Abstract
Tumor responses to PD-1 blockade therapy are mediated by T cells, which we characterized in 102 tumor biopsies obtained from 53 patients treated with pembrolizumab, an antibody to PD-1. Biopsies were dissociated and single cell infiltrates were analyzed by multicolor flow cytometry using two computational approaches to resolve the leukocyte phenotypes at the single cell level. There was a statistically significant increase in the frequency of T cells in patients who responded to therapy. The frequency of intratumoral B cells and monocytic myeloid-derived suppressor cells (moMDSCs) significantly increased in patients’ biopsies taken on treatment. The percentage of cells with a T regulatory phenotype, monocytes, and NK cells did not change while on PD-1 blockade therapy. CD8+ T memory cells were the most prominent phenotype that expanded intratumorally on therapy. However, the frequency of CD4+ T effector memory cells significantly decreased on treatment, whereas CD4+ T effector cells significantly increased in nonresponding tumors on therapy. In peripheral blood, an unusual population of blood cells expressing CD56 were detected in two patients with regressing melanoma. In conclusion, PD-1 blockade increases the frequency of T cells, B cells, and MDSCs in tumors, with the CD8+ T effector memory subset being the major T-cell phenotype expanded in patients with a response to therapy.
Keywords: anti–PD1 antibody, T cells, melanoma, flow cytometry
Introduction
The programmed cell death protein 1 (PD-1) is an immune checkpoint protein expressed in T cells. PD-1 inhibits T cell responses to cancer after binding to one of its ligands, PD-1 ligand 1 (PD-L1or B7-H1) or PDL-2 (also called B2-DC) (1-4). PD-1 limits the activity of T cells by inducing a phosphatase that inhibits T cell receptor (TCR) downstream signaling (2, 4, 5). In addition, effects on other lymphocyte subsets have been described, including an enhancement of T regulatory (Treg) cell proliferation and suppressive activity (6), and a decrease in the activity of both B and natural killer (NK) cells (7).
Therapeutic blockade of PD-1 or PD-L1 with monoclonal antibodies leads to durable tumor regressions in patients with several cancer types (8-12). These emerging clinical data have led to the approval by the US Food and Drug Administration of two antibodies to PD-1 for the treatment of metastatic melanoma and lung cancer, the humanized IgG4 antibody pembrolizumab (MK-3475) and nivolumab (BMS-936558).
Clinical responses to PD-1 blockade are associated with increased PD-L1 expression on tumor-resident cells, induced by pre-existing tumor-infiltrating lymphocytes (TILs) , in what is termed “adaptive immune resistance” (1, 10, 13).Patient biopsies obtained before and on therapy with pembrolizumab showed that intratumoral CD8+ T cells proliferated only in patients with an objective response to therapy, as assessed by quantitative immunohistochemistry (IHC). However, it is currently unclear which T cell functional subsets are involved in this response, and other tumor microenvironment hematopoietic lineage cells have not been well characterized in patient samples.
In the current study, we undertook a comprehensive analysis using multicolor flow cytometry and single-cell multiparametric data interpretation of immune cell infiltrates, particularly in T cells, in biopsies of patients with metastatic melanoma treated with PD-1 blockade.
Material and Methods
Clinical Trial and Study Samples
We collected baseline and at least one tumor biopsy while patients were on treatment with pembrolizumab, taken at a mean time of 74 days (range 15 to 230 days) from 53 patients with metastatic melanoma (stage M1a to M1c; Table 1) treated with pembrolizumab within a phase I clinical trial at UCLA (UCLA IRB# 11-003066; NCT01295827) between January of 2012 through May 2013. Patients received single agent pembrolizumab intravenously at one of three dosing regimens, 0 mg/kg every 2 weeks (Q2W), or 2 mg/kg or 10 mg/kg every 3 weeks (Q3W). Three patients had two baseline biopsies and one patient had three baseline biopsies in different metastatic lesions, for a total of 62 baseline biopsies. Tumor response was assessed at 3 months, with scans performed at 3 month intervals thereafter.
Table 1.
Patient Characteristics
| Total patients 53, mean age 65 | ||
|---|---|---|
| Responders | Non-Responders | |
|
| ||
| Number of patients (M/F) | 19 (16/3) | 34 (24/10) |
|
| ||
| Mean age (M/F) | 68 (69/65) | 54 (66/58) |
|
| ||
| Stage | ||
| M0 | 0 | 0 |
| M1a | 3 | 4 |
| M1b | 8 | 7 |
| M1c | 8 | 23 |
|
| ||
| Brain metastasis | ||
| Yes | 5 | 4 |
| No | 14 | 30 |
|
| ||
| LDH | ||
| Normal | 17 | 18 |
| Elevated | 2 | 16 |
|
| ||
| BRAF status | ||
| Wild type | 14 | 22 |
| V600E positive | 3 | 8 |
| V600E heterozygous | 1 | 0 |
| K601E positive | 0 | 1 |
| Others (NRAS mutated + monosomy 3) | 1 | 3 |
|
| ||
| Prior treatment | ||
| None | 4 | 3 |
| Radiation only (R) | 1 | 4 |
| Chemotherapy only (C) | 0 | 0 |
| Immunotherapy only (I) | 4 | 10 |
| BCG | 1 | 0 |
| IFN (interferon) | 0 | 2 |
| Ipilimumab | 2 | 2 |
| Ipilimumab + IFN | 0 | 1 |
| Ipilimumab + IL-2 | 0 | 5 |
| Targeted therapy only (T) | 0 | 0 |
| Adoptive cell therapy (A) | 2 | 0 |
| Combined treatment | 10 | 17 |
| I + C | 1 | 4 |
| I + R | 5 | 4 |
| I + T | 0 | 1 |
| R + C | 0 | 1 |
| I + R + C | 0 | 1 |
| I + T + A | 1 | 0 |
| I + R + T | 2 | 3 |
| I + T + C | 0 | 1 |
| I + C + T + R | 0 | 2 |
| I + C + T + R + A | 1 | 0 |
|
| ||
| Toxicities (Adverse effects) | ||
| Fatigue (grade 1-2) | 2 | 2 |
| Colitis (grade 1-3) | 2 | 1 |
| Pneumonitis (grade 1-2) | 1 | 1 |
| Myalgia (grade 1-2) | 0 | 1 |
| Vitiligo | 3 | 1 |
| Diverticulitis (grade 1-2) | 1 | 0 |
| Liver function test abnormality, grade 3 | 0 | 1 |
| Acute kidney injury, grade 4 | 1 | 0 |
|
| ||
| Dose of PD-1 antibody | ||
| 2 mg/kg | 4 | 14 |
| 10 mg/kg | 15 | 18 |
|
| ||
| Response | ||
| Partial response (PR) | 12 | - |
| Stable disease (SD) | 3 | 1 |
| Complete response (CR) | 4 | - |
| Partial response followed by progression | - | 1 |
| Progression of disease (PD) | - | 32 |
|
| ||
| Reason for study discontinuation | ||
| Adverse events | 2 | - |
| PD | - | 31 |
| CR | 1 | - |
| Investigator decision | 1 | - |
Isolation of single cell leukocytes from biopsies and peripheral blood
Pieces of tumors from dermatological, surgical, or image-guided biopsies were mechanically dissociated, centrifuged at 500g for 5 minutes, and the pellets collected. Cells were either stained immediately, or in a few cases, cryopreserved as described (14). Peripheral blood mononuclear cells (PBMCs) were isolated as described in Ibarrondo et al. (14).
Flow Cytometry Surface Staining
TILs and PBMCs were stained and acquired in an LSR II Flow Cytometer (BD Biosciences) as described by Ibarrondo and colleagues (14). A description of flow antibody reagents used is on Supplementary Table S1. Panel 1 in that table defines white blood cell (WBC) subpopulations, such as B cells, NK cells, T cells, Tregs, monocytic myeloid-derived suppressor cells (moMDSC), and monocytes. Panel 2 characterized different T cell subsets. Panels 3 and 4 designated putative T memory stem cells (TMSC) (15, 16). A healthy donor sample of PBMCs (IRB# 10-001598) or PBMCs from patients was stained and run in parallel with the TIL sample as an internal quality control for staining and gating strategies. Of note, although we analyzed the PD-1 marker by flow cytometry (clone MIH4) (Affimetrix, CA, USA), it was discarded from the final analysis after noting that MIH4 and pembrolizumab cross-reacted on the same binding site.
Flow Cytometry Analysis
All flow data analyses were done with either FlowJo (Tree Star Inc., Asland, OR) or cyt, software for visualizing viSNE maps (17). The gating strategy is described in Supplemental Figs. S1-S3. Biexponential display was used in the analyses. The CytoAnalytics program Vasco (version 1.1.3) is based on exhaustive expansion software (18). One of its functions allows the analysis of all of the possible combinations (positive, negative, and unspecified) from the results of the FlowJo analysis. The statistic parameters of the filter utilized were a delta minimum of 2 and baseline readout ≥ 1, baseline versus treated, or responder versus non-responders P value of ≤ 0.05; excluding null P values. Delta was defined as day of treatment minus baseline serves to prevent large fold changes when the baseline is small (18). We also used the viSNE software program (17), where we gated for live lymphocytes and then removed all of the events found to be negative for all phenotypical markers. Then we used the viSNE algorithm with the cyt software package on the remaining cells.
Statistical Analysis
Descriptive statistical analyses were done with GraphPad Prism (GraphPad, San Diego, CA), and/or the Vasco software program. Pearson’s chi-square test was used for testing difference in the percentage of responders in two dosage groups. Mann Whitney (unpaired samples) and Wilcoxon matched-pairs signed rank (paired samples) test was utilized to compare the pre- and on-treatment effect, and/or the Vasco software program. Confidence intervals (CI) were calculated by the Clopper-Pearson method.
Results
Patient demographics and treatment
Fifty three patients receiving pembrolizumab underwent biopsies for intratumoral cell analyses from February 2012 to May 2013. Table 1 displays the patient characteristics, treatment administered and clinical outcome. Seven (13%) had stage M1a, 15 (28%) had stage M1b, and 31 (58%) had stage M1c metastatic melanoma. Fourteen patients (26%) had prior immunotherapy only, 27 (51%) had previously received other treatments, and 7 (13%) were treatment-naive. There was no correlation between the two different doses of pembrolizumab and patient response (P = 0.18). One patient was treated under Keynote 002 and his/her dose still remains blinded. Three (4%) patients had grade 3 or 4 toxicities on pembrolizumab (one with grade 3 elevation of liver function test, one with grade 3 colitis and the other with grade 4 acute kidney injury). The rest of the toxicities were grade 1 or 2 in 14 (28%) patients including vitiligo, myalgia, diverticulitis, fatigue, colitis, and pneumoniti. Nineteen (36%) patients had an objective tumor response, whereas 34 (64%) were nonresponders by the Response Evaluation Criteria in Solid Tumors 1.1 (RECIST) criteria (19).
Intratumoral T cell, B cell, and moMDSC frequency on PD-1 blockade
Twenty seven baseline and 24 on-therapy tumor biopsies were analyzed to study changes in tumor infiltrating leukocyte (WBC) subsets (Supplemental Fig. S1). The percentage of cells expressing leukocyte common antigen (CD45+) in tumor biopsies increased, independent of clinical response, on PD-1 blockade (Fig. 1A). Of these CD45+ cells, the percentage of T cells (CD3+; P = 0.01) and B cells (CD19+CD3− and CD20+CD3−; P = 0.04) increased in biopsies taken on treatment. Tumors from responding patients on therapy contained a higher percentage of T cells. The percentage of monocytes (CD14+) and CD56+CD3− (NK) cells showed no significant change on treatment (Fig. 1B). Among T cells, there was a nonsignificant increase in the ratio of CD8+/CD4+ T cells when examining 22 pairs of tumors pre- and on-treatment (P = 0.054, Fig. 1C). The frequency of the late activation marker HLA-DR, but not the CD25 early activation marker (20, 21) (gating strategy described on Supplemental Fig. S2A and C), was slightly increased in both CD4+ and CD8 (CD4−) T cell subsets (CD4+: P = 0.024; CD4− P > 0.05, Supplemental Fig. S2B). There was a marginal increase in B cells expressing the activation marker HLA-DR in tumors from patients who were treated (Supplemental Fig. S2D).
Figure 1. Changes in leukocyte subpopulations on PD-1 blockade therapy in tumor samples.
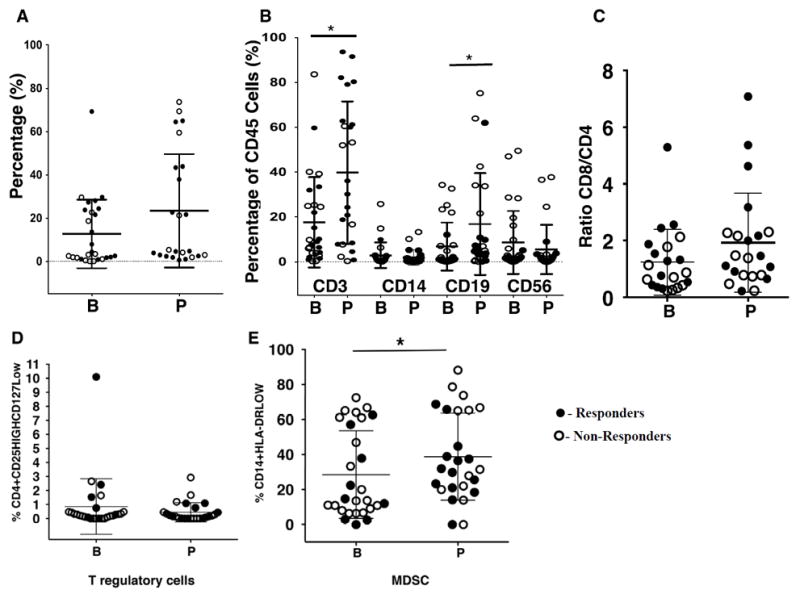
(A)Frequency of leukocytes (CD45+) before (B, n = 27) and on (n = 24) anti–PD-1 therapy. (B) Among leukocytes, percentage of T cells (CD3+; * P = 0.02), monocytes (CD14+; P = 0.476), NK (CD56+; P = 0.47) and B cells (CD19/20+; * P = 0.04). (n=29 before therapy; n=25, on therapy). (C) Proportion of the ratio for CD8/CD4 cells in paired samples (n= 22 pairs; P = 0.0542; Wilcoxon test). (D) Changes in the percentage of T reg. (n=42 before, n=35 on therapy; P = 0.54). (E) Changes in the percentage of moMDSC.(n=27 before, n=25 on therapy; *, P = 0.04). Mean and ± SD are provided. Solid circles represent responders; open circles represent non-responders. B = before treatment; P = on anti–PD-1 therapy; n = number of biopsies analyzed.
Two types of immune suppressor cells were studied. Tregs were defined by the phenotype of CD45+CD3+CD4+CD25HighCD127Low (22); the proportion of Tregs changed little on treatment (Fig. 1D). In addition, we assessed the percentage of moMDSC, based on the phenotype of CD45+CD14+HLA-DRLow (23-25) (Supplemental Fig. S1), and found them significantly increased on treatment (P = 0.04; Fig. 1E). No differences between responders and non-responders were detected for either population of immune suppressor cells within the TILs, at baseline or while on PD-1 blockade.
Baseline T cell infiltrates
To analyze the status of T cells at baseline, we assessed the CD3+ T cell population for relevant immune phenotype markers (Fig. 2). Most T cells had a phenotype of previous exposure to cognate antigen, revealed by expression of CD45RO. Some T cells had a naïve phenotype expressing CD45RA, IL7 receptor α (CD127), and CD62L, but few cells expressed CCR7. The costimulatory marker CD28 was expressed more frequently than CD27. We also analyzed three markers (CD95, CD57, and PD-1) that are usually expressed by effector, terminally differentiated T cells or exhausted T cells (26); we observed a high expression of CD95, but lower CD57 and PD-1 at baseline.
Figure 2. Phenotype analysis of T cells from baseline tumors.
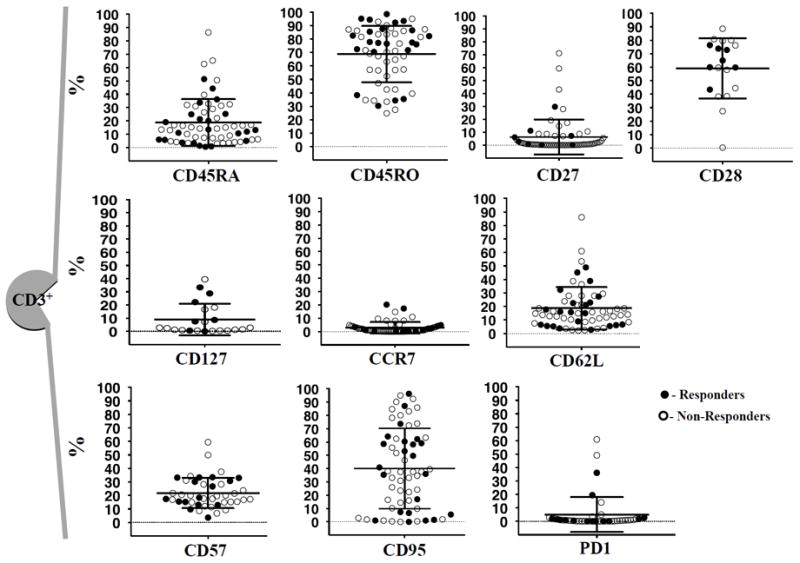
The following CD3+ T cells markers were plotted prior to any treatment. In order: CD45RA, n = 62; CD45RO, n = 58; CD27, n = 62; CD28, n = 19; CD127, n = 19; CCR7, n = 62; CD62L; n = 62; CD57, n = 43; CD95, n = 62; and PD-1, n = 44. All the markers expressed in percentages. Mean and ± SD are provided. n = number of biopsies analyzed. Solid circles represent responders; open circles represent non-responders.
Tumor-infiltrating CD45RO+CD8+ T cells increased on anti–PD-1 therapy
In order to further characterize T cell subsets, we analyzed the 14-parameter flow cytometry data (Supplemental Fig. S3), using multiparametric single cell resolution in 46 baseline and 37 on treatment biopsies. Of the 19,683 possible phenotypes analyzed, we discarded all phenotypes with either P values higher than 0.05 and less than 0.05, but with upper or lower 95% CI close to zero. We found a significant increase in the frequency of CD8+ T cells expressing the memory marker CD45RO in combination with the absence of CD57, CCR7, and CD27, which corresponds to a T effector memory (TEM) phenotype (Fig. 3A) in biopsies obtained while on anti–PD-1. Figure 3B and C show the phenotype with least number of immune markers (CD8+CD4−CD45RO+) and the phenotype with the highest number of immune markers (CD8+CD4−CD45RO+CCR7−CD27−CD57−) TEM phenotypes comparing baseline and on-therapy biopsies. Biopsies from patients who responded had an enrichment of T memory cells on treatment (Fig. 3B and C, right graph; P = 0.002 for CD8+CD4−CD45RO+; P = 0.006 for CD8+CD45RO+CCCR7−CD27−CD57−). Expression of other phenotypes did not change over time or were lower than 1%. These data demonstrate that CD8+ TEM is the main characterized functional phenotype present in biopsies from patients on anti–PD-1 therapy, in particular in tumors of patients who responded to therapy.
Figure 3. CD8+ T cell subsets with significant differences in biopsies on PD-1 blockade therapy.
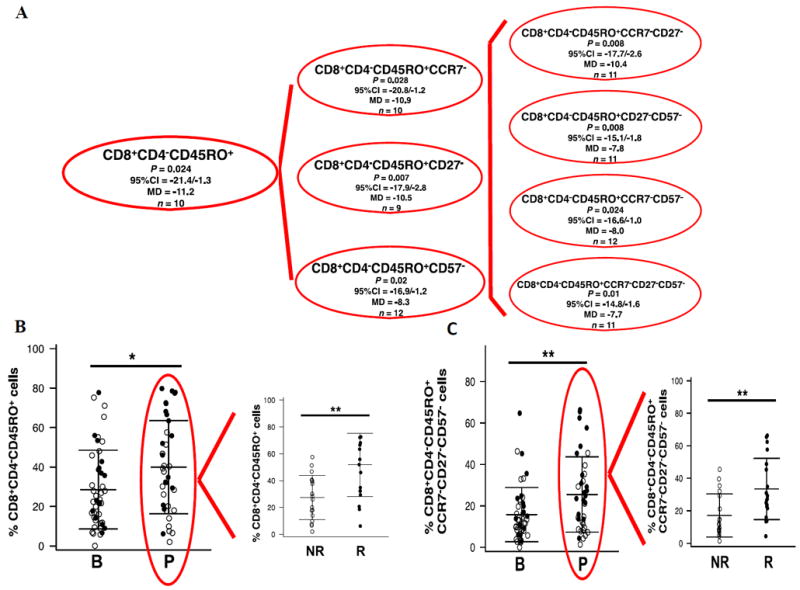
(A) Dendogram illustrating the frequency of CD8+ T cell subsets that present lower P value (P ≤ 0.05) with higher 95% confidence interval (95% CI) using CytoAnalysis program. The program presented an increase of the mean difference (MD) and the 95% CI of the baseline and post-dose samples as a negative value. (n) describes how many patients of the 18 paired TILs present with that particular phenotype. (B) Analysis of the percentage of CD8+CD4-CD45RO+ phenotype (*P = 0.02), displaying a higher percentage the responders (**P = 0.002). (C) Analysis of the percentage of the more extended combination of phenotype markers CD8+CD4-CD45RO+CCR7-CD27-CD57- (*P = 0.01), being augmented in responding patients (**P = 0.006) (right plot).
The on-going treatment (red circle) was arranged by responder and non-responders tumors (right plot). Mean and ± SD are provided. B = before treatment; P = on anti–PD-1 therapy; n = number of biopsies
Frequency of tumor-infiltrating CD45RO+CD4+ T cells on anti–PD-1 therapy
Within the CD4+ T cell subset (CD3+CD8−CD4+), we found a dominant population of CD4+ T cells expressing the memory marker CD45RO that were CD57 negative, in combination with the absence of CCR7, CD62L, and CD45RA (Fig. 4A). The CD4+ TEM phenotypes significantly decreased on anti–PD-1 therapy. On the contrary, the CD4+ T effector-like phenotypes CD8−CD4+CD45RO+CD57+ (P = 0.047), and two variations of this phenotype, CD8−CD4+CD45RO+CCR7−CD57+ (P = 0.03) and CD8−CD4+CD45RO+CCR7−CD62L−CD57+ (P = 0.05), significantly increased on therapy (Fig. 4B, left graph). Tumors from nonresponding patients had greater percentages of the following phenotypes: CD8−CD4+CD45RO+CD57+ (P = 0.024), CD8−CD4+CD45RO+CCR7−CD57+ (P = 0.022), and CD8−CD4+CD45RO+CCR7−CD62L−CD57+ (p>0.05) (Fig.4B, right plots) (27, 28). Taken together, these results show a decrease in the percentage of CD4+ TEM in tumors on pembrolizumab treatment in both responders and nonresponders, and an augmented percentage of CD4+ T effector cells on anti–PD1 treatment only in tumors of nonresponding patients.
Figure 4. CD4+ T cell subsets with significant differences in biopsies on PD-1 blockade therapy.
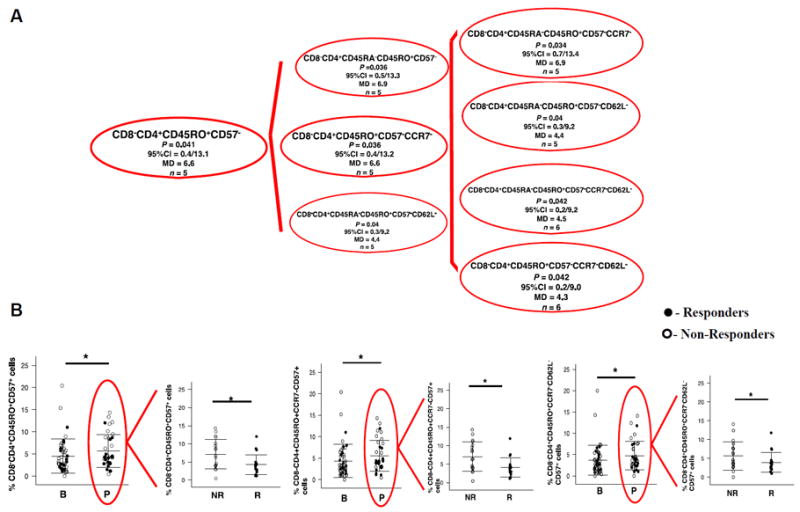
(A) Dendogram illustrating all CD4+ T cells that present with a lower P value (P ≤ 0.05) with higher 95%CI using CytoAnalysis program. The program presents as positive values a decrease of the mean difference (MD) and the 95% CI of the baseline and post-dose samples as a positive value. (n) describes how many patients of the 18 paired TILs show that particular phenotype. (B) The percentage of a phenotype of T effector CD8-CD4+CD45RO+CD57+ T cells (*P = 0.05) plus two related more extended phenotypes, CD8-CD4+CD45RO+CCR7-CD57+ (*P = 0.003) and CD8−CD4+CD45RO+CCR7-CD62L-CD57+ (*P = 0.05). The on-going treatment (red circle) was compared between responder and non-responders tumors (right plot), being the P value: CD8-CD4+CD45RO+CD57+: * P = 0.024; CD8-CD4+CD45RO+CCR7-CD57+: *P = 0.022; and CD8-CD4+CD45RO+CCR7-CD62L-CD57+ (p>0.05)). B = before treatment; P = on anti–PD-1 therapy. Mean and ± SD are provided. Solid circles represent responders; open circles represent non-responders (n = 46 baseline; n = 37 treated).
Visualization of changes in CD8+ CD45RO+ T cells in paired biopsies
We then focused the analysis on the nine paired biopsies obtained from patients who responded to therapy. The analysis of changes in CD8+CD45RO+ T cells were suggestive of a T memory phenotype (Fig. 5A). The starting lesions were of different sizes and the post-dose tumors were obtained at different time points on-therapy (range 19 and 186 days, median of 39 days). Five of the biopsies showed an apparent increase in the percentage of CD8+CD45RO+ cells, while three showed a clear decrease. Only one of those patients (patient #47) had both biopsies (before and on-going treatment) performed from the same lesion, a left suprarenal metastasis. We applied viSNE analysis to better visualize the changes in T cells in this biopsy (Fig. 5B), which provided a global view of all cell subsets simultaneously. This analysis allowed us to conclude that the decrease in the frequency of CD45RO observed in Fig. 5A could be explained by a strong decrease in the total number of T cells in the post-dose tumor as observed in Fig. 5B (lower row), likely due to taking the biopsy from a residual regressed tumor.
Figure 5. Comparison of CD8+ T memory phenotypes in paired biopsies from patients with a response to anti–PD-1 therapy.
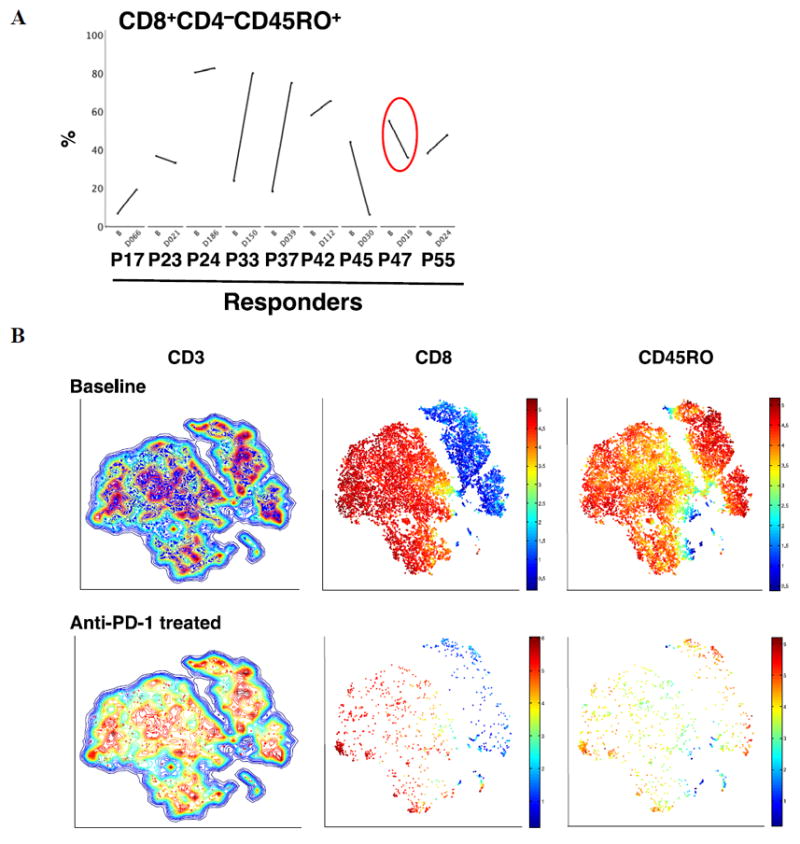
(A) Paired longitudinal analysis of the frequency of CD8+CD4−CD45RO+ at baseline and on anti–PD-1 treatment by exhaustive expansion software. PXX = patient number. B = baseline. DXXX = day of blood draw from the start of anti–PD-1 treatment. n= 9 patients. (B) Visualization of the CD45RO T cells from the suprarenal biopsy from patient #47 nineteen days on therapy. Contour plots of the viSNE maps for double positive for CD3 and live gated cells (excluding CD16 and CD19). The contour plots show cell density in each region of the map. Each dot in the viSNE map depicts the location of an individual cell and its color represents the expression of its immune cell marker. CD3+ T cells (left column) contours plots before (blue), and on anti–PD-1 blockade therapy (red). The expression of CD8+ T (in red) and CD4+ T (in blue) cells inside the CD3 T cell contour plot are shown in the middle column. On the right column, CD45RO (in red) is shown. Red color means high, orange means medium and blue represents low expression of the marker on the cell surface.
Analysis of T naïve and TMSC cells
We also explored the presence and potential of on-treatment changes in the frequency of naïve-like T-cell (29) and TMSC phenotypes described originally by Gattinoni and colleagues (29) and modified by Cieri and colleagues (16). Both of these populations were present at very low frequencies among tumor-infiltrating lymphocytes at baseline and did not change with treatment (Supplemental Fig. S4).
Changes in WBC subpopulations in peripheral blood
We performed similar analyses in peripheral blood samples from 9 baseline and 14 on-therapy blood draws. The percentage of leukocytes slightly decreased on anti–PD-1 treatment, mainly due to a decrease in CD3+ T cells. Monocytes, B cells, and NK cells did not change. The ratio of CD8/CD4 increased slightly (Fig. 6A). We did not observe any change in cells with the Treg or moMDSC (Fig. 6B). There was no statistically significant change in any particular T cell phenotype comparing baseline and on-therapy blood draws (Fig. 6C).
Figure 6. Analysis of peripheral blood samples at baseline and on anti–PD1 therapy.
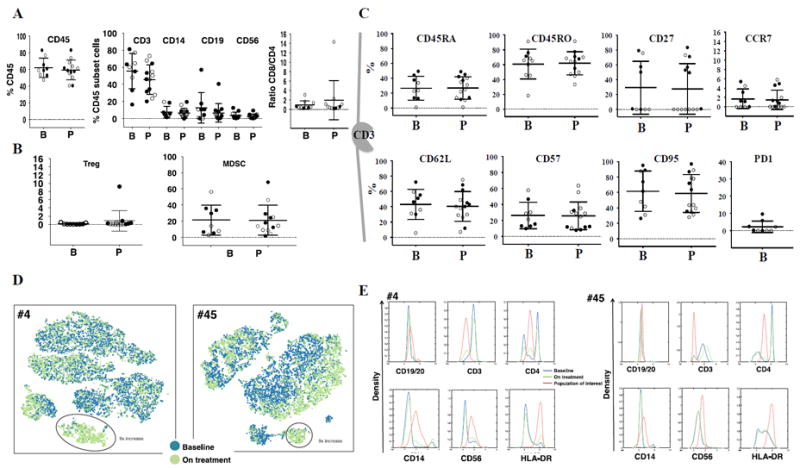
(A) Dot plots depicting the percentage of leukocytes (CD45+) on the left, the percentage of T (CD3+), monocytes (CD14+), B (CD19+/CD20+) and NK (CD56+) in the center, and the ratio CD8/CD4 on the right (B, n = 9; P n = 13). (B) Dot plots of suppressor cells. Tregs on the left, and moMDSC on the right. (C) Dot plots of the T cell subset, CD45RA, CD45RO, CD27, CCR7, CD62L, CD57, CD95 (B, n = 9; P n = 14), and PD-1 only at baseline (n = 9). Mean and ± SD are provided. B = Before treatment; P = on anti–PD-1 therapy; n = number of biopsies analyzed. Solid circles, responders; open circles, nonresponders (D, E) viSNE analysis from blood from two patients who responded to anti–PD-1 (patients #4 and #45) identified differences between the baseline and on treatment samples. (D) The viSNE map of the baseline (blue) and on treatment (green) sample showed the presence of a distinct population (events within the red circle). (E) Histogram representation of the population of interest (red) identified from the viSNE plot at baseline (blue) and on treatment (green). The CD19, CD56, CD14, CD3, CD4, CD127, CD25, and HLA-DR channels of the baseline and on treatment samples were chosen for analysis by viSNE. The marker expression is shown on the x-axis and the density of cells is shown on the y-axis.
To better analyze any potential changes in leukocyte populations upon PD-1 blockade that were not evident by the combinatorial gating analysis, we applied viSNE program. viSNE is not limited to high-low thresholds and can discern mid-level phenotypes, as well as non-linear relations between markers and hence is capable of searching for more complex phenotypes. We mapped the T cells of two responders and nonresponders facilitating a comprehensive comparison of all the cell phenotypes (Fig. 6D and E). Responders were patients #4 and #45, with blood taken at 59 and 41 days from study start. Nonresponders were patients #12 and #14 with the blood collected 42 and 60 days from study start. A distinct population of leukocytes had high expression of HLA-DR and CD56, and mid-level expression of CD14 and CD4. This population of cells increased by 9 times in both on-treatment peripheral blood samples from the patients with a response to therapy.
Discussion
In this study we performed a thorough characterization of TILs in tumors from patients treated with PD-1 blockade therapy. The major finding is the increase in the frequency of T memory cells in patients with a tumor response, where the principal immune marker was CD8+CD4−CD45RO+ and combinations of it, giving a final most common phenotype of TEM cells. This finding is consistent with the anticipated mechanism of action of this therapy. However, we would anticipate an increase in the frequency of CD8+ T cells with an effector phenotype, which we were not able to document. As opposed to the changes in CD8+ T cells, we did find CD4+ T cells expressing CD57, a T effector phenotype also called proliferation-incompetent CD4 T cells, as they have been reported to produce IFNγ but have limited proliferation capacity (30). CD4+CD57+ T cells increased in tumors from patients with disease progression as opposed to responding lesions. Tumor-infiltrating TEM have been found in other studies and may have good prognostic implications. Pagès et al. reported a TEM phenotype in primary tumors of patients with colorectal cancer analyzed by flow cytometry (31). Furthermore, Koelzer et al. reported the presence of CD8 and CD45RO positive TILs, which correlated with improved survival in patients, based on the IHC analysis of colorectal carcinoma samples (32).
Our findings of an enhanced proportion of cells with a phenotype of moMDSC (24) in patients’ on-therapy biopsies from both responders and nonresponders could be interpreted as a by-product of inflammation in the tumor microenvironment on PD-1 blockade (33, 34). In fact, IHC and transcriptome analyses of on-therapy tumor biopsies suggest that several inflammatory cells and markers increase with anti–PD-1 or anti–PD-L1 treatment (10, 13). The same process may attract B cells into tumors obtained from patients on treatment. The significant enrichment of B cells in the tumor microenvironment may have a role in local antigen presentation (35). However, we could not link these B cells to tertiary lymphoid structures (36, 37) as our approach, based on single cell flow cytometry analysis of tumor lysates, does not preserve tissue morphology (38) as does IHC (39).
Polychromatic flow cytometry has flourished over the last decade (40, 41), and it has been one of the primary factors contributing to the ability to document the heterogeneity of immune cells at the single cell level (42). However, until recently, the ability to plot multidimensional flow data has remained a challenge despite efforts to simplify it with different computational methods (17, 43-45). We chose to work with the Vasco (18) and the viSNE programs (17). The former has the advantage of the simplicity of the plots, the flexibility of the filters used to scan results across the sets, and the ability to study the weight of each immune marker from one at a time to all simultaneously (18). The latter has the ability to visualize the multidimensional data in simple two dimension plots maintaining the nonlinearity and geometry of the sample at the single cell level. Thus, strengths of the approach are the visualization of the samples and the simultaneous integration of all of the dimensions (17). In our studies, the application of both programs provided complementary data and improved our understanding of the immune cells in biopsies from anti–PD-1 treated patients.
The effects of PD-1 blockade in cells circulating in peripheral blood were minimal compared to the changes in tumors. No major characterized blood cell subtype changed significantly. However, a population of cells with both monocyte and T cell markers was detected in on-treatment blood samples from two of four patients. Monocytes expressing CD56 have been previously described in peripheral blood by Gruenbacher et al. (46). Moreover, we cannot rule out the possibility that these cells may be the human counterpart of the mice iKDC or pre-mature NK cells. In a study of patients with refractory gastrointestinal stromal tumors, HLA-DR+CD56+ cells correlated with overall survival and progression-free survival after treatment with imatinib mesyate and IL2 (47).
In conclusion, anti–PD1 therapy results in an intratumoral increase in the frequency of T cells, B cells, and MDSCs. T memory cell phenotypes were found specifically increased in patients whose tumors responded to therapy. In contrast, changes were minor in peripheral blood cells, although we could detect an unusual population of what may be a subset of dendritic cells or HLA-DR NK cells in some responding patients. Despite being subjected to challenges derived from tumor heterogeneity that can drive different human immune signatures (48, 49), the different anatomical locations of the biopsies, the relatively small sample size, and the different collection time points, we have found some common characteristics in the intratumoral T cell populations in melanoma biopsies from the responding patients. Our results help advance the knowledge of the effects of PD-1 blockade on the human T cells of the immune system.
Supplementary Material
Acknowledgments
Flow cytometry was performed in the UCLA Jonsson Comprehensive Cancer Center (JCCC) and Center for AIDS Research. The authors thank Rongqing Guo and Li Wang for technical support.
Financial Support: A. Ribas has received NIH grants R35 CA197633, P01 CA168585, and Stand Up To Cancer – Cancer Research Institute (SU2C-CRI) Cancer Immunology Dream Team Translational ResearchGrant (SU2C-AACR-DT1012). A.Ribas and D. Pe’er were supported by a Stand Up To Cancer Phillip A. Sharp Innovation in Collaboration Award (SU2C-AACR-PS04). Stand Up To Cancer is a program of the Entertainment Industry Foundation administered by the American Association for Cancer Research. D.S. Shin was supported by Oncology (5T32CA009297-30), Dermatology (5T32AR058921-05), and Tumor Immunology (5T32CA009120-39) training grants and a Tower Cancer Research Foundation Grant. The Flow Cytometry Core Facility is supported by NIH grants CA16042 and AI 28697, and by the JCCC, the UCLA AIDS Institute, and the David Geffen School of Medicine at UCLA.
The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked advertisement in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
Footnotes
Competing interests: Competing interests: Dr. A. Ribas has served as consultant for Merck, with the honoraria paid to UCLA. B. Chmielowski has received honoraria from BMS, Genentech, and Prometheus, and has served as a consultant for or on the advisory boards of Genentech, Amgen, Lilly, Astellas, Merck, and BMS.
References
- 1.Pardoll DM. The blockade of immune checkpoints in cancer immunotherapy. Nat Rev Cancer. 2012;12(4):252–64. doi: 10.1038/nrc3239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ishida Y, Agata Y, Shibahara K, Honjo T. Induced expression of PD-1, a novel member of the immunoglobulin gene superfamily, upon programmed cell death. EMBO J. 1992;11(11):3887–95. doi: 10.1002/j.1460-2075.1992.tb05481.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ahmadzadeh M, Johnson LA, Heemskerk B, Wunderlich JR, Dudley ME, White DE, et al. Tumor antigen-specific CD8 T cells infiltrating the tumor express high levels of PD-1 and are functionally impaired. Blood. 2009;114(8):1537–44. doi: 10.1182/blood-2008-12-195792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sheppard KA, Fitz LJ, Lee JM, Benander C, George JA, Wooters J, et al. PD-1 inhibits T-cell receptor induced phosphorylation of the ZAP70/CD3zeta signalosome and downstream signaling to PKCtheta. FEBS Lett. 2004;574(1-3):37–41. doi: 10.1016/j.febslet.2004.07.083. [DOI] [PubMed] [Google Scholar]
- 5.Okazaki T, Maeda A, Nishimura H, Kurosaki T, Honjo T. PD-1 immunoreceptor inhibits B cell receptor-mediated signaling by recruiting src homology 2-domain-containing tyrosine phosphatase 2 to phosphotyrosine. Proc Natl Acad Sci U S A. 2001;98(24):13866–71. doi: 10.1073/pnas.231486598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Francisco LM, Salinas VH, Brown KE, Vanguri VK, Freeman GJ, Kuchroo VK, et al. PD-L1 regulates the development, maintenance, and function of induced regulatory T cells. J Exp Med. 2009;206(13):3015–29. doi: 10.1084/jem.20090847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Terme M, Ullrich E, Aymeric L, Meinhardt K, Desbois M, Delahaye N, et al. IL-18 induces PD-1-dependent immunosuppression in cancer. Cancer Res. 2011;71(16):5393–9. doi: 10.1158/0008-5472.CAN-11-0993. [DOI] [PubMed] [Google Scholar]
- 8.Topalian SL, Sznol M, McDermott DF, Kluger HM, Carvajal RD, Sharfman WH, et al. Survival, durable tumor remission, and long-term safety in patients with advanced melanoma receiving nivolumab. J Clin Oncol. 2014;32(10):1020–30. doi: 10.1200/JCO.2013.53.0105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hamid O, Robert C, Daud A, Hodi FS, Hwu WJ, Kefford R, et al. Safety and tumor responses with lambrolizumab (anti–PD-1) in melanoma. N Engl J Med. 2013;369(2):134–44. doi: 10.1056/NEJMoa1305133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Herbst RS, Soria JC, Kowanetz M, Fine GD, Hamid O, Gordon MS, et al. Predictive correlates of response to the anti–PD-L1 antibody MPDL3280A in cancer patients. Nature. 2014;515(7528):563–7. doi: 10.1038/nature14011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Powles T, Eder JP, Fine GD, Braiteh FS, Loriot Y, Cruz C, et al. MPDL3280A (anti–PD-L1) treatment leads to clinical activity in metastatic bladder cancer. Nature. 2014;515(7528):558–62. doi: 10.1038/nature13904. [DOI] [PubMed] [Google Scholar]
- 12.Ansell SM, Lesokhin AM, Borrello I, Halwani A, Scott EC, Gutierrez M, et al. PD-1 blockade with nivolumab in relapsed or refractory Hodgkin’s lymphoma. N Engl J Med. 2015;372(4):311–9. doi: 10.1056/NEJMoa1411087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tumeh PC, Harview CL, Yearley JH, Shintaku IP, Taylor EJ, Robert L, et al. PD-1 blockade induces responses by inhibiting adaptive immune resistance. Nature. 2014;515(7528):568–71. doi: 10.1038/nature13954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ibarrondo FJ, Yang OO, Chodon T, Avramis E, Lee Y, Sazegar H, et al. Natural killer T cells in advanced melanoma patients treated with tremelimumab. PLoS One. 2013;8(10):e76829. doi: 10.1371/journal.pone.0076829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lugli E, Gattinoni L, Roberto A, Mavilio D, Price DA, Restifo NP, et al. Identification, isolation and in vitro expansion of human and nonhuman primate T stem cell memory cells. Nat Protoc. 2013;8(1):33–42. doi: 10.1038/nprot.2012.143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cieri N, Camisa B, Cocchiarella F, Forcato M, Oliveira G, Provasi E, et al. IL-7 and IL-15 instruct the generation of human memory stem T cells from naive precursors. Blood. 2013;121(4):573–84. doi: 10.1182/blood-2012-05-431718. [DOI] [PubMed] [Google Scholar]
- 17.Amir el AD, Davis KL, Tadmor MD, Simonds EF, Levine JH, Bendall SC, et al. viSNE enables visualization of high dimensional single-cell data and reveals phenotypic heterogeneity of leukemia. Nat Biotechnol. 2013;31(6):545–52. doi: 10.1038/nbt.2594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Siebert JC, Wang L, Haley DP, Romer A, Zheng B, Munsil W, et al. Exhaustive expansion: A novel technique for analyzing complex data generated by higher-order polychromatic flow cytometry experiments. J Transl Med. 2010;8:106. doi: 10.1186/1479-5876-8-106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Eisenhauer EA, Therasse P, Bogaerts J, Schwartz LH, Sargent D, Ford R, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1) Eur J Cancer. 2009;45(2):228–47. doi: 10.1016/j.ejca.2008.10.026. [DOI] [PubMed] [Google Scholar]
- 20.Heeren AM, Koster BD, Samuels S, Ferns DM, Chondronasiou D, Kenter GG, et al. High and Interrelated Rates of PD-L1+CD14+ Antigen-Presenting Cells and Regulatory T Cells Mark the Microenvironment of Metastatic Lymph Nodes from Patients with Cervical Cancer. Cancer Immunol Res. 2015;3(1):48–58. doi: 10.1158/2326-6066.CIR-14-0149. [DOI] [PubMed] [Google Scholar]
- 21.Chao KH, Wu MY, Yang JH, Chen SU, Yang YS, Ho HN. Expression of the interleukin-2 receptor alpha (CD25) is selectively decreased on decidual CD4+ and CD8+ T lymphocytes in normal pregnancies. Mol Hum Reprod. 2002;8(7):667–73. doi: 10.1093/molehr/8.7.667. [DOI] [PubMed] [Google Scholar]
- 22.Seddiki N, Santner-Nanan B, Martinson J, Zaunders J, Sasson S, Landay A, et al. Expression of interleukin (IL)-2 and IL-7 receptors discriminates between human regulatory and activated T cells. J Exp Med. 2006;203(7):1693–700. doi: 10.1084/jem.20060468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tarhini AA, Butterfield LH, Shuai Y, Gooding WE, Kalinski P, Kirkwood JM. Differing patterns of circulating regulatory T cells and myeloid-derived suppressor cells in metastatic melanoma patients receiving anti-CTLA4 antibody and interferon-alpha or TLR-9 agonist and GM-CSF with peptide vaccination. J Immunother. 2012;35(9):702–10. doi: 10.1097/CJI.0b013e318272569b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Filipazzi P, Valenti R, Huber V, Pilla L, Canese P, Iero M, et al. Identification of a new subset of myeloid suppressor cells in peripheral blood of melanoma patients with modulation by a granulocyte-macrophage colony-stimulation factor-based antitumor vaccine. J Clin Oncol. 2007;25(18):2546–53. doi: 10.1200/JCO.2006.08.5829. [DOI] [PubMed] [Google Scholar]
- 25.Poschke I, Mougiakakos D, Hansson J, Masucci GV, Kiessling R. Immature immunosuppressive CD14+HLA-DR-/low cells in melanoma patients are Stat3hi and overexpress CD80, CD83, and DC-sign. Cancer Res. 2010;70(11):4335–45. doi: 10.1158/0008-5472.CAN-09-3767. [DOI] [PubMed] [Google Scholar]
- 26.Apetoh L, Smyth MJ, Drake C, Abastado J-P, Apte RN, Ayyoub M, et al. Consensus nomeclature for CD8+ T cell phenotypes in cancer. OncoImmunology. 2015 doi: 10.1080/2162402X.2014.998538. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chattopadhyay PK, Betts MR, Price DA, Gostick E, Horton H, Roederer M, et al. The cytolytic enzymes granyzme A, granzyme B, and perforin: expression patterns, cell distribution, and their relationship to cell maturity and bright CD57 expression. J Leukoc Biol. 2009;85(1):88–97. doi: 10.1189/jlb.0208107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gattinoni L, Powell DJ, Jr, Rosenberg SA, Restifo NP. Adoptive immunotherapy for cancer: building on success. Nat Rev Immunol. 2006;6(5):383–93. doi: 10.1038/nri1842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gattinoni L, Lugli E, Ji Y, Pos Z, Paulos CM, Quigley MF, et al. A human memory T cell subset with stem cell-like properties. Nat Med. 2011;17(10):1290–7. doi: 10.1038/nm.2446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Palmer BE, Blyveis N, Fontenot AP, Wilson CC. Functional and phenotypic characterization of CD57+CD4+ T cells and their association with HIV-1-induced T cell dysfunction. J Immunol. 2005;175(12):8415–23. doi: 10.4049/jimmunol.175.12.8415. [DOI] [PubMed] [Google Scholar]
- 31.Pages F, Berger A, Camus M, Sanchez-Cabo F, Costes A, Molidor R, et al. Effector memory T cells, early metastasis, and survival in colorectal cancer. N Engl J Med. 2005;353(25):2654–66. doi: 10.1056/NEJMoa051424. [DOI] [PubMed] [Google Scholar]
- 32.Koelzer VH, Lugli A, Dawson H, Hadrich M, Berger MD, Borner M, et al. CD8/CD45RO T-cell infiltration in endoscopic biopsies of colorectal cancer predicts nodal metastasis and survival. J Transl Med. 2014;12:81. doi: 10.1186/1479-5876-12-81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ostrand-Rosenberg S, Sinha P. Myeloid-derived suppressor cells: linking inflammation and cancer. J Immunol. 2009;182(8):4499–506. doi: 10.4049/jimmunol.0802740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Umansky V, Sevko A, Gebhardt C, Utikal J. Myeloid-derived suppressor cells in malignant melanoma. J Dtsch Dermatol Ges. 2014;12(11):1021–7. doi: 10.1111/ddg.12411. [DOI] [PubMed] [Google Scholar]
- 35.Nelson BH. CD20+ B cells: the other tumor-infiltrating lymphocytes. J Immunol. 2010;185(9):4977–82. doi: 10.4049/jimmunol.1001323. [DOI] [PubMed] [Google Scholar]
- 36.Carragher DM, Rangel-Moreno J, Randall TD. Ectopic lymphoid tissues and local immunity. Semin Immunol. 2008;20(1):26–42. doi: 10.1016/j.smim.2007.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Drayton DL, Liao S, Mounzer RH, Ruddle NH. Lymphoid organ development: from ontogeny to neogenesis. Nat Immunol. 2006;7(4):344–53. doi: 10.1038/ni1330. [DOI] [PubMed] [Google Scholar]
- 38.Ruffell B, Au A, Rugo HS, Esserman LJ, Hwang ES, Coussens LM. Leukocyte composition of human breast cancer. Proc Natl Acad Sci U S A. 2012;109(8):2796–801. doi: 10.1073/pnas.1104303108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Fridman WH, Pages F, Sautes-Fridman C, Galon J. The immune contexture in human tumours: impact on clinical outcome. Nat Rev Cancer. 2012;12(4):298–306. doi: 10.1038/nrc3245. [DOI] [PubMed] [Google Scholar]
- 40.Perfetto SP, Chattopadhyay PK, Roederer M. Seventeen-colour flow cytometry: unravelling the immune system. Nat Rev Immunol. 2004;4(8):648–55. doi: 10.1038/nri1416. [DOI] [PubMed] [Google Scholar]
- 41.Bendall SC, Nolan GP, Roederer M, Chattopadhyay PK. A deep profiler’s guide to cytometry. Trends Immunol. 2012;33(7):323–32. doi: 10.1016/j.it.2012.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bendall SC, Nolan GP. From single cells to deep phenotypes in cancer. Nat Biotechnol. 2012;30(7):639–47. doi: 10.1038/nbt.2283. [DOI] [PubMed] [Google Scholar]
- 43.Lugli E, Roederer M, Cossarizza A. Data analysis in flow cytometry: the future just started. Cytometry A. 2010;77(7):705–13. doi: 10.1002/cyto.a.20901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Aghaeepour N, Finak G, Flow CAPC, Consortium D, Hoos H, Mosmann TR, et al. Critical assessment of automated flow cytometry data analysis techniques. Nat Methods. 2013;10(3):228–38. doi: 10.1038/nmeth.2365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Schadt EE, Linderman MD, Sorenson J, Lee L, Nolan GP. Computational solutions to large-scale data management and analysis. Nat Rev Genet. 2010;11(9):647–57. doi: 10.1038/nrg2857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Gruenbacher G, Gander H, Rahm A, Nussbaumer W, Romani N, Thurnher M. CD56+ human blood dendritic cells effectively promote TH1-type gammadelta T-cell responses. Blood. 2009;114(20):4422–31. doi: 10.1182/blood-2009-06-227256. [DOI] [PubMed] [Google Scholar]
- 47.Chaput N, Flament C, Locher C, Desbois M, Rey A, Rusakiewicz S, et al. Phase I clinical trial combining imatinib mesylate and IL-2: HLA-DR NK cell levels correlate with disease outcome. Oncoimmunology. 2013;2(2):e23080. doi: 10.4161/onci.23080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Jamal-Hanjani M, Quezada SA, Larkin J, Swanton C. Translational implications of tumor heterogeneity. Clin Cancer Res. 2015;21(6):1258–66. doi: 10.1158/1078-0432.CCR-14-1429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Comin-Anduix B, Lee Y, Jalil J, Algazi A, de la Rocha P, Camacho LH, et al. Detailed analysis of immunologic effects of the cytotoxic T lymphocyte-associated antigen 4-blocking monoclonal antibody tremelimumab in peripheral blood of patients with melanoma. J Transl Med. 2008;6:22. doi: 10.1186/1479-5876-6-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


