Abstract
Objective
This cross-sectional study evaluated event-related potentials (ERPs) across three groups: naïve, novice, and experienced meditators as potential physiological markers of mindfulness meditation competence.
Methods
Electroencephalographic (EEG) data was collected during a target tone detection task and a Breath Counting task. The Breath Counting task served as the mindfulness meditation condition for the novice and experienced meditator groups. Participants were instructed to respond to target tones with a button press in the first task (Tones), and then ignore the primed tones while breath counting. The primary outcomes were ERP responses to target tones, namely the N2 and P3, as markers of stimulus discrimination and attention, respectively.
Results
As expected, P3 amplitudes elicited by target tones were attenuated within groups during the Breath Counting task in comparison to the Tones task (p < .001). There was a task by group interaction for P3 (p = .039). Both meditator groups displayed greater change in peak-to-trough P3 amplitudes, with higher amplitudes during the Tones condition and more pronounced reductions in P3 amplitudes during the Breath Counting meditation task in comparison to the naïve group.
Conclusions
Meditators had stronger P3 amplitude responses to target tones when instructed to attend to the tones, and a greater attenuation of P3 amplitudes when instructed to ignore the same tones during the Breath Counting task. This study introduces the idea of identifying ERP markers as a means of measuring mindfulness meditation competence, and results suggest this may be a valid approach. This information has the potential to improve mindfulness meditation interventions by allowing objective assessment of mindfulness meditation quality.
Keywords: Meditation, mindfulness, event-related potentials, attention
Mindfulness meditation is an effective complementary medicine technique in the treatment of anxiety, depression, and chronic pain (Kabat-Zinn et al., 1992; Miller et al., 1995; Teasdale et al., 2000; Zeidan et al., 2010; Chiesa & Serretti, 2010; Grossman et al., 2004). However, much remains to be learned about the neurophysiological mechanisms underlying the benefits of mindfulness meditation (Ospina et al., 2007; Wahbeh et al., 2008), despite reported changes in electroencephalography (EEG) and magnetic resonance imaging (MRI) (Chiesa & Serretti, 2010; Fox et al., 2014; Dickenson et al., 2013; Kilpatrick et al., 2011; Cahn & Polich, 2006). A more straightforward EEG marker could enable researchers to more accurately assess the efficacy of mindfulness meditation trainings, as more and more meditation interventions are emerging. More accurate assessment would further elucidate the brain mechanisms in mindfulness meditation, assist in the development of effective dose in clinical trials, and facilitate mindfulness meditation training in the future.
Mindfulness meditation competence, for the purposes of this experiment, is delineated as the ability to attend to breathing while ignoring other stimuli. EEG is appealing way to assess brain changes in meditation because it allows for brain activity to be inexpensively, noninvasively, and unobtrusively recorded. Considering that mindfulness meditation can improve aspects of cognition (Brefczynski-Lewis et al., 2007; Lutz et al., 2009), EEG may be of additional benefit since it can evaluate these changes.
The aim of this study was to identify an electrophysiological correlate of mindfulness meditation competence using event-related potentials (ERPs). To achieve this aim, we investigated brain changes with EEG during mindfulness meditation practice among participants with differing levels of experience: naïve, novice, and experienced.
Jon Kabat-Zinn’s (1994) definition of mindfulness is “paying attention in a particular way: on purpose, in the present moment, and non-judgmentally.” We examined the ERP correlates of “paying attention”, not the motivational or emotional processes that accompany paying attention. We acknowledge that there are many definitions and components of mindfulness. In this particular experiment we are delineating the ability to keep awareness in the present moment because we were interested in developing a biomarker for the focused attention aspect of mindfulness. During mindfulness meditation practice and interventions such as mindfulness-based stress reduction and mindfulness-based cognitive therapy, participants both practice mindful attention to the present moment during daily living and cultivate concentrative meditational techniques. The Body Scan and Sitting Meditation, which are the two primary practices in the aforementioned secular interventions, are both concentrative techniques. Mindfulness meditation competence must be associated with a cognitive surrogate in order to be evaluated, and attention is an ideal marker given the results of past studies (Brefcyznski-Lewis et al. 2007; Chan & Woollacott 2007; Chiesa et al., 2011; Lutz et al., 2008). We used a breath counting task because non-meditators can do it and also because it has shown potential as an index of mindfulness (Levinson et al., 2014; Milz et al., 2014).
ERP components are predictable patterns of brain activity that occur in response to specific stimuli, and we expected two components in particular to display meditation experience-related differences during a quiet, potentially meditative state. The first was the P3, which generally indicates stronger attentional focus on a particular stimulus. We predicted that meditators would have greater attentional expertise, as observed in earlier work (Brefcyznski-Lewis et al. 2007; Chan & Woollacott 2007; Chiesa et al., 2011; Lutz et al., 2008). Thus we hypothesized that meditators would also have greater P3 amplitudes than non-meditators, as previously shown (Lutz et al., 2009). Differences in attentional focus and engagement have been previously observed between meditators and non-meditators (Cahn & Polich 2009; Chiesa et al. 2011; Delgado-Pastor et al. 2013; Tang et al., 2009), although the relationship between meditation training and attentional priming effects remains unknown. We also predicted that P3 amplitudes would differ between experienced and novice meditators, expecting that participants with the most meditation experience would also have the most attentional control.
The second component of interest in this study was the N2, which is elicited by stimuli similar to those that elicit the P3, specifically those that involve greater task-related attention or novelty (Luck, 2014). The N2 is elicited in response to target tones in an oddball task. We hypothesized that N2 amplitudes in the Tones task would be greater in meditators due to meditation-related attentional training. Specifically, experienced meditators were expected to have the most pronounced difference from naïve participants while novices’ performance would fall in the middle. To test these hypotheses, we compared the N2 and P3 components of experienced, novice, and naïve meditators during an odd-ball paradigm, initially while participants were instructed to attend to the target tones and then while they were told to ignore the tones and focus on their breathing. We were particularly interested in the effects of primed ERP responses to target tones during the Breath Counting task and how these responses differed from those in the Tones task.
Methods
Participants
Forty-two participants were recruited from the Portland metropolitan area utilizing internet-based, flyer, and word-of-mouth strategies. All underwent a 30-minute telephone screening. Inclusion criteria for participants in all groups were as follows: 1) Age 25–75 years; 2) Good general past and present medical health; 3) Stable on all medications for at least two months; and 4) Cognitively intact, as determined by a score of ≥31 on the Telephone Interview for Cognitive Status (TICS) (Welsh et al., 1993). Exclusion criteria were as follows: 1) Significant medical or neurological disorder/disease; 2) Significant visual or hearing impairment; 3) Medications that would affect outcome measures (e.g. benzodiazepines or neuroleptics); and 4) Significant untreated depression, as determined by a score of ≥10 on the 10-item Center for Epidemiologic Studies Depression Scale (CESD-10) (Andresen et al., 1994).
The naïve, novice, and experienced groups were carefully defined, and each subject’s telephone screening was thoroughly reviewed by the principal investigator. Information about lifetime hours of practice was collected via verbal self-report. Meditation was defined as any practice of attention training, in which one is intentionally working to improve his or her ability to pay attention to the present moment. Further details about the types of practice are described by group below. Mind-body movement practices were defined as any activity in which one is practicing attention training. Mind-body movement-based practices included yoga, tai chi, martial arts, qi gong, mindful eating, walking, running, and swimming. The practices included foci of attention were reported to be breath, physical sensations, and sound (Table 1).
Table 1.
Participant Characteristics
| All Participants (SD) |
Non- Meditators (SD) |
Novice Meditators (SD) |
Experienced Meditators (SD) |
|
|---|---|---|---|---|
| N | 42 | 13 | 15 | 14 |
| Age (M) | 49 (13) | 48 (11) | 50 (13) | 49 (15) |
| Female | 62% | 69% | 73% | 43% |
| Minority | 24% | 23% | 27% | 21% |
| Practice in Years (M) | -- | -- | 2.4 years (2.5) | 22.6 years (13.2) |
There were no significant demographic differences among groups.
The three groups were evaluated by specific criteria. Potential naïve subjects were excluded if they had taken a meditation or other mind-body movement class within the past two years or if they had a daily meditation practice of five minutes or longer in the past 30 days. Novice subjects were included if they had some formal training, meaning they attended classes that were described and approved by the principal investigator, practiced at least three days a week, and had ≤1,000 hours’ lifetime experience of combined meditation and mind-body practices. All novice meditators took a meditation class and continued practicing meditation on the breath, as well as a myriad of other methods, including the body scan, compassion practices, and mindfulness of sounds. To qualify as an experienced meditator, a subject needed ≥5,000 lifetime meditation and mind-body movement practice hours. All had ≥4,000 hours of meditation experience, and all self-identified as either Tibetan or Zen Buddhists. Experienced meditators reported many different types of meditation practice in addition to those practiced by the novices, including but not limited to the following: mantra and prayer recitation, visualization, analytical meditation on philosophical topics, non-conceptual awareness, and open awareness. The protocol for this study was approved by the OHSU Institutional Review Board. Participants attended a 3.5-hour laboratory session where they were connected to physiological data collection equipment and instructed in the tasks described below.
Our experiment was designed to measure changes in brain activity during Breath Counting meditation and assess priming as a means of attentional focus; therefore, our primary measures were the attentional N2/P3 ERP components and P3b specifically in response to infrequent target tones across tasks. Given the age of our sample (M age = 49 years), it is worth noting that P3a and P3b tend to overlap as age increases (Alperin et al., 2014) and that general P3 anteriorization occurs as age increases (Kopp et al., 2014; Oken & Kaye 1992).
Tones Task
For the first cognitive task, participants were seated with eyes closed for 15 minutes and asked to press a button when they identified an infrequent high-pitched target tone (2000Hz). Participants also heard infrequent, non-target low tones (low-500Hz) and frequent standard tones (1000Hz). Tones were presented at 1500–2500ms second intervals. In each block of 10 tones, there was 1 high frequency target tone, 1 low frequency distractor tone, and 8 standard tones. The minimum number of standard tones between each target tone was 2. On average, there were 45 target tones presented in 15 minutes. Participants practiced this task before beginning the experiment.
Breath Counting Task
To enable some experimental matching of state between meditators and non-meditators, there was a Breath Counting session with the eyes closed. This task was described simply as breath counting to naïve participants and as a breath counting meditation for meditators. The rationale for using Breath Counting as a mindfulness meditation task is that it is easy to comprehend and attempt by participants in all three groups, and all novice and experienced meditators had experience with meditation focusing on the breath. The same tones were presented in the background during the Breath Counting task as during the Tones task, but participants were instructed to ignore the tones and keep track of their breath count. The prior exposure to the tones served as a prime.
Importantly, the purpose of the Breath Counting task is to allow for between-subjects comparisons of priming effects during a task that could be performed by both meditators and non-meditators and also be considered a meditative state. Participants were instructed to press a button if they lost count of their breaths and continue counting using their best estimate of their breath count to that point. The Meditation Depth Index (MEDI) (Piron, 2001) and Stanford Sleepiness Scale (SSS) (Herscovitch, 1981) were administered after the Breath Counting session. The MEDI assessed the subjective quality of attentional focus to breath counting. The Stanford Sleepiness Scale was administered after the Breath Counting sessions to assess potential differences that might be attributed to drowsiness from prolonged eye closure, although none were found. Additionally, we calculated theta and alpha power for one-second epochs prior to all stimulus tones to evaluate drowsiness.
Physiological Recordings
EEG was collected using a BioSemi 32- channel ActiveTwo system using passive-sensor Ag-AgCl electrodes (BioSemi, Amsterdam, the Netherlands). Electrodes were placed according to a Modified Combinatorial Nomenclature (MCN) at AF3/4, F7/8, F3/4, FC1/2, FC5/6, T7/8, C3/4, CP1/2, CP5/6, P7/8, P3/4, P03/4, O1/2, Oz, Fz, Cz, and Pz. All EEG data were acquired at a sampling rate of 1024Hz with a resolution of 24 bits. Amplification occurred with a bandwidth of 0.1–70.0Hz. Online recordings referenced a common mode sense (CMS) and driven right leg (DRL) electrodes, halfway between Cz and C3/4.
Respiration has been shown to be important for meditation in general (Ahani et al., 2014) and also important for the specific nature of the Breath Counting meditative task. It was measured with a light elastic piezoelectric belt (Ambu-Sleepmate, Maryland) around the participant’s chest near the diaphragm. Respiration rate was analyzed in BrainVision Analyzer (Version 2.0.1.3931, Professional Edition). Breaths were labeled semi-automatically using a voltage trigger to label peak values. Specifically, a new breath was counted whenever the respiratory voltage reading crossed zero in a positive direction.
Electrophysiological Analyses
Data were analyzed from the Fz, Cz, and Pz midline electrode sites. During offline processing data were re-referenced to linked mastoids. Data were filtered offline using a 70Hz low pass filter, a 0.1Hz high pass filter, and a 60Hz notch filter and analyzed in BrainVision Analyzer. Visual, semi-automatic artifact rejection was employed to remove data segments contaminated by non-neuronal activity such as muscle activity, electrode malfunction, or abrupt head movements. Criteria for semi-automatic artifact rejections were as follows: maximal allowed voltage step of 50µV/ms; maximal allowed absolute difference 125µV within interval length of 50ms; maximum EEG amplitudes allowed was +/− 75 µV and the lowest allowed EEG activity was .5µV within interval length of 100ms. The EEG activity 200ms before and 200ms after artifact events were eliminated.
To generate the ERPs for processing of the N2 and P3 peaks, data were segmented −200ms before to 1000ms after stimulus presentations. The tone types were then separated using distinct markers, averaged, and baseline corrected using 200ms before stimulus presentation. A semi-automated peak detection tool was implemented using these intervals N2 (200–300ms) and P3 (275–450ms). All peak estimates were subject to visual inspection, and temporal windows were adjusted and reapplied if necessary. These criteria were decided on a case-by-case basis for target tones. Inter-rater reliability was achieved by having two researchers separately view the data and agree upon window settings/peak identifications. Automated peak analyses revealed one naïve participant with no discernible N1, P2, or N2 during Breath Counting, although there was no issue with this person’s P3. Two experienced meditators had no discernible N2 or P3 during Breath Counting. In these three cases, automated approximate values were kept in an effort to represent a complete, objective data set. Following detection, discrete peak voltage values were exported from Brain Vision Analyzer for further processing. Final N2 and P3 values were computed as the change in voltage from the previous peak estimate (i.e., N2final = P1raw − N2raw, and P3final = P3raw − N2raw). This computation was intended to control for deceptive individual differences in absolute peak amplitudes resulting from general positive or negative drift across the multiple ERP components.
EEG frequency analysis was utilized to assess drowsiness and thus ensure ERP differences were not simply related to differences in levels of alertness. The analysis was performed on one-second epochs prior to all stimulus tones using the same pre-processing and filtering techniques as the ERPs. A periodic Hanning window was applied with a tapering of 20%. The frequency spectrum was generated using the Fast Fourier Transformation (FFT). The resulting epoch FFTs were averaged and exported, then the square root of power in frontal-midline theta (4–8 Hz) and posterior alpha (8–13 Hz) were calculated. The square root transform was used to improve normality of FFT data (Oken & Chiappa, 1988).
Statistical Analyses
Results were assessed using mixed design ANOVAs for N2 and P3 amplitudes and total task respiration counts (SPSS v.21). Mixed 2 (Tones or Breath Counting, within subjects) by 3 (naïve, novice, or experienced meditator, between subjects) ANOVAs were conducted to assess changes in event-related potentials by task and by group. Univariate ANOVAs were used to assess N2 and P3 difference values by group. Independent t-tests were conducted to follow up on potential group differences in these measures as well as the MEDI and SSS. A separate 2×2 mixed ANOVA that pooled novice and experienced meditator groups was also calculated.
Results
The overall sample had an underrepresented racial and ethnic representation of 7% African American, 7% Asian American, 2% Native American, 2% Hispanic, and 5% of more than one race (Table 1). A one-way ANOVA determined there were no significant demographic differences among the groups. Novices had been practicing meditation and/or mind-body activities for an average of 2.4 (SD = 2.5) years and currently practiced 12.6 (SD = 7.7) minutes per day. Experienced meditators had been practicing meditation and/or mind-body activities for an average of 22.6 (SD = 13.1) years and presently practiced an average of 122.1 (SD = 180.9) minutes per day. High-pitched target tones were the focus of ERP analyses from the Fz, Cz, and Pz sites because we were looking for N2/P3 priming effects from the Tones condition to the Breath Counting condition (see Table 2 for task summary). The non-target infrequent and standard tone results as well as the N1/P2 components are discussed separately. Please note that one novice participant was excluded from Pz analyses due to electrode failure at that particular site.
Table 2.
Tone and Breath Counting Tasks
|
Tones Tasks Length: 15 minutes Stimuli: ~45 target tones 10% High Frequency Target Tone 10% Low Frequency Distracter Tone 80% Standard Tone Instructions: Sit still with eyes closed and press a button for the High Frequency Target Tones only. Hypothesis: Participants’ neurophysiological responses (N2 and P3 amplitudes) to the Target Tones would correlate with amount of attention training—the more training, the higher the amplitude. |
Breath Counting Task Length: 15 minutes Stimuli: Same as Tones Task Instructions: Sit still with eyes closed, ignore all tones, and count breaths. If count is lost, press a button and resume counting. Hypothesis: N2 and P3 amplitudes elicited by High Frequency Tones (previous target) would be most attenuated in experienced meditators, less attenuated in novice meditators, and least attenuated in naive subjects. |
P3 ERP
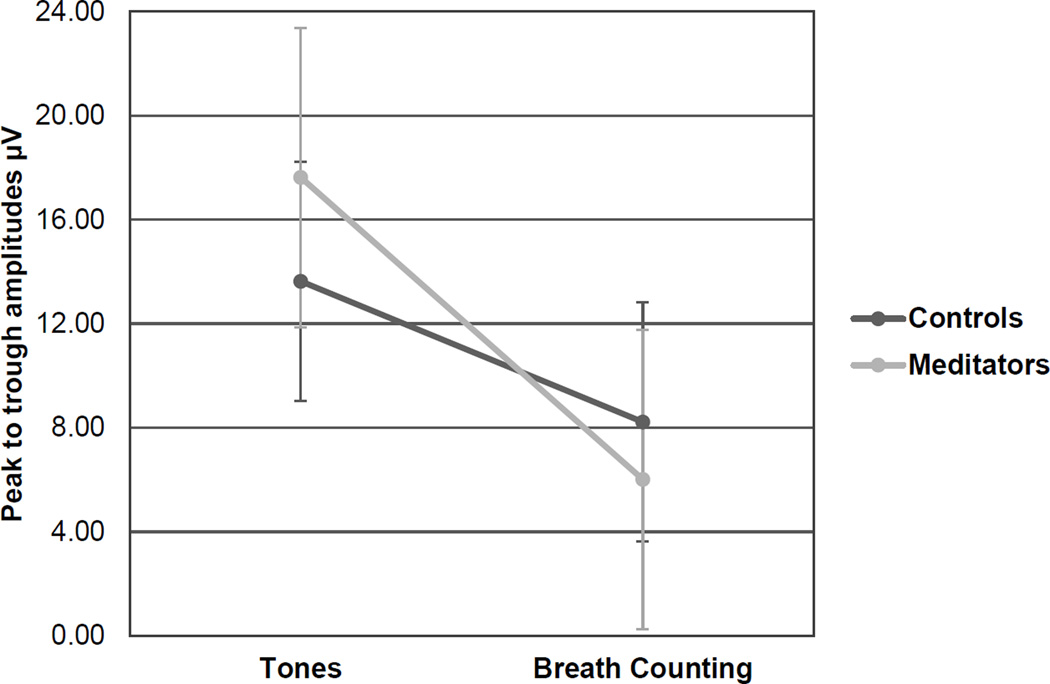
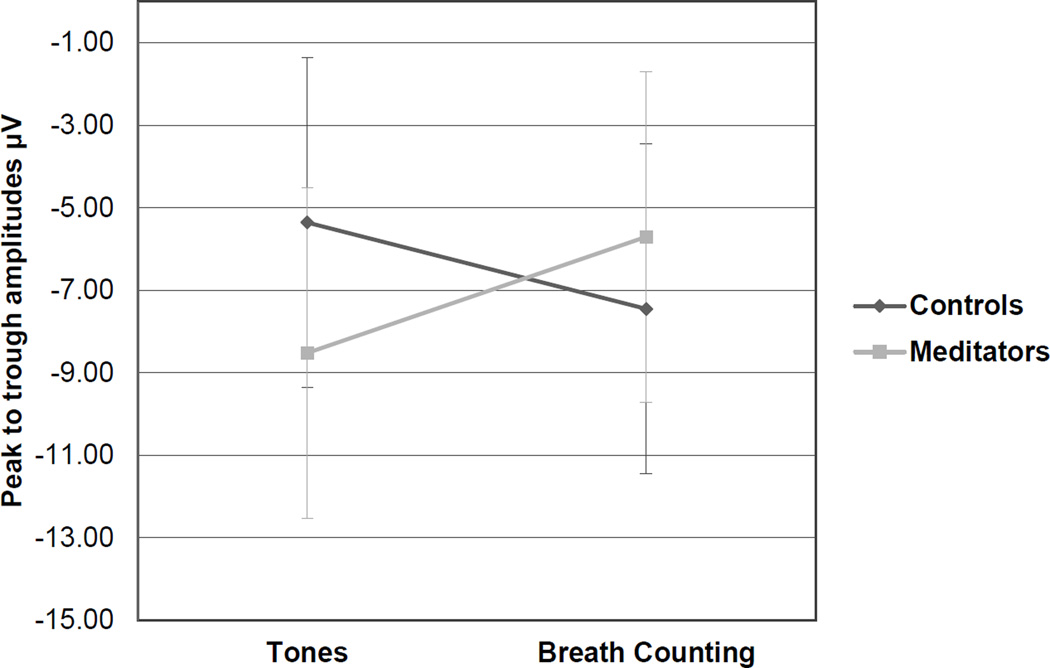
IN all three groups there was a main within-subjects effect of task on P3 amplitudes elicited by infrequent target tones, F(1,39) = 58.02, p < .001, with primed P3 amplitudes attenuated during the Breath Counting task (M = 5.12µV) compared to the Tones task (M = 5.96µV) across the Fz, Cz, and Pz midline electrode sites. There were no significant differences between the novice and experienced groups, so in order to further assess the effects of meditation experience, we pooled novice and experienced meditators into one meditation group and repeated the above analyses. For the pooled meditator group, there was a priming effect on P3 amplitude following infrequent target tones at the Fz site: F(1,40) = 42.34, p < .001; Cz site: F(1,40) = 34.31; p < .001; and Pz site: F(1,40) = 42.23, p < .001. There was an interaction for target P3s at the Cz site as well, F(1,40) = 4.56, p = .039 (Figure 1), with meditators showing a greater change in peak-to-trough P3 amplitudes from the Tones task to the Breath Counting task. See Figure 2 for average ERP waveforms elicited by target tones at the Cz site. Figures 3 and 4 contain summaries of topographic distributions and general ERP amplitudes across Tone conditions.
Figure 1.
P3 responses to targets, Cz
*For target tones this interaction was significant, p = .039, with meditators showing a greater change in peak-to-trough P3 amplitudes from the Tones to the Breath Counting task.
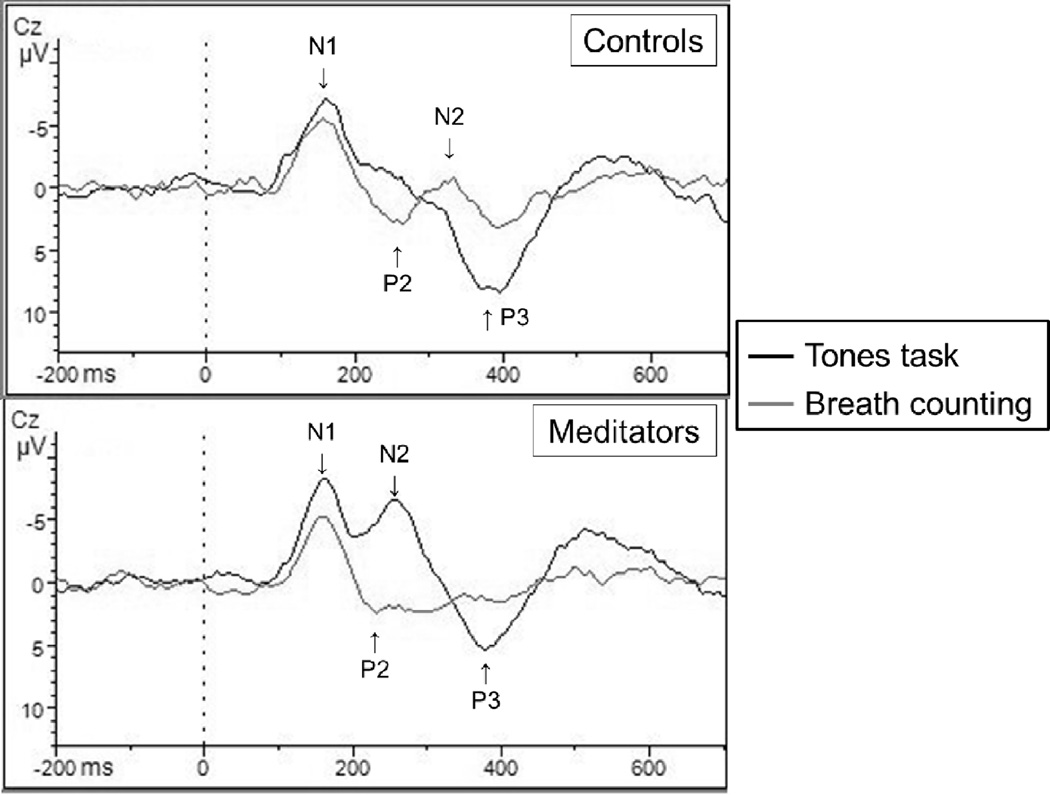
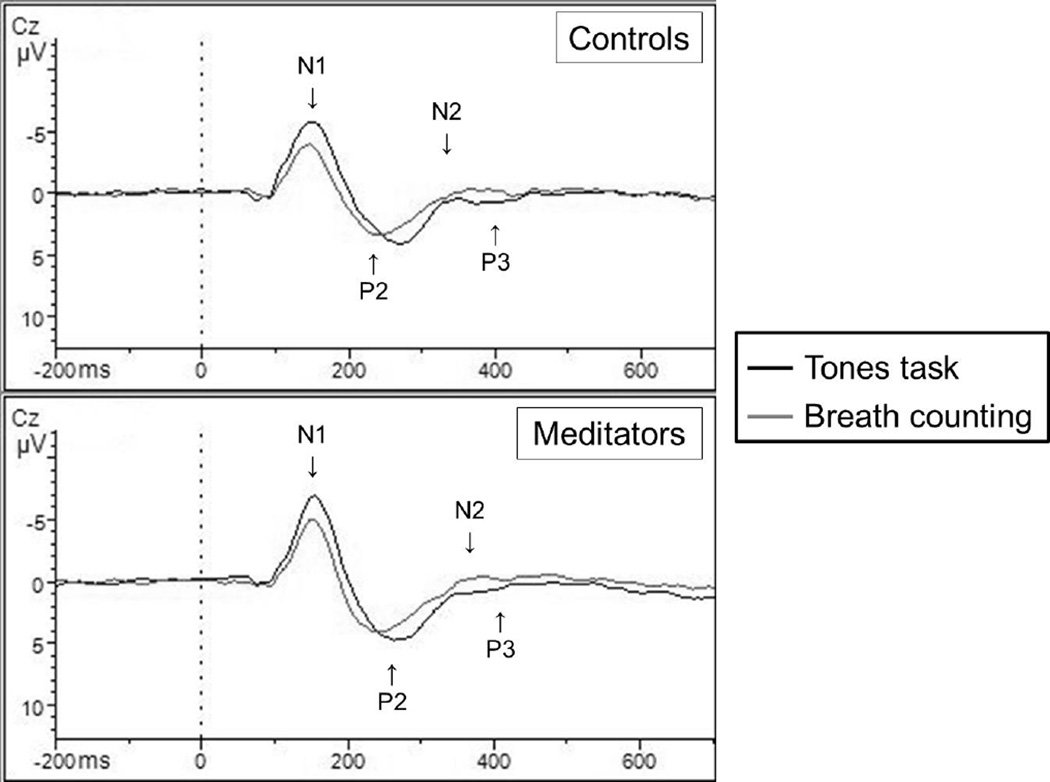
Figure 2.
Target tone event-related potential responses
* With novice and experienced groups combined, meditators showed a greater peak-to-trough change in P3 and N2 across tasks in comparison to non-meditators when responding to infrequent target tones. Data here are reported from the Cz electrode site. Please note that the ERP component labels are approximate denotations of general areas that may not apply to both overlapping conditions.
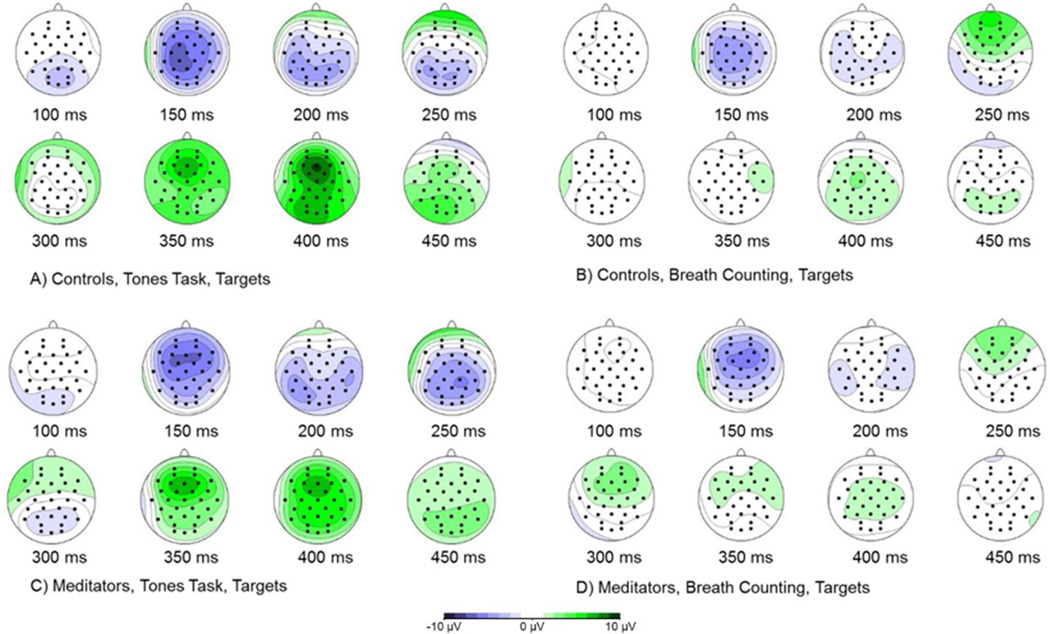
Figure 3.
Topographic maps of brain activity in response to target tones across conditions
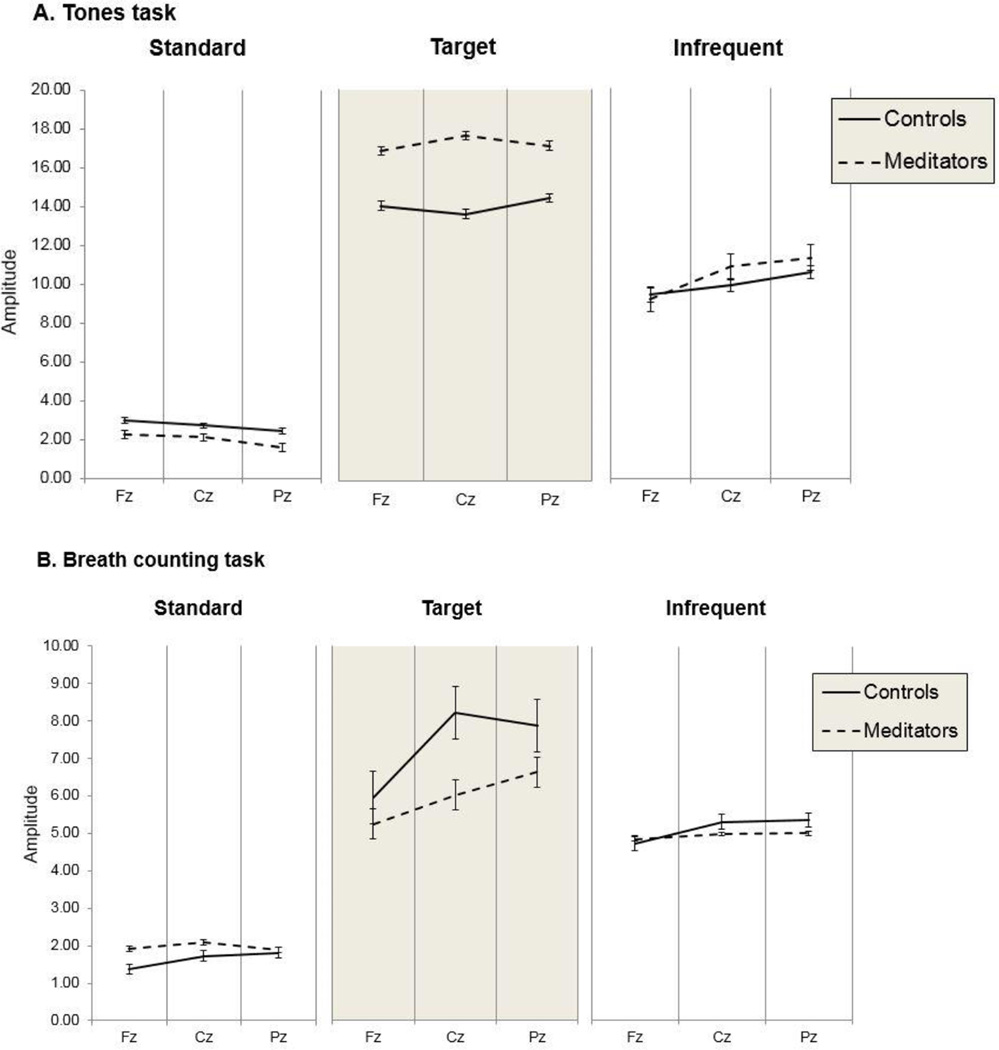
Figure 4.
P3 amplitudes across all conditions (group, task, tone type)
N2 ERP
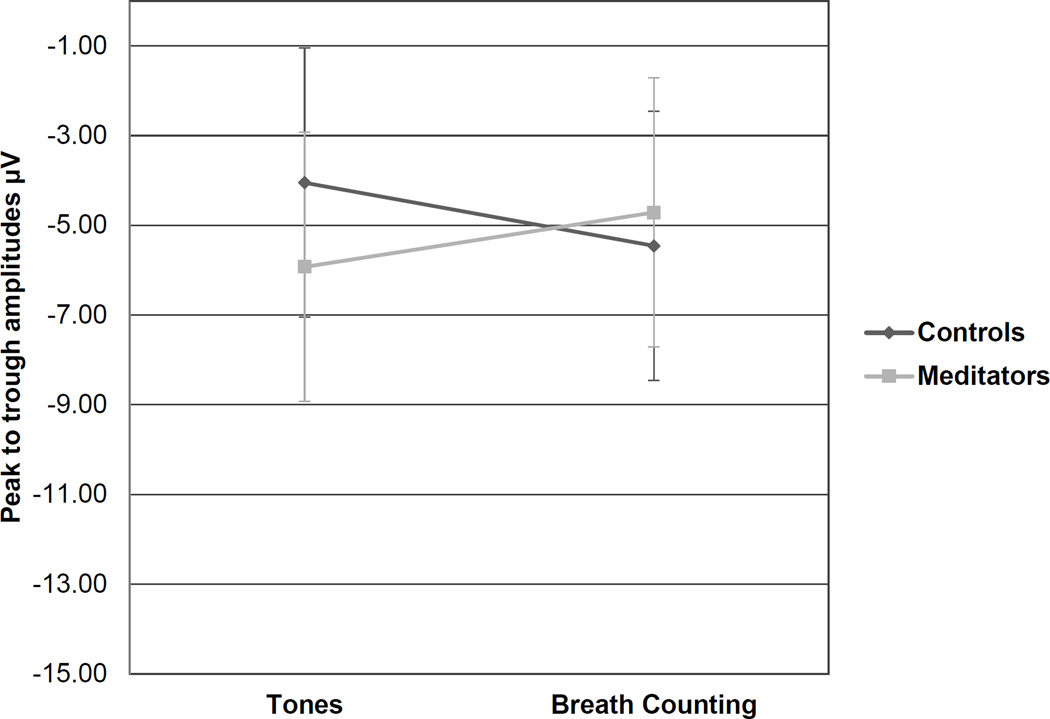
With three groups there were interactions for N2 responses to target tones at the Cz site, F(2,39) = 3.96, p = .027, and Pz site, F(2,38) = 3.25, p = .05. With pooled meditators, these group interactions were still present at the Cz site, F(1,40) = 6.25, p = .017, and Pz site, F(1,39) = 4.32, p = .044, for high/infrequent target tones (Figures 5 & 6). At the Cz electrode site the effect was more pronounced, with meditators again showing a greater difference in N2 peak-to-trough amplitudes from the Tones task to the Breath Counting task. In both cases meditators on average had greater N2 amplitudes during the Tones task and more attenuated N2 amplitudes during the Breath Counting task in comparison to naïve participants.
Figure 5.
N2 responses to targets, Cz
*This interaction was significant at the Cz site, p = .017, with a more pronounced effect than at the Pz site and meditators exhibiting a greater difference in N2 peak-to-trough amplitudes from the Tones Task to Breath Counting.
Figure 6.
N2 reponses to targets, Pz
*This interaction was significant at the Pz site, p = .044, with meditators on average showing greater peak-to-trough N2 amplitudes during the Tones task and attenuated N2 amplitudes during the Breath Counting task in comparison to controls.
Exploratory ERPs
This section will cover results for N1 and P2 ERPs as well as responses to low infrequent non-target tones and frequent standard tones. These analyses are exploratory, as N2/P3 responses to target tones were our primary analyses. Thus, only group effects are of interest with these variables. We will report pooled group results (meditators versus naïve participants) as they were the strongest.
Additional analyses on standard and low tones for P3 revealed no group effects or interactions regarding low or standard tones. For N2 at the Pz site, there was a significant group effect in which meditators (M = −5.98µV) had higher amplitude responses to low tones than naive participants (M = −3.46µV) during the Tones task. Meditators (M = −6.43µV) also had higher amplitude N2s than naïve participants (M = −4.27µV) during Breath Counting. There were no significant results for N2 responses to standard tones.
For N1, there was a general reduction in amplitude from the Tones task to the Breath Counting task for all tone types (standard, high, low) at all electrode sites of interest (Fz, Cz, Pz), all p values < .005, with the exception of Pz low tones, for which there was no task effect. A group difference in N1 amplitudes between naïve participants and meditators was observed for low tones at the Cz midline site, F(1,40) = 5.94, p = .019. During Tones, meditators (M = −11.67µV) exhibited higher amplitude N1s than naïve participants (M = −6.83µV).
P2 results were more scattered. P2 amplitudes went down from Tones to Breath Counting but only for standard tones across Fz, Cz, and Pz, with all p values < .008. Like N1, there was a group effect for low tones at the Cz site, F(1,40) = 4.53, p = .040, but the effect was for the Breath Counting task as meditators (M = 15.72µV) exhibited higher amplitude P2s in comparison to naïve participants (M = 11.39µV). ERP waveform graphs are included for standard and low tones (Figures 7 & 8). These results will not be discussed further since they were exploratory and separate from our original research question.
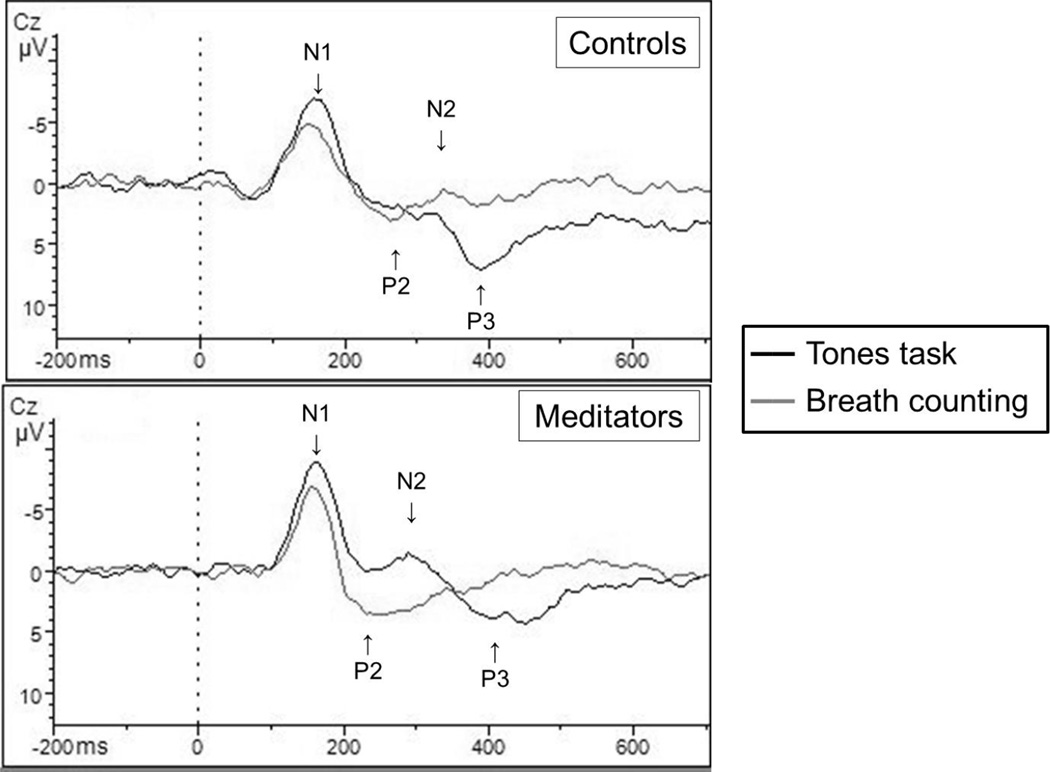
Figure 7.
Frequent standard tone event-related potential responses
*For frequent standard tones there were significant changes in N1 and P2 peak-to-trough values from the Tones task to the Breath Counting task, but there were no group effects, as expected.
Figure 8.
Infrequent non-target tone event-related potential responses
*At the Cz site, there were significant changes in amplitude in N1 and P3 responses to infrequent non-targets as well as a group effect for N1 responses, with meditators showing higher amplitudes during the Tones task, p = .02. There was an additional group effect for P2 with meditators showing higher amplitudes during the Breath Counting task, p = .04.
Respiration
Respiration data were assessed to supplement ERP results. Two (Tones or Breath Counting, within subjects) by 3 (experienced or novice or naïve meditator, between subjects) mixed ANOVAs revealed a main effect of task on total automated respiration count for the Breath Counting session after the Tones task, F(1,41) = 51.78, p < 001. Total respiration count decreased during the Breath Counting task (M = 128.67) in comparison to the Tones task (M = 203.70). These tasks were equivalent in length at 15 minutes. When the tasks were further divided into three equivalent segments, there were significant respiration differences between the tasks at each time point for the first segment, F(1,41) = 49.10, p < 001, second segment, F(1,41) = 34.75, p < 001, and final segment, F(1,39) = 40.79, p < 001. Overall, there were no interactions or group effects.
Drowsiness
There were no differences between groups after the Tones or Breath Counting tasks in the frequency content analyzed. We also assessed theta/alpha ratios to assess drowsiness in an objective way using a 2 (task, Tones or Breath Counting) by 3 (group, naïve, novice, or experienced meditator) mixed ANOVA. Again, there were no differences across groups in drowsiness measures. When theta and alpha levels were measured separately however, there was a significant task by group theta interaction, F(2,36) = 5.12, p = 01. When broken down by t-tests, we see that naïve participants had greater theta (Theta M = .94) than experienced meditators (Theta M = .54) during the Breath Counting task, potentially suggesting a less vigilant state (Oken et al., 2006), although drowsiness does not explain the N2/P3 difference between groups.
Meditation Questionnaire
Finally, we assessed group differences on the (Meditation Depth Index = MEDI) using t-tests. After the Breath Counting session, naïve participants reported the lowest scores (M = 24), novices were in the middle (M = 27), and experienced meditators had the highest MEDI scores (M = 28). The difference between the naïve participants and experienced meditators was significant, p = .024, and the naïve/novice difference bordered on significance, p = .054.
Behavioral Measures
We assessed accuracy on the Tones task and found a ceiling effect. The average correct identification rate for target tones was 98% for meditators and 97% for naïve participants, as measured by button press responses. Misses due to time outs and false alarms were both taken into account in error rates. There were no significant group differences in any of these factors. We did not assess reaction time for the Tones task because participants were not instructed to press the button as quickly as they could. One naïve group participant had to be excluded after behavioral analyses revealed that they did not follow instructions. This person responded to the infrequent low tones as targets in addition to high tones as targets, thus their data had to be removed from all ERP analyses since the goal of the priming task was compromised. Importantly, P3 data were only calculated from correct hits/button presses on the Tones task.
Discussion
This experiment attempted to identify an objective measure of mindfulness meditation competence through event-related potential indices of attention. To accomplish this, we compared N2 and P3 component responses to target tones when they were relevant versus irrelevant in order to capture mindful cognitive states across naïve, novice, and experienced meditation groups. The Breath Counting session accomplished a controlled state that was meditative as assessed by the self-rated MEDI scale.
Previous work strongly suggests that attentional control increases with meditation experience (Cahn & Polich, 2006). On average, meditators in this experiment had greater N2 and P3 amplitudes than non-meditators while attending to targets during the Tones task, and greater attenuation of N2 and P3 while engaged in the breath counting meditation and ignoring the primed tones. This strongly suggests that meditators had greater attentional control both while they were specifically attending to target tones as well as while ignoring them during the Breath Counting meditative task.
The lack of differences between the novice and experienced groups might indicate that proficiency in the attentional training aspect of mindfulness can be achieved relatively early on in practice, although the benefits of longer term meditative practices may not be sensitive to this particular attentional aspect of mindfulness, or perhaps the group definitions and/or sample sizes weakened the results. The findings presented here are not conclusive on this matter. The novices in this experiment were actually quite practiced in meditation with an average of 2.4 years of experience, and some novices had rather extensive formal training. Perhaps greater differences would be more apparent with less experienced novices.
Drowsiness was also an a priori concern in all of our groups since drowsiness attenuates P3, and increased theta is associated with drowsiness. However, our drowsiness analyses provided some evidence that the decreased P3 amplitude in meditators during Breath Counting is not due to drowsiness.
This study had some limitations. All novice meditators reported practicing meditation on breath, and for most, it was their primary practice. In contrast, meditation on breath was just one among many practices for the experienced meditators. An experienced Zen meditator participant also provided valuable feedback that the Breath Counting meditation was essentially a dual performance task since, in Zen meditation, eyes are usually open without a focus on breath counting. Thus, although we were constrained by the experimental need to create a replicable and simple meditation task, Breath Counting may not have captured the more profound meditation states that experienced meditators may achieve. This potential meditation state difference between novice and experienced mediators during the Breath Counting meditation was documented by self-report, but this issue may have contributed to the lack of differences between novice and experienced meditators. Additionally, experienced meditators may have other habitual conditions used for their meditation practice that were not present in this study, and these issues may have also contributed to the lack of differences between experienced and novice meditators in our study. In the future, it may be worthwhile to work with more homogenous meditation groups.
In summary, this study set out to identify objective markers of mindfulness meditation competence. Using ERP techniques, we found that meditators of all experience levels were better able to direct their attention than naïve participants. This was reflected by self-rated (MEDI) scores as well as increased N2 and P3 component responses to target tone stimuli when they were relevant to the task and decreased responses when irrelevant. Intriguingly, meditators demonstrated these effects regardless of experience level. The results of this study have important implications for improving mindfulness meditation interventions by introducing an initial step toward objective measurement of mindfulness competence.
Meditators were better able to direct their attention while on-task
Meditators were better able to redirect their attention after priming
Identified event-related potential markers of mindfulness competence
This information can be used to improve mindfulness training and interventions
Acknowledgments
This study was funded by NIH-NCCIH T32 AT002688. Roger Ellingson, MS, provided programming and engineering support.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Ahani A, Wahbeh H, Nezamfar H, Miller M, Erdogmus D, Oken B. Quantitative change of EEG and respiration signals during mindfulness meditation. Journal of neuroengineering and rehabilitation. 2014;11:87. doi: 10.1186/1743-0003-11-87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Alperin BR, Mott KK, Holcomb PJ, Daffner KR. Does the age-related “anterior shift” of the P3 reflect an inability to habituate the novelty response? Neuroscience Letters. 2014;577:6–10. doi: 10.1016/j.neulet.2014.05.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Andresen EM, Malmgren JA, Carter WB, Patrick DL. Screening for depression in well older adults: Evaluation of a short form of the CES-D. American journal of preventative medicine. 1994;10:77–84. [PubMed] [Google Scholar]
- 4.Brefczynski-Lewis JA, Lutz A, Schaefer HS, Levinson DB, Davidson RJ. Neural correlates of attentional expertise in long-term meditation practitioners. Proc Natl Acad Sci U S A. 2007;104:11483–11488. doi: 10.1073/pnas.0606552104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cahn BR, Polich J. Meditation states and traits. EEG, ERP, and neuroimaging studies. Psychological Bulletin. 2006;132:180–211. doi: 10.1037/0033-2909.132.2.180. [DOI] [PubMed] [Google Scholar]
- 6.Cahn BR, Polich J. Meditation (Vipassana) and the P3a event-related brain potential. International Journal of Pscyhophysiology. 2009;72:51–60. doi: 10.1016/j.ijpsycho.2008.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chan D, Woollacott M. Effects of level of meditation experience on attentional focus: is the efficiency of executive or orientation networks improved? J Altern Complement Med. 2007;13:651–657. doi: 10.1089/acm.2007.7022. [DOI] [PubMed] [Google Scholar]
- 8.Chiesa A, Calati R, Serretti A. Does mindfulness training improve cognitive abilities? a systematic review of neuropsychological findings. Clinical Psychology Review. 2011;32:449–464. doi: 10.1016/j.cpr.2010.11.003. [DOI] [PubMed] [Google Scholar]
- 9.Chiesa A, Serretti A. A systematic review of neurobiological and clinical features of mindfulness meditations. Psychological Medicine. 2010;40:1239–1252. doi: 10.1017/S0033291709991747. [DOI] [PubMed] [Google Scholar]
- 10.Delgado-Pastor LC, Perakakis P, Subramanya P, Telles S, Vila J. Mindfulness (Vipassana) meditation: Effects on P3b event-related potential and heart rate variability. Internation Jounral of Psychophysiology. 2013;90:207–214. doi: 10.1016/j.ijpsycho.2013.07.006. [DOI] [PubMed] [Google Scholar]
- 11.Dickenson J, Berkman ET, Arch J, Lieberman MD. Neural correlates of focused attention during a brief mindfulness induction. Soc Cogn Affect Neurosci. 2013;8:40–47. doi: 10.1093/scan/nss030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fox KC, Nijeboer S, Dixon ML, Floman JL, Ellamil M, Rumak SP, Sedlmeier P, Christoff K. Is meditation associated with altered brain structure? A systematic review and meta-analysis of morphometric neuroimaging in meditation practitioners. Neurosci Biobehav Rev. 2014;43:48–73. doi: 10.1016/j.neubiorev.2014.03.016. [DOI] [PubMed] [Google Scholar]
- 13.Grossman P, Niemann L, Schmidt S, Walach H. Mindfulness-based stress reduction and health benefits. A meta-analysis. J Psychosom Res. 2004;57:35–43. doi: 10.1016/S0022-3999(03)00573-7. [DOI] [PubMed] [Google Scholar]
- 14.Herscovitch J, Broughton R. Sensitivity of the Stanford Sleepiness Scale to the effects of cumulative partial sleep deprivation and recovery oversleeping. Sleep. 1981;4:83–92. doi: 10.1093/sleep/4.1.83. [DOI] [PubMed] [Google Scholar]
- 15.Kabat-Zinn J. Wherever you go, there you are: Mindfulness meditation in everyday life. New York: Hyperion; [Google Scholar]
- 16.Kabat-Zinn J, Massion AO, Kisteller J, Peterson LG, Fletcher KE, Pbert L, Lenderking WR, Santorelli SF. Effectiveness of a meditation-based stress reduction program in the treatment of anxiety disorders. Am J Psychiatry. 1992;149:936–943. doi: 10.1176/ajp.149.7.936. [DOI] [PubMed] [Google Scholar]
- 17.Kilpatrick LA, Suyenobu BY, Smith SR, Bueller JA, Goodman T, Creswell JD, Tillisch K, Mayer EA, Naliboff BD. Impact of Mindfulness-Based Stress Reduction training on intrinsic brain connectivity. Neuroimage. 2011;56:290–298. doi: 10.1016/j.neuroimage.2011.02.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kopp B, Lange F, Howe J, Wessel K. Age-related changes in neural recruitment for cognitive control. Brain and Cognition. 2014;85:209–219. doi: 10.1016/j.bandc.2013.12.008. [DOI] [PubMed] [Google Scholar]
- 19.Luck SJ. An introduction to the event-related potential technique. 2nd. Cambridge: The MIT Press; 2014. [Google Scholar]
- 20.Levinson DB, Stoll EL, Kindy SD, Merry HL, Davidson RJ. A mind you can count on: validating breath counting as a behavioral measure of mindfulness. Frontiers in psychology. 2014;5:1202. doi: 10.3389/fpsyg.2014.01202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lutz A, Slatger HA, Dunne JD, Davidson RJ. Attention regulation and monitoring in meditation. Trends Cogn Sci. 2008;12:163–169. doi: 10.1016/j.tics.2008.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lutz A, Slagter HA, Rawlings NB, Francis AD, Greischar LL, Davidson RJ. Mental training enhances attentional stability: neural and behavioral evidence. J Neurosci. 2009;29:13418–13427. doi: 10.1523/JNEUROSCI.1614-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Miller JJ, Fletcher K, Kabat-Zinn J. Three-year follow-up and clinical implications of a mindfulness meditation-based stress reduction intervention in the treatment of anxiety disorders. Gen Hosp Psychiatry. 1995;17:192–200. doi: 10.1016/0163-8343(95)00025-m. [DOI] [PubMed] [Google Scholar]
- 24.Milz P, Faber PL, Lehmann D, Kochi K, Pascual-Marqui RD. sLORETA intracortical lagged coherence during breath counting in meditation-naïve participants. Frontiers in Human Neuroscience. 2014;8:303. doi: 10.3389/fnhum.2014.00303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Oken BS, Chiappa KH. Short-term variability in EEG frequency analysis. Electroencephalography and clinical Neurophysiology. 1988;69:191–198. doi: 10.1016/0013-4694(88)90128-9. [DOI] [PubMed] [Google Scholar]
- 26.Oken BS, Kaye JA. Electrophysiological function in the healthy, extremely old. Neurology. 1992;42:519–526. doi: 10.1212/wnl.42.3.519. [DOI] [PubMed] [Google Scholar]
- 27.Oken BS, Salinsky MC, Elsas SM. Vigilance, alertness, or sustained attention: physiological basis and measurement. Clinical Neurophysiology. 2006;117:1885–1901. doi: 10.1016/j.clinph.2006.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ospina MB, Bond K, Karkhaneh M, Tjosvold L, Vandermeer B, Liang Y, Bialy L, Hooton N, Buscemi N, Dryden DM, Klassen TP. Meditation practices for health: state of the research (AHRQ) Evid Rep Technol Assess (Full Rep) 2007;155:1–263. [PMC free article] [PubMed] [Google Scholar]
- 29.Piron H. The meditation depth index (MEDI) and the meditation depth questionnaire (MEDEQ) Journal for meditation and meditation research. 2001;1:69–92. [Google Scholar]
- 30.Tang YY, Ma Y, Fan Y, Feng H, Wang J, Feng S, Lu Q, Hu B, Lin Y, Li J, Zhang Y, Wang Y, Zhou L, Fan M. Central and autonomic nervous system interaction is altered by short-term meditation. Proc Natl Acad Sci U S A. 2009;106:8865–8870. doi: 10.1073/pnas.0904031106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Teasdale JD, Segal ZV, Williams JM, Ridgeway VA, Soulsby JM, Lau MA. Prevention of relapse/recurrence in major depression by mindfulness-based cognitive therapy. J Consult Clin Psychol. 2000;68:615–623. doi: 10.1037//0022-006x.68.4.615. [DOI] [PubMed] [Google Scholar]
- 32.Wahbeh H, Elsas S-M, Oken B. Mind-body interventions: applications in neurology. Neurology. 2008;70:2321–2328. doi: 10.1212/01.wnl.0000314667.16386.5e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Welsh KA, Breitner JC, Magruder-Habib KM. Detection of demetia in the elderly using telephone screening of cognitive stats. Cognitive and behavioral neurology. 1993;6:103–110. [Google Scholar]
- 34.Zeidan F, Gordon NS, Merchant J, Goolkasian P. The effects of brief mindfulness meditation training on experimentally induced pain. J Pain. 2010;3:199–209. doi: 10.1016/j.jpain.2009.07.015. [DOI] [PubMed] [Google Scholar]