Abstract
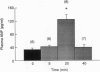
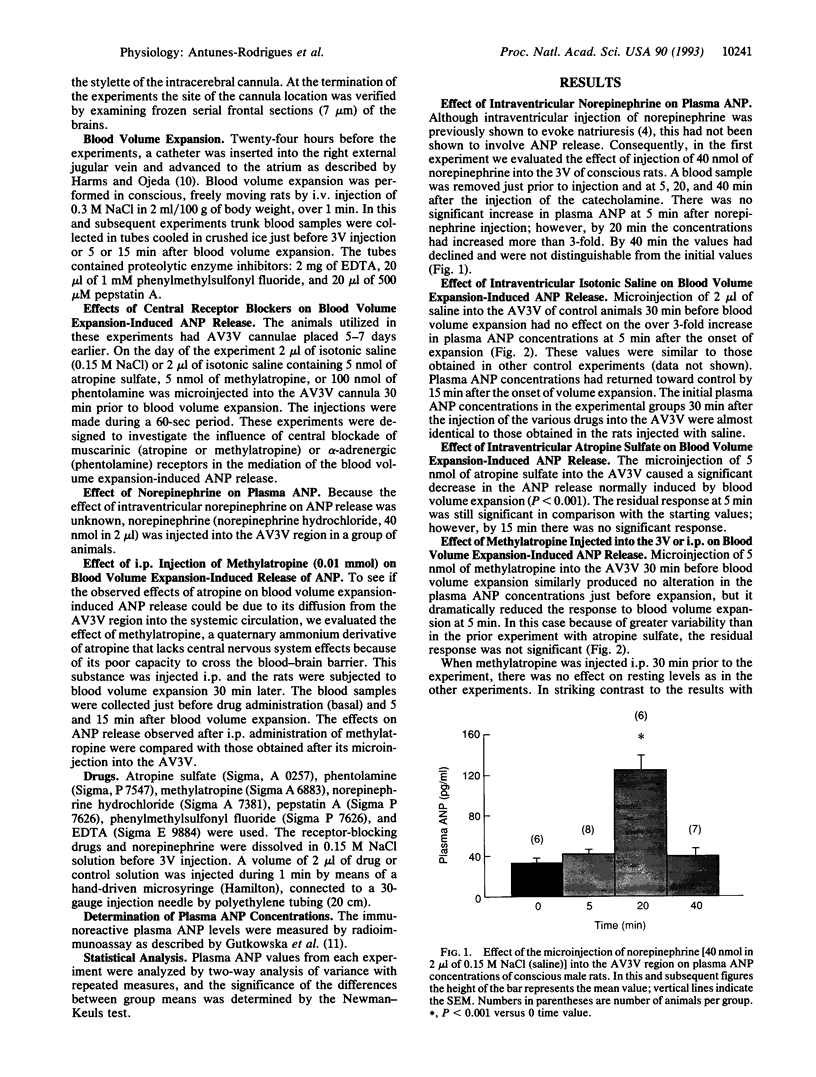
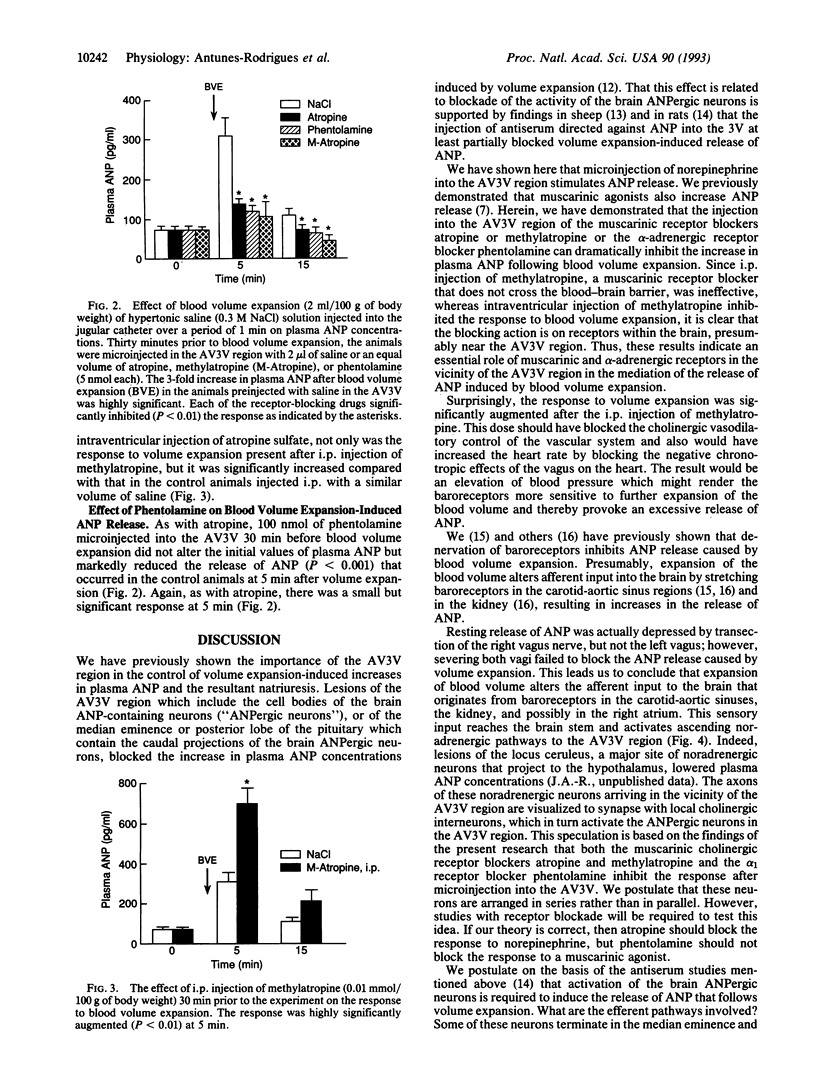
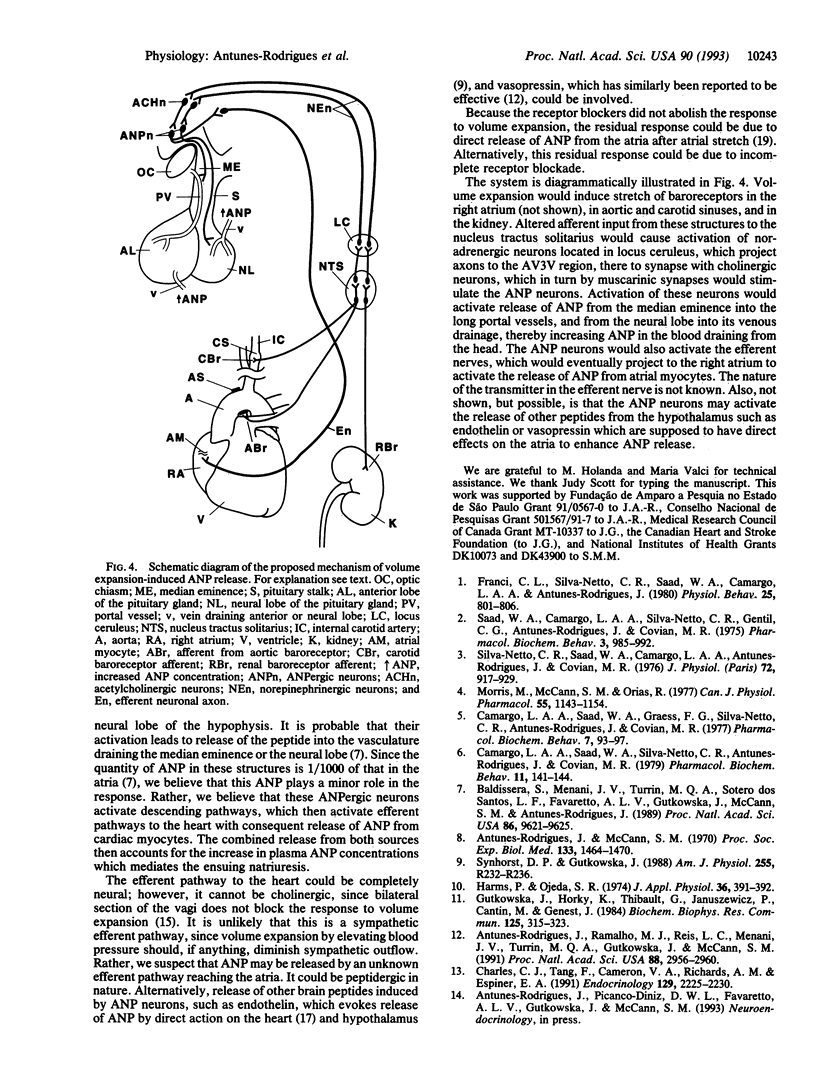
Expansion of the blood volume induces natriuresis, which tends to return the blood volume to normal. This response is mediated at least in part by the release of atrial natriuretic peptide (ANP) into the circulation. Previous experiments have shown the participation of the anterior ventral third ventricular (AV3V) region of the hypothalamus in the ANP release that follows volume expansion. When injected into the AV3V region, the cholinergic drug carbachol induces natriuresis and the release of ANP. In the present experiments, microinjection of norepinephrine into the AV3V region induced natriuresis and an increase in plasma ANP. To determine whether cholinergic and alpha-adrenergic pathways are crucial to the volume expansion-induced release of ANP, certain receptor-blocking drugs were injected into the AV3V region of conscious rats. Thirty minutes later blood volume was expanded by intravenous injection of 2.0 ml/100 g of body weight of hypertonic saline (0.3 M NaCl). Microinjection of isotonic saline (2 microliters) into AV3V region of control animals 30 min prior to volume expansion had no effect on the 3-fold increase in plasma ANP concentrations measured 5 min after volume expansion. In contrast, although the receptor-blocking drugs did not alter the initial concentrations of plasma ANP 30 min later, just prior to volume expansion, blockade of muscarinic cholinergic receptors by intraventricular injection of 5 nmol (2 microliters) of atropine sulfate or methylatropine markedly reduced the response to volume expansion but did not obliterate it. Microinjection of the alpha receptor blocker phentolamine (5 nmol) into the AV3V 30 min prior to volume expansion also markedly suppressed the ANP response. Intraperitoneal (i.p.) injection of methylatropine (0.01 mmol/100 g of body weight), which does not cross the blood-brain barrier, also did not affect the basal levels of ANP 30 min after i.p. injection. But, in striking contrast with the blockade of the response to volume expansion induced by intraventricular injection of methylatropine, the response to volume expansion was markedly enhanced by i.p. injection of methylatropine. The results therefore indicate that hypothalamic muscarinic and alpha-adrenergic synapses are essential to release of ANP in response to volume expansion. These results are consistent with a hypothetical pathway for physiological control of ANP release which involves distension of baroreceptors within the right atria, carotid and aortic sinuses, and kidney which alters afferent input to brain stem noradrenergic neurons with axons projecting to the AV3V region. There they activate cholinergic interneurons by an alpha 1-adrenergic synapse. The cholinergic neurons in turn stimulate ANP neurons in this brain region via muscarinic receptors. The stimulation of these neurons activates efferent pathways which induce the release of ANP.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Antunes-Rodrigues J., Machado B. H., Andrade H. A., Mauad H., Ramalho M. J., Reis L. C., Silva-Netto C. R., Favaretto A. L., Gutkowska J., McCann S. M. Carotid-aortic and renal baroreceptors mediate the atrial natriuretic peptide release induced by blood volume expansion. Proc Natl Acad Sci U S A. 1992 Aug 1;89(15):6828–6831. doi: 10.1073/pnas.89.15.6828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antunes-Rodrigues J., McCann S. M. Water, sodium chloride, and food intake induced by injections of cholinergic and adrenergic drugs into the third ventricle of the rat brain. Proc Soc Exp Biol Med. 1970 Apr;133(4):1464–1470. doi: 10.3181/00379727-133-34713. [DOI] [PubMed] [Google Scholar]
- Antunes-Rodrigues J., Ramalho M. J., Reis L. C., Menani J. V., Turrin M. Q., Gutkowska J., McCann S. M. Lesions of the hypothalamus and pituitary inhibit volume-expansion-induced release of atrial natriuretic peptide. Proc Natl Acad Sci U S A. 1991 Apr 1;88(7):2956–2960. doi: 10.1073/pnas.88.7.2956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baldissera S., Menani J. W., dos Santos L. F., Favaretto A. L., Gutkowska J., Turrin M. Q., McCann S. M., Antunes-Rodrigues J. Role of the hypothalamus in the control of atrial natriuretic peptide release. Proc Natl Acad Sci U S A. 1989 Dec;86(23):9621–9625. doi: 10.1073/pnas.86.23.9621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Camargo L. A., Saad W. A., Graeff F. G., Silva Neto C. R., Antunes-Rodrigues J., Covian M. R. Role played by the adenylcyclase-cAMP system of the rat septal area on Na+, K+ and water renal excretion. Pharmacol Biochem Behav. 1977 Aug;7(2):93–97. doi: 10.1016/0091-3057(77)90190-3. [DOI] [PubMed] [Google Scholar]
- Camargo L. A., Saad W. A., Silva Neto C. R., Antunes-Rodrigues J., Covian M. R. Effect of beta-adrenergic stimulation of the septal area on renal excretion of electrolytes and water in the rat. Pharmacol Biochem Behav. 1979 Aug;11(2):141–144. doi: 10.1016/0091-3057(79)90004-2. [DOI] [PubMed] [Google Scholar]
- Charles C. J., Tang F., Cameron V. A., Richards A. M., Espiner E. A. Intracerebroventricular atrial natriuretic factor (ANF) antiserum inhibits volume-induced ANF in sheep: evidence for the brain's regulation of ANF secretion. Endocrinology. 1991 Oct;129(4):2225–2230. doi: 10.1210/endo-129-4-2225. [DOI] [PubMed] [Google Scholar]
- Franci C. R., Silva-Netto C. R., Saad W. A., Camargo L. A., Antunes-Rodrigues J. Interaction between the lateral hypothalamic area (LHA) and the medial septal area (MSA) in the control of sodium and potassium excretion in rats. Physiol Behav. 1980 Dec;25(6):801–806. doi: 10.1016/0031-9384(80)90297-8. [DOI] [PubMed] [Google Scholar]
- Gutkowska J., Horký K., Thibault G., Januszewicz P., Cantin M., Genest J. Atrial natriuretic factor is a circulating hormone. Biochem Biophys Res Commun. 1984 Nov 30;125(1):315–323. doi: 10.1016/s0006-291x(84)80370-8. [DOI] [PubMed] [Google Scholar]
- Harms P. G., Ojeda S. R. A rapid and simple procedure for chronic cannulation of the rat jugular vein. J Appl Physiol. 1974 Mar;36(3):391–392. doi: 10.1152/jappl.1974.36.3.391. [DOI] [PubMed] [Google Scholar]
- Morris M., Alexander N. Baroreceptor influences on plasma atrial natriuretic peptide (ANP): sinoaortic denervation reduces basal levels and the response to an osmotic challenge. Endocrinology. 1988 Jan;122(1):373–375. doi: 10.1210/endo-122-1-373. [DOI] [PubMed] [Google Scholar]
- Morris M., McCann S. M., Orias R. Role of transmitters in mediating hypothalamic control of electrolyte excretion. Can J Physiol Pharmacol. 1977 Oct;55(5):1143–1154. doi: 10.1139/y77-157. [DOI] [PubMed] [Google Scholar]
- Saad W. A., Camargo L. A., Netto C. R., Gentil C. G., Antunes-Rodrigues J., Covian M. R. Natriuresis, kaliuresis and diuresis in the rat following microinjections of carbachol into the septal area. Pharmacol Biochem Behav. 1975 Nov-Dec;3(6):985–992. doi: 10.1016/0091-3057(75)90006-4. [DOI] [PubMed] [Google Scholar]
- Synhorst D. P., Gutkowska J. Atrial distension of isolated rabbit hearts and release of atrial natriuretic factor. Am J Physiol. 1988 Aug;255(2 Pt 2):R232–R236. doi: 10.1152/ajpregu.1988.255.2.R232. [DOI] [PubMed] [Google Scholar]
- Yoshizawa T., Shinmi O., Giaid A., Yanagisawa M., Gibson S. J., Kimura S., Uchiyama Y., Polak J. M., Masaki T., Kanazawa I. Endothelin: a novel peptide in the posterior pituitary system. Science. 1990 Jan 26;247(4941):462–464. doi: 10.1126/science.2405487. [DOI] [PubMed] [Google Scholar]