Abstract
β-globin gene disorders are the most prevalent inherited diseases worldwide and result from abnormal β-globin synthesis or structure. Novel therapeutic approaches are being developed in an effort to move beyond palliative management. Gene therapy, by ex vivo lentiviral transfer of a therapeutic β-globin gene derivative (βAT87Q-globin) to hematopoietic stem cells, driven by cis-regulatory elements that confer high, erythroid-specific expression, has been evaluated in human clinical trials over the past 8 years. βAT87Q-globin is used both as a strong inhibitor of HbS polymerization and as a biomarker. While long-term studies are underway in multiple centers in Europe and in the United States, proof-of-principle of efficacy and safety has already been obtained in multiple patients with β-thalassemia and sickle cell disease.
Introduction
Sickle cell disease (SCD) and β-thalassemia major (β-TM), the latter defined clinically as transfusion-dependent cases regardless of the underlying genotype, are the most common monogenic disorders worldwide with approximately 400,000 affected conceptions or births each year.1,2 These disorders fall into two large groups of β-globin gene mutations that result in either abnormal hemoglobin structure (SCD) or massively reduced/absent production of β-globin chains (β-TM). The clinical manifestations of these inherited disorders typically appear several months after birth, when gene expression switches from the fetal γ-globin chain, which forms fetal hemoglobin (HbF), to the adult βA-globin chain forming hemoglobin (HbA).3 Note that adult βA-globin is also simply referred to as β-globin when no confusion with other β-like globin chains is possible. In low-income countries, most of the affected children succumb in early childhood, whereas in developed countries, neonatal diagnosis and supportive care have greatly improved survival. However, even with modern and specialized care, life expectancy is still reduced by several decades,4–6 and quality of life greatly suffers.7–9
Allogenic hematopoietic stem cell transplantation (AHSCT) as a curative option is currently recommended for β-TM if a human leukocyte antigen (HLA)-matched sibling donor is available.10,11 Disease-free survival after AHSCT is approximately 88% in pediatric subjects12 and 65% in adults.13 Recent transplantation trials conducted during the last 15 years for young SCD patients reported a disease free-survival rate of 85–90%.11 However, fewer than 25% of patients have a suitable intrafamilial donor.14 In the absence of matched sibling donors, AHSCT from HLA-matched unrelated or haploidentical donors or minimally mismatched cord blood products may be used as the source of donor cells, although these approaches exhibit a lower benefit/risk ratio and thus remain experimental.15 Although outcomes are improving, AHSCT continues to carry a substantial risk of severe adverse events and mortality,16,17 both increasing with recipient age and disease severity.18,19 Severe adverse events include graft failure, graft-versus-host disease (GVHD), early or late side effects from conditioning regimens that are both myeloablative and immunosuppressive (infections, hemorrhages, secondary malignancies), and aggravation of preexisting organ damage.20–22
For patients who lack a suitable HLA-matched donor, ex vivo gene therapy using autologous HSCs brings hope as a potential curative treatment option. If proven safe and effective, gene therapy may then be extended to most β-TM and severe SCD patients, as there is no concern here for histocompatibility-related complications and the conditioning regimen does not need to include immunosuppressive drugs. However, gene therapy shares with AHSCT the risks associated with the transplant procedure and the toxicity of the myeloablative agent. Globin gene addition to HSCs by means of lentiviral vectors (LVs) is a promising approach under investigation (Fig. 1). Several clinical trials of gene therapy for β-TM and severe SCD are ongoing in France and in the United States (Tables 1 and 2). Other recent approaches under study for the gene therapy of the β-hemoglobinopathies include pharmacological23 or genetic induction of γ-globin production through interference with the BCL11A pathway24,25 or disruption of the BCL11A erythroid enhancer by CRISPR/CAS9 technology as well as zinc finger or transcription activator-like effector nuclease,26,27 or even attempts at repairing the defective βA-globin gene in HSCs by genome editing.28–30 These approaches are at the stage of collecting evidence of efficacy in relevant cell and animal models and scoring potentially untoward off-target events. Even if these approaches are ultimately successful, gene addition has the advantage of making use of a single product applicable to all cases of β-TM and SCD regardless of the genotype, whereas gene repair will have to tackle separately the hundreds of mutations known to cause β-thalassemia in humans.
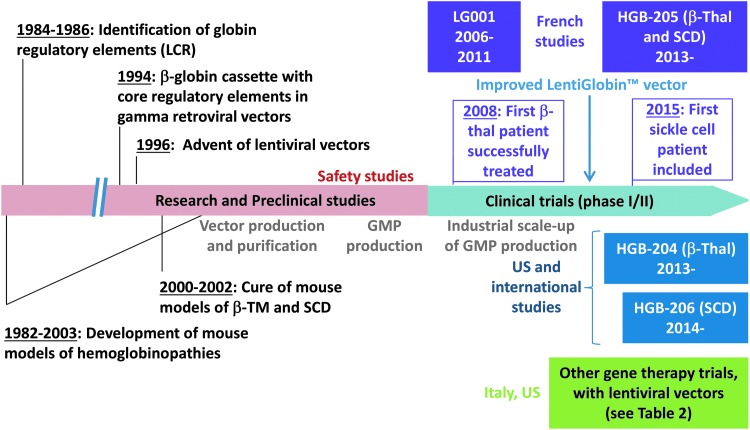
Figure 1.
The milestones of ex vivo gene therapy research and development for hemoglobin disorders. LG001, HGB204, HGB205, and HGB206 clinical studies are conducted with our lentiviral vectors (Table 1). Gene therapy trials using other lentiviral vectors are summarized in Table 2.
Table 1.
Human clinical trials to date for gene therapy of β-TM and/or severe SCD in France and internationally with our lentiviral vectors (HPV569 and then BB305)
| Gene | Vector | Location | Protocol number | Sponsor | Condition | Conditioning | Intervention | Phase | Title | Start date | Results as of December 2015 | Estimated primary completion |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| βA-T87Q-globin | HPV569 | France | LG001 study159 | bluebird bio (formerly Genetix Pharmaceuticals) | β-thalassemia major and severe sickle cell disease | Myeloablative conditioning | Transplantation of HSCs transduced ex vivo with a lentiviral vector | I/II | A Phase I/II Open Label Study with Anticipated Benefit Evaluating Genetic Therapy of the β-Hemoglobinopathies (Sickle Cell Anemia and β-Thalassemia Major) by Transplantation of Autologous CD34+ Stem Cells Modified ex-vivo with a Lentiviral bA-T87Q Globin (Lentiglobin™) Vector | Sept 2006 | First βE/β0-treated patient in the world, independent of transfusions for more than 7 years | Terminated |
| βA-T87Q-globin | BB305 | France | NCT02151526 (HGB-205 study)159 | bluebird bio | β-thalassemia major and severe sickle cell disease | Myeloablative conditioning | Transplantation of HSCs transduced ex vivo with a lentiviral vector | I/II | A Phase 1/2 Open Label Study Evaluating the Safety and Efficacy of Gene Therapy of the β-Hemoglobinopathies (Sickle Cell Anemia and β-Thalassemia Major) by Transplantation of Autologous CD34+ Stem Cells Transduced Ex Vivo with a Lentiviral βA-T87Q-Globin Vector (LentiGlobin® BB305 Drug Product) | July 2013 | First βS/βS-treated patient in the world, with >50% βT87Q-globin2 βE/β0 patients independent of transfusions, 1 β0/β0 treated recently | December 2017 |
| βA-T87Q-globin | BB305 | USA, Thailand, Australia | NCT01745120 (HGB-204 study)163 | bluebird bio | β-Thalassemia major | Myeloablative conditioning | Transplantation of HSCs transduced ex vivo with a lentiviral vector | I/II | A Phase 1/2 Open Label Study Evaluating the Safety and Efficacy of Gene Therapy in Subjects with β-Thalassemia Major by Transplantation of Autologous CD34+ Cells Transduced Ex Vivo with a Lentiviral β-A(T87Q)-Globin Vector (LentiGlobin® BB305 Drug Product) | August 2013 | 10 subjects infused: 5 β0/β0, 3 β0/βE, 1 β0/β+, and 1 with another genotypeTransfusion independence for the majority | September 2017 |
| βA-T87Q-globin | BB305 | USA | NCT02140554 (HGB-206 study)164 | bluebird bio | Severe sickle cell disease | Myeloablative conditioning | Transplantation of HSCs transduced ex vivo with a lentiviral vector | I | Phase 1 Study Evaluating Gene Therapy by Transplantation of Autologous CD34+ Stem Cells Transduced Ex Vivo with the LentiGlobin BB305 Lentiviral Vector in Subjects with Severe Sickle Cell Disease | August 2014 | 3 βS/βS subjects treated. No clinical results available yet | March 2019 |
Table 2.
Human clinical trials for gene therapy of β-TM or severe SCD with other lentiviral vectors
| Gene | Vector | Location | Protocol number | Sponsor | Condition | Conditioning | Intervention | Phase | Title | Start date | Results | Estimated primary completion |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| β-globin | TNS9.3.55 | USA | NCT01639690165 | Memorial Sloan Kettering Cancer Center | β-Thalassemia major | Partial cytoreduction (Bu 8 mg/kg) for 3 patients, myeloablative conditioning (Bu 14 mg/kg) for 1 patient | Transplantation of HSCs transduced ex vivo with a lentiviral vector | I | A Phase I Clinical Trial for the Treatment of β-Thalassemia Major with Autologous CD34+ Hematopoietic Progenitor Cells Transduced with TNS9.3.55 a Lentiviral Vector Encoding the Normal Human β-Globin Gene | July 2012 | Four patients treated. Three β0/β+ and one β0/β0. One patient had a significant decrease in transfusion requirements. | July 2016 |
| γ-globin | sGbG | USA | NCT02186418a | Children's Hospital Medical Center, Cincinnati | Severe sickle cell disease | Unknown | Transplantation of HSCs transduced ex vivo with a lentiviral vector | I/II | Gene Transfer for Patients with Sickle Cell Disease Using a Gamma Globin Lentivirus Vector: An Open Label Phase I/II Pilot Study | July 2014 | No results available yet | July 2017 |
| βAS3-globin (T87Q, G16D, E22A) | Lenti/βAS3-FB | USA | NCT02247843a | University of California, Children's Hospital, Los Angeles | Severe sickle cell disease | Unknown | Transplantation of HSCs transduced ex vivo with a lentiviral vector | I | Clinical Research Study of Autologous Bone Marrow Transplantation for Sickle Cell Disease (SCD) Using Bone Marrow CD34+ Cells Modified with the Lenti/βAS3-FB Lentiviral Vector | August 2014 | No results available yet | April 2017 |
| β-globin | GLOBE | Italy | NCT02453477166,a | IRCCS San Raffaele | β-Thalassemia major | Myeloablative conditioning | Transplantation of HSCs transduced ex vivo with a lentiviral vector (intrabone injection) | I/II | A Phase I/II Study Evaluating Safety and Efficacy of Autologous Hematopoietic Stem Cells Genetically Modified with GLOBE Lentiviral Vector Encoding for the Human Beta Globin Gene for the Treatment of Patients Affected by Transfusion Dependent Beta-Thalassemia | May 2015 | First patient recently treated | August 2019 |
While we recognize the important contributions from other laboratories, this review will largely focus on our own experience with the development of the HPV569 and BB305 vectors, self-inactivating (SIN) LVs bearing a human βA-globin mini-gene encoding an “antisickling” β-globin with amino-acid substitution (T87Q) and driven by cis-regulatory elements of the human β-globin gene locus (promoter, locus control region [LCR] elements), designed in the Leboulch laboratory in collaboration with bluebird bio (formerly Genetix Pharmaceuticals).
The β-Hemoglobinopathies
The β-thalassemias
β-TM is a microcytic hemolytic anemia that is rapidly fatal in the absence of palliative life-long red blood cell (RBC) transfusions and iron chelation.31 The disease results from absence (β0) or massive reduction (e.g., β+, βE/β0) in βA-globin gene expression. While complete absence of βA-globin expression is not compatible with RBC production, even a severe diminution in its expression also results in β-TM. This is because the protein component of adult hemoglobin comprises two α- and two β-globin chains, and massive decrease in β-globin expression results in a relative excess of unpaired and toxic free α-chains. Free α-chains precipitate, damage the cell membrane,32 and sequester the chaperone heat shock protein 70 (HSP70), which is no longer available to protect the GATA-1 erythroid maturation transcription factor from caspase-3 cleavage.33 α/β-chain imbalance results in cell death and ineffective erythropoiesis (dyserythropoiesis)34 in bone marrow as well as reduced erythrocyte lifespan and hemolysis.35
Among the severe β-thalassemias, the βE/β0 genotype presents interesting features and is highly frequent. In this disease, one β-globin allele is completely silent (β0) while the other encodes the missense mutation (26AAG>GAG) that results both in an amino-acid substitution (GLU26LYS) and in abnormal RNA splicing.36 Part of the transcribed βE mRNA is not translated because of abnormal splicing, whereas the small amount of mutated (GLU26LYS) protein produced is functional but slightly unstable.37 Thus, when the βE allele is compounded with a β0 allele, a profound decrease in β-like globin production is observed.38 Approximately 50% of patients with βE/β0-thalassemia have transfusion-dependent β-TM, depending on the patient's genetic makeup in modulators of disease severity.39 Hemoglobin E is one of the world's most common mutations40 and is especially prevalent in Southeast Asia. The frequency can approach 60% of the population in some parts of Thailand, Cambodia, and Laos,40 where it is estimated that 100,000 new cases of βE/β0 are expected in the next few decades. It is also found at high frequency in other Asian nations (India, Sri Lanka, Malaysia, and southern China) and increasingly in Europe and North America through immigration.41,42
Sickle cell disease
SCD is a multisystem disorder that results from a single mutation in the βA-globin gene, changing a glutamic acid into a valine at the sixth position of the β-globin chain, known as the βS mutation.43 In homozygous patients (βS/βS), the most frequent genotype, or in the compound heterozygous states (βS/βC, βS/βThalassemia) of SCD, the chronic hemolytic anemia is complicated by painful vaso-occlusive crises (VOC), acute chest syndrome (ACS), increased risk of infections, as well as organ vasculopathy and dysfunctions, particularly affecting brain, kidney, lung, heart, bone, eye, and the skin.44 At the protein level, the single amino-acid substitution at codon 6 (βS chain) prompts the formation of HbS polymers at low oxygen pressure,45 the rate of which is proportional to the corpuscular concentration of HbS, the extent of hemoglobin deoxygenation in the microcirculation,46 and the quantity of HbF, which inhibits HbS polymerization.47 Vaso-occlusion, resulting from erythrocyte stiffness and the adhesive interaction of abnormal erythrocytes to endothelial cells and leukocytes,48 leads to organ infarction and inflammation, which in turn enhances adhesive interactions,49 microvascular occlusion, and ischemia. The increased number of activated white blood cells produces high levels of reactive oxygen species (ROS) and other factors contributing actively to micro-vessel clogging, increased local hypoxia, and increased proportions of rigid RBCs containing HbS polymers.50 The cycles of ischemia/reperfusion cause oxidative stress51 that contributes to the proinflammatory phenotype,52 worsening the vicious circle. Furthermore, acute and chronic intravascular hemolysis impairs endothelial functions, causing progressive systemic and pulmonary vasculopathy.53
Challenges for Effective Gene Therapy
The therapeutic βA(T87Q)-globin gene
With regard to the gene therapy of SCD by gene addition, wild-type human βA-globin is a relatively weak inhibitor of HbS polymerization because it acts by mere dilution. When PO2 is relatively low, as in capillary vessels, phenylalanine 85 (βF85) and leucine 88 (βL88) form an acceptor hydrophobic pocket on one α2βS2 tetramer that binds to the mutated valine (βV6) of a close α2βS2 complex.54 This phenomenon is responsible for HbS fiber polymerization after further aggregation and fiber elongation.46 HbA (α2βA2) has these same hydrophobic residues and thus does not inhibit polymer formation when it is incorporated.54–56 In contrast, other human “β-like”-globin chains such as γ-globin and δ-globin are stronger inhibitors.57,58 This inhibition of polymerization is best exemplified by cases of hereditary persistence of HbF (HPFH), especially of the pan-cellular type (within all erythrocytes), where as little as 20% HbF is sufficient to inhibit HbS formation in vivo and to alleviate substantially the clinical manifestations of SCD in homozygous βS/βS patients.59,60 In these cases, the γ-chain excludes the heterotetramer α2γβS from the HbS polymer.61–63
However, neither γ- or δ-globin genes are highly expressed in the “adult” (postglobin switch) RBC environment. Because the βA-, γ-, and δ-globin chains are co-linear and comprise only a few differences in amino-acid residues between them, especially between βA and δ with only 10 differences out of 146, biochemical studies were performed to determine which positions were critical to inhibit HbS polymerization. Within the acceptor hydrophobic pocket, a critical residue differs at position 87 between βA (T87) and γ or δ (Q87), and has been determined to be responsible for most of the inhibitory effect of γ- or δ-globins on HbS polymerization.55 A few other residues co-contribute to a lesser degree, and in particular Ala-22.64 In an effort to express a strong antisickling β-globin derivative at high levels in adult RBCs, a modified βA-globin gene was designed (Leboulch Laboratory) that comprises a point mutation within a human β-globin mini-gene suitable for retroviral transfer, so that the expressed therapeutic protein is βA(T87Q). The resulting βA(T87Q)-globin chain appears as efficient as γ-globin to inhibit HbS polymerization in biochemical assays.65 The oxygen affinity of the mutated tetramer (HbAT87Q: α2βT87Q2) is in the range of that of HbA,65 whereas the oxygen affinity of HBF is substantially higher than that of HbA.66
Furthermore, the β-globin T87Q is a valuable biomarker of biological efficacy in human clinical trials. The βT87Q-globin chain can be distinguished and quantified from βA- and γ-globin chains by high-performance reverse-phase liquid chromatography (HPLC).67,68 In the case of β+ thalassemia or when RBC transfusions are still provided, there would otherwise be no possibility of quantifying vector-derived gene expression if wild-type βA-globin was used as the therapeutic gene.
Therapeutic levels of βA(T87Q)-globin gene expression
The threshold level of therapeutic βA(T87Q)-globin expression required for effective gene therapy of β-TM will vary depending on residual levels, if any, of endogenous β-globin production. It will also depend on genetic modulators that contribute to reducing the severity of the disease in each individual patient. These modulators include associated α-thalassemia69,70 or the ability to produce substantial amounts of γ-globin after birth,71,72 both reducing the magnitude of free α-chains in erythroid cells and the severity of the disease.
With respect to SCD, one can surmise that the antisickling properties of βA(T87Q)-globin will be comparable to or slightly lower than that of γ-globin.65 HPFH with HbF levels of 30% or even lower are consistently associated with complete absence of clinical and biological signs of SCD in otherwise homozygous βS/βS patients, when HbF expression is well distributed among RBCs (pan-cellular HPFH).73 When HbF distribution is more heterogeneous, the antisickling effect is less pronounced, although SCD symptoms of homozygous βS/βS were alleviated in a few reported cases of stable mixed chimerism as low as 11% after AHSCT.74 It is thus likely that expression of βA(T87Q)-globin between 20% and 30%, preferably with moderate variegation in expression will meet the minimum threshold to prevent most of the clinical manifestations and complications of SCD in homozygous βS/βS patients.
Cis-regulatory control elements to be incorporated in vectors
Most of the genetic elements that control tissue specificity and developmental switching of β-globin gene expression are located within or near the transcription unit in the promoter region, downstream of the polyadenylation signal, and within the second intron.75–78 Nevertheless, introducing these elements to control β-globin gene expression in gamma-retroviral vectors (γ-RV) has resulted in very low levels of human β-globin gene expression in erythroid cells of mice transplanted with transduced HSCs.79,80 The discovery of chromatin domains, referred to as DNase I hypersensitive sites (HS), several kilobases upstream of the β-globin gene,81,82 has shed light on the regulatory organization of the locus. The ability of the 15 kb LCR to confer physiological levels of human β-globin, when inserted immediately 5′ to the β-globin gene in transgenic mice,83,84 has yielded clues for designing effective expression systems. The most important HS sites to cis-link to the human β-globin gene are HS2, HS3, and HS4 that have enhancer activity when tested individually in cell culture and in transgenic mice.85,86 Several laboratories have undertaken to reduce the size of each of the HS sites while maintaining most of their enhancer effect. However, while the so-called “core elements” of 250–350 bp long show useful activity when combined in γ-RV,87–89 expanded elements appear necessary for near-optimal enhancer effect.84,85,90,91
Gene transfer vectors suitable for the gene therapy of the β-hemoglobinopathies
Sustained gene therapy of inherited hematological disorders by ex vivo gene addition requires integration of the vector in the chromosomes of HSCs to obtain proper replication and segregation in the daughter cells for the lifespan of the recipient. To date, only retroviral vectors have reproducibly shown this capability in animal models. Efforts have first made use of γ-RV derived from the Moloney murine leukemia virus (M-MuLV). The first M-MuLV vectors containing a human β-globin gene and its promoter were reported by the Mulligan and Nienhuis laboratories.92,93 However, β-globin expression was erythroid specific but well below a possible therapeutic effect.79,80,92,93
Incorporation of core LCR elements resulted in low-titer γ-RV that were also highly unstable with multiple rearrangements of the transferred proviral structures.94,95 Reducing the size of the LCR to minimal elements is unsatisfactory as β-globin expression levels are too low.96,97 Leboulch et al.88 identified untoward polyadenylation and splicing of the genomic viral RNA before packaging as the main mechanisms of both low titers and provirus rearrangements (internal splicing and polyadenylation). LCR elements, the promoter, and the actual globin gene were placed in reverse orientation to avoid splicing of the bona fide introns before packaging, which resulted in the unmasking of consensus polyadenylation and splicing signals along the vector genomic viral RNA. A small internal deletion of sequence repeats within the second (minigene), together with site-directed mutagenesis eliminating those sequences, resulted in stable proviral transmission.88 Sadelain et al.89 achieved a similar result by applying the same internal deletion within the second intron while permutating LCR elements, possibly resulting in a conformational change in the viral genomic RNA that also inhibited untoward splicing. In spite of these advances, vector-encoded β-globin remained below therapeutic levels, and transduction of HSCs by γ-RV was suboptimal in vivo.98,99
Grants from the National Institutes of Health (NIH) were awarded in the mid-1990s to focus on making further progress in this area. Sadelain and Leboulch (together with R. Nagel, I. Mondon, C. Eaves, and K. Humphries) were awarded grants to focus on the gene therapy of β-TM and SCD, respectively. With the discovery that the Rev responsive element (RRE) system of lentiviruses, including the human immunodeficiency virus (HIV), allows for efficient nucleocytoplasmic export and subsequent packaging of full-length, unspliced genomic viral RNA100 and the subsequent advent of lentiviral vectors (LVs),101,102 there appeared a great opportunity to apply this class of vectors to the complex genomic β-globin structures. An additional benefit is that LVs pseudotyped with the envelope of the G protein of the vesicular stomatitis virus (VSV-G) are amenable to concentration and are much more effective than γ-RV at transducing cells arrested at the G1-S boundary of the cell cycle101–103 and in quiescent HSCs.104,105 Sadelain and colleagues published the first correction of murine β-thalassemia by an LV containing the β-globin mini-gene described above,106,107 although with substantially larger LCR elements than those incorporated in previous γ-RV (LV referred to as TNS9). Leboulch and colleagues published the first correction of transgenic mouse models of SCD,65 by making use of their own vector, also containing the β-globin mini-gene together with similarly larger LCR elements (644 bp for HS2, 845 bp for HS3, and 1153 bp for HS4). Importantly, this LV contains the antisickling βA(T87Q)-globin gene. Human CD34+ cells transduced with a SIN version of this vector generated long-term hematopoietic reconstitution in immunodeficient mice,108 indicating effective transduction of human HSCs. This vector was modified further in the Leboulch lab to yield HPV569109 and BB305110 vectors, which have become the basis for our own clinical trials described in this review. We and other groups have subsequently published other β-globin- or γ-globin-based LVs and reported efficient transduction of mouse HSCs and high levels of β-globin expression.111–114
Regulatory Approval Process in the United States and the European Union
In spite of existing regulatory frameworks for gene therapy projects,115,116 there are still many unknowns, in part because of the lack of precedents. Innovative regulatory science can help foster a supportive ecosystem for gene therapy products and accelerate their development in a sustainable manner. An example of such a framework is the adaptive biomedical innovation framework.117
Frequent, science-based, transparent, and proactive interactions with regulators can also contribute to the overall development strategy for gene therapy products by reducing regulatory uncertainty. The majority of the regulatory mechanisms that exist to accelerate the development of medicinal products, including gene therapy products, require (1) targeting a serious or life-threatening condition with significant unmet medical need, (2) having the potential for outstanding efficacy with an acceptable safety profile, and (3) having a robust development plan. A clear mechanism of action and early proof-of-concept studies are also helpful.
Typically, the first interaction with regulatory agencies in the development of a gene therapy product is to discuss the nonclinical development (traditionally referred to as pharmacology and toxicology) and the design of the planned first-in-human study. In the United States, this first interaction is a pre–Investigational New Drug (IND) meeting with the Food and Drug Administration (FDA). In the European Union (EU), this first interaction can be a presubmission meeting before filing a clinical trial application (CTA) with national authorities and/or a scientific advice meeting with the European Medicines Agency (EMA). In addition, “pre-pre-IND” informal discussions with FDA and meetings with the EMA Innovation Task Force can be useful to discuss the early development of very innovative products, including innovative gene therapy products. Seeking orphan designation for products developed for rare diseases (defined as fewer than 5/10,000 affected individuals in the EU118 and fewer than 200,000 prevalent cases in the United States),119 as well as seeking EU gene therapy,120 can also be helpful early on for sponsors to define their products and their active substance, clarify their mechanism of action, and outline their potential for significant clinical benefit.
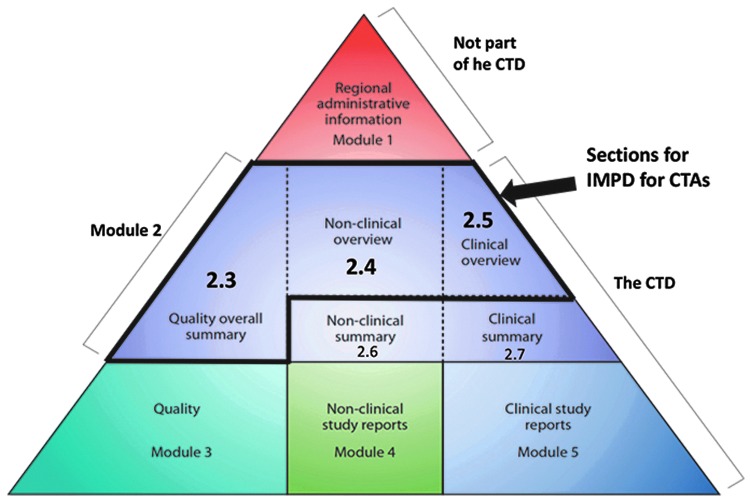
Clinical trials are regulated under Directive 2001/20/EC in the EU and under 21 CFR Part 312 in the United States. In the EU, a new regulation121 will come into force in 2016 that will replace the current directive. This regulation should streamline the process and allow for centralized submissions via a new electronic portal system to one reference member state. For gene therapy products, seeking approval to initiate a clinical trial in the EU currently requires filing a CTA including an investigational medicinal product dossier (IMPD) in each member state involved, and requires filing a genetically modified organism (“GMO”) submission sometimes in advance of the CTA. GMO submissions for gene therapy product are cumbersome and often unclear. In the future, specific applications customized for gene therapy medicinal products should be created and these should be managed by the same regulatory agencies receiving the CTA applications. In the United States, filing of an IND application is required. INDs follow the common technical dossier (CTD) format of the international conference harmonization (ICH). IMPDs headings are also consistent at a high level with the CTD organization (Fig. 2).122 IMPDs and INDs must contain information on manufacturing and quality, on the nonclinical studies conducted, and on the planned clinical trial, including a protocol and Investigator's Brochure. For gene therapy products, filing a pediatric investigational plan (PIP) with EMA early in the development is advisable if the targeted condition affects pediatric patients. During this process, sponsors can receive advice on their preliminary long-term development plans.
Figure 2.
The common technical document, adapted from www.ich.org/products/ctd.html The common technical document is organized into five modules. Module 1 is region specific, and modules 2–5 are intended to be common for all regions.
Other key regulatory mechanisms that can be leveraged to accelerate the development of gene therapy products include seeking fast-track designation or breakthrough therapy designation in the United States.123 In the EU, in addition to scientific advice, applying for an EMA nonclinical and quality/manufacturing certification procedure should be considered (for small and medium-size enterprises), as well as seeking the recently created priority medicines (PRIME) designation,91 and leveraging the flexibility of the risk-based approach and of the advanced therapy medicinal product (ATMP) Regulation No. 1394/2007/EC, particularly for the quality/manufacturing development.124,125
In the specific case study of HPV569 and BB305 drug candidates, a number of above-mentioned existing regulatory mechanisms were used to support and accelerate development, with an emphasis on evaluating early the entire life-span of the product in a creative way, including leveraging the potential of real-world evidence and “Safe Harbor” discussions with multiple stakeholders, including patients' representatives.
Looking forward, in order to obtain marketing authorization, a biological license application (BLA) must be submitted to the FDA in the United States in accordance with 21 CRF Part 601.126 In the EU, a marketing authorization application (MAA) must be submitted to the EMA in accordance with Directive 2001/83/EC and the advanced therapy medicinal product (ATMP) regulation 1394/2007/EC.127 In both regions, mechanisms exist to accelerate the review of the BLA or MAA: priority review and accelerated assessment, respectively.123,128 Mechanisms also exist to provide flexibility in the timing to obtain approval. In the United States, “accelerated approval” is the only mechanism based on the use of a surrogate or intermediate endpoint.123 In the EU, centralized marketing authorizations that are meant to accelerate access of medicines to patients in need for serious conditions can be granted “under exceptional circumstances” or as a “conditional” MAA.129,130
Gene therapy products provide unique opportunities to contribute to the evolution of regulatory science because of their transformative efficacy potential, their increasing complexity, and their pending individualized nature.
Hpv569 and Bb305 Vector Development
The HPV569 lentiviral vector was assessed in the first human trial LG001
The original LVs we used to achieve long-term correction of SCD and β-thalassemia in mice65,112 comprised (1) HIV1's RRE and central polypurine tract; (2) the human βA-globin gene, in reverse orientation, either wild-type or with the βT87Q mutation; (3) the human βA-globin promoter, and (4) a mini-LCR composed of HS2, HS3, and HS4 of 644, 845, and 1153 bp length, respectively.
To prepare for human clinical trials, safety modifications were made to the original vector, as follows, to yield the vector referred to as HPV569.109,112 These include mutating the GAG gene and deletion of the viral enhancer and promoter elements in the U3 region of the 3′LTR to generate a SIN vector (Fig. 3). The SIN modification reduces the likelihood of propagation of replication-competent recombinant LV in the vector producer and target cells,105,131 decreases the risk of mobilization of the vector genome upon HIV secondary infection,132 and reduces the residual activation of cellular oncogenes by enhancer/promoter activities of integrated LTRs.133,134 Another modification has concerned the U5 region of the 3′LTR, which was replaced by an artificial polyadenylation/termination signal derived from the rabbit β-globin gene.135 This latter modification yielded higher viral titers for the SIN versions of the HPV569 vector because of more efficient polyadenylation/termination of the viral transcript.136 In an effort to protect transduced cells against cis activation of adjacent genes by enhancer activities present within the β-globin LCR,137 two copies of the 250 bp core elements of the chicken β-globin chromatin insulator (chicken β-globin locus DNase I hypersensitive site 4: cHS4) were inserted in place of the deleted U3 region of the vector.
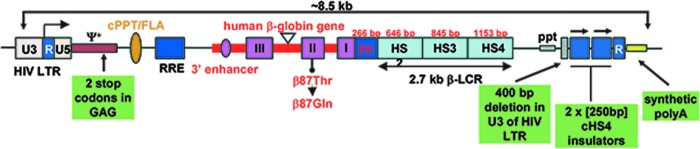
Figure 3.
Diagram of the HPV569 β-globin (βA-T87Q) lentiviral vector. The 3′ β-globin enhancer, the 372 bp IVS2 deletion, the βA-T87Q mutation (ACA[Thr] to CAG[Gln]), and DNase I hypersensitive sites (HS) 2, HS3, and HS4 of the human β-globin locus control region (LCR) are indicated. Safety modifications, including the 2 stop codons in the ψ+ packaging signal, the 400 bp deletion in the U3 of the right HIV LTR, the rabbit β-globin polyA signal, and the 2 × 250 bp cHS4 chromatin insulators, are indicated. In the BB305 lentiviral vector, U3 promoter/enhancer has been replaced by cytomeglovirus (CMV) promoter/enhancer and the 2 × 250 bp cHS4 insulator elements have been removed. cPPT/flap, central polypurine tract; HIV LTR, human immunodeficiency type-1 virus long terminal repeat; ppt, polypurine tract; RRE, Rev-responsive element; βp, human β-globin promoter.
The HPV569 vector109 was produced at clinical grade68 and used to evaluate therapeutic efficacy and safety in a mouse model of β-thalassemia.138 The gene-corrected mice showed normalization of their phenotype. Consistent with other LV studies,139,140 the HPV569 vector was shown to favor targeting of transcription units (≈70%) and gene-dense regions. Importantly, no enrichment of integration sites within proto-oncogenes was found posttransplantation, and no relationship was seen between the proximity of integration sites to oncogenes and site abundance in mice.138 A variety of complementary efficacy and safety studies were performed in human CD34+ cells from patients and in several mouse models before filing a CTA dossier with the French regulatory agency, as described in the section Regulatory approval process in the United States and the European Union. The first clinical trial with the HPV569 drug product candidate vector (LG001) was initiated in 2006 in France. Three subjects with β-TM were treated and one (subject No. 1003) became transfusion independent (see details below).141
The BB305 lentiviral vector
Although the LG001 trial brought the proof-of-principle of clinical efficacy in a human patient (subject No. 1003), partial clonal dominance, which subsequently resulted, was observed upon vector integration within the HMGA2 gene. This integration triggered abnormal splicing of the endogenous HMGA2 RNA using a cryptic acceptor site within the cHS4 insulator core in the left LTR. Studies performed in mouse samples showed that most of the integrated provectors had lost one of the two 250 bp cHS4 insulator cores at each end.138 This phenomenon was observed in subject No. 1003 as well.141 Data reported by several contributors also showed that inclusion of cHS4 chromatin insulator elements in the 3′LTR of LVs reduced their titers and transduction efficacy for human CD34+ cells.142–144 Moreover, their presence had limited efficacy on transgene expression in human hematopoietic cells in vitro and in vivo,144 while the protection provided was dependent on the location of cHS4 integration in the genome.145 For all these reasons (decreased titers and transduction efficiency, insulator loss, untoward splicing, and limited enhancer blocking activity), removal of the cHS4 insulator from our HPV569 vector was warranted.
In addition, we decided to replace the Tat-dependent U3 promoter/enhancer of the 5′LTR by that of the cytomegalovirus (CMV) (Fig. 3) in order to further increase the titer of the vector.105 Overall, the modified vector, referred to as the BB305 vector,110 is identical to HPV569 after chromosomal integration, except for the absence of the cHS4 insulator (Fig. 3). In plasmid form, however, as used in producer cells, the CMV promoter now drives transcription instead of the 5′LTR.
The new-generation BB305 vector was produced, purified, and tested on human CD34+ cells as well as in a mouse model of β-thalassemia.110 Side-by-side comparison of HPV569 and BB305 showed that the vector changes resulted in both increased vector titers (3–4-fold) and increased transduction efficiency (2–3-fold). Comprehensive toxicological mouse studies and vector comparison showed β-thalassemic phenotype correction with no evidence of toxic effect related to any of the vectors, and similar integration patterns (mostly within RefSeq genes), without any sign of clonal outgrowth or in vivo selection.110 In vitro immortalization (IVIM) assays, developed by the Baum lab,146 were run in order to evaluate the risk of hematopoietic cell transformation upon vector integration. Both vectors (HPV569 and BB305) exhibited a strongly reduced risk of immortalization of murine hematopoietic cells as compared with control retroviral vectors, and LVs containing the strong SFFV viral promoter.110
Vector and Drug Product Manufacturing
Production of lentiviral vectors
Guidance on development and manufacturing of LVs, which are considered starting materials by both U.S. and EU regulatory agencies when used for ex vivo transduction, is provided by FDA and EMA. This guidance provides recommendations for LV design, manufacturing, and characterization, including transducing activity, LV particle quantification, and testing for replication-competent lentiviruses (RCLs). bluebird bio has developed a GMP manufacturing process for large-scale production (>40 liters) of third-generation VSV-G-pseudotyped HIV-1-based LVs. Clinical-grade vesicular stomatitis virus glycoprotein-pseudotyped lentiviral particles are produced by a plasmid-based co-transfection method. Purification is done by chromatography and buffer is exchanged by ultrafiltration before final filtration according to published protocols.147,148 This method is used to produce large-scale clinical-grade LV lots to support our clinical trials.
Release tests performed on the manufactured LV include potency and identity, safety, and purity. The LV potency is determined using assays that measure both the concentration of viral particles and infectious titer. The concentration of vector particles is assessed by measuring the HIV-1 p24 antigen. The titer is defined as the number of functional transduction units per milliliter (TU/ml).149 Vector infectivity (or specific transducing activity) is defined as the ratio between transduction unit per milliliter and the concentration of p24 [(TU/ml)/(ng/ml)]. This ratio gives a reliable parameter to evaluate the quality of the vector preparation.150 High-quality vector preparations with an infectious titer >108 TU/ml and a particle/infectious ratio between 100 and 500 are obtained.151
The ability of transduced cells to produce the therapeutic βT87Q-globin is verified after erythroid differentiation of transduced CD34+ hematopoietic cells. It is assessed by reverse-phase HPLC analysis of globin chains in differentiated erythroid cells. The therapeutic βT87Q-globin can be easily distinguished from the normal β-chain, as well as from the βE and βS polypeptides.67,68
The generation of RCL is highly improbable because of the multiplasmid packaging system and the U3-deletion. Nevertheless, RCL testing is performed. It relies upon the permissive cell line C8166-45, allowing the amplification and the detection of replicative-competent particles.152,153
Drug product (genetically modified CD34+ cells)
Once subjects have been screened and eligibility has been determined, autologous hematopoietic CD34+ cells are procured either by apheresis of mobilized peripheral blood cells (for β-TM subjects) or by bone marrow harvest (for subjects with SCD). Multiple harvests may be undertaken if needed to meet the minimum cell dose required for drug product manufacturing and untransduced backup. Mobilization is performed with filgrastim, a recombinant form of granulocyte-colony stimulating factor (G-CSF), in combination with plerixafor. The combination of plerixafor and filgrastim may be the most effective mobilization strategy for subjects with β-thalassemia.154,155 Apheresis is performed on the fifth day of mobilization. The objective is to collect sufficient cells for both manufacturing and for rescue therapy. After procurement, the CD34+ cell population is enriched via purification. A portion of the product is cryopreserved for rescue therapy. CD34+ cells are grown in serum-free medium supplemented with recombinant human cytokines stem cell factor (SCF), thrombopoietin (TPO), and FMS-like tyrosine kinase receptor-3 (Flt3-L) for approximately two days. The cells are then incubated for an additional day for the lentiviral transduction step. After transduction, a portion of the cells and supernatant are removed for release testing. The reminder of the cells is cryopreserved. After the completion of release testing and disposition, the drug product, defined as CD34+ hematopoietic stem cells transduced with the BB305 LV, is infused after the patient has undergone myeloablative conditioning.
Clinical Trials: Interim Results From France
The HPV569 and BB305 vectors are designed to be used as a single product for the gene therapy of the β-hemoglobinopathies (SCD and β-TM). Vector design is intended to overcome the deficit of β-globin chains in β-thalassemia and to provide anti-HbS polymerizing activity in SCD. The HPV569 vector is the first vector to have been tested worldwide in an approved human trial for the gene therapy of the β-hemoglobinopathies, with the first patient transplanted with transduced cells in 2006.
The French trials led the way in the testing of the HPV569 and BB305 drug products and are the primary focus of this review article. The BB305 program has now expanded internationally, and initial results are summarized in Table 1 and in the section Multicenter U.S. and international trials for β-TM (HGB-204) and SCD (HGB-206) with the BB305 drug product. Other ongoing trials of other gene therapies for hemoglobinopathies are summarized in Table 2.
Study design and pretransplant conditioning
Clinical studies of HPV569 and BB305 drug products run in France are nonrandomized, open-label, single-dose, phase 1/2 studies and only enroll patients with no sibling donor. The first trial performed, with regulatory approval obtained in 2006, was termed LG001 and aimed to assess the HPV569 drug product, as described in the section, “The HPV569 lentiviral vector was assessed in the first human trial LG001” above, in 10 subjects with β-TM or severe SCD. After 3 subjects had been treated, the HPV569 vector was replaced with the improved BB305 vector and manufacturing thereof, and a new clinical trial protocol, termed HGB-205, was implemented to treat the remaining 7 subjects. The HGB-205 study is currently ongoing in France for β-TM and SCD.
In addition to the characteristics of the clinical-grade vector (high-titer, high purity of the manufactured lot, intrinsic therapeutic globin expression properties), key parameters to success include (1) the dose of CD34+ cells infused, (2) the mean vector copy number (VCN) in CD34+ cells preinfusion, and (3) efficient myeloablative conditioning. Because there is little convincing evidence in animal models and clinical trials that effective lentiviral gene transfer to HSCs can be achieved with a high degree of sustained chimerism for vector-bearing cells in the absence of extensive myeloablation, when a CD34+ cell dose similar to that applicable to human autologous settings is used, we decided to apply a full dose of myeloablative agent with intravenous (IV) busulfan as pretransplant conditioning regimen. However, the additional use of immunosuppressive agent (e.g., cyclophosphamide) is not required in this autologous setting. Monitoring of busulfan conditioning to optimize HSC transplantation is an important component of the success156 and is meticulously performed. When the drug product manufacture is complete, the subject receives myeloablative conditioning with IV busulfan at a starting dose of 3.2 mg/kg/day for 4 days with pharmacokinetics analysis. The dose and schedule of busulfan is monitored daily and may be adjusted based upon busulfan plasma levels in order to maintain appropriate levels for myeloablation (AUC exposure of 4500–5000 [μM•min]/day for a daily dosing regimen, over 4 days). After busulfan washout for several days after the end of IV busulfan administration, the drug product is infused. The subject remains hospitalized until engraftment occurs (absolute neutrophil count [ANC] ≥0.5 × 109/liter for 3 consecutive days) and the patient is medically stable. Subjects are followed monthly for the first 6 months posttransplant, and then every 3 months through 24 months posttransplant. Subjects are then enrolled in a long-term follow-up study for an additional 13 years.
Endpoints
The primary study objective is to assess the safety, tolerability, and success of engraftment with autologous CD34+ HSCs transduced with the vectors encoding the human βA-T87Q-globin gene after conditioning with intravenous busulfan in subjects with β-TM and severe SCD. The primary outcome measures are success and kinetics of HSC engraftment, incidence of transplant-related mortality through 100 days posttreatment, overall survival, detection of RCL, characterization of any events of insertional mutagenesis, and monitoring of laboratory parameters, and frequency and severity of clinical adverse events (AEs).
Secondary endpoints include therapeutic globin (HbAT87Q) expression quantified by HPLC analysis, VCN levels in peripheral blood (and in bone marrow, if collected), and RBC transfusion requirements posttransplant. They also include the assessment of dyserythropoiesis for β-TM subjects and VOC and ACS frequencies for SCD subjects.
Inclusion criteria
All subjects must be between 5 and 35 years old. They must have transfusion-dependent (≥100 ml/kg/year of packed RBCs for β-TM) or severe SCD, confirmed by Hb studies. Subjects must be eligible for AHSCT based on institutional medical guidelines, but without a suitable, willing HLA-identical sibling donor. In addition, subjects with SCD must have failed to achieve adequate clinical benefit after hydroxyurea treatment for at least 4 months unless this treatment was not indicated or not well tolerated, and have a history of 1 or more of the following poor prognostic risk factors: recurrent VOC, ACS (at least 2 episodes), significant cerebral abnormality on magnetic resonance imaging (MRI), stroke, antierythrocyte alloimmunization (>2 antibodies), presence of SCD cardiomyopathy documented by Doppler echocardiography, or osteonecrosis of 2 or more joints.
All subjects must have been treated and followed for at least the past 2 years in a specialized center that maintained detailed medical records, including transfusion history. Sperm preservation or testis or ovary biopsy is offered to subjects enrolled on the study. All subjects or their parents or guardians must provide written informed consent.
Exclusion criteria
Subjects meeting any of the following criteria cannot be enrolled in the study: (1) availability of HLA-identical sibling hematopoietic cell donor, (2) clinically significant, active bacterial, viral, parasitic, or fungal infection, (3) prior or current malignancy, myeloproliferative disorder or immunodeficiency, (4) contraindication to anesthesia for bone marrow harvesting, (5) white blood cell count lower than 3 × 109/liter and/or platelet count lower than 120 × 109/liter, and (6) history of major organ damage.
Trial results to date
The LG001 and HGB-205 were the first gene therapy studies worldwide to treat β-TM and SCD subjects, respectively, achieving the first conversion to long-term transfusion independence of a β-TM subject15,141,157,158 and the first evidence of clinical benefit in SCD.159
β-TM patients in the first French trial LG001 with HPV569 drug product
Four subjects with β-TM were enrolled, 3 of whom were treated between 2006 and 2011, under the LG001 protocol. Subject 1002 was 29 years old with β+/β0 genotype and received a dose of 0.93 × 106 cells/kg with a mean VCN of 1.3. Subject No. 1003 was 18 years old with βE/β0 genotype and received a dose of 3.9 × 106 cells/kg with a mean VCN of 0.6. Subject 1004 was 22 years old with βE/β0 genotype and received a dose of 4.3 × 106 cells/kg and a VCN of 0.3. Transplantation was uneventful in all subjects. Transduced cells did not successfully engraft in subject 1002, although gene marking was detected for a few months; this subject received backup hematopoietic cells for rescue and remains transfusion dependent. Neutrophil engraftment was reported for subject No. 1003 at day +27 and for subject 1004 at day +22 postinfusion. The HPV569 drug product was well tolerated, with no nonhematologic serious AE and no drug product-related AEs reported. In addition, no subject has developed vector-derived RCL, leukemia, or lymphoma.
Clinical benefit of treatment with HPV569 drug product was observed in 1 of the 2 treated subjects who did not receive backup cells. Subject No. 1003 sustained clinical benefit as evidenced by long-term transfusion independence that was achieved approximately 1 year posttransplant15,141 and sustained through approximately 8 years posttransplant. The hemoglobin level stabilized 1.5 years posttreatment, and the mean VCN per myeloid cell was close to 0.2. A detailed report on this subject after 33 months of follow-up has been published.15,141 After approximately 7 years of transfusion independence, this subject received a few transfusions to address symptoms of anemia. Ongoing follow-up will determine whether or not periodic transfusion support will again be required. His total hemoglobin and levels of HbAT87Q transgenic hemoglobin have remained generally consistent from years 2 through 8 postinfusion at around 8 g/dl total Hb of which 30% is HbAT87Q.
Integration-site analysis revealed the relative dominance of a clone bearing the vector inside intron 3 of the HMGA2 gene, the proportion of which reached a maximum representation of ∼30% of the transduced myeloid cells (i.e., ∼4% of hematopoietic cells) 15 months posttransplantation. Molecular analyses revealed that the integrated vector had caused transcriptional activation of the HMGA2 promoter in erythroid cells. Furthermore, a cryptic splice acceptor site (GTAT(C)6AG), located within the cHS4 insulator core of the 5′LTR, generated a truncated mRNA containing HMGA2 exons 1, 2, and 3. Cleavage/polyadenylation occurred within the adjacent R region of the 5′LTR that leads to increased stability of the RNA because of deletion of the Let7 microRNA binding sites (located in exon 5) and an excess of truncated HMGA2 mRNA. The clone has remained under homeostatic control, with a peak at ∼4% of hematopoietic cells 4 years after transplantation, gradually decreasing to approximately 1% of total nucleated blood cells at 5 years posttransplant.158 The clone is absent from lymphocytes. Despite declining levels of this clone, the amount of βT87Q-globin has remained stable, indicating that the observed therapeutic benefit is not dependent on this specific clone. The insertion of viral vectors in the HMGA2 gene has been observed in other gene therapy studies, with no case of leukemia or lymphoma related to this insertion site in any patient.158,160–162
Transduced cells successfully engrafted in subject No. 1004, but this subject did not achieve sustained clinical benefit and remains transfusion dependent. Hemoglobin levels containing βT87Q-globin account for ∼5% of total hemoglobin.158
β-TM and SCD patients in French trial HGB-205 with BB305 drug product
As of November 2015, four subjects with β-TM and one subject with SCD have been treated with BB305 drug product under the HGB-205 protocol. Initial results have been presented at scientific meetings but not yet published, and the trial is ongoing.157–159 All of the four treated β-TM subjects have so far become transfusion independent, and the treated SCD subject shows early clinical benefit.
Multicenter U.S. and International Trials for β-TM (HGB-204) and SCD (HGB-206) with the BB305 Drug Product
Additional subjects with β-TM and SCD are currently being treated under the HGB-204 and HGB-206 protocols, in two separate international and U.S. studies (Table 1).163,164 Early initial results have been presented at scientific meetings but not yet published.
Conclusions
Gene therapy for hemoglobin disorders has made major progress, from early discovery of β-globin regulatory elements; development of LVs, including the HPV569 and BB305 vectors described here; efficient transduction of hematopoietic stem cells; proof-of-principle of efficacy in mouse models; the first conversion to multiyear transfusion independence of a patient with β-TM; and early clinical benefit now observed in SCD.
Successfully implementing a gene therapy strategy for the β-hemoglobinopathies involves an integrated approach of regulatory, manufacturing, and clinical trial design, and execution. The promise of bringing this therapeutic modality to a large number of patients will require the successful industrialization and commercialization as well as regulatory product approval.
To date, no serious adverse effects have been attributed to ex vivo LV-based HSC gene therapy for hemoglobinopathies, but long-term data in a larger number of patients are needed to assess the risks fully. Current vectors are designed to minimize the possibility of genotoxicity, including oncogenesis, but this risk cannot yet be conclusively quantified or excluded. Ongoing and future clinical studies will allow for a more complete understanding of the benefit–risk profile of this therapeutic approach. Implementation of gene therapy for β-TM and SCD to a large extent will depend on the benefit/risk/cost ratios.
Acknowledgments
We thank Eliane Gluckman (Saint-Louis hospital, Paris, France), the principal investigator at the beginning of the LG001 study; Françoise Bernaudin (CHIC, Créteil, France) for contributions to study design and for clinical care; and Olivier Hermine and Felipe Suarez (Necker Hospital, Paris), the transplanters for the HGB-205 study. We thank Christof von Kalle, Manfred Schmidt (National Center for Tumor Disease, Heidelberg, Germany) and Frederic Bushman (University of Pennsylvania, PA) for vector insertion site analyses. We thank Laure Caccavelli (Necker hospital, Paris, France) and the bluebird bio France team (CEA, Fontenay aux Roses, France) for technical assistance. We thank Philipp Gregory, Sandeep Soni, Kate Lewis, Gabor Veres, Michael Paglia, John Pierciey, and Tim Douros (bluebird bio, Cambridge) for manuscript proofreading. The work was supported by an Industrial Chair from France's Agence Nationale pour la Recherche (ANR) awarded to Philippe Leboulch. bluebird bio is the sponsor of the clinical trials and provided funds to Necker Hospital, Assistance Publique-Hôpitaux de Paris.
Author Disclosure
O.N. and A.-V.E. are employees of bluebird bio, Inc. Y.B., P.L., and E.P. have financial relationships with bluebird bio, Inc. All other authors have no competing interests to disclose.
References
- 1.Modell B, Darlison M. Global epidemiology of haemoglobin disorders and derived service indicators. Bull World Health Organ 2008;86:480–487 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Piel FB, Patil AP, Howes RE, et al. Global epidemiology of sickle haemoglobin in neonates: A contemporary geostatistical model-based map and population estimates. Lancet 2013;381:142–151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Weatherall DJ. The genetic control of protein synthesis: The haemoglobin model. J Clin Pathol Suppl (R Coll Pathol) 1974;8:1–11 [PMC free article] [PubMed] [Google Scholar]
- 4.Modell B, Khan M, Darlison M, et al. Improved survival of thalassaemia major in the UK and relation to T2* cardiovascular magnetic resonance. J Cardiovasc Magn Reson 2008;10:42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Platt OS, Brambilla DJ, Rosse WF, et al. Mortality in sickle cell disease. Life expectancy and risk factors for early death. N Engl J Med 1994;330:1639–1644 [DOI] [PubMed] [Google Scholar]
- 6.Telfer PT, Warburton F, Christou S, et al. Improved survival in thalassemia major patients on switching from desferrioxamine to combined chelation therapy with desferrioxamine and deferiprone. Haematologica 2009;94:1777–1778 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Borgna-Pignatti C. The life of patients with thalassemia major. Haematologica 2010;95:345–348 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Roseff SD. Sickle cell disease: A review. Immunohematology 2009;25:67–74 [PubMed] [Google Scholar]
- 9.Taylor LE, Stotts NA, Humphreys J, et al. A review of the literature on the multiple dimensions of chronic pain in adults with sickle cell disease. J Pain Symptom Manage 2010;40:416–435 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Isgro A, Gaziev J, Sodani P, et al. Progress in hematopoietic stem cell transplantation as allogeneic cellular gene therapy in thalassemia. Ann N Y Acad Sci 2010;1202:149–154 [DOI] [PubMed] [Google Scholar]
- 11.Bernaudin F, Socie G, Kuentz M, et al. Long-term results of related myeloablative stem-cell transplantation to cure sickle cell disease. Blood 2007;110:2749–2756 [DOI] [PubMed] [Google Scholar]
- 12.Sabloff M, Chandy M, Wang Z, et al. HLA-matched sibling bone marrow transplantation for beta-thalassemia major. Blood 2011;117:1745–1750 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lucarelli G, Isgro A, Sodani P, et al. Hematopoietic stem cell transplantation in thalassemia and sickle cell anemia. Cold Spring Harb Perspect Med 2012;2:a011825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pennings G, Schots R, Liebaers I. Ethical considerations on preimplantation genetic diagnosis for HLA typing to match a future child as a donor of haematopoietic stem cells to a sibling. Hum Reprod 2002;17:534–538 [DOI] [PubMed] [Google Scholar]
- 15.Payen E, Leboulch P. Advances in stem cell transplantation and gene therapy in the beta-hemoglobinopathies. Hematology 2012;2012:276–283 [DOI] [PubMed] [Google Scholar]
- 16.Caocci G, Efficace F, Ciotti F, et al. Prospective assessment of health related quality of life in pediatric beta-thalassemia patients following hematopoietic stem cell transplantation. Biol Blood Marrow Transplant 2011;17:861–866 [DOI] [PubMed] [Google Scholar]
- 17.Luznik L, Jones RJ, Fuchs EJ. High-dose cyclophosphamide for graft-versus-host disease prevention. Curr Opin Hematol 2010;17:493–499 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gaziev J, Sodani P, Polchi P, et al. Bone marrow transplantation in adults with thalassemia: Treatment and long-term follow-up. Ann N Y Acad Sci 2005;1054:196–205 [DOI] [PubMed] [Google Scholar]
- 19.Lucarelli G, Clift RA, Galimberti M, et al. Bone marrow transplantation in adult thalassemic patients. Blood 1999;93:1164–1167 [PubMed] [Google Scholar]
- 20.Faraci M, Bekassy AN, De Fazio V, et al. Non-endocrine late complications in children after allogeneic haematopoietic SCT. Bone Marrow Transplant 2008;41 Suppl 2:S49–S57 [DOI] [PubMed] [Google Scholar]
- 21.Gaziev D, Galimberti M, Lucarelli G, et al. Bone marrow transplantation from alternative donors for thalassemia: HLA-phenotypically identical relative and HLA-nonidentical sibling or parent transplants. Bone Marrow Transplant 2000;25:815–821 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bernard F, Auquier P, Herrmann I, et al. Health status of childhood leukemia survivors who received hematopoietic cell transplantation after BU or TBI: An LEA study. Bone Marrow Transplant 2014;49:709–716 [DOI] [PubMed] [Google Scholar]
- 23.Chou YC, Chen RL, Lai ZS, et al. Pharmacological Induction of Human Fetal Globin Gene in Hydroxyurea-Resistant Primary Adult Erythroid Cells. Mol Cell Biol 2015;35:2541–2553 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Guda S, Brendel C, Renella R, et al. miRNA-embedded shRNAs for Lineage-specific BCL11A Knockdown and Hemoglobin F Induction. Mol Ther 2015;23:1465–1474 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Xu J, Peng C, Sankaran VG, et al. Correction of sickle cell disease in adult mice by interference with fetal hemoglobin silencing. Science 2011;334:993–996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bauer DE, Orkin SH. Hemoglobin switching's surprise: The versatile transcription factor BCL11A is a master repressor of fetal hemoglobin. Curr Opin Genet Dev 2015;33:62–70 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Vierstra J, Reik A, Chang KH, et al. Functional footprinting of regulatory DNA. Nat Methods 2015;12:927–930 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Huang X, Wang Y, Yan W, et al. Production of gene-corrected adult beta globin protein in human erythrocytes differentiated from patient iPSCs after genome editing of the sickle point mutation. Stem Cells 2015;33:1470–1479 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Xu P, Tong Y, Liu XZ, et al. Both TALENs and CRISPR/Cas9 directly target the HBB IVS2-654 (C>T) mutation in beta-thalassemia-derived iPSCs. Sci Rep 2015;5:12065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hoban MD, Cost GJ, Mendel MC, et al. Correction of the sickle cell disease mutation in human hematopoietic stem/progenitor cells. Blood 2015;125:2597–2604 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rachmilewitz EA, Giardina PJ. How I treat thalassemia. Blood 2011;118:3479–3488 [DOI] [PubMed] [Google Scholar]
- 32.Rouyer Fessard P, Leroy Viard K, Domenget C, et al. Mouse beta thalassemia, a model for the membrane defects of erythrocytes in the human disease. J Biol Chem 1990;265:20247–20251 [PubMed] [Google Scholar]
- 33.Arlet JB, Ribeil JA, Guillem F, et al. HSP70 sequestration by free alpha-globin promotes ineffective erythropoiesis in beta-thalassaemia. Nature 2014;514:242–246 [DOI] [PubMed] [Google Scholar]
- 34.Mathias LA, Fisher TC, Zeng L, et al. Ineffective erythropoiesis in beta-thalassemia major is due to apoptosis at the polychromatophilic normoblast stage. Exp Hematol 2000;28:1343–1353 [DOI] [PubMed] [Google Scholar]
- 35.Vigi V, Volpato S, Gaburro D, et al. The correlation between red-cell survival and excess of alpha-globin synthesis in beta-thalassemia. Br J Haematol 1969;16:25–30 [DOI] [PubMed] [Google Scholar]
- 36.Orkin SH, Kazazian HH, Jr., Antonarakis SE, et al. Abnormal RNA processing due to the exon mutation of beta E-globin gene. Nature 1982;300:768–769 [DOI] [PubMed] [Google Scholar]
- 37.Rees DC, Clegg JB, Weatherall DJ. Is hemoglobin instability important in the interaction between hemoglobin E and beta thalassemia? Blood 1998;92:2141–2146 [PubMed] [Google Scholar]
- 38.Fucharoen S, Weatherall DJ. The hemoglobin E thalassemias. Cold Spring Harb Perspect Med 2012;2 pii: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rund D, Fucharoen S. Genetic modifiers in hemoglobinopathies. Curr Mol Med 2008;8:600–608 [DOI] [PubMed] [Google Scholar]
- 40.Vichinsky E. Hemoglobin e syndromes. Hematology 2007:79–83 [DOI] [PubMed] [Google Scholar]
- 41.Lorey F. Asian immigration and public health in California: Thalassemia in newborns in California. J Pediatr Hematol Oncol 2000;22:564–566 [DOI] [PubMed] [Google Scholar]
- 42.Vichinsky EP, MacKlin EA, Waye JS, et al. Changes in the epidemiology of thalassemia in North America: A new minority disease. Pediatrics 2005;116:e818–e825 [DOI] [PubMed] [Google Scholar]
- 43.Marotta CA, Wilson JT, Forget BG, et al. Human beta-globin messenger RNA. III. Nucleotide sequences derived from complementary DNA. J Biol Chem 1977;252:5040–5053 [PubMed] [Google Scholar]
- 44.Rees DC, Williams TN, Gladwin MT. Sickle-cell disease. Lancet 2010;376:2018–2031 [DOI] [PubMed] [Google Scholar]
- 45.Brittenham GM, Schechter AN, Noguchi CT. Hemoglobin S polymerization: Primary determinant of the hemolytic and clinical severity of the sickling syndromes. Blood 1985;65:183–189 [PubMed] [Google Scholar]
- 46.Bunn HF. Pathogenesis and treatment of sickle cell disease. N Engl J Med 1997;337:762–769 [DOI] [PubMed] [Google Scholar]
- 47.Noguchi CT, Rodgers GP, Serjeant G, et al. Levels of fetal hemoglobin necessary for treatment of sickle cell disease. N Engl J Med 1988;318:96–99 [DOI] [PubMed] [Google Scholar]
- 48.Turhan A, Weiss LA, Mohandas N, et al. Primary role for adherent leukocytes in sickle cell vascular occlusion: A new paradigm. Proc Natl Acad Sci U S A 2002;99:3047–3051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Belcher JD, Bryant CJ, Nguyen J, et al. Transgenic sickle mice have vascular inflammation. Blood 2003;101:3953–3959 [DOI] [PubMed] [Google Scholar]
- 50.Amer J, Ghoti H, Rachmilewitz E, et al. Red blood cells, platelets and polymorphonuclear neutrophils of patients with sickle cell disease exhibit oxidative stress that can be ameliorated by antioxidants. Br J Haematol 2006;132:108–113 [DOI] [PubMed] [Google Scholar]
- 51.Szocs K. Endothelial dysfunction and reactive oxygen species production in ischemia/reperfusion and nitrate tolerance. Gen Physiol Biophys 2004;23:265–295 [PubMed] [Google Scholar]
- 52.Wood KC, Hebbel RP, Granger DN. Endothelial cell NADPH oxidase mediates the cerebral microvascular dysfunction in sickle cell transgenic mice. FASEB J 2005;19:989–991 [DOI] [PubMed] [Google Scholar]
- 53.Gladwin MT. Revisiting the hyperhemolysis paradigm. Blood 2015;126:695–696 [DOI] [PubMed] [Google Scholar]
- 54.Adachi K, Reddy LR, Surrey S. Role of hydrophobicity of phenylalanine beta 85 and leucine beta 88 in the acceptor pocket for valine beta 6 during hemoglobin S polymerization. J Biol Chem 1994;269:31563–31566 [PubMed] [Google Scholar]
- 55.Adachi K, Konitzer P, Surrey S. Role of gamma 87 Gln in the inhibition of hemoglobin S polymerization by hemoglobin F. J Biol Chem 1994;269:9562–9567 [PubMed] [Google Scholar]
- 56.Reddy LR, Reddy KS, Surrey S, et al. Role of beta87 Thr in the beta6 Val acceptor site during deoxy Hb S polymerization. Biochemistry 1997;36:15992–15998 [DOI] [PubMed] [Google Scholar]
- 57.Goldberg MA, Husson MA, Bunn HF. Participation of hemoglobins A and F in polymerization of sickle hemoglobin. J Biol Chem 1977;252:3414–3421 [PubMed] [Google Scholar]
- 58.Poillon WN, Kim BC, Rodgers GP, et al. Sparing effect of hemoglobin F and hemoglobin A2 on the polymerization of hemoglobin S at physiologic ligand saturations. Proc Natl Acad Sci U S A 1993;90:5039–5043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Powars DR, Weiss JN, Chan LS, et al. Is there a threshold level of fetal hemoglobin that ameliorates morbidity in sickle cell anemia? Blood 1984;63:921–926 [PubMed] [Google Scholar]
- 60.Thomas PW, Higgs DR, Serjeant GR. Benign clinical course in homozygous sickle cell disease: A search for predictors. J Clin Epidemiol 1997;50:121–126 [DOI] [PubMed] [Google Scholar]
- 61.Benesch RE, Edalji R, Benesch R, et al. Solubilization of hemoglobin S by other hemoglobins. Proc Natl Acad Sci U S A 1980;77:5130–5134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Bookchin RM, Nagel RL, Balazs T. Role of hybrid tetramer formation in gelation of haemoglobin S. Nature 1975;256:667–668 [DOI] [PubMed] [Google Scholar]
- 63.Sunshine HR, Hofrichter J, Eaton WA. Gelation of sickle cell hemoglobin in mixtures with normal adult and fetal hemoglobins. J Mol Biol 1979;133:435–467 [DOI] [PubMed] [Google Scholar]
- 64.Nagel RL, Bookchin RM, Johnson J, et al. Structural bases of the inhibitory effects of hemoglobin F and hemoglobin A2 on the polymerization of hemoglobin S. Proc Natl Acad Sci U S A 1979;76:670–672 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Pawliuk R, Westerman KA, Fabry ME, et al. Correction of sickle cell disease in transgenic mouse models by gene therapy. Science 2001;294:2368–2371 [DOI] [PubMed] [Google Scholar]
- 66.Maurer HS, Behrman RE, Honig GR. Dependence of the oxygen affinity of blood on the presence of foetal or adult haemoglobin. Nature 1970;227:388–390 [DOI] [PubMed] [Google Scholar]
- 67.Amin A, Bourget P, Gourmel B, et al. A sensitive and rapid HPLC assay for semi-quantitative analysis of globin chain levels in blood after transplantation of autologous hematopoietic stem cells transduced by a lentiviral bA-T87Q globin vector in b-thalassemia major and sickle cell disease. EBMT 2015: 41st Annual Meeting of the European Society for Blood and Marrow Transplantation, Istanbul (Turkey) [Google Scholar]
- 68.Payen E, Colomb C, Negre O, et al. Lentivirus vectors in beta-thalassemia. Methods Enzymol 2012;507:109–124 [DOI] [PubMed] [Google Scholar]
- 69.Camaschella C, Kattamis AC, Petroni D, et al. Different hematological phenotypes caused by the interaction of triplicated alpha-globin genes and heterozygous beta-thalassemia. Am J Hematol 1997;55:83–88 [DOI] [PubMed] [Google Scholar]
- 70.Winichagoon P, Fucharoen S, Weatherall D, et al. Concomitant inheritance of alpha-thalassemia in beta 0-thalassemia/Hb E disease. Am J Hematol 1985;20:217–222 [DOI] [PubMed] [Google Scholar]
- 71.Cappellini MD, Fiorelli G, Bernini LF. Interaction between homozygous beta (0) thalassaemia and the Swiss type of hereditary persistence of fetal haemoglobin. Br J Haematol 1981;48:561–572 [DOI] [PubMed] [Google Scholar]
- 72.Winichagoon P, Thonglairoam V, Fucharoen S, et al. Severity differences in beta-thalassaemia/haemoglobin E syndromes: Implication of genetic factors. Br J Haematol 1993;83:633–639 [DOI] [PubMed] [Google Scholar]
- 73.Akinsheye I, Alsultan A, Solovieff N, et al. Fetal hemoglobin in sickle cell anemia. Blood 2011;118:19–27 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Walters MC, Patience M, Leisenring W, et al. Stable mixed hematopoietic chimerism after bone marrow transplantation for sickle cell anemia. Biol Blood Marrow Transplant 2001;7:665–673 [DOI] [PubMed] [Google Scholar]
- 75.Chada K, Magram J, Raphael K, et al. Specific expression of a foreign beta-globin gene in erythroid cells of transgenic mice. Nature 1985;314:377–380 [DOI] [PubMed] [Google Scholar]
- 76.Kollias G, Wrighton N, Hurst J, et al. Regulated expression of human A gamma-, beta-, and hybrid gamma beta-globin genes in transgenic mice: Manipulation of the developmental expression patterns. Cell 1986;46:89–94 [DOI] [PubMed] [Google Scholar]
- 77.Magram J, Chada K, Costantini F. Developmental regulation of a cloned adult beta-globin gene in transgenic mice. Nature 1985;315:338–340 [DOI] [PubMed] [Google Scholar]
- 78.Townes TM, Lingrel JB, Chen HY, et al. Erythroid-specific expression of human beta-globin genes in transgenic mice. EMBO J 1985;4:1715–1723 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Dzierzak EA, Papayannopoulou T, Mulligan RC. Lineage-specific expression of a human beta-globin gene in murine bone marrow transplant recipients reconstituted with retrovirus-transduced stem cells. Nature 1988;331:35–41 [DOI] [PubMed] [Google Scholar]
- 80.Karlsson S, Bodine DM, Perry L, et al. Expression of the human beta-globin gene following retroviral-mediated transfer into multipotential hematopoietic progenitors of mice. Proc Natl Acad Sci U S A 1988;85:6062–6066 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Forrester WC, Thompson C, Elder JT, et al. A developmentally stable chromatin structure in the human beta-globin gene cluster. Proc Natl Acad Sci U S A 1986;83:1359–1363 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Tuan D, Solomon W, Li Q, et al. The “beta-like-globin” gene domain in human erythroid cells. Proc Natl Acad Sci U S A 1985;82:6384–6388 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Grosveld F, van Assendelft GB, Greaves DR, et al. Position-independent, high-level expression of the human beta-globin gene in transgenic mice. Cell 1987;51:975–985 [DOI] [PubMed] [Google Scholar]
- 84.Talbot D, Collis P, Antoniou M, et al. A dominant control region from the human beta-globin locus conferring integration site-independent gene expression. Nature 1989;338:352–355 [DOI] [PubMed] [Google Scholar]
- 85.Fraser P, Hurst J, Collis P, et al. DNaseI hypersensitive sites 1, 2 and 3 of the human beta-globin dominant control region direct position-independent expression. Nucleic Acids Res 1990;18:3503–3508 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Fraser P, Pruzina S, Antoniou M, et al. Each hypersensitive site of the human beta-globin locus control region confers a different developmental pattern of expression on the globin genes. Genes Dev 1993;7:106–113 [DOI] [PubMed] [Google Scholar]
- 87.Emery DW, Chen H, Li Q, et al. Development of a condensed locus control region cassette and testing in retrovirus vectors for A gamma-globin. Blood Cells Mol Dis 1998;24:322–339 [DOI] [PubMed] [Google Scholar]
- 88.Leboulch P, Huang GM, Humphries RK, et al. Mutagenesis of retroviral vectors transducing human beta-globin gene and beta-globin locus control region derivatives results in stable transmission of an active transcriptional structure. EMBO J 1994;13:3065–3076 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Sadelain M, Wang CH, Antoniou M, et al. Generation of a high-titer retroviral vector capable of expressing high levels of the human beta-globin gene. Proc Natl Acad Sci U S A 1995;92:6728–6732 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Collis P, Antoniou M, Grosveld F. Definition of the minimal requirements within the human beta-globin gene and the dominant control region for high level expression. EMBO J 1990;9:233–240 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Forrester WC, Novak U, Gelinas R, et al. Molecular analysis of the human beta-globin locus activation region. Proc Natl Acad Sci U S A 1989;86:5439–5443 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Cone RD, Weber-Benarous A, Baorto D, et al. Regulated expression of a complete human beta-globin gene encoded by a transmissible retrovirus vector. Mol Cell Biol 1987;7:887–897 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Karlsson S, Papayannopoulou T, Schweiger SG, et al. Retroviral-mediated transfer of genomic globin genes leads to regulated production of RNA and protein. Proc Natl Acad Sci U S A 1987;84:2411–2415 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Gelinas R, Frazier A, Harris E. A normal level of beta-globin expression in erythroid cells after retroviral cells transfer. Bone Marrow Transplant 1992;9 Suppl 1:154–157 [PubMed] [Google Scholar]
- 95.Novak U, Harris EA, Forrester W, et al. High-level beta-globin expression after retroviral transfer of locus activation region-containing human beta-globin gene derivatives into murine erythroleukemia cells. Proc Natl Acad Sci U S A 1990;87:3386–3390 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Chang JC, Liu D, Kan YW. A 36-base-pair core sequence of locus control region enhances retrovirally transferred human beta-globin gene expression. Proc Natl Acad Sci U S A 1992;89:3107–3110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Philipsen S, Talbot D, Fraser P, et al. The beta-globin dominant control region: Hypersensitive site 2. EMBO J 1990;9:2159–2167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Raftopoulos H, Ward M, Leboulch P, et al. Long-term transfer and expression of the human beta-globin gene in a mouse transplant model. Blood 1997;90:3414–3422 [PubMed] [Google Scholar]
- 99.Rivella S, Sadelain M. Genetic treatment of severe hemoglobinopathies: The combat against transgene variegation and transgene silencing. Semin Hematol 1998;35:112–125 [PubMed] [Google Scholar]
- 100.Cullen BR. Retroviruses as model systems for the study of nuclear RNA export pathways. Virology 1998;249:203–210 [DOI] [PubMed] [Google Scholar]
- 101.Naldini L, Blomer U, Gage FH, et al. Efficient transfer, integration, and sustained long-term expression of the transgene in adult rat brains injected with a lentiviral vector. Proc Natl Acad Sci U S A 1996;93:11382–11388 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Naldini L, Blomer U, Gallay P, et al. In vivo gene delivery and stable transduction of nondividing cells by a lentiviral vector. Science 1996;272:263–267 [DOI] [PubMed] [Google Scholar]
- 103.Lewis P, Hensel M, Emerman M. Human immunodeficiency virus infection of cells arrested in the cell cycle. Embo J 1992;11:3053–3058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Case SS, Price MA, Jordan CT, et al. Stable transduction of quiescent CD34(+)CD38(-) human hematopoietic cells by HIV-1-based lentiviral vectors. Proc Natl Acad Sci U S A 1999;96:2988–2993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Miyoshi H, Smith KA, Mosier DE, et al. Transduction of human CD34+ cells that mediate long-term engraftment of NOD/SCID mice by HIV vectors. Science 1999;283:682–686 [DOI] [PubMed] [Google Scholar]
- 106.May C, Rivella S, Callegari J, et al. Therapeutic haemoglobin synthesis in beta-thalassaemic mice expressing lentivirus-encoded human beta-globin. Nature 2000;406:82–86 [DOI] [PubMed] [Google Scholar]
- 107.Rivella S, May C, Chadburn A, et al. A novel murine model of Cooley anemia and its rescue by lentiviral-mediated human beta-globin gene transfer. Blood 2003;101:2932–2939 [DOI] [PubMed] [Google Scholar]
- 108.Imren S, Fabry ME, Westerman KA, et al. High-level beta-globin expression and preferred intragenic integration after lentiviral transduction of human cord blood stem cells. J Clin Invest 2004;114:953–962 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Bank A, Dorazio R, Leboulch P. A phase I/II clinical trial of beta-globin gene therapy for beta-thalassemia. Ann N Y Acad Sci 2005;1054:308–316 [DOI] [PubMed] [Google Scholar]
- 110.Negre O, Bartholomae C, Beuzard Y, et al. Preclinical evaluation of efficacy and safety of an improved lentiviral vector for the treatment of beta-thalassemia and sickle cell disease. Curr Gene Ther 2015;15:64–81 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Hanawa H, Hargrove PW, Kepes S, et al. Extended beta-globin locus control region elements promote consistent therapeutic expression of a gamma-globin lentiviral vector in murine beta-thalassemia. Blood 2004;104:2281–2290 [DOI] [PubMed] [Google Scholar]
- 112.Imren S, Payen E, Westerman KA, et al. Permanent and panerythroid correction of murine beta thalassemia by multiple lentiviral integration in hematopoietic stem cells. Proc Natl Acad Sci U S A 2002;99:14380–14385 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Miccio A, Cesari R, Lotti F, et al. In vivo selection of genetically modified erythroblastic progenitors leads to long-term correction of beta-thalassemia. Proc Natl Acad Sci U S A 2008;105:10547–10552 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Puthenveetil G, Scholes J, Carbonell D, et al. Successful correction of the human beta-thalassemia major phenotype using a lentiviral vector. Blood 2004;3:3. [DOI] [PubMed] [Google Scholar]
- 115.Maeda D, Yamaguchi T, Ishizuka T, et al. Regulatory frameworks for gene and cell therapies in Japan. Adv Exp Med Biol 2015;871:147–162 [DOI] [PubMed] [Google Scholar]
- 116.Martins JS, Abreu SC, Araujo ME, et al. [Strategies and results of the oral cancer prevention campaign among the elderly in Sao Paulo, Brazil, 2001 to 2009]. Revista Panamericana De Salud Publica [Pan Am J Public Health] 2012;31:246–252 [DOI] [PubMed] [Google Scholar]
- 117.Eichler HG, Oye K, Baird LG, et al. Adaptive licensing: Taking the next step in the evolution of drug approval. Clin Pharmacol Ther 2012;91:426–437 [DOI] [PubMed] [Google Scholar]
- 118.European Medicines Agency, Orphan drugs and rare diseases at a glance. Doc. Ref. EMEA/290072/2007. www.ema.europa.eu/docs/en_GB/document_library/Other/2010/01/WC500069805.pdf
- 119.U.S. Food and Drug Administration. Developing products for rare disease and conditions. www.fda.gov/ForIndustry/DevelopingProductsforRareDiseasesConditions/default.htm
- 120.EMA. Summaries of scientific recommendations on classification of advanced-therapy medicinal products. www.ema.europa.eu/ema/indexjsp?curl=pages/regulation/general/general_content_000301.jsp&mid=WC0b01ac05800862c0
- 121.European Commission. Q&A: New rules for clinical trials conducted in the EU. http://europa.eu/rapid/press-release_MEMO-14–254_en.htm
- 122.ICH. M4: The common technical document. www.ich.org/products/ctd.html
- 123.FDA. Guidance for Industry—Expedited Programs for Serious Conditions—Drugs and Biologics. www.fda.gov/downloads/drugs/guidancecomplianceregulatoryinformation/guidances/ucm358301.pdf
- 124.EMA. Guideline on the Risk-Based Approach According to Annex I, Part IV of Directive 2001/83/EC Applied to Advanced Therapy Medicinal Products. www.emaeuropa.eu/docs/en_GB/document_library/Scientific_guideline/2013/03/WC500139748.pdf
- 125.EC. Consultation Document—Good Manufacturing Practice for Advanced Therapy Medicinal Products. http://ec.europa.eu/health/files/advtherapies/2015_pc/publ_cons_doc_2015.pdf
- 126.FDA. Titel 21—Food and Drugs Chapter I—Food and drug administration department of health and human services subchapter F—Biologics—Part 601 Licensing. www.accessdatafdagov/scripts/cdrh/cfdocs/cfCFR/CFRSearchcfm?CFRPart=601
- 127.EC. Regulation (EC) No. 1394/2007 of the European Parliament and of the Council of 13 November 2007 on Advanced Therapy Medicinal Products and Amending Directive 2001/83/EC and Regulation (EC) No 726/2004. http://ec.europa.eu/health/files/eudralex/vol-1/reg_2007_1394/reg_2007_1394_en.pdf
- 128.EC. Guideline on the Scientific Application and the Practical Arrangements Necessary to Implement the Procedure for Accelerated Assessment Pursuant to Article 14(9) of Regulation (EC) No 726/2004. http://ec.europa.eu/health/files/committee/stamp/2015–10_stamp3/stamp_3_16_3b_guideline_on_accelerated_assessment_-_public_consultation.pdf
- 129.EMA. Guideline on Procedures for the Granting of a Marketing Authorization under Exceptional Circumsances, Pursuant to Article 14 (8) of Regulation (EC) No 726/2004. www.ema.europa.eu/docs/en_GB/document_library/Regulatory_and_procedural_guideline/2009/10/WC500004883.pdf
- 130.EMA. Guideline on the Scientific Application and the Practical Arrangements Necessary to Implement Commission Regulation (EC) No 507/2006 on the Conditional Marketing Authorisation for Medicinal Products for Human use Falling within the Scope of Regulation (EC) No 726/2004. www.ema.europa.eu/docs/en_GB/document_library/Regulatory_and_procedural_guideline/2015/07/WC500190555.pdf
- 131.Zufferey R, Dull T, Mandel RJ, et al. Self-inactivating lentivirus vector for safe and efficient in vivo gene delivery. J Virol 1998;72:9873–9880 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Hanawa H, Persons DA, Nienhuis AW. Mobilization and mechanism of transcription of integrated self-inactivating lentiviral vectors. J Virol 2005;79:8410–8421 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Cesana D, Ranzani M, Volpin M, et al. Uncovering and dissecting the genotoxicity of self-inactivating lentiviral vectors in vivo. Mol Ther 2014;22:774–785 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Zychlinski D, Schambach A, Modlich U, et al. Physiological promoters reduce the genotoxic risk of integrating gene vectors. Mol Ther 2008;16:718–725 [DOI] [PubMed] [Google Scholar]
- 135.Levitt N, Briggs D, Gil A, et al. Definition of an efficient synthetic poly(A) site. Genes Dev 1989;3:1019–1025 [DOI] [PubMed] [Google Scholar]
- 136.Iwakuma T, Cui Y, Chang LJ. Self-inactivating lentiviral vectors with U3 and U5 modifications. Virology 1999;261:120–132 [DOI] [PubMed] [Google Scholar]
- 137.Hargrove PW, Kepes S, Hanawa H, et al. Globin lentiviral vector insertions can perturb the expression of endogenous genes in beta-thalassemic hematopoietic cells. Mol Ther 2008;16:525–533 [DOI] [PubMed] [Google Scholar]
- 138.Ronen K, Negre O, Roth S, et al. Distribution of lentiviral vector integration sites in mice following therapeutic gene transfer to treat beta-thalassemia. Mol Ther 2011;19:1273–1286 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Schroder AR, Shinn P, Chen H, et al. HIV-1 integration in the human genome favors active genes and local hotspots. Cell 2002;110:521–529 [DOI] [PubMed] [Google Scholar]
- 140.Wang GP, Ciuffi A, Leipzig J, et al. HIV integration site selection: Analysis by massively parallel pyrosequencing reveals association with epigenetic modifications. Genome Res 2007;17:1186–1194 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Cavazzana-Calvo M, Payen E, Negre O, et al. Transfusion independence and HMGA2 activation after gene therapy of human beta-thalassaemia. Nature 2010;467:318–322 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Hanawa H, Yamamoto M, Zhao H, et al. Optimized lentiviral vector design improves titer and transgene expression of vectors containing the chicken beta-globin locus HS4 insulator element. Mol Ther 2009;17:667–674 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Nielsen TT, Jakobsson J, Rosenqvist N, et al. Incorporating double copies of a chromatin insulator into lentiviral vectors results in less viral integrants. BMC Biotechnol. 2009;9:13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Uchida N, Washington KN, Lap CJ, et al. Chicken HS4 insulators have minimal barrier function among progeny of human hematopoietic cells transduced with an HIV1-based lentiviral vector. Mol Ther 2011;19:133–139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Desprat R, Bouhassira EE. Gene specificity of suppression of transgene-mediated insertional transcriptional activation by the chicken HS4 insulator. PLoS One 2009;4:e5956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Modlich U, Navarro S, Zychlinski D, et al. Insertional transformation of hematopoietic cells by self-inactivating lentiviral and gammaretroviral vectors. Mol Ther 2009;17:1919–1928 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Kutner RH, Puthli S, Marino MP, et al. Simplified production and concentration of HIV-1-based lentiviral vectors using HYPERFlask vessels and anion exchange membrane chromatography. BMC Biotechnol. 2009;9:10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Kutner RH, Zhang XY, Reiser J. Production, concentration and titration of pseudotyped HIV-1-based lentiviral vectors. Nat Protoc 2009;4:495–505 [DOI] [PubMed] [Google Scholar]
- 149.Skorik C, Gorman WC, Finer M, et al. Development of a validated method offering a potentially standardized biological assay for the titration of HIV-1 based lentiviral vectors. Mol Ther 2013;21:S99 [Google Scholar]
- 150.Follenzi A, Naldini L. Generation of HIV-1 derived lentiviral vectors. Methods Enzymol 2002;346:454–465 [DOI] [PubMed] [Google Scholar]
- 151.Gorman WC, Skorik C, Finer M, et al. Developmenent of a scale-down manufacturing model for generation of HIV-1 based lentiviral vectors. Mol Ther 2013;21:S100 [Google Scholar]
- 152.Cornetta K, Yao J, Jasti A, et al. Replication-competent lentivirus analysis of clinical grade vector products. Mol Ther 2011;19:557–566 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Sastry L, Xu Y, Johnson T, et al. Certification assays for HIV-1-based vectors: Frequent passage of gag sequences without evidence of replication-competent viruses. Mol Ther 2003;8:830–839 [DOI] [PubMed] [Google Scholar]
- 154.Yannaki E, Karponi G, Zervou F, et al. Hematopoietic stem cell mobilization for gene therapy: Superior mobilization by the combination of granulocyte-colony stimulating factor plus plerixafor in patients with beta-thalassemia major. Hum Gene Ther 2013;24:852–860 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Karponi G, Psatha N, Lederer CW, et al. Plerixafor+G-CSF-mobilized CD34+ cells represent an optimal graft source for thalassemia gene therapy. Blood 2015;126:616–619 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Bourget P, Falaschi L, Suarez F, et al. [A medical-pharmaceutical partnership model as a contributor to the success in conditioning regimen for allogenic hematopoietic stem cell transplantation in adults: A cross-reflection on our organizations]. Bull Cancer 2012;99:643–653 [DOI] [PubMed] [Google Scholar]
- 157.Cavazzana M, Ribeil JA, Payen E, et al. Study Hgb-205: Outcomes of gene therapy for hemoglobinopathies via transplantation of autologous hematopoietic stem cells transduced ex vivo with a lentiviral βA-T87Q-globin vector (LentiGlobin BB305 drug product) [abstract]. Blood 2014;124:4797 [Google Scholar]
- 158.Cavazzana M, Ribeil JA, Payen E, et al. Outcomes of gene therapy for beta-thalassemai major via transplantation of autologous hematopoietic stem cells transduced ex vivo with a lentiviral beta globin vector. Haematologica 2014;99:abstract S742 [Google Scholar]
- 159.Cavazzana M, Ribeil JA, Payen E, et al. Outcomes of gene therapy for severe sickle disease and beta-thalassemia major via transplantation of autologous hematopoietic stem cells transduced ex vivo with a lentiviral beta AT87Q-globin vector. Blood 2015;126: Abstract 202 [Google Scholar]
- 160.Wang GP, Berry CC, Malani N, et al. Dynamics of gene-modified progenitor cells analyzed by tracking retroviral integration sites in a human SCID-X1 gene therapy trial. Blood 2010;115:4356–4366 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Adair JE, Beard BC, Trobridge GD, et al. Extended survival of glioblastoma patients after chemoprotective HSC gene therapy. Sci Transl Med 2012;4:133ra157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Braun CJ, Boztug K, Paruzynski A, et al. Gene therapy for Wiskott-Aldrich syndrome—long-term efficacy and genotoxicity. Sci Transl Med 2014;6:227ra233. [DOI] [PubMed] [Google Scholar]
- 163.Walters M, Rasko JE, Hongeng S, et al. Uptdate of results from the northstar study (HGB-204): A phase 1/2 study of gene therapy for b-thalassemia major via transplantation of autologous hematopoietic stem cells transduced ex vivo with a lentiviral bA-T87Q-globin vector (Lentiglobin BB305 drug product). Blood 2015;126): Abstract 201 [Google Scholar]
- 164.Kanter J, Walters M, Hsieh M, et al. Initial results from study HGB-206: A phase 1 study evaluating gene therapy by transplantation of autologous CD34+ stem cells transduced ex vivo with the Lentiglobin BB305 lentiviral vector in subjects with severe sickle cell disease. Blood 2015;126: Abstract 3233 [Google Scholar]
- 165.Boulad F, Maggio A, Wang X, et al. Gene therapy for beta-thalassemia major: The Memorial Sloan Kettering experience. Cooley's Anemia Foundation Symposium, October 2015 [Google Scholar]
- 166.Lidonnici MR, Aprile A, Paleari Y, et al. Update on gene therapy clinical trial for the treatment of beta thalassemia major in Italy. Cooley's Anemia Foundation Symposium, October 2015 [Google Scholar]