Abstract
Wolbachia endosymbionts are potentially useful tools for suppressing disease transmission by Aedes aegypti mosquitoes because Wolbachia can interfere with the transmission of dengue and other viruses as well as causing deleterious effects on their mosquito hosts. Most recent research has focused on the wMel infection, but other infections also influence viral transmission and may spread in natural populations. Here, we focus on the wAlbB infection in an Australian outbred background and show that this infection has many features that facilitate its invasion into natural populations including strong cytoplasmic incompatibility, a lack of effect on larval development, an equivalent mating success to uninfected males and perfect maternal transmission fidelity. On the other hand, the infection has deleterious effects when eggs are held in a dried state, falling between wMel and the more virulent wMelPop Wolbachia strains. The impact of this infection on lifespan also appears to be intermediate, consistent with the observation that this infection has a titer in adults between wMel and wMelPop. Population cage experiments indicate that the wAlbB infection establishes in cages when introduced at a frequency of 22%, suggesting that this strain could be successfully introduced into populations and subsequently persist and spread.
Introduction
Wolbachia bacteria are endosymbionts of insects that form the basis of novel approaches for suppressing disease transmission by mosquitoes. In particular, Wolbachia from Drosophila transferred to Aedes aegypti mosquitoes are being utilized in current releases aimed at suppressing dengue transmission.1,2 The main reason for this suppression comes from the fact that the presence of the bacteria reduces virus titer, particularly in tissues that the virus needs to invade for transmission between people.3,4 In addition, Wolbachia can influence disease transmission by causing deleterious effects on its host and triggering embryo mortality or cytoplasmic incompatibility (CI) when Wolbachia-infected males mate with uninfected females, potentially reducing vector population size.5,6 Effects on viral transmission interference and host fitness may last if the Wolbachia infection reaches a high and stable frequency in host populations after invasion, which has now been achieved in some field populations of Ae. aegypti.7
The ability of Wolbachia strains to generate viral blockage and influence host fitness depends on the nature of the Wolbachia strain and background host genome.4,8,9 This has become evident from research in Drosophila where multiple combinations of hosts and Wolbachia have now been generated and challenged with viruses.10,11 So far, in Ae. aegypti mosquitoes, three Wolbachia strains have been stably introduced and characterized: the wMel and wMelPop infections from Drosophila melanogaster12,13 and the wAlbB infection from Aedes albopictus.14 As in Drosophila, there are likely to be differences in fitness effects and blockage exhibited by these strains, which requires the strains to be compared in the same genetic background. The wMelPop strain generates very high viral blockage, whereas blockage by wMel is somewhat weaker.4,13 Blockage by wAlbB appears to be strong,15 but a direct comparison to the other strains has not yet been undertaken. The wMelPop infection generates large deleterious effects on adult longevity, egg viability particularly after quiescence, larval viability under high density, and other traits.12,16,17 These deleterious effects are either much weaker or absent in wMel.13 Less comprehensive data for wAlbB also suggest limited deleterious effects on a genetic background different from that used in experiments with wMel and wMelPop.14 The magnitude of deleterious effects as well as viral blockage may be partly related to bacterial titer and tissue distribution, with the wMelPop infection occurring at a higher titer and having a wider tissue distribution than wMel.13
Although strong viral blockage is clearly desirable from the perspective of curtailing disease transmission, large deleterious effects may be sufficient to prevent Wolbachia from invading into wild Aedes populations16 and subsequently spreading into surrounding areas.18 Invasion of uninfected populations depends on the process of CI, where infected males cause the death of embryos or immature stages when they mate with uninfected females (or females carrying an incompatible Wolbachia strain). Although CI is very strong for all three Wolbachia infections introduced into Ae. aegypti albeit with different genetic backgrounds,12–14 the presence of large deleterious effects can mean that invasion by wMelPop is difficult because of a high invasion threshold, particularly in the dry season when eggs have to persist in a quiescent phase.6 This is borne out by the difficulty of achieving invasion by this infection in semi-field cages13,19 and in field releases into relatively isolated areas.20 On the other hand, invasion by wMel has now been repeatedly achieved, and results in high and stable frequencies.7 The wAlbB infection has not yet been used for invasion in field releases although it has been shown to invade small laboratory population cages.14
In this article, we consider the three infections for fitness effects on a common genetic background with the aim of comparing their invasibility and also potential use in population suppression.8 We focus particularly on the wAlbB infection that has not been characterized in detail previously, and contrast its host effects and bacterial density with that of the wMel and wMelPop infections. We also provide estimates of maternal transmission and CI to help assess the likely frequency of wAlbB in natural populations after invasion.
Materials and Methods
Colony maintenance.
Aedes aegypti infected with wAlbB, wMel, or wMelPop were reared in a laboratory at 26 ± 1°C with a 12:12-hour (day:night) photoperiod, which included 1-hour dusk/dawn periods. Colonies of 450–500 adults (not differing significantly from a 1:1 sex ratio) were housed in 19.7-L BugDorm-1® cages (MegaView Science Co., Ltd., Taichung City, Xitun District, Taiwan), covered with plastic bags to maintain high humidity (∼85%). Adults were provided with access to a 10% sucrose solution supplied by capillary action through a cotton wool–braided cord (7 × 0.5 cm) inserted through the lid of a 30-mL cup. Females were blood-fed by human volunteers for 15 minutes, 8 days after eclosion, to allow maturation and fertilization. Females oviposited on Norton® Master Painters P80 sandpaper (3.8 × 18 cm; Saint -Gobain Abrasives Pty. Ltd., Thomastown, Victoria, Australia) for routine maintenance or conical filter paper (Whatman® qualitative circles—15 cm Ø; GE Healthcare Australia Pty. Ltd., Parramatta, New South Wales, Australia) lining the inside of a plastic cup containing 150 mL reverse osmosis (RO) water. Eggs were conditioned by removing excess moisture with paper towel for 30 seconds on the second day post-oviposition, and then almost completely dried on the third day. Egg strips were then sealed in plastic zip-lock bags with a moist paper towel square (2 × 2 cm) to prevent desiccation. Egg hatching occurred in RO water (3 L for colony maintenance, but see specific methods for experimental volumes), deoxygenated with active dried yeast to stimulate hatching (∼0.02 mg/L), and containing crushed TetraMin® fish food tablets (Tetra, Melle, Germany; hereafter referred to as hatching water). Immature stages were fed ad libitum with the fish food. Colonies were maintained by controlling the density of second instar larvae at 450–500 individuals per 4 L of RO water using a glass pipette and clicker counter. Colony pupae were collected 5 days later into 500 mL fresh RO water and placed in 19.7-L BugDorm-1 cages for eclosion. Colonies were routinely screened for Wolbachia to confirm their infection status (see section Wolbachia detection and primers) and maintained at a size of several hundred individuals.
Aedes aegypti strains had been transinfected with the wAlbB, wMel, and wMelPop strains of Wolbachia by embryonic microinjection as described elsewhere.12–14 However, both the wMel and wMelPop cultures used in the experiments had been sourced from field material subsequent to releases.16 Colonies infected with each strain were maintained in our laboratory alongside an uninfected colony (CNS), sourced from eggs oviposited around Cairns. Host nuclear background effects9 were controlled by backcrossing16 females from all infected lines to CNS males for six generations before experimentation.
Fecundity.
Reductions in female fecundity because of the wAlbB infection were tested relative to uninfected CNS females. Colonies were blood-fed by a single human volunteer. Twenty engorged females from infected and uninfected colonies were then isolated into 70-mL specimen cups with mesh lids using an aspirator. Access to a 10% sucrose solution was provided through a soaked cotton wool bud placed on the mesh. Cups were lined with a sand paper strip (2 × 12 cm, see section Colony maintenance) and filled with 20 mL water from larval rearing trays to promote oviposition. Eggs were collected daily and counted by eye under a dissecting microscope using a clicker counter.
Quiescent egg viability.
The long-term viability of wAlbB-infected eggs in quiescence was assessed in comparison to eggs from the uninfected CNS colony. Eggs were collected daily en masse for 4 days from each line on a filter paper substrate (see section Colony maintenance). On the third day post-oviposition (day 0), filter papers were stored in a plastic environmental chamber, sealed with Blu-Tack (Bostik, Thomastown, Victoria, Australia). Relative humidity (RH) inside the chamber was maintained at 75% using a saturated solution of sodium chloride in a cup, which was monitored for 1 week with a hygrochron (1-wire; iButton.com) before the introduction of eggs. Ten replicate batches of at least 25 eggs from each line were hatched at days 0, 3, 10, 17, 24, 31, 61, 90, and 124. All batches were captured with a digital camera just before hatching and eggs were counted using the Cell Counter plugin21 in ImageJ.22 To avoid underestimating viability, eggs that hatched early (egg cap clearly detached) before immersion were removed from the analysis. Batches were immersed in plastic cups containing 140 mL hatching water (see section Colony maintenance). After 6 days, all individuals (dead or alive) were counted using a glass pipette and clicker counter.
Larval development time, survival, and adult body size.
To test for any effects of Wolbachia on immature development, we reared cohorts of wAlbB-infected and uninfected CNS larvae under both high- and low-stress conditions. Eggs from both lines were submerged synchronously in RO water, and first instar larvae were added to treatments within 6 hours of hatching. Cohorts of 200 wAlbB-infected or 200 uninfected larvae were reared independently in either 4,000 mL (low density, one larva per 20 mL) or 200 mL (high density, one larva per 1 mL) of RO water. Containers were provided with either 0.25 mg per larva (high nutrition) or 0.05 mg per larva (low nutrition) of crushed TetraMin tablets daily. Each combination of density, nutrition, and Wolbachia infection status was replicated six times.
Cohorts were monitored to determine mean development time, survival, and adult body size. Pupae were transferred to separate containers of RO water as they appeared, and emerging adults were collected twice daily, in the morning and evening, and stored in absolute ethanol. Adults that emerged around the median development time for a particular level of nutrition and density were measured for their wing length to provide an estimate of body size.23,24 At least 25 males and 25 females sampled across all containers were measured for each treatment. The right wing was dissected from each adult and fixed under a 10-mm coverslip with Hoyer's solution (distilled water:gum arabic:chloral hydrate:glycerin in the ratio 5:3:20:2).25 The distance between the alular notch and the intersection of the radius 3 vein and outer margin (excluding the fringe scales) provided a measure of wing length.20 Two independent measurements of each wing were averaged to give the final length. Damaged or folded wings were not measured.
Adult survival in groups.
Adult survival of wAlbB, wMel, and CNS was assayed using groups of 50 individuals (1:1 sex ratio), replicated eight times. Pupae were sexed and added to 25 mL of RO water in plastic cups and allowed to eclose in 3-L plastic containers with stocking lids and mesh sides. A filter paper oviposition site and 10% sucrose solution were provided and refreshed twice a week (see section Colony maintenance). To prevent desiccation, containers were maintained at high humidity (∼85% RH) in white plastic garbage bags. Females were blood-fed weekly for the duration of the experiment, and mortality was scored for males and females three times a week until at least 50% of females from the longest surviving line had died.
Mating.
The mating success of wAlbB males competing with CNS males for mates was estimated. Infected and uninfected males were established in 19.7-L cages before the introduction of 80 virgin CNS females in the following groups: 1) 80 CNS males (negative control, 0% infected), 2) 40 wAlbB and CNS males (treatment, 50% infected), and 3) 80 wAlbB males (positive control, 100% infected). Each group was replicated five times. Males and females were allowed to mature for 1 week before release. During the release, cages were tapped to ensure males were distributed throughout the cage. Mosquitoes were left to mate for 1 week before providing females with a blood meal. Eggs were collected en masse. Because Ae. aegypti females are known to exhibit skip oviposition,26,27 three sandpaper oviposition sites per cage were provided (see section Colony maintenance). Eggs were collected 2 and 4 days later, and counted digitally on the third day post-oviposition using a Canon LiDE 110 flatbed scanner (Canon Australia, Macquarie Park, New South Wales, Australia) and the Cell Counter plugin21 in ImageJ.22 Egg strips were hatched separately within 1 week of oviposition in 3 L hatching water (see section Colony maintenance) and all individuals were counted 6 days later using a glass pipette and clicker counter.
Cytoplasmic incompatibility.
The wAlbB line was reciprocally crossed with CNS to test for CI. Reciprocal crosses between wAlbB and wMel were included to test for bidirectional incompatibility. Incompatible crosses involved wAlbB males mated to wMel or CNS females (or wMel males mated to wAlbB females for the bidirectional test). Compatible crosses were performed between wAlbB females and CNS males and within each infected or uninfected parental line. All crosses were replicated eight times.
For each cross, 14 pupae were sexed (1:1 sex ratio) and added to 20 mL of RO water in 30-mL cups. Pupae eclosed within 1.5-L plastic containers with mesh sides and covered with a stocking. Adults were provided with access to a 10% sucrose solution (see section Colony maintenance). Adults were left to mate for 1 week; they were then fed, and 3 days later they were provided with a filter paper oviposition site. Eggs were collected daily over 4 days, photographed with a digital camera and counted in ImageJ22 using the Cell Counter plugin21 to determine the number of eggs. Within 1 week of embryonation, eggs were hatched in plastic containers with 500 mL hatching water (see section Colony maintenance). To test for age-related effects on CI, two further blood meals were provided weekly and hatch rates determined for three gonotrophic cycles in total.
Maternal transmission.
The maternal transmission efficiency of the wAlbB infection was estimated by testing the proportion of infected offspring produced by an infected female. Females from the wAlbB line were crossed to CNS males en masse and blood fed by a single human volunteer within 1 week of emergence. Engorged females were isolated (see section Fecundity) and later stored in absolute ethanol at 4°C after completing oviposition. Within 1 week of collection, eggs were hatched in plastic trays containing 500 mL hatching water and larvae were reared to adulthood (see section Colony maintenance), then stored in absolute ethanol at 4°C. A minimum of 11 progeny each from 29 females was then tested to detect the presence of the wAlbB infection (see section Wolbachia detection and primers).
Population cages.
The unstable equilibrium threshold (UET) is the proportion of infected to uninfected individuals in a population above which a particular strain of Wolbachia is likely to spread.28 A UET of 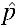 = 0.15 for the successful invasion of the wAlbB infection was estimated by Xi and others14 based on an absence of maternal transmission leakage (μ = 0) with perfect CI (sh = 1) and a fitness cost (sf) of 0.15. However, although our experiments have confirmed μ = 0 and sh = 1 (see Cytoplasmic Incompatibility and Maternal Transmission results), sf seemed to be somewhat lower; the mean relative egg viability for the first month for wAlbB was 0.97 with no reduction in fecundity (sf = 0.03, see Fecundity and Quiescent egg viability results), giving rise to a UET lower than 0.15. The null hypothesis chosen for this experiment was therefore H0:
= 0.15 for the successful invasion of the wAlbB infection was estimated by Xi and others14 based on an absence of maternal transmission leakage (μ = 0) with perfect CI (sh = 1) and a fitness cost (sf) of 0.15. However, although our experiments have confirmed μ = 0 and sh = 1 (see Cytoplasmic Incompatibility and Maternal Transmission results), sf seemed to be somewhat lower; the mean relative egg viability for the first month for wAlbB was 0.97 with no reduction in fecundity (sf = 0.03, see Fecundity and Quiescent egg viability results), giving rise to a UET lower than 0.15. The null hypothesis chosen for this experiment was therefore H0: 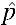 = 0.15 while the alternative was H1:
= 0.15 while the alternative was H1: 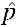 = 0.03. A power function was written in R 3.1.0.29 to determine the likelihood of invasion given a UET of 0.03 with an initial invasion frequency (p0) of 0.1. An existing model of Wolbachia invasion30 was used within the power test, incorporating sample size stochasticity and discrete generations, which is applicable to laboratory colony cages (see Supplemental Appendix 1). The number of generations was set to 10 and population sizes (N) of 200, 400, 600, and 800 were included in the test. The criterion set for successful invasion was 1) the final infection frequency was > p0 and 2) at least two-thirds of the 10 observed generations possessed an infection frequency > p0. On the basis of H1:
= 0.03. A power function was written in R 3.1.0.29 to determine the likelihood of invasion given a UET of 0.03 with an initial invasion frequency (p0) of 0.1. An existing model of Wolbachia invasion30 was used within the power test, incorporating sample size stochasticity and discrete generations, which is applicable to laboratory colony cages (see Supplemental Appendix 1). The number of generations was set to 10 and population sizes (N) of 200, 400, 600, and 800 were included in the test. The criterion set for successful invasion was 1) the final infection frequency was > p0 and 2) at least two-thirds of the 10 observed generations possessed an infection frequency > p0. On the basis of H1: 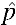 = 0.03, establishing five replicates of 400 individuals (1:1 sex ratio) with p0 = 0.1 and observing invasion within 10 generations was expected to provide sufficient power to reject the null hypothesis while keeping an appropriate type I error rate, α < 0.05. For this set up, we set an observation of at least three invasions of five as the critical point of rejection as it has a probability of less than 0.05 if H0:
= 0.03, establishing five replicates of 400 individuals (1:1 sex ratio) with p0 = 0.1 and observing invasion within 10 generations was expected to provide sufficient power to reject the null hypothesis while keeping an appropriate type I error rate, α < 0.05. For this set up, we set an observation of at least three invasions of five as the critical point of rejection as it has a probability of less than 0.05 if H0: 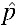 = 0.15 is true, but a probability near or above 0.8 if H1:
= 0.15 is true, but a probability near or above 0.8 if H1: 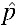 = 0.03 is true (see Supplemental Figure 1). We also established five replicate positive control cages with a p0 of 0.22 (22% group) alongside the 10% treatment group.
= 0.03 is true (see Supplemental Figure 1). We also established five replicate positive control cages with a p0 of 0.22 (22% group) alongside the 10% treatment group.
Separate 12-L cages were populated with male or female pupae from each line. In the 10% group, wAlbB males and females totaled 20 each, whereas they totaled 44 each in the 22% group. Sex was confirmed post-eclosion and dead individuals were replaced to ensure that p0 was exactly maintained, leading up to the initial invasion event. Adults matured for 1 week before an exposure of 4°C for 1 minute. Comatose males were combined from wAlbB and CNS into 19.7-L BugDorm-1 cages whereas females were combined into 500-mL containers. Once all adults were capable of flight again, females were released into the cages containing the males.
Colonies were maintained following the methods described earlier (see section Colony maintenance). After egg collection was complete, 50 females were sampled from each cage and stored in absolute ethanol at 4°C for Wolbachia detection (see section Wolbachia detection and primers). Discrete generations were maintained in new cages, which were established by randomly selecting 400 offspring from the previous generation.
Wolbachia detection and primers.
Tests for Wolbachia infection were conducted using a previously described quantitative real-time polymerase chain reaction assay7 with the addition of wAlbB-specific primers (Table 1). Specific strains were detected based on the combinations of crossing point (Cp) and melting point (Tm) values of the PCR products as determined in a Light Cycler 480 (Roche Applied Science, Castle Hill, New South Wales, Australia). Wolbachia load was also determined in 25 females sampled from the wAlbB, wMel, and wMelPop colonies.
Table 1.
Primers used in qPCR
| Name | Sequence (5′-3′) | Reference | |
|---|---|---|---|
| Aedes | mRpS6_F | AGTTGAACGTATCGTTTCCCGCTAC | 32 |
| mRpS6_R | GAAGTGACGCAGCTTGTGGTCGTCC | ||
| Aedes aegypti | aRpS6_F | ATCAAGAAGCGCCGTGTCG | 32 |
| aRpS6_R | CAGGTGCAGGATCTTCATGTATTCG | ||
| wMel | w1_F | AAAATCTTTGTGAAGAGGTGATCTGC | 32 |
| w1_R | GCACTGGGATGACAGGAAAAGG | ||
| wMelPop | wMelpop_F | CTCATCTTTACCCCGTACTAAAATTTC | 19 |
| wMelpop_R | TCTTCCTCATTAAGAACCTCTATCTTG | ||
| wAlbB | wAlbB_F | CCTTACCTCCTGCACAACAA | * |
| wAlbB_R | GGATTGTCCAGTGGCCTTA |
qPCR = quantitative real-time polymerase chain reaction.
Inaki Iturbe-Ormaetxe, personal communication.
Analysis.
All data were analyzed and graphed in R 3.1.0.29 Fecundity was scored as the number of eggs laid per female, and egg viability (or hatch rate) was defined as the proportion of eggs that hatched by counting the number of third or fourth instar larvae. Proportional data were arcsine square root transformed if they failed Shapiro–Wilk tests of normality and tested again. We checked for heteroscedasticity between groups being compared using F tests. Depending on whether we could assume equal variance and normality, we used the Student's t test and Welch t test for comparing means. For data that could not be transformed to meet assumptions of parametric tests, we used nonparametric Mann–Whitney U tests.
Development time was calculated as the time in days from hatching to adult emergence while larval survival was defined as the proportion of larvae that reached adulthood. Larval development time, survival to adulthood, and wing length data were analyzed by analysis of variance (ANOVA) and Tukey's honest significant difference post hoc tests or by nonparametric Kruskal–Wallis tests depending on normality. To identify deviations from a 1:1 sex ratio, χ2 tests were run.
The equality of adult survival curves (separated by sex) was compared using the Cox regression procedure. Replicates were initially compared, and replicates detected as significant outliers were removed from the analysis. Strains were then compared for pooled data. CNS was set as the baseline, thus the hazard ratio (eβ) for each infected line is the average relative mortality rate of infected to uninfected CNS for the entire monitoring period. We tested the proportional hazards assumption for each line. If nonproportionality was suspected, time was partitioned into blocks. Blocks were decided based on survival curves by visually identifying cutoff time points where hazard ratios were expected to change. The final blocks met the proportional hazard assumption test.
The degree of CI induced by wAlbB males in crosses to CNS or wMel females (and the reciprocal cross) was determined by computing the proportion of eggs that hatched (cages that laid ≤ 10 eggs were excluded). We also tested for the effects of cross and gonotrophic cycle (a proxy of female age), treated as factors, on hatch rates in an ANOVA if data were distributed normally or a Kruskal–Wallis test for non-normal data.
Assortative mating data were analyzed by applying a linear model on mean hatch rates resulting from cages possessing 0%, 50%, or 100% infection rates. Hatch rate was used as a proxy for the proportionate contribution of infected versus uninfected males.31 The mating competitiveness term, βam, represents the deviation between observed and expected hatch rates given a particular rate of CI (see Supplemental Appendix 2). A Student's t test was then performed against the null model (βam = 1) to test for significant deviations, where βam > 1 or βam < 1 indicates an advantage or disadvantage, respectively, in wAlbB male–mating success relative to CNS.
The rate of wAlbB maternal transmission from parent to offspring was calculated as the mean proportion of infected progeny produced by infected mothers. Binomial 95% confidence intervals (95% CIs) were also computed.
Bacterial density, an indicator of Wolbachia load, was quantified using ΔCp (= CpAe. aegypti − CpWolbachia), which corresponds to the difference in log concentration of template DNA between host and parasite.32 Differences in Wolbachia load (ΔCp) between the strains wAlbB, wMel and wMelPop were compared with a Kruskal–Wallis test because of a lack of normality. A post hoc (Dunn) test for multiple comparisons was also performed.
Results
Fecundity.
No difference was found in the number of eggs laid (P > 0.9, t test) between wAlbB females (mean = 60.14, standard deviation [SD] = 7.63, N = 14) and CNS females (mean = 59.72, SD = 12.16, N = 18, relative fecundity = 1.007).
Quiescent egg viability.
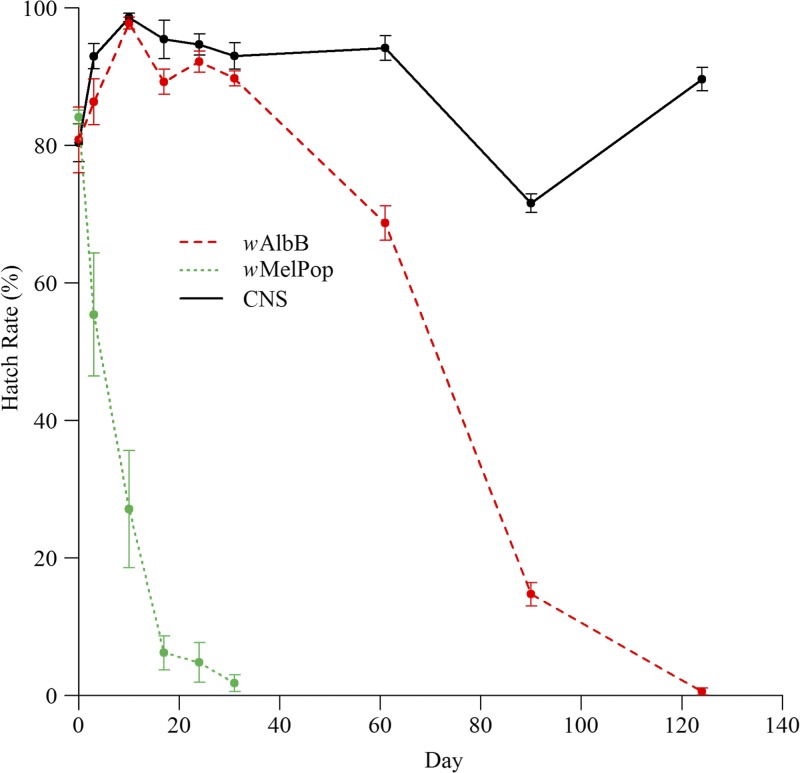
Egg batches ranged from 25 to 78 eggs. Student's t tests were performed on hatch proportions (arcsine transformed). The average percentage of wAlbB eggs surviving at days 0, 3, 10, 17, 24, and 31 was not significantly different from equivalent time points for the CNS strain (Figure 1 , P > 0.05). However, after day 31, the wAlbB infection caused a 27% reduction in mean egg hatch by day 61, 79% by day 90, and 99% by day 124, compared with CNS. The wAlbB egg hatch rates were significantly different from those for CNS (day 61: t = 6.85, df = 18, P < 0.001; day 90: t = 25.39, df = 18, P < 0.001; day 124: t = 28.86, df = 18, P < 0.001). In contrast, the wMelPop strain had a strong negative effect on quiescent egg viability in Ae. aegypti outbred to the CNS background (plotted in Figure 1 for comparison),16 while wMel-infected Ae. aegypti outbred to the same genetic background did not affect quiescent egg viability within a period of 1 month.13
Figure 1.
Percentage hatch rate of wAlbB-infected (red, dashed line), wMelPop-infected (green, dotted line), and uninfected CNS (black, solid line) Aedes aegypti eggs after 0, 3, 10, 17, 24, 31, 61, 90, and 124 days of quiescence. Error bars are standard error of means. Ten replicates per time point. Egg batches ranged from 25 to 78 eggs. The wMelPop curve, generated from eggs outbred to the Cairns genetic background, is taken from the work of Yeap and others.16
Larval development time, survival, and adult body size.
Larval to adult survival was generally high (Table 2). In an analysis of the overall data, we found significant effects of nutrition (F1,40 = 14.38, P < 0.001) and larval density (F1,40 = 10.50, P = 0.002) but not Wolbachia infection status (F1,40 = 0.37, P = 0.548, relative wAlbB survival = 1.002) on survival to adulthood. There were no significant departures from a 1:1 sex ratio in both wAlbB-infected and uninfected adults for any treatment (P > 0.05, χ2 test).
Table 2.
Larval survival, development time, and wing length (mean ± SE) for wAlbB-infected and CNS reared under two nutrition regimes and at two densities
| Nutrition* | Density† | Infection | Survival to adulthood (%)‡ | Development time (days)‡ | Wing length (mm)‡ | ||
|---|---|---|---|---|---|---|---|
| Male | Female | Male | Female | ||||
| High | High | wAlbB | 98.92 ± 0.154 a | 8.32 ± 0.06 a | 9.53 ± 0.05 ab | 1.992 ± 0.012 c | 2.600 ± 0.015 f |
| CNS | 97.83 ± 0.792 ab | 8.30 ± 0.08 a | 9.77 ± 0.10 ab | 2.000 ± 0.008 c | 2.587 ± 0.015 f | ||
| Low | wAlbB | 98.17 ± 0.803 a | 9.83 ± 0.09 ab | 10.99 ± 0.08 b | 2.027 ± 0.011 c | 2.678 ± 0.016 g | |
| CNS | 97.33 ± 0.703 abc | 9.56 ± 0.09 ab | 10.93 ± 0.07 b | 2.017 ± 0.008 c | 2.655 ± 0.014 fg | ||
| Low | High | wAlbB | 96.50 ± 1.252 abc | 17.23 ± 0.29 c | 21.65 ± 0.44 d | 1.869 ± 0.010 ab | 2.379 ± 0.012 d |
| CNS | 97.75 ± 0.793 ab | 17.29 ± 0.31 c | 21.63 ± 0.45 d | 1.916 ± 0.012 b | 2.462 ± 0.013 e | ||
| Low | wAlbB | 93.00 ± 1.494 bc | 24.46 ± 0.56 e | 28.21 ± 0.88 f | 1.837 ± 0.011 a | 2.344 ± 0.024 d | |
| CNS | 93.00 ± 0.707 c | 23.64 ± 0.91 de | 28.85 ± 1.04 f | 1.864 ± 0.017 ab | 2.327 ± 0.025 d | ||
SE = standard error.
High- and low-nutrition regimes consisted of 0.05 and 0.25 mg, respectively, of TetraMin per larva per day.
High- and low-density treatments consisted of 200 larvae in 200 mL (one larva per 1 mL) and 200 larvae in 4,000 mL (1 larva per 20 mL), respectively.
For each trait, values with the same letter are not significantly different from each other (P > 0.05, Tukey's honest significant difference test).
We also found that nutrition (F1,80 = 3,082.52, P < 0.001), larval density (F1,80 = 295.36, P < 0.001), and sex (F1,80 = 144.95, P < 0.001) but not Wolbachia infection status (F1,80 = 0.02, P = 0.891) affected larval development time. Relative to CNS, larval development time for wAlbB was 1.015 days for males and 0.99 days for females (average across nutrition and density). Low larval densities increased development time to a greater extent when nutrition was also low (Table 2). Males reached adulthood around 1.3 days earlier than females at high nutrient levels (mean ± standard error (SE) for males = 9.002 ± 0.151 days, females = 10.302 ± 0.142 days), while this difference was extended to 4.4 days in the low-nutrition treatments (males = 20.653 ± 0.759 days, females = 25.085 ± 0.800 days). No pairwise comparisons for development time between wAlbB-infected and uninfected larvae were significant (Table 2).
We estimated body size in the larval development experiment by measuring wing length. As expected, female wings (mean ± SE = 2.502 mm ± 0.011, N = 213) were considerably larger than male wings (1.941 ± 0.006 mm, N = 222, F1,419 = 6,078.57, P < 0.001). We also found a significant effect of nutrition on wing length (F1,419 = 725.89, P < 0.001), while Wolbachia infection (F1,419 = 3.09, P = 0.080) and larval density (F1,419 = 0.91, P = 0.340) had no significant effects. Relative to CNS, wing length for wAlbB males was 0.99 and 1.00 for females. Larval density affected size differentially at each level of nutrition. For the high nutrition level, the low-density treatment resulted in larger wings, while for the low-nutrition level, the high-density treatment resulted in larger wings (Table 2). No pairwise comparisons between wAlbB-infected and uninfected wings were significant, with the exception of the low-nutrition, high-density containers where uninfected females were significantly larger than wAlbB-infected females (relative wAlbB wing length = 0.97, Table 2).
Adult survival in groups.
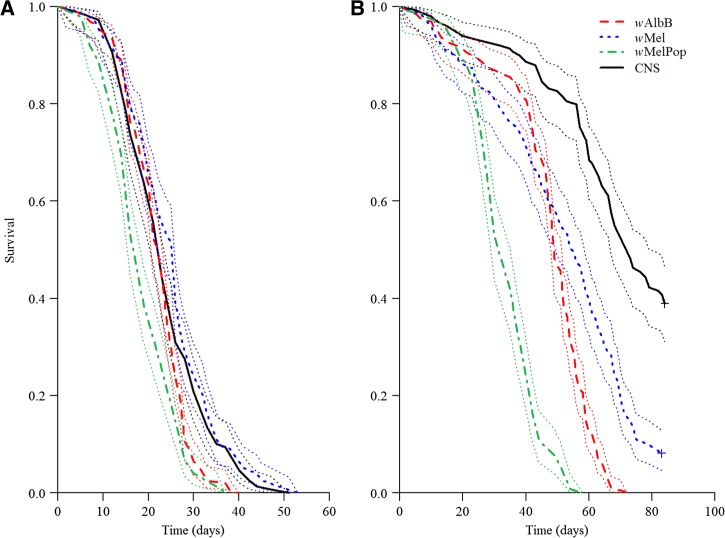
Shortly after CNS had passed 50% survival, this experiment was terminated at day 86. Log-rank tests on unpooled data identified CNS replicates 1 and 2 and wMel replicate 5 as significant outliers, which were excluded before proceeding to analyze pooled data. Within infected lines, wMel males survived the longest followed by wAlbB and then wMelPop (Figure 2A ). Hazard ratios for males from each infected line remained proportional throughout the monitoring period. The mortality rate of wAlbB males differed significantly from CNS (Figure 2, z = 3.24, eβ = 1.43 [95% CI = 1.15–1.78], P = 0.001), as did wMelPop (z = 5.77, eβ = 1.98 [95% CI = 1.57–2.50], P < 0.001), whereas wMel and CNS did not differ significantly (z = −1.01, eβ = 0.89 [95% CI = 0.71–1.12], P = 0.312). Within the infected lines, wAlbB females performed the best up to around 40 days before a sudden increase in mortality rate, which led to a significantly lower survival overall compared with wMel, but this was still higher than for wMelPop (Figure 2B, Supplemental Table 3). Hazard ratios were nonproportional for wAlbB and wMelPop females; however, wMel was proportional throughout the monitoring period. Separate Cox regressions were performed on days 0–20 (block 1), 21–40 (block 2), and > 40 days (block 3) as the hazards appeared to be different across blocks (Figure 2, see Supplemental Table 3). The mortality rate of wAlbB females was significantly different from CNS in block 3 only (z = 13.27, eβ = 8.54 [95% CI = 6.22–11.73], P < 0.001), whereas wMel females became significantly different from CNS in blocks 2 (z = 3.34, eβ = 3.77 [95% CI: 1.73–8.23], P < 0.001) and 3 (z = 6.34, eβ = 2.64 [95% CI = 1.95–3.56], P < 0.001). Females infected with wMelPop had the highest mortality rate, becoming significantly different from CNS in blocks 2 (z = 8.29, eβ = 21.63 [95% CI = 10.45–44.75], P < 0.001) and 3 (z = 12.85, eβ = 21.87 [95% CI = 13.66–35.01], P < 0.001) (see Supplemental Table 4).
Figure 2.
Survival of adult Aedes aegypti males (A) and females (B) infected with wAlbB (red, dashed line), wMel (blue, dotted line), or wMelPop (green, dot–dash line) outcrossed to the Cairns genetic background, represented by CNS (black, solid line). “+” represents right-censored data. Thin dotted lines are 95% confidence intervals.
Mating.
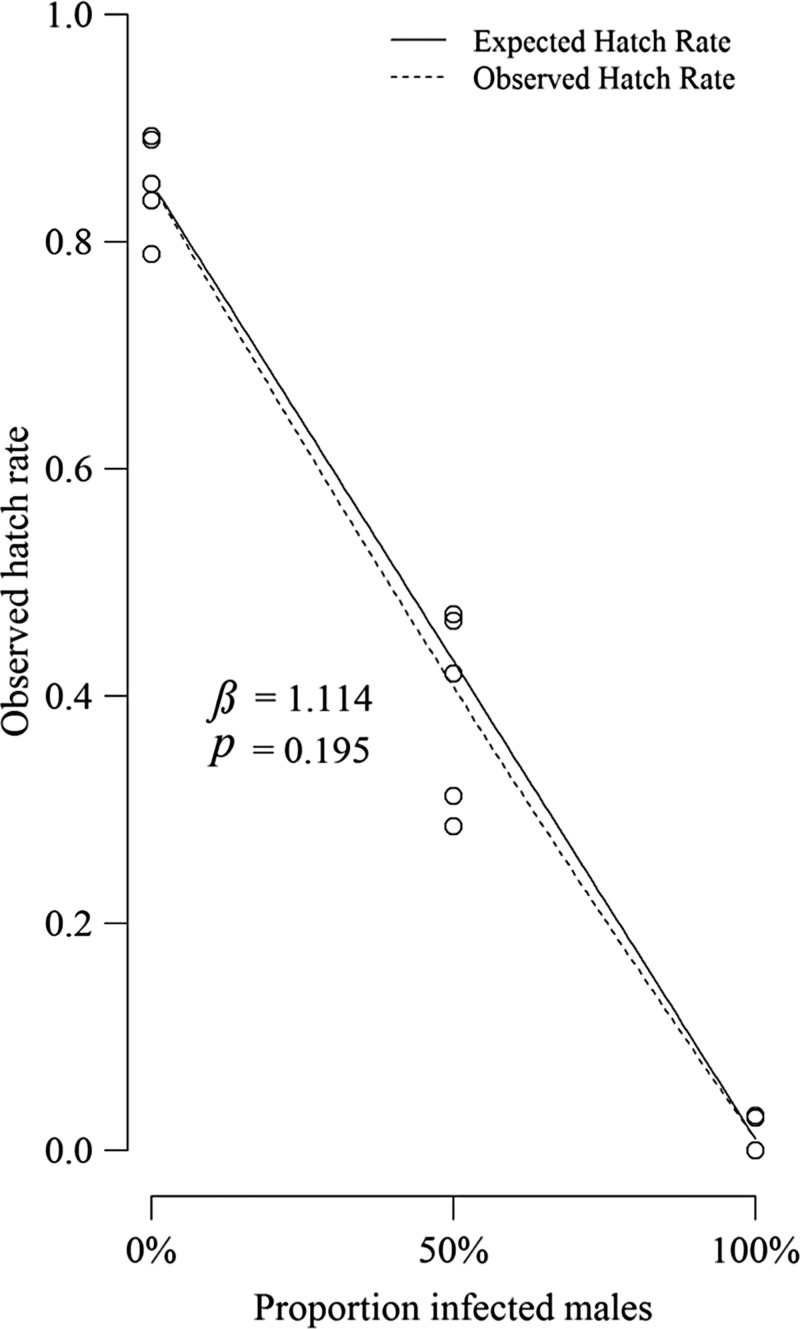
The average number of eggs oviposited by CNS females in cages possessing 0%, 50%, and 100% infection frequencies was 2,364, 2,471, and 2,346, respectively, and their mean hatch rates were 0.01, 0.39, and 0.85, respectively (Figure 3 ). The number of eggs that hatched early and died on the paper (egg cap clearly detached) was negligible (< 40 per replicate). The relative mating success of wAlbB males to CNS males (βam) was 1.114, but this did not differ significantly from the null hypothesis (βam = 1) in a t test (t = 0.89, df = 14, P = 0.195). Therefore, there was no strong evidence for assortative mating in favor of males from either strain.
Figure 3.
Mean hatch rates of Aedes aegypti eggs oviposited by 80 CNS females exposed to populations of males in the following groups: 0% infected (80 CNS males), 50% infected (40 wAlbB and CNS males), and 100% infected (80 wAlbB males). The solid line denotes the null model for the expected hatch rate (no difference in mating ability), whereas the dotted line represents the observed hatch rate and relative mating success (β) of wAlbB to CNS males and its probability (p).
Cytoplasmic incompatibility.
Complete CI was observed between wAlbB males and CNS females resulting in sterility, regardless of gonotrophic cycle (Table 3). Similarly, reciprocal crosses between wMel and wAlbB exhibited complete bidirectional incompatibility (Table 3). One-way ANOVAs on arcsine transformed hatch rates of the compatible crosses indicated a nonsignificant effect of gonotrophic cycle (F2,88 = 0.35, P = 0.708), but a significant effect of cross (F3,88 = 28.16, P < 0.001). Fecundity did not differ between incompatible and compatible crosses in a t test (t = 0.28, df = 141, P = 0.782), but showed a consistent decrease between gonotrophic cycles 1 and 3, most likely due to age effects (Table 3). Average hatch rates of ≥ 79% were observed for all control crosses. Tests on Cairns outbred wMel and wMelPop males crossed to Cairns wild-type females also indicated very strong CI.7,13,16
Table 3.
Crosses performed between infected and uninfected lines to test for CI
| Gonotrophic cycle* | |||
|---|---|---|---|
| 1 | 2 | 3 | |
| Incompatible | |||
| CNS (♀) × wAlbB (♂) | 0 (319.75) | 0 (334) | 0 (286.38) |
| wMel (♀) × wAlbB (♂) | 0 (214.25) | 0 (221.75) | 0 (206.25) |
| wAlbB (♀) × wMel (♂) | 0 (332.43) | 0 (294.75) | 0 (268.38) |
| Compatible | |||
| CNS (♀) × CNS (♂) | 0.97 (484.86) | 0.99 (364) | 0.93 (434.25) |
| wAlbB (♀) × CNS (♂) | 0.94 (227.75) | 0.96 (241.13) | 0.94 (214) |
| wMel (♀) × wMel (♂) | 0.81 (286.38) | 0.81 (288.75) | 0.79 (200.13) |
| wAlbB (♀) × wAlbB (♂) | 0.88 (381.25) | 0.83 (384.63) | 0.84 (285.38) |
CI = cytoplasmic incompatibility.
Mean hatch rate (mean number of eggs scored).
Maternal transmission.
Out of the 319 offspring produced by 29 wAlbB-infected females, 319 were positively infected with the wAlbB strain (maternal transmission rate = 1, lower 95% CI = 0.99).
Population cages.
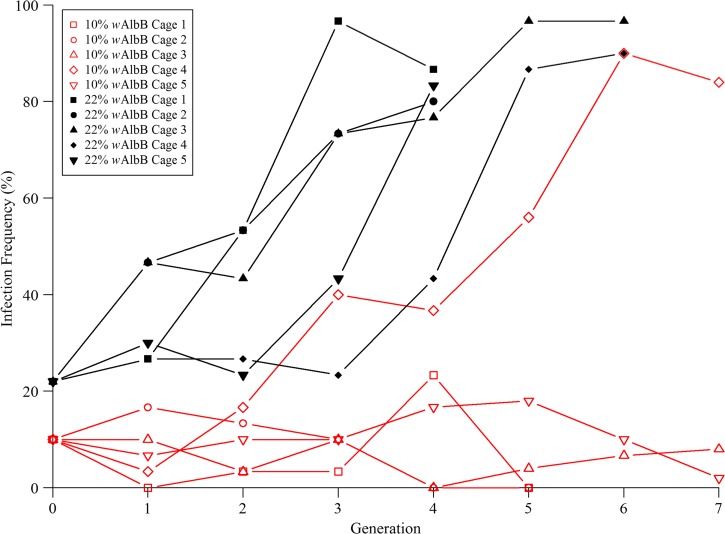
As outlined in the Materials and methods section, replicates were terminated from four to seven generations after it was clear that the wAlbB infection had successfully invaded in either the 10% or 22% groups (Figure 4
). All remaining colonies were terminated at generation 7 when the trajectory of the populations was clear. By the seventh generation, one of the five cages in the 10% group had successfully invaded. Because we observed less than three invasions, the critical threshold to achieve α < 0.05, we could not reject the null hypothesis that 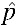 = 0.15 (P = 0.482; Figure 4, Supplemental Figure 1). The remaining four cages in the 10% group failed to invade, with infection frequencies ranging from 0% to 23% over seven generations. All of the 22% control cages successfully invaded uninfected populations in four to six generations (Figure 4).
= 0.15 (P = 0.482; Figure 4, Supplemental Figure 1). The remaining four cages in the 10% group failed to invade, with infection frequencies ranging from 0% to 23% over seven generations. All of the 22% control cages successfully invaded uninfected populations in four to six generations (Figure 4).
Figure 4.
Wolbachia infection frequency per generation after an initial release of wAlbB females and males (red, open markers: 10%; black, solid markers: 22%) into 10 cages possessing CNS populations.
Wolbachia load.
The median difference in log concentration of template DNA between Wolbachia and its host genome (ΔCp) in order of highest to lowest was wMelPop (6.83), wAlbB (5.09), and wMel (3.33) (Figure 5 ). Significant differences between groups were found in a Kruskal–Wallis test (χ2 = 53.19, df = 2, P < 0.001) and all pairwise comparisons between strains in a post hoc test (P < 0.001, Dunn test).
Figure 5.
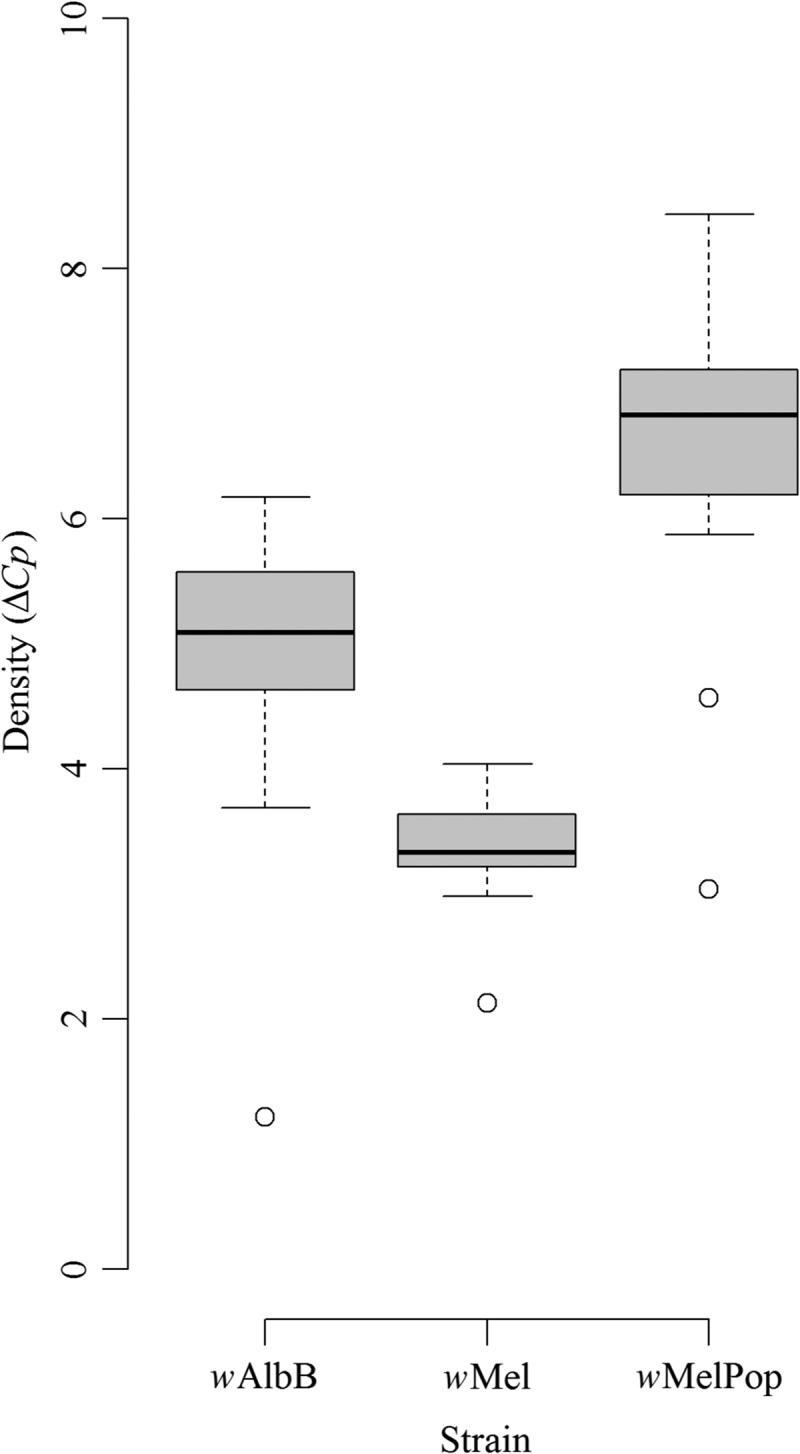
Box plot of Wolbachia load for wAlbB, wMel, and wMelPop in laboratory-reared Aedes aegypti (N = 25). Outliers are represented as circles. Medians are indicated by horizontal lines.
Discussion
We have characterized fitness and reproductive effects of the wAlbB infection in an outbred Ae. aegypti population. Previously, this infection was placed into an Ae. aegypti line originating from Texas33 through microinjection after having been sourced from Ae. albopictus.14 In this study, we have crossed wAlbB-infected Ae. aegypti into a common Australian background to allow comparisons to other Wolbachia-infected strains of Ae. aegypti. The effects of wAlbB on fitness appear to fall between those of wMel and wMelPop, infections that both originated from D. melanogaster.13,16,34 We find costs associated with the wAlbB infection on egg hatch when the eggs are maintained in a quiescent state for more than 31 days. These costs are greater than those imposed by the wMel infection but the costs are not as severe as for wMelPop, where the majority of infected eggs do not last longer than 1 month.19
In terms of adult survival, we found that wAlbB shows a reduction in lifespan that is intermediate between the pattern for wMel and wMelPop. Females infected with wAlbB reached 50% survival 29 days earlier than uninfected females, in contrast to wMel and wMelPop, which reached the same point at 17 and 43 days earlier than uninfected females, respectively. We also found nonproportionality in the hazard ratio for wAlbB females due to an increase in mortality after around 40 days for five of eight replicates. This may represent an underlying feature of its growth within Ae. aegypti adults as they age; wAlbB densities tend to increase with adult age in both its native hosts35 and in an experimental infection of Anopheles stephensi.36 The nonproportional hazard ratio of females infected with wMelPop may reflect its overreplication in host cells, which causes early death as demonstrated in Drosophila.37 The fitness effects of Wolbachia infections (but not their levels of CI) appear to be at least partly related to the density of the Wolbachia in tissues although only a modest number of infections have so far been tested.8,10
We confirmed the absence of any costs in terms of fecundity14 and also male mating success for wAlbB; wMelPop does exhibit fecundity costs,12,16 but neither this infection nor wMel show mating costs.31 We also confirmed the maternal transmission of wAlbB was 100%14 and complete CI was shown in crosses with uninfected females from Cairns, which was also reported for wMel and wMelPop.13,16 Complete bidirectional incompatibility was found between wAlbB and wMel; given the relative fitness effects found here, wMel may outcompete wAlbB should these two infections be released in the same population at an equal frequency. However, a population that has been invaded by wAlbB is not expected to be invaded by wMel because of bidirectional incompatibility and frequency dependence; CI will be induced in crosses between wAlbB males and wMel females, and, when present at a low frequency, most wMel females will inevitably mate with wAlbB males. If two infected strains are present in a population, the outcome will depend mostly on the nature of incompatibility patterns among the strains.28 If strains are bidirectionally incompatible, both strains may persist when they have invaded different areas because common strains have an advantage in an area; females from the common strain will be more likely to engage in compatible matings with males from the same strain. If males from one strain show CI but males from the other strain do not, the CI-inducing strain is likely to invade as occurred in Australian populations of Drosophila simulans where the wAu strain that did not induce CI was replaced rapidly by the wRi strain that did induce it.38
A decade ago, the wAlbB infection was reported as generating strong CI and perfect maternal transmission while having minimal effects on host fitness in terms of fecundity and egg hatch.14 The wAlbB infection was shown to invade small population cages when introduced at a starting frequency of 20%.14 We confirmed these results in a larger cage population with greater replication, suggesting relative fitness costs that are comparable to the frequency of Wolbachia required to obtain invasion28 are most likely greater than 10% but lower than 20%. However, we found no significant fitness costs for male mating competitiveness, adult survival within 1 month, or larval development and survival to adulthood. Previous experiments on the natural host of the wAlbB infection, Ae. albopictus, suggest that the wAlbB infection in combination with the wAlbA infection can generate a fitness advantage for its host.39 Our results suggest that releasing wAlbB-infected adults so that they comprise 20% of the target population may be sufficient to achieve rapid invasion, but this threshold may well be higher in natural populations, particularly given the costs we have found at the quiescent egg stage.
In summary, the wAlbB infection might be suitable for invasion into Ae. aegypti populations given that this infection appears to have moderate fitness costs that place it between wMel, which can successfully invade populations where it is stably maintained,1,7 and wMelPop, which can be invaded into semi-field populations13,19 but which has substantial fitness costs that make invasion into natural populations difficult. Given that wAlbB in Ae. aegypti can block arbovirus transmission, this strain may be suitable for release alongside wMel, although its ability to block different serotypes of dengue and other viruses remains to be established (cf.4). Because it influences the hatch rate of quiescent eggs, the wAlbB strain may also have utility in releases aimed at suppressing or eliminating populations of Ae. aegypti during the dry season.6,19
Supplementary Material
ACKNOWLEDGMENTS
We acknowledge the McGraw lab at Monash University for providing the wAlbB-infected line and Steve Dobson for permission to run experiments with this line. We also thank Thomas Walker and Meg Woolfit from the O'Neill lab at Monash for providing the wAlbB primers and Taqman probe information.
Footnotes
Financial support: This study was supported by a program grant from the National Health and Medical Research Council and fellowship from the Australian Research Council.
Authors' addresses: Jason K. Axford, Perran A. Ross, Ashley G. Callahan, and Ary A. Hoffmann, Pest and Environmental Adaptation Research Group, Bio21 Institute and School of BioSciences, University of Melbourne, Parkville, Australia, E-mails: jkaxford@unimelb.edu.au, paross@student.unimelb.edu.au, ashleygc@unimelb.edu.au, and ary@unimelb.edu.au. Heng Lin Yeap, CSIRO, Acton, Australia, E-mail: henglin.yeap@csiro.au.
References
- 1.Hoffmann AA, Montgomery BL, Popovici J, Iturbe-Ormaetxe I, Johnson PH, Muzzi F, Greenfield M, Durkan M, Leong YS, Dong Y, Cook H, Axford J, Callahan AG, Kenny N, Omodei C, McGraw EA, Ryan PA, Ritchie SA, Turelli M, O'Neill SL. Successful establishment of Wolbachia in Aedes populations to suppress dengue transmission. Nature. 2011;476:454–457. doi: 10.1038/nature10356. [DOI] [PubMed] [Google Scholar]
- 2.McGraw EA, O'Neill SL. Beyond insecticides: new thinking on an ancient problem. Nat Rev Microbiol. 2013;11:181–193. doi: 10.1038/nrmicro2968. [DOI] [PubMed] [Google Scholar]
- 3.Moreira LA, Iturbe-Ormaetxe I, Jeffery JA, Lu GJ, Pyke AT, Hedges LM, Rocha BC, Hall-Mendelin S, Day A, Riegler M, Hugo LE, Johnson KN, Kay BH, McGraw EA, van den Hurk AF, Ryan PA, O'Neill SL. A Wolbachia symbiont in Aedes aegypti limits infection with dengue, chikungunya, and Plasmodium. Cell. 2009;139:1268–1278. doi: 10.1016/j.cell.2009.11.042. [DOI] [PubMed] [Google Scholar]
- 4.Ferguson NM, Hue Kien DT, Clapham H, Aguas R, Trung VT, Bich Chau TN, Popovici J, Ryan PA, O'Neill SL, McGraw EA, Long VT, Dui LT, Nguyen HL, Vinh Chau NV, Wills B, Simmons CP. Modeling the impact on virus transmission of Wolbachia-mediated blocking of dengue virus infection of Aedes aegypti. Sci Transl Med. 2015;7:279ra37. doi: 10.1126/scitranslmed.3010370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mains JW, Brelsfoard CL, Crain PR, Huang YX, Dobson SL. Population impacts of Wolbachia on Aedes albopictus. Ecol Appl. 2013;23:493–501. doi: 10.1890/12-1097.1. [DOI] [PubMed] [Google Scholar]
- 6.Rašić G, Endersby EM, Williams C, Hoffmann AA. Using Wolbachia-based releases for suppression of Aedes mosquitoes: insights from genetic data and population simulations. Ecol Appl. 2014;24:1226–1234. doi: 10.1890/13-1305.1. [DOI] [PubMed] [Google Scholar]
- 7.Hoffmann AA, Iturbe-Ormaetxe I, Callahan A, Phillips BL, Billington K, Axford JK, Montgomery B, Turley AP, O'Neill SL. Stability of the wMel Wolbachia infection following invasion into Aedes aegypti populations. PLoS Negl Trop Dis. 2014;8:e3115. doi: 10.1371/journal.pntd.0003115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hoffmann AA, Ross PA, Rašić G. Wolbachia strains for disease control: ecological and evolutionary considerations. Evol Appl. 2015;8:751–768. doi: 10.1111/eva.12286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dean MD. A Wolbachia-associated fitness benefit depends on genetic background in Drosophila simulans. Proc R Soc Lond B Biol Sci. 2006;273:1415–1420. doi: 10.1098/rspb.2005.3453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chrostek E, Marialva MSP, Esteves SS, Weinert LA, Martinez J, Jiggins FM, Teixeira L. Wolbachia variants induce differential protection to viruses in Drosophila melanogaster: a phenotypic and phylogenomic analysis. PLoS Genet. 2013;9:e1003896. doi: 10.1371/journal.pgen.1003896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Martinez J, Longdon B, Bauer S, Chan YS, Miller WJ, Bourtzis K, Teixeira L, Jiggins FM. Symbionts commonly provide broad spectrum resistance to viruses in insects: a comparative analysis of Wolbachia strains. PLoS Pathog. 2014;10:e1004369. doi: 10.1371/journal.ppat.1004369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.McMeniman CJ, Lane RV, Cass BN, Fong AWC, Sidhu M, Wang YF, O'Neill SL. Stable introduction of a life-shortening Wolbachia infection into the mosquito Aedes aegypti. Science. 2009;323:141–144. doi: 10.1126/science.1165326. [DOI] [PubMed] [Google Scholar]
- 13.Walker T, Johnson PH, Moreira LA, Iturbe-Ormaetxe I, Frentiu FD, McMeniman CJ, Leong YS, Dong Y, Axford J, Kriesner P, Lloyd AL, Ritchie SA, O'Neill SL, Hoffmann AA. The wMel Wolbachia strain blocks dengue and invades caged Aedes aegypti populations. Nature. 2011;476:450–453. doi: 10.1038/nature10355. [DOI] [PubMed] [Google Scholar]
- 14.Xi ZY, Khoo CCH, Dobson SL. Wolbachia establishment and invasion in an Aedes aegypti laboratory population. Science. 2005;310:326–328. doi: 10.1126/science.1117607. [DOI] [PubMed] [Google Scholar]
- 15.Bian GW, Xu Y, Lu P, Xie Y, Xi ZY. The endosymbiotic bacterium Wolbachia induces resistance to dengue virus in Aedes aegypti. PLoS Pathog. 2010;6:e1000833. doi: 10.1371/journal.ppat.1000833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yeap HL, Mee P, Walker T, Weeks AR, O'Neill SL, Johnson P, Ritchie SA, Richardson KM, Doig C, Endersby NM, Hoffmann AA. Dynamics of the “Popcorn” Wolbachia infection in outbred Aedes aegypti informs prospects for mosquito vector control. Genetics. 2011;187:583–595. doi: 10.1534/genetics.110.122390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ross PA, Endersby NM, Yeap HL, Hoffmann AA. Larval competition extends developmental time and decreases adult size of wMelPop Wolbachia-infected Aedes aegypti. Am J Trop Med Hyg. 2014;91:198–205. doi: 10.4269/ajtmh.13-0576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Barton NH, Turelli M. Spatial waves of advance with bistable dynamics: cytoplasmic and genetic analogues of allee effects. Am Nat. 2011;178:E48–E75. doi: 10.1086/661246. [DOI] [PubMed] [Google Scholar]
- 19.Ritchie SA, Townsend M, Paton CJ, Callahan AG, Hoffmann AA. Application of wMelPop Wolbachia strain to crash local populations of Aedes aegypti. PLoS Negl Trop Dis. 2015;9:e0003930. doi: 10.1371/journal.pntd.0003930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yeap HL, Axford JK, Popovici J, Endersby NM, Iturbe-Ormaetxe I, Ritchie SA, Hoffmann AA. Assessing quality of life-shortening Wolbachia-infected Aedes aegypti mosquitoes in the field based on capture rates and morphometric assessments. Parasit Vectors. 2014;7:58. doi: 10.1186/1756-3305-7-58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.De Vos K. Cell Counter v2.0. 2005. http://rsb.info.nih.gov/ij/plugins/cell-counter.html Available at. Accessed June 10, 2013.
- 22.Schneider CA, Rasband WS, Eliceiri KW. NIH Image to ImageJ: 25 years of image analysis. Nat Methods. 2012;9:671–675. doi: 10.1038/nmeth.2089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Briegel H. Metabolic relationship between female body size, reserves, and fecundity of Aedes aegypti. J Insect Physiol. 1990;36:165–172. [Google Scholar]
- 24.Nasci RS. Relationship of wing length to adult dry-weight in several mosquito species (Diptera, Culicidae) J Med Entomol. 1990;27:716–719. doi: 10.1093/jmedent/27.4.716. [DOI] [PubMed] [Google Scholar]
- 25.Anderson LE. Hoyer's solution as a rapid permanent mounting medium for bryophytes. Bryologist. 1954;57:242–244. [Google Scholar]
- 26.Colton YM, Chadee DD, Severson DW. Natural skip oviposition of the mosquito Aedes aegypti indicated by codominant genetic markers. Med Vet Entomol. 2003;17:195–204. doi: 10.1046/j.1365-2915.2003.00424.x. [DOI] [PubMed] [Google Scholar]
- 27.Oliva LO, Correia JC, Albuquerque CMR. How mosquito age and the type and color of oviposition sites modify skip-oviposition behavior in Aedes aegypti (Diptera: Culicidae)? J Insect Behav. 2014;27:81–91. [Google Scholar]
- 28.Hoffmann AA, Turelli M. Cytoplasmic incompatibility in insects. In: O'Neill S, Hoffmann AA, Werren J, editors. Influential Passengers: Microorganisms and Invertebrate Reproduction. Oxford, UK: Oxford University Press; 1997. pp. 42–80. [Google Scholar]
- 29.R Core Team . R: A Language and Environment for Statistical Computing. Vienna, Austria: R Foundation for Statistical Computing; 2015. http://www.R-project.org/ Available at. [Google Scholar]
- 30.Hoffmann AA, Turelli M, Harshman LG. Factors affecting the distribution of cytoplasmic incompatibility in Drosophila simulans. Genetics. 1990;126:933–948. doi: 10.1093/genetics/126.4.933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Segoli M, Hoffmann AA, Lloyd J, Omodei GJ, Ritchie SA. The effect of virus-blocking Wolbachia on male competitiveness of the dengue vector mosquito, Aedes aegypti. PLoS Negl Trop Dis. 2014;8:10. doi: 10.1371/journal.pntd.0003294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lee SF, White VL, Weeks AR, Hoffmann AA, Endersby NM. High-throughput PCR assays to monitor Wolbachia infection in the dengue mosquito (Aedes aegypti) and Drosophila simulans. Appl Environ Microbiol. 2012;78:4740–4743. doi: 10.1128/AEM.00069-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Duhrkopf RE, Benny H. Differences in the larval alarm reaction in populations of Aedes aegypti and Aedes albopictus. J Am Mosq Control Assoc. 1990;6:411–414. [PubMed] [Google Scholar]
- 34.McMeniman CJ, O'Neill SL. A virulent Wolbachia infection decreases the viability of the dengue vector Aedes aegypti during periods of embryonic quiescence. PLoS Negl Trop Dis. 2010;4:e748. doi: 10.1371/journal.pntd.0000748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tortosa P, Charlat S, Labbé P, Dehecq J-S, Barré H, Weill M. Wolbachia age-sex-specific density in Aedes albopictus: a host evolutionary response to cytoplasmic incompatibility. PLoS One. 2010;5:e9700. doi: 10.1371/journal.pone.0009700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Murdock CC, Blanford S, Hughes GL, Rasgon JL, Thomas MB. Temperature alters Plasmodium blocking by Wolbachia. Sci Rep. 2014;4:3932. doi: 10.1038/srep03932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Min KT, Benzer S. Wolbachia, normally a symbiont of Drosophila, can be virulent, causing degeneration and early death. Proc Natl Acad Sci USA. 1997;94:10792–10796. doi: 10.1073/pnas.94.20.10792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kriesner P, Hoffmann AA, Lee SF, Turelli M, Weeks AR. Rapid sequential spread of two Wolbachia variants in Drosophila simulans. PLoS Pathog. 2013;9:e1003607. doi: 10.1371/journal.ppat.1003607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Dobson SL, Marsland EJ, Rattanadechakul W. Mutualistic Wolbachia infection in Aedes albopictus: accelerating cytoplasmic drive. Genetics. 2002;160:1087–1094. doi: 10.1093/genetics/160.3.1087. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.