Abstract
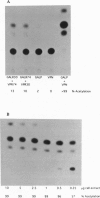
Specific interaction between the nucleocapsid protein (N) and the phosphoprotein (P) of vesicular stomatitis virus (VSV), an important step in the life-cycle of the virus, was studied by using a two-hybrid system. Plasmids encoding P fused with the yeast GAL4 DNA-binding domain (pGALP) and N fused with the herpes simplex virus VP16 transactivating region (pVPN) were transfected into CHO cells along with a reporter plasmid encoding chloramphenicol acetyltransferase (CAT). The ability of N and P to associate in vivo was measured by activation of the CAT gene by the VP16 transactivating region. Transfection of plasmids pGALP and pVPN resulted in a high level of CAT activity, indicating that the N and P portions of the fusion proteins associated very strongly with each other. Progressive C-terminal deletions of the P protein revealed two regions that are important for association with the N protein: the N-terminal acidic domain and the C-terminal basic domain. Phosphorylation of P protein was not required for N-P association. Various deletions and mutations of the N protein revealed the C-terminal 5 amino acids (Val-Glu-Phe-Asp-Lys), in particular the amino acids Val-Glu-Phe, to be critical for N association with P. This two-hybrid system can be used in other viral systems to study the interaction between proteins involved in transcription and replication.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aso T., Vasavada H. A., Kawaguchi T., Germino F. J., Ganguly S., Kitajima S., Weissman S. M., Yasukochi Y. Characterization of cDNA for the large subunit of the transcription initiation factor TFIIF. Nature. 1992 Jan 30;355(6359):461–464. doi: 10.1038/355461a0. [DOI] [PubMed] [Google Scholar]
- Banerjee A. K., Barik S. Gene expression of vesicular stomatitis virus genome RNA. Virology. 1992 Jun;188(2):417–428. doi: 10.1016/0042-6822(92)90495-b. [DOI] [PubMed] [Google Scholar]
- Barik S., Banerjee A. K. Phosphorylation by cellular casein kinase II is essential for transcriptional activity of vesicular stomatitis virus phosphoprotein P. Proc Natl Acad Sci U S A. 1992 Jul 15;89(14):6570–6574. doi: 10.1073/pnas.89.14.6570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chattopadhyay D., Banerjee A. K. NH2-terminal acidic region of the phosphoprotein of vesicular stomatitis virus can be functionally replaced by tubulin. Proc Natl Acad Sci U S A. 1988 Nov;85(21):7977–7981. doi: 10.1073/pnas.85.21.7977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chattopadhyay D., Banerjee A. K. Phosphorylation within a specific domain of the phosphoprotein of vesicular stomatitis virus regulates transcription in vitro. Cell. 1987 May 8;49(3):407–414. doi: 10.1016/0092-8674(87)90293-5. [DOI] [PubMed] [Google Scholar]
- Chattopadhyay D., Banerjee A. K. Two separate domains within vesicular stomatitis virus phosphoprotein support transcription when added in trans. Proc Natl Acad Sci U S A. 1987 Dec;84(24):8932–8936. doi: 10.1073/pnas.84.24.8932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crysler J. G., Lee P., Reinders M., Prevec L. The sequence of the nucleocapsid protein (N) gene of Piry virus: possible domains in the N protein of vesiculoviruses. J Gen Virol. 1990 Sep;71(Pt 9):2191–2194. doi: 10.1099/0022-1317-71-9-2191. [DOI] [PubMed] [Google Scholar]
- Dang C. V., Barrett J., Villa-Garcia M., Resar L. M., Kato G. J., Fearon E. R. Intracellular leucine zipper interactions suggest c-Myc hetero-oligomerization. Mol Cell Biol. 1991 Feb;11(2):954–962. doi: 10.1128/mcb.11.2.954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Das T., Banerjee A. K. Role of the phosphoprotein (P) in the encapsidation of presynthesized and de novo synthesized vesicular stomatitis virus RNA by the nucleocapsid protein (N) in vitro. Cell Mol Biol. 1992 Feb;38(1):17–26. [PubMed] [Google Scholar]
- Davis N. L., Arnheiter H., Wertz G. W. Vesicular stomatitis virus N and NS proteins form multiple complexes. J Virol. 1986 Sep;59(3):751–754. doi: 10.1128/jvi.59.3.751-754.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fearon E. R., Finkel T., Gillison M. L., Kennedy S. P., Casella J. F., Tomaselli G. F., Morrow J. S., Van Dang C. Karyoplasmic interaction selection strategy: a general strategy to detect protein-protein interactions in mammalian cells. Proc Natl Acad Sci U S A. 1992 Sep 1;89(17):7958–7962. doi: 10.1073/pnas.89.17.7958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fields S., Song O. A novel genetic system to detect protein-protein interactions. Nature. 1989 Jul 20;340(6230):245–246. doi: 10.1038/340245a0. [DOI] [PubMed] [Google Scholar]
- Gill D. S., Chattopadhyay D., Banerjee A. K. Identification of a domain within the phosphoprotein of vesicular stomatitis virus that is essential for transcription in vitro. Proc Natl Acad Sci U S A. 1986 Dec;83(23):8873–8877. doi: 10.1073/pnas.83.23.8873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howard M., Wertz G. Vesicular stomatitis virus RNA replication: a role for the NS protein. J Gen Virol. 1989 Oct;70(Pt 10):2683–2694. doi: 10.1099/0022-1317-70-10-2683. [DOI] [PubMed] [Google Scholar]
- La Ferla F. M., Peluso R. W. The 1:1 N-NS protein complex of vesicular stomatitis virus is essential for efficient genome replication. J Virol. 1989 Sep;63(9):3852–3857. doi: 10.1128/jvi.63.9.3852-3857.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lang I., Scholz M., Peters R. Molecular mobility and nucleocytoplasmic flux in hepatoma cells. J Cell Biol. 1986 Apr;102(4):1183–1190. doi: 10.1083/jcb.102.4.1183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopata M. A., Cleveland D. W., Sollner-Webb B. High level transient expression of a chloramphenicol acetyl transferase gene by DEAE-dextran mediated DNA transfection coupled with a dimethyl sulfoxide or glycerol shock treatment. Nucleic Acids Res. 1984 Jul 25;12(14):5707–5717. doi: 10.1093/nar/12.14.5707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luban J., Alin K. B., Bossolt K. L., Humaran T., Goff S. P. Genetic assay for multimerization of retroviral gag polyproteins. J Virol. 1992 Aug;66(8):5157–5160. doi: 10.1128/jvi.66.8.5157-5160.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma J., Ptashne M. Deletion analysis of GAL4 defines two transcriptional activating segments. Cell. 1987 Mar 13;48(5):847–853. doi: 10.1016/0092-8674(87)90081-x. [DOI] [PubMed] [Google Scholar]
- Martin K. J., Lillie J. W., Green M. R. Evidence for interaction of different eukaryotic transcriptional activators with distinct cellular targets. Nature. 1990 Jul 12;346(6280):147–152. doi: 10.1038/346147a0. [DOI] [PubMed] [Google Scholar]
- Masters P. S., Banerjee A. K. Complex formation with vesicular stomatitis virus phosphoprotein NS prevents binding of nucleocapsid protein N to nonspecific RNA. J Virol. 1988 Aug;62(8):2658–2664. doi: 10.1128/jvi.62.8.2658-2664.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masters P. S., Banerjee A. K. Resolution of multiple complexes of phosphoprotein NS with nucleocapsid protein N of vesicular stomatitis virus. J Virol. 1988 Aug;62(8):2651–2657. doi: 10.1128/jvi.62.8.2651-2657.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moyer S. A. Replication of the genome RNAs of defective interfering particles of vesicular stomatitis and Sendai viruses using heterologous viral proteins. Virology. 1989 Sep;172(1):341–345. doi: 10.1016/0042-6822(89)90136-0. [DOI] [PubMed] [Google Scholar]
- Peluso R. W., Moyer S. A. Viral proteins required for the in vitro replication of vesicular stomatitis virus defective interfering particle genome RNA. Virology. 1988 Feb;162(2):369–376. doi: 10.1016/0042-6822(88)90477-1. [DOI] [PubMed] [Google Scholar]
- Ruppert S., Wang E. H., Tjian R. Cloning and expression of human TAFII250: a TBP-associated factor implicated in cell-cycle regulation. Nature. 1993 Mar 11;362(6416):175–179. doi: 10.1038/362175a0. [DOI] [PubMed] [Google Scholar]
- Sadowski I., Ptashne M. A vector for expressing GAL4(1-147) fusions in mammalian cells. Nucleic Acids Res. 1989 Sep 25;17(18):7539–7539. doi: 10.1093/nar/17.18.7539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silver P. A., Keegan L. P., Ptashne M. Amino terminus of the yeast GAL4 gene product is sufficient for nuclear localization. Proc Natl Acad Sci U S A. 1984 Oct;81(19):5951–5955. doi: 10.1073/pnas.81.19.5951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takacs A. M., Barik S., Das T., Banerjee A. K. Phosphorylation of specific serine residues within the acidic domain of the phosphoprotein of vesicular stomatitis virus regulates transcription in vitro. J Virol. 1992 Oct;66(10):5842–5848. doi: 10.1128/jvi.66.10.5842-5848.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takacs A. M., Perrine K. G., Barik S., Banerjee A. K. Alteration of specific amino acid residues in the acidic domain I of VSV phosphoprotein (P) converts a GAL4-P(I) hybrid into a transcriptional activator. New Biol. 1991 Jun;3(6):581–591. [PubMed] [Google Scholar]
- Wertz G. M., Howard M. B., Davis N., Patton J. The switch from transcription to replication of a negative-strand RNA virus. Cold Spring Harb Symp Quant Biol. 1987;52:367–371. doi: 10.1101/sqb.1987.052.01.042. [DOI] [PubMed] [Google Scholar]