Abstract
In the present study, we tested the hypothesis that the potent and selective dopamine-β-hydroxylase (DβH) inhibitor nepicastat would have minimal effects on cardiovascular and pharmacokinetic parameters associated with cocaine administration and would reduce the positive subjective effects produced by cocaine. We conducted a double-blind, placebo-controlled, inpatient study of oral nepicastat (0, 80 and 160 mg) concurrent with intravenous (IV) cocaine (0, 10, 20 and 40 mg) in non-treatment seeking participants who metcriteria for cocaine use disorder. Safety analyses revealed that nepicastat was well-tolerated and there were no differences in adverse events observed after nepicastat plus cocaine vs. cocaine alone. In addition, the pharmacokinetic properties of cocaine administration were not altered by nepicastat treatment. Cocaine-induced cardiovascular and subjective effects were evaluated for completers in the cohort randomized to nepicastat (n = 13) using a within-subjects statistical analysis strategy. Specifically, the cardiovascular and subjective effects of cocaine were assessed in the presence of placebo (0 mg), 80 mg of nepicastat or 160 mg of nepicastat on study Days 4, 8 and 12, respectively. Analyses revealed a main effect of nepicastat to reduce several cocaine-induced positive subjective effects. Taken together, these data indicate that nepicastat is safe when co-administered with cocaine and may suppress its positive subjective effects, and may be viable as a pharmacotherapy for treatment of cocaine use disorder.
Keywords: Cocaine, Dopamine-β-hydroxylase, Nepicastat, SYN117
1. Introduction
A number of medications have been evaluated in clinical trials for cocaine use disorder, including antidepressants, antipsychotics, stimulants, and anticonvulsants (Haile and Kosten, 2013; Moeller et al., 2008; Vocci and Elkashef, 2005), although no medication has gained approval from the Food and Drug Administration (FDA) for this indication. Disulfiram (Antabuse®), the first FDA-approved medication for the treatment of alcohol use disorder, was initially reported to reduce concomitant cocaine and alcohol use (Carroll et al., 1998; Carroll et al., 2000; Higgins et al., 1993). Subsequently, disulfiram was shown to be at least equally effective, and possibly even more beneficial, at reducing cocaine use (self-report and/or cocaine-positive urine screens) in subjects not meetingalcohol use disorder criteria (Carroll et al., 2004; George et al., 2000; Petrakis et al., 2000).More recent data show that disulfiram reduced the positive subjective effects produced by cocaine (Baker et al., 2007) and higher doses of disulfiram (mg/kg basis) reduced cocaine self-administration (Haile et al., 2012a, 2012b). These studies suggested that the efficacy of disulfiram to suppress alcohol vs. cocaine consumption may involve differential underlying mechanisms. Disulfiram suppresses alcohol intake by inhibiting hepatic aldehyde dehydrogenase which is required to effectively metabolize alcohol; in the face of this inhibition, the accumulated acetaldehyde burden causes the unpleasant disulfiram ethanol reaction (e.g., flushing, nausea, vomiting, sweating, headache) which deters continued drinking (Haley, 1979). However, disulfiram is a non-selective, irreversible inhibitor of sulfhydryl-containing enzymes, which encompass several plasma esterases, including those that metabolize cocaine. It also inhibits the enzyme dopamine β hydroxylase (DβH) (Goldstein et al., 1964; Musacchio et al., 1966) and this is specifically hypothesized to account for its efficacy for reducing cocaine consumption (Gaval-Cruz and Weinshenker, 2009).
The enzyme DβH, expressed in synaptic vesicles of central nervous system noradrenergic neurons, converts dopamine (DA) into norepinephrine (NE) (Kaufman and Friedman, 1965), two neurotransmitters important in mediating the subjective and rewarding properties of cocaine (Sofuoglu and Sewell, 2009; Haile et al., 2012a, 2012b). Thus, while the exact mechanism by which DβH inhibition reduces cocaine use remains elusive (Gaval-Cruz and Weinshenker, 2009), decreased NE production and enhanced DA levels that occur as a result of DβH inhibition (Bourdelat-Parks et al., 2005; Goldstein et al., 1964; Musacchio et al., 1966) are likely involved. Indeed, disulfiram blocked cocaine-primed reinstatement of cocaine-seeking in rats extinguished from repeated self-administration at a dose that significantly reduced brain NE levels (Schroeder et al., 2010). Blockade of post-synaptic NE α1a receptors by doxazosin significantly reduced the positive subjective effects produced by cocaine in an inpatient study (Newton et al., 2012) and reduced cocaine use in an outpatient study (Shorter et al., 2013),while theα1 antagonist prazosin attenuates cocaine-seeking behavior in rats (Zhang and Kosten, 2005). Thus, clinical and preclinical findings suggest that pharmacological inhibition of DβH may offer a viable therapeutic strategy for the treatment of cocaine use disorder.
Proof-of-concept studies with disulfiram suggest the potential utility of DβH inhibitors for treatment of cocaine use disorder. In a study of 74 subjects stabilized on methadone and randomized into disulfiram and placebo groups for 10 weeks, disulfiram treatment reduced cocaine-positive urines, and disulfiram efficacy differed by DβH genotype (Kosten et al., 2013). These outcomes were similar to a recent report in 177 buprenorphine-treated participants that received 12 weeks of treatment with disulfiram or placebo, and participants treated with disulfiram reported significantly less frequent cocaine use (Schottenfeld et al., 2014). The lack of selectivity of disulfiram for DβH over other enzymes and its extensive metabolism into additional enzyme inhibitors (e.g., diethylthiocarbamate) (Lipsky et al., 2001) contributes to a wide range of severe adverse side effects (e.g., respiratory depression, peripheral neuropathy, cardiovascular complications, compromised liver function) (Chick, 1999; Wright et al., 1993). Disulfiram also inhibits plasma esterases to slow the elimination of cocaine, resulting in elevated peak plasma cocaine levels and, therefore, the potential for cocaine toxicity (Baker et al., 2007; McCance-Katz et al., 1998a, 1998b). Additionally, intravenous (IV) administration of alcohol in cocaine-dependent participants during disulfiram treatment produced adverse electrocardiogram (ECG) changes and a pronounced disulfiram ethanol reaction (Roache et al., 2011). Taken together, the practicality of employing disulfiram pharmacotherapy for cocaine use disorder is limited by its overall lack of specificity of action.
Nepicastat (SYN117; RS-25560-197) demonstrates significantly higher potency as a DβH inhibitor (IC50 = 9 nM) as compared to disulfiram (IC50 ≅ 1000 nM) and greater selectivity over other enzymes (Stanley et al., 1997) (K. Walker, Roche Biosciences, personal communication). In line with its actions as a DβH inhibitor, nepicastat effectively reduces NE and enhances levels of DA in the brain (Bourdelat-Parks et al., 2005). A recent microdialysis study in rats revealed that nepicastat differentially affects DA and NE release in the corticostriatal circuit (Devoto et al., 2014), reducing NE release both in the medial prefrontal cortex (mPFC) and in the nucleus accumbens (NAc), and increasing DA release in the mPFC but not in the NAc. In addition, the authors reported that nepicastat markedly potentiated cocaine- and amphetamine-induced extracellular DA accumulation in the mPFC, but not in the NAc (Devoto et al., 2014).
Like disulfiram, nepicastat blocks cocaine-primed reinstatement of cocaine-seeking in rats at a dose that significantly reduced brain NE levels (Schroeder et al., 2010). In a more recent study, nepicastat significantly lowered the breakpoint for cocaine in rats, and attenuated cue-, footshock-, or yohimbine-induced reinstatement (Schroeder et al., 2013). These data indicate that nepicastat reduces the reinforcing properties of cocaine under a stringent schedule and attenuates relapse-like behavior produced by cocaine, formerly cocaine-paired cues, and physiological and pharmacological stressors. In a drug discrimination study, pretreatment with either disulfiram or nepicastat produced leftward shifts in the cocaine dose–response function and also conferred cocaine-like stimulus effects to the selective NE transporter inhibitor reboxetine. These results suggest that pharmacological inhibition of DβH functionally enhances the interoceptive stimulus effects of cocaine possibly due to facilitated increases in DA released from noradrenergic terminals (Manvich et al., 2013).
Nepicastat is well tolerated in healthy adults and in patients with congestive heart failure (Hegde and Friday, 1998) (Roche Biosciences, unpublished observations) and may represent a better tolerated, more efficacious treatment for cocaine use disorder than disulfiram. As such, the present study was initiated to evaluate the safety of administration of oral nepicastat in combination with IV cocaine to participants who metcriteria for cocaine use disorder in an inpatient, hospital-based setting. We hypothesized that nepicastat would have minimal effects on the pharmacokinetic and cardiovascular properties of cocaine and would reduce the positive subjective effects produced by cocaine.
2. Methods
2.1. Subjects
Twenty non-treatment seeking participants who metcriteria for cocaine use disorder were recruited through advertisements and randomized into the study. All subjects metDiagnostic and Statistical Manual (DSM) of Mental Disorders criteria for cocaine use disorder, determined using the MINI neuropsychiatric interview (Sheehan et al., 1998), and did not meetuse disorder criteria for other psychotropic drugs, other than nicotine or marijuana. DSM-IV-TR diagnosis criteria for “substance dependence” has been shown to correlate with DSM-5 diagnostic criteria for “substance use disorders”, therefore we use DSM-5 nomenclature throughout this manuscript for consistency purposes (Compton et al., 2013). Subjects were required to have recently used cocaine by the smoking or IV route, and all randomized subjects supplied a cocaine positive urine sample collected within the 30 days prior to admission. Subjects were required to be 18–50 years of age, and to be otherwise healthy, as confirmed by physical examination and routine laboratory testing, including an ECG. Exclusion criteria included a history of seizure disorder, brain injury, neurological or psychiatric disorders, organic brain disease, dementia, clinically significant heart disease, hypertension, early cardiovascular morbidity or mortality in first-degree relatives, or other serious medical conditions; history of a previous adverse reaction to cocaine; currently pregnant or nursing; asthma or current use of alpha or beta agonists, theophylline, or other sympathomimetics; current status as parolee; or inadequate veins to allow peripheral access. Subjects were compensated for study participation. The study was approved by The University of Texas Medical Branch at Galveston (UTMB) Institutional Review Board. All procedures were in accordance with the ethical standards of the responsible committee on human experimentation (institutional or regional) or with the Helsinki Declaration of 1975 (as revised in 1983) and all subjects provided written informed consent after being fully informed about potential risks of participation. Subject confidentiality was protected by a Certificate of Confidentiality issued by the FDA.
2.2. Study design
This randomized, double-blind, placebo-controlled study was conducted in the UTMB General Clinical Research Center (GCRC). Subjects were admitted to the GCRC and remained hospitalized for a period of 15 days to allow close monitoring of adverse events (AEs), prevent unauthorized drug use and ensure daily compliance with medications and all study procedures.
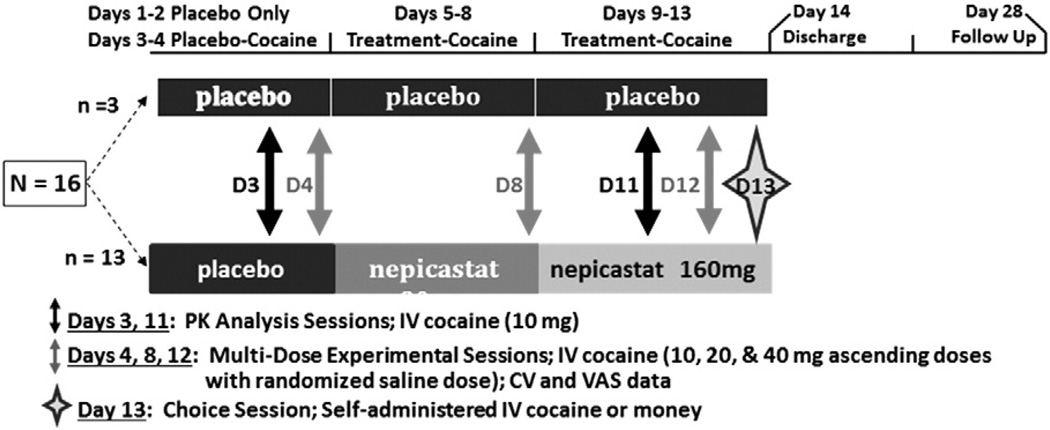
Among study completers, participants were randomized to study medication (n = 13) using an escalating design (placebo on Days 1–4; 80 mg nepicastat on Days 5–8; 160 mg nepicastat on Days 9–13) or administered placebo (n = 3; control condition included to maintain the blind) throughout the study (13 days). Placebo or nepicastat was dosed once daily and the 4-day interval was chosen to reach steady state levels. As noted in Fig. 1, each participant who completed the study was exposed to five cocaine infusion sessions, differentiated by three sets of procedures: a cocaine pharmacokinetic analysis [Day 3 (prior to receiving nepicastat) and Day 11]; a multi-dose experimental session (Days 4, 8, and 12) and a choice session (Day 13).
Fig. 1.
Study design. The study schema depicts the entire 28-day procedure. Subjects randomized to the placebo group (n = 3) received placebo for 13 days, while those randomized to the nepicastat group (n = 13) received placebo on Days 1–4, 80 mg of nepicastat on Days 5–8, and 160 mg of nepicastat on Days 9–13. Pharmacokinetic analyses were conducted following 10 mg of cocaine IV on Days 3 and 11. Multi-dose experimental sessions for safety and efficacy were conducted on Days 4, 8, and 12. Data were also obtained during Day 13 for the Choice Session.
2.3. Drugs
Nepicastat hydrochloride [(S)-5-aminomethyl-1-(5,7-difluoro- 1,2,3,4-tetrahydronaphth-2-yl)-1,3-dihydroimidazole-2-thione hydrochloride monohydrate], supplied as 40 mg (free base equivalent) capsules, and matched placebo (containing microcrystalline cellulose) were packaged, labeled and distributed by Aptuit, Inc. (Kansas City, MO). Human use cocaine HCl was provided in 2 mL vials containing 20 mg/mL by Research Triangle Institute International (Research Triangle Park, NC). On infusion days, the UTMB pharmacist diluted the cocaine stock solution with sterile saline (0.9% sodium chloride) to create the required dosages (0, 10, 20, and 40 mg); each contained in a volume of 10 mL. This volume of cocaine solution or placebo (sterile saline) was administered IV over 2 min by the study cardiologist. The use of nepicastat in this study was under an IND at the FDA.
2.4. Safety
AEs were summarized by Medical Dictionary for Drug Regulatory Affairs system organ class, preferred term and observation period (placebo-only period, placebo-cocaine period and treatment-cocaine period) for overall incidence, incidence by severity, incidence by relationship to study drug and incidence by relationship to cocaine.
In addition, vital signs were obtained prior to and 15, 30, and 60 min after daily nepicastat/placebo administration (data not shown). ECG monitoring occurred continuously and was recorded prior to each cocaine dose and 60 min after the last infusion (data not shown).
A study cardiologist (D.L.W., J.B.J.) was present during all cocaine infusion sessions to carefully monitor heart rate, blood pressure and ECG wave form. Stopping rules were in place to halt cocaine administration under conditions in which cardiovascular indices exceeded preset values, although cocaine was not withheld for subjects during any of the multi-dose experimental sessions (Day 4, 8 or 12). This included clinically significant arrhythmias, resting heart rate >130 beats/min, blood pressure >165 mm Hg systolic and 100 mm Hg diastolic. In addition, repeated doses of cocaine were not administered (and the study physician halted continued cocaine delivery) if behavioral manifestations of cocaine toxicity (e.g., agitation, psychosis) or inability to cooperate with study procedures were noted by the cardiologist.
2.5. Pharmacokinetic analyses
The interaction effects of nepicastat on pharmacokinetics (PK) of cocaine and its major metabolites were assessed by measuring plasma PK parameters following IV infusion of 10 mg of cocaine during treatment with placebo (on Day 3) versus treatment with 160 mg nepicastat or placebo (Day 11). At the time of cocaine PK assessments on study Day 11, subjects randomized to nepicastat had received seven daily doses of nepicastat [four doses of 80 mg (Days 5–8); three doses of 160 mg (Days 9–11)]. Blood samples were collected at −15, 5, 10, 20, 30, 45, 60, 90, 120, 180, 240, 300, 360, 420 and 480 min following dosing of 10 mg of cocaine. Blood was collected into Vacutainer tubes containing potassium oxalate and sodium fluoride to inhibit cocaine hydrolysis by plasma cholinesterases. After separation of plasma by routine centrifugation, samples were frozen at −70 °C until analysis. Plasma levels of cocaine and metabolites were assayed by liquid chromatography–tandem mass spectrometry (LC/MS/MS) with a lower limit of quantitation of 2.5 ng/mL at the University of Utah Center for Human Toxicology under the direction of David E. Moody, Ph.D. Cocaine, benzoylecgonine, ecgonine methyl ester, and norcocaine (where detectable) were measured. Residual samples were destroyed after analyses. To determine nepicastat plasma concentrations, additional blood samples were collected on Day 3, 15 min prior to cocaine administration and on Day 11 at approximately 2 and 6 h after the morning dose of nepicastat or placebo, corresponding to 15 min prior to and 240 min following cocaine administration. Bioanalysis was performed using validated LC/MS/MS methodology, with a linear quantitation range of 0.5–500 ng/mL at Frontage Laboratories, Malvern, PA.
2.6. Cardiovascular, subjective and reinforcement measures
Cocaine-induced cardiovascular and subjective effects were evaluated for completers in the cohort randomized to nepicastat (n = 13) using a within-subjects statistical analysis strategy. Specifically, the cardiovascular and subjective effects of cocaine were assessed in the presence of placebo (0 mg), 80 mg of nepicastat or 160 mg of nepicastat on study Days 4, 8 and 12, respectively. Beginning at 10:00 am, at hourly intervals, subjects received receive three ascending doses of cocaine (10, 20, and 40 mg, IV) with a saline dose (0 mg cocaine) randomly administered before, between or after to maintain the blind.
Heart rate and blood pressure were recorded at 15 min prior to each of the four infusions, 5, 10, 15, 30 and 45 min following each cocaine dose, and 60, 90, 120, 180, and 240 min following the fourth infusion (extended time course—60–240 min not shown).
To assess subjective effects, on Days 4, 8 and 12, visual analogue scales (VAS) were completed before each cocaine dose, and 10, 15, 30 and 45 min after every infusion. VAS data were collected for ratings of “Any Drug Effect”, “Good Effects”, “Pay” (i.e., “Howmuch are you willing to pay for the infusion you just received-in dollars?”), “Like”, “Desire Cocaine”, “Want Cocaine”, “Crave Cocaine”, “High”, “Likely to Use if Given Access”, “Stimulating”, “Anxious”, “Bad Effects”, and “Depressed”. These scales ranged from 0 (no effect) to 100 (greatest effect ever).
To evaluate reinforcing effects, on Day 13, subjects participated in two self-administration sessions making 10 choices for cocaine (0 or 20 mg, IV) or money. The first session began at 10:00 am and the second session began at 1:00 pm. In one session, subjects made 10 choices for 0 mg cocaine, IV or money. During the other self-administration session, subjects made 10 choices between 20 mg cocaine, IV and money. The subject made a series of choices between ascending value money options ($.05, $.05, $.05, $.05, $1, $4, $7, $10, $13, and $16) or cocaine (0 or 20 mg/IV/infusion) using a patient-controlled analgesia (PCA) pump (Lynch et al., 2006; Sughondhabirom et al., 2005). Infusions took place over 2-min followed by a 13-min time-out period.
2.7. Statistical analyses
Data were analyzed using StatView 5.0 (SAS Institute Inc., Cary, NC). For all measures, statistical significance was set at p < 0.05. All data are presented as mean ± standard deviation (S.D.). Descriptive statistics were compiled for demographic information, drug use variables, and daily measures (Beck Depression Inventory and Brief Substance Craving Scale) and analyzed using appropriate parametric or non-parametric tests.
Pharmacokinetic parameters were calculated based on the plasma concentration of cocaine and the metabolites benzoylecgonine and ecgonine methyl ester for the baseline cocaine infusion (10 mg, IV, on Day 3) and the post-treatment cocaine infusion (10 mg, IV, on Day 11). Plasma concentration-time profiles were analyzed to obtain PK parameter estimates of cocaine, including maximum concentration (Cmax), area under curve (AUC), apparent half-life (t½), clearance and volume of distribution. Within and between group comparisons were performed using Wilcoxon signed-rank tests.
To determine peak effects (the maximum response observed at any time-point), cardiovascular and VAS data from the nepicastat group (n = 13) only were analyzed using one way ANOVA as a function of nepicastat dose (0, 80, or 160 mg) and cocaine dose (0, 10, 20 or 40 mg). To determine effects across the time-course, cardiovascular and VAS data were analyzed using repeated measures ANOVA as a function of nepicastat dose (0, 80, or 160 mg), cocaine dose (0 or 20 mg) and time (in minutes).
To evaluate the reinforcing effects of cocaine, self-administration data were analyzed using one way ANOVA as a function of nepicastat dose (0 or 160 mg) and cocaine dose (0 or 20 mg).
3. Results
3.1. Demographics and drug use
Demographic information and drug-use data for randomized subjects (nepicastat; N = 15 and placebo; N = 5) are provided in Table 1. In brief, participants randomized to treatment groups were statistically similar. The majority of participants were males who were African American, preferred using cocaine by the smoked route of administration, and also used alcohol and marijuana on occasion.
Table 1.
Demographics and drug use.
| Nepicastat (n = 15) | Placebo (n = 5) | |
|---|---|---|
| Gender (n) | ||
| Male | 13 | 4 |
| Female | 2 | 1 |
| Race/ethnicity (n) | ||
| Caucasian | 7 | 2 |
| Hispanic | 1 | 0 |
| African American | 7 | 3 |
| Age (years) | 40.46 ± 1.3 | 37.8 ± 2.2 |
| Education (years) | 11.90 ± 0.2 | 12.2 ± 0.2 |
| Cocaine use | ||
| Years of use | 16.9 ± 1.9 | 16.8 ± 2.9 |
| Last 30 days usea | 22.5 ± 1.2 | 26.4 ± 2.8 |
| Cocaine primary route of admin (n) | ||
| Smoke | 13 | 5 |
| IV | 2 | 0 |
| Alcohol use (n) | 15/15 | 5/5 |
| Years of use | 15.1 ± 2.1 | 15.6 ± 5.7 |
| Last 30 days use | 10.1 ± 2.4 | 17.8 ± 7.3 |
| Marijuana use (n) | 13/15 | 4/5 |
| Years of use | 12.4 ± 2.7 | 13.2 ± 4.6 |
| Last 30 days use | 7.2 ± 2.7 | 9.4 ± 6.6 |
Data reflect number of subjects (n) or mean ± S.E.M.
Last 30 days use reflected the 30 days preceding screening for the current study.
The demographic data for study completers (n = 13), used for analysis of cardiovascular and subjective effects data, were statistically similar to that described for the n = 15 participants in the nepicastat group included in the safety analyses shown in Table 1.
Of the 20 randomized subjects, 15 subjects completed the entire study (“completers” comprising n = 13 in the nepicastat and n = 3 in the placebo groups, which was one more than planned in the nepicastat group and one less in the placebo group). Four subjects discontinued the study prior to completion (two randomized to nepicastat and two to placebo). Of these, three subjects withdrew consent for personal reasons and one subject was discontinued by the investigators on study Day 4 due to viral gastroenteritis.
3.2. Adverse events (AEs)
Details of AEs (present in two or more subjects) are shown for randomized subjects (Table 2). In brief, the majority were mild with only one event reported as moderate (symptoms of cocaine intoxication) and no AEs were reported as severe or very severe/life-threatening. One subject reported paresthesia during baseline cocaine infusion. In addition, there were no serious AEs experienced in this study and no discontinuations from the study due to AEs.
Table 2.
Incidence of adverse events reported in >2 subjects by preferred term, period & treatment group.
| Preferred term | Placebo-only | Placebo–Cocaine | Treatment–Cocaine | |||
|---|---|---|---|---|---|---|
| Nepicastat (n = 15) |
Placebo (n = 5) |
Nepicastat (n = 15) |
Placebo (n = 5) |
Nepicastat (n = 14)a |
Placebo (n = 4)b |
|
| Insomnia | 2 (13) | 0 (0) | 1 (6) | 1 (20) | 4 (29) | 1 (25) |
| Vessel puncture site pain | 0 (0) | 0 (0) | 2 (13) | 1 (20) | 4 (29) | 0 (0) |
| Dyspepsia | 0 (0) | 0 (0) | 1 (6) | 0 (0) | 2 (14) | 1 (25) |
| Gastroesophageal reflux disease | 1 (6) | 0 (0) | 0 (0) | 0 (0) | 3 (21) | 0 (0) |
| Headache | 0 (0) | 1 (20) | 1 (6) | 0 (0) | 2 (14) | 1 (25) |
| Neck pain | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 2 (14) | 1 (25) |
| Arthralgia | 0 (0) | 0 (0) | 1 (6) | 0 (0) | 2 (14) | 0 (0) |
| Dizziness | 0 (0) | 0 (0) | 0 (0) | 1 (20) | 0 (0) | 2 (50) |
| Back pain | 1 (6) | 1 (20) | 0 (0) | 0 (0) | 0 (0) | 1 (25) |
| Myalgia | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 2 (50) |
| Nausea | 1 (6) | 0 (0) | 0 (0) | 0 (0) | 1 (7) | 1 (25) |
| Depressed mood | 1 (6) | 0 (0) | 0 (0) | 0 (0) | 1 (7) | 1 (25) |
| Abnormal dreams | 2 (13) | 0 (0) | 0 (0) | 1 (20) | 0 (0) | 1 (25) |
| Vessel puncture site hematoma | 0 (0) | 0 (0) | 2 (13) | 0 (0) | 0 (0) | 0 (0) |
Values reflect the number (%) of subjects.
Subject 117 completed the “placebo only” and “placebo–cocaine” periods only.
Subject 112 completed the “placebo-only” period and a partial “placebo–cocaine” period.
3.3. Pharmacokinetics
Summary statistics of key pharmacokinetic parameters for cocaine determined from plasma samples collected following IV administration of 10 mg of cocaine during the placebo phase (study Day 3) or following dosing with 160 mg nepicastat or placebo (study Day 11) are presented in Table 3. In the nepicastat group, mean Cmax decreased from 98 ng/mL measured at the end of the placebo run-in period (Day 3) to 75 ng/mL at Day 11 (following 7 days of dosing with nepicastat), a 23% decrease. The placebo group had a similar decrease in magnitude. The AUC0-last and AUC∞ showed similar decreases between the two time points in both treatment groups. The apparent terminal elimination half-life remained unchanged in the nepicastat group with mean values of 82 and 81 min at Day 3 and Day 11, respectively. Consistent with decreasing AUC and stable T1/2, clearance increased somewhat in both treatment groups between Days 3 and 11 (a 12% increase in the nepicastat group, from 2.1 L/min to 2.4 L/min, respectively). None of these differences were statistically significant by the Wilcoxon signed-rank test.
Table 3.
Pharmacokinetic parameters following cocaine infusion (10 mg, IV).
| Study Day 3a | Study Day 11 | |||
|---|---|---|---|---|
| Parameter | Nepicastat 0 mg (n = 13) |
Placebo (n = 3) |
Nepicastat 160 mg (n = 13) |
Placebo (n = 3) |
| Cmax [ng/mL] | ||||
| Mean (SD) | 97.97 (46.48) | 86.20 (18.57) | 75.37 (29.35) | 62.80 (23.11) |
| Median | 93.40 | 94.00 | 70 | 65 |
| CV (%) | 47 | 22 | 39 | 37 |
| AUC0–last [min * ng/mL] | ||||
| Mean (SD) | 5080 (2437) | 4607 (1351) | 3975 (807) | 3931 (1365) |
| Median | 4867 | 4778 | 4196 | 3691 |
| CV (%) | 48 | 29 | 20 | 35 |
| AUC∞ [min * ng/mL] | ||||
| Mean (SD) | 5469 (2439) | 5033 (1412) | 4361 (846) | 4192 (1416) |
| Median | 5180 | 5135 | 4540 | 3977 |
| CV (%) | 45 | 28 | 19 | 34 |
| T1/2 [min] | ||||
| Mean (SD) | 81.9 (23.6) | 91.6 (35.1) | 80.7 (37.0) | 66.6 (13.8) |
| Median | 76.9 | 82.5 | 70.3 | 70.7 |
| CV (%) | 29 | 38 | 46 | 21 |
| Clearance [mL/min] | ||||
| Mean (SD) | 2068 (652) | 2104 (632) | 2387 (530) | 2574 (852) |
| Median | 1930 | 1947 | 2202 | 2515 |
| CV (%) | 32 | 30 | 22 | 33 |
| Volume of distribution [L] | ||||
| Mean (SD) | 234.6 (75.7) | 258.8 (32.1) | 267.5 (99.0) | 236.2 (34.0) |
| Median | 211.9 | 250.5 | 226.8 | 255.2 |
| CV (%) | 32 | 12 | 37 | 14 |
Abbreviations: AUC, area under the curve; Cmax, maximum concentration; CV, coefficient of variability (= standard deviation / mean); SD, standard deviation; T1/2, apparent half-life.
The nepicastat group (n = 13) was dosed with placebo from Study Day 1 to 4.
None of the PK parameters for benzoylecgonine or ecgonine methyl ester showed statistically significant changes in response to nepicastat (data not shown). The major metabolite of nepicastat, N-acetyl-nepicastat, was also measured; however, these data are not presented as there was no difference in this metabolite with treatment. Lastly, tests showed that there was no analytical interference between cocaine and nepicastat during their respective LC/MS/MS analyses.
3.4. Cardiovascular effects
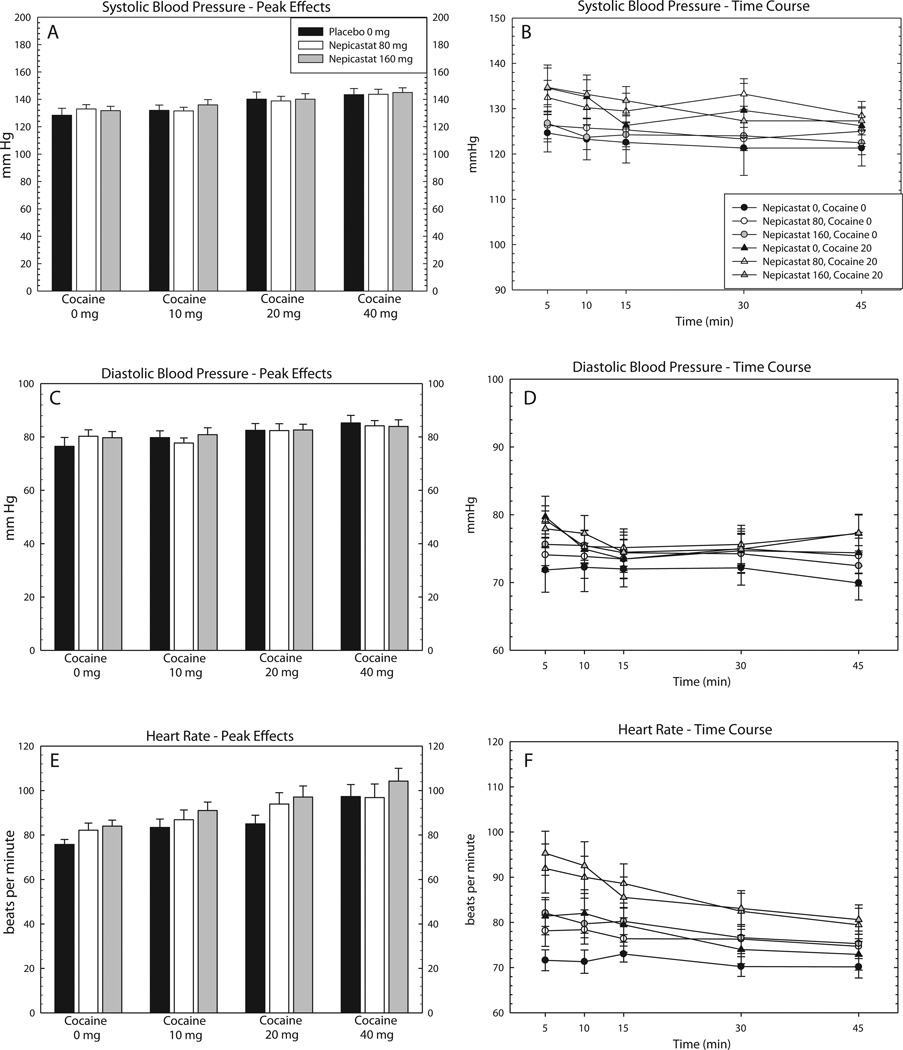
For peak effects, ANOVA did not reveal a main effect of nepicastat on systolic or diastolic blood pressure, though one was detected for heart rate (Table 4). In addition, ANOVA revealed main effects of Cocaine on all three cardiovascular measures, though there were no effects on the Nepicastat × Cocaine interaction. Peak effects data for cardiovascular measures are shown in Fig. 2, panels A, C and E.
Table 4.
Statistical outcomes for peak effect data.
| Nepicastat | Cocaine | Nep × Coc | |
|---|---|---|---|
| Systolic BP | F = 7.1, p = .0002 | ||
| Diastolic BP | F = 3.4, p = .021 | ||
| Heart Rate | F = 3.9, p = .023 | F = 9.6, p < .0001 | |
| Any | F = 5.2, p = .0067 | F = 13.7, p < .0001 | |
| High | F = 3.4, p = .036 | F = 12.0, p < .0001 | |
| Like | F = 3.1, p = .05 | F = 5.4, p = .0014 | |
| Good Effects | F = 6.9, p = 0.0002 | ||
| Stimulated | F = 5.7, p = .004 | F = 5.1, p = .002 | |
| Want | F = 3.2, p = .04 | ||
| Bad Effects | |||
| Desire | F = 3.6, p = .03 | ||
| Refuse | |||
| Depressed | |||
| Anxious | F = 4.7, p = .0108 | ||
| Crave | F = 3.3, p = .04 | ||
| Access | F = 6.6, p = .0018 | ||
| Pay | F = 9.5, p < .0001 | F = 3.5, p = .0182 |
F and p values are shown only for those that reached statistical significance, p < 0.05.
Fig. 2.
Effects of nepicastat plus cocaine on cardiovascular measures. Data are presented for the nepicastat group only (n = 13) as the mean (±S.E.M.) for systolic blood pressure peak effects [A] and full-time course [B], diastolic blood pressure peak effects [C] and full-time course [D], and heart rate peak effects [E] and full-time course [F]. Peak effects are shown following infusions of cocaine (0, 10, 20, and 40 mg) as a function of nepicastat dose (0, 80, and 160 mg), and full time-course data are shown following infusions of cocaine (0 and 20 mg) as a function of nepicastat dose (0, 80, and 160 mg), and time (5, 10, 15, 30 and 45 min).
For time-course, repeated measures ANOVA revealed a main effect of Time for all cardiovascular measures (Table 5). In addition, a main effect of Cocaine × Time detected for systolic blood pressure and heart rate, though there were no Time × Nepicastat × Cocaine interactions. Time course data for cardiovascular measures are shown in Fig. 2, panels B, D and F.
Table 5.
Statistical outcomes for time course data.
| Time | Nep × Time | Coc × Time | Nep × Coc × Time | |
|---|---|---|---|---|
| Systolic BP | F = 22.8, p < .0001 | F = 2.1, p = .0185 | ||
| Diastolic BP | F = 11.3, p < .0001 | |||
| Heart Rate | F = 65.6, p < .0001 | F = 3.4, p < .0001 | ||
| Any | F = 118.9, p < .0001 | F = 4.9, p < .0001 | F = 9.6, p < .0001 | |
| High | F = 113.7, p < .0001 | F = 3.8, p < .0001 | F = 9.7, p < .0001 | |
| Like | F = 40.6, p < .0001 | F = 2.7, p = .0126 | F = 6.1, p < .0001 | |
| Good Effects | F = 52.2, p < .0001 | F = 4.2, p = .0004 | F = 6.6, p < .0001 | |
| Stimulated | F = 38.5, p < .0001 | F = 7.7, p < .0001 | ||
| Want | F = 7.2, p < .0001 | F = 2.7, p = .0042 | ||
| Bad Effects | ||||
| Desire | F = 5.5, p < .0001 | |||
| Refuse | ||||
| Depressed | ||||
| Anxious | F = 2.7, p = .0426 | F = 2.7, p = .0043 | ||
| Crave | ||||
| Access | F = 2.7, p = .0431 | F = 2.2, p = .0409 | ||
| Pay | F = 12.3, p < .0001 | F = 4.3, p = .0003 | F = 2.9, p = .0025 |
F and p values are shown only for those that reached statistical significance, p < 0.05.
3.5. Subjective effects
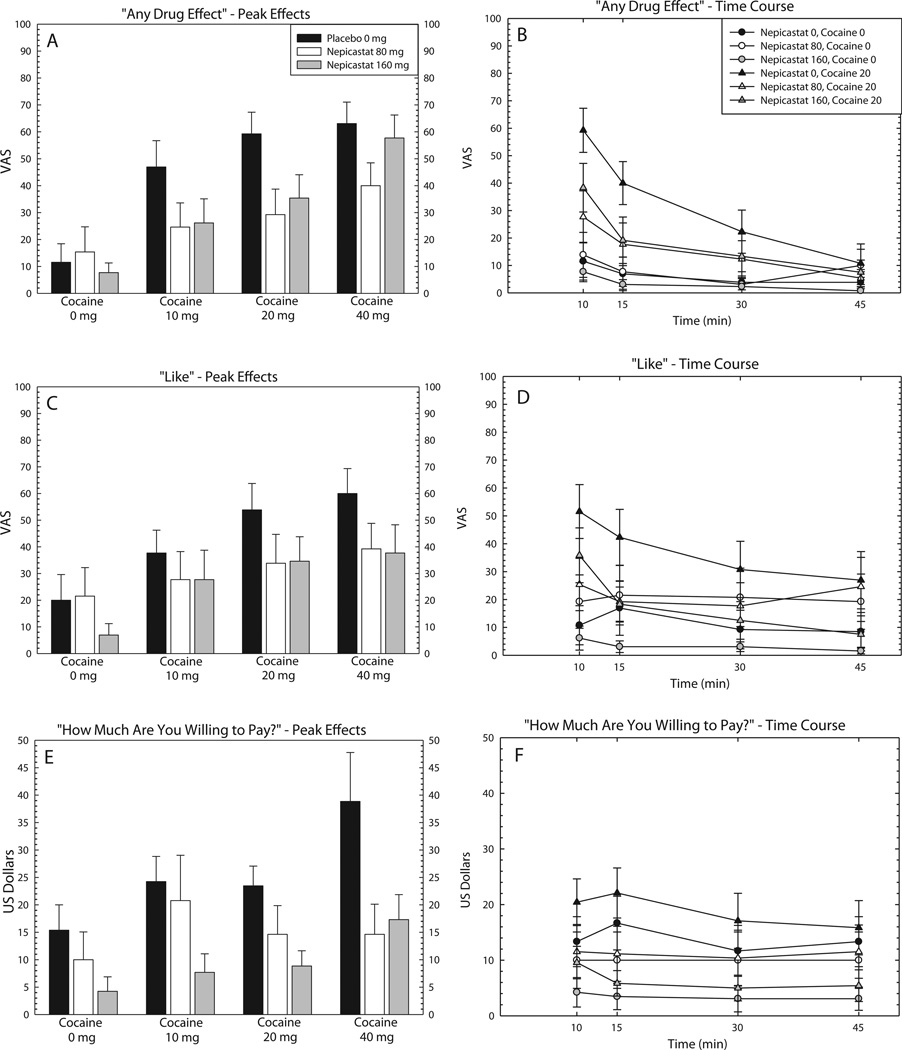
For peak effects, ANOVA revealed a main effect of Nepicastat on several positive subjective effects (Table 4). In addition, ANOVA revealed main effects of Cocaine on several positive subjective effects, although there was no Nepicastat × Cocaine interaction. Peak effects data are shown for “Any Drug Effect”, “Like”, and “How Much Are You Willing to Pay?” in Fig. 3, panels A, C and E.
Fig. 3.
Effects of nepicastat plus cocaine on subjective measures. Data are presented for the nepicastat group only (n = 13) as the mean (±S.E.M.) for “Any Drug Effect” peak effects [A] and full-time course [B], “Good Effects” peak effects [C] and full-time course [D], and “How Much Are You Willing to Pay” peak effects [E] and full-time course [F]. Peak effects are shown following infusions of cocaine (0, 10, 20, and 40 mg) as a function of nepicastat dose (0, 80, and 160 mg), and full time-course data are shown following infusions of cocaine (0 and 20 mg) as a function of nepicastat dose (0, 80, and 160 mg), and time (10, 15, 30 and 45 min).
For time-course, repeated measures ANOVA revealed a main effect of Time for almost all positive subjective effects and the negative subjective effect Anxious (Table 5). In addition, a main effect of Nepicastat × Time and a main effect of Cocaine × Time was detected for several positive subjective effects, though there were no Time × Nepicastat × Cocaine interactions. Time course data are shown for the same adjectives above in Fig. 3, panels B, D and F
3.6. Reinforcing effects
An ANOVA did not reveal a main effect of Nepicastat (F1,28 = .002, p = 0.96) or Cocaine (F1,28 = 2.53, p = 0.12), and no Nepicastat × Cocaine interaction (F1,28 = .013, p = 0.91).
4. Discussion
The primary aim of this study was to determine the safety of treatment with the selective DβH inhibitor nepicastat during cocaine exposure in a controlled clinical setting. The number, type, severity and duration of AEs during placebo and nepicastat treatment were comparable, indicating that nepicastat was safe and well-tolerated in this cohort of individuals who metcriteria for cocaine use disorder. Of importance, nepicastat did not significantly alter the cardiovascular effects or pharmacokinetic characteristics of cocaine. Together, these data suggest that cocaine intake experienced during nepicastat pharmacotherapy would not result in deleterious adverse effects.
Preclinical data indicate that nepicastat modulates sympathetic drive to the cardiovascular system with a mild pressor effect in various animal models of hypertension (Stanney et al., 1998). However in this clinical study, the doses of nepicastat employed did not significantly alter systolic or diastolic blood pressure or heart rate alone, and did not affect the anticipated transient increase in heart rate and blood pressure evoked by cocaine. Furthermore, unlike disulfiram, nepicastat did not change the kinetics, metabolism or clearance of cocaine or its metabolites. The lack of effects of nepicastat upon the cardiovascular and pharmacokinetic profile evoked by cocaine suggests that the selective targeting of nepicastat for the DβH enzyme improves the safety profile of this compound as compared to the nonselective DβH inhibitor disulfiram. Disulfiram treatment alone has been reported to significantly elevate heart rate and, when combined with cocaine, significantly enhanced the effects of cocaine to increase heart rate and blood pressure (McCance-Katz et al., 1998a, 1998b), although another group did not report these effects (Baker et al., 2007). Additionally, disulfiram consistently produces elevated plasma cocaine levels (Baker et al., 2007; Hameedi et al., 1995; McCance-Katz et al., 1998a, 1998b), likely due to its inhibition of plasma cholinesterases and plasma and microsomal carboxylesterases, which are critical for cocaine metabolism (Benowitz, 1993). These potentially dangerous cardiovascular and metabolic interactions of disulfiram with cocaine along with its medically significant interactions with alcohol (Roache et al., 2011) have limited the utility of disulfiram as a pharmacotherapy. The present findings suggest that nepicastat represents a safer alternative to disulfiram for evaluation in cocaine pharmacotherapy trials, as was recently completed (NCT01704196).
A secondary goal of the present study was to gain insight into the potential efficacy of nepicastat as a treatment for cocaine use disorder. To this end, we evaluated the potential for nepicastat to alter the subjective effects produced by cocaine. As demonstrated previously (De La Garza et al., 2014; Hameedi et al., 1995; Newton et al., 2005; Verrico et al., 2014), acute cocaine exposure in this study increased several positive subjective effects. Analyses revealed a main effect of nepicastat to reduce several cocaine-induced positive subjective effects, though the cocaine by nepicastat interaction did not reach statistical significance. Although the attenuation of subjective effects of cocaine produced by nepicastat is encouraging, it is important to concede that these measures can produce false positives (Comer et al., 2008; Haney and Spealman, 2008). Previous studies have described variable effects of disulfiram upon the subjective effects produced by cocaine. Specifically, disulfiram decreased (Baker et al., 2007), had no effect (McCance-Katz et al., 1998a, 1998b), or evoked a non-significant trend to increase cocaine “high” (McCance-Katz et al., 1998a, 1998b). The exact nature of this variability across studies is unknown, but may be due to differences in drug dosing and/or route of cocaine administration (Baker et al., 2007). An additional factor that may have contributed to the variability observed when evaluating the effects of DβH inhibitors in humans is the existence of polymorphisms in the DβH gene that alter the function of the enzyme. For example, the single nucleotide polymorphism (SNP) C-1021T (-1021C>T) leads to decreased basal levels of DβH (Zabetian et al., 2001), and this SNP appears to control disulfiram response in cocaine-dependent subjects (Kosten et al., 2013; Schottenfeld et al., 2014). Thus, the specific allel expression and associated differences in DβH enzyme levels among individual subjects may produce varied responses to administration of the DβH inhibitor, thereby contributing to the variability in subjective effects reported in the current study. In the current study, DβH genotype appeared to influence responses to cocaine and also responses produced by nepicastat (data to be presented elsewhere). The current data add further credence to the theory that selective inhibition of DβH with nepicastat will be effective at reducing cocaine use in patients with cocaine use disorder. The observed effects for nepicastat to reduce (~25%) the positive subjective effects produced by cocaine are encouraging and support further evaluation of this drug as a pharmacotherapy for cocaine use disorder.
In the current study, cocaine did not significantly increase negative subjective effects and nepicastat did not increase these responses. This outcome is somewhat surprising given that the most commonly reported effect to date has been to increase the aversive properties of cocaine such as anxiety, paranoia, and psychosis in humans or in rodent models (Gaval-Cruz et al., 2012; Mutschler et al., 2009; Schank et al., 2006). Furthermore, genetic reduction of DBH enhances cocaine-induced paranoia in humans (Cubells et al., 2000; Kalayasiri et al., 2007) and transforms a cocaine conditioned place preference into a place aversion in mice (Schank et al., 2006). This is important since a preferential increase in the aversive effects of cocaine could explain why a medication that enhances cocaine responses (e.g., Haile et al., 2003; Hameedi et al., 1995; Manvich et al., 2013;McCance-Katz et al., 1998a, 1998b) reduces cocaine use. The reason for a lack of effect nepicastat on cocaine-induced negative subjective effects in the current study may be a function of the small sample size, but also the relatively low doses of cocaine evaluated.
The primary limitation of the present study was a small sample size, although the final N is typical of many inpatient studies. The sample size contributes to the possibility that the study was underpowered to detect an effect of nepicastat on some outcome measures. A second limitation was that efficacy data were based solely on participants randomized to nepicastat using a within-subjects study design for statistical analyses. While a more favorable comparison would have been with a group of participants who received placebo for the same study duration (13 days), the within subject comparison was prospectively planned, and placebo was included in the design to maintain the double-blind. A final limitation was that subjects received multiple randomized doses of cocaine at 1-hour intervals within the same session, thus the report for each individual dose of cocaine may have been influenced by previous infusions.
5. Conclusions
The present results demonstrate minimal pharmacokinetic and cardiovascular interactions of nepicastat with cocaine and a main effect of nepicastat to reduce several cocaine-induced positive subjective effects. Taken together, these data support further evaluation of this drug as a pharmacotherapy for cocaine use disorder.
Abbreviations
- AE
Adverse Event
- ANOVA
Analysis of Variance
- AUC
Area Under the Curve
- BP
Blood Pressure
- Cmax
Maximum Concentration
- DA
Dopamine
- DβH
Dopamine-β-hydroxylase
- DSM
Diagnostic and Statistical Manual
- ECG
Electrocardiogram
- FDA
Food and Drug Administration
- GCRC
General Clinical Research Center
- IV
Intravenous
- LC/MS/MS
Liquid Chromatography Tandem Mass Spectrometry
- mPFC
Medial Prefrontal Cortex
- PK
Pharmacokinetics
- NAc
Nucleus Accumbens
- NE
Norepinepherine
- PCA
Patient Controlled Analgesia
- SD
standard deviation
- UTMB
University of Texas Medical Branch
- VAS
Visual Analog Scale
References
- Baker JR, Jatlow P, McCance-Katz EF. Disulfiram effects on responses to intravenous cocaine administration. Drug Alcohol Depend. 2007;87:202–209. doi: 10.1016/j.drugalcdep.2006.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benowitz NL. Clinical pharmacology and toxicology of cocaine. Pharmacol Toxicol. 1993;72:3–12. doi: 10.1111/j.1600-0773.1993.tb01331.x. [DOI] [PubMed] [Google Scholar]
- Bourdelat-Parks BN, Anderson GM, Donaldson ZR, Weiss JM, Bonsall RW, Emery MS, et al. Effects of dopamine beta-hydroxylase genotype and disulfiram inhibition on catecholamine homeostasis in mice. Psychopharmacology (Berl) 2005;183:72–80. doi: 10.1007/s00213-005-0139-8. [DOI] [PubMed] [Google Scholar]
- Carroll KM, Nich C, Ball SA, McCance E, Rounsavile BJ. Treatment of cocaine and alcohol dependence with psychotherapy and disulfiram. Addiction. 1998;93:713–727. doi: 10.1046/j.1360-0443.1998.9357137.x. [DOI] [PubMed] [Google Scholar]
- Carroll KM, Nich C, Ball SA, McCance E, Frankforter TL, Rounsaville BJ. One-year follow-up of disulfiram and psychotherapy for cocaine-alcohol users: sustained effects of treatment. Addiction. 2000;95:1335–1349. doi: 10.1046/j.1360-0443.2000.95913355.x. [DOI] [PubMed] [Google Scholar]
- Carroll KM, Fenton LR, Ball SA, Nich C, Frankforter TL, Shi J, et al. Efficacy of disulfiramand cognitive behavior therapy in cocaine-dependent outpatients: a randomized placebo-controlled trial. Arch Gen Psychiatry. 2004;61:264–272. doi: 10.1001/archpsyc.61.3.264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chick J. Safety issues concerning the use of disulfiram in treating alcohol dependence. Drug Saf. 1999;20:427–435. doi: 10.2165/00002018-199920050-00003. [DOI] [PubMed] [Google Scholar]
- Comer SD, Ashworth JB, Foltin RW, Johanson CE, Zacny JP, Walsh SL. The role of human drug self-administration procedures in the development of medications. Drug Alcohol Depend. 2008 Jul 1;96(1–2):1–15. doi: 10.1016/j.drugalcdep.2008.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Compton WM, Dawson DA, Goldstein RB, Grant BF. Crosswalk between DSM-IV dependence and DSM-5 substance use disorders for opioids, cannabis, cocaine and alcohol. Drug Alcohol Depend. 2013;132:387–390. doi: 10.1016/j.drugalcdep.2013.02.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cubells JF, Kranzler HR, McCance-Katz E, Anderson GM, Malison RT, Price LH, et al. A haplotype at the DBH locus, associated with low plasma dopamine beta-hydroxylase activity, also associates with cocaine-induced paranoia. Mol Psychiatry. 2000 Jan;5(1):56–63. doi: 10.1038/sj.mp.4000657. [DOI] [PubMed] [Google Scholar]
- De La Garza R, II, Galloway GP, Newton TF, Mendelson J, Haile CN, Dib E, et al. Assessment of safety, cardiovascular and subjective effects after intravenous cocaine and lofexidine. Prog Neuropsychopharmacol Biol Psychiatry. 2014;50:44–52. doi: 10.1016/j.pnpbp.2013.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devoto P, Flore G, Saba P, Bini V, Gessa GL. The dopamine beta-hydroxylase inhibitor nepicastat increases dopamine release and potentiates psychostimulant-induced dopamine release in the prefrontal cortex. Addict Biol. 2014;19:612–622. doi: 10.1111/adb.12026. [DOI] [PubMed] [Google Scholar]
- Gaval-Cruz M, Weinshenker D. Mechanisms of disulfiram-induced cocaine abstinence: Antabuse and cocaine relapse. Mol Interv. 2009;9:175–187. doi: 10.1124/mi.9.4.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaval-Cruz M, Liles LC, Iuvone PM, Weinshenker D. Chronic inhibition of dopamine β-hydroxylase facilitates behavioral responses to cocaine in mice. PLoS One. 2012;7(11):e50583. doi: 10.1371/journal.pone.0050583. http://dx.doi.org/10.1371/journal.pone.0050583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- George TP, Chawarski MC, Pakes J, Carroll KM, Kosten TR, Schottenfeld RS. Disulfiram versus placebo for cocaine dependence in buprenorphine-maintained subjects: a preliminary trial. Biol Psychiatry. 2000;47:1080–1086. doi: 10.1016/s0006-3223(99)00310-8. [DOI] [PubMed] [Google Scholar]
- Goldstein M, Anagnoste B, Lauber E, McKeregham MR. Inhibition of dopamine-beta-hydroxylase by disulfiram. Life Sci. 1964;3:763–767. doi: 10.1016/0024-3205(64)90031-1. [DOI] [PubMed] [Google Scholar]
- Haile CN, Kosten TR. Pharmacotherapy for stimulant-related disorders. Curr Psychiatry Rep. 2013;15:415. doi: 10.1007/s11920-013-0415-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haile CN, During MJ, Jatlow PI, Kosten TR, Kosten TA. Disulfiram facilitates the development and expression of locomotor sensitization to cocaine in rats. Biol Psychiatry. 2003 Nov 1;54(9):915–921. doi: 10.1016/s0006-3223(03)00241-5. [DOI] [PubMed] [Google Scholar]
- Haile CN, De La Garza R, II, Mahoney JJ, III, Nielsen DA, Kosten TR, Newton TF. The impact of disulfiram treatment on the reinforcing effects of cocaine: a randomized clinical trial. PLoS One. 2012a;7:e47702. doi: 10.1371/journal.pone.0047702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haile CN, Mahoney JJ, III, Newton TF, De La Garza R., II Pharmacotherapeutics directed at deficiencies associated with cocaine dependence: focus on dopamine, norepinephrine and glutamate. Pharmacol Ther. 2012b;134:260–277. doi: 10.1016/j.pharmthera.2012.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haley TJ. Disulfiram (tetraethylthioperoxydicarbonic diamide): a reappraisal of its toxicity and therapeutic application. Drug Metab Rev. 1979;9:319–335. doi: 10.3109/03602537908993897. [DOI] [PubMed] [Google Scholar]
- Hameedi FA, Rosen MI, McCance-Katz EF, McMahon TJ, Price LH, Jatlow PI, et al. Behavioral, physiological, and pharmacological interaction of cocaine and disulfiram in humans. Biol Psychiatry. 1995;37:560–563. doi: 10.1016/0006-3223(94)00361-6. [DOI] [PubMed] [Google Scholar]
- Haney M, Spealman R. Controversies in translational research: drug self-administration. Psychopharmacology (Berl) 2008 Aug;199(3):403–419. doi: 10.1007/s00213-008-1079-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hegde SS, Friday KF. Dopamine-beta-hydroxylase inhibition: a novel sympathomodulatory approach for the treatment of congestive heart failure. Curr Pharm Des. 1998;4:469–479. [PubMed] [Google Scholar]
- Higgins ST, Budney AJ, Bickel WK, Hughes JR, Foerg F. Disulfiram therapy in patients abusing cocaine and alcohol. Am J Psychiatry. 1993;150:675–676. doi: 10.1176/ajp.150.4.675b. [DOI] [PubMed] [Google Scholar]
- Kalayasiri R, Sughondhabirom A, Gueorguieva R, Coric V, Lynch WJ, Lappalainen J, et al. Dopamine beta-hydroxylase gene (DbetaH) -1021C--> T influences self-reported paranoia during cocaine self-administration. Biol Psychiatry. 2007 Jun 1;61(11):1310–1313. doi: 10.1016/j.biopsych.2006.08.012. [DOI] [PubMed] [Google Scholar]
- Kaufman S, Friedman S. Dopamine-beta-hydroxylase. Pharmacol Rev. 1965;17:71–100. [PubMed] [Google Scholar]
- Kosten TR, Wu G, Huang W, Harding MJ, Hamon SC, Lappalainen J, et al. Pharmacogenetic randomized trial for cocaine abuse: disulfiram and dopamine beta-hydroxylase. Biol Psychiatry. 2013;73:219–224. doi: 10.1016/j.biopsych.2012.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lipsky JJ, Shen ML, Naylor S. Overview—in vitro inhibition of aldehyde dehydrogenase by disulfiram and metabolites. Chem Biol Interact. 2001;130–132:81–91. doi: 10.1016/s0009-2797(00)00224-6. [DOI] [PubMed] [Google Scholar]
- Lynch WJ, Sughondhabirom A, Pittman B, Gueorguieva R, Kalayasiri R, Joshua D, et al. Paradigmto investigate the regulation of cocaine self−administration in human cocaine users: a randomized trial. Psychopharmacology (Berl) 2006 Apr;185(3):306–314. doi: 10.1007/s00213-006-0323-5. [DOI] [PubMed] [Google Scholar]
- Manvich DF, DePoy LM, Weinshenker D. Dopamine beta-hydroxylase inhibitors enhance the discriminative stimulus effects of cocaine in rats. J Pharmacol Exp Ther. 2013;347:564–573. doi: 10.1124/jpet.113.207746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCance-Katz EF, Kosten TR, Jatlow P. Chronic disulfiram treatment effects on intranasal cocaine administration: initial results. Biol Psychiatry. 1998a;43:540–543. doi: 10.1016/S0006-3223(97)00506-4. [DOI] [PubMed] [Google Scholar]
- McCance-Katz EF, Kosten TR, Jatlow P. Disulfiram effects on acute cocaine administration. Drug Alcohol Depend. 1998b;52:27–39. doi: 10.1016/s0376-8716(98)00050-7. [DOI] [PubMed] [Google Scholar]
- Moeller FG, Schmitz JM, Herin D, Kjome KL. Use of stimulants to treat cocaine and methamphetamine abuse. Curr Psychiatry Rep. 2008;10:385–391. doi: 10.1007/s11920-008-0062-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Musacchio JM, Goldstein M, Anagnoste B, Poch G, Kopin IJ. Inhibition of dopamine-beta-hydroxylase by disulfiram in vivo. J Pharmacol Exp Ther. 1966;152:56–61. [PubMed] [Google Scholar]
- Mutschler J, Diehl A, Kiefer F. Pronounced paranoia as a result of cocaine-disulfiram interaction: case report and mode of action. J Clin Psychopharmacol. 2009 Feb;29(1):99–101. doi: 10.1097/JCP.0b013e3181934451. [DOI] [PubMed] [Google Scholar]
- Newton TF, De La Garza R, II, Kalechstein AD, Nestor L. Cocaine and methamphetamine produce different patterns of subjective and cardiovascular effects. Pharmacol Biochem Behav. 2005;82:90–97. doi: 10.1016/j.pbb.2005.07.012. [DOI] [PubMed] [Google Scholar]
- Newton TF, De La Garza R, II, Brown G, Kosten TR, Mahoney JJ, III, Haile CN. Noradrenergic alpha(1) receptor antagonist treatment attenuates positive subjective effects of cocaine in humans: a randomized trial. PLoS One. 2012;7:e30854. doi: 10.1371/journal.pone.0030854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrakis IL, Carroll KM, Nich C, Gordon LT, McCance-Katz EF, Frankforter T, et al. Disulfiram treatment for cocaine dependence in methadone-maintained opioid addicts. Addiction. 2000;95:219–228. doi: 10.1046/j.1360-0443.2000.9522198.x. [DOI] [PubMed] [Google Scholar]
- Roache JD, Kahn R, Newton TF, Wallace CL, Murff WL, De La Garza R, II, et al. A double-blind, placebo-controlled assessment of the safety of potential interactions between intravenous cocaine, ethanol, and oral disulfiram. Drug Alcohol Depend. 2011;119:37–45. doi: 10.1016/j.drugalcdep.2011.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schank JR, Ventura R, Puglisi-Allegra S, Alcaro A, Cole CD, Liles LC, et al. Dopamine beta-hydroxylase knockout mice have alterations in dopamine signaling and are hypersensitive to cocaine. Neuropsychopharmacology. 2006 Oct;31(10):2221–2230. doi: 10.1038/sj.npp.1301000. [DOI] [PubMed] [Google Scholar]
- Schottenfeld RS, Chawarski MC, Cubells JF, George TP, Lappalainen J, Kosten TR. Randomized clinical trial of disulfiram for cocaine dependence or abuse during buprenorphine treatment. Drug Alcohol Depend. 2014;136:36–42. doi: 10.1016/j.drugalcdep.2013.12.007. [DOI] [PubMed] [Google Scholar]
- Schroeder JP, Cooper DA, Schank JR, Lyle MA, Gaval-Cruz M, Ogbonmwan YE, et al. Disulfiram attenuates drug-primed reinstatement of cocaine seeking via inhibition of dopamine beta-hydroxylase. Neuropsychopharmacology. 2010;35:2440–2449. doi: 10.1038/npp.2010.127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schroeder JP, Alisha Epps S, Grice TW, Weinshenker D. The selective dopamine beta-hydroxylase inhibitor nepicastat attenuates multiple aspects of cocaine-seeking behavior. Neuropsychopharmacology. 2013;38:1032–1038. doi: 10.1038/npp.2012.267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheehan DV, Lecrubier Y, Sheehan KH, Amorim P, Janavs J, Weiller E, et al. The mini-international neuropsychiatric interview (M.I.N.I.): the development and validation of a structured diagnostic psychiatric interview for DSM-iv and ICD-10. J Clin Psychiatry. 1998;59(Suppl. 20):22–33. [quiz 34–57]. [PubMed] [Google Scholar]
- Shorter D, Lindsay JA, Kosten TR. The alpha-1 adrenergic antagonist doxazosin for treatment of cocaine dependence: a pilot study. Drug Alcohol Depend. 2013;131:66–70. doi: 10.1016/j.drugalcdep.2012.11.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sofuoglu M, Sewell RA. Norepinephrine and stimulant addiction. Addict Biol. 2009;14:119–129. doi: 10.1111/j.1369-1600.2008.00138.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanley WC, Li B, Bonhaus DW, Johnson LG, Lee K, Porter S, et al. Catecholamine modulatory effects of nepicastat (rs-25560-197), a novel, potent and selective inhibitor of dopamine-beta-hydroxylase. Br J Pharmacol. 1997;121:1803–1809. doi: 10.1038/sj.bjp.0701315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanney K, Salvendy G, Deisinger J, DiZio P, Ellis S, Ellison J, et al. After effects and sense of presence in virtual environments: formulation of a research and development agenda. Int J Hum Comput Interact. 1998;10:135–187. doi: 10.1207/s15327590ijhc1002_3. [DOI] [PubMed] [Google Scholar]
- Sughondhabirom A, Jain D, Gueorguieva R, Coric V, Berman R, Lynch WJ. A paradigm to investigate the self−regulation of cocaine administration in humans. Psychopharmacology (Berl) 2005 Jul;180(3):436–446. doi: 10.1007/s00213-005-2192-8. [DOI] [PubMed] [Google Scholar]
- Verrico CD, Haile CN, Mahoney JJ, III, Thompson-Lake DG, Newton TF, De La Garza R. Treatment with modafinil and escitalopram, alone and in combination, on cocaine-induced effects: a randomized, double blind, placebo-controlled human laboratory study. Drug Alcohol Depend. 2014;141:72–78. doi: 10.1016/j.drugalcdep.2014.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vocci FJ, Elkashef A. Pharmacotherapy and other treatments for cocaine abuse and dependence. Curr Opin Psychiatry. 2005;18:265–270. doi: 10.1097/01.yco.0000165596.98552.02. [DOI] [PubMed] [Google Scholar]
- Wright C, Moore RD, Grodin DM, Spyker DA, Gill EV. Screening for disulfiram-induced liver test dysfunction in an inpatient alcoholism program. Alcohol Clin Exp Res. 1993;17:184–186. doi: 10.1111/j.1530-0277.1993.tb00745.x. [DOI] [PubMed] [Google Scholar]
- Zabetian CP, Anderson GM, Buxbaum SG, Elston RC, Ichinose H, Nagatsu T, et al. A quantitative-trait analysis of human plasma-dopamine beta-hydroxylase activity: evidence for a major functional polymorphism at the DBH locus. Am J Hum Genet. 2001;68:515–522. doi: 10.1086/318198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang XY, Kosten TA. Prazosin, an alpha-1 adrenergic antagonist, reduces cocaine-induced reinstatement of drug-seeking. Biol Psychiatry. 2005;57:1202–1204. doi: 10.1016/j.biopsych.2005.02.003. [DOI] [PubMed] [Google Scholar]