Abstract
Exocytosis is a highly regulated intercellular communication process involving various membrane proteins, lipids, and cytoskeleton restructuring. These components help control granule fusion with the cell membrane, creating a pore through which granular contents are released into the extracellular environment. Platelets are an ideal model system for studying exocytosis due to their lack of a nucleus, resulting in decreased membrane regulation in response to cellular changes. In addition, platelets contain fewer granules than most other exocytosing cells, allowing straightforward measurement of individual granule release with carbon-fiber microelectrode amperometry. This technique monitors the concentration of serotonin, an electroactive molecule found in the dense-body granules of platelets, released as a function of time, with 50 μs time resolution, revealing biophysical characteristics of the fundamental exocytotic process. Variations in fusion pore formation and closure cause deviations from the classic current versus time spike profile and may influence diffusion of serotonin molecules from the site of granule fusion. Physiologically, the delivery of smaller packets of chemical messengers or the prolonged delivery of chemical messengers may represent how cells/organisms tune biological response. The goals of this work are twofold: 1) to categorize secretion features that deviate from the traditional mode of secretion and 2) to examine how changing the cholesterol composition of the platelet membrane results in changes in the pore formation process. Results herein indicate that the expected traditional mode of release is actually in the minority of granule content release events. In addition, results indicate that as the cholesterol content of the plasma membrane is increased, pore opening is less continuous.
Introduction
To maintain proper physiological function, cell-cell communication occurs through the highly regulated exocytotic process wherein granules dock on the cell membrane, using SNARE proteins to assist in regulating attachment. These proteins are localized to cholesterol-rich microdomains throughout the cell membrane (1). The amount of cholesterol in these domains not only plays a role in the proper clustering of SNARE proteins, but also helps stabilize the negative curvature needed for formation and stabilization of the fusion pore between the docked and primed granule and the cell membrane. Cholesterol levels and membrane viscosity are directly correlated, and increasing viscosity is known to result in delayed movement of lipids between the leaflets, which has been hypothesized to increase fusion pore formation and closing times (2, 3). Chemical messenger molecules can be released through this dynamic fusion pore structure into the extracellular space. Literature precedent has shown that cholesterol has a role in controlling exocytosis, but the extent to which it dictates the opening and closing of the fusion pore structure requires more exploration, ideally with a method that can quantitatively assess this dynamic structure (2, 3).
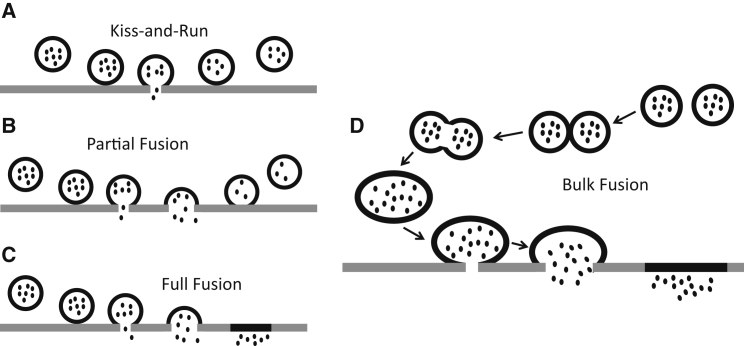
In recent years, it has become clear that the process of granule fusion is more intricate than originally thought. Traditionally, exocytosis was considered to be an all-or-nothing process, but in the last decade the definition has changed to include kiss-and-run events where a granule temporarily fuses with the cell membrane before detaching. More recently, it has become apparent that most granule release events are in fact extended kiss-and-run events, which do not result in full chemical messenger content release (4, 5, 6, 7). Schematic portrayals of these various exocytosis events can be seen in Fig. 1, A–C. Further countering the traditional view of exocytosis, individual granules have also been observed combining with one another, either before or during release, resulting in a process termed compound exocytosis (8, 9). However, in platelets, our chosen exocytosis model due to its lack of nucleus and therefore decreased ability to up- or down-regulate proteins, granules can also fuse to the open canalicular system (OCS). The OCS is a tubular system located throughout the platelet, which is hypothesized to assist in the release of granular content into the extracellular environment (10). At the OCS-cytosol interface, it is possible for two granules to attach near one another and release their contents into the OCS where the contents can combine and release simultaneously into the extracellular environment. This phenomenon could lead to chemical messenger release measurements characteristic of compound exocytosis. Unfortunately, current measurement technology does not allow us to distinguish between these two forms. Therefore, we will describe all events with large amounts of serotonin release, typically larger than the average amount released in two separate granules (>960 fC) and a 10/90 slope of six or greater, as bulk fusion (Fig. 1 D).
Figure 1.
Various proposed modes of exocytosis, from least to most chemical messenger release. (A) Kiss-and-Run: in kiss-and-run events, the granule fuses briefly with the plasma membrane, releasing only a small portion of its contents without significant dilation of the fusion pore. (B) Partial fusion: in partial fusion events the granule initially fuses with the plasma membrane, releases some of its contents, and then closes and returns to the platelet cytoplasm. (C) Full fusion: in full fusion events, the granule fully fuses with the plasma membrane and releases all of its contents. (D) Bulk fusion: in bulk fusion, multiple granules are hypothesized to fuse with one another before fusing with the plasma membrane, this is commonly referred to as compound fusion. However, platelet granules can adhere to the OCS and release their contents into the extracellular environment together, an event indistinguishable from compound fusion using CFMA, therefore, we termed all of these events as bulk fusion.
Even with this limitation, carbon-fiber microelectrode amperometry (CFMA) is an ideal technique for studying the dynamic variations in granule release events. In platelets, the only electroactive compound at 700 mV versus Ag/AgCl is serotonin, which has been confirmed by cyclic voltammetry (11, 12). Statistical analysis has demonstrated that due to the size of the cell relative to the electrode and the distance between the electrode and platelet, nearly 100% of all serotonin is detected (11). Electrical fouling is also checked by monitoring the root mean-square current (IRMS) noise on the electrode and replacing the electrode if IRMS changes significantly. Therefore, monitoring the oxidation of serotonin, found in the dense-body granules, gives us a means to study changes in the pore dynamics when microelectrodes are placed in immediate contact with individual platelets. As each granule fusion pore opens, an increase in current is detected as secreted serotonin is oxidized, resulting in a current spike. In some cases, an individual current spike will be flanked either before or after the current maximum by a smaller current feature known as a “foot” event; these foot events correspond to the relatively small number of serotonin molecules released when a fusion pore is not fully dilated. Due to the small number of granules in platelets and length of time between granule release events, it is possible to distinguish the start and end of each granule event, including feet events, without overlap from other granule release events. This event resolution allows analysis of the variations in each spike profile, revealing mechanistic biophysical information about pore fusion behavior for each granular release event. Because cells do not typically release their entire content and there is a limited quantity of dense-body granules, it is possible that several of the recorded spikes in each cell amperometric trace are from the same granule. This work examines the modes of pore formation and closure and how membrane cholesterol levels impact these modes. Results indicate that as the cholesterol membrane content increases, there is a corresponding increase in the number of release events with distinguishing characteristics. In particular, an increase in the number of nontraditional events (NTEs) was observed. It was also noted that, at greater levels of cholesterol, the NTEs have a slower fusion pore opening, increased release of serotonin molecules during each release event, and a longer duration in which the fusion pore remains open.
Materials and Methods
Amperometric traces from individual rabbit platelets were recorded previously by Shencheng Ge using CFMA (2). Briefly, platelet cholesterol levels were manipulated by exposing platelets either to methyl-β-cyclodextrin (MβCD) (Sigma-Aldrich St. Louis, MO) to achieve cholesterol depletion or MβCD complexed with cholesterol (Sigma-Aldrich) to achieve cholesterol enrichment. Platelets with varying levels of cholesterol were stimulated using 10 μM ionomycin and 2 mM Ca2+ in Tyrode’s buffer. The released serotonin was oxidized by an in-house fabricated carbon-fiber microelectrode held at 700 mV versus a Ag/AgCl reference electrode held by an Axopatch 200B potentiostat (Molecular Devices, Sunnyvale, CA), and the resulting current was measured as a function of time. Each collected current versus time trace was filtered at 5 KHz by a four-pole Bessel filter ahead of further analysis.
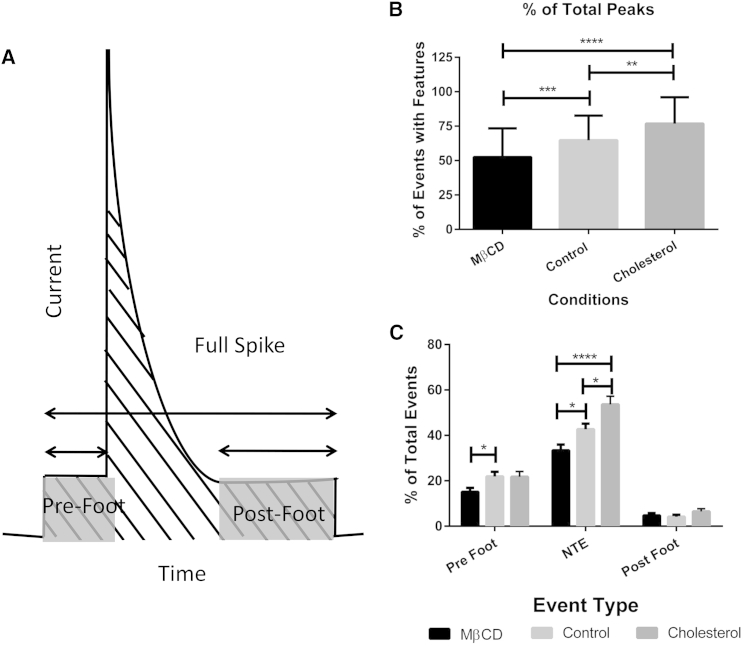
Each amperometric trace (N = 75, 72, and 50 for cholesterol depleted, control, and cholesterol-loaded platelets, respectively) was then digitally filtered at 1000 Hz and analyzed using Mini Analysis software (Snyptosoft, Fort Lee, NJ) for pre- and postspike feet. The peaks were analyzed visually by using a gain of 15 and a block of 20 unless the current spike was too large for the frame, in which case the gain was decreased so that the entire peak was visible within the frame. After examining each trace, the various features were divided into categories based on the general shape of the release event, resulting in pre- and postfeet and NTEs (Figs. S1 A and S3 A in the Supporting Material; see also Fig. 6 A). Each peak was analyzed manually using the group analysis and curve fitting option to determine the duration and area of both the foot alone and the peak and foot features combined. Schematic representation of these features can be seen in Fig. 2 A. For NTEs, the total event area and duration were measured. The percent frequency of each feature (prefoot, postfoot, or NTE) was determined by dividing the total number of events with that feature by the total number of release events in each trace. Within each feature type, the percent frequency was accounted for with respect to the total number of events with that feature (for example, within each trace, a prefoot type was compared to the total number of granule release events with prefoot features). Significance (p < 0.05) was determined between conditions by one-way analysis of variance with all error given as the standard error of the mean.
Figure 6.
Nontraditional events. (A) Representative traces of the different types of NTE peaks. The representative traces are all on the same current and timescale. (B) Relative frequency for each type of NTE under the different cholesterol treatments.
Figure 2.
Classes of peaks. (A) Diagram indicating the parameters used to categorize and quantify various peaks. (B) The frequency of events with features upon the different cholesterol treatments. (C) Relative frequency of classes of peaks with the different cholesterol treatments. ∗p ≤ 0.05, ∗∗p ≤ 0.01, ∗∗∗p ≤ 0.001, ∗∗∗∗p ≤ 0.0001.
Nontraditional events
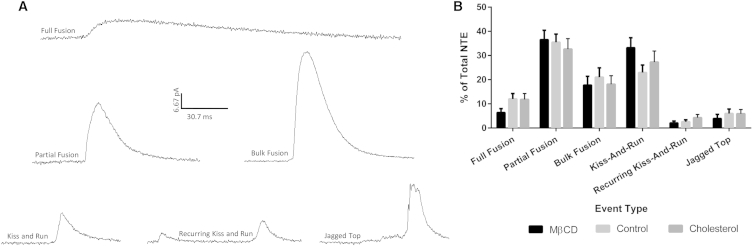
Nontraditional events are current spikes that do not display the typical quick current rise due to serotonin release followed by a gradual current decay as serotonin diffuses away. These NTEs were subdivided into six different categories (full fusion, partial fusion, bulk fusion, kiss-and-run, recurring kiss-and-run, and jagged top) based on the area (charge, Q) under each peak, which is proportional, through Faraday’s Law, to the amount of serotonin released, and how quickly the fusion pore opened relative to its maximum release (slope10/90, the slope between 10% and 90% of maximal current intensity) (Table 1). Jagged top features were distinguished by continuous up and down fluctuations of serotonin release at maximum opening, and recurring kiss-and-run displayed several kiss-and-run events within a second of one another.
Table 1.
Nontraditional Events Were Characterized by Their 10/90 Slope and Area
| Name | 10/90 Slope | Area (fC) | Percent Release | Release Mode |
|---|---|---|---|---|
| Kiss-and-Run | <6 | <100 | 0–20 | small release |
| Partial Fusion | <6 | 100 < area < 385 | 20–80 | partial release |
| Full Fusion | <6 | 385 < area | 80–200 | full release |
| Bulk Fusion | >6 | 960 < area | >200 | OCS or combining granule release |
As NTEs are often characterized by slower pore opening, the 10/90 slope was determined to be a good indicator of NTEs. To distinguish between the different types of NTEs the extent of release, or area under the current spike, was used. Commonly, events releasing greater than twice the amount of expected serotonin, had steep 10/90 slopes that were most likely due to more serotonin occupying the larger free space outside the dense-body core. Therefore, bulk fusion events had a 10/90 slope > 6. All slopes and areas were calculated using Mini Analysis software.
The cutoff criteria categorizing events with a 10/90 slope under 6 pA/ms was chosen empirically based on the manual analysis of many spikes that represented traditional and nontraditional release events. Nontraditional release events were further categorized based upon the amount of serotonin released. To set an upper limit on the release that could be attributed to kiss-and-run, we averaged the peaks in the control platelets considered to be kiss-and-run and found the highest value within one standard deviation of the mean, and then made this our maximum release value. Previous data obtained by high-performance liquidity chromatography indicated that a 5-min ionomycin stimulation on rabbit platelets resulted in the average release of 52% of the total serotonin content (13). The average area (250 fC per granule in control platelets) was thus assumed to be 52% content release. Full fusion was set as a minimum of 80% content release, or 385 fC/event. Release events where granule fusion resulted in at least 200% content release, or >970 fC, and contained a 10/90 slope of 6 pA/ms or greater were categorized as bulk fusion events. For NTEs, the event area and event duration of the entire peak was analyzed. Example traces of NTEs can be found in Fig. 6 with additional details in Table 1.
Results and Discussion
Based upon previously established methods of release, exocytosis events have been categorized herein into four types: 1) kiss-and-run, 2) partial fusion for both normal pore opening rates (10/90 slope > 6 pA/ms) and extended pore opening rates (10/90 slope < 6 pA/ms) (when discussing partial fusion for the remainder of the work, we are referring to the extended pore opening rates and not the traditional spike formation), 3) full fusion, and 4) bulk fusion (including both granule-granule fusion and bulk OCS fusion). The different models are illustrated schematically in Fig. 1 (4, 5, 6, 7, 8, 9).
After platelets were enriched in cholesterol or depleted of cholesterol by platelet exposure to MβCD (132% and 68%, respectively, compared to control platelet cholesterol levels) (2), the total percentage of events with either a foot or NTE release profile increased with increasing concentrations of cholesterol (52.6 ± 2.4%, 64.7 ± 2.2%, and 76.8 ± 2.8% for depleted, normal, and cholesterol-enriched platelets, respectively, Fig. 2 B). Normal levels of foot events reported with CFMA are traditionally around 10–30% (2, 13, 14), which is nominally lower than what we report; this discrepancy is likely due to our inclusion of nontraditional type events and postfoot-containing events when determining the percentage of events with features of interest. When only events with prefoot characteristics are taken into account, the percentage of events falls in previously described ranges with 15.2 ± 1.8%, 21.9 ± 2.0%, and 21.8 ± 2.3% for increasing cholesterol concentrations, respectively (Fig. 2 C). Postfoot events, which have been known to only occur ∼2% of the time in PC-12 cells (6), occur relatively infrequently (4.8 ± 1.0%, 4.2 ± 0.8%, and 6.5 ± 1.2% for increasing cholesterol concentrations) in platelets and do not change significantly as the cholesterol content is changed. Changes in platelet membrane cholesterol have the largest impact on secretion via NTEs, with statistically significant increases from 33.5 ± 2.5% for cholesterol-depleted platelets to 53.6 ± 3.6% of the events for cholesterol-enriched platelets, with a value of 42.7 ± 2.5% in control platelets. This suggests that cholesterol has a large impact on the whole fusion event rather than just the initial opening or closing of the pore. This difference is not attributable to a change in the number of granules undergoing exocytosis as the membrane cholesterol levels change. By counting the number of peaks in each trace, on average, each platelet released 12.7 ± 0.7, 12.6 ± 0.9, and 13.1 ± 0.9 granules per cholesterol-depleted, control, and cholesterol-enriched platelet, respectively (p > 0.05).
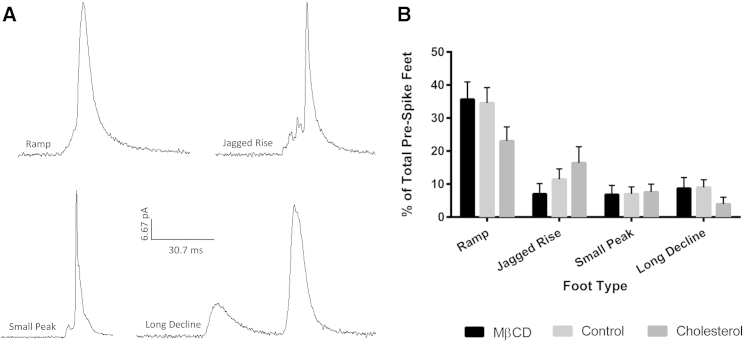
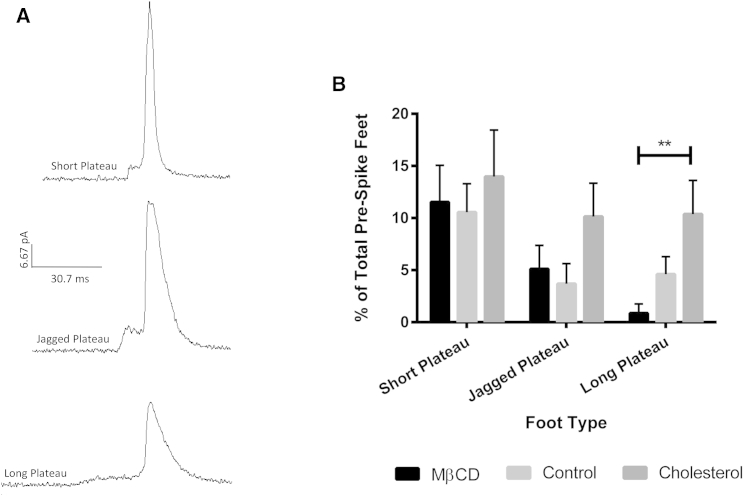
The occurrence of prespike foot events only changes significantly as seen in a decrease for cholesterol-depleted platelets (p ≤ 0.05). When the prefoot events are broken down into subtypes, the majority of the prefeet were present in two forms: those where the initial release took some sort of ramp (an initial slow gradual opening) or those where the initial release took the form of a plateau (an initial opening that is held steady over an extended period of time) (Figs. 3 and 4, respectively). These two general characteristics have been reported before in several works (6, 14, 15). The most commonly reported and most discussed prespike foot type is the ramp. Amatore and colleagues reported that in chromaffin cells, of their prespike feet events, 70% were ramps, 20% were ramps with plateaus, and 10% were unclassified. They then found a positive correlation between the ramps’ maximum current and the maximum current given by the spike (14). Our data show that the ramp type prespike foot was the most frequently occurring of the prespike foot types, at 35.7 ± 5.3%, 34.6 ± 4.7%, and 23.0 ± 3.8% for cholesterol-depleted, control, and cholesterol-enriched platelets, respectively. For the depleted and control conditions, the percent occurrence of the ramp was significantly enhanced (p ≤ 0.0001) compared to the other prespike foot types. The ramp frequency in cholesterol-enriched platelets was only significantly different from four out of the nine other prespike feet types (p ranging from 0.05 to 0.0001). This, along with previous work in other groups, leads us to believe that this ramp characteristic is representative of the traditional entryway for granular fusion. The ramp-like variants, such as jagged rise, could potentially correspond to the opening and closing of the fusion pore during formation, suggesting that the cell is not able to easily transition to the type of curvature needed in the membrane as the fusion pore opens. This inability to rapidly change the membrane curvature paired with increased membrane stiffness is also implicated in the significant increase in the occurrence of long plateau foot events between the cholesterol-depleted and cholesterol-enriched conditions (p ≤ 0.01). In addition, the increase in the duration of the short plateau foot relative to the whole event as cholesterol content increases suggests that increasing membrane rigidity results in a barrier to the pore opening to the proper form (Fig. S2 B).
Figure 3.
Prespike foot events with ramp-like character. (A) Representative traces of the different types of prespike feet that had a ramp-like characteristic. The representative traces are all on the same current and timescale. (B) Relative frequency for each type of ramp-like prespike foot with the different cholesterol treatments.
Figure 4.
Prespike foot events with plateau-like character. (A) Representative traces of the different types of prespike feet that had a plateau-like characteristic. The representative traces are all on the same current and timescale. (B) Relative frequency for each type of plateau-like prespike foot with the different cholesterol treatments. ∗∗p ≤ 0.01.
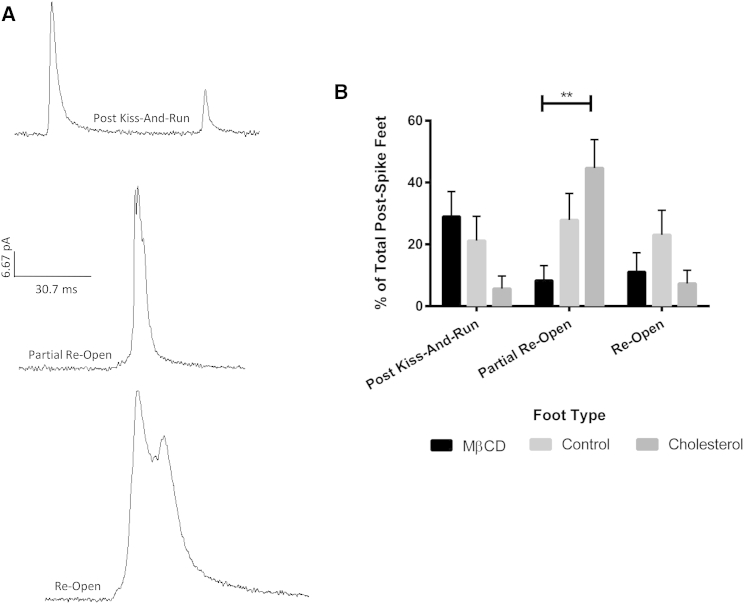
As the postspike feet were not very prevalent, it was difficult to identify variation in their forms. In total, eight different types of postspike feet were identified, three of which were categorized as different forms of reopening (Figs. S3 and 5). Of the reopening type postspike feet, the partial reopening type foot showed a significant difference in frequency between the cholesterol-depleted and cholesterol-enriched conditions, with the foot occurring 8.3 ± 4.8% and 46.5 ± 9.4% of the time, respectively. Because the significant increase is only seen in the partial reopen postfoot event, where the pore begins to reopen directly after beginning to close, it indicates that the pore initially has difficulty closing, but after closure has been initiated, the cholesterol levels do not impact the process to the extent they do for prefoot events.
Figure 5.
Events characterized by reopening. (A) Representative traces of peaks containing post kiss-and-run, partial reopen, and reopen postfoot characteristics. The representative traces are all on the same current and timescale. (B) Frequency of occurrence for the different postspike foot types. ∗∗p ≤ 0.01.
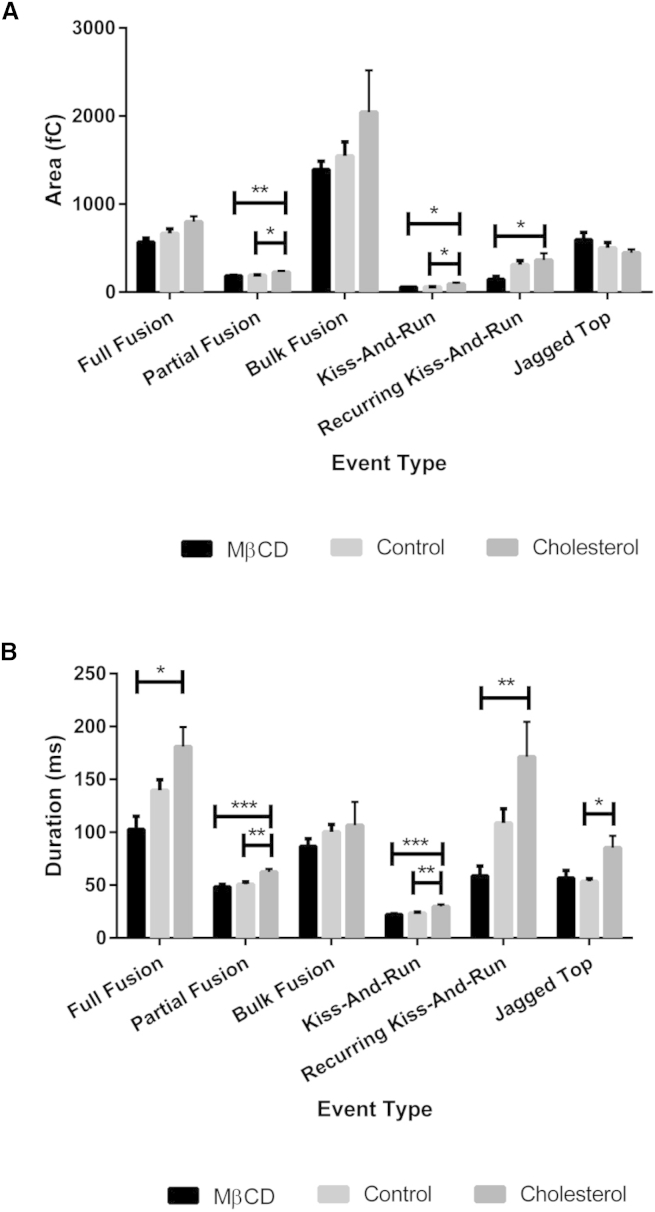
The most interesting results arising from categorization of the spikes observed upon platelet activation was the prevalence of spikes that were not in the form of traditional release events, meaning that they lacked the typical fast rising current due to pore opening followed by a slow current decrease as serotonin diffuses out of the closing pore. These release events were sorted into six categories by examining a combination of the following characteristics: 10/90 slope, the lack of a foot character, and the area (Fig. 6). Table 1 gives the 10/90 slope and area characteristics for four out of the six types of NTEs observed. Although the NTEs as a category showed significant increases in percent frequency of total events as the cholesterol increases (Fig. 2 C), when the NTEs are broken down into the various exhibited types, no significant changes are observed where one specific category is affected by the amount of cholesterol (Fig. 6). However, there are significant changes in the area and duration for the various features as the membrane-cholesterol levels are changed (Fig. 7). Most surprisingly, these changes in duration and area are seen in events that have set upper and lower limits for the amount of serotonin released, including partial fusion and kiss-and-run events. When comparing the average amount of serotonin released per ms during kiss-and-run events, there is no significant difference between cholesterol conditions (Fig. S5 A). This suggests that the cholesterol helps the pore stay open longer, but does not change the size of the pore, assuming that the serotonin diffusion characteristics are unchanged. Therefore, with the small standard deviation associated with each event type’s duration and amount of serotonin released and the fact that there is no difference in the rate of serotonin release between conditions, this suggests that all granules going through kiss-and-run will follow a similar pattern of release. In comparison, for partial fusion, there were no significant differences in rate of release, but a downward trend was noted as membrane cholesterol content increased (Fig. S5 B). This suggests that once the pore opens past the kiss-and-run stage, it may not open as wide at higher cholesterol levels. However, due to the longer length of time it stays open, more serotonin is released, which serves to make up for this decreased rate of release. Finally, there is an apparent upward trend in percent occurrence for full fusion events and a corresponding apparent downward trend for partial fusion events as the conditions contribute to higher membrane cholesterol levels. These trends can all be explained by increased membrane rigidity due to the additional cholesterol. As previously hypothesized, an increase in cholesterol would lead to longer inversion times for the phospholipids needing to change the curvature along the fusion pore, biasing toward full release events.
Figure 7.
Area and duration of nontraditional events. (A) Average area of each NTE type under the different cholesterol treatments. (B) Average duration of each NTE type under the different cholesterol treatments. ∗p ≤ 0.05, ∗∗p ≤ 0.01, ∗∗∗p ≤ 0.001.
Conclusions
Close examination of the prefoot and postfoot spike features from individual platelets with manipulated outer membrane cholesterol levels indicated a few styles of pore opening and closing. Prefoot features tended to fall into two subcategories: ramp-like and plateau-like, with the ramp occurring most frequently. There was a slight decrease in the occurrence of prefoot features in cholesterol-depleted platelets, indicating that the pore opens more quickly at low cholesterol levels, bypassing the slower foot-inducing features. The postfoot features occurred fairly infrequently and had primarily reopening characteristics. The most prevalent category of nontraditional secretion events showed an increase in frequency as the cholesterol content of the membrane increased. These events are characterized by an increase in the amount of serotonin released, increased duration of the pore opening, and a slowing in the opening of the pore. From these analyses, we conclude that as the cholesterol concentration in platelets is increased, the membrane-granule fusion curvature transitions are slowed due to increased rigidity, leading to a slower pore opening and closing.
Even though the fusion pore dynamics of human platelets have not been studied directly with CFMA, it has been shown that platelets from other species, particularly the cow, which has comparatively increased levels of cholesterol, demonstrate similar fusion pore dynamics as the rabbit platelets incubated with cholesterol. These included increased Trise and T1/2 times compared to rabbit platelets without cholesterol modification, which are often associated with NTEs (2, 13). In addition, because platelets are anucleate, the up- and down-regulation of proteins and receptors is limited, suggesting that the only difference between the conditions was the concentration of cholesterol. Therefore, we believe these results, though they are measured in a model system, can be generalized to human platelets and other cell types that undergo exocytosis.
Human platelets have been shown to undergo changes in cholesterol membrane content due to various diseases, drugs, or predispositions (16, 17). In addition, platelets are known to increase cholesterol uptake in vitro when suspended in cholesterol-rich environments (18). Previous literature indicates that cholesterol plays a role in human platelet sensitivity, microtubule ring formation, and aggregate size due to cholesterol levels (18, 19). Thus, it is likely that the results presented here can inform understanding of human platelets and the diseases affecting them.
Author Contributions
Peak analysis, data discussion, article writing, tables, and figures were equally worked on by S.M.G. and S.F.Q. Graphing and analyzing final cholesterol-depleted and control data was done by S.M.G. Graphing and analyzing cholesterol-loaded data and initial figure design was done by S.F.Q. Guidance, funding, and article writing was done by C.L.H.
Acknowledgments
The authors acknowledge Dr. Shencheng Ge for data collection.
The authors also acknowledge the National Institutes of Health (NIH) New Innovator award (DP2 OD004258-01) and the University of Minnesota for funding, as well as the National Institute of General Medical Science (NIGMS) Biotechnology Training Program Fellowship (5T32GM008347-23) for support to S.M.G.
Editor: Edward Stuenkel.
Footnotes
Solaire A. Finkenstaedt-Quinn and Sarah M. Gruba contributed equally to this work.
Five figures are available at http://www.biophysj.org/biophysj/supplemental/S0006-3495(16)00009-6.
Supporting Material
References
- 1.Salaün C., James D.J., Chamberlain L.H. Lipid rafts and the regulation of exocytosis. Traffic. 2004;5:255–264. doi: 10.1111/j.1600-0854.2004.0162.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ge S., White J.G., Haynes C.L. Critical role of membrane cholesterol in exocytosis revealed by single platelet study. ACS Chem. Biol. 2010;5:819–828. doi: 10.1021/cb100130b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wang N., Kwan C., Tse F.W. Influence of cholesterol on catecholamine release from the fusion pore of large dense core chromaffin granules. J. Neurosci. 2010;30:3904–3911. doi: 10.1523/JNEUROSCI.4000-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wightman R.M., Haynes C.L. Synaptic vesicles really do kiss and run. Nat. Neurosci. 2004;7:321–322. doi: 10.1038/nn0404-321. [DOI] [PubMed] [Google Scholar]
- 5.Omiatek D.M., Dong Y., Ewing A.G. Only a fraction of quantal content is released during exocytosis as revealed by electrochemical cytometry of secretory vesicles. ACS Chem. Neurosci. 2010;1:234–245. doi: 10.1021/cn900040e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mellander L.J., Trouillon R., Ewing A.G. Amperometric post spike feet reveal most exocytosis is via extended kiss-and-run fusion. Sci. Rep. 2012;2:907–912. doi: 10.1038/srep00907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mellander L.J., Kurczy M.E., Cans A.S. Two modes of exocytosis in an artificial cell. Sci. Rep. 2014;4:3847. doi: 10.1038/srep03847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cabeza J.M., Acosta J., Alés E. Mechanisms of granule membrane recapture following exocytosis in intact mast cells. J. Biol. Chem. 2013;288:20293–20305. doi: 10.1074/jbc.M113.459065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Staal R.G., Mosharov E.V., Sulzer D. Dopamine neurons release transmitter via a flickering fusion pore. Nat. Neurosci. 2004;7:341–346. doi: 10.1038/nn1205. [DOI] [PubMed] [Google Scholar]
- 10.White J. Platelet structure. In: Michelson A., editor. Platelets. Academic/Elsevier; Amsterdam: 2007. pp. 45–74. [Google Scholar]
- 11.Ge S., White J.G., Haynes C.L. Quantal release of serotonin from platelets. Anal. Chem. 2009;81:2935–2943. doi: 10.1021/ac8024202. [DOI] [PubMed] [Google Scholar]
- 12.Ge S., Wittenberg N.J., Haynes C.L. Quantitative and real-time detection of secretion of chemical messengers from individual platelets. Biochemistry. 2008;47:7020–7024. doi: 10.1021/bi800792m. [DOI] [PubMed] [Google Scholar]
- 13.Gruba S.M., Koseoglu S., Haynes C.L. Platelet membrane variations and their effects on δ-granule secretion kinetics and aggregation spreading among different species. Biochim. Biophys. Acta. 2015;1848:1609–1618. doi: 10.1016/j.bbamem.2015.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Amatore C., Arbault S., Guille M. Quantitative investigations of amperometric spike feet suggest different controlling factors of the fusion pore in exocytosis at chromaffin cells. Biophys. Chem. 2009;143:124–131. doi: 10.1016/j.bpc.2009.04.007. [DOI] [PubMed] [Google Scholar]
- 15.Cookson E.A., Conte I.L., Carter T. Characterisation of Weibel-Palade body fusion by amperometry in endothelial cells reveals fusion pore dynamics and the effect of cholesterol on exocytosis. J. Cell Sci. 2013;126:5490–5499. doi: 10.1242/jcs.138438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hochgraf E., Levy Y., Cogan U. Lovastatin decreases plasma and platelet cholesterol levels and normalizes elevated platelet fluidity and aggregation in hypercholesterolemic patients. Metabolism. 1994;43:11–17. doi: 10.1016/0026-0495(94)90151-1. [DOI] [PubMed] [Google Scholar]
- 17.Lijnen P., Echevaría-Vázquez D., Petrov V. Influence of cholesterol-lowering on plasma membrane lipids and function. Methods Find. Exp. Clin. Pharmacol. 1996;18:123–136. [PubMed] [Google Scholar]
- 18.Shattil S.J., Anaya-Galindo R., Cooper R.A. Platelet hypersensitivity induced by cholesterol incorporation. J. Clin. Invest. 1975;55:636–643. doi: 10.1172/JCI107971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Grgurevich S., Krishnan R., Jennings L.K. Role of in vitro cholesterol depletion in mediating human platelet aggregation. J. Thromb. Haemost. 2003;1:576–586. doi: 10.1046/j.1538-7836.2003.00087.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.