Abstract
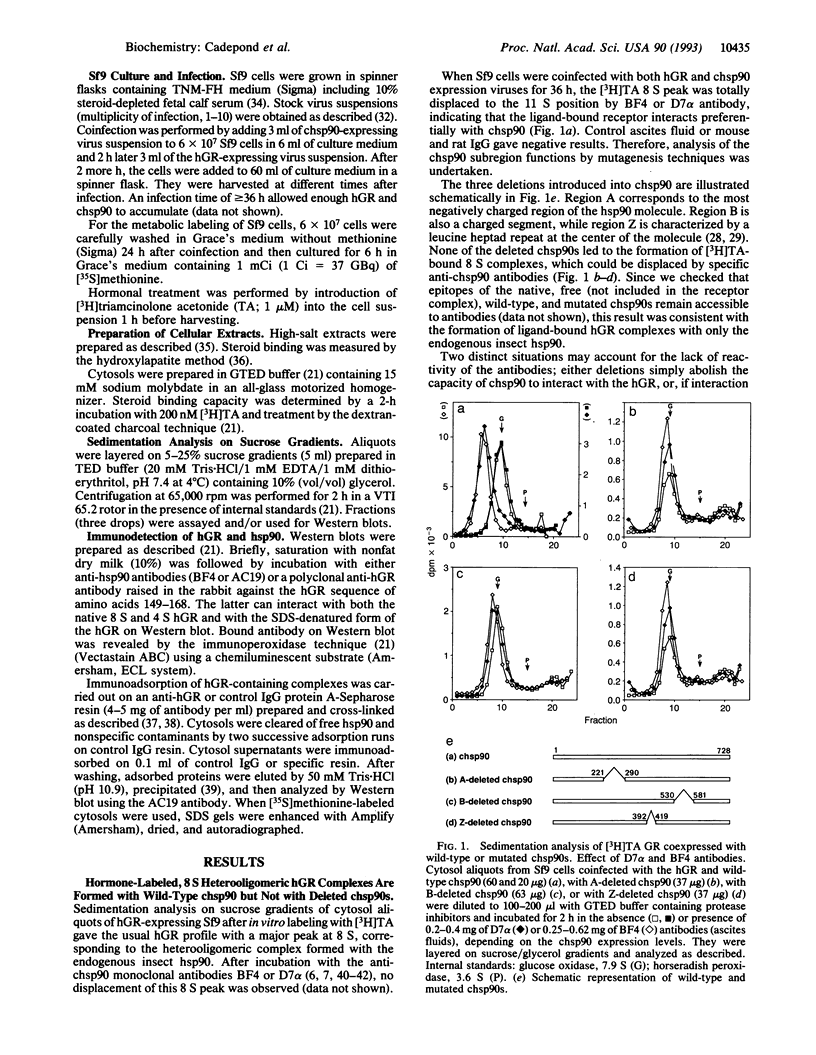
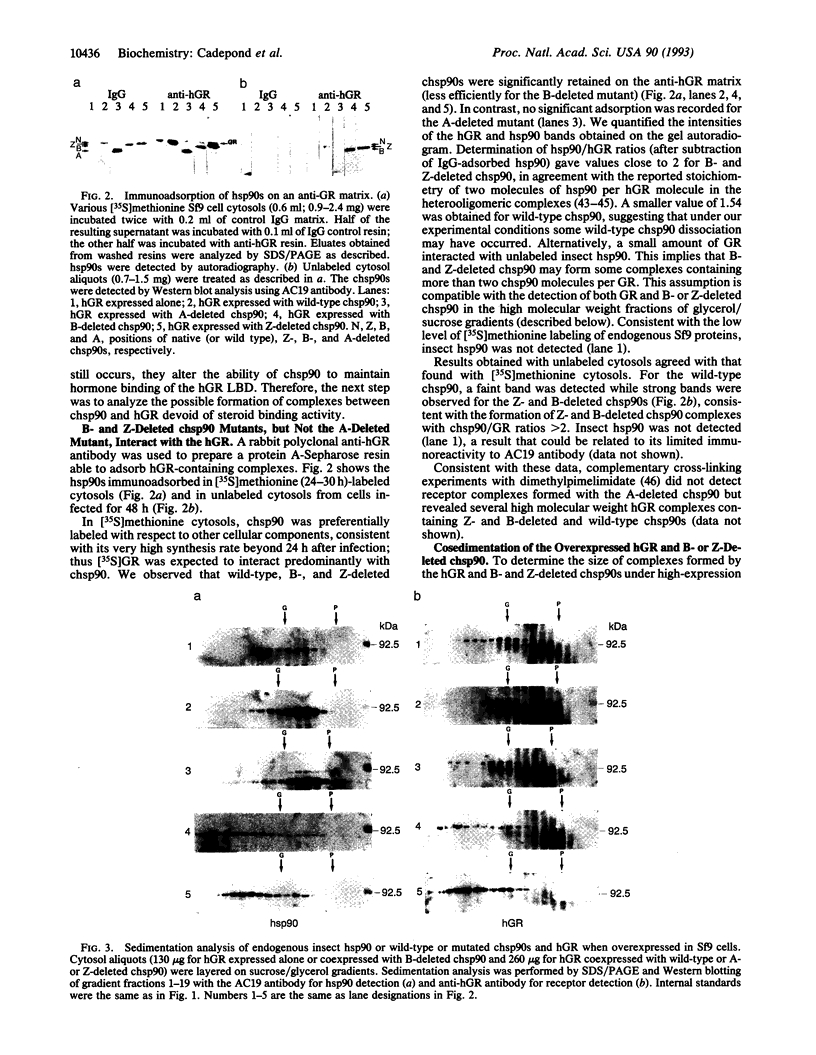
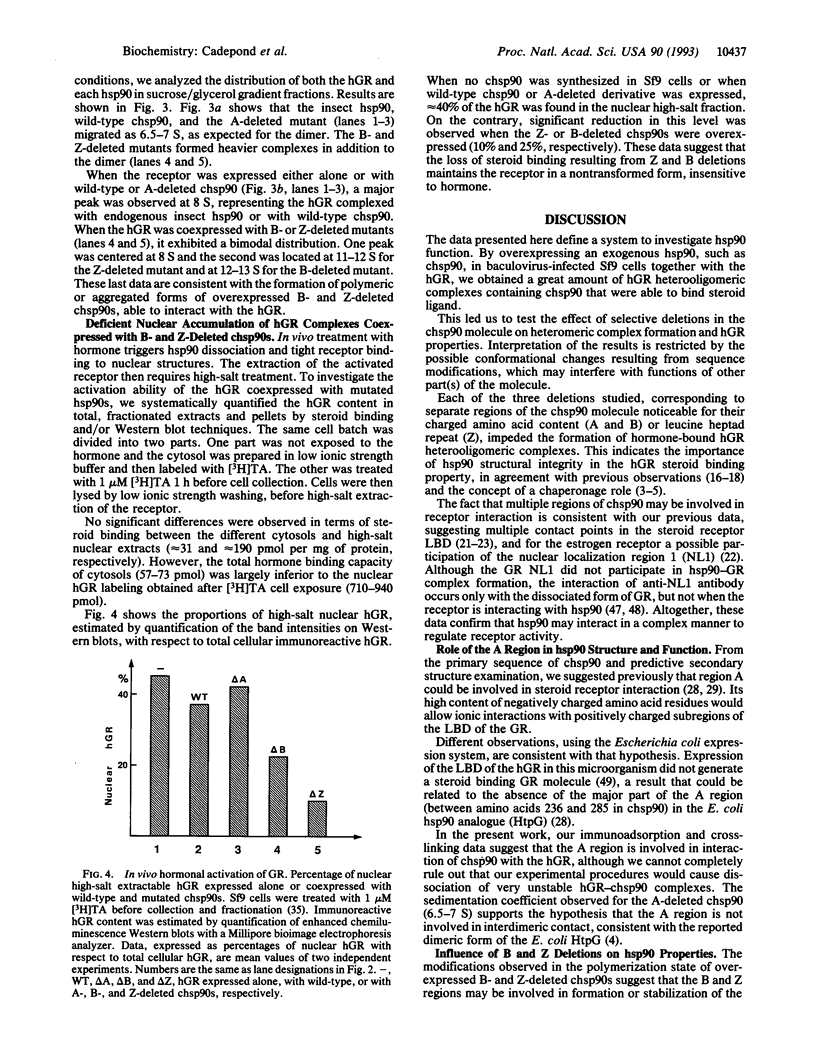
Coexpression of the human glucocorticosteroid receptor (hGR) and chicken 90-kDa heat shock protein alpha (chsp90) in recombinant baculovirus-infected Sf9 cells is a system that provides a large quantity of wild-type chsp90-hGR complexes able to bind hormone ([3H]triamcinolone acetonide; TA), sedimenting at 8 S, and displaceable to 11 S by BF4 and D7 alpha anti-chsp90 monoclonal antibodies. Thus, we were able to examine the effects of selective chsp90 mutations on hetero-oligomeric complex formation. Two deletions involved hydrophilic regions, A between amino acids 221 and 290 and B between amino acids 530 and 581, and the third, Z, removed a central leucine heptad repeat region (amino acids 392-419). When these chsp90 mutants were expressed, the lack of displacement of [3H]TA receptor complexes on sucrose gradient by specific chsp90 antibodies was consistent with the formation of [3H]TA receptor complexes containing only endogenous insect hsp90. By using an immunoadsorption method and sedimentation analysis, we found that the deletion of region A precluded the interaction of chsp90 with the hGR, while B and Z deletions led to formation of abnormal complexes with the hGR, which displayed large forms (> 10 S), were unable to bind hormone, and apparently formed only small amounts of tightly bound nuclei hGR upon in vivo hormone treatment. As a whole, the data are consistent with distinct roles of hsp90 regions in hGR function.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Binart N., Chambraud B., Dumas B., Rowlands D. A., Bigogne C., Levin J. M., Garnier J., Baulieu E. E., Catelli M. G. The cDNA-derived amino acid sequence of chick heat shock protein Mr 90,000 (HSP 90) reveals a "DNA like" structure: potential site of interaction with steroid receptors. Biochem Biophys Res Commun. 1989 Feb 28;159(1):140–147. doi: 10.1016/0006-291x(89)92415-7. [DOI] [PubMed] [Google Scholar]
- Binart N., Chambraud B., Levin J. M., Garnier J., Baulieu E. E. A highly charged sequence of chick hsp90: a good candidate for interaction with steroid receptors. J Steroid Biochem. 1989;34(1-6):369–374. doi: 10.1016/0022-4731(89)90110-6. [DOI] [PubMed] [Google Scholar]
- Borkovich K. A., Farrelly F. W., Finkelstein D. B., Taulien J., Lindquist S. hsp82 is an essential protein that is required in higher concentrations for growth of cells at higher temperatures. Mol Cell Biol. 1989 Sep;9(9):3919–3930. doi: 10.1128/mcb.9.9.3919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bresnick E. H., Dalman F. C., Sanchez E. R., Pratt W. B. Evidence that the 90-kDa heat shock protein is necessary for the steroid binding conformation of the L cell glucocorticoid receptor. J Biol Chem. 1989 Mar 25;264(9):4992–4997. [PubMed] [Google Scholar]
- Brugge J. S., Erikson E., Erikson R. L. The specific interaction of the Rous sarcoma virus transforming protein, pp60src, with two cellular proteins. Cell. 1981 Aug;25(2):363–372. doi: 10.1016/0092-8674(81)90055-6. [DOI] [PubMed] [Google Scholar]
- Cadepond F., Gasc J. M., Delahaye F., Jibard N., Schweizer-Groyer G., Segard-Maurel I., Evans R., Baulieu E. E. Hormonal regulation of the nuclear localization signals of the human glucocorticosteroid receptor. Exp Cell Res. 1992 Jul;201(1):99–108. doi: 10.1016/0014-4827(92)90352-9. [DOI] [PubMed] [Google Scholar]
- Cadepond F., Schweizer-Groyer G., Segard-Maurel I., Jibard N., Hollenberg S. M., Giguère V., Evans R. M., Baulieu E. E. Heat shock protein 90 as a critical factor in maintaining glucocorticosteroid receptor in a nonfunctional state. J Biol Chem. 1991 Mar 25;266(9):5834–5841. [PubMed] [Google Scholar]
- Callebaut I., Renoir J. M., Lebeau M. C., Massol N., Burny A., Baulieu E. E., Mornon J. P. An immunophilin that binds M(r) 90,000 heat shock protein: main structural features of a mammalian p59 protein. Proc Natl Acad Sci U S A. 1992 Jul 15;89(14):6270–6274. doi: 10.1073/pnas.89.14.6270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Catelli M. G., Binart N., Feramisco J. R., Helfman D. M. Cloning of the chick hsp 90 cDNA in expression vector. Nucleic Acids Res. 1985 Sep 11;13(17):6035–6047. doi: 10.1093/nar/13.17.6035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Catelli M. G., Binart N., Jung-Testas I., Renoir J. M., Baulieu E. E., Feramisco J. R., Welch W. J. The common 90-kd protein component of non-transformed '8S' steroid receptors is a heat-shock protein. EMBO J. 1985 Dec 1;4(12):3131–3135. doi: 10.1002/j.1460-2075.1985.tb04055.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Csermely P., Kajtár J., Hollósi M., Jalsovszky G., Holly S., Kahn C. R., Gergely P., Jr, Söti C., Mihály K., Somogyi J. ATP induces a conformational change of the 90-kDa heat shock protein (hsp90). J Biol Chem. 1993 Jan 25;268(3):1901–1907. [PubMed] [Google Scholar]
- Denis M., Gustafsson J. A., Wikström A. C. Interaction of the Mr = 90,000 heat shock protein with the steroid-binding domain of the glucocorticoid receptor. J Biol Chem. 1988 Dec 5;263(34):18520–18523. [PubMed] [Google Scholar]
- Denis M., Wikström A. C., Gustafsson J. A. Subunit composition of the molybdate-stabilized non-activated glucocorticoid receptor from rat liver. J Steroid Biochem. 1988;30(1-6):271–276. doi: 10.1016/0022-4731(88)90105-7. [DOI] [PubMed] [Google Scholar]
- Elliston J. F., Beekman J. M., Tsai S. Y., O'Malley B. W., Tsai M. J. Hormone activation of baculovirus expressed progesterone receptors. J Biol Chem. 1992 Mar 15;267(8):5193–5198. [PubMed] [Google Scholar]
- Erdos T., Best-Belpomme M., Bessada R. A rapid assay for binding estradiol to uterine receptor(s). Anal Biochem. 1970 Oct;37(2):244–252. doi: 10.1016/0003-2697(70)90044-8. [DOI] [PubMed] [Google Scholar]
- Gething M. J., Sambrook J. Protein folding in the cell. Nature. 1992 Jan 2;355(6355):33–45. doi: 10.1038/355033a0. [DOI] [PubMed] [Google Scholar]
- Giguère V., Hollenberg S. M., Rosenfeld M. G., Evans R. M. Functional domains of the human glucocorticoid receptor. Cell. 1986 Aug 29;46(5):645–652. doi: 10.1016/0092-8674(86)90339-9. [DOI] [PubMed] [Google Scholar]
- Groyer A., Le Bouc Y., Joab I., Radanyi C., Renoir J. M., Robel P., Baulieu E. E. Chick oviduct glucocorticosteroid receptor. Specific binding of the synthetic steroid RU 486 and immunological studies with antibodies to chick oviduct progesterone receptor. Eur J Biochem. 1985 Jun 3;149(2):445–451. doi: 10.1111/j.1432-1033.1985.tb08945.x. [DOI] [PubMed] [Google Scholar]
- Groyer A., Schweizer-Groyer G., Cadepond F., Mariller M., Baulieu E. E. Antiglucocorticosteroid effects suggest why steroid hormone is required for receptors to bind DNA in vivo but not in vitro. Nature. 1987 Aug 13;328(6131):624–626. doi: 10.1038/328624a0. [DOI] [PubMed] [Google Scholar]
- Hutchison K. A., Czar M. J., Pratt W. B. Evidence that the hormone-binding domain of the mouse glucocorticoid receptor directly represses DNA binding activity in a major portion of receptors that are "misfolded" after removal of hsp90. J Biol Chem. 1992 Feb 15;267(5):3190–3195. [PubMed] [Google Scholar]
- Joab I., Radanyi C., Renoir M., Buchou T., Catelli M. G., Binart N., Mester J., Baulieu E. E. Common non-hormone binding component in non-transformed chick oviduct receptors of four steroid hormones. 1984 Apr 26-May 2Nature. 308(5962):850–853. doi: 10.1038/308850a0. [DOI] [PubMed] [Google Scholar]
- Lindquist S., Craig E. A. The heat-shock proteins. Annu Rev Genet. 1988;22:631–677. doi: 10.1146/annurev.ge.22.120188.003215. [DOI] [PubMed] [Google Scholar]
- Mendel D. B., Ortí E. Isoform composition and stoichiometry of the approximately 90-kDa heat shock protein associated with glucocorticoid receptors. J Biol Chem. 1988 May 15;263(14):6695–6702. [PubMed] [Google Scholar]
- Miyata Y., Yahara I. The 90-kDa heat shock protein, HSP90, binds and protects casein kinase II from self-aggregation and enhances its kinase activity. J Biol Chem. 1992 Apr 5;267(10):7042–7047. [PubMed] [Google Scholar]
- Nadeau K., Das A., Walsh C. T. Hsp90 chaperonins possess ATPase activity and bind heat shock transcription factors and peptidyl prolyl isomerases. J Biol Chem. 1993 Jan 15;268(2):1479–1487. [PubMed] [Google Scholar]
- Nemoto T., Ohara-Nemoto Y., Denis M., Gustafsson J. A. The transformed glucocorticoid receptor has a lower steroid-binding affinity than the nontransformed receptor. Biochemistry. 1990 Feb 20;29(7):1880–1886. doi: 10.1021/bi00459a031. [DOI] [PubMed] [Google Scholar]
- Ohara-Nemoto Y., Strömstedt P. E., Dahlman-Wright K., Nemoto T., Gustafsson J. A., Carlstedt-Duke J. The steroid-binding properties of recombinant glucocorticoid receptor: a putative role for heat shock protein hsp90. J Steroid Biochem Mol Biol. 1990 Nov 30;37(4):481–490. doi: 10.1016/0960-0760(90)90391-w. [DOI] [PubMed] [Google Scholar]
- Oppermann H., Levinson W., Bishop J. M. A cellular protein that associates with the transforming protein of Rous sarcoma virus is also a heat-shock protein. Proc Natl Acad Sci U S A. 1981 Feb;78(2):1067–1071. doi: 10.1073/pnas.78.2.1067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perdew G. H., Whitelaw M. L. Evidence that the 90-kDa heat shock protein (HSP90) exists in cytosol in heteromeric complexes containing HSP70 and three other proteins with Mr of 63,000, 56,000, and 50,000. J Biol Chem. 1991 Apr 15;266(11):6708–6713. [PubMed] [Google Scholar]
- Picard D., Khursheed B., Garabedian M. J., Fortin M. G., Lindquist S., Yamamoto K. R. Reduced levels of hsp90 compromise steroid receptor action in vivo. Nature. 1990 Nov 8;348(6297):166–168. doi: 10.1038/348166a0. [DOI] [PubMed] [Google Scholar]
- Pratt W. B., Jolly D. J., Pratt D. V., Hollenberg S. M., Giguere V., Cadepond F. M., Schweizer-Groyer G., Catelli M. G., Evans R. M., Baulieu E. E. A region in the steroid binding domain determines formation of the non-DNA-binding, 9 S glucocorticoid receptor complex. J Biol Chem. 1988 Jan 5;263(1):267–273. [PubMed] [Google Scholar]
- Radanyi C., Renoir J. M., Sabbah M., Baulieu E. E. Chick heat-shock protein of Mr = 90,000, free or released from progesterone receptor, is in a dimeric form. J Biol Chem. 1989 Feb 15;264(5):2568–2573. [PubMed] [Google Scholar]
- Scherrer L. C., Picard D., Massa E., Harmon J. M., Simons S. S., Jr, Yamamoto K. R., Pratt W. B. Evidence that the hormone binding domain of steroid receptors confers hormonal control on chimeric proteins by determining their hormone-regulated binding to heat-shock protein 90. Biochemistry. 1993 May 25;32(20):5381–5386. doi: 10.1021/bi00071a013. [DOI] [PubMed] [Google Scholar]
- Schuh S., Yonemoto W., Brugge J., Bauer V. J., Riehl R. M., Sullivan W. P., Toft D. O. A 90,000-dalton binding protein common to both steroid receptors and the Rous sarcoma virus transforming protein, pp60v-src. J Biol Chem. 1985 Nov 15;260(26):14292–14296. [PubMed] [Google Scholar]
- Smith D. F., Schowalter D. B., Kost S. L., Toft D. O. Reconstitution of progesterone receptor with heat shock proteins. Mol Endocrinol. 1990 Nov;4(11):1704–1711. doi: 10.1210/mend-4-11-1704. [DOI] [PubMed] [Google Scholar]
- Smith D. F., Stensgard B. A., Welch W. J., Toft D. O. Assembly of progesterone receptor with heat shock proteins and receptor activation are ATP mediated events. J Biol Chem. 1992 Jan 15;267(2):1350–1356. [PubMed] [Google Scholar]
- Smith D. F., Toft D. O. Steroid receptors and their associated proteins. Mol Endocrinol. 1993 Jan;7(1):4–11. doi: 10.1210/mend.7.1.8446107. [DOI] [PubMed] [Google Scholar]
- Urda L. A., Yen P. M., Simons S. S., Jr, Harmon J. M. Region-specific antiglucocorticoid receptor antibodies selectively recognize the activated form of the ligand-occupied receptor and inhibit the binding of activated complexes to deoxyribonucleic acid. Mol Endocrinol. 1989 Feb;3(2):251–260. doi: 10.1210/mend-3-2-251. [DOI] [PubMed] [Google Scholar]
- Vecsei P., Abdelhamid S., Mittelstädt G. V., Lichtwald K., Haack D., Lewicka S. Aldosterone metabolites and possible aldosterone precursors in hypertension. J Steroid Biochem. 1983 Jul;19(1A):345–351. [PubMed] [Google Scholar]
- Vialard J., Lalumière M., Vernet T., Briedis D., Alkhatib G., Henning D., Levin D., Richardson C. Synthesis of the membrane fusion and hemagglutinin proteins of measles virus, using a novel baculovirus vector containing the beta-galactosidase gene. J Virol. 1990 Jan;64(1):37–50. doi: 10.1128/jvi.64.1.37-50.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiech H., Buchner J., Zimmermann R., Jakob U. Hsp90 chaperones protein folding in vitro. Nature. 1992 Jul 9;358(6382):169–170. doi: 10.1038/358169a0. [DOI] [PubMed] [Google Scholar]