Abstract
The 5- and 12/15- lipoxygenase (LOX) isozymes have been implicated to contribute to disease development in CNS disorders such as Alzheimer's disease. These LOX isozymes are distinct in function, with differential effects on neuroinflammation, and the impact of the distinct isozymes in the pathogenesis of Parkinson's disease (PD) has not as yet been evaluated. To determine whether the isozymes contribute differently to nigrostriatal vulnerability, the effects of 5- and 12/15-LOX deficiency on dopaminergic tone under na ve and toxicant-challenged conditions were tested. In na ve mice deficient in 5-LOX expression, a modest but significant reduction (18.0% reduction vs. wildtype (WT)) in striatal dopamine (DA) was detected (n=6-8 per genotype). A concomitant decline in striatal tyrosine hydroxylase (TH) enzyme was also revealed in null 5-LOX vs. WT mice (26.2%); however, no changes in levels of DA or TH immunoreactivity were observed in null 12/15-LOX vs. WT mice. When challenged with the selective dopaminergic toxin, 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP), WT mice showed marked reduction in DA (31.9%) and robust astrocytic and microglial activation as compared to saline-treated animals. In contrast, null 5-LOX littermates demonstrated no significant striatal DA depletion or astrogliosis (as noted by Western blot analyses for GFAP immunoreactivity). In na ve null 12/15-LOX mice, no significant change in striatal DA values was observed compared to WT, and following MPTP treatment, the transgenics revealed striatal DA reduction similar to the challenged WT mice. Taken together, these data provide the first evidence that: (i) LOX isozymes are involved in the maintenance of normal dopaminergic function in the striatum, and (ii) the 5- and 12/15-LOX isozymes contribute differentially to striatal vulnerability in response to neurotoxicant challenge.
Keywords: Parkinson's disease, nigrostriatal, dopamine, lipoxygenase, MPTP, inflammation
1. Introduction
Parkinson's disease (PD) is a progressive CNS disorder characterized behaviorally by motor impairment and neurochemically by a loss of nigrostriatal dopaminergic tone. Fundamental processes thought to underlie ongoing degeneration in PD include oxidative damage, mitochondrial dysfunction, protein clearance abnormalities and protein misfolding (Imai and Lu, 2011; Plowey and Chu, 2011; Breydo et al., 2012; McCoy and Cookson, 2012). Inflammation has been implicated to contribute to PD pathogenesis as revealed by epidemiological and pathological studies as well as experimental modeling but whether these are primary or secondary processes is not well understood (Hunot and Hirsch, 2003;Tansey et al., 2008; Ouchi et al., 2009; Ton et al., 2012). Glial involvement (i.e. astrogliosis and microglial activation) in cell death and dysfunction have been demonstrated to play a key role (Ton et al., 2006; Tansey et al., 2008; Tansey and Goldberg, 2010; Ton et al., 2012), and eicosanoids, the collective name for products of arachidonic acid (AA) metabolism, have been widely studied in mediating activation at a cellular level (Minghetti, 2004; Farooqui and Horrocks, 2006; Farooqui et al., 2007; Adibhatla and Hatcher, 2008). The lipoxygenases (LOX) are a family of distinct isozymes that catalyze oxidation of AA and consequently contribute to toxicity through the generation of free reactive oxygen species (ROS), toxic lipid hydroperoxides and potent cytokines (Serhan and Samuelsson, 1988; Zaleska and Wilson, 1989; Simonian and Coyle, 1996; Manev, 2000; Sugaya et al., 2000; Mytilineou et al., 2002; Parkinson, 2006; Serhan, 2006; Yang et al., 2010). The LOX enzymes have been shown to play a central role in pathogenesis in a variety of disease states including diabetes, asthma, cancer, heart disease, and most recently, neurodegenerative conditions (Serhan and Samuelsson, 1988; Parkinson, 2006; Serhan, 2006; Cheng et al., 2008; van Leyen et al., 2008; Weaver et al., 2012; Yeung et al., 2012) including Alzheimer's disease (AD) (Pratico et al., 2004; Manev and Manev, 2006; Listi et al., 2010; Yang et al., 2010; Chu and Pratico, 2011; Manev et al., 2011; Puccio et al., 2011) and Creutzfeldt-Jakob disease (Stewart et al., 2001; Phillis et al., 2006). Despite that LOX isozymes are well-established in lipid-mediated cellular responses, understanding of their role(s) in the nigrostriatal pathway represents a gap in knowledge.
All LOX isozymes are expressed in the brain (Bendani et al., 1995; Manev et al., 2000a, 2001; Qu et al., 2000; Uz et al., 2001a; Uz et al., 2001b). including the ventral midbrain which contains the nigrostriatal neuronal cell bodies affected in PD (Mytilineou et al., 1999). The expression of LOX is enhanced in the brain with aging and in models of neurodegeneration and inflammation (Uz et al., 1998; Manev et al., 2000b; Qu et al., 2000; Firuzi et al., 2008; Basselin et al., 2010). Recent studies related to CNS disease have focused primarily on the 5- and 12/15-LOX isozymes (Chu and Pratico, 2011; Hashimoto, 2011; Manev et al., 2011; Puccio et al., 2011; Chu et al., 2012a,b). The LOX isozymes catalyze stereospecific modification of AA at either carbon position 5, 12 or 15 or both 12 and 15, and the site of molecular oxygen insertion correspondingly defines the isozyme (i.e. 5-, 12-, 15- or a dual specificity 12/15-LOX, respectively). The reaction product of AA oxidation is hydroperoxyeicosatetraenoic acid (HPETE); this unstable compound is subsequently reduced by glutathione peroxidase to the more stable hydroxyeicosatetraenoic acid (HETE) (Brash, 1999). While 5-, 12-, 15- and 12/15-LOX can generate HETE forms, the distinct products have selective downstream effects. For example, 12-HETE, but not 5-HETE, antagonizes the activity of the peroxisome proliferator activator receptor gamma (PPARγ) to promote glial cell activation (Lopez-Parra et al., 2005; Limor et al., 2008). Interestingly, 5-HETE is also a substrate for the 5-LOX isozyme, catalyzing the formation of the epoxide, leukotriene A4, which is subsequently converted to leukotriene B4 (LTB4). LTB4 is a highly potent inflammatory factor and promotes enhanced expression of IL-12, IL-6 and TNFα (Phillis et al., 2006; Tassoni et al., 2008).
Although inhibition of 5- and 12/15-LOX has been shown to protect against the detrimental outcomes in disease models of stroke and AD (Klegeris and McGeer, 2002; Wang et al., 2006; Jin et al., 2008; Sobrado et al., 2009; Cui et al., 2010; Tu et al., 2010), the role of these isozymes in the nigrostriatal pathway has not been fully evaluated. The development and availability of mouse models with targeted gene deletions provide critical tools to better understand the role of LOX in degeneration related to PD. To determine if the 5- and 12/15-LOX isozymes selectively contribute to nigrostriatal vulnerability, the impact of 5- or 12/15-LOX deficiency on striatal dopaminergic tone and injury was tested in mouse models under na ve and toxicant-challenged conditions.
2. Experimental Procedures
2.1 Animals
Transgenic mice deficient in 5-LOX (004155; sex-matched and aged 8 weeks) or 12/15-LOX (002778; males aged 8 weeks) and respective sex-matched strain controls were purchased from The Jackson Laboratory (Bar Harbor, ME). All mice were housed on a 12-h light–dark cycle and given free access to food and drinking water as well as environmental enrichment. All animal procedures and animal care methods were approved by the Institutional Animal Care and Usage Committees for SRI International.
2.1.1 MPTP exposure
The dopaminergic neurotoxin 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP) (Sigma, St. Louis, MO) was dissolved in sterile saline. Mice received saline or 15 mg/kg MPTP, i.p., daily for 5 days. All animals were weighed daily during MPTP administration and monitored until 72 hours after the last MPTP exposure. Animals were euthanized by cervical dislocation 7 days following the last MPTP injection.
2.2 Tissue Processing
Midbrain was immersion-fixed in 4% paraformaldehyde overnight, cryoprotected in graded sucrose solutions and frozen at −80°C. Coronal sections were collected on a Leica cryostat (Buffalo Grove, IL) at 40 μm thickness for histology. Striatal tissue was dissected from a forebrain slice (1-2 mm thick) at the level of the anterior commissure and frozen immediately on dry ice. Tissues were sonicated and centrifuged, and homogenate fractions collected for biochemical analyses by Western blot and neurochemical analyses of dopamine (DA) and metabolite levels.
2.2.1 Neurochemistry
Striatal samples were placed in 1 ml ice-cold 0.4 M perchloric acid, sonicated and centrifuged at 15,000 × g for 12 min. The supernatant was collected for assay of DA and its metabolites, dihydroxyphenylacetic acid (DOPAC) and homovanillic acid (HVA) by HPLC with electrochemical detection (Coulochem III detector; Dionex/Thermo Scientific) using a reverse phase C18 column (MD-150; Dionex/Thermo Scientific) (Kilpatrick et al., 1986). The pellet was dried and reconstituted in 0.5N NaOH and briefly sonicated, and total protein determined using the Lowry method (Manning-Bog et al., 2007).
2.2.2 Immunoblotting
Proteins separated by the sample preparation were used for immunoblotting experiments. Following sonication in Tris-EDTA buffer with protease and phosphatase inhibitors (Sigma), samples were centrifuged at 1,000 × g for 10 min, the supernatant aspirated, and the pellet reconstituted in sample buffer. The protein concentration was measured by Pierce BCA assay (Thermo Scientific, Rockford, IL) and supernatant proteins separated by SDS-PAGE (12% Tris-glycine, Invitrogen/Life Technologies, Carlsbad, CA) then transferred to nitrocellulose. Blots were blocked in 5% non-fat milk and incubated overnight at 4°C with rabbit anti-tyrosine hydroxylase (TH; Pel-Freez Biologicals, Rogers, AK; and Chemicon/Millipore, Billerica, MA), rabbit anti-β-actin (Sigma), and mouse anti-glial acidic fibrillary protein (GFAP; Covance Inc., Princeton, NJ). Next, appropriate peroxidase-conjugated secondary antibodies were applied, and signal visualized following incubation with Pierce chemiluminescent substrate (Thermo Scientific). Blots were placed in plastic sleeves and exposed to CL-XPosure Film (Thermo Scientific), and the immunoreactivity was quantified by Image J software.
2.2.3 Immunohistochemistry
Coronal tissue sections (40 μm) were obtained as described above and stored in cryoprotectant solution (0.01M phosphate buffer, 30% sucrose, 30% ethylene glycol, pH 7.4) at −20°C until further use. The standard protocol that was used to detect primary antibodies has been described previously (Manning-Bog et al., 2003). Incubation times varied depending on the primary and secondary antibodies that were utilized. Antibodies used were rabbit and sheep anti-TH (Pel-Freez and Chemicon/Millipore), rabbit anti- GFAP (Chemicon/Millipore), and rabbit anti-Iba1 (Biocare Medical, Walnut Creek, CA). IgG from the primary species served as the negative control. Immunohistochemistry was either by immunofluorescence with secondary antibodies conjugated to Alexa Fluor 568 (AF568; Invitrogen) or FITC (Jackson ImmunoResearch Laboratories, West Grove, PA), or by 3,3′-diaminobenzidine (DAB) visualization (avidin-biotin system from Vector Laboratories, Burlingame, CA). Immunofluorescent sections were mounted using anti-fade mounting medium with DAPI (Vector Laboratories). DAB sections were lightly counterstained with 0.5% cresyl violet (FD Neurotechnologies, Ellicott City, MD), dehydrated successively in alcohols and xylenes and coverslipped with Permount (Fisher Scientific, Atlanta, GA). Positive immunoreactivity was observed by a light microscope (Eclipse E400; Nikon, Melville, NY) equipped for epi-fluorescence.
2.3 Statistics
All experiments were repeated to ensure reproducibility. Differences among means were analyzed using one-way analysis of variance (ANOVA) when comparing differences between genotype and two-way ANOVA when comparing differences between genotype and toxicant treatment. Tukey's HSD post hoc analysis was employed when differences were observed in ANOVA testing (p≤0.05).
3. Results
To determine whether LOX isozymes contribute to nigrostriatal dopaminergic function and vulnerability to toxicity, two transgenic mouse lines deficient in 5- or 12/15-LOX were utilized. In striatal homogenates from null 5-LOX mice, a significant reduction (18.0%) in DA values was measured as compared to wildtype littermates (WT; Fig. 1A). No change was detected in the metabolite DOPAC, but levels of HVA were significantly lower (25.7%) in the 5-LOX-deficient vs. WT mice (Fig. 1A). To determine if this effect were specific to the 5-LOX isozyme, or rather a common feature to general LOX deficiency, striatal samples were obtained from null 12/15-LOX vs. WT mice and tested for DA and metabolite levels. No differences in striatal DA or HVA were measured in the animals lacking 12/15-LOX (Fig. 1B). However, a modest decrease was discovered in DOPAC (9.3%) in the 12/15-LOX vs. WT mice (Fig. 1B).
Fig. 1. LOX isozyme-selective effects on striatal dopaminergic tone.
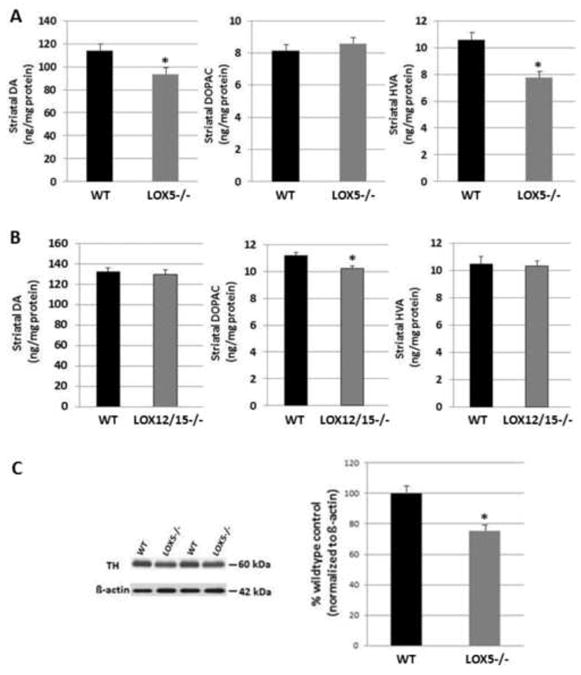
(A) DA and metabolites DOPAC and HVA were measured in striatal homogenates by reverse phase HPLC from WT and null 5-LOX littermates (n=6-8/group). (B) DA and metabolites DOPAC and HVA were measured in striatal homogenates from WT and null 12/15-LOX littermates (n=6-8/group). (C) The rate-limiting synthetic enzyme for DA, TH, was semi-quantitatively assessed using Western blot analyses of striatal homogenate from WT and null 5-LOX littermates (n=6-8/group). Immunoreactivity for TH was measured by optical density and normalized to the housekeeper β-actin. Data are shown as means±SEM. * P<0.01.
One possibility for the difference in DA values in the null 5-LOX mice is a change in synthesis machinery, such as levels of TH protein, the rate-limiting enzyme in DA synthesis. Consequently, TH protein expression measurement was performed using samples obtained from the other striatal hemisphere. Western blot analyses revealed that the TH level was significantly lower (26.2%) in null 5-LOX vs. WT striatum (Fig. 1C). No difference in TH protein immunoreactivity was observed in striatum between null 12/15-LOX vs. WT littermates (data not shown). These data indicate differential effects on striatal TH expression and DA synthesis and metabolism by the two isozymes.
The transgenic lines were subsequently utilized to address whether LOX isozymes differentially contribute to nigrostriatal vulnerability. Null 5-LOX and WT were exposed to the dopaminergic neurotoxin, MPTP using a subacute paradigm (Manning-Bog et al., 2007). In WT mice, treatment with 15 mg/kg MPTP, i.p., once daily for five consecutive days resulted in a 31.9% reduction in striatal DA values as compared to WT mice injected with saline. In null 5-LOX mice, MPTP treatment did not significantly change DA levels vs. saline-treated animals (Fig. 2A). Similarly, the metabolite HVA was significantly reduced in MPTP-exposed WT mice as compared to saline exposed (8.24 ± 0.79 vs. 10.57 ± 1.31 ng/mg protein, respectively), but MPTP toxicity was not observed in the null 5-LOX transgenics which revealed HVA values equivalent to saline-treated null 5-LOX mice (7.02 ± 1.39 vs. 7.74 ± 1.00 ng/mg protein, respectively) (Fig. 2C). Although no change in striatal DOPAC was measured (Fig. 2B), increased DA turnover (37.6%) was revealed in MPTP vs. saline-injected WT mice whereas no significant difference in turnover was noted in MPTP vs. saline-exposed null 5-LOX transgenics (Fig. 2D).
Fig. 2. 5-LOX isozyme effects on striatal neurochemistry following toxic insult.
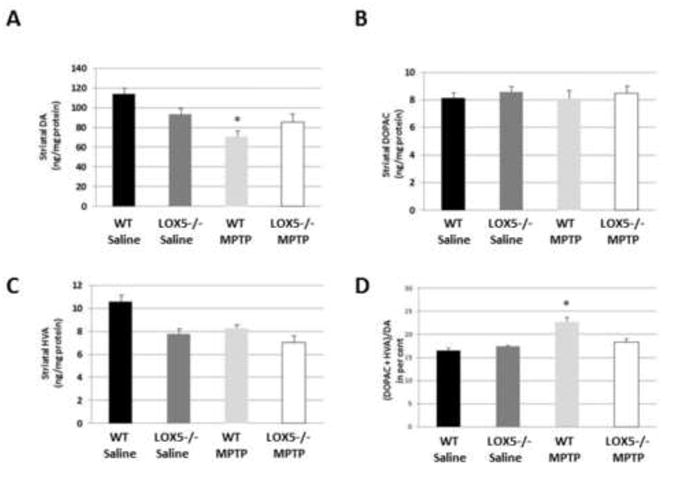
(A) DA was measured by reverse phase HPLC in WT and null 5-LOX littermates administered saline or MPTP (n=6-8/group). (B) DOPAC was measured by reverse phase HPLC in WT and null 5-LOX littermates administered saline or MPTP (n=6-8/group). (C) HVA was measured by reverse phase HPLC in WT and null 5-LOX littermates administered saline or MPTP (n=6-8/group). (D) DA turnover was assessed in WT and null 5-LOX littermates administered saline or MPTP (n=6-8/group). Data are shown as means±SEM. * P<0.05.
Null 12/15-LOX and WT littermate mice were also challenged with MPTP. However, with this LOX transgenic line, no significant difference in MPTP-mediated injury was observed (Fig. 3). Striatal DA was depleted by 70.4% in WT mice and 70.5% in 12/15-LOX-deficient littermates (Fig. 3A); similar reductions in DOPAC and HVA were observed (Fig. 3B, C).
Fig. 3. 12/15-LOX isozyme effects on striatal neurochemistry following toxic insult.
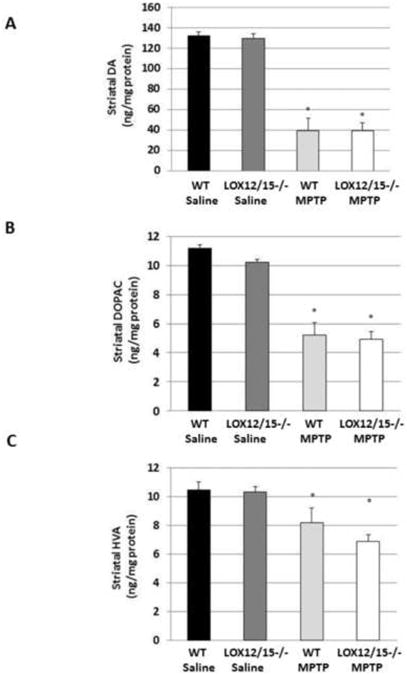
(A) DA, (B) DOPAC and (C) HVA were measured by reverse phase HPLC in WT and null 12/15-LOX littermates administered saline or MPTP (n=6-8/group). Data are shown as means±SEM. * P<0.01.
To confirm protection from MPTP-induced damage, TH expression was measured using homogenates obtained from the other striatal hemisphere. A 38.0% reduction in TH immunoreactivity, as determined by semi-quantitative Western blot analyses, occurred in WT mice treated with MPTP compared to mice administered saline; however, in 5-LOX-deficient mice, striatal TH immunoreactivity was not significantly different between the saline- and MPTP-challenged groups (Fig. 4A). Representative images of immunofluorescence in the substantia nigra show a dramatic depletion of FITC-labeled TH-positive dopaminergic neurons in the WT MPTP cohort, but not the null 5-LOX MPTP group (Fig. 4B).
Fig. 4. 5-LOX isozyme effects on TH following toxic insult.
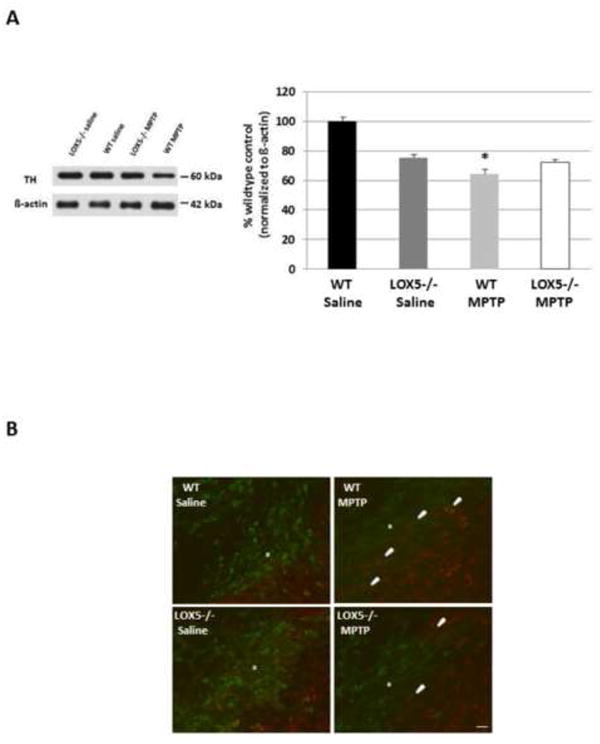
(A) The rate-limiting synthetic enzyme for DA, TH, was semi-quantitatively assessed using Western blot analyses of striatal homogenate from WT and null 5-LOX littermates treated with saline or MPTP (n=6-8/group). Immunoreactivity for TH was measured by optical density and normalized to the housekeeper β-actin. Data are shown as means±SEM. * P<0.05. (B) Dual label immunofluorescence staining for TH (FITC) or GFAP (AF568) was performed in coronal brain sections containing substantia nigra from WT and null 5-LOX littermates treated with saline or MPTP. *indicates substantia nigra pars compacta; arrowheads show GFAP-immunoreactive astrocytes within the substantia nigra pars compacta. Bar = 25 μm.
A marker for astrocytic activation (i.e. neuroinflammation), GFAP, was also assessed. While little to no difference in striatal GFAP immunoreactivity was detected in WT and 5-LOX-deficient littermates administered saline, a marked increase in GFAP levels was revealed in WT treated with MPTP. This change was absent from the MPTP-challenged null 5-LOX animals (Fig. 5A); GFAP immunoreactivity in this cohort was analogous to mice exposed to saline. Consistent with this finding, more robust labeling by anti-Iba-1 was apparent in nigral microglia in WT than null 5-LOX animals treated with the toxin (Fig. 5B, right panels). Additionally, nigral microglia in the MPTP-treated null 5-LOX cohort demonstrated a ramified appearance with soma similar to the saline-treated groups (Fig. 5B). In contrast, microglia in the WT MPTP group demonstrated morphology indicative of the activated state, more ovoid cell body and less ramified with retracted processes (Kettenmann et al., 2011) (Fig. 5B, top right panel).
Fig. 5. 5-LOX isozyme effects on inflammatory markers following toxic insult.
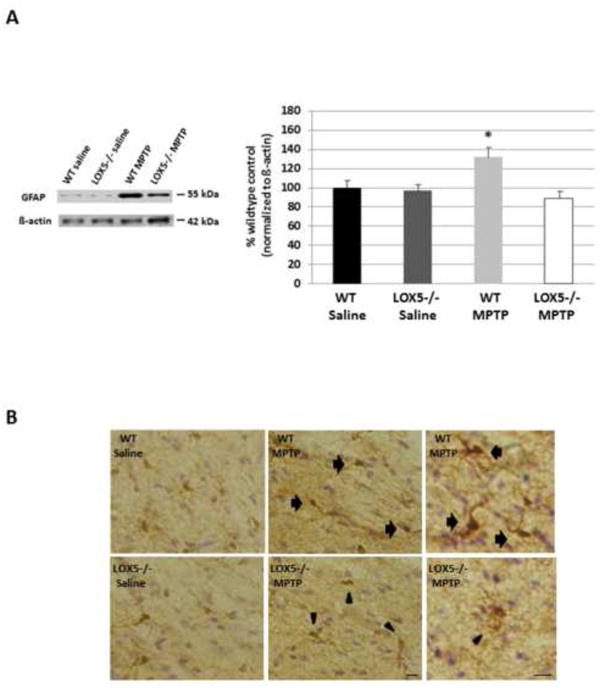
(A) The astrocytic protein GFAP was semi-quantitatively assessed using Western blot analyses of striatal homogenate from WT and null 5-LOX littermates treated with saline or MPTP (n=6-8/group). Immunoreactivity for GFAP was measured by optical density and normalized to the housekeeper β-actin. Data are shown as means±SEM. * P<0.05. (B) Immunohistochemical staining for the microglial protein Iba-1 was visualized using the brown chromogen DAB with counterstaining by cresyl violet. Coronal brain sections containing substantia nigra from WT and null 5-LOX littermates treated with saline or MPTP were utilized. Panel on far right shows microglial morphology: arrows indicate rounded cell bodies with short, thick processes; arrowheads denote ramified cell bodies with long, branching processes. Bar = 25 μm.
4. Discussion
The purpose of this study was to investigate LOX isozyme-specific modifications to the nigrostriatal pathway that may contribute to pathophysiology in PD. While extensively studied for pro- and anti-inflammatory effects in other conditions such as diabetes, cancer, asthma, cardiovascular disorders and most recently AD (Marks et al., 2000; Werz, 2002; Yao et al., 2005; Gubitosi-Klug et al., 2008; Ikonomovic et al., 2008; Planaguma et al., 2008; Liu et al., 2009; Menna et al., 2010; Neilson et al., 2012), LOX enzymes have not been widely explored in the context of PD etiology and progression (Mosca et al., 1996; Li et al., 1997; Mytilineou et al., 1999, 2002; Klegeris and McGeer, 2002; Canals et al., 2003; de Bernardo et al., 2004; Kramer et al., 2004; Scholz et al., 2008; Gupta et al., 2010; Dobrian et al., 2011). Further, although products of distinct LOX-mediated metabolism have differential effects on activation pro- and anti-inflammatory cellular pathways, no study has compared isozyme-specific effects on nigrostriatal dopamine regulation and vulnerability. In summary, these studies show that: (1) the 5- and 12/15- LOX isozymes have distinct effects within the nigrostriatal pathway on DA metabolism and response to neurotoxic insult, and (2) the functional impact of 5-LOX activity changes depending on the health condition (i.e. normal or injured) in the mouse model.
The differential impact of 5- and 12/15-LOX deficiency on dopaminergic neuroprotection in the nigrostriatal pathway following toxicant exposure (Figs. 2-4) is not entirely surprising as isozyme-selective effects have been reported previously with these LOX-deficient transgenic lines (Gubitosi-Klug et al., 2008). Consistent with our findings, in diabetic retinopathy studies, mice lacking 5-LOX demonstrated significantly reduced capillary degeneration as compared to null 12/15-LOX transgenics and WT mice. Furthermore, the histopathology in the diabetic 12/15-LOX genotype was not significantly different from diabetic WT animals (Gubitosi-Klug et al., 2008). In the present study, striatal MPTP toxicity was attenuated in null 5-LOX transgenics as evidenced by higher striatal DA and metabolite values (Fig. 2) and TH immunoreactivities in the striatum (Fig. 4) and substantia nigra (Fig. 5). Neuroprotection was not observed in MPTP-challenged 12/15-LOX mice, however, as these transgenics responded similarly to exposed WT mice (Fig. 3). Taken together, these data demonstrate LOX isozyme-selective effects on vulnerability of the nigrostriatal pathway to injury.
Both 5- and 12-LOX have been previously shown to impact CNS dopaminergic systems, particularly in response to agents that selectively target dopaminergic neurons. The isozymes, for example, affect the behavioral and biochemical responses to the dopamine re-uptake inhibitor cocaine (Kurtuncu et al., 2008, Chen and Manev, 2011). Mice deficient in 5- or 12-LOX exposed to repeated cocaine exposures do not develop locomotor sensitization as is noted in wildtype control mice (Kurtuncu et al., 2008). This effect is likely attributable to enhanced site-specific GluR1 phosphorylation which occurs in striatum of 5-LOX-deficient mice (Chen and Manev, 2011), that impacts membrane insertion of the receptor and its activity; similar alterations have been observed using a pharmacological model of 5-LOX inhibition, minocycline (Imbesi et al., 2008). Thus, it is possible that products of the isozyme's activity, through interactions with AMPA receptors, contribute to a 5-LOX-mediated response to dopaminergic agents.
Although LOX-isozyme activity can promote damage via oxidative stress-producing events such as lipid hydroperoxide formation and glutathione depletion (Roy et al., 1994, 1995; Pratico et al., 2004; Sun et al., 2012), LOX toxicity is most commonly associated with eicosanoid production that elicits cytotoxic inflammatory responses (Manev et al., 2011). Indeed, markers of inflammation, including both astrocytic and microglial activation were depressed in the toxicant-challenged null 5-LOX mice as compared to WT littermates (Fig. 5). The 5-LOX product, 5-HETE, is converted to LTB4, a highly potent endogenous leukocyte attractant that promotes enhanced expression of IL-12, IL-6 and TNFα (Phillis et al., 2006; Tassoni et al., 2008). While our findings of glial activation support the hypothesis that 5-LOX-activated pro-inflammatory cytokine cascades promote nigrostriatal damage in the mouse MPTP model, the precise AA metabolites that contribute to degeneration remain unknown and a role for oxidative damage cannot be ruled out. In fact, DA turnover, which promotes the formation of reactive and cytotoxic DA quinones (Graham, 1978; Ogawa et al., 2000; Sulzer et al., 2000), is lowered in null 5-LOX vs. WT mice exposed to MPTP (Fig. 2D) suggesting that oxidative stress may be a factor in 5-LOX-related vulnerability to exogenous insult.
Deficiency in 5-LOX function appears to be protective from MPTP challenge in the mouse line; however, evaluation under control conditions revealed provocative results: striatal DA, HVA and TH protein levels are significantly lower in null 5-LOX vs. WT littermates (Fig. 1A, C). These data demonstrate a relationship between 5-LOX activity and regulation of DA synthesis and metabolism, suggesting that 5-LOX activity contributes to normal striatal DA homeostasis. A previous ex vivo study revealed that AA-derived eicosanoids increase DA release in striatal slice cultures (Davidson, 2003); our finding is the first to show that 5-LOX activity is necessary for normal striatal levels of DA as well as its metabolite HVA and its synthetic enzyme TH.
Other studies indirectly provide evidence for a link between LOX activity and DA synthesis. For example, increases in 12-HETE inhibits protein kinase II (Piomelli et al., 1989), an enzyme which phosphorylates and activates TH (Fujisawa and Okuno, 1989). Whether 5-HETE has a similar effect on TH activity is unknown, but given the change in TH expression in null 5-LOX mice, it is likely additional mechanisms are at play. Reduced DA and TH expression were selective with 5-LOX isozyme deficiency; no change in either marker was revealed in mice lacking 12/15-LOX, although a subtle but consistent decrease in DOPAC was measured (Fig. 2B). These data support isozyme-specific functional activities, and importantly indicate that the 5-LOX isozyme elicits a beneficial or detrimental influence on the nigrostriatal dopaminergic pathway depending on cellular state (i.e. normal or distressed).
The seemingly paradoxical effects of 5-LOX activity can be reconciled, however. Previous studies in vitro revealed LOX catalyzes peroxidation of catecholamines to form toxic reactive species, but this deleterious activity was dependent on the presence of an oxidative insult (i.e. hydrogen peroxide) (Rosei et al., 1994). Extrapolating this finding, one interpretation is that the mitochondrial dysfunction caused by MPTP exposure may drive toxic LOX-mediated catecholamine modification or other deleterious effects and subsequent nigrostriatal damage.
Alternatively, LOX-mediated AA catabolism generates a variety of lipid products; these congeners have diverse function and can elicit pro- or anti-inflammatory cellular responses depending on their downstream targets. Increased expression and activity of 12-LOX accelerates pro-inflammatory gene expression including Cox2 (Prasad et al., 2008). 12-HETE activates signaling pathways related to localized oxidative stress, including glutathione depletion (Badr, 1997; Funk and Cyrus, 2001; Kuhn and O'Donnell, 2006), and antagonizes the antiinflammatory actions of PPARγ. Pro-inflammatory leukotrienes are also generated by 5-LOX; however, other downstream products of 5- and 15-LOX, lipoxins, act as agonists for PPARγ (Planaguma et al., 2002; Kuhn and O'Donnell, 2006). PPARγ agonists mediate protection through activating ROS-detoxifying enzymes and diminishing toxicant-induced increased expression of cytokines in animal models of disease, including MPTP (St-Pierre et al., 2006; Schintu et al., 2009; Sobrado et al., 2009; Carta et al., 2011). These results suggest that future studies should regard the 5-LOX isozyme and its products as potential targets in the study of nigrostriatal vulnerability.
Highlights.
Deficiency in 5-, but not 12/15-, lipoxygenase is associated with reduced striatal dopamine and tyrosine hydroxylase levels
5-lipoxygenase deficiency protects against nigrostriatal damage from MPTP
Diminished inflammatory response is noted in null 5-lipoxygenase vs. wildtype mice after intoxication by MPTP
Acknowledgments
The Authors thank Ms. Novie Ko, Ms. Martha Isla-Karpov and Ms. Lisa Jack for technical assistance and Drs. Maryka Quik, Donato A. Di Monte and Gary E. Swan for critical discussions. This work was funded by NIH-GM056062.
Abbreviations
- LOX
lipoxygenase(s)
- AA
arachidonic acid
- PD
Parkinson's disease
- AD
Alzheimer's disease
- WT
wildtype
- TH
tyrosine hydroxylase
- MPTP
1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine
- GFAP
glial acidic fibrillary protein
- ROS
reactive oxygen species
- HPETE
hydroxyeicosatetraenoic acid
- HETE
hydroxyeicosatetraenoic acid
- PPARγ
peroxisome proliferator activator receptor gamma
- LTB4
leukotriene B4
- DA
dopamine
- DOPAC
dihydroxyphenylacetic acid
- HVA
homovanillic acid
- ANOVA
analysis of variance
Footnotes
Amy B. Manning-Bog, Ph.D., Center for Health Sciences, SRI International, 333 Ravenswood Avenue BN118, Menlo Park, CA 94025 USA, Phone: +1 (650) 859-3293, Fax: +1 (650) 859-5099, amy.manningbog@sri.com
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Vivian P. Chou, Email: vivian.chou@sri.com.
Theodore R. Holman, Email: holman@ucsc.edu.
References
- Adibhatla RM, Hatcher JF. Altered lipid metabolism in brain injury and disorders. Subcell Biochem. 2008;49:241–268. doi: 10.1007/978-1-4020-8831-5_9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Badr KF. Glomerulonephritis: roles for lipoxygenase pathways in pathophysiology and therapy. Curr Opin Nephrol Hypertens. 1997;6:111–118. [PubMed] [Google Scholar]
- Basselin M, Kim HW, Chen M, Ma K, Rapoport SI, Murphy RC, Farias SE. Lithium modifies brain arachidonic and docosahexaenoic metabolism in rat lipopolysaccharide model of neuroinflammation. J Lipid Res. 2010;51:1049–1056. doi: 10.1194/jlr.M002469. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Bendani MK, Palluy O, Cook-Moreau J, Beneytout JL, Rigaud M, Vallat JM. Localization of 12-lipoxygenase mRNA in cultured oligodendrocytes and astrocytes by in situ reverse transcriptase and polymerase chain reaction. Neurosci Lett. 1995;189:159–162. doi: 10.1016/0304-3940(95)11482-c. [DOI] [PubMed] [Google Scholar]
- Brash AR. Lipoxygenases: occurrence, functions, catalysis, and acquisition of substrate. J Biol Chem. 1999;274:23679–23682. doi: 10.1074/jbc.274.34.23679. [DOI] [PubMed] [Google Scholar]
- Breydo L, Wu JW, Uversky VN. Alpha-synuclein misfolding and Parkinson's disease. Biochim Biophys Acta. 2012;1822:261–285. doi: 10.1016/j.bbadis.2011.10.002. [DOI] [PubMed] [Google Scholar]
- Canals S, Casarejos MJ, de Bernardo S, Rodriguez-Martin E, Mena MA. Nitric oxide triggers the toxicity due to glutathione depletion in midbrain cultures through 12-lipoxygenase. J Biol Chem. 2003;278:21542–21549. doi: 10.1074/jbc.M213174200. [DOI] [PubMed] [Google Scholar]
- Carta AR, Frau L, Pisanu A, Wardas J, Spiga S, Carboni E. Rosiglitazone decreases peroxisome proliferator receptor-gamma levels in microglia and inhibits TNF-alpha production: new evidences on neuroprotection in a progressive Parkinson's disease model. Neuroscience. 2011;194:250–261. doi: 10.1016/j.neuroscience.2011.07.046. [DOI] [PubMed] [Google Scholar]
- Chen H, Manev H. Effects of minocycline on cocaine sensitization and phosphorylation of GluR1 receptors in 5-lipoxygenase deficient mice. Neuropharmacology. 2011;60:1058–1063. doi: 10.1016/j.neuropharm.2010.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng Y, He G, Mu X, Zhang T, Li X, Hu J, Xu B, Du G. Neuroprotective effect of baicalein against MPTP neurotoxicity: behavioral, biochemical and immunohistochemical profile. Neurosci Lett. 2008;441:16–20. doi: 10.1016/j.neulet.2008.05.116. [DOI] [PubMed] [Google Scholar]
- Chu J, Giannopoulos PF, Ceballos-Diaz C, Golde TE, Pratico D. Adeno-associated virus-mediated brain delivery of 5-lipoxygenase modulates the AD-like phenotype of APP mice. Mol Neurodegener. 2012a;7:1. doi: 10.1186/1750-1326-7-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chu J, Pratico D. Pharmacologic blockade of 5-lipoxygenase improves the amyloidotic phenotype of an Alzheimer's disease transgenic mouse model involvement of gamma-secretase. Am J Pathol. 2011;178:1762–1769. doi: 10.1016/j.ajpath.2010.12.032. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Chu J, Zhuo JM, Pratico D. Transcriptional regulation of beta-secretase-1 by 12/15-lipoxygenase results in enhanced amyloidogenesis and cognitive impairments. Ann Neurol. 2012b;71:57–67. doi: 10.1002/ana.22625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cui L, Zhang X, Yang R, Liu L, Wang L, Li M, Du W. Baicalein is neuroprotective in rat MCAO model: role of 12/15-lipoxygenase, mitogen-activated protein kinase and cytosolic phospholipase A2. Pharmacol Biochem Behav. 2010;96:469–475. doi: 10.1016/j.pbb.2010.07.007. [DOI] [PubMed] [Google Scholar]
- Davidson BC. Eicosanoid precursor polyenoic fatty acids modulate synaptic levels of dopamine in ex-vivo slices of rat brain striatum. In Vivo. 2003;17:83–88. [PubMed] [Google Scholar]
- de Bernardo S, Canals S, Casarejos MJ, Solano RM, Menendez J, Mena MA. Role of extracellular signal-regulated protein kinase in neuronal cell death induced by glutathione depletion in neuron/glia mesencephalic cultures. J Neurochem. 2004;91:667–682. doi: 10.1111/j.1471-4159.2004.02744.x. [DOI] [PubMed] [Google Scholar]
- Dobrian AD, Lieb DC, Cole BK, Taylor-Fishwick DA, Chakrabarti SK, Nadler JL. Functional and pathological roles of the 12- and 15-lipoxygenases. Prog Lipid Res. 2011;50:115–131. doi: 10.1016/j.plipres.2010.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farooqui AA, Horrocks LA. Phospholipase A2-generated lipid mediators in the brain: the good, the bad, and the ugly. Neuroscientist. 2006;12:245–260. doi: 10.1177/1073858405285923. [DOI] [PubMed] [Google Scholar]
- Farooqui AA, Horrocks LA, Farooqui T. Interactions between neural membrane glycerophospholipid and sphingolipid mediators: a recipe for neural cell survival or suicide. J Neurosci Res. 2007;85:1834–1850. doi: 10.1002/jnr.21268. [DOI] [PubMed] [Google Scholar]
- Firuzi O, Zhuo J, Chinnici CM, Wisniewski T, Pratico D. 5-Lipoxygenase gene disruption reduces amyloid-beta pathology in a mouse model of Alzheimer's disease. FASEB J. 2008;22:1169–1178. doi: 10.1096/fj.07-9131.com. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujisawa H, Okuno S. Regulation of the activity of tyrosine hydroxylase in the central nervous system. Adv Enzyme Regul. 1989;28:93–110. doi: 10.1016/0065-2571(89)90066-6. [DOI] [PubMed] [Google Scholar]
- Funk CD, Cyrus T. 12/15-lipoxygenase, oxidative modification of LDL and atherogenesis. Trends Cardiovasc Med. 2001;11:116–124. doi: 10.1016/s1050-1738(01)00096-2. [DOI] [PubMed] [Google Scholar]
- Graham DG. Oxidative pathways for catecholamines in the genesis of neuromelanin and cytotoxic quinones. Mol Pharmacol. 1978;14:633–643. [PubMed] [Google Scholar]
- Gubitosi-Klug RA, Talahalli R, Du Y, Nadler JL, Kern TS. 5-Lipoxygenase, but not 12/15-lipoxygenase, contributes to degeneration of retinal capillaries in a mouse model of diabetic retinopathy. Diabetes. 2008;57:1387–1393. doi: 10.2337/db07-1217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta A, Kumar A, Kulkarni SK. Licofelone attenuates MPTP-induced neuronal toxicity: behavioral, biochemical and cellular evidence. Inflammopharmacology. 2010;18:223–232. doi: 10.1007/s10787-010-0052-6. [DOI] [PubMed] [Google Scholar]
- Hashimoto K. Can minocycline prevent the onset of Alzheimer's disease? Ann Neurol. 2011;69:739. doi: 10.1002/ana.22378. author reply 739-740. [DOI] [PubMed] [Google Scholar]
- Hunot S, Hirsch EC. Neuroinflammatory processes in Parkinson's disease. Ann Neurol. 2003;53(3):S49–58. doi: 10.1002/ana.10481. discussion S58-60. [DOI] [PubMed] [Google Scholar]
- Ikonomovic MD, Abrahamson EE, Uz T, Manev H, Dekosky ST. Increased 5-lipoxygenase immunoreactivity in the hippocampus of patients with Alzheimer's disease. J Histochem Cytochem. 2008;56:1065–1073. doi: 10.1369/jhc.2008.951855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imai Y, Lu B. Mitochondrial dynamics and mitophagy in Parkinson's disease: disordered cellular power plant becomes a big deal in a major movement disorder. Curr Opin Neurobiol. 2011;21:935–941. doi: 10.1016/j.conb.2011.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imbesi M, Uz T, Manev R, Sharma RP, Manev H. Minocycline increases phosphorylation and membrane insertion of neuronal GluR1 receptors. Neurosci Lett. 2008;447:134–137. doi: 10.1016/j.neulet.2008.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin G, Arai K, Murata Y, Wang S, Stins MF, Lo EH, van Leyen K. Protecting against cerebrovascular injury: contributions of 12/15-lipoxygenase to edema formation after transient focal ischemia. Stroke. 2008;39:2538–2543. doi: 10.1161/STROKEAHA.108.514927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kettenmann H, Hanisch UK, Noda M, Verkhratsky A. Physiology of microglia. Physiol Rev. 2011;91:461–553. doi: 10.1152/physrev.00011.2010. [DOI] [PubMed] [Google Scholar]
- Kilpatrick IC, Jones MW, Phillipson OT. A semiautomated analysis method for catecholamines, indoleamines, and some prominent metabolites in microdissected regions of the nervous system: an isocratic HPLC technique employing coulometric detection and minimal sample preparation. J Neurochem. 1986;46:1865–1876. doi: 10.1111/j.1471-4159.1986.tb08506.x. [DOI] [PubMed] [Google Scholar]
- Klegeris A, McGeer PL. Cyclooxygenase and 5-lipoxygenase inhibitors protect against mononuclear phagocyte neurotoxicity. Neurobiol Aging. 2002;23:787–794. doi: 10.1016/s0197-4580(02)00021-0. [DOI] [PubMed] [Google Scholar]
- Kramer BC, Yabut JA, Cheong J, Jnobaptiste R, Robakis T, Olanow CW, Mytilineou C. Toxicity of glutathione depletion in mesencephalic cultures: a role for arachidonic acid and its lipoxygenase metabolites. Eur J Neurosci. 2004;19:280–286. doi: 10.1111/j.1460-9568.2004.03111.x. [DOI] [PubMed] [Google Scholar]
- Kuhn H, O'Donnell VB. Inflammation and immune regulation by 12/15-lipoxygenases. Prog Lipid Res. 2006;45:334–356. doi: 10.1016/j.plipres.2006.02.003. [DOI] [PubMed] [Google Scholar]
- Kurtuncu M, Battista N, Uz T, D'Agostino A, Dimitrijevic N, Pasquariello N, Manev R, Maccarrone M, Manev H. Effects of cocaine in 5-lipoxygenase-deficient mice. J Neural Transm. 2008;115:389–395. doi: 10.1007/s00702-007-0848-8. [DOI] [PubMed] [Google Scholar]
- Li Y, Maher P, Schubert D. A role for 12-lipoxygenase in nerve cell death caused by glutathione depletion. Neuron. 1997;19:453–463. doi: 10.1016/s0896-6273(00)80953-8. [DOI] [PubMed] [Google Scholar]
- Limor R, Sharon O, Knoll E, Many A, Weisinger G, Stern N. Lipoxygenase-derived metabolites are regulators of peroxisome proliferator-activated receptor gamma-2 expression in human vascular smooth muscle cells. Am J Hypertens. 2008;21:219–223. doi: 10.1038/ajh.2007.39. [DOI] [PubMed] [Google Scholar]
- Listi F, Caruso C, Lio D, Colonna-Romano G, Chiappelli M, Licastro F, Candore G. Role of cyclooxygenase-2 and 5-lipoxygenase polymorphisms in Alzheimer's disease in a population from northern Italy: implication for pharmacogenomics. J Alzheimers Dis. 2010;19:551–557. doi: 10.3233/JAD-2010-1260. [DOI] [PubMed] [Google Scholar]
- Liu C, Xu D, Liu L, Schain F, Brunnstrom A, Bjorkholm M, Claesson HE, Sjoberg J. 15-Lipoxygenase-1 induces expression and release of chemokines in cultured human lung epithelial cells. Am J Physiol Lung Cell Mol Physiol. 2009;297:L196–203. doi: 10.1152/ajplung.00036.2008. [DOI] [PubMed] [Google Scholar]
- Lopez-Parra M, Claria J, Titos E, Planaguma A, Parrizas M, Masferrer JL, Jimenez W, Arroyo V, Rivera F, Rodes J. The selective cyclooxygenase-2 inhibitor celecoxib modulates the formation of vasoconstrictor eicosanoids and activates PPARgamma. Influence of albumin J Hepatol. 2005;42:75–81. doi: 10.1016/j.jhep.2004.09.011. [DOI] [PubMed] [Google Scholar]
- Manev H. 5-Lipoxygenase gene polymorphism and onset of Alzheimer's disease. Med Hypotheses. 2000;54:75–76. doi: 10.1054/mehy.1998.0824. [DOI] [PubMed] [Google Scholar]
- Manev H, Chen H, Dzitoyeva S, Manev R. Cyclooxygenases and 5-lipoxygenase in Alzheimer's disease. Progress in neuro-psychopharmacology & biological psychiatry. 2011;35:315–319. doi: 10.1016/j.pnpbp.2010.07.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manev H, Manev R. 5-Lipoxygenase (ALOX5) and FLAP (ALOX5AP) gene polymorphisms as factors in vascular pathology and Alzheimer's disease. Med Hypotheses. 2006;66:501–503. doi: 10.1016/j.mehy.2005.09.031. [DOI] [PubMed] [Google Scholar]
- Manev H, Uz T, Manev R, Zhang Z. Neurogenesis and neuroprotection in the adult brain. A putative role for 5-lipoxygenase? Ann N Y Acad Sci. 2001;939:45–51. doi: 10.1111/j.1749-6632.2001.tb03610.x. [DOI] [PubMed] [Google Scholar]
- Manev H, Uz T, Qu T. 5-Lipoxygenase and cyclooxygenase mRNA expression in rat hippocampus:early response to glutamate receptor activation by kainate. Exp Gerontol. 2000a;35:1201–1209. doi: 10.1016/s0531-5565(00)00152-2. [DOI] [PubMed] [Google Scholar]
- Manev H, Uz T, Sugaya K, Qu T. Putative role of neuronal 5-lipoxygenase in an aging brain. FASEB J. 2000b;14:1464–1469. doi: 10.1096/fj.14.10.1464. [DOI] [PubMed] [Google Scholar]
- Manning-Bog AB, Caudle WM, Perez XA, Reaney SH, Paletzki R, Isla MZ, Chou VP, McCormack AL, Miller GW, Langston JW, Gerfen CR, Dimonte DA. Increased vulnerability of nigrostriatal terminals in DJ-1-deficient mice is mediated by the dopamine transporter. Neurobiol Dis. 2007;27:141–150. doi: 10.1016/j.nbd.2007.03.014. [DOI] [PubMed] [Google Scholar]
- Manning-Bog AB, McCormack AL, Purisai MG, Bolin LM, Di Monte DA. Alpha-synuclein overexpression protects against paraquat-induced neurodegeneration. J Neurosci. 2003;23:3095–3099. doi: 10.1523/JNEUROSCI.23-08-03095.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marks F, Muller-Decker K, Furstenberger G. A causal relationship between unscheduled eicosanoid signaling and tumor development: cancer chemoprevention by inhibitors of arachidonic acid metabolism. Toxicology. 2000;153:11–26. doi: 10.1016/s0300-483x(00)00301-2. [DOI] [PubMed] [Google Scholar]
- McCoy MK, Cookson MR. Mitochondrial quality control and dynamics in Parkinson's disease. Antioxid Redox Signal. 2012;16:869–882. doi: 10.1089/ars.2011.4019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menna C, Olivieri F, Catalano A, Procopio A. Lipoxygenase inhibitors for cancer prevention: promises and risks. Curr Pharm Des. 2010;16:725–733. doi: 10.2174/138161210790883822. [DOI] [PubMed] [Google Scholar]
- Minghetti L. Cyclooxygenase-2 (COX-2) in inflammatory and degenerative brain diseases. J Neuropathol Exp Neurol. 2004;63:901–910. doi: 10.1093/jnen/63.9.901. [DOI] [PubMed] [Google Scholar]
- Mosca L, Foppoli C, Coccia R, Rosei MA. Pheomelanin production by the lipoxygenase-catalyzed oxidation of 5-S-cysteinyldopa and 5-S-cysteinyldopamine. Pigment Cell Res. 1996;9:117–125. doi: 10.1111/j.1600-0749.1996.tb00099.x. [DOI] [PubMed] [Google Scholar]
- Mytilineou C, Kokotos Leonardi ET, Kramer BC, Jamindar T, Olanow CW. Glial cells mediate toxicity in glutathione-depleted mesencephalic cultures. J Neurochem. 1999;73:112–119. doi: 10.1046/j.1471-4159.1999.0730112.x. [DOI] [PubMed] [Google Scholar]
- Mytilineou C, Kramer BC, Yabut JA. Glutathione depletion and oxidative stress. Parkinsonism Relat Disord. 2002;8:385–387. doi: 10.1016/s1353-8020(02)00018-4. [DOI] [PubMed] [Google Scholar]
- Neilson AP, Ren J, Hong YH, Sen A, Smith WL, Brenner DE, Djuric Z. Effect of fish oil on levels of R- and S-enantiomers of 5-, 12-, and 15-hydroxyeicosatetraenoic acids in mouse colonic mucosa. Nutr Cancer. 2012;64:163–172. doi: 10.1080/01635581.2012.630168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogawa N, Tanaka K, Asanuma M. Bromocriptine markedly suppresses levodopa-induced abnormal increase of dopamine turnover in the parkinsonian striatum. Neurochem Res. 2000;25:755–758. doi: 10.1023/a:1007530720544. [DOI] [PubMed] [Google Scholar]
- Ouchi Y, Yagi S, Yokokura M, Sakamoto M. Neuroinflammation in the living brain of Parkinson's disease. Parkinsonism Relat Disord. 2009;15(3):S200–204. doi: 10.1016/S1353-8020(09)70814-4. [DOI] [PubMed] [Google Scholar]
- Parkinson JF. Lipoxin and synthetic lipoxin analogs: an overview of anti-inflammatory functions and new concepts in immunomodulation. Inflamm Allergy Drug Targets. 2006;5:91–106. doi: 10.2174/187152806776383125. [DOI] [PubMed] [Google Scholar]
- Phillis JW, Horrocks LA, Farooqui AA. Cyclooxygenases, lipoxygenases, and epoxygenases in CNS: their role and involvement in neurological disorders. Brain Res Rev. 2006;52:201–243. doi: 10.1016/j.brainresrev.2006.02.002. [DOI] [PubMed] [Google Scholar]
- Piomelli D, Wang JK, Sihra TS, Nairn AC, Czernik AJ, Greengard P. Inhibition of Ca2+/calmodulin-dependent protein kinase II by arachidonic acid and its metabolites. Proc Natl Acad Sci U S A. 1989;86:8550–8554. doi: 10.1073/pnas.86.21.8550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Planaguma A, Kazani S, Marigowda G, Haworth O, Mariani TJ, Israel E, Bleecker ER, Curran-Everett D, Erzurum SC, Calhoun WJ, Castro M, Chung KF, Gaston B, Jarjour NN, Busse WW, Wenzel SE, Levy BD. Airway lipoxin A4 generation and lipoxin A4 receptor expression are decreased in severe asthma. Am J Respir Crit Care Med. 2008;178:574–582. doi: 10.1164/rccm.200801-061OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Planaguma A, Titos E, Lopez-Parra M, Gaya J, Pueyo G, Arroyo V, Claria J. Aspirin (ASA) regulates 5-lipoxygenase activity and peroxisome proliferator-activated receptor alpha-mediated CINC-1 release in rat liver cells: novel actions of lipoxin A4 (LXA4) and ASA-triggered 15-epi-LXA4. FASEB J. 2002;16:1937–1939. doi: 10.1096/fj.02-0224fje. [DOI] [PubMed] [Google Scholar]
- Plowey ED, Chu CT. Synaptic dysfunction in genetic models of Parkinson's disease: a role for autophagy? Neurobiol Dis. 2011;43:60–67. doi: 10.1016/j.nbd.2010.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prasad VV, Nithipatikom K, Harder DR. Ceramide elevates 12-hydroxyeicosatetraenoic acid levels and upregulates 12-lipoxygenase in rat primary hippocampal cell cultures containing predominantly astrocytes. Neurochem Int. 2008;53:220–229. doi: 10.1016/j.neuint.2008.07.002. [DOI] [PubMed] [Google Scholar]
- Pratico D, Zhukareva V, Yao Y, Uryu K, Funk CD, Lawson JA, Trojanowski JQ, Lee VM. 12/15-lipoxygenase is increased in Alzheimer's disease: possible involvement in brain oxidative stress. The American journal of pathology. 2004;164:1655–1662. doi: 10.1016/S0002-9440(10)63724-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puccio S, Chu J, Pratico D. Involvement of 5-lipoxygenase in the corticosteroid- dependent amyloid beta formation: in vitro and in vivo evidence. PLoS One. 2011;6:e15163. doi: 10.1371/journal.pone.0015163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qu T, Uz T, Manev H. Inflammatory 5-LOX mRNA and protein are increased in brain of aging rats. Neurobiol Aging. 2000;21:647–652. doi: 10.1016/s0197-4580(00)00167-6. [DOI] [PubMed] [Google Scholar]
- Rosei MA, Blarzino C, Foppoli C, Mosca L, Coccia R. Lipoxygenase-catalyzed oxidation of catecholamines. Biochem Biophys Res Commun. 1994;200:344–350. doi: 10.1006/bbrc.1994.1454. [DOI] [PubMed] [Google Scholar]
- Roy P, Roy SK, Mitra A, Kulkarni AP. Superoxide generation by lipoxygenase in the presence of NADH and NADPH. Biochim Biophys Acta. 1994;1214:171–179. doi: 10.1016/0005-2760(94)90041-8. [DOI] [PubMed] [Google Scholar]
- Roy P, Sajan MP, Kulkarni AP. Lipoxygenase-mediated glutathione oxidation and superoxide generation. J Biochem Toxicol. 1995;10:111–120. doi: 10.1002/jbt.2570100208. [DOI] [PubMed] [Google Scholar]
- Schintu N, Frau L, Ibba M, Caboni P, Garau A, Carboni E, Carta AR. PPAR-gamma-mediated neuroprotection in a chronic mouse model of Parkinson's disease. Eur J Neurosci. 2009;29:954–963. doi: 10.1111/j.1460-9568.2009.06657.x. [DOI] [PubMed] [Google Scholar]
- Scholz M, Ulbrich HK, Dannhardt G. Investigations concerning the COX/5-LOX inhibiting and hydroxyl radical scavenging potencies of novel 4,5-diaryl isoselenazoles. Eur J Med Chem. 2008;43:1152–1159. doi: 10.1016/j.ejmech.2007.09.007. [DOI] [PubMed] [Google Scholar]
- Serhan CN. Novel chemical mediators in the resolution of inflammation: resolvins and protectins. Anesthesiol Clin. 2006;24:341–364. doi: 10.1016/j.atc.2006.01.003. [DOI] [PubMed] [Google Scholar]
- Serhan CN, Samuelsson B. Lipoxins: a new series of eicosanoids (biosynthesis, stereochemistry, and biological activities) Adv Exp Med Biol. 1988;229:1–14. doi: 10.1007/978-1-4757-0937-7_1. [DOI] [PubMed] [Google Scholar]
- Simonian NA, Coyle JT. Oxidative stress in neurodegenerative diseases. Annu Rev Pharmacol Toxicol. 1996;36:83–106. doi: 10.1146/annurev.pa.36.040196.000503. [DOI] [PubMed] [Google Scholar]
- Sobrado M, Pereira MP, Ballesteros I, Hurtado O, Fernandez-Lopez D, Pradillo JM, Caso JR, Vivancos J, Nombela F, Serena J, Lizasoain I, Moro MA. Synthesis of lipoxin A4 by 5-lipoxygenase mediates PPARgamma-dependent, neuroprotective effects of rosiglitazone in experimental stroke. J Neurosci. 2009;29:3875–3884. doi: 10.1523/JNEUROSCI.5529-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- St-Pierre J, Drori S, Uldry M, Silvaggi JM, Rhee J, Jager S, Handschin C, Zheng K, Lin J, Yang W, Simon DK, Bachoo R, Spiegelman BM. Suppression of reactive oxygen species and neurodegeneration by the PGC-1 transcriptional coactivators. Cell. 2006;127:397–408. doi: 10.1016/j.cell.2006.09.024. [DOI] [PubMed] [Google Scholar]
- Stewart LR, White AR, Jobling MF, Needham BE, Maher F, Thyer J, Beyreuther K, Masters CL, Collins SJ, Cappai R. Involvement of the 5-lipoxygenase pathway in the neurotoxicity of the prion peptide PrP106-126. J Neurosci Res. 2001;65:565–572. doi: 10.1002/jnr.1186. [DOI] [PubMed] [Google Scholar]
- Sugaya K, Uz T, Kumar V, Manev H. New anti-inflammatory treatment strategy in Alzheimer's disease. Jpn J Pharmacol. 2000;82:85–94. doi: 10.1254/jjp.82.85. [DOI] [PubMed] [Google Scholar]
- Sulzer D, Bogulavsky J, Larsen KE, Behr G, Karatekin E, Kleinman MH, Turro N, Krantz D, Edwards RH, Greene LA, Zecca L. Neuromelanin biosynthesis is driven by excess cytosolic catecholamines not accumulated by synaptic vesicles. Proc Natl Acad Sci U S A. 2000;97:11869–11874. doi: 10.1073/pnas.97.22.11869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun L, Yang L, Xu YW, Liang H, Han J, Zhao RJ, Cheng Y. Neuroprotection of hydroxysafflor yellow A in the transient focal ischemia: Inhibition of protein oxidation/nitration, 12/15-lipoxygenase and blood-brain barrier disruption. Brain Res. 2012;1473:227–235. doi: 10.1016/j.brainres.2012.07.047. [DOI] [PubMed] [Google Scholar]
- Tansey MG, Frank-Cannon TC, McCoy MK, Lee JK, Martinez TN, McAlpine FE, Ruhn KA, Tran TA. Neuroinflammation in Parkinson's disease: is there sufficient evidence for mechanism-based interventional therapy? Frontiers in bioscience: a journal and virtual library. 2008;13:709–717. doi: 10.2741/2713. [DOI] [PubMed] [Google Scholar]
- Tansey MG, Goldberg MS. Neuroinflammation in Parkinson's disease: its role in neuronal death and implications for therapeutic intervention. Neurobiol Dis. 2010;37:510–518. doi: 10.1016/j.nbd.2009.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tassoni D, Kaur G, Weisinger RS, Sinclair AJ. The role of eicosanoids in the brain. Asia Pac J Clin Nutr. 2008;17(1):220–228. [PubMed] [Google Scholar]
- Ton TG, Heckbert SR, Longstreth WT, Jr, Rossing MA, Kukull WA, Franklin GM, Swanson PD, Smith-Weller T, Checkoway H. Nonsteroidal anti-inflammatory drugs and risk of Parkinson's disease. Mov Disord. 2006;21:964–969. doi: 10.1002/mds.20856. [DOI] [PubMed] [Google Scholar]
- Ton TG, Jain S, Biggs ML, Thacker EL, Strotmeyer ES, Boudreau R, Newman AB, Longstreth WT, Jr, Checkoway H. Markers of inflammation in prevalent and incident Parkinson's disease in the Cardiovascular Health Study. Parkinsonism Relat Disord. 2012;18:274–278. doi: 10.1016/j.parkreldis.2011.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tu XK, Yang WZ, Wang CH, Shi SS, Zhang YL, Chen CM, Yang YK, Jin CD, Wen S. Zileuton reduces inflammatory reaction and brain damage following permanent cerebral ischemia in rats. Inflammation. 2010;33:344–352. doi: 10.1007/s10753-010-9191-6. [DOI] [PubMed] [Google Scholar]
- Uz T, Dwivedi Y, Qeli A, Peters-Golden M, Pandey G, Manev H. Glucocorticoid receptors are required for up-regulation of neuronal 5-lipoxygenase (5LOX) expression by dexamethasone. FASEB J. 2001a;15:1792–1794. doi: 10.1096/fj.00-0836fje. [DOI] [PubMed] [Google Scholar]
- Uz T, Manev R, Manev H. 5-Lipoxygenase is required for proliferation of immature cerebellar granule neurons in vitro. Eur J Pharmacol. 2001b;418:15–22. doi: 10.1016/s0014-2999(01)00924-4. [DOI] [PubMed] [Google Scholar]
- Uz T, Pesold C, Longone P, Manev H. Aging-associated up-regulation of neuronal 5-lipoxygenase expression: putative role in neuronal vulnerability. FASEB J. 1998;12:439–449. doi: 10.1096/fasebj.12.6.439. [DOI] [PubMed] [Google Scholar]
- van Leyen K, Arai K, Jin G, Kenyon V, Gerstner B, Rosenberg PA, Holman TR, Lo EH. Novel lipoxygenase inhibitors as neuroprotective reagents. J Neurosci Res. 2008;86:904–909. doi: 10.1002/jnr.21543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang ZJ, Liang CL, Li GM, Yu CY, Yin M. Neuroprotective effects of arachidonic acid against oxidative stress on rat hippocampal slices. Chem Biol Interact. 2006;163:207–217. doi: 10.1016/j.cbi.2006.08.005. [DOI] [PubMed] [Google Scholar]
- Weaver JR, Holman TR, Imai Y, Jadhav A, Kenyon V, Maloney DJ, Nadler JL, Rai G, Simeonov A, Taylor-Fishwick DA. Integration of pro-inflammatory cytokines, 12-lipoxygenase and NOX-1 in pancreatic islet beta cell dysfunction. Mol Cell Endocrinol. 2012;358:88–95. doi: 10.1016/j.mce.2012.03.004. [DOI] [PubMed] [Google Scholar]
- Werz O. 5-lipoxygenase: cellular biology and molecular pharmacology. Curr Drug Targets Inflamm Allergy. 2002;1:23–44. doi: 10.2174/1568010023344959. [DOI] [PubMed] [Google Scholar]
- Yang H, Zhuo JM, Chu J, Chinnici C, Pratico D. Amelioration of the Alzheimer's disease phenotype by absence of 12/15-lipoxygenase. Biol Psychiatry. 2010;68:922–929. doi: 10.1016/j.biopsych.2010.04.010. [DOI] [PubMed] [Google Scholar]
- Yao Y, Clark CM, Trojanowski JQ, Lee VM, Pratico D. Elevation of 12/15 lipoxygenase products in AD and mild cognitive impairment. Ann Neurol. 2005;58:623–626. doi: 10.1002/ana.20558. [DOI] [PubMed] [Google Scholar]
- Yeung J, Apopa PL, Vesci J, Kenyon V, Rai G, Jadhav A, Simeonov A, Holman TR, Maloney DJ, Boutaud O, Holinstat M. Protein kinase C regulation of 12-lipoxygenase-mediated human platelet activation. Mol Pharmacol. 2012;81:420–430. doi: 10.1124/mol.111.075630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaleska MM, Wilson DF. Lipid hydroperoxides inhibit reacylation of phospholipids in neuronal membranes. J Neurochem. 1989;52:255–260. doi: 10.1111/j.1471-4159.1989.tb10925.x. [DOI] [PubMed] [Google Scholar]


