Abstract
BACKGROUND & AIMS
Despite the significant association between obesity and several cancers, it has been difficult to establish an association between obesity and hepatocellular carcinoma (HCC). Patients with HCC often have ascites, making it a challenge to accurately determine body mass index (BMI), and many factors contribute to the development of HCC. We performed a case–control study to investigate whether obesity early in adulthood affects risk, age of onset, or outcomes of patients with HCC.
METHODS
We interviewed 622 patients newly diagnosed with HCC from January 2004 through December 2013, along with 660 healthy controls (frequency-matched by age and sex) to determine weights, heights, and body sizes (self-reported) at various ages before HCC development or enrollment as controls. Multivariable logistic and Cox regression analyses were performed to determine the independent effects of early obesity on risk for HCC and patient outcomes, respectively. BMI was calculated, and patients with a BMI ≥30 kg/m2 were considered obese.
RESULTS
Obesity in early adulthood (age, mid-20s to mid-40s) is a significant risk factor for HCC. The estimated odds ratios (OR) and 95% confidence intervals (CI) were 2.6 (1.4–4.4), 2.3 (1.2–4.4), and 3.6 (1.5–8.9) for the entire population, men, and women, respectively. Each unit increase in BMI at early adulthood was associated with a 3.89-month decrease in age at HCC diagnosis (P<.001). Moreover, there is a synergistic interaction between obesity and hepatitis virus infection. However, we found no effect of obesity on the overall survival of patients with HCC.
CONCLUSION
Early adulthood obesity is associated with increased risk of developing HCC at a young age in the absence of major HCC risk factors, with no effect on outcomes of patients with HCC.
Keywords: obesity, HCC, case-control, risk factor
INTRODUCTION
Overweight and obesity are major public health problems in both economically developed and developing countries. Between 1980 and 2013, the global prevalence of overweight and obesity combined increased by 27.5% for adults and 47.1% for children.1 The increase was higher in developed than in developing countries. If such trends continue, by 2030, up to 57.8% of the world’s adult population could be either overweight or obese.2
Concurrent with the increased rate of obesity in the United States, the incidence of hepatocellular carcinoma (HCC) has significantly increased over the past 3 decades,3, 4 with a positive correlation observed between prevalence of obesity and incidence of HCC.5, 6
Despite the reported significant association between obesity and several cancers in the United States,7 the association between obesity and HCC8, 9 has been difficult to confirm for the following reasons: 1) rarity and poor prognosis of HCC, making large-scale studies difficult to conduct; 2) underlying cirrhosis associated with portal hypertension and ascites that can preclude accurate assessment of body mass index (BMI) at the time of HCC diagnosis; 3) missing BMI estimates in medical records of HCC patients; and 4) the multifactorial origin of HCC, necessitating adjustments for the confounding effects of the major HCC risk factors including hepatitis C virus (HCV), hepatitis B virus (HBV), diabetes mellitus, and alcohol consumption.
To investigate the association between HCC and obesity before HCC development, we embarked on a large case-control study in which we integrated clinical and epidemiological data with obesity data to assess 1) the independent effect of excess body weight across an individual’s life cycle on HCC risk, 2) the synergistic interaction between obesity and other HCC risk factors, and 3) the effect of obesity on age at HCC onset or on overall survival rate of HCC patients.
METHODS
This investigation was part of an active hospital-based case-control study, which was approved by the Institutional Review Board at The University Texas MD Anderson Cancer Center (Protocol # ID00-083). Written informed consent for participation was obtained from each participant.
Case patients were recruited from the population of patients with newly diagnosed HCC who were evaluated and treated at MD Anderson Cancer Center’s gastrointestinal medical oncology and surgical oncology outpatient clinics. The inclusion criteria were a pathologically or radiologically confirmed diagnosis of HCC and U.S. residency. The exclusion criteria were the presence of other types of primary liver cancer (such as cholangiocarcinoma or fibrolamellar hepatocarcinoma), unknown primary tumors, and concurrent or past history of cancer at another organ site.
Control subjects were healthy (cancer-free) and genetically unrelated family members (such as spouses) of cancer patients at MD Anderson. However, we excluded family members and spouses of patients with liver, gastrointestinal, lung, or head and neck cancer. The reason for such exclusion was to prevent the introduction of selection bias connected with shared environmental and genetic factors that are highly associated with HCC, e.g., alcohol consumption, smoking, family history of cancer, and hepatitis virus infection. Cases and controls were frequency-matched by age (±5 years) and sex. Between January 2004 and December 2013, 622 HCC case patients and 660 control subjects participated in this investigation. HCC patients and controls were recruited simultaneously and were interviewed in person for demographic features and HCC risk factors (Table 1) with use of a structured and validated questionnaire. We defined cigarette smokers as subjects who had smoked ≥100 cigarettes during their lifetime. Heavy smokers were defined as those who had >20 pack-years of smoking. We defined ever-alcohol drinkers as subjects who had consumed at least 4 alcoholic drinks each month for 6 months in their lifetime. We further classified ever-drinkers according to the total lifetime volume of ethanol consumed in milliliters, which was computed according to the frequency of drinking, type of serving (glass, bottle, or can), number and size of each serving, and duration of consumption, summed over the whole period of alcohol use. Heavy alcohol consumption was defined as consumption of more than 60 mL of ethanol/day during the subject’s period of alcohol drinking.10
Table 1.
Multivariate-adjusted odds ratios (AORs) and 95% confidence intervals (CIs) of hepatocellular carcinoma for demographic and other factors
| Demographic variables | No. | (%) | No. | (%) | AOR* (95% CI) | P value |
|---|---|---|---|---|---|---|
|
|
|
|||||
| HCC patients (N = 622) | Controls (N = 660) | |||||
| Sex | ||||||
|
| ||||||
| Female | 149 | 24 | 257 | 38.9 | 1 (reference) | |
|
| ||||||
| Male | 473 | 76 | 403 | 61.1 | .9 (.6–1.2) | .5 |
|
| ||||||
| Age (years) | ||||||
|
| ||||||
| <60 | 229 | 36.8 | 314 | 47.6 | 1 (reference) | |
|
| ||||||
| ≥60 | 393 | 63.2 | 346 | 52.4 | 2.7 (1.8–4) | .001 |
|
| ||||||
| Ethnicity | ||||||
|
| ||||||
| Non-Hispanic white | 421 | 67.7 | 596 | 90.3 | 1 (reference) | |
|
| ||||||
| Hispanic | 88 | 14.1 | 41 | 6.2 | 2.5 (1.5–4.4) | .001 |
|
| ||||||
| African American | 67 | 10.8 | 19 | 2.9 | 3.9 (1.8–8.8) | .001 |
|
| ||||||
| Asian | 46 | 7.4 | 4 | 0.6 | 12.7 (3.7–43.4) | <.001 |
|
| ||||||
| Educational level | ||||||
|
| ||||||
| < College Education | 268 | 43.1 | 178 | 27.0 | 1 (reference) | |
|
| ||||||
| ≥ College Education | 354 | 56.9 | 482 | 73.0 | 1.3 (.9–1.9) | .06 |
|
| ||||||
| Hepatitis virus infection | ||||||
|
| ||||||
| No virus infection | 311 | 50 | 635 | 96.2 | 1 (reference) | |
|
| ||||||
| HCV alone | 141 | 22.7 | 2 | 0.3 | 169.9 (40.4–715.6) | <.001 |
|
| ||||||
| HBV alone | 83 | 13.3 | 21 | 3.2 | 8.3 (4.4–15.4) | <.001 |
|
| ||||||
| HCV and HBV | 87 | 14 | 2 | 0.3 | 94.5 (22.1–403.5) | <.001 |
|
| ||||||
| Cigarette smoking | ||||||
|
| ||||||
| No smoking | 225 | 36.2 | 353 | 53.5 | 1(reference) | |
|
| ||||||
| ≤20 pack-years | 171 | 27.5 | 142 | 21.5 | .9 (.6–1.3) | .5 |
|
| ||||||
| >20 pack-years | 226 | 36.2 | 165 | 25.0 | 1.5 (1.1–1.9) | .006 |
|
| ||||||
| Alcohol drinking | ||||||
|
| ||||||
| No drinking | 178 | 28.6 | 295 | 44.7 | 1 (reference) | |
|
| ||||||
| <60 ml ethanol/day | 310 | 49.8 | 329 | 49.8 | 1.7 (1.2–2.4) | .002 |
|
| ||||||
| ≥ 60 ml ethanol/day | 134 | 21.5 | 36 | 5.5 | 4.5 (2.5–8.1) | <.001 |
|
| ||||||
| Prior history of diabetes | ||||||
|
| ||||||
| No diabetes mellitus | 411 | 66.1 | 581 | 88 | 1 (reference) | |
|
| ||||||
| Diabetes ≤I year | 14 | 2.3 | 18 | 2.7 | 1.9 (.9–4.4) | .2 |
|
| ||||||
| Diabetes >1 year | 197 | 31.7 | 61 | 9.2 | 4.7 (3.2–7.1) | <.001 |
|
| ||||||
| Family history of cancer | ||||||
|
| ||||||
| No | 117 | 18.8 | 228 | 34.5 | 1 (reference) | |
|
| ||||||
| Yes | 505 | 81.2 | 432 | 65.5 | 3.7 (2.6–5.1) | <.001 |
|
| ||||||
| State of residency | ||||||
|
| ||||||
| TX, LA, AK, NM, OK † | 448 | 72 | 497 | 75.3 | 1 (reference) | |
|
| ||||||
| Other states | 174 | 28 | 163 | 24.7 | .8 (.7–1.1) | .2 |
Abbreviations: HBV, hepatitis B virus; HCV, hepatitis C virus.
The AORs were estimated from a multiple logistic regression model that included sex, age, ethnicity, education level, hepatitis virus infection, alcohol drinking, cigarette smoking, history of diabetes, and family history of cancer.
States of Texas (TX), Louisiana (LA), Arkansas (AK), New Mexico (NM), and Oklahoma (OK)
Participants were interviewed for history of diabetes mellitus, type of diabetes, age at diagnosis, and duration of diabetes. Subjects with a history of diabetes were questioned about medications used for diabetes control and the duration of treatment, and reported results of HbA1. Information about prior history of chronic liver diseases (CLDs) was obtained including cirrhosis, hemochromatosis, primary biliary cirrhosis, Wilson disease, autoimmune hepatitis, and alpha 1 antitrypsin deficiency. A detailed questionnaire about obesity was included during the interview to obtain information about self-reported height (inches) and weight (pounds) across the life cycle before cancer diagnosis (HCC patients) or before recruitment (controls), including current weight as well as weight when patients and controls were in their mid-20s, mid-30s, mid-40s, mid-50s, and mid-60s. In addition, self-reported body size across the same ages using the validated Stunkard pictograms was obtained from each participant. BMI was calculated [(weight (kg)/height (m)2] and classified as a four-level categorical variable: underweight (<18.5 kg/m2), normal weight (18.5–24.9 kg/m2), overweight (25–29.9 kg/m2), or obese (≥30 kg/m2). Only 4 HCC patients were classified as underweight and were included among the normal weight category.
All participants were questioned to classify their past engagement in physical activity (at work or free time), as well as the type, frequency, and duration of activities during the past 5 years. Vigorous physical activity was described as enough to get sweaty, experience fast heart beats, or get out of breath. All cases and controls recalled their family history of all cancers among first- and second-degree relatives.
Detailed clinical variables were retrieved from HCC patients’ medical records; these variables included information about different HCC staging scores, HCC treatment exposure, pathological differentiation, underlying cirrhosis, vascular invasion, metastasis, lymph node involvement, tumor nodularity and size, and alpha fetoprotein level.11–13 Overall survival (OS) was defined as the time between HCC diagnosis and death (as a result of all causes) or end of follow-up (censored observations). Underlying cirrhosis was determined by pathological findings (diagnostic biopsies) and by computed tomography scans. In addition, all HCC patients were examined for the signs of cirrhosis including manifestations related to portal hypertension, e.g. ascites, bleeding from esophageal varices, and hepatic encephalopathy. Minor signs were also noted clinically, such as palmar erythema, spider angioma, and clubbing of the fingers.
Blood samples from cases and controls were tested for HBV and HCV. HCV antibodies, hepatitis B surface antigen, and antibodies to hepatitis B core antigen were detected by use of a third-generation enzyme-linked immunosorbent assay (ELISA) (Abbott Laboratories, North Chicago, IL). Positive results prompted repeated confirmatory ELISA testing.
Stata software (Stata Corp, College Station, TX) was used for statistical analysis. We performed multivariate unconditional logistic regression analyses. For each risk factor, we calculated the adjusted odds ratio (OR) and 95% confidence interval (CI) values, using maximum likelihood estimation. All ORs for the association between BMI and HCC were adjusted for age, sex, race, educational level, smoking, alcohol, diabetes, family history of cancer, physical activity, and HBV or HCV infection. Hazard ratios (HRs) and 95% CIs were calculated by using Cox proportional hazard models. The population-attributable risk percentage (PAR%) of HCC was calculated, as follows: , in which OR is the adjusted OR for the relationship between being obese and having HCC, and Pe is the prevalence of being obese in the control population in the early adulthood period before enrollment.
Analysis of covariance was used to analyze patients’ mean age at HCC onset by BMI status. Linear regression models were used to estimate the mean differences in age at HCC onset associated with BMI after adjusting for other factors associated with age at onset in this study population.
We used multiple logistic regression models to investigate possible interactions on an additive scale of prior adulthood history of obesity with hepatitis virus infection (HCV and HCV), alcohol consumption, and diabetes mellitus. To assess deviation from the additive model (which assumes no interaction between variables), we calculated the synergism index , in which OR11 = OR of the joint effect of two risk factors, and OR10 and OR01 = OR of each risk factor in the absence of the other. A value of S equal to unity was indicative of additivity, whereas a value greater than unity was indicative of superadditivity and synergism.14, 15
RESULTS
The baseline demographic characteristics of patients and controls are summarized in table 1. Most study subjects were non-Hispanic white men; the men-to-women ratio was 3.2 to 1 for HCC patients. Case patients were slightly older than control subjects, with a mean difference of 3 years (95% CI, 2 to 5); the mean [± standard error (SE)] ages were 63 ± .4 years for HCC patients and 60 ± .4 years for controls. Higher education (≥ college degree) was more frequent among control subjects than among HCC patients. Cases and controls had a similar distribution of geographical region (US state of residency) where 369 (59.3%) cases and 401 (60.8%) controls were from state of Texas, P =.1.
This study continued to support the association between HCC and several risk factors reported previously by us16–19 and by other investigators, including alcohol drinking, cigarette smoking, diabetes mellitus, HCV, HBV, and family history of cancer.20–24
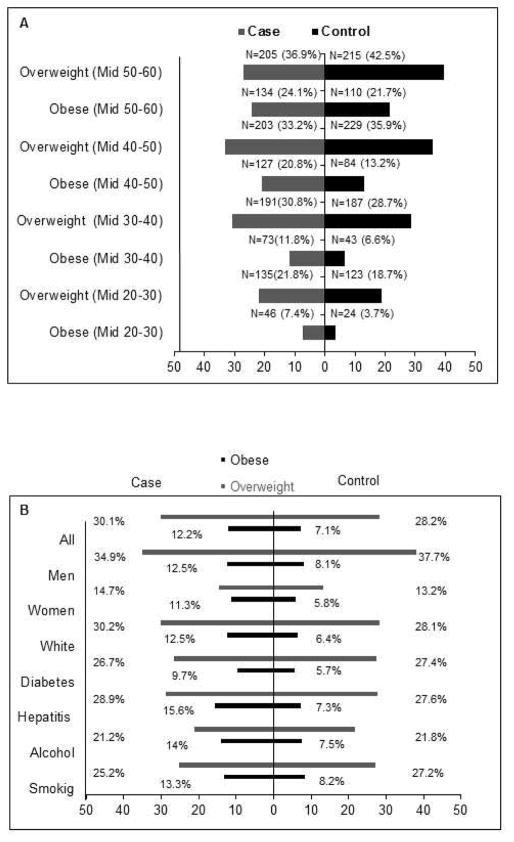
Figure 1A shows the distribution of overweight and obesity among HCC cases and controls at ages ranging from their mid-20s to mid-60s.
Figure 1.
(A) Frequency of overweight and obesity at various ages during the life cycle before hepatocellular carcinoma diagnosis or control recruitment.
(B) Distribution of body mass index status by percentages (overweight, obese) by HCC risk factors in cases and controls in their mid-20s to mid-40s.
The prevalence of ever experience of obesity during lifetime was recalled by 38.4% (95% CI, 33.7%–43.4%) of HCC case patients and by 30.6% (95% CI, 27.1%–34.3%) of healthy controls (P = .03).
We calculated the average BMI during early adulthood (mid-20s to mid-40s) and then classified BMI into normal, overweight, and obese. The prevalence of obesity (BMI ≥30) in early adulthood (mid-20s to mid-40s) was significantly higher in HCC cases than in controls (Figure 1B; P = .002). Table 2 shows that among all study subjects, more cases (21.8%) than controls (18.7%) reported overweight in their mid-20s (P = .02). A prior history of obesity in the mid-20s, mid-30s, and mid-40s was significantly associated with increased HCC risk in the whole study population and in absence of major HCC risk factors (Table 2).
Table 2.
AOR (95% CI)* for the associations between prior history of overweight/obesity and risk of hepatocellular carcinoma in all population, men, women, and in absence of risk factors (HCV, HBV, Alcohol Drinking, Diabetes)
| Previous BMI | Overweight (BMI 24–29.9) | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| All | Men | Women | ||||||||||
| Cases | Controls | AOR (95% CI) | P | Cases | Controls | AOR (95% CI) | P | Cases | Controls | AOR (95% CI) | P | |
| Mid-20s | 135 | 123 | 1.6 (1.1–2.3) | .02 | 124 | 109 | 1.5 (.9–2.3) | .078 | 11 | 14 | 2.4 (.9–3.0) | .089 |
| Mid-30s | 191 | 187 | 1.2 (.9–1.8) | .27 | 172 | 158 | 1.3 (.9–2.1) | .174 | 19 | 29 | 1.2 (.5–2.6) | .734 |
| Mid-40s | 203 | 229 | .9 (.6–1.2) | .41 | 174 | 178 | .9 (.6–1.4) | .709 | 29 | 51 | .8 (.4–1.6) | .589 |
| Mid-50s | 205 | 215 | .6 (.4–1.1) | .10 | 170 | 156 | .5 (.3–.9) | .014 | 35 | 59 | .9 (.5–1.7) | .788 |
| Overweight (BMI 24–29.9) | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| No Diabetes | No-HCV/HBV infection | No Alcohol Drinking | ||||||||||
| Cases | Controls | AOR (95% CI) | P | Cases | Controls | AOR (95% CI) | P | Cases | Controls | AOR (95% CI) | P | |
| Mid-20s | 82 | 107 | 1.5 (.9–2.3) | .093 | 74 | 118 | 1.6 (1.1–2.4) | .024 | 22 | 36 | 1.7 (.8–3.6) | .139 |
| Mid-30s | 113 | 160 | 1.3 (.8–1.9) | .279 | 94 | 178 | 1.4 (.9–1.9) | .118 | 35 | 61 | 1.4 (.8–2.7) | .277 |
| Mid-40s | 132 | 200 | .9 (.6–1.3) | .512 | 99 | 214 | 1.2 (.8–1.7) | .430 | 39 | 91 | .7 (.4–1.3) | .268 |
| Mid-50s | 127 | 194 | .6 (.4–.9) | .012 | 108 | 204 | .8 (.5–1.2) | .237 | 50 | 89 | .7 (.4–1.3) | .265 |
| Obesity (BMI ≥30) | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| All | Men | Women | ||||||||||
| Cases | Controls | AOR (95% CI) | P | Cases | Controls | AOR (95% CI) | P | Cases | Controls | AOR (95% CI) | P | |
| Mid-20s | 46 | 24 | 2.5 (1.3–4.8) | .009 | 33 | 18 | 1.8 (.8–4.1) | .174 | 13 | 6 | 5.2 (1.6–7.2) | .007 |
| Mid-30s | 73 | 43 | 2.9 (1.7–5.1) | <.001 | 58 | 31 | 3.1(1.6–6) | .001 | 15 | 12 | 3.3 (1.3–8.6) | .013 |
| Mid-40s | 127 | 84 | 2.1 (1.3–3.3) | .002 | 101 | 56 | 2.2 (1.2–4) | .001 | 26 | 28 | 2.1 (1.1–4.5) | .0489 |
| Mid-50s | 134 | 110 | .9 (.6–1.5) | .7 | 104 | 70 | .8 (.4–1.4) | .381 | 30 | 40 | 1.2 (.5–2.5) | .715 |
| Obesity (BMI ≥30) | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| No diabetes | No-HCV/HBV infection | No alcohol drinking | ||||||||||
| Cases | Controls | AOR (95% CI) | P | Cases | Controls | AOR (95% CI) | P | Cases | Controls | AOR (95% CI) | P | |
| Mid-20s | 22 | 15 | 2.7 (1.1–6.6) | .038 | 28 | 23 | 2.7 (1.3–5.5) | .006 | 17 | 12 | 3.9 (1.6–9.7) | .004 |
| Mid-30s | 39 | 31 | 2.9 (1.5–5.7) | .002 | 49 | 41 | 3.4 (1.9–5.9) | <.001 | 27 | 19 | 2.5 (1.2–4.9) | .01 |
| Mid-40s | 66 | 63 | 2.2 (1.3–3.9) | .006 | 72 | 82 | 2.5 (1.5–4.1) | <.001 | 41 | 40 | 1.6 (.8–2.9) | .166 |
| Mid-50s | 65 | 80 | .9 (.5–1.6) | .817 | 80 | 106 | 1.1 (.7–1.8) | .763 | 41 | 58 | .7 (.4–1.5) | .381 |
Abbreviations: AOR, multivariate-adjusted odds ratio; BMI, body mass index; HBV, hepatitis B virus; HCV, hepatitis C virus.
AOR for sex, age, ethnicity, education level, HCV, HBV, alcohol drinking, cigarette smoking, history of diabetes, physical activity, and family history of cancer
Table 3 shows that the mean age at HCC onset among case patients who recalled a prior history of early adulthood obesity in their mid-20s to mid-40s was significantly lower than the mean age at onset of those with normal BMIs at the same life cycle (P = .01, <.001, <.001, respectively). For example, in those with obesity history in their mid-20s, HCC was diagnosed more than 3 years sooner than in those at normal weight. The mean ages (years ± SD) at diagnosis were 63.4 (±11.19), 63.0 (±10.6), and 60.1 (±11.6) years for those in their mid-20s who were normal weight, overweight, and obese, respectively. We observed similar results when we examined the mean age at HCC onset in patients in their mid-30s and mid-40s, comparing those who were obese/overweight with those with normal weight. The mean difference in age at HCC onset between obese individuals and those with normal body weight was determined to be statistically significant after adjusting for other factors associated with age at HCC onset (Table 3). We estimated that each 1-unit increase in BMI at early adulthood (mid-20s to mid-40s) before HCC diagnosis was associated with a 3.89-month decrease in the age at HCC diagnosis (P = <.001). The estimated coefficient = −3.89 and 95% CI (−5.60 to −2.18) after controlling for the confounding effect of HCC risk factors (P < .0001).
Table 3.
Association between BMI status before HCC diagnosis and age at onset of HCC (Multiple Linear Regression)
| Age (Range) | Patients (N) | Age at HCC onset (years) | Mean difference (95% CI) | P Value | |
|---|---|---|---|---|---|
| Median (IQR) | Mean (±SD) | ||||
| Mid-20s | |||||
| Normal weight | 438 | 70 (62–79) | 63.4 (±11.19) | ||
| Overweight | 135 | 62 (56–70) | 63.0 (±10.6) | −2.0 (−4.1 to .06) | .057 |
| Obese | 46 | 61 (55–70) | 60.1 (±11.6) | −4.1 (−7.2 to .85) | .013 |
| Mid-30s | |||||
| Normal weight | 356 | 63 (56–72) | 63.7 (±11.3) | ||
| Overweight | 191 | 64 (56–70) | 63.2 (±10.5) | −1.6 (−3.5 to 21) | .084 |
| Obese | 73 | 60 (55–69) | 61.3 (±10.2) | −5.1 (−7.7 to 2.4) | <.001 |
| Mid-40s | |||||
| Normal weight | 281 | 65 (56–73) | 64.3 (±11.9) | ||
| Overweight | 203 | 64 (57–71) | 64.3 (±9.1) | −.5 (−2.3 to 1.3) | .589 |
| Obese | 127 | 60 (56–68) | 61.7 (±9.1) | −4.14 (−6.2 to 2.1) | <.001 |
| Mid-50s | |||||
| Normal weight | 217 | 64 (56–74) | 64.7 (±12.3) | .9 (−.8 to 2.7) | .3 |
| Overweight | 205 | 66 (60–72) | 66.5 (± 8.5) | −1.7 (−3.8 to .3) | .09 |
| Obese | 134 | 63 (59–69) | 64.3 (±6.9) | ||
Abbreviations: BMI, body mass index; CI, confidence interval; HCC, hepatocellular carcinoma; IQR, interquartile range
Only 12% of obese cases and 4% of obese controls recalled weight reduction over time; among whom 6% of the case patients and 2% of the controls experienced ≥ 10% weight reduction. These reductions had no significant effect on the risk of HCC development.
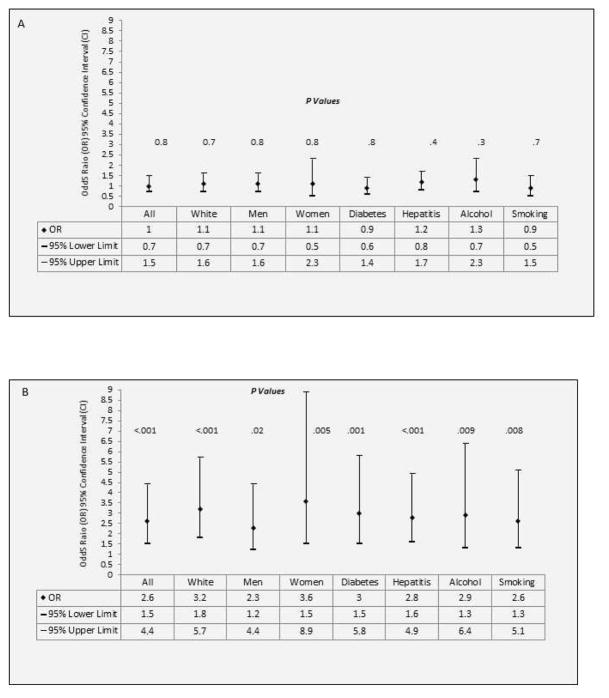
Restricted analyses among white subjects, men, women, nondrinkers, non-HCV/-HBV–infected subjects, nondiabetics, and nonsmokers indicated no significant association between early adulthood overweight (mid-20s to mid-40s) with HCC development (Figure 2A). However, the odds for developing HCC are approximately 2- to 4-fold greater for subjects with early adulthood obesity than for subjects with normal BMI (Figure 2B). A total of 21 HCC cases and 0 controls recalled a prior history of CLD; excluding these cases from analysis did not meaningfully change the significant association between early adulthood obesity and HCC.
Figure 2.
(A) Odds Ratio, 95% Confidence Interval for the association between adulthood overweight (mid-20s to mid-40s) with hepatocellular carcinoma risk in the absence of major risk factors and with adjustment of confounding factors.
(B) Odds Ratio, 95% Confidence Interval for the association between adulthood obesity (mid-20s to mid-40s) with hepatocellular carcinoma risk in the absence of major risk factors and with adjustment of confounding factors.
Example: The estimated odds ratios, 95% confidence intervals for the association between adulthood overweight/obesity and HCC among non-diabetics were .9 (.6–1.4), P=.8 and 3.0 (1.5–5.8), P=.001 respectively, after adjustment for confounding factors including age, ethnicity, HCV, HBV, education level, alcohol drinking, cigarette smoking, physical activity, and family history of cancer.
Table 4 shows the relative excess risk for patients having prior history of early adulthood obesity (mid-20s to mid-40s) and HCV/HBV, alcohol consumption, or diabetes mellitus. By crossing each risk factor with early adulthood obesity (mid-20s to mid-40s), a dummy variable of 4 categories was obtained: two for the presence of each factor in the absence of the other, one indicating the presence of joint factors, and one for unexposed to either factor. The “unexposed to either factor” category was used as the reference category in the regression model. For example, the ORs (95% CI) for hepatitis virus infection in the absence of obesity, obesity in the absence of virus infection, and combined virus infection and obesity were 31.7 (19.3–52.3), 2.5 (1.5–4.3), and 72.5 (9.2–574.2), respectively. Using the OR as an estimate for the relative risk of disease development, the relative excess risk for patients having early adulthood obesity plus hepatitis virus infection exceeded the sum of the relative excess risks for the virus infection and obesity alone, that is, 72.5−1.0 > (31.7−1.0) + (2.5−1.0), indicating a departure from additivity in the joint effect of early adulthood obesity with HCV/HBV (S = 2.2; 95% CI, 1.2–3.9). This may suggest that obesity, in addition to its own direct effects, may exacerbate the effect of chronic hepatitis virus infection on HCC. A similar approach was performed for the joint effect of diabetes and alcohol consumption with early adulthood obesity. Unlike with hepatitis virus infection (HCV/HBV), we found no risk modification for the joint effect between alcohol consumption or diabetes mellitus with early adulthood obesity (mid-20s to mid-40s) (Table 4).
Table 4.
Risk modification of early adulthood obesity (mid-20s to mid-40s) by hepatitis virus infection, alcohol consumption, and diabetes mellitus on HCC development: AOR* (95% CI) using multivariate logistic regression analyses
| Variables | Cases N=622 |
Controls N=660 |
Model | AOR (95% CI) | P | |
|---|---|---|---|---|---|---|
| HCV/HBV* | Early Adulthood Obesity | (1)† | ||||
| No | No | 266 | 589 | 1 (reference) | ||
| Yes | No | 281 | 24 | 31.7 (19.3–52.3) | <.0001 | |
| No | Yes | 48 | 46 | 2.5 (1.5–4.3) | <.0001 | |
| Yes | Yes | 27 | 1 | 72.5 (9.2–574.2) | <.0001 | |
| Diabetes | Early Adulthood Obesity | (2)‡ | ||||
| No | No | 375 | 548 | 1 (reference) | ||
| Yes | No | 172 | 65 | 3.9 (2.6–5.7) | <.0001 | |
| No | Yes | 39 | 33 | 3.3 (1.7–6.4) | <.0001 | |
| Yes | Yes | 36 | 14 | 6.5 (3.2–13.5) | <.0001 | |
| Alcohol | Early Adulthood Obesity | (3)§ | ||||
| No | No | 155 | 273 | 1 (reference) | ||
| Yes | No | 392 | 340 | 2.1 (1.5–3.0) | <.0001 | |
| No | Yes | 25 | 22 | 2.7 (1.2–5.9) | <.0001 | |
| Yes | Yes | 50 | 25 | 5.0 (2.4–10.1) | <.0001 |
AOR= Adjusted Odds Ratio; HBV, hepatitis B virus; HCV, hepatitis C virus
Model (1) adjustment for age, ethnicity, education level, alcohol drinking, cigarette smoking, history of diabetes, physical activity, and family history of cancer
Model (2) adjustment for age, ethnicity, HCV, HBV, education level, alcohol drinking, cigarette smoking, physical activity, and family history of cancer
Model (3) adjustment for age, ethnicity, HCV, HBV, education level, history of diabetes, cigarette smoking, physical activity, and family history of cancer
In our control group, the prevalence of diabetes, early adulthood obesity, and combined diabetes with early adulthood obesity were 9.85%, 5%, and 2.12%, respectively. Therefore, according to the ORs values in Table 4, the estimated PARs% were 21% for diabetes, 10% for early adulthood obesity, and 11% for the combination of diabetes and early adulthood obesity.
The clinical features of HCC did not significantly vary by the status of early adulthood BMI (normal, overweight, obese) (Table 5). The estimated median OS (95% CI) values were 21.9 (18.7–25.2), 18.9 (13.2–24.8), and 19.9 (16.7–23.2) for normal BMI, overweight, and obesity, respectively (P = .6). In addition, multivariate Cox regression analysis indicated that early adulthood obesity (mid-20s to mid-40s) was not associated with a significantly increased total mortality (HR = .9; 95% CI, .6–1.4) (P = .6).
Table 5.
Distribution of hepatocellular carcinoma clinical features by BMI status at early adulthood (mid-20s to mid-40s)
| Clinical Feature* | Normal N=360 (%) |
Overweight N=187 (%) |
Obese N= 75(%) |
P value† |
|---|---|---|---|---|
| Presence of Cirrhosis | .1 | |||
| Yes | 214 (59.6) | 128 (68.4) | 43 (57.3) | |
| No | 145 (40.4) | 59 (31.6) | 32 (42.7) | |
| Evidence of Vascular Invasion | 0.2 | |||
| Yes | 107 (29.8) | 70 (37.4) | 25 (33.3) | |
| No | 252 (70.2) | 117 (62.6) | 50 (66.7) | |
| Evidence of Portal Thrombosis | 0.7 | |||
| Yes | 82 (22.8) | 49 (26.2) | 19 (25.3) | |
| No | 277 (77.2) | 138 (73.8) | 56 (74.7) | |
| Extra-hepatic Metastasis | 0.9 | |||
| Yes | 95 (26.5) | 47 (25.1) | 21 (28) | |
| No | 264 (73.5) | 140 (74.9) | 54 (72) | |
| Lymph Node Involvement | ||||
| Yes | 60 (16.8) | 45 (24.1) | 17 (22.7) | 0.1 |
| No | 298 (83.2) | 142 (75.9) | 58 (77.3) | |
| Tumor Involvement | ||||
| >50% | 71 (20.4) | 41 (22.2) | 23 (31.1) | 0.2 |
| ≤50% | 272 (78.2) | 143 (77.3) | 51 (68.9) | |
| Tumor Nodularity | 0.4 | |||
| Multi-nodular | 222 (62) | 109 (58.3) | 50 (66.7) | |
| Solitary-nodule | 119 (33.2) | 75 (40.1) | 24 (32) | |
| Performance Status | 0.1 | |||
| (≥ 2) | 44 (12.2) | 24 (12.8) | 9 (12) | |
| (< 2) | 316 (87.8) | 163 (87.2) | 66 (88) | |
| Tumor Differentiation | 0.1 | |||
| Well-Differentiated | 83 (23.1) | 49 (26.4) | 19 (25.3) | |
| Moderate-Differentiated | 114 (31.7) | 54 (29) | 24 (32) | |
| Poor-Differentiated | 44 (12.2) | 35 (18.8) | 8 (10.7) | |
| Not Reported | 113 (31.4) | 48 (25.8) | 24 (32) | |
| CLIP | 0.2 | |||
| CLIP Score (0–2) | 276 (79.5) | 138 (74.6) | 50 (67.6) | |
| CLIP Score (3) | 40 (11.5) | 29 (15.7) | 14 (18.9) | |
| CLIP Score (4–6) | 27 (7.8) | 17 (9.2) | 10 (13.5) | |
| Okuda | 0.1 | |||
| Stage-I | 211 (58.9) | 93 (49.7) | 34 (45.3) | |
| Stage-II | 132 (36.9) | 88 (47.1) | 37 (49.3) | |
| Stage-III | 11 (3.1) | 5 (2.7) | 4 (5.3) | |
| BCLC | 0.3 | |||
| Early Stage (0-A) | 27 (7.5) | 10 (5.3) | 3 (4) | |
| Intermediate Stage (B) | 66 (18.3) | 35 (18.7) | 13 (17.3) | |
| Advances Stage (C) | 241 (66.9) | 136 (72.7) | 52 (69.3) | |
| End Stage (D) | 17 (4.7) | 4 (2.1) | 7 (9.3) | |
| TNM | 0.8 | |||
| I–II | 119 (33) | 60 (32.1) | 21 (28) | |
| IIIA-IIIB-IIIC | 93 (25.9) | 55 (29.4) | 24 (31.9) | |
| IVA–IVB | 131(36.4) | 67 (35.8) | 27 (36) | |
| Treatment | 0.4 | |||
| Surgery and Transplant | 58 (16.1) | 21 (11.2) | 8 (10.7) | |
| Ablation Therapy | 11 (3.1) | 7 (3.7) | 3 (4) | |
| Local Therapy | 118 (32.8) | 69 (36.9) | 19 (25.3) | |
| Systemic Therapy | 153 (42.5) | 78 (41.7) | 37 (49.3) | |
| No therapy | 20 (5.6) | 12 (6.4) | 8 (10.7) | |
| AFP (> 400 ng/ml) | 125 (34.7) | 56 (29.9) | 34 (45.3) | 0.1 |
Abbreviations: Tumor–Nodes–Metastases (TNM), Cancer of the Liver Italian Program (CLIP), Barcelona Clinic Liver Cancer (BCLC), alpha-fetoprotein levels (AFP)
Some baseline (at time of diagnosis) clinical information were missing from patients’ medical records including (cirrhosis, 1; vascular invasion, 1; portal thrombosis, 1; extra-hepatic metastasis, 1; lymph node metastasis, 2; tumor involvement, 21; tumor nodularity, 23; tumor differentiation, 7; CLIP score, 21; OKUDA stage, 7; BCLC stage, 11; TNM stage, 25)
P value using chi-square test
DISCUSSION
The current study is, to our knowledge, the first to show that obesity in early adulthood (mid-20s to mid-40s) in both men and women is associated with increased risk of HCC development and with early onset of HCC, regardless of the confounding effects of the established risk factors of HCC such as HCV, HBV, alcohol consumption, cigarette smoking, and diabetes mellitus.
Approximately 42% of our HCC cases could be explained by obesity and diabetes, which was comparable to the 38.9% reported by the McGlynn group.25 However, the uniqueness of our study is its ability to analyze diabetes and obesity separately, specifically, in determining that 10% of the HCC cases in our study could be attributed to early adulthood obesity independent from diabetes mellitus or other HCC risk factors.
The association between increased body weight and liver cancer has been determined through meta-analyses and systematic reviews.8, 9, 25–28 One potential limitation of these review studies, which is highlighted by their authors, is that many of the individual studies did not adjust for the major risk factors of HCC, including HCV, HBV, diabetes, and alcohol consumption.29 In addition, population selection in the individual studies in these reviews was not exclusive to a diagnosis of HCC. Also, relying on BMI at the time of case ascertainment (HCC diagnosis) from case-control studies is subject to miscalculation (overestimation) because of the presence of ascites among many HCC patients with underlying cirrhosis. Moreover, in the included cohort studies, BMI had been estimated at initial enrollment and weight change monitored for only a small number of patients with HCC.
The potential biological mechanism for the association between obesity and HCC can be related to a number of physiological changes.30–32 Obese individuals often experience hepatic steatosis with potential progression to steatohepatitis and cirrhosis.33, 34 Key transcription factors in fatty acid oxidation, such as peroxisome proliferator-activated receptors, may play a dual role in hepatocellular proliferation and in cyclooxygenase-2 expression, which may explain terminal disease progression from steatohepatitis to HCC.35 Yet, cirrhosis is not the only explanation for the association between obesity and HCC. In this study, we found that ~62% of HCC patients had underlying cirrhosis and that among these patients, there were no significant differences in the proportions with normal weight, overweight, or obesity. Many obese individuals develop some degree of insulin resistance with elevated insulin-like growth factors,36 which may have tumorigenic activity and have a role in cell growth/proliferation and fatty degeneration.37
Several studies have examined the association between obesity and clinical outcome in various cancers including HCC.38–42 However, HCC studies have shown that mortality was not influenced by BMI.40, 43 Moreover, the association between obesity and life-threatening morbidities and complications after hepatic resection has not been conclusive, with some studies suggesting that hepatic resection in overweight and obese HCC patients is safe.39, 41, 42
The reasons for the poor prognosis observed in obese cancer patients is unclear but can be correlated with comorbidities or cancer consequences typically seen in obese patients such as heart diseases.41 Other pathways have been hypothesized including the observed low level of adiponectin in men and women with high BMI44; in fact, a more favorable prognosis was observed in HCC patients with higher expression of adiponectin.45, 46 In addition, angiogenesis dysregulation induced by the adipose tissue through leptin expression has been suggested.47 Siegel and colleagues showed that obesity is associated with microvascular invasion, resulting in poor HCC survival.48 In our study, vascular invasion was observed more frequently in overweight and obese patients than in normal-weight patients, but the differences were not statistically significant.
Increased risk of HCC associated with obesity was previously reported for chronic carriers of HBV and HCV49 and for alcohol drinkers,50 suggesting that obesity-induced oxidative stress may increase the liver’s susceptibility to chronic inflammation, DNA damage, fatty liver, and cirrhosis progression.50,34
The current study has some limitations, specifically, 1) the use of hospital-based case patients, many of whom were diagnosed as having advanced-stage disease, and 2) the use of weight and height data recalled from the distant past. Given the poor prognosis of this cancer, it is difficult to rely on a population-based design for recruiting patients with newly diagnosed HCC for a large-scale clinico-epidemiological study. To minimize ascertainment or selection bias related to misdiagnosis of case patients, we chose to use a hospital-based design, in which all cases had a confirmed diagnosis of HCC. Similar to the natural history of HCC where majority of the patients are presented with advanced stage,51–54 approximately 64% of our patients are diagnosed as having advanced-stage disease (TNM III–IV) at time of initial evaluation.
Control subjects were selected to represent the population from which the HCC patients were ascertained. Only U.S. patients and controls were included, and the geographic distribution of their residential states was similar. Therefore, it is unlikely that our findings were confounded by selection bias of cases or controls. The prevalence of ever history of obesity during lifetime in the control group was 30.6%, which is consistent with the recently reported U.S. estimate by Ng and colleagues.1
All participants were personally interviewed to obtain information about self-reported weight across the life cycle before cancer diagnosis (HCC patients) or before recruitment (controls). We observed consistent agreement in interview response between self-reported weight and body size (Stunkard pictograms) across different ages for HCC cases and healthy controls. Moreover, we found no discrepancy between interview information and patients’ records with respect to HCC risk factors. There was strong evidence supporting the reliability and validity of self-reported diabetes mellitus, where agreement between self-reported disease diagnosis and medical conditions was observed.55–57 In addition, several studies reported high correlations between recalled and measured weight and height in young adulthood among middle-aged and older men and women.58–62
The current study continues to show the multifactorial origin of HCC. Variation in the magnitude of ORs for the association between environmental exposures and HCC in various epidemiological studies, including our early study, could be partially explained by the type of controls included in the study, that is, healthy versus sick controls.19 Another explanation was lack of quantitative assessment of environmental exposures including alcohol consumption and cigarette smoking by some studies. Consideration of diabetes duration with the exclusion of patients with a recent diagnosis may have affected the magnitude of reported ORs of HCC risk factors.
In conclusion, this study provides robust epidemiological evidence to support the association between obese adults in their mid-20s to mid-40s and risk of HCC in American men and women, with obese subjects more susceptible than non-obese subjects to early-onset HCC.
Educational interventions and public awareness may be key to reducing the incidence of obesity at a young age. Behavioral modification, including abstaining from alcohol and restricting diet, especially among patients with chronic viral infection, may reduce the incidence of end-stage CLDs.
The effect of obesity on viral activity and the treatment response of HCV and HCC among these high-risk patients has not yet been investigated. In addition, experimental studies are warranted to describe the underlying mechanisms responsible for the effect of obesity on HCC in the absence of cirrhosis. Finally, future investigation of the preventive and favorable prognostic role of metformin and statin in patients with CLDs including HCC should be initiated.
Acknowledgments
Grant Support: Supported by National Institutes of Health NIH R03 grant ES11481 (to MMH) and CA106458-01 (to MMH).
Abbreviations
- BMI
Body Mass Index
- OR
Odds Ratio
- S
synergetic Index
- HCV
hepatitis C Virus
- HBV
hepatitis B Virus
- HCC
hepatocellular carcinoma
- IQR
interquartile range R
- AOR
Adjusted Odds Ratio
- TNM
Tumor–Nodes–Metastases
- CLIP
Cancer of the Liver Italian Program
- BCLC
Barcelona Clinic Liver Cancer
- AFP
alpha-fetoprotein levels
- X2
chi-square test
- PAR%
population-attributable risk percentage
- S
synergism index
Footnotes
The authors disclose no conflicts
No references with co-first authorship
- Study concept and design: Manal Hassan, Ahmed Kaseb, Alexandria T. Phan, Hashem B. El-Serag, Ernest Hawk, Jeff Morris, Kanwal Pratap Singh Raghav, Ju-Seog Lee, Jean-Nicolas Vauthey, Harrys A Torres, Christopher I. Amos, Robert A Wolf, and Donghui Li
- Acquisition of data: Manal Hassan, Reham Abdel-Wahab, Ahmed Shalaby, Gehan Botrus
- Analysis and interpretation of data: Manal Hassan, Reham Abdel-Wahab, Christopher I. Amos, Jeff Morris, Ahmed A Kaseb, Robert A Wolff, Donghui Li
- Drafting of the manuscript: Manal Hassan, Ahmed Kaseb, Reham Abdel-Wahab, Alexandria T. Phan, Hashem B. El-Serag, Ernest Hawk, Jeff Morris, Kanwal Pratap Singh Raghav, Ju-Seog Lee, Jean-Nicolas Vauthey, Harrys A Torres, Christopher I. Amos, Robert A Wolf, and Donghui Li
- Critical revision of the manuscript for important intellectual content: Manal Hassan, Ahmed Kaseb, Alexandria T. Phan, Hashem B. El-Serag, Ernest Hawk, Jeff Morris, Kanwal Pratap Singh Raghav, Ju-Seog Lee, Jean-Nicolas Vauthey, Harrys A Torres, Christopher I. Amos, Robert A Wolf, and Donghui Li
- Statistical analysis: Manal Hassan, Reham Abdel-wahab, Christopher I. Amos, Jeff Morris
- Obtained funding: Manal Hassan
- Administrative, technical, or material support: Manal Hassan, Ahmed Kaseb, Alexandria T. Phan, Hashem B. El-Serag, Ernest Hawk, Kanwal Pratap Singh Raghav, Jean-Nicolas Vauthey, Harrys A Torres,Gehan Botrus, and Donghui Li, Robert A Wolf
- Study supervision: Manal Hassan
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Reference List
- 1.Ng M, Fleming T, Robinson M, et al. Global, regional, and national prevalence of overweight and obesity in children and adults during 1980–2013: a systematic analysis for the Global Burden of Disease Study 2013. Lancet. 2014;384:766–781. doi: 10.1016/S0140-6736(14)60460-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wild S, Roglic G, Green A, et al. Global prevalence of diabetes: estimates for the year 2000 and projections for 2030. Diabetes Care. 2004;27:1047–1053. doi: 10.2337/diacare.27.5.1047. [DOI] [PubMed] [Google Scholar]
- 3.Venook AP, Papandreou C, Furuse J, et al. The incidence and epidemiology of hepatocellular carcinoma: a global and regional perspective. Oncologist. 2010;15 (Suppl 4):5–13. doi: 10.1634/theoncologist.2010-S4-05. [DOI] [PubMed] [Google Scholar]
- 4.Altekruse SF, McGlynn KA, Reichman ME. Hepatocellular carcinoma incidence, mortality, and survival trends in the United States from 1975 to 2005. J Clin Oncol. 2009;27:1485–1491. doi: 10.1200/JCO.2008.20.7753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Alhyas L, McKay A, Balasanthiran A, et al. Prevalences of overweight, obesity, hyperglycaemia, hypertension and dyslipidaemia in the Gulf: systematic review. JRSM Short Rep. 2011;2:55. doi: 10.1258/shorts.2011.011019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ramirez AG, Munoz E, Holden AE, et al. Incidence of Hepatocellular Carcinoma in Texas Latinos, 1995–2010: an update. PLoS One. 2014;9:e99365. doi: 10.1371/journal.pone.0099365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Renehan AG, Tyson M, Egger M, et al. Body-mass index and incidence of cancer: a systematic review and meta-analysis of prospective observational studies. Lancet. 2008;371:569–578. doi: 10.1016/S0140-6736(08)60269-X. [DOI] [PubMed] [Google Scholar]
- 8.Saunders D, Seidel D, Allison M, et al. Systematic review: the association between obesity and hepatocellular carcinoma - epidemiological evidence. Aliment Pharmacol Ther. 2010;31:1051–1063. doi: 10.1111/j.1365-2036.2010.04271.x. [DOI] [PubMed] [Google Scholar]
- 9.Larsson SC, Wolk A. Overweight, obesity and risk of liver cancer: a meta-analysis of cohort studies. Br J Cancer. 2007;97:1005–1008. doi: 10.1038/sj.bjc.6603932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Donato F, Tagger A, Gelatti U, et al. Alcohol and hepatocellular carcinoma: the effect of lifetime intake and hepatitis virus infections in men and women. Am J Epidemiol. 2002;155:323–331. doi: 10.1093/aje/155.4.323. [DOI] [PubMed] [Google Scholar]
- 11.Hassan MM, Kaseb A, Etzel CJ, et al. Genetic variation in the PNPLA3 gene and hepatocellular carcinoma in USA: risk and prognosis prediction. Mol Carcinog. 2013;52 (Suppl 1):E139–E147. doi: 10.1002/mc.22057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kaseb AO, Morris JS, Hassan MM, et al. Clinical and prognostic implications of plasma insulin-like growth factor-1 and vascular endothelial growth factor in patients with hepatocellular carcinoma. J Clin Oncol. 2011;29:3892–3899. doi: 10.1200/JCO.2011.36.0636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kaseb AO, Xiao L, Hassan MM, et al. Development and validation of insulin-like growth factor-1 score to assess hepatic reserve in hepatocellular carcinoma. J Natl Cancer Inst. 2014:106. doi: 10.1093/jnci/dju088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rothman KJ, Greenland S, Walker AM. Concepts of interaction. Am J Epidemiol. 1980;112:467–470. doi: 10.1093/oxfordjournals.aje.a113015. [DOI] [PubMed] [Google Scholar]
- 15.Rothman KJ. The estimation of synergy or antagonism. Am J Epidemiol. 1976;103:506–511. doi: 10.1093/oxfordjournals.aje.a112252. [DOI] [PubMed] [Google Scholar]
- 16.Hassan MM, Spitz MR, Thomas MB, et al. Effect of different types of smoking and synergism with hepatitis C virus on risk of hepatocellular carcinoma in American men and women: case-control study. Int J Cancer. 2008;123:1883–1891. doi: 10.1002/ijc.23730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hassan MM, Curley SA, Li D, et al. Association of diabetes duration and diabetes treatment with the risk of hepatocellular carcinoma. Cancer. 2010;116:1938–1946. doi: 10.1002/cncr.24982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hassan MM, Spitz MR, Thomas MB, et al. The association of family history of liver cancer with hepatocellular carcinoma: a case-control study in the United States. J Hepatol. 2009;50:334–341. doi: 10.1016/j.jhep.2008.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hassan MM, Hwang LY, Hatten CJ, et al. Risk factors for hepatocellular carcinoma: synergism of alcohol with viral hepatitis and diabetes mellitus. Hepatology. 2002;36:1206–1213. doi: 10.1053/jhep.2002.36780. [DOI] [PubMed] [Google Scholar]
- 20.Marrero CR, Marrero JA. Viral hepatitis and hepatocellular carcinoma. Arch Med Res. 2007;38:612–620. doi: 10.1016/j.arcmed.2006.09.004. [DOI] [PubMed] [Google Scholar]
- 21.El-Serag HB, Hampel H, Javadi F. The association between diabetes and hepatocellular carcinoma: a systematic review of epidemiologic evidence. Clin Gastroenterol Hepatol. 2006;4:369–380. doi: 10.1016/j.cgh.2005.12.007. [DOI] [PubMed] [Google Scholar]
- 22.Yu MC, Yuan JM, Lu SC. Alcohol, cofactors and the genetics of hepatocellular carcinoma. J Gastroenterol Hepatol. 2008;23 (Suppl 1):S92–S97. doi: 10.1111/j.1440-1746.2007.05293.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Persson EC, Schwartz LM, Park Y, et al. Alcohol consumption, folate intake, hepatocellular carcinoma, and liver disease mortality. Cancer Epidemiol Biomarkers Prev. 2013;22:415–421. doi: 10.1158/1055-9965.EPI-12-1169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bosetti C, Turati F, La VC. Hepatocellular carcinoma epidemiology. Best Pract Res Clin Gastroenterol. 2014;28:753–770. doi: 10.1016/j.bpg.2014.08.007. [DOI] [PubMed] [Google Scholar]
- 25.Welzel TM, Graubard BI, Quraishi S, et al. Population-attributable fractions of risk factors for hepatocellular carcinoma in the United States. Am J Gastroenterol. 2013;108:1314–1321. doi: 10.1038/ajg.2013.160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ohki T, Tateishi R, Sato T, et al. Obesity is an independent risk factor for hepatocellular carcinoma development in chronic hepatitis C patients. Clin Gastroenterol Hepatol. 2008;6:459–464. doi: 10.1016/j.cgh.2008.02.012. [DOI] [PubMed] [Google Scholar]
- 27.Chen CL, Yang HI, Yang WS, et al. Metabolic factors and risk of hepatocellular carcinoma by chronic hepatitis B/C infection: a follow-up study in Taiwan. Gastroenterology. 2008;135:111–121. doi: 10.1053/j.gastro.2008.03.073. [DOI] [PubMed] [Google Scholar]
- 28.Nair S, Mason A, Eason J, et al. Is obesity an independent risk factor for hepatocellular carcinoma in cirrhosis? Hepatology. 2002;36:150–155. doi: 10.1053/jhep.2002.33713. [DOI] [PubMed] [Google Scholar]
- 29.Czaja AJ, Carpenter HA, Santrach PJ, et al. Host- and disease-specific factors affecting steatosis in chronic hepatitis C. J Hepatol. 1998;29:198–206. doi: 10.1016/s0168-8278(98)80004-4. [DOI] [PubMed] [Google Scholar]
- 30.Khandekar MJ, Cohen P, Spiegelman BM. Molecular mechanisms of cancer development in obesity. Nat Rev Cancer. 2011;11:886–895. doi: 10.1038/nrc3174. [DOI] [PubMed] [Google Scholar]
- 31.Polonsky KS. Dynamics of insulin secretion in obesity and diabetes. Int J Obes Relat Metab Disord. 2000;24 (Suppl 2):S29–S31. doi: 10.1038/sj.ijo.0801273. [DOI] [PubMed] [Google Scholar]
- 32.Meyer MR, Clegg DJ, Prossnitz ER, et al. Obesity, insulin resistance and diabetes: sex differences and role of oestrogen receptors. Acta Physiol (Oxf) 2011;203:259–269. doi: 10.1111/j.1748-1716.2010.02237.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Qian Y, Fan JG. Obesity, fatty liver and liver cancer. Hepatobiliary Pancreat Dis Int. 2005;4:173–177. [PubMed] [Google Scholar]
- 34.Wood PA. Connecting the dots: obesity, fatty acids and cancer. Lab Invest. 2009;89:1192–1194. doi: 10.1038/labinvest.2009.99. [DOI] [PubMed] [Google Scholar]
- 35.Xu L, Han C, Lim K, et al. Cross-talk between peroxisome proliferator-activated receptor delta and cytosolic phospholipase A(2)alpha/cyclooxygenase-2/prostaglandin E(2) signaling pathways in human hepatocellular carcinoma cells. Cancer Res. 2006;66:11859–11868. doi: 10.1158/0008-5472.CAN-06-1445. [DOI] [PubMed] [Google Scholar]
- 36.Frystyk J, Skjaerbaek C, Vestbo E, et al. Circulating levels of free insulin-like growth factors in obese subjects: the impact of type 2 diabetes. Diabetes Metab Res Rev. 1999;15:314–322. doi: 10.1002/(sici)1520-7560(199909/10)15:5<314::aid-dmrr56>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- 37.Sohda T, Kamimura S, Iwata K, et al. Immunohistochemical evidence of insulin-like growth factor II in human small hepatocellular carcinoma with hepatitis C virus infection: relationship to fatty change in carcinoma cells. J Gastroenterol Hepatol. 1997;12:224–228. doi: 10.1111/j.1440-1746.1997.tb00412.x. [DOI] [PubMed] [Google Scholar]
- 38.Li D, Morris JS, Liu J, et al. Body mass index and risk, age of onset, and survival in patients with pancreatic cancer. JAMA. 2009;301:2553–2562. doi: 10.1001/jama.2009.886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Utsunomiya T, Okamoto M, Kameyama T, et al. Impact of obesity on the surgical outcome following repeat hepatic resection in Japanese patients with recurrent hepatocellular carcinoma. World J Gastroenterol. 2008;14:1553–1558. doi: 10.3748/wjg.14.1553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mathur AK, Ghaferi AA, Osborne NH, et al. Body mass index and adverse perioperative outcomes following hepatic resection. J Gastrointest Surg. 2010;14:1285–1291. doi: 10.1007/s11605-010-1232-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tanaka S, Iimuro Y, Hirano T, et al. Safety of hepatic resection for hepatocellular carcinoma in obese patients with cirrhosis. Surg Today. 2013;43:1290–1297. doi: 10.1007/s00595-013-0706-2. [DOI] [PubMed] [Google Scholar]
- 42.Balzan S, Nagarajan G, Farges O, et al. Safety of liver resections in obese and overweight patients. World J Surg. 2010;34:2960–2968. doi: 10.1007/s00268-010-0756-1. [DOI] [PubMed] [Google Scholar]
- 43.Tateishi R, Okanoue T, Fujiwara N, et al. Clinical characteristics, treatment, and prognosis of non-B, non-C hepatocellular carcinoma: a large retrospective multicenter cohort study. J Gastroenterol. 2014 doi: 10.1007/s00535-014-0973-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Haluzik M, Parizkova J, Haluzik MM. Adiponectin and its role in the obesity-induced insulin resistance and related complications. Physiol Res. 2004;53:123–129. [PubMed] [Google Scholar]
- 45.Shin E, Yu YD, Kim DS, et al. Adiponectin receptor expression predicts favorable prognosis in cases of hepatocellular carcinoma. Pathol Oncol Res. 2014;20:667–675. doi: 10.1007/s12253-014-9747-0. [DOI] [PubMed] [Google Scholar]
- 46.Duan XF, Tang P, Li Q, et al. Obesity, adipokines and hepatocellular carcinoma. Int J Cancer. 2013;133:1776–1783. doi: 10.1002/ijc.28105. [DOI] [PubMed] [Google Scholar]
- 47.Wang SN, Lee KT, Ker CG. Leptin in hepatocellular carcinoma. World J Gastroenterol. 2010;16:5801–5809. doi: 10.3748/wjg.v16.i46.5801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Siegel AB, Wang S, Jacobson JS, et al. Obesity and microvascular invasion in hepatocellular carcinoma. Cancer Invest. 2010;28:1063–1069. doi: 10.3109/07357907.2010.483500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kawaguchi Y, Mizuta T. Interaction between hepatitis C virus and metabolic factors. World J Gastroenterol. 2014;20:2888–2901. doi: 10.3748/wjg.v20.i11.2888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Loomba R, Yang HI, Su J, et al. Synergism between obesity and alcohol in increasing the risk of hepatocellular carcinoma: a prospective cohort study. Am J Epidemiol. 2013;177:333–342. doi: 10.1093/aje/kws252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Llovet JM, Burroughs A, Bruix J. Hepatocellular carcinoma. Lancet. 2003;362:1907–1917. doi: 10.1016/S0140-6736(03)14964-1. [DOI] [PubMed] [Google Scholar]
- 52.Bruix J, Llovet JM. Prognostic prediction and treatment strategy in hepatocellular carcinoma. Hepatology. 2002;35:519–524. doi: 10.1053/jhep.2002.32089. [DOI] [PubMed] [Google Scholar]
- 53.Llovet JM, Ricci S, Mazzaferro V, et al. Sorafenib in advanced hepatocellular carcinoma. N Engl J Med. 2008;359:378–390. doi: 10.1056/NEJMoa0708857. [DOI] [PubMed] [Google Scholar]
- 54.Thomas MB. Systemic therapy for hepatocellular carcinoma. Cancer J. 2008;14:123–127. doi: 10.1097/PPO.0b013e31816a6058. [DOI] [PubMed] [Google Scholar]
- 55.Simpson CF, Boyd CM, Carlson MC, et al. Agreement between self-report of disease diagnoses and medical record validation in disabled older women: factors that modify agreement. J Am Geriatr Soc. 2004;52:123–127. doi: 10.1111/j.1532-5415.2004.52021.x. [DOI] [PubMed] [Google Scholar]
- 56.Bush TL, Miller SR, Golden AL, et al. Self-report and medical record report agreement of selected medical conditions in the elderly. Am J Public Health. 1989;79:1554–1556. doi: 10.2105/ajph.79.11.1554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Cavanaugh KL, Merkin SS, Plantinga LC, et al. Accuracy of patients’ reports of comorbid disease and their association with mortality in ESRD. Am J Kidney Dis. 2008;52:118–127. doi: 10.1053/j.ajkd.2008.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Houston DK, Ding J, Nicklas BJ, et al. The association between weight history and physical performance in the Health, Aging and Body Composition study. Int J Obes (Lond) 2007;31:1680–1687. doi: 10.1038/sj.ijo.0803652. [DOI] [PubMed] [Google Scholar]
- 59.Casey VA, Dwyer JT, Berkey CS, et al. Long-term memory of body weight and past weight satisfaction: a longitudinal follow-up study. Am J Clin Nutr. 1991;53:1493–1498. doi: 10.1093/ajcn/53.6.1493. [DOI] [PubMed] [Google Scholar]
- 60.Stevens J, Keil JE, Waid LR, et al. Accuracy of current, 4-year, and 28-year self-reported body weight in an elderly population. Am J Epidemiol. 1990;132:1156–1163. doi: 10.1093/oxfordjournals.aje.a115758. [DOI] [PubMed] [Google Scholar]
- 61.Kuczmarski MF, Kuczmarski RJ, Najjar M. Effects of age on validity of self-reported height, weight, and body mass index: findings from the Third National Health and Nutrition Examination Survey, 1988–1994. J Am Diet Assoc. 2001;101:28–34. doi: 10.1016/S0002-8223(01)00008-6. [DOI] [PubMed] [Google Scholar]
- 62.Gunnell D, Berney L, Holland P, et al. How accurately are height, weight and leg length reported by the elderly, and how closely are they related to measurements recorded in childhood? Int J Epidemiol. 2000;29:456–464. [PubMed] [Google Scholar]