.8) μm diam, distinctly darkened-refractive and thickened; microcyclic conidiogenesis not observed.
Culture characteristics: Colonies on PDA attaining 39–55 mm after 14 d, iron-grey to olivaceous-black, reverse leaden-black to olivaceous-black, felty, margins white, narrow, glabrous to somewhat feathery, regular, aerial mycelium loose, diffuse to fluffy, growth flat. Colonies on MEA reaching 23–32 mm, olivaceous-grey to iron-grey, grey-olivaceous due to abundant sporulation, somewhat zonate. Colonies on OA reaching 42–55 mm, grey-olivaceous, aerial mycelium loose diffuse.
Specimen examined: Australia, Queensland, Fraser Island, isol. from Eucalyptus sp. (Myrtaceae), 29 Jul. 2009, P.W. Crous (CBS H-22358, holotype; ex-type culture CBS 140487 = CPC 17193).
Substrate and distribution: On Eucalyptus; Australia.
Notes: Cladosporium parapenidielloides was named after C. penidielloides due to its similar morphology although the two species are phylogenetically not allied. Cladosporium penidielloides, which clusters with C. salinae, deviates in having more frequently septate, somewhat wider and longer conidia. Cladosporium ipereniae is also similar but has wider conidia, 2.5–4(–4.5) μm, and shorter small terminal and intercalary conidia.
Cladosporium parapenidielloides, which belongs in the cladosporioides species complex, clusters with C. exasperatum, also described from Australia on Eucalyptus, but the conidia of the latter species are shorter and narrower with a different surface ornamentation. Cladosporium australiense, another species on Eucalyptus in Australia, is distinct in having longer conidiophores, wider conidiogenous loci and hila [(0.5–)0.8–2 μm diam] as well as smooth, longer and wider secondary ramoconidia [(7–)11–25(–27) × 3–4 μm] (Bensch et al. 2010). Morphological differences between C. parapenidielloides and the closely allied C. longicatenatum are discussed under the latter species.
Cladosporium penidielloides Bensch, Crous & U. Braun, sp. nov. MycoBank MB814633. Fig. 23, Fig. 24.
Fig. 23.
Cladosporium penidielloides (CBS 140489). A–F. Macro- and micronematous conidiophores and conidial chains. G. Ramoconidium and conidia. Scale bars = 10 μm.
Fig. 24.
Cladosporium penidielloides (CBS 140489). A. Overview on the agar surface also containing slender conidiophores. B–D, F, H. Short and long conidiophores erumpent from structures beneath the agar or arising from plagiotropous hyphae with conidial chains. E. Conidiogenous cell with a cluster of four pronounced loci and secondary ramoconidia. G, I–J. Conidial chains showing details of conidial hila. Ornamentation is missing in most of the conidia, few show some irregular reticulate structures. Note the large scar / contact region between spores in E and G. Also note the conidium initials in (J). Scale bars = 2 (J), 5 (E–F, I–J), 10 (B–D, H), 20 (A) μm.
Etymology: This species was provisionally placed in the genus Penidiella on the basis of morphology. Name refers to its morphological similarity and this initial identification.
Mycelium loosely branched, filiform to subcylindrical, 1–3.5(–5) μm wide, septate, neither constricted nor swollen, but often with a short lateral outgrowth, subhyaline to pale olivaceous-brown, smooth or minutely verruculose in wider hyphae, walls unthickened, forming ropes of a few hyphae. conidi-ophores macro- and micronematous, arising terminally and laterally from hyphae, solitary, filiform to narrowly cylindrical-oblong, sometimes with unilateral swellings or short lateral prolongations at the apex, then appearing somewhat irregular in outline, unbranched or branched, branches mostly as short lateral outgrowths, 8–55(–80) × (2–)2.5–4 μm, septate, sometimes densely multiseptate, sometimes seceding at one of these septa and forming ramoconidia, subhyaline or pale olivaceous-brown, smooth or almost so, walls unthickened. Conidiogenous cells integrated, mostly terminal, but also intercalary, loci situated at the apex, on short peg-like lateral prolongations or on small lateral shoulders, sometimes forming a small cluster of up to six pronounced conidiogenous loci, 8–25 μm long, loci protuberant, 1–1.5(–2) μm diam, thickened and darkened-refractive. Ramoconidia occurring, often multiseptate, 8–25 μm long. Conidia catenate, in unbranched or often dichotomously branched chains, 1–6 conidia in the terminal unbranched part of the chain, straight, sometimes swollen, small terminal conidia obovoid or ellipsoid, 4–6.5(–7.5) × 2.5–3.5 μm [av. (± SD) 5.8 (± 0.8) × 3.0 (± 0.4)], 0–1-septate, apex rounded, intercalary conidia ellipsoid, 5–13 × 2.5–3.5 μm [av. (± SD) 9.6 (± 2.5) × 3.1 (± 0.3)], 0–1(–2)-septate, secondary ramoconidia ellipsoid or subcylindrical, 7–22(–29) × 3–4 μm [av. (± SD) 16.4 (± 6.0) × 3.4 (± 0.4)], (0–)1–3(–6)-septate, septa refractive, becoming sinuous with age, some conidia with up to 12 septa, with 1–3 distal hila, smooth or almost so or loosely verruculose, smooth or occasionally irregularly reticulate under SEM, pale olivaceous-brown, walls unthickened or slightly thick-walled; microcyclic conidiogenesis occasionally occurring.
Culture characteristics: Colonies on PDA, OA and MEA attaining 15–20 mm after 14 d. On PDA surface and reverse olivaceous-grey. On MEA surface grey-olivaceous, reverse olivaceous-grey. On OA surface grey-olivaceous. Colonies spreading with aerial mycelium sparse to absent, and smooth, even margins.
Specimen examined: Australia, Victoria, Melbourne, stop 2 in the vicinity of the Twelve Apostles, isol. from Acacia verticillata (Fabaceae), 18 Oct. 2009, P.W. Crous, as ‘Penidiella’ (CBS H-22360, holotype; ex-type culture CBS 140489 = CPC 17674).
Substrate and distribution: On Acacia; Australia.
Notes: The genus Penidiella (Capnodiales, Teratosphaeriaceae) is a cladosporium-like genus which is characterised by penicillate conidiophores with a quite distinct branching system consisting of a single terminal conidiogenous cell giving rise to several ramoconidia that form several secondary ramoconidia or the branched apparatus is composed of several terminal and sometimes lateral conidiogenous cells giving rise to sequences of ramoconidia (Crous et al. 2007a). Cladosporium penidielloides is reminiscent of this genus and was originally identified as a Penidiella.
Phylogenetically it is allied to C. salinae which clusters with C. halotolerans and together with a few other species they form a sister clade to C. sphaerospermum and closely allied species (Fig. 2). In previous phylogenetic analyses, C. salinae had a more separate position within the genus Cladosporium being distantly related to any other described species (Zalar et al. 2007) which is also true for C. aphidis (Bensch et al. 2012). The phylogenetic position of C. salinae is sensitive to the species sampling included in the phylogenetic analysis and might be an example of long-branch attraction.
Cladosporium penidielloides and C. salinae have usually short, poorly differentiated conidiophores sometimes forming small clusters of pronounced scars and smooth conidia but globose or subglobose terminal conidia typical for members of the sphaerospermum species complex are lacking. They rather resemble species of the genus Fusicladium. Cladosporium penidielloides is distinguishable from C. salinae by longer and more frequently septate intercalary conidia and secondary ramoconidia.
Cladosporium perangustum Bensch et al., Stud. Mycol. 67: 65. 2010.
Specimens examined: Panama, isol. from Ananas comosus (Bromeliaceae), CPC 18494 = 97.1; from the same plant, CPC 18496 = 110.1.
Note: These isolates represent the first records of C. perangustum from Central America.
Cladosporium pseudochalastosporoides Bensch, Crous & U. Braun, sp. nov. MycoBank MB814634. Fig. 25.
Fig. 25.
Cladosporium pseudochalastosporoides (CBS 140490). A–E. Conidiophores and conidial chains. F–G. Tip of conidiophores and conidia. Scale bars = 10 μm.
Etymology: Name refers to its morphological similarity to Cladosporium chalastosporoides.
Mycelium sparingly branched, filiform or cylindrical, 2.5–5.5 μm wide, septate, in wider hyphae constricted at septa, pale to medium olivaceous-brown, smooth to verruculose, walls somewhat thickened, occasionally swollen at the base of conidi-ophores. Conidiophores macronematous, solitary or in pairs, formed terminally or laterally from hyphae, cylindrical or slightly to distinctly attenuated towards the apex, non-nodulose, sometimes once geniculate towards or at the apex, rarely up to three times, unbranched, 20–120 μm long, 2.5–3.5 μm wide at the apex, 3.5–5.5 μm towards the base, 1–4(–5)-septate, not constricted at septa, pale to medium olivaceous-brown, somewhat paler towards the apex, smooth or minutely verruculose, walls slightly thickened, apex sometimes swollen, slightly capitate, reminding one of species belonging to the herbarum species complex. Conidiogenous cells integrated, terminal, subcylindrical, sometimes geniculate due to sympodial proliferation, 20–33 μm long, with 1–3 loci around the apex, loci 1–1.5 μm diam, slightly thickened and refractive. Ramoconidia occasionally formed, subcylindrical or cylindrical, 20–39 × 3–4 μm, 0–1-septate, base broadly truncate, 3–3.5 μm wide, not thickened, somewhat refractive. Conidia catenate, in long, often dichotomously branched chains, up to 10 conidia in the terminal unbranched part of the chain, small terminal conidia obovoid or ellipsoid, (4–)5–7 × (1.5–)2–2.5(–3) μm [av. (± SD) 6.0 (± 0.8) × 2.4 (± 0.3)], aseptate, basal hilum 0.8–1 μm diam, intercalary conidia fusiform or subcylindrical, 6.5–10(–12.5) × 2–3 μm [av. (± SD) 8.2 (± 1.7) × 2.7 (± 0.4)], aseptate, with 1–2(–3) distal hila, 0.8–1(–1.2) μm diam, secondary ramoconidia ellipsoid or subcylindrical, 9–16(–27) × 3–4 μm [av. (± SD) 13.9 (± 4.1) × 3.5 (± 0.4)], 0(–1)-septate, pale olivaceous or pale olivaceous-brown, smooth, walls unthickened or slightly thick-walled in secondary ramoconidia, with 1–3(–5) distal hila, 1–1.5(–2) μm diam, somewhat thickened and darkened-refractive; microcyclic Conidiogenous not observed.
Culture characteristics: Colonies on PDA reaching 45–52 mm after 14 d, iron-grey to olivaceous-black, reverse olivaceous-black, fluffy due to aerial mycelium, margins white, glabrous or somewhat feathery, narrow, aerial mycelium olivaceous-grey, dense, high, growth flat to low convex, without prominent exudates, sporulation profuse. Colonies on MEA attaining 43–50 mm, olivaceous-grey to pale olivaceous-grey, reverse olivaceous-grey, fluffy, margins white, glabrous, radially furrowed, aerial mycelium abundant, covering almost the whole colony, dense, growth flat to low convex, sporulating. Colonies on OA reaching 34–37 mm, olivaceous-grey to pale olivaceous-grey, reverse leaden-grey to olivaceous-grey, fluffy to wooly, margins glabrous, regular, white, aerial mycelium loose diffuse to fluffy, growth flat, without exudates, sporulating.
Specimen examined: Mexico, isol. from pine needles (Pinus sp., Pinaceae), 30 Nov. 2009, coll. M. de Jesús Yáñez-Morales, isol. P.W. Crous (CBS H-22361, holotype; ex-type culture CBS 140490 = CPC 17823).
Substrate and distribution: Isolated from pine needles; Mexico.
Notes: This species, which belongs to the cladosporioides species complex, is both morphologically and phylogenetically close to C. chalastosporoides (Bensch et al. 2010) but differs in having longer conidiophores which are wider at the base (3.5–5.5 μm) and attenuated towards the apex (2.5–3.5 μm), and longer ramoconidia, 20–39 μm. Furthermore, secondary ramoconidia are not distinctly darker than small terminal and intercalary conidia, and microcyclic conidiogenesis seems to be lacking. The name C. chalastosporoides refers to the fusiform conidia formed in long, mostly unbranched chains which are reminiscent of the genus Chalastospora, especially the species Ch. gossypii (with Alternaria malorum as a synonym) representing an asexual lineage in the Pleosporales, Pleosporaceae (Simmons 2007, Crous et al., 2009a).
Cladosporium pseudocladosporioides Bensch et al., Stud. Mycol. 67: 71. 2010.
Specimen examined: South Africa, isol. from Rooibos (Aspalathus linearis, Fabaceae), wild population, code R 8263K, 8 Sep. 2009, G. Marais, CPC 18014.
Cladosporium puyae Bensch, Crous & U. Braun, sp. nov. MycoBank MB814635. Fig. 26, Fig. 27.
Fig. 26.
Cladosporium puyae (CBS 274.80A). A–G. Conidiophores and conidial chains. Scale bars = 10 μm.
Fig. 27.
Cladosporium puyae (CBS 274.80A). A. Survey of colony development with numerous conidiophores arising from the agar surface. B–C, E. Conidiophores, hyphae and conidial chains. D. Conidial chain showing the characteristic dispersed surface ornamentation. Note that several of the verrucae are irregularly enlarged. F. Details of conidial surface ornamentation and hila. Note the conidium initial. Scale bars = 2 (F), 10 (C–E), 20 (B), 50 (A) μm.
Etymology: Named after the host from which it was isolated, Puya.
Mycelium loosely branched, plagiotropous and ascending, cylindrical-oblong, sometimes with swellings and slightly constricted at septa, 2–5 μm wide, septate, pale to medium olivaceous-brown, smooth or almost so to minutely verruculose or loosely verrucose or irregularly rough-walled, walls slightly thickened. Conidiophores macronematous, solitary, in pairs or in loose groups, arising from plagiotropous and ascending hyphae, cylindrical-oblong, non-nodulose, often once or a few times geniculate-sinuous at the outermost apex, unbranched or once branched, 8–140 × (2–)2.5–4.5 μm, up to 4-septate, septa often somewhat darkened where ramoconidia are seceded, pale to medium olivaceous-brown, somewhat paler towards the apex, almost smooth to asperulate or verruculose, walls slightly thickened. Conidiogenous cells integrated, mostly terminal but also intercalary, terminal ones often seceding, forming ramoconidia, in intercalary ones loci situated on small lateral prolongations just below the septum, cylindrical-oblong, with two or few conidiogenous loci crowded at or towards the apex, sometimes once or a few times geniculate due to sympodial proliferation, 10–41 μm long, loci conspicuous, 1–2(–2.5) μm diam, thickened and darkened-refractive. Ramoconidia up to 35 μm long, 3–4 μm wide, base 2.5–3.5 μm wide, 0–1-septate, base usually unthickened and not darkened, however delimitation from secondary ramoconidia sometimes difficult with light microscopy. Conidia catenate, in branched chains, up to 4(–5) conidia in the terminal unbranched part of the chain, asperulate to verruculose, several of the verrucae irregularly enlarged (SEM, Fig. 27D), small terminal conidia ovoid, obovoid to ellipsoid, 3.5–6 × 2.5–3 μm [av. (± SD) 4.9 (± 0.8) × 2.8 (± 0.3)], aseptate, apex rounded, intercalary conidia ellipsoid to subcylindrical, only slightly attenuated towards apex and base, 5.5–16(–19) × 2.5–3.5(–4) μm [av. (± SD) 9.8 (± 3.8) × 3.3 (± 0.4)], 0(–1)-septate, with 1–3 distal hila, secondary ramoconidia ellipsoid to cylindrical, 10–33 × (3–)3.5–4(–5) μm [av. (± SD) 20.4 (± 6.7) × 3.7 (± 0.5)], 0–1(–2)-septate, with 2–3(–4) distal hila, not constricted at septa, pale olivaceous-brown, walls more or less unthickened, hila conspicuous, protuberant, (0.5–)1–2.5 μm diam, thickened, darkened-refractive.
Culture characteristics: Colonies on PDA attaining 34–38 mm after 14 d, greenish olivaceous to grey-olivaceous due to abundant sporulation, olivaceous-black, reverse olivaceous-black, velvety to powdery, margins white, somewhat feathery, narrow, regular, aerial mycelium loose, diffuse, growth flat, sporulation profuse. Colonies on MEA reaching 23–35 mm, grey-olivaceous, olivaceous-black towards margins, reverse olivaceous-grey to iron-grey, velvety to powdery, margins white, narrow, somewhat feathery, regular, aerial mycelium sparse, diffuse, growth flat, radially furrowed and folded in colony centre, sporulation profuse. Colonies on OA grey-olivaceous, olivaceous-black towards margins, reverse leaden-grey to leaden-black, velvety to powdery, margins regular, entire edge, glabrous, aerial mycelium loose, diffuse, growth flat, and sporulation profuse.
Specimen examined: Colombia, Páramo de Boquerón, alt. 3 420 m, isol. from Puya goudotiana (Bromeliaceae) coll. by W. Gams, depos. May 1980, ident. as “C. tenuissimum” (CBS H-10372, holotype; ex-type culture CBS 274.80A).
Substrate and distribution: On Puya; Colombia.
Notes: Cladosporium puyae resembles C. cladosporioides and C. tenuissimum but differs in having asperulate to verruculose conidia, which is typical for species belonging to the herbarum species complex. It clusters close to C. spinulosum but the conidia of the latter species are shorter and wider [small terminal and intercalary conidia 4–7(–8) × 3–4.5(–5) μm and secondary ramoconidia (6–)7–15(–18) × 4–5(–6) μm] as well as conspicuously digitate (Zalar et al., 2007, Bensch et al., 2012).
Two other CBS strains were isolated from Colombian plant material, both collected in the same year, CBS 274.80B and CBS 274.80C. CBS 274.80B, isolated from a dead leaf of Cortaderia, represents the ex-type of Cladosporium colombiae (Schubert et al. 2009) which differs in having macro- and micronematous conidiophores, conidia formed in long branched chains with up to 10 conidia in the terminal unbranched part of the chain and shorter intercalary conidia and secondary ramoconidia. CBS 274.80C, isolated from the same host as C. puyae, clusters close to C. lycoperdinum and is tentatively maintained in that species although the morphology is slightly different (Bensch et al. 2010).
Cladosporium ramotenellum K. Schub. et al., Stud. Mycol. 58: 137. 2007, emend.
Holotype: Slovenia, Sečovlje, isolated from hypersaline water from reverse ponds, salterns, 2005, P. Zalar, CBS H-19862. Isotype: HAL 2026 F. Ex-type culture: CBS 121628 = CPC 12043 = EXF-454.
Mycelium unbranched or only sparingly branched, 1.5–4 μm wide, septate, without swellings and constrictions, hyaline or subhyaline, smooth, sometimes irregularly rough-walled, walls unthickened. Conidiophores solitary, macronematous and micronematous, arising as lateral branches of plagiotropous hyphae or terminally from ascending hyphae, erect, straight or slightly flexuous, cylindrical, neither geniculate nor nodulose, without capitate apices or intercalary swellings, unbranched, sometimes branched, branches often only as short lateral prolongations, mainly formed below a septum, 14–120(–230) × 2–4(–5) μm, septate, not constricted at the septa, subhyaline to pale olivaceous or brown, smooth to minutely verruculose, walls unthickened, sometimes guttulate. Conidiogenous cells integrated, terminal, sometimes also intercalary, cylindrical, not geniculate, non-nodulose, 10–28(–50) μm long, proliferation sympodial, sometimes swollen, up to 7 μm wide, with few conidiogenous loci, mostly 1–3, loci sometimes situated on small lateral prolongations, protuberant, 0.5–1.5(–2) μm diam, thickened and somewhat darkened-refractive. Ramoconidia cylindrical-oblong, 15–50 × 2–4(–5) μm, 0–1(–3)-septate, rarely up to 4-septate, subhyaline to very pale olivaceous, smooth or almost so, with a broadly truncate base lacking a dome and raised rim, 2–3 μm wide, not thickened but somewhat refractive. Conidia numerous, polymorphous, catenate, in branched chains with 2–5 conidia in the terminal unbranched part of the chain, straight, sometimes slightly curved, small terminal conidia numerous, globose, subglobose or ovoid, obovoid or limoniform, 2.5–7 × 2–4(–4.5) μm [av. ± SD, 4.5 (± 1.2) × 2.9 (± 0.6) μm], aseptate, without distal hilum or with a single apical hilum, intercalary conidia ellipsoid, limoniform to subcylindrical, 5–12(–15) × 3–4(–5) μm [av. ± SD, 8.8 (± 2.5) × 3.7 (± 0.4) μm], 0–1-septate; secondary ramoconidia ellipsoid, subcylindrical to cylindrical-oblong, (7–)13–30(–39) × 3–4(–5) μm [av. ± SD, 17.8 (± 6.0) × 4.1 (± 0.5) μm], sometimes swollen up to 7 μm, 0–1(–3)-septate, usually not constricted at septa, sometimes distinctly constricted at the median septum, subhyaline to very pale olivaceous, minutely verruculose (granulate under SEM), walls unthickened or almost so, apex broadly rounded or slightly attenuated towards apex and base, sometimes guttulate, hila protuberant, conspicuous, 0.8–1.5(–2) μm diam, somewhat thickened and darkened-refractive; microcyclic conidiogenesis occurring.
Culture characteristics: Colonies on PDA reaching 46–49 mm diam after 14 d, olivaceous to grey-olivaceous due to abundant sporulation, appearing zonate in forming concentric zones, margin entire edge to slightly undulate, white, glabrous, aerial mycelium absent or sparse, growth flat with a somewhat folded and wrinkled colony centre, without prominent exudates, sporulation profuse. Colonies on MEA reaching 48–49 mm diam, grey-olivaceous to olivaceous-grey, velvety, olivaceous-grey to iron-grey reverse, margin entire edge to undulate, radially furrowed, glabrous to feathery, aerial mycelium sparse, diffuse, growth flat with slightly elevated colony centre, distinctly wrinkled, prominent exudates not formed, abundantly sporulating. Colonies on OA attaining 40 mm diam, grey-olivaceous, margin entire edge, hyaline or white, glabrous, aerial mycelium absent or sparse, growth flat, without exudates, sporulation profuse.
Specimens examined: Australia, Victoria, Geelong, Sheraton Hotel, isol. from Eucalyptus sp. (Myrtaceae), 1 Oct. 2005, P.W. Crous, CPC 12385. Cyprus, Polis, Akamas Nature Reserve, isol. from Quercus infectoria (Fagaceae), 20 Mar. 2007, coll. A. van Iperen, isol. P.W. Crous, CPC 13943. Denmark, isol. from food, garfish gill, 2007, B.A. Andersen, CPC 14306 = BA1705; isol. from indoor building material, school, 2007, B.A. Andersen, CPC 14300 = BA 1699; isol. from cheese, J. Frisvad, deposited in CBS in Oct. 2000, CBS 109031 = JBT 13731, identified as C. cladosporioides. Germany, Essen, Botanical Garden, isol. from Rosa sp. (Rosaceae), 2005, N. Ale-Agha, CPC 12313. Italy, isol. from Paeonia sp. (Paeoniaceae) by M. Curzi as C. paeoniae, CBS 118.24 = ATCC 36972 = MUCL 10098. New Zealand, Auckland, −37.00, 175.00, isol. from leaf spots of Yucca gigantea (= Y. elephantipes) (Asparagaceae), 20 May 2005, C.F. Hill, CPC 12126 = Hill 1192. Portugal, Aveiro, Aveiro campus, isol. from Ginkgo biloba (Ginkgoaceae), 11 Oct. 2006, P.W. Crous, CPC 13407–13409. Slovenia, Ljubljana, isol. from an air conditioning system (bathroom), 2004, M. Butala, CBS 121627 = CPC 12047 = EXF-967. South Korea, isol. from Dioscorea tenuipes (Dioscoreaceae), 2004, coll. H.D. Shin, isol. P.W. Crous, CPC 11395; isol. from Weigela subsessilis (Caprifoliaceae), 2004, coll. H.D. Shin, isol. P.W. Crous, CPC 11401. Spain, isol. from margarine, depos. by A.M. Jansen, CBS 261.80; Tenerife, isol. from an unknown plant, 1 Mar. 2007, P.W. Crous, CPC 13792; isol. from leaves of Leucadendron sp. (Proteaceae), 1 Mar., P.W. Crous, CPC 13798; isol. from Leucospermum sp. (Proteaceae), 1 Mar. 2007, P.W. Crous, CPC 13795, 13801; isol. from Protea sp. (Proteaceae), 1 Mar. 2007, P.W. Crous, CPC 13789. Turkey, Istanbul, isol. from man, deep mycosis of patient, isol. by A.S. Kantarcioglu, CBS 109501 = dH 12343. UK, Kew, isol. from Arundo leaf (Poaceae) by G.R. Bisby, CBS H-6933, CBS 169.54 = CBS 170.54 = IMI 025324 = NCTC 6740 = dH 15462. USA, Washington, Seattle, University of Washington campus, isol. from chasmothecia of Phyllactinia guttata (Erysiphales) on leaves of Corylus sp. (Betulaceae), 2 Dec. 2004, D. Glawe, CPC 11826, 11827, 11832. Unknown, isol. from leaf spot of Populus tremuloides (Salicaceae), dep. by C.L. Shear in Aug 1929, CBS 133.29 = ATCC 36970.
Substrate and distribution: Hypersaline water, air, indoor environments, food and plant material; Australasia (Australia, New Zealand), Asia (South Korea), Europe (Cyprus, Denmark, Germany, Italy, Portugal, Slovenia, Spain, Turkey, UK), North America (USA).
Notes: Cladosporium ramotenellum was previously only known from two Slovenian isolates (Schubert et al. 2007b), one being the type isolated from hypersaline water and an additional strain isolated from an air conditioning system. Since present molecular and morphological studies indicate that this species is a quite common saprobic species occurring on various substrates with a wider geographic distribution, its species description needs to be emended. Bensch et al. (2012) and Jang et al. (2013) already drew attention to a broadening of the range of several characters for C. ramotenellum. Jang et al. (2013) recorded the species for Korea in a study on moulds inhabiting wood. Furthermore, it was also reported from Korea by Lee et al. (2011) causing sapwood discoloration. Samson (2014) recently showed that C. ramotenellum is also quite common in indoor environments.
Strain CBS 169.54 = CBS 170.54, Bisby's “standard culture”, which was invalidly and erroneously chosen by de Vries (1952) as lectotype for C. cladosporioides, proved to belong to C. ramotenellum. It deviates from typical C. ramotenellum in having geniculate conidiophores with numerous conidiogenous loci crowded at the apex, missing ramoconidia and somewhat narrower small terminal and intercalary conidia.
Cladosporium rhusicola Bensch, Crous & U. Braun, sp. nov. MycoBank MB814636. Fig. 28, Fig. 29.
Fig. 28.
Cladosporium rhusicola (CBS 140492). A–D, F–G. Macro-, semimacro- and micronematous conidiophores and conidial chains. E. Conidial chains. Scale bars = 10 μm.
Fig. 29.
Cladosporium rhusicola (CBS 140492). A. Survey of colony development with swollen hyphal cells that give rise to young conidiophores. The ornamentation, most probably of conidia, is also visible. B. Overview on agar surface with several intact conidiophores arising from the surface level and forming conidial chains. C–D. Conidial chains on conidiophores with scars visible in (C). Note the septated aerial hyphae. Note also the relatively thin connection between the conidia and the absence of ornamentation in the hilar region. E. Several conidial chains form a stabilised superstructure. Note the conidium initial. F, I. Young intact conidiophores with three secondary ramoconidia on the tapered smooth conidiophore. G. Details of strong somewhat dispersed ornamentation and the hilar region with scar. H. Branching pattern of conidial chains and conidium initial. J. Details of ornamentation and connection of spores. Scale bars = 2 (J), 5 (F–G, I), 10 (A, C–E, H), 20 (B) μm.
Etymology: Epithet composed of the name of the host genus, Rhus, and -cola, dweller.
Mycelium sparingly branched, filiform to cylindrical-oblong, 1–4 μm wide, subhyaline to pale olivaceous-brown, smooth to asperulate or verruculose, walls unthickened, sometimes irregular in outline due to small swellings and constrictions, some cells distinctly swollen, up to 8 μm diam. Conidiophores micronematous, semimacronematous to macronematous, arising terminally or laterally from plagiotropous or ascending hyphae or from bulbous swollen hyphal cells, starting as very short lateral outgrowths of hyphae; macronematous ones straight to somewhat flexuous, cylindrical, unbranched, usually with a somewhat capitate apex, sometimes once geniculate-sinuous, 6–45(–95) × 2.5–3.5 μm, aseptate or only a few septa, septa neither darkened nor thickened, medium olivaceous-brown, smooth or almost so, walls unthickened; micronematous to semimacronematous ones filiform to narrowly cylindrical-oblong, occasionally subnodulose at the apex, rarely branched, not geniculate, length variable, 1.5–2.5 μm wide, septate, septa not constricted but often distinctly darkened and appearing somewhat thickened, subhyaline to pale olivaceous-brown, minutely verruculose to verruculose or somewhat irregularly rough-walled, walls unthickened. Conidiogenous cells integrated, terminal, sometimes intercalary in semi- and macronematous conidiophores, often slightly swollen at the apex, sometimes once geniculate-sinuous with conidiogenous loci being situated on unilateral or multilateral small swellings, in semimacronematous ones apex not or only slightly swollen, conidiogenous loci conspicuous, protuberant, 1–1.5 μm diam, thickened and darkened-refractive. Conidia catenate in branched chains, (1–)2–5 conidia in the terminal unbranched part of the chain, small terminal conidia globose, subglobose, obovoid to ellipsoid, 3–9 × 2.5–4 μm [av. (± SD) 5.3 (± 1.9) × 3.3 (± 0.5)], aseptate, apex usually broadly rounded, intercalary conidia broadly ellipsoid-ovoid, limoniform, 6–13 × (3–)3.5–4.5(–5) μm [av. (± SD) 8.9 (± 2.3) × 3.9 (± 0.5)], 0(–1)-septate, with 1–2(–3) distal hila, secondary ramoconidia ellipsoid to subcylindrical or obclavate, obclavate ones commonly formed by semimacronematous conidiophores, 9–18.5(–21) × (3.5–)4–6 μm [av. (± SD) 14.1 (± 3.3) × 4.5 (± 0.7)], 0–1-septate, septa median or often somewhat in the upper half, not very conspicuous, with (1–)2–3 distal hila, pale to medium olivaceous-brown, verruculose to verrucose or echinulate, walls slightly thickened, hila conspicuous, 0.5–1.5 μm diam, thickened and darkened-refractive; microcyclic conidiogenesis occasionally occurring.
Culture characteristics: Colonies on PDA attaining 40–58 mm after 14 d, olivaceous-grey to iron-grey, grey-olivaceous towards margins, reverse greyish blue or olivaceous-black, fluffy; margins regular, white, narrow, glabrous to somewhat feathery; aerial mycelium abundantly formed, fluffy, dense and high; sporulating. Colonies on MEA reaching 42–63 mm, grey-olivaceous to pale olivaceous-grey or olivaceous-grey at margins, reverse olivaceous-grey or iron-grey, velvety to wooly-felty; margins regular to undulate, glabrous, narrow to broad, white; aerial mycelium abundant, pale olivaceous-grey, loose to dense, fluffy, growth flat, radially furrowed and wrinkled, sporulation profuse. Colonies on OA attaining 38–50 mm, grey-olivaceous, pale olivaceous-grey to smoke-grey due to aerial mycelium, reverse leaden-grey to iron-grey, fluffy to wooly-felty; margins regular, narrow, glabrous; aerial mycelium abundant, fluffy, loose to dense, covering large parts of the colony; growth flat; sporulation profuse.
Specimen examined: South Africa, Western Cape Province, Stellenbosch, Assegaaibos, isol. from Rhus sp. (Anacardiaceae), 16 Apr. 2008, coll. F. Roets, isol. P.W. Crous (CBS H-22363, holotype; ex-type culture CBS 140492 = CPC 15219).
Substrate and distribution: On Rhus; South Africa.
Notes: Cladosporium rhusicola, a member of the herbarum species complex, reminds one of C. herbarum with the conidiophores having terminal swellings. However, the conidial surface ornamentation in the new species is more prominent than in C. herbarum being verrucose to echinulate, and conidi-ophores and conidia are shorter (Schubert et al., 2007b, Bensch et al., 2012).
Cladosporium ruguloflabelliforme Bensch, Crous & U. Braun, sp. nov. MycoBank MB814637. Fig. 30, Fig. 31.
Fig. 30.
Cladosporium ruguloflabelliforme (CBS 140494). A–E. Conidiophores with conidial chains. F. Tip of a conidiophore with dichotomously branched conidial chains. G. Conidial chain. Scale bars = 10 μm.
Fig. 31.
Cladosporium ruguloflabelliforme (CBS 140494). A. Conidiophore showing several secondary ramoconidia with hila. Note the shape and loci on the conidiophore itself. Note also the irregularly reticulate surface ornamentation of conidia. B. Terminal conidia and ornamentation that seems to be less prominent at forming (young) conidia. C. Dislodged conidial chain, conidiophore, aerial and substrate hyphae in the colony. D–G. Conidiophores, terminal, intercalary and secondary ramoconidia, details of scars and notable ornamentation in G. Scale bars = 5 (B, E–G), 10 (A, C–D) μm.
Etymology: Named after its morphological similarity with Cladosporium flabelliforme and the surface ornamentation of its conidia being rugulose or distinctly rugose (visible with SEM).
Mycelium unbranched or sparingly branched, 1–3.5 μm wide, subhyaline, pale or pale medium olivaceous-brown, with scattered irregular loose, rugulose to rugose structures, sometimes outer walls seem to detach; sometimes forming anastomoses between two hyphae. Conidiophores macro-, semimacro- and micronematous, solitary, arising terminally or laterally from hyphae, narrowly cylindrical-oblong, sometimes once geniculate-sinuous towards the apex due to sympodial proliferation, somewhat flexuous, unbranched, occasionally once branched, 40–140(–250) × (2–)2.5–3.5 μm, multiseptate, not constricted, subhyaline, pale or pale medium olivaceous-brown, almost smooth, loosely verruculose or irregularly rough-walled, walls somewhat thickened; micro- and semimacronematous hyphae hardly distinguishable from hyphae. Conidiogenous cells integrated, usually terminal, occasionally intercalary when geniculate due to sympodial proliferation, then conidiogenous cells with a single conidiogenous locus situated on a small lateral shoulder, terminal cells with (1–)2–3(–4) loci at the apex giving rise to a whirl of secondary ramoconidia, subcylindrical, 10–24 μm long, loci 1–1.5 μm diam., thickened and darkened-refractive. Ramoconidia 19–35 × 3–3.5 μm, often 1-septate, base somewhat darkened-refractive. Conidia catenate, in long, mostly basely dichotomously branched chains, up to 12 conidia per chain, 2–9 conidia in the unbranched part of the chain; small terminal conidia ovoid or narrowly ellipsoid, 5.5–7(–8) × 2–3 μm [av. (± SD) 6.6 (± 0.7) × 2.3 (± 0.4)], intercalary conidia ellipsoid or fusiform, 6–14 × (2–)2.5–3.5(–4) μm [av. (± SD) 9.2 (± 2.4) × 3.0 (± 0.6)], 0–1-septate, with 1–2 distal hila, secondary ramoconidia ellipsoid, fusiform or subcylindrical, (8–)11.5–22(–26) × (2.5–)3–4 μm [av. (± SD) 16.6 (± 4.7) × 3.5 (± 0.6)], 0–2(–3)-septate, septum median or somewhat in the upper half, septa somewhat darkened, with 1–2(–3) distal hila, pale or pale medium olivaceous-brown, surface ornamentation variable, almost smooth, minutely rugulose to rugose, distinctly reticulate with SEM, walls slightly thickened in larger ones, attenuated towards apex and base, hila 0.5–1.5(–2) μm diam, slightly thickened and darkened-refractive; microcyclic Conidi-ogenous occasionally occurring.
Culture characteristics: Colonies on PDA attaining 40–47 mm after 14 d, olivaceous-grey to iron-grey, olivaceous-black at margins, reverse iron-grey, greyish blue at margins, velvety to fluffy-felty; margins hyaline, somewhat feathery, colony centre somewhat elevated, radially furrowed and folded; aerial mycelium low or high, fluffy-felty, abundantly formed; numerous small but prominent exudates formed; sporulation profuse. Colonies on MEA reaching 45–51 mm, greenish black, shiny, in colony centre olivaceous-grey due to aerial mycelium and sporulation, reverse olivaceous-grey, velvety; margin white, narrow, glabrous, radially furrowed, colony centre wrinkled and folded, elevated; aerial mycelium dense, fluffy-felty, a single prominent exudate formed. Colonies on OA reaching 35–43 mm, greenish black, olivaceous-grey due to dense and fluffy aerial mycelium and sporulation, reverse leaden-grey to iron-grey; margin narrow, glabrous; growth low convex, without prominent exudates.
Specimen examined: South Africa, Eastern Cape Province, Grahamstown, Grahamstown Botanical Garden, isol. from Diatrapaceae sp. on Aloe sp. (Xanthorrhoeaceae), 26 Jul. 2011, P.W. Crous (CBS H-22365, holotype; ex-type culture CBS 140494 = CPC 19707).
Substrate and distribution: Isolated from Diatrapaceae on Aloe; South Africa.
Notes: The conidial chains of this species are reminiscent of C. flabelliforme, but the conidia are smooth in the latter species. In the phylogenetic analyses it clusters close to C. dominicanum but based on its morphology it is not a member of the sphaerospermum but the cladosporioides species complex. Cladosporium dominicanum differs in having subglobose and shorter small terminal conidia [(2–)3–3.5(–5.5) μm long] and shorter intercalary conidia (3–8.5 μm long) (Bensch et al. 2012).
Cladosporium rugulovarians Bensch, Crous & U. Braun, sp. nov. MycoBank MB814638. Fig. 32, Fig. 33.
Fig. 32.
Cladosporium rugulovarians (CBS 140495). A–C. Conidiophores with conidial chains. D–E. Branched conidiophores. F–H. Tips of conidiophores, with ramoconidia, secondary ramoconidia and conidia. Scale bars = 10 μm.
Fig. 33.
Cladosporium rugulovarians (CBS 140495). A. Overview of a colony with a fascicle of long conidiophores arising from the agar. B. Conidiophore with dislodged conidia and conidium initial. C. Conidiophores with secondary ramoconidia forming large amounts of globose, subglobose and ovoid intercalary and small terminal conidia with a fine dispersed reticular ornamentation. D. Conidiophore (left) and conidia showing ornamentation and the hilar region. All structures show scars. E. Intercalary and small terminal conidia showing the irregularly reticulate surface ornamentation. Scale bars = 2 (B, D–E), 10 (C), 100 (A) μm.
Etymology: Name refers to the rugulose surface ornamentation of conidia (visible with SEM) and its morphological similarity to C. varians.
Mycelium sparsely formed, 4–5.5 μm wide, septate, medium or dark olivaceous-brown, verruculose. Conidiophores macronematous, erect, arising terminally and laterally from hyphae, very long, geniculate, several times dichotomously branched towards the apex, branchlets also one or several times dichotomously branched, mostly at an angle of 5–45°, up to 475 μm long or even longer, at the base 3.5–5 μm wide and dark olivaceous-brown, becoming distinctly narrower and paler towards the apex, at the apex about 2 μm wide and subhyaline, multiseptate, often seceding at one of the lower septa, smooth, walls thickened at the base, thin-walled at the apex. Conidiogenous cells integrated, terminal and intercalary, often geniculate, initiation of conidiogenesis connected with onset of formation of several secondary ramoconidia (2–4), then growth stopping, often laterally succeeding after resurgent sympodial proliferation, loci then situated on small lateral shoulders, up to 60 μm long, conidiogenous loci prominent, subdenticulate, 1–1.5 μm diam, somewhat thickened and darkened-refractive. Ramoconidia commonly formed, 20–55 × 3–4.5 μm, 0–1-septate, base 2.5 μm wide. Conidia numerous, formed in branched chains, straight, small terminal conidia globose, subglobose, obovoid, 3–6.5 × 3–5 μm [av. (± SD) 4.6 (± 1.1) × 3.8 (± 0.8)], intercalary conidia ovoid or ellipsoid, 5–8.5(–10) × 3.5–5(–6) μm [av. (± SD) 7.1 (± 1.4) × 4.4 (± 0.9)], aseptate, very rarely 1-septate, with 1–3 distal hila, secondary ramoconidia narrowly or broadly ellipsoid or subcylindrical, width depending on the place of formation at the conidiophore, those formed at narrower and paler apical parts also narrower and paler, (9–)11–24(–30) μm long, (3–)4–5(–5.5) μm wide when formed at the lower and wider parts of conidiophores, (2–)2.5–3 μm wide when formed at apical parts of conidiophores [av. (± SD) 17.9 (± 5.6) × 3.7 (± 1.0)], subhyaline or pale olivaceous, (1–)2–4(–5) distal hila, 0–2-septate, sometimes constricted at the median septum; surface ornamentation variable, reaching from smooth or almost so to verruculose–rugulose, verrucose–rugose or irregularly rough-walled (LM), with light microscopy reminiscent of C. verrucocladosporioides (irregularly wrinkled-striate, reticulate structures in SEM), pigmentation variable, wider conidia medium or even dark olivaceous-brown, narrower ones subhyaline or pale olivaceous, walls thickened in wider conidia, unthickened in narrower ones, conidiogenous hila conspicuous, 0.5–1.5 μm diam; microcyclic conidiogenesis occurring.
Culture characteristics: Colonies on PDA olivaceous-grey to iron-grey, reverse olivaceous-black to leaden-black, velvety to floccose; margins feathery; aerial mycelium loose, diffuse; growth low convex to convex; without prominent exudates; sporulation profuse. Colonies on MEA grey-olivaceous, pale olivaceous-grey due to aerial mycelium, reverse iron-grey to olivaceous-grey, velvety to floccose; margins white, somewhat feathery, narrow; aerial mycelium mainly in colony centre, loose, fluffy; growth low convex to convex, radially furrowed and folded; without prominent exudates; sporulation profuse. Colonies on OA grey-olivaceous or olivaceous-grey, pale olivaceous-grey due to aerial mycelium, reverse leaden-grey to olivaceous-grey, velvety to floccose or fluffy; margins glabrous, sometimes somewhat rhizoid; aerial mycelium loosely floccose; growth flat; without prominent exudates; sporulation profuse.
Specimen examined: Brazil, Mato Grosso, Chapada dos Guimarães, Salgadeira, isol. from leaf sheaths of unidentified Poaceae, 18 Aug. 2010, P.W. Crous (CBS H-22366, holotype; ex-type culture CBS 140495 = CPC 18444).
Substrate and distribution: Isolated from leaf sheaths of unidentified Poaceae; Brazil.
Notes: Cladosporium rugulovarians is quite unique and only comparable with C. varians. In both species, the conidiophores are very long and several times dichotomously branched towards the apex. However, the conidia in C. varians are usually smooth with small terminal conidia being narrower, 2.5–3 μm wide, and secondary ramoconidia being longer [(8–)11–33(–40) μm]. The surface ornamentation in C. rugulovarians reminds one of C. verrucocladosporioides, which displays rugulose as well as verrucose ornamentation (Bensch et al. 2012). In the phylogenetic analyses it forms a basal sister species to the cladosporioides species complex; based on ITS it is identical to numerous other species belonging to the cladosporioides species complex (Fig. 1). In the individual gene trees it is included within the complex but at a basal position (see TreeBASE).
Cladosporium sinuosum K. Schub. et al., Stud. Mycol. 58: 141. 2007, emend. Fig. 34, Fig. 35, Fig. 36.
Fig. 34.
Cladosporium sinuosum (CPC 14000 and CPC 17632). A–G, I–M (CPC 17632). Geniculate-sinuous conidiophores and conidia, either formed solitary or in short chains. H. Conidia. Scale bars = 10 μm.
Fig. 35.
Cladosporium sinuosum (CPC 14000). A. Overview of a part of a colony on SNA, with numerous aerial hyphae. B. Detailed overview with hyphae, aerial hyphae, conidiophores and ornamented conidia. Note the continuous growth of the conidiophore that forms conidial chains as it goes (see also Figure G). C–F. Aerial hyphae, conidiophores and conidia. Note the aerial hyphal bundles with an anastomosis in Fig. E. Note also the shape of the conidiophore in this Figure. The area of contact between the conidia is not characterised by an absence in ornamentation. G–H. Details of conidia and conidiophores. Geniculate conidiophore with a single conidium formed. H. Details of ornamentation. Scale bars = 5 (H), 10 (C–G), 20 (A–B) μm.
Fig. 36.
Cladosporium sinuosum (CPC 17632) A. Overview showing a very dense area of conidium formation. Some aerial hyphae are visible. B–F. Conidial chains on geniculate conidiophores. The shape of conidia and conidiophores is the same as in Fig. 35. Note the conidium initial in (B), and the geniculate growth of the conidiophore in (F). G. Details of conidia, conidiophores and scars. Note the fine ornamentation of the conidiophore. Scale bars = 5 (G), 10 (B–F), 50 (A) μm.
Mycelium loosely branched, 1–5(–7) μm wide, irregular in outline due to swellings and constrictions, sometimes swollen up to 7 μm, subhyaline to pale olivaceous-brown, smooth, minutely verruculose or irregularly rough-walled, walls unthickened, sometimes forming loose stromatic hyphal aggregations of swollen hyphal cells, hyphal cells up to 15 μm diam, medium brown or olivaceous-brown, walls somewhat thickened; sterile hyphae sometimes forming ropes. Conidiophores macronematous, erect, straight to often flexuous, arising terminally and laterally from hyphae or from swollen bulbous hyphal cells, long, subnodulose or nodulose, with uni- or multilateral swellings, several times slightly to distinctly geniculate-sinuous due to sympodial proliferation, sometimes even zig-zag-like (see Bensch et al. 2012, fig. 282B), unbranched or branched, up to 380 μm long, (3.5–)4–6(–7) μm wide, swellings up to 10 μm wide, multiseptate, medium olivaceous-brown, smooth or minutely verruculose, walls thickened, sometimes even distinctly two-layered, 1(–1.5) μm thick. Conidiogenous cells integrated, terminal and intercalary, cylindrical-oblong, with 1–2 uni- or multilateral swellings per cell, rarely more, geniculate-sinuous, 8–31(–45) μm long, loci confined to swellings, up to four loci per nodule, loci conspicuous, prominent, 1–2(–2.2) μm diam, thickened and darkened-refractive. Conidia solitary or in short unbranched or branched chains, up to four conidia in a chain, conidia without a distal hilum ovoid, obovoid to broadly ellipsoid or doliiform, (5–)8–15 × (4–)5–8(–9) μm [av. (± SD) 10.9 (± 2.8) × 6.8 (± 1.1)], 0–1-septate, basal and intercalary conidia ellipsoid–ovoid to subcylindrical, 11–19(–24) × (5–)6–9(–11) μm [av. (± SD) 16.0 (± 2.8) × 7.7 (± 1.0)], 0–1(–2)-septate, septa median or somewhat in the upper half, becoming curved or sinuous with age, pale olivaceous to medium olivaceous-brown or pale greyish brown, densely verrucose to echinulate, walls appearing to be thick-walled due to surface ornamentation, 1–2 μm wide, with 1–2(–3) distal hila, hila protuberant, more or less conspicuous, sometimes immersed in surface ornamentation and therefore not very prominent, 1–2 μm diam, thickened and darkened-refractive; microcyclic Conidi-ogenous not observed on SNA but occurring while growing on PDA, MEA and OA.
Culture characteristics: Colonies on PDA attaining 28–47 mm after 14 d, smoke-grey to pale olivaceous-grey due to aerial mycelium, grey-olivaceous towards margins, reverse leaden-grey or olivaceous-black, fluffy-felty, margins somewhat feathery, aerial mycelium high, loose to dense, fluffy, growth low convex, without prominent exudates. Colonies on MEA reaching 38–55 mm, greenish grey to grey-olivaceous, white or smoke-grey due to abundant aerial mycelium, reverse olivaceous-grey, wooly-felty, margins white, narrow, glabrous to somewhat feathery, radially furrowed and folded, aerial mycelium loose to dense, fluffy to wooly or diffuse, growth flat or effuse, sporulation profuse. Colonies on OA attaining 18–37 mm, white, smoke-grey to pale olivaceous-grey, olivaceous-grey at margins, reverse iron-grey or leaden-grey, wooly-felty, margins crenate, aerial mycelium abundant, covering almost the whole colony, wooly-felty, dense, low to high, growth flat, sporulation profuse.
Specimens examined: France, isol. from an unidentified moss, isol. by J. Nicot-Toulouse, CBS 164.48 = ATCC 11285, stored as C. macrocarpum. Germany, Dierhagen, isol. from Eryngium maritimum (Asteraceae), 2 Oct. 2009, U. Damm, CPC 17632. Netherlands, Schiermonnikoog, isol. from air, isol. by A. Kikstra, CBS 393.68, stored as C. macrocarpum; Utrecht, De Uithof, isol. from Iris pseudacorus (Iridaceae), 26 Jun. 2010, P.W. Crous, CPC 18365. New Zealand, South Canterbury, Fairlie, State Highway 8, isol. from Crocus sativus (Iridaceae), 14 Jul. 2008, J. Rennie, CPC 15454. South Africa, Free State, Bethlehem, isol. from wheat (Poaceae), 1982, MRC 02998 = CPC 14000.
Substrate and distribution: Isolated from various plants, air and mosses; Europe (France, Germany, Netherlands), New Zealand and South Africa.
Notes: Cladosporium sinuosum, introduced by Schubert et al. in 2007 as a member of the herbarum species complex, was described on living leaves of Fuchsia excorticata from New Zealand. It is a heterosporium-like species, morphologically similar to C. macrocarpum but its conidia are formed either singly or in short unbranched chains being 0–1(–2)-septate. Terminal conidia without a distal hilum are longer and wider, broadly ellipsoid-ovoid and doliiform and micronematous conidiophores as occurring in C. macrocarpum are missing. Cladosporium herbarum differs in having distinctly narrower conidia. Until now, it was only known from the type. In the present phylogenetic study, several isolates from different substrates from Europe and South Africa are shown to belong to this species (see Fig. 2). Therefore, the species description, host range and distribution of C. sinuosum need to be emended.
Cladosporium subinflatum K. Schub. et al., Stud. Mycol. 58: 153. 2007.
Specimen examined: Ukraine, Svjatie Gory, isol. from Iris sp. (Iridaceae), 18 Jul. 2008, coll. A. Akulov, isol. P.W. Crous, CPC 15565.
Notes: Until now C. subinflatum has only been known from hypersaline environments, but it can also be saprobic as shown by the isolate from Iris sp. The isolate fits the species concept of C. subinflatum although the small swellings of the conidiophores are not as prominent as in the type.
Cladosporium subuliforme Bensch et al., Stud. Mycol. 67: 77. 2010.
Specimens examined: Brazil, Mato Grosso, on cotton (Gossypium sp.) leaves, coll. D.B. da Silva, isol. P.W. Crous, CPC 18243. Mexico, Tamanlipas, isol. from Agave tequilana var. azul (Agavaceae), 16. Oct. 2009, coll. M. de Jesús Yáñez-Morales, isol. P.W. Crous, CPC 15838; isol. from orange (Citrus sp., Rutaceae), 16 Oct. 2008, coll. M. de Jesús Yáñez-Morales, isol. P.W. Crous, CPC 15833. South Africa, Western Cape Province, Jonkershoek, isol. from Eucalyptus sp. (Myrtaceae), 12 Jan. 2009, coll. A.R. Wood, isol. P.W. Crous, CPC 16318.
Notes: Until now, this species has been recorded only from the type locality in Thailand (Bensch et al. 2010). It is herewith reported from Mexico and South Africa and probably has an even wider distribution. The conidiophores are macro-, semimacro- or micronematous, 2–3.5(–4) μm wide with the base up to 5 μm, occasionally they can be subnodulose or nodulose, both intercalary or at the apex. Superficial mycelium is abundantly formed, and the hyphae are up to 6 μm wide at the base of conidiophores. Secondary ramoconidia of the isolates cited above are slightly longer than in the type specimen [(6–)8–27(–31) μm].
Cladosporium velox Zalar et al., Stud. Mycol. 58: 181. 2007.
Specimen examined: Brazil, Chapada dos Guimaraes, Capim colonias, stop 1, −15.3621°, −55.0322°, isol. from Zea mays (Poaceae), 18 Aug. 2010, P.W. Crous, CPC 18450.
Notes: This is the first record of C. velox for South America, which has until now been known from Europe and Asia. In the specimen from Brazil, which fits the species circumscription of C. velox very well, ramoconidia were observed being 28–50 × 2.5–3 μm, 0–1-septate with a base of about 2.5 μm width.
Cladosporium versiforme Bensch, Crous & U. Braun, sp. nov. MycoBank MB814639. Fig. 37, Fig. 38.
Fig. 37.
Cladosporium versiforme (CBS 140491). A–G. Macro-, semimacro- and micronematous conidiophores and conidial chains. Scale bars = 10 μm.
Fig. 38.
Cladosporium versiforme (CBS 140491). A. Conidiophores sprouting from hyphae or swollen intercalary cells on the agar surface or arising from an aerial hypha. B–D. Conidiophores with a characteristic rounded shape on which relatively narrow loci can be observed. Note the near geniculate shape of a conidiophore in figure (B). Conidi-ogenous loci which are confined to these areas are shown in more detail together with some conidial hila. E. Conidial chains with triangular hilus. F. Detail of the prominent dense ornamentation on conidia. Note the small area on the spore that remains smooth (see also E) and the strong ornamentation of terminal conidia that are still very small. Scale bars = 5 (D–F), 10 (B–C), 20 (A) μm.
Etymology: Name refers to the different polymorph shapes of conidiophores and conidia and the variable forms of conidial surface ornamentation.
Mycelium commonly formed, creeping or ascending, unbranched or loosely branched, narrow or broad, 1–6 μm wide, subhyaline or pale olivaceous-brown, surface ornamentation variable, almost smooth or loosely verruculose, walls unthickened, sometimes forming ropes of a few hyphae, rarely anastomosing. Conidiophores polymorphic, arising terminally from ascending hyphae or laterally, solitary, shape, colour and surface ornamentation very variable, macro-, semimacro- and micronematous, filiform or narrowly cylindrical, mainly unbranched, occasionally once branched at a lower level, in macronematous ones apex usually broadened or swollen, laterally proliferated, occasionally with additional intercalary swellings or lateral shoulders, in micro- and semimacronematous conidiophores usually without lateral shoulders or swellings, sometimes apically subnodulose, maximum length ambiguous especially in conidiophores arising terminally from hyphae, up to 150 μm long or even longer, with few septa, those arising laterally from hyphae usually shorter, 7–61 μm long, 1–2-septate, macronematous conidiophores 3–4 μm wide, swellings or shoulders 5–7 μm wide, micro- and semimacronematous conidiophores 2–3 μm wide, subhyaline, pale or medium olivaceous-brown, smooth, minutely verruculose or loosely verruculose, walls unthickened, slightly thick-walled in macronematous ones. Conidiogenous cells variable in shape, mainly terminal, sometimes intercalary, in terminal cells subnodulose, capitate or with lateral proliferation and therefore irregular in shape, conidiogenous loci confined to these proliferations and swellings, usually 3–6 loci, rarely more, crowded at the apex, in unswollen cells usually 1–2 loci at the apex, 7–30 μm long, loci conspicuous, prominent, sometimes subdenticulate, 1–2 μm diam, thickened and darkened-refractive. Ramoconidia up to 45 μm long and with up to five septa, hardly distinguishable from secondary ramoconidia of the second type (see below). Conidia very variable in shape, size and colour, two different types formed in unbranched or loosely branched chains with 2–5 conidia in the terminal unbranched part of the chain, conidia of the first type herbarum-like usually formed by macronematous conidiophores, broader and darker, medium or dark olivaceous-brown, small terminal conidia globose, subglobose, broadly obovoid, 4.5–9 × 4–7 μm [av. ± SD, 6.9 (± 1.5) × 5.4 (± 1.0) μm], aseptate, intercalary conidia broadly ellipsoid, 7–15 × (4–)4.5–6(–7) μm [av. ± SD, 9.9 (± 1.9) × 5.7 (± 0.9) μm], 0(–1)-septate, secondary ramoconidia broadly ellipsoid or subcylindrical, 9–30 × 5–7.5 μm [av. ± SD, 17.9 (± 5.8) × 6.3 (± 0.8) μm], 0–3-septate; conidia of the second type paler and narrower, more commonly septate, pale olivaceous-brown or subhyaline, small terminal conidia subglobose, ovoid or ellipsoid, 4–18 × 3–5 μm [av. ± SD, 8.4 (± 5.9) × 3.7 (± 0.4) μm], intercalary conidia ellipsoid or subcylindrical, 6–21 × 3.5–5 μm [av. ± SD, 14.9 (± 6.1) × 3.7 (± 0.3) μm], 0–1-septate, secondary ramoconidia subcylindrical or cylindrical, often curved, 14–38 × 4–6 μm [av. ± SD, 28.2 (± 8.3) × 4.6 (± 0.6) μm], 1–4-septate, sometimes slightly to distinctly constricted at single septa in both types, becoming sinuous with age, surface ornamentation ranging from almost smooth to minutely verruculose, verruculose or verrucose, walls unthickened or slightly thick-walled, hila conspicuous, 0.5–2 μm diam, thickened and darkened-refractive; microcyclic conidiogenesis occurring with conidia forming secondary conidiophores.
Culture characteristics: Colonies on PDA reaching up to 70 mm after 14 d, olivaceous-black, olivaceous-grey due to sporulation, pale vinaceous due to aerial mycelium, releasing a vinaceous pigment into the agar, reverse olivaceous-grey or dark vinaceous, fluffy-felty; margins feathery; aerial mycelium dense, covering large parts oft he colony surface, felty; very small exudates formed. Colonies on MEA attaining 62–72 mm, grey-olivaceous or olivaceous where sporulating profusely, whitish or rosy buff due to abundant, felty or fluffy aerial mycelium, reverse olivaceous-grey, brick or cinnamon; margin broad, somewhat feathery, radially furrowed, colony centre folded and wrinkled; small exudates start to be formed after 14 d. Colonies on OA reaching 55–62 mm, grey-olivaceous or olivaceous where sporulating abundantly, smoke-grey or rosy-vinaceous due to felty aerial mycelium, releasing some vinaceous soluble pigment into the agar, reverse dark vinaceous and livid-red, olivaceous-grey towards margins, velvety to fluffy-felty; margins glabrous, regular, growth flat, without prominent exudates.
Specimen examined: Iran, isol. from Hordeum sp. (Poaceae), 12 May 2009, P.W. Crous (CBS H-22362, holotype; ex-type culture CBS 140491 = CPC 19053).
Substrate and distribution: On Hordeum; Iran.
Notes: Shape of conidia and conidiophores is very polymorphic in C. versiforme, which is also true for surface ornamentation and colour. This new species belongs to the herbarum species complex (Fig. 2). Cladosporium herbaroides, isolated from hypersaline water in Israel, is characterised by also having two different types of conidia but is easily distinguishable from the new species in having longer and somewhat wider conidiophores (30–230 μm long or even longer, 3–5 μm wide), longer conidiogenous cells, and narrower conidia in both conidial types [(2–)3–6(–7) μm and 2–3.5 μm] (Schubert et al., 2007b, Bensch et al., 2012). Cladosporium basiinflatum, isolated from Hordeum in Germany, and C. herbarum, epitypified by material isolated from Hordeum in Belgium, are morphologically and phylogenetically quite distinct.
Cladosporium xylophilum Bensch et al., Stud. Mycol. 67: 77. 2010.
Specimen examined: Mexico, Chiapas, isol. from banana (Musa sp., Musaceae), 16 Dec. 2008, coll. M. de Jesús Yáñez-Morales, isol. P.W. Crous, CPC 16356.
Notes: With its typical clusters of pronounced conidiogenous loci in intercalary conidia and secondary ramoconidia, this specimen matches the species concept of C. xylophilum. It is the first report of this species from Mexico.
Discussion
Cladosporium represents one of the most common, widely distributed genera of fungi, occurring in and on all kinds of materials, from soil to plants, food, paint, textiles and air, even causing human infections (Bensch et al., 2012, Sandoval-Denis et al., 2015). The first DNA phylogeny of Cladosporium s. lat. (Braun et al. 2003), clearly confirmed the genus as polyphyletic. Sexual morphs of Cladosporium s. str. were originally included in Mycosphaerella, but later the genus Davidiella (Davidiellaceae) was subsequently introduced to accommodate them (Schoch et al. 2006). Species of Davidiella have ascospores with irregular cellular inclusions (lumina), which are absent in species of Mycosphaerella (Aptroot 2006), along with periphysoids and pseudoparaphyses (Schubert et al. 2007b). Since this study, the International Code of Nomenclature for algae, fungi and plants changed, having profound implications for fungi, most importantly being the end of dual nomenclature (Wingfield et al., 2012, Crous et al., 2015), meaning that Davidiella is no longer recognised, being a synonym of Cladosporium (Cladosporiaceae). Cladosporiaceae took preference over the younger Davidiellaceae (Bensch et al. 2012), while Mycosphaerella has become a synonym of Ramularia (Mycosphaerellaceae) (Videira et al. 2015). Other major changes that occurred related to the separation of cladosporium-like genera into separate entities, e.g. Seifert et al. (2004) established Devriesia (Teratosphaeriaceae, Crous et al. 2007b) to accommodate a group of heat-resistant species. Heuchert et al. (2005), separated Digitopodium (incertae sedis) and Parapericoniella (incertae sedis), while Crous et al. (2006) introduced Metulocladosporiella (Chaetothyriales, Herpotrichiellaceae) for the taxa causing freckle disease of banana. Further studies separated several species into newly established genera, e.g. Penidiella (Capnodiales, Teratosphaeriaceae), Rachicladosporium (Capnodiales, Cladosporiaceae), Toxicocladosporium (Capnodiales, Cladosporiaceae), Verrucocladosporium (Capnodiales, Cladosporiaceae), Hyalodendriella (Helotiales, incertae sedis), Ochrocladosporium (Pleosporales, incertae sedis), Rhizocladosporium (Helotiales, incertae sedis) (Crous et al. 2007c) and Graphiopsis (Capnodiales, Cladosporiaceae) (Schubert et al., 2007a, Braun et al., 2008). Seifert et al. (2007) also delineated Amorphotheca (Leotiomycetes, Myxotrichaceae) and Hormodendrum (as Sorocybe; Chaetothyriales, incertae sedis) from Cladosporium. Finally, Quaedvlieg et al. (2014) allocated several lineages that were penidiella- and devriesia-like to novel genera, namely Neopenidiella (Capnodiales, Mycosphaerellaceae), Neodevriesia (Capnodiales, Neodevriesiaceae), Apenidiella, Eupenidiella, Myrtapenidiella, Queenslandipenidiella and Xenopenidiella (Capnodiales, Teratosphaeriaceae). Several species originally placed in Cladosporium also proved to belong to Passalora s. lat., Pseudocercospora, and Zasmidium s. lat., respectively (Crous and Braun, 2003, Schubert and Braun, 2005a, Schubert and Braun, 2005b, Schubert and Braun, 2007, Braun and Schubert, 2007).
Although Cladosporium has always been recognised as one of the largest and most heterogeneous genera of hyphomycetes encompassing more than 772 names (Dugan et al. 2004), the recent monographic treatment by Bensch et al. (2012) only recognised 170 true Cladosporium species. In spite of this, revisions of established morphological species revealed some of them to represent species complexes, namely C. herbarum s. lat., C. sphaerospermum s. lat. and C. cladosporioides s. lat. (Zalar et al., 2007, Schubert et al., 2007b, Bensch et al., 2010). The description of several novel species in the present study is therefore not that surprising. These are the result of continuous isolations from a range of substrates, collected in various continents. The polyphasic approach delineated by Bensch et al. (2012), employing morphology and a range of phylogenetic markers has proven to work well for species delimitation within the genus Cladosporium.
The current concept of the three species complexes is based on morphology only and does not always agree with the phylogenetic position of the included species. The concept of species complexes is used for practical purposes to indicate a morphological similarity of species and not to divide the genus into smaller phylogenetic entities. Furthermore, a species complex is not a taxonomic term ruled by the ICN but can freely be defined and used. Morphological features describing the three recognised species complexes within Cladosporium can be summarised as follows:
Species belonging to the herbarum complex are characterised by possessing conidia which are ornamentated ranging from minutely verruculose to verrucose, echinulate or spinulose. The surface ornamentation varies based on the length of surface protuberances and in the density of ornamentation. Most of the species possess nodulose conidiophores with the Conidi-ogenous confined to the usually lateral swellings. However, this phenetic trend is not expressed in all of the species belonging to the herbarum complex, e.g. C. subtilissimum and C. limoniforme (Schubert et al. 2007b). The ITS locus is not very successful in distinguishing species in this complex (3 / 19 species resolved), whereas act and tef1 are more or less equal in their ability to distinguish species in the complex (19 / 19 and 18 / 19, respectively).
Species of the cladosporioides species complex usually have narrowly cylindrical or cylindrical-oblong, non-nodulose, mostly non-geniculate conidiophores. Nodose conidiophores with distinct regular, more pronounced swellings, clearly separated and distant from each other, are formed only in C. colocasiae, C. oxysporum and partly also in C. tenuissimum. However, the process of conidiogenesis differs from that in the herbarum complex where the conidiophores often possess multilateral swellings round about the stalks, usually formed in quick succession which give the conidiophores a somewhat gnarled or knotty appearance. Surface ornamentation of conidia in the cladosporioides complex is quite variable ranging from smooth or almost so to irregularly verrucose–rugose or rough-walled (Bensch et al. 2010). The most prominent surfaces are formed by C. acalyphae, C. exasperatum, C. rugulovarians and C. verrucocladosporioides. What appears to be lightmicroscopically verrucose–rugose represents irregularly reticulate structures or embossed stripes (ridges) under SEM. True verrucose conidia are not known for species belonging to the cladosporoides complex until now. The ITS locus is not very successful in distinguishing species in this complex (3 / 44 species resolved), whereas act and tef1 are more or less equal in their ability to distinguish species in the complex (39 / 44 and 38 / 44, respectively).
The most remarkable feature of species belonging to the sphaerospermum complex is the formation of numerous globose or subglobose terminal and intercalary conidia. Surface ornamentation is very variable; all types of ornamentation occurring in the other two species complexes are expressed here ranging from almost smooth (C. dominicanum) to minutely verruculose (C. fusiforme, C. langeronii), verrucose (C. halotolerans) or rugose (C. ruguloflabelliforme). Conidiophores of all sphaerospermum-like species, are usually ascending, neither nodulose nor geniculate and can sometimes be poorly differentiated from their supporting hyphae. The ITS locus is rather successful in distinguishing species in this complex (10 / 14 species resolved), whereas act and tef1 are more or less equal in their ability to distinguish species in the complex (13 / 14 and 14 / 14, respectively).
Although all species belonging to the herbarum and cladosporioides complex are also phylogenetically allied, species of the sphaerospermum complex are phylogenetically not monophyletic. The complex is becoming increasingly more paraphyletic as more species with these morphological features are added to the molecular phylogeny, which means that this type of morphology has evolved several times. This is also mirrored in the resolution of ITS for the identification of a species to the species complex level in Cladosporium – ITS is actually quite successful in resolving individual species in the sphaerospermum species complex, whereas it fares rather poorly in the other two species complexes, which indicates that species of the sphaerospermum species complex are genetically quite diverse compared to the other two species complexes.
Based on their morphology most of the Cladosporium species can be referred to one of the three species complexes. There are however, a few exceptions, e.g. C. aciculare which is phylogenetically allied to C. sphaerospermum or C. ruguloflabelliforme which clusters with C. dominicanum. These two species morphologically rather resemble species of the cladosporioides complex.
In spite of the novel taxa introduced here, the continuous collection of isolates also meant that the species concepts, host ranges and distributions of some species could be emended and expanded, most notably that of C. ramotenellum and C. sinuosum. Furthermore, as we continue to collect and culture more isolates of Cladosporium spp., we expect to reveal even more species within the genus. Further collections would hopefully also shed more light on their ecology, as species of Cladosporium exhibit an interesting range of life styles, ranging from saprobes to endophytes, mycophylic species, biocontrol agents, as well as human and plant pathogens.
Acknowledgements
The authors thank the technical staff, Mieke Starink-Willemse and Patrick Arensman (DNA isolation and sequencing), Arien van Iperen (cultures), Trix Merkx (deposit of isolates) and Marjan Vermaas (photographic plates) for their invaluable assistance.
Footnotes
Peer review under responsibility of CBS-KNAW Fungal Biodiversity Centre.
References
- Allescher A. Einige weniger bekannte Pilze aus den Gewächshäusern des Kgl. Botan. Gartens in München. Hedwigia. 1895;34:215–221. [Google Scholar]
- Aptroot A. Mycosphaerella and its anamorphs: 2. Conspectus of Mycosphaerella. CBS Biodiversity Series. 2006;5:1–231. [Google Scholar]
- Bensch K., Braun U., Groenewald J.Z. The genus Cladosporium. Studies in Mycology. 2012;72:1–401. doi: 10.3114/sim0003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bensch K., Groenewald J.Z., Dijksterhuis J. Species and ecological diversity within the Cladosporium cladosporioides complex (Davidiellaceae, Capnodiales) Studies in Mycology. 2010;67:1–94. doi: 10.3114/sim.2010.67.01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braun U., Crous P.W., Dugan F.M. Phylogeny and taxonomy of cladosporium-like hyphomycetes, including Davidiella gen. nov., the teleomorph of Cladosporium s.str. Mycological Progress. 2003;2(1):3–18. [Google Scholar]
- Braun U., Crous P.W., Schubert K. Taxonomic revision of the genus Cladosporium s. lat. 8. Reintroduction of Graphiopsis (= Dichocladosporium) with further reassessments of cladosporioid hyphomycetes. Mycotaxon. 2008;103:207–216. [Google Scholar]
- Braun U., Cunnington J., Priest M.J. Annotated checklist of Ramularia species in Australia. Australasian Plant Pathology. 2005;34:1–7. [Google Scholar]
- Braun U., Schubert K. Taxonomic revision of the genus Cladosporium s. lat. 7. Descriptions of new species, a new combination and further new data. Schlechtendalia. 2007;16:61–76. [Google Scholar]
- Crous P.W., Braun U. Mycosphaerella and its anamorphs: 1. Names published in Cercospora and Passalora. CBS Biodiversity Series. 2003;1:1–571. [Google Scholar]
- Crous P.W., Braun U., Groenewald J.Z. Mycosphaerella is polyphyletic. Studies in Mycology. 2007;58:1–32. doi: 10.3114/sim.2007.58.01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crous P.W., Braun U., Schubert K., editors. The genus Cladosporium and similar dematiaceous hyphomycetesStudies in Mycology. 2007;58:1–253. doi: 10.3114/sim.2007.58.02. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crous P.W., Braun U., Schubert K. Delimiting Cladosporium from morphologically similar genera. Studies in Mycology. 2007;58:33–56. doi: 10.3114/sim.2007.58.02. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crous P.W., Braun U., Wingfield M.J. Phylogeny and taxonomy of obscure genera of microfungi. Persoonia. 2009;22:139–161. doi: 10.3767/003158509X461701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crous P.W., Gams W., Stalpers J.A. MycoBank: an online initiative to launch mycology into the 21st century. Studies in Mycology. 2004;50:19–22. [Google Scholar]
- Crous P.W., Hawksworth D.L., Wingfield M.J. Identifying and naming plant-pathogenic fungi: past, present, and future. Annual Review of Phytopathology. 2015;53:12.1–12.21. doi: 10.1146/annurev-phyto-080614-120245. [DOI] [PubMed] [Google Scholar]
- Crous P.W., Schoch C.L., Hyde K.D. Phylogenetic lineages in the Capnodiales. Studies in Mycology. 2009;64:17–47. doi: 10.3114/sim.2009.64.02. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crous P.W., Schroers H.-J., Groenewald J.Z. Metulocladosporiella gen. nov. for the causal organism of Cladosporium speckle disease of banana. Mycological Research. 2006;110:264–275. doi: 10.1016/j.mycres.2005.10.003. [DOI] [PubMed] [Google Scholar]
- Crous P.W., Shivas R.G., Quaedvlieg W. Fungal Planet description sheets 214–280. Persoonia. 2014;32:184–306. doi: 10.3767/003158514X682395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crous P.W., Verkley G.J.M., Groenewald J.Z., editors. Fungal biodiversity. Centraalbureau voor Schimmelcultures; Utrecht, Netherlands: 2009. pp. 1–269. (CBS laboratory manual series 1). [Google Scholar]
- Crous P.W., Wingfield M.J., Park R.F. Mycosphaerella nubilosa a synonym of M. molleriana. Mycological Research. 1991;95:628–632. [Google Scholar]
- David J.C. A contribution to the systematics of Cladosporium. Revision of the fungi previously referred to Heterosporium. Mycological Papers. 1997;172:1–157. [Google Scholar]
- De Vries GA (1952). Contribution to the knowledge of the genus Cladosporium Link ex Fr. CBS, Baarn.
- Dugan F.M., Schubert K., Braun U. Check-list of Cladosporium names. Schlechtendalia. 2004;11:1–103. [Google Scholar]
- Groenewald M., Groenewald J.Z., Crous P.W. Distinct species exist within the Cercospora apii morphotype. Phytopathology. 2005;95:951–959. doi: 10.1094/PHYTO-95-0951. [DOI] [PubMed] [Google Scholar]
- Groenewald J.Z., Nakashima C., Nishikawa J. Species concepts in Cercospora: spotting the weeds among the roses. Studies in Mycology. 2013;75:115–170. doi: 10.3114/sim0012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heuchert B., Braun U., Schubert K. Morphotaxonomic revision of fungicolous Cladosporium species (hyphomycetes) Schlechtendalia. 2005;13:1–78. [Google Scholar]
- Jang Y., Lee Y.M., Kim G.H. Two species of Cladosporium associated with wood discoloration in Korea. Mycotaxon. 2013;124:21–29. [Google Scholar]
- Lee Y.M., Jang Y., Kim G.H. Phylogenetic analysis and discoloration characteristics of major molds inhabiting woods. Part 3. Genus Cladosporium. Holzforschung. 2011;66(4):537–541. [Google Scholar]
- Lombard L., Serrato-Diaz L.M., Cheewangkoon R. Phylogeny and taxonomy of the genus Gliocephalotrichum. Persoonia. 2014;32:127–140. doi: 10.3767/003158514X680261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quaedvlieg W., Binder M., Groenewald J.Z. Introducing the Consolidated Species Concept to resolve species in the Teratosphaeriaceae. Persoonia. 2014;33:1–40. doi: 10.3767/003158514X681981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rayner R.W. CMI and British Mycological Society; Kew, Surrey, England: 1970. A mycological colour chart. [Google Scholar]
- Samson R.A. 2014. New insights into the biodiversity and ecology of the indoor mycobiota.http://microbe.net/wp-content/uploads/2014/10/Samson-Sloan-Berkeley-Rob-PC3_v2.pdf [Google Scholar]
- Sandoval-Denis M.P., Sutton D.A., Martin-Vicente A. Cladosporium species recovered from clinical samples in the United States. Journal of Clinical Microbiology. 2015;53:2990–3000. doi: 10.1128/JCM.01482-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schoch C., Shoemaker R.A., Seifert K.A. A multigene phylogeny of the Dothideomycetes using four nuclear loci. Mycologia. 2006;98:1041–1052. doi: 10.3852/mycologia.98.6.1041. [DOI] [PubMed] [Google Scholar]
- Schoch C.L., Crous P.W., Groenewald J.Z. A class-wide phylogenetic assessment of Dothideomycetes. Studies in Mycology. 2009;64:1–15. doi: 10.3114/sim.2009.64.01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schoch C.L., Sung G.H., López-Giráldez F. The Ascomycota Tree of Life: a phylum-wide phylogeny clarifies the origin and evolution of fundamental reproductive and ecological traits. Systematic Biology. 2009;58:224–239. doi: 10.1093/sysbio/syp020. [DOI] [PubMed] [Google Scholar]
- Schubert K., Braun U. Taxonomic revision of the genus Cladosporium s. lat. 1. Species reallocated to Fusicladium, Parastenella, Passalora, Pseudocercospora and Stenella. Mycological Progress. 2005;4(2):101–109. [Google Scholar]
- Schubert K., Braun U. Taxonomic revision of the genus Cladosporium s. lat. 4. Species reallocated to Asperisporium, Dischloridium, Fusicladium, Passalora, Pseudoasperisporium and Stenella. Fungal Diversity. 2005;20:187–208. [Google Scholar]
- Schubert K., Braun U. Taxonomic revision of the genus Cladosporium s. lat. 6. New species, reallocations to and synonyms of Cercospora, Fusicladium, Passalora, Septonema and Stenella. Nova Hedwigia. 2007;84:189–208. [Google Scholar]
- Schubert K., Braun U., Groenewald J.Z. Cladosporium leaf-blotch and stem rot of Paeonia spp. caused by Dichocladosporium gen. nov. Studies in Mycology. 2007;58:95–104. doi: 10.3114/sim.2007.58.04. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schubert K., Greslebin A., Groenewald J.Z. New foliicolous species of Cladosporium from South America. Persoonia. 2009;22:111–122. doi: 10.3767/003158509X449381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schubert K., Groenewald J.Z., Braun U. Biodiversity in the Cladosporium herbarum complex (Davidiellaceae, Capnodiales), with standardisation of methods for Cladosporium taxonomy and diagnostics. Studies in Mycology. 2007;58:105–156. doi: 10.3114/sim.2007.58.05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seifert K.A., Nickerson N.L., Corlett M. Devriesia, a new hyphomycete genus to accommodate heat-resistant, cladosporium-like fungi. Canadian Journal of Botany. 2004;82:914–926. [Google Scholar]
- Seifert K.A., Hughes S.J., Boulay H. Taxonomy, nomenclature and phylogeny of three cladosporium-like hyphomycetes, Sorocybe resinae, Seifertia azalea and the Hormoconis anamorph of Amorphotheca resinae. Studies in Mycology. 2007;58:235–245. doi: 10.3114/sim.2007.58.09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simmons E.G. Alternaria. An identification manual. CBS Biodiversity Series. 2007;6:1–775. [Google Scholar]
- Videira S.I.R., Groenewald J.Z., Verkley G.J.M. The rise of Ramularia from the Mycosphaerella labyrinth. Fungal Biology. 2015;119(9):823–843. doi: 10.1016/j.funbio.2015.06.003. [DOI] [PubMed] [Google Scholar]
- Wingfield M.J., De Beer Z.W., Slippers B. One fungus, one name promotes progressive plant pathology. Molecular Plant Pathology. 2012;13:604–613. doi: 10.1111/j.1364-3703.2011.00768.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zalar P., Hoog G.S. de, Schroers H.-J. Phylogeny and ecology of the ubiquitous saprobe Cladosporium sphaerospermum, with descriptions of seven new species from hypersaline environments. Studies in Mycology. 2007;58:157–183. doi: 10.3114/sim.2007.58.06. [DOI] [PMC free article] [PubMed] [Google Scholar]