Abstract
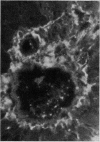
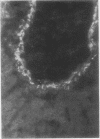
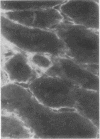
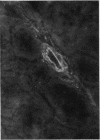
Reticulin antibodies have been classified by immunofluorescence into five types reacting with distinct antigens of intra- and extracellular components in mesenchyme. Two types of fibrillar antigens can be distinguished on the basis of the staining patterns, anatomical distribution, and species specificity. A third antibody reacts with either small fibres, amorphous proteins, or mucopolysaccharides lining the hepatic sinusoids (ground substance antigens). In addition, at least two kinds of intrasinusoidal cells show cytoplasmic fluorescence, ie, Kupffer cells and glass-adherent, blood-borne cells antigenically related to peritoneal macrophages. Some sera may contain antibodies reacting with sinusoidal endothelial cells though this has not yet been proven.
It has been confirmed that all these distinct antibodies related to reticulin antigens are most frequent in dermatitis herpetiformis and coeliac disease, but they are also found with increased frequency in chronic heroin addicts and in rheumatoid and Sjögren's syndromes. About 5% of normal individuals had such antibodies and no significant increase could be demonstrated in autoimmune disorders or in liver cirrhosis. The antibodies appear to be stimulated by bacterial or nutritional antigens and are likely to represent anamnestic responses rather than direct cross reactions with a multiplicity of foreign antigens.
Full text
PDF








Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alp M. H., Wright R. Autoantibodies to reticulin in patients with idiopathic steatorrhoea, coeliac disease, and Crohn's disease, and their relation to immunoglobulins and dietary antibodies. Lancet. 1971 Sep 25;2(7726):682–685. doi: 10.1016/s0140-6736(71)92249-5. [DOI] [PubMed] [Google Scholar]
- Ammann A. J., Hong R. Unique antibody to basement membrane in patients with selective IgA deficiency and coeliac disease. Lancet. 1971 Jun 19;1(7712):1264–1266. doi: 10.1016/s0140-6736(71)91779-x. [DOI] [PubMed] [Google Scholar]
- Avrameas S., Ternynck T. The cross-linking of proteins with glutaraldehyde and its use for the preparation of immunoadsorbents. Immunochemistry. 1969 Jan;6(1):53–66. doi: 10.1016/0019-2791(69)90178-5. [DOI] [PubMed] [Google Scholar]
- Doniach D. Autoimmunity in liver diseases. Prog Clin Immunol. 1972;1:45–70. [PubMed] [Google Scholar]
- Holborow E. J. Smooth-muscle autoantibodies, viral infections and malignant disease. Proc R Soc Med. 1972 May;65(5):481–484. [PMC free article] [PubMed] [Google Scholar]
- Huber H., Fudenberg H. H. Receptor sites of human monocytes for IgG. Int Arch Allergy Appl Immunol. 1968;34(1):18–31. doi: 10.1159/000230091. [DOI] [PubMed] [Google Scholar]
- Huberman A., Recio A., Rojkind M. Collagen biosynthesis in normal and cirrhotic rat liver slices. Proc Soc Exp Biol Med. 1969 May;131(1):200–203. doi: 10.3181/00379727-131-33839. [DOI] [PubMed] [Google Scholar]
- Ireton H. J., Muller H. K., McGiven A. R. Human antibody against rat gastric parietal cells and kidney brush border. Clin Exp Immunol. 1971 May;8(5):783–789. [PMC free article] [PubMed] [Google Scholar]
- Ito T., Shibasaki S. Electron microscopic study on the hepatic sinusoidal wall and the fat-storing cells in the normal human liver. Arch Histol Jpn. 1968 Mar;29(2):137–192. doi: 10.1679/aohc1950.29.137. [DOI] [PubMed] [Google Scholar]
- Jones G., Torrigiani G., Roitt I. M. Immunoglobulin determinants on mouse lymphocytes. J Immunol. 1971 Jun;106(6):1425–1430. [PubMed] [Google Scholar]
- Kingston D., Glynn L. E. A cross-reaction between Str. pyogenes and human fibroblasts, endothelial cells and astrocytes. Immunology. 1971 Dec;21(6):1003–1016. [PMC free article] [PubMed] [Google Scholar]
- Pisano J. C., Filkins J. P., Di Luzio N. R. Phagocytic and metabolic activities of isolated rat Kupffer cells. Proc Soc Exp Biol Med. 1968 Jul;128(3):917–922. doi: 10.3181/00379727-128-33157. [DOI] [PubMed] [Google Scholar]
- Popper H., Uenfriend S. Hepatic fibrosis. Correlation of biochemical and morphologic investigations. Am J Med. 1970 Nov;49:707–721. doi: 10.1016/s0002-9343(70)80135-8. [DOI] [PubMed] [Google Scholar]
- Seah P. P., Fry L., Hoffbrand A. V., Holborow E. J. Tissue antibodies in dermatitis herpetiformis and adult coeliac disease. Lancet. 1971 Apr 24;1(7704):834–836. doi: 10.1016/s0140-6736(71)91499-1. [DOI] [PubMed] [Google Scholar]
- Seah P. P., Fry L., Holborow E. J., Rossiter M. A., Doe W. F., Magalhaes A. F., Hoffbrand A. V. Antireticulin antibody: incidence and diagnostic significance. Gut. 1973 Apr;14(4):311–315. doi: 10.1136/gut.14.4.311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seah P. P., Fry L., Rossiter M. A., Hoffbrand A. V., Holborow E. J. Anti-reticulin antibodies in childhood coeliac disease. Lancet. 1971 Sep 25;2(7726):681–682. doi: 10.1016/s0140-6736(71)92248-3. [DOI] [PubMed] [Google Scholar]
- Shiner M., Ballard J. Antigen-antibody reactions in jejunal mucosa in childhood coeliac disease after gluten challenge. Lancet. 1972 Jun 3;1(7762):1202–1205. doi: 10.1016/s0140-6736(72)90924-5. [DOI] [PubMed] [Google Scholar]
- Spiro R. G. Biochemistry of the renal glomerular basement membrane and its alterations in diabetes mellitus. N Engl J Med. 1973 Jun 21;288(25):1337–1342. doi: 10.1056/NEJM197306212882506. [DOI] [PubMed] [Google Scholar]
- Steffen C. Antigenicity and autoantigenicity of collagen. Ann N Y Acad Sci. 1965 Jun 30;124(2):570–585. doi: 10.1111/j.1749-6632.1965.tb18988.x. [DOI] [PubMed] [Google Scholar]
- Steffen C., Timpl R., Wolff I. Immunogenicity and specificity of collagen. V. Demonstration of three different antigenic determinants on calf collagen. Immunology. 1968 Jul;15(1):135–144. [PMC free article] [PubMed] [Google Scholar]
- Triger D. R., Wright R. Hyperglobulinaemia in liver disease. Lancet. 1973 Jun 30;1(7818):1494–1496. doi: 10.1016/s0140-6736(73)91827-8. [DOI] [PubMed] [Google Scholar]
- Widmann J. J., Cotran R. S., Fahimi H. D. Mononuclear phagocytes (Kupffer cells) and endothelial cells. Identification of two functional cell types in rat liver sinusoids by endogenous peroxidase activity. J Cell Biol. 1972 Jan;52(1):159–170. doi: 10.1083/jcb.52.1.159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zembala M., Asherson G. L. The rapid purification of peritoneal exudate macrophages by ficoll (polysucrose) density gradient centrifugation. Immunology. 1970 Oct;19(4):677–681. [PMC free article] [PubMed] [Google Scholar]