Abstract
Background
The aim of this study is to describe the major evolutionary historical events among Leishmania, sandflies, and the associated animal reservoirs in detail, in accordance with the geographical evolution of the Earth, which has not been previously discussed on a large scale.
Methodology and Principal Findings
Leishmania and sandfly classification has always been a controversial matter, and the increasing number of species currently described further complicates this issue. Despite several hypotheses on the origin, evolution, and distribution of Leishmania and sandflies in the Old and New World, no consistent agreement exists regarding dissemination of the actors that play roles in leishmaniasis. For this purpose, we present here three centuries of research on sandflies and Leishmania descriptions, as well as a complete description of Leishmania and sandfly fossils and the emergence date of each Leishmania and sandfly group during different geographical periods, from 550 million years ago until now. We discuss critically the different approaches that were used for Leishmana and sandfly classification and their synonymies, proposing an updated classification for each species of Leishmania and sandfly. We update information on the current distribution and dispersion of different species of Leishmania (53), sandflies (more than 800 at genus or subgenus level), and animal reservoirs in each of the following geographical ecozones: Palearctic, Nearctic, Neotropic, Afrotropical, Oriental, Malagasy, and Australian. We propose an updated list of the potential and proven sandfly vectors for each Leishmania species in the Old and New World. Finally, we address a classical question about digenetic Leishmania evolution: which was the first host, a vertebrate or an invertebrate?
Conclusions and Significance
We propose an updated view of events that have played important roles in the geographical dispersion of sandflies, in relation to both the Leishmania species they transmit and the animal reservoirs of the parasites.
Introduction
Leishmaniases are vector-borne diseases caused by obligate protozoan parasites from the genus Leishmania (Trypanosomatida: Trypanosomatidae). Leishmaniases are endemic in large areas of the tropics, subtropics, and the Mediterranean basin, including more than 98 countries, where there are a total of 350 million people at risk and 12 million cases of infection. Canine leishmaniasis is a serious problem, and it is estimated that 2.5 million dogs are infected in the Mediterranean basin only [1]. Among the endemic regions on five continents, there is an estimated incidence of 0.7–1.2 million cases of cutaneous leishmaniasis (CL) and 0.2–0.4 million cases of visceral leishmaniasis (VL) in these countries [2]. The disease is absent in New Zealand and the southern Pacific. Leishmaniasis is transmitted by the bite of infected female sandflies, whose hosts are animals such as canids, rodents, marsupials, hyraxes, or human beings. Approximately 53 Leishmania species have been described (without considering the synonyms and including all five subgenera and complexes: Leishmania, Viannia, Sauroleishmania, L. enriettii complex, and Paraleishmania); of these, 31 species are known to be parasites of mammals and 20 species are pathogenic for human beings. Leishmania parasites cause four main clinical forms of the disease—according to the location of the parasite in mammalian tissues—referred to as visceral, cutaneous, diffuse cutaneous, and mucocutaneous leishmaniasis. The most common form is cutaneous disease, and the ten countries of Afghanistan, Algeria, Colombia, Brazil, Iran, Syria, Ethiopia, North Sudan, Costa Rica, and Peru together account for 70% to 75% of the global estimated CL incidence [2]. Regarding visceral leishmaniasis, more than 90% of all cases occur in just the six countries of India, Bangladesh, Sudan, South Sudan, Brazil, and Ethiopia [2]. Leishmaniasis currently constitutes a major global public health problem, showing an increasing burden over the last decade [2].
Leishmaniasis has a long history, dating to 2,500 B.C., with several primitive descriptions of the disease having been found in ancient writings and recent molecular findings from ancient archeological material. A detailed history of Leishmania descriptions is gven in Table 1.
Table 1. History of Leishmania descriptions.
| Century | Author (Year): Description |
|---|---|
| B.C. | (2,500 to 1,500 B.C.): First description of conspicuous lesions similar to current cutaneous leishmanisis (CL) lesions. (2,000 B.C.): Leishmania donovani infection in ancient Egyptian and Christian Nubian mummies. (1,500 B.C.): Report of Leishmania DNA in northern Sudan. (800 B.C.): Leishmania infection in a 6-year-old girl mummy in Peru. (700 B.C.): Similar descriptions of CL discovered on tablets from King Ashurbanipal. (650 B.C.): Records of what seems to be CL in the Tigris–Euphrates basin. |
| A.D. | (First century A.D.): Evidence for the presence of the cutaneous form of the disease in Ecuador and Peru, South America. Avicenna (10th century A.D.): Description of cutaneous lesions called Balakh sore and probability of mosquito intervention. (15th and 16th centuries A.D.: Inca period): Notification of "valley sickness," "Andean sickness," or "white leprosy," which are likely to be South American CL. |
| 18th century | Russell (1756): First detailed clinical description of the disease. Indian physicians (1756): Description of kala azar clinical symptoms (kālā āzār: kālā meaning black and āzār meaning fever or disease). Cosme Bueno (1764): First suspicions reporting the probable role of phlebotomine sandflies in disease transmission in the New World. |
| 19th century | Villar (1859): Earliest traceable clinical description of the Peruvian ‘‘uta,” similar to the "Aleppo button." Borovsky (1898): First accurate description of the causative agent of the oriental sore, reference to Protozoa. |
| first half of 20th century | Leishman (1901): Identification of organisms, as "trypanosomes," in smears from the spleen of an Indian patient deceased from "dum-dum fever." Donovan (1901): Confirms the presence of what became known as Leishman-Donovan bodies in the smears from Indian patients. First description of the link between Leishman-Donovan bodies and kala azar. Ross (1903): Proposed the name of Leishmania donovani for the Leishman-Donovan bodies. Wright (1903): Description of Helcosoma tropica (L. tropica). Leishman and Rogers (1904): Demonstrated oval intracellular amastigotes can differentiate into flagellated promastigotes. Rogers (1904): First successful in vitro cultivation of the flagellated forms. Laveran and Chatoin (1904): First case of kala azar in the Mediterranean region. Sergent and colleagues (1905): First report of CL transmited by sandflies of the Phlebotomus genus. Patton (1907): Evidence of the presence of Leishman-Donovan bodies in peripheral blood lymphocytes and its flagellated forms in the sandfly's gut. Nicolle (1908): Isolation of Leishmania parasites from a child or "infant," leading to name Leishmania infantum. Differentiation between the Mediterranean visceral leishmaniasis caused by L. infantum and the Indian kala azar due to L. donovani. Nicolle and Comple (1908): Isolation of Leishmania parasites from infected dogs. Lindenberg, Carini, and Paranhos (1909): Confirm the presence of autochthonous cutaneous leishmaniasis, "Baurú ulcer," in the Americas. Wenyon (1911): Incrimination of Phlebotomus as the probable vector of diseases caused by Leishmania in the Old World. Splendore (1911): Leishmania as the causative agent of mucocutaneous lesions "Espundia." Vianna (1911): Description of L. braziliensis. Migone (1913): First report of visceral leishmaniasis in the Americas. Yakimoff and Schokhor (1914): Proposition of the names L. tropica minor and L. tropica major to separate parasites causing "dry urban" and "wet rural" cutaneous leishmaniasis. Casteliani and Chalmers (1919): L. donovani archibaldi as the ethiological agent of a lethal form of visceral leishmaniasis. Aragão (1922): Reproduced in a dog the clinical signs of leishmaniasis by injecting squashed infected sandflies. Montenegro (1923): Experimental inoculation of L. braziliensis, introduction of the intradermal test (Montenegro skin test), still in use for the diagnosis of leishmaniasis. Penna (1934): First record of the Amazonian visceral leishmanaisis. Chagas (1936): Description of visceral leishmaniasis in Brazil. Cunha and Chagas (1937): Isolation of L. chagasi from Brazilian VL. Swaminath and colleagues (1942): Demontrated the process of Leishmania transmition to humans by sandflies using a group of volunteers. Hoare (1948): Demonstrated the Leishmania circulation in sandflies, indicating the flagellates being set free and multiplying in the sandfly intestine; the infection later is caused through the posterior station (like Trypanosoma cruzi). Kirk (1949): Classification of Leishmania according to their morphology, culture characteristics, clinical and epidemiological aspects of infections in human and other natural hosts, cross-immunity, serological tests, and xenodifferentiation. Propose a complete nomenclature of the Leishmania genus and their synonyms. |
| second half of 20th century | Biagi (1953): Discription of various Leishmania species. Pessôa (1961): Present the first list of known Leishmania species in the Americas. Use of the trinomial system for Leishmania. Adler (1962): Reports transient cryptic infections in mice by L. adleri, which usually infects lizards, that lead to the proposal of the evolution of Leishmania species infecting mammals from reptilian parasites. Adler (1963 and 1964): Differentiates L. tropica, L. mexicana, and L. braziliensis with serological techniques. Proposed a taxonomy for Leishmania infecting hummans and lizards. Shaw (1964): Demostrates the transmission of Endotrypanum schaudinni by Phlebotomus species. Hoare and Wallace (1966): Introduced new terms for the description of the Leishmania developmental stages. Lainson and Shaw (1970): Subdivide Leishmania species into two groups: "fast-growing (L. mexicana)" and "slow-growing (L. braziliensis)." Lainson and Shaw (1972): First proposal of complexes of species for Neotropical Leishmania causing CL: the mexicana complex and the braziliensis complex. Schnur and colleagues (1972): serotype Leishmania with promastigotes excreted factors. Ranquein (1973): First proposal of a separate genus for Sauroleishmania. Bray (1973): Use the systematic concept for description of Leishmania species. Vickerman (1976): Proposed Leishmania that do not infect mammals as “not strictly being” Leishmania species, giving the status "Incertae sedis" to Leishmania isolated from reptiles. Gardener (1977): Proposed a taxonomy of the Leishmania genus that includes nomenclature, classification, and synonomies for the principal species and a list of species that do not normally infect humans. Hommel (1978), Wilson and Southgate (1979): Consider the identification and nomenclature under two titles of “traditional” and “modern” taxonomic criteria. Consider parasites that do not infect mammals as “not strictly being” Leishmania species. Lainson and Shaw (1979): Proposed a revised classification for American Leishmania species, based on their developmental patterns in Lutzomyia longipalpis. Subdivision into three groups: (i) Hypopylaria (L. agamae and and L. ceramodactyli), (ii) Peripylaria (L. braziliensis complex), (iii) Suprapylaria (L. donovani, L. mexicana, L. hertigi, and L. tropica complexes). Tait (1980): Suggests sexual recombination in trypanosomatids. Saf'janova (1982): Created a subgenus of Leishmania and proposed the term of Sauroleishmania Ranque, 1973 for parasites infecting lizards. Le Blancq and Peters (1986): Consider isoenzyme electrophoresis as a discriminatory system for Leishmania identification. Lainson and Shaw (1987): Division of Leishmania genus into two subgenera, based on the developmental pattern of Leishmania in the sand fly's gut: Leishmania (Suprapylorian) and Viannia (Peripylorian). Rioux and colleagues (1990): New classification of the Leishmania genus based on the use of intrinsic and extrinsic characters with Linnean and Adansonian methods. WHO (1990): Categorised the Leishmania species into three subgenera: Leishmania, Sauroleishmania, and Viannia. Momen (1993): Proposes the synonimy of L. chagasi (responsible for VL in the New World) and L. infantum. Shaw (1994): Proposes that the genus Leishmania encompass 30 species infecting mammals and 21 species infecting human. Cupolillo and colleagues (1994): Describe the monophyly of the subgenus Viannia. Dedet and colleagues (1999): Categorize the history of Leishmania classification into four periods of Linnean classifications, Adansonian classifications, phenetic classifications, and phylogenetic classifications. |
| 2000 until now | Cupolillo and colleagues (2000), Schoenian and colleagues (2010): Leishmania genus composed of two groups: (i) Euleishmania (Leishmania and Viannia subgenera) and (ii) Paraleishmania (L. hertigi, L. deanei, L. colombiensis, L. equatorensis, L. herreri, and Endotrypanum species). Moreira and colleagues (2004): Present an updated classification of kinetoplastid protists. Fraga and colleagues (2010): New concepts, based on molecular data, concerning the reduction of the number of species, suppression of some species, and downgrading some to subspecies level. Kuhls and colleagues (2011), Leblois and colleagues (2011): Import of L. infantum (ca. 500 years ago) from the Old World (namely Portugal) to the New World as a result of finding a suitable vector there. Lukeš and colleagues (2014): Trypanosomatidae family consists of 13 genera: Trypanosoma, Phytomonas, Leishmania, Leptomonas, Crithidia, Blastocrithidia, Herpetomonas, Sergeia, Wallacemonas, Blechomonas, Angomonas, Strigomonas, and Kentomonas. |
Comprehension of the evolutionary relationships between sandflies and Leishmania is crucial for the future prediction of Leishmania transmission patterns, leishmaniasis epidemiology, and for developing intervention and control strategies. To achieve such an understanding, better information on the worldwide distribution of Leishmania parasites in relation to their sandfly vectors and intermediate hosts will be required. It is therefore necessary to obtain information on the origin of Leishmania and phlebotomine sandflies and their chronological history of coevolution. In this paper, we present a detailed review of the relevant literature on the Phlebotominae and Leishmania and update and discuss theories on their classification, origin, evolution, and dispersion.
Sandflies
Among more than 800 recognized sandfly species, approximately 464 species are found in the New World and 375 in the Old World [3,4]. The classification of both Old and New World sandflies has historically been based mainly on a phenetic approach to identifying overall similarity relationships between genera and subgenera, rather than on ancestor–descendant relationships. This approach has led to a proliferation of taxa, particularly at the subgeneric level, and to the simplification and incorporation of higher taxonomic categories into species. Sandflies belong to the order Diptera, suborder Nematocera, family Psychodidae, and subfamily Phlebotominae. Initially, studies on phlebotomine sandfly taxonomy were exclusively based on morphological aspects of dead specimens. Because of the introduction of several new methods, such as chromosome analysis, multivariate morphometrics, laboratory rearing and colonization, isoenzyme, molecular and phylogenetic analysis and, more recently, mass spectrometry, our knowledge of phlebotomine sandfly systematics has increased. These advances have led to better identification and classification of sandfly specimens, which together with an appreciation of sandfly flight range (approximately 1.5 km per day), have helped to clarify the intraspecific and interspecific variations within sandfly subgenera and populations. A large portion of the literature regarding phlebotomine sandfly systematics addresses their general classification and relationships with other groups [3,5–8] as well as the phylogenetics of the Psychodidae, based on insect fossils [9], phlebotomine sandfly evolution [5], phenetic and phylogenetic analyses of phlebotomine sandflies [10], and the molecular systematics and phylogenetic relationships of phlebotomines using DNA analysis [11]. Many classification systems for phlebotomine sandflies have been proposed since that of Newstead 1911, including those of Abonnenc, Davidson, Fairchild, Leng, Lewis, Quate, and Theodor. However, despite this extensive literature, there is no universal agreement regarding the ranking of taxa above the species level.
The history of sandfly taxonomy can be roughly divided into two distinct periods (Table 2). During the first period, taxa were distinguished according to the analysis of certain external structures (e.g., the structure of the male genitalia, wing venation indices and other external measurements, known as phlebotometry). In the second period, descriptions of internal structures such as the spermathecae, cibarium, and the pharynx were employed [12]. Based on the classification performed by Theodor [6,13], Lewis et al. [14] have proposed subdivision of the phlebotomine sandflies into two genera for Old World species, Phlebotomus (Rondani) and Sergentomyia (França), and three genera for New World species, Lutzomyia (França), Brumptomyia (França and Parrot), and Warileya (Hertig). The genus Chinius (Leng, 1987) belongs to a distinct taxon that is used for some Chinese sandfly species with primitive characters [15]. Rispail and Léger [10] proposed a new genus and subgenus classification for Old World sandflies, based on a morphological study suggesting their division into seven genera, including Phlebotomus, Australophlebotomus, Idiophlebotomus, Spelaeophlebotomus, Sergentomyia, Spelaeomyia, and Chinius (Table 2). In addition to the mentioned classification, some subgenera from the genus Phlebotomus, such as Abonnencius and Legeromyia, have been recently described and could be retained until a complete classification is proposed for the entire genus Phlebotomus.
Table 2. History of sandfly descriptions.
| Century | Author (Year): Description |
|---|---|
| 17th century | Bonanni (1691): First recognizable description of a sandfly as a species of Culex, or mosquito. |
| 18th century | Linnaeus (1735): Description of Angioptera in the insect order that includes the Tipulary flies. Scopoli (1786): Description of Phlebotomus papatasi (Bibio papataci) as first species of described "Psychodidae," with no mention of a particular classification level. Latreille (1796): Description of the "Pschoda" genus that diverges from Bibio and Tipula. |
| first half of 19th century | Meigen (1818): Description of the Muchen (Tipularia) family that encompasses: Eulermuchen, Gallmucken (Gallicolae). Latreille (1825): Changed Tipulariae into Nemocera (Nematocera) family that included the tribe of Tipulariae and the group of Gallicolae (Psychode). Newman (1834): Gathered Psychoda genus in the order of Psychodite (Currently known as Psychodidae). Rondani (1840): Named sandflies as "Flebotomus" and put them into the tribe of Flebotomidae, family of Flebotominae. Renamed later as "Phlebotomus" by Lewis (1845). Rondani (1843): Includes sandflies in the tribe of Tipulidae, family of Hebotomina. Loew (1844): Description of Haemasson minutus (Sergentomyia minuta) that belongs to the family of "Tipularia gallicola," Psychodina. Walker (1848): Gathered Psychoda and Sycorax in the family of Tipularia, Noctuaeforme. Zetterstedt (1850): Includes Psychoda genus into the Psychodidae family. |
| second half of 19th century | Walker (1851): Considered the Phlebotomidae as a family belonging to Diptera. Bigot (1854), Rondani (1864), Schiner (1864): Gathered Phloebotomus, Psychoda, and some other genera in the Psychodidae family. Rondani (1856): Separation of the Phloebotomidae into Phloebotomina and Psychodina. Walker (1856): Gathered Sycorax and Psychoda and some other genera in the Phlebotomidae family. Loew (1862): Subdivided the Psychodidae family into Psychodina and Phlebotomina. Philippi (1865): Included the Psychoda genus into the "Tipularia gallicola" family. Hennig (1872): Proposed to use the name "Psychodites" as the generic name of fossil sandflies. Rondani (1873): Classification of sandflies into the Tipulidae tribe, family of Hebotomina (probably a syntax error). Eaton (1895), Kertesz (1902): Subdivided the Psychodidae into the Psychodinae and Phlebotominae subfamilies. |
| first half of 20th century | Kertesz (1903): Includes Phlebotomus and Sycorax into the Phlebotominae subfamily. Newstead (1911): First systematic study of the Phlebotomus genus. Subdivision of sandflies based on the dorsum hairs of the abdomen: erected or recumbent. Franca (1919, 1920): Subdivided sandfly species into Phlebotomus and Prophlebotomus subgenera. Formation of the first New World subgenus "Lutzia," encompassing Phlebotomus longipalpis Lutz and Neiva, 1912. Franca and Parrot (1921): Use phlebotometry to subdivide the Phlebotomus genus into five subgenera: Phlebotomus, Prophlebotomus, Brumptomyia, Lutzia (Lutzomyia), and Sergentomyia. Franca (1921): Proposed three subgenera; Phlebotomus, Sergentomyia, and Lutzia. Tonnoir (1922): Separated Trichomyia and Sycorax from the Phlebotominae and included them into the Trichomyiinae subfamily. France (1924): Substituted the name Lutzia for Lutzomyia. Adler and Theodor (1926): Highlighted the taxonomic value of the pharyngeal armatures and the spermathecae morphology. Sinton (1928): Noted a correlation between species defined by Newstead on the basis of erected or recumbent hairs and the female spermathecae morphology. Divided sandflies into three groups: erect-haired, recumbent-haired, and intermediate species. Dyar (1929): Updated the knowledge of the American flebotomíneos, proposing Brumptomyia (type species: P. brumpti), Lutzomyia, Neophlebotomus (type species: P. malabaricus), and Shannonomyia (type species: P. panamensis) subgenera. Adler and Theodor (1929): Defined sandflies as a formal member of the Phlebotomidae family. Nitzulescu (1931): Description of Larroussius and Adlerius subgenera, based on the pharyngeal armature and spermathecae structure. Proposed five subgenera: Phlebotomus s. str., Larroussius (type species: P. major), Adlerius (type species: P. chinensis), Sintonius (type species: P. hospittii), and Brumptius (type species: P. minutus). Sinton (1931): First illustrated identification keys for the Indian subcontinent sandflies. Theodor (1932): Phlebotominae subfamily composed of three tribes, further subdivided into genera and subgenera. Parrot (1934): Phlebotomus genus with two subgenera: Phlebotomus and Prophlebotomus. Raynal (1935): Tentative classification based on the spermathecae structure, male genitalia, and pharynx morphology. Mangabeira (1942): Created five subgenera for American sandfly species: Evandromyia, Psychodopigus, Viannamyia, Pressatia, and Castromyia. Dampf (1944): Put Prophlebotomus and Brumplills in synonymy with Sergentomyia, agreed with the subgenera Brumptomyia, Shannonomyia, Castromyia, and Pintomyia. Addis (1945): Created Dampfomyia as a new Neotropical subgenus. Kirk and Lewis (1946): Modified Parrot's (1934) classification and proposed three subgenera: Phlebotomus, Sintonius, and Prophlebotomus. Theodor (1948): Noted that two distinct periods characterize the progress in sandflies taxonomy: the first one that uses external morphological characters (phlebotometry) and the second one that uses internal characters. Four genera: Phlebotomus and Sergentomyia in the Old World, Lutzomyia and Brumptomyia in the New World. Description of six subgenera (Paraphlebotomus, Synphlebotomus, Euphlebotomus, Anaphlebotomus, Australophlebotomus, and Spelaeophlebotomus) that with three previously described (Phlebotomus, Larroussius, and Adlerius) made nine subgenera in total. Subdivided the Sergentomyia genus into three subgenera (Sergentomyia, Sintonius, and Spelaeomyia). Hertig (1948), Fairchild (1949): Description of Warileya (type species: W. phlebotomanica) and Hertigia (type species: H. hertigi) genera. |
| second half of 20th century | Jung (1954): Defines the Sycoracinae subfamily. Barretto (1955): Challenges Theodor's classification, proposed Brumptomyia and Warileya genera as being constitutive of New World species (166 species for the Old World and 199 from New World). Fairchild (1955): Subdivided Psychodidae into Phlebotominae (Nemopalpus and Bruchomyia), Trichomyiinae (Horaiella and Sycorax and others), and Psychodina. Theodor (1958): Erection of Parrotomyia, Rondanomyia, and Grassomyia as new subgenera of the Sergentomyia genus. Quate and Fairchild (1961): Addition of Idiophlebotomus as a new subgenus of the Phlebotomus genus. Barretto (1961): Stated that New and Old World sandflies must be phylogenetically distinct. Creation of the subgenus Trichopygomyia in the Lutzomyia genus. Barretto (1962): Confirmation of Warileya, Brumptomyia, and Lutzomyia genera in the New World and subdivision of Lutzomyia into fifteen subgenera: Lutzomyia s.str., Pintomyia, Evandromyia, Psychodopygus, Viannamyia, Pressatia, Dampfomyia, Micropygomyia, Sciopemyia, Helcocyrtomyia, Trichophoromyia, Coromyia, Trichopygomyia, Nyssomyia, and Psathyromyia. Theodor and Mesghali (1964): Erection of Parvidens as a new subgenus of Sergentomyia. Rohdendorf (1964): Included sandflies in the Phlebotomidae family. Separated sandflies from other Psychodidae because of their blood feeding habit. Theodor (1965): Hertigia, Warileya, Brumptomyia, and Lutzomyia genera for the New World. Subdivision of Lutzomyia into eight subgenera and 16 species groups. Perfil'ev (1966): Proposed a taxonomy based on external characters (phlebotometry) and internal structures (e.g., cibarium, pharynx, or spermathecae). Lewis (1971): Agrees with Perfil'ev (1966), divided Phlebotomidae into six genera (two in the Old World and four in the New World). Subdivided the Phlebotomus genus into 11 subgenera and Sergentomyia into six. Forattini (1971): Proposed seven genera for New World sandflies: Brumptomyia, Lutzomyia, Pintomyia, Psychodopygus, Viannamyia, Pressatia, and Warileya. Divides the Lutzomyia genus into six subgenera: Lutzomyia, Dampfomyia, Micropygomyia, Coromyia, Trichopygomyia, and Barretomyia. Hennig (1972): Considered Phlebotominae as a monophyletic group composed of three monophyletic genera: Phlebotomus, Sergentomyia (without Parvidens), and a genus gathering species from the Brumptomyia and Lutzomyia genera. Recognized the subfamilies Bruchomyiinae, Phlebotominae, Trichomyiinae, and Psychodinae within the Psychodidae family. Trichomyiinae familly encompasses three extinct genera (Eophlebotomus, Eatonisca, Pasthon) and three extant genera (Horaiella, Sycorax, Trichomyia). Abonnenc (1972): Agreed with Fairchild’s (1955) classification, recognised only three genera: Phlebotomus, Warileya, and Hertigia. Gathered Spelaeophlebotomus and Idiophlebotomus into the Phlebotomus genus. Raised the Phlebotomus, Sergentomyia, and Lutzomyia subgenera to a generic rank. Hennig (1973): Considered the Psychodoidae superfamily as a monophyletic infraorder of Psychodomorpha. Duckhouse (1973): Six subfamilies for the Psychodidae family: Phlebotominae, Bruchomyiinae, Sycoracinae, Trichomyiinae, Horaellinae, and Psychodinae. Forattini (1973): Considered Phlebotomus, Sergentomyia, and Lutzomyia as genera. Gathered the Hertigia genus within the Bruchomyiinae subfamily. Proposed ten genera for the Phlebotominae subfamily. Lewis (1973): included Hertigia (currently known as Warileya) into the Phlebotominae subfamily. Young and Fairchild (1974): Proposed a classification similar to Theodor (1965), with some modifications. Lewis (1974): Six genera for the Phlebotomidae subfamily (two for Old World species and four for the New World ones). Lewis (1975): 11 subgenera for Phlebotomus and six for Sergentomyia. Abonnenc and Leger (1976): The Phlebotomidae family with three subfamilies: Euphlebotominae (only Old World), Neophlebotominae (only New World), and Disphlebotominae (Old and New World). Lewis and colleagues (1977), Lewis (1978): First stable classification of Phlebotominae with five genera: Warileya (two subgenera), Phlebotomus (ten subgenera), Sergentomyia (seven subgenera with 54 unplaced species), Brumptomyia, and Lutzomyia (26 subgenera and 19 unplaced species). Ready and colleagues (1980): Proposed a “flexible” classification with “exclusive” characters supporting the proposed genera of Phlebotomus, Sergentomyia, Brumptomyia, Warileya, and Psychodopygus, without considering Lutzomyia. Lewis (1982): Described and added a new subgenus, Kasaulius. Published a distribution map for Old World sandflies. Artemiev and Neronov (1984): 14 genera for Phlebotominae: Australophlebotomus, Brumptomyia, Demeillonius, Grassomyia, Hertigia, Idiophlebotomus, Lutzomyia, Parvidens, Phlebotomus, Psychodopygus, Sergentomyia, Spelaeomyia, Spelaeophlebotomus, and Warileya. Description of the Transphlebotomus subgenus. Leng (1987): Description of new genus of Chinius. Artemiev (1991): Two tribes, seven subtribes, 24 genera, 40 subgenera, and 70 species constitute the Phlebotominae subfamily. Divided Old World sandflies into Phlebotomus, Australophleotomus, Idiophlebotomus, Spelaeophlebotomus, Sergentomyia, Spelaeomyia, Chinius, and Parvidens. Lane (1993): Genus Phlebotomus composed of 12 subgenera. Added the genus Chinius into the Phlebotominae subfamily. Young and Duncan (1994): Neotropical sandflies composed of Lutzomyia, Brumptomyia, and Warileya. Galati (1995): Created a new subtribe (Sergentomyiina) that gathered species from the Sergentomyia genus and some reptile-biting species from the Lutzomyia genus. Division of Phlebotominae into Hertigiini (Hertigiina, Idiophlebotomina) and Phlebotomini (Phlebotomina, Australophlebotomina, Brumptomyiina, Sergentomyiina, Lutzomyiina, and Psychodopygina) tribes. Rispail and Leger (1998): Proposed seven genera for Phlebotominae sandflies: Phlebotomus, Australophlebotomus, Idiophlebotomus, Spelaeophlebotomus, Sergentomyia, Spelaeomyia, and Chinius. The Phlebotomus genus includes nine subgenera: Adlerius, Anaphlebotomus, Euphlebotomus, Kasaulius, Larroussius, Paraphlebotomus, Phlebotomus, Synphlebotomus, and Transphlebotomus. The Sergentomyia genus includes six subgenera: Demeillonius, Grassomyia, Neophlebotomus, Parrotomyia, Sergentomyia, and Sintonius. |
| 2000 until now | Galati (2003): Proposed to subdivide the Phlebotominae familly into two tribes: Hertigiini (subtribes of Hertigiina and Idiophlebotomina) and Phlebotomini (subtribes of Phlebotomina, Australophlebotomina, Brumptomyiina, Sergentomyiina, Lutzomyiina, and Psychodopygina). Galati (2009): Upgraded many of the Lutzomyia subgenera, cited in Young and Duncan, 1994, to a generic status. Galati (2014): Revised the classification proposed by Galati, 2003, leading to an increase in genera numbers. |
A classification first proposed by Lewis et al. [14] and later reviewed by Young and Duncan [8] subdivides the Neotropical sandflies into Lutzomyia, Brumptomyia, and Warileya. This classification is still accepted by a majority of sandfly taxonomists. A new system of classification has been proposed by Galati [3], who revised the existing proposals for New World sandflies. The system recognized 464 species of Neotropical phlebotomine sandflies, grouped into 23 genera, 20 subgenera, three species groups, and 28 series. This classification includes a complete review and reorganization of the subfamily Phlebotominae, which is further classified into two tribes, Hertigiini (Hertigiina and Idiophlebotomina subtribes) and Phlebotomini (Phlebotomina, Australophlebotomina, Brumptomyiina, Sergentomyiina, Lutzomyiina, and Psychodopygina subtribes).
In 2014, Galati revised her previous publication and proposed a new version of classification for Phlebotominae sandflies [3,16]. Based on her classification, the Phlebotomini tribe includes 931 extant species (916 valid species and 15 with uncertain taxonomic status) classified in six subtribes:
Phlebotomina (Phlebotomus genus, 110 spp.)
Australophlebotomina (Australophlebotomus genus, ten spp.)
Brumptomyiina (Brumptomyia [26 spp.] and Oligodontomyia [three spp.] genera)
Sergentomyiina (Sergentomyia [310 spp.], Deanemyia [five spp.], and Micropygomyia [55 spp.] genera)
Lutzomyiina (Sciopemyia [eight spp.], Lutzomyia [74 spp.], Migonemyia [seven spp.], Pintomyia [57 spp.], Dampfomyia [20 spp.], Expapillata [two spp.], Pressatia [eight spp.], Trichopygomyia [16 spp.], and Evandromyia [42 spp.] genera)
Psychodopygina (Psathyromyia [43 spp.], Viannamyia [four spp.], Martinsmyia [11 spp.], Bichromomyia [six spp.], Psychodopygus [40 spp.], Nyssomyia [20 spp.], and Trichophoromyia [39 spp.] genera).
The genus Edentomyia, including one species (Edentomyia piauiensis), was described by Galati [3] without indicating the taxonomic position in the Phlebotomini tribe.
The Hertigiini tribe contained two subtribes of Hertigiina (Warileya and Hertigia genera) and Idiophlebotomina (Spelaeophlebotomus, Idiophlebotomus, and Chinius genera), with five genera and 28 extant species.
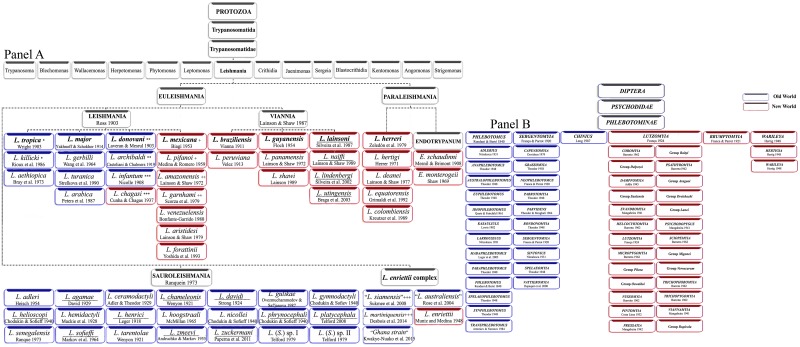
Currently, a conservative approach based on practical criteria has led to subdivision of the Phlebotominae into six genera: three genera from the Old World (Phlebotomus [13 subgenera], Sergentomyia [ten subgenera], and Chinius [four species]) and three from the New World (Lutzomyia [26 subgenera and groups], Brumptomyia [24 species], and Warileya [six species]) (Fig 1) [8,17]. This classification is currently widely used.
Fig 1. Updated classification of Leishmania and sandfly.
Panel A. Classification of Leishmania species. Panel B. Phlebotominae sandfly classification, according to Theodor [6,13], Quate and Fairchild [163], Theodor and Mesghali [22], Lewis [5], Leng [15], and Young and Duncan [8].
Old World Sandflies
The Old World sandflies include three genera: Phlebotomus, Sergentomyia, and Chinius, which are found in the Palaearctic, Afrotropical, Malagasy, Oriental, and Australian regions.
Genus Phlebotomus (Rondani and Berté, 1840) includes 13 subgenera: Adlerius, Anaphlebotomus, Australophlebotomus, Euphlebotomus, Idiophlebotomus, Kasauliuls, Larroussius, Madaphlebotomus, Paraphlebotomus, Phlebotomus, Spelaeophlebotomus, Synphlebotomus, and Transphlebotomus (Fig 1). They are present only in the Old World and are particularly prevalent in the Palaearctic region, which is the main temperate area of the Old World. Most Phlebotomus species are inhabitants of semiarid and savannah areas rather than forests. Therefore, the geographical distribution of the genus Phlebotomus extends from the Mediterranean, Afrotropical, Middle East, and Oriental regions to central Asia. They are found in a wide range of altitudes, from Jericho of Palestine (~300 metres below sea level) to Mashad in Iran (3,600 metres above sea level). In tropical areas, only a few species of Phlebotomus are present, such as in sub-Saharan Africa, Southeast Asia, or the Pacific region. They feed mainly on mammals, although there are some exceptions. This genus includes many human blood feeders and some endophilic species. All of the vectors of human cutaneous and visceral leishmaniasis found in Eurasia and Africa belong to this genus.
Genus Sergentomyia (Franca and Parrot, 1920) is subdivided into ten subgenera: Capensomyia, Grassomyia, Neophlebotomus, Parrotomyia, Parvidens, Rondonomyia, Sergentomyia, Sintonius, Spelaeomyia, and Vattieromyia (Fig 1). This genus contains some ungrouped species. Members of this genus are widespread in the Old World and are dominant in tropical areas where Phlebotomus species are scarce. Their distribution comprises Afrotropical, Oriental, and Australasian regions, the Indian subregion, sub-Saharan Africa, and Asia. Most species are likely to feed chiefly on cold-blooded vertebrates, but some species occasionally bite mammals [18]. Some Sergentomyia specimens have been found to contain Sauroleishmania (a subgenus of Leishmania) and Trypanosoma parasites that are often identified as parasites from lizards [19], but current evidence indicates human Leishmania parasites are not transmissible by Sergentomyia flies [20].
Genus Chinius (Leng, 1987) includes four known species: Chinius junlianensis, C. barbazani, C. eunicegalatiae, and C. samarensis. The geographical repartitioning of Chinius corresponds to the classical Oriento-Australasian track, and they are found in caves in high mountainous regions.
The geographical distribution of the currently known Old World sandfly species encompasses the following areas:
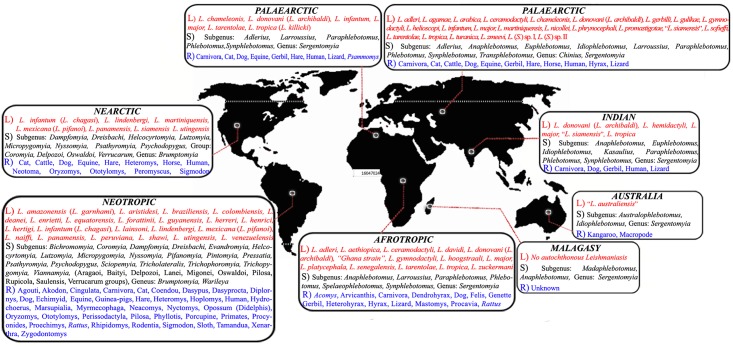
The Palaearctic region: species belonging to the Phlebotomus genus are dominant in the Palaearctic region, as it is the main temperate area of the Old World. Nearly 200 sandfly species belong to various Phlebotomus subgenera; Adlerius, Anaphlebotomus, Euphlebotomus, Idiophlebotomus, Larroussius, Paraphlebotomus, Phlebotomus, Synphlebotomus, and Transphlebotomus, as well as the Chinius and Sergentomyia genera, are found in the Palaearctic region. (Iran [6,21,22], Pakistan [23], the former U.S.S.R. [12], France [24], Turkey [25], Morocco [26], Yemen [27], Spain [28], Tunisia [29], Afghanistan [30], Saudi Arabia [31], Iraq [32], Algeria [33], Egypt [34], Greece [35], China [15,40], Jordan [4,10,36–39].)
The Afrotropical region: subgenera of Anaphlebotomus, Larroussius, Paraphlebotomus, Phlebotomus, Spelaeophlebotomus, and Synphlebotomus from the genus Phlebotomus, together with the genus Sergentomyia, are distributed in this region. Surprisingly, however, some Phlebotomus species that are known to be inhabitants of this region are absent from western Afrotropical regions. (Gabon [41], Sudan [17], Central African Republic [4,10,39,42], Ethiopia [43], Southern Africa [44].)
The Malagasy region (Madagascar and nearby Indian Ocean islands): Species belonging to the genera of Phlebotomus (Anaphlebotomus and Madaphlebotomus subgenera) and Sergentomyia are present in this region. Despite their presence, no sandfly species has been reported as a disease vector in this region [45].
The Oriental region: Approximately 122 sandfly species belonging to the Phlebotomus, Chinius, and Sergentomyia genera are present in this region. In the mainly dry western area, the sandfly fauna is essentially Eremian (The Eremian zone has an arid climate, and its vegetation ranges from barely vegetated desert and hills to a variety of semiarid shrub savannas, semiarid tussock grasslands, and hummock grasslands). In eastern India, Phlebotomus argentipes is an important vector of kala azar. In the far eastern area, including Vietnam, sandflies known to bite humans are rare or absent, and there appear to be rather few phlebotomine species in this area, with the exception of the Philippines [46,47,48].
The Australian region: the Australasian phlebotomine fauna is bipolar in origin, with the genus Phlebotomus (Australophlebotomus: eight spp.) originating from the south and the subgenus Idiophlebotomus (one sp.) and Sergentomyia (24 spp.) from the north [49]. The co-occurrence of some sandfly species (e.g., S. hoogstraali, S. vanella) in both Australia and New Guinea supports the hypothesis proposed by Schodde and Calaby [50] regarding the simultaneous development of the New Guinea sandfly fauna along with the eastern Australia sandflies. Sandflies are generally abundant in both regions where there is rainfall of less than 635 mm, as well as in the wetter northern zone, where the dry season is long. These areas, unlike the Eremian zone of the northern hemisphere, support only a few Phlebotomus species, and humans and livestock are rarely attacked (New Guinea [49,51–53]).
New World Sandflies
The New World sandflies include three genera: Lutzomyia, Warileya, and Brumptomyia, which are found in the Nearctic and Neotropical regions:
Genus Lutzomyia Franca, 1924. This is a large genus, with nearly 434 species and several subgenera, including the Coromyia (Delpozoi group), Dampfomyia (Saulensis group), Evandromyia, Helcocyrtomyia, Lutzomyia, Micropygomyia (Pilosa and Oswaldoi groups), Nyssomyia, Pintomyia, Pressatia (Baityi group), Psathyromyia (Aragaoi, Dreisbachi, and Lanei groups), Psychodopygus, Sciopemyia (Migonei and Verrucarum groups), Trichophoromyia, Trichopygomyia, and Viannamyia (Rupicola group), as well as some ungrouped species (Fig 1). The Lutzomyia genus is more diverse than its Old World counterparts. Nevertheless, vector species are found only in some subgenera (Nyssomyia, Psychodopygus, and Lutzomyia s.str.). Sandflies are of little importance in temperate North America but are abundant in tropical America. Lutzomyia is the most important genus in terms of species diversity and medical importance and exhibits a wide dispersion area. Species of this genus are found only in the New World, with a distribution ranging from the southern areas of the Nearctic region throughout the Neotropical ecozone. Sandflies are found mainly in forest areas in Central and South America. Wide morphological variations have been described for Lutzomyia species, which are greater than those of the Old World species. Therefore, the classification of Lutzomyia species remains largely unresolved and relies on divisions based on morphological taxonomic characters that are still controversial.
Genus Warileya (Hertig, 1948) includes six species, which are mainly found in the Neotropical ecozone.
Genus Brumptomyia (Franca and Parrot, 1921) comprises approximately 24 species, which are broadly distributed in Central and South America. None of these species are known to bite humans. Brumptomyia species constitute a group of sandflies commonly associated with armadillo burrows and sometimes tree trunks. The specific identification of species belonging to this genus is based entirely on male structures [3,54,55].
Sandflies from the New World are present only in Nearctic and Neotropical ecozones:
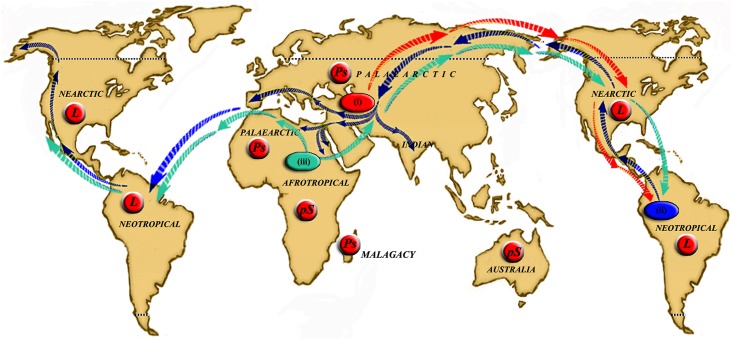
The Nearctic region: only 14 species, a majority of which come from the Micropygomyia subgenus, are present in the Nearctic, but five are restricted to this ecozone. Most of these species exhibit a preference for hot temperatures and humidity. The temperate climate found in the Nearctic is unfavourable for phlebotomine development, particularly for immature stages. This characteristic supports the idea that phlebotomine sandflies might have originated in the tropics, with only a few species dispersing into temperate regions. The sandfly species that are currently found in North America likely arose from the Palaearctic or from South America during the arid phase in the Tertiary period. Therefore, their decreased presence may be a consequence of the constant climatic fluctuations that have occurred during the Quaternary period, causing many sandfly species to become extinct or displaced into the tropics, where hotter and more humid conditions are present [3,56,57].
The Neotropical region: approximately 450 sandfly species are found in this ecozone. The distribution centre of the present-day Lutzomyia genus in the Neotropics is thought to be the forested lowlands present in the east of the Andes. This situation is probably a consequence of the dry periods that occurred during the Pleistocene that isolated conspecific populations, some of which became reproductively isolated and have colonized more humid areas present in the northern and western parts of the subcontinent [10]. The varied sandfly fauna present in wet areas includes many potential sandflies that feed on the blood of human beings. However, only a few are endophilic species (Colombia [58], Ecuador [59], Costa Rica [60], Peru [61], Brazil [62], French Guiana [63], Venezuela [3,8,55,64–67]).
Sandfly Fossil Evidence
Fossils, including the remains of living organisms from the past, are one of the best forms of evolutionary evidence. They allow for comparisons with current organisms and are of particular importance in allowing knowledge of primitive character states (plesiomorphic) and derived specialized states (apomorphic) to be obtained. Fossils provide information about the origin of vector flies in relation to infectious agents, host coevolution, and geographic locations. Therefore, research on sandfly fossils is of great importance for highlighting the evolution and phylogeny of these insects. As mentioned above, phlebotomine sandflies are found in a wide range of ecozones, which could be due to their long evolutionary history with their origins in the Palaeozoic or Mesozoic eras [68].
Arthropods first arose towards the end of the Precambrian period, approximately 550 million years ago (MYA). The first Parainsecta appeared in the Devonian (408 MYA), and the earliest insect orders emerged during the subsequent Carboniferous period. Variegation continued to occur in the Permian (286 MYA), which was the period during which the Diptera arose. Psychodidae emerged later, during either the Jurassic [69] or the Triassic period [70]. This group was likely well diversified by the Cretaceous, and the majority of these species were likely to have been blood feeders. These observations together support the theory of a hypothetical phlebotomine-like ancestor for Psychodidae [9]. The sandflies most likely emerged during the Carboniferous and, thus, before the mammalian hosts of Leishmania. A common ancestor for Phlebotominae is thought to have occurred in the Triassic period (248 MYA) (Table 3).
Table 3. Evolution history of Leishmania, sandfly, and reservoir over the time along the geographical evolution of the Earth.
| Geographical time (MYA: Million Years Ago) | PALEOZOIC | MESOZOIC | CAENOZOIC | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| PRECAMBRIAN (>544) | CAMBRIAN (544–505) | ORDOVICIAN (505–440) | SILURIAN (440–410) | DEVONIAN (410–360) | CARBONIFEROUS (360–286) | PERMIAN (286–245) | TRIASSIC (245–208) | JURASSIC (208–146) | CRETACEOUS (146–65) | PALAEOCENE (65–55) | EOCENE (55–38) | OLIGOCENE (38–25) | MIOCENE (25–5) | PLIOCENE (5–2.5) | PLEISTOCENE (2.5MYA-12TYA) | HOLOCENE (12TYA until now) | |
| GEOGRAPHICAL EVENTS | Emergence of Atlas Mountains | Melting of the large glacial formations Emergence of the Land Plants |
Continents joined (Pangea) | Seperation of the continents (235) | Separation of Gondwana from Pangea (180) Separation of Laurasia from Gondwana (180) Formation of Andes Mountains (200) |
Separation of Africa and South America Formation of Bering straits Emergence of Rocky Mountains (70) |
Formation of McKinley (Denali) Mountains (56) | Histricomorpha of Neotropics Emergence of Alps Mountains (50) Emergence of Himalayas Mountains (40) |
Separation of Africa and Saudi Arabia plate Breaking the Bering land bridge |
Cooling of the North Pacific | Formation of Panamamian Isthmus and physical unification between Nearctic and Neotropic | GlaciationsCooling and drying the earth (1.5–2.5) Emergence of Kilimanjaro mountain (750 TYA) |
Warming trend of the earth (600–900) | ||||
| LEISHMANIA sp. | Emergence of Protozoa (750) | Emergence of eukaryote supergroup Excavata Appearence of the descendant of Leishmania |
Emergence of the first digenetic protozoa, ancestor of other Trypanosoma, not Leishmania | Emergence of Trypanasomes (300) | Division of Trypanosomatidae following the evolution of Diptera (vector of Leishmania) | First digenetic protozoa, a possible ancestor of Leishmania | First Leishmania decendent in a reptile host, Evolving of Sauroleishmania from other genera of Leishmania First fossil of the genus Leishmania (Paleoleishmania proterus) (Burmese amber) (100) Divergence of Old World and New World Leishmania(90) Dixenous life cycle of Leishmania (85) |
Distribution of Leishmania species after rodents’ emergence during Paleocene (after emergence of primitive mammals) | Predecessor of L. donovani group and L. major evolved from South America (36–46) Complete life cycle of Leishmaia (50) |
Leishmania migration from Palearctic to Nearctic or inversly based on the hypotheses of Palearctic or Nearctic origin of Leishmania Adaptation of Sauroleishmania to the lizards Paleoleishmania neotropicum (Dominican amber) (20) Diverging the ancestor of L. donovani from other Leishmania species (14–24) |
Dispersion of Leishmania into or out of the Neotropic region throught the Panamamian Isthmus | Divergence of L. donovani from L. infantum (1) Origination of L. chagasi from L. infantum in South America (500) |
|||||
| SANDFLY | Emergence of the first Arthropodes (550) | Dispensation of the Arthropodes | Emergence of Parainsecta (408) | Emergence of Insects (360) First winged insect (300) |
Emergence of Diptera (286) Earliest Psychodids |
Emergence of Phlebotominae, common ancestor of the Old and New World sandfliesDifferentiation of the tribes Hertigiini and Phlebotomini | Emergence of Psychodids First record of the presence of the true sandflies (180) Separation of Old World and New World sandflies (200) |
First hematophagus winged insect, ancestor of Phlebotomus, Sergentomyia, Lutzomyia (140) Phlebotomites longifilis, P. brevifilis, Mesophlebotomites hennigi, and Libanophlebotomus lutfallahi, fossil records from Lebanon amber (120) Palaeomyia burmitis (Burmese amber) (100) Emegence of the ancestor of Phlebotomus and Sergentomyia in the Palaearctic region |
Emergence of the genus Phlebotomus Phlebotomiella/Sergentomyia succini (Baltic amber) |
Separation of Lutzomyia and Phlebotomus genera |
P. (Phlebotomiella) tipuliformis (Baltic amber) (20) Lutzomyia (Helcocyrtomyia) paterna (Mexican amber) (20) Lutzomyia adiketis (Dominican amber) (20) Pintomyia falcaorum (Dominican amber) (20) Phlebotomus pungens (Jordanian amber) |
||||||
| RESERVOIR | Emergence of Animalia kingdom (700) | Emergence of Reptiles (285) | Emergence of Mammals (210) | Emergence of Lizards | Emergence of Marsupials (Opossums) Spread of Leishmania into the Neartic by primitive mammals through the Bering Strait |
Placental mammals Primates (60) Rodents (55) Xenarthrans (55) |
Emergence of the sloths | Emergence of Rodents (25) Emergence of Canides (dogs) Emergence of Caviomorph rodents (25) |
Spread of the Leishmania from Palearctic to the New World, probably by an infected rodent Sigmodontinae (Cricetids) (20) |
Emergence of Human being, genus Homo (3) | Homo sapiens (200 TYA) | ||||||
To date, sixteen fossils representative of New World species have been described (15 from Dominican and one from Mexican amber). These fossils correspond to the Lutzomyia genus, including subgenera of Lutzomyia (one sp.), Micropygomyia (two spp.), Pintomyia (12 spp.), and Psathyromyia (one sp.) [71]. Additionally, some old amberic records of phlebotomine-like species have been recorded from the Old World, including some fossils deposited in France [72], Germany [73], Spain [74], Burma [75], and Lebanon [76], although the taxonomic placement of some of these species into the Phlebotominae is still unclear. The oldest known species of Phlebotominae are Phlebotomites longifilis (Hennig, 1972), P. brevifilis (Hennig, 1972), Mesophlebotomites hennigi (Azar, Solignac, Paicheler, and Bouchet, 1999), and Libanophlebotomus lutfallahi (Azar, Solignac, Paicheler, and Bouchet, 1999), for which there are fossil records described from Lebanon, in the south of the Tethys Sea, dated to approximately 120 MYA [5,9]. Since that time, the evolution of the Phlebotominae was likely to have been driven by major tectonic events and related climatic changes that affected the break up of Pangaea. Prior to 120 MYA, the Phlebotominae had likely remained on Pangaea for quite some time, from which separated sandfly faunas could have developed in the Old World and New World [5]. Sandfly fossil records as well as data on systematics strongly indicate that the current genera existed quite some time before the Mesozoic, 250 MYA [73]. Palaeomyia burmitis was found in Burmese amber dated from the Cretaceous period (100 MYA). Trypanosomatids associated with a fungal food source were discovered in the alimentary tract of sandfly larva. Another sandfly fossil, P. (Phlebotomiella) tipuliformis (Meunier, l905), was found in Baltic amber dated from the Eocene (20 MYA). This species may have lived in the forest and fed on thin-skinned reptiles [9,77]. Sergentomyia succini (Stuckenberg, 1975), is another sandfly fossil found in Baltic amber [77]. Additionally, Phlebotomus pungens (Loew, 1845), and P. khludae (Kaddumi, 2005) [78], reported from the Old World, both were discovered in Jordanian fossil amber. Sandflies from Mexican ambers from Chiapas were identified as Micropygomyia patterna (= Lutzomyia paterna [Quate, 1963]) and dated to the Miocene (20 MYA). This species is the first known phlebotomine among the current reptile-feeding species to exhibit narrow wings and to feed on blood [9,79]. A sandfly fossil found in Dominican amber was identified as a female of Lutzomyia adiketis and was dated to approximately 20 MYA. This discovery supports the hypothesis of the radiation of Lutzomyia species throughout the Neotropics. In addition to Lutzomyia adiketis, Pintomyia falcaorum, Trichopygomyia killickorum, L. filipalpis, L. succini, L. miocena, L. paleopestis, L. schleei, P. brazilorum, P. paleotownsendi, P. paleotrichia, and M. brandaoi were also found in this Miocene Dominican amber. Two other groups of fossils were found by Young and Lawyer [56] and Antoine et al. [80] in Dominican (14 specimens) and Peruvian (one specimen) ambers, dating from the Miocene. These specimens were not described by the authors that discovered the ambers.
Currently, there are two hypotheses that attempt to explain how the worldwide dispersion of sandfly ancestors occurred. The first hypothesis assumes that sandflies evolved in the Palaearctic ecozone during the Cretaceous period and were then isolated because of the breakup of Pangaea and underwent independent evolution, resulting in two subgenera, Phlebotomus (that has evolved during the Eocene) and Lutzomyia (which evolved during the Oligocene, after the breaking of the Bering bridge). These two genera include species that are involved in the transmission of Leishmania in the Old and New Worlds, respectively [81,82]. According to the second hypothesis, the similarities between the current sandfly taxa and those recorded in fossils, as well as their external positions on phenetic or cladistic trees, support the hypothesis that they existed in Gondwana before the continental separation [83].
Leishmania
The Trypanosomatidae family consists of three dixenous genera (life cycle in vertebrates or plants and invertebrates)—Trypanosoma, Phytomonas, and Leishmania—11 monoxenous genera (life cycle in invertebrates only)—Leptomonas, Crithidia (together with Leishmania form the subfamily Leishmaniinae), Blastocrithidia, Herpetomonas, Sergeia, Wallacemonas, Blechomonas, and Jaenimonas—and three genera that are characterized by the presence of endosymbiotic bacteria and form the subfamily Strigomonadinae: Angomonas, Strigomonas, and Kentomonas [84–88].
Leishmania parasites belong to the Kingdom Protista (Haeckel, 1866), Class Kinetoplastea (Honigberg, 1963 emend. Vickerman, 1976), Subclass Metakinetoplastina (Vickerman, 2004), Order Trypanosomatida (Kent, 1880), Family Trypanosomatidae (Döflein, 1901), Subfamily Leishmaniinae (Maslov and Lukeš 2012), and Genus Leishmania (Ross, 1903).
Leishmania species are heteroxenous, meaning that they are able to colonize two hosts. They live in the phagocytes of the reticulo-endothelial system of mammals and in the intestinal tract of phlebotomine sandflies, although Forcipomyia spp. (Diptera: Ceratopogonidae) as well as some tick species have been reported as the potential vectors of Leishmania sp. [89–91]. Mammalian Leishmania species exhibit a worldwide distribution (Table 4). They are present in tropical and subtropical areas, including North, Central, and South America, as well as in the Mediterranean basin, Southeast Europe, the Middle East, Central and Southeast Asia, the Indian subcontinent, Africa, and recent reports also demonstrate their presence in Australia (Table 4). In the Malagasy region, with the exception of one case of canine leishmaniasis reported by Buck et al. [92], no autochthonous case of leishmaniasis has been reported. Alvar et al. [2] presented an overview of the occurrence of leishmaniasis and causative species in all affected countries. In the Old World, most Leishmania transmissions occur peridomestically in semiarid areas modified by humans, whereas New World parasites are often associated with sylvatic habitats, though some species exhibit predominantly peridomestic transmission. Host preference is also a major factor that affects the modality of Leishmania transmission by a vector that can occur among wild animals, from animals to man, or among people. Although predominantly gut-dwelling, Leishmania parasites were rarely detected also in salivary glands of sand flies. The presence of parasites in the glands was correlated with heavy infections of metacyclic promastigotes in the stomodaeal valve and thoracic midgut of the fly. Therefore, there was a strong correlation between infected glands and the intensity of infection in the midgut, linked to the presence of numerous metacyclic forms [93].
Table 4. Different Leishmania species of Old and New World, their synonymies, distributions, reservoirs, and their potential or proven vectors.
| Leishmania sp.(synonymes) | Old and/or New World | Clinical Disease | Reservoir | Sandfly Vector (potential or proven) | Distribution | References | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Mammal | Human | Lizard | Insect | ||||||||
| EULEISHMANIA | LEISHMANIA (growth in the midgut and foregut of sandfly) | L. aethiopica | OW | CL, DCL | X | X | P. (Lar.) longipes*, P. (Lar.) pedifer*, P. (Par.) sergenti* | East Africa (Ethiopia, Kenya) | [172] | ||
| L. amazonensis (syn. of L. garnhami) | NW | CL, DCL, MCL | X | X | Lu. (Lu.) diabolica, Lu. (N.) flaviscutellata*, Lu. (Lu.) longipalpis*, Lu. (Lu.) nuneztovari anglesi*, Lu. (N.) olmeca novica*, Lu. (N.) olmeca reducta*, Lu. (V.) townsendi, Lu. (N.) ylephiletor, Lu. (V.) youngi | South America (Bolivia, Brazil, Venezuela) | [173,174] | ||||
| L. arabica | OW | _ | X | P. (P.) papatasi | Saudi Arabia | [175] | |||||
| L. aristidesi | NW | _ | XR | Lu. (N.) olmeca bicolor, Lu. (N.) trapidoi | Panama | [176] | |||||
| L. donovani (syn. of L. archibaldi) | OW | VL, PKDL | X | X | P. (Pa.) alexandri*, P. (Eu.) argentipes*, P. (Syn.) celiae*, P. (Ad.) chinensis, P. (Ad.) longiductus, P. (Syn.) martini*, P. (La.) orientalis*, P. (Ad.) sichuanensis, P. (Sy.) vansomerenae | Central Africa, South Asia, Middle East, India, China | [4,40,177] | ||||
| L. gerbilli | OW | _ | X | P. (P.) papatasi | Central Asia, South Mongolia, Iran | [178] | |||||
| L. forattinii | NW | _ | XR | Lu. (Lu.) gasparviannai | Brazil | [179] | |||||
| L. infantum (syn. of L. chagasi) | OW, NW | VL, CL | X | X | P. (Pa.) alexandri, Lu. (Lu.)almerioi*, P. (La.) ariasi*, L. (Lu.) atunesi, P. (Ad.) balcanicus*, P. (Ad.) brevis, P. (Ad.) chinensis*, Lu. (Lu.) cruzi*, Lu. (Pf.) evansi*, Lu. (Lu.) forattenii, P. (Ad.) halepensis, P. (La.) kandelakii*, P. (Ad.) Kyreniae, P. (La.) langeroni*, P.(La.) longicuspis, P. (Ad.) longiductus*, Lu. (Lu.) longipalpis*, P. (La.) major s.l.*, Lu. (Lu.) migonei, Lu. (N.) olmeca olmeca, Lu. (V.) ovallesi, P. (La.) perfiliewi s.l.*, P. (La.) perniciosus*, Lu. (Lu.) pseudolongipalpis, Lu. (Lu.) sallesi, P. (Ad.) simici, P. (Ad.) sichuanensis*, P. (La.) smirnovi*, P. (La.) tobbi*, P. (Ad.) turanicus*, P. (La.) wui* | North Africa, Mediterranean countries (Europe and North Africa), Southeast Europe, Middle East, Central Asia, North, Central and South America (Brazil, Venezuela, Bolivia, Mexico) | [157,180, 181,182] | ||||
| L. major | OW | CL | X | X | P. (Syn.) ansarii, P. (P.) bergeroti, P. (Par.) caucasicus*, P. (P.) duboscqi*, P. (Par.) mongolensis, P. (P.) papatasi*, P. (P.) salehi* | Central and North Africa, Middle East, Central Asia | [183,184, 218] | ||||
| L. mexicana (syn. of L. pifanoi) | NW | CL, DCL | X | X | Lu. (D.) anthophora, Lu. (Hel.) ayacuchenisis*, Lu. (C.) christophei, Lu. (V.) columbiana, Lu. (Lu.) cruciata, Lu. (Lu.) diabolica, Lu. (N.) flaviscutellata, Lu. (Lu.) gomezi, Lu. (Lu.) longipalpis, Lu. (Lu.) migonei, Lu. (N.) olmeca olmeca*, Lu. (V.) ovallesi*, Lu. (Psy.) panamensis, Lu. (Ps.) shannoni, Lu. (N.) ylephiletor | United States of America, Ecuador, Peru, Venezuela | [56,94, 185,186, 187,188,189] | ||||
| L. tropica (syn. of L. killicki) | OW | CL, VL | X | X | P. (La.) aculeatus, P. (Ad.) arabicus*, P. (Par.) chabaudi, P. (La.) guggisbergi*, P. (Syn.) rossi*, P. (Pa.) saevus*, P. (Par.) sergenti* | Central and North Africa, Middle East, Central Asia, India | [81,190,191] | ||||
| L. turanica | OW | _ | X | P. (P.) papatasi | Central Asia, South Mongolia, Iran | [192,193] | |||||
| L.venezuelensis | NW | CL | X | X | Lu. (Lu.) lichyi, Lu. (N.) olmeca bicolor, Lu. (Ps.) panamnsis, Lu. (V.) spinicrassa | Northern South America, Venezuela | [19] | ||||
| VIANNIA (growth in the hindgut of sandfly) | L. braziliensis | NW | CL, MCL | X | X | Lu. (N.) anduzei, Lu. (Psy.) ayrozai, Lu. (Ps.) carrerai*, Lu. (V.)columbiana, Lu. (Ps.) complexa*, Lu. (Lu.)cruciata, Lu. (Lu.)edwardsi, Lu. (Pi.) fischeri*, Lu. (Lu.) gomezi*, Lu. (N.) intermedia, Lu. (Lu.) lichyi, Lu. (Ps.) llanosmartinsi*, Lu. (Lu.) longipalpis, Lu. (Lu.) migonei*, Lu. (N.)neivai*, Lu. (Lu.)nuneztovari anglesi*, Lu. (V.) ovallesi*, Lu. (Psy.)panamensis*, Lu. (Psy.)paraensis, Lu. (V.)pescei, Lu. (Lu.) pessoai, Lu. (V.)pia, Lu. (X.) shawi*, Lu. (V.) spinicrassa*, Lu. (Psy.) squamiventris, Lu. (Hel.) tejadai, Lu. (Lu.) townsendi, Lu. (Lu.) trinidadensis, Lu. (N.) trapidoi, Lu. (N.) umbralitis, Lu. (N.) whitmani*, Lu. (Ps.) wellcomei*, Lu. (N.) ylephiletor*, Lu. (Lu.) youngi, Lu.(Psy.) yucumensis* | Western Amazon basin, South America, Brazil,Bolivia, Peru Guatemala, Venezuela | [19,64,172,174,194,195,196,197,198] | |||
| L. guyanensis | NW | CL, MCL | X | X | Lu. (N.) anduzei*, Lu. (Hel.) ayacuchensis*, Lu. (N.) flaviscutellata, Lu. (V.) longiflocosa, Lu. (Psy.) llanosmartinsi, Lu. (Lu.) migonei, Lu. (V.) ovallesi, Lu. (N.) shawi*, Lu. (N.) umbratilis*, Lu. (N.) whitmani* | Northern South America, Bolivia, Brazil, French Guiana, Suriname | [38,172,174,199,200] | ||||
| L. lainsoni | NW | CL | X | X | Lu. (V.) nuneztovari anglesi*, Lu. (N.) olmeca bicolor, Lu. (T.) ubiquitalis*, Lu. (N.) whitmani | Brazil, Bolivia, Peru | [201] | ||||
| L. lindenbergi | NW | CL | X | X | L. (Lu.) atunesi | Brazil | [39] | ||||
| L. naiffi | NW | CL | X | X | Lu. (Psy.) amazonensis, Lu. (Ps.) ayrozai*, Lu. (Lu.) gomezi, Lu. (Psy.) paraensis, Lu. (Ps.) squamiventris*, Lu. (N.) trapidoi | Brazil, French Guyana | [172,199,202] | ||||
| L. panamensis | NW | CL, MCL | X | X | Lu. (T.) cruciata, Lu. (N.) flaviscutellata, Lu. (Lu.) gomezi*, Lu. (Hel.) hartmanni*, Lu. (Mig.) migonei, Lu. (V.) ovallesi, Lu. (Psy.) panamensis*, Lu. (Hel.) sanguinaria, Lu. (V.) spinicrassa, Lu. (N.) trapidoi*, Lu. (N.) umbratilis, Lu. (N.) ylephiletor, Lu. (N.) yuilli* | Central and South America, Brazil, Panama, Venezuela, Colombia | [19,174,203,204,205] | ||||
| L. peruviana | NW | CL, MCL | X | X | Lu. (Hel.) ayacuchensis*, Lu. (Hel.) noguchii, Lu. (Hel.) peruensis*, Lu. (Hel.) tejadai, Lu. (V.) verrucarum* | Peru, Bolivia | [19,172,174] | ||||
| L. shawi | NW | CL | X | X | Lu. (N.) whitmani* | Brazil | [201] | ||||
| L. utingensis | NW | Unknown | X | Lu. (Vi.) tuberculata | Brazil | [206] | |||||
| SAUROLEISHMANIA (growth in the hindgut of sandfly) | L. adleri | OW | _ | X | S. (Si.) clydei, S. (S.) dentata | Iran, Kenya | [104,207,208, 217] | ||||
| L. agamae | OW | _ | X | P. (Pa.) caucasicus, P. (P.) papatasi, S. (S.) sintoni | Eastern Mediterranean, Palestine, Lebanon, Israel, Turkmenistan | [104,209,210,211] | |||||
| L. ceramodactyli | OW | _ | X | P. (Pa.) caucasicus, P. (P.) papatasi, S. (S.) sintoni | Eastern Mediterranean, Iraq, Sudan, Turkmenistan | [104,209] | |||||
| L. chameleonis | OW | _ | X | Unknown | Egypt, Israel | [104,210] | |||||
| L. davidi | OW | _ | X | Unknown | Central Africa | [104,210] | |||||
| L. gulikae | OW | _ | X | Unknown | Turkmenistan | [213] | |||||
| L. gymnodactyli | OW | _ | X | P. (Pa.) caucasicus, S. (Si.) clydei, S. (S.) dentata, P. (P.) papatasi, S. (S.) sintoni | Sudan, Iran, Turkmenistan | [102,150,209,212] | |||||
| L. helioscopi | OW | _ | X | Unknown | Turkmenistan | [214] | |||||
| L. hemidactyli | OW | _ | X | Unknown | India | [104] | |||||
| L. henrici | OW | _ | X | Unknown | Martinique island (?) | [104] | |||||
| L. hoogstraali | OW | _ | X | S. (Si.) clydei | Sudan, Senegal | [104,215,216] | |||||
| L. nicollei | OW | _ | X | Unknown | Turkmenistan | [214] | |||||
| L. phrynocephali | OW | _ | X | Unknown | Turkmenistan | [214] | |||||
| L. platycephala | OW | _ | X | Unknown | Tanzania | [128] | |||||
| L. senegalensis | OW | _ | X | S. (S.) dubia | Senegal | [104,213,215] | |||||
| L. sofieffi | OW | _ | X | Unknown | Russia (Caspian Sea) | [102] | |||||
| L. tarentolae | OW | _ | X | S. (S.) antennata, S. (S.) minuta, P. (P.) papatasi | North Africa, Malta, Sudan, Algeria, Italy, France, Malta | [24,104,175,211,219,220, 221] | |||||
| L. zmeevi | OW | _ | X | S. (S.) arpaklensis, P. (P.) papatasi | Turkmeistan | [209,222] | |||||
| L. zuckermani | OW | _ | X | Unknown | Sudan, South Africa | [223] | |||||
| L. (S.) sp. I | OW | _ | X | Unknown | Pakistan | [210] | |||||
| L. (S.) sp. II | OW | _ | X | Unknown | Pakistan | [210] | |||||
| UNCLEAR | L. enrietti complex | L. enrietti | NW | _ | X | Lu. (Lu.) gasparviannai, Lu. (Lu.) gomezi, Lu. (Pf.) monticola | Brazil | [174,229] | |||
| L. martiniquensis | NW, OW | CL, VL | X | X | Unknown | Martinique, Thailand | [230,231] | ||||
| “L. siamensis” | OW, NW | VL, CL | X | X | S. (Ne.) gemmea | Central Europe, Thailand, USA | [232,233,234] | ||||
| “L. australiensis” | Australia | _ | XM | Midges | Australia | [228] | |||||
| PARALEISHMANIA | L. colombiensis | NW | CL, VL | X | X | Lu. (Lu.) gomezi, Lu. (Hel.) hartmanni*, Lu. (Psy.) Panamensis | Colombia | [101,224] | |||
| L. deanei | NW | _ | XP | Lu. (Vi.) furcata | South America, Brazil | [225] | |||||
| L. equatorensis | NW | _ | XS | Lu. (Hel.) hartmanni | Ecuador | [226] | |||||
| L. herreri | NW | _ | XS | Lu. (Ps.) shannoni, Lu. (N.) trapidoi, Lu. (N.) ylephiletor | Costa Rica | [149] | |||||
| L. hertigi | NW | _ | XP | Lu. (Psy.) chagasi, Lu. (Psy.) claustrei, Lu. (Psy.) davisi, Lu. (Psy.) squamiventris | Panama, Costa Rica | [174,227] | |||||
*: Proven vector, Ad.: Adlerius, C.: Coromyia, CL: Cutaneous Leishmaniasis, DCL: Diffuse Cutaneous Leishmaniasis, Eu.: Euphlebotomus, Hel.: Helcocyrtomyia, L.: Leishmania, La.: Larroussius, Lu.: Lutzomyia, Mig.: Migonei, N.: Nyssomyia, Ne.: Neophlebotomus, P.: Phlebotomus, Pa.: Paraphlebotomus, Pf.: Pifanomyia, Pi: Pintomyia, Ps.: Psathyromyia, Psy.: Psychodopygus, S.: Sergentomyia, Si.: Sintonius, Sy.: Synphlebotomus, T.: Tricholateralis, V.: Verrucarum, Vi.: Viannamyia, VL: Visceral Leishmaniasis, XM: Mammal (Marsupials), XP: Mammal (Porcupines), XR: Mammal (Rodent), XS: Mammal (sloth)
First attempts at the classification of Leishmania were monothetic Linnean classifications that were proposed between 1916 and 1961, based on extrinsic characters only (Table 1). An early Leishmania classification was suggested by Nicolle in 1908, which separated L. infantum, the etiological agent of Mediterranean visceral leishmaniasis, from L. donovani, the causative agent of Indian kala azar. Then, Biagi proposed the separation of various New World Leishmania species [94] (see Table 1). In 1964 [95], Adler discussed the difficulties in accepting a clinically based taxonomy, as leishmaniasis may demonstrate the same clinical symptoms but by two different Leishmania species, e.g., visceral leishmaniasis with cutaneous symptoms. The most intensive and extensive investigations on these parasites were carried out in the Turkmenian USSR (reviewed by Belova, [96]). Other attempts to classify mammalian Leishmania in the traditional way (that is, by naming and defining species and subspecies) were presented by Lainson and Shaw [97,98] and Bray et al. [99]. In 1976 [100], Vickerman proposed the recognition of four species complexes within the genus: the donovani complex, the tropica complex, the mexicana complex and the braziliensis complex (adapted later partially by Lainson and Shaw). In 1979 [101], Lainson and colleagues described three sections of Leishmania, according to the intravectorial development of the parasite: Hypopylaria (saurian Leishmania developing in the hindgut), Peripylaria (developing in the hindgut and pylorus), and Suprapylaria (all development anterior to the pylorus). In 1982 [102], the Russian researcher Saf'janova proposed separation of Leishmania infecting lizards from other Leishmania species that infect mammals, and she proposed the name Sauroleishmania for these species [103]. The saurian Leishmania species were then assigned to a separate genus Sauroleishmania by Killick-Kendrick et al. [104]. A milestone for Leishmania classification was the system presented by Lainson and Shaw in 1987, who divided the genus Leishmania into two subgenera, L. (Leishmania) for the section Suprapylaria and L. (Viannia) for the section Peripylaria. In the early 1970s, intrinsic characteristics (immunological, biochemical, and molecular) of Leishmania were identified and used to develop new classification systems. Isoenzyme electrophoresis, developed in the 1970s, has been widely used as a typing system and was accepted over decades as the gold standard for identification and is still a valuable tool as a reference technique for parasite characterization. Since the 1980s, Adansonian phenetic classification, based on the multiple similarity-weighted characters (absence of hierarchy) applied simultaneously (polythetic classification) without an a priori hypothesis, has been employed for Leishmania classification. Subsequently, phylogenetic analyses revealed a parental relationship between different species of Leishmania. The phenetic and, especially, the cladistic classification confirmed the majority of the taxonomic groups previously established through Linnean classifications, particularly that of Lainson and Shaw [19]. Pioneering phenetic classifications based on izoenzymes have been proposed by Moreno et al. [105], Thomas-Soccol et al. [106], and Cupolillo et al. [107] for the New World and by Lanotte et al. [108] and Le Blanq et al. [109] for the Old World. Rioux et al. [110] combined all New and Old World taxa in one classification system. Several of these authors also applied a phylogenetic concept of classification [111] that is based on the concepts of monophyletism, parsimony of changes, and nonconvergence of characters [106,112]. The concordance between these classifications mutually validated both the extrinsic (geographic distribution, associated clinical syndrome, and developmental features in the sandfly gut) and intrinsic (biochemical, immunological, and molecular markers) identification criteria applied. However, cladistic analyses allowed a more detailed analysis of some groups and led to the establishment of some new complexes of species (L. infantum, L. turanica, L. guyanensis). However, some of these complexes were later rejected by molecular data. In addition, these cladistic analyses led to the proposal to place previously separated species in the same complex (L. guyanensis, L. panamensis, L. shawi) [113].
Recently, a new classification for Leishmania has been proposed based on combined molecular data, which divides Leishmania species into two major phylogenetic lineages referred to as sections Euleishmania and Paraleishmania [114]. The section Euleishmania comprises four subgenera: Leishmania (type strain: L. donovani), Viannia (type strain: L. braziliensis), Sauroleishmania (type strain: L. tarentolae), and L. enriettii complex (type strain: L. enriettii). Section Paraleishmania includes L. hertigi, L. deanei, L. herreri, L. equatorensis, and L. colombiensis as well as the former Endotrypanum genus. Of this group, only L. colombiensis was found to be pathogenic to humans. The evolutionary history of the section Paraleishmania has not been yet resolved, and it is so far a polyphyletic clade within the genus Leishmania. Based on izoenzyme data, the genus Leishmania was shown to be monophyletic, but inference of its origin and evolution is complicated by its disjunct geographic distribution [106]. Especially with respect to the position of Endotrypanum, with its intraerythrocyte developmental stage as well as distinct morphology (epimastigote or trypomastigote form) within section Paraleishmania, as shown by molecular data, this remains questionable and has to be carefully reevaluated. The subgenus Viannia is restricted to the Neotropics, while the subgenus Leishmania occurs in both the New and Old World. Fifty-three named species (without synonyms, including all five subgenera and complexes: Leishmania, Viannia, Sauroleishmania, L. enrittii complex, and Paraleishmania) are recognized, 29 of which are present in the Old World, 20 in the New World, three species (“L. siamensis,” L. martiniquensis, and L. infantum) in both Old and New World, and one species in Australia (“L. australiensis”). Among these recognized species, 20 (without synonyms) are known to infect humans (updated information from Maroli et al. [39]).
Synonymy was shown for several species using molecular typing, e.g., L. tropica (syn. L. killicki) [117,118,119] and L. donovani (syn. L. archibaldi) [120,121,122]. Synonymy was also suggested for L. mexicana (syn. L. pifanoi) and L. amazonensis (syn. L. garnhami). However, in all published studies, only a few representatives for these synonyms have been included, and they should be studied using an adequate sampling strategy. It was also shown by multilocus microsatellite typing (MLMT) that one species (L. infantum/L. chagasi) was only recently (ca. 500 years ago) brought from the Old World (namely Portugal) to the New World and that it found a suitable vector there [123,124]. For a number of species, the phylogenetic status is not yet resolved (species or subspecies or even synonyms), mainly because of the limited number of included isolates, e.g., for L. amazonensis, L. garnhami, L. pifanoi, L. venezuelensis, L. aristidesi, L. forattinii, L. arabica, L. utingensis (represented by only a single sample), L. lindenbergi, L. enrietti, and those belonging to the Paraleishmania section. Moreover, molecular data based mainly on hsp70 [125] proved the existence of only nine monophyletic groups. These groups might represent distinct species, and several other species should be treated as subspecies within these main groups, which was also confirmed by MLMT studies, e.g., for L. braziliensis and L. peruviana as subspecies, L. donovani and L. infantum as subspecies, L. guyanensis, L. shawi, and L. panamensis as subspecies, L. mexicana and L. amazonensis as subspecies, L. tropica and L. aethiopica as subspecies, etc. [126]. However, not all known species have been included in these studies, especially for the L. mexicana complex.
In conclusion, molecular data based on sequences of different targets and on MLMT do not support the concept of species complexes presented by Lainson and Shaw [19,127], and the classification should be revised, including both suppression of several species and also downgrading some species to the level of subspecies. Ongoing whole-genome sequencing and SNP analysis as well as further analysis by multilocus sequence typing (MLST) and MLMT and an adequate sampling and inclusion of representatives of all species (with sufficient numbers of isolates from different areas of distribution) will contribute to further improvement of the classification of the Leishmania genus.
Sauroleishmania was originally described by Ranque in 1973 [103] as a separate genus. It includes 19 named and two unnamed species (L. [S.] sp. I, L. [S.] sp. II; Telford [210]), according to Ovezmukhammedov and Saf'janova [213], Killick-Kendrick et al. [104], and Telford [128], without specifying their taxonomic positions. Among these, ten species were considered as valid by Ovezmukhammedov and Saf'janova (Fig 1) [213]. They [213] also reported one species as L. (S.) sp. without any additional information about its descriptor (author) and taxonomic position. During the 1980s, Leishmania that infect lizards were placed in a new genus, Sauroleishmania, which was also primarily based on the use of extrinsic characters [104]. In 1986 [129], Saf’janova proposed that Leishmania species diverged from Leptomonas and that such parasites were present in primitive sandflies during the Mesozoic period. This idea was supported later by molecular data [85,130]. The two subgenera that encompass Leishmania infecting mammals were regarded as having been separated by continental drift during the Mesozoic, and it was suggested that Sauroleishmania developed only in the Old World because the presence of the sandfly vectors for these parasites is strictly restricted to the Old World [129].
The L. enriettii complex and related parasites form a well-supported monophyletic group (L. enriettii complex) that most likely represents a new subgenus (Pothirat et al. [115]; Kwakye-Nuako et al. [116]). The only two formally described and named members of this group are L. enriettii, described in 1948 and repeatedly isolated from domestic guinea pigs, and Leishmania martiniquensis, described in 2014 as a causative agent of human diseases. Another three members that have been accommodated into the L. enriettii complex are: (i) never formally described "L. siamensis" from human patients; (ii) unnamed species sometimes called "L. australiensis" from Australia marsupials, most likely transmitted by midges; and (iii) very recently (2015) introduced unnamed Leishmania species from human cases in Ghana. At the moment, the names of “L. siamensis” and “L. australiensis” are not taxonomically valid names. For this, these names have been used in this paper with quotation marks.
The Endotrypanum genus belonging to the Paraleishmania group is known as a parasite of sloths that is transmitted by Lutzomyia species in Central and South America. These parasites are found within the erythrocytes of the Choloepus and Bradypus sloth genera. Only two species, Endotrypanum schaudinni and E. monterogeii, have been described in this genus [131]. The parasites that have been obtained through the in vitro culture of infected blood from sloths and from Lutzomyia sandfly guts are promastigotes that are indistinguishable from Leishmania promastigotes. Sloths also serve as a reservoir of L. braziliensis, L. guyanensis, L. herreri, L. equatoriensis, and L. panamensis, which are transmitted by sandfly vectors. They could be one of the first vertebrate hosts in which the dixenous life cycle of Leishmania could have emerged.
Leishmania Fossil Evidence
Leishmania belongs to the phylum Kinetoplastida, which is likely related to the phylum of Euglenids [132]. Both of these groups belong to the eukaryotic supergroup Excavata, for which fossil evidence suggests emergence during the Ordovician [133]. Leishmania might have originated during the Mesozoic, prior to the separation of Gondwana [106]. The first Leishmania fossil record was Paleoleishmania proterus, a digenetic Leishmania species associated with a blood-filled female of the sandfly P. burmitis in Burmese fossil amber (Cretaceous, 100 MYA) (Table 3) [134]. Within the alimentary canal of this sandfly, amastigotes (n = 20), promastigotes (n = 393), and paramastigotes (n = 64) of digenetic leishmanial trypanosomatids were observed. The observation of these different parasitic stages in the alimentary tract of the insect suggests that their presence was likely the result of a blood meal and that they were multiplying within the midgut. The blood cells were later identified as being of reptilian origin. They also described the development of putative amastigotes within whitish, spherical-to-oval vacuoles associated with some blood cells. The second fossil of Paleoleishmania species described was P. neotropicum, which was found in Dominican fossil amber (20 MYA). A large number of promastigotes (n = 20) and amastigotes (n = 20) were found in the gut of L. adiketis. Additionally, four promastigotes, two paramastigotes, and several amastigotes of P. neotropicum were found in the proboscis of L. adiketis. The presence of amastigotes demonstrated the digenetic life cycle of P. neotropicum, as this parasitic life stage is considered to be present only in the vertebrate host, and no monogenetic flagellates are known to colonize sandflies.
The kingdom Animalia appeared 700 MYA, and the first Leishmania host ancestor likely also appeared at this time. In this period, the Earth was covered by water with a lower oxygen concentration [135]. The definitive hosts for primitive Leishmania may therefore have been reptiles or primitive mammals. It was initially suggested that the Leishmania genus originated in the Palaeocene, following the emergence of the first placental mammals. The ancestors of Leishmania emerged during the Ordovician [130,136], while winged insects appeared during the Carboniferous (300 MYA), and the first hematophagous winged insect appeared during the Cretaceous (140 MYA) [137]. The separation between primitive Phlebotomus and Lutzomyia arose approximately 200 MYA [138]. While trypanosomatids were present during the Palaeozoic, free-living forms were likely more diverse in the past than today. In this period, the Leishmania ancestor was separated into Sauroleishmania (reptile-infecting Leishmania) and the current Leishmania genus (mammal-infecting Leishmania) [139]. Subsequently, the division of Leishmania into L. (Leishmania) and L. (Viannia) occurred approximately between 54 to 25 MYA, after the separation of Africa from South America [140]. Geologically, the Earth experienced a cooling and drying period (1.5–2.5 MYA). The grassland biomes required for the development of the earliest murid rodents likely shifted towards the equator and the tropical forest biomes [141]. Along with their required biome, sigmodontine rodents (Rodentia: Muridae: Sigmodontinae) travelled across the Panamanian land bridge into South America.
The observation of sandfly larvae that develop in habitats containing trypanosomatid flagellates led to the hypothesis that sandflies host monoxenous trypanosomatids, and that these flagellates were carried through the pupal into the adult stage. This corresponds with the fact that Leishmania parasites evolved originally from Leptomonas monoxenous trypanosomatids [85], which are rarely transmitted to mammalian hosts, including humans [130]. The transmission of flagellates by an adult sandfly to a vertebrate host, establishing a continuing cycle between the vector and vertebrate species, likely occurred before the appearance of placental mammals during the Palaeocene. Thus, the appearance of placental mammals appears to have occurred after the appearance of the currently known Leishmania vectors, i.e., Phlebotomus and Lutzomyia species. Hence, the vector, mammalian host, and fossil record all suggest that leishmaniasis may have been established during the Palaeocene (65–31 MYA).
Palaearctic Origin of Leishmania
A Palaearctic origin of the genus Leishmania was proposed by Lysenko in 1971 [142]. Fossil evidence indicates that both phlebotomine sandflies and murid rodents originated in the Palaearctic [5,143], making it likely that Leishmania, along with its vectors and reservoirs, could have evolved in the Palaearctic during the Cenozoic period and dispersed to the Nearctic during the Oligocene (Eocene), when the Bering land bridge was intact. These species then dispersed into the Neotropics across the Panamanian land bridge during the Pliocene, when the climate was sufficiently warm to permit further dispersal of Leishmania (Fig 2) [82,142,144,145].
Fig 2. Possible routes of dissemination of Leishmania.
(i). Red arrow: Palearctic origin of Leishmania (Lysenko [142], Kerr [136,144], Kerr et al. [145]). (ii) Blue arrow: Neotropical origin of Leishmania (Croan et al. [150], Noyes [149], Noyes et al. [83], Lukeš et al. [146]). (iii) Green arrow: Neotropical/African origin of Leishmania (Momen and Cupolillo [139]). Distribution of medically important sandflies is highlighted by red symbols. L: Lutzomyia, P: Phlebotomus, S: Sergentomyia, PS: Relative density and diversity of Phlebotomus as compared to Sergentomyia.
Molecular analyses of Leishmania strains coming from various Old World endemic areas suggest that L. donovani and L. infantum, which are responsible for VL, likely diverged approximately 1 MYA. Leishmania donovani subsequently invaded India and Africa [146], and 500 years ago, Leishmania infantum was transported to South America and was named L. chagasi, which is now considered to be synonymous with L. infantum [146–148].
P. proterus found in sandflies fed with reptile blood in the Palaearctic during the Cretaceous period led to the hypothesis that reptiles were likely the original hosts of Leishmania. Sauroleishmania may have then diverged from L. (Leishmania) in the Old World as a consequence of its adaption to reptiles. Sauroleishmania could have originated in Cretaceous reptiles residing in the Palaearctic region and subsequently declined during the Cenozoic period because of cooling of the Earth, as mammals radiated. Thus, the successful establishment of Leishmania appears to have been assisted by first infecting reptiles. This evolutionary scenario is supported by some molecular data and the numerous reptilian trypanosomes that are transmitted by today’s sandflies. The infections then shifted to the murid rodents, which are now the most significant reservoirs of Leishmania strains causing CL. Murid rodents likely appeared in the Palaearctic during the Oligocene era and then dispersed across the Bering land bridge to Nearctic regions during the Eocene era. Mice and rats from the New World evolved in the Nearctic ecozone before crossing the Panamanian land bridge to the Neotropics during the Pliocene, after which they underwent a rapid radiation, leading to the introduction of parasites to caviomorth rodents, sloths, armadillos, and anteaters [136,141,144]. All of these species act as reservoirs and play an important role in the persistence and dispersal of the parasites because of their relatively long lifespan compared with sandflies [136,141]. The origin and dispersion of murid rodents has been taken as essential evidence that Leishmania originated in the Palaearctic region. Around this time, phlebotomine species ancestral to both Phlebotomus and Lutzomyia adapted to feed on rodents instead of reptiles, likely because their burrows offer humidity and shelter from cold for both rodents and sandflies. The fossil record indicates that the phlebotomine sandfly ancestor evolved in the Palaearctic (Cretaceous, 120 MYA) and that Phlebotomus also evolved in the Palaearctic (Eocene, Baltic amber), and Lutzomyia diverged from Phlebotomus (Oligocene, Mexican amber) after the breaking of the Bering land bridge [136,141,145].
Neotropical Origin of Leishmania
In 1998 [149], Noyes suggested a Neotropical origin of Leishmania during the Palaeocene or Eocene period (36–46 MYA). Subsequently, the parasites invaded the Nearctic ecozone via the Panamanian land bridge and the Palaearctic via the Bering land bridge during the Miocene. The greater diversity observed among New World Leishmania species compared with those from the Old World provides some circumstantial evidence arguing for a Neotropical origin of Leishmania [19,150]. Nevertheless, if this hypothesis is true, then Sauroleishmania might have evolved later during the Miocene, either in the Nearctic or the Palaearctic area, as a result of adaptation to reptiles [149]. Sloths (Xenarthra) might have served as the first vertebrate reservoirs of Leishmania in the Neotropics. Also, it has been suggested that a number of monogenetic and digenetic trypanosomatids can grow in the rectal glands of marsupials. After adaptation to rodents during the Eocene, infected porcupines would have carried the parasites across the Panamanian land bridge to the Nearctics and across the Bering land bridge to the Palaearctic during the Miocene in an unspecified mammalian reservoir (Fig 2) [83,149,150].
Climate change, in combination with the topographic diversity found in the Central and South America, has certainly played a role in the vicariance of the sigmodontine rodents and their accelerated speciation. The cricetids (sigmodontines) encompass approximately 40 genera and more than 200 species that evolved within approximately 2.5 MYA [141]. A similarly rapid rate of evolution is observed in New World Leishmania [141,151].
Neotropical/African Origin of Leishmania
According to this theory, the genus Leishmania is divided into two sections: Euleishmania (Leishmania and Viannia subgenera and Sauroleishmania) and Paraleishmania (L. hertigi, L. deanei, L. colombiensis, L. equatorensis, and L. herreri) [114,139]. It is also speculated that the separation of Gondwana in the Mesozoic resulted in the evolution of the Leishmania genus into Leishmania and Sauroleishmania in Africa, and Viannia and Paraleishmania in South America [139]. The origin and the evolution of Leishmania would have been related to the origin of humans in eastern Africa, with Leishmania following the dynamics of the human population in the Palaearctic (Asia, Africa, and Europe) ecozone. An African origin of Leishmania was emphasized by Momen and Cupolillo [139], based on the importance of the origins of its vectors and reservoirs as evidence for this hypothesis and citing the restricted habitat of Arvicanthis rodents and Phlebotomus sandflies in Africa. According to this hypothesis, the Old World Leishmania species (e.g., L. donovani/L. infantum, L. tropica, L. major, and L. aethiopica) exhibit an African origin. L. aethiopica is present only in the Ethiopian and Kenyan highlands. Because of its restricted geographical distribution, it is reasonable to assume an African origin for this species as well as for the other L. (Leishmania)–hyrax systems that occur in Africa [128]. The origin of humans from eastern Africa suggests that Leishmania species with anthroponotic transmission, i.e., L. tropica and L. donovani, may also have originated in eastern Africa (Fig 2) [152].
Relationship between Sandflies and Leishmania
The term “coevolution” was first used to demonstrate a particular type of relationship between Leishmania and sandfly species in the Old World [147]. Leishmania and sandflies have survived over many millions of years under selective pressure, depending on natural ecological changes. A close relationship has been demonstrated between some sandfly and Leishmania species, such as L. major and P. papatasi. This longstanding evolutionary history of Leishmania and sandflies has resulted in a similar distribution. However, there is not always a clear distinction between coevolution and certain other concepts, such as coassociation (meaning that the transmission cycle exhibits a distinctive landscape epidemiology), interaction (the molecular and immunological relationship between the sandfly midgut and the parasite’s external surface), or vector–parasite cospeciation or co-cladogenesis [37]. Most Leishmania parasites are more restricted regarding the range of sandfly vectors that can transmit them than in the range of mammalian hosts/reservoirs they are able to infect, suggesting a much closer coevolutionary relationship with sandflies than with their vertebrate hosts, although it is sometimes difficult to interpret this coevolutionary relationship [153]. For example, there is a specific relationship between P. papatasi and L. major because of the presence of specific midgut receptors [154], and these two species show strong distribution sympatry. Nevertheless, such high specificity of Leishmania for its sandfly vector appears to be restricted to P. papatasi or P. duboscqi and P. sergenti. However, the appearance of Leishmania interspecies hybrids might have consequences in terms of specificity and transmission efficiency [155,156].
The incrimination of sandflies as proven or potential vectors of Leishmania is a controversial and debated matter. Five criteria stated by Killick-Kendrick [104] are required to incriminate a particular sandfly species as a vector, which include the observation of corresponding epidemiological data, feeding behaviour of the sandflies on the animal intermediate host, the isolation of promastigote parasites from the sandflies, the occurrence of the complete life cycle of the parasite in its putative vector, and experimental transmission of the parasite through the bite of the infected species. Since the 1990s, with PCR invention and advances in molecular parasitology, molecular evidence was added to the mentioned criteria, and reports regarding the presence of Leishmania DNA in various sandfly species have dramatically increased. Nevertheless, according to the above-mentioned criteria, the presence of Leishmania DNA within sandflies should certainly not be considered to be a sufficient criterion to incriminate a sandfly species as a proven vector. Further evidence highlighting the presence of metacyclic promastigotes within the insect’s gut as well as demonstration of the insect’s capacity to retransmit Leishmania are essential criteria that need to be investigated to indicate the vectorial competence of sandflies. Approximately 166 species have been reported to be proven or potential vectors of different Leishmania species in the Old and New World (Table 4). Among these species, 78 are reported as the proven vectors of Leishmania. In the Old World, Leishmania are transmitted by sandflies belonging to the Phlebotomus genus (49 species, 31 are reported as proven), while Sauroleishmania are transmitted by sandflies of the Sergentomyia genus. In the New World, Leishmania, Viannia, and Endotrypanum species are transmitted by sandflies belonging to the Lutzomyia genus (118 species, 47 are reported as proven). Among the above-mentioned sandfly vectors, seven are involved in the transmission of L. major, seven in the transmission of L. tropica, 31 in the transmission of L. infantum, and nine in the transmission of L. donovani. New World sandflies (genus Lutzomyia) are involved in the transmission of different species (see Table 4, updated information from various publications). The stronger restriction of vectors to cutaneous Leishmania species than to vectors of either the visceralizing donovani/infantum group [147] or L. (Viannia) [19] provides support for the hypothesis that cutaneous species evolved first.
Cutaneous leishmaniasis (CL) is a vector-borne zoonotic disease, involving various wild rodents and humans as vertebrate hosts and different sandfly species as vectors playing a role in Leishmania transmission. In the Old World, a large majority of CL cases are geographically restricted to the arid and semiarid areas of the North, Central sub-Saharan, and East African regions; the Near East and Middle East; and Central Asia and India. New World CL occurs in tropical and subtropical areas of Mexico and Central and South America. The Leishmania species responsible for CL differ between the Old and New World. In the Old World, the etiological agents of CL include L. tropica, L. major, and L. aethiopica, whereas New World CL is caused by parasites of the L. mexicana complex (L. mexicana, L. amazonensis, L. pifanoi, L. garnhami, and L. venezuelensis) or the subgenus Viannia (L. braziliensis, L. guyanensis, L. panamensis, L. naiffi, L. shawi, L. lainsoni, and L. peruviana). In the Old World, the proven vectors of CL are mainly classified in the subgenera Phlebotomus and Paraphlebotomus, even though some species of the Adlerius and Larroussius subgenera are thought to be vectors of parasites causing Old World CL [81,157]. In the New World, the main vectors of CL belong to the subgenera Nyssomyia, Psychodopygus, Lutzomyia s.str., and Verrucarum (Fig 3) (Table 4).
Fig 3. Geographical distributions of various Leishmania spp.; sandflies and animal reservoirs in the Old and New World.
L: Leishmania (species), S: Sandfly (genus or subgenus), R: Reservoir (genus or family).
Diffuse cutaneous leishmaniasis (DCL) was first reported in Kenya in 1969. This disease is an anergic variant of localized CL, in which lesions are disseminated. The causative agent is L. aethiopica, which is transmitted by P. pedifer and P. longipes. Nevertheless, DCL caused by L. amazonensis, transmitted by Lutzomyia-group Olmeca in the New World, has also been reported.
Mucocutaneous leishmaniasis (MCL), or espundia, occurs exclusively in South America, showing a greater incidence in Peru, Bolivia, Paraguay, Ecuador, Colombia, and Venezuela. L. braziliensis (Viannia subgenus) is the main causative agent, and to a lesser extent, L. guyanensis, L. panamensis, and L. amazonensis are also known to be responsible for MCL in this region. The vectors of this disease mainly belong to the subgenus Psychodopygus (e.g., L. (Ps.) wellcomei) [158].
Visceral leishmaniasis (VL) is usually a systemic disease that affects internal organs, particularly the spleen, liver, and bone marrow. L. donovani and L. infantum are the agents responsible for Old World VL, whereas L. chagasi (synonym with L. infantum) is responsible for New World VL. Some VL cases caused by L. tropica or L. amazonensis have also been reported [159]. The main VL vectors belong to the Euphlebotomus, Larroussius, and Synphlebotomus subgenera [160], but some species of the Adlerius and Paraphlebotomus subgenera have also been reported as vectors of L. infantum and L. donovani. The vectors involved in the transmission of New World VL belong to the Lutzomyia sensu stricto, Migonemyia, Nyssomyia, Pifanomyia, Psychodopygus, and Verrucarum subgenera (Fig 3) [161].
Discussion and Conclusion
Phlebotomine sandfly systematics, particularly at the supraspecific level, have always been controversial [34,53]. Originally, this family was composed of a single genus: Phlebotomus Rondani. In 1948, Theodor proposed subdivision of the sandfly family into four genera: Phlebotomus and Sergentomyia in the Old World and Lutzomyia and Brumptomyia in the New World. A "stable" classification of the phlebotomine sandflies was proposed in 1977 by Lewis and colleagues [14], who retained the well-known family, subfamily, and genus names. It was also proposed that the subgenera and species groups be used as a model to put forward a new proposal. A “flexible” classification was proposed by Ready and colleagues in 1980 [162]. These researchers challenged the “stable” classification through a comparative analysis of characters that were described as “exclusive” characters for their proposed genera, e.g., Phlebotomus, Sergentomyia, Brumptomyia, Warileya, and Psychodopygus, but no such characters were found for Lutzomyia. The absence of unique characters for the genus Lutzomyia is certainly the weakest point in their comparative character analysis. New discoveries in later years led to the erection of new subgenera or genera. One of the difficulties in sandfly classification concerns the position of sandfly species at the genus or subgenus level. There is no general agreement regarding the definition of some groups at the genus or subgenus level. Idiophlebotomus in Phlebotomus, as well as Parrotomyia, Rondanomyia, and Grassomyia in Sergentomyia were classified by Quate and Fairchild [163] at the subgenus level, whereas Abonnenc [164] considered Idiophlebotomus to be genus and Sergentomyia to be a subgenus. Abonnenc and Minter [165] did not include Parvidens as a subgenus of Sergentomyia, whereas Abonnenc [164] considered Parvidens to be a subgenus of the Phlebotomus genus. Lewis [5] declined to recognize generic status for Spelaeophlebotomus and Idiophlebotomus, whereas Artemiev and Neronov [166] considered them at the genus level. Similarly, for New World sandfly species, Young and Duncan [8] classified Bichromomyia, Dampfomyia, Deanemyia, Evandromyia, Expapillata, Martinsmyia, Micropigomyia, Migonemyia, Nyssomyia, Pintomyia, Psathyomyia, Psychodopigus, Trichophoroymyia, Trichopigomyia, and Viannamyia to be subgenera of the Lutzomyia genus, whereas Galati et al. [66] elevated these groups to the genus level. These conflicts in classification are mainly due to (i) differences or variations in the criteria and the methods used for classification, such as criteria that are now considered to be outdated or scarce, e.g., the presence of erected or recumbent abdominal setae; (ii) morphological similarities between species and some uncertainty in species identification, such as the existence of cryptic or sibling species and the similarity of morphological characters among females that makes species identification dependent on male characters (e.g., Adlerius); (iii) the inadequacy of the reported species descriptions; and (iv) the massive increase in the number of sandfly species described. The construction of a well-supported phylogeny of the generic and subgeneric groups in the Phlebotominae subfamily will likely require a supermatrix analysis. This matrix must include molecular information on several nuclear genes combined with mitochondrial genes—as well as other criteria related to biology—and ecology, which has been successfully applied for the classification of the Drosophilidae family [167]. This type of analysis would provide a firmer basis for the classification of Phlebotominae sandflies, in addition to resolving the problem of the proposal of classifications suggested for the Old World and New World sandflies. Therefore, a more extensive molecular phylogenetic analysis, e.g., focussing on gene flow and the phenotypes of specimens, awaits the development of an accurate and valid protocol for sandfly classification.
A reliable taxonomy of Leishmania species will represent a keystone for biological and epidemiological research programs. There is still no universal agreement regarding the classification of Leishmania, especially concerning the criteria defined for species definition, or the method used to address phylogenetic classification. The greatest inconsistency concerns the assignment of Leishmania at the specific or subspecific level. Although the clustering of Leishmania at the subgeneric level and the definition of “complexes” in Leishmania classification have gained rather wide acceptance since being reported by Lainson and Shaw [98], there are still serious challenges in terms of the genus composition. Various molecular methods have been introduced to elucidate the taxonomy of Leishmania, though defining a Leishmania species or accepting all of the described species is still not straightforward. The currently accepted classification of Leishmania proposes the division of this genus into three subgenera: Leishmania, Viannia, and Sauroleishmania. Under this proposal, species that cannot be classified into any of these subgenera are included in the Paraleishmania section, such as yet-unclear-status Leishmania parasites. A question that remains open to debate is the position and classification of Sauroleishmania. Because this group is of low medical importance, there is little information about the reliability of its classification at present. Its placement in the Leishmania phylogeny therefore remains highly debated. Contradictorily, Kerr [144] proposed that the mammalian Leishmania evolved from lizard Sauroleishmania in the Palaearctic, whereas Noyes [149] controversially suggested that lizard Sauroleishmania evolved from mammalian parasites. This group has been placed both at the crown of the phylogeny [83,139,150] and at its root [136,144,145]. It appears more likely that the position of Sauroleishmania external to all L. (Leishmania) is a consequence of a faster rate of evolution in this subgenus, as suggested by a molecular phylogenetic analysis performed on the RNA and DNA polymerase genes [150]. Therefore, the systematic position of many Leishmania infecting reptiles remains unresolved. This difficulty in assigning a phylogenetic position is likely due to (i) the paucity of information about the life cycle of Sauroleishmania; (ii) the fact that all of the flagellates found in reptiles have been studied mainly at the light-optical level (except some submitted sequences in Genbank), without additional study methods being applied (serological, biochemical, and others), whereas some flagellates from reptiles belong to Trypanosoma and are also transmitted by sand flies; and (iii) the existence of a priori notions that every flagellate detected in a reptile’s body should be attributed to Leishmania promastigotes without further study of their true identity. Therefore, to avoid any doubt in the classification of Leishmania as well as Sauroleishmania, emphasis on the exploration of new isolates via molecular biology and phylogenetic (DNA analysis) methods is suggested. Finally, to clarify the position of Leishmania species in this classification, it is proposed that assignment to major groups across the entire genus Leishmania should be based on gene sequences, which are remarkably congruent and uncontroversial. For classification within the major groups, more highly discriminatory markers, such as MLST markers, microsatellites, or genome-wide single nucleotide polymorphisms, are considered to be better suited.
Knowledge about the origin and dispersal of Leishmania will help us to more precisely understand the factors that have and will continue to influence the circulation of leishmaniasis, in relation to its etiological parasitic agents, the vectors that transmit them, and their reservoirs. The dissemination of Leishmania has followed the migration of its vectors and hosts together [168]. Concerning the origin of Leishmania species, several hypotheses have been proposed, which were described above. These hypotheses profit from significant fossil, molecular, ecological, and biochemical data supporting them. Nevertheless, the debate is still open. To gather more information to support hypotheses of the origin and evolution of Leishmania, more evidence must be considered. Such evidence will include the following:
Molecular phylogenies: based on several independent genes that display different evolutionary constraints, e.g., the elongation factor (EF-1α), heat shock protein gene (hsp70), and glyceraldehyde dehydrogenase (GAPDH), SSU (small subunit of ribosomal DNAs), DNA Polymerase α (POLA), cytochrome b (cytb), cysteine proteases, RNA polymerase II large subunit, gp63, mini-exon, and internal transcribed spacer of rDNA (ITS) (at lower taxonomical level) and spliced leader (SL) genes. Some of these genes are single-copy, protein-coding genes and are therefore suitable candidates for studying the molecular systematics and phylogeny of Leishmania [169].
Biogeographical and ecological evidence: geographical, ecological, and climatic aspects as well as geological periods of the Earth and the presence of natural environmental pressures or geographical barriers must be investigated to obtain insight into the origin, evolution, and dispersion of Leishmania. It is worth considering that the absence or emergence of geographical barriers, such as mountains, in the past few million years (or even today), has resulted in a wider or restricted distribution of Leishmania parasites and their sandfly vectors and animal hosts at a worldwide scale.
Entomological evidence: considering that leishmaniasis is a vector-borne disease, it is of course essential to more precisely understand the origin and the evolution of sandfly vectors along with Leishmania development, considering their coevolution and sympatry in different periods of time.
Mammalogical evidence: considering that leishmaniasis is a zoonotic disease, the origin, conservation, and dispersion of Leishmania is highly dependent on animal reservoirs.
Three hypotheses have been proposed concerning the origin of Leishmania (Fig 2). Kerr [144] proposed a Palaearctic origin of Leishmania, based on a study carried out by Lysenko in 1971 [142]. He used fossil evidence of mammalian taxa and sandflies previously reported by Nowak [143] and Lewis [5], respectively, to support his hypothesis. Nevertheless, this hypothesis has been proposed based on a biogeographical study, which must be tested against other independent datasets. In 2000 [144], based on biogeographical evidence, fossil records of mammals and sandflies, and ecological data, Kerr also proposed a revision of the Leishmania/Sauroleishmania clade, but the lack of an independent phylogenetic analysis undermined the reliability of this hypothesis. Several factors argue against a Neotropical origin of Leishmania. Based on this theory, (i) porcupines did not move from the Neotropic to the Nearctic, whereas the fossil record demonstrates that such migration occurred after the formation of the Panamanian land bridge during the Pliocene [143]; (ii) porcupines did not travel across the Bering land bridge; (iii) the use of nonmolecular evidence, such as data based on biogeography, epidemiology, ecology, and historical events, is controversial; and (iv) there is an inconsistency between the current classifications of phlebotomine sandflies and the proposed Neotropical origin of Leishmania as well as a discrepancy between a Palaearctic origin of the murid rodents and a Neotropical origin of the parasite [7,139,144]. The third hypothesis considers Leishmania to exhibit a Neotropical/African origin. Despite reported evidence, this theory does not consider human dispersion into the Neotropics [139]. Finally, based on this hypothesis, a serious question remains regarding the Sauroleishmania phylogeny at the crown of the phylogenic tree and the dispersal of Leishmania from Africa to the Neotropics before the separation of Pangaea when considering the lack of evidence concerning the presence of Sauroleishmania in the Neotropics.
The question about Leishmania evolution has classically been centred on two opposing theories related to the original host for Leishmania as a digenetic parasite; i.e., was the first host a vertebrate or an invertebrate? Such information will certainly help us to better understand the origin and factors that play an important role in Leishmania dispersion and therefore in the epidemiology of leishmaniasis. The Phlebotominae ancestor emerged in the Triassic period, before the appearance of Leishmania (Jurassic) and placental mammals (Palaeocene). This hypothesis is further supported by an SSU rRNA data analysis indicating that Leishmania diverged from a trypanosomatid line of monogenetic insect parasites [140]. The oldest fossil ancestors of the modern sandflies date from the Cretaceous period (120 MYA, Lebanon), followed by Burmese fossil amber (Cretaceous, 100 MYA). A gap of approximately 80 MYA is present from this Burmese fossil amber specimen until the next fossil found in Baltic amber (20 MYA), meaning that there is a serious gap in knowledge. According to the Burmese fossil amber specimen, ingested and free-living flagellates of P. proterus were found in habitats containing P. burmitis sandflies. In the Jurassic period, the reptiles were the predominant vertebrate fauna for many years. Despite their presence, there is no strong evidence, such as fossils, linking the sandfly lineage with ancient cold-blooded vertebrates. This absence or rarity of Leishmania in older reptiles suggests that sandflies with haematophagous habits were likely to be the first host of Leishmania. In addition, the greater range restriction of the sandfly vectors than the animal hosts of Leishmania parasites supports the much closer coevolutionary relationship of Leishmania and sandflies. Considering the above observations, it appears that monogenetic parasites of sandflies adapted to mammals some 90 MYA, giving rise to Leishmania. This adaptation likely took place during a period when mammals were diversifying into different orders during the separation of Africa and South America. Kerr [144] proposed a Palaearctic origin of Leishmania, suggesting that reptiles were the first vertebrate hosts of Leishmania, whereas Noyes [149] considered rodents to be the first vertebrate host. With the exception of the Sauroleishmania group, no human pathogenic Leishmania have been reported from reptiles. One the other hand, regarding some characteristics of sandflies, such as their restricted flight distance, short life cycle, slow larval development, and greater blood feeding preference for warm-blooded animals compared with cold-blooded species, it is assumed that these insects were the first host of Leishmania, but they have not played a major role in the Leishmania dispersion, particularly in regions that are unsuitable for sandfly survival. Hence, it is assumed that Leishmania were transferred by infected sandflies to local vertebrates, in which the parasite can survive for long period, after which the vertebrates, particularly the murid rodents, were the responsible for disease dispersion in the Old and New World. Muroids are a large superfamily of rodents. They have diversified into a large superfamily comprising over 1,500 species, including hamsters, gerbils, true mice, and rats as well as many other relatives. They now make up nearly one-third of all mammalian species, and they occupy a vast variety of habitats on every continent except for Antarctica. Comparison of the origin and distribution pattern of rodents proposed by Schenk et al. [170] (Steppan [171]) with the hypotheses of Leishmania appearance and dispersion suggests a close similarity in the distribution patterns of these groups, supporting the theory that they might be responsible for Leishmania dispersion in both the Old and New World.
Concluding Remarks
The evolutionary relationship between sandflies and Leishmania has implications for leishmaniasis interventions and control. It is therefore necessary to obtain information on the origin of Leishmania and the Phlebotominae sandflies and their chronological history of coevolution. Understanding these evolutionary relationships between different Leishmania and sandfly species is of epidemiological importance for the future prediction of Leishmania transmission patterns.
Key Learning Points
Understanding the current hypotheses of the origin and dispersion of Leishmania and sandflies, based on the available fossil evidence and molecular studies and the factors that play important role in these dispersions
To have a knowledge about three-century history of sandflies and Leishmania classification as well as a complete description of Leishmania and sandfly fossils, with biological emergence date of each Leishmania and sandfly groups during different geographical periods from 550 million years ago until now
An update of information on the current distribution and dispersion of different species of Leishmania (53 species), sandfly vectors (More than 800 species), and animal reservoirs in each geographical regions of Palearctic, Nearctic, Neotropic, Afrotropical, Oriental, Madagascar, and Australia
A critical discussion on the different approaches that were used for Leishmana and sandfly classification, their advantages and disadvantages, their synonymy, and proposal of an updated classification for each species of Leishmania and sandfly
Suggesting a complete list of the potential and proven sandfly vectors for each Leishmania species in the Old and New World
Top Five Papers
Lainson, R., Shaw, J. J. (1987) Evolution, classification and geographical distribution. In: W Peters, R Killick-Kendrick, editors. The Leishmaniases in Biology and Medicine, Academic Press, London, p. 1–120.
Cupolillo, E., Medina-Acosta, E., Noyes, H., Momen, H., Grimaldi, G. Jr. (2000) A revised classification for Leishmania and Endotrypanum. Parasitology Today, 16, 142–144.
Kerr, S. F. (2000) Palaearctic Origin of Leishmania. Memorias de Instituto Oswaldo Cruz, 95, 75–80.
Poinar, Jr. G. O. (2004) Palaeomyia burmitis (Diptera: Phlebotomidae), a new genus and species of Cretaceous sandflies with evidence of blood-sucking habits. Proceedings of the Entomological Society of Washington, 106, 598–605.
Lewis, D. J. (1982) A taxonomic review of the genus Phlebotomus (Diptera: Psychodidae). Bulletin of the British Museum (Natural History), 45, 121–209. http://www.sandflycatalog.org/pdfs/116803.pdf
Acknowledgments
The authors are grateful for Prof. Petr Volf for his critical comments, which improved this manuscript. We also thank Prof. Paul Bates and two anonymous reviewers for their comments that improved greatly the manuscript.
Funding Statement
The author(s) received no specific funding for this work.
References
- 1.Moreno J, Alvar J. Canine leishmaniasis: epidemiological risk and the experimental model. Trends Parasitol. 2002; 18: 399–405. [DOI] [PubMed] [Google Scholar]
- 2.Alvar J, Vélez ID, Bern C, Herrero M, Desjeux P, Cano J, et al. Leishmaniasis Worldwide and Global Estimates of Its Incidence. PLoS ONE. (2012); 7(5): e35671 10.1371/journal.pone.0035671 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Galati EAB. Classificacao de Phlebotominae In: Rangel ER, Lainson R, editors. Flebotomineos do Brasil. Editora Fiocruz, Rio de Janeiro, Brazil: 2003; p. 23–52. 367pp. [Google Scholar]
- 4.Seccombe AK, Ready PD, Huddleston LM. A catalogue of Old World Phlebotomine sandflies (Diptera: Psychodidae). Occas Pap Syst Entomol. 1993; 8: 1–57. [Google Scholar]
- 5.Lewis DJ. A taxonomic review of the genus Phlebotomus (Diptera: Psychodidae). Bull Br Mus nat. 1982; 45: 121–209. [Google Scholar]
- 6.Theodor O. Psychodidae, Phlebotominae, p. 1–55. In E Lindner, Die Fliegen der Palaearktischen Region, 1958; Vol. 9, Psychodidae, E. Schweizerbart’scheVerlag, Stuttgart.
- 7.Williams P. Relationships of phlebotomine sandflies (Diptera). Mem Inst Oswaldo Cruz. 1993; 88: 177–183. [DOI] [PubMed] [Google Scholar]
- 8.Young DG, Duncan MA. Guide to the Identification and Geographic Distribution of Lutzomyia Sandflies in Mexico, the West Indies, Central and South America (Diptera: Psychodidae) Mem Amer Inst Entomol 54, Associate Publishers, Gainesville, 1994; 881 pp. [Google Scholar]
- 9.Hennig W. Insektenfossilien aus der unteren Kreide. IV. Psychodidae (Phlebotominae), mit einer kritischen Übersicht über das phylogenetische System der familie und die bisher beschriebenen Fossilien (Diptera). Stutt Beit Natur (B).1972; 241: 1–69. [Google Scholar]
- 10.Rispal R, Leger N. Numerical taxonomy of Old World Phlebotominae (Diptera: Psychodidae). I. Considerations of Morphological Characters in the genus Phlebotomus Rondani & Berte. Mem Inst Oswaldo Cruz. 1998; 93: 793–785. [DOI] [PubMed] [Google Scholar]
- 11.Aransay AM, Scoulica E, Tselentis Y, Ready PD. Phylogenetic relationships of Phlebotomine sandflies inferred from small subunit nuclear ribosomal DNA. Insect Mol Biol. 2000; 9: 157–168. [DOI] [PubMed] [Google Scholar]
- 12.Perfil’ev PP. Phlebotomidae. Translation of Perfil’ev, 1966 Diptera: Family Phlebotomidae. Fauna SSSR. 1968; 93: 1–382. [Google Scholar]
- 13.Theodor O. Classification of the Old World species of the subfamily Phlebotominae (Diptera, Psychodidae). Bull Entomol Res.1948; 39: 85–115. [DOI] [PubMed] [Google Scholar]
- 14.Lewis DJ, Young DG, Fairchild GB, Minter DM. Proposal for a stable classification of the phlebotomine sandflies (Diptera: Psychodidae). Sys Entomol. 1977; 2: 319–332. [Google Scholar]
- 15.Leng YJ. A preliminary survey of phlebotomine sandflies in limestone caves of Sichuan and Guizhou Provinces, South-West China, and description and discussion of a primitive new genus Chinius. Ann Trop Med Parasitol. 1987; 81: 311–317. [DOI] [PubMed] [Google Scholar]
- 16.Galati E.A.B. Classificação, morfologia, terminologia e identificação de Adultos: Bioecologia e Identificação de Phlebotominae In: Rangel E.F., Lainson R., editors. Flebotomíneos do Brasil. FIOCRUZ, Río de Janeiro, 2014. p. 367. [Google Scholar]
- 17.Lane RP. Sandflies (Phlebotominae) 1993; p. 78–113. In: Lane RP, Crosskey RW, editors. Medical Insects and Arachnids, Chapman & Hall, London, 723 pp. [Google Scholar]
- 18.Lewis DJ. Functional morphology of the mouth parts in World phlebotomine sandflies. Proc R Soc London. 1975; 126: 497–532. [Google Scholar]
- 19.Lainson R, Shaw JJ. Evolution, classification and geographical distribution In: Peters W, Killick-Kendrick R editors. The Leishmaniases in Biology and Medicine, Academic Press, London, 1987; p. 1–120. [Google Scholar]
- 20.Sadlova J, Dvorak V, Seblova V, Warburg A, Votypka J, Volf P. Sergentomyia schwetzi is not a competent vector for Leishmania donovani and other Leishmania species pathogenic to humans. Parasites Vectors. 2013; 6: 186–196. 10.1186/1756-3305-6-186 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Javadian E, Mesghali A. Checklist of phlebotominae sandflies (Diptera; Psychodidae) of Iran. Diptera, sandflies, Phlebotomus sp. checklist, Iran. Bull Soc Pathol Exot Filiales. 1975; 68, 207–9. [PubMed] [Google Scholar]
- 22.Theodor O, Mesghali A. On the Phlebotominae of Iran. J Med Entomol. 1964; 1: 285–300. [DOI] [PubMed] [Google Scholar]
- 23.Lewis DJ. The Phlebotomine sandflies of West Pakistan (Diptera: Psychodidae). Bull. Br.Mus.nat (Natural History). 1967; 19: 1–57. [Google Scholar]
- 24.Rioux JA, Golvan YJ. Epidémiologie des leishmanioses dans le sud de la France. Monographie de l’INSERM (Paris). 1969; 37: 1–223. [Google Scholar]
- 25.Houin R, Abonnenc E, Denial M. Phlebotomes du sud de la Turquie. Resultats d’un sondage. Annales de parasitologie humaine et comparée. 1971; 46: 633–652. [PubMed] [Google Scholar]
- 26.Bailly-Chaumara H, Abonnec E, Pastre J. Contribution à l’étude des phlébotomes du Maroc (Diptera: Psychodidae). Données faunistiques et écologiques. Cahiers ORSTOM. Série Entomologie Médicale et Parazitologie. 1971; 4: 431–460. [Google Scholar]
- 27.Lewis DJ. The Phlebotomid sandflies of Yemen Arab Republic. Tropenmed Parasitol. 1974; 25: 187–197. [PubMed] [Google Scholar]
- 28.Gil Collado J. Phlebotomes et Leishmanioses en Espagne. In: Colloques Internationaux du C. N. R. S.: Ecologie des Leishmaniose. Ed. C. N. R. S. Paris, 1977; 177–190.
- 29.Croset H, Rioux JA, Maistre M, Bayar N. The phlebotomines of Tunisia (Diptera-Phlebotominae). A revision of the systematics, distribution and behaviour. Ann Parasitol Hum Comp. 1978; 53: 711–749. [PubMed] [Google Scholar]
- 30.Artemiev MM. Sandflies (Diptera, Psychodidae. Phlebotominae) of Afghanistan, Ministry of Health: Malaria and Leishmania Institute; Kabul, 1978; 87pp. [Google Scholar]
- 31.Lewis DJ, Buttiker W. Diptera: Fam. Psychodidae, subfam. Phlebotominae, pp. 252–285. In: Wittmer W, Buttiker W, editors. Fauna of Saudi Arabia. 1980; Vol. 2., 443 pp. Basle, Pro Entomologia/Ciba-Geigy. [Google Scholar]
- 32.Abul-Hab J, Ahmed SA. Revision of the family Phlebotomidae (Diptera) in Iraq. J Biol Sci Res. 1984; 7: 1–64. [Google Scholar]
- 33.Dedet JP, Addadi K, Belazzoug S. Les phlébotomes (Diptera, Psychodidae) d’Algérie. Cahier ORSTOM, série Entomologie Médicale et Parasitologie. 1984; 22: 99–127. [Google Scholar]
- 34.Lane RP. Recent advances in the systematics of Phlebotomine sandflies. Insect Sci Appl. 1986; 7: 225–230. [Google Scholar]
- 35.Léger N, Pesson B, Madulo-Leblond G. Les phlebotomes de Grece. Biol Gallo-hellenica. 1986; 11: 165–192. [Google Scholar]
- 36.Lane RP, Abdel-Hafez S, Kamhawi S. The distribution of Phlebotomine sandflies in the principal ecological of Jordan. Med Vet Entomol J. 1988; 2: 237–246. [DOI] [PubMed] [Google Scholar]
- 37.Esseghir S, Ready PD, Ben-Ismail R. Speciation of Phlebotomus sandflies of the subgenus Larroussius coincided with the late Miocene-Pliocene aridification of the Mediterranean subregion. Biol J Linnean Soc. 2000; 70: 189–219. [Google Scholar]
- 38.Killick-Kendrick R, Killick-Kendrick M. Biology of sandfly vectors of Mediterranean canine leishmaniasis In: Killick-Kendrick R, editor. Canine leishmaniasis: an update. Proc. Int. Can. Leishm. Forum, Barcelona, Spain, Intervet Int., Boxmeer, The Netherlands, 1999; pp 26–31. [Google Scholar]
- 39.Maroli M, Feliciangeli D, Bichaud L, Charrel R, Gradoni L. Phlebotomine sandflies and the spreading of leishmaniases and other diseases of public health concern. Medical Vet Entomol J. 2012; 27: 123–47. [DOI] [PubMed] [Google Scholar]
- 40.Zhang L, Ma Y, Xu J. Genetic differentiation between sandfly populations of Phlebotomus chinensis and Phlebotomus sichuanensis (Diptera: Psychodidae) in China inferred by microsatellites. Parasites Vectors. 2013; 6: 115 10.1186/1756-3305-6-115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Galliard H, Nitzulescu V. Contribution à l'etude des phlebotomes du Gabon. Phlebotomus sanneri n. sp. Ann Parasitol Hum Comp. 1931; 9: 233–246. [Google Scholar]
- 42.Grepin G. A Check-List of Sandflies (Diptera-Phlebotominae) of Central African Republic. Ann Parasitol Hum Comp. 1983; 58: 85–90. [PubMed] [Google Scholar]
- 43.Ashford RW. Sandflies (Diptera: Phlebotomidae) from Ethiopia: taxonomi and biological notes. J Med Entomol. 1974; 11: 605–616. [PubMed] [Google Scholar]
- 44.Davidson IH. The subgenus Anaphlebotomus of Phlebotomus (Diptera: Psychodidae) in southern Africa. J Entomol Soc South Africa. 1981; 44: 259–264. [Google Scholar]
- 45.Léger N, Depaquit J, Robert V. les phlébotomes de madagascar (Diptera: Psychodidae). iv–description de Sergentomyia (rondanomyia) goodmani n. sp. rétablissement du sous-genre Rondanomyia theodor. Parasite. 2005; 12: 51–57. [DOI] [PubMed] [Google Scholar]
- 46.Léger N, Depaquit J, Gay F. Description of the sandfly species Chinius samarensis n. sp. (Psychodidae; Diptera) from the Philippines. Pathog Glob Health. 2012; 106: 346–351. 10.1179/2047773212Y.0000000041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Léger N, Depaquit J, Gay F. Chinius eunicegalatiae n. sp. (Diptera; Psychodidae), a cavernicolous sandfly from Laos. Ann TropMed Parasitol. 2010; 104: 595–600. [DOI] [PubMed] [Google Scholar]
- 48.Lewis DJ. The Phlebotomine sandflies (Diptera: Psychodidae) of the Oriental Region. Bull Br Mus (Natural History). 1978; 37: 217–343. [Google Scholar]
- 49.Lewis DJ, Dyce A. Taxonomy of the Australasian Phlebotominae (Diptera: Psychodidae) with revision of genus Sergentomyia from the region. Invertebrate Taxonomy. 1988; 2(6): 755–804. [Google Scholar]
- 50.Schodde R, Calaby JH. The biogeography of the Australo-Papuan Bird and mammal faunas in relation to Torres Strait, p. 257–300. In: Bridge and barrier: the natural and cultural history of Torres Strait. Australian Nat. Univ., 1972; Canberra, Australia. [Google Scholar]
- 51.Quate LW, Quate SH. A monograph of Papuan Psychodidae, including Phlebotomus. Pac Insects. 1967; 15: 1–216. [Google Scholar]
- 52.Dyce AL, Wellings G. Phlebotomine sandflies (Diptera: Psychodidae) from caves in Australia. Parasitologia. 1991; 33: 193–8. [PubMed] [Google Scholar]
- 53.Lewis DJ, Dyce AL. The subgenus Australophlebotomus Theodor of Phlebotomus Rondani and Berté (Diptera: Psychodidae). J Aust Entomol Soc. 1982; 21: 37–54. [Google Scholar]
- 54.Galati EAB, Nunes VLB, Rego FA, Oshiro ET, Chang MR. Estudo de flebotomíneos (Diptera: Psychodidae) em foco de leishmaniose visceral no estado de Mato Grosso do Sul, Brasil. Cad Saúde Pública. 1997; 31: 378–390. [DOI] [PubMed] [Google Scholar]
- 55.Martins AV, Williams P, Falcao AL. (1978) American Sandflies (Diptera: Psychodidae, Phleboiominae). Academia Brasiliera de Ciencias, Rio de Janeiro. [Google Scholar]
- 56.Young DG, Lawyer PG. New World vectors of the leishmaniases In: Harris KF, Current Topics in Vector Research, Vol. 4 Springer-Verlag, New York, 1987; p. 29–37. [Google Scholar]
- 57.Young DG, Perkins PV. Phlebotomine sandflies of North America (Diptera: Psychodidae). Mosq News. 1984; 44: 263–304. [Google Scholar]
- 58.Young DG. A review of the bloodsucking psychodid flies of Colombia (Diptern: Phlebotominae and Sycoracinae) Tech. Bull. 806, Agric. Exp. Station, IFAS, Univ. Florida, Gainesville: 1979; 226 p. [Google Scholar]
- 59.Young DG, Rogers TE. The Phlebotomine sandfly fauna of Ecuador. J Med Entomol. 1984; 24: 651–665. [Google Scholar]
- 60.Murillo J, Zeledon R. Flebotomos de Costa Rica. Brenesia: Rev. Cienc. Nat. Museo Nac. Costa Rica, 1985; 137 p.
- 61.Young DG, Murillo J. A new Phlebotomine sandfly, Lutzomyia zeledoni, n. sp. (Diptera: Psychodidae) from Central America. J Med Entomol. 1984; 21: 711–713. [Google Scholar]
- 62.Ryan L. Flebótomos do Estado do Pará, Brasil (Diptera: Psychodidae: Phlebotominae), Tech. Doc. No. 1, Instituto Evandro Chagas, Belém, 1986; 154 pp. [Google Scholar]
- 63.Lebbe J, Vignes R, Dedet JP. Computer Aided Identification of Phlebotomine Sandflies of French Guiana (Diptera: Psychodidae). Publication de l'Institut Pasteur de la Guyane française, Cayenne, 1987; 165 pp. [Google Scholar]
- 64.Feliciangeli MD, Rabinovich J. Abundance of Lutzomyia ovallesi but not Lu. gomezi (Diptera: Psychodidae) correlated with cutaneous leishmaniasis incidence in north-central Venezuela. M Vet Entomol J. 1998; 12: 121–131. [DOI] [PubMed] [Google Scholar]
- 65.Forattini OP. Entomologia Médica Psychodidae. Phlebotominae. Leismanioses. Bartonelose, 1973; Vol 4, Edgar Blücher Ltda., São Paulo. [Google Scholar]
- 66.Galati EAB, Fonseca MB, Marassá AM, Bueno EFM. Dispersal and survival of Nyssomyia intermedia and Nyssomyia neivai (Diptera: Psychodidae: Phlebotominae) in a cutaneous leishmaniasis endemic area of the speleological province of the Ribeira Valley, State of São Paulo, Brazil. Mem Inst Oswaldo Cruz. 2009; 104: 1148–1158. [DOI] [PubMed] [Google Scholar]
- 67.Theodor O. On the classification of American Phlebotominae. J Med Entomol. 1965; 2: 171–197. [DOI] [PubMed] [Google Scholar]
- 68.Andrade Filho JD, Brazil RP. Relationships of new word Phlebotomine sandflies (Diptera: Psychodidae) based on fossil evidence. Mem Inst Oswaldo Cruz. 2003; 98: 145–149. [DOI] [PubMed] [Google Scholar]
- 69.Krzeminski W, Krzeminska E. Triassic Diptera: descriptions, revisions and phylogenetic relations. Acta Zool Cracov. 2003; 46: 153–184. [Google Scholar]
- 70.Blagoderov VA, Grimaldi DA, Fraser NC. How time flies for flies: diverse Diptera from the Triassic of Virginia and early radiation of the order. Am Mus Novit. 2007; 3572: 1–39. [Google Scholar]
- 71.Poinar G. Lutzomyia adiketis sp. n. (Diptera: Phlebotomidae), a vector of Paleoleishmania neotropicum sp. n. (Kinetoplastida: Trypanosomatidae) in Dominican amber. Parasites Vectors. 2008; 1, 22 10.1186/1756-3305-1-22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Perrichot V, Neraudeau D, Nel A, de Ploeg G. A reassessment of the Cretaceous amber deposits from France and their palaeontological significance. Afr Invertebr. 2007; 48: 213–227. [Google Scholar]
- 73.Ansorge J. Tanyderidae and Psychodidae (Insecta: Diptera) from the lower Jurassic of Northeastern Germany. Palaontologische Zeitschrift. 1994; 68: 199–210. [Google Scholar]
- 74.Declòs X, Arillo A, Penalver E, Barron E, Soriano C, Lopez del Valle R, et al. Fossiliferous amber deposits from Cretaceous (Albian) of Spain. Comptes Rendus de l’Academie des Sciences—Series IIA—Earth and Planetary Science (Comptes Rendus Palevol). 2007; 6: 135–149. [Google Scholar]
- 75.Poinar G. O. Jr Palaeomyia burmitis (Diptera: Phlebotomidae), a new genus and species of Cretaceous sandflies with evidence of blood-sucking habits. Proc Entomol Soc Wash. 2004; 106: 598–605. [Google Scholar]
- 76.Azar D, Nel A, Solignac M, Paicheler JC, Bouchet F. New genera and species of phlebotomid and psychodid flies from the Lower Cretaceous amber of Lebanon (Insecta: Diptera: Phlebotomidae, Psychodidae). Palaeontol. 1999; 42: 1131–1136. [Google Scholar]
- 77.Stuckenberg BR. New fossil species of Phlebotomus and Haematopota in Baltic Amber (Diptera: Psychodidae, Tabinidae). Ann Natal Mus. 1975; 22: 455–464. [Google Scholar]
- 78.Kaddumi H.F.. 2005. Amber of Jordan The oldest prehistoric insects in fossilised resins. Eternal River Museum of Natural History, Jordan: 168 pp. [Google Scholar]
- 79.Solorzano-Kraemer M. Systematic, palaeoecology, and palaeobiogeography of the insect fauna from the Mexican amber. Palaeontogr Abt. 2007; 282: 1–133. [Google Scholar]
- 80.Antoine PO, Franceschi D, Flynn JJ, Nel A, Baby P, Benammi M, et al. Amber from western Amazonia reveals Neotropical diversity during the middle Miocene. PNAS. 2006; 113: 13595–13600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Killick-Kendrick R. Phlebotomine vectors of the leishmaniases: a review. Med Vet Entomol J. 1990; 4: 1–24. [DOI] [PubMed] [Google Scholar]
- 82.Tuon FF, Neto VA, Amato VS. Leishmania: origin, evolution and future since the Precambrian. FEMS Immunol Med Microbiol. 2008; 54: 158–66. 10.1111/j.1574-695X.2008.00455.x [DOI] [PubMed] [Google Scholar]
- 83.Noyes HA, Morrison DA, Chance ML, Ellis JT. Evidence for a Neotropical Origin of Leishmania. Mem Inst Oswaldo Cruz. 2000; 95: 575 [DOI] [PubMed] [Google Scholar]
- 84.Flegontov P, Votýpka J, Skalick´y T, Logacheva MD, Penin AA, Tanifuji G, et al. Paratrypanosoma is a novel early-branching trypanosomatid. Curr Biol. 2013; 23: 1787–93. 10.1016/j.cub.2013.07.045 [DOI] [PubMed] [Google Scholar]
- 85.Maslov DA, Votýpka J, Yurchenko V, Lukeš J. Diversity and phylogeny of insect trypanosomatids: all that is hidden shall be revealed. Trends Parasitol. 2013; 1: 43–52. [DOI] [PubMed] [Google Scholar]
- 86.Teixeira MM, Borghesan TC, Ferreira RC, Santos MA, Takata CS, Campaner M, et al. Phylogenetic validation of the genera Angomonas and Strigomonas of trypanosomatids harboring bacterial endosymbionts with the description of new species of trypanosomatids and of proteobacterial symbionts. Protist. 2011; 162: 503–524. 10.1016/j.protis.2011.01.001 [DOI] [PubMed] [Google Scholar]
- 87.Votýpka J, Suková E, Kraeva N, Ishemgulova A, Duží I, Lukeš J, et al. Diversity of Trypanosomatids (Kinetoplastea: Trypanosomatidae) Parasitizing Fleas (Insecta: Siphonaptera) and Description of a New Genus Blechomonas gen. n. Protist. 2013; 164: 763–781. 10.1016/j.protis.2013.08.002 [DOI] [PubMed] [Google Scholar]
- 88.Hamilton PT, Votýpka J, Dostálová A, Yurchenko V, Bird NH, Lukeš J, et al. Infection Dynamics and Immune Response in a Newly Described Drosophila-Trypanosomatid Association. MBio. 2015; 6: pii: e01356–15. 10.1128/mBio.01356-15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Dantas-Torres F, Lorusso V, Testini G, de Paiva-Cavalcanti M, Figueredo L A, Stanneck D, et al. Detection of Leishmania infantum in Rhipicephalus sanguineus ticks from Brazil and Italy. Parasitol Res. 2010; 106: 857–60. 10.1007/s00436-010-1722-4 [DOI] [PubMed] [Google Scholar]
- 90.Slama D, Haouas N, Remadi L, Mezhoub H, Babba H, Chaker E. First detection of Leishmania infantum (Kinetoplastida: Trypanosomotidae) in Culicoides spp. (Diptera: Ceratopogonidae). Parasits Vectors. 2014; 7: 51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Solano-Gallego L, Rossi L, Scroccaro AM, Montarsi F, Caldin M, Furlanello T, et al. Detection of Leishmania infantum DNA mainly in Rhipicephalus sanguineus male ticks removed from dogs living in endemic areas of canine leishmaniosis. Parasites Vectors. 2012; 5: 98 10.1186/1756-3305-5-98 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Buck G, Courdurier J, Dorel R, Quesnel JJ. The first case of canine leishmaniasis in Madagascar. Bull Soc Pathol Exot. 1951; 44: 428–30. [PubMed] [Google Scholar]
- 93.Killick-Kendrick R, Killick-Kendrick M, Tang Y, Bastien P. Metacyclic promastigotes of Leishmania in the salivary glands of experimentally infected phlebotomine sandflies. Parasite. 1996; 3: 55–60. [Google Scholar]
- 94.Lainson R. The Neotropical Leishmania species: a brief historical review of their discovery, ecology and taxonomy. Revista Pan-Amazônica de Saúde. 2010; 1: 13–32. [Google Scholar]
- 95.Adler S. Leishmania. Adv Parasitol. 1964; 2: 35–96. [DOI] [PubMed] [Google Scholar]
- 96.Belova EM. Reptiles and their importance in epidemiology of leishmaniasis. Bull World Health Organ. 1971; 44: 553–560. [PMC free article] [PubMed] [Google Scholar]
- 97.Lainson R, Shaw JJ. Leishmanias and leishmaniasis of the New World, with particular reference to Brazil. Bull Pan Am Health Organ.1973; 7: 1. [Google Scholar]
- 98.Lainson R, Shaw JJ. Leishmaniasis of the New World: Taxonomic problems. British med Bull. 1972; 28: 44. [DOI] [PubMed] [Google Scholar]
- 99.Bray RS, Ashford RW, Bray MA. The parasite causing cutaneous leishmaniasis in Ethiopia. Trans R Soc Trop Med Hyg. 1973; 67: 345–8. [DOI] [PubMed] [Google Scholar]
- 100.Vickerman K. (1976) The diversity of the kinetoplastid flagellates In: Lumsden WHR, Evans DA, editors. Biology of the kinetoplastida. London: Academic Press; p. 1–34. [Google Scholar]
- 101.Lainson R, Ready PD, Shaw JJ. Leishmania in phlebotomid sandflies. VII. On the taxonomic status of Leishmania peruviana, causative agent of Peruvian “uta”, as indicated by its development in the sandfly, Lutzomyia longipalpis. Proc R Soc London. 1979; 206: 307–318. [DOI] [PubMed] [Google Scholar]
- 102.Saf'janova VM. Classification of the genus Leishmania Ross. Chapter 11 (in Russian). In: The Leishmaniasis. Protozoology, Academy of Sciences, USSR All Union Society of Protozoologists, Lennigrad, Part 7, 1982; p. 95–101.
- 103.Ranque P. Etude morphologique et biologique de quelques Trypanosomides recoltes au senegal. These sciences Marseille, 1973; 378, pp.
- 104.Killick-Kendrick R, Lainson R, Rioux JA, Saf'janova VM. The taxonomy of Leishmania-like parasites of reptiles In: Rioux JA. Leishmania: Taxonomie et Phylogenèse. Application Éco-epidemiologiques (Colloque International du CNRS/INSERM, 1984), 1986; IMEE, Montpellier. [Google Scholar]
- 105.Moreno G, Rioux JA, Lanotte G, Pratlong F, Serres E. Le complexe Leishmania donovani s.l. Analyse enzymatique et traitement nume´rique. Individualisation du complexe Leishmania infantum In: Rioux JA, editor. Leishmania: Taxonomie et Phylogenese. Application Éco-epidemiologiques (Colloque International du CNRS/INSERM, 1984), 1986; IMEE, Montpellier: pp. 105–117. [Google Scholar]
- 106.Thomaz-Soccol V, Lanotte G, Rioux JA, Pratlong F, Martini-Dumas A, Serres E. Monophyletic origin of the genus Leishmania Ross, 1903. Ann Parasitol Hum Comp. 1993; 68: 107–108. [PubMed] [Google Scholar]
- 107.Cupolillo E, Grimaldi G Jr, Momen H. A general classification of New World Leishmania using numerical zymotaxonomy. Am J Trop Med Hyg. 1994; 50: 250–311. [DOI] [PubMed] [Google Scholar]
- 108.Lanotte G, Rioux JA, Maazoun R, Pasteur N, Pratlong F, Lepart J. The application of a numerical method to the taxonomy of the genus Leishmania Ross, 1903. The recognition of 146 original lines in the Old World. Use of allozymic characters. Epidemiological and phyletic significance. Ann Parasitol Hum Comp. 1981; 56: 575–91. [PubMed] [Google Scholar]
- 109.Le Blanq SM, Belehu A, Peters W. Leishmania in the Old world: 3 the distribution of L. aethiopica zymodemes. Trans R Soc Trop Med Hyg. 1986; 80: 360–366. [DOI] [PubMed] [Google Scholar]
- 110.Rioux JA. Taxonomy of Leishmania. Use of isoenzymes. Suggestions for a new classification. Ann Parasitol Hum Comp. 1990; 65: 11–125. [DOI] [PubMed] [Google Scholar]
- 111.Hennig W. Phylogenetic systematics. Ann Rev Entomol. 1965; 10: 97–116. [Google Scholar]
- 112.Rioux JA, Lanotte G. Apport de la cladistique à l’analyse du genre Leishmania Ross 1903 (Kinetoplastida: Trypanosomatidae). Corollaires epidemiologiques. Biosyst. 1993; 8: 79–80. [Google Scholar]
- 113.Dedet JP. Current status of epidemiology of leishmaniases In: Farrell JP editor. Leishmania series: World class parasites, Vol. 4 London: Kluwer academic press; 2002. p. 1–10. [Google Scholar]
- 114.Cupolillo E, Medina-Acosta E, Noyes H, Momen H, Grimaldi G Jr. A revised classification for Leishmania and Endotrypanum. Parasitol Today. 2000; 16: 142–144. [DOI] [PubMed] [Google Scholar]
- 115.Pothirat T, Tantiworawit A, Chaiwarith R, Jariyapan N, Wannasan A, et al. First Isolation of Leishmania from Northern Thailand: Case Report, Identification as Leishmania martiniquensis and Phylogenetic Position within the Leishmania enriettii Complex. PLoS Negl Trop Dis. 2014; 8: e3339 10.1371/journal.pntd.0003339 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Kwakye-Nuako G, Mosore MT, Duplessis C, Bates MD, Puplampu N, Mensah-Attipoe I, Desewu K, Afegbe G, Asmah RH, Jamjoom MB, Ayeh-Kumi PF, Boakye DA, Bates PA. First isolation of a new species of Leishmania responsible for human cutaneous leishmaniasis in Ghana and classification in the Leishmania enriettii complex. Int J Par. 2015; 45: 679–684. [DOI] [PubMed] [Google Scholar]
- 117.Asato Y, Oshiro M, Myint CK, Yamamoto Y, Kato H, Marco JD, et al. Phylogenic analysis of the genus Leishmania by cytochrome b gene sequencing. Exp Parasitol. 2009; 121: 352–361. 10.1016/j.exppara.2008.12.013 [DOI] [PubMed] [Google Scholar]
- 118.Schönian G, El Fari M, Lewin S, Schweynoch C, Presber W. Molecular epidemiology and population genetics in Leishmania. Med Microb Immunol. 2001; 190: 61–63. [DOI] [PubMed] [Google Scholar]
- 119.Schwenkenbecher JM, Wirth T, Schnur LF, Jaffe CL, Schallig H, Al-Jawabreh A, et al. Microsatellite analysis reveals genetic structure of Leishmania tropica. Int J Parasitol. 2006; 36: 237–246. [DOI] [PubMed] [Google Scholar]
- 120.Jamjoom MB, Ashford RW, Bates PA, Chance ML, Kemp SJ, Watts PC, et al. Leishmania donovani is the only cause of visceral leishmaniasis in East Africa; previous descriptions of L. infantum and "L. archibaldi" from this region are a consequence of convergent evolution in the isoenzyme data. Parasitol. 2004; 129: 399–409. [DOI] [PubMed] [Google Scholar]
- 121.Kuhls K, Keilonat L, Ochsenreither S, Schaar M, Schweynoch C, Presber W, et al. Multilocus microsatellite typing (MLMT) reveals genetically isolated populations between and within the main endemic regions of visceral leishmaniasis. Microbes Infect. 2007; 9: 334–343. [DOI] [PubMed] [Google Scholar]
- 122.Kuhls K, Mauricio IL, Pratlong F, Presber W, Schonian G. Analysis of ribosomal DNA internal transcribed spacer sequences of the Leishmania donovani complex. Microbes Infect. 2005; 7: 1224–1234. [DOI] [PubMed] [Google Scholar]
- 123.Kuhls K, Alam MZ, Cupolillo E, Ferreira GEMF, Mauricio IL, Oddone R, et al. Comparative microsatellite typing of New World Leishmania infantum reveals low heterogeneity among populations and its recent Old World origin. PLoS Negl Trop Dis.2011; 5: e1155 10.1371/journal.pntd.0001155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Leblois R, Kuhls K, François O, Schönian G, Wirth T. Guns, germs and dogs: on the origin of Leishmania chagasi. Infect Genet Evol. 2011; 11: 1091–1095. 10.1016/j.meegid.2011.04.004 [DOI] [PubMed] [Google Scholar]
- 125.Fraga J, Montalvo AM, De Doncker S, Dujardin JC, Van der Auwera G. Phylogeny of Leishmania species based on the heat-shock protein 70 gene. Infect Genet Evol. 2010; 10: 238–245. 10.1016/j.meegid.2009.11.007 [DOI] [PubMed] [Google Scholar]
- 126.Schönian G, Mauricio I, Cupolillo E. Is it time to revise the nomenclature of Leishmania? Trends Parasitol. 2010; 26: 466–9. 10.1016/j.pt.2010.06.013 [DOI] [PubMed] [Google Scholar]
- 127.Schönian G, Cupolillo E, Mauricio I. Molecular evolution and phylogeny of Leishmania In: Ponte-Sucre A, Diaz E, Padrón-Nieves M, editors. Drug Resistance in Leishmania Parasites: Consequences, Molecular Mechanisms and Possible Treatments. Springer; Wien Heidelberg New York, Dordrecht London; 2013. p.15–44. [Google Scholar]
- 128.Telford SR Jr. The kinetoplastid hemoflagellates of reptiles, p. 161–223. In: Kreier JP, Baker JR, editors. Parasitic Protozoa, 2nd ed., 1995; Vol. 10, Academic Press, San Diego. [Google Scholar]
- 129.Saf'janova WM. The problems of classification and phylogeny of the Leishmania In: Rioux JA., editor. Leishmania: Taxonomie et Phylogenese. Applications Eco-epidemiologiques 1986; pp. 247–255. Montpellier: 1MEEE. [Google Scholar]
- 130.Lukeš J, Skalický T, Týč J, Votýpka J, Yurchenko V. Evolution of parasitism in kinetoplastid flagellates. Mol Biochem Parasitol. 2014; 195: 115–122. 10.1016/j.molbiopara.2014.05.007 [DOI] [PubMed] [Google Scholar]
- 131.Franco AMR, Machado GMC, Moreira CFS, Grimaldi G. Minicircle kDNA Microheterogeneity in Endotrypanum Indicate Diversity within this Genus. Mem Inst Oswaldo Cruz. 2000; 95: 189–191. [DOI] [PubMed] [Google Scholar]
- 132.Dooijes D, Chaves I, Kieft R, Dirks-Mulder A, Martin W, Borst P. Base J originally found in kinetoplastida is also a minor constituent of nuclear DNA of Euglena gracilis. Nucleic Acids Res. 2000; 28: 3017–3021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Roger A, Hug L. The origin and diversification of eukaryotes: problems with molecular phylogenetics and molecular clock estimation. Phil Trans R Soc A. 2006; 361: 1039–1054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Poinar GO Jr, Poinar R. Fossil evidence of insect pathogens. J Invertebr Pathol. 2004; 89: 243–250. [DOI] [PubMed] [Google Scholar]
- 135.Cavalier-Smith T. Rooting the tree of life by transition analyses. Biol Direct. 2006; 1: 19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Kerr SF. Molecular trees of trypanosomes incongruent with fossil records of hosts. Mem Inst Oswaldo Cruz. 2006; 101: 25–30. [DOI] [PubMed] [Google Scholar]
- 137.Azar D, Nel A. Fossil Psychodoid flies and their relation to parasitic diseases. Mem Inst Oswaldo Cruz. 2003; 97: 35–37. [DOI] [PubMed] [Google Scholar]
- 138.Gullan PJ, Cranston PS. The Insects: An Outline of Entomology, 2nd Edition Wiley-Blackwell, London, 2000; 470 pp. [Google Scholar]
- 139.Momen H, Cupolillo E. Speculations on the Origin and Evolution of the Genus Leishmania. Mem Inst Oswaldo Cruz. 2000; 95: 583 [DOI] [PubMed] [Google Scholar]
- 140.Fernandes AP, Nelson K, Beverley SM. Evolution of nuclear ribosomal RNAs in kinetoplastid protozoa—perspectives on the age and origins of parasitism. PNAS. 1993; 90: 11608–11612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Vrba ES. Mammals as a key to evolutionary theory. J Mammal. 1992; 73: 1–28. [Google Scholar]
- 142.Lysenko AJ. Distribution of leishmaniasis in the Old World. Bull World Health Organization. 1971; 44, 515–520. [PMC free article] [PubMed] [Google Scholar]
- 143.Nowak RM. Walker’s Mammals of the World, 5th ed., Vol. II The Johns Hopkins University Press, Baltimore and London, 1991; p. 643–1629. [Google Scholar]
- 144.Kerr SF. Palaearctic Origin of Leishmania. Mem Inst Oswaldo Cruz. 2000; 95: 75 [DOI] [PubMed] [Google Scholar]
- 145.Kerr SF, Merkelz R, MacKinnon C. Further support for a palaearctic origin of Leishmania. Mem Inst Oswaldo Cruz. 2000; 95: 579–581. [DOI] [PubMed] [Google Scholar]
- 146.Lukeš J, Mauricio IL, Schönian G, Dujardin JC, Soteriadou K, Dedet JP, et al. Evolutionary and geographical history of the Leishmania donovani complex with a revision of current taxonomy. PNAS. 2007; 104: 9375–9380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Killick-Kendrick R. Some epidemiological consequences of the evolutionary fit between Leishmaniae and their phlebotomine vectors. Bull Soc Pathol Exot. 1985; 78: 747–755. [PubMed] [Google Scholar]
- 148.Mauricio IL, Stothard JR, Miles MA. The strange case of Leishmania chagasi. Parasitol Today. 2000; 16: 188–189. [DOI] [PubMed] [Google Scholar]
- 149.Noyes H. Implications of a Neotropical origin of the genus Leishmania. Mem Inst Oswaldo Cruz. 1998; 93: 657–661. [DOI] [PubMed] [Google Scholar]
- 150.Croan DG, Morrison DA, Ellis JT. Evolution of the genus Leishmania revealed by comparison of DNA and RNA polymerase gene sequences. Mol Biochem Parasitol. 1997; 89: 149–159. [DOI] [PubMed] [Google Scholar]
- 151.Wirth DF, McMahon-Pratt D. Rapid identification of Leishmania species by specific hybridization of kinetoplast DNA in cutaneous lesions. PNAS. 1982; 79: 6999–7003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Sang DK, Njeru WK, Ashford RW. A zoonotic focus of cutaneous leishmaniasis due to Leishmania tropica at Utut, Fift Valley Province, Kenya. Trans Roy Soci Trop Med Hyg. 1994; 88: 35–37. [DOI] [PubMed] [Google Scholar]
- 153.Ready PD, Pesson B. Hybridization, introgression and distribution of vectorial traits. International Symposium on Phlebotomine Sandflies III, 1999; Montpellier, France.
- 154.Kamhawi S, Ramalho-Ortigao M, Pham VM, Kumar S, Lawyer PG, Turco SJ, Barillas-Mury C, Sacks DL, Valenzuela JG. A role for insect galectins in parasite survival. Cell. 2004; 119: 329–41. [DOI] [PubMed] [Google Scholar]
- 155.Dostálová A, Volf P. Leishmania development in sandflies: parasite-vector interactions overview. Parasite Vector. 2012; 5: 276–288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Volf P, Benkova I, Myskova J, Sadlova J, Campino L, Ravel C. Increased transmission potential of Leishmania major/Leishmania infantum hybrids. Int J Parasitol. 2007; 37: 589–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Akhoundi M, Parvizi P, Baghaei A, Depaquit J. The subgenus Adlerius Nitzulescu (Diptera, Psychodidae, Phlebotomus) in Iran. Acta Trop. 2011; 122: 7–15. 10.1016/j.actatropica.2011.10.012 [DOI] [PubMed] [Google Scholar]
- 158.Strazzulla A, Cocuzza S, Pinzone MR, Postorino MC, Cosentino S, Serra A, et al. Mucosal Leishmaniasis: An Underestimated Presentation of a Neglected Disease. Bio Med Res Int. 2013; 10: 1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Alborzi A, Rasouli M, Shamsizadeh A. Leishmania tropica-isolated patient with visceral leishmaniasis in southern Iran. Am J Trop Med Hyg. 2006; 74: 306–307. [PubMed] [Google Scholar]
- 160.Gállego M, Pratlong F, Fisa R, Riera C, Rioux JA, Dedet JP, et al. The life-cycle of Leishmania infantum MON-77 in the Priorat (Catalonia, Spain) involves humans, dogs and sandflies; also literature review of distribution and hosts of L. infantum zymodemes in the old world. Trans R Soc Trop Med Hyg. 2001; 95: 269–271. [DOI] [PubMed] [Google Scholar]
- 161.Ready PD. Epidemiology of visceral leishmaniasis. Clinic Epidemiol. 2014; 6: 147–154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Ready PD, Fraiha H, Lainson R, Shaw J. Psychodopygus as a genus: reasons for a flexible classification of the Phlebotomine sandflies (Diptera: Psychodidae). J Med Entomol. 1980; 17: 75–88. [Google Scholar]
- 163.Quate LW, Fairchild GB. Phlebotomus sandflies of Malaya and Borneo. Pac Insects. 1961; 3: 203–222. [Google Scholar]
- 164.Abonnenc E. Les phlebotomes de la region ethiopienne (Diptera: Psychodidae). Mémoire de l'ORSTOM. 1972; No.55.
- 165.Abonnenc E, Minter DM. Bilingual Keys for the identification of the sandflies of the Ethiopian region. Cahier ORSTOM, série Entomologie Médicale et Parasitologie. 1965; 5: 24–63. [Google Scholar]
- 166.Artemiev MM, Neronov V. Distribution and ecology of sandflies of the world (genus Phlebotomus). Institute of Evolution, Morphology and Animal Ecology, Moscow, 1984; pp 1–208. [Google Scholar]
- 167.Van der Linde K, Houle D, Spicer GS, Steppan SJ. A supermatrix-based molecular phylogeny of the family Drosophilidae. Gen Res. 2010; 92: 25–38. [DOI] [PubMed] [Google Scholar]
- 168.Perrotey S, Mahamdallie SS, Pesson B, Richardson KJ, Gallego M, Ready PD. Postglacial dispersal of Phlebotomus perniciosus into France. Parasite. 2005; 12: 283–291. [DOI] [PubMed] [Google Scholar]
- 169.Philippe H. Molecular phylogeny of kinetoplastids In: Coombs GH, Vickerman K, Sleigh MA, Warren A, editors. Evolutionary Relationships among Protozoa. Kluwer Academic Publishers, Dordrecht/Boston/London: 1998; p. 195–212. [Google Scholar]
- 170.Schenk JJ, Rowe KC, Steppan SJ. Ecological Opportunity and Incumbency in the Diversification of Repeated Continental Colonizations by Muroid Rodents. Syst biol. 2013; 62: 837–864. 10.1093/sysbio/syt050 [DOI] [PubMed] [Google Scholar]
- 171.Steppan, S. Passways of continental Muroid immigration, Map: Scott Steppan, Florida State University, Source: Florida State University (personal document), 2013; http://museumvictoria.com.au/about/media-centre/news/january-2014/story3/.
- 172.Peters W, Pasvol G. Atlas of Tropical Medicine and Parasitology, sixième Edition. 2007. Elsevier Mosby. [Google Scholar]
- 173.Añez N, Nieves E, Scorza JV. The taxonomic status of Leishmania garnhami, indicated by its pattern of development in the vector. Mem Inst Oswaldo Cruz. 1985; 80: 113–119. [DOI] [PubMed] [Google Scholar]
- 174.Marsella R, Ruiz de Gopegui R. Leishmaniasis: a re-emerging zoonosis. Int J Dermatol. 1998; 37: 801–814. [DOI] [PubMed] [Google Scholar]
- 175.Hamarsheh O. Distribution of Leishmania major zymodemes in relation to populations of Phlebotomus papatasi sandflies. Parasites Vectors. 2011; 4: 9 10.1186/1756-3305-4-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Saenz RE, Paz HM, Johnson CM, Marr JJ, Nelson DJ, Pattishall KH, et al. Treatment of American cutaneous leishmaniasis with orally administered allopurinol riboside. J Infect Dis. 1989; 160: 153–158. [DOI] [PubMed] [Google Scholar]
- 177.Guerbouj S, Guizani I, De Doncker S, Dujardin JC, Nuwayri-Salti N. Identification of Lebanese dermotropic putative Leishmania archibaldi isolates by gp63 PCR-RFLP. Trans R Soc Trop Med Hyg. 2001; 95: 687–688. [DOI] [PubMed] [Google Scholar]
- 178.Ardehali S, Moattari A, Hatam GR, Hosseini SM, Sharifi I. Characterization of Leishmania isolated in Iran: Serotyping with species specific monoclonal antibodies. Acta Trop. 2000; 75: 301–307. [DOI] [PubMed] [Google Scholar]
- 179.Falqueto A, Cupolillo E, Machado GM, de Carvalho-Paes LE, Grimaldi G Júnior. A new enzymatic variant of Leishmania (Leishmania) forattinii isolated from Proechimys iheringi (Rodentia, Echimydae) in Espírito Santo, Brazil. Mem Inst Oswaldo Cruz. 1998; 93: 795–798. [DOI] [PubMed] [Google Scholar]
- 180.De Pita-Pereira D, Cardoso MAB, Alves CR, Brazil RP, Britto C. Detection of natural infection in Lutzomyia cruzi and Lutzomyia forattinii (Diptera: Psychodidae: Phlebotominae) by Leishmania infantum chagasi in an endemic area of visceral leishmaniasis in Brazil using a PCR multiplex assay. Acta Trop. 2008; 107: 66–69. 10.1016/j.actatropica.2008.04.015 [DOI] [PubMed] [Google Scholar]
- 181.Lainson R, Ward RD, Shaw JJ. Experimental transmission of Leishmania chagasi, causative agent of neotropical visceral leishmaniasis, by the sandfly Lutzomyia longipalpis. Nature. 1977; 266: 628–630. [DOI] [PubMed] [Google Scholar]
- 182.Rassi Y, Abai MR, Oshaghi MA, Javadian E, Sanei A, Rafidzadeh S, Arzamani K. First detection of Leishmania infantum in Phlebotomus kandelakii using molecular methods in north-eastern Islamic Republic of Iran. East Mediterr Health J. 2012; 18: 387.–. [DOI] [PubMed] [Google Scholar]
- 183.Akhoundi M, Baghaei A, Depaquit J, Parvizi P. Molecular Characterization of Leishmania Infection from Naturally Infected Sandflies Caught in a Focus of Cutaneous Leishmaniasis (Eastern Iran). J Arthropod Borne Dis. 2013; 7: 122–131. [PMC free article] [PubMed] [Google Scholar]
- 184.Kassiri H, Javadian E, Sharififard M. Monthly activity of Phlebotominae sandflies in Sistan-Baluchistan Province, Southeast Iran. J Insect Sci. 2013; 13: 153 10.1673/031.013.15301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Fairchild GB, Theodor O. On Lutzomyia flaviscutellata (Mangabeira) and L. Olmeca (Vargas and Diaz-Najera) (Diptera: Psychodidae). J Med Entomol. 1971; 8: 153–159. [DOI] [PubMed] [Google Scholar]
- 186.Feliciangeli MD, Rodriguez N, Bravo A, Arias F, Guzman B. Vectors of cutaneous leishmaniasis in north-central Venezuela. Med Vet Entomol J. 1994; 8: 317–324. [DOI] [PubMed] [Google Scholar]
- 187.Gómez EA, Hashiguchi Y. Monthly variation in natural infection of the sandfly Lutzomyia ayacuchensis with Leishmania mexicana in an endemic focus in the Ecuadorian Andes. Ann Trop Med Parasitol. 1991; 85: 407–411. [DOI] [PubMed] [Google Scholar]
- 188.Lawyer PG, Young DG, Butler JF, Akin DE. Development of Leishmania mexicana in Lutzomyia diabolica and Lutzomyia shannoni (Diptera: Psychodidae). J Med Entomol. 1987; 24: 347–355. [DOI] [PubMed] [Google Scholar]
- 189.Saldaña A, Chaves LF, Rigg CA, Wald C, Smucker JE, Calzada JE. Clinical cutaneous leishmaniasis rates are associated with household Lutzomyia gomezi, Lu. panamensis, and Lu. trapidoi abundance in Trinidad de Las Minas, western Panama. Am J Trop Med Hyg. 2013; 88: 572–574. 10.4269/ajtmh.12-0579 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Akhoundi M, Hajjaran H, Baghaei A, Mohebali M. Geographical Distribution of Leishmania Species of Human Cutaneous Leishmaniasis in Fars Province, Southern Iran. Iran J Parasitol. 2013; 8: 85–91. [PMC free article] [PubMed] [Google Scholar]
- 191.Svobodova M, Votypka J, Peckova J, Dvorak V, Nasseredin A, Baneth G, et al. Distinct transmission cycles of Leishmania tropica in 2 adjacent foci, Northern Israel. Emerg Infect Dis. 2006; 12: 1860.–. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Akhoundi M, Mohebali M, Asadi M, Mahmodi MR, Amraei K, Mirzaei A. Molecular characterization of Leishmania spp. in reservoir hosts in endemic foci of zoonotic cutaneous leishmaniasis in Iran. Folia Parasitol. 2013; 60: 218–224. [DOI] [PubMed] [Google Scholar]
- 193.Rassi Y, Oshaghi MA, Azani SM, Abaie MR, Rafizadeh S, Mohebai M, et al. Molecular detection of Leishmania infection due to Leishmania major and Leishmania turanica in the vectors and reservoir host in Iran. Vector Borne Zoonotic Dis. 2011; 11: 145–150. 10.1089/vbz.2009.0167 [DOI] [PubMed] [Google Scholar]
- 194.De Queiroz RG, Vasconcelos IA, Vasconcelos AW, Pessoa FA, de Sousa RN, David JR. Cutaneous leishmaniasis in Ceara state in northeastern Brazil: incrimination of Lutzomyia whitmani (Diptera: Psychodidae) as a vector of Leishmania braziliensis in baturite municipality. Am J Trop Med Hyg. 1994; 50: 693–698. [DOI] [PubMed] [Google Scholar]
- 195.De Souza A, Ishikawa E, Braga R, Silveira F, Lainson R, Shaw J. Psychodopygus complexus, a new vector of Leishmania braziliensis to humans in Pará State, Brazil. Trans R Soc Trop Med Hyg. 1996; 90, 112–113. [DOI] [PubMed] [Google Scholar]
- 196.Forattini OP, Pattoli DB, Rabello EX, Ferreira OA. Natural infection of Phlebotominae in an enzootic focus of cutaneous leishmaniasis in São Paulo State, Brazil. Rev Saude Publ. 1972; 6: 431–433. [DOI] [PubMed] [Google Scholar]
- 197.Le Pont F, Desjeux P. Leishmaniasis in Bolivia. II. The involvement of Psychodopygus yucumensis and Psychodopygus llanosmartinsi in the selvatic transmission cycle of Leishmania braziliensis braziliensis in a lowland subandean region. Mem Inst Oswaldo Cruz. 1986; 81: 311–318. [DOI] [PubMed] [Google Scholar]
- 198.Rangel EF, de Souza NA, Wermelinger ED, Barbosa AF. Natural infection of Lutzomyia intermedia Lutz & Neiva, 1912, in an endemic area of visceral leishmaniasis of Rio de Janeiro. Mem Inst Oswaldo Cruz. 1984; 79: 395–396. [DOI] [PubMed] [Google Scholar]
- 199.Fouque F, Gaborit P, Issaly J, Carinci R, Gantier JC, Ravel C, et al. Phlebotomine sandflies (Diptera: Psychodidae) associated with changing patterns in the transmission of the human cutaneous leishmaniasis in French Guiana. Mem. Inst. Oswaldo Cruz. 2007; 102: 35–40. [DOI] [PubMed] [Google Scholar]
- 200.Lainson R, Shaw JJ, Ready PD, Miles MA, Póvoa M. Leishmaniasis in Brazil: XVI. Isolation and identification of Leishmania species from sandflies, wild mammals and man in north Para State, with particular reference to L. braziliensis guyanensis causative agent of “pian-bois.” Trans R Soc Trop Med Hyg. 1981; 75: 530–536. [DOI] [PubMed] [Google Scholar]
- 201.Lainson R, Shaw JJ, Souza AA, Silveira FT, Falqueto A. Further observations on Lutzomyia ubiquitalis (Psychodidae: Phlebotominae), the sandfly vector of Leishmania (Viannia) lainsoni. Mem Inst Oswaldo Cruz.1992; 87: 437–439. [DOI] [PubMed] [Google Scholar]
- 202.Azpurua J, De La Cruz D, Valderama A, Windsor D. Lutzomyia sandfly diversity and rates of infection by Wolbachia and an exotic Leishmania species on Barro Colorado Island, Panama. PLoS Negl Trop Dis. 2010; 4: e627 10.1371/journal.pntd.0000627 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Christensen HA, Fairchild GB, Herrer A, Johnson CM, Young DG, de Vásquez AM. The ecology of cutaneous leishmaniasis in the Republic of Panama. J Med Entomol. 1983; 20: 463–484. [DOI] [PubMed] [Google Scholar]
- 204.Christensen HA, Herrer A. Attractiveness of sentinel animals to vectors of leishmaniasis in Panama. Am J Trop Med Hyg. 1973; 22: 578–584. [DOI] [PubMed] [Google Scholar]
- 205.Morales A, Corredor A, Cáceres E, Ibagos AL, Rodríguez CDe. Aislamiento de tres cepas de Leishmanía à partir de Lutzomyia trapidoi en Colombia. Bioméd. 1981; 1: 198. [Google Scholar]
- 206.Braga RR, Lainson R, Ishikawa EAY, Shaw JJ. Leishmania (Viannia) utingensis n. sp., a parasite from the sandfly Lutzomyia (Viannamyia) tuberculata in Amazonian Brazil. Parasite. 2003; 10: 111–118. [DOI] [PubMed] [Google Scholar]
- 207.Heisch RB. On Leishmania adleri sp. nov. from lacertid lizards (Latastia sp.) in Kenya. Ann Trop Med Parasitol. 1958; 52: 68–71. [DOI] [PubMed] [Google Scholar]
- 208.Maleki N, Javadian E, Mohebali M, Dalimiasl AAH, Sadraei J, Zarei ZA, et al. Natural infection of sandflies Sergentomyia dentata in ardebil to lizard Leishmania. Modares J Med Sciences (pathobiol). 2007; 10: 65–73. [Google Scholar]
- 209.Garnham PC. The genus Leishmania. Bull World Health Organ. 1971; 44: 477–489. [PMC free article] [PubMed] [Google Scholar]
- 210.Telford SR. Evolutionary implications of Leishmania amastigotes in circulating blood cells of lizards. Parasitol. 1979; 79: 317–324. [DOI] [PubMed] [Google Scholar]
- 211.Wilson VCLC, Southgate BA. Lizard Leishmania In: Lumsden W.H.R., Evans D.A., editors. Biology of the Kinetoplastida, Vol. 2 Academic Press; London: 1979. pp. 241–268. [Google Scholar]
- 212.Motazedian H, Noyes H, Maingon R. Leishmania and Sauroleishmania: the use of random amplified polymorphic DNA for the identification of parasites from vertebrates and invertebrates. Exp Parasitol. 1996; 83: 150–154. [DOI] [PubMed] [Google Scholar]
- 213.Ovezmukhammedov A, Saf'janova VM. Taxonomic problems of the Leishmania of reptiles. Parazitol. 1989; 23: 334–43. [PubMed] [Google Scholar]
- 214.Hodukin NI, Sofiev MS. Tr. Uzbek. Inst. Eksp. Med. 1940; 5: 185–217. [Google Scholar]
- 215.Desjeux P, Waroqw L. Mise en evidence du cycle évolutif de la leishmaniose du Gecko Tareentola annulans (Geoffroy Saint-Hilaire, 1823) au Sénégal. Rôle Vecteur de Sergentomyia dubia (Parrot, Mornet & Cadenat, 1945). Afrique Médicale; 1981: 19, 439–442. [Google Scholar]
- 216.McMillan B. Leishmaniasis in the Sudan Republic. 22. Leishmania hoogstraali p. n. in the gecko. J Parasitol. 1965; 51: 336–339. [PubMed] [Google Scholar]
- 217.Kazemi B, Tahvildar-Bideroni Gh, Hashemi Feshareki SR, Javadian E. Isolation a Lizard Leishmania promastigote from its natural host in Iran. Int J Biol Sci. 2004; 4: 620–623. [Google Scholar]
- 218.Nadim A, Seyedi-Rashti MA, Mesghali A. Epidemiology of cutaneous leishmaniasis in Turkeman Sahara, Iran. J Trop Med Hyg. 1968; 71: 238–9. [PubMed] [Google Scholar]
- 219.Adler S, Theodor O. The distribution of sandflies and leishmaniasis in Palestine, Syria and Mesopotamia. Ann Trop Med Parasit. 1929; 23: 269–306. [Google Scholar]
- 220.Elwasila M. Leishmania tarentolae Wenyon, 1921 from the gecko Tarentola annularis in the Sudan. Parasitol Res. 1988; 74: 591–592. [DOI] [PubMed] [Google Scholar]
- 221.Parrot C. Nouvelles recherches sur I’evolution de Leishmania tarentolae chez Phlebotomus minutus Rondani. Bull Soc Pathol Exot. 1935; 28: 958–960. [Google Scholar]
- 222.Andrushko AM, Markov GS. The new finding of Leishmania in reptiles of Central Asia. Vestnik Leningrad University, 1995; 1: 55–59. [Google Scholar]
- 223.Paperna I, Boulard Y, Hering–Hagenback SH, Landau I. Description and ultrastructure of Leishmania zuckermani n. sp. amastigotes dandected within the erythrocytes of the South African gecko Pachydactylus turneri Gray, 1864. Parasite. 2001; 8: 349–353. [DOI] [PubMed] [Google Scholar]
- 224.Kreutzer RD, Corredor A, Grimaldi G, Grogl M, Rowton ED, Young D G, et al. Characterization of Leishmania colombiensis sp. n (Kinetoplastida: Trypanosomatidae), a new parasite infecting humans, animals, and Phlebotomine sandflies in Colombia and Panama. Am J Trop Med Hyg. 1991; 44: 662–675. [DOI] [PubMed] [Google Scholar]
- 225.Miles MA, Póvoa MM, de Souza AA, Lainson R, Shaw JJ. Some methods for the enzymic characterization of Latin-American Leishmania with particular reference to Leishmania mexicana amazonensis and subspecies of Leishmania hertigi. Trans R Soc Trop Med Hyg. 1980; 74: 243–252. [DOI] [PubMed] [Google Scholar]
- 226.Furuya M, Motonari S, Akimaru Y, Mimori T, Gomez EA, Hashiguchi Y. Natural infection of Lutzomyia hartmanni with Leishmania (V.) equatorensis in Ecuador. Parasitol Int. 1998; 47: 121–126. [Google Scholar]
- 227.Herrer A. Leishmania hertigi sp. n., from the tropical porcupine, Coendou rothschildi Thomas. J Parasitol. 1971; 57: 626–629. [PubMed] [Google Scholar]
- 228.Rose K. Cutaneous leishmaniasis in red kangaroos. Aust Vet J. 2004; 82: 440 [DOI] [PubMed] [Google Scholar]
- 229.Marcondes CB, Santos-Neto LG., Lozovei AL. Ecology of phlebotomine sandflies (Diptera, Psychodidae) in Brazilian Atlantic Forest. Rev Soc Bras Med Trop. 2001; 34: 255–260. [DOI] [PubMed] [Google Scholar]
- 230.Desbois N, Pratlong F, Quist D, Dedet J. Leishmania (Leishmania) martiniquensis n. sp. (Kinetoplastida: Trypanosomatidae), description of the parasite responsible for cutaneous leishmaniasis in Martinique Island (French West Indies). Parasite. 2014; 21: 12 10.1051/parasite/2014011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Pothirat T, Tantiworawit A, Chaiwarith R, Jariyapan N, Wannasan A, Siriyasatien P, et al. First Isolation of Leishmania from Northern Thailand: Case Report, Identification as Leishmania martiniquensis and Phylogenetic Position within the Leishmania enriettii Complex. PLoS Negl Trop Dis.2014; 8: e3339 10.1371/journal.pntd.0003339 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Bualert L, Charungkiattikul W, Thongsuksai P, Mungthin M, Siripattanapipong S, Khositnithikul R, et al. Autochthonous disseminated dermal and visceral leishmaniasis in an AIDS patient, southern Thailand, caused by Leishmania siamensis. Am J Trop Med Hyg. 2012; 86: 821–824. 10.4269/ajtmh.2012.11-0707 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Kanjanopas K, Siripattanapipong S, Ninsaeng U, Hitakarun A, Jitkaew S, Kaewtaphaya P, et al. Sergentomyia (Neophlebotomus) gemmea, a potential vector of Leishmania siamensis in southern Thailand. BMC Infect Dis. 2013; 13, 333 10.1186/1471-2334-13-333 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Sukmee T, Siripattanapipong S, Mungthin M, Worapong J, Rangsin R, Samung Y, et al. A suspected new species of Leishmania, the causative agent of visceral leishmaniasis in a Thai patient. Int J Parasitol. 2008; 38: 617–22. 10.1016/j.ijpara.2007.12.003 [DOI] [PubMed] [Google Scholar]