Abstract
The objectives of this study were to design a pharmacokinetic (PK) study by using information about adults and evaluate the robustness of the recommended design through a case study of mefloquine. PK data about adults and children were available from two different randomized studies of the treatment of malaria with the same artesunate-mefloquine combination regimen. A recommended design for pediatric studies of mefloquine was optimized on the basis of an extrapolated model built from adult data through the following approach. (i) An adult PK model was built, and parameters were estimated by using the stochastic approximation expectation-maximization algorithm. (ii) Pediatric PK parameters were then obtained by adding allometry and maturation to the adult model. (iii) A D-optimal design for children was obtained with PFIM by assuming the extrapolated design. Finally, the robustness of the recommended design was evaluated in terms of the relative bias and relative standard errors (RSE) of the parameters in a simulation study with four different models and was compared to the empirical design used for the pediatric study. Combining PK modeling, extrapolation, and design optimization led to a design for children with five sampling times. PK parameters were well estimated by this design with few RSE. Although the extrapolated model did not predict the observed mefloquine concentrations in children very accurately, it allowed precise and unbiased estimates across various model assumptions, contrary to the empirical design. Using information from adult studies combined with allometry and maturation can help provide robust designs for pediatric studies.
INTRODUCTION
Pediatrics have long been poorly investigated in drug development for ethical, practical, and methodological reasons (1). Given these limitations, the dose given to children is often derived mostly from the adult dose by linear adjustment for body weight. However, a number of studies have shown that this crude approach could be misleading, prompting scientists and physicians to consider children less as small adults (2, 3) and more as a specific population with different drug metabolism and sensitivity. Recognizing this challenge, regulatory authorities have sought to bolster the efforts of the industry through the pediatric investigation plan (PIP) (4), and drug development for children has become an independent field, creating new challenges in medicine. An increasing number of clinical trials are being performed to allow the proper evaluation of drug pharmacokinetics (PK) in children, holding the promise that a better balance between toxicity and efficacy may be found for drugs in pediatrics (5). However, the precise characterization of a drug's PK is a difficult task that requires careful choice of the dose regimen and the time to sample observations, which together form the design of the study. This is particularly problematic in pediatrics, where ethical constraints dramatically reduce the number of measurements possible, making PK parameter estimation a particularly difficult endeavor and the choice of an appropriate design a decision even more critical than for adults (6). Contrary to the first-in-humans trials, where no prior clinical information is available, the first study of children is often performed after studies with adults have been performed. When properly leveraged, the data from adults could be used to build an appropriate design for a pediatric study, and they are often the only information available at this early stage (7). Within the PIP, incorporating prior knowledge from adults is also a way of streamlining pediatric drug development in the global development program (8).
To optimize the available information, PK are often analyzed by using nonlinear mixed-effect (NLME) models, an approach that allows the use of sparse and heterogeneous designs (9). In that framework, design optimization based on the Fisher information matrix (FIM) has become an increasingly popular tool to maximize the information collected in a study and determine the times for sampling measurements that are most likely to provide precise estimation of PK parameters (10, 11).
In the present work, we investigated the process of designing a pediatric study by using information about adults. Mefloquine, an antimalarial drug, served as a case study, with data from two clinical trials with adults and children (12). We used adult data to obtain the PK model of mefloquine in adults and leveraged this information for children through allometric and maturation functions while taking into account changes in body size and metabolic processes that occur with age (13). We then used the extrapolated model to design a study for a pediatric population with different age groups. We show that this approach provides a framework that may dramatically improve the design of a study of PK in children, allowing precise estimation of PK parameters while limiting the number of sampling measurements.
MATERIALS AND METHODS
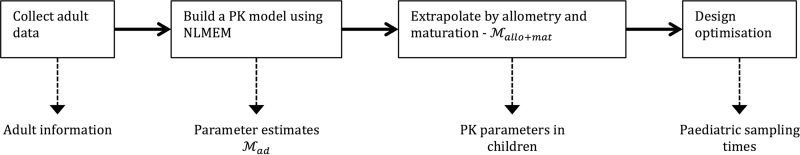
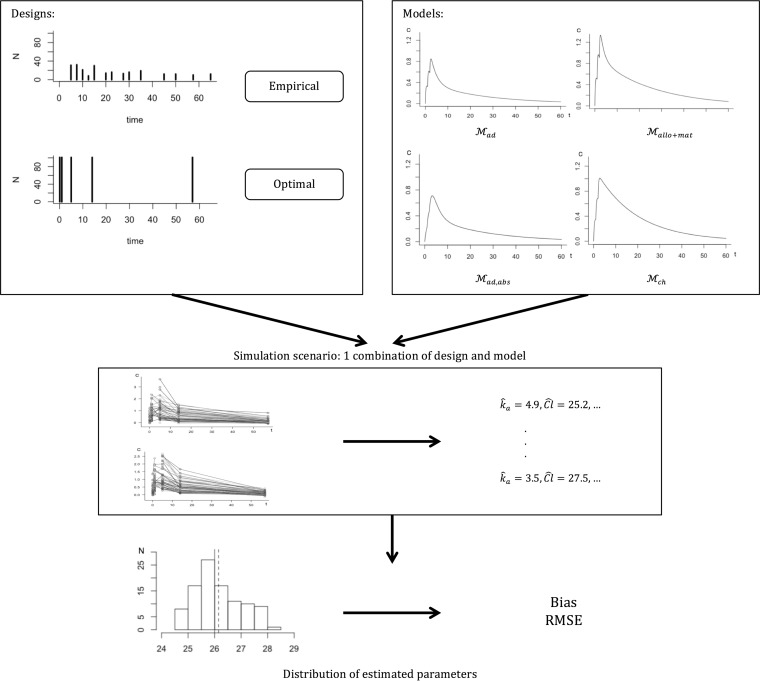
In the present work, we considered the following methodological workflow, which is summarized in Fig. 1. First, on the basis of data collected from an adult population, we built a PK model. Extrapolation using allometry and maturation was then applied to the resulting model in order to derive the PK model and parameters for children. The extrapolated model was then used to optimize the design for children. The performance of the optimized design was evaluated by assessing its ability to estimate the population parameters correctly through a simulation study under different model assumptions to assess its robustness. The evaluation process is illustrated separately in Fig. 2. The optimized design was compared to the design of the pediatric database, which we call an empirical design. Simulations were performed for four different models to ensure robustness. An external evaluation was also performed by fitting the pediatric data with the different models used for simulations and comparing their predictive abilities.
FIG 1.
Framework used to design a pediatric study by using adult information.
FIG 2.
Schema of simulation study. For both the optimal design and the empirical design from the pediatric database and for each model tested, 100 data sets are simulated. For each data set, PK parameters are estimated and then compared to the theoretical value of the original model with bias and RMSE. Mad is the adult model, Mallo+mat is the maturation model using the adult model with allometry and maturation, Mad,abs is the adult model with a modified absorption of 1, and Mch is a model resulting from the PK of the pediatric data.
Adult data.
The first study included data from adults taking part in a phase I-II clinical trial in India (http://www.isrctn.com/ISRCTN70618692). This multicenter, single-arm clinical trial was carried out to assess the safety, efficacy, and population PK of a fixed-dose combination of artesunate-mefloquine in Indian adults infected with acute uncomplicated Plasmodium falciparum malaria. Seventy-seven patients were included. Subjects received two tablets containing 100 mg of artesunate and 200 mg of mefloquine orally once daily for 3 consecutive days. Blood samples for the analysis of mefloquine PK and laboratory evaluation were collected before the first dose, within 72 h of the first dose, and on study days 7, 28, 35, and 42.
Child data.
The second study included children under 15 years old enrolled in a phase I-II clinical trial in Thailand (14). This randomized trial was carried out to assess the safety and efficacy of a new artesunate-mefloquine coformulation for the treatment of acute uncomplicated P. falciparum malaria in children. A total of 101 children under 15 years old were included in this study. Pediatric patients were administered a weight-related dose, approximately 4 mg/kg/day, of artesunate for 3 days of treatment and 25 mg/kg of mefloquine split into 15 mg/kg on the second day and 10 mg/kg on the third day. The following PK samples were scheduled from the first day of administration and during follow-up: three or four samples randomly selected from day 1, 2, or 3 or days 7 to 14 and one or two additional samples taken on day 21, 28, 35, 42, 49, 56, or 63.
Modeling of the PK of mefloquine in adults.
The PK of mefloquine in adults were analyzed by using NLME. Designating yi = (yi1, yi2,…yini)T the ni vector of observations for individual i (i = 1…N) collected at sampling times ti = (ti1, ti2,…tini)T, we have the statistical model yi = f(ϕi, ti) + εi, where f is a mathematical function representing the evolution of the concentration with time. The vector ϕi is the vector of individual parameters of i and εi, a ni vector of random errors distributed as εi∼N(0, Σi). We assume that the distribution of the parameters can be described through a log-normal distribution. For the kth component of ϕ, k = 1…K, we write the individual parameter ϕ(k) as a function of a fixed effect μ(k) and an individual i random effect bi(k): ϕ(k) = μ(k)ebi(k).
The distribution of the random effects was assumed to be multivariate normal, with a variance-covariance matrix designated Ω2.
The parameters of the NLME model were estimated by using the stochastic approximation expectation-maximization algorithm (15) implemented in the Monolix software (version 4.2.2) (16). The likelihood was computed by using importance sampling. Model building was based on the likelihood ratio test for nested models and the Bayesian information criteria for nonnested models. We investigated first the structural model by comparing different compartment models, then interindividual variability by testing whether Ω2 could be assumed to be diagonal or not, and finally residual variability. Different residual error models were considered, i.e., a constant-error model, Var(εij) = a2; a proportional-error model, Var(εij) = b2 × f(ϕi, tij)2; and a combined-error model, Var(εij) = [a + b × f(ϕi, tij)]2. To evaluate the stability of the estimates, the run assessment feature in Monolix was used; this consists of performing the evaluation five times while changing the initial conditions and seed for the random number generators and comparing the estimates of the parameters and the log likelihood across the five runs.
The final adult PK model was called Mad, and the adult population PK parameters were called μadult. The model was evaluated through goodness-of-fit plots, including visual predictive checks (VPC), predictions of individual concentration profiles, plots of observations versus predictions, and residual scatterplots involving normalized prediction distribution errors (NPDE) (17). Empirical Bayesian estimates of the individual parameters were obtained for each subject as the conditional mean of the individual conditional distribution and used for diagnostic plots. VPC and NPDE were obtained by using 1,000 data sets simulated under the model tested with the design of the original data set (18). Estimates of the standard errors and residual standard errors were obtained through a linear approximation of the FIM. The predictive ability of Mad was evaluated by computing the bias and root mean square errors (RMSE) between predicted and observed concentrations:
| (1) |
| (2) |
where μ̂ are the estimated population parameters and
| (3) |
is the variance of the predicted concentrations.
Extrapolation from adults to children.
Mad, the PK model developed for adults, was then modified for the child population. The structural model was left unchanged, but we scaled the values of the parameters by using either allometry alone (Mallo) or both allometry and maturation (Mallo+mat) as detailed in the rest of this section.
Body size is a major determinant of metabolic rates, diffusion and transfer processes, and organ size throughout the animal kingdom and beyond. Allometric theory models these processes throughout fractal geometry and proposes a general scaling for many processes (19). Designating BW body size, the parameter μ would vary as μ = α × BWβ, where α is a constant characterizing the type of organism and β is a scaling component. In particular, volumes of distribution tend to increase linearly with size (β = 1) while clearances, which are related to blood flow, increase nonlinearly with a coefficient of 3/4 (β = 0.75) derived from geometric considerations.
The Mallo model was derived from Mad by introducing allometry into the population value of the parameters to account for size through the relationship μchild,allo = μadult × (BWchild/BWadult)β, where BWadult is the mean adult body weight, BWchild is the mean body weight of a child, and β is 0.75 for clearances and 1 for volumes.
However, size differences do not explain all of the differences between adults and children. Many physiological processes evolve slowly toward adult functionality during childhood. The Mallo+mat model was developed from the Mallo allometric model by introducing the maturation factor Kmat,child into the previous equation as follows: μchild,allo+mat = μadult × (BWchild/BWadult)β × Kmat,child.
Maturation is highly correlated with age and has been studied for many physiological processes, including absorption, first-pass effect, metabolism, and transport. We derived maturation equations for mefloquine and used them to adjust the individual clearances and volumes of each child. These equations are described in Appendix 1.
For both Mallo and Mallo+mat, we assumed the same interindividual variability for all parameters, as well as the same residual errors as those estimated for adult populations. Because we had access to pediatric data in this work, we used them as an external evaluation data set to assess the extrapolation process for both Mallo and Mallo+mat. The predictive capacity of these two models was evaluated by computing bias and RMSE on the pediatric data. We also evaluated the predictive capacity of the model without extrapolation (Mad). For comparison, we also performed a population PK analysis of the pediatric data alone by using the same approach as for the adults. This led to the Mch model.
Optimal design for a pediatric population.
Optimization of the model design was performed by using both allometry and maturation (Mallo+mat). Design optimization consists of selecting the best dose regimen and sampling times, given constraints such as the total number of samples or the times when samples can be taken, in order to allow precise estimation of the parameters. In this work, we will focus on sampling times only because the doses were fixed for children. This is generally achieved through D optimality, which consists of maximizing the determinant of the FIM (6). Although the FIM in NLME has no closed-form solution, it can be approximated by first-order linearization around the mean of the random effects. This method is implemented in PFIM, which we used here (PFIM version 4.0, running in R version 3.0) (20), and in most software for design optimization.
Because the design may be different depending on age, optimization was performed for four different age groups that were represented in the Thai study: an infant-toddler group (up to 3 years old) that included only one infant in the actual study, a preschool child group (4 to 5 years old), a school age group (6 to 11 years old), and an adolescent group (12 to 15 years old).
We therefore first performed optimization for these four different groups by using the parameters μchild,allo+mat with the average weight and age of each group in the real pediatric study. For each group, the dose was set to the average dose for the group, yielding fixed parameters for Mallo+mat for each group. We used the Fedorov-Wynn algorithm (21), which optimizes over a discrete set of times, by using the sampling times from the original pediatric protocol (0.1, 0.5, 1, 2, 5, 10, 15, 25, 35, 55, and 65 days) in the first step. We also set a constraint on the number of sampling points, performing several optimizations with three to six samples per subject. We refined this first design by running the Simplex algorithm, adjusting the set of possible times to include more informative time points and running the Fedorov-Wynn algorithm again. This led to an optimal design for each age group, from which we derived the final optimal design by choosing the closest sample times across groups.
The resulting optimal design is exact, with fixed days, which may be difficult to implement. We can relax this assumption by using sampling windows to add flexibility to its practical implementation. As this cannot be implemented prospectively in PFIM, we derived sensible windows for the optimized design by assuming that patients can come in at any time of day and for several days on later visits.
Evaluation of pediatric design.
To illustrate the expected performance and robustness of the optimal pediatric design, we evaluated its ability to estimate the PK parameters of children across a range of scenarios corresponding to different models and model parameters through a simulation study. Figure 2 summarizes the different stages of the evaluation.
We evaluated the design over the four different models previously introduced, (i) the extrapolated model with maturation (Mallo+mat), which was used to optimize the design; (ii) the adult model (Mad) without extrapolation; (iii) a model derived from Mad, called Mad,abs, with an absorption rate constant (ka) modified to a value of 1 to mimic the much slower drug absorption of children; and (iv) the PK model obtained in the analysis of the pediatric data alone (Mch).
In each scenario, we simulated L = 100 data sets under the related model for sampling times corresponding to the optimized design. The covariate distributions, doses, and number of subjects were kept identical to those in the real pediatric study. Therefore, the simulated population was identical to the pediatric population in the database. We then re-estimated model parameters by using Monolix for each simulation. Finally, we computed the relative bias and empirical relative standard errors (RSE) for each estimated parameter compared to the theoretical model value over the 100 simulations as follows:
| (4) |
| (5) |
where θ̂k(l) is the estimate of the kth parameter in simulation l = 1…L and θk,th is the theoretical value. The same simulations were also performed for the empirical design to compare the performance of the optimal design with the design that was in fact implemented in the child study. The same parameters were used to simulate the concentrations in both designs (optimal and empirical).
We also evaluated the performance of the design when relaxing the fixed times through sampling windows. We again simulated 100 data sets, but this time, the sampling times for each visit were drawn according to a uniform distribution from the sampling windows chosen. Evaluation was performed in a manner similar to that used for the optimal design.
RESULTS
Characteristics of both populations.
Table 1 shows the demographic characteristics and biological measurements in the adult (left) and pediatric (right) data sets used in the present analysis. The adult population was almost exclusively male (one woman), while the recruitment was more balanced in the pediatric study (51 girls and 60 boys, 59% males).
TABLE 1.
Summary of demographic and covariate dataa
| Parameter | Adults (n = 77) | Children (n = 101) |
|---|---|---|
| Wt (kg) | 53.2 (7.3)–52.0 [48.0, 58.0] | 24.6 (10.8)–23.0 [15.0, 35.0] |
| Age (yr) | 28.2 (8.8)–25.0 [21.0, 35.0] | 8.8 (4.2)–10.0 [5.0, 13.0] |
| Hemoglobin (g/dl) | 13.1 (2.14)–13.3 [11.7, 14.9] | 10.9 (1.9)–11.0 [9.7, 12.4] |
| ASATb (UI/liter) | 34.4 (14.1)–21.0 [25.0, 41.0] | 34.9 (38.6)–22.0 [18.0, 29.0] |
| ALATc (UI/liter) | 26.2 (17.1)–21.0 [15.0, 31.0] | 17.3 (27.0)–8.0 [6.0, 12.8] |
The values are the means of the variables, with standard deviation in parentheses, followed by the median and the interquartile interval ([Q1, Q3]).
ASAT, aspartate aminotransferase.
ALAT, alanine aminotransferase.
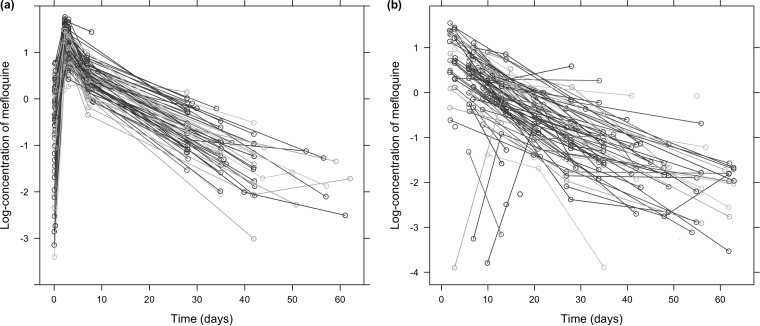
Figure 3 shows the evolution of mefloquine concentrations with time in the two populations. Most adults were sampled four or five times during the study. On average, the first sample was taken 4 h after the first dose and the next was taken on day 2, 3, 11, 36, or 56, with a few concentrations measured up to 62 days after the first dose. Four patients had only one sample taken. Concentration profiles show accumulation over the first 3 days, when mefloquine is administered once daily, followed by a slow biphasic decline.
FIG 3.
Concentrations of mefloquine in blood (in milligrams per liter) shown on a log scale. Panels: a, adults; b, children.
In children, the design was more sparse and variable (Fig. 3b) and fewer samples were collected. Most (51%) of the children contributed three concentrations, and 37% had only two concentrations taken. The first sample was usually taken on day 8, long after the end of the absorption phase. The second sample was taken around day 23, and then further samples were taken on days 35 and 45.
Modeling of the PK of mefloquine in adults.
The final PK model was found to be a two-compartment model with first-order absorption because of significant tissular distribution. Absorption and elimination were found to be linear. The parameters in this model are the rate of absorption, the central and intercompartmental clearances, and the volumes of the two compartments, so that ϕi = (kai, CLi, V1i, Qi, V2i). The residual error was best described as a combined-error model. We found that we could remove the variability in V2 from the model. This may be due to either low interindividual variability in that parameter or, more likely, a lack of information to estimate that parameter.
Table 2 shows the population parameters estimated for the adult model (Mad). The residual variability was low, indicating that the model explained most of the variability. Values were well estimated, with small standard errors. Absorption (ka) and intercompartmental clearance (Q) showed the highest interindividual variability.
TABLE 2.
Estimates of the parameters in the Mad modela
| Parameter | Population value (% RSE) | % Variability (% RSE) |
|---|---|---|
| ka (day−1) | 4.2 (12) | 81 (12) |
| CL (liters/day) | 26.0 (5) | 34 (11) |
| V1 (liters) | 248.0 (5) | 25 (17) |
| Q (liters/day) | 41.6 (15) | 70 (18) |
| V2 (liters) | 282.0 (7) | |
| a | 0.07 (24) | |
| b | 0.14 (11) |
The first column shows the value of the fixed effect, while the second column shows the variabilities expressed as percentages. The RSE of estimation is shown in parentheses.
There was no bias in predicting the adult concentrations (bias = 0.06), showing no systematic model misspecification, and the RMSE was estimated to be 1.14.
Extrapolation from adults to children.
Mad was then used as a basis for individual extrapolation to the pediatric population, yielding the Mallo+mat model.
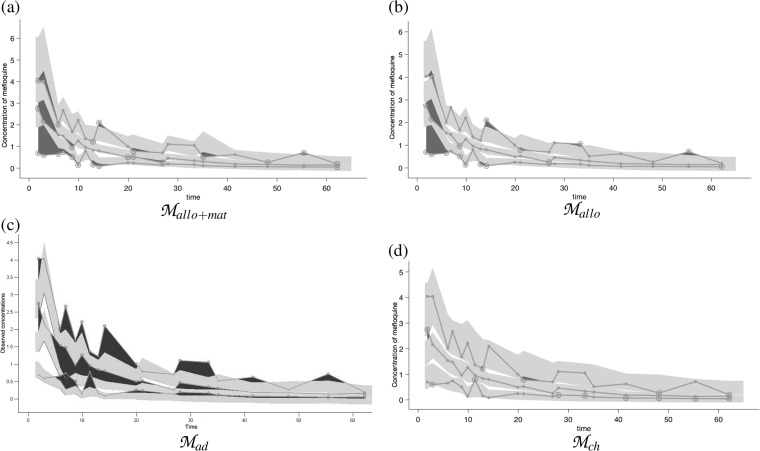
Extrapolation was assessed by using the pediatric data as an external evaluation data set on the Mallo+mat, Mallo, Mad, and Mch models. VPC are shown in Fig. 4. Mallo+mat (Fig. 4a) clearly overpredicts the observed concentrations in children during the first days of the trial, suggesting some discrepancy in absorption between the adult and child populations in the rate of absorption, in bioavailability, or in both. On the other hand, the elimination and distribution phases are not inconsistent with the prediction ranges, and the variability, shown by the breadth of the shaded areas, appears to be similar in children and adults.
FIG 4.
VPC for extrapolation models based on pediatric data. The 95% confidence interval for the median of the model corresponds to the middle shaded area, other shaded areas correspond to the 95% prediction bands of the upper and lower limits of the 80% predictive interval, and the dark areas characterize outlier data points. Panels: a, extrapolation from the Mallo+mat adult model with allometry and maturation; b, extrapolation from the Mallo adult model with allometry; c, extrapolation from the Mad adult model; d, extrapolation from the Mch model constructed from the child database.
To assess the impact of the different extrapolations involved in Mallo+mat, we compared the predictive abilities of the other models. The Mch model was obtained by using a PK analysis similar to that used for adults and constitutes the best possible fit to the data. In our analysis, it served as the gold standard for assessment of the accuracy of model predictions, as it was the only model derived directly from the pediatric data. In children, we could not identify a distribution phase; therefore, the Mch model was a one-compartment model. The absorption phase was unidentifiable, and the estimates of ka were unstable. Therefore, the absorption rate constant (ka) was fixed to the value obtained from the adult population without interindividual variability. As expected, there was no bias for Mch (0.06); the precision measured by RMSE was 0.89. The bias was significant for the other three models; the model with allometry (Mallo), in fact, has a slightly lower bias (−0.15) than the model with maturation (Mallo+mat) (−0.27). Both of these models tended to underpredict child drug concentrations, while the adult model (Mad) systematically overpredicted drug concentrations in children (bias = 0.34), as shown in Fig. 4. The RMSE for the two extrapolated models was quite high (1.2 and 1.1 with and without maturation, respectively). It was lower for Mad (0.8) than for Mch (0.9).
Optimal design for the pediatric population.
Mallo+mat was then used to design a sampling schedule for the pediatric population. We first attempted to optimize designs with three or four sampling times, as this was close to the design in the pediatric database, which we call an empirical design. But optimization failed, indicating that the model was not identifiable with so few samples. We therefore increased the number of samples to five or six. Table 3 shows the optimal times found for each group for designs with five sampling points; several sampling times were found to be quite similar across the designs, with three samples in the first 4 days and two after 65 days. The parameters were well estimated in each group, according to the RSE predicted by PFIM, with RSE of around 5% for CL, V1, and V2 and around 10% for ka and Q. Intersubject random effects should have somewhat higher RSE, between 20 and 30%, but the designs would still allow proper estimation of the variabilities. Designs with six sampling times gave similar results in terms of RSE, suggesting that the use of five sampling times was sufficient in our case.
TABLE 3.
Optimal sampling times for each age group and for the optimal design across groups
| Groupa | Age (yr) | Doseb (ml/day) | Optimized times (days) |
|---|---|---|---|
| Infant-toddler | <3 | 87 | 0.1, 0.9, 4.5, 12, 57 |
| Preschool age | 4–5 | 113 | 0.1, 0.9, 4.5, 13, 55 |
| School age | 5–11 | 178 | 0.1, 2, 5, 14, 57 |
| Adolescent | 12–15 | 342 | 0.2, 2, 6, 16, 66 |
| Overall (optimal design) | 0.1, 1, 5, 14, 57 |
The four age groups correspond to an infant-toddler group including only one infant (13%), a group of preschool children (17%), a school age group (37%), and an adolescent group (33%).
The dose is the average quantity of mefloquine given per day.
The optimal design merged the four designs, and the corresponding times are shown in the last row of Table 3.
Design evaluation.
In order to assess robustness, we performed a set of simulations under different model assumptions. Table 4 summarizes the results of the evaluation for each combination of model (rows) and design (columns). For each model, we recall the values of the parameters used in the simulation, and for each design, we show the relative bias and the empirical RSE, expressed in percentages. Simulated patients had the same covariate distribution as in the real study. For the data sets simulated with the optimal design, parameter estimation was successful for all 100 data sets. The design in the pediatric database, or the empirical design, on the other hand, generated a few simulations for which we were unable to estimate all of the standard errors, mostly for absorption, intercompartmental clearance, and the respective random effects. Because only the estimated values, not their RSE, were used to compute the relative bias and empirical RSE, all of the values in Table 4 were computed over all of the corresponding runs. As shown in Table 4, there was no bias in the parameter estimates when the data were simulated according to the optimal design, regardless of the actual model. For the first model (Mallo+mat), this only shows that the estimation algorithm provides unbiased estimates, as expected. For the other models, it reflects the fact that there is enough information in this design to estimate the parameters under different model misspecifications. The empirical RSE were also in line with predictions from PFIM, ranging from 3 to 15% for the Mallo+mat model, which was used to establish the optimal design. More interestingly, parameter precision was also similar for the other models, showing that the optimal design allows unbiased and precise estimates to be obtained over a range of model changes.
TABLE 4.
Validation of optimal design on different models
| Modela and parameter | Value | Optimal design |
Empirical design |
||
|---|---|---|---|---|---|
| Relative bias (%) | Empirical RSE (%) | Relative bias (%) | Empirical RSE (%) | ||
| Mallo+mat | |||||
| ka (day−1) | 4.16 | −1.29 | 7.90 | 469.43 | 486.60 |
| CL (liters/day) | 26.00 | 0.58 | 2.67 | −0.73 | 3.72 |
| V1 (liters) | 248.00 | −2.33 | 4.39 | −6.85 | 10.82 |
| Q (liters/day) | 41.60 | 4.21 | 9.86 | 6.56 | 21.78 |
| V2 (liters) | 282.00 | 2.30 | 4.98 | 0.91 | 7.13 |
| ωka | 0.81 | −2.22 | 8.10 | 16.11 | 34.97 |
| ωCL | 0.34 | −0.31 | 5.66 | −2.37 | 8.11 |
| ωV1 | 0.25 | −1.71 | 11.45 | 18.02 | 29.94 |
| ωQ | 0.70 | −0.03 | 15.37 | −1.24 | 20.71 |
| a (mg/kg) | 0.07 | −1.32 | 7.47 | 1.16 | 11.16 |
| b | 0.14 | −2.07 | 9.48 | −8.63 | 14.01 |
| Mad | |||||
| ka (day−1) | 4.16 | −2.75 | 8.33 | 219.15 | 240.32 |
| CL (liters/day) | 26.00 | −0.52 | 3.73 | −1.69 | 3.98 |
| V1 (liters) | 248.00 | −1.46 | 4.08 | −11.27 | 13.39 |
| Q (liters/day) | 41.60 | 5.54 | 14.08 | 22.60 | 31.75 |
| V2 (liters) | 282.00 | 2.78 | 5.34 | 5.79 | 9.30 |
| ωka | 0.81 | −2.61 | 8.38 | 15.17 | 33.64 |
| ωCL | 0.34 | −1.12 | 7.89 | −2.43 | 8.93 |
| ωV1 | 0.25 | 0.59 | 14.18 | 14.58 | 30.73 |
| ωQ | 0.70 | 3.74 | 17.12 | 5.95 | 23.87 |
| a (mg/kg) | 0.07 | −1.73 | 6.10 | 0.14 | 7.38 |
| b | 0.14 | −4.15 | 12.62 | −15.82 | 23.08 |
| Mad,abs | |||||
| ka (day−1) | 1.00 | −1.67 | 12.11 | 319.11 | 337.19 |
| CL (liters/day) | 26.00 | −0.28 | 3.58 | −1.60 | 4.15 |
| V1 (liters) | 248.00 | −2.35 | 8.70 | −3.54 | 14.92 |
| Q (liters/day) | 41.60 | 2.45 | 15.95 | 40.62 | 53.55 |
| V2 (liters) | 282.00 | 3.03 | 7.08 | 2.44 | 11.33 |
| ωka | 0.81 | −2.93 | 9.09 | 1.63 | 32.17 |
| ωCL | 0.34 | 0.17 | 8.68 | −1.65 | 10.18 |
| ωV1 | 0.25 | 4.68 | 19.47 | 31.07 | 39.23 |
| ωQ | 0.70 | 0.72 | 21.54 | 30.07 | 42.85 |
| a (mg/kg) | 0.07 | −0.53 | 4.55 | −0.88 | 7.99 |
| b | 0.14 | −8.68 | 15.15 | −13.45 | 26.38 |
| Mch | |||||
| ka (day−1) | 4.16 | 3.77 | 10.23 | 13.51 | 50.28 |
| CL (liters/day) | 14.30 | 1.82 | 5.54 | 1.92 | 7.32 |
| V (liters) | 263.00 | 0.64 | 5.43 | −0.62 | 7.81 |
| ωka | 0.81 | −1.74 | 14.36 | 52.26 | 53.87 |
| ωCL | 0.63 | −2.33 | 8.69 | −0.41 | 8.80 |
| ωV | 0.66 | 0.18 | 6.93 | −4.48 | 10.43 |
| a (mg/kg) | 0.08 | −0.84 | 7.66 | 3.05 | 11.80 |
| b | 0.35 | −0.04 | 5.32 | −4.18 | 9.98 |
The models are Mallo+mat based the adult model Mad with allometry and maturation; Mad, the adult model; Mad,abs, the adult model with different absorption; and Mch, the model built from the child data. Relative bias and empirical RSE are expressed in percentages.
We can contrast this behavior with the performance of the empirical design. Across all four models, we found that this design had relatively high bias for ka, its variability (ωka), or both, even when the true model was the much simpler one-compartment model that was estimated to best describe the real data collected from children. In addition, this design was less robust when the model assumptions were changed, as other parameters, such as ωQ and ωV1, proved difficult to estimate, yielding very large and implausible values or very large RSE.
Although the optimal design gives good results, actually respecting the exact sampling times may be difficult to implement in practice. We therefore also evaluated a design with the following sampling windows, which relaxes the exact optimized design. The first sample was taken between 1 and 5 h after the first dose, and the second was taken between 1 h before and 12 h after the second dose. For the third to fifth sampling times, we allowed for 12-h sampling windows over several days, as the concentrations changed more slowly over this period; the third time was assumed to be in daytime during day 4 or 5, the fourth during days 13 to 16, and the final sampling window was from day 55 to day 60. The evaluation of this design over 100 simulated data sets gave similar results for every model compared to the optimal design, in terms of empirical RSE and relative bias. Full numerical results for simulations of the sampling window design are shown in Appendix 2 and Table 5.
TABLE 5.
Evaluation of the design with sampling windows derived from the optimized design
| Modela and parameter | Value | Optimal design |
|
|---|---|---|---|
| Relative bias (%) | Empirical RSE (%) | ||
| Mallo+mat | |||
| ka (day−1) | 4.16 | −1.23 | 9.12 |
| CL (liters/day) | 26.00 | −0.39 | 3.08 |
| V1 (liters) | 248.00 | −1.61 | 3.93 |
| Q (liters/day) | 41.60 | 4.28 | 11.19 |
| V2 (liters) | 282.00 | 1.28 | 4.12 |
| ωka | 0.81 | 0.51 | 7.81 |
| ωCL | 0.34 | −0.08 | 6.66 |
| ωV1 | 0.25 | −1.79 | 10.39 |
| ωQ | 0.70 | −0.71 | 14.87 |
| a (mg/kg) | 0.07 | −2.45 | 8.81 |
| b | 0.14 | −2.08 | 7.75 |
| Mad | |||
| ka (day−1) | 4.16 | −3.01 | 9.26 |
| CL (liters/day) | 26.00 | 0.67 | 3.57 |
| V1 (liters) | 248.00 | −1.34 | 4.37 |
| Q (liters/day) | 41.60 | 2.27 | 12.50 |
| V2 (liters) | 282.00 | 1.36 | 5.62 |
| ωka | 0.81 | −2.58 | 7.25 |
| ωCL | 0.34 | −0.48 | 7.12 |
| ωV1 | 0.25 | 0.09 | 15.32 |
| ωQ | 0.70 | 0.69 | 17.64 |
| a (mg/kg) | 0.07 | −1.94 | 6.30 |
| b | 0.14 | −3.05 | 10.99 |
| Mad,abs | |||
| ka (day−1) | 1.00 | −0.72 | 11.57 |
| CL (liters/day) | 26.00 | −0.48 | 3.78 |
| V1 (liters) | 248.00 | −1.28 | 7.92 |
| Q (liters/day) | 41.60 | 1.21 | 16.88 |
| V2 (liters) | 282.00 | 2.71 | 7.95 |
| ωka | 0.81 | −1.17 | 8.01 |
| ωCL | 0.34 | 0.36 | 8.19 |
| ωV1 | 0.25 | 1.69 | 20.19 |
| ωQ | 0.70 | −0.15 | 21.64 |
| a (mg/kg) | 0.07 | −0.83 | 4.88 |
| b | 0.14 | −5.86 | 13.53 |
| Mch | |||
| ka (day−1) | 4.16 | 0.48 | 9.45 |
| CL (liters/day) | 14.30 | 0.53 | 5.60 |
| V (liters) | 263.00 | 1.43 | 5.15 |
| ωka | 0.81 | −0.29 | 12.86 |
| ωCL | 0.63 | −0.91 | 6.75 |
| ωV | 0.66 | −1.13 | 6.98 |
| a (mg/kg) | 0.08 | −0.84 | 8.31 |
| b | 0.35 | −0.10 | 5.10 |
The models are Mallo+mat based the adult model Mad with allometry and maturation; Mad, the adult model; Mad,abs, the adult model with different absorption; and Mch, the model built from the child data.
DISCUSSION
The objective of the present work was to design a PK pediatric study by using adult malaria information. To this end, we investigated the impact of design on the information gained from the child study, exploring models taking into account prior adult information through extrapolation by allometry and maturation. We used the pediatric data both as an external evaluation data set and to suggest alternative models to test the robustness of both the empirical design actually performed with children and the optimized design. We assessed their performance with regard to changes in parameter assumptions.
In the adult PK analysis, a two-compartment model was found to best describe the PK of mefloquine. In previous studies (22, 23, 24, 25), both one- and two-compartment models have been used to describe its PK. However, a more appropriate sampling schedule shows evidence of tissular distribution (26, 27) both in patients (28) and in a large population of healthy military personnel administered mefloquine for malaria prophylaxis (29). The parameter estimates we obtained in the present analysis were consistent with the estimates from these two studies. In particular, we found slow elimination of mefloquine, with a terminal half-life of 17 days, in line with previous estimates of 14 to 16 days.
In our study, we derived the PK parameters of children from the parameters of adults by using simple methods combining allometry and maturation functions. Allometric scaling to predict structural and functional properties of vertebrate cardiovascular and respiratory systems was formally introduced by West et al. in 1997 (19). As the etymology underlines, the purpose of allometry was initially to find measurements working across and within species. The allometric coefficients (e.g., 0.75 for clearances and 1 for volumes [19]) have been estimated in human populations and found to be compatible with the theory (30). Allometric coefficients can also be estimated in specific PK studies, although conclusive evidence that they differ from the theoretical values is questionable and may, in fact, reflect model misspecification. On the other hand, there is mounting evidence that allometric relationships may need to be adjusted in early childhood. For example, Peeters et al. found differences in clearance exponents in a study including 98 subjects ranging from neonates to adults and suggested the use of an exponent varying with weight (3). This discrepancy between size-based scaling and effective changes in model parameters in neonates and very young children can be partially explained by additional maturational changes in physiological processes that occur during this period. Maturation functions have been proposed for several drugs (31, 32), and we adapted them to the characteristics of mefloquine, such as binding properties and first-pass metabolism. A similar approach was used by Anderson and Holford in several studies (30, 33, 34, 35). In particular, their work on paracetamol involved different physiological processes such as renal and hepatic clearance (13). In the present work, we applied their methods with formulae specific to mefloquine by considering the maturation of the cytochromes and of albumin concentrations.
The extrapolated models were evaluated by using the data collected in the pediatric study as an external evaluation data set to assess how well the child data could be predicted by considering only information about adults. The results were not particularly good, as the model was found to systematically underpredict the early drug concentrations in children. Using the adult parameters directly was, of course, also not appropriate, as not taking into account the body size factor led to systematic overprediction. Compared to the impact of allometry, the contribution of maturational changes here was small and even slightly increased the prediction bias. This may be due to the fact that the major impact of maturation for mefloquine occurs in neonates and infants, and our population included only six very young children (less than 2 years old).
Other methods could be used to extrapolate from adults to children. A physiological approach, describing the intricacies of biological processes is provided by the physiologically based PK (PBPK) models. The model equations rely on principles of mass transport and fluid dynamics and require knowledge of the exact drug process. Although very rich, the PBPK models often contain a large number of unknown parameters, the determination of which requires many specific studies. PBPK models have not yet been established for mefloquine. Knibbe et al. (36) proposed an alternative model combining both PBPK models and maturation with the development of semiphysiological functions for specific processes. They applied this method to the glomerular filtration rate in a study of gentamicin, tobramycin, and vancomycin including 1,760 patients ranging from preterm infants to adults. The present work could benefit from such an approach, using biological system-specific rather than drug-specific information. Approaching a physiological process such as maturation of cytochrome, in particular, CYP3A, in childhood would give more precise results. However, it would require more covariates, which were not available in our pediatric study.
Despite the lackluster performance of the maturation model in terms of predictive ability, in the present work, we used the full extrapolated model, including both maturation and allometry, to produce the optimal design. We wanted to reproduce the actual clinical process, where the child data would not be available to assess which model performs best, and to take into account all of the prior knowledge about the drug. The recommended design, blending the four age group-specific optimal designs, performed very well in our simulations, yielding low RSE for all parameters, confirming that the blended recommended design is appropriate for the entire pediatric data set. Even in this complex study with a distribution of ages and weights, PFIM predicted the range of standard errors found in the simulation study quite well. Optimization of the design of a clinical trial of mefloquine has already been addressed in adults (24, 37), and our results here are in agreement with these previous studies. In particular, Jamsen et al. (24) considered optimal designs for various combinations of mefloquine and another malaria drug but for a mixed population including adults, pregnant women, and children. The optimal designs consisted of two groups of subjects with five samples each, including an early sample (2 or 3 h after dosing), samples taken on days 2 and 7, and two additional samples taken at times that differed between the two groups. In our own work, we focused only on the pediatric population, but the results over the different age groups in the study, including adolescents, suggested that there is not much difference in the sampling schedule recommended over a large span of ages. Indeed, the similar RSE found in that study (24) suggest that their design would also be quite robust.
We assessed the performance of the optimal design in a simulation study including four different sets of model assumptions designed to test model departures from the predicted PK in children. Of course, we cannot expect a design to perform well when the PK change completely, but the range of scenarios we simulated reflected changes that could be expected when moving from adults to children. Overall, the optimal design performed much better than the empirical design from the real pediatric study in all scenarios. With the empirical design, absorption parameters were always poorly estimated because of the lack of early time points, and this seemed to have an impact also on the distribution parameters. If we were then performing a real analysis of the pediatric data, we would need to simplify the model, to fix some parameters to the adult value, or to perform a joint analysis of adult and child data together, risking biased estimates if the populations are, in fact, different. Here, in the analysis of the pediatric data alone, we had to use a simplified one-compartment model with fixed absorption (Mch), illustrating the choices that poor designs will lead to.
In this particular case, the empirical design also reflected logistic and practical constraints. Indeed, most children did not have as many measurements as originally planned per protocol, which specified that three or four samples were supposed to be randomly collected during the first 3 days and during the second week, with an additional one or two samples taken on different days between the 21st and 63rd days. In the empirical design, most patients only had three samples and the first sample was usually taken after 5 days, yielding no information about the absorption phase. Because mefloquine has a long half-life, late follow-up requires additional visits to the treatment centers, which may not be convenient or cheap enough for the families to afford. However, samples from these late time points are crucial for good estimation of the distribution and terminal phases.
A few studies of the PK of mefloquine included children (22), but there has been no specific pediatric study of mefloquine with an informative design. Here, when we analyzed the pediatric data separately, we could not identify a two-compartment model. But the poor performance of the empirical design in the simulations also suggested that a more informative design could have been obtained if the available adult information had been taken into account, even if the pediatric PK differed substantially from the adult PK.
In order to get around some of the logistic and practical constraints of a fixed design, a solution is to propose time windows around the sampling times found for the optimal design. In the present study, we evaluated a relaxed design with the same simulation setting as for the optimal and empirical designs and found similar performances. The windows were chosen empirically, with sensible assumptions, and a similar approach could be implemented in practice with the physicians of the trial, who are generally aware of the logistic constraints they need to respect. Evaluating relaxed designs through simulations as we did in the present study is possible for a limited number of designs, but this approach can also be implemented prospectively. Sampling windows can be specified for instance in the PopED software, which could be used instead of PFIM to further develop the method presented (38). Here, however, we obtained good results with sensible sampling windows derived from the optimal design.
An interesting finding of our work is the message that the design need not be perfect, as long as it is robust enough. As is always the case in optimal design, the model we are trying to estimate is unknown prior to performing the study but needs to be specified to design that study, and the design will be appropriate only if the model is correct. A way to enhance robustness is to ensure that the design performs well across different model and parameter assumptions. Here, we show how a cycle of simulation-evaluation can be integrated into the decision-making process to safeguard against reasonable departures from candidate model assumptions, by comparing the performances of the optimized design for different models. In the case of mefloquine, the optimized design performed well both for the extrapolated model (Mallo+mat) and for the real model derived from child data (Mch). Here, we used D optimality, which relies on prior knowledge of the parameters, but we could enhance robustness through ED optimality, which allows the incorporation of uncertainty in the prior parameter specifications (39). These methods could be investigated in order to obtain more robust designs for pediatrics studies, where parameters are usually unknown and the interindividual variability is very high.
In our study, we used data from an adult population and extrapolated the estimated parameters to children through allometric and maturation considerations. A similar method could be applied to estimates obtained from the literature. Another interesting approach in this context is adaptive design, where the initial design is refined through one or several intermediate analyses. Dumont et al. (7) applied optimal two-stage designs in a pediatric context and showed that such designs can correct initial model misspecifications. In their work, the prior information about children was obtained by extrapolating a PBPK model developed with adults to a child population and performing a population PK analysis of simulated data from a virtual pediatric population, an alternative to extrapolation models.
In the present study, we use repeated optimization and simulation to evaluate the optimized and alternative designs before implementation, chalking them across different model assumptions. The framework presented in Fig. 2 can therefore be implemented in the clinical development process as a way of qualifying prospective designs to gauge the probability of success of a future trial, as well as convey to clinical teams the importance of implementing the designs in a rigorous way. Because logistic constraints can be elicited prior to the study to be taken into account both at the design stage and at the implementation stage, it is a powerful way of ensuring that the constraints are well accepted and that the design is applicable in practice.
In conclusion, the present work supports the use of information about adults for design optimization in pediatrics. Optimal design methodology combined with allometry and maturation allowed the determination of sampling schedules appropriate for children. The optimal design was more robust and provided better estimates of PK parameters for pediatrics, taking into account age specificities.
APPENDIX 1.
MATURATION AND ALLOMETRY.
Mechanisms of absorption, distribution and elimination of mefloquine during treatment involve different physiological processes. Mefloquine is well absorbed, with an estimated bioavailability of around 85% (40), but little is known about the exact mechanism of its absorption. Molecules of mefloquine bind strongly to albumin (98% in adults), resulting in slow diffusion. Unbound molecules of mefloquine are metabolized by cytochrome CYP3A4. Afterward, mefloquine is eliminated through renal clearance.
These processes are slightly modified for children because of ongoing maturation. Indeed, in parallel with the size differences from adults that warrant a first adjustment, metabolism functions are not fully developed until a certain age. Therefore, drug metabolism has a distinct evolution, which is characterized by differences in PK parameter values. Analysis of metabolism processes makes it possible to identify those that induce a difference from adult values and to adjust PK parameters with a maturation factor.
During absorption, bioavailability is the first process susceptible to maturation. The bioavailability of mefloquine, as a substrate of CYP3A, will decrease with the available quantity of CYP3A during intestinal and hepatic first-pass effects. Each first pass is characterized by its own extraction coefficient, Egut for intestinal and Ehepa for hepatic. Consequently, the overall bioavailability (F) represents the amount of mefloquine that, once absorbed, is not metabolized during intestinal and hepatic first passes and reaches the circulatory system. Adult bioavailability is defined as follows: Fad = (1 − Egut)(1 − Ehepa). However, in children, both processes are modulated by the quantity of CYP3A. Indeed, depending on age, CYP3A is not produced in the same amount in children as in adults. Gut and hepatic CYP3A abundances are characterized by their own maturation function (32). Designating KCYP3A the maturation of CYP3A and KCYP3A4/5 the maturation of CYP3A4/5, the bioavailability for children can be written as follows: Fch = (1 − EgutKCYP3A)(1 − EhepaKCYP3A4/5).
With oral drugs, bioavailability is a key value in the estimation of PK parameters, which are estimated as apparent, that is, relative to bioavailability. Therefore, it has an impact on all clearance and volume parameters. Let CLad be apparent adult clearance related to real clearance CLad,real through CLad = CLad,real/Fad, where Fad is adult bioavailability. Likewise, we express apparent clearance from children as follows: CLch = CLch,real/Fch. As for volume, we have Vad = Vad,real/Fad with Vad the apparent volume and Vad,real the real volume. Likewise, for children, we have Vch = Vch,real/Fch.
In the bloodstream, mefloquine binds strongly to albumin, leaving only a small fraction of mefloquine unbound. Let fu,ch be this fraction in children. While bound to albumin, mefloquine cannot be eliminated from the bloodstream and only the unbound fraction can be eliminated. Let CLch,u be the clearance of the unbound fraction of mefloquine in the blood. Therefore, we have CLch,real = CLch,u × fu,ch, leading to CLch = (fu,chCLch,u)/Fch.
In adults, 98% of mefloquine is bound to albumin, such that the adult unbound fraction (fu,ad) is 0.02. In children, the fraction of unbound mefloquine can be related to the adult unbound fraction of mefloquine (fu,ad) and to the albumin concentration, which varies from Cad (40 g/liter, on average) and the corresponding value in children, Cch, respectively (32). The following relationship links the unbound fraction of mefloquine in children to the albumin concentration: fu,ch = 1/[1 + ({1 − fu,ad}/fu,ad)(Cch/Cad)].
Moreover, the albumin concentration in children can be expressed as a function of age (32) as follows: Cch = 1.1287 ln(age) + 33.746.
Therefore, we have Cch = CLch,u/{Fch[1.383 ln(age) + 42.339]}.
Unbound mefloquine is metabolized by CYP3A4/5. Again, the quantity of CYP3A4/5 influences the extent of metabolism and its lower value in children needs to be taken into account. Moreover, clearance is also related to weight and an allometric factor needs to be introduced. Therefore, clearance of the unbound fraction of mefloquine from children is related to the adult value (CLad) according to the following equation: CLch,u = CLad,u × KCYP3A4/5 × (W/70)0.75.
As previously stated, we deduce that clearance of the unbound fraction by adults is CLad,real/0.02 = CLad × Fad/0.02. Therefore, CLch = CLad/{0.02[1.383 ln(age) + 42.339]} × (Fad/Fch) × KCYP3A4/5 × (W/70)0.75, with Fad/Fch = [(1 − Egut)(1 − Ehepa)]/[(1 − EgutKCYP3A4)(1 − EhepaKCYP3A4/5)].
As mefloquine extraction coefficients are unknown, we arbitrary chose Egut = Ehepa = 0.05.
We then need to evaluate the maturation of the cytochrome. Its maturation has been characterized by Johnson et al. (32) as follows: KCYP3A4/5 = age0.83/(0.31 + age0.83) and KCYP3A4 = 0.42 + [(0.639 × age)/(2.35 + age)].
Contrary to clearance, no maturation process interferes with the volume in the blood. However, as previously stated, estimated volumes are apparent volumes. Therefore, adjustment with bioavailability is appropriate. Although there is no maturation, size adjustment is still warranted and we have Vch,real = Vad,real × (W/70). Therefore, Vch = Vad × (Fad/Fch) × (W/70), where Fad/Fch is determined as described above.
APPENDIX 2.
EVALUATION OF SAMPLING WINDOW DESIGN.
Table A5 presents the results of the evaluation of the design with sampling windows that were derived empirically from the optimized design. It shows the same evaluation metrics presented in the text for the optimized and empirical designs.
ACKNOWLEDGMENTS
Caroline Petit was supported during this work by a grant IDEX from the Université Sorbonne Paris Cité (2013, project 24). Sarah Zohar and Emmanuelle Comets were funded by the InSPiRe (Innovative Methodology for Small Populations Research) Project of the European Union Seventh Framework Programme for Research, Technological Development, and Demonstration under grant agreement FP HEALTH 2013-602144.
We thank the Drugs for Neglected Diseases Initiative for making its data sets available for this project.
Funding Statement
IDEX Paris Cité Sorbonne provided funding for this project under grant number 24. InSPiRe provided funding to Sarah Zohar and Emmanuelle Comets under grant agreement FP HEALTH 2013-602144.
REFERENCES
- 1.Roberts R, Rodriguez W, Murphy D, Crescenzi T. 2003. Pediatric drug labeling: improving the safety and efficacy of pediatric therapies. JAMA 290:905–911. doi: 10.1001/jama.290.7.905. [DOI] [PubMed] [Google Scholar]
- 2.Anderson BJ, Woollard GA, Holford NH. 2000. A model for size and age changes in the pharmacokinetics of paracetamol in neoates, infants and children. Br J Clin Pharmacol 50:125–134. doi: 10.1046/j.1365-2125.2000.00231.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Peeters MYM, Allegaert K, Blussé van Oud-Albas HJ, Cella M, Tibboel D, Danhof M, Knibbe CAJ. 2010. Prediction of propofol clearance in children from an allometric model developed in rats, children and adults versus a 0.75 fixed-exponent allometric model. Clin Pharmacokinet 49:269–275. doi: 10.2165/11319350-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 4.European Medicines Agency (EMA). 2012. Policy on the determination of the condition(s) for a paediatric investigation plan/waiver (scope of the PIP/waiver). European Medicines Agency, London, United Kingdom: http://www.ema.europa.eu/docs/en_GB/document_library/Other/2012/09/WC500133065.pdf. [Google Scholar]
- 5.Institute of Medicine (US) Forum on Drug Discovery Development and Translation. 2008. Addressing the barriers to pediatric drug development. Workshop summary. National Academies Press, Washington, DC. [PubMed] [Google Scholar]
- 6.Mentré F, Baccar D, Mallet A. 1997. Optimal design in random-effects regression models. Biometrika 84:429–442. doi: 10.1093/biomet/84.2.429. [DOI] [Google Scholar]
- 7.Dumont C, Chenel M, Mentré F. 2014. Two-stage adaptive designs in nonlinear mixed effects models: application to pharmacokinetics in children. Commun Stat Simul Comput doi: 10.1080/03610918.2014.930901. [DOI] [Google Scholar]
- 8.Food and Drug Administration. 2010. Clinical path initiative—reports on projects receiving critical path support. Food and Drug Administration, Silver Spring, MD: http://www.fda.gov/downloads/ScienceResearch/SpecialTopics/CriticalPathInitiative/UCM249262.pdf. [Google Scholar]
- 9.Ette E, Williams PJ. 2007. Pharmacometrics: the science of quantitative pharmacology. Wiley-Interscience, Hoboken, NJ. [Google Scholar]
- 10.Mentré F, Dubruc C, Thénot JP. 2001. Population pharmacokinetic analysis and optimization of the experimental design for mizolastine solution in children. J Pharmacokinet Pharmacodyn 28:299–319. doi: 10.1023/A:1011583210549. [DOI] [PubMed] [Google Scholar]
- 11.Mentré F, Chenel M, Comets E, Grevel J, Hooker A, Karlsson MO, Lavielle M, Gueorguieva I. 2013. Current use and developments needed for optimal design in pharmacometrics: a study performed among DDMoRe's European Federation of Pharmaceutical Industries and Associations members. CPT Pharmacometrics Syst Pharmacol 2:e46. doi: 10.1038/psp.2013.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jullien V, Valecha N, Srivastava B, Sharma B, Kiechel J-R. 2014. Population pharmacokinetics of mefloquine, administered as a fixed-dose combination of artesunate-mefloquine in Indian patients for the treatment of acute uncomplicated Plasmodium falciparum malaria. Malar J 13:187. doi: 10.1186/1475-2875-13-187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Anderson BJ, Holford NHG. 2009. Mechanistic basis of using body size and maturation to predict clearance in humans. Drug Metab Pharmacokinet 24:25–36. doi: 10.2133/dmpk.24.25. [DOI] [PubMed] [Google Scholar]
- 14.Taylor W. 2008. A single dose two-phase crossover study to assess the tolerability and pharmacokinetic parameters of a fixed dose formulation of artesunate-mefloquine and standard dose artesunate and mefloquine as loose tablets in healthy normal volunteers (Thailand). BioMed Central, London, United Kingdom: http://www.isrctn.com/ISRCTN22508774. [Google Scholar]
- 15.Kuhn E, Lavielle M. 2005. Maximum likelihood estimation in nonlinear mixed effects models. Comput Statist Data Anal 49:1020–1038. doi: 10.1016/j.csda.2004.07.002. [DOI] [Google Scholar]
- 16.Lixoft. 2013. Monolix methodology, version 4.2.2. Lixoft, Antony, France. [Google Scholar]
- 17.Brendel K, Comets E, Laffont C, Laveille C, Mentré F. 2006. Metrics for external model evaluation with an application to the population pharmacokinetics of gliclazide. Pharm Res 23:2036–2049. doi: 10.1007/s11095-006-9067-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bergstrand M, Hooker AC, Wallin JE, Karlsson MO. 2011. Prediction-corrected visual predictive checks for diagnosing nonlinear mixed-effects models. AAPS J 13:143–151. doi: 10.1208/s12248-011-9255-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.West GB, Brown JH, Enquist BJ. 1997. A general model for the origin of allometric scaling laws in biology. Science 276:122–126, April. doi: 10.1126/science.276.5309.122. [DOI] [PubMed] [Google Scholar]
- 20.PFIM Group. 2014. PFIM user guide. PFIM Group, Paris, France: http://www.pfim.biostat.fr/download/PFIM4.0_UserGuide.pdf. [Google Scholar]
- 21.Retout S, Comets E, Samson A, Mentré F. 2007. Design in nonlinear mixed effects models: optimization using the Fedorov-Wynn algorithm and power of the Wald test for binary covariates. Stat Med 26:5162–5179. doi: 10.1002/sim.2910. [DOI] [PubMed] [Google Scholar]
- 22.Staehli Hodel E, Guidi M, Zanolari B, Mercier T, Duong S, Kabanywanyi A, Ariey F, Buclin T, Beck H-P, Decosterd L, Olliaro P, Genton B, Csajka C. 2013. Population pharmacokinetics of mefloquine, piperaquine and artemether-lumefantrine in Cambodian and Tanzanian malaria patients. Malar J 12:235. doi: 10.1186/1475-2875-12-235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Simpson JA, Watkins ER, Price RN, Aarons L, Kyle DE, White NJ. 2000. Mefloquine pharmacokinetics-pharmacodynamic models: implications for dosing and resistance. Antimicrob Agents Chemother 44:3414–3424. doi: 10.1128/AAC.44.12.3414-3424.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jamsen K, Duffull S, Tarning J, Lindegarth N, White N, Simpson J. 2012. Optimal designs for population pharmacokinetic studies of the partner drugs co-administered with artemisinin derivatives ni patients with uncomplicated falciparum malaria. Malar J 11:143–152. doi: 10.1186/1475-2875-11-143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Na Bangchang K, Davis T, Looareesuwan S, White N, Bunnag D, Karbwang J. 1994. Mefloquine pharmacokinetics in pregnant women with acute falciparum malaria. Trans R Soc Trop Med Hyg 88:321–323. doi: 10.1016/0035-9203(94)90101-5. [DOI] [PubMed] [Google Scholar]
- 26.Hung LQ, De Vries PJ, Binh TQ, Giao PT, Nam NV, Holman R, Kager PA. 2004. Artesunate with mefloquine at various intervals for non-severe Plasmodium falciparum malaria. Am J Trop Med Hyg 71:160–166. [PubMed] [Google Scholar]
- 27.Svensson US, Alin MH, Karisson MO, Bergqvist Y, Ashton M. 2002. Population pharmacokinetic and pharmacodynamic modelling of artemisinin and mefloquine enantiomers in patients with falciparum malaria. Eur J Clin Pharmacol 58:339–351. doi: 10.1007/s00228-002-0485-y. [DOI] [PubMed] [Google Scholar]
- 28.Reuter S, Upton R, Evans A, Navaratnam V, Olliaro P. 2015. Population pharmacokinetics of orally administered mefloquine in healthy volunteers and patients with uncomplicated Plasmodium falciparum malaria. J Antimicrob Chemother 70:868–876. doi: 10.1093/jac/dku430. [DOI] [PubMed] [Google Scholar]
- 29.Charles B, Blomgren A, Nasveld P, Kitchener S, Jensen A, Gregory R, Robertson B, Harris I, Reid M, Edstein M. 2007. Population pharmacokinetics of mefloquine in military personnel for prophylaxis against malaria infection during field deployment. Eur J Clin Pharmacol 63:271–278. doi: 10.1007/s00228-006-0247-3. [DOI] [PubMed] [Google Scholar]
- 30.Anderson BJ, Holford NHG. 2008. Mechanism-based concepts of size and maturity in pharmacokinetics. Annu Rev Pharmacol Toxicol 48:303–332. doi: 10.1146/annurev.pharmtox.48.113006.094708. [DOI] [PubMed] [Google Scholar]
- 31.Edginton AN, Schmitt W, Voith B, Willmann S. 2006. A mechanistic approach for the scaling of clearance in children. Clin Pharmacokinet 45:683–704. doi: 10.2165/00003088-200645070-00004. [DOI] [PubMed] [Google Scholar]
- 32.Johnson TN, Rostami-Hodjegan A, Tucker GT. 2006. Prediction of the clearance of eleven drugs and associated variability in neonates, infants and children. Clin Pharmacokinet 45:931–956. 35. doi: 10.2165/00003088-200645090-00005. [DOI] [PubMed] [Google Scholar]
- 33.Anderson BJ, Allegaert K, Holford NHG. 2006. Population clinical pharmacology of children: modelling covariate effects. Eur J Pediatr 165:819–829. doi: 10.1007/s00431-006-0189-x. [DOI] [PubMed] [Google Scholar]
- 34.Anderson BJ, Allegaert K, Van den Anker JN, Cossey V, Holford NH. 2007. Vancomycin pharmacokinetics in preterm neonates and the prediction of adult clearance. Br J Clin Pharmacol 63:75–84. doi: 10.1111/j.1365-2125.2006.02725.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Anderson BJ, Holford NH. 2011. Tips and traps analyzing pediatric PK data. Paediatr Anaesth 21:222–237, March. doi: 10.1111/j.1460-9592.2011.03536.x. [DOI] [PubMed] [Google Scholar]
- 36.De Cock R, Allegaert K, Sherwin C, Nielsen E, de Hoog M, van den Anker J, Danhof M, Knibbe C. 2014. A neonatal amikacin covariate model can be used to predict ontogeny of other drugs eliminated through glomerular filtration in neonates. Pharm Res 31:754–767. doi: 10.1007/s11095-013-1197-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Simpson J, Jamsen K, Price R, White N, Lindegardh N, Tarning J, Duffull S. 2009. Towards optimal design of anti-malarial pharmacokinetic studies. Malar J 8:189–196. doi: 10.1186/1475-2875-8-189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.The PopED development team. 2015. PopED manual, version 2.13. Department of Pharmaceutical Biosciences, Uppsala University, Uppsala, Sweden: http://poped.sourceforge.net/manual.pdf. [Google Scholar]
- 39.Dodds M, Hooker A, Vicini P. 2005. Robust population pharmacokinetic experiment design. J Pharmacokinet Pharmacodyn 32:33–63. doi: 10.1007/s10928-005-2102-z. [DOI] [PubMed] [Google Scholar]
- 40.Roche Laboratories. 2004. Lariam brand of mefloquine hydrochloride. Roche Laboratories, Indianapolis, IN: http://www.accessdata.fda.gov/drugsatfda_docs/label/2008/019591s023lbl.pdf Accessed 3 March 2015. [Google Scholar]