Abstract
The percentage of time that free drug concentrations remain above the MIC (fT>MIC) that is necessary to prevent mortality among cefepime-treated patients with Gram-negative bloodstream infections (GNBSI) is poorly defined. We conducted a retrospective study of adult patients with GNBSI. Eligible cases were frequency matched to ensure categorical representation from all MICs. Organism, MIC, infection source, gender, age, serum creatinine, weight, antibiotic history, and modified APACHE II score were collected from hospital records. Two population pharmacokinetic models (models 1 and 2) were used to impute exposures over the first 24 h in each patient from mean model parameters, covariates, and dosing history. From the imputed exposures, survival thresholds for fT>MIC were identified using classification and regression tree (CART) analysis and analyzed as nominal variables for univariate and multivariate regressions. A total of 180 patients were included in the analysis, of whom 13.9% died and 86.1% survived. Many patients (46.7% [n = 84/180]) received combination therapy with cefepime. Survivors had higher mean (standard deviation [SD]) fT>MIC than those who died (model 1, 74.2% [29.6%] versus 52.1% [33.8%], P < 0.001; model 2, 85.9% [24.0%] versus 64.4% [31.4%], P < 0.001). CART identified fT>MIC threshold values for greater survival according to models 1 and 2 at >68% and >74%, respectively. Survival was improved for those with fT>MIC of >68% (model 1 adjusted odds ratio [aOR], 7.12; 95% confidence interval [CI], 1.90 to 26.7; P = 0.004) and >74% (model 2 aOR, 6.48; 95% CI, 1.90 to 22.1) after controlling for clinical covariates. Similarly, each 1% increase in cefepime fT>MIC resulted in a 2% improvement in multivariate survival probability (P = 0.015). Achieving a cefepime fT>MIC of 68 to 74% was associated with a higher odds of survival for patients with GNBSI. Regimens targeting this exposure should be aggressively pursued.
INTRODUCTION
Antimicrobial resistance among contemporary Gram-negative (GN) isolates has eroded the efficacy of many first-line antibiotics. Increasing beta-lactam MICs have been correlated with increasing antimicrobial failures in the treatment of serious bacterial infections (1–4). Elevated beta-lactam MICs can be expected to reduce the probability of achieving pharmacokinetic-pharmacodynamic (PK/PD) targets for beta-lactams. As the percentage of time in 24 hours that free drug concentrations are above the MIC (fT>MIC) is the PK/PD target predictive of microbiologic efficacy for beta-lactams (5), decreasing fT>MIC is expected to result in worse patient outcomes (6).
Cefepime, a broad-spectrum fourth-generation cephalosporin, is widely prescribed as the primary therapy for serious Gram-negative infections, including bloodstream infections (termed GNBSIs) (7). Several clinical studies have associated elevated cefepime MICs with an increased risk of treatment failure and mortality for cefepime-treated patients (1, 3, 8, 9), while other investigations have shown improved clinical outcomes among patients receiving aggressive cefepime dosing for bloodstream infections (BSIs) (10, 11). These previous studies lacked PK/PD data, which could be highly useful in interpreting observed outcomes. Very few studies have analyzed patient outcomes according to fT>MIC in cefepime-treated patients (12–14). As such, the necessary fT>MIC to prevent mortality for cefepime-treated patients with GNBSI is not well defined.
We sought to analyze the cefepime fT>MIC to see if a threshold existed for improved survival among patients treated with cefepime for GNBSIs. Secondarily, we sought to examine if candidate clinical threshold values for cefepime fT>MIC were predictive of other outcomes, such as hospital and intensive care unit (ICU) lengths of stay (LOS) and 30-day readmission rates.
MATERIALS AND METHODS
This retrospective cohort study was conducted at Northwestern Memorial Hospital (NMH) in Chicago, Illinois. Study methods were reviewed and approved by the Institutional Review Boards at Northwestern University and Midwestern University.
Clinical cohort.
Patients with at least one positive GNBSI who were treated with at least 24 h of cefepime therapy between 1 September 2006 and 3 June 2014 were reviewed for inclusion in the clinical cohort. Patients were included only once, and the index culture was the first GNBSI during the study period. Patients were excluded if they did not receive cefepime in an empirical or directed manner for the index bacteremia (i.e., if cefepime was initiated >96 h after or completed >72 h before the index bacteremia). Patient cases also were excluded if (i) no cefepime MIC was documented for the infecting pathogen, (ii) only 1 dose was administered and amounted to less than 24 h of therapy, or (iii) the culture was polymicrobial (i.e., mixed Gram-positive and Gram-negative organisms in the same culture). Patients were frequency matched and randomly selected to fill MIC categories, such that a maximum of 3 bloodstream isolates with a cefepime MIC of ≤1 mg/liter were included for every isolate with a cefepime MIC of ≥2 mg/liter (i.e., 2, 4, 8, 16, 32, and 64 mg/liter) to balance power and avoid overrepresentation of the more commonly encountered low MICs (15). None of the patients within the clinical cohort had measured cefepime concentrations available. Thus, concentration data were imputed using population pharmacokinetic models and patient covariates, as described below.
Gram-negative organisms were identified and tested for antibiotic susceptibility using the Vitek 2 system (bioMérieux, Balmes-les-Grottes, France). Cefepime MICs were quantified from 1 to 64 mg/liter. Antibiotic susceptibility was interpreted for clinicians according to CLSI guidelines at the time of culture (16). In cases of mixed GNBSI, the most resistant organism (i.e., the highest cefepime MIC) was used for analyses.
Variables were collected from medical, pharmacy, and microbiology records by trained reviewers. Variables collected included demographics (age, gender, height, body weight, and race), ICU versus non-ICU admission, modified APACHE II score (17, 18), renal dysfunction, hepatic dysfunction, solid-organ or hematologic transplant, receipt of immunosuppression therapy or prior hospitalization within 12 months before culture, hospital LOS postinfection, presence of multiple-Gram-negative-pathogen bacteremia, infecting organism, infection source, all Gram-negative antibiotics received (including drug name, dose, and schedule), and organism cefepime MIC. Outcomes included in-hospital all-cause mortality, requirement for ICU-level care, hospital and ICU LOS for survivors, and 30-day readmission. Of note, cefepime was dosed prospectively and adjusted by clinical pharmacists according to institutional protocols (9).
Definitions.
ICU-onset infection was defined as patient ICU residence at the time of the GNBSI. Concurrent renal dysfunction was defined as acute or chronic renal dysfunction. Acute renal dysfunction was an increase in serum creatinine of 0.5 mg/dl or 50% from baseline to immediately before the infection (19). Chronic renal dysfunction was defined as a physician diagnosis of chronic kidney disease (CKD). Hepatic dysfunction was defined as any liver enzyme level greater than three times the upper normal limit at the time of culture, chronic hepatitis, or documented cirrhosis (2, 9, 20). Prior immunosuppression was defined as steroid, chemotherapy, or immunosuppressant use within the 12 months prior to the index culture (2, 9, 20). Patient comorbidities were considered to be present if they were documented in the admission history and physical. The source of the BSI was determined from the attending physician's written diagnosis. The first day of positive blood cultures was considered the first day of infection. Cefepime dose intensity was classified according to approved product labeling as 2 g every 8 h, 2 g every 12 h, 1 g every 12 h, 500 mg every 12 h, or the renal dysfunction-adjusted equivalent doses (21). Charts also were reviewed for receipt of active antimicrobials other than cefepime during the index admission, including amikacin, cefazolin, ceftazidime, ceftriaxone, ciprofloxacin, colistin, ertapenem, gentamicin, imipenem, levofloxacin, meropenem, moxifloxacin, piperacillin-tazobactam, polymyxin B, tigecycline, and tobramycin. Combination antimicrobial therapy was defined as the administration of any other active antimicrobial (per CLSI criteria) concurrent to cefepime within the same 24-h period (excluding other beta-lactams).
Pharmacokinetic models.
We utilized two population PK models to impute individual cefepime exposures within the clinical cohort. First, we refit data from a previously published study (22) using a two-compartment model of cefepime clearance (model 1). The original report created a model using data from 26 acutely ill, hospitalized adult patients with an additional validation subset of 6 patients. Patients had a median (interquartile [IQR]) creatinine clearance (CLCR) distribution of 114 ml/min (75 to 145 ml/min) (range, 26 to 298 ml/min) and were treated with high-dose, prolonged-infusion cefepime for ventilator-associated pneumonia. For this study, all 32 patients and their attendant data were fit to a body weight- and creatinine clearance-adjusted structural model, as was done previously (22). We utilized the nonparametric adaptive grid (NPAG) algorithm within the Pmetrics package for R (Los Angeles, CA) for model-fitting procedures related to these data (23–25). The fully fit model was utilized for Bayesian estimations as described below. The model was parameterized with intercompartmental transfer rate constants (K12 and K21), central volume of distribution (VC), and elimination rate (Ke) constant using the following equations:
| (1) |
| (2) |
| (3) |
| (4) |
where Ke was scaled to CLCR (milliliters per minute) as calculated using the Cockcroft-Gault formula (26) using linear regression with slope (KS) and intercept (Ki) terms and where VC was scaled to total body weight (TBW) to obtain a scaled volume of distribution (V0). RateIV is the rate of drug input (mg/h). Model fitting and comparative performance procedures are described in Table S1 in the supplemental material.
To ensure that imputations were robust, a second population PK model was applied (from a previously fit population model) (model 2). Model 2 was previously fit using data from 164 individuals (27). Briefly, pooled PK data were obtained from 8 separate phase I single- or multiple-dose studies of cefepime disposition among individuals with various levels of renal dysfunction with a median (IQR) CLCR distribution of 85 ml/min/1.73 m2 (50 to 100 ml/min/1.73 m2) (range, 0 to 135 ml/min/1.73 m2) in the analysis population (27). The three-compartment structural model was parameterized with nonrenal clearance (CLNR), intercompartmental clearance rates (CLd1 and CLd2), and central and peripheral distribution volumes (Vc, Vp1, and Vp2) using the following equations:
| (5) |
| (6) |
| (7) |
| (8) |
where total renal clearance (CLR) was scaled to CLCR (milliliters per minute) as calculated using the Cockcroft-Gault formula (26) standardized to body surface area (i.e., ml/min/1.73/m2) using a Hill-type function. Model fitting for these data were conducted using the first-order conditional estimation method with η-ε interaction as implemented in NONMEM version 6.2. Model fitting and comparative performance procedures are described in Table S2 in the supplemental material.
Simulations and calculation of predicted fT>MIC.
As no concentrations were available within our clinical cohort (n = 180), we utilized each patient's individual TBW and CLCR together with the mean PK parameter estimates from each population PK model (i.e., models 1 and 2). Plasma concentration-time data were generated every half hour for the first 24 h of therapy. These plasma cefepime concentration-time profiles subsequently were corrected for protein binding predictions (i.e., 80% unbound drug was assumed [21]), generated every half hour for the first 24 h of therapy to determine free drug concentrations. In all simulations, the actual patient administration times, CLCR estimates, body weights, and organism MICs concurrent to cefepime administration were utilized to calculate fT>MIC for the first 24 h following the start of therapy. All simulations were conducted using the Monte Carlo simulator engine available within Pmetrics.
Statistical analysis.
The primary outcome was the rate of in-hospital survival. Cefepime fT>MIC was assessed as the primary predictor (13, 28, 29). The determination of a clinical cefepime fT>MIC threshold was completed using the recursive partitioning function within the classification and regression tree (CART) package tree (http://CRAN.R-project.org/package=tree) for R version 3.1 (30). The cefepime fT>MIC was handled as a linear variable bounded by 0 and 100% of the dosing interval and as a categorical variable for the CART-derived threshold fT>MIC. All other analyses were performed using Intercooled Stata version 14.0 (StataCorp, College Station, TX). Secondary outcomes (i.e., additional dependent variables) were LOS postculture among survivors, 30-day readmission rates for survivors, and durations of antibiotic therapy postculture.
Continuous variables were evaluated with Student's t test or Wilcoxon rank-sum test as appropriate. Categorical variables were evaluated with the Chi-square or Fisher's exact test as appropriate. Logistic regression was performed in a stepwise fashion. Candidate covariates (Table 1) at a bivariate level of significance of P < 0.2 were further assessed as possible independent predictors of the primary outcome (2, 9). Variables were retained in models if the objective function value changed by >3.84 with each iterative addition (n + 1 model). Final models were checked secondarily for optimal parsimony utilizing the Akaike information criterion (31). Outcome probabilities, adjusting for comodeled covariates, were calculated from adjusted odds ratios (aORs).
TABLE 1.
Baseline characteristics by model 1 and model 2 CART-derived fT>MIC thresholds
| Predictorb | Value(s) for: |
|||||
|---|---|---|---|---|---|---|
| Model 1 |
Model 2 |
|||||
| fT>MIC ≤ 68 % | fT>MIC > 68 % | P valuea | fT>MIC ≤ 74 % | fT>MIC > 74 % | P valuea | |
| Total no. (%) of patients | 67 (37.2) | 113 (62.3) | 46 (25.6) | 134 (74.4) | ||
| Age in yr, mean (SD) | 51.4 (15.3) | 61.3 (14.3) | <0.001 | 54.3 (14.6) | 58.8 (15.6) | 0.09 |
| Male, n (%) | 39 (58.2) | 63 (55.8) | 0.75 | 33 (71.7) | 69 (51.5) | 0.02 |
| Race, n (%) | 0.43 | 0.82 | ||||
| White | 37 (55.2) | 72 (63.7) | 0.26 | 25 (54.4) | 84 (62.7) | 0.32 |
| Black | 14 (20.9) | 26 (23.0) | 0.74 | 12 (26.1) | 28 (20.9) | 0.47 |
| Asian | 3 (4.5) | 3 (2.7) | 0.67 | 2 (4.4) | 4 (3.0) | 0.65 |
| Hispanic | 10 (14.9) | 8 (7.1) | 0.09 | 5 (10.9) | 13 (9.7) | 0.78 |
| Other | 3 (4.5) | 4 (3.5) | 0.71 | 2 (4.4) | 5 (3.7) | >0.99 |
| Modified APACHE II score on day of culture, mean (SD) | 13.6 (4.7) | 15.2 (4.9) | 0.03 | 14.0 (4.8) | 14.8 (4.9) | 0.34 |
| Creatinine clearance on day of culture (ml/min), median (IQR) | 105 (79.1–148) | 58.5 (34.8–84.3) | <0.001 | 105 (79.1–140) | 62.1 (37.4–95.4) | <0.001 |
| ICU at culture, n (%) | 18 (26.9) | 21 (18.6) | 0.19 | 14 (30.4) | 25 (18.7) | 0.09 |
| ANC of <500 cells/mm3 at admission, n (%) | 28 (41.8) | 54 (47.8) | 0.44 | 22 (47.8) | 60 (44.8) | 0.72 |
| Medical history, n (%) | ||||||
| Mechanical ventilation within prior 12 mo | 15 (22.4) | 23 (20.4) | 0.75 | 13 (28.3) | 25 (18.7) | 0.17 |
| Previous surgical procedure within prior 12 mo | 1 (1.5) | 13 (11.5) | 0.02 | 1 (2.2) | 13 (9.7) | 0.12 |
| History of leukemia | 14 (20.9) | 12 (10.6) | 0.06 | 10 (21.7) | 16 (11.9) | 0.10 |
| History of lymphoma | 6 (9.0) | 16 (14.2) | 0.30 | 6 (13.0) | 16 (12.0) | 0.84 |
| History of myeloma | 5 (7.5) | 13 (11.5) | 0.38 | 5 (10.9) | 13 (9.7) | 0.82 |
| History of renal transplant | 2 (3.0) | 13 (11.5) | 0.05 | 2 (4.4) | 13 (9.7) | 0.36 |
| History of liver transplant | 0 (0) | 7 (6.2) | 0.05 | 1 (2.2) | 6 (4.5) | 0.68 |
| Renal dysfunction within 30 days of culture | 24 (35.8) | 55 (48.7) | 0.09 | 20 (43.5) | 59 (44.0) | 0.95 |
| Receipt of prior immune suppressants (12 mo) | 42 (62.7) | 82 (72.6) | 0.17 | 30 (65.2) | 94 (70.2) | 0.53 |
| Days to positive culture from admission, median (IQR) | 3.9 (1.74–20.3) | 1.9 (1.6–10.7) | 0.01 | 10.9 (1.8–20.3) | 1.9 (1.6–10.7) | 0.01 |
| Days of cefepime therapy, median (IQR) | 4 (2–8) | 4 (3–8) | 0.30 | 4 (3–9) | 4 (3–8) | 0.91 |
| Received active combination therapy with cefepime, n (%) | 25 (37.3) | 59 (52.2) | 0.62 | 18 (39.1) | 66 (49.3) | 0.45 |
| Source known, n (%) | 45 (67.2) | 70 (62.0) | 0.48 | 28 (60.9) | 87 (64.9) | 0.62 |
| Source, n (%) | 0.18 | 0.74 | ||||
| Central line (n = 59) | 29 (43.3) | 30 (26.6) | 0.02 | 18 (39.1) | 41 (30.6) | 0.29 |
| CSF (n = 1) | 0 (0) | 1 (0.9) | >0.99 | 0 (0) | 1 (0.8) | >0.99 |
| GI/intra-abdominal (n = 14) | 2 (3.0) | 12 (10.6) | 0.09 | 3 (6.5) | 11 (8.2) | >0.99 |
| GU/urinary (n = 31) | 10 (14.9) | 21 (18.6) | 0.53 | 5 (10.9) | 26 (19.4) | 0.19 |
| Respiratory (n = 9) | 4 (6.0) | 5 (4.4) | 0.65 | 3 (6.5) | 6 (4.5) | 0.70 |
| Skin/wound (n = 3) | 1 (1.5) | 2 (1.8) | >0.99 | 0 (0) | 3 (2.2) | 0.57 |
| Unclear (n = 63) | 21 (31.3) | 42 (37.2) | 0.43 | 17 (37.0) | 46 (34.4) | 0.75 |
| Organism genera, n (%) | 0.01 | 0.08 | ||||
| Achromobacter spp. (n = 2) | 2 (3.0) | 0 (0) | 0.14 | 2 (4.4) | 0 (0) | 0.06 |
| Acinetobacter spp. (n = 4) | 4 (6.0) | 0 (0) | 0.02 | 3 (6.5) | 1 (0.8) | 0.05 |
| Aeromonas spp. (n = 1) | 0 (0) | 1 (0.9) | >0.99 | 0 (0) | 1 (0.8) | >0.99 |
| Citrobacter spp. (n = 5) | 2 (3.0) | 3 (2.7) | >0.99 | 1 (2.2) | 4 (3.0) | >0.99 |
| Delftia spp. (n = 1) | 1 (1.5) | 0 (0) | 0.37 | 0 (0) | 1 (0.8) | >0.99 |
| Enterobacter spp. (n = 15) | 3 (4.5) | 12 (10.6) | 0.18 | 1 (2.2) | 14 (10.5) | 0.12 |
| Escherichia coli (n = 58) | 15 (22.4) | 43 (38.1) | 0.03 | 13 (28.3) | 45 (33.6) | 0.51 |
| Klebsiella spp. (n = 34) | 17 (25.4) | 17 (15.0) | 0.09 | 11 (23.9) | 23 (17.2) | 0.31 |
| Proteus spp. (n = 3) | 2 (3.0) | 1 (0.9) | 0.56 | 0 (0) | 3 (2.2) | 0.57 |
| Pseudomonas spp. (n = 51) | 20 (29.9) | 31 (27.4) | 0.73 | 15 (32.6) | 36 (26.9) | 0.46 |
| Salmonella spp. (n = 2) | 0 (0) | 2 (1.8) | 0.53 | 0 (0) | 2 (1.5) | >0.99 |
| Serratia spp. (n = 4) | 1 (1.5) | 3 (2.7) | >0.99 | 0 (0) | 4 (3.0) | 0.57 |
| Enterobacteriaceae, n (%) | 41 (61.2) | 81 (71.7) | 0.15 | 26 (56.5) | 96 (71.6) | 0.06 |
| ESBL positive, n (%) | 23 (34.3) | 9 (8.0) | <0.001 | 20 (43.5) | 12 (9.0) | <0.001 |
For categorical variables, P values are from Fisher's exact test when expected cell counts were from <6 observations. Boldfaced P values are significant at the P < 0.2 level and eligible for inclusion in multivariate models.
ANC, absolute neutrophil count; ESBL, extended-spectrum beta-lactamase; GI, gastrointestinal; GU, genitourinary; ICU, intensive care unit; IQR, interquartile range; SD, standard deviation.
We evaluated competing multivariate models of imputed cefepime fT>MIC as a predictor of survival. First, we assessed whether the CART-derived threshold fT>MIC was a significant predictor of survival, adjusting for clinical covariates. Second, we assessed whether increasing cefepime fT>MIC was a significant predictor of survival, adjusting for clinical covariates. Modified APACHE II score (as either a continuous variable or as a CART-derived categorical variable) was forced into all models a priori, as it is a known predictor of mortality (17, 18). Exploratory analyses were conducted to evaluate the contribution of combination therapy by adding additional variables to the final multivariate survival models using the same procedure and retention criteria as those described above. All tests were two tailed, with an a priori level of alpha set at 0.05 for statistical significance.
RESULTS
Demographics of clinical outcomes cohort.
A total of 357 patient charts were screened; 180 were included and 177 were excluded. Of the charts excluded, 107 (60.5%) were excluded as cefepime was not given for the index bacteremia, 32 (18.1%) were excluded as less than 24 h of cefepime treatment was documented, 26 (14.7%) were excluded as cefepime was initiated >96 h after the index culture or was completed >72 h before the index culture, 10 (5.6%) were excluded as no cefepime MIC was documented, and 2 (1.1%) were excluded due to polymicrobial infection. Patients in the clinical cohort were mostly male (56.7%) and had a mean (standard deviation [SD]) age of 57.6 (15.4) years, a mean (SD) body weight of 79.8 (24.2) kg, and a mean (SD) modified APACHE II score of 14.6 (4.9) on the day of infection. Patients had a mean (SD) estimated CLCR of 85 (59) ml/min on the day of infection and a mean (SD) body surface area (BSA)-standardized CLCR of 96 (68) ml/min/1.73 m2. The range of estimated CLCR on the day of infection was 7 to 112 ml/min. Many patients had concurrent neutropenia (45.6%), with a significant number of patients presenting with a history of hematologic malignancy (36.7%), including leukemia (14.4%), lymphoma (12.2%), and multiple myeloma (10.0%). The majority of patients had extensive prior health care experience, with 83.3% having been hospitalized within the previous 12 months and 21.1% having required mechanical ventilation within the previous 12 months. Receipt of prior immunosuppressive therapy also was highly prevalent, with 68.9% of patients having received some form of therapeutic immune suppression within the previous 12 months. With respect to organism MIC distributions, the overall MIC50 and MIC90 values were 1 and 16 mg/liter, respectively.
Of the 180 patients included, 13.9% died and 86.1% survived. Durations of cefepime therapy were similar between patients who survived and those who died in the hospital (median [IQR] days of therapy of 4 [3 to 9] days versus 4 [3 to 8] days; P = 0.43), as shown in Table 1. Many patients (46.7% [n = 84/180]) received combination therapy with cefepime for the index episode of bacteremia. Similar proportions of survivors and nonsurvivors received combination therapy (44.5% [n = 69/155] versus 60.0% [n = 15/25]; P = 0.15).
Pharmacokinetic model fitness. (i) Model 1.
The NPAG-refitted population model using the full 32 patients' concentrations identified 20 support points. The population PK model produced acceptable fits of the observed concentrations (R2 = 51%), as shown in Fig. S1 in the supplemental material. Population parameter estimates for bias, imprecision, and coefficient of determination were 6.60 μg/ml, 109 μg2/ml2, and 51.0%, respectively. Likewise, the Bayesian individual posterior fits for the observed data were good (R2 = 96.8%). Bayesian individual posterior parameter estimates for bias, imprecision, and coefficient of determination were −0.289 μg/ml, 4.92 μg2/ml2, and 96.8%, respectively. Model 1 accurately characterized total cefepime clearance over a wide range of CLCR (see Fig. S2 in the supplemental material). Thus, the refit model was considered acceptable for predicting cefepime concentrations in our clinical cohort of acutely ill adult patients in whom only dosing history, demographic, and CLCR data were available for analysis. The mean (SD) fT>MIC imputed by model 1 within the clinical cohort was 71.1% (31.1%), while the median (IQR) fT>MIC imputed by model 1 was 81.3% (47.9% to 100%).
(ii) Model 2.
The previously fit model demonstrated excellent agreement between the observed plasma cefepime concentrations and both the population mean predictions (R2 = 91%) and the individual post hoc predictions (R2 = 98%), as shown in Fig. S3 in the supplemental material. Likewise, model 2 accurately characterized total cefepime clearance over a wide range of CLCR (data not shown). Given that the population cefepime predictions agreed so well with the observed plasma cefepime concentrations, the model was considered suitable for estimating cefepime exposure in patients in whom only dosing history, demographic, and CLCR data were available for analysis. The mean (SD) fT>MIC imputed by model 2 within the clinical cohort was 82.9% (26.2%), while the median (IQR) fT>MIC imputed by model 2 was 100% (72.9% to 100%).
Clinical outcomes according to model-predicted fT>MIC.
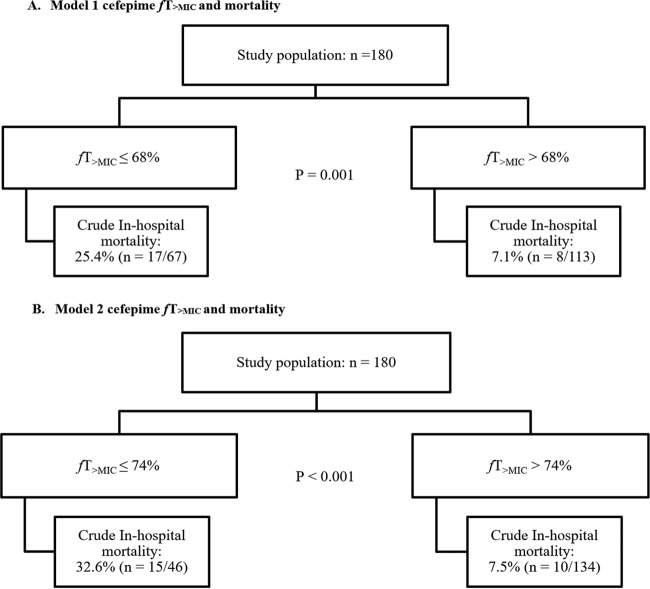
The CART-derived mortality thresholds for fT>MIC using model 1 and model 2 simulated imputations are shown in Fig. 1. CART identified a threshold for greater in-hospital survival at an fT>MIC of >68% (model 1) and >74% (model 2). CART also identified greater survival at modified APACHE II scores of <17. When splitting the patient sample into patients that achieved an fT>MIC of >68% and those that did not, several patient variables differed. Among them, age (P < 0.001), total body weight on admission (P < 0.001), modified APACHE II score on the day of infection (P = 0.03), being in the ICU at the time of culture (P < 0.001), CLCR on the day of infection (P < 0.001), length of stay prior to infection (P = 0.01), history of surgery in the previous 30 days (P = 0.02), history of leukemia (P = 0.06), history of liver (P = 0.05) or renal transplant (P = 0.05), organism genera (P = 0.01), and infection with an extended-spectrum β-lactamase (ESBL)-positive organism (P < 0.001) differed between the two groups (Table 1). Similar findings generally were noted using the model 2 threshold fT>MIC of >74%. Likewise, demographics also were stratified according to the outcome of in-hospital mortality or survival (Table 2). All differences in baseline demographics according to fT>MIC or outcome were considered for inclusion in multivariate models (statistical analysis).
FIG 1.
Incidence of in-hospital death according to classification and regression tree-determined cefepime fT>MIC threshold. (A) Model 1 cefepime fT>MIC and mortality. (B) Model 2 cefepime fT>MIC and mortality.
TABLE 2.
Baseline characteristics by in-hospital survival
| Predictor | Value(s) by category |
Univariate OR (95% CI) for survivala |
P valueb | |
|---|---|---|---|---|
| Died in hospital | Survived in hospital | |||
| Total no. (%) of patients | 25 (13.9) | 155 (86.1) | ||
| Age in yr, mean (SD) | 59.3 (14.2) | 57.4 (15.6) | 0.99 (0.96–1.02) | 0.56 |
| Male, n (%) | 14 (56.0) | 88 (56.7) | 1.03 (0.44–2.42) | 0.94 |
| Race | 0.16 | |||
| White, n (%) | 12 (48.0) | 97 (62.6) | 1.81 (0.77–4.24) | 0.17 |
| Black, n (%) | 7 (28.0) | 33 (21.3) | 0.70 (0.27–1.81) | 0.45 |
| Asian, n (%) | 1 (4.0) | 5 (3.2) | 0.80 (0.09–7.15) | >0.99 |
| Hispanic, n (%) | 2 (8.0) | 16 (10.3) | 1.32 (0.29–6.14) | >0.99 |
| Other, n (%) | 3 (12.0) | 4 (2.6) | 0.19 (0.04–0.93) | 0.06 |
| Modified APACHE II on day of culture, mean (SD) | 17.5 (4.5) | 14.1 (4.8) | 0.86 (0.78–0.95) | 0.001 |
| Creatinine clearance on day of culture (ml/min), median (IQR) | 83.0 (34.8–140) | 74.9 (43.1–110) | 0.99 (0.99–1.00) | 0.44 |
| ICU at culture, n (%) | 14 (56.0) | 25 (16.1) | 0.15 (0.06–0.37) | <0.001 |
| ANC of <500 cells/mm3 at admission, n (%) | 16 (64.0) | 66 (42.6) | 0.42 (0.17–1.00) | 0.05 |
| Medical history, n (%) | ||||
| Mechanical ventilation within prior 12 mo | 5 (20.0) | 33 (21.3) | 1.08 (0.38–3.10) | 0.88 |
| Previous surgical procedure within prior 12 mo | 2 (8.0) | 12 (7.7) | 0.97 (0.20–4.59) | >0.99 |
| History of leukemia | 9 (36.0) | 17 (11.0) | 0.22 (0.08–0.57) | 0.001 |
| History of lymphoma | 3 (12.0) | 19 (12.3) | 1.02 (0.28–3.75) | >0.99 |
| History of myeloma | 3 (12.0) | 15 (9.7) | 0.79 (0.21–2.94) | 0.72 |
| History of renal transplant | 2 (8.0) | 13 (8.4) | 1.05 (0.22–4.97) | >0.99 |
| History of liver transplant | 1 (4.0) | 6 (3.9) | 0.97 (0.11–8.38) | >0.99 |
| Renal dysfunction within 30 days of culture | 15 (60.0) | 64 (41.3) | 0.47 (0.20–1.11) | 0.08 |
| Receipt of prior immune suppressants (12 mo) | 22 (88.0) | 102 (65.8) | 0.26 (0.08–0.92) | 0.03 |
| Days to positive culture from admission, median (IQR) | 15.3 (3.4–28.8) | 1.9 (1.6–11.1) | 0.95 (0.93–0.98) | <0.001 |
| Days of cefepime therapy, median (IQR) | 4 (3–9) | 4 (3–8) | 0.97 (0.90–1.05) | 0.43 |
| Received active combination therapy with cefepime, n (%) | 15 (60.0) | 69 (44.5) | 0.53 (0.23–1.26) | 0.15 |
| Source known, n (%) | 11 (44.0) | 104 (67.1) | 2.60 (1.10–6.12) | 0.03 |
| Source, n (%) | 0.08 | |||
| Central line (n = 59) | 4 (16.0) | 55 (35.5) | 2.89 (0.94–8.84) | 0.07 |
| CSF (n = 1) | 0 (0) | 1 (0.7) | —b | >0.99 |
| GI/intra-abdominal (n = 14) | 3 (12.0) | 11 (7.1) | 0.56 (0.14–2.17) | 0.42 |
| GU/urinary (n = 31) | 2 (8.0) | 29 (18.7) | 2.65 (0.59–11.9) | 0.26 |
| Respiratory (n = 9) | 3 (12.0) | 6 (3.9) | 0.30 (0.07–1.27) | 0.11 |
| Skin/wound (n = 3) | 0 (0) | 3 (1.9) | — | >0.99 |
| Unclear (n = 63) | 13 (52.0) | 50 (32.3) | 0.44 (0.19–1.03) | 0.06 |
| Organism genera, n (%) | 0.66 | |||
| Achromobacter spp. (n = 2) | 1 (4.0) | 1 (0.7) | 0.16 (0.01–2.58) | 0.26 |
| Acinetobacter spp. (n = 4) | 1 (4.0) | 3 (1.9) | 0.47 (0.05–4.74) | 0.45 |
| Aeromonas spp. (n = 1) | 0 (0) | 1 (0.7) | — | >0.99 |
| Citrobacter spp. (n = 5) | 1 (4.0) | 4 (2.6) | 0.64 (0.07–5.93) | 0.53 |
| Delftia spp. (n = 1) | 0 (0) | 1 (0.7) | — | >0.99 |
| Enterobacter spp. (n = 15) | 2 (8.0) | 13 (8.4) | 1.05 (0.22–4.97) | >0.99 |
| Escherichia coli (n = 58) | 5 (20.0) | 53 (34.2) | 2.08 (0.74–5.85) | 0.16 |
| Klebsiella spp. (n = 34) | 6 (24.0) | 28 (18.1) | 0.70 (0.26–1.91) | 0.48 |
| Proteus spp. (n = 3) | 0 (0) | 3 (1.9) | — | >0.99 |
| Pseudomonas spp. (n = 51) | 9 (36.0) | 42 (27.1) | 0.66 (0.27–1.61) | 0.36 |
| Salmonella spp. (n = 2) | 0 (0) | 2 (1.3) | — | >0.99 |
| Serratia spp. (n = 4) | 0 (0) | 4 (2.6) | — | >0.99 |
| Enterobacteriaceae, n (%) | 14 (56.0) | 108 (69.7) | 1.81 (0.76–4.27) | 0.17 |
| ESBL positive, n (%) | 5 (20.0) | 27 (17.4) | 0.84 (0.29–2.45) | 0.75 |
Univariate odds ratios are presented where estimates were possible. A dash indicates the low precision of the odds ratio estimates.
For categorical variables, P values are from Fisher's exact test when cell counts were from <6 observations. Boldfaced P values are significant at the P < 0.2 level and eligible for inclusion in multivariate models.
Clinical outcomes according to the fT>MIC CART-derived thresholds are shown in Table 3. Crude in-hospital mortality was significantly higher among patients achieving cefepime at an fT>MIC of ≤68% compared to those patients achieving an fT>MIC of >68% (25.4% [n = 17/67] versus 7.1% [n = 8/113]; P = 0.001), as imputed using model 1. Neither median (IQR) hospital LOS for survivors (for fT>MIC ≤68% versus >68%, 8.4 [5.9 to 18.1] days versus 9.1 [5.9 to 18.4] days; P = 0.94) nor median (IQR) ICU LOS for survivors (fT>MIC ≤68% versus >68%, 6.7 [2.0 to 13.0] days versus 3.0 [2.0 to 7.7] days; P = 0.22) differed significantly according to fT>MIC. The 30-day readmission rate for survivors was numerically, but not significantly, higher among patients achieving an fT>MIC of >68% compared to those achieving an fT>MIC of ≤68% (39% [n = 41/105] versus 30% [n = 15/50]; P = 0.27). The median (IQR) total duration of antibiotics in days received postculture was similar between patients achieving an fT>MIC of >68% and those achieving an fT>MIC of ≤68% (8.6 [5.9 to 16.4] days versus 9.1 [5.2 to 17] days; P = 0.91). Outcomes were very similar for the fT>MIC threshold of >74%, as imputed using model 2.
TABLE 3.
Clinical outcomes by CART-derived threshold group and in-hospital survival
| Parameter | Value(s) for each PK model of estimated exposure: |
|||||
|---|---|---|---|---|---|---|
| Model 1 |
Model 2 |
|||||
| fT>MIC ≤ 68 % | fT>MIC > 68 % | P valuea | fT>MIC ≤ 74 % | fT>MIC > 74 % | P valuea | |
| Total no. (%) of patients | 67 (37.2) | 113 (62.3) | 46 (25.6) | 134 (74.4) | ||
| Died, n (%) | 17 (25.4) | 8 (7.1) | 0.001 | 15 (32.6) | 10 (7.5) | <0.001 |
| ICU transfer postculture, n (%) | 26 (38.8) | 39 (34.5) | 0.56 | 19 (41.3) | 46 (34.3) | 0.40 |
| Hospital LOS postculture for survivors (n = 155), median (IQR) | 8.4 (5.9–18) | 9.1 (5.9–18) | 0.94 | 9.5 (6.0–21) | 8.9 (5.7–18) | 0.37 |
| ICU LOS postculture for survivors (n = 47), median (IQR) | 6.7 (2.0–13) | 3.0 (2.0–7.7) | 0.22 | 9.0 (2.0–16) | 3.0 (2.0–7.9) | 0.22 |
| Readmission within 30 days, n (%) | 15 (30.0) | 41 (39.1) | 0.27 | 10 (32.3) | 46 (37.1) | 0.62 |
| Inpatient total duration of antibiotics post culture, median (IQR) | 9.1 (5.2–17) | 8.6 (5.9–16.4) | 0.91 | 9.9 (5.9–21) | 8.4 (5.6–15.4) | 0.26 |
For categorical variables, P values are from Fisher's exact test when cell counts were from <6 observations.
Multivariate models of survival.
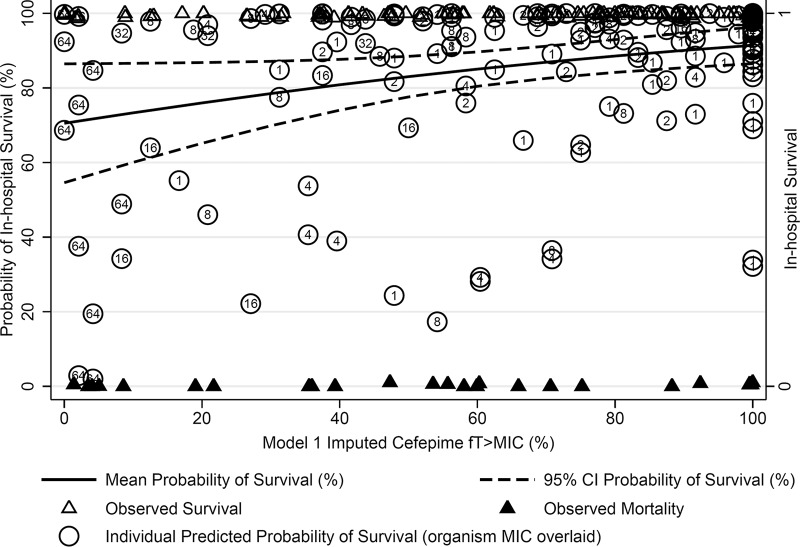
Multivariate logistic models for survival according to imputed cefepime fT>MIC are shown in Table 4. The first logistic model (LR1) evaluated the influence of the fT>MIC > 68% threshold on the outcome of in-hospital survival while controlling for the CART-derived modified APACHE II threshold of >17.5, LOS prior to positive culture (log10-normalized days), receipt of previous immunosuppression therapy, and being in the ICU at the time of culture. Patients with an fT>MIC of >68% were significantly more likely to survive (aOR, 7.12; 95% confidence interval [CI], 1.90 to 26.7; P = 0.004). The second logistic model (LR2) evaluated the influence of each incremental increase in the model 1-predicted cefepime fT>MIC (as a percentage of the dosing interval), adjusting for the same covariates as LR1, on the outcome of in-hospital survival. Increasing the cefepime fT>MIC (each 1% increase) independently predicted a higher odds of survival (aOR, 1.02; 95% CI, 1.00 to 1.04; P = 0.015) (graphically displayed in Fig. 2). In the univariate and multivariate models of survival, neutropenia status, receipt of combination therapy, and Gram-negative species were not independently associated with the outcome of survival (data not shown). Similar multivariate adjusted estimates for the odds of survival were seen with a model 2 fT>MIC threshold of 74% (Table 4).
TABLE 4.
Multivariate model of the contribution of cefepime fT>MIC to the odds of in-hospital survival
| Model parameter | Value(s) for each PK model of estimated exposurea: |
|||
|---|---|---|---|---|
| Model 1 (fT>MIC threshold of 68%) |
Model 2 (fT>MIC threshold of 74%) |
|||
| Univariate analysis, OR (95% CI) | Multivariate analysis, aOR (95% CI) | Univariate analysis, OR (95% CI) | Multivariate analysis, aOR (95% CI) | |
| Cefepime fT>MIC > threshold | 4.46 (1.80–11.0) | 7.12 (1.90–26.7) | 6.00 (2.46–14.6) | 6.48 (1.90–22.1) |
| Modified APACHE II (day 0) > 17.5 | 0.20 (0.08–0.49) | 0.08 (0.02–0.32) | 0.20 (0.08–0.49) | 0.11 (0.03–0.41) |
| Log10 days to positive culture | 0.19 (0.08–0.44) | 0.33 (0.12–0.96) | 0.19 (0.08–0.44) | 0.30 (0.03–0.89) |
| Receipt of prior immune suppressants (12 mo) | 0.26 (0.08–0.92) | 0.25 (0.05–1.15) | 0.26 (0.08–0.92) | 0.23 (0.05–1.11) |
| History of leukemia | 0.22 (0.08–0.57) | 0.17 (0.04–0.70) | 0.22 (0.08–0.57) | 0.16 (0.04–0.68) |
| ICU at time of culture | 0.15 (0.06–0.37) | 0.09 (0.02–0.31) | 0.15 (0.06–0.37) | 0.09 (0.03–0.33) |
Model 1, in-hospital survival according to an fT>MIC threshold of 68%. Model 2, in-hospital survival according to an fT>MIC threshold of 74%.
FIG 2.
Adjusted probabilities of survival and actual in-hospital survival rates according to model 1-imputed cefepime fT>MIC over the first 24 h of therapy. The probability of death was adjusted for regression covariates as shown in Table 4. The fT>MIC was regressed against the outcome of survival while holding constant the modified APACHE II score, the log10-normalized length of stay prior to culture, prior receipt of immunosuppression, and being in the ICU at the time of culture at their mean values. Individual death and survival observations are jittered for visual appreciation of the count across levels of fT>MIC; organism MIC is displayed within each individual prediction of mortality.
Cefepime dosing strategies used and exploration of the impact of concurrent therapy.
The dosing intensity of patients according to renal-equivalent dose was similar between cefepime fT>MIC threshold groups. Overall, 67.3% of patients who achieved an fT>MIC of >68% (model 1) received the renal equivalent of 2 g every 8 h compared to 56.7% of patients who failed to achieve an fT>MIC of >68% (P = 0.16). All other renal dose categories were similar between the fT>MIC CART-derived thresholds. The receipt of combination treatment was more common among patients achieving an fT>MIC of >68% than among those who did not (52% [n = 59/113] versus 37% [n = 25/67]; P = 0.05). Likewise, receipt of combination therapy plus directed cefepime was numerically more common among patients achieving an fT>MIC of >68% than among those who did not (52% [n = 55/106] versus 39% [n = 21/54]; P = 0.12). However, exploratory multivariate models of survival evaluating the impact of cefepime combination therapy revealed that combination therapy was not an independent predictor of in-hospital survival (data not shown). Additionally, a restricted logistic regression analysis limited to patients who received only cefepime monotherapy (n = 96) revealed that a model 1 imputed cefepime fT>MIC of >68% and a model 2 imputed cefepime fT>MIC of >74% remained significantly predictive of survival at the univariate level (P < 0.05 for each threshold value).
Impact of pathogen MIC for cefepime and Gram-negative species.
The distribution of pathogen MICs according to increasing cefepime fT>MIC is displayed within the individual predictions of survival shown in Fig. 2. The MIC50 and MIC90 among those who survived were 1 and 16 mg/liter, while the MIC50 and MIC90 among those who died were 4 and 64 mg/liter. The proportion of patients achieving an fT>MIC of >68% was significantly greater at a MIC of ≤2 mg/liter than at a MIC of >2 mg/liter (79% [n = 101/128] versus 23% [n = 12/52]; P < 0.001). GNBSIs due to non-Enterobacteriaceae (e.g., Acinetobacter and Pseudomonas spp.) were numerically but not significantly less likely to be associated with achieving a cefepime fT>MIC of >68% (28% [n = 32/113] versus 39% [n = 26/67]; P = 0.15). The subgroup of patients with GNBSIs due to Escherichia coli was significantly more likely to achieve a cefepime fT>MIC of >68% (38% [n = 43/113] versus 22% [15/67]; P = 0.03). The subgroup of patients with GNBSIs due to Acinetobacter spp. were significantly less likely to achieve an fT>MIC of >68% (0% [n = 0/113] versus 6% [4/67]; P = 0.02). Similar results were seen for an fT>MIC of >74% (model 2) (data not shown).
DISCUSSION
Increasing cefepime fT>MIC predicted improved hospital survival among patients with GNBSI treated with cefepime. This study is unique in that it is the first to define a clinical PK/PD threshold for cefepime in bloodstream infections. Our analysis is strengthened in that the patient population was highly comorbid (with a mean [SD] modified APACHE II score on the day of infection of 14.6 [4.9]), likely defining a worst-case scenario similar to that which is mimicked in neutropenic animal models. To that end, our population often was neutropenic (46%; n = 82/180). Additionally, this study analyzed a heterogeneous representation of organisms with cefepime MICs between 1 and 64 mg/liter.
Our PK/PD data, imputed using two distinct pharmacokinetic models, support a clinical threshold for decreased mortality at an fT>MIC of >68 to 74%. Although many patients in our cohort received combination therapy, it appears not to have affected our results, as indicated in our multivariate analyses. A restricted analysis limited to patients who received cefepime monotherapy at the time of or after the initial blood culture was drawn also found that the CART-derived threshold of an fT>MIC of >68 to 74% remained a significant predictor of in-hospital survival. Thus, our method for relating fT>MIC to clinical outcomes may have broad implications for patient care and supports the need for cefepime dose optimization in real time.
The results presented here generally are consistent with other studies of clinical outcomes according to fT>MIC. Roberts et al. conducted a prospective, multinational, point-prevalence study (DALI) of patients receiving eight different beta-lactam antibiotics and found that fT>MIC of 50% and 100% were associated with clinical cure after adjusting for patient severity of illness measures (OR of 1.03 [95% CI, 1.01 to 1.04] and OR of 1.56 [95% CI, 1.15 to 2.13], respectively) (4). Similarly in our cohort, we observed a significant survival benefit for patients achieving an fT>MIC of >68 to 74%. Notably, our cohort had baseline modified APACHE II scores similar to those in the DALI study (means of 14.6 and 18, respectively). Additionally, Chapuis et al. conducted a PK and clinical outcomes study of critically ill patients receiving empirical cefepime in the ICU (12). The authors found that cefepime regimens of 2 g every 12 h (for a CLCR of ≥50 ml/min) and 2 g every 24 h (for a CLCR of <50 ml/min) yielded 100% T>MIC (first dose and steady state) when the MIC was ≤4 mg/liter, the but T>MIC fell to 67% (first dose) and 44% (steady state between days 4 and 6) when the MIC was 8 mg/liter. The authors noted 95.2% survival, whereas we noted 86.1% survival. Differences in outcomes may have been partially explained by the lower severity of illness within the Chapuis et al. cohort.
Our findings also are similar to those of in vitro PK/PD studies of cefepime. Andes and Craig evaluated the pharmacodynamics of cephalosporins, including cefepime, in a series of murine thigh infection model experiments (32). In these experiments (33–35), animals were infected with Enterobacteriaceae (e.g., E. coli, Klebsiella spp., Enterobacter spp., and Serratia spp.) that were either phenotypically susceptible or resistant (i.e., organisms either were ESBL producing or exhibited other known resistance mechanisms). The authors examined bacterial counts (in log10 CFU) stratified by T>MIC achieved over 24 h of exposure. The authors observed net bacterial stasis at T>MIC values of 40 to 50% and maximal bacterial killing at a T>MIC value of 60%. However, incremental decreases (1 to 3 log10 CFU reductions) in organism burden also were observed between T>MIC values of 50 to 60%. The observed decreases in organism burden were observed irrespective of whether or not the organism was an ESBL producer. In another recent study of ceftobiprole pharmacodynamics, the T>MIC values needed to net bacterial stasis or a 2 log10 CFU reduction in Gram-negative bacilli were 36.5 and 54.3%, respectively (36). When examining Enterobacteriaceae in particular, the authors noted the T>MIC needed to produce a 2 log10 CFU reduction was 64.5% ± 25.1% for ceftobiprole. Our observed clinical threshold fT>MIC of 68 to 74% for cefepime suggests that reductions in bacterial burden of ≥2 log10 CFU may be necessary to prevent mortality among acutely ill, highly comorbid patients.
Additionally, our CART-derived threshold for improved clinical outcomes at fT>MIC values of 68 to 74% among patients with Gram-negative bloodstream infections is similar to that of other PK/PD thresholds previously identified for cefepime in other clinical infections. Crandon et al. identified a CART-derived threshold for microbiologic success at an fT>MIC of >60% for patients with mixed pseudomonal infections (i.e., 66% respiratory, 25% skin, and 9% blood) treated with cefepime (28). Likewise, MacVane et al. identified a CART-derived threshold of microbiologic success at an fT>MIC of >53% for nosocomial pneumonia patients treated with cefepime or ceftazidime (29). Our study demonstrates the complex interplay between the achievement of PK goals and the attainment of PD outcomes. Importantly, patient severity of illness quantified using modified APACHE II scores in our study was a major modifier of treatment outcomes. As can be garnered from probabilities of survival between 0 and 100% fT>MIC shown in Fig. 2, the impact of ideal PK provides a 20% absolute reduction in the probability of in-hospital mortality. For every incremental 1% increase in fT>MIC, we observed a marginal relative increase in survival of 1.8 to 2.1%.
Limitations to our study must be considered. First, this was a single-center, retrospective cohort and is subject to inherent biases. Second, imputations of cefepime fT>MIC were based on administrations within the first 24 h. We chose the first 24 h as the most critical time to establish active therapy, as each hour of delay in achieving adequate therapy is a well-known contributor to mortality (37, 38). Third, several PK/PD studies have shown that the achievement of adequate beta-lactam concentrations is subject to fluctuations and significant intrapatient variability (39). However, renal clearance and volume of distribution are the most important modifiers (22), and we controlled for these patient-specific factors (CLCR and TBW) when deriving our imputed estimates of cefepime exposure. Similar results were noted regardless of which PK model was used. Fourth, no plasma PK samples were collected from our outcome patients; however, we believe our methods of imputed concentrations represent the best available methods to try to establish a link between mortality and fT>MIC. Fifth, many patients in our study had concurrent neutropenia, which qualifies for maximal dosing of cefepime at our institution. As neutropenia status did not significantly improve the multivariate models for death, we have attempted to control for confounding by adjusting for modified APACHE II scores and other risk factors as assessed in the multivariate model. Our findings may be less applicable to healthier subjects. Sixth, no statistical differences were noted between outcome groups according to the source of infection. However, our findings may be more applicable to populations with neutropenia, where line-related infections and unclear sources are more common. Seventh, MICs were obtained from automated susceptibility testing using the Vitek 2 system as opposed to agar dilution or broth microdilution; however, Vitek 2 is an FDA-approved method.
We have shown that there is clinical evidence that achieving an adequate fT>MIC (>68 to 74%) among severely ill and comorbid patients can lower the risk of in-hospital mortality due to GNBSI. As we have shown, the achievement of this fT>MIC threshold cannot be predicted from dose alone. Our results suggest that clinicians should utilize aggressive prolonged-infusion schemes until the pathogen MIC is known. We suggest that until additional studies are conducted in less acute populations, with a larger representation of MICs between 4 and 32 mg/liter and with a more diverse representation of organisms at each MIC, dosing regimens targeting an fT>MIC of >68 to 74% should be aggressively pursued in the setting of GNBSI.
Supplementary Material
ACKNOWLEDGMENTS
We acknowledge the following individuals who supported the development of the manuscript and provided reviews of the data presented here: Sonia Rao, Milena McLaughlin, and Sheila Wang. We thank Brandon Chiu and Ryan Heraty for their assistance with data collection.
No external funding was received.
We have no relevant conflicts of interest to disclose.
Footnotes
Supplemental material for this article may be found at http://dx.doi.org/10.1128/AAC.01956-15.
REFERENCES
- 1.Bhat SV, Peleg AY, Lodise TP, Shutt KA, Capitano B, Potoski BA, Paterson DL. 2007. Failure of current cefepime breakpoints to predict clinical outcomes of bacteremia caused by gram-negative organisms. Antimicrob Agents Chemother 51:4390–4395. doi: 10.1128/AAC.01487-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Esterly JS, Wagner J, McLaughlin MM, Postelnick MJ, Qi C, Scheetz MH. 2012. Evaluation of clinical outcomes in patients with bloodstream infections due to Gram-negative bacteria according to carbapenem MIC stratification. Antimicrob Agents Chemother 56:4885–4890. doi: 10.1128/AAC.06365-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lee NY, Lee CC, Huang WH, Tsui KC, Hsueh PR, Ko WC. 2013. Cefepime therapy for monomicrobial bacteremia caused by cefepime-susceptible extended-spectrum beta-lactamase-producing Enterobacteriaceae: MIC matters. Clin Infect Dis 56:488–495. doi: 10.1093/cid/cis916. [DOI] [PubMed] [Google Scholar]
- 4.Roberts JA, Paul SK, Akova M, Bassetti M, De Waele JJ, Dimopoulos G, Kaukonen KM, Koulenti D, Martin C, Montravers P, Rello J, Rhodes A, Starr T, Wallis SC, Lipman J, DALI Study. 2014. DALI: defining antibiotic levels in intensive care unit patients: are current beta-lactam antibiotic doses sufficient for critically ill patients? Clin Infect Dis 58:1072–1083. doi: 10.1093/cid/ciu027. [DOI] [PubMed] [Google Scholar]
- 5.Craig WA. 1995. Interrelationship between pharmacokinetics and pharmacodynamics in determining dosage regimens for broad-spectrum cephalosporins. Diagn Microbiol Infect Dis 22:89–96. doi: 10.1016/0732-8893(95)00053-D. [DOI] [PubMed] [Google Scholar]
- 6.Drusano GL. 2004. Antimicrobial pharmacodynamics: critical interactions of “bug and drug.” Nat Rev Microbiol 2:289–300. doi: 10.1038/nrmicro862. [DOI] [PubMed] [Google Scholar]
- 7.Kelesidis T, Braykov N, Uslan DZ, Morgan DJ, Gandra S, Johannsson B, Schweizer ML, Weisenberg SA, Young H, Cantey J, Perencevich E, Septimus E, Srinivasan A, Laxminarayan R. 2015. Indications and types of antibiotic agents used in 6 acute care hospitals, 2009-2010: a pragmatic retrospective observational study. Infect Control Hosp Epidemiol 37:70–79. doi: 10.1017/ice.2015.226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chopra T, Marchaim D, Veltman J, Johnson P, Zhao JJ, Tansek R, Hatahet D, Chaudhry K, Pogue JM, Rahbar H, Chen TY, Truong T, Rodriguez V, Ellsworth J, Bernabela L, Bhargava A, Yousuf A, Alangaden G, Kaye KS. 2012. Impact of cefepime therapy on mortality among patients with bloodstream infections caused by extended-spectrum-beta-lactamase-producing Klebsiella pneumoniae and Escherichia coli. Antimicrob Agents Chemother 56:3936–3942. doi: 10.1128/AAC.05419-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rhodes NJ, Liu J, McLaughlin MM, Qi C, Scheetz MH. 2015. Evaluation of clinical outcomes in patients with Gram-negative bloodstream infections according to cefepime MIC. Diagn Microbiol Infect Dis 82:165–171. doi: 10.1016/j.diagmicrobio.2015.03.005. [DOI] [PubMed] [Google Scholar]
- 10.Siedner MJ, Galar A, Guzman-Suarez BB, Kubiak DW, Baghdady N, Ferraro MJ, Hooper DC, O'Brien TF, Marty FM. 2014. Cefepime vs other antibacterial agents for the treatment of Enterobacter species bacteremia. Clin Infect Dis 58:1554–1563. doi: 10.1093/cid/ciu182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Alves MD, Ribeiro VB, Tessari JP, Mattiello F, De Bacco G, Luz DI, Vieira FJ, Behle TF, Pasqualotto AC, Zavascki AP. 2014. Effect of cefepime dose on mortality of patients with Gram-negative bacterial bloodstream infections: a prospective cohort study. J Antimicrob Chemother 69:1681–1687. doi: 10.1093/jac/dku001. [DOI] [PubMed] [Google Scholar]
- 12.Chapuis TM, Giannoni E, Majcherczyk PA, Chioléro R, Schaller MD, Berger MM, Bolay S, Décosterd LA, Bugnon D, Moreillon P. 2010. Prospective monitoring of cefepime in intensive care unit adult patients. Crit Care 14:R51. doi: 10.1186/cc8941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lee SY, Kuti JL, Nicolau DP. 2007. Cefepime pharmacodynamics in patients with extended spectrum beta-lactamase (ESBL) and non-ESBL infections. J Infect 54:463–468. doi: 10.1016/j.jinf.2006.09.004. [DOI] [PubMed] [Google Scholar]
- 14.McKinnon PS, Paladino JA, Schentag JJ. 2008. Evaluation of area under the inhibitory curve (AUIC) and time above the minimum inhibitory concentration (T>MIC) as predictors of outcome for cefepime and ceftazidime in serious bacterial infections. Int J Antimicrob Agents 31:345–351. doi: 10.1016/j.ijantimicag.2007.12.009. [DOI] [PubMed] [Google Scholar]
- 15.Ury HK. 1975. Efficiency of case-control studies with multiple controls per case: continuous or dichotomous data. Biometrics 31:643–649. doi: 10.2307/2529548. [DOI] [PubMed] [Google Scholar]
- 16.Clinical and Laboratory Standards Institute. 2014. Performance standards for antimicrobial susceptibility testing. M100-S24. CLSI, Wayne, PA. [Google Scholar]
- 17.Hamilton KW, Bilker WB, Lautenbach E. 2007. Controlling for severity of illness in assessment of the association between antimicrobial-resistant infection and mortality: impact of calculation of Acute Physiology and Chronic Health Evaluation (APACHE) II scores at different time points. Infect Control Hosp Epidemiol 28:832–836. doi: 10.1086/518751. [DOI] [PubMed] [Google Scholar]
- 18.Thom KA, Shardell MD, Osih RB, Schweizer ML, Furuno JP, Perencevich EN, McGregor JC, Harris AD. 2008. Controlling for severity of illness in outcome studies involving infectious diseases: impact of measurement at different time points. Infect Control Hosp Epidemiol 29:1048–1053. doi: 10.1086/591453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bellomo R, Ronco C, Kellum JA, Mehta RL, Palevsky P, Acute Dialysis Quality Initiative Workgroup. 2004. Acute renal failure–definition, outcome measures, animal models, fluid therapy and information technology needs: the Second International Consensus Conference of the Acute Dialysis Quality Initiative (ADQI) Group. Crit Care 8:R204–R212. doi: 10.1186/cc2872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.McLaughlin MM, Advincula MR, Malczynski M, Barajas G, Qi C, Scheetz MH. 2014. Quantifying the clinical virulence of Klebsiella pneumoniae producing carbapenemase Klebsiella pneumoniae with a Galleria mellonella model and a pilot study to translate to patient outcomes. BMC Infect Dis 14:31. doi: 10.1186/1471-2334-14-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hospira. 2013. Maxipime (cefepime hydrochloride) IV package insert. Hospira, Inc., Lake Forest, IL. [Google Scholar]
- 22.Nicasio AM, Ariano RE, Zelenitsky SA, Kim A, Crandon JL, Kuti JL, Nicolau DP. 2009. Population pharmacokinetics of high-dose, prolonged-infusion cefepime in adult critically ill patients with ventilator-associated pneumonia. Antimicrob Agents Chemother 53:1476–1481. doi: 10.1128/AAC.01141-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Leary R, Jelliffe R, Schumitzky A, Van Guilder M. 2001. An adaptive grid nonparametric approach to pharmacokinetic and dynamic (PK/PD) population models, p 389–394. In Proceedings of the 14th IEEE Symposium on Computer-Based Medical Systems, Bethesda, MD. [Google Scholar]
- 24.Tatarinova T, Neely M, Bartroff J, van Guilder M, Yamada W, Bayard D, Jelliffe R, Leary R, Chubatiuk A, Schumitzky A. 2013. Two general methods for population pharmacokinetic modeling: non-parametric adaptive grid and non-parametric Bayesian. J Pharmacokinet Pharmacodyn 40:189–199. doi: 10.1007/s10928-013-9302-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Neely MN, van Guilder MG, Yamada WM, Schumitzky A, Jelliffe RW. 2012. Accurate detection of outliers and subpopulations with Pmetrics, a nonparametric and parametric pharmacometric modeling and simulation package for R. Ther Drug Monit 34:467–476. doi: 10.1097/FTD.0b013e31825c4ba6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cockcroft DW, Gault MH. 1976. Prediction of creatinine clearance from serum creatinine. Nephron 16:31–41. doi: 10.1159/000180580. [DOI] [PubMed] [Google Scholar]
- 27.Van Wart SA, Bhavnani SM, Jones RN, Forrest A, Bulik CC, Pfister M, Bertz RJ, Ambrose PG. 2011. Pharmacokinetic-pharmacodynamic (PK-PD) target attainment (TA) analyses to evaluate susceptibility breakpoints (SB) for cefepime (CPM) in renal impairment. 51st Abstr Intersci Conf Antimicrob Agents Chemother, abstr A-1100. [Google Scholar]
- 28.Crandon JL, Bulik CC, Kuti JL, Nicolau DP. 2010. Clinical pharmacodynamics of cefepime in patients infected with Pseudomonas aeruginosa. Antimicrob Agents Chemother 54:1111–1116. doi: 10.1128/AAC.01183-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.MacVane SH, Kuti JL, Nicolau DP. 2014. Clinical pharmacodynamics of antipseudomonal cephalosporins in patients with ventilator-associated pneumonia. Antimicrob Agents Chemother 58:1359–1364. doi: 10.1128/AAC.01463-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.R Core Team. 2015. R: a language and environment for statistical computing, 3rd ed R Foundation for Statistical Computing, Vienna, Austria. [Google Scholar]
- 31.Hosmer DW, Lemeshow S. 2000. Applied logistic regression, 2nd ed Wiley, New York, NY. [Google Scholar]
- 32.Andes D, Craig WA. 2005. Treatment of infections with ESBL-producing organisms: pharmacokinetic and pharmacodynamic considerations. Clin Microbiol Infect 11(Suppl 6):S10–S17. [DOI] [PubMed] [Google Scholar]
- 33.Andes D, Jacoby G, Craig W. 1995. Impact of extended-spectrum beta-lactamase production in K. pneumoniae on activity of four beta-lactams in an animal infection model. 35th Abstr Intersci Conf Antimicrob Agents Chemother, p 205. [Google Scholar]
- 34.Craig WA, Kiem S, Andes D, Ambrose P, Jones R. 2003. Impact of ESBLs on in vivo activity of four cephalosporins in the neutropenic mouse-thigh infection model. 43rd Abstr Intersci Conf Antimicrob Agents Chemother, abstr A1318. [Google Scholar]
- 35.Andes D, Craig W, Bhavnani SM, Jones RN, Thye D, Wikler MA, Ambrose PG. 2003. PK-PD evaluation of doripenem (DOR) against extended spectrum b-lactamase (ESBL) producing Enterobacteriaceae. Abstr 41st Infect Dis Soc Am, p 25. [Google Scholar]
- 36.Craig WA, Andes DR. 2008. In vivo pharmacodynamics of ceftobiprole against multiple bacterial pathogens in murine thigh and lung infection models. Antimicrob Agents Chemother 52:3492–3496. doi: 10.1128/AAC.01273-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kumar A, Roberts D, Wood KE, Light B, Parrillo JE, Sharma S, Suppes R, Feinstein D, Zanotti S, Taiberg L, Gurka D, Kumar A, Cheang M. 2006. Duration of hypotension before initiation of effective antimicrobial therapy is the critical determinant of survival in human septic shock. Crit Care Med 34:1589–1596. doi: 10.1097/01.CCM.0000217961.75225.E9. [DOI] [PubMed] [Google Scholar]
- 38.Kumar A, Haery C, Paladugu B, Kumar A, Symeoneides S, Taiberg L, Osman J, Trenholme G, Opal SM, Goldfarb R, Parrillo JE. 2006. The duration of hypotension before the initiation of antibiotic treatment is a critical determinant of survival in a murine model of Escherichia coli septic shock: association with serum lactate and inflammatory cytokine levels. J Infect Dis 193:251–258. doi: 10.1086/498909. [DOI] [PubMed] [Google Scholar]
- 39.Roberts DM, Roberts JA, Roberts MS, Liu X, Nair P, Cole L, Lipman J, Bellomo R, Renal Replacement Therapy Study Investigators. 2012. Variability of antibiotic concentrations in critically ill patients receiving continuous renal replacement therapy: a multicentre pharmacokinetic study. Crit Care Med 40:1523–1528. doi: 10.1097/CCM.0b013e318241e553. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.