Abstract
Background
Chronic heart failure (HF) remains a leading cause of cardiovascular (CV) mortality and morbidity worldwide. The aim of the study was to investigate whether the pattern of angiogenic endothelial progenitor cells (EPCs) and apoptotic endothelial cell-derived microparticles (EMPs) would be able to differentiate HF with reduced (HFrEF) and preserved (HFpEF) ejection fraction.
Methods
One hundred sixty four chronic HF subjects met inclusion criteria. Patients with global left ventricular ejection fraction ≥ 50% were categorized as the HFpEF group (n = 79) and those with ≤ 45% as the HFrEF group (n = 85). Therefore, to compare the circulating levels of biological markers 35 control subjects without HF were included in the study. All control individuals were age- and sex-matched chronic HF patients. The serum level of biomarkers was measured at baseline. The flow cytometric technique was used for predictably distinguishing circulating cell subsets depending on expression of CD45, CD34, CD14, Tie-2, and CD309 antigens and determining endothelial cell-derived microparticles. CD31+/annexin V+ was defined as apoptotic endothelial cell-derived MPs, MPs labeled for CD105+ or CD62E+ were determined as MPs produced due to activation of endothelial cells.
Results
In multivariate logistic regression model T2DM (R2 = 0.26; P = 0.001), obesity (R2 = 0.22; P = 0.001), previous MI (R2 = 0.17; P = 0.012), galectin-3 (R2 = 0.67; P = 0.012), CD31+/annexin V+ EMPs (R2 = 0.11; P = 0.001), NT-proBNP (R2 = 0.11; P = 0.046), CD14+ CD309+ cells (R2 = 0.058; P = 0.001), and CD14+ СD309+ Tie-2+ cells (R2 = 0.044; P = 0.028) were found as independent predictors of HFpEF. Using multivariate Cox-regression analysis adjusted etiology (previous myocardial infarction), cardiovascular risk factors (obesity, type 2 diabetes mellitus) we found that NT-proBNP (OR 1.08; 95% CI = 1.03–1.12; P = 0.001) and CD31+/annexin V+ EMPs to CD14+ CD309+ cell ratio (OR 1.06; 95% CI = 1.02–1.11; P = 0.02) were independent predictors for HFpEF.
Conclusion
We found that CD31+/annexin V+ EMPs to CD14+ CD309+ cell ratio added to NT-proBNP, clinical data, and cardiovascular risk factors has exhibited the best discriminate value and higher reliability to predict HFpEF compared with NT-proBNP and clinical data/cardiovascular risk factors alone.
Keywords: Chronic heart failure, Preserved left ventricular function, Biomarkers, Endothelial progenitor cells, Endothelial cell-derived microvesicles
Highlights
-
•
Endothelial progenitors and apoptotic endothelial microparticles contribute in regulation of endothelial function
-
•
Heart failure associates with decreased endothelial progenitors and increased apoptotic endothelial microparticles
-
•
Imbalance between endothelial progenitors and apoptotic endothelial microparticles reflects impaired immune phenotype
-
•
Endothelial progenitors to apoptotic endothelial microparticles ratio might be a surrogate predictor of heart failure
We have searched the clinical studies depicted predictive value of progenitor mononuclears and apoptotic endothelial microparticles in heart failure with preserved (HFpEF) and reduced (HFpEF) left ventricular ejection fraction. Our results have shown that simple measurement of circulating pool of endothelial-derived progenitor cells or apoptotic endothelial microparticles were not probably allowed to create novel model for risk stratification of patients with HEpEF. We suggest that CD31+/annexin V+ EMPs to CD14+ CD309+ cell ratio might be considered as an essential tool for prediction and individualized medical care of HFpEF patients.
1. Introduction
Chronic heart failure (HF) remains a leading cause of cardiovascular (CV) mortality and morbidity worldwide (Go et al., 2014). Although over the last decades the incidence of newly HF in developed countries have been substantially declined particularly for HF with reduced ejection fraction (HFrEF) (Gerber et al., 2015), there is marked increase in hospital admissions, CV and non-CV death rate predominance of HF with preserved ejection fraction (HFpEF) (Dunlay et al., 2015, Jorge et al., 2015). As expected, the routine use of biomarkers on diagnosis of HFrEF and HFpEF might help stratify the patients at higher risk of death and clinical outcomes. In fact, both 2012 European Society of Cardiology (ESC) Guidelines for the Diagnosis and Treatment of Acute and Chronic Heart Failure and 2013 American College of Cardiology Foundation/American Heart Association (ACCF/AHA) Guideline for the Management of Heart Failure are well accepted by many clinicians regarding HFrEF diagnosis. Indeed, the HFpEF is that one that really needs improvement of biomarkers for diagnosis and prognosis (McMurray et al., 2012, Yancy et al., 2013). In this context, many biological markers, which reflect several faces of pathogenesis of HF, have been investigated in detail, but by now natriuretic peptides, soluble ST2, galectin-3, and high-sensitive cardiac specific troponins were validated only. However, there was not a large body of evidence regarding perspectives that may provide clinically useful prognostic information both concerning the future risk of HFpEF/HFrEF manifestation in asymptomatic subjects, the risk of fatal events and primary/re-admissions in the hospital in individuals for those have already established symptomatic acute, acutely decompensated/advanced, and chronic stable HF related to ischemic and non-ischemic causes (D'Elia et al., 2015). It is suggested that multimorbidity in HF may limit the diagnostic and predictive utility of biomarkers (Chamberlain et al., 2015).
Recent studies showed that endothelium injury is common for HF onset and development beyond etiology (Fujisue et al., 2015). Endothelial dysfunction closely associates with activation and/or apoptosis of endothelial cells lead to release of newly detectable circulating biomarkers related to endothelial dysfunction called endothelial cell-derived microparticles (EMPs) (Dignat-George and Boulanger, 2011, Burger and Touyz, 2012). Human CD34+ primitive progenitors and CD14+ monocytic progenitors have exhibited pro-angiogenic capacities mediated through increased sensitivity to vascular endothelial growth factor and cell-to-cell cooperation via secretion of endothelial cell-derived microparticles (Awad et al., 2006, Burger and Touyz, 2012).
Therefore, endothelial progenitor cells (EPCs) labeled as CD14+ CD309+(VEGFR2) and CD14+ CD309+(VEGFR2) Tie-2 + cells were found a marker of endothelial dysfunction and reparation ability (Dignat-George and Boulanger, 2011). It has been suggested that imbalance between EPCs with angiogenic capacity and apoptotic EMPs contributed in cell injury and endothelial dysfunction may reflect impaired reparative phenotype that is suitable for several CV diseases including HF (Berezin, 2015a, Berezin and Kremzer, 2015a, Berezin and Kremzer, 2015b, Berezin et al., 2015a). Indeed, endogenous deficiency of angiopoetic stimuli mediated by secretion of pro-inflammatory cytokines, neurohormones, growth factors, might lead to worsening endothelium reparation and HF progression (Singh et al., 2012, Berezin et al., 2015b). Recently we have reported that apoptotic EMP to EPC ratio might independently predict clinical outcomes in advanced chronic HFrEF patients (Berezin et al., 2015c). However, whether impaired reparative phenotype might reflect a development of HFpEF and HFpEF is still not clear. The aim of the study was to investigate whether the pattern of endothelial progenitor cells with angiogenic capacity and apoptotic endothelial cell-derived microparticles would be able to associate with HFpEF and HFpEF phenotypes.
2. Methods
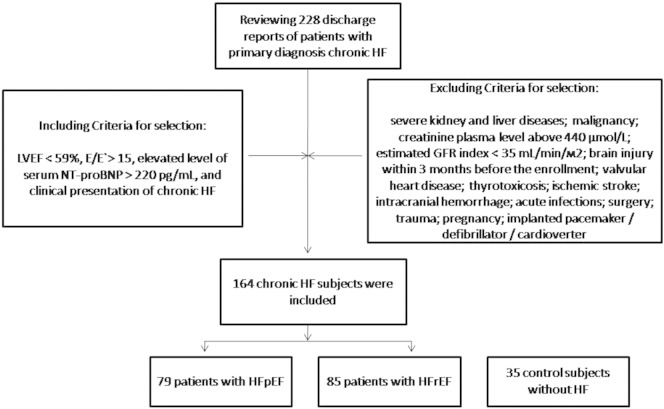
A total of 228 subjects suspected chronic HF were selected in this study after reviewing discharge reports. All these persons were treated in Zaporozhye Regional Hospital, City Hospital #6, City Hospital #10, Zaporozhye Regional Center of Cardiovascular Diseases from April 2010 to June 2015 with primary diagnosis chronic HF.
Chronic HF was defined according to contemporary criteria provided by actual clinical recommendation (McMurray et al., 2012). HFrEF (LVEF ≤ 45%) and HFpEF (LVEF ≥ 50%) were determined accordingly this recommendation. T2DM was diagnosed with revised criteria provided by American Diabetes Association when source documents were reviewed (Executive summary, 2013). When one or more of the following components were found (glycated hemoglobin [HbA1c] ≥ 6.5%; fasting plasma glucose ≥ 7 mmol/L; 2-h plasma glucose ≥ 11.1 mmol/L during an oral glucose tolerance test; a random plasma glucose ≥ 11.1 mmol/L; exposure of insulin or oral anti-diabetic drugs; a previous diagnosis of T2DM) T2DM was determined. Dyslipidemia was checked and determined according to NCEP Adult Treatment Panel III (National Cholesterol Education Program) (National Cholesterol Education Program Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults, 2002).
Including criteria for selection of the HF patients in the study were LVEF < 59%, ratio of mitral peak velocity of early filling (E) to early diastolic mitral annular velocity (E’) [E/E’] ratio > 15 units, elevated level of serum NT-proBNP > 220 pg/mL, and clinical presentation of chronic HF. Excluding criteria were severe kidney and liver diseases; malignancy; creatinine plasma level above 440 μmol/L; estimated GFR index < 35 mL/min/m2; brain injury within 3 months before the enrollment; valvular heart disease; thyrotoxicosis; ischemic stroke; intracranial hemorrhage; acute infections; surgery; trauma; pregnancy; implanted pacemaker/defibrillator/cardioverter.
The flow chart representing patient in the study is reported in Fig. 1.
Fig. 1.
The flow chart representing patients in the study.
Among these 228 prescreened subjects, only 164 chronic HF subjects were included in the study accordingly inclusion/exclusion criteria. Patients with global left ventricular ejection fraction > 55% were categorized as the HFpEF group (n = 79) and those with ≤ 45% as the HFrEF group (n = 85). Therefore, to compare the circulating levels of biological markers 35 control subjects without HF were included in the study. To compare EPCs and microparticles between healthy subjects, HFpEF and HFrEF individuals control group was made. Control subjects are defined as individuals with normal global cardiac function (LVEF > 55%, E/E′ ratio < 8 units) assessed by transthoracic echocardiography and Tissue Doppler Imaging, serum NT-proBNP level < 125 pg/mL, and without any signs and symptoms of symptomatic HF (Fig. 2).
Fig. 2.
The sample of flow cytometry picture represented CD14+ CD309+ cells (panel A) and CD31+/annexin V+ EMPs (panel B).
2.1. Ethical Statement
The study protocol was approved by the local Ethics Committee Review Board (IRB # 3/2010), State Medical University of Zaporozhye (Ukraine) prior to the study initiation. The study complied with the Declaration of Helsinki and voluntary informed written consent was obtained from all patients included in this study. All individuals included in the study have given voluntary informed written consent.
2.2. Anthropometric Measurements
Anthropometric measurements were made using standard procedures.
2.3. Echocardiography and Doppler Imaging
Transthoracic B-mode echocardiography and Tissue Doppler Imaging were performed according to a conventional procedure on ACUSON scanner (Siemens, Germany) using phased probe with modulated frequency of 2.5–5 МHz. Left ventricular end-diastolic and end-systolic volumes, and LVEF were measured by modified Simpson's method (Quiñones et al., 2003). E/E′ ratio was measured using pulsed wave Tissue Doppler Imaging according contemporary protocol (Paulus et al., 2007).
2.4. Glomerular Filtration Rate Measurement
Calculation of glomerular filtration rate (GFR) was calculated by CKD-EPI formula (Levey et al., 2009).
2.5. Blood Sampling
After an overnight fast blood samples were drawn in the morning (at 7–8 a.m.) into cooled silicone test tubes wherein 2 mL of 5% Trilon B solution were added; then they were immediately centrifuged upon permanent cooling at 6000 rpm for 10 min. Then, plasma was refrigerated immediately to be stored at a temperature − 70 °С. All laboratory tests were performed using standard methods to measure the serum fasting plasma glucose, fasting lipid profiles and other biomarkers.
N-terminal pro-brain natriuretic peptide (NT-pro-BNP) level was measured by immunoelectrochemoluminescent assay using sets by R&D Systems (USA) on Elecsys 1010 analyzer (Roche, Mannheim, Germany). High-sensitive C-reactive protein (hs-CRP) was measured by commercially available standard kit (R&D Systems GmbH, Wiesbaden-Nordenstadt, Germany). Galectin-3 was measured using an ELISA kit (BG Medicine, Germany). Concentrations of total cholesterol (TC), cholesterol of high-density lipoproteins (LDL-C), and cholesterol of high-density lipoproteins (HDL-C) were measured by enzymatic colorimetric method according standardized methodology on Beckman Synchron LX20 chemistry analyzer.
2.6. Assay of Circulating Endothelial Progenitor Cell Subsets
The flow cytometric technique (FCT) was used for predictably distinguishing circulating cell subsets, which depend on expression of CD45, CD34, CD14, Tie-2, and CD309 (VEGFR2), using High-definition Fluorescence Activated Cell Sorter (HD-FACS) methodology (Tung et al., 2004). Accordingly, the cells were labeled on the basis of their forward scatter characteristic (FSC) and side scatter characteristic (SSC) profiles, and standardize and calibrate instruments, fluorescence and light scatter resolution, and sensitivity were determined according to standard protocol (Hoffman, 2005).
The cells were directly stained and analyzed for phenotypic expression of surface proteins using anti-human monoclonal antibodies, including anti-CD45 FITS (BD Biosciences, San Jose, CA, USA), anti-CD34 FITS (BD Biosciences), anti-VEGFR-2 known as anti-CD309 (BD Biosciences), anti-Tie-2 (BD Biosciences) and anti-CD14 (BD Biosciences). The fluorescence minus one technique was used to provide negative controls and establish positive stain boundaries. After lysis of erythrocytes with Utilize wash solution, the samples were centrifuged at 200 × g for 15 min. Then the samples were washed twice with PBS and fixed immediately. Double- or triple-positive events were determined using Boolean principles (“and”, “not”, “or”, etc.).
2.7. Determination of Circulating Endothelial Progenitor Cells
Circulating EPCs were defined as CD34/СD309 (VEGFR2) positive cells with lack of CD45 expression. From each tube 500,000 events were analyzed. For CD14+ populations, co-expression with Tie-2- and/or VEGFR-2- was determined using quadrant analysis. Standardized cell counts were presented as a percentage of the total of the white blood cell count, identified as the total number of all CD45+ cells. The FITC-labeled isotype control was analyzed with the same gate and window settings. Pro-angiogenic phenotype for EPCs was determined as CD14+ СD309+ (VEGFR2) Tie-2+ antigen presentation. The reproducibility of EPC measurements using the standard protocol was 3.5%.
2.8. Assay of Circulating Microparticles
Circulating MPs were isolated from 5 mL of venous citrated blood drawn from the fistula-free arm. To prevent contamination of samples platelet-free plasma (PFP) was separated from whole blood. PFP was centrifuged at 20,500 × rpm for 90 min. MP pellets were washed with DMEM (supplemented with 10 μg/mL polymyxin B, 100 UI of streptomycin, and 100 U/mL penicillin) and centrifuged again (20,500 rpm for 60 min). The obtained supernatant was extracted, and MP pellets were re-suspended into the remaining 200 μL of supernatant. PFP, MPs, and supernatant were diluted five-, 10-, and five-fold in PBS, respectively. Only 100 μL of supernatant was prepared for further analysis through incubation with different fluorochrome-labeled antibodies or their respective isotypic immunoglobulins (Beckman Coulter).
2.9. Determination of Endothelial Cell-derived Microparticles
MPs were labeled and characterized by flow cytometry technique per HD-FACS (High-definition Fluorescence Activated Cell Sorter) methodology independently after supernatant diluted without freeze (Orozco and Lewis, 2010). Two size gates were defined based on forward angle light scattering from polystyrene microsphere (0.5–0.9 μm) according to standard protocol (Shah et al., 2008). Accordingly, MPs' gate was defined less than a 0.4 μm polystyrene microsphere extending down to the noise threshold level that is equivalent to cell-derived MPs < 1 μm diameter (Lacroix et al., 2010).
CD31 antigen was determined as essential marker for endothelial cells. CD31+/annexin V+ was defined as apoptotic endothelial cell-derived MPs, MPs labeled for CD105+ or CD62E+ were determined as MPs produced due to activation of endothelial cells (Lacroix et al., 2013). We used anti-CD31 [(platelet endothelial cell adhesion molecule [PECAM]-1)]-phycoerythrin (PE; 20 μL/test), anti-CD62E [E-selectin]-FITC (20 μL/test) antibodies obtained from Beckman Coulter. MPs that expressed phosphatidylserine were labeled using fluorescein-conjugated Annexin V solution (20 μL/test; BD Biosciences, USA) in the presence of CaCl2 (5 mM) according to the recommendation of the supplier.
The samples were incubated in the dark for 15 min at room temperature according to the manufacturer's instructions. The analysis of area, height, and width forward scatter (FSC) and side scatter (SSC) parameters was performed as well as side scatter width (SSC-W). The gate for MPs was defined by size, using 0.5 and 1.0 μm beads (Sigma, St Louis, MO, USA). For each sample, 500 thousand events have been analyzed. Compensation tubes were used with similar reagents as were used in the sample tubes. Data were constructed as numerous of MPs depending on marker presentation (positive or negative) and determination of MP populations.
Calculation of the number of MPs per liter plasma was based upon the particle count per unit time, the flow rate of the flow cytometer, and the net dilution during sample preparation of the analyzed MP suspension. MP-exposed antigen concentrations were calculated in each sample by multiplying the total concentration of positive MPs by the mean fluorescence intensity of the antigen exposure of the total positive MP population. The reproducibility of EPCs using standard protocol was 4.5%.
2.10. Statistical Analysis
Data were analyzed using SPSS 20.0 (SPSS, IBM Corporation, NY, USA) and Prism v.6 (GraphPad Software Inc., La Jolla, CA, USA). Quantitative variables were expressed as mean (M) and standard deviation (± SD), median and interquartile range (IQR), estimated marginal mean (95% confidence interval [CI]) or number (percentage). An independent group t-test was used to compare all the interval parameters matching the criteria of normality and homogeneity of variance. For interval parameters that fail to match these criteria, the non-parametric Mann–Whitney test was used to compare variables. Categorical variables and frequencies were compared using Chi2 test and Fisher exact test of independence. Comparisons of control group with a combined population of both HF groups are done using ANOVA. The potential factors that may be associated with HFpEF were identified first with the univariate analysis (ANOVA), and then the independent predictors of HFpEF were searched with the multivariate one-step backward logistic regression analysis, initially including variables for which a P value of < 0.1 was achieved from the univariate analysis. R2, B-coefficient were calculated for all regression models. The odds ratio (OR) and 95% CI were calculated for factors independently predicted HFpEF vs HFrEH in Cox-regression model. For each model that is able to differentiate HFpEF from HFrEF OR (95% CI) and AUC [Area Under Curve] (95% CI) were calculated. A calculated difference of P < 0.05 was considered significant.
3. Results
The study population consisted of 164 HF subjects (86 males and 78 females), with mean age of 52.13 ± 7.80 years, and 35 healthy volunteers as control for biomarker examination. All control individuals were age- and sex-matched chronic HF patients. The baseline data of eligible individuals are listed in Table 1. We did not find any significant difference between both HF cohorts in age, sex, NYHA class representation, hypertension, adherence to smoke, body mass index (BMI), systolic and diastolic blood pressure (BP) and heart rate. Previous myocardial infarction (MI), dilated cardiomyopathy, dyslipidemia were determined frequently in subjects with HFrEF compared with persons with HFpEF. Contrary, obesity, and type 2 diabetes mellitus (T2DM) patients were found frequently in patients with HFpEF then in HFpEF patient cohort.
Table 1.
The characteristics of participants in the study.
| Variables | Healthy volunteers (n = 35) | Entire patient group (n = 164) | P value between healthy volunteers and entire HF group | Subjects with HFrEF (n = 85) | Subjects with HFpEF (n = 79) | P value between HF cohorts |
|---|---|---|---|---|---|---|
| Age, years | 54.85 ± 5.20 | 56.13 ± 7.80 | 0.44 | 57.50 ± 6.70 | 54.79 ± 6.62 | 0.78 |
| Male | 18 (51.4%) | 86 (52.4%) | 0.24 | 49 (57.6%) | 37 (46.8%) | 0.24 |
| II NYHA class | – | 57 (34.8%) | – | 29 (34.1%) | 28 (35.4%) | 0.96 |
| III NYHA class | – | 65 (39.6%) | – | 36 (42.4%) | 29 (36.7%) | 0.66 |
| IV NYHA class | – | 42 (25.6%) | – | 20 (23.5%) | 22 (27.8%) | 0.84 |
| Previous MI | – | 112 (62.3%) | – | 66 (77.6%) | 46 (58.2%) | 0.01 |
| Dilated cardiomyopathy | – | 21 (12.8%) | – | 18 (21.2%) | 3 (3.8%) | 0.001 |
| Hypertension | – | 104 (63.4%) | – | 53 (62.4%) | 51 (64.6%) | 0.88 |
| Dyslipidemia | – | 116 (70.7%) | – | 68 (80.0%) | 48 (70.8%) | 0.01 |
| T2DM | – | 38 (23.2%) | – | 15 (17.6%) | 23 (29.1%) | 0.01 |
| Obesity | – | 62 (37.8%) | – | 26 (30.5%) | 36 (45.6%) | 0.01 |
| Adherence to smoke | 9 (25.7%) | 35 (21.3%) | 0.68 | 18 (21.2%) | 17 (21.5%) | 0.98 |
| BMI, kg/m2 | 22.3 (20.1–23.5) | 24.5 (21.2–28.9) | 0.01 | 22.5 (20.6–25.2) | 25.1 (20.7–27.6) | 0.72 |
| Systolic BP, mm Hg | 121 ± 4 | 132 ± 9 | 0.01 | 130 ± 7 | 133 ± 6 | 0.88 |
| Diastolic BP, mm Hg | 68 ± 4 | 77 ± 6 | 0.01 | 76 ± 5 | 78 ± 5 | 0.88 |
| Heart rate, beat per min | 70.25 ± 4.12 | 72.35 ± 6.95 | 0.88 | 76.20 ± 5.11 | 66.70 ± 5.24 | 0.12 |
| LVEF, % | 67.2 (61.9–72.8) | 45.5 (30.4–55.3) | 0.01 | 36.50 (30.7–42.1) | 55.1 (50.9–58.4) | 0.026 |
Notes: Data are expressed as mean (M) and standard deviation (± SD), numerous (n) and frequencies (%). Comparisons of control group with a combined population of both HF groups are done using ANOVA. Statistical comparisons between both HF groups are made using Mann–Whitney test with significance levels of < 0.05 (for 2-tailed).
Abbreviations: NYHA — New York Heart Association; T2DM — type 2 diabetes mellitus, MI — myocardial infarction; LVEF — left ventriculat ejection fraction.
Subjects with HFrEF compared with HFpEF have demonstrated higher levels of creatinine, total cholesterol, high-density lipoproteins, uric acid, NT-proBNP, galectin-3, and lower estimated GFR and hemoglobin (Table 2). However, level of circulating biomarkers have sufficiently differenced between healthy volunteers and HF group patients apart from hemoglobin. Therefore, numerous of CD14+ CD309+ cells were significantly higher (P = 0.001) in HFpEF patient cohort than in HFrEF patient cohort. Contrary, HFpEF patients have exhibited lower levels of CD31+/annexin V+ EMPs compared with HFrEF patients. However, healthy volunteers have exhibited a lower level of CD31+/annexin V+ EMPs compared with entire HF patient cohort and both HFpEF and HFrEF individuals. There were not found significant changes between HF cohorts in numerous of CD14+ СD309+ Tie-2+ cells and CD62E+ EMPs, while in the control healthy subjects the level of both biomarkers was significantly higher.
Table 2.
The biomarkers in the patient study population.
| Variables | Healthy volunteers (n = 35) | Entire patient cohort (n = 164) | P value between healthy volunteers and entire HF group | Subjects with HFrEF (n = 85) | Subjects with HFpEF (n = 79) | P value between HF cohorts |
|---|---|---|---|---|---|---|
| GFR, mL/min/1.73 m2 | 112.4 (102.2–123.4) | 82.3 (68.7–102.6) | 0.01 | 79.6 (63.1–92.3) | 88.2 (77.1–102.1) | 0.046 |
| Hemoglobin, g/L | 138.3 (129.8–151.2) | 135.4 (128.5–142.1) | 0.94 | 128.1 (124.2–133.1) | 138.5 (126.2–141.8) | 0.001 |
| Fasting glucose, mmol/L | 4.24 (3.6–4.9) | 5.17 (3.5–9.6) | 0.01 | 4.98 (3.8–8.1) | 5.27(3.6–9.3) | 0.28 |
| HbA1c, % | 4.78 (4.2–5.15) | 6.8 (4.1–9.5) | 0.01 | 6.4 (4.6–8.0) | 6.9 (4.3–9.2) | 0.22 |
| Creatinine, μmol/L | 65.4 (58.2–81.2) | 72.3 (58.7–92.6) | 0.01 | 82.1 (64.9–90.5) | 67.7 (59.1–84.1) | 0.01 |
| Total cholesterol, mmol/L | 4.56 (3.25–4.88) | 5.1 (3.9–6.1) | 0.01 | 5.3 (4.6–6.0) | 5.0 (3.5–5.9) | 0.02 |
| HDL cholesterol, mmol/L | 1.03 (0.98–1.08) | 0.92 (0.88–1.13) | 0.01 | 0.97 (0.92–1.08) | 0.88 (0.83–1.03) | 0.042 |
| LDL cholesterol, mmol/L | 2.77 (2.33–3.10) | 3.23 (3.11–4.40) | 0.01 | 3.71 (3.50–4.20) | 3.50 (3.10–3.96) | 0.05 |
| Uric acid, μmol/L | 295 (210–367) | 345 (253–420) | 0.06 | 357 (253–412) | 311 (206–369) | 0.01 |
| NT-pro-BNP, pg/mL | 33.1 (18.3–63.6) | 2336.2 (988.5–3552.8) | 0.001 | 2774.5 (1520.4–3870.2) | 2130.8 (954.5–3056.2) | 0.02 |
| hs-CRP, mg/L | 3.27 (0–5.33) | 7.10 (6.25–8.20) | 0.001 | 7.05 (6.09–8.03) | 7.14 (6.22–8.32) | 0.46 |
| Galectin-3, μg/L | 4.36 (0.98–9.37) | 18.92 (14.25–23.15) | 0.001 | 19.03 (15.80–23.96) | 16.99 (13.77–19.20) | 0.022 |
| CD14+ CD309+, cells/μL | 0.426 (0.370–0.574) | 0.296 (0.225–0.351) | 0.001 | 0.236 (0.202–0.325) | 0.325 (0.233–0.407) | 0.001 |
| CD14+ СD309+ Tie-2+, cells/μL | 0.0465 (0.0253–0.0710) | 0.032 (0.025–0.410) | 0.001 | 0.030 (0.021–0.403) | 0.036 (0.019–0.465) | 0.26 |
| CD31+/annexin V+ EMPs, n/mL | 0.154 (0.03–0.21) | 0.48 (0.29–0.64) | 0.001 | 0.59 (0.42–0.65) | 0.32 (0.25–0.43) | 0.001 |
| CD62E+ EMPs, n/mL | 1.35 (0.95–1.68) | 0.98 (0.87–1.12) | 0.001 | 1.02 (0.81–1.25) | 0.96 (0.82–1.17) | 0.66 |
Note: The values correspond to medians and IQR of 25%–75%. Comparisons of control group with a combined population of both HF groups are done using ANOVA. Statistical comparisons between both HF groups are made using Mann–Whitney test with significance levels of < 0.05 (for 2-tailed).
Abbreviations: GFR — glomerular filtration rate; BMP — brain natriuretic peptide; BP — blood pressure; LVEF — left ventricular ejection fraction; BMI — body mass index, EMPs — endothelial cell-derived microparticles; EPCs — endothelial progenitor cells; HbA1c — glycated hemoglobin, HDL — high-density lipoprotein; LDL — low-density lipoprotein,
Table 3 is reported the concomitant study medication. As one can see, all HF patients were treated with ACE inhibitors or ARBs in combination with loop diuretics. Proportions of the beta-blocker treated patients in both HF cohorts were similar. Aspirin, ivabradine, mineralocorticoid receptor antagonists, and statins were used frequently in HFrEF subjects. Contrarily, metformin and anti-platelet drugs distinguished as aspirin were prescribed frequently in HFpEF patients.
Table 3.
The medications in the patient study population.
| Entire patient cohort (n = 164) | Subjects with HFrEF (n = 85) | Subjects with HFpEF (n = 79) | P value | |
|---|---|---|---|---|
| ACE inhibitors or ARBs, n (%) | 164 (100%) | 85 (100%) | 79 (100%) | 1.0 |
| Aspirin, n (%) | 128 (78.0%) | 77 (90.5%) | 51 (64.6%) | 0.022 |
| Other anti-platelet drugs, n (%) | 36 (22.0%) | 8 (9.5%) | 28 (35.4%) | 0.001 |
| Beta-adrenoblockers, n (%) | 135 (82.3%) | 71 (83.5%) | 64 (81.0%) | 0.76 |
| Dihydropyridine calcium channel blockers, n (%) | 27 (16.5%) | 15 (17.7%) | 12 (15.2%) | 0.24 |
| Ivabradine, n (%) | 48 (29.3%) | 38 (44.7%) | 10 (12.7%) | 0.001 |
| Mineralocorticoid receptor antagonists, n (%) | 57 (34.8%) | 43 (50.5%) | 14 (17.7%) | 0.001 |
| Loop diuretics, n (%) | 164 (100%) | 85 (100%) | 79 (100%) | 0.043 |
| Statins, n (%) | 116 (70.7%) | 68 (80.0%) | 48 (70.8%) | 0.01 |
| Metformin, n (%) | 38 (23.2%) | 15 (17.6%) | 23 (29.1%) | 0.01 |
| Sitagliptin, n (%) | 21 (12.8%) | 9 (10.6%) | 12 (15.2%) | 0.12 |
Notes: Data are expressed as numerous (n) and frequencies (%). Comparisons of control group with a combined population of both HF groups are done using ANOVA.
Abbreviations: ACE — angiotensin-converting enzyme; ARBs — angiotensin-2 receptor blockers.
We did not find any sufficient association between CD14+ CD309+ cells, CD14+ СD309+ Tie-2+ cells, CD31+/annexin V+ EMPs, CD62E+ EMPs, and age, sex, GFR, NT-pro-BNP, galectin-3 and hs-CRP in control healthy volunteers. However, there were significant associations between CD31+/annexin V+ EMPs with adherence to smoke (r = 0.31; P = 0.001) in healthy individuals.
In HFrEF cohort serum galectin-3 associated positively NYHA class of CHF (r = 0.27, P = 0.001), CD31+/annexin V+ EMPs (r = 0.23; P = 0.001), and negatively with CD14+ CD309+ cells (r = − 0.28; P = 0.003), CD14+ СD309+ Tie-2+ cells (r = − 0.23; P = 0.001), GFR (r = − 0.23; P = 0.001), hemoglobin (r = − 0.21; P = 0.001). Therefore, NT-pro-BNP positively associated with NYHA class of CHF (r = 0.43, P = 0.001), CD31+/annexin V+ EMPs (r = 0.23; P = 0.001), and negatively with LVEF (r = − 0.43, P = 0.001), GFR (r = − 0.28; P = 0.001), obesity (r = − 0.25; P = 0.001).
In HFpEF galectin-3 positively associated with type 2 diabetes mellitus (r = 0.26; P = 0.001), CD31+/annexin V+ EMPs (r = 0.23; P = 0.002), and negatively with CD14+ CD309+ cells (r = − 0.32; P = 0.001), CD14+ СD309+ Tie-2+ cells (r = − 0.29; P = 0.001). NT-pro-BNP positively associated with NYHA class of CHF (r = 0.36, P = 0.002), CD31+/annexin V+ EMPs (r = 0.23; P = 0.001), and negatively with LVEF (r = − 0.28, P = 0.001), CD14+ CD309+ cells (r = − 0.26, P = 0.003), obesity (r = − 0.24, P = 0.001). Additionally, hs-CRP associated significantly with type 2 diabetes mellitus (r = 0.22; P = 0.001) in entire group of HF patients. No associations between numerous EPCs and EMPs with medications were found.
In multivariate logistic regression model type 2 diabetes mellitus (R2 = 0.26; P = 0.001), obesity (R2 = 0.22; P = 0.001), previous MI (R2 = 0.17; P = 0.012), galectin-3 (R2 = 0.67; P = 0.012), CD31+/annexin V+ EMPs (R2 = 0.11; P = 0.001), NT-proBNP (R2 = 0.11; P = 0.046), CD14+ CD309+ cells (R2 = 0.058; P = 0.001), and CD14+ СD309+ Tie-2+ cells (R2 = 0.044; P = 0.028) were found as independent predictors of HFpEF (Table 4).
Table 4.
An association of demographics, risk factors and biomarkers with dependent variable: HFpEF. The results of univariate and multivariate one-step backward logistic regression models.
| Variables | Univariate logistic regression model |
Multivariate logistic regression model |
||||
|---|---|---|---|---|---|---|
| R2 | B-coefficient | P value | R2 | B-coefficient | P value | |
| Previous MI | 0.19 | 3.77 | 0.001 | 0.17 | 2.05 | 0.012 |
| Dilated cardiomyopathy | 0.05 | 0.135 | 0.26 | – | ||
| Obesity | 0.27 | 9.23 | 0.001 | 0.22 | 8.97 | 0.001 |
| GFR | 0.053 | − 0.017 | 0.18 | – | ||
| T2DM | 0.26 | 11.92 | 0.003 | 0.26 | 9.54 | 0.001 |
| Hemoglobin | 0.04 | − 0.036 | 0.56 | – | ||
| hs-CRP | 0.073 | 0.98 | 0.044 | 0.057 | 0.54 | 0.12 |
| NT-proBNP | 0.19 | 6.24 | 0.001 | 0.11 | 5.12 | 0.046 |
| Galectin-3 | 0.09 | 3.15 | 0.001 | 0.67 | 2.97 | 0.012 |
| CD14+ CD309+ cells | 0.096 | − 1.47 | 0.003 | 0.058 | − 2.14 | 0.001 |
| CD14+ СD309+ Tie-2+ cells | 0.084 | − 2.55 | 0.001 | 0.044 | − 1.06 | 0.028 |
| CD31+/annexin V+ EMPs | 0.12 | 3.25 | 0.001 | 0.12 | 2.97 | 0.001 |
| CD62E+ EMPs | 0.063 | − 0.37 | 0.044 | 0.058 | − 0.22 | 0.26 |
Abbreviations: T2DM — type 2 diabetes mellitus, GFR — glomerular filtration rate; BMP — brain natriuretic peptide; LVEF — left ventricular ejection fraction; EMPs — endothelial cell-derived microparticles; EPCs — endothelial progenitor cells.
Using multivariate Cox-regression analysis adjusted etiology (previous myocardial infarction), cardiovascular risk factors (obesity, type 2 diabetes mellitus) we found that NT-proBNP (OR 1.08; 95% CI = 1.03–1.12; P = 0.001) and CD31+/annexin V+ EMPs to CD14+ CD309+ cell ratio (OR 1.06; 95% CI = 1.02–1.11; P = 0.02) were independent predictors for HFpEF (Table 5).
Table 5.
Predictive value of biomarkers on dependent variable: HFpEF vs HFrEF. The Cox-regression analysis adjusted etiology (previous myocardial infarction), cardiovascular risk factors (obesity, type 2 diabetes mellitus).
| Variables | Univariate Cox regression |
Multivariate Cox regression |
||||
|---|---|---|---|---|---|---|
| OR | 95% CI | P value | OR | 95% CI | P value | |
| NT-proBNP | 1.12 | 1.06–1.27 | 0.001 | 1.08 | 1.03–1.12 | 0.001 |
| Galectin-3 | 1.08 | 1.03–1.12 | 0.002 | 1.04 | 1.00–1.09 | 0.12 |
| CD31+/annexin V+ EMPs to CD14+ CD309+ cell ratio | 1.09 | 1.04–1.16 | 0.001 | 1.06 | 1.02–1.11 | 0.02 |
| CD31+/annexin V+ EMPs | 1.04 | 1.01–1.09 | 0.024 | 1.02 | 0.98–1.04 | 0.26 |
| CD31+/annexin V+ EMPs to CD14+ CD309+ Tie-2+ cell ratio | 1.05 | 1.02–1.09 | 0.002 | 1.04 | 1.00–1.09 | 0.064 |
| CD14+ CD309+ cells | 1.04 | 1.01–1.06 | 0.044 | 1.02 | 0.99–1.05 | 0.34 |
| CD14+ СD309+ Tie-2+ cells | 1.03 | 1.00–1.05 | 0.12 | – | ||
Abbreviations: OR — odds ration; BMP — brain natriuretic peptide; EMPs — endothelial cell-derived microparticles.
Then we have compared various predictive models based on clinical data, cardiovascular risk factors, and biomarkers. We found that Model 7 constructed on combination of standard model + NT-proBNP + CD31+/annexin V+ EMPs to CD14+ CD309+ cell ratio has exhibited the best discriminative value and reliability to predict HFpEF (Table 6).
Table 6.
Comparison of predictive value of models expressed HFpEF vs HFrEF.
| Models | OR | 95% CI | P value | AUC | 95% CI | P value |
|---|---|---|---|---|---|---|
| Model 1 (standard model) | 1.05 | 1.02–1.08 | 0.001 | 0.62 | 0.59–0.66 | 0.044 |
| Model 2: NT-proBNP | 1.08 | 1.03–1.12 | 0.001 | 0.68 | 0.61–0.74 | 0.012 |
| Model 3: standard model + NT-proBNP | 1.11 | 1.06–1.17 | 0.001 | 0.71 | 0.65–0.79 | 0.001 |
| Model 4: CD31+/annexin V+ EMPs to CD14+ CD309+ cell ratio | 1.06 | 1.02–1.11 | 0.02 | 0.69 | 0.63–0.76 | 0.016 |
| Model 4: standard model + CD31+/annexin V+ EMPs to CD14+ CD309+ cell ratio | 1.12 | 1.05–1.21 | 0.001 | 0.73 | 0.64–0.81 | 0.001 |
| Model 6: NT-proBNP + CD31+/annexin V+ EMPs to CD14+ CD309+ cell ratio | 1.10 | 1.04–1.17 | 0.001 | 0.70 | 0.63–0.76 | 0.001 |
| Model 7: standard model + NT-proBNP + CD31+/annexin V+ EMPs to CD14+ CD309+ cell ratio | 1.17 | 1.10–1.25 | 0.001 | 0.81 | 0.69–0.93 | 0.001 |
Note: Standard model — previous myocardial infarction, obesity, type 2 diabetes mellitus.
Abbreviations: OR — odds ration; AUC — area under curve; CI — confidence interval; BMP — brain natriuretic peptide; EMPs — endothelial cell-derived microparticles.
4. Discussion
The results of our study have revealed for the first time that combination of NT-proBNP and CD31+/annexin V+ EMPs to CD14+ CD309+ cell ratio is a useful biomarker for chronic HF, which contributes in the differentiation of HFpEF from HFrEF. We have demonstrated that HF patients have exhibited elevated levels of EPCs with angiopoetic capacities, apoptotic-derived EMCs, as well as decreased level of CD62E+ EMPs secreted by activated endothelial cells. Recently higher level of CD62+ EMP secreted from activated endothelial cells in the healthy subjects versus HF patients was related to rather endothelial dysfunction than endothelial cell injury) (Dignat-George and Boulanger, 2011, Burger and Touyz, 2012). In our study we did not find any significance changes between HFrEF and HFpEF patients, probably due to similar molecular mechanisms that might lead to endothelial cell activation. We have suggested that lack of sufficient difference between co-morbidities' presentation among HFrEF and HFpEF groups might express similar finding. Interestingly, because of the number of existing CV risk factors which are variable between HF patients, simple EPC counts do not adequately describe vascular disease risk in all clinical conditions and, as such, the CV risk remains (Sen et al., 2011). However, the imbalance in pattern of circulating EMPs and EPCs might affect endothelium ability to repair and previously we have described similar changes as “impaired” phenotype (Berezin, 2015b). The concept of “impaired” phenotype as imbalance between factors originating from endothelium with innate angiogenic and/or injury capacities directly contributed in the endothelial dysfunction and requires further investigation because the molecular mechanism of their release into circulation still remains elusive.
Because biomarkers' levels might suggest different amounts of activation of several pathophysiological pathways between HFpEF and HFrEF development (Sanders-van Wijk et al., 2015, Kaila et al., 2012), we have hypothesized that numerous apoptotic EMPs to EPCs might be distinguished in HFpEF and HFpEF individuals. It is important to note that recent clinical studies have shown increased serum level of NT-proBNP as powerful predictive factor in both HFpEF and HFrEF, although patients with HFpEF exhibits lower NT-proBNP levels (Kang et al., 2015). Several biomarkers, i.e. soluble suppression of tumorigenicity 2 protein, galectin-3, cardiac specific troponins, provides robust prognostic information in HFrEF, but not for HFpEF, while they could improve the prognostication pattern via adding to NT-proBNP in predictive model (Friões et al., 2015). In the LUdwigshafen Risk and Cardiovascular Health (LURIC) study high-sensitive C-reactive protein was found to be an independent and strong predictor of mortality in HFpEF (Koller et al., 2014), but this biomarker even after adding to predictive model based on NT-proBNP was not able to predict HFpEF development. Overall, discriminative values of several cardiac biomarkers including NT-proBNP to stratify patients with HFpEF and HFrEF are not fully adequate.
Apoptotic EMPs are considered a marker of endothelial cell injury and a factor that contributed in transferring biological information, active molecules, hormones, proteins, lipid components, as well as regulating cell homeostasis and cell response (Sansone et al., 2015, Bank et al., 2015). Interestingly, apoptotic EMPs may directly injure endothelium (Montoro-García et al., 2015). In contrast, EPCs labeled as CD14+ CD309+ and CD14+ СD309+ Tie-2+ have citoprotective action; they are capable of repairing endothelium and restoring of endothelial function (Sandri et al., 2015, Berezin and Kremzer, 2015a, Berezin and Kremzer, 2015b). In this context, decreased CD31+/annexin V+ EMPs and increased CD14+ CD309+ cells might elucidate reparative ability of endothelium.
Recent clinical studies have shown that numerous of EPCs defined as CD34+ CD309+ have failed to associate with CV risk factors, medications, HF etiology, age or gender of the patients, while low EPCs' number was associated with an increased likelihood of abnormal left ventricular mass and worse cardiac remodeling (Michelucci et al., 2015, António et al., 2014). Numerous of CD31+/annexin V+ EMPs strongly correlate with endothelial function and CV outcomes in stable CAD patients (Sinning et al., 2011, Werner et al., 2006). Moreover, Huang et al. (2010) reported that increased circulating CD31+/annexin V+ EMPs and decreased circulating EPCs predict target organ damage in hypertensive patients. In our study HFpEF patients have exhibited lower CD31+/annexin V+ EMPs to CD14+ CD309+ cell ratio and CD31+/annexin V+ EMPs to CD14+ CD309+ Tie-2+ cell ratio in comparison with HFrEF patients. The result of Cox-regression analysis adjusted etiology (previous myocardial infarction), cardiovascular risk factors (obesity, type 2 diabetes mellitus) has revealed that NT-proBNP and CD31+/annexin V+ EMPs to CD14+ CD309+ cell ratio are independent predictors of HFpEF. Additionally, we found that the discriminative value of NT-proBNP as a HF diagnostic biomarker might improve by use in combination with CD31+/annexin V+ EMPs to CD14+ CD309+ cell ratio, whereas increased number of CD31+/annexin V+ EMPs alone and decreased number of CD14+ CD309+ cells alone were not able to improve the discriminative value of standard models based on combination of clinical features (previous myocardial infarction, obesity, type 2 diabetes mellitus) or NT-proBNP.
However, it is assumed to take into consideration the high costs of the of CD31+/annexin V+ EMPs to CD14+ CD309+ cell ratio versus traditional circulating biomarkers' assessment using ELISA tests. Probably, this might be a limitation for a wide implementation of the assay in the routine clinical practice, although clinical utility of expected results is not completely determined and in future similar investigations might consider as an essential tool for prediction and probably individualized medical care in predominantly patients with HFpEF.
Thus, there is a large body of evidence regarding participation of angiogenic EPCs and apoptotic EMPs in the regulation of cardiac regeneration and endothelial function. The results reflect the role of imbalance between angiopoetic EPCs and apoptotic EMPs in HF development, in particular an ability of CD31+/annexin V+ EMPs to CD14+ CD309+ cell ratio to improve the discriminative value of NT-proBNP to associate with HFpEF vs HFrEF was found. We believe that this novel biomarkers' approach could become highly relevant for providing effect to risk stratify patients with HFpEF. Probably, biomarkers that reflect reparative capacity of endothelium may support diagnostic strategies in subpopulations of patients with chronic HF. The identification of these biomarkers might be useful for monitoring disease progression and potential therapeutic targets. To assay well balanced cutoff points regarding NT-proBNP and CD31+/annexin V+ EMPs to CD14+ CD309+ cell ratio as predictors of HFpEF, more investigations are required.
Conclusion: CD31+/annexin V+ EMPs to CD14+ CD309+ cell ratio was added to NT-proBNP, clinical data, and cardiovascular risk factors have exhibited the best discriminate value and higher reliability to predict HFpEF compared with NT-proBNP and clinical data/cardiovascular risk factors alone.
5. Study Limitations
This study has some limitations. The first limitation affects the methods of isolation and determination of EMPs and EPCs. Although HD-FACS methodology is widely used, this method is not standardized. Therefore, there are some overlaps between centrifugation velocity and further determination of EMPs that might reflect some obstacles for results' interpretation. Moreover, annexin V binding by MPs is a calcium dependent process and the marker has a limited role in assessment of apoptotic MPs. However, annexin V+ MPs remain a well investigated marker of comprehensive description of apoptotic-derived MPs received from peripheral blood in healthy individuals as well as in HF patients. The next limitation is a small cohort of patients included in the study. Therefore, we used regression models for adjusted clinical data, CV risk, previous MI, metabolic co-morbidities, to analyze the discriminative ability of NT-proBNP alone, CD31+/annexin V+ EMPs to CD14+ CD309+ cell ratio alone and their combination as predictors of HFpEF. Greater cohort patients in observation study might help increase statistical power. The high cost of the biomarker assay might be considered as a limitation of the study as of now. Further investigations with a greater cohort of patients explaining the role of novel biomarker to differentiate both phenotypes of chronic HF are required. The authors suppose that these restrictions might have no significant impact on the study data interpretation.
Abbreviations
- ACEI
angiotensin-converting enzyme inhibitors
- ARBs
angiotensin receptor blockers,
- AUC
area under curve
- BMI
body mass index
- BNP
brain natriuretic peptide
- CHF
chronic heart failure
- CV
cardiovascular
- EMPs
endothelial cell-derived microparticles
- HF
heart failure
- HFpEF
chronic HF with preserved ejection fraction
- HFrEF
chronic HF with reduced ejection fraction
- GFR
glomerular filtration rate
- hs-CRP
high-sensitive C-reactive protein
- HDL-C
high-density lipoprotein cholesterol
- LDL-C
low-density lipoprotein cholesterol
- LVEF
left ventricular ejection fraction
Author contributions
AB initiated the hypothesis and designed the study protocol, contributed to the collection, analysis and interpretation of the data, performed statistical analysis, wrote the manuscript and approved the final version of the paper. AK enrolled the patients; collected and analyzed the data and reviewed the source documents. TB contributed to the enrollment of the patients in the study and collected the data. YM performed of cytofluorometry and interpreted the obtained results. EG collected blood samples, contributed in the cytofluorometry assay of endothelial cell-derived microparticles and endothelial progenitor cells, performed biomarker assay, and interpreted the obtained results. All authors read the manuscript before submitting and agree with the final version of the paper.
Conflicts of Interest
None of the authors has any conflict of interest related to the content of this study. This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Acknowledgments
We thank all patients for their participation in the investigation, staff of the Regional Zaporozhye Hospital (Ukraine) and the doctors, nurses, and administrative staff intCity Hospital # 6 (Zaporozhye, Ukraine), City Hospital #10 (Zaporozhye, Ukraine), Regional Center of Cardiovascular Diseases (Zaporozhye, Ukraine) general practices, and site-managed organizations that assisted with the study.
References
- António N., Soares A., Carvalheiro T., Fernandes R., Paiva A., Ventura M. Circulating endothelial progenitor cells as a predictor of response to cardiac resynchronization therapy: the missing piece of the puzzle? Pacing Clin. Electrophysiol. 2014;37(6):731–739. doi: 10.1111/pace.12334. [DOI] [PubMed] [Google Scholar]
- Awad O., Dedkov E.I., Jiao C., Bloomer S., Tomanek R.J., Schatteman G.C. Differential healing activities of CD34 + and CD14 + endothelial cell progenitors. Arterioscler. Thromb. Vasc. Biol. 2006;26(4):758–764. doi: 10.1161/01.ATV.0000203513.29227.6f. [DOI] [PubMed] [Google Scholar]
- Bank I.E., Timmers L., Gijsberts C.M., Zhang Y.N., Mosterd A., Wang J.W. The diagnostic and prognostic potential of plasma extracellular vesicles for cardiovascular disease. Expert. Rev. Mol. Diagn. 2015;1-12 doi: 10.1586/14737159.2015.1109450. [DOI] [PubMed] [Google Scholar]
- Berezin A.E. Endothelial derived micro particles: biomarkers for heart failure diagnosis and management. J. Clin. Trial Cardiol. 2015;2(3):1–3. [Google Scholar]
- Berezin A.E. Impaired phenotype of circulating endothelial-derived microparticles: novel marker of cardiovascular risk. J. Cardiol. Ther. 2015;2(4):273–278. [Google Scholar]
- Berezin A.E., Kremzer A.A. Impaired phenotype of circulating endothelial microparticles in chronic heart failure patients: relevance to body mass index. Diabetol. Metab. Syndr. 2015;9(4):230–236. doi: 10.1016/j.dsx.2015.04.003. [DOI] [PubMed] [Google Scholar]
- Berezin A.E., Kremzer A.A. Content of circulating endothelial progenitor cells in patients with chronic ischemic heart failure with preserved left ventricular ejection fraction. Kardiologiia. 2015;55(1):14–22. doi: 10.18565/cardio.2015.1.14-22. [DOI] [PubMed] [Google Scholar]
- Berezin A.E., Kremzer A.A., Samura T.A., Berezina T.A., Kruzliak P. Impaired immune phenotype of circulating endothelial-derived microparticles in patients with metabolic syndrome and diabetes mellitus. J. Endocrinol. Investig. 2015;38(8):865–874. doi: 10.1007/s40618-015-0273-z. [DOI] [PubMed] [Google Scholar]
- Berezin A.E., Kremzer A.A., Samura T.A., Martovitskaya Y.V., Malinovskiy Y.V., Oleshko S.V. Predictive value of apoptotic microparticles to mononuclear progenitor cells ratio in advanced chronic heart failure patients. J. Cardiol. 2015;65:403–411. doi: 10.1016/j.jjcc.2014.06.014. [DOI] [PubMed] [Google Scholar]
- Berezin A., Zulli A., Kerrigan S., Petrovic D., Kruzliak P. Predictive role of circulating endothelial-derived microparticles in cardiovascular diseases. Clin. Biochem. 2015;48(9):562–568. doi: 10.1016/j.clinbiochem.2015.02.003. [DOI] [PubMed] [Google Scholar]
- Burger D., Touyz R.M. Cellular biomarkers of endothelial health: microparticles, endothelial progenitor cells and circulating endothelial cells. J. Am. Soc. Hypertens. 2012;6:85–99. doi: 10.1016/j.jash.2011.11.003. [DOI] [PubMed] [Google Scholar]
- Chamberlain A.M., St Sauver J.L., Gerber Y., Manemann S.M., Boyd C.M., Dunlay S.M. Multimorbidity in heart failure: a community perspective. Am. J. Med. 2015;128(1):38–45. doi: 10.1016/j.amjmed.2014.08.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D'Elia E., Vaduganathan M., Gori M., Gavazzi A., Butler J., Senni M. Role of biomarkers in cardiac structure phenotyping in heart failure with preserved ejection fraction: critical appraisal and practical use. Eur. J. Heart Fail. 2015 doi: 10.1002/ejhf.430. (Epub ahead of print) [DOI] [PubMed] [Google Scholar]
- Dignat-George F., Boulanger C.M. The many faces of endothelial microparticles. Arterioscler. Thromb. Vasc. Biol. 2011;31:27–33. doi: 10.1161/ATVBAHA.110.218123. [DOI] [PubMed] [Google Scholar]
- Dunlay S.M., Manemann S.M., Chamberlain A.M., Cheville A.L., Jiang R., Weston S.A. Activities of daily living and outcomes in heart failure. Circ. Heart Fail. 2015;8(2):261–267. doi: 10.1161/CIRCHEARTFAILURE.114.001542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Executive summary Standards of medical care in diabetes — 2013. Diabetes Care. 2013;36(1):S4–10. doi: 10.2337/dc13-S004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friões F., Lourenço P., Laszczynska O., Almeida P.B., Guimarães J.T., Januzzi J.L. Prognostic value of sST2 added to BNP in acute heart failure with preserved or reduced ejection fraction. Clin. Res. Cardiol. 2015;104(6):491–499. doi: 10.1007/s00392-015-0811-x. [DOI] [PubMed] [Google Scholar]
- Fujisue K., Sugiyama S., Matsuzawa Y., Akiyama E., Sugamura K., Matsubara J. Prognostic significance of peripheral microvascular endothelial dysfunction in heart failure with reduced left ventricular ejection fraction. Circ. J. 2015;79(12):2623–2631. doi: 10.1253/circj.CJ-15-0671. [DOI] [PubMed] [Google Scholar]
- Gerber Y., Weston S.A., Redfield M.M., Chamberlain A.M., Manemann S.M., Jiang R. A contemporary appraisal of the heart failure epidemic in Olmsted County, Minnesota, 2000 to 2010. JAMA Intern. Med. 2015;175(6):996–1004. doi: 10.1001/jamainternmed.2015.0924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Go A.S., Mozaffarian D., Roger V.L., Benjamin E.J., Berry J.D., Blaha M.J. Heart disease and stroke statistics—2014 update: a report from the American Heart Association. Circulation. 2014;129(3):e28–e292. doi: 10.1161/01.cir.0000441139.02102.80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffman R.A. Standardization, calibration, and control in flow cytometry. Curr. Protoc. Cytom. 2005 doi: 10.1002/0471142956.cy0103s32. (chapter 1: unit 1.3) [DOI] [PubMed] [Google Scholar]
- Huang P.H., Huang S.S., Chen Y.H., Lin C.P., Chiang K.H., Chen J.S. Increased circulating CD31 +/annexin V + apoptotic microparticles and decreased circulating endothelial progenitor cell levels in hypertensive patients with microalbuminuria. J. Hypertens. 2010;28(8):1655–1665. doi: 10.1097/HJH.0b013e32833a4d0a. [DOI] [PubMed] [Google Scholar]
- Jorge A.L., Rosa M.L., Martins W.A., Correia D.M., Fernandes L.C., Costa J.A. the prevalence of stages of heart failure in primary care: a population-based study. J. Card. Fail. 2015 doi: 10.1016/j.cardfail.2015.10.017. (Epub ahead of print) [DOI] [PubMed] [Google Scholar]
- Kaila K., Haykowsky M.J., Thompson R.B., Paterson D.I. Heart failure with preserved ejection fraction in the elderly: scope of the problem. Heart Fail. Rev. 2012;17(4–5):555–562. doi: 10.1007/s10741-011-9273-z. [DOI] [PubMed] [Google Scholar]
- Kang S.H., Park J.J., Choi D.J., Yoon C.H., Oh I.Y., Kang S.M. Registry. prognostic value of NT-proBNP in heart failure with preserved versus reduced EF. Heart. 2015;101(23):1881–1888. doi: 10.1136/heartjnl-2015-307782. [DOI] [PubMed] [Google Scholar]
- Koller L., Kleber M., Goliasch G., Sulzgruber P., Scharnagl H., Silbernagel G. C-reactive protein predicts mortality in patients referred for coronary angiography and symptoms of heart failure with preserved ejection fraction. Eur. J. Heart Fail. 2014;16(7):758–766. doi: 10.1002/ejhf.104. [DOI] [PubMed] [Google Scholar]
- Lacroix R., Judicone C., Mooberry M., Boucekine M., Key N.S., Dignat-George F. The ISTH SSC Workshop. Standardization of pre-analytical variables in plasma microparticle determination: results of the International Society on Thrombosis and Haemostasis SSC collaborative workshop. J. Thromb. Haemost. 2013 doi: 10.1111/jth.12207. (Epub ahead of print) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lacroix R., Robert S., Poncelet P., Dignat-George F. Overcoming limitations of microparticle measurement by flow cytometry. Semin. Thromb. Hemost. 2010;36:807–818. doi: 10.1055/s-0030-1267034. [DOI] [PubMed] [Google Scholar]
- Levey A.S., Stevens L.A., Schmid C.H. For the CKD-EPI (chronic kidney disease epidemiology collaboration). A new equation to estimate glomerular filtration rate. Ann. Intern. Med. 2009;150:604–612. doi: 10.7326/0003-4819-150-9-200905050-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMurray J.J., Adamopoulos S., Anker S.D., Auricchio A., Bohm M., Dickstein K. ESC Guidelines for the Diagnosis and Treatment of Acute and Chronic Heart Failure 2012: the Task Force for the Diagnosis and Treatment of Acute and Chronic Heart Failure 2012 of the European Society of Cardiology. Developed in collaboration with the Heart Failure Association (HFA) of the ESC. Eur. J. Heart Fail. 2012;14(8):803–869. doi: 10.1093/eurjhf/hfs105. [DOI] [PubMed] [Google Scholar]
- Michelucci A., Cesari F., Ricciardi G., Attanà P., Pieragnoli P., Ristalli F. Left ventricular mass and progenitor cells in chronic heart failure patients. Intern. Emerg. Med. 2015;10(3):329–335. doi: 10.1007/s11739-014-1149-5. [DOI] [PubMed] [Google Scholar]
- Montoro-García S., Shantsila E., Wrigley B.J., Tapp L.D., Abellán Alemán J., Lip G.Y. Small-size microparticles as indicators of acute decompensated state in ischemic heart failure. Rev. Esp. Cardiol. (Engl. Ed.) 2015;68(11):951–958. doi: 10.1016/j.rec.2014.11.016. [DOI] [PubMed] [Google Scholar]
- National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III) third report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III) final report. Circulation. 2002;106(25):3143–3421. [PubMed] [Google Scholar]
- Orozco A.F., Lewis D.E. Flow cytometric analysis of circulating microparticles in plasma. Cytometry. 2010;77(6):502–514. doi: 10.1002/cyto.a.20886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paulus W.J., Tschöpe C., Sanderson J.E., Rusconi C., Flachskampf F.A., Rademakers F.E. How to diagnose diastolic heart failure: a consensus statement on the diagnosis of heart failure with normal left ventricular ejection fraction by the Heart Failure and Echocardiography Associations of the European Society of Cardiology. Eur. Heart J. 2007;28(20):2539–2550. doi: 10.1093/eurheartj/ehm037. [DOI] [PubMed] [Google Scholar]
- Quiñones M.A., Douglas P.S., Foster E., Gorcsan J.I.I.I., Lewis J.F., Pearlman A.S., American College of Cardiology, American Heart Association, American College of Physicians, American Society of Internal Medicine Task Force on Clinical Competence American College of Cardiology/American Heart Association clinical competence statement on echocardiography: a report of the American College of Cardiology/American Heart Association/American College of Physicians — American Society of Internal Medicine Task Force on Clinical Competence. Circulation. 2003;107(7):1068–1089. doi: 10.1161/01.cir.0000061708.42540.47. [DOI] [PubMed] [Google Scholar]
- Sanders-van Wijk S., van Empel V., Davarzani N., Maeder M.T., Handschin R., Pfisterer M.E. et al; TIME-CHF Investigators. Circulating biomarkers of distinct pathophysiological pathways in heart failure with preserved vs. reduced left ventricular ejection fraction. Eur. J. Heart Fail. 2015;17(10):1006–1014. doi: 10.1002/ejhf.414. [DOI] [PubMed] [Google Scholar]
- Sandri M., Viehmann M., Adams V., Rabald K., Mangner N., Höllriegel R. Chronic heart failure and aging — effects of exercise training on endothelial function and mechanisms of endothelial regeneration: results from the Leipzig exercise intervention in chronic heart failure and aging (LEICA) study. Eur. J. Prev. Cardiol. 2015 doi: 10.1177/2047487315588391. (pii: 2047487315588391. [Epub ahead of print]) [DOI] [PubMed] [Google Scholar]
- Sansone R., Stanske B., Keymel S., Schuler D., Horn P., Saeed D. Macrovascular and microvascular function after implantation of left ventricular assist devices in end-stage heart failure: role of microparticles. J. Heart Lung Transplant. 2015;34(7):921–932. doi: 10.1016/j.healun.2015.03.004. [DOI] [PubMed] [Google Scholar]
- Sen S., McDonald S.P., Coates P.T., Bonder C.S. Endothelial progenitor cells: novel biomarker and promising cell therapy for cardiovascular disease. Clin. Sci. (Lond.) 2011;120(7):263–283. doi: 10.1042/CS20100429. [DOI] [PubMed] [Google Scholar]
- Shah M.D., Bergeron A.L., Dong J.F., Lopez J.A. Flow cytometric measurement of microparticles: pitfalls and protocol modifications. Platelets. 2008;19:365–372. doi: 10.1080/09537100802054107. [DOI] [PubMed] [Google Scholar]
- Singh N., Van Craeyveld E., Tjwa M., Ciarka A., Emmerechts J., Droogne W. Circulating apoptotic endothelial cells and apoptotic endothelial microparticles independently predict the presence of cardiac allograft vasculopathy. J. Am. Coll. Cardiol. 2012;60:324–331. doi: 10.1016/j.jacc.2012.02.065. [DOI] [PubMed] [Google Scholar]
- Sinning J.M., Losch J., Walenta K., Böhm M., Nickenig G., Werner N. Circulating CD31 +/annexin V + microparticles correlate with cardiovascular outcomes. Eur. Heart J. 2011;32(16):2034–2041. doi: 10.1093/eurheartj/ehq478. [DOI] [PubMed] [Google Scholar]
- Tung J.W., Parks D.R., Moore W.A. New approaches to fluorescence compensation and visualization of FACS Data. Clin. Immunol. 2004;110:277–283. doi: 10.1016/j.clim.2003.11.016. [DOI] [PubMed] [Google Scholar]
- Werner N., Wassmann S., Ahlers P., Kosiol S., Nickenig G. Circulating CD31 +/annexin V + apoptotic microparticles correlate with coronary endothelial function in patients with coronary artery disease. Arterioscler. Thromb. Vasc. Biol. 2006;26(1):112–116. doi: 10.1161/01.ATV.0000191634.13057.15. [DOI] [PubMed] [Google Scholar]
- Yancy C.W., Jessup M., Bozkurt B., Butler J., Casey D.E., Jr., Drazner M.H. 2013 ACCF/AHA Guideline for the Management of Heart Failure: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. J. Am. Coll. Cardiol. 2013;62(16):e147–e239. doi: 10.1016/j.jacc.2013.05.019. [DOI] [PubMed] [Google Scholar]