Abstract
Management of acute diarrhea remains a global challenge, particularly in resource-limiting countries. Oral rehydration solution (ORS), a passive rehydrating therapy developed approximately 40 years ago, remains the mainstay treatment. Although ORS is effective for hydration, since it does not inhibit enterotoxin-mediated excessive secretion, reduced absorption and compromised barrier function - the primary mechanisms of diarrhea, ORS does not offer a rapid relief of diarrhea symptom. There are a few alternative therapies available, yet the use of these drugs is limited by their expense, lack of availability and/or safety concerns. Novel anti-diarrheal therapeutic approaches, particularly those simple affordable therapies, are needed. This article explores intestinal calcium-sensing receptor (CaSR), a newly uncovered target for therapy of diarrhea. Unlike others, targeting this host antidiarrheal receptor system appears “all-inclusive”: it is anti-secretory, pro-absorptive, anti-motility, and anti-inflammatory. Thus, activating CaSR reverses changes of both secretory and inflammatory diarrheas. Considering its unique property of using simple nutrients such as calcium, polyamines, and certain amino acids/oligopeptides as activators, it is possible that through targeting of CaSR with a combination of specific nutrients, novel oral rehydrating solutions that are inexpensive and practical to use in all countries may be developed.
Keywords: Secretory diarrhea, Inflammatory diarrhea, Oral rehydration solution, Anti-secretory, Pro-absorptive, Intestinal permeability, Intestinal barrier function, Enteric nervous system, Cholera toxin, Escherichia coli heat stable toxin
Core tip: Diarrheal disease remains a leading cause of death in children and the elderly throughout the world. The cause of death is dehydration secondary to severe diarrhea. Intestinal calcium-sensing receptor (CaSR) is a newly uncovered ancient antidiarrheal receptor system that appears to exert profound effects not only on intestinal secretion, absorption and motility but also on gut permeability and inflammatory responses. Activating this unusual machinery reverses pathophysiological changes of both secretory and inflammatory diarrheas. Considering its unique property of using simple nutrients as activators, it is now possible that through targeting of CaSR and developing novel oral rehydrating solutions that are inexpensive and practical to use in all countries, these diarrhea-associated deaths are reduced or eliminated.
THERE IS NEED FOR NEW TREATMENTS FOR ACUTE DIARRHEA
Problem
Despite advances and improvements in health care over the past century, diarrheal diseases continue to exert a staggering health burden worldwide, particularly in children and the elderly[1-3]. Globally, there are nearly 1.7 billion cases per year of diarrheal disease in children under 5 years old. Although this number is slightly declining compared to 1.9 billion cases per year 20 years ago[4], it remains a huge challenge. In some developing countries, children may be plagued with 12 or more episodes (a median of 6 episodes) of diarrhea per year by the time they reach 5 years old. In the United States, the costs spent on diarrhea outpatient visits and hospitalizations in between 1993-1996 were $1.2 and 2.2 billion/year, respectively[5]; these costs increased to $3.5 and $4.6 billion/year in between 2001-2006[6]. Adding the costs of ED visits of $1.8 billion/year, the total estimated expense for diarrhea management was nearly $10 billion/year, excluding indirect costs from parents and deaths[6].
Also, diarrheal disease is the second leading cause of child death[1-3]. According to the World Health Organization (WHO), 9%-34% of childhood mortality in developing countries is due to diarrheal diseases[1-3]. Worldwide, approximately 1.5 million people including 620000 children under 5 years old and 320000 adults over 70 years old die each year from diarrhea[3]. Importantly, the majority of these deaths do not result from infection, the most common cause of diarrhea; instead, they are due to associated dehydration, acidosis and other metabolic derangements. These latter are very preventable and treatable. Thus, new methods that effectively reduce the fluid and electrolyte losses from acute diarrhea would offer a major opportunity for improving human health globally.
Success and failure of ORS
Oral rehydration solution (ORS) is currently the only oral therapy that is recommended for children with acute diarrhea. A mixture of simple salts and glucose in specific proportions, ORS was developed in 1968 by researchers from US government-funded institutes in Calcutta and Dhaka. This therapy is a result of the basic science discoveries that show that sodium (Na+) transport and glucose transport are coupled in the intestine so that glucose accelerates fluid absorption. What leads to the proposal and forms the physiological basis for efficacy of ORS in treating diarrhea-associated dehydration is the discovery that the glucose stimulated Na+ absorption is a cAMP-independent process, which means it remains intact without being inhibited in diarrhea (cholera) while most other absorptive processes are shut down by enterotoxins (e.g., cholera toxin)[7]. Initially, ORS was used as a treatment for cholera. Later, it was found that this solution is efficacious to correcting dehydration by other forms of acute diarrhea, both in adults and in children. Because of the simplicity, inexpensiveness and availability, ORS was rapidly spread worldwide and was used by over 90% of the population who need it. Since it was effective in reducing the morbidity and mortality of acute diarrhea, it was established as the mainstay of therapy for dehydration, especially in developing countries where hundreds and thousands of patients were affected by large-volume diarrhea and IV hydration was not always available. For this reason, the invention of ORS is considered one of the most important medical advancements in the 20th Century. Since its inception, it has saved a minimum of one million lives a year.
Although it is valuable for hydration, ORS neither suppresses intestinal fluid secretion or overly active enteric nerve activity that occurs in secretory diarrhea, nor does it reduce gut permeability or inflammation that are the primary contributors of inflammatory diarrhea. Consequently, ORS does not decrease diarrhea over the short term. It may even paradoxically make diarrhea worse as the patient is rehydrated[8,9]. Because of this perceived failure, caregivers have become reluctant to continue to use ORS but resort to antimicrobial or other agents for treating diarrhea. It is estimated that ORS is currently used by parents and practitioners in only one third of cases that need it[10].
Alternative antidiarrheal therapies
In addition to ORS, there are a few alternative anti-diarrheal therapies in use (Table 1). Based upon their mechanisms of action, these therapies are classified into four types, namely, proabsorptive, antisecretory, antimotility, and anti-inflammatory (Table 1). These four types of therapies target changes in four corresponding host diarrhea-forming mechanisms seen in four types of diarrhea (Figure 1).
Table 1.
Current antidiarrheal therapies and their mechanisms of action
| Therapies | Mechanisms of action | Comments | Ref. |
| Proabsorptive | |||
| ORS/RS-ORS | ↑glucose/SCFA absorption | Limited efficacy to reduce diarrhea | [98-100] |
| Antisecretory | |||
| Crofelemer | ↓CFTR and CaCC→↓ secretion | Less efficacious as anticipated | [15] |
| Cholestyramine resin | ↓bile salt in lumen→↓secretion | Concerns for vitamin malabsorption | [101] |
| Bismuth subsalicylate | ↓PG synthesis→↓secretion | Safety concerns in children (Reye syndrome) | [102,103] |
| Antimotility/ENS modulatory | |||
| Loperamide/Diphenoxylate | ↑μ opioid receptor →↓motility | Safety concerns in children (ileus) | [13,14] |
| Hyoscyamine/Dicyclomine | ↓Ach action→↓muscle contraction | Safety concerns in children (seizure) | |
| Alosetron | ↓5-HT3R →↓motility and secretion | Safety concerns in children (ischemic colitis) | [104] |
| Racecadotril | ↑encephalin→↓secretion | Not widely available | [105,106] |
| Clonidine | ↑α2 adrenoceptor→↑absorption | No efficacy and safety study in children | |
| Anti-inflammatory | |||
| TEN | Unknown mechanism of action | Slow action | [107] |
| Corticosteroids | Immunosuppression (↓PGs, ↑IL-10) | Concerns for adverse effects | |
| Anti-TNFα | ↓blood and tissue TNFα | Limited by expenses and adverse effects |
ACh: Acetylcholine; CaCC: Ca2+ activated chloride channels; CFTR: Cystic fibrosis transmembrane conductance regulator; ORS: Oral rehydration solution; PG: Prostaglandins; RS-ORS: Resistant starch-based oral rehydration solution; SCFA: Short-chain fatty acids; VIP: Vasoactive intestinal polypeptide; TEN: Total enteral nutrition.
Figure 1.
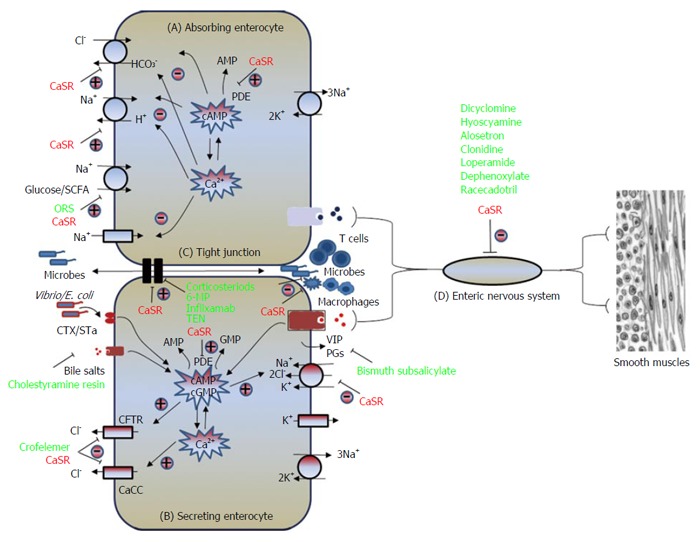
Illustrates the four common pathways leading to formation of diarrhea. The four pathways are: A: Impaired absorption (cause of osmotic diarrhea); B: Excessive secretion (leading to secretory diarrhea); C: Breakdown of intestinal barrier and enhanced inflammation (implicated in inflammatory diarrhea); D: Overly active enteric nervous system (presumed cause of neurogenic diarrhea seen in irritable bowel syndrome). Note that while most current antidiarrheal therapeutics (green-colored) target one individual pathway, CaSR activators (red-colored) have the potential of correcting changes in all the four diarrhea-causing pathways. CaCC: Calcium-activated chloride channel; CaSR: Calcium-sensing receptor; CFTR: Cystic fibrosis transmembrane conductance regulator; CTX: Cholera toxin; ORS: Oral rehydration solution; PDE: Phosphodiesterase; PG: Prostaglandin; SCFA: Short-chain fatty acid; STa: Heat stable toxin; VIP: Vasoactive intestinal peptide.
Normally, fluid moves across and along the intestine; both processes contribute to diarrhea formation when they are disturbed. While these are inter-connected and not separated processes, the fluid movement across the intestine (absorption or secretion) is driven by active epithelium transport of electrolytes, mainly Na+, Cl- and HCO3-, and solutes, mainly glucose in the small intestine and short-chain fatty acids (SCFA) in the large intestine (Figure 1), and the fluid moving along the intestine (anterograde or retrograde) is governed by gut motility. Enteric nervous system (ENS), the brain of the gut, controls both processes, with absorption/secretion being primarily controlled by the submucosal Meissner’s plexus whereas motility by the myenteric Auerbach’s plexus. Diarrhea results when excessive secretion, impaired absorption and/or overly active motility/ENS activity occur. All these changes have been described in secretory diarrheas, exemplified in Vibro cholera, enterotoxigenic Escherichia coli, and rotavirus infections.
A fourth mechanism that leads to formation of diarrhea is compromised intestinal barrier function, a mechanism commonly seen in inflammatory diarrheas (e.g., Shigella, Salmonella, Campylobacter, enteroinvasive and enterohemorrhagic Escherichia coli infections as well as inflammatory bowel disease). Situated between adjacent intestinal epithelial cells of the mucosa is the apical junctional complex, i.e., the tight junction (Figure 1) and the adherens junction. These intercellular structures along with the layer of epithelium composing the intestinal mucosa act as a barrier separating the luminal contents from the submucosal compartment, which is home to gut immune system. Breaching of this barrier function may result in excessive exposure of submucosal immune system to luminal microbes and foreign antigens, leading to intestinal inflammation. Thus, in addition to the aforementioned mechanisms, breakdown of intestinal barrier is a primary mechanism that causes inflammatory diarrheas. Detailed description of the diarrhea-forming mechanisms can be found in a recent review by Thiagarajah et al[11].
While alternative anti-diarrheal therapies, described in Table 1, are helpful for symptomatic treatment of diarrhea, the use of these drugs is limited by their expense, lack of availability, toxicities, and other safety concerns, particularly in pediatric age patients (Table 1). For example, the use of antimotility agents in children should be considered with caution[12-14]. These agents are generally not recommended for any child at any age when acute infectious diarrhea or colitis is suspected as there are reports, albeit rare, of toxic megacolon associated with the use of these agents. Antimotility agents are suspected, though not proven, to increase the risk of hemolytic-uretic syndrome in children with Escherichia coli O157:H7 infection. Many of the proabsorptive/antisecretory drugs lack pediatric studies determining efficacy and safety (e.g., clonidine) or optimal dosing for children (e.g., racecadotril). Also, some of the newly developed antisecretory drugs (e.g., crefelemer) are not as efficacious as originally anticipated[15].
Use of antibiotics: While anti-microbial therapy is useful in some cases (e.g., dysentery), selection for appropriate antibiotics requires lab detection of the organisms, which is often unavailable. Misuse or abuse of antibiotics can lead to development of resistance, and antibiotics are contraindicated in certain enteric infections (e.g., salmonella). Due to their delayed onset of action, antibiotics do not prevent immediate dehydration; in fact, it is generally not the enteric infections but the dehydration and electrolyte imbalance that cause most of the diarrhea-associated morbidity and deaths.
In addition to diarrhea-related dehydration, repeated diarrheal episodes in children often cause malnutrition, which in turn leads to more severe and more frequent diarrhea. This diarrhea-malnutrition-diarrhea cycle causes almost half of the deaths associated with diarrheal diseases in children under five. Except for TEN (total enteral nutrition), none of the current diarrhea therapies, including the proabsorptive ORS, the antisecretory, the antiinflammatory and the antimotility therapies, have the notable capacity to break this vicious cycle. Therefore, novel anti-diarrheal therapies, particularly those simple nutrient-based “child-friendly” therapeutic approaches are needed.
UNDERSTANDING OF THE PHYSIOLOGICAL ROLES FOR CASR IN GASTROINTESTINAL BIOLOGY MAY LEAD TO THE DEVELOPMENT OF NOVEL COST-EFFECTIVE THERAPIES FOR TREATING DIARRHEA
Extracellular CaSR[16] is a well-conserved ancient G protein-coupled cell surface receptor (GPCR) of class C, originally cloned from bovine parathyroid[16] and subsequently found to be expressed in diverse tissues in mammalian[17], birds[18], amphibians[19,20], and marine species[21,22]. As the name implies, CaSR is a key regulator of tissue responses for calcium homeostasis[16]. Later, it was found that CaSR also plays a crucial role for fluid balance[23,24] and osmotic regulation[21]. The primary physiological ligand for CaSR is extracellular ionized calcium (Ca2+o), providing a mechanism for Ca2+o to function as a first messenger. Importantly, CaSR also functions as a general sensor of the extracellular milieu due to allosteric modification of Ca2+o affinity and efficacy by polyamines, L-amino acids, oligo-peptides, pH and ionic strength[25].
In marine species, CaSR is expressed in the intestine and tissues that are critical for water preservation[21]. There, it acts as a calcium/osmo/salinity-sensor[21], helping fish preserve water from loss to their hyperosmotic surroundings and protect against calcium overload from high calcium sea water[26].
CaSR is also highly expressed in the mammalian gut, including the transporting epithelial cells[27-30], the fluid/motility-modulating enteric nerves[27,31], and the cells that regulate gut inflammation[32,33]. Over the past several years, studies have shown that the water preserving, or anti-dehydrating function, of CaSR is conserved along with its calcium homeostatic function in terrestrial animals[28,29,31,34-36]. These data strongly support the notion that targeting of CaSR may be a new approach for the development of novel anti-diarrheal therapies.
Activation of intestinal CaSR reverses changes in epithelial transport in diarrhea
Increased anion secretion and decreased salt absorption are two major abnormalities found in electrolyte handling by the intestine during diarrhea, particularly in secretory diarrhea[37,38]. CaSR is expressed in both absorbing surface cells and secreting crypts of the intestine, suggesting critical roles in regulating intestinal absorption and secretion. In enteric epithelial cells, CaSR has been identified on both the apical and basolateral membranes of human[29,39] and rat colonocytes[27,29]. Receptors in both membrane domains of these polarized epithelia are functionally active and can be activated by Ca2+o[28,29], amino acids and peptides[40,41], polyamines[28,29] and the specific pharmacological CaSR agonist (also called calcimimetic) R568[34].
CaSR agonists inhibit anion secretion
In rat colonic crypts, CaSR activation from either mucosal or serosal side by extracellular calcium, spermine or R568 inhibits net fluid secretion[28,29,34] and cyclic nucleotide accumulation[34] induced by synthetic/natural secretagogues. These secretagogues include forskolin[42] and guanylin[43], which generate cAMP and cGMP, respectively. CaSR activation also blocks the effects of bacterial enterotoxins[34] such as cholera toxin[44], a potent activator of membrane bound adenylyl cyclase leading to elevated intracellular levels of cAMP, and STa[45], which enhances cytosolic cGMP accumulation through the guanylyl cyclase C-type guanylin receptor. Similarly, activation of CaSR by extracellular Ca2+ or R568 inhibits net fluid secretion induced by cholera toxin and guanylin in colon mucosa of wild type mice; such effects are abolished in CaSR null mice[34]. Pharmacological inhibitor studies show that these CaSR anti-secretory effects depends on receptor-mediated increases in intracellular Ca2+ and require the presence of phosphodiesterase (PDE)[34] suggesting that CaSR activation may reverse secretagogue-stimulated fluid secretion through a signaling pathway that activates phospholipase C (PLC) and degrades cyclic nucleotides by PDE.
CaSR agonists inhibit apical anion channel activity: Fluid secretion is driven primarily by transepithelial anion secretion[37,46,47] (Figure 1). Anion secretion into the lumen of colonic crypts depends on movement of anions across the luminal plasma membrane through anion channels such as cystic fibrosis transmembrane conductance regulator chloride channels (CFTR), and mice deficient in CFTR lack a secretory response to cholera toxin[48]. Secretagogue-induced increases in cellular accumulation of cAMP and cGMP enhance PKA and PKG phosphorylation processes, respectively, which drives translocation of activated CFTR channels to the luminal plasma membrane[37]. Because CaSR agonists reduce cyclic nucleotide accumulation, activation of CaSR would reverse increased apical anion channel activity induced by secretagogues. Indeed, by measuring short circuit current responses to pharmacological inhibitors of anion channels in the apical membrane of colonic mucosa mounted in Ussing chambers, it has been shown that the cyclic nucleotide-dependent NPPB/glibenclamide-sensitive apical anion channel activity is inhibited by activation of CaSR[49], although it remains unknown whether it is directly inhibited by CaSR or indirectly via the reversal of changes in cyclic nucleotide by the activation of the receptor.
CaSR agonists inhibit basolateral anion entry pathway mediated by NKCC1: Equally critical for transepithelial anion (Cl-) transport during secretagogue-stimulated fluid secretion is increased Cl- entry into cells from the basolateral fluid via the bumetanide-sensitive Na+-K+-2Cl- cotransporter (NKCC1; ref[37]). Mice lacking NKCC1 exhibit impaired secretory responses to cAMP and STa[50]. Using perfused colonic crypt model and by measuring Cl--sensitive MQAE fluorescence, it has been shown that basolateral addition of bumetanide abolishes forskolin stimulated basolateral Cl- entry into colonic crypt cells consistent with bumetanide inhibition of cAMP activated NKCC1[34]. Addition of R568 to the basolateral fluid also significantly reduces the rate of forskolin-stimulated Cl- entry[34]. A similar inhibition of Cl- entry via NKCC1 is seen in the presence of cholera toxin with increasing extracellular calcium[34], demonstrating that activation of the CaSR inhibits NKCC1 activity.
CaSR agonists inhibit secretagogue-induced HCO3- secretion: In addition to Cl- secretion, HCO3- secretion is markedly increased in cholera and other secretagogue-induced diarrheal diseases[51,52]. This enhanced intestinal HCO3- secretion can result in not only fluid loss and dehydration but also HCO3- deficit and metabolic acidosis[51,52], another most common cause (additional to dehydration and systemic volume depletion) of the morbidity and mortality associated with these clinical conditions. To assess if CaSR activation inhibits secretagogue-induced HCO3- secretion as it does for the secretagogue-induced Cl- secretion, CaSR effect was examined in a tissue model (colonic mucosa) of secretagogue-induced secretory diarrhea. In this study, forskolin was used as a secretagogue to stimulate HCO3- secretion, and HCO3- secretory response was monitored by measuring HCO3- secretory rate (JHCO3) and by recording Isc. Forskolin stimulated both JHCO3 and Isc in colon mucosa of rats, wild type mice, and CaSR null mice; subsequent addition of R568 to either luminal or basolateral fluid decreased forskolin-induced HCO3- secretion in colon mucosa of rats and wild type mice but not in colon mucosa of CaSR null mice[49]. The results indicate that targeting of CaSR may be useful in arresting not only intestinal Cl- but also HCO3- losses associated with diarrheal diseases.
CaSR agonists enhance absorption
Secretory diarrhea results not only from enhanced fluid secretion, but also from reduced fluid absorption[37,38] (Figure 1). CaSR is expressed in absorbing villus/surface cells, suggesting critical roles in regulating intestinal absorption. To address this, colonic mucosa epithelia from rat and mice were isolated and used as models[34]. Since both absorption and secretion can occur in the same epithelial cells, to minimize interference from secretion, tissues were first treated with basolateral bumetanide to block secretion before absorption was studied. In the absence of secretagogues, addition of bumetanide to the basolateral fluid of perfused crypts slightly increased the absorptive netJV due to inhibition of a small remaining fluid secretion. This basal fluid secretion is likely due to the low levels of cell cyclic nucleotides that remain even in the absence of secretagogues. Thus, in the presence of bumetanide, netJV measurements represent the absorptive component of fluid transport. This absorptive fluid movement was substantially reduced by addition of cAMP or forskolin, which would importantly contribute to secretagogue-induced diarrhea. Either increasing extracellular calcium and/or addition of R568 to the basolateral bath significantly abrogated the cAMP-mediated reduction in fluid absorption, demonstrating that activation of CaSR is able to reverse the reduced absorption caused in secretagogue-induced diarrhea.
CaSR agonists stimulate apical NHE activity: A major component of fluid absorption in the colon (and small intestine) is mediated by parallel Na+/H+ (sodium-hydrogen exchanger, NHE)[37,38] and Cl-/HCO3- exchange[56,57] located at the apical plasma membranes. Cyclic nucleotides reduce this Na+-dependent fluid absorption in ileum and colon by inhibiting NHE activity and Cl-/HCO3- exchange (Figure 1; also ref[11]), and this event contributes importantly to severity of fluid and electrolyte losses in secretory diarrheas[11,37,38]. To examine whether CaSR agonists reverse the cyclic nucleotide diminished NHE activity, the effects of Ca2+ or R568 on Na+-dependent proton extrusion from colonocytes in the presence of forskolin were examined[34]. NHE activity was found significantly increased by raising basolateral bath Ca2+ from 0.1 to 2 mmol/L, and addition of R568 to the 2 mmol/L Ca2+-containing bath had resulted in a further increase in NHE activity[34].
CaSR agonists stimulate apical Cl-/HCO3- exchange: To address whether CaSR regulates Cl-/HCO3- exchange, colonic mucosa were isolated, mounted into Ussing chamber and perfused, and Cl- absorption mediated by Cl-/HCO3- exchange was then recorded by measuring lumen Cl--dependent HCO3- secretion using pH stat technique[49]. The latter technique measures the amount of acid delivered per unit time per surface area to neutralize the secreted HCO3- in order to maintain a constant lumen pH. A luminal Cl--dependent DIDS-sensitive HCO3- secretory mechanism (Cl-/HCO3- exchange) was observed in colon mucosa of rats and mice. Activation of CaSR by R568 stimulated Cl-/HCO3- exchange activity in colons of rats and wild type mice; such an effect was abolished in CaSR null mice[49], suggesting that activation of CaSR also stimulates Cl-/HCO3- exchange.
CaSR agonists stimulate apical SCFA/HCO3- exchange: SCFA are the major anion or solute in stool. SCFA is produced in colon by bacteria fermentation of unabsorbed carbohydrates. SCFA absorption stimulates Na+, Cl- and water absorption and this occurs via a process involving apical membrane Na+/H+, Cl-/HCO3- and SCFA/HCO3- exchanges[53,54]. Thus, SCFA production and absorption represents another major mechanism in the colon to conserve fluid and electrolytes, and is target for some modified forms of ORS (e.g., resistant starch-based ORS). To examine if activating CaSR affects absorption of this physiologically and clinically important solute in the colon, SCFA absorption mediated by SCFA/HCO3- exchange has recently been studied by measuring lumen isobutyrate-dependent HCO3- secretion using the same Ussing chamber-pH stat technique mentioned above, and its responses to absence or presence of R568 compared[49]. Similar to the R568 effects on Na+/H+ and Cl-/HCO3- exchanges, isobyturate-dependent HCO3- secretion is found significantly stimulated by R568 in colons of rats and wild type mice but not CaSR null mice[49].
Although the effects on these transporters can be explained by reversal changes in second messengers, as increases in cAMP/cGMP-dependent PKA/PKG activity are associated with phosphorylation, and thereby stimulation, of these transporters in transport epithelia (Figure 1), the stimulation of CaSR also appears to directly affect their function. Besides the aforementioned transporters, apical Na+ and K+ channels as well as basolateral K+ channels and Na+, K+-ATPase (Figure 1) also play critical roles in epithelial absorption and secretion. For example, in Cl- secreting epithelia, the basolateral K+ channels facilitate basolateral Cl- entry (via cycling back the K+ for NKCC1) as well as apical Cl- exit (by maintaining a favorable transepithelial electrical gradient) whereas the Na+, K+-ATPase pumps the Na+ entered by NKCC1 out of the cell. Whether CaSR also affects these transporters activity and function remains to be determined.
ACTIVATION OF INTESTINAL CASR REDUCES OVERLY ACTIVE ENTERIC NERVE ACTIVITY AND MOTILITY
In humans and rodents, at least 50% of the fluid secreted in cholera, rotavirus, and other forms of infectious diarrhea are caused and mediated by activation of the enteric nervous system (ENS)[38,55-58]. For example, cholera toxin-induced fluid secretion/diarrhea is blocked by tetrodotoxin (TTX)[38,55], an inhibitor of neurotransmission[38,55]. Rotavirus-induced diarrhea is blocked by lidocaine[57,59], an inhibitor of voltage-gated Na+ channels in the ENS. This ENS-evoked secretion is also found to contribute to diarrhea formation in patients with irritable bowel syndrome[60], inflammatory bowel disease[61], and intestinal allergies. On this basis, a dual-pathway model for fluid secretion in intestine is proposed: (1) a non-neuronal fluid secretory response due to the binding of enterotoxins directly to enterocytes, leading to generation of cyclic nucleotides, which is TTX/lidocaine-insensitive; and (2) a neuronal secretory response that is mediated by stimulation of the ENS, which is TTX/lidocaine-sensitive. The antidiarrheal CaSR is expressed in both non-neuronal and neuronal tissues, and recent studies have suggested that CaSR agonists also appear to produce their antidiarrheal effects in two ways: (1) direct inhibition of epithelium-mediated diarrheal responses, which is TTX/lidocaine-insensitive (see previous section); and (2) indirectly via inhibiting the ENS, which is TTX/lidocaine-sensitive (see below).
CaSR agonists inhibit ENS-mediated secretion
By measuring TTX-sensitive short-circuit current (Isc) responses of intact ENS-containing colon segments in Ussing chambers, it has been shown that ENS-mediated secretion is abolished by R568[31]. First set of experiments are performed to test diarrhea “treatment” effect of CaSR agonist. In these experiments, forskolin or cholera toxin is added to stimulate secretion before R568 is added. TTX-sensitive Isc is employed as a measure of ENS-mediated anion secretion. Consistent with active regulation of secretion by the ENS, a significant proportion of Isc in the proximal and distal colon is inhibited by serosal TTX, both at basal and under cAMP (forskolin or cholera toxin)-stimulated conditions. TTX-sensitive Isc is substantially increased by addition of forskolin or cholera toxin; subsequent addition of R568 to the basolateral bath abolishes the secretagogue-stimulated TTX-sensitive Isc.
Second set of experiments are performed to test diarrhea “prevention” effect of CaSR agonist. In these experiments, R568 is added prior to the secretagogue stimulation. R568 pretreatment reduces both basal and stimulated secretion. Thus, CaSR agonist may be useful not only for diarrhea treatment but also for diarrhea prevention.
CaSR agonists inhibit motility
CaSR is present in the ENS[27,31], not only in the submucosal Meissner’s plexus that mainly controls fluid secretion by the epithelium, but also in the myenteric Auerbach’s plexus that is thought to primarily control the gut motility. Thus, CaSR may have important roles in gastrointestinal motility and constipation. Indeed, calcium, the primary ligand of CaSR, is well-known for its constipation-causing effects in humans. People taking high calcium diets are often constipated as are patients with hypercalcemia. Chronic opiate use inhibits the motility of the gastrointestinal tract; opiate withdrawal reverses the inhibited motility, causing diarrhea. In mice, co-administration of calcium and magnesium effectively blocks the signs of morphine withdrawal[62]. Polyamines, another class of agonists for CaSR, are also shown to have a profound impact on the motility[63,64] and are effective in slow down of the gastrointestinal transit in several rodent models of diarrhea-dominant irritable bowel syndrome[65-67]. Using LoxP and Nestin-Cre conditional gene targeting technology, mice lacking the neuronal CaSR in ENS are generated. We are now characterizing these mice. By comparing fecal pellet output rates, it appears that mice deficient in the neuronal CaSR have enhanced colonic propulsive activity, as evidenced by significantly faster bowel movements than their wild-type littermates (unpublished observation). Also useful of these mice is to determine how much of the effects of calcium and polyamines are mediated through activation of CaSR.
ACTIVATION OF INTESTINAL CASR SUPPRESSES GUT INFLAMMATION
Depending on the presence or absence of inflammation, diarrhea can be pathologically grouped into inflammatory (as observed in enterocolitis caused by Salmonella and Shigella, as well as inflammatory bowel diseases) and non-inflammatory (e.g., osmotic as seen in lactose intolerance; secretory as seen in cholera, travelers’ diarrhea, and rotavirus). The CaSR is also expressed in inflammatory cells, as well as other cells that regulate inflammatory diarrhea[32,33], suggesting a potential protective role in this setting. Indeed, by characterizing the gut-specific CaSR knockout mice, it has been shown that this highly conserved, nutrient-sensing mechanism also plays a critical role in maintaining intestinal barrier function integrity and reducing gut permeability[35] - central to the pathogenesis of inflammatory diarrhea.
CaSR regulates claudin-2 expression and intestinal barrier function integrity
Mice lacking intestinal CaSR have a decreased colonic expression of tight junction molecules (e.g., claudin-2) and diminished intestinal barrier function, with the transepithelial electrical resistance reduced and the permeability to FITC-dextran increased, the results that are consistent with CaSR regulation of tight junction assembly in cultured MDCK cells[68]. Moreover, microflora composition in CaSR null mice is altered; abundance of beneficial flora (e.g., Lactobacilli and Clostridia) is reduced and of harmful phylum (e.g., Deferribacteres) increased.
CaSR regulates Reg3b and Reg3g expression and bacterial translocation and dissemination
Importantly, mice lacking CaSR have significantly decreased epithelial expression of Reg3b and Reg3g, which encode secreted C-type lectins that bind and protect against translocation and dissemination of Gram-negative[69] and Gram-positive bacteria[70,71], respectively. As a consequence, more bacteria are found to translocate and disseminate into peripheral organs of CaSR null mice compared to CaSR wild type mice, and immune responses (e.g., CD11b+ dendritic cell, Th1 and Th17 responses) are activated and are skewed to pro-inflammatory, both locally and systemically[35].
CaSR regulates Wnt5a-Ror2-TNFR1 expression
The colon is an organ in a constant state of inflammation. The latter is largely controlled by the integrity of intestinal barrier function. In addition to its direct action on intestinal barrier shown in 3.1, CaSR can produce its effect indirectly via epithelial receptors for inflammatory mediators[72], such as TNFR1, a known modulator of barrier function. MacLeod has compared wild type and “global” CaSR knockout mice and found that TNFR1 signaling is inhibited by CaSR[72]. This occurs by two distinct mechanisms: CaSR increases secretion of wnt5a from subepithelial myofibroblasts, which interacts with Ror2, an orphan tyrosine kinase and receptor for Wnt5a in epithelial cells and leads to a decrease in TNFR1 expression. CaSR also inhibits secretion of TNFα from macrophages, thereby interrupting TNFR1 signaling.
CaSR regulates intestinal inflammation
Because of the aforementioned anti-inflammatory properties of intestinal CaSR, mice lacking intestinal CaSR are found to have more severe spontaneous and induced colitis compared to their littermate counterparts[35,72].
These studies demonstrate that CaSR is a key molecule expressed in gut epithelial and other cells that contributes to the preservation of intestinal epithelial cell integrity, and maintenance of immune homeostasis in the gut, the disruption of which results in intestinal inflammation. Consistent with this, dietary supplementation with calcium, spermine and tryptophan, activators of CaSR, delay the onset, reduce the severity, and accelerate recovery of animals with DSS colitis[73], whereas inhibition of the receptor by depletion of dietary calcium enhances gut inflammation in animal models of induced colitis[74], even if a recent study suggests a different role for the CaSR in murine bone marrow-derived macrophages/monocytes[75].
EFFECTS OF CaSR-/- MICE
While based on observations made from isolated perfused crypts and intestinal tissues in Ussing chambers CaSR certainly seems to have an array of effects on many intestinal aspects leading to diarrhea, it should be noted that so far there has been no report of any associating mutations or SNPs in CaSR for diarrheal conditions. This may suggest redundancy with other pathways. Given these limitations and the fact that studying these effects in isolation can be artificial, it is critical that future studies should be directed in the organismal and systemic levels using whole animals and mice lacking CaSR in order to better define the effects of under and over activation of this gene.
Currently, there are three types of CaSR null mouse models that are available: single global, double global, and intestine-specific. Since deletion of the CaSR gene results in early death from the toxic effects of unregulated release of parathyroid hormone (PTH) from parathyroid chief cells as well as from the pathological effects of the consequent hypercalcemia[76], double knockouts with simultaneous ablation of additional PTH gene (as in CaSR-/- PTH-/- double knockout mice[77]) or gene that regulates PTH (e.g., Gcm2 as in CaSR-/- Gcm2-/- double knockout mice[78]) are generated that “rescue” the lethal CaSR-deficient phenotype. Also available are intestinal-specific CaSR knockouts in the floxed mice[79].
Preliminary studies in global[72] and intestinal-specific CaSR knockout mice[35] have shown development of spontaneous intestinal inflammation and exaggerated immune responses in these CaSR-/- animals (see above sections for details). So far, no study has ever provided evidence of developing diarrhea in these animals. Given the redundancy of mechanisms/pathways implicated in regulation of diarrhea formation as well as the global nature and multiple confounding factors involved in double CaSR knockout mice, the use of these global CaSR knockout animals may be difficult to discern the intestinal fluid changes that are attributed to intestinal CaSR. For example, besides being a Ca2+-regulating hormone, cAMP-stimulating PTH also activates CFTR-mediated anion secretion in intestinal epithelial cells[80]. Thus, both CaSR-/-PTH-/- and CaSR-/-Gcm2-/- double mice deficient in both cAMP-stimulating PTH and cAMP-inactivating CaSR may not be developing diarrhea. Additionally, in order to sustain their systemic serum calcium these animals are normally maintained in high calcium diet, which may well generate constipation via CaSR independent mechanisms. Accordingly, use of conditional CaSR knockout mice for characterization of intestinal fluid movement would be more appropriate. In this regard, hyperplastic elongated secreting crypts have been noted in intestinal epithelium-specific CaSR-deficient mice[79]. In a preliminary study, we show that mice deficient in neuronal CaSR display enhanced colonic propulsive activity, as evidenced by significantly faster bowel movements than their wild-type littermates (unpublished observation). While the result from the former study is in keeping with the anti-secretory effect of epithelial cell CaSR, the data from the latter are consistent with the active control of colonic motility by neuronal CaSR. We are now performing studies to further characterize these animals both under basal vs challenged conditions with vs without presence of CaSR activators and inhibitors.
In summary, intestinal CaSR is an antidiarrheal GPCR receptor in the gut that, when activated, appears to exert profound effects not only on intestinal secretion, absorption and motility but also on gut permeability and inflammatory responses. As such, activating intestinal CaSR may reverse changes in both secretory and inflammatory diarrheas in animals and humans.
DATA FROM ANIMALS AND CLINICAL TRIALS ON HUMANS
In rodents, increased dietary calcium intake is found to reduce diarrheas that are caused by infectious pathogens (e.g., Salmonella enterocolitis[81-84]) or induced chemically (e.g., DSS colitis[73]) or immune-mediated (e.g., colitis in HLA-B27 transgenic rats[85]). In contrast, lowering dietary calcium intake in mice was found to increase the severity of diarrhea, at least in DSS colitis[74] (Table 2).
Table 2.
Clinical evidence for dietary calcium-sensing receptor activators as antidiarrheals in animals and humans1
| CaSR agonists | Antidiarrheal efficacy | Ref. |
| Calcium | ↑intestinal resistance, ↓bacterial colonization and translocation to Salmonella infection in rats | [81-84] |
| ↓intestinal permeability in rats | [108] | |
| ↓diarrhea severity in Salmonella enterocolitis in rats | [82] | |
| ↓diarrhea onset, ↓severity, ↑ recovery in DSS colitis in rodents | [73,74] | |
| ↓gut permeability and diarrhea in immune-mediated colitis in HLA-B27 transgenic rats | [85] | |
| ↓induced intestinal inflammation in mice | [35,72] | |
| ↓stool volume and duration of diarrheas by viruses or parasites in humans (children) | [36] | |
| ↓stool weight and duration of diarrhea by ETEC in humans (adults) | [87] | |
| ↓diarrhea frequency in patients with calcitonin-secreting medullary thyroid cancer | [88] | |
| Calcium and magnesium | ↓intestinal motility and diarrhea symptoms of morphine withdrawal in mice | [62] |
| Polyamines | ↓intestinal motility in mice | [63,64] |
| ↓gastrointestinal transit and diarrhea of irritable bowel syndrome in mice | [65-67] | |
| ↓DSS colitis in rodents | [73] | |
| Tryptophan | ↓intestinal inflammation in mice | [109] |
| ↓DSS colitis in rodents | [73] |
1The naturally occurring calcium-sensing receptor (CaSR) activators described are all friendly minerals or nutrients and generally safe. Except for chemically synthesized polyamines, no adverse events other than mild GI discomforts (e.g., constipation[36,88], flatulence[88] and bloating[88]) were reported. DSS: Dextran sodium sulfate; ETEC: Enterotoxigenic Escherichia coli.
Three human studies also support the concept that CaSR agonists have the anti-diarrheal potential. The 1st study tested the primary agonist calcium in children with viral or parasitic diarrhea[36]. These children who were immune-compromised, presented with persistent enteric infections, hypocalcemia, and protracted diarrhea. When hypocalcemia was corrected, diarrhea stops[36]. In this study, calcium equivalent to 1x RDA (recommended daily allowance) was used. Within 12-24 h following administration, diarrhea stops or significantly reduces. It is safe within the period of 10 d treatment without causing hypercalcemia[36]. In keeping with the unique CaSR agonist-induced receptor feed-forward mechanism[86] (also see below for further explanation), calcium therapy can be repeated in a same patient multiple times without reduction in efficacy or development of resistance. Although the number of the patients tested in this study is small, the result proved the principle.
The 2nd study is a randomized controlled trial in young adult volunteers who ingested attenuated live enterotoxigenic Escherichia coli (ETEC)[87]. In this study, 32 subjects were randomized to receive placebo or calcium (about 1x RDA, in the form of cow’s milk) for 10 d before they were infected with ETEC. Diarrhea developed in both groups. However, diarrhea recovery was significantly faster in calcium treated vs untreated groups: it recovered within one day in calcium treatment group vs more than two days in the placebo control group. The authors have also generated evidence that the bulk of the calcium ingested remain in the lumen of the gut unabsorbed, producing local effect, and are subsequently excreted in feces. Urinary calcium measurements did not show any significant difference between the two groups, demonstrating the safety of this treatment. The only adverse effect associated with this treatment was mild reversible constipation. Neither hypercalcemia nor kidney stone formation were evidenced.
Secretory diarrhea is common in hormone-secreting neuroendocrine tumors. Recently, a group from M.D. Anderson Cancer Center in Houston, TX, tested the efficacy of oral calcium (in the form of aluminosilicate salt) in reducing secretory diarrhea associated with calcitonin-secreting medullary thyroid cancer[88]. Of the 7 patients evaluated, 5 were considered calcium antidiarrheal a success. The mean number of bowel movements/day reduced from baseline by 7%-99%. Adverse effects were mild and include flatulence, bloating, heartburn, and constipation.
Despite the excitement and promise of the proof-of-concept studies, so far, no human studies have tested antidiarrheal efficacy and safety of CaSR pharmaconutritional agonists other than calcium. Clearly, more randomized controlled trials are warranted on this new simple and promising antidiarrheal therapy.
TRANSLATION ADVANTAGES OF CASR-BASED ANTIDIARRHEAL THERAPEUTICS
Compared to other antidiarrheal therapies in use and in development, CaSR-based antidiarrheals have many advantages. First, as discussed in aforementioned sections, CaSR is an inclusive antidiarrheal mechanism that is both anti-secretory and pro-absorptive while being anti-motility and anti-inflammatory. This is in contrast to other antidiarrheal agents that target only one individual transporter or diarrhea-causing pathway (Figure 1). Accordingly, activating this mechanism may reverse pathophysiological changes of both secretory and inflammatory diarrheas.
Second, CaSR is a unique GPCR that uses simple nutrients as agonists. It uses calcium as a primary or orthosteric activator and polyamines, L-amino acids, and oligo-peptides as secondary or allosteric activators. Thus, unlike most other GPCRs, CaSR must function in and respond to the continuous presence of nutrients/activators. This is possible because CaSR adopts unusual mechanisms regulating its receptor expression, trafficking, and degradation[86]. For example, instead of inducing internalization and causing desensitization in other GPCRs, continuous elevation of the primary activator/ligand or addition of allosteric activators is found to increase the number of plasma membrane-localized CaSRs and sensitizes receptor function[86]. This unique property of not inducing receptor desensitization would make CaSR agonists potentially useful as potent antidiarrheals. Indeed, addition of as low as 1 pmol/L of R568 to the lumen or bath perfusate of perfused colonic crypts is found to inhibit the forskolin-stimulated fluid secretion, with the EC50 values being only 5 (if added luminally) to 20 (if added from blood side) pmol/L[34]. Also, raising [Ca2+]o to a slightly supra-physiological concentration of 2 mmol/L, either luminally or basolaterally, completely reverses the forskolin or cholera toxin-induced cyclic nucleotide accumulation[34] and secretion[29,34]. Similar potency and efficacy are observed for polyamines[28]. For example, in the presence of physiological or near physiological concentrations of 0.5-1 mmol/L Ca2+o, as low as 1 nmol/L of spermine, added luminally or basolaterally, is able to reverse the secretagogue-induced secretion[28], with the 50% of maximal reversal effect (EC50) of the polyamine being achieved in only 0.5-2 μmol/L[28]. The latter are the polyamine concentrations most often seen in breast milk[89-91] but not in infant formulas [in which the polyamine concentration is at least 1 order of magnitude lower than in breast milk and 2-3 orders of magnitude lower than the polyamine concentration in the lumen of the intestine shortly after ingestion of a typical adult human meal (see reviews[92-94]). Thus, it is conceivable that supplementation of ORS or infant formulas with polyamines and/or other CaSR agonists may be beneficial in treating children with diarrhea and is currently under investigation. A significant challenge for drugs targeting the enterocyte extracellular surface is convective washout in which secreted fluid in intestinal crypts washes away antidiarrheal drugs, preventing the drugs from diffusing into the target in the surface of enterocyte to effect. To overcome this barrier, an orally administered, surface-targeted antidiarrheal agent requires high drug affinity or low EC50 to its target in order to obtain sufficiently high luminal drug concentration (usually > 100-fold EC50)[95]. In this regard, the calcimimetic R568 and the polyamine have extremely low EC50 values, and washout would not be a concern.
Third, in contrast to many other GPCRs that exist in either an “on” or “off” conformation, there is evidence that CaSR adopts multiple active conformations stabilized by different agonists to generate a set of distinct intracellular signals and biological effects[96]. Consequently, super-agonism (i.e., more than 100% efficacy) and biased-agonism (i.e., selective activation or inactivation of one function over others) may occur when a combination of different agonists are used to influence the receptor function. These are important because the unusual properties enable exploration of different CaSR agonist combinations to design an ideal anti-diarrheal therapy - a therapy that produces maximal therapeutic and minimal unwanted outcomes[97].
Finally, naturally occurring nutrients represent an attractive source of antidiarrheal therapeutics, because they are safe, widely available and generally inexpensive, and have the potential for rapid translation into clinical practice. The CaSR agonists are naturally occurring nutrients. Because they are all child-friendly, they may be particularly useful in pediatric population. Malnutrition is one of the sequelae of diarrhea and contributes significantly to the morbidity and mortality. Except for total enteral nutrition, none of the current diarrhea therapies has the notable capacity of treating this important diarrheal complication. Considering the unique property of CaSR using simple nutrients as activators, it is now possible that through targeting of CaSR with child-friendly nutrients, used alone or in combination with the calcimimetics, diarrhea and malnutrition may both be treated.
CONCLUSION
Based upon recent studies, it appears that the intestinal calcium-sensing receptor (CaSR) has an inclusive antidiarrheal function. Activating CaSR is anti-secretory, pro-absorptive, anti-motility, and anti-inflammatory. As such, diarrhea therapies that are based on CaSR may have potentials in reversing pathophysiological changes of both secretory and inflammatory diarrheas and reducing morbidity and mortality associated with these diarrheal diseases. Considering its unique property of using simple nutrients as activating ligands, it is now possible that through targeting of CaSR with nutrients, alone or in combination with calcimimetics, novel oral rehydrating solutions that target central diarrhea-forming pathways that are inexpensive and practical to use in all countries can be developed. Clearly, randomized clinical trials using these pharmaconutritional agonists are warranted.
ACKNOWLEDGMENTS
The author would like to extend his gratitude to Dr. Scott Rivkees, MD, Dr. Joel Andres, MD, and Dr. Richard Wagner, PhD, for critical review of this manuscript.
Footnotes
Supported by The National Institute of Health NICHD, award No. K08HD079674; the CDNHF/NASPGHAN foundation, award No. 00102979, and the Children’s Miracle Network.
Conflict-of-interest statement: The author declares no conflicts of interest for this article.
Open-Access: This article is an open-access article which was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/
Peer-review started: October 14, 2015
First decision: November 13, 2015
Article in press: December 8, 2015
P- Reviewer: Shimoyama S, Wine E S- Editor: Qi Y L- Editor: A E- Editor: Zhang DN
References
- 1.Black RE, Cousens S, Johnson HL, Lawn JE, Rudan I, Bassani DG, Jha P, Campbell H, Walker CF, Cibulskis R, et al. Global, regional, and national causes of child mortality in 2008: a systematic analysis. Lancet. 2010;375:1969–1987. doi: 10.1016/S0140-6736(10)60549-1. [DOI] [PubMed] [Google Scholar]
- 2.Moore SR, Lima AA, Guerrant RL. Infection: Preventing 5 million child deaths from diarrhea in the next 5 years. Nat Rev Gastroenterol Hepatol. 2011;8:363–364. doi: 10.1038/nrgastro.2011.103. [DOI] [PubMed] [Google Scholar]
- 3.World Health Organization. Global Health Observatory Data Repository. Available from: http://apps.who.int/gho/data/node.main.CODWORLD?lang=en.2015.
- 4.Fischer Walker CL, Perin J, Aryee MJ, Boschi-Pinto C, Black RE. Diarrhea incidence in low- and middle-income countries in 1990 and 2010: a systematic review. BMC Public Health. 2012;12:220. doi: 10.1186/1471-2458-12-220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zimmerman CM, Bresee JS, Parashar UD, Riggs TL, Holman RC, Glass RI. Cost of diarrhea-associated hospitalizations and outpatient visits in an insured population of young children in the United States. Pediatr Infect Dis J. 2001;20:14–19. doi: 10.1097/00006454-200101000-00004. [DOI] [PubMed] [Google Scholar]
- 6.Cortes JE, Curns AT, Tate JE, Parashar UD. Trends in healthcare utilization for diarrhea and rotavirus disease in privately insured US children & lt; 5 years of age, 2001-2006. Pediatr Infect Dis J. 2009;28:874–878. doi: 10.1097/INF.0b013e3181a653cd. [DOI] [PubMed] [Google Scholar]
- 7.Carpenter CC. The treatment of cholera: clinical science at the bedside. J Infect Dis. 1992;166:2–14. doi: 10.1093/infdis/166.1.2. [DOI] [PubMed] [Google Scholar]
- 8.Halaihel N, Liévin V, Ball JM, Estes MK, Alvarado F, Vasseur M. Direct inhibitory effect of rotavirus NSP4(114-135) peptide on the Na(+)-D-glucose symporter of rabbit intestinal brush border membrane. J Virol. 2000;74:9464–9470. doi: 10.1128/jvi.74.20.9464-9470.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yin L, Vijaygopal P, MacGregor GG, Menon R, Ranganathan P, Prabhakaran S, Zhang L, Zhang M, Binder HJ, Okunieff P, et al. Glucose stimulates calcium-activated chloride secretion in small intestinal cells. Am J Physiol Cell Physiol. 2014;306:C687–C696. doi: 10.1152/ajpcell.00174.2013. [DOI] [PubMed] [Google Scholar]
- 10.Donowitz M, Alpers DH, Binder HJ, Brewer T, Carrington J, Grey MJ. Translational approaches for pharmacotherapy development for acute diarrhea. Gastroenterology. 2012;142:e1–e9. doi: 10.1053/j.gastro.2012.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Thiagarajah JR, Donowitz M, Verkman AS. Secretory diarrhoea: mechanisms and emerging therapies. Nat Rev Gastroenterol Hepatol. 2015;12:446–457. doi: 10.1038/nrgastro.2015.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Thomas TJ, Pauze D, Love JN. Are one or two dangerous? Diphenoxylate-atropine exposure in toddlers. J Emerg Med. 2008;34:71–75. doi: 10.1016/j.jemermed.2007.03.051. [DOI] [PubMed] [Google Scholar]
- 13.Pulling M, Surawicz CM. Loperamide use for acute infectious diarrhea in children: safe and sound? Gastroenterology. 2008;134:1260–1262. doi: 10.1053/j.gastro.2008.02.052. [DOI] [PubMed] [Google Scholar]
- 14.Li ST, Grossman DC, Cummings P. Loperamide therapy for acute diarrhea in children: systematic review and meta-analysis. PLoS Med. 2007;4:e98. doi: 10.1371/journal.pmed.0040098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.FDA approves first anti-diarrheal drug for HIV/AIDS patients. 2012. Available from: http://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm333701.htm.
- 16.Brown EM, Gamba G, Riccardi D, Lombardi M, Butters R, Kifor O, Sun A, Hediger MA, Lytton J, Hebert SC. Cloning and characterization of an extracellular Ca(2+)-sensing receptor from bovine parathyroid. Nature. 1993;366:575–580. doi: 10.1038/366575a0. [DOI] [PubMed] [Google Scholar]
- 17.Brown EM, MacLeod RJ. Extracellular calcium sensing and extracellular calcium signaling. Physiol Rev. 2001;81:239–297. doi: 10.1152/physrev.2001.81.1.239. [DOI] [PubMed] [Google Scholar]
- 18.Diaz R, Hurwitz S, Chattopadhyay N, Pines M, Yang Y, Kifor O, Einat MS, Butters R, Hebert SC, Brown EM. Cloning, expression, and tissue localization of the calcium-sensing receptor in chicken (Gallus domesticus) Am J Physiol. 1997;273:R1008–R1016. doi: 10.1152/ajpregu.1997.273.3.R1008. [DOI] [PubMed] [Google Scholar]
- 19.Cima RR, Cheng I, Klingensmith ME, Chattopadhyay N, Kifor O, Hebert SC, Brown EM, Soybel DI. Identification and functional assay of an extracellular calcium-sensing receptor in Necturus gastric mucosa. Am J Physiol. 1997;273:G1051–G1060. doi: 10.1152/ajpgi.1997.273.5.G1051. [DOI] [PubMed] [Google Scholar]
- 20.Caroppo R, Gerbino A, Debellis L, Kifor O, Soybel DI, Brown EM, Hofer AM, Curci S. Asymmetrical, agonist-induced fluctuations in local extracellular [Ca(2+)] in intact polarized epithelia. EMBO J. 2001;20:6316–6326. doi: 10.1093/emboj/20.22.6316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nearing J, Betka M, Quinn S, Hentschel H, Elger M, Baum M, Bai M, Chattopadyhay N, Brown EM, Hebert SC, et al. Polyvalent cation receptor proteins (CaRs) are salinity sensors in fish. Proc Natl Acad Sci USA. 2002;99:9231–9236. doi: 10.1073/pnas.152294399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hentschel H, Nearing J, Harris HW, Betka M, Baum M, Hebert SC, Elger M. Localization of Mg2+-sensing shark kidney calcium receptor SKCaR in kidney of spiny dogfish, Squalus acanthias. Am J Physiol Renal Physiol. 2003;285:F430–F439. doi: 10.1152/ajprenal.00081.2002. [DOI] [PubMed] [Google Scholar]
- 23.Riccardi D, Park J, Lee WS, Gamba G, Brown EM, Hebert SC. Cloning and functional expression of a rat kidney extracellular calcium/polyvalent cation-sensing receptor. Proc Natl Acad Sci USA. 1995;92:131–135. doi: 10.1073/pnas.92.1.131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sands JM, Naruse M, Baum M, Jo I, Hebert SC, Brown EM, Harris HW. Apical extracellular calcium/polyvalent cation-sensing receptor regulates vasopressin-elicited water permeability in rat kidney inner medullary collecting duct. J Clin Invest. 1997;99:1399–1405. doi: 10.1172/JCI119299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tfelt-Hansen J, Brown EM. The calcium-sensing receptor in normal physiology and pathophysiology: a review. Crit Rev Clin Lab Sci. 2005;42:35–70. doi: 10.1080/10408360590886606. [DOI] [PubMed] [Google Scholar]
- 26.Whittamore JM, Cooper CA, Wilson RW. HCO (3)(-) secretion and CaCO3 precipitation play major roles in intestinal water absorption in marine teleost fish in vivo. Am J Physiol Regul Integr Comp Physiol. 2010;298:R877–R886. doi: 10.1152/ajpregu.00545.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chattopadhyay N, Cheng I, Rogers K, Riccardi D, Hall A, Diaz R, Hebert SC, Soybel DI, Brown EM. Identification and localization of extracellular Ca(2+)-sensing receptor in rat intestine. Am J Physiol. 1998;274:G122–G130. doi: 10.1152/ajpgi.1998.274.1.G122. [DOI] [PubMed] [Google Scholar]
- 28.Cheng SX, Geibel JP, Hebert SC. Extracellular polyamines regulate fluid secretion in rat colonic crypts via the extracellular calcium-sensing receptor. Gastroenterology. 2004;126:148–158. doi: 10.1053/j.gastro.2003.10.064. [DOI] [PubMed] [Google Scholar]
- 29.Cheng SX, Okuda M, Hall AE, Geibel JP, Hebert SC. Expression of calcium-sensing receptor in rat colonic epithelium: evidence for modulation of fluid secretion. Am J Physiol Gastrointest Liver Physiol. 2002;283:G240–G250. doi: 10.1152/ajpgi.00500.2001. [DOI] [PubMed] [Google Scholar]
- 30.Gama L, Baxendale-Cox LM, Breitwieser GE. Ca2+-sensing receptors in intestinal epithelium. Am J Physiol. 1997;273:C1168–C1175. doi: 10.1152/ajpcell.1997.273.4.C1168. [DOI] [PubMed] [Google Scholar]
- 31.Cheng SX. Calcium-sensing receptor inhibits secretagogue-induced electrolyte secretion by intestine via the enteric nervous system. Am J Physiol Gastrointest Liver Physiol. 2012;303:G60–G70. doi: 10.1152/ajpgi.00425.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kelly JC, Lungchukiet P, Macleod RJ. Extracellular Calcium-Sensing Receptor Inhibition of Intestinal EpithelialTNF Signaling Requires CaSR-Mediated Wnt5a/Ror2 Interaction. Front Physiol. 2011;2:17. doi: 10.3389/fphys.2011.00017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yamaguchi T, Olozak I, Chattopadhyay N, Butters RR, Kifor O, Scadden DT, Brown EM. Expression of extracellular calcium (Ca2+o)-sensing receptor in human peripheral blood monocytes. Biochem Biophys Res Commun. 1998;246:501–506. doi: 10.1006/bbrc.1998.8648. [DOI] [PubMed] [Google Scholar]
- 34.Geibel J, Sritharan K, Geibel R, Geibel P, Persing JS, Seeger A, Roepke TK, Deichstetter M, Prinz C, Cheng SX, et al. Calcium-sensing receptor abrogates secretagogue- induced increases in intestinal net fluid secretion by enhancing cyclic nucleotide destruction. Proc Natl Acad Sci USA. 2006;103:9390–9397. doi: 10.1073/pnas.0602996103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cheng SX, Lightfoot YL, Yang T, Zadeh M, Tang L, Sahay B, Wang GP, Owen JL, Mohamadzadeh M. Epithelial CaSR deficiency alters intestinal integrity and promotes proinflammatory immune responses. FEBS Lett. 2014;588:4158–4166. doi: 10.1016/j.febslet.2014.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cheng SX, Bai HX, Gonzalez-Peralta R, Mistry PK, Gorelick FS. Calcium ameliorates diarrhea in immunocompromised children. J Pediatr Gastroenterol Nutr. 2013;56:641–644. doi: 10.1097/MPG.0b013e3182868946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kunzelmann K, Mall M. Electrolyte transport in the mammalian colon: mechanisms and implications for disease. Physiol Rev. 2002;82:245–289. doi: 10.1152/physrev.00026.2001. [DOI] [PubMed] [Google Scholar]
- 38.Field M. Intestinal ion transport and the pathophysiology of diarrhea. J Clin Invest. 2003;111:931–943. doi: 10.1172/JCI18326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sheinin Y, Kállay E, Wrba F, Kriwanek S, Peterlik M, Cross HS. Immunocytochemical localization of the extracellular calcium-sensing receptor in normal and malignant human large intestinal mucosa. J Histochem Cytochem. 2000;48:595–602. doi: 10.1177/002215540004800503. [DOI] [PubMed] [Google Scholar]
- 40.Liou AP, Sei Y, Zhao X, Feng J, Lu X, Thomas C, Pechhold S, Raybould HE, Wank SA. The extracellular calcium-sensing receptor is required for cholecystokinin secretion in response to L-phenylalanine in acutely isolated intestinal I cells. Am J Physiol Gastrointest Liver Physiol. 2011;300:G538–G546. doi: 10.1152/ajpgi.00342.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wang Y, Chandra R, Samsa LA, Gooch B, Fee BE, Cook JM, Vigna SR, Grant AO, Liddle RA. Amino acids stimulate cholecystokinin release through the Ca2+-sensing receptor. Am J Physiol Gastrointest Liver Physiol. 2011;300:G528–G537. doi: 10.1152/ajpgi.00387.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Seamon KB, Padgett W, Daly JW. Forskolin: unique diterpene activator of adenylate cyclase in membranes and in intact cells. Proc Natl Acad Sci USA. 1981;78:3363–3367. doi: 10.1073/pnas.78.6.3363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Currie MG, Fok KF, Kato J, Moore RJ, Hamra FK, Duffin KL, Smith CE. Guanylin: an endogenous activator of intestinal guanylate cyclase. Proc Natl Acad Sci USA. 1992;89:947–951. doi: 10.1073/pnas.89.3.947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Flores J, Sharp GW. The activation of adenylate cyclase by cholera toxin: possible interaction with the nucleotide regulatory site. Ciba Found Symp. 1976;(42):89–108. doi: 10.1002/9780470720240.ch6. [DOI] [PubMed] [Google Scholar]
- 45.Field M, Graf LH, Laird WJ, Smith PL. Heat-stable enterotoxin of Escherichia coli: in vitro effects on guanylate cyclase activity, cyclic GMP concentration, and ion transport in small intestine. Proc Natl Acad Sci USA. 1978;75:2800–2804. doi: 10.1073/pnas.75.6.2800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Barrett KE, Keely SJ. Chloride secretion by the intestinal epithelium: molecular basis and regulatory aspects. Annu Rev Physiol. 2000;62:535–572. doi: 10.1146/annurev.physiol.62.1.535. [DOI] [PubMed] [Google Scholar]
- 47.Kere J, Höglund P. Inherited disorders of ion transport in the intestine. Curr Opin Genet Dev. 2000;10:306–309. doi: 10.1016/s0959-437x(00)00088-5. [DOI] [PubMed] [Google Scholar]
- 48.Gabriel SE, Brigman KN, Koller BH, Boucher RC, Stutts MJ. Cystic fibrosis heterozygote resistance to cholera toxin in the cystic fibrosis mouse model. Science. 1994;266:107–109. doi: 10.1126/science.7524148. [DOI] [PubMed] [Google Scholar]
- 49.Tang L, Peng M, Liu L, Chang W, Binder HJ, Cheng SX. Calcium-sensing receptor stimulates Cl(-)- and SCFA-dependent but inhibits cAMP-dependent HCO3(-) secretion in colon. Am J Physiol Gastrointest Liver Physiol. 2015;308:G874–G883. doi: 10.1152/ajpgi.00341.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Flagella M, Clarke LL, Miller ML, Erway LC, Giannella RA, Andringa A, Gawenis LR, Kramer J, Duffy JJ, Doetschman T, et al. Mice lacking the basolateral Na-K-2Cl cotransporter have impaired epithelial chloride secretion and are profoundly deaf. J Biol Chem. 1999;274:26946–26955. doi: 10.1074/jbc.274.38.26946. [DOI] [PubMed] [Google Scholar]
- 51.Powell DW, Solberg LI, Plotkin GR, Catlin DH, Maenza RM, Formal SB. Experimental diarrhea. 3. Bicarbonate transport in rat salmonella enterocolitis. Gastroenterology. 1971;60:1076–1086. [PubMed] [Google Scholar]
- 52.Fordtran JS. Speculations on the pathogenesis of diarrhea. Fed Proc. 1967;26:1405–1414. [PubMed] [Google Scholar]
- 53.Ruppin H, Bar-Meir S, Soergel KH, Wood CM, Schmitt MG. Absorption of short-chain fatty acids by the colon. Gastroenterology. 1980;78:1500–1507. [PubMed] [Google Scholar]
- 54.Binder HJ. Role of colonic short-chain fatty acid transport in diarrhea. Annu Rev Physiol. 2010;72:297–313. doi: 10.1146/annurev-physiol-021909-135817. [DOI] [PubMed] [Google Scholar]
- 55.Burleigh DE, Borman RA. Evidence for a nonneural electrogenic effect of cholera toxin on human isolated ileal mucosa. Dig Dis Sci. 1997;42:1964–1968. doi: 10.1023/a:1018835815627. [DOI] [PubMed] [Google Scholar]
- 56.Lorrot M, Vasseur M. How do the rotavirus NSP4 and bacterial enterotoxins lead differently to diarrhea? Virol J. 2007;4:31. doi: 10.1186/1743-422X-4-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Lundgren O, Peregrin AT, Persson K, Kordasti S, Uhnoo I, Svensson L. Role of the enteric nervous system in the fluid and electrolyte secretion of rotavirus diarrhea. Science. 2000;287:491–495. doi: 10.1126/science.287.5452.491. [DOI] [PubMed] [Google Scholar]
- 58.Farthing MJ. Antisecretory drugs for diarrheal disease. Dig Dis. 2006;24:47–58. doi: 10.1159/000090308. [DOI] [PubMed] [Google Scholar]
- 59.Lundgren O. Enteric nerves and diarrhoea. Pharmacol Toxicol. 2002;90:109–120. doi: 10.1034/j.1600-0773.2002.900301.x. [DOI] [PubMed] [Google Scholar]
- 60.Wood JD. Histamine, mast cells, and the enteric nervous system in the irritable bowel syndrome, enteritis, and food allergies. Gut. 2006;55:445–447. doi: 10.1136/gut.2005.079046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Margolis KG, Stevanovic K, Karamooz N, Li ZS, Ahuja A, D’Autréaux F, Saurman V, Chalazonitis A, Gershon MD. Enteric neuronal density contributes to the severity of intestinal inflammation. Gastroenterology. 2011;141:588–598, 598.e1-2. doi: 10.1053/j.gastro.2011.04.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Rabbani M, Hajhashemi V, Vadizadeh A. Co-administration of calcium gluconate and magnesium acetate effectively blocks the signs of morphine withdrawal in mice. Magnes Res. 2012;25:40–48. doi: 10.1684/mrh.2012.0303. [DOI] [PubMed] [Google Scholar]
- 63.Tansy MF, Martin JS, Landin WE, Kendall FM, Melamed S. Spermine and spermidine as inhibitors of gastrointestinal motor activity. Surg Gynecol Obstet. 1982;154:74–80. [PubMed] [Google Scholar]
- 64.Belair EJ, Carlson GR, Melamed S, Moss JN. Effects of spermine and spermidine on gastric emptying in rats. J Pharm Sci. 1981;70:347. doi: 10.1002/jps.2600700338. [DOI] [PubMed] [Google Scholar]
- 65.Bergeron RJ, Wiegand J, Fannin TL. Control of irritable bowel syndrome with polyamine analogs: a structure-activity study. Dig Dis Sci. 2001;46:2615–2623. doi: 10.1023/a:1012750723644. [DOI] [PubMed] [Google Scholar]
- 66.Bergeron RJ, Wiegand J, McManis JS, Weimar WR, Smith RE, Algee SE, Fannin TL, Slusher MA, Snyder PS. Polyamine analogue antidiarrheals: a structure-activity study. J Med Chem. 2001;44:232–244. doi: 10.1021/jm000277+. [DOI] [PubMed] [Google Scholar]
- 67.Bergeron RJ, Yao GW, Yao H, Weimar WR, Sninsky CA, Raisler B, Feng Y, Wu Q, Gao F. Metabolically programmed polyamine analogue antidiarrheals. J Med Chem. 1996;39:2461–2471. doi: 10.1021/jm950827h. [DOI] [PubMed] [Google Scholar]
- 68.Jouret F, Wu J, Hull M, Rajendran V, Mayr B, Schöfl C, Geibel J, Caplan MJ. Activation of the Ca²+-sensing receptor induces deposition of tight junction components to the epithelial cell plasma membrane. J Cell Sci. 2013;126:5132–5142. doi: 10.1242/jcs.127555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.van Ampting MT, Loonen LM, Schonewille AJ, Konings I, Vink C, Iovanna J, Chamaillard M, Dekker J, van der Meer R, Wells JM, et al. Intestinally secreted C-type lectin Reg3b attenuates salmonellosis but not listeriosis in mice. Infect Immun. 2012;80:1115–1120. doi: 10.1128/IAI.06165-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Brandl K, Plitas G, Schnabl B, DeMatteo RP, Pamer EG. MyD88-mediated signals induce the bactericidal lectin RegIII gamma and protect mice against intestinal Listeria monocytogenes infection. J Exp Med. 2007;204:1891–1900. doi: 10.1084/jem.20070563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Cash HL, Whitham CV, Behrendt CL, Hooper LV. Symbiotic bacteria direct expression of an intestinal bactericidal lectin. Science. 2006;313:1126–1130. doi: 10.1126/science.1127119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.MacLeod RJ. Extracellular calcium-sensing receptor/PTH knockout mice colons have increased Wnt/β-catenin signaling, reduced non-canonical Wnt signaling, and increased susceptibility to azoxymethane-induced aberrant crypt foci. Lab Invest. 2013;93:520–527. doi: 10.1038/labinvest.2013.51. [DOI] [PubMed] [Google Scholar]
- 73.Cheng SX. Calcium-sensing receptor in the gut: evidence for its role in mediating known nutritional therapy for inflammatory bowel disease (abstract) JPGN. 2012;55(suppl 1):E70. [Google Scholar]
- 74.Pele LC, Thoree V, Mustafa F, He S, Tsaprouni L, Punchard NA, Thompson RP, Evans SM, Powell JJ. Low dietary calcium levels modulate mucosal caspase expression and increase disease activity in mice with dextran sulfate sodium induced colitis. J Nutr. 2007;137:2475–2480. doi: 10.1093/jn/137.11.2475. [DOI] [PubMed] [Google Scholar]
- 75.Lee GS, Subramanian N, Kim AI, Aksentijevich I, Goldbach-Mansky R, Sacks DB, Germain RN, Kastner DL, Chae JJ. The calcium-sensing receptor regulates the NLRP3 inflammasome through Ca2+ and cAMP. Nature. 2012;492:123–127. doi: 10.1038/nature11588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Ho C, Conner DA, Pollak MR, Ladd DJ, Kifor O, Warren HB, Brown EM, Seidman JG, Seidman CE. A mouse model of human familial hypocalciuric hypercalcemia and neonatal severe hyperparathyroidism. Nat Genet. 1995;11:389–394. doi: 10.1038/ng1295-389. [DOI] [PubMed] [Google Scholar]
- 77.Kos CH, Karaplis AC, Peng JB, Hediger MA, Goltzman D, Mohammad KS, Guise TA, Pollak MR. The calcium-sensing receptor is required for normal calcium homeostasis independent of parathyroid hormone. J Clin Invest. 2003;111:1021–1028. doi: 10.1172/JCI17416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Tu Q, Pi M, Karsenty G, Simpson L, Liu S, Quarles LD. Rescue of the skeletal phenotype in CasR-deficient mice by transfer onto the Gcm2 null background. J Clin Invest. 2003;111:1029–1037. doi: 10.1172/JCI17054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Rey O, Chang W, Bikle D, Rozengurt N, Young SH, Rozengurt E. Negative cross-talk between calcium-sensing receptor and β-catenin signaling systems in colonic epithelium. J Biol Chem. 2012;287:1158–1167. doi: 10.1074/jbc.M111.274589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Laohapitakworn S, Thongbunchoo J, Nakkrasae LI, Krishnamra N, Charoenphandhu N. Parathyroid hormone (PTH) rapidly enhances CFTR-mediated HCO3- secretion in intestinal epithelium-like Caco-2 monolayer: a novel ion regulatory action of PTH. Am J Physiol Cell Physiol. 2011;301:C137–C149. doi: 10.1152/ajpcell.00001.2011. [DOI] [PubMed] [Google Scholar]
- 81.Bovee-Oudenhoven IM, Termont DS, Heidt PJ, Van der Meer R. Increasing the intestinal resistance of rats to the invasive pathogen Salmonella enteritidis: additive effects of dietary lactulose and calcium. Gut. 1997;40:497–504. doi: 10.1136/gut.40.4.497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Bovee-Oudenhoven IM, Wissink ML, Wouters JT, Van der Meer R. Dietary calcium phosphate stimulates intestinal lactobacilli and decreases the severity of a salmonella infection in rats. J Nutr. 1999;129:607–612. doi: 10.1093/jn/129.3.607. [DOI] [PubMed] [Google Scholar]
- 83.Bovee-Oudenhoven IM, Termont DS, Weerkamp AH, Faassen-Peters MA, Van der Meer R. Dietary calcium inhibits the intestinal colonization and translocation of Salmonella in rats. Gastroenterology. 1997;113:550–557. doi: 10.1053/gast.1997.v113.pm9247475. [DOI] [PubMed] [Google Scholar]
- 84.Bovee-Oudenhoven I, Termont D, Dekker R, Van der Meer R. Calcium in milk and fermentation by yoghurt bacteria increase the resistance of rats to Salmonella infection. Gut. 1996;38:59–65. doi: 10.1136/gut.38.1.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Schepens MA, Schonewille AJ, Vink C, van Schothorst EM, Kramer E, Hendriks T, Brummer RJ, Keijer J, van der Meer R, Bovee-Oudenhoven IM. Supplemental calcium attenuates the colitis-related increase in diarrhea, intestinal permeability, and extracellular matrix breakdown in HLA-B27 transgenic rats. J Nutr. 2009;139:1525–1533. doi: 10.3945/jn.109.105205. [DOI] [PubMed] [Google Scholar]
- 86.Breitwieser GE. The calcium sensing receptor life cycle: trafficking, cell surface expression, and degradation. Best Pract Res Clin Endocrinol Metab. 2013;27:303–313. doi: 10.1016/j.beem.2013.03.003. [DOI] [PubMed] [Google Scholar]
- 87.Bovee-Oudenhoven IM, Lettink-Wissink ML, Van Doesburg W, Witteman BJ, Van Der Meer R. Diarrhea caused by enterotoxigenic Escherichia coli infection of humans is inhibited by dietary calcium. Gastroenterology. 2003;125:469–476. doi: 10.1016/s0016-5085(03)00884-9. [DOI] [PubMed] [Google Scholar]
- 88.Dadu R, Hu MI, Cleeland C, Busaidy NL, Habra M, Waguespack SG, Sherman SI, Ying A, Fox P, Cabanillas ME. Efficacy of the Natural Clay, Calcium Aluminosilicate Anti-Diarrheal, in Reducing Medullary Thyroid Cancer-Related Diarrhea and Its Effects on Quality of Life: A Pilot Study. Thyroid. 2015;25:1085–1090. doi: 10.1089/thy.2015.0166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Romain N, Dandrifosse G, Jeusette F, Forget P. Polyamine concentration in rat milk and food, human milk, and infant formulas. Pediatr Res. 1992;32:58–63. doi: 10.1203/00006450-199207000-00011. [DOI] [PubMed] [Google Scholar]
- 90.Buts JP, De Keyser N, De Raedemaeker L, Collette E, Sokal EM. Polyamine profiles in human milk, infant artificial formulas, and semi-elemental diets. J Pediatr Gastroenterol Nutr. 1995;21:44–49. doi: 10.1097/00005176-199507000-00007. [DOI] [PubMed] [Google Scholar]
- 91.Pollack PF, Koldovsky O, Nishioka K. Polyamines in human and rat milk and in infant formulas. Am J Clin Nutr. 1992;56:371–375. doi: 10.1093/ajcn/56.2.371. [DOI] [PubMed] [Google Scholar]
- 92.Ralph A, Englyst K, Bardocz S. Polyamine content of the human diet. In: Bardocz S, White A, editors. Polyamines in health and nutrition. London, UK: Kluwer Academic Publishers; 1999. pp. 123–137. [Google Scholar]
- 93.Milovic V. Polyamines in the gut lumen: bioavailability and biodistribution. Eur J Gastroenterol Hepatol. 2001;13:1021–1025. doi: 10.1097/00042737-200109000-00004. [DOI] [PubMed] [Google Scholar]
- 94.Bardócz S, Duguid TJ, Brown DS, Grant G, Pusztai A, White A, Ralph A. The importance of dietary polyamines in cell regeneration and growth. Br J Nutr. 1995;73:819–828. doi: 10.1079/bjn19950087. [DOI] [PubMed] [Google Scholar]
- 95.Jin BJ, Thiagarajah JR, Verkman AS. Convective washout reduces the antidiarrheal efficacy of enterocyte surface-targeted antisecretory drugs. J Gen Physiol. 2013;141:261–272. doi: 10.1085/jgp.201210885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Thomsen AR, Hvidtfeldt M, Bräuner-Osborne H. Biased agonism of the calcium-sensing receptor. Cell Calcium. 2012;51:107–116. doi: 10.1016/j.ceca.2011.11.009. [DOI] [PubMed] [Google Scholar]
- 97.Thomsen AR, Smajilovic S, Bräuner-Osborne H. Novel strategies in drug discovery of the calcium-sensing receptor based on biased signaling. Curr Drug Targets. 2012;13:1324–1335. doi: 10.2174/138945012802429642. [DOI] [PubMed] [Google Scholar]
- 98.Ramakrishna BS, Subramanian V, Mohan V, Sebastian BK, Young GP, Farthing MJ, Binder HJ. A randomized controlled trial of glucose versus amylase resistant starch hypo-osmolar oral rehydration solution for adult acute dehydrating diarrhea. PLoS One. 2008;3:e1587. doi: 10.1371/journal.pone.0001587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Ramakrishna BS, Venkataraman S, Srinivasan P, Dash P, Young GP, Binder HJ. Amylase-resistant starch plus oral rehydration solution for cholera. N Engl J Med. 2000;342:308–313. doi: 10.1056/NEJM200002033420502. [DOI] [PubMed] [Google Scholar]
- 100.Raghupathy P, Ramakrishna BS, Oommen SP, Ahmed MS, Priyaa G, Dziura J, Young GP, Binder HJ. Amylase-resistant starch as adjunct to oral rehydration therapy in children with diarrhea. J Pediatr Gastroenterol Nutr. 2006;42:362–368. doi: 10.1097/01.mpg.0000214163.83316.41. [DOI] [PubMed] [Google Scholar]
- 101.West RJ, Lloyd JK. The effect of cholestyramine on intestinal absorption. Gut. 1975;16:93–98. doi: 10.1136/gut.16.2.93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Lewis TV, Badillo R, Schaeffer S, Hagemann TM, McGoodwin L. Salicylate toxicity associated with administration of Percy medicine in an infant. Pharmacotherapy. 2006;26:403–409. doi: 10.1592/phco.26.3.403. [DOI] [PubMed] [Google Scholar]
- 103.Jungreis AC, Schaumburg HH. Encephalopathy from abuse of bismuth subsalicylate (Pepto-Bismol) Neurology. 1993;43:1265. doi: 10.1212/wnl.43.6.1265. [DOI] [PubMed] [Google Scholar]
- 104.Cappell MS. Colonic toxicity of administered drugs and chemicals. Am J Gastroenterol. 2004;99:1175–1190. doi: 10.1111/j.1572-0241.2004.30192.x. [DOI] [PubMed] [Google Scholar]
- 105.Hamza H, Ben Khalifa H, Baumer P, Berard H, Lecomte JM. Racecadotril versus placebo in the treatment of acute diarrhoea in adults. Aliment Pharmacol Ther. 1999;13 Suppl 6:15–19. doi: 10.1046/j.1365-2036.1999.00002.x-i1. [DOI] [PubMed] [Google Scholar]
- 106.Salazar-Lindo E, Santisteban-Ponce J, Chea-Woo E, Gutierrez M. Racecadotril in the treatment of acute watery diarrhea in children. N Engl J Med. 2000;343:463–467. doi: 10.1056/NEJM200008173430703. [DOI] [PubMed] [Google Scholar]
- 107.Day AS, Whitten KE, Sidler M, Lemberg DA. Systematic review: nutritional therapy in paediatric Crohn’s disease. Aliment Pharmacol Ther. 2008;27:293–307. doi: 10.1111/j.1365-2036.2007.03578.x. [DOI] [PubMed] [Google Scholar]
- 108.Schepens MA, Rijnierse A, Schonewille AJ, Vink C, Brummer RJ, Willemsen LE, van der Meer R, Bovee-Oudenhoven IM. Dietary calcium decreases but short-chain fructo-oligosaccharides increase colonic permeability in rats. Br J Nutr. 2010;104:1780–1786. doi: 10.1017/S0007114510002990. [DOI] [PubMed] [Google Scholar]
- 109.Hashimoto T, Perlot T, Rehman A, Trichereau J, Ishiguro H, Paolino M, Sigl V, Hanada T, Hanada R, Lipinski S, et al. ACE2 links amino acid malnutrition to microbial ecology and intestinal inflammation. Nature. 2012;487:477–481. doi: 10.1038/nature11228. [DOI] [PMC free article] [PubMed] [Google Scholar]


