Abstract
Deficits in N-methyl-D-aspartate receptor (NMDAR) function are increasingly linked to persistent negative symptoms and cognitive deficits in schizophrenia. Accordingly, clinical studies have been targeting the modulatory site of the NMDA receptor, based on the decreased function of NMDA receptor, to see whether increasing NMDA function can potentially help treat the negative and cognitive deficits seen in the disease. Glycine and D-serine are endogenous ligands to the NMDA modulatory site, but since high doses are needed to affect brain levels, related compounds are being developed, for example glycine transport (GlyT) inhibitors to potentially elevate brain glycine or targeting enzymes, such as D-amino acid oxidase (DAAO) to slow the breakdown and increase the brain level of D-serine. In the present study we further evaluated the effect of DAAO inhibitors 5-chloro-benzo[d]isoxazol-3-ol (CBIO) and sodium benzoate (NaB) in a phencyclidine (PCP) rodent mouse model to see if the inhibitors affect PCP-induced locomotor activity, alter brain D-serine level, and thereby potentially enhance D-serine responses. D-Serine dose-dependently reduced the PCP-induced locomotor activity at doses above 1000 mg/kg. Acute CBIO (30 mg/kg) did not affect PCP-induced locomotor activity, but appeared to reduce locomotor activity when given with D-serine (600 mg/kg); a dose that by itself did not have an effect. However, the effect was also present when the vehicle (Trappsol®) was tested with D-serine, suggesting that the reduction in locomotor activity was not related to DAAO inhibition, but possibly reflected enhanced bioavailability of D-serine across the blood brain barrier related to the vehicle. With this acute dose of CBIO, D-serine level in brain and plasma were not increased. Another weaker DAAO inhibitor sodium benzoate (NaB) (400 mg/kg), and NaB plus D-serine also significantly reduced PCP-induced locomotor activity, but without affecting plasma or brain D-serine level, arguing against a DAAO-mediated effect. However, NaB reduced plasma L-serine and based on reports that NaB also elevates various plasma metabolites, for example aminoisobutyric acid (AIB), a potential effect via the System A amino acid carrier may be involved in the regulation of synaptic glycine level to modulate NMDAR function needs to be investigated. Acute ascorbic acid (300 mg/kg) also inhibited PCP-induced locomotor activity, which was further attenuated in the presence of D-serine (600 mg/kg). Ascorbic acid may have an action at the dopamine membrane carrier and/or altering redox mechanisms that modulate NMDARs, but this needs to be further investigated. The findings support an effect of D-serine on PCP-induced hyperactivity. They also offer suggestions on an interaction of NaB via an unknown mechanism, other than DAAO inhibition, perhaps through metabolomic changes, and find unexpected synergy between D-serine and ascorbic acid that supports combined NMDA glycine- and redox-site intervention. Although mechanisms of these specific agents need to be determined, overall it supports continued glutamatergic drug development.
Introduction
Schizophrenia is a severe and complex neuropsychiatric disorder that has been linked to hyperactivity of brain dopaminergic systems that may, in turn, reflect an underlying dysfunction of N-methyl-D-aspartate receptor (NMDAR)-mediated neurotransmission (1). Hypofunction of the NMDAR has been implicated as one feature in the pathophysiology of schizophrenia. Phencyclidine (PCP), an NMDA receptor antagonist, induces schizophrenia-like cognitive dysfunction and psychoses by blocking the NMDA receptor-mediated transmission, and has been frequently used in rodents to model aspects of the disease, since PCP or ketamine treatment induces the enhancement of amphetamine-induced dopamine release that is also seen schizophrenics (2, 3).
Current antipsychotic medications are only partially effective towards all the symptoms of the disease, with 30% responding and enter full remission, another 30% showing some response, and 20–30% do not respond at all (4–6). In the search for more efficacious treatments, many studies have focused on testing a number of glutamate receptor (NMDAR)-based modulators as novel drugs for schizophrenia. Many of these trials have been evaluating adjunctive therapies to ongoing antipsychotic treatments, especially compounds that target the glycine modulatory site of the NMDA receptor (7, 8). For example, glycine and D-serine are endogenous modulators of NMDA receptors that can therefore be used as supplemental agents. These drugs have been used in several clinical studies with mixed results across single-site and multi-center studies (9–11), but with significant meta-analytic findings (12). Nevertheless, use of these compounds is limited by the high-doses needed for efficacy, in addition to (for D-serine) potential high-dose nephrotoxicity (13).
Other approaches seek to modulate glycine and/or D-serine levels indirectly by affecting regulatory mechanisms (14). For example, glycine (GlyT1) transport inhibitors prevent removal of glycine from the synapse (15). These compounds were found to be effective in several preclinical schizophrenia models, including the ability to reverse PCP-induced hyperactivity and alterations in amphetamine-induced dopamine release (16). Nevertheless, clinical experience with these agents has also been mixed with some studies showing significant beneficial effects using the GlyT1 inhibitor sarcosine (17, 18), but other studies reporting non-significant effects (19).
Another potential approach is through manipulation of D-serine metabolism. D-Serine is degraded in the brain by D-amino acid oxidase (DAAO), an enzyme that is also increased in post mortem brain tissue from patients with schizophrenia (20, 21). As such, DAAO inhibitors may be novel treatments as ways to enhance NMDA activity via increasing D-serine concentrations. The DAAO inhibitor 5-chloro-benzo[d]isoxazol-3-ol (CBIO) has been reported to enhance the efficacy of D-serine in attenuating prepulse inhibition (PPI) deficits after administration of a NMDA antagonist (22), but studies with the compound remain limited.
An alternative compound, sodium benzoate (NaB) has also recently been tested in both preclinical (23) and clinical (19, 24) studies with encouraging results. Nevertheless, the affinity of this compound for DAAO is low, so the degree to which effects were mediated by DAAO inhibition remains uncertain. Given uncertainty regarding mechanism of action, the present study evaluates plasma and brain levels of D-serine produced by treatment with these agents alone or in combination with D-serine at doses that produce putative pro-therapeutic activity in animal models of dopaminergic hyperactivity. Finally, in addition to the glycine modulatory site, NMDA receptors are sensitive to the redox state within the brain, with some studies suggesting that dysfunction of NMDA receptors in brain may result from impairments in redox balance (25). In order to evaluate the potential effects of acute manipulations of redox state on NMDA receptor function, we combined D-serine administration here with acute administration of ascorbic acid. We hypothesized that agents would be effective in reversal of NMDA receptor antagonist (PCP)-induced behavioral effects to the extent that they modulated NMDA receptor function through either the glycine- or redox-modulatory sites.
Material and Methods
Material
Phencyclidine hydrochloride (PCP) was provided by NIDA (Reference Number 013603), 6 chloro-3-hydroxy-1,2-benzisoxazole (CBIO, CAS 61977-29-5) from Carbocore, TX, Trappsol® (hydroxypropyl BCD, cat. THPB-EC) from Cyclodextrin Technologies Development, Inc., FL., cis-4,7,10,13,16,19-Docosahexaenoic acid (CAS 6217-54-5, cat. D2534) and sodium benzoate (CAS 532-32-1, cat. B3420) D-amphetamine hemisulfate (CAS 51-63-8, cat. A5880), ascorbic acid (CAS 50-81-7, cat. A0278), and dimethyl sulfoxide (DMSO, CAS 67-68-5, cat. D2650) from Sigma-Aldrich (St. Louis, MO).
Animals
C57BL/6 mice, males 8 to 9 weeks of age, were use, and locomotor activity measured in activity boxes equipped with infrared beams. In brief, an Opto-Varimex Auto-Track System (version4.10, 8 Opto-Varimex-3 photocell activity monitors; Columbia Instruments, Columbia Station, OH) was used. Mice were housed singly in a standard mouse cage (7 × 12 × 5 inches) 1 day before activity monitoring. The cage was placed in the activity monitor, and activity was measured starting after intraperitoneal drug injection. Locomotor activity was calculated based on total ambulatory counts (consecutive beams broken during ambulation; single beams broken repeatedly were not counted). Data were expressed as ambulatory beam breaks over a 60- to 120-min period (in 10-min segments or total counts).
Drug treatment schedules are described in the figure legends. Generally, CBIO was given 60 minutes or sodium benzoate 90 minutes before, and D-serine 30 minutes before PCP administration. Locomotor activity was measured during drug administration and 60–90 minutes after PCP or as indicated in the legend.
In Vitro NMDA-Induced [3H]Dopamine Release-Fractional Release
Mice were decapitated and the striatal tissue (approximately 8–10 mg block of tissue) was dissected out and incubated for 60 min in Mg2+-containing 0.5 ml of Krebs bicarbonate buffer containing 1.25–3.0 μCi [3H]dopamine (New England Nuclear, Boston, MA) as previously described (16). The reaction mixture was continuously gassed with an O2/CO2 mixture (95%/5%). After prelabeling, tissue was transferred to superfusion chambers (12 × 0.3 ml chambers, Brandel Superfusion 1200, MD) and pre-perfused at a rate of 0.8 ml/min for 60 min in the above buffer minus Mg2+. Effluent was discarded during this period and thereafter 3 or 4 min fractions were collected for an additional 45–60 min, depending on the experiment. D-Serine was added before indicated fractions and maintained until the end of the experiment. Mice were also first treated with CBIO (30 mg/kg, i.p.) and killed 1 hour later for release measurements. NMDA (300 μM)-induced [3H]dopamine release was defined as the area under the fractional release curve for 24 min following NMDA exposure (fractions 6–11), relative to surrounding fractions. Fractional release (FRS) was defined as radioactivity content of each fraction divided by the amount of radioactivity remaining in the tissue at the time the fraction was collected. Following the perfusion period (fraction 15), the tissue was removed from the perfusion chamber along with the remaining perfusion buffer, sonicated, and assayed for tissue radioactivity. To determine radioactivity released from the tissue, the perfusate (2.4 ml) was mixed with 3ml Liquiscint scintillation fluid (National Diagnostics, Atlanta, Georgia) and counted in a Packard Scintillation Counter (Model 1500).
D-Serine Measurement
Plasma and brain d/l-serine was determined using liquid chromatography with fluorescence detection. The procedure is based upon the formation of a fluorescent diastereomeric derivative formed from the pre-column addition of o-phthalaldehyde and BOC-L-Cys (26)
For the CBIO treated-tissue, to 100 μl of plasma or brain homogenate sample, the internal standard (d-homocysteic acid) is added, with acetonitrile, vortexed and centrifuged. Following evaporation of the supernatant, the residue was re-dissolved in borate buffer and allowed to react with the derivatizing reagent. Immediately following the reaction, the mixture was injected onto a C-18 column (LUNA C18, 150 × 4.6mm, 3μ, Phenomenex) and eluted using a complex gradient elution program consisting of an acetate buffer, tetrahydrofuran and acetonitrile. The separated components were detected using an Agilent Model 1321A Fluorescence Detector set at λexcit. = 245nm and λemiss. = 470nm. The resulting retention times for the internal standard, l-serine and d-serine were 12.4, 28.5, and 34.3 min., respectively. The total run time between samples was about 70 minutes, including equilibration time and on-line derivatization. Intra-assay variability did not exceed 6.4% for the 7 calibration concentrations of d-serine (n = 8 for each concentration). Inter-assay variation did not exceed 9.1% based on 2 sets of quality controls included with each run (n = 13 days).
D-Serine was assayed by a slightly different method (27) with the NaB-treated tissue. Tissues were rapidly removed, weighed, and homogenized in 3% perchloric acid. After centrifugation the supernatant was stored at-80 degrees until assayed. Chiral derivatives of the amino acids were made with orthophthalaldehyde and Ntert-butoxycarbonyl-L-cysteine. Sample (5 mL) is mixed with 0.4 M borate buffer, pH 9.7 (25 mL) and reagent (25 mL of 10 mg/mL of orthophthalaldehyde and tert-butoxycarbonyl-L-cysteine per mL methanol). After 2 min the sample was chromatographed on a Supelco discovery HS C18 column under isocratic conditions, 1 mL/min, using a buffer containing 15% acetonitrile and 25 mM sodium phosphate and 25 mM sodium acetate, pH 5.7. D-serine, eluting at 48 minutes, was monitored on a fluorescence meter (excitation at 340 nm and emission at 455 nm).
Statistical Analysis
Date were analyzed by using repeated measures ANOVA followed by post-hoc t-test. Two-tailed statistics with preset alpha level for significance of p<0.05 are used throughout. Data in text represent the average ± S.D. or S.E.M. of indicated n-values shown.
Results
Effect of D-Serine on PCP-induced Locomotor Activity
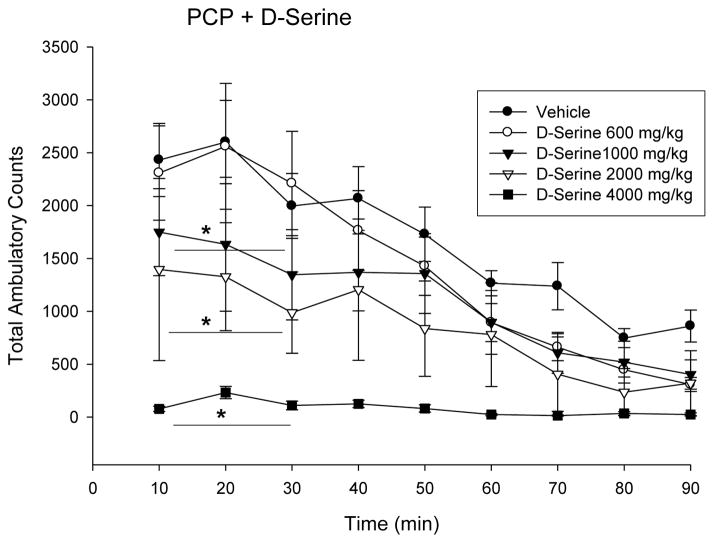
NMDA-receptor (NMDA-R) blockade with phencyclidine (PCP) has been used to model schizophrenia in experimental animals. As such, many studies have been conducted to evaluate the co-agonist site of the NMDA receptor as a potential target for drug treatment. The effect of D-serine (600–4000 mg/kg i.p) to block the behavioral stimulation produced by acute phencyclidine administration in mice was measured. PCP (0.5 mg/kg) increases locomotor activity with a dose-dependent decrease in activity with D-serine administration (Fig. 1). Inhibition of locomotor activity was significant starting at 1000 mg/kg and higher doses of D-serine (P < 0.05 -0.01).
Fig 1.
D-Serine, at the dose indicated (600–4000 mg/kg), was administered to mice 60 min before PCP (5 mg/kg i.p.). Activity was measured starting after PCP administration. Results are the mean Total Ambulatory Counts in 10-min segments (n = 8, mean ± S.E.), T-test p-values for 10–30 minute segment after PCP administration < 0.05 1000 mg vs vehicle; < 0.01 2000 mg and 4000 mg vs vehicle.
Effect of CBIO ± D-Serine on PCP-Induced Locomotor Activity and on Plasma and Brain D-Serine Level
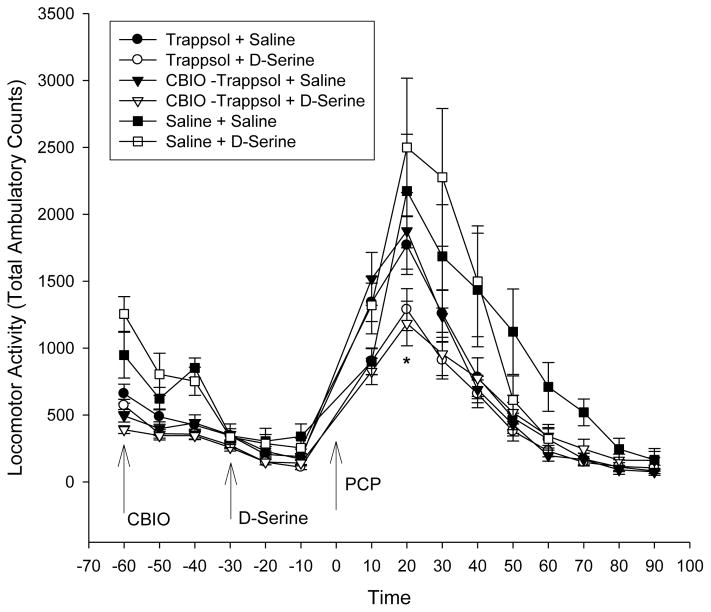
CBIO (5-chloro-benzo[d]isoxazol-3-ol) is a D-amino acid oxidase (DAAO) inhibitor. CBIO was administered at a dose of 30 mg/kg (prepared in Trappsol® (cyclodextrin) 12% at 3 mg/ml CBIO) ± D-serine (600 mg/kg). CBIO was administered, followed 30 min later with D-serine (600 mg/kg) or saline. After another 30 minutes, PCP (5 mg/kg) was given and locomotor behavior monitored for another 90 minutes (Fig 2). CBIO (in Trappsol) + D-serine showed an apparent decrease of PCP-induced behavior (p<0.05), but the effect was also present with Trappsol (no CBIO) + D-serine (p<0.05), suggesting that the response was not related to DAAO inhibition.
Fig 2.
Mice were treated with the DAAO inhibitor CBIO (5-Chlorobenzo[d]isoxazol-3-ol, 30 mg/kg, i.p.) solubilized in 12% Trappsol® (Hydroxypropyl Beta Cyclodextrin). Thirty minutes later, the mice were given D-serine (600 mg/kg i.p.) followed 30 minutes later with PCP (5 mg/kg i.p.) and locomotor activity monitored for another 90 minutes. Values are Total Ambulatory counts taken in 10 minute segments (n = 15, mean ± S.E.) (p values over segments 10–40 minutes <0.05 Trappsol + D- serine ○ and CBIO + Trappsol + D-serine △ vs Trappsol + saline ●).
With this dose and acute administration of CBIO, there were no changes in plasma or brain D-serine level (Table1) and plasma and brain D-serine was not higher in CBIO + D-serine (600 mg/kg) versus D-serine (600 mg/kg) only-treated mice. In mice treated with CBIO in their drinking water for 3 days, plasma and kidney D-serine level increased (p< 0.05), but brain level was unchanged (Table 2).
Table 1.
Effect of CBIO + D-Serine on Plasma and Brain L-Serine and D-Serine Level
| D-Serine
|
||||||
|---|---|---|---|---|---|---|
| Control | 600 mg/kg | 1,000 mg/kg | Trapp + D-Serine (600 mg/kg) | CBIO/Trapp | CBIO/Trapp + D-Serine (600 mg/kg) | |
| Plasma (nmol/ml) | ||||||
| L-Serine | 93 ± 8.2 | 105 ± 5.6 | 96.3 ± 16.4 | 94.6 ± 6.2 | 88.2 ± 11.0 | 113 ± 27.9 |
| D-Serine | 3.6 ± 0.3 | 2132 ± 337 | 4702 ± 1315 | 2288 ± 283 | 2.5 ± 2.0 | 1998 ± 483 |
| Brain (μmol/g) | ||||||
| L-Serine | 1.20 ± 0.04 | 1.18 ± 0.01 | 1.12 ± 0.59 | 1.32 ± 0.29 | 1.24 ± 0.10 | 1.17 ± 0.09 |
| D-Serine | 0.29 ± 0.01 | 0.43 ± 0.02 | 0.63 ± 0.04 | 0.42 ± 0.09 | 0.31 ± 0.01 | 0.45 ± 0.07 |
Groups of mice were treated with vehicle (controls, Trappsol), D-serine (600 and 1000 mg/kg), Trappsol + D-serine (600 mg/kg), CBIO (30 mg/kg, i.p. in 12% Trappsol) and CBIO-treated mice + D-serine (600 mg/kg) D-serine was given 1 hour after CBIO and killed and killed 30 minutes later. Trunk blood was collected and plasma isolated, and brain cortex removed and processed for L- and D-serine measurement. Values are means ± S.D., n=5.
Table 2.
Effect of CBIO in drinking water on Plasma and Brain D-Serine Level
| D-Serine | Plasma (nmol/ml) | Brain (μmol/g) | Kidney (μmol/g) |
|---|---|---|---|
| Control | 3.36 ± 0.66 | 0.30 ± 0.02 | 0.04 ± 0.02 |
| CBIO-treated | 5.17 ± 0.40* | 0.34 ± 0.01 | 0.12 ± 0.05* |
Mice were treated with CBIO (1.5 mg/ml) in their drinking water for 3 days. Based of fluid consumption, the CBIO dose consumed was about 8 mg/mouse/day. Values are means ± S.D., n =3, p < 0.05.
Effect of D-serine ± CBIO on NMDA-Induced Fractional Release of Labeled [3H]Dopamine from Mouse Striatum
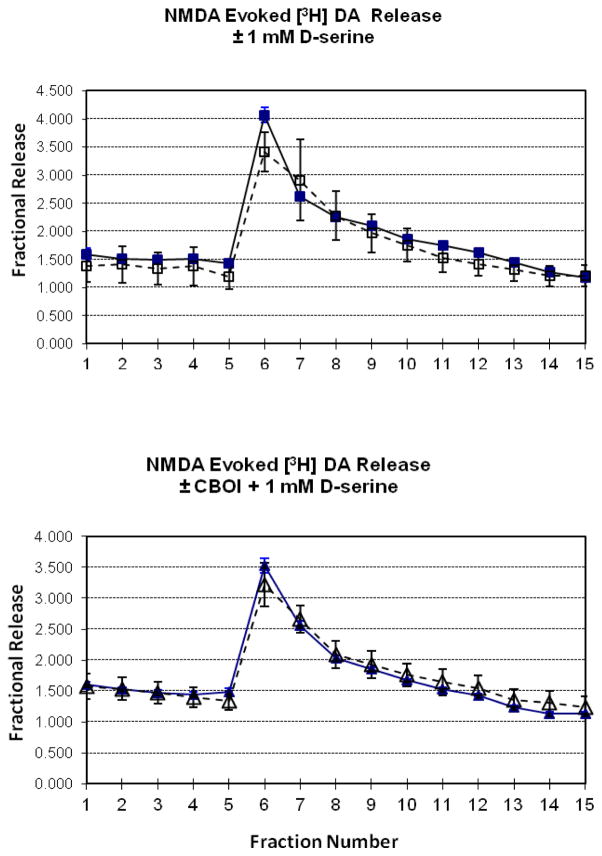
The effect of 300 μM NMDA on [3H]dopamine release was measured in mouse striatal tissue in vitro, in the presence of D-serine in the perfusion buffer. Release of tritium was expressed as fractional release (FRS)- a percentage of amount of radioactivity in the tissue when the release was measured as previously described (28). NMDA (added for fraction 5) induced an increase in dopamine efflux that peaked immediately following NMDA exposure and decayed over the next 7 fractions. NMDA-induced release (FRS fractions 4–8 = 2.35 ± 0.55) was not affected in the presence of 1 mM D-serine in the perfusion buffer (added starting at fraction 1) (FRS fractions 4–8 = 2.80 ± 0.37) (Fig 3 top). Similarly, striatal tissue from mice treated with CBIO (30 mg/kg 1 hour prior), did not show a difference in NMDA-induced dopamine release in the presence of D-serine in the perfusion buffer (FRS fractions 4–8 = 2.24 ± 0.50 (saline-treated mice) vs 2.59 ± 0.34 (CBIO-treated mice) (Fig 3, bottom).
Fig 3.
Effect of D-serine (1 mM) on NMDA (300 μM)-induced [3H]dopamine release from mouse striatal tissue (Top Panel). D-Serine was added to the perfusion buffer at fraction 1 and maintained throughout the collection period. NMDA was added at fraction 5 for one fraction. NMDA induced [3H]dopamine release (■), but D-serine did not affect the release (□). Values are expressed as mean fractional release ± S.E., n =6. Mice were treated with CBIO (30 mg/kg) and killed one hour later and striatal tissue incubated as above (Bottom Panel). NMDA-induced [3H]dopamine was measured (bottom). NMDA + D-serine induced [3H]dopamine release (▲), but prior CBIO treatment (△) did not affect the fractional release. Values are expressed as mean fractional release ± S.E., n = 6.
Effect of Sodium Benzoate ± D-Serine on PCP-Induced Locomotor Activity and on Plasma and Brain D-Serine Level
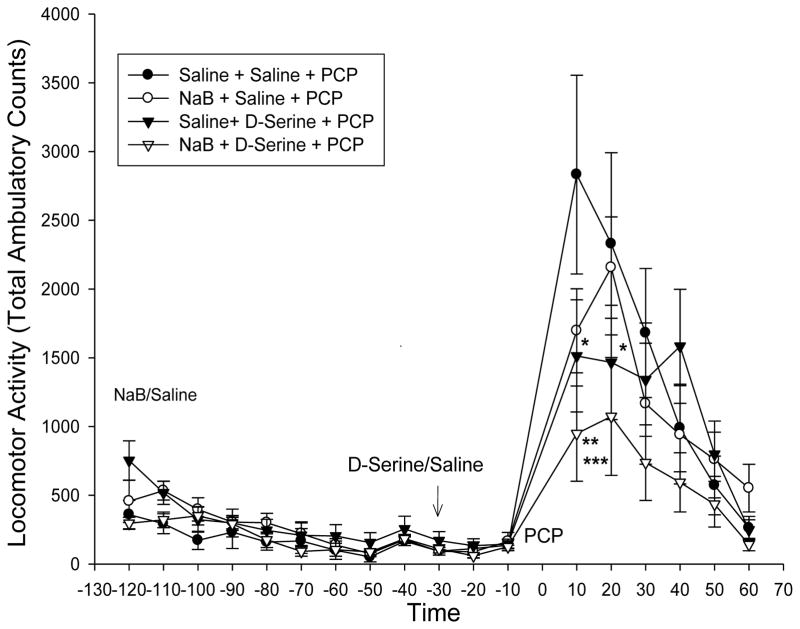
Sodium benzoate (NaB) is also a modest DAAO inhibitor and has been used as add-on treatment therapy in schizophrenia (24). Locomotor activity was measured after sodium benzoate acute administration (NaB, 400 mg/kg, i.p.). D-serine (600 mg/kg, i.p) or saline was given 90 min later, and followed 30 minutes later by PCP (5 mg/kg), and activity monitored for another 60 minutes. NaB or D-serine by themselves did not significantly affect PCP-induced locomotor activity, but NaB + D-serine did inhibit the PCP-induced locomotor activity (p< 0.05 vs D-serine or P < 0.01 vs saline) (Fig 4).
Fig 4.
In the first treatment groups, mice (n=8) were treated with Na-benzoate (400 mg/kg, i.p.) or saline, followed by D-serine (600 mg/kg, i.p.) or saline 90 min later. After an additional 30 min, PCP (10 mg/kg, i.p.) was given and locomotor activity monitored for an additional hour. Na benzoate and D-serine did not increase locomotor behavior during the pre-PCP injection. The PCP-induced locomotor response was slightly decreased by D-serine alone (saline + D-serine (▲) versus saline + saline (●) * T-test p< 0.05 over time segments 10–20 minutes). Na benzoate (○) by itself was not significantly different from control (saline + saline (●)), but when co-administered with D-serine (NaB + D-serine (△)) further reduced the PCP-induce locomotor response (**P<0.05 △ vs ▲ or ***P<0.01 △ vs ● for time segments 10–30 minutes). Values are Total Ambulatory counts taken in 10 minute segments (n = 15, mean ± S.E.)
Administration of NaB did not increase plasma or brain D-serine level compared to D-serine (100 mg/kg) alone administration, but decreased plasma, but not brain L-serine (p < 0.05) (Table 3)
Table 3.
Effect of Sodium Benzoate (400 mg/kg) + D-Serine (100 mg/kg) on Plasma and Brain L-Serine and D-Serine Level
| Control | D-Serine (100 mg/kg) | Na-Benzoate + D-Serine (100 mg/kg) | |
|---|---|---|---|
| Plasma (nmol/ml) | |||
| L-Serine | 163 ± 25.37 | 89.6 ± 10 | 50.8 ± 5.7* |
| D-Serine | 3.36 ± 0.66 | 462 ± 138 | 327 ± 19 |
| Brain (μmol/g) | |||
| L-Serine | 0.74 ± 0.08 | 1.03 ± 0.12 | 0.93 ± 0.07 |
| D-Serine | 0.30 ± 0.02 | 0.45 ± 0.14 | 0.45 ± 0.10 |
Mice were given sodium benzoate (NaB; 400 mg/kg) followed 90 min later with D-serine (100 mg/kg) and killed 30 minutes after D-serine administration. Another group of mice were given D-serine (100 mg/kg) without NaB and killed 30 min later. Values are means ± S.D., n =4. P < 0.0005 t-test versus D-serine alone.
Effect of Ascorbic Acid and Docosahexanoic Acid ± D-Serine on PCP- and Amphetamine-Induced Locomotor Activity
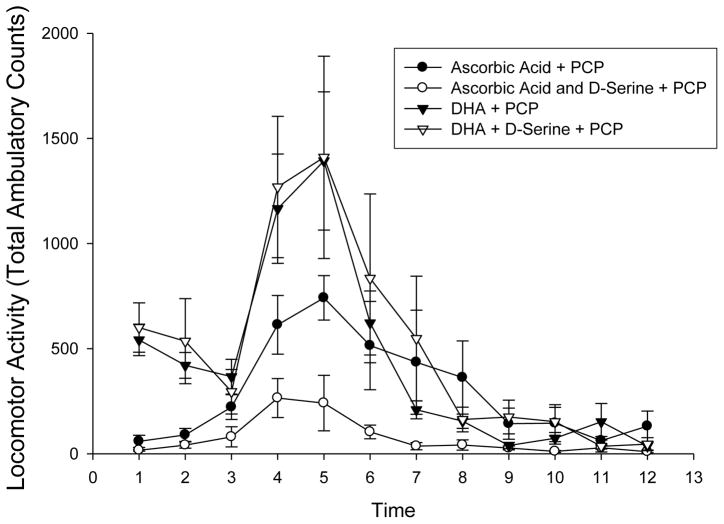
Polyunsaturated fatty acid metabolism deficits have been suggested in psychiatric disorders, and omega-3 fatty acid and vitamins (Vitamin E and C) supplementation have been evaluated as added to antipsychotics, but with mixed findings on symptom reductions (29–31). Docosahexanoic (DHA) (50 mg/kg) or DHA + D-serine (600 mg/kg) had no effect on PCP-induced locomotor activation, but ascorbic acid (300 mg/kg, i.p.) reduced, and ascorbic acid + D-serine further inhibited the PCP-induced locomotor response (p<0.01 vs saline from Fig 2) (Fig 5).
Fig 5.
Effect of ascorbic acid (300 mg/kg, i.p.) and docosahexanoic acid (DHA 50 mg/kg in 25% DMSO, i.p.) on PCP (5 mg/kg) induced locomotor activity. DHA + D-serine (▲) was not different than DHA alone (△), nor did DHA have an effect on locomotor activity (versus sal-sal +PCP (■) in figure 5). Ascorbic acid (●) and ascorbic acid + D-serine (○) further reduced the PCP-induced locomotor activity. Values are mean Total Ambulatory Counts taken in 10 minute segments (n =8) (p values for time segment 4–6, < 0.001 ascorbic acid vs saline (□ from Fig. 2); <0.01 ascorbic acid ● vs ascorbic acid + D-serine ○).
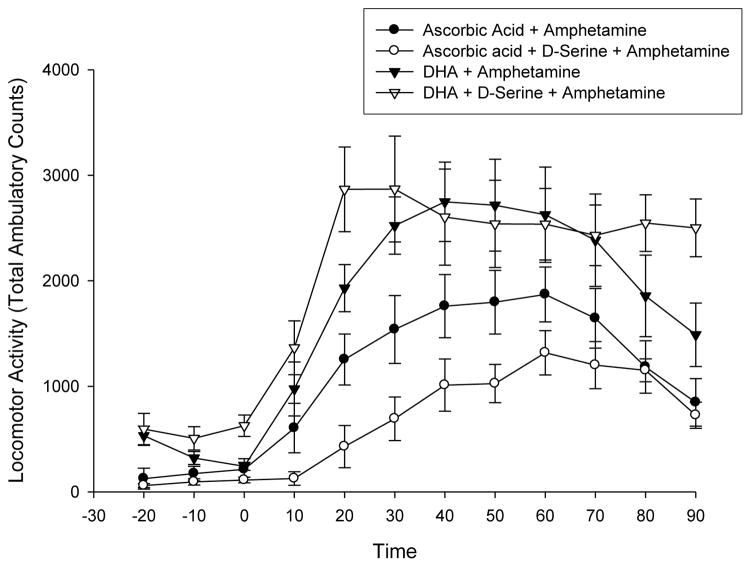
Similarly, when tested against amphetamine-induced locomotor activation, there was no difference in locomotor activity after administration of either DHA or DHA + D-serine (Fig. 6). Amphetamine-induced locomotor activity was reduced in the presence of ascorbic acid and further reduced with administration of D-serine (p < 0.01 vs DHA) (Fig 6).
Fig 6.
Effect of ascorbic acid (300 mg/kg, i.p.) and docosahexanoic acid (DHA 50 mg/kg in 25% DMSO, i.p.) on amphetamine (5 mg/kg, i.p.) induced locomotor activity. DHA + D-serine (△) was not different than DHA alone (▲). Ascorbic acid (●) and ascorbic acid + D-serine (○) further reduced the amphetamine-induced locomotor activity. Values are mean Total Ambulatory Counts taken in 10 minute segments (n =8) (p values over time segments 20–60 minutes ● and ○ vs △ or ▲ <0.01).
Discussion
Deficits in NMDAR function are well-established in schizophrenia, but ideal approaches for reversal of these deficits remains to be determined. We have previously demonstrated significant inter-correlated reversal of PCP-enhanced amphetamine-induced locomotor activity and subcortical dopamine release by glycine and high-affinity GlyT1 inhibitors (32–35). NMDAR in the brain are regulated by glutamate, which serves as the primary agonist, and glycine and D-serine, which bind to an allosteric modulatory site (36–38). Over the past decade, several well tolerated, high affinity GlyT1 inhibitors have been developed and shown to potentiate NMDA receptor-mediated neurotransmission in animal models relevant to schizophrenia. In addition, clinical trials have been conducted with sarcosine (N-methylglycine), a naturally occurring GlyT1 inhibitor, and with the high affinity compound bitopertin (RG1678) (39). Many studies have initially evaluated potential treatments, for example GlyT1 inhibitors, in a PCP-induced locomotor activity model; the ability to inhibit PCP-induced locomotor hyperactivity in mice, or the effect of potential compounds on NMDA-stimulated dopamine release in brain tissue preparations as a screening method (16, 40, 41). However, there have been two major clinical trials – Roche and Merck, that show questionable or disappointing outcome effects of the GlyT1 inhibitor bitopertin (42, 43).
D-Serine has also been suggested to have efficacy in the treatment of negative symptoms in a recent small double-blind trial (10). Although in a larger multi-center trial, with lower achieved doses of D-serine, no significant differences were seen between placebo and D-serine (2 g/day) groups, suggesting that further studies may be needed with higher doses (11). In the present study, the dose of D-serine (1000 mg/kg and higher) that inhibited PCP-induced locomotor activity agrees with the reported behaviorally efficacious dose (1280 mg) in rat studies (44). High doses of D-serine were effective in reducing PCP-induced locomotor activity (Fig 1), but CBIO did not enhance the effect of D-serine when given at a dose (600 mg/kg) that did not behaviorally affect PCP-induced locomotor activity (Fig 2). The attenuated response appeared to be mediated by the cyclodextrin Trappsol, used to solubilize CBIO. With a similar CBIO dose and administration time to the Hashimoto study (22), we also did not see an increase in brain cortex D-serine after CBIO or CBIO and D-serine (600 mg/kg) (Table 1). However, in the Hashimoto study (22), they reported an increase in extracellular, not tissue, D-serine measured in frontal cortex by microdialysis with CBIO plus D-serine versus D-serine alone (22). In the present study, total brain cortex D-serine was measured. Strick et al. (45) also did not see an increase in forebrain D-serine other than cerebellum, after DAAO inhibition (Compound 1) in CD-1 mice. With a longer exposure to CBIO in the drinking water (3 days), mouse plasma and kidney D-serine were elevated, but not brain D-serine (Table 2). Species differences in D-serine metabolism has been suggested, for example between mouse, monkey, and dog, when DAAO inhibition did not influence exposure to D-serine, but elevated plasma and brain D-serine in CD-1 mice (46). It is possible that mouse strain differences also exist (CD-1 mouse and C57Bl/6 in present study), and that elevations of brain D-serine from acute inhibition of DAAO are only modest. DAAO may also play less a role in modulating cortical D-serine level than serine racemase (45). With a different DAAO inhibitor (AS057278), brain D-serine only showed a modest increase and not enough to produce behavioral effects in the test employed in that study (amphetamine-induced locomotor activity) (47).
Since CBIO (a low affinity DAAO antagonist) had no significant behavioral effects either alone or in combination with D-serine once control was made for use of the cyclodextrin Trappsol vehicle, suggests that permeability or bioavailability of D-serine through the blood brain barrier may be a major factor controlling effectiveness during treatment. Cyclodextrins, their interaction with plasma membranes and extraction of different lipids are relevant at the level of the BBB (48), and may alter the delivery of D-serine into the brain.
An even lower affinity DAAO inhibitor, sodium benzoate (NaB), did show additive behavioral effects with D-serine (Fig 4), as also reported in other recent preclinical studies (49). Furthermore, NaB dose-dependently attenuated PPI deficits in mice after administration of PCP, but was not affected in the presence of a glycine site antagonist, and NaB given orally at 1000 mg/kg did not change brain or plasma D-serine (49). Consistent with prior studies, NaB has no significant effect on either plasma or brain D-serine levels arguing against a DAAO-mediated effect. Interestingly, in contrast to the lack of effect of NaB on D-serine levels, NaB administration did lead to a highly significant reduction in L-serine (Table 3), suggesting potentially relevant effects on small amino acid metabolism. However, in the Matsuura et al. (49) study, the slight decrease (~20%) in plasma L-serine (mice at 1 hour, Table 1) was not indicated as significantly different, nor the decrease (~40%) in plasma glutamine, but striatum L-serine was reported as significantly decreased. In other studies, NaB administration to rats has been reported to decrease plasma glycine, serine, and alanine 2 hours after administration (50), and in humans a significant decrease in plasma glycine was seen after oral administration (51), in which the present study is in more agreement. Interestingly, Lennerz et al. (52) recently evaluated the effect of NaB on glucose homeostasis, and reported an influence on circulating metabolites- anthranilic acid increased, and acetylglycine and possibly glycine decreased, and although not highlighted, also an increase in aminoisobutyric acid (AIB, Fig. 4). The increase in AIB might indicate a possible effect on System A transport carriers, which may play a role in the regulation of synaptic glycine level and by extension NMDA receptor function, as we had suggested as a potential mechanism for the clinical efficacy of clozapine- System A antagonism (53). Although NaB did not affect D-serine metabolism, suggesting a relatively weak effect at DAAO, it did affect plasma serine level suggesting that it may affect more general levels of amino acid homeostasis - potentially by conversion to AIB and interference with system A-mediated small neutral amino acid transport (53) Disturbances in glycine/serine metabolism have been noted previously in schizophrenia (54, 55) and shown to correlate with severity of negative symptoms, in addition to response to clozapine (56). Clozapine, in turn, may mediate its actions in part through modulation of system A transport (53). To the extent that NaB has positive clinical effects in schizophrenia (19, 24), future studies investigating its effect on regulation of small neutral amino acids, such as glycine or L-serine, are warranted.
D-Serine did not affect NMDA-induced [3H]dopamine release from mouse striatal tissue (Fig 3); however, we have shown that a GlyT inhibitor ALX5311 does inhibit dopamine release (16), and D-serine has been shown to inhibit NMDA-induced endogenous dopamine efflux from rat striatal tissue (57). In the latter study, D-serine and glycine stimulated endogenous dopamine release; whereas, in our earlier studies glycine did not affect labeled dopamine release (16, 28). Similarly, NMDA-evoked release of dopamine was not potentiated by glycineb agonist, while these agonists stimulate the spontaneous release of dopamine in the absence of NMDA, suggesting different populations of NMDA receptors are involved in the effects of NMDA and glycine on striatal dopamine release (57) and also possibly for D-serine. Although the present study did not measure dopamine levels directly, we have previously shown that locomotor activity correlates with prefrontal cortex dopamine (35, 58) but future studies are required to verify.
Omega-3 fatty acids like docosahexaenoic acid (DHA) are being tested as an add-on therapy in schizophrenic patients (59). Although DHA may have action directly on the NMDA receptor or indirectly by altering its lipid environment (60), it had no effect on PCP- or amphetamine-induced locomotor response (Fig. 6).
Ascorbic acid also inhibited the PCP-induced locomotor activity and it was further reduced in the presence of D-serine (Fig 5). A similar inhibition was seen with amphetamine-induced locomotor activity (Fig 6). This effect could involve the dopamine transporter, since ascorbic acid may have a modulatory role involving dopamine carrier-membrane translocation; both dopamine and ascorbic acid being transported by the same carrier (61–63). The effect may also be directly related to enhanced glutamate. Ascorbic acid has also been shown to modulate glutamate transmission, involving a hetero-exchange mechanism where high level of extracellular ascorbic acid prevents glutamate uptake, resulting in elevated extracellular glutamate (64).
Redox phenomena have also been reported to modulate the activity of the NMDAR, for example by ascorbate (65–68). Several biochemical steps of NMDA receptor activation are redox-regulated and via volume transmission, the redox property of dopamine, may also play a role in the development of dysconnectivity between brain regions in schizophrenia, which can cause abnormal modulation of NMDA-dependent synaptic plasticity (69), which should be further investigated.
NMDA receptors are located at synaptic and extrasynaptic locations and mediate distinct physiological and pathological processes. The regionalized availability of co-agonists also matches the preferential affinity of synaptic NMDAR for D-serine and extrasynaptic NMDRs for glycine, in which glycine and D-serine inhibit NMDAR surface trafficking in a sub-unit dependent manner, and can have roles in either synaptic plasticity and neuroprotection/excitotoxicity (70). As such, the ability to alter the availability of the supply of either d-serine or glycine at or outside the synapse, for example with DAAO inhibitors or transport inhibitors, may influence the subunit composition of region-specific NMDARs and thereby have an important role in the symptoms associated with schizophrenia.
The present study does not rule of the potential of using a DAAO inhibitor to elevate brain D-serine levels, nor does it rule out elevating brain D-serine at higher doses than currently attained as a pharmacological method for clinical treatments. There are still questions of differences in D-serine metabolism between different species or methodological differences in responses to account for the discrepancies in outcome results. Many of the behavioral assays that measure antipsychotic potential and pro-cognitive effects in animal studies have to date been inconsistent (71). Secondly, some of the add-on drugs targeting DAAO inhibition may have action at other sites (other than DAAO inhibition), can have potential relevance. The effects seen in the present mouse animal models do not necessarily predict or translate to potential efficacies in human treatments, as seen in the outcome of some multi-center trials, but the potential for their use cannot be completely eliminated. As with the clinical trials, elevating brain D-serine in several rodent models also shows some inconsistencies, but nevertheless still suggestive of being potentially efficacious in modulating NMDA function.
In summary, although NMDAR-based theories are well established, ideal approaches for NMDAR enhancement remain to be determined. In the present study, both D-serine and NaB were effective in reversing PCP-induced behavioral effects, supporting therapeutic efficacy. Moreover, effects of these two agents were additive suggesting potential synergistic effects. Effects of NaB, however, did not appear to be mediated by DAAO, it did affect aspects of amino acid metabolism, consistent with prior reports. Various metabolites (e.g. acteylglycine, AIB) may be of relevance, and future studies should evaluate the mechanism of effect. Ascorbic acid also showed additive effects with D-serine, suggesting potential benefits of combined redox- and glycine-site intervention. Beta-cyclodextrin (Trappasol) also markedly facilitated D-serine effect, potentially by facilitating bioavailability into brain. Because of the limitations of each of these agents given individually, synergistic nutritional intervention using a combination of approaches for NMDAR enhancement may be more effective than treatment with any single agent individually.
Acknowledgments
Supported in part by grant P50 MH086385 (D.C.J).
Contributor Information
Henry Sershen, Email: sershen@nki.rfmh.org, Nathan S. Kline Institute for Psychiatric Research, 140 Old Orangeburg Rd., Orangeburg, NY 10962, USA. NYU Langone Medical Center, Department of Psychiatry, New York, NY 10016, USA.
Audrey Hashim, Nathan S. Kline Institute for Psychiatric Research, 140 Old Orangeburg Rd., Orangeburg, NY 10962, USA.
David S. Dunlop, Nathan S. Kline Institute for Psychiatric Research, 140 Old Orangeburg Rd., Orangeburg, NY 10962, USA
Raymond F. Suckow, Nathan S. Kline Institute for Psychiatric Research, 140 Old Orangeburg Rd., Orangeburg, NY 10962, USA. New York State Psychiatric Institute, 1051 Riverside Dr., New York, NY 10032, USA
Tom B. Cooper, Nathan S. Kline Institute for Psychiatric Research, 140 Old Orangeburg Rd., Orangeburg, NY 10962, USA. New York State Psychiatric Institute, 1051 Riverside Dr., New York, NY 10032, USA
Daniel C. Javitt, Email: Javitt@nki.rfmh.org, Nathan S. Kline Institute for Psychiatric Research, 140 Old Orangeburg Rd., Orangeburg, NY 10962, USA. Columbia University College of Physicians and Surgeons, New York, New York 10032, USA
Reference List
- 1.Javitt DC. Twenty-five years of glutamate in schizophrenia: are we there yet? Schizophr Bull. 2012;38:911–913. doi: 10.1093/schbul/sbs100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Laruelle M, Abi-Dargham A, van Dyck CH, Gil R, D’Souza CD, Erdos J, McCance E, Rosenblatt W, Fingado C, Zoghbi SS, Baldwin RM, Seibyl JP, Krystal JH, Charney DS, Innis RB. Single photon emission computerized tomography imaging of amphetamine-induced dopamine release in drug-free schizophrenic subjects. Proc Natl Acad Sci U S A. 1996;93:9235–9240. doi: 10.1073/pnas.93.17.9235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kegeles LS, Abi-Dargham A, Zea-Ponce Y, Rodenhiser-Hill J, Mann JJ, Van Heertum RL, Cooper TB, Carlsson A, Laruelle M. Modulation of amphetamine-induced striatal dopamine release by ketamine in humans: implications for schizophrenia. Biol Psychiatry. 2000;48:627–640. doi: 10.1016/s0006-3223(00)00976-8. [DOI] [PubMed] [Google Scholar]
- 4.Steeds H, Carhart-Harris RL, Stone JM. Drug models of schizophrenia. Ther Adv Psychopharmacol. 2015;5:43–58. doi: 10.1177/2045125314557797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mouchlianitis E, Bloomfield MA, Law V, Beck K, Selvaraj S, Rasquinha N, Waldman A, Turkheimer FE, Egerton A, Stone J, Howes OD. Treatment-Resistant Schizophrenia Patients Show Elevated Anterior Cingulate Cortex Glutamate Compared to Treatment-Responsive. Schizophr Bull. 2015 doi: 10.1093/schbul/sbv151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lally J, MacCabe JH. Antipsychotic medication in schizophrenia: a review. Br Med Bull. 2015;114:169–179. doi: 10.1093/bmb/ldv017. [DOI] [PubMed] [Google Scholar]
- 7.Heresco-Levy U, Silipo G, Javitt DC. Glycinergic augmentation of NMDA receptor-mediated neurotransmission in the treatment of schizophrenia. Psychopharmacol Bull. 1996;32:731–740. [PubMed] [Google Scholar]
- 8.Woods SW, Walsh BC, Hawkins KA, Miller TJ, Saksa JR, D’Souza DC, Pearlson GD, Javitt DC, McGlashan TH, Krystal JH. Glycine treatment of the risk syndrome for psychosis: report of two pilot studies. Eur Neuropsychopharmacol. 2013;23:931–940. doi: 10.1016/j.euroneuro.2012.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kantrowitz JT, Malhotra AK, Cornblatt B, Silipo G, Balla A, Suckow RF, D’Souza C, Saksa J, Woods SW, Javitt DC. High dose D-serine in the treatment of schizophrenia. Schizophr Res. 2010;121:125–130. doi: 10.1016/j.schres.2010.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kantrowitz JT, Woods SW, Petkova E, Cornblatt B, Corcoran CM, Chen H, Silipo G, Javitt DC. D-serine for the treatment of negative symptoms in individuals at clinical high risk of schizophrenia: a pilot, double-blind, placebo-controlled, randomised parallel group mechanistic proof-of-concept trial. Lancet Psychiatry. 2015;2:403–412. doi: 10.1016/S2215-0366(15)00098-X. [DOI] [PubMed] [Google Scholar]
- 11.Weiser M, Heresco-Levy U, Davidson M, Javitt DC, Werbeloff N, Gershon AA, Abramovich Y, Amital D, Doron A, Konas S, Levkovitz Y, Liba D, Teitelbaum A, Mashiach M, Zimmerman Y. A multicenter, add-on randomized controlled trial of low-dose d-serine for negative and cognitive symptoms of schizophrenia. J Clin Psychiatry. 2012;73:e728–e734. doi: 10.4088/JCP.11m07031. [DOI] [PubMed] [Google Scholar]
- 12.Moller HJ, Czobor P. Pharmacological treatment of negative symptoms in schizophrenia. Eur Arch Psychiatry Clin Neurosci. 2015;265:567–578. doi: 10.1007/s00406-015-0596-y. [DOI] [PubMed] [Google Scholar]
- 13.Williams RE, Lock EA. D-serine-induced nephrotoxicity: possible interaction with tyrosine metabolism. Toxicology. 2004;201:231–238. doi: 10.1016/j.tox.2004.05.001. [DOI] [PubMed] [Google Scholar]
- 14.Javitt DC. Current and emergent treatments for symptoms and neurocognitive impairment in schizophrenia. Curr Treat Options Psychiatry. 2015;1:107–120. doi: 10.1007/s40501-014-0010-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Supplisson S, Bergman C. Control of NMDA receptor activation by a glycine transporter co-expressed in Xenopus oocytes. J Neurosci. 1997;17:4580–4590. doi: 10.1523/JNEUROSCI.17-12-04580.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Javitt DC, Hashim A, Sershen H. Modulation of striatal dopamine release by glycine transport inhibitors. Neuropsychopharmacology. 2005;30:649–656. doi: 10.1038/sj.npp.1300589. [DOI] [PubMed] [Google Scholar]
- 17.Amiaz R, Kent I, Rubinstein K, Sela BA, Javitt D, Weiser M. Safety, tolerability and pharmacokinetics of open label sarcosine added on to anti-psychotic treatment in schizophrenia - preliminary study. Isr J Psychiatry Relat Sci. 2015;52:12–15. [PubMed] [Google Scholar]
- 18.Strzelecki D, Podgorski M, Kaluzynska O, Gawlik-Kotelnicka O, Stefanczyk L, Kotlicka-Antczak M, Gmitrowicz A, Grzelak P. Supplementation of Antipsychotic Treatment with the Amino Acid Sarcosine Influences Proton Magnetic Resonance Spectroscopy Parameters in Left Frontal White Matter in Patients with Schizophrenia. Nutrients. 2015;7:8767–8782. doi: 10.3390/nu7105427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lin CY, Liang SY, Chang YC, Ting SY, Kao CL, Wu YH, Tsai GE, Lane HY. Adjunctive sarcosine plus benzoate improved cognitive function in chronic schizophrenia patients with constant clinical symptoms: A randomised, double-blind, placebo-controlled trial. World J Biol Psychiatry. 2015:1–12. doi: 10.3109/15622975.2015.1117654. [DOI] [PubMed] [Google Scholar]
- 20.Kapoor R, Lim KS, Cheng A, Garrick T, Kapoor V. Preliminary evidence for a link between schizophrenia and NMDA-glycine site receptor ligand metabolic enzymes, d-amino acid oxidase (DAAO) and kynurenine aminotransferase-1 (KAT-1) Brain Res. 2006;1106:205–210. doi: 10.1016/j.brainres.2006.05.082. [DOI] [PubMed] [Google Scholar]
- 21.Madeira C, Freitas ME, Vargas-Lopes C, Wolosker H, Panizzutti R. Increased brain D-amino acid oxidase (DAAO) activity in schizophrenia. Schizophr Res. 2008;101:76–83. doi: 10.1016/j.schres.2008.02.002. [DOI] [PubMed] [Google Scholar]
- 22.Hashimoto K, Fujita Y, Horio M, Kunitachi S, Iyo M, Ferraris D, Tsukamoto T. Co-administration of a D-amino acid oxidase inhibitor potentiates the efficacy of D-serine in attenuating prepulse inhibition deficits after administration of dizocilpine. Biol Psychiatry. 2009;65:1103–1106. doi: 10.1016/j.biopsych.2009.01.002. [DOI] [PubMed] [Google Scholar]
- 23.Matsuura A, Fujita Y, Iyo M, Hashimoto K. Effects of sodium benzoate on pre-pulse inhibition deficits and hyperlocomotion in mice after administration of phencyclidine. Acta Neuropsychiatr. 2015;27:159–167. doi: 10.1017/neu.2015.1. [DOI] [PubMed] [Google Scholar]
- 24.Lane HY, Lin CH, Green MF, Hellemann G, Huang CC, Chen PW, Tun R, Chang YC, Tsai GE. Add-on treatment of benzoate for schizophrenia: a randomized, double-blind, placebo-controlled trial of D-amino acid oxidase inhibitor. JAMA Psychiatry. 2013;70:1267–1275. doi: 10.1001/jamapsychiatry.2013.2159. [DOI] [PubMed] [Google Scholar]
- 25.Do KQ, Cabungcal JH, Frank A, Steullet P, Cuenod M. Redox dysregulation, neurodevelopment, and schizophrenia. Curr Opin Neurobiol. 2009;19:220–230. doi: 10.1016/j.conb.2009.05.001. [DOI] [PubMed] [Google Scholar]
- 26.Hashimoto A, Nishikawa T, Oka T, Takahashi K, Hayashi T. Determination of free amino acid enantiomers in rat brain and serum by high-performance liquid chromatography after derivatization with N-tert-butyloxycarbonyl-L-cysteine and o-phthaldialdehyde. J Chromatogr. 1992;582:41–48. doi: 10.1016/0378-4347(92)80300-f. [DOI] [PubMed] [Google Scholar]
- 27.Neidle A, Dunlop DS. Allosteric regulation of mouse brain serine racemase. Neurochem Res. 2002;27:1719–1724. doi: 10.1023/a:1021607715824. [DOI] [PubMed] [Google Scholar]
- 28.Javitt DC, Sershen H, Hashim A, Lajtha A. Inhibition of striatal dopamine release by glycine and glycyldodecylamide. Brain Res Bull. 2000;52:213–216. doi: 10.1016/s0361-9230(00)00258-6. [DOI] [PubMed] [Google Scholar]
- 29.McNamara RK, Strawn JR. Role of Long-Chain Omega-3 Fatty Acids in Psychiatric Practice. PharmaNutrition. 2013;1:41–49. doi: 10.1016/j.phanu.2012.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sivrioglu EY, Kirli S, Sipahioglu D, Gursoy B, Sarandol E. The impact of omega-3 fatty acids, vitamins E and C supplementation on treatment outcome and side effects in schizophrenia patients treated with haloperidol: an open-label pilot study. Prog Neuropsychopharmacol Biol Psychiatry. 2007;31:1493–1499. doi: 10.1016/j.pnpbp.2007.07.004. [DOI] [PubMed] [Google Scholar]
- 31.Bentsen H, Osnes K, Refsum H, Solberg DK, Bohmer T. A randomized placebo-controlled trial of an omega-3 fatty acid and vitamins E+C in schizophrenia. Transl Psychiatry. 2013;3:e335. doi: 10.1038/tp.2013.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Javitt DC, Sershen H, Hashim A, Lajtha A. Reversal of phencyclidine-induced hyperactivity by glycine and the glycine uptake inhibitor glycyldodecylamide. Neuropsychopharmacology. 1997;17:202–204. doi: 10.1016/S0893-133X(97)00047-X. [DOI] [PubMed] [Google Scholar]
- 33.Javitt DC, Balla A, Burch S, Suckow R, Xie S, Sershen H. Reversal of phencyclidine-induced dopaminergic dysregulation by N-methyl-D-aspartate receptor/glycine-site agonists. Neuropsychopharmacology. 2004;29:300–307. doi: 10.1038/sj.npp.1300313. [DOI] [PubMed] [Google Scholar]
- 34.Javitt DC, Hashim A, Sershen H. Modulation of striatal dopamine release by glycine transport inhibitors. Neuropsychopharmacology. 2005;30:649–656. doi: 10.1038/sj.npp.1300589. [DOI] [PubMed] [Google Scholar]
- 35.Balla A, Schneider S, Sershen H, Javitt DC. Effects of novel, high affinity glycine transport inhibitors on frontostriatal dopamine release in a rodent model of schizophrenia. Eur Neuropsychopharmacol. 2012;22:902–910. doi: 10.1016/j.euroneuro.2012.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Dingledine R, Kleckner NW, McBain CJ. The glycine coagonist site of the NMDA receptor. Adv Exp Med Biol. 1990;268:17–26. doi: 10.1007/978-1-4684-5769-8_3. [DOI] [PubMed] [Google Scholar]
- 37.Reynolds IJ, Miller RJ. Allosteric modulation of N-methyl-D-aspartate receptors. Adv Pharmacol. 1990;21:101–126. doi: 10.1016/s1054-3589(08)60340-3. [DOI] [PubMed] [Google Scholar]
- 38.Fadda E, Danysz W, Wroblewski JT, Costa E. Glycine and D-serine increase the affinity of N-methyl-D-aspartate sensitive glutamate binding sites in rat brain synaptic membranes. Neuropharmacology. 1988;27:1183–1185. doi: 10.1016/0028-3908(88)90015-9. [DOI] [PubMed] [Google Scholar]
- 39.Javitt DC. Glycine transport inhibitors in the treatment of schizophrenia. Handb Exp Pharmacol. 2012:367–399. doi: 10.1007/978-3-642-25758-2_12. [DOI] [PubMed] [Google Scholar]
- 40.Harsing LG, Jr, Gacsalyi I, Szabo G, Schmidt E, Sziray N, Sebban C, Tesolin-Decros B, Matyus P, Egyed A, Spedding M, Levay G. The glycine transporter-1 inhibitors NFPS and Org 24461: a pharmacological study. Pharmacol Biochem Behav. 2003;74:811–825. doi: 10.1016/s0091-3057(02)01078-x. [DOI] [PubMed] [Google Scholar]
- 41.Harsing LG, Jr, Timar J, Szabo G, Udvari S, Nagy KM, Marko B, Zsilla G, Czompa A, Tapolcsanyi P, Kocsis A, Matyus P. Sarcosine-Based Glycine Transporter Type-1 (GlyT-1) Inhibitors Containing Pyridazine Moiety: A Further Search for Drugs with Potential to Influence Schizophrenia Negative Symptoms. Curr Pharm Des. 2015;21:2291–2303. doi: 10.2174/1381612821666150109125623. [DOI] [PubMed] [Google Scholar]
- 42.Umbricht D, Alberati D, Martin-Facklam M, Borroni E, Youssef EA, Ostland M, Wallace TL, Knoflach F, Dorflinger E, Wettstein JG, Bausch A, Garibaldi G, Santarelli L. Effect of bitopertin, a glycine reuptake inhibitor, on negative symptoms of schizophrenia: a randomized, double-blind, proof-of-concept study. JAMA Psychiatry. 2014;71:637–646. doi: 10.1001/jamapsychiatry.2014.163. [DOI] [PubMed] [Google Scholar]
- 43.Singer P, Dubroqua S, Yee BK. Inhibition of glycine transporter 1: The yellow brick road to new schizophrenia therapy? Curr Pharm Des. 2015;21:3771–3787. doi: 10.2174/1381612821666150724100952. [DOI] [PubMed] [Google Scholar]
- 44.Smith SM, Uslaner JM, Yao L, Mullins CM, Surles NO, Huszar SL, McNaughton CH, Pascarella DM, Kandebo M, Hinchliffe RM, Sparey T, Brandon NJ, Jones B, Venkatraman S, Young MB, Sachs N, Jacobson MA, Hutson PH. The behavioral and neurochemical effects of a novel D-amino acid oxidase inhibitor compound 8 [4H-thieno [3,2-b]pyrrole-5-carboxylic acid] and D-serine. J Pharmacol Exp Ther. 2009;328:921–930. doi: 10.1124/jpet.108.147884. [DOI] [PubMed] [Google Scholar]
- 45.Strick CA, Li C, Scott L, Harvey B, Hajos M, Steyn SJ, Piotrowski MA, James LC, Downs JT, Rago B, Becker SL, El-Kattan A, Xu Y, Ganong AH, Tingley FD, III, Ramirez AD, Seymour PA, Guanowsky V, Majchrzak MJ, Fox CB, Schmidt CJ, Duplantier AJ. Modulation of NMDA receptor function by inhibition of D-amino acid oxidase in rodent brain. Neuropharmacology. 2011;61:1001–1015. doi: 10.1016/j.neuropharm.2011.06.029. [DOI] [PubMed] [Google Scholar]
- 46.Rojas C, Alt J, Ator NA, Thomas AG, Wu Y, Hin N, Wozniak K, Ferraris D, Rais R, Tsukamoto T, Slusher BS. D-Amino-Acid Oxidase Inhibition Increases D-Serine Plasma Levels in Mouse But not in Monkey or Dog. Neuropsychopharmacology. 2015 doi: 10.1038/npp.2015.319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Adage T, Trillat AC, Quattropani A, Perrin D, Cavarec L, Shaw J, Guerassimenko O, Giachetti C, Greco B, Chumakov I, Halazy S, Roach A, Zaratin P. In vitro and in vivo pharmacological profile of AS057278, a selective d-amino acid oxidase inhibitor with potential anti-psychotic properties. Eur Neuropsychopharmacol. 2008;18:200–214. doi: 10.1016/j.euroneuro.2007.06.006. [DOI] [PubMed] [Google Scholar]
- 48.Vecsernyes M, Fenyvesi F, Bacskay I, Deli MA, Szente L, Fenyvesi E. Cyclodextrins, blood-brain barrier, and treatment of neurological diseases. Arch Med Res. 2014;45:711–729. doi: 10.1016/j.arcmed.2014.11.020. [DOI] [PubMed] [Google Scholar]
- 49.Matsuura A, Fujita Y, Iyo M, Hashimoto K. Effects of sodium benzoate on pre-pulse inhibition deficits and hyperlocomotion in mice after administration of phencyclidine. Acta Neuropsychiatr. 2015;27:159–167. doi: 10.1017/neu.2015.1. [DOI] [PubMed] [Google Scholar]
- 50.Palekar AG, Kalbag SS. Amino acids in the rat liver and plasma and some metabolites in the liver after sodium benzoate treatment. Biochem Med Metab Biol. 1991;46:52–58. doi: 10.1016/0885-4505(91)90050-u. [DOI] [PubMed] [Google Scholar]
- 51.de VA, Alexander B, Quamo Y. STUDIES ON AMINO ACID METABOLISM III PLASMA GLYCINE CONCENTRATION AND HIPPURIC ACID FORMATION FOLLOWING THE INGESTION OF BENZOATE. J Clin Invest. 1948;27:665–668. doi: 10.1172/JCI102014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Lennerz BS, Vafai SB, Delaney NF, Clish CB, Deik AA, Pierce KA, Ludwig DS, Mootha VK. Effects of sodium benzoate, a widely used food preservative, on glucose homeostasis and metabolic profiles in humans. Mol Genet Metab. 2015;114:73–79. doi: 10.1016/j.ymgme.2014.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Javitt DC, Duncan L, Balla A, Sershen H. Inhibition of system A-mediated glycine transport in cortical synaptosomes by therapeutic concentrations of clozapine: implications for mechanisms of action. Mol Psychiatry. 2005;10:275–287. doi: 10.1038/sj.mp.4001552. [DOI] [PubMed] [Google Scholar]
- 54.Neeman G, Blanaru M, Bloch B, Kremer I, Ermilov M, Javitt DC, Heresco-Levy U. Relation of plasma glycine, serine, and homocysteine levels to schizophrenia symptoms and medication type. Am J Psychiatry. 2005;162:1738–1740. doi: 10.1176/appi.ajp.162.9.1738. [DOI] [PubMed] [Google Scholar]
- 55.Sumiyoshi T, Anil AE, Jin D, Jayathilake K, Lee M, Meltzer HY. Plasma glycine and serine levels in schizophrenia compared to normal controls and major depression: relation to negative symptoms. Int J Neuropsychopharmacol. 2004;7:1–8. doi: 10.1017/S1461145703003900. [DOI] [PubMed] [Google Scholar]
- 56.Sumiyoshi T, Jin D, Jayathilake K, Lee M, Meltzer HY. Prediction of the ability of clozapine to treat negative symptoms from plasma glycine and serine levels in schizophrenia. Int J Neuropsychopharmacol. 2005;8:451–455. doi: 10.1017/S1461145705005237. [DOI] [PubMed] [Google Scholar]
- 57.Bennett S, Gronier B. Modulation of striatal dopamine release in vitro by agonists of the glycineB site of NMDA receptors; interaction with antipsychotics. Eur J Pharmacol. 2005;527:52–59. doi: 10.1016/j.ejphar.2005.10.015. [DOI] [PubMed] [Google Scholar]
- 58.Balla A, Nattini ME, Sershen H, Lajtha A, Dunlop DS, Javitt DC. GABAB/NMDA receptor interaction in the regulation of extracellular dopamine levels in rodent prefrontal cortex and striatum. Neuropharmacology. 2009;56:915–921. doi: 10.1016/j.neuropharm.2009.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Pawelczyk T, Grancow M, Kotlicka-Antczak M, Trafalska E, Gebski P, Szemraj J, Zurner N, Pawelczyk A. Omega-3 fatty acids in first-episode schizophrenia - a randomized controlled study of efficacy and relapse prevention (OFFER): rationale, design, and methods. BMC Psychiatry. 2015;15:97. doi: 10.1186/s12888-015-0473-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Nishikawa M, Kimura S, Akaike N. Facilitatory effect of docosahexaenoic acid on N-methyl-D-aspartate response in pyramidal neurones of rat cerebral cortex. J Physiol. 1994;475:83–93. doi: 10.1113/jphysiol.1994.sp020051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Sershen H, Debler EA, Lajtha A. Effect of ascorbic acid on the synaptosomal uptake of [3H]MPP+, [3H]dopamine, and [14C]GABA. J Neurosci Res. 1987;17:298–301. doi: 10.1002/jnr.490170315. [DOI] [PubMed] [Google Scholar]
- 62.Debler EA, Hashim A, Lajtha A, Sershen H. Ascorbic acid and striatal transport of [3H] 1-methyl-4-phenylpyridine (MPP+) and [3H] dopamine. Life Sci. 1988;42:2553–2559. doi: 10.1016/0024-3205(88)90323-2. [DOI] [PubMed] [Google Scholar]
- 63.Debler EA, Sershen H, Hashim A, Lajtha A, Reith ME. Carrier-mediated efflux of [3H]dopamine and [3H]1-methyl-4-phenylpyridine: effect of ascorbic acid. Synapse. 1991;7:99–105. doi: 10.1002/syn.890070203. [DOI] [PubMed] [Google Scholar]
- 64.Sandstrom MI, Rebec GV. Extracellular ascorbate modulates glutamate dynamics: role of behavioral activation. BMC Neurosci. 2007;8:32. doi: 10.1186/1471-2202-8-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Majewska MD, Bell JA, London ED. Regulation of the NMDA receptor by redox phenomena: inhibitory role of ascorbate. Brain Res. 1990;537:328–332. doi: 10.1016/0006-8993(90)90379-p. [DOI] [PubMed] [Google Scholar]
- 66.Rebec GV, Pierce RC. A vitamin as neuromodulator: ascorbate release into the extracellular fluid of the brain regulates dopaminergic and glutamatergic transmission. Prog Neurobiol. 1994;43:537–565. doi: 10.1016/0301-0082(94)90052-3. [DOI] [PubMed] [Google Scholar]
- 67.Javitt DC, Frusciante MJ, Zukin SR. Activation-related and activation-independent effects of polyamines on phencyclidine receptor binding within the N-methyl-D-aspartate receptor complex. J Pharmacol Exp Ther. 1994;270:604–613. [PubMed] [Google Scholar]
- 68.Javitt DC, Zukin SR. Interaction of [3H]MK-801 with multiple states of the N-methyl-D-aspartate receptor complex of rat brain. Proc Natl Acad Sci U S A. 1989;86:740–744. doi: 10.1073/pnas.86.2.740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Bokkon I, Antal I. Schizophrenia: redox regulation and volume neurotransmission. Curr Neuropharmacol. 2011;9:289–300. doi: 10.2174/157015911795596504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Papouin T, Ladepeche L, Ruel J, Sacchi S, Labasque M, Hanini M, Groc L, Pollegioni L, Mothet JP, Oliet SH. Synaptic and extrasynaptic NMDA receptors are gated by different endogenous coagonists. Cell. 2012;150:633–646. doi: 10.1016/j.cell.2012.06.029. [DOI] [PubMed] [Google Scholar]
- 71.Smith SM, Uslaner JM, Hutson PH. The Therapeutic Potential of D-Amino Acid Oxidase (DAAO) Inhibitors. Open Med Chem J. 2010;4:3–9. doi: 10.2174/1874104501004020003. [DOI] [PMC free article] [PubMed] [Google Scholar]