Abstract
Psoriasis vulgaris is a chronic, common skin disease, which affects the patient’s quality of life to the highest degree. Several exogenous factors and endogenous hormonal changes may act as triggers for psoriasis.
The skin possesses a true endocrine system, which is very important in multiple systemic diseases. A number of conditions are associated with psoriasis, and its severity can also be influenced by hormones. Even though the sex hormones and prolactin have a major role in psoriasis pathogenicity, there are a lot of other hormones which can influence the psoriasis clinical manifestations: glucocorticoids, epinephrine, thyroid hormones, and insulin.
Keywords: psoriasis vulgaris, sex hormones, prolactin, glucocorticoids
Introduction
Psoriasis vulgaris, an immunologically mediated skin disease, is a common disorder, having as main pathogenetic mechanisms the chronic inflammation and keratinocytes hyperproliferation [1]. The keratinocytes differentiation is altered and the expression of genes in the psoriasis plaque may also be affected [2]. The antimicrobial peptides and proteins production during the innate and adaptive immune response reflect the genetic susceptibility to psoriasis [3].
Psoriasis vulgaris is not a life threatening disease, but it affects severly the quality of life; there is still no causative treatment [4].
Due to the psoriasis chronicity and the absence of a curative treatment [5], the disease prevalence remains a question [6]: it was estimated to more than 3% in the United States and Canada [7] and between 0.6 to 6.5% in Europe [8].
The onset of psoriasis vulgaris may be at any age, but two peaks were observed, around 20–30 and over 50 years of age [9]; pediatric psoriasis can reach about 30% of all cases [10]. The early onset of chronic plaque psoriasis in white population was associated with 36 genetic loci [11], a finding which supports the assumption that the age of onset is, at least partially, genetically determined [12].
Triggering factors of psoriasis
Psoriasis can be precipitated by multiple factors, exogenous or endogenous.
Among the exogenous triggers there are physical factors (friction, injury, injection site, surgical scar, pressure points, scalding, burning, ultraviolet or X radiation), seasonal variations [13], chemical factors (cauterization, chronic alkaline damage, toxic agents) [13,14]. Other exogenous precipitating factors can be alcohol consumption [15,16], smoking [5], drugs (gold salts [17], lithium, antihypertensives – beta-blockers, antimalarials, antifungals, which have all a strong causal relationship and can trigger or worsen the psoriatic clinical phenomena [18,19,20]; even diet can precipitate symptoms [5].
Infections with one of the following agents can trigger or exacerbate psoriasis: Streptococcus Beta Haemolyticus [21], Streptococcus pyogenes [22], Hepatitis C virus [23,24], Varicella zoster virus [25], herpes simplex virus [26], Human immunodeficiency virus [27], Candida albicans [28], upper respiratory pathogens [29].
Other risk factors for psoriasis onset are skin injuries, in the context of Köbner phenomenon [30,31].
The endogenous factors are also triggers for psoriasis. Here can be mentioned the allergies [32,33] and hormonal changes [5], but the most common trigger for several inflammatory skin diseases, including psoriasis, is the emotional stress [34,35].
Skin: hormones target and synthesis organ
The skin, the central nervous system and the endocrine system have a common embryological origin and they all express the same, numerous mediators [36]. For example, human skin produces, activates or inactivates neuropeptides like serotonin [37]; some opioid peptides and their receptors are also expressed in the skin [38].
The normal skin development and function is influenced by hormones: for example, the growth hormone stimulates keratinocyte proliferation [39], and thyroid hormones act directly on hair follicles [40]. The skin is a neuroendocrine organ, capable of hormone synthesis and release [41]: corticosteroids and sex hormones are synthesized and transformed [42] (with sebocytes playing a central role in cutaneous androgen metabolism [43]); catecholamines are synthesized by keratinocytes and melanocytes [36].
Dermal fibroblasts present strong circadian rhythm [44] and melatonin is implicated in the regulation of hair growth cycle [45] and is metabolized [46]. Prolactin is also implicated in hair growth regulation, and scalp skin and hair follicles are sources of prolactin [47].
The cutaneous endocrine system is highly important in multiple systemic diseases [48].
Conditions associated with psoriasis
A large spectrum of diseases with hormonal involvement are associated with psoriasis: depression [49], arterial hypertension [50] and other cardiovascular disorders: atherothrombotic disease or transient ischemic attack [51], nonalcoholic steatohepatitis [52], chronic inflammatory bowel disease [53] and Crohn’s disease [54], dyslipidemia, obesity, metabolic syndrome [55], insulin resistance [56] and diabetes mellitus, respiratory diseases, including asthma [57], chronic kidney disease [58], uveitis [54], malignant lymphoma [55]. The treatment of those associated diseases might aggravate psoriasis [57].
The role of hormones in psoriasis
The hormones also have an important influence on the severity of psoriasis clinical manifestations. This fact is indicated by the disease frequency peaks during puberty [59] and menopause [60], besides the peaks around the age of 30 and 50 years [61]. Therefore, the hormonal variances, major changes and the hormonal diseases could represent risk, triggering or modulating factors in the evolution of psoriasis.
1. Sex hormones and psoriasis
Psoriasis is a chronic inflammatory disease, characterized mainly by the involvement of Th1 type of T lymphocytes [62], and also by neutrophils, dendritic cells, and mast cells, all major inflammatory cytokines producers: interferon gamma (IFN-γ), tumor necrosis factor alpha (TNF-α), and interleukins (IL-2, IL-12, IL-17 and IL-23) [63,64].
Estrogens influence the immune responses, modulating the development and activation of immune cells, through the influence and control exerted upon the expression of different cytokines [65].
In vitro and in vivo studies demonstrated the anti-inflammatory effects of estrogens: they decrease the neutrophil’s blood level and keratinocytes production of some macrophage-attracting cytokines [66], and they increase the production of IL-10 by B lymphocytes and dendritic cells [67]. Those estrogens effects may also decrease the psoriatic inflammation. Other effect of estrogens is the decreasing of matrix metalloproteinase activity in fibroblasts [66], which lowers the destruction of extracellular matrix and the release of growth factors, another pathogenic psoriatic link.
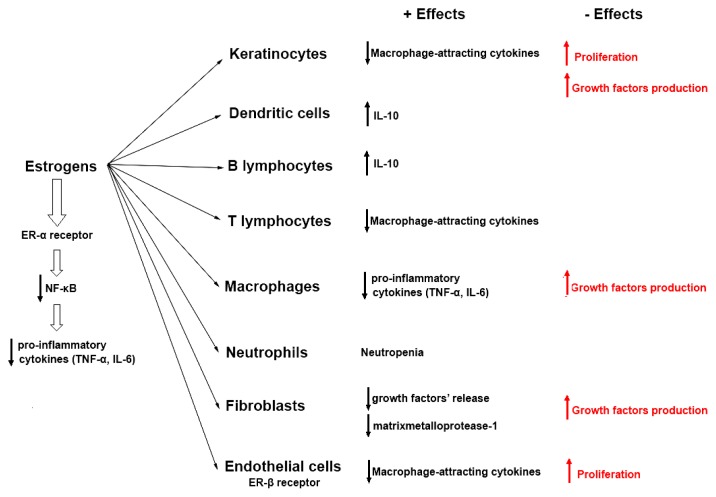
The effects of estrogen are differently mediated by estrogens receptor-α (ER-α) and β (ER-β) [68,69,70] (Figure 1). Among negative effects of estrogens in psoriasis, some should be mentioned: the suppression of apoptosis and the stimulation of proliferation at keratinocyte level, the stimulation of growth factors production in macrophages, keratinocytes and fibroblasts [66], which can stimulate the development of neovascularization, a pathogenic way in psoriasis [71].
Figure 1.
Positive and negative effects of estrogens, differently mediated by ER-α and ER-β [62–71].
At the same time, Progesterone, which is a commonly indicated drug, can precipitate some forms of psoriasis (pustular) [20].
1.1. Pregnancy
Extremely high levels of hormonal and immunological changes occur during pregnancy, as maternal adaptations to the developing fetus.
The evolution of psoriasis is variable during pregnancy [72]. At mid pregnancy (around 30th week of gestation), the patients’ psoriatic symptoms can diminish (in >50%) or worsening (in >20%) [73]. A possible explanation could be that at this moment, there is an immunity shift from Th2 to Th1, mainly due to the increased levels of estrogen, progesterone and cortisol [74].
Other chronic immune diseases Th1-driven were shown to improve during pregnancy, such as multiple sclerosis [75] and rheumatoid arthritis [76]. Therefore, it was supposed that the increased hormone levels improve the psoriatic symptoms [77].
Due to the decrease in hormonal levels after parturition and during menopause, the psoriasis severity seems to accentuate [63]. In these conditions, it was assumed that the flares occurring in the postpartum period represent rather a return to the initial level than a true worsening [73].
A moderate or severe form of psoriasis can increase the risk for a poor outcome in pregnancy [78], abortions, eclampsia, premature rupture of membranes, or macrosomia [79], but this can be due to the multiple comorbidities associated with psoriasis, which can represent risk factors in pregnancy.
Definitely, psoriasis does not represent a contraindication for a pregnancy [77]. If a systemic treatment is necessary, mothers are told not to nurse because the drugs may be excreted in milk [80].
1.2. Menopause
During menopause, the estrogen level decline and a low-grade inflammation may appear [81], meaning that menopause may aggravate the psoriasis evolution [82]. This fact is in concordance with the observation that after menopause the incidence of chronic inflammatory diseases in women became closer or higher than the incidence in males [83].
1.3. Androgens
The epidermis, dermis and hair follicle and the associated sebaceous glands express androgen receptors, and the skin is an important androgen target [84]. Androgen hormones influence the homeostasis of the epidermal barrier, the growth and differentiation of the hair and the sebaceous gland [85]. They also antagonize the macrophage’s production of vascular endothelial growth factor (VEGF), which can prolong the inflammation and the wound healing [69]. The adrenal androgens decrease in chronic inflammatory diseases [86] and the therapies based on androgen can aggravate/exacerbate psoriasis [87].
2. Stress hormones and exercise
Endocrine and immune reactions are both highly influenced by stress. At the same time, the evolution is marked by the important stressful moments in life [63]. The hypothalamic-pituitary-suprarenal axis controls the stress hormones, cortisol and epinephrine, which are antagonists and have important effects on immune system.
Immune cells (macrophages, lymphocytes T and B) express beta-adrenergic receptor [88] and epinephrine induces multiple but dual immune responses: promotes macrophages responses through increasing secretion of cytokines TNF-α, IL-1, IL-10 [89], and regulates the level of T and B lymphocyte function. Also, it has bee observed that an acute activation of the sympathetic nervous system attenuates the innate immune response [90].
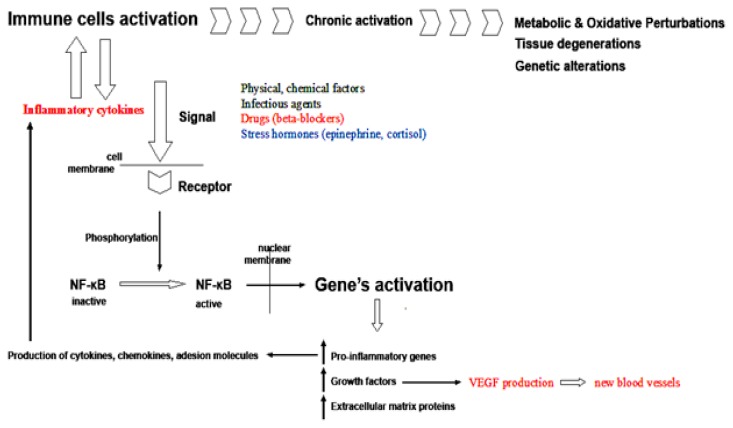
The immunomodulation in stress takes place through intersections in signaling cascade at different levels: for example, stress signals in immune response are regulated through the key Nuclear Factor-kappaB (NF-κB) and the epinephrine stimulation of β2-adrenergic receptors, expressed on immune cells, intersect with the NF-κB signaling cascade [91] (Figure 2).
Figure 2.
Loop of keratinocytes activation: stressing factors activate the immune cells, which produce cytokines that cause inflammation, which in turn activates more cytokine-producing cells, altering the keratinocytes proliferations and genetic expression. VEGF production is a major pathogenic factor in psoriasis.
Glucocorticoids prevent the inflammatory changes by inhibiting the migration of leukocytes and suppressing T cells [92]. The stimulation of epinephrine and adrenergic receptors may also exert some corticosteroid effects [93].
The cortisol response to stress is diminished in psoriasis [94]. The psoriatic patients present higher levels of epinephrine and adrenocorticotropic hormone and lower levels of cortisol and corticotrophin releasing factor [95]. Epinephrine can modulate the remission phase and cortisol the eruption phase [22].
The stress link in psoriasis is sustained also by the fact that a regular physical activity may have favorable effect on psoriasis evolution. Through metabolic and psycho-neuro-immune effects, a regular exercise influences positively the metabolic comorbidities, lowers the risk and the onset of psoriasis [53].
3. Prolactin
Prolactin (PRL) is the pituitary hormone of lactation and reproduction. The epidermal keratinocytes express receptors for prolactin [96] and the hormone has proliferative effects on keratinocytes [97], epithelial cells, and lymphocytes [98].
There is a true “PRL–skin connection” [99]. PRL exerts a variety of immunostimulative effects which may promote the development of psoriasis [100]: it enhances keratinocytes chemokine production (IL-17) and favors infiltration with Th lymphocytes [96,101], stimulates IFN-γ production and promotes angiogenesis [102], regulates the maturation of T cells [103]. According to those multiple immune roles, hyperprolactinemia was observed in several autoimmune diseases (lupus erythematosus, rheumatoid arthritis, Sjogren’s syndrome, Hashimoto’s thyroiditis, multiple sclerosis) [104].
PRL is involved in the psoriasis etiopathogenesis [105] and this role of prolactin is sustained by the observations that psoriasis can be aggravated during the development of prolactinoma [106]. A significant decrease in prolactin level in patients with psoriasis was noticed after systemic treatment [98]. Several studies have shown a positive relation between serum prolactin levels and psoriasis severity [107,108,109]. Bromocriptine, used in prolactinoma, may be useful in the psoriasis treatment [108].
4. Thyroid hormones
The thyroid hormones present receptors expressed in the skin and they are important factors that can stimulate skin proliferation [110], by increasing the level of epidermal growth factor [111].
Arguments for the aggravating effect of thyroid hormones in psoriasis are the following: the psoriasis can be intensified by an excessive production of thyroid hormones [112], the free thyroxine is increased significantly in the psoriatic patients [113], in severe psoriasis, there are increased levels of thyroid-stimulating hormone [111], and the patients with thyroiditis had longer disease periods [113].
Also, it has been shown that the antithyroid drugs have an anti-proliferative effect on psoriasis [112]. For example, propylthiouracil, exhibits antiproliferative and immunomodulatory effects and is beneficial in psoriasis [114]. There were reported cases of psoriasis resolution after thyroidectomy [115,116].
5. Leptin, ghrelin, insulin & obesity
The metabolic syndrome, including obesity and diabetes mellitus, are strongly associated with psoriasis [57].
Leptin is an adipose cell hormone with long-term action that decreases the appetite and suppresses food intake. Ghrelin is released primarily in the stomach and is a fast-acting hormone, increasing the appetite and playing a role in meal initiation [117].
Ghrelin can antagonize the insulin system [118] and there is a negative correlation between severity of psoriasis and ghrelin level [119].
Also, there is a relationship between leptin, obesity and psoriasis. But there are contradictive reports regarding the leptin levels in psoriasis, significantly decreased [120] or higher [121]. But, an argument for the leptin molecular link between psoriasis and metabolic comorbidities is represented by the higher serum levels of leptin in overweight or obese psoriatic patients [122].
Strongly associated with insulin resistance are both psoriasis [123] and obesity [124]. In both, there are also common pro-inflammatory pathways involved (TNF-α, IL-6). Reducing the obesity chronic low-level inflammation may improve insulin sensitivity and leptin levels [125].
Conclusions
Even though the sex hormones and prolactin are the most implicated in psoriasis pathogenesis and clinical manifestations, there are a lot of other hormonal mechanisms with significant influence on the evolution of psoriasis, therefore new therapeutic ways need to be explored.
At the same time, a hormonal assessment should be performed in patients with psoriasis, in order to correctly diagnose and treat pathologies that may be related with psoriasis exacerbations.
Due to the pathogenic complexity, there a curative treatment for psoriasis is still missing and hormonal therapeutic interventions may relieve the clinical phenomena in psoriasis.
Acknowledgments
Ackowledgement
The research was funded by POSDRU grant no. 159/1.5/S/138776 grant with title: “Model colaborativ instituţional pentru translatarea cercetării ştiinţifice biomedicale în practica clinică – TRANSCENT”[Institutional collaborative model for the translation of biomedical research into practice].
References
- 1.Gudjonsson JE, Elder JT. Psoriasis: epidemiology. Clin Dermatol. 2007;25(6):535–546. doi: 10.1016/j.clindermatol.2007.08.007. [DOI] [PubMed] [Google Scholar]
- 2.Liu Y, Krueger JG, Bowcock AM. Psoriasis: genetic associations and immune system changes. Genes Immun. 2007;8(1):1–12. doi: 10.1038/sj.gene.6364351. [DOI] [PubMed] [Google Scholar]
- 3.Batycka-Baran A, Maj J, Wolf R, Szepietowski JC. The new insight into the role of antimicrobial proteins-alarmins in the immunopathogenesis of psoriasis. J Immunol Res. 2014 doi: 10.1155/2014/628289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Schafer T. Epidemiology of psoriasis. Review and the German perspective. Dermatology. 2006;212(4):327–337. doi: 10.1159/000092283. [DOI] [PubMed] [Google Scholar]
- 5.Naldi L. Epidemiology of psoriasis. Curr Drug Targets Inflamm Allergy. 2004;3(2):121–128. doi: 10.2174/1568010043343958. [DOI] [PubMed] [Google Scholar]
- 6.Christophers E. Psoriasis--epidemiology and clinical spectrum. Clin Exp Dermatol. 2001;26(4):314–320. doi: 10.1046/j.1365-2230.2001.00832.x. [DOI] [PubMed] [Google Scholar]
- 7.Kurd SK, Gelfand JM. The prevalence of previously diagnosed and undiagnosed psoriasis in US adults: results from NHANES 2003–2004. J Am Acad Dermatol. 2009;60(2):218–224. doi: 10.1016/j.jaad.2008.09.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chandran V, Raychaudhuri SP. Geoepidemiology and environmental factors of psoriasis and psoriatic arthritis. J Autoimmun. 2010;34(3):J314–J321. doi: 10.1016/j.jaut.2009.12.001. [DOI] [PubMed] [Google Scholar]
- 9.Xhaja A, Shkodrani E, Frangaj S, Kuneshka L, Vasili E. An epidemiological study on trigger factors and quality of life in psoriatic patients. Mater Sociomed. 2014;26(3):168–171. doi: 10.5455/msm.2014.26.168-171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Raychaudhuri SP, Gross J. A comparative study of pediatric onset psoriasis with adult onset psoriasis. Pediatr Dermatol. 2000;17(3):174–178. doi: 10.1046/j.1525-1470.2000.01746.x. [DOI] [PubMed] [Google Scholar]
- 11.Hébert HL, Bowes J, Smith RL, Flynn E, Parslew R, Alsharqi A, et al. Identification of loci associated with late-onset psoriasis using dense genotyping of immune-related regions. Br J Dermatol. 2015;172(4):933–939. doi: 10.1111/bjd.13340. [DOI] [PubMed] [Google Scholar]
- 12.Lønnberg AS, Skov L, Duffy DL, Skytthe A, Kyvik KO, Pedersen OB, et al. Genetic Factors Explain Variation in the Age at Onset of Psoriasis: A Population-based Twin Study. Acta Derm Venereol. 2016;96(1):35–38. doi: 10.2340/00015555-2171. [DOI] [PubMed] [Google Scholar]
- 13.Islam MT, Paul HK, Zakaria SM, Islam MM, Shafiquzzaman M. Epidemiological determinants of psoriasis. Mymensingh Med J. 2011;20(1):9–15. [PubMed] [Google Scholar]
- 14.Braun-Falco O, Plewig G, Wolff HH, Winkelmann RK, editors. Dermatology. Vol. 14. Berlin: Springer Verlag; 1991. Erythematous and erythematosquamous skin diseases; pp. 417–435. [Google Scholar]
- 15.Poikolainen K, Reunala T, Karvonen J, Lauharanta J, Karkkainen P. Alcohol intake: a risk factor for psoriasis in young and middle aged men? BMJ. 1990;300(6727):780–783. doi: 10.1136/bmj.300.6727.780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Farkas A, Kemény L. Psoriasis and alcohol: is cutaneous ethanol one of the missing links? Br J Dermatol. 2010;162(4):711–716. doi: 10.1111/j.1365-2133.2009.09595.x. [DOI] [PubMed] [Google Scholar]
- 17.Milavec-Puretić V, Mance M, Ceović R, Lipozenčić J. Drug induced psoriasis. Acta Dermatovenerol Croat. 2011;19(1):39–42. [PubMed] [Google Scholar]
- 18.Rongioletti F, Fiorucci C, Parodi A. Psoriasis induced or aggravated by drugs. J Rheumatol Suppl. 2009;83:59–61. doi: 10.3899/jrheum.090227. [DOI] [PubMed] [Google Scholar]
- 19.Kim GK, Del Rosso JQ. Drug-provoked psoriasis: is it drug induced or drug aggravated?: understanding pathophysiology and clinical relevance. J Clin Aesthet Dermatol. 2010;3(1):32–38. [PMC free article] [PubMed] [Google Scholar]
- 20.Keerthi S, Rangaraj M, Karthikeyan K. Telmisartan aggravates pustular psoriasis. J Pharmacol Pharmacother. 2015;6(2):107–109. doi: 10.4103/0976-500X.155492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wu W, Debbaneh M, Moslehi H, Koo J, Liao W. Tonsillectomy as a treatment for psoriasis: a review. J Dermatolog Treat. 2014;25(6):482–486. doi: 10.3109/09546634.2013.848258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Weigl BA. The significance of stress hormones (glucocorticoids, catecholamines) for eruptions and spontaneous remission phases in psoriasis. Int J Dermatol. 2000;39(9):678–688. doi: 10.1046/j.1365-4362.2000.00800.x. [DOI] [PubMed] [Google Scholar]
- 23.Halawani MR. Dermatological manifestations of hepatitis C virus infection in Saudi Arabia. Saudi Med J. 2014;35(6):531–537. [PubMed] [Google Scholar]
- 24.Imafuku S, Naito R, Nakayama J. Possible association of hepatitis C virus infection with late-onset psoriasis: a hospital-based observational study. J Dermatol. 2013;40(10):813–818. doi: 10.1111/1346-8138.12240. [DOI] [PubMed] [Google Scholar]
- 25.Ito T, Furukawa F. Psoriasis guttate acuta triggered by varicella zoster virus infection. Eur J Dermatol. 2000;10(3):226–227. [PubMed] [Google Scholar]
- 26.Wiemers S, Krutmann J, Kapp A, Schöpf E. Postherpetic erythema exsudativum multiforme with concomitant exacerbation of psoriasis vulgaris. Hautarzt. 1990;41(9):506–508. [PubMed] [Google Scholar]
- 27.Bhalerao J, Bowcock AM. The genetics of psoriasis: a complex disorder of the skin and immune system. Hum Mol Genet. 1998;7(10):1537–1545. doi: 10.1093/hmg/7.10.1537. [DOI] [PubMed] [Google Scholar]
- 28.Picciani BL, Michalski-Santos B, Carneiro S, Sampaio AL, Avelleira JC, Azulay DR, et al. Oral candidiasis in patients with psoriasis: correlation of oral examination and cytopathological evaluation with psoriasis disease severity and treatment. J Am Acad Dermatol. 2013;68(6):986–991. doi: 10.1016/j.jaad.2012.11.033. [DOI] [PubMed] [Google Scholar]
- 29.Silverberg NB. Pediatric psoriasis: an update. Ther Clin Risk Manag. 2009;5:849–856. doi: 10.2147/tcrm.s4908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Camargo CM, Brotas AM, Ramos-e-Silva M, Carneiro S. Isomorphic phenomenon of Koebner: facts and controversies. Clin Dermatol. 2013;31(6):741–749. doi: 10.1016/j.clindermatol.2013.05.012. [DOI] [PubMed] [Google Scholar]
- 31.Ockenfels HM. Trigger factors for psoriasis. Hautarzt. 2003;54(3):215–223. doi: 10.1007/s00105-003-0494-5. [DOI] [PubMed] [Google Scholar]
- 32.Jacob SE, Butler D, Herro E. Corticosteroid and fragrance allergy exacerbating scalp psoriasis. J Clin Aesthet Dermatol. 2014;7(2):54–55. [PMC free article] [PubMed] [Google Scholar]
- 33.Lipozencić J, Milavec-Puretić V, Pasić A. Contact allergy and psoriasis. Arh Hig Rada Toksikol. 1992;43(3):249–254. [PubMed] [Google Scholar]
- 34.Mallbris L, Larsson P, Bergqvist S, Vingard E, Granath F, Stahle M. Psoriasis phenotype at disease onset: clinical characterization of 400 adult cases. J Invest Dermatol. 2005;124(3):499–504. doi: 10.1111/j.0022-202X.2004.23611.x. [DOI] [PubMed] [Google Scholar]
- 35.Huynh M, Gupta R, Koo JY. Emotional stress as a trigger for inflammatory skin disorders. Semin Cutan Med Surg. 2013;32(2):68–72. doi: 10.12788/j.sder.0003. [DOI] [PubMed] [Google Scholar]
- 36.Slominski AT, Zmijewski MA, Skobowiat C, Zbytek B, Slominski RM, Steketee JD. Sensing the environment: regulation of local and global homeostasis by the skin’s neuroendocrine system. Adv Anat Embryol Cell Biol. 2012;212:v, vii, 1–115. doi: 10.1007/978-3-642-19683-6_1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Nordlind K, Azmitia EC, Slominski A. The skin as a mirror of the soul: exploring the possible roles of serotonin. Exp Dermatol. 2008;17(4):301–311. doi: 10.1111/j.1600-0625.2007.00670.x. [DOI] [PubMed] [Google Scholar]
- 38.Tachibana T, Nawa T. Immunohistochemical reactions of receptors to met-enkephalin, VIP, substance P, and CGRP located on Merkel cells in the rat sinus hair follicle. Arch Histol Cytol. 2005;68:383–391. doi: 10.1679/aohc.68.383. [DOI] [PubMed] [Google Scholar]
- 39.Safari M, Ghahari L, Zoroufchi MD. Effects of epidermal growth factor, platelet derived growth factor and growth hormone on cultured rat keratinocytes cells in vitro. Pak J Biol Sci. 2014;17(7):931–936. doi: 10.3923/pjbs.2014.931.936. [DOI] [PubMed] [Google Scholar]
- 40.van Beek N, Bodó E, Kromminga A, Gáspár E, Meyer K, Zmijewski MA, et al. Thyroid hormones directly alter human hair follicle functions: anagen prolongation and stimulation of both hair matrix keratinocyte proliferation and hair pigmentation. J Clin Endocrinol Metab. 2008;93(11):4381–4388. doi: 10.1210/jc.2008-0283. [DOI] [PubMed] [Google Scholar]
- 41.Zouboulis CC. The human skin as a hormone target and an endocrine gland. Hormones (Athens) 2004;3(1):9–26. doi: 10.14310/horm.2002.11109. [DOI] [PubMed] [Google Scholar]
- 42.Slominski AT, Manna PR, Tuckey RC. On the role of skin in the regulation of local and systemic steroidogenic activities. Steroids. 2015;103:72–88. doi: 10.1016/j.steroids.2015.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Haag M, Hamann T, Kulle AE, Riepe FG, Blatt T, Wenck H, et al. Age and skin site related differences in steroid metabolism in male skin point to a key role of sebocytes in cutaneous hormone metabolism. Dermatoendocrinol. 2012;4(1):58–64. doi: 10.4161/derm.19201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sandu C, Liu T, Malan A, Challet E, Pévet P, Felder-Schmittbuhl MP. Circadian clocks in rat skin and dermal fibroblasts: differential effects of aging, temperature and melatonin. Cell Mol Life Sci. 2015;72(11):2237–2248. doi: 10.1007/s00018-014-1809-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Fischer TW, Slominski A, Tobin DJ, Paus R. Melatonin and the hair follicle. J Pineal Res. 2008;44(1):1–15. doi: 10.1111/j.1600-079X.2007.00512.x. [DOI] [PubMed] [Google Scholar]
- 46.Kim TK, Lin Z, Li W, Reiter RJ, Slominski AT. N1-Acetyl-5-Methoxykynuramine (AMK) is produced in the human epidermis and shows antiproliferative effects. Endocrinology. 2015;156(5):1630–1636. doi: 10.1210/en.2014-1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Langan EA, Vidali S, Pigat N, Funk W, Lisztes E, Bíró T, et al. Tumour necrosis factor alpha, interferon gamma and substance P are novel modulators of extrapituitary prolactin expression in human skin. PLoS One. 2013;8(4):e60819. doi: 10.1371/journal.pone.0060819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Reichrath J. The skin: A powerful hormone factory. Dermato Endocrinol. 2012;4(1):1. doi: 10.4161/derm.20016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Jensen P, Ahlehoff O, Egeberg A, Gislason G, Hansen PR, Skov L. Psoriasis and New-onset Depression: A Danish Nationwide Cohort Study. Acta Derm Venereol. 2016;96:39–42. doi: 10.2340/00015555-2183. [DOI] [PubMed] [Google Scholar]
- 50.Irimie M, Oanţă A, Irimie CA, Fekete LG, Minea DI, Pascu A. Cardiovascular risk factors in patients with chronic plaque psoriasis: a case-control study on the Brasov County population. Acta Dermatovenerol Croat. 2015;23(1):28–35. [PubMed] [Google Scholar]
- 51.Skiveren J, Philipsen P, Therming G. Patients with psoriasis have insufficient knowledge of their risk of atherothrombotic disease and metabolic syndrome. Clin Exp Dermatol. 2015;40(6):600–604. doi: 10.1111/ced.12628. [DOI] [PubMed] [Google Scholar]
- 52.Abedini R, Salehi M, Lajevardi V, Beygi S. Patients with psoriasis are at a higher risk of developing nonalcoholic fatty liver disease. Clin Exp Dermatol. 2015;40:722–727. doi: 10.1111/ced.12672. [DOI] [PubMed] [Google Scholar]
- 53.Piérard-Franchimont C, Piérard GE, Delvenne P, Hermanns-Lê T. Psoriasis syndrome with its comorbidities. Rev Med Liege. 2014;69(10):555–558. [PubMed] [Google Scholar]
- 54.de Oliveira MF, de Rocha BO, Duarte GV. Psoriasis: classical and emerging comorbidities. An Bras Dermatol. 2015;90(1):9–20. doi: 10.1590/abd1806-4841.20153038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Radtke MA, Mrowietz U, Feuerhahn J, Härter M, von Kiedrowski R, Nast A, et al. Early detection of comorbidity in psoriasis: recommendations of the National Conference on Healthcare in Psoriasis. J Dtsch Dermatol Ges. 2015;13(7):674–690. doi: 10.1111/ddg.12643. [DOI] [PubMed] [Google Scholar]
- 56.Gyldenløve M, Storgaard H, Holst JJ, Vilsbøll T, Knop FK, Skov L. Patients with psoriasis are insulin resistant. J Am Acad Dermatol. 2015;72(4):599–605. doi: 10.1016/j.jaad.2015.01.004. [DOI] [PubMed] [Google Scholar]
- 57.Trojacka E, Zaleska M, Galus R. Influence of exogenous and endogenous factors on the course of psoriasis. Pol Merkur Lekarski. 2015;38(225):169–173. [PubMed] [Google Scholar]
- 58.Chi CC, Wang J, Chen YF, Wang SH, Chen FL, Tung TH. Risk of incident chronic kidney disease and end-stage renal disease in patients with psoriasis: A nationwide population-based cohort study. J Dermatol Sci. 2015;78(3):232–238. doi: 10.1016/j.jdermsci.2015.03.012. [DOI] [PubMed] [Google Scholar]
- 59.Lysell J, Tessma M, Nikamo P, Wahlgren CF, Ståhle M. Clinical characterisation at onset of childhood psoriasis- a cross sectional study in Sweden. Acta DermVenereol. 2015;95(4):457–461. doi: 10.2340/00015555-1986. [DOI] [PubMed] [Google Scholar]
- 60.Ceovic R, Mance M, Bukvic Mokos Z, Svetec M, Kostovic K, Stulhofer Buzina D. Psoriasis: female skin changes in various hormonal stages throughout life--puberty, pregnancy, and menopause. Biomed Res Int. 2013;2013:571912. doi: 10.1155/2013/571912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Swanbeck G, Inerot A, Martinsson T, Wahlström J, Enerbäck C, Enlund F, et al. Age at onset and different types of psoriasis. Br J Dermatol. 1995;133(5):768–773. doi: 10.1111/j.1365-2133.1995.tb02753.x. [DOI] [PubMed] [Google Scholar]
- 62.Gaspari AA. Innate and adaptive immunity and the pathophysiology of psoriasis. J Am Acad Dermatol. 2006;54(3 Suppl 2):S67–S80. doi: 10.1016/j.jaad.2005.10.057. [DOI] [PubMed] [Google Scholar]
- 63.Mashiko S, Bouguermouh S, Rubio M, Baba N, Bissonnette R, Sarfati M. Human mast cells are major IL-22 producers in patients with psoriasis and atopic dermatitis. J Allergy Clin Immunol. 2015;136(2):351–359.e1. doi: 10.1016/j.jaci.2015.01.033. [DOI] [PubMed] [Google Scholar]
- 64.Biedermann T, Kneilling M, Mailhammer R, Maier K, Sander CA, Kollias G, et al. Mast cells control neutrophil recruitment during T cell-mediated delayed-type hypersensitivity reactions through tumor necrosis factor and macrophage inflammatory protein 2. J Exp Med. 2000;192(10):1441–1452. doi: 10.1084/jem.192.10.1441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Dong G, You M, Fan H, Ji J, Ding L, Li P, et al. 17β-estradiol contributes to the accumulation of myeloid-derived suppressor cells in blood by promoting TNF-α secretion. Acta Biochim Biophys Sin (Shanghai) 2015;47(8):620–629. doi: 10.1093/abbs/gmv053. [DOI] [PubMed] [Google Scholar]
- 66.Kanda N, Watanabe S. Regulatory roles of sex hormones in cutaneous biology and immunology. J Dermatol Sci. 2005;38(1):1–7. doi: 10.1016/j.jdermsci.2004.10.011. [DOI] [PubMed] [Google Scholar]
- 67.Zhang J, Lapato A, Bodhankar S, Vandenbark AA, Offner H. Treatment with IL-10 producing B cells in combination with E2 ameliorates EAE severity and decreases CNS inflammation in B cell-deficient mice. Metab Brain Dis. 2015;30:1117–1127. doi: 10.1007/s11011-015-9661-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Miller CN, Brown LM, Rayalam S, Della-Fera MA, Baile CA. Estrogens, inflammation and obesity: an overview. Frontiers in Biology. 2012;7(1):40–47. [Google Scholar]
- 69.Jobe SO, Ramadoss J, Koch JM, Jiang Y, Zheng J, Magness RR. Estradiol-17beta and its cytochrome P450- and catechol-O-methyltransferase-derived metabolites stimulate proliferation in uterine artery endothelial cells: role of estrogen receptor-alpha versus estrogen receptor-beta. Hypertension. 2010;55(4):1005–1011. doi: 10.1161/HYPERTENSIONAHA.109.146399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Thornton MJ. Estrogens and aging skin. Dermatoendocrinol. 2013;5(2):264–270. doi: 10.4161/derm.23872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Heidenreich R, Röcken M, Ghoreschi K. Angiogenesis drives psoriasis pathogenesis. Int J Exp Pathol. 2009;90(3):232–248. doi: 10.1111/j.1365-2613.2009.00669.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Schmutz JL. Dermatological diseases influenced by pregnancy. Presse Med. 2003;32(38):1809–1812. [PubMed] [Google Scholar]
- 73.Murase JE, Chan KK, Garite TJ, Cooper DM, Weinstein GD. Hormonal effect on psoriasis in pregnancy and post partum. Arch Dermatol. 2005;141(5):601–606. doi: 10.1001/archderm.141.5.601. [DOI] [PubMed] [Google Scholar]
- 74.Piccinni M, Scaletti C, Maggi E, Romagnani S. Role of hormone-controlled Th1- and Th2-type cytokines in successful pregnancy. J Neuroimmunol. 2000;109(1):30–33. doi: 10.1016/s0165-5728(00)00299-x. [DOI] [PubMed] [Google Scholar]
- 75.Airas L. Hormonal and gender-related immune changes in multiple sclerosis. Acta Neurol Scand Suppl. 2015;132(199):62–70. doi: 10.1111/ane.12433. [DOI] [PubMed] [Google Scholar]
- 76.Falcón CR, Martínez FF, Carranza F, Cervi L, Motrán CC. In vivo expression of recombinant pregnancy-specific glycoprotein 1a inhibits the symptoms of collagen-induced arthritis. Am J Reprod Immunol. 2014;72(6):527–533. doi: 10.1111/aji.12307. [DOI] [PubMed] [Google Scholar]
- 77.Woidacki K, Zenclussen AC, Siebenhaar F. Mast cell-mediated and associated disorders in pregnancy: a risky game with an uncertain outcome? Front Immunol. 2014;5:231. doi: 10.3389/fimmu.2014.00231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.De Simone C, Caldarola G, Corbeddu M, Moro F, Tropea A, Moretta G, et al. A possible role of polycystic ovary syndrome for pregnancy complications in women with psoriasis. Drug Dev Res. 2014;75(Suppl 1):S64–S66. doi: 10.1002/ddr.21199. [DOI] [PubMed] [Google Scholar]
- 79.Cohen-Barak E, Nachum Z, Rozenman D, Ziv M. Pregnancy outcomes in women with moderate-to-severe psoriasis. J Eur Acad Dermatol Venereol. 2011;25(9):1041–1047. doi: 10.1111/j.1468-3083.2010.03917.x. [DOI] [PubMed] [Google Scholar]
- 80.Ryan C, Korman NJ, Gelfand JM, Lim HW, Elmets CA, Feldman SR, et al. Research gaps in psoriasis:opportunities for future studies. J Am Acad Dermatol. 2014;70(1):146–167. doi: 10.1016/j.jaad.2013.08.042. [DOI] [PubMed] [Google Scholar]
- 81.Figueroa-Vega N, Moreno-Frías C, Malacara JM. Alterations in adhesion molecules, pro-inflammatory cytokines and cell-derived microparticles contribute to intima-media thickness and symptoms in postmenopausal women. PLoS One. 2015;10(5):e0120990. doi: 10.1371/journal.pone.0120990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Pietrzak A, Czuczwar P, Mosiewicz J, Paszkowski T, Chodorowska G, Bartosinska J, et al. Cardiovascular disease in psoriatic post-menopausal women. J Eur Acad Dermatol Venereol. 2015;29(6):1231–1234. doi: 10.1111/jdv.12620. [DOI] [PubMed] [Google Scholar]
- 83.Gubbels Bupp MR. Sex, the aging immune system, and chronic disease. Cell Immunol. 2015;294(2):102–110. doi: 10.1016/j.cellimm.2015.02.002. [DOI] [PubMed] [Google Scholar]
- 84.Alesci S, Bornstein SR. Neuroimmunoregulation of androgens in the adrenal gland and the skin. Horm Res. 2000;54(5–6):281–286. doi: 10.1159/000053272. [DOI] [PubMed] [Google Scholar]
- 85.Makrantonaki E, Zouboulis CC. Androgens and ageing of the skin. Curr Opin Endocrinol Diabetes Obes. 2009;16(3):240–245. doi: 10.1097/MED.0b013e32832b71dc. [DOI] [PubMed] [Google Scholar]
- 86.Atzeni F, Straub RH, Cutolo M, Sarzi-Puttini P. Psoriatic arthritis: clinical improvement and correlation with hormone axes in etanercept-treated patients. Ann N Y Acad Sci. 2010;1193:176–178. doi: 10.1111/j.1749-6632.2009.05363.x. [DOI] [PubMed] [Google Scholar]
- 87.Ziółkowska E, Biedka M, Zyromska A, Makarewicz R. Psoriasis exacerbation after hormonotherapy in prostate cancer patient-Case report. Rep Pract Oncol Radiother. 2010;15(4):103–106. doi: 10.1016/j.rpor.2010.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Sanders VM. The beta2-adrenergic receptor on T and B lymphocytes: do we understand it yet? Brain Behav Immun. 2012;26(2):195–200. doi: 10.1016/j.bbi.2011.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Zhou J, Yan J, Liang H, Jiang J. Epinephrine enhances the response of macrophages under LPS stimulation. Biomed Res Int. 2014;2014:254686. doi: 10.1155/2014/254686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Kox M, van Eijk LT, Zwaag J, van den Wildenberg J, Sweep FC, van der Hoeven JG, et al. Voluntary activation of the sympathetic nervous system and attenuation of the innate immune response in humans. Proc Natl Acad Sci U S A. 2014;111(20):7379–7384. doi: 10.1073/pnas.1322174111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Kolmus K, Tavernier J, Gerlo S. β2-Adrenergic receptors in immunity and inflammation: stressing NF-κB. Brain Behav Immun. 2015;45:297–310. doi: 10.1016/j.bbi.2014.10.007. [DOI] [PubMed] [Google Scholar]
- 92.Sembulingam K, Sembulingam P. Essentials of Medical Physiology. 6th ed. Vol. 70. New Delhi: Jaypee Brothers Medical Publishers; 2012. p. 432. [Google Scholar]
- 93.Wong DL, Tai TC, Wong-Faull DC, Claycomb R, Meloni EG, Myers KM, et al. Epinephrine: a short- and long-term regulator of stress and development of illness: a potential new role for epinephrine in stress. Cell Mol Neurobiol. 2012;32(5):737–748. doi: 10.1007/s10571-011-9768-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Evers AW, Verhoeven EW, Kraaimaat FW, de Jong EM, de Brouwer SJ, Schalkwijk J, et al. How stress gets under the skin: cortisol and stress reactivity in psoriasis. Br J Dermatol. 2010;163(5):986–991. doi: 10.1111/j.1365-2133.2010.09984.x. [DOI] [PubMed] [Google Scholar]
- 95.Zangeneh FZ, Fazeli A. The significance of stress hormones in psoriasis. Acta Med Iran. 2008;46(6):485–488. [Google Scholar]
- 96.Langan EA, Griffiths CE, Paus R. Exploring the role of prolactin in psoriasis. Arch Dermatol Res. 2012;304(2):115–118. doi: 10.1007/s00403-012-1208-6. [DOI] [PubMed] [Google Scholar]
- 97.Handjani F, Saki N, Ahrari I, Ebrahimi M, Khorrami MM, Nematollahi P. Serum prolactin levels in psoriasis vulgaris. ISRN Dermatol. 2014;2014:586049. doi: 10.1155/2014/586049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Robati RM, Toossi P, Rahmati-Roodsari M, Khalilazar S, Abolhasani E, Namazi N, et al. Association of psoriasis severity with serum prolactin, thyroid hormones, and cortisol before and after treatment. ScientificWorldJournal. 2013;2013:921819. doi: 10.1155/2013/921819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Foitzik K, Langan EA, Paus R. Prolactin and the skin: a dermatological perspective on an ancient pleiotropic peptide hormone. J Invest Dermatol. 2009;129(5):1071–1087. doi: 10.1038/jid.2008.348. [DOI] [PubMed] [Google Scholar]
- 100.Kanda N, Hau CS, Tada Y, Watanabe S. Prolactin May Promote the Development of Psoriasis: Reawakened Issue. J Clin Exp Dermatol Res. 2013;4(5):1000198. [Google Scholar]
- 101.Kanda N, Shibata S, Tada Y, Nashiro K, Tamaki K, Watanabe S. Prolactin enhances basal and IL-17-induced CCL20 production by human keratinocytes. Eur J Immunol. 2009;39(4):996–1006. doi: 10.1002/eji.200838852. [DOI] [PubMed] [Google Scholar]
- 102.De Bellis A, Bizzarro A, Pivonello R, Lombardi G, Bellastella A. Prolactin and autoimmunity. Pituitary. 2005;8(1):25–30. doi: 10.1007/s11102-005-5082-5. [DOI] [PubMed] [Google Scholar]
- 103.Peeva E, Zouali M. Spotlight on the role of hormonal factors in the emergence of autoreactive B-lymphocytes. Immunol Lett. 2005;101(2):123–143. doi: 10.1016/j.imlet.2005.05.014. [DOI] [PubMed] [Google Scholar]
- 104.Orbach H, Shoenfeld Y. Hyperprolactinemia and autoimmune diseases. Autoimmun Rev. 2007;6:537–542. doi: 10.1016/j.autrev.2006.10.005. [DOI] [PubMed] [Google Scholar]
- 105.Keen MA, Hassan I. Serum prolactin levels in psoriasis and its association with disease activity: a case-control study. Indian J Dermatol. 2014;59(6):562–566. doi: 10.4103/0019-5154.143512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Sanchez-Regaña M, Umbert MP. Psoriasis in association with prolactinoma: three cases. Br J Dermatol. 2000;143:864–867. doi: 10.1046/j.1365-2133.2000.03792.x. [DOI] [PubMed] [Google Scholar]
- 107.Azizzadeh M, Malek M, Amiri M, Ghorbani R. Does Prolactin Indicate Severity of Psoriasis? Iran J Dermatol. 2009;12(3):79–81. [Google Scholar]
- 108.Abou Khedr NA, Abulfotooh Eid A. Clinical efficacy of bromocriptine and the influence of serum prolactin levels on disease severity in patients with chronic plaque-type psoriasis. Alexandria J Med. 2013;49:385–389. [Google Scholar]
- 109.Kato AM, Gheida SF, El-Bendary AS, Badawy AA, El Fakharany RE. Serum level of prolactin in psoriatic patients. Egypt Dermatol Online J. 2012;8(2):1–11. [Google Scholar]
- 110.Contreras-Jurado C, García-Serrano L, Gómez-Ferrería M, Costa C, Paramio JM, Aranda A. The thyroid hormone receptors as modulators of skin proliferation and inflammation. J Biol Chem. 2011;286(27):24079–24088. doi: 10.1074/jbc.M111.218487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Zoabi A, Ziv M, Rozenman D, Lovoshitski R. Prevalence of thyroid abnormalities among psoriatic patients. Harefuah. 2012;151(10):566–569. 605–606. [PubMed] [Google Scholar]
- 112.Arican O, Bilgic K, Koc K. The effect of thyroid hormones in psoriasis vulgaris. Indian J Dermatol Venereol Leprol. 2004;70(6):354–356. [PubMed] [Google Scholar]
- 113.Gul U, Gonul M, Kaya I, Aslan E. Autoimmune thyroid disorders in patients with psoriasis. Eur J Dermatol. 2009;19(3):221–223. doi: 10.1684/ejd.2009.0632. [DOI] [PubMed] [Google Scholar]
- 114.Utaş S, Köse K, Yazici C, Akdaş A, Keleştimur F. Antioxidant potential of propylthiouracil in patients with psoriasis. Clin Biochem. 2002;35(3):241–246. doi: 10.1016/s0009-9120(02)00294-1. [DOI] [PubMed] [Google Scholar]
- 115.Kano Y, Chiba M, Yagita A, Shiohara T. Complete resolution of psoriasis vulgaris after excision of thyroid cancer. Int J Dermatol. 1997;36(4):280–282. doi: 10.1111/j.1365-4362.1997.tb03044.x. [DOI] [PubMed] [Google Scholar]
- 116.Kuchel J, Barakate M, Delbridge L, Agarwal G, Beattie J, Barnetson R. Short-term resolution of psoriasis after total thyroidectomy for euthyroid multinodular goitre. Australas J Dermatol. 2002;43(3):214–217. doi: 10.1046/j.1440-0960.2002.00599.x. [DOI] [PubMed] [Google Scholar]
- 117.Klok MD, Jakobsdottir S, Drent ML. The role of leptin and ghrelin in the regulation of food intake and body weight in humans: a review. Obes Rev. 2007;8(1):21–34. doi: 10.1111/j.1467-789X.2006.00270.x. [DOI] [PubMed] [Google Scholar]
- 118.Kunath N, van Groen T, Allison DB, Kumar A, Dozier-Sharpe M, Kadish I. Ghrelin agonist does not foster insulin resistance but improves cognition in an Alzheimer’s disease mouse model. Sci Rep. 2015;5:11452. doi: 10.1038/srep11452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Ucak H, Demir B, Cicek D, Erden I, Aydin S, Dertlioglu SB, et al. Metabolic changes and serum ghrelin level in patients with psoriasis. Dermatol Res Pract. 2014;2014:175693. doi: 10.1155/2014/175693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Baran A, Flisiak I, Jaroszewicz J, Świderska M. Effect of psoriasis activity on serum adiponectin and leptin levels. Postepy Dermatol Alergol. 2015;32(2):101–106. doi: 10.5114/pdia.2014.40960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Oh YJ, Lim HK, Choi JH, Lee JW, Kim NI. Serum leptin and adiponectin levels in Korean patients with psoriasis. J Korean Med Sci. 2014;29(5):729–734. doi: 10.3346/jkms.2014.29.5.729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Xue K, Liu H, Jian Q, Liu B, Zhu D, Zhang M, et al. Leptin induces secretion of pro-inflammatory cytokines by human keratinocytes in vitro--a possible reason for increased severity of psoriasis in patients with a high body mass index. Exp Dermatol. 2013;22(6):406–410. doi: 10.1111/exd.12162. [DOI] [PubMed] [Google Scholar]
- 123.Napolitano M, Megna M, Monfrecola G. Insulin resistance and skin diseases. Scientific World Journal. 2015;2015:479354. doi: 10.1155/2015/479354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Tangvarasittichai S, Pongthaisong S, Meemark S, Tangvarasittichai O. Abdominal Obesity Associated with Elevated Serum Butyrylcholinesterase Activity, Insulin Resistance and Reduced High Density Lipoprotein-Cholesterol Levels. Indian J Clin Biochem. 2015;30(3):275–280. doi: 10.1007/s12291-014-0443-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Hamminga EA, van der Lely AJ, Neumann HA, Thio HB. Chronic inflammation in psoriasis and obesity: implications for therapy. Med Hypotheses. 2006;67(4):768–773. doi: 10.1016/j.mehy.2005.11.050. [DOI] [PubMed] [Google Scholar]