Summary
Male risk taking and decision making are affected by sex-related cues, with men making poorer and riskier decisions in the presence of females and, or their cues. In non-human species, female cues can also increase male risk taking, reducing their responses to predator threat. As estrogen receptors α and β (ERα and ERβ) are involved in the mediation of social and sexual responses, we investigated their roles in determining the effects of female-associated cues on male risk taking. We examined the effects of brief pre-exposure to the odors of either a novel or familiar estrous female on the avoidance of, and aversive responses to, predator threat (cat odor) in ERα and ERβ wild type (αERWT, βERWT) and gene-deleted (knockout, αERKO, βERKO) male mice. Exposure of αERWT and βERWT males to the odors of a novel, but not a familiar, estrous female mouse resulted in enhanced risk taking with the males displaying reduced avoidance of, and analgesic responses to, cat odor. In contrast, αERKO male mice failed to show any changes in risk taking, while βERKO males, although displaying greater risk taking, did not distinguish between novel and familiar females, displaying similarly reduced avoidance responses to cat odor after exposure to either a novel or familiar female odor. These findings indicate that the gene for ERα is associated with the sexual mechanisms (response to estrous female) and the genes for ERβ and ERα with the social (recognition of novel female) mechanisms underlying the effects of female cues on male risk taking.
Keywords: Boldness, Decision making, Predator odor, Anxiety, Fear, Social behavior, Social recognition, Sexual motivation
1. Introduction
Sex-related cues have a significant impact on male behavior. Men are reported to make “poorer” and riskier decisions when female-related cues are present (Roney et al., 2003, 2007; Wilson and Daly, 2004; Ariely and Lowenstein, 2006; Van den Bergh and Dewitte, 2006). In non-humans, predation threat provides an ethologically relevant risk for examining male decision taking (Lima and Dill, 1990; Kavaliers and Choleris, 2001). For example, the presence of a female leads to a greater risk taking and boldness by male guppies towards a predator (Godin and Dugatkin, 1996).
Animals generally respond to the threat of predation risk with a number of defensive behaviors including either immobilization or fleeing, an increase in corticosterone levels, as well as a decrease in nociceptive sensitivity and the induction of analgesia (Blanchard et al., 1990, 1998; Kavaliers and Colwell, 1991; Kavaliers and Choleris, 2001). In rodents, where chemical signals play a key role in social behavior and communication (e.g. Hurst and Benyon, 2004; Hurst et al., 2001; Beynon and Hurst, 2003), male mice that are exposed to female odor show reduced fear responses and greater risk taking. Brief exposure to the urinary odors of a novel, though not a familiar, estrous female, enhances the risk taking and boldness displayed by male mice reducing their avoidance of predator odor as well as the predator-induced rises in corticosterone and analgesic responses (Kavaliers et al., 2001).
This risk-enhancing phenomenon is, thus, composed of two aspects. One is a sexual component involving a response to a sexually receptive female and her cues, while the other consists of a social response involving the distinction between a novel and familiar individual. Only a novel estrous female will induce this enhanced risk taking by a male. These sexual and social components allow males to selectively respond to sexually receptive and potentially accessible novel females, thereby, potentially increasing their reproductive fitness.
The neurobiological mechanisms that underlie social and sexual behaviors and responses (e.g. Choleris et al., 2003, 2004; Insel and Fernald, 2004; Keverne and Curley, 2004) as well as those that affect risk taking and boldness (e.g. Montague and Berns, 2002; Trepel et al., 2005; Ditto et al., 2006) are coming under increased scrutiny. There is mounting evidence that estrogens and estrogen receptors (ERs) have an important role in determining various aspects of social and sexual behavior in males as well as in females. Mice in which the genes encoding either estrogen receptor α (ERα) or estrogen receptor β (ERβ) had been disrupted (ER-knockout mice (ERKO, αERKO, βERKO)) were impaired in their olfactory-mediated social recognition (Imwalle et al., 2002; Choleris et al., 2003, 2006; Kavaliers et al., 2004). In addition, ERα has been associated with the mediation of male aggression and sexual behavior. ERα wild-type (αERWT) male mice displayed normal sexual behavior and mating with estrous females while αERKO males failed to do so. αERKO males were, however, reported to show normal responses to, and interests in, the odors of estrous females (Ogawa et al., 1998, 2000, 2002; see, however, Rissman et al., 1999; Wersinger and Rissman, 2000). In contrast to ERα, the lack of a functional ERβ, while affecting social recognition (Choleris et al., 2003), did not impair normal expression of adult sexual behavior or preferences for females by male mice. Both the wild-type (βERWT) and βERKO male mice expressed an interest in, and responses to, female olfactory cues and exhibited normal sexual behavior, with the βERKO mice also displaying enhanced inter-male aggression (Ogawa et al., 1999, 2000; Nomura et al., 2002). Thus, ERα seems to be involved in the mediation of both social responses and sexual behaviors, while ERβ seems to be involved with social responses but not sexual behaviors. These findings raise the possibility that the genes for ERα and ERβ may also be differentially involved in mediating the sexual and social components of the impact of female cues on male risk-related behaviors. Here we examined the effects of brief exposure to the odors of either a novel or a familiar female on the subsequent avoidance and aversive responses of αERWT, βERWT, αERKO and βERKO male mice to predator odor.
2. Methods
2.1. Animals
Gonadally intact male αERKO and βERKO mice and their wild-type (WT) littermates (αERWT and βERWT); 25–30 g; 7–12 months of age) were used. They were obtained from the breeding colony maintained at The Rockefeller University (New York, NY, USA) by mating heterozygous male and female mice. The genotype of each mouse was determined by PCR amplification of tail DNA. Both colonies of mice were developed in a mixed 129/SvJ and C57BL/6J background and back-crossed into C57BL/6J. The original breeding pairs were obtained from the National Institute of Environmental Health Sciences (Lubhan et al., 1993; Krege et al., 1998). Female Swiss Webster mice ((Charles River NY) 20–30 g, 2–3 months of age) were housed in a separate room. Prior to testing, all of the mice were individually housed for 2–3 weeks in Plexiglas cages under a 12 h:12 h light–dark cycle (lights off at 10:00 h) at 22±1 °C with food and water available ad libitum. All procedures were conducted in accordance with the Institutional Animal Care and Use Committee of The Rockefeller University.
2.2. Experiment 1. Effects of pre-exposure to a female odor on the avoidance responses of males to predator odor
2.2.1. Apparatus
Odor (predator, non-predator) responses of individual male mice (αERWT, βERWT, αERKO, and βERKO) were tested during the mid-dark period in a translucent Plexiglas Y-maze apparatus (5 cm diameter) with 30 cm arms. The stimulus compartments at the end of the two arms of the Y-maze in which odor cues were placed, and the start box in which a male mouse was placed, were each 14 cm long. A solid Plexiglas barrier restricted the mouse to the start box, while perforated Plexiglas barriers at the ends of the two stimulus arms prevented contact with the odor sources while allowing detection of the odor. Removable solid Plexiglas barriers, present at ‘seams’ 8 cm into each of the stimulus arms, prevented exposure of the mice to the odor cues until the designated test times.
2.2.2. Procedures
To minimize novelty responses, male mice (n = 10, per group) were placed in the apparatus and allowed to explore the various arms (after being held in the start box for 5 min) for 30 min on 2 consecutive days prior to testing. On the test day, a male mouse was placed in the start box of the apparatus for 15 min after which the solid barrier was removed, allowing the mouse access to the two arms of the Y-maze. Two minutes later, the Plexiglas barriers in the arms were removed exposing the mouse to the stimulus odors in each arm. During the subsequent 5 min, the duration of the time spent by a mouse in each arm within 8 cm of an odor source was recorded. ‘Preference’ as used hereafter is defined as the duration of time the mouse spent in the one stimulus arm of interest divided by the total time spent in the two stimulus arms. Preference here implies actively going to and inspecting/approaching the potentially threatening stimulus (cat odor) and as such providing a measure of risk taking and boldness. Although “increased preference” can be interpreted as a reduced “avoidance response”, preference rather than avoidance ratios (corrected for total time actively responding to both odors) are considered as the appropriate measure to be used for statistical analyses (Hardy and Field, 1998; Wagner, 1998; Kavaliers et al., 2003, 2006).
The stimulus odor choice conditions were of a predator (cat) odor vs non-predator (control novel odor) odor. Cat odor was provided by a 2 cm2 strip of cloth collar worn by a cat for 2 weeks, while novel odor was provided by a 2 cm2 strip of clean collar treated with dilute (10%) natural almond extract. Results of prior studies showed that the almond odor had no evident aversive effects on male mice (Kavaliers et al., 2004).
Testing of male mice in the Y-maze was carried out after a 1 min exposure to the odors of either an estrous or non-estrous female. During the odor exposures, male mice (n = 10, in each case) were individually placed in a Plexiglas partitioned area (12.5 × 15 × 10 cm3) that was provided with a vented Plexiglas tube, 10 cm in length, 3 cm in diameter and sealed at each end with fine plastic mesh across which a mouse could neither traverse nor reach. The tube contained the urine and associated odorous secretions of either a familiar or a novel estrous or non-estrous female to which the male could come into close olfactory contact. When a male needed to be familiarized with female odor for experimental purposes, he was exposed for 24 h to the soiled bedding of a female collected over 24 h. This included a 1 h exposure to the actual female across a wire mesh through which direct olfactory contact was possible, further ensuring that a male was exposed to non-volatile odor cues that are considered to be important for individual recognition (Cheetham et al., 2007). Testing of males occurred on the following day after the 24 h exposure.
Freshly deposited urine and associated odors were obtained from single females that were placed for 1 h in a clean cage lined with blank filter paper (Whatman No. 4, England). Examination under an ultraviolet light confirmed that the filter papers were marked by the females, who generally urinated within 5 min of arriving into the cage. The filter paper that lined a single cage was cut into strips and inserted into to the exposure tubes. Additional urine-marked filter paper that was collected over several days and frozen was added to the tube to ensure that estrous female odor was present. Each male was presented with the odor of a single female. Isolated females were primed with substrate from the cages of Swiss Webster males to stimulate estrous cycling (Marsden and Bronson, 1965). Non-primed females were used to obtain non-estrous odors. Wet mount vaginal smears were used to determine the estrous state of the females.
2.2.3. Data analyses
All of the preference ratios were transformed to natural log (ln) values prior to analysis by analysis of variance (ANOVA) with Tukey’s post hoc tests with a 0.05 significance level.
2.3. Experiment 2. Effects of pre-exposure to female odor on predator-induced analgesia in males
2.3.1. Procedures
Nociceptive responses of the individual WT and KO mice were carried out for a minimum of 4 days after the choice tests. During the mid-to-late light period in a room separate from their holding rooms, male mice (αERWT, βERWT, αERKO, and βERKO) that had been exposed for 24 h to the odors of a familiar estrous female (as described for Experiment 1) were individually placed in clean cages (25 × 15 × 20 cm3) and exposed for 1 min to the urinary odors of either a familiar (female odor that a male was exposed to for 24 h) or novel estrous or non-estrous female using the vented tube described in Experiment 1. The familiar and novel female odors were different from those used in Experiment 1. After the female odor source was removed the males were exposed to a second vented Plexiglas tube (10 cm long, 3 cm diameter and sealed at each end with plastic mesh) containing the cat odor.
Prior to any odor exposure, immediately after exposure to the odor of a female, and after exposure to the predator odor for 1 min, the nociceptive responses of individual mice were determined using the ‘hot-plate’ test. Animals were placed individually on a warmed surface (analgesiometer, AccuScan Instruments, Columbus, OH, USA) maintained at 50±0.5 °C and the latency of the first foot lift or lick, whichever came first, was recorded. After this response was displayed, or after 60 s, the mouse was quickly removed from the surface and returned to his cage. Pilot and previous investigations showed that repeated handling procedures, including assessments of nociceptive responses, had no significant effects on nociceptive sensitivity (Kavaliers et al., 2001).
2.3.2. Data analyses
Data were analyzed with a mixed-design repeated measures ANOVA with Tukey’s post hoc tests. All analyses were performed using SPPS with 0.05 level of significance.
3. Results
3.1. Experiment 1. Predator odor avoidance
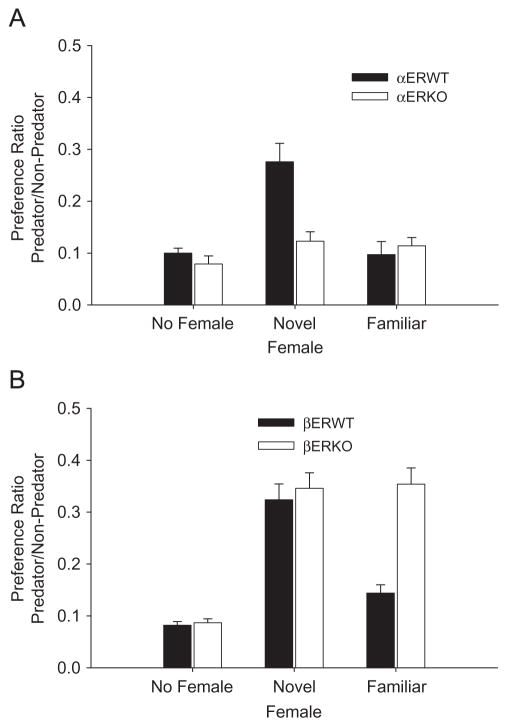
All of the male mice displayed a similar marked overall preference for non-predator odor and avoidance of the predator odor when presented with the predator (cat odor) and non-predator (control odor) stimulus odor combination, with only 15–22% of their time being spent in the arm holding the predator odor (Figure 1A, B). This relative preference for non-predator odor and avoidance of predator odor was affected by pre-exposure to estrous female odor with a significant main effect of female odor condition (F (2,108) 50.25, p<0.001), genotype (F (3, 108) = 18.08, p<0.001) and a significant interaction of male genotype and female pre-exposure (F (6,108) = 7.157, p<0.001).
Figure 1.
(A–B) Effects of a 1 min pre-exposure to the odors of either a familiar or novel (unfamiliar) estrous female on the subsequent responses of (A) αERWT, αERKO and (B) βERWT, βERKO male mice in a Y-maze odor choice apparatus to a predator (cat odor) and non-predator (novel odor, almond), odor combinations. The responses of mice receiving no prior odor exposures (no female) are also shown. Responses are given as preference ratios (e.g. time spent in the vicinity of the predator odor/time spent in the vicinity of the predator odor+time spent in the vicinity of the non-predator odor). Increased preference indicates an augmented interest in, and approach to, the predator odor and is indicative of a reduced avoidance of the predator odor. Preferences were determined over a 5 min period. N = 10 in all cases. Vertical lines denote a standard error of the mean.
The responses of the WT males were significantly affected by the female odor condition (αERWT, F (2, 27) = 15.93, p<0.01); βERWT, F (2, 27) = 40.14, p<0.001). Brief (1 min) pre-exposure to the odors of a novel estrous female significantly decreased the avoidance and increased the inspection of and preference for the predator odor by the αERWT and βERWT male mice (all p’s<0.001) who now spent 28–35% of their time active in the predator arm. Exposure to the odors of the familiar female had no significant effects on the avoidance of the predator odor.
The responses of the αERKO males were not affected (F (2, 27) = 1.963, p = 0.180) by the female odor exposure condition. Neither the familiar nor novel estrous female odor exposure had any significant effect on their responses to, and avoidance of, the predator odor (Figure 1A). In contrast, the βERKO males displayed a significantly reduced avoidance of and greater interest in the predator odor (F (2, 27) = 71.25, p<0.001) after pre-exposure to estrous female odor. However, the βERKO males displayed equivalent, significantly reduced avoidance responses to the cat odor after exposure to either the familiar (p<0.001) or novel (p<0.001) female odor (Figure 1B). In both cases the responses were not significantly different from those of the βERWT males that received a brief exposure to the odor of a novel female.
Brief (1 min) pre-exposure to the odors of either a familiar or novel non-estrous female or an empty tube had no significant effects on the avoidance of the predator odor displayed by any of the WT or KO male mice (not shown). Their avoidance responses were not significantly different from those displayed by the WT and KO males that received no prior exposure to female odor (Figure 1A, B).
3.2. Experiment 2. Predator and female-odor-induced analgesia
3.2.1. Effects of exposure to cat odor
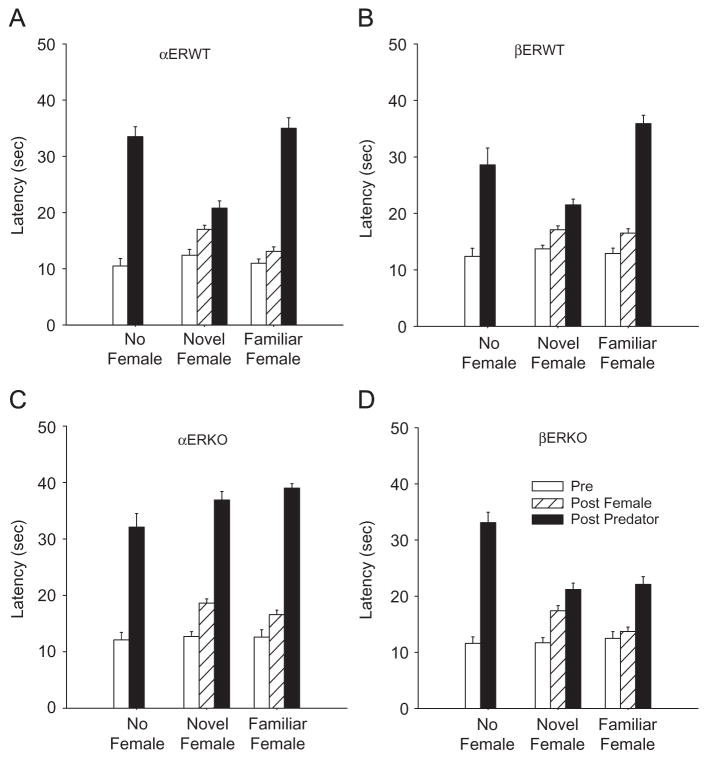
All of the WT and KO mice that were exposed to the odor of a predator (cat) for 1 min in the absence of female odor showed increased thermal response latencies (F (1,27)> 4.04; p<0.03), indicative of the induction of analgesia (Figure 2A–D). There were no significant differences in the levels of cat-odor-induced analgesia displayed by the four genotypes. Exposure to the novel almond odor (not shown) had no significant effects on nociceptive sensitivity. There were also no significant differences in basal nociceptive sensitivity between the WTs and KOs, consistent with the results of prior investigations (Spooner et al., 2007).
Figure 2.
(A–D) Nociceptive responses of: (A) αERWT; (B) βERWT; (C) αERKO; and (D) βERKO male mice that were exposed for either 1 min to the odors of either a familiar or unfamiliar (novel) estrous female and then exposed for 1 min to predator (cat) odor. Responses of mice (control) receiving no female odor exposure are also shown. Nociceptive sensitivity, as measured by the latency of response to a 50 °C thermal surface was determined before any odor exposures (baseline), after exposure a female (post-female) and after exposure to the predator odor (post-predator). N = 10, in all cases. Vertical lines denote a standard error of the mean.
3.2.2. Effects of exposure to female odor
Both the WT and KO males that were exposed to estrous female odors displayed analgesic responses with the magnitude of these responses being dependent on prior familiarity with the female odor (all Fs (1,18)>8.77, p’s<0.03). The odors of novel females elicited significant analgesic responses in all of the genotypes (p<0.03) with the levels of analgesia being markedly lower (all p’s<0.001) than those elicited by predator odor (Figure 2A–D). Exposure to the odors of a familiar female elicited significant analgesic responses in only the αERKO males (p<0.01). Exposure to the odors of non-estrous females had no significant effects on the thermal response latencies of any of the WT or KO males (data not shown). Their response latencies were equivalent to the pre-exposure response latencies shown in Figure 2.
3.2.3. Effects of pre-exposure to female odor on the responses to predator odor
The analgesic responses of the WTs to the predator odor were significantly affected by female odor condition (αERWT, F (2, 36) = 48.542, p<0.001; βERWT, F (2, 36 = 44.85, p<0.001). Pre-exposure of the WT males to the odors of a familiar estrous female had no significant effect on the level of cat-odor-induced analgesia. However, βERWT and αERWT males that were pre-exposed for 1 min to the odor of a novel estrous female and then exposed for 1 min to the odor of a predator displayed significantly (all p’s<0.001) reduced analgesic responses. Their response latencies were significantly (all p’s<0.02) lower than those of the males with either no female odor pre-exposure, or that had been pre-exposed to the odors of a familiar female (αERWT, βERWT, p<0.001) before being presented the predator odor (Figure 2A–C).
The analgesic responses of the αERKO males were not significantly affected by estrous female odor pre-exposure (F (2, 36) = 30.1, p = 0.09). Pre-exposure to the odors of either a novel, or a familiar estrous female, had no significant effects on the level of cat-odor-induced analgesia displayed by the αERKO males. Their predator-odor-induced increased response latencies were not significantly different from those seen in the absence of a female. In contrast, the level of cat-odor-induced analgesia displayed by βERKO males was significantly reduced by female odor pre-exposure (F (2, 36) = 40.53, p = 0.03). However, the attenuated responses of the βERKO males were not affected by prior familiarity with the female. The levels of analgesia displayed by βERKO males pre-exposed to either familiar or novel female odors were equivalent and not significantly different from those displayed by βERWT males that were pre-exposed to the odors of a novel female. Pre-exposure to the odors of an unfamiliar non-estrous female had no significant effects on the levels of cat-odor-induced analgesia displayed by any of the males, with the levels of analgesia elicited being equivalent to that seen in the various KOs and WTs following just predator odor exposure (no female, Figure 2).
4. Discussion
Here we show that the genes for ERα and ERβ are differentially involved in mediating the facilitatory effects of female cues on risk taking by males. Male mice are “emboldened” in their responses to a predator after brief exposure to the odors of a novel female with the gene for ERα associated with the sexual mechanisms (response to estrous female) and the genes for ERβ, and likely ERα, with the social (recognition of a novel female) mechanisms of this effect. As “sex-related cues” have a parallel impact on decision making and risk taking in human males (Blanton and Gerrard, 1997; Wilson and Daly, 2004; Ariely and Lowenstein, 2006; Van den Bergh and Dewitte, 2006) our findings suggest that the genes for ERα and ERβ may also have a modulating role on sexually motivated risk taking and decision making in men.
Both WT and KO male mice displayed marked fear, anxiety and stress responses to cat odor. This was assessed in two ways. (i) In an odor choice test where test male mice displayed an intense aversion to, and avoidance of, cat odor (Figure 1). This predator-odor avoidance involves a heightened anxiety that is sensitive to anxiolytic agents (e.g. Blanchard et al., 1993; Dielenberg et al., 1999). (ii) By the assessment of nociceptive sensitivity. Brief exposure of the WT and KO mice to cat-odor-elicited decreases in nociceptive sensitivity (Figure 2), indicative of the induction of analgesia. This analgesia is not simply a reflection of increased fearfulness and “freezing”. Rather, it is associated with stress-induced activation of opioid and non-opioid neurochemical mechanism (Kavaliers and Colwell, 1991; Kavaliers and Choleris, 2001). This stress-induced analgesia reflects a motivational shift that facilitates the expression of various active and passive defensive behavioral responses (Fields, 2004), thereby reducing the risk of predation.
Our results show that it is not just the presence of the odors of a female per se that elicits reduced aversive responses to predator odor and a greater risk taking by the WT males. The female has to be both novel and in estrous. Exposure to the odors of a novel non-estrous female had no significant effects on male responses. This supports the presence of both sexual (sexually receptive estrous female) and social (novel estrous female) components in the adaptive expression of this risk facilitatory, emboldening, response. Brief exposure to a novel estrous female may signal the likelihood of an immediate, though temporally limited, availability of a sexually responsive female. The presence of this sexual incentive could facilitate a rapid motivational shift in the males from defensive responses to a search for a sexually receptive female. Human decisions that are made under risk have also been found to be malleable (Fong and McCabe, 1999). In men, sexual cues can affect decision making, facilitating the expression of sexually motivated behaviors (Roney, 2003; Wilson and Daly, 2004; Ariely and Lowenstein, 2006).
Brief exposure to the odors of estrous females also reduced the predator-induced avoidance and analgesic responses of the βERKO males who, however, seemed to be unable to distinguish between the odors of familiar and novel estrous females. Although displaying riskier behaviors, their responses were no longer linked to novel sexually receptive females and increased reproductive chances. In contrast, the αERKO males failed to show any reduction in their aversive responses to predator odor after exposure to either novel or familiar female odors. This indicates that the sexual components of emboldening involve only the gene for ERα while the social (recognition) components involve the gene for ERβ and possibly ERα.
The present findings are in agreement with other studies with ERKO mice showing a differential involvement of the two ERs in sexual and social behavior. ERα and ERβ often have antagonistic actions and transcription effects leading to potentially different behavioral effects (Vasudevan et al., 2001; Lindberg et al., 2003). Loss of ERα is reported to result in a decrease of both male and female sexual behaviors with no evident effects of ERβ deletion (Ogawa et al., 1997, 1999, 2000; Wersinger and Rissman, 2000; Kudwa et al., 2006; Nomura et al., 2006). Although, in both cases the KOs could still distinguish between males and females of various sexual conditions, there are some suggestions that αERKOs may show a decrease in sexual incentive motivation (Wersinger and Rissman, 2000). Regarding the social recognition, female αERKO and βERKO mice have also been shown to differ in the extent of their attenuation of social memory (Choleris et al., 2006). In addition, αERKO males displayed reduced aggressive behavior towards other males while βERKO males displayed enhanced inter-male aggressiveness relative to their WTs (Ogawa et al., 1998, 2000, 2002; Imwalle et al., 2002; Nomura et al., 2002; Rissman et al., 1999; Scordalakes and Rissman, 2003; Dominguez-Salazar et al., 2004). In this regard, it has been suggested that ERβ activation may exert an attenuating effect on male aggression induced by estrogen through ERα-mediated mechanisms (Nomura et al., 2006).
These findings support sexual motivation as a possible underlying factor driving the male behavior in our choice test. The enhanced risk taking elicited by brief exposure to female odors may facilitate mate search and aggressive interactions with other male competitors. The greater risk taking or male boldness may be a “side-effect” of the lower fear and stress responses and greater sexual motivation and “searching” for the briefly available novel female. Augmented sexual motivation is similarly speculated to contribute to the greater risk taking seen in men exposed to sex-related cues of women (Ariely and Lowenstein, 2006; Van den Bergh and Dewitte, 2006).
The responses of the male mice may be elicited by either relatively short-lived highly volatile and/or non-volatile female odor cues that are detected upon close inspection of the urinary cue (Hurst et al., 2001; Hurst and Beynon, 2004; Cheetham et al., 2007). Volatile and non-volatile odors associated with the major histocompatibility complex and the major urinary proteins provide information about the condition and individual identity of the scent owner (Hurst et al., 2001; Beynon and Hurst, 2003; Hurst and Beynon, 2004). Together these signals provide information about the condition and identity of the female.
Brief pre-exposure to these odor components of a novel female significantly reduced the predator-odor-induced fear and stress responses in the WT males (Figure 1). This reduction is unlikely to be due to changes in male testosterone levels in that in previous studies it was shown that exposure to the odors of a novel female blunted predator-odor-induced rises in corticosterone levels, but was not associated with an immediate increase in testosterone levels (Kavaliers et al., 2001). The impairments of the KO mice also cannot be attributed to differences in basal testosterone levels in that the various WTs and KOs examined here are reported to display similar basal testosterone levels (Ogawa et al., 1998, 2002; Nomura et al., 2002, 2006). Furthermore, increases in testosterone levels in male mice generally occur 15–30 min after exposure to females or their odors (Coquelin and Desjardins, 1982; Smith et al., 1996; Kavaliers et al., 2001) suggesting that the emboldening responses seen 1 min after exposure to female odor are not directly associated with changes in testosterone. The testosterone responses of men to brief interaction with women or their cues are suggested to follow similar temporal patterns (Roney et al., 2003, 2007). Thus, in men, like in male mice, short-term increases in risk taking induced by female cue may occur independent of any rise in testosterone.
While unlikely to be due to changes in testosterone levels, the “emboldening” effects of female odor may involve alterations in the metabolism of testosterone. Increased aromatase activity with subsequent alterations in testosterone metabolism, shifts in central estrogen levels, and possibly ER function, have been proposed to be associated with augmented male sexual interest (Bakker et al., 2002; Balthazar et al., 2005; Taziaux et al., 2007). This is further supported by the findings that non-copulating rats, while having similar testosterone levels as copulating rats, display reduced neuronal aromatase activity and levels of ERα (Portillo et al., 2006, 2007).
These findings are consistent with a proposed “micronet” involving genes for ERα, ERβ, oxytocin (OT), and the OT receptor (OTR) as the regulatory basis for olfactory-mediated social recognition (Choleris et al., 2003, 2004). Oxytocin has been shown to augment “trust” and the use of information provided by others in mice and humans (Kosfeld et al., 2005; Kavaliers et al., 2006). Both of these actions could influence sexually motivated decision making and risk taking. Olfactory signals from the main and accessory olfactory pathways converge at the medial amygdala where the identity of the odor source is most likely determined. In the medial amygdala ERα is needed for the induction of OT receptors and, thus, for the normal action of OT at this level. OT production in the hypothalamus is also under estrogen control, through ERβ. Disruptions at the level of either OT, ER-α, or ER-β genes and their products could lead to impaired processing and/or integration of odor information at the level of the medial amygdale. This could result in impaired discrimination between familiar and unfamiliar female odor, thus, modifying sexual motivation. This is supported by the findings that exposure to the signals of an estrous female results in the activation of brain OT at the level of the paraventricular nucleus of the hypothalamus (Waldherr and Neumann, 2007). Moreover, this OT activation was associated with an anxiolytic response and reduced emotional response to anxiogenic stimuli that is consistent with an enhanced risk taking and boldness.
The use of selective KOs has allowed us to distinguish the sexual and social components of male risk taking from a neurobiological perspective. We have shown that the effects of cues from sexually receptive females on male boldness and risk taking, and likely decision making, involves at the sexual level the gene for ERα and at the social level the gene for ERβ and likely ERα. This raises the possibility that ERα and ERβ may similarly be part of the mechanism(s) whereby sex-related cues impact decision making and risk taking by human males.
Acknowledgments
Roles of the funding sources
This study has been supported by the Natural Sciences and Engineering Research Council of Canada RO557A01(MK), 045881 (EC), NIH MH36273 (DWD), and NIMH 62147 (SO).
Footnotes
Conflict of interest statement
Jan-Ake Gustafsson is a shareholder, research grant receiver and consultant of KaroBio AB.
References
- Ariely D, Lowenstein G. The heat of the moment: the effect of sexual arousal on sexual decision making. J Behav Decis Making. 2006;19:87–98. [Google Scholar]
- Bakker J, Honda S, Harda N, Balthazart J. Sexual partner preference requires a functional aromatase (Cyp 19) gene in male mice. Horm Behav. 2002;42:158–171. doi: 10.1006/hbeh.2002.1805. [DOI] [PubMed] [Google Scholar]
- Balthazar J, Cornil CA, Taziaux M, Charlier TD, Ballien M, Ball GF. Rapid changes in production and behavioral action of estrogens. Neuroscience. 2005;138:783–791. doi: 10.1016/j.neuroscience.2005.06.016. [DOI] [PubMed] [Google Scholar]
- Beynon R, Hurst JL. Multiple roles of major urinary proteins in the house mouse, Mus domesticus. Biochem Soc Trans. 2003;31:142–146. doi: 10.1042/bst0310142. [DOI] [PubMed] [Google Scholar]
- Blanchard DC, Blanchard RJ, Rodgers RJ, Weiss SM. The characterization and modeling of antipredator defensive behavior. Neurosci Biobehav Rev. 1990;14:463–472. doi: 10.1016/s0149-7634(05)80069-7. [DOI] [PubMed] [Google Scholar]
- Blanchard RJ, Shepherd JK, Rodgers RJ, Magee L, Blanchard DC. Attenuation of antipredator defensive behavior in rats following chronic treatment with imipramine. Psychopharmacology. 1993;110:245–253. doi: 10.1007/BF02246981. [DOI] [PubMed] [Google Scholar]
- Blanchard RJ, Nikulina JN, Sakai RR, Mckittrick C, Mcewen B, Blanchard C. Behavioral and endocrine changes following chronic predatory stress. Physiol Behav. 1998;63:561–569. doi: 10.1016/s0031-9384(97)00508-8. [DOI] [PubMed] [Google Scholar]
- Blanton H, Gerrard M. Effects of sexual motivation on men’s risk perception for sexually transmitted disease: there must be 50 ways to justify a lover. Health Psychol. 1997;16:374–379. doi: 10.1037//0278-6133.16.4.374. [DOI] [PubMed] [Google Scholar]
- Cheetham SA, Thom MD, Jury F, Oiller WER, Benyon RJ, Hurst JL. The genetic basis of individual-recognition signals in mice. Curr Biol. 2007;17:1771–1777. doi: 10.1016/j.cub.2007.10.007. [DOI] [PubMed] [Google Scholar]
- Choleris E, Gustafsson J-Å, Korach KS, Muglia LJ, Pfaff D, Ogawa SW. An estrogen-dependent four-gene micronet regulating social recognition: a study with oxytocin and estrogen receptor -α and -β knockout mice. Proc Natl Acad Sci USA. 2003;100:6192–6197. doi: 10.1073/pnas.0631699100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choleris E, Kavaliers M, Pfaff DW. Functional genomics of social recognition. J Neuroendocrinol. 2004;16:383–389. doi: 10.1111/j.0953-8194.2004.01178.x. [DOI] [PubMed] [Google Scholar]
- Choleris E, Ogawa S, Kavaliers M, Gustafsson JA, Korach KS, Muglia J, Pfaff DW. Differential involvement of estrogen receptor α, β and oxytocin in social discrimination: a detailed behavioral analysis with knockout mice. Genes Brain Behav. 2006;5:528–539. doi: 10.1111/j.1601-183X.2006.00203.x. [DOI] [PubMed] [Google Scholar]
- Coquelin A, Desjardins C. Luteinizing hormone and testosterone secretion in young and old male mice. Am J Physiol (Endocrinol Metab) 1982;243:E257–E263. doi: 10.1152/ajpendo.1982.243.3.E257. [DOI] [PubMed] [Google Scholar]
- Dielenberg RA, Arnold JC, McGregor IS. Low-dose midazolam attenuates predatory odor avoidance in rats. Pharmacol Biochem Behav. 1999;62:197–201. doi: 10.1016/s0091-3057(98)00064-1. [DOI] [PubMed] [Google Scholar]
- Ditto PH, Pizarro DA, Epstein EB, Jacobson JA, Macdonald TK. Visceral influences on risk-taking behavior. J Behav Decis Making. 2006;19:99–113. [Google Scholar]
- Dominguez-Salazar E, Bateman HL, Rissman EF. Background matters: the effects of estrogen receptor α gene disruption on male sexual behavior are modified by background strain. Horm Behav. 2004;46:482–490. doi: 10.1016/j.yhbeh.2004.05.006. [DOI] [PubMed] [Google Scholar]
- Fields H. State-dependent opioid control of pain. Nature Neurosci. 2004;5:565–575. doi: 10.1038/nrn1431. [DOI] [PubMed] [Google Scholar]
- Fong C, McCabe K. Are decisions under risk malleable? Proc Natl Acad Sci USA. 1999;96:10927–10932. doi: 10.1073/pnas.96.19.10927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Godin JG, Dugatkin LA. Female mating preferences for bold males in the guppy, Poecilia reticulata. Proc Natl Acad Sci USA. 1996;93:10262–10267. doi: 10.1073/pnas.93.19.10262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardy ICW, Field SA. Logistic analysis of animal contests. Anim Behav. 1998;56:767–792. doi: 10.1006/anbe.1998.0833. [DOI] [PubMed] [Google Scholar]
- Hurst JL, Beynon RJ. Scent wars: the chemobiology of competitive signaling in mice. BioEssays. 2004;26:1288–1298. doi: 10.1002/bies.20147. [DOI] [PubMed] [Google Scholar]
- Hurst JL, Payne CE, Nevison CM, Maries AD, Humphries RE, Robertson DHL, Cavaggioni A, Beynon RJ. Individual recognition in mice mediated by major urinary proteins. Nature. 2001;414:631–634. doi: 10.1038/414631a. [DOI] [PubMed] [Google Scholar]
- Imwalle DB, Scordalakales EM, Rissman EF. Estrogen receptor α influences socially motivated behaviors. Horm Behav. 2002;42:484–491. doi: 10.1006/hbeh.2002.1837. [DOI] [PubMed] [Google Scholar]
- Insel TR, Fernald RD. How the brain processes social information: searching for the social brain. Annu Rev Neurosci. 2004;27:697–722. doi: 10.1146/annurev.neuro.27.070203.144148. [DOI] [PubMed] [Google Scholar]
- Kavaliers M, Choleris E. Antipredator responses and defensive behavior: ecological and ethological approaches for the neurosciences. Neurosci Biobehav Rev. 2001;25:577–586. doi: 10.1016/s0149-7634(01)00042-2. [DOI] [PubMed] [Google Scholar]
- Kavaliers M, Colwell DD. Sex differences in opioid and non-opioid mediated predator-induced analgesia in mice. Brain Res. 1991;568:173–177. doi: 10.1016/0006-8993(91)91394-g. [DOI] [PubMed] [Google Scholar]
- Kavaliers M, Choleris E, Colwell DD. Brief exposure to a novel oestrous female emboldens male mice by reducing predator-induced behavioral and hormonal responses. Horm Behav. 2001;40:497–509. doi: 10.1006/hbeh.2001.1714. [DOI] [PubMed] [Google Scholar]
- Kavaliers M, Colwell DD, Braun JW, Choleris E. Brief exposure to the odour of a parasitized male alters the subsequent mate odour responses of female mice. Anim Behav. 2003;65:59–68. [Google Scholar]
- Kavaliers M, Choleris E, Agmo A, Pfaff DW. Olfactory mediated parasite recognition and avoidance: linking genes to behavior. Horm Behav. 2004;46:272–283. doi: 10.1016/j.yhbeh.2004.03.005. [DOI] [PubMed] [Google Scholar]
- Kavaliers M, Choleris E, Agmo A, Braun WJ, Colwell DD, Muglia LJ, Ogawa S, Pfaff DW. Inadvertent social information and the avoidance of parasitized male mice: a role for oxytocin. Proc Natl Acad Sci USA. 2006;103:4293–4298. doi: 10.1073/pnas.0600410103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keverne EB, Curley JP. Vasopressin, oxytocin and social behavior. Curr Opin Neurobiol. 2004;14:777–783. doi: 10.1016/j.conb.2004.10.006. [DOI] [PubMed] [Google Scholar]
- Kosfeld M, Heinrichs M, Zak PJ, Fischbacher U, Fehr E. Oxytocin increases trust in humans. Nature. 2005;453:673–676. doi: 10.1038/nature03701. [DOI] [PubMed] [Google Scholar]
- Krege JH, Hodgin JB, Couse JF, Enmark E, Warner M, Mahler JF, Sar M, Korach KS, Gustafsson JA, Smithies O. Generation and reproductive phenotypes of mice lacking estrogen receptor β. Proc Natl Acad Sci USA. 1998;95:15677–15682. doi: 10.1073/pnas.95.26.15677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kudwa AE, Michopoulos V, Gatewood JD, Rissman EF. Roles of estrogen receptors α and β in differentiation of mouse sexual behavior. Neuroscience. 2006;138:921–928. doi: 10.1016/j.neuroscience.2005.10.018. [DOI] [PubMed] [Google Scholar]
- Lima SL, Dill LM. Behavioral decisions made under the risk of predation: a review and prospectus. Can J Zool. 1990;68:619–640. [Google Scholar]
- Lindberg MK, Moverare S, Skrtic S, Gao H, Dahlman-Wright K, Gustafsson JA, Ohlsonn C. Estrogen receptor (ER)-β reduces ERα-regulated gene transcription supporting a ‘ying yang’ relationship between ERα and ERβ in mice. Mol Endocrinol. 2003;17:203–208. doi: 10.1210/me.2002-0206. [DOI] [PubMed] [Google Scholar]
- Lubhan DB, Moyer JS, Golding TS, Couse JF, Korach KS, Smithies O. Alteration of reproductive function but not prenatal sexual development after insertional disruption of the mose estrogen receptor gene. Proc Natl Acad Sci USA. 1993;90:11162–11166. doi: 10.1073/pnas.90.23.11162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marsden HM, Bronson FI. The synchrony of oestrous mice and the relative roles of the male and female environment. J Endocrinol. 1965;32:313–319. doi: 10.1677/joe.0.0320313. [DOI] [PubMed] [Google Scholar]
- Montague RP, Berns GS. Neural economics and the biological substrates of valuation. Neuron. 2002;36:265–284. doi: 10.1016/s0896-6273(02)00974-1. [DOI] [PubMed] [Google Scholar]
- Nomura M, Durbak Chen J, Smithies O, Gustafsson J-A, Korach KS, Pfaff DW, Ogawa S. Genotype/age interactions on aggressive behavior in gonadally intact estrogen receptor β knockout (βERKO) male mice. Horm Behav. 2002;41:288–296. doi: 10.1006/hbeh.2002.1773. [DOI] [PubMed] [Google Scholar]
- Nomura M, Andersson S, Korach KS, Gustafsson JA, Pfaff DW, Ogawa S. Estrogen receptor-β gene disruption potentiates estrogen-inducible aggression but not sexual behavior in male mice. Eur J Neurosci. 2006;23:1860–1868. doi: 10.1111/j.1460-9568.2006.04703.x. [DOI] [PubMed] [Google Scholar]
- Ogawa S, Lubahn DB, Korach KS, Pfaff DW. Behavioral effects of estrogen receptor gene disruption in male mice. Proc Natl Acad Sci USA. 1997;94:1476–1481. doi: 10.1073/pnas.94.4.1476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogawa S, Washburn TS, Taylor J, Lubahn DB, Korach KS, Pfaff DW. Modification of testosterone-dependent behaviours by estrogen receptor-α disruption in male mice. Endocrinology. 1998;139:5058–5069. doi: 10.1210/endo.139.12.6358. [DOI] [PubMed] [Google Scholar]
- Ogawa S, Chan J, Chester AE, Gustafsson JA, Korach KS, Pfaff DW. Survival of reproductive behaviors in estrogen receptor β gene-deficient (βERKO) male and female mice. Proc Natl Acad Sci USA. 1999;96:12887–12892. doi: 10.1073/pnas.96.22.12887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogawa S, Chester AE, Hewitt SC, Walker VR, Gustafsson JA, Smithies O, Korach KS, Pfaff DW. Abolition of male sexual behaviors in mice lacking estrogen receptors α and β (αβERKO) Proc Natl Acad Sci USA. 2000;97:14737–14741. doi: 10.1073/pnas.250473597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogawa S, Korach KS, Pfaff DW. Differential roles of two types of estrogen receptors in reproductive behavior. Curr Opin Endocrinol. 2002;9:224–229. [Google Scholar]
- Portillo W, Diaz NF, Cabera EA, Ferandez-Guasti A, Paeredes RG. Comparative analysis of immunoreactive cells for androgen and oestrogen receptor α in copulating and non-copulating male rats. J Neuroendocrinol. 2006;18:168–176. doi: 10.1111/j.1365-2826.2005.01401.x. [DOI] [PubMed] [Google Scholar]
- Portillo W, Catillo CG, Retana-Marquez EC, Roselli CE, Paredes CG. Neuronal activity of aromatase enzyme in non-copulating male rats. J Neuroendocrinol. 2007;19:139–141. doi: 10.1111/j.1365-2826.2006.01513.x. [DOI] [PubMed] [Google Scholar]
- Rissman EF, Wersinger SR, Fugger HN, Foster TC. Sex with knockout models: behavioral studies with estrogen receptor α. Brain Res. 1999;835:80–90. doi: 10.1016/s0006-8993(99)01452-3. [DOI] [PubMed] [Google Scholar]
- Roney JR. Effects of visual exposure to the opposite sex: cognitive aspects of mate attraction in human males. Pers Social Psychol Bull. 2003;29:393–404. doi: 10.1177/0146167202250221. [DOI] [PubMed] [Google Scholar]
- Roney JR, Mahler SV, Maestripieri D. Behavioral and hormonal responses of men to brief interactions with women. Evol Human Behav. 2003;21:1–9. [Google Scholar]
- Roney JR, Lukaszewski AW, Simmons ZL. Rapid endocrine responses of young men to social interactions with young women. Horm Behav. 2007;52:326–333. doi: 10.1016/j.yhbeh.2007.05.008. [DOI] [PubMed] [Google Scholar]
- Scordalakes EM, Risssman EF. Aggression in male mice lacking functional estrogen receptor α. Behav Neurosci. 2003;117:38–45. [PubMed] [Google Scholar]
- Smith FV, Barnard CJ, Behnke JM. Social odours, hormone modulation and resistance to disease in male laboratory mice, Mus musculus. Anim Behav. 1996;52:141–153. [Google Scholar]
- Spooner MF, Robichaud P, Carrier JC, Marchand S. Endogenous pain modulation during the formalin test in estrogen receptor beta knockout mice. Neuroscience. 2007;150:675–680. doi: 10.1016/j.neuroscience.2007.09.037. [DOI] [PubMed] [Google Scholar]
- Taziaux M, Keller M, Bakker J, Balthazart J. Sexual behavior activity tracks rapid changes in brain estrogen concentrations. J Neurosci. 2007;27:663–672. doi: 10.1523/JNEUROSCI.1797-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trepel C, Fox CR, Poldrack RA. Prospect theory on the brain? Toward a cognitive neuroscience of decisions under risk. Cognitive Brain Res. 2005;23:34–50. doi: 10.1016/j.cogbrainres.2005.01.016. [DOI] [PubMed] [Google Scholar]
- Van den Bergh B, Dewitte S. Digit ratio (2D:4D) moderates the impact of sexual cues on men’s decision in ultimatum games. Proc Roy Soc B. 2006;273:2091–2095. doi: 10.1098/rspb.2006.3550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vasudevan N, Koibuchi N, Chin WW, Pfaff DW. Differential crosstalk between estrogen receptor (ER)α and ERβ and the thyroid hormone receptor isoforms results in flexible regulation of the consensus ERE. Mol Brain Res. 2001;95:9–17. doi: 10.1016/s0169-328x(01)00165-6. [DOI] [PubMed] [Google Scholar]
- Wagner WE., Jr Measuring female mating preferences. Anim Behav. 1998;55:1029–1043. doi: 10.1006/anbe.1997.0635. [DOI] [PubMed] [Google Scholar]
- Waldherr M, Neumann ID. Centrally released oxytocin mediates mating-induced anxiolysis in male rats. Proc Natl Acad Sci USA. 2007;104:16681–16684. doi: 10.1073/pnas.0705860104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wersinger SR, Rissman EF. Oestrogen receptor α is essential for female-directed chemo-investigatory behaviour but is not required for the pheromone-induced luteinizing hormone surge. J Neuroendocrinol. 2000;12:103–110. doi: 10.1046/j.1365-2826.2000.00418.x. [DOI] [PubMed] [Google Scholar]
- Wilson M, Daly M. Do pretty women inspire men to discount the future? Proc R Soc B. 2004;271(Suppl 4):S177–S179. doi: 10.1098/rsbl.2003.0134. [DOI] [PMC free article] [PubMed] [Google Scholar]