An exploratory, single-blind RCT with clients receiving outpatient occupational therapy services provides early support for the use of CO–OP to improve performance and remediate cognitive and arm movement impairments after stroke.
MeSH TERMS: activities of daily living, cognition, movement, occupational therapy, recovery of function, stroke
Abstract
The purpose of this study was to estimate the effect of Cognitive Orientation to Daily Occupational Performance (CO–OP) compared with usual occupational therapy on upper-extremity movement, cognitive flexibility, and stroke impact in people less than 3 mo after stroke. An exploratory, single-blind randomized controlled trial was conducted with people referred to outpatient occupational therapy services at two rehabilitation centers. Arm movement was measured with the Action Research Arm Test, cognitive flexibility with the Delis–Kaplan Executive Function System Trail Making subtest, and stroke impact with subscales of the Stroke Impact Scale. A total of 35 participants were randomized, and 26 completed the intervention. CO–OP demonstrated measurable effects over usual care on all measures. These data provide early support for the use of CO–OP to improve performance and remediate cognitive and arm movement impairments after stroke over usual care; however, future study is warranted to confirm the effects observed in this trial.
Although the death rate from stroke continues to decline in the United States and globally, the number of people living with chronic symptoms is rising. More than 7 million people have experienced stroke, and it is now the leading cause of long-term disability in the United States (National Stroke Association, 2015).
Although current best evidence has shown that repetitive practice of functional tasks is associated with improvements, such as better gait speed and upper-extremity function (French et al., 2008), most occupational therapy practitioners continue to use impairment-based approaches that emphasize remediation of impairments (Connell, McMahon, Eng, & Watkins, 2014; Teasell, Foley, Salter, & Jutai, 2008; Veerbeek et al., 2014). The expectation with these impairment-based rehabilitation approaches, also called bottom-up approaches, is that gains in the targeted component (e.g., motor function) will translate into improvement in performance in everyday life activity. Unfortunately, evidence suggests that gains do not occur; almost half of the people living in the community with stroke are still dependent in the activities necessary to support their daily lives (Appelros, Samuelsson, Karlsson‐Tivenius, Lokander, & Terént, 2007). Most of them have significant restrictions in their everyday life participation compared with their age-matched peers (Alzahrani, Ada, & Dean, 2011; Hackett, Glozier, Jan, & Lindley, 2012; Mayo, Wood-Dauphinee, Côté, Durcan, & Carlton, 2002).
For occupational therapy practitioners, this lack of association between impairment-based approaches and improvement in everyday activity should not be surprising. During the inception of the profession of occupational therapy in the early 1900s, Adolph Meyer established the philosophical foundation for occupational therapy. He challenged the profession to view disease not merely as a demon that had to be excised from the body, but rather as an affliction of maladaptation that can be addressed with a well-fitted use of time and occupation (Meyer, 1922). Mary Reilly (1962) further supported this foundation of occupational therapy in her 1962 Eleanor Clarke Slagle lecture when she declared the hypothesis of occupational therapy to be that “man, through the use of his hands, as energized through mind and will, can influence the state of his own health” (p. 92). Thus, for more than a century, occupational therapy theorists have espoused top-down approaches with the assumption that engagement in occupation can improve health and remediate impairment. Although increasing evidence has shown that bottom-up approaches do not improve occupation, little information exists on the impact of top-down approaches on impairment.
Cognitive Orientation to Daily Occupational Performance (CO–OP; McEwen, Polatajko, Huijbregts, & Ryan, 2010) is a top-down approach that reduces impairments and improves health. CO–OP is defined as “a client-centered, performance-based, problem solving approach that enables skill acquisition through a process of strategy use and guided discovery” (Polatajko et al., 2001, p. 108). The goal of CO–OP is to focus treatments directly on improving performance in everyday life activity rather than treating the underlying impairments and hoping for secondary improvement in meaningful activities. CO–OP was originally developed for use with pediatric populations, but a growing body of literature has supported its use to improve performance in people with stroke. Early evidence from two single-case experimental series in people with chronic stroke showed improvement in trained and untrained activities (McEwen et al., 2010; McEwen, Polatajko, Huijbregts, & Ryan, 2009). This improvement in activity performance was also demonstrated in an early-phase pilot clinical trial that compared people with chronic stroke with an active control group (Polatajko, McEwen, Ryan, & Baum, 2012). Although these early results with people with chronic stroke were promising, the general consensus is that rehabilitation early after stroke can have a greater effect on outcomes (Ploughman, 2002; Salter, Foley, & Teasell, 2010; Teasell et al., 2008).
With this in mind, our research group undertook an early-phase clinical trial using CO–OP with people less than 3 mo after stroke compared with usual and customary care delivered in the occupational therapy outpatient setting. On the primary outcome, objective performance of meaningful, functional activities as measured by the Performance Quality Rating Scale (PQRS; Martini, Rios, Polatajko, Wolf, & McEwen, 2015), CO–OP was found to have a medium effect (d = 0.5) over usual care on self-selected trained activities and a large effect (d = 1.2) on untrained activities. The effect on trained activities increased at 3 mo after intervention (d = 1.6), whereas the effect on untrained activities was maintained (d = 1.1; McEwen et al., 2015). Because of previous preliminary evidence that CO–OP may affect impairments and components of stroke impact (McEwen et al., 2010), we also sought to explore its effect on a group of secondary outcomes postulated to be affected by this complex, client-centered, performance-based, problem-solving approach. Specifically, our objective was to estimate the effect of CO–OP compared with usual occupational therapy on immediate and longer term secondary outcomes for upper-extremity movement, cognitive flexibility, and stroke impact in people less than 3 mo after stroke.
Method
This study was a single-blind, exploratory, randomized controlled trial with participants referred to outpatient stroke rehabilitation. The study was conducted at two rehabilitation centers collaborating with university investigators. All participants in this study were randomized to receive either usual outpatient occupational therapy provided at the institution (usual care) or CO–OP. All participants received other health care services as was typical at the centers, including, but not limited to, physical therapy, speech–language pathology, counseling, and nursing. Eligibility for participation in this study, however, was related only to occupational therapy services. The study was reviewed and approved by the Washington University in St. Louis School of Medicine Human Research Protection Office and the research ethics board of Sunnybrook Health Sciences Centre. A complete description of this study’s methods can be found in the previously published primary results article (McEwen et al., 2015).
Participants
Patients with ischemic stroke who were referred for outpatient therapy services at either Sunnybrook–St. John’s Rehab (Toronto) or The Rehabilitation Institute of St. Louis were recruited for participation in this study. Anyone who met these criteria was considered eligible. Patients were excluded on the basis of the following criteria: (1) more than 3 mo after stroke at the time of enrollment, (2) not referred to receive occupational therapy, (3) any prior neurological diagnoses other than stroke, (4) any major psychiatric illness, (5) moderate or greater aphasia as determined by combined scores of 6 or less on the Canadian Institute for Health Information (CIHI) National Rehabilitation Reporting System (NRS) Listing of Data Elements (CIHI, 2009) Items 64 and 66, and (6) significant cognitive impairment as determined by a score of 21 or less on the Montreal Cognitive Assessment (MoCA; Nasreddine et al., 2005).
A blocked randomization procedure stratified by site was used to determine group allocation. A statistician external to the study team developed the randomization sequence. A block size of 6 and an allocation ratio of 1:1 were used to ensure equal distribution in groups of 6 at each site. Group allocation was completed after consent and final determination of eligibility. The entire study team was blinded to the randomization procedure and block size.
Procedures
Participants with consent completed a baseline assessment (Time 1) with a rater blinded to allocation before starting the intervention. The baseline assessment also included the MoCA and the CIHI to determine final eligibility. After the baseline assessment, eligible participants completed an occupational interview using the Canadian Occupational Performance Measure (COPM; Law et al., 1998) with an occupational therapist, who was also blinded to group allocation, to establish goals for participation in this study.
During the COPM interview, participants selected functional activity goals that would become the focus of CO–OP treatment. After completion of the COPM, participants completed a third assessment to obtain PQRS scores. To complete the PQRS, participants were videotaped performing the self-selected goals from the COPM; the videos were later scored by a rater who was blinded to group allocation.
Next, participants were randomized to either CO–OP or usual care by the study coordinator at each site, and the results of the COPM were distributed to each participant’s treating occupational therapist. Participants had a range of stroke impairments and rehabilitation needs; therefore, the number of treatment sessions varied. However, the CO–OP group received a maximum of 10 CO–OP intervention sessions, and any additional sessions received were through usual care. After completion of the intervention, participants completed a postintervention assessment (Time 2) and a 3-mo follow-up assessment (Time 3) that included all the outcome measures described subsequently.
Intervention
Cognitive Orientation to Daily Occupational Performance.
Complete details about the theoretical underpinnings and implementation of the CO–OP approach have been previously published (Polatajko et al., 2001). In this study, CO–OP treatment was delivered in a separate location and with separate occupational therapists from those who administered the usual-care intervention. The first therapy session included a review of the CO–OP treatment model and the global strategy of Goal–Plan–Do–Check, adapted from Meichenbaum and Goodman (1971). In the first session, the participant and therapist also collaborated using results from the COPM to select three goals to address in treatment.
Throughout the subsequent therapy sessions, the participant used the problem-solving strategy Goal–Plan–Do–Check to address and master each goal activity. After the participant identified a goal for each session, the therapist used the process of guided discovery to help the participant establish a plan to accomplish the goal. The plan could have many parts and address aspects of the environment, the person, and how the activity was done. Performance problems related to the activity were assessed collaboratively by the participant and the therapist, and the therapist encouraged problem solving to allow the participant to self-discover domain-specific strategies specific to the activity such as modifying the task or changing body position to resolve the performance problem. Next, the participant executed the selected strategy (do the plan) and then checked to evaluate the success of the plan. The check presents an opportunity to refine or modify the plan or to decide that the goal was met. To achieve the goal, the Plan–Do–Check portion was often repeated more than once. The participant decided when the goal was achieved and the preferred plan to achieve the goal, not the therapist.
Usual Care.
Usual-care therapy took place at one of two freestanding rehabilitation centers and was done by the usual staff, who were unaffiliated with the research group. The clinicians received no direction from the research study staff. They were provided with the COPM results and personalized activity goals for each participant; however, no information was given to the usual-care therapist about how to use the COPM results. During study preparation, a survey conducted at both sites with a sample of experienced therapists indicated that usual-care stroke rehabilitation consisted of a combination of functional, task-based training, such as practicing dressing, and component-based training, such as grasping objects, chosen by each individual therapist to meet individual patient needs.
Outcome Measures
Table 1 provides an overview of the study outcome measures. All measures were administered at baseline (Time 1), postintervention (Time 2), and at 3 mo after completion of the postintervention assessment (Time 3). Although the total number of intervention sessions varied, therapists in both arms of this study were instructed to inform the study coordinator when the participant was being discharged from occupational therapy or when the maximum of 10 intervention sessions was reached, whichever came first, to complete the posttreatment assessment. This instruction was given to ensure dose equivalence at Time 2, considering the necessary variability that was expected in the number of treatment sessions in this population.
Table 1.
Description of Outcome Measures
| Instrument | Construct | Description |
| Delis–Kaplan Executive Function System (D–KEFS) Trail Making subtest (Condition 4) | Cognitive function (executive function) | The D–KEFS is a standardized executive function battery with subtests to assess 9 components of executive function, all of which have adequate test–retest reliability and internal consistency. For the purposes of this study, Condition 4 of the Trail Making subtest was used as a gross measure of executive function. It assesses visual–motor skills, visual scanning abilities, number and letter sequencing, and cognitive flexibility. |
| Action Research Arm Test (ARAT) | Upper-extremity function | The ARAT measures upper-extremity impairment and activity limitation by assessing upper-extremity capacity. It has 19 items with four subscales: Grasp, Grip, Pinch, and Gross Movement. |
| Stroke Impact Scale (SIS) | Health status | The SIS is a self-report measure to evaluate the impact that stroke has had on a participant’s function. The following domains were evaluated in this study: Strength, Hand Function, Mobility, Activities of Daily Living, Memory, Emotion, Recovery, and Communication. The Participation domain was not evaluated because it was previously reported on. |
| Canadian Occupational Performance Measure (COPM) | Self-reported occupational performance: trained and untrained goals | The COPM is a standardized instrument for eliciting performance issues from the client's perspective and for capturing perceived changes in performance over time. The COPM was used to elicit 4–6 participant-selected goals and to rate self-perceived performance and performance satisfaction for each goal on a 10-point scale for each participant. |
| Performance Quality Rating Scale (PQRS) | Objective rating of performance of COPM goals | The PQRS rates the video-recorded performance of participant-selected activities on a 10-point scale (ranging from 1 = can’t do the skill at all to 10 = does the skill very well ). The activities performed and video recorded are determined using the COPM, and most, but not all, goals selected by participants are amenable to video recording. |
Note. From “Combined Cognitive-Strategy and Task-Specific Training Improve Transfer to Untrained Activities in Subacute Stroke: An Exploratory Randomized Controlled Trial,” by S. McEwen, H. Polatajko, C. Baum, J. Rios, D. Cirone, M. Doherty, & T. Wolf, 2015, in Neurorehabilitation and Neural Repair, 29, 529. http://dx.doi.org/10.1177/1545968314558602. Copyright © 2015 by the American Society of Neurorehabilitation. Adapted with permission.
The video of the PQRS was rated on a scale ranging from 1 (can’t do the activity at all) to 10 (does the activity very well) by an independent blinded rater who viewed and scored the PQRS videos in randomized order (by participant, goal, and time). All raters were trained by the study investigators and had to demonstrate competency in administration of all the assessments with people with stroke before they were qualified to administer the assessments to study participants.
Analysis
After data cleaning and accuracy checks, analyses were conducted using IBM SPSS Statistics (Version 21; IBM Corporation, Armonk, NY) and Excel (Microsoft, Redmond, WA). Normal distribution was verified using the Shapiro–Wilk test. Descriptive statistics for the primary outcomes, PQRS, COPM, and participation were previously reported (McEwen et al., 2015). Descriptive statistics for Action Research Arm Test (ARAT; Lyle, 1981), Delis–Kaplan Executive Function System (D–KEFS; Delis, Kaplan, & Kramer, 2001), and Stroke Impact Scale (SIS; Duncan, Bode, Min Lai, & Perera, 2003) domains related to function were calculated, and baseline comparisons were made between sites and between groups. Time 1 to Time 2 and Time 1 to Time 3 mean change scores and standard deviations were calculated for normally distributed data, and Cohen’s d effect sizes and confidence intervals were calculated. For non-normally distributed data, medians and ranges were determined, and a nonparametric effect size r was calculated using the formula r2 = z2/N (Fritz, Morris, & Richler, 2012).
Results
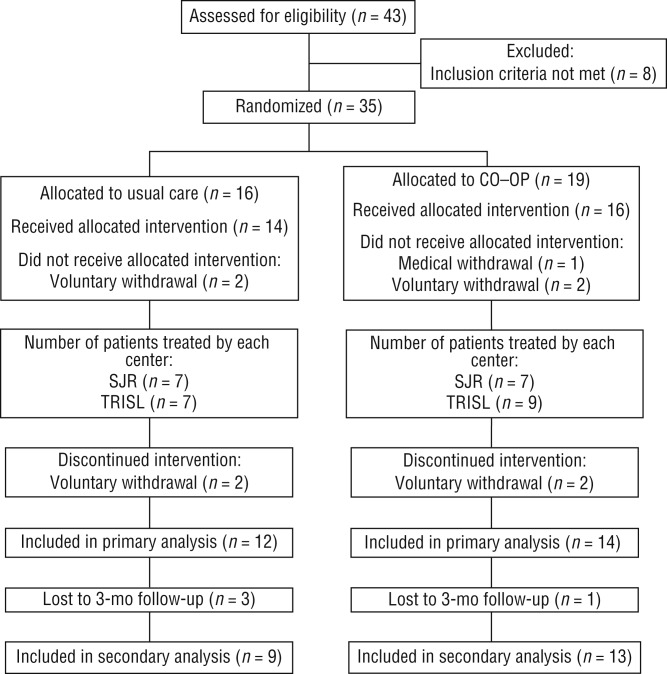
A CONSORT (CONsolidated Standards Of Reporting Trials) diagram depicts participant flow through the study (Figure 1). A total of 35 participants were randomized between The Rehabilitation Institute of St. Louis (n = 20) and St. John’s Rehab in Toronto (n = 15). Of these 35 participants, 26 completed the intervention and the postintervention assessment (Time 2). Table 2 provides demographic data for participants by group and site. Overall, participants in St. Louis started outpatient occupational therapy services sooner than participants in Toronto (p = .04); however, participants had no other significant differences between sites at baseline. Table 2 shows that participants in both groups received almost an equal number of occupational therapy sessions; however, for this analysis, two outliers were removed, one from each treatment group, who had received more than 100 sessions of occupational therapy, perhaps related to their ability to pay privately for services.
Figure 1.
CONSORT (CONsolidated Standards Of Reporting Trials) diagram of participant flow through the study.
Note. CO–OP = Cognitive Orientation to Daily Occupational Performance; SJR = St. John’s Rehab; TRISL = The Rehabilitation Institute of St. Louis. From “Combined Cognitive-Strategy and Task-Specific Training Improve Transfer to Untrained Activities in Subacute Stroke: An Exploratory Randomized Controlled Trial,” by S. McEwen, H. Polatajko, C. Baum, J. Rios, D. Cirone, M. Doherty, & T. Wolf, 2015, Neurorehabilitation and Neural Repair, 29, 531. http://dx.doi.org/10.1177/1545968314558602. Copyright © 2015 by the American Society of Neurorehabilitation. Used with permission.
Table 2.
Participant Demographics (N = 35)
| M (SD) or n (%) | ||||
| Characteristic and Group | St. Louis | Toronto | Total | |
| Days since stroke | ||||
| UC | 41.6 (17.1) | 52.0 (25.4) | 46.5 (21.3) | |
| CO–OP | 30.5 (10.7) | 53.1 (23.9) | 40.1 (20.4) | |
| Therapy, hr | ||||
| UC | 10.2 (2.8) | 17.0 (13.0) | 13.3 (9.2) | |
| CO–OP | 7.9 (2.3) | 14.4 (8.7) | 10.9 (6.8) | |
| Age, yr | ||||
| UC | 50.7 (14.5) | 59.3 (12.7) | 54.4 (14.0) | |
| CO–OP | 57.4 (15.5) | 57.6 (12.7) | 57.5 (14.0) | |
| Education, yr | ||||
| UC | 12.2 (1.5) | 14.4 (1.9) | 13.2 (2.0) | |
| CO–OP | 14.3 (3.0) | 15.3 (5.6) | 14.7 (4.2) | |
| Female | ||||
| UC | 5 (44.4) | 2 (28.6) | 7 (43.8) | |
| CO–OP | 4 (36.4) | 2 (25.0) | 6 (31.6) | |
| Right-side stroke | ||||
| UC | 7 (77.8) | 6 (85.7) | 13 (81.3) | |
| CO–OP | 6 (54.5) | 5 (62.5) | 11 (57.9) | |
| Right-handedness | ||||
| UC | 8 (88.9) | 6 (85.7) | 14 (87.5) | |
| CO–OP | 9 (81.8) | 8 (100) | 17 (89.5) | |
| Living with others | ||||
| UC | 8 (88.9) | 7 (100) | 15 (93.8) | |
| CO–OP | 7 (63.6) | 7 (87.5) | 14 (73.7) | |
| Ethnicity | ||||
| White | ||||
| UC | 2 (25.0) | 5 (71.4) | 7 (46.7) | |
| CO–OP | 5 (45.5) | 4 (50.0) | 9 (47.4) | |
| African-American | ||||
| UC | 6 (75.0) | 0 | 6 (40.0) | |
| CO–OP | 6 (54.4) | 4 (50.0) | 10 (52.6) | |
| Asian | ||||
| UC | 0 | 1 (14.3) | 1 (6.7) | |
| CO–OP | 0 | 0 | 0 | |
| Other | ||||
| UC | 0 | 1 (14.3) | 1 (6.7) | |
| CO–OP | 0 | 0 | 0 | |
Note. CO–OP = Cognitive Orientation to Daily Occupational Performance; M = mean; SD = standard deviation; UC = usual care. From “Combined Cognitive-Strategy and Task-Specific Training Improve Transfer to Untrained Activities in Subacute Stroke: An Exploratory Randomized Controlled Trial,” by S. McEwen, H. Polatajko, C. Baum, J. Rios, D. Cirone, M. Doherty, & T. Wolf, 2015, Neurorehabilitation and Neural Repair, 29, 532. http://dx.doi.org/10.1177/1545968314558602. Copyright © 2015 by the American Society of Neurorehabilitation. Used with permission.
Table 3 displays score and change score means and standard deviations and effect sizes for all normally distributed data for stroke impact and cognitive flexibility between the CO–OP and the usual-care groups. Cohen’s d effect size interpretation is as follows: 0.2 = small effect, 0.5 = medium effect, and 0.8 = large effect. At Time 2, CO–OP had a large effect over usual care for SIS Recovery (d = 0.8) and a medium effect over usual care for changes in the SIS Physical summary score (strength, hand function, mobility, and ADL/IADL scores), SIS Hand Function, and the D–KEFS Trail Making subtest (d = 0.5). At Time 3, there was a medium effect for SIS Hand Function (d = 0.6) and the D–KEFS Trail Making subtest (d = 0.5). ARAT and SIS Communication, Physical summary score, Emotion, and Memory domains were all found to be non-normally distributed, thus nonparametric analyses were conducted.
Table 3.
Comparisons Between Groups for Stroke Impact and Cognitive Flexibility
| M (SD) | M (SD) | |||||||
| Outcome Measure and Group | Time 1 | Time 2 | Time 1–2 Change Score | da [95% CI] | Time 3 | Time 1–3 Change Score | da [95% CI] | |
| SIS ADLs | ||||||||
| UC | 59.6 (13.8) | 58.2 (15.6) | −0.5 (18.6) | 0.6 [–0.2, 1.4] | 64.7 (10.8) | 7.3 (14.1) | 0.1 [–0.8, 1.0] | |
| CO–OP | 54.8 (14.6) | 64.4 (15.0) | 8.4 (10.9) | 64.9 (11.7) | 8.5 (10.1) | |||
| SIS Mobility | ||||||||
| UC | 57.6 (14.7) | 64.4 (15.2) | 4.6 (19.7) | 0.2 [–0.6, 0.9] | 64.9 (11.2) | 9.6 (16.3) | 0.1 [–0.8, 1.0] | |
| CO–OP | 54.4 (46.9) | 62.4 (17.1) | 7.0 (7.9) | 69.3 (11.8) | 10.7 (10.8) | |||
| SIS Hand Function | ||||||||
| UC | 38.3 (26.7) | 39.0 (30.2) | 3.7 (16.4) | 0.5 [–0.3, 1.3] | 44.4 (30.2) | 9.8 (17.1) | 0.6 [–0.3, 1.5] | |
| CO–OP | 32.8 (27.0) | 46.0 (27.6) | 11.7 (15.9) | 51.6 (24.8) | 20.7 (16.9) | |||
| SIS | ||||||||
| UC | 60.0 (11.4) | 62.4 (12.7) | 1.6 (15.3) | 0.5 [–0.3, 1.3] | 65.8 (8.1) | 7.4 (12.1) | 0.2 [–0.7, 1.1] | |
| CO–OP | 55.9 (14.8) | 64.2 (14.0) | 7.8 (7.7) | 67.7 (10.7) | 9.9 (11.4) | |||
| SIS Recovery | ||||||||
| UC | 58.1 (22.2) | 50.0 (18.3) | −5.5 (16.3) | 0.8 [0.0, 1.6] | 66.7 (11.7) | 11.1 (18.3) | 0.0 [–0.9, 0.9] | |
| CO–OP | 58.9 (19.1) | 67.5 (15.2) | 7.1 (14.8) | 72.3 (17.7) | 11.4 (13.4) | |||
| D–KEFS Condition 4 | ||||||||
| UC | 5.4 (4.2) | 6.4 (4.9) | 0.9 (2.0) | 0.5 [–0.3, 1.3] | 6.9 (4.5) | 1.3 (3.8) | 0.5 (–0.4, 1.4) | |
| CO–OP | 5.3 (3.8) | 8.1 (3.3) | 2.6 (4.3) | 9.0 (3.3) | 3.4 (4.6) | |||
Note. ADLs = activities of daily living; CI = confidence interval; CO–OP = Cognitive Orientation to Daily Occupational Performance; D–KEFS = Delis–Kaplan Executive Function System; M = mean; SD = standard deviation; SIS = Stroke Impact Scale; UC = usual care.
Effect size: 0.2 = small effect, 0.5 = medium effect, and 0.8 = large effect.
Table 4 displays score and change score medians and ranges and the nonparametric effect size r (r2 = z2/N) for stroke impact and arm movement between the CO–OP and the usual-care groups. Effect size r interpretation is as follows: 0.1 = small effect, 0.3 = medium effect, and 0.5 = large effect (Fritz et al., 2012). At Time 2, changes in the ARAT and SIS domains all showed a small to medium effect of CO–OP over usual care. At Time 3, changes in SIS Communication showed a medium effect of CO–OP over usual care, and changes in the ARAT showed a small effect of CO–OP.
Table 4.
Comparison Between Groups for Stroke Impact and Arm Movement
| Median (Range) | Median (Range) | |||||||
| Outcome Measure and Group | Time 1 | Time 2 | Time 1–2 Change Score | ra | Time 3 | Time 1–3 Change Score | ra | |
| SIS Physical | ||||||||
| UC | 42.5 (10–80) | 37.5 (10–80) | −2.5 (–70 to 20) | .3 | 45 (25–80) | 5.0 (–20 to 20) | .2 | |
| CO–OP | 45 (15–80) | 50 (20–80) | 2.5 (–5 to 20) | 55 (20–80) | 10.0 (–5.0 to 25.0) | |||
| SIS Memory | ||||||||
| UC | 67.1 (34.3–80) | 71.4 (48.6–80) | 0 (–25.7 to 40) | .3 | 62.9 (51.4–74.3) | −2.9 (–17.1 to 40) | .2 | |
| CO–OP | 62.9 (11.4–80) | 67.1 (2.9–80) | 4.3 (–8.6 to 40) | 77.1 (0–80) | 5.7 (–14.3 to 37.1) | |||
| SIS Emotion | ||||||||
| UC | 61.1 (28.9–77.8) | 66.7 (17.8–80) | −1.1 (–22.2 to 11.1) | .3 | 66.7 (57.8–77.8) | 2.2 (–13.3 to 13.3) | .2 | |
| CO–OP | 60 (24.4–80) | 66.7 (26.7–80) | 7.8 (–35.6 to 13.3) | 71.1 (20–77.8) | 6.7 (–42.2 to 17.8) | |||
| SIS Communication | ||||||||
| UC | 77.1 (40–80) | 78.6 (54.3–80) | 0 (–20 to 2.9) | .4 | 74.3 (54.3–80) | 0 (–11.4 to 8.6) | .4 | |
| CO–OP | 65.1 (22.9–80) | 72.9 (28.6–80) | 1.43 (–11.4 to 20) | 71.4 (42.9–80) | 2.9 (–5.7 to 34.3) | |||
| ARAT | ||||||||
| UC | 55 (0–57) | 50 (0–57) | 0 (–24 to 5) | .2 | 55 (4–57) | 1 (–5 to 10) | .1 | |
| CO–OP | 50 (0–57) | 55 (0–57) | 0 (–5 to 11) | 55 (2–57) | 2.5 (–1 to 18) | |||
| ARAT (Impairment)b | ||||||||
| UC | 22 (0–47) | 5 (0–47) | –1.5 (–24 to 5) | .3 | 30 (4–55) | 8 (1 to 10) | .3 | |
| CO–OP | 4 (0–45) | 10 (0–49) | 0 (–5 to 11) | 22 (2–56) | 12 (–1 to 18) | |||
Note. ARAT = Action Research Arm Test; CO–OP = Cognitive Orientation to Daily Occupational Performance; SIS = Stroke Impact Scale; UC = usual care.
Effect size: 0.1 = small effect, 0.3 = medium effect, 0.5 = large effect.
UC group, n = 7; CO-OP group, n = 8.
Given that the primary outcome of this study was to evaluate the effect of CO–OP on performance, no inclusion or exclusion criteria were related to upper-extremity function. Therefore, as the data show, the sample in both intervention groups included a wide range of upper-extremity function. Therefore, we also evaluated the effect of the interventions only in participants who had an impairment as defined by the ARAT (i.e., score <49). This criterion for impairment was established in an ongoing clinical trial, conducted by Catherine Lang and colleagues (NCT01146379), to evaluate the necessary dose of task-specific training to improve upper-extremity function. In participants with upper-extremity impairment (CO–OP group, n = 8; usual-care group, n = 7), a medium effect of CO–OP over usual care was still maintained at follow-up (see Table 4).
Discussion
The results of this study suggest that CO–OP, a top-down intervention focused primarily on skill performance, may have a broader positive effect on stroke recovery than usual care and a broader effect than might be expected of a top-down approach. In addition to positive gains in the targeted areas, subjective and objective occupational performance (McEwen et al., 2015), these data support a positive effect of CO–OP over usual occupational therapy on upper-extremity function, cognitive flexibility, and perceived body functions, areas not directly targeted during treatment. Note that the effect size estimates reported are the effect of CO–OP compared with usual occupational therapy services, rather than the absolute effect of CO–OP.
Although overall the differential effect on some secondary measures lessened between Time 2 and follow-up, there was still a measureable positive effect of CO–OP over usual care on all of the outcomes except the single SIS question related to self-report global recovery. This question asks participants to rate overall recovery from stroke on a scale ranging from 0 to 100. The CO–OP participants reported consistently better overall recovery over time, whereas the usual-care group decreased from baseline to postintervention assessment before improving at follow-up. Overall, the mean change scores for overall recovery for the CO–OP group were consistently higher than those for the usual-care group. The differential and positive effect of CO–OP on the objective measure of cognitive flexibility (D–KEFS Trail Making subtest) was maintained at a moderate level through the 3-mo follow-up. The differential and positive effect of CO–OP was moderate (r = .3) on the objective measure of arm movement (ARAT) postintervention and lessened minimally to r = .2 at 3-mo follow-up.
The explanation for the differential and positive effect of CO–OP over usual care remains unknown; however, some potential hypotheses could explain the results. First, CO–OP’s focus on skill performance, cognitive strategy use, and guided discovery could serve as a catalyst that provides an initial increase in both effect and efficiency (rate of effect) over usual care, as seen at Time 2. This hypothesis could be tested in a future study and would provide support for the use of CO–OP to expedite recovery poststroke. Second, almost all of the CO–OP participants received some usual-care occupational therapy after their 10 CO–OP sessions, which may have influenced the results at follow-up. We postulate that this situation may have caused confusion and mixed messages because participants went from a largely self-directed approach to a more traditional therapist-led approach. Future studies could evaluate whether additional CO–OP sessions instead of usual care would lead to maintenance of the larger differential effect observed postintervention.
CO–OP’s stated clinical objectives also include cognitive strategy use and transfer and generalization beyond the specific activities trained. Therefore, some of the moderate effects on secondary outcomes are perhaps expected. It can be postulated that using the CO–OP approach that implements global cognitive strategy in combination with guided discovery is linked to changes in cognitive flexibility. In CO–OP, participants are trained to use the global strategy Goal–Plan–Do–Check to develop a specific plan to improve performance of a self-selected goal, review performance, and modify the plan accordingly if performance is not satisfactory. This process is consistent with psychology theories of cognitive flexibility that describe cognitive flexibility as necessary to assess a situation when a nonroutine response is required, plan a new response or action to be taken, and use strategies to deal with the demands of the novel environment (Cañas, Quesada, Antolí, & Fajardo, 2003; Norman & Shallice, 1986). Therefore, the CO–OP process, in essence, helps to retrain cognitive flexibility in a way that usual care, which most likely focuses on remediation of habitual activities and function, does not. This effect could explain the gains in cognitive flexibility in the CO–OP group and the relative stability in such flexibility in the usual-care group. It is also consistent with at least one other study that has evaluated the use of key CO–OP components with people with stroke (Skidmore et al., 2014).
CO–OP has been found to have a medium effect over usual care on self-efficacy in ability to perform daily activities (McEwen et al., 2015). It can be postulated that this increase in self-efficacy leads to increased motivation to participate in everyday life activities and thereby increased use of the impaired upper extremity. This improvement in self-efficacy could also explain the differential effect on self-perception of function observed between the two groups. Regardless, the measurable effect of CO–OP over usual care provides preliminary evidence to support the use of CO–OP to improve occupational performance and remediate impairments in arm movement and cognitive flexibility. These results bring into question the continued clinical practice of focusing treatment on impairments with the hopes that reducing impairments will lead to meaningful occupational changes. The causal relationship between the effects observed remains unknown and is an area for future investigation.
This study has several limitations on the generalizability of the findings. First, all CO–OP participants were eligible to receive additional occupational therapy services after their completion of 10 CO–OP sessions. The majority of participants did receive additional usual-care occupational therapy services between the postintervention and the follow-up assessment that could not be controlled for in the data analysis because of the relatively small sample. Although dosage data were collected and were comparable between groups, there were unequal numbers of sessions between sites. In addition, the content of these additional sessions is largely unknown and may have biased the results. These additional sessions may potentially be responsible for the decrease in the differential effect between CO–OP and usual care at follow-up.
Second, for unknown reasons, there were more dropouts between Time 2 and 3 in the usual-care group (i.e., 3 participants) than in the CO–OP group (i.e., 1 participant); therefore, effect sizes between Time 2 and Time 3 could not be directly compared. Third, this early-phase feasibility study had a small sample with the aim to identify within-group and between-groups effects on a wide range of outcomes. The study was not powered for hypothesis testing, and further research is required to confirm the effects found. Fourth, little information was available to evaluate what specifically constituted usual-care occupational therapy at each site. The study team was limited to self-report from the therapists and daily notes; however, these items were not sufficiently descriptive. Future studies will have to document and classify usual-care occupational therapy more thoroughly. Fifth, upper-extremity dysfunction was not a specified inclusion criterion for this study. Therefore, the final sample had unequal upper-extremity function between groups. A future study that targets upper-extremity function as a primary outcome will need to take this function into account in the design of the study.
Finally, the effect of the interventions on impairment remediation was considered a secondary outcome in this study. Therefore, data were limited to evaluate the effect of the interventions on impairment and function. Future studies should more thoroughly evaluate potential outcome measures to try to capture this effect with more sensitive and comprehensive tools.
Implications for Occupational Therapy Practice
This study’s findings have the following implications for occupational therapy practice:
CO–OP, a top-down performance-based approach, has a positive effect on performance and impairment reduction in this early-phase study.
The generalizability of these findings is currently limited, and future research is necessary to confirm them.
These findings call into question the continued use of impairment-based rehabilitation methods to improve occupational performance and reduce impairment after stroke.
Conclusion
CO–OP, a top-down intervention targeting performance, cognitive strategy use, and transfer and generalization has a measureable effect not only on performance but also on impairment reduction and stroke impact. Specifically, measurable effects of CO–OP were seen in cognitive flexibility, arm function, and most self-reported stroke impact domains compared with usual-care occupational therapy. This early-phase feasibility study provides support for the use of CO–OP over usual care; however, it has several limitations on generalizability of the results. Future investigation is warranted to confirm and expand these findings.
Acknowledgments
This study was funded by a Canadian Institutes of Health Research Open Operating Grant (Funding Reference No. 111200) and was supported by the St. John’s Rehab Hospital and The Rehabilitation Institute of St. Louis. The authors acknowledge the assistance of Alisa Grigorovich, Tanya Ramsey, Carin Roth, Tammy Craig, Kimberly Walker, and Duana Russell-Thomas and of the staff in the Performance, Participation, and Neurorehabilitation Laboratory in the Program in Occupational Therapy at Washington University in St. Louis in assessment, evaluation, and treatment. Sara McEwen’s salary support came from the St. John’s Rehab Foundation. Timothy J. Wolf received salary support from the National Center for Medical Rehabilitation Research, National Institute of Child Health and Human Development, National Institutes of Health under award numbers K12HD055931 and K23HD073190. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
References
- Alzahrani M. A., Ada L., & Dean C. M. (2011). Duration of physical activity is normal but frequency is reduced after stroke: An observational study. Journal of Physiotherapy, 57, 47–51. http://dx.doi.org/10.1016/S1836-9553(11)70007-8 [DOI] [PubMed] [Google Scholar]
- Appelros P., Samuelsson M., Karlsson-Tivenius S., Lokander M., & Terént A. (2007). A national stroke quality register: 12 years experience from a participating hospital. European Journal of Neurology, 14, 890–894. http://dx.doi.org/10.1111/j.1468-1331.2007.01826.x [DOI] [PubMed] [Google Scholar]
- Canadian Institute for Health Information. (2009). CIHI national rehabilitation reporting system, listing of data elements. Retrieved from http://www.cihi.ca/CIHI-ext-portal/pdf/internet/
- Cañas J., Quesada J. F., Antolí A., & Fajardo I. (2003). Cognitive flexibility and adaptability to environmental changes in dynamic complex problem-solving tasks. Ergonomics, 46, 482–501. http://dx.doi.org/10.1080/0014013031000061640 [DOI] [PubMed] [Google Scholar]
- Connell L. A., McMahon N. E., Eng J. J., & Watkins C. L. (2014). Prescribing upper limb exercises after stroke: A survey of current UK therapy practice. Journal of Rehabilitation Medicine, 46, 212–218. http://dx.doi.org/10.2340/16501977-1268 [DOI] [PubMed] [Google Scholar]
- Delis D. C., Kaplan E., & Kramer J. H. (2001). Delis–Kaplan Executive Function System. San Antonio, TX: Psychological Corporation. [Google Scholar]
- Duncan P. W., Bode R. K., Min Lai S., & Perera S. (2003). Rasch analysis of a new stroke-specific outcome scale: The Stroke Impact Scale. Archives of Physical Medicine and Rehabilitation, 84, 950–963. http://dx.doi.org/10.1016/S0003-9993(03)00035-2 [DOI] [PubMed] [Google Scholar]
- Duncan P. W., Lai S. M., Bode R. K., Perera S., & DeRosa J. (2003). Stroke Impact Scale–16: A brief assessment of physical function. Neurology, 60, 291–296. http://dx.doi.org/10.1212/01.WNL.0000041493.65665.D6 [DOI] [PubMed] [Google Scholar]
- French B., Leathley M., Sutton C., McAdam J., Thomas L., Forster A., . . . Watkins C. (2008). A systematic review of repetitive functional task practice with modelling of resource use, costs and effectiveness. Health Technology Assessment, 12(30), 1–117. http://dx.doi.org/10.3310/hta12300 [DOI] [PubMed] [Google Scholar]
- Fritz C. O., Morris P. E., & Richler J. J. (2012). Effect size estimates: Current use, calculations, and interpretation. Journal of Experimental Psychology: General, 141, 2–18. http://dx.doi.org/10.1037/a0024338 [DOI] [PubMed] [Google Scholar]
- Hackett M. L., Glozier N., Jan S., & Lindley R. (2012). Returning to paid employment after stroke: The Psychosocial Outcomes in StrokE (POISE) cohort study. PLoS One, 7, e41795 http://dx.doi.org/10.1371/journal.pone.0041795 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Law M., Baptiste S., Carswell A., McColl M., Polatajko H., & Pollock N. (1998). Canadian Occupational Performance Measure. Ottawa, ON: CAOT Publications. [Google Scholar]
- Lyle R. C. (1981). A performance test for assessment of upper limb function in physical rehabilitation treatment and research. International Journal of Rehabilitation Research, 4, 483–492. http://dx.doi.org/10.1097/00004356-198112000-00001 [DOI] [PubMed] [Google Scholar]
- Martini R., Rios J., Polatajko H., Wolf T., & McEwen S. (2015). The Performance Quality Rating Scale (PQRS): Reliability, convergent validity, and internal responsiveness for two scoring systems. Disability and Rehabilitation, 37, 1–8. [DOI] [PubMed] [Google Scholar]
- Mayo N. E., Wood-Dauphinee S., Côté R., Durcan L., & Carlton J. (2002). Activity, participation, and quality of life 6 months poststroke. Archives of Physical Medicine and Rehabilitation, 83, 1035–1042. http://dx.doi.org/10.1053/apmr.2002.33984 [DOI] [PubMed] [Google Scholar]
- McEwen S., Polatajko H., Baum C., Rios J., Cirone D., Doherty M., & Wolf T. (2015). Combined cognitive-strategy and task-specific training improve transfer to untrained activities in subacute stroke: An exploratory randomized controlled trial. Neurorehabilitation and Neural Repair, 29, 526–536. http://dx.doi.org/10.3109/09638288.2014.913702 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McEwen S. E., Polatajko H. J., Huijbregts M. P., & Ryan J. D. (2009). Exploring a cognitive-based treatment approach to improve motor-based skill performance in chronic stroke: Results of three single case experiments. Brain Injury, 23, 1041–1053. http://dx.doi.org/10.3109/02699050903421107 [DOI] [PubMed] [Google Scholar]
- McEwen S. E., Polatajko H. J., Huijbregts M. P., & Ryan J. D. (2010). Inter-task transfer of meaningful, functional skills following a cognitive-based treatment: Results of three multiple baseline design experiments in adults with chronic stroke. Neuropsychological Rehabilitation, 20, 541–561. http://dx.doi.org/10.1080/09602011003638194 [DOI] [PubMed] [Google Scholar]
- Meichenbaum D. H., & Goodman J. (1971). Training impulsive children to talk to themselves: A means of developing self-control. Journal of Abnormal Psychology, 77, 115–126. http://dx.doi.org/10.1037/h0030773 [DOI] [PubMed] [Google Scholar]
- Meyer A. (1922). The philosophy of occupational therapy. Archives of Occupational Therapy, 1, 1–10. [Google Scholar]
- Nasreddine Z. S., Phillips N. A., Bédirian V., Charbonneau S., Whitehead V., Collin I., . . . Chertkow H. (2005). The Montreal Cognitive Assessment, MoCA: A brief screening tool for mild cognitive impairment. Journal of the American Geriatrics Society, 53, 695–699. http://dx.doi.org/10.1111/j.1532-5415.2005.53221.x [DOI] [PubMed] [Google Scholar]
- National Stroke Association. (2015). Survivors: Are you a stroke survivor? Retrieved from http://www.stroke.org/we-can-help/survivors
- Norman D. A., & Shallice T. (1986). Attention to action: Willed and automatic control of behavior. In Davidson R. J., Schwartz G. E., & Shapiro D. (Eds.), Consciousness and self-regulation (Vol. 4, pp. 1–18). New York: Plenum Press; http://dx.doi.org/10.1007/978-1-4757-0629-1_1 [Google Scholar]
- Ploughman M. (2002). A review of brain neuroplasticity and implications for the physiotherapeutic management of stroke. Physiotherapy Canada, 54, 164–176. [Google Scholar]
- Polatajko H., Mandich A., Missiuna C., Miller L. T., Macnab J. J., Malloy-Miller T., & Kinsella E. (2001). Cognitive Orientation to Daily Occupational Performance: Part III—The protocol in brief. Physical and Occupational Therapy in Pediatrics, 20, 108. [PubMed] [Google Scholar]
- Polatajko H. J., McEwen S. E., Ryan J. D., & Baum C. M. (2012). Pilot randomized controlled trial investigating cognitive strategy use to improve goal performance after stroke. American Journal of Occupational Therapy, 66, 104–109. http://dx.doi.org/10.5014/ajot.2012.001784 [DOI] [PubMed] [Google Scholar]
- Reilly M. (1962). Occupational therapy can be one of the great ideas of 20th century medicine. American Journal of Occupational Therapy, 16, 1–9. [PubMed] [Google Scholar]
- Rennie D.(2001)CONSORT revised—Improving the reporting of randomized trials. JAMA, 285, 2006–2007. http://dx.doi.org/10.1001/jama.285.15.2006 [DOI] [PubMed] [Google Scholar]
- Salter K., Foley N., & Teasell R. (2010). Social support interventions and mood status post stroke: A review. International Journal of Nursing Studies, 47, 616–625. http://dx.doi.org/10.1016/j.ijnurstu.2009.12.002 [DOI] [PubMed] [Google Scholar]
- Skidmore E. R., Dawson D. R., Butters M. A., Grattan E. S., Juengst S. B., Whyte E. M., . . . Becker J. T. (2014). Strategy training shows promise for addressing disability in the first 6 months after stroke. Neurorehabilitation and Neural Repair, 29, 668–676 http://dx.doi.org/10.1177/1545968314562113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teasell R. W., Foley N. C., Salter K. L., & Jutai J. W. (2008). A blueprint for transforming stroke rehabilitation care in Canada: The case for change. Archives of Physical Medicine and Rehabilitation, 89, 575–578. http://dx.doi.org/10.1016/j.apmr.2007.08.164 [DOI] [PubMed] [Google Scholar]
- Veerbeek J. M., van Wegen E., van Peppen R., van der Wees P. J., Hendriks E., Rietberg M., & Kwakkel G. (2014). What is the evidence for physical therapy poststroke? A systematic review and meta-analysis. PLoS One, 9, e87987 http://dx.doi.org/10.1371/journal.pone.0087987 [DOI] [PMC free article] [PubMed] [Google Scholar]