Abstract
Background
Visceral and subcutaneous adipose tissue (VAT and SAT) vary in volume and quality. We evaluated whether fat volume or attenuation (indirect measure of quality) predicts metabolic risk factor changes.
Methods and Results
Framingham Heart Study Multi-detector Computed Tomography Substudy participants (n=1730, 45% women) were followed over a mean of 6.2 years. Baseline VAT and SAT volume (in cm3) and attenuation (in Hounsfield units, HU) were assessed. Outcomes included blood pressure, lipids and glucose. We constructed multivariable regression models predicting change from baseline to follow-up. Baseline VAT was associated with metabolic risk factors at follow-up. Per 500 cm3 increment in baseline VAT, glucose was 2.34 mg/dL higher (95% CI 1.71–2.97) and HDL was 1.62 mg/dL lower (95% CI 0.97–2.28) in women (p<0.0001 for both). These findings remained significant after adjustment for BMI. Results for SAT were similar, although less striking. Lower (more negative) fat attenuation was associated with more adverse metabolic profiles at follow-up. For example, per 5 unit decrease in baseline VAT HU, log triglycerides increased by 0.08 mg/dL (95% CI 0.05–0.12,p=0.005), which remained significant after adjustment for baseline VAT. Among men, VAT and SAT HU were associated with changes in CVD risk factors, but were mostly attenuated after baseline volume adjustment.
Conclusions
VAT and SAT volume are associated with incident metabolic risk factors beyond their contributions to overall adiposity. Decrements in fat attenuation are also associated with incident risk factors. These findings suggest that both volume and quality of VAT and SAT contribute to metabolic risk.
Keywords: Epidemiology, Population, Prevention, Adipose Depots
Introduction
VAT and SAT are distinct adipose depots that can be quantified using advanced radiographic techniques such as computed tomography (CT) and magnetic resonance imaging (MRI). While not routinely measured clinically, both VAT and SAT have been correlated with metabolic risk factors. In particular, VAT is associated with insulin resistance and markers of oxidative stress and inflammation.1–4 Obesity is known to be a heterogeneous condition, and metabolic risk can vary widely among obese individuals.5 A better understanding of the risks associated with distinct adipose depots may help explain some of this heterogeneity.
While both VAT and SAT are associated with an adverse metabolic risk profile, less is known about whether VAT or SAT predict future development of cardiovascular risk factors. Prior work has suggested that VAT, but not SAT or total fat mass predicted incident diabetes.6 VAT has been reported to predict development of cardiovascular disease or risk factors among certain ethnicities or specific age groups.7–9 Moreover, whether fat quality in different depots contributes to incident metabolic risk factors has not been widely explored. Large prospective studies evaluating both the volume and quality of distinct fat depots and their associations with development of metabolic risk factors are lacking. Thus, we sought to evaluate whether VAT or SAT volume and attenuation (as an indirect measure of fat quality) predicts development of a broad array of traditional cardiovascular risk factors over and above what is accounted for by BMI in a large population-based study.
Research Methods
Study Participants
The Framingham Heart Study is a longitudinal study of cardiovascular risk factors that began in 1948 with the enrollment of the original cohort.10, 11 Participants included in the current study were drawn from a subset of the Third Generation cohort that underwent multi-detector computed tomography (MDCT) scanning. A total of 1765 Third Generation participants who underwent MDCT and attended both exam 1 (2002–2005) and exam 2 (2008–2011) were included; the median time between CT exams was 6.2 years with an interquartile range of 6.0–6.4 years. After excluding individuals with missing MDCT measures or missing covariates, a total sample of 1730 (45% women) was available for analysis. Participants gave written informed consent; the FHS procedures and protocols were approved by the Institutional Review Boards at the Boston University Medical Center and the Massachusetts General Hospital..
VAT and SAT Measurements
Participants underwent MDCT of the chest and abdomen using Discovery VCT 64-slice PET/CT scanner (GE Healthcare). Thirty contiguous 5-mm thick slices (120kVP; 100–300mA dependent on BMI) were acquired, beginning 2 cm above the S1 vertebra. Subcutaneous adipose tissue (SAT) and visceral adipose tissue (VAT) volumes were measured using the Aquarius 3D Workstation (TeraRecon Inc., San Mateo, CA). SAT and VAT attenuation in Hounsfield units (HU) were measured using a previously described protocol.12 Briefly, the abdominal muscular wall separating the visceral and subcutaneous compartments was traced manually, and fat tissue was identified by HU attenuation between −195 and −45. Average HU of each fat depot generated the fat attenuation measure. In a previous study, our group reported high inter-reader and intra-reader correlation (0.997 for SAT, 0.992 for VAT).13
Metabolic Risk Factor Assessment
Metabolic risk factors were assessed at baseline and follow-up examinations. Seated systolic blood pressure (SBP) and diastolic blood pressure (DBP) were measured as part of the physician examinations. Plasma glucose, HDL cholesterol and triglycerides were measured on fasting blood samples.
Hypertension was defined as systolic blood pressure ≥ 140 mm Hg, diastolic blood pressure ≥ 90mm Hg, or on treatment. Hypertriglyceridemia was defined as triglycerides ≥ 150 mg/dL or on lipid-lowering treatment. Low HDL was defined as < 40 mg/dL in men or < 50 mg/dL in women. Type 2 diabetes was defined as a fasting glucose ≥ 126 mg/dL or on diabetes treatment. Metabolic syndrome was defined using modified Adult Treatment Panel III criteria.14
Covariate Assessment
Covariates were measured at the baseline exam. Height, weight and waist circumference at the level of the umbilicus were measured on site as part of each examination cycle. BMI was calculated as the weight in kilograms divided by the square of the height in meters. A technician-administered physical activity questionnaire yielded a physical activity index based on average number of hours of daily sleep and level of reported activity (sedentary, slight, moderate or high). Alcohol intake was assessed at the physician interview; moderate to heavy alcohol intake was defined as > 14 drinks/week in men and > 7 drinks/week in women. Participants were considered current smokers (if they had smoked at least 1 cigarette per day for the previous year), former smokers or never smokers. Women were classified as being menopausal if they had no menstrual bleeding for at least 1 year. Use of hormone replacement was assessed at the physician interview.
Statistical Analysis
We constructed sex-specific linear regression models predicting changes in systolic and diastolic blood pressure (SBP and DBP), fasting glucose, log transformed triglycerides and HDL-cholesterol. Estimates are given per 500 cm3 increment in SAT or VAT volume. For each metabolic risk factor, a multivariable model (Model 1) adjusted for age, physical activity, alcohol intake, smoking status, menopausal status (women only), and hormone replacement therapy (women only). A second model additionally adjusted Model 1 for BMI. The SBP and DBP models excluded individuals on anti-hypertensive medications at baseline; 73/771 women [9.5%] and 110/958 men [11.5%] were excluded. Weadded 10 mm Hg to the follow up value of SBP and 5 mm Hg to the follow up value of DBP if a participant was on anti-hypertensive medications at follow up.15 At follow-up, 78/698 women (11.2%) and 133/848 men (15.7%) reported use of anti-hypertensive medication. The glucose model excluded participants on diabetes medications at baseline; 11 of 772 women (1.4%) and 20 of 954 men (2.1%) were excluded due to use of diabetes medications at baseline. The triglyceride model excluded participants with hypertriglyceridemia at baseline; 40 of 772 women (5.2%) and 133 of 954 men (13.9%) were excluded due to use of lipid-lowering medication at baseline. Similar imputation methods for glucose and lipids do not exist and therefore were not used. The HDL model excluded participants with low HDL at baseline. Sex-interaction p-values were calculated for each outcome. Due to exclusions based on the baseline parameters, the sample sizes varied for each outcome.
Sex-specific logistic regression models were also constructed predicting incidence at the follow-up examination of diabetes, hypertension, low HDL, hypertriglyceridemia and metabolic syndrome for a 500 cm3 increment in baseline SAT or VAT volume. For each outcome, a multivariable model (Model 1) adjusted for age, physical activity, alcohol intake, smoking status, menopausal status (women only) and hormone replacement therapy. A second model additional adjusted Model 1 for BMI. The hypertension model was additionally adjusted for baseline SBP and baseline DBP. The diabetes model was additionally adjusted for baseline fasting glucose. Low HDL models and hypertriglyceridemia models were additionally adjusted for baseline HDL and baseline log triglycerides, respectively.
For the same outcomes described above, sex-specific linear and logistic regression models were also constructed predicting outcomes based on a 5 HU decrement in baseline fat attenuation. A multivariable model (Model 1) was constructed identical to the modeling structures described above for fat volume. A second model adjusted Model 1 for the corresponding fat volume.
All statistical analyses were conducted using SAS version 9.3. A two-sided 0.05 level of significance was used to declare statistical significance; there were no adjustments made for multiple comparisons.
Results
Study Sample Characteristics
Sex-specific baseline characteristics of study participants are shown in Table 1. Of the 1730 included individuals, nearly half (45%) were women. Mean age at baseline was 46.0 years among women and 44.1 years among men. Mean BMI was in the overweight range for both women and men.
Table 1.
Baseline Study Sample Characteristics. Continuous data shown are mean (SD) except for triglycerides (median with interquartile range); Fat volume and attenuation data are mean (SD) followed by median with interquartile range. Categorical data are shown as % (n).
| Women n=772 |
Men n = 958 |
|
|---|---|---|
| Age (years) | 46.0 (5.7) | 44.1 (6.3) |
| Current Smoking (%) | 12.7 (98) | 14.3 (137) |
| Physical Activity Index | 36.4 (5.9) | 38.3 (9.1) |
| Moderate - Heavy Alcohol Use (%)* | 14.5 (112) | 15.7 (150) |
| Postmenopausal (%) | 24.0 (185) | N/A |
| Hormone Replacement (%) | 8.4 (65) | N/A |
| BMI (kg/m^2) | 26.3 (5.8) | 28.0 (4.4) |
| Normal Weight (BMI< 25 kg/m2) (%) | 51.3 | 24.1 |
| Overweight (BMI 25 to < 30 kg/m2) (%) | 26.4 | 49.7 |
| Obese (BMI ≥30 kg/m2) (%) | 22.3 | 26.2 |
| Waist Circumference (cm) | 90 (15) | 99 (12) |
| Systolic Blood Pressure (mmHg) | 117 (16) | 121 (13) |
| Diastolic Blood Pressure (mmHg) | 74 (9) | 79 (9) |
| Fasting Glucose (mg/dl) | 93 (16) | 100 (21) |
| HDL Cholesterol (mg/dl) | 63 (18) | 47 (12) |
| Triglycerides (mg/dl) | 81.0 (61.0,113.5) | 112.0 (75.0,168.0) |
| Hypertension (%)† | 15.7 (121) | 24.0 (230) |
| Diabetes (%)‡‡ | 2.3 (18) | 3.9 (37) |
| Low HDL Cholesterol (%)†† | 23.6 (182) | 29.8 (285) |
| Hypertriglyceridemia (%)‡ | 15.8 (122) | 40.3 (386) |
| Metabolic Syndrome (%)** | 17.0 (131) | 31.9 (305) |
| VAT Volume (cm^3) | 1103 (715) 885 (537, 1493) |
1977 (877) 1897 (1334, 2533) |
| SAT Volume (cm^3) | 2954 (1548) 2627 (1828, 3893) |
2566 (1227) 2334 (1745, 3162) |
| VAT Attenuation (HU) | −91.9 (4.3) −91.4 (−95.5, −88.5) |
−95.5 (4.5) −96.4 (−98.8, −92.5) |
| SAT Attenuation (HU) | −101.9 (5.3) −103.2 (−105.4, −99.6) |
−99.8 (4.6) −100.6 (−102.9, −98.2) |
Defined as > 14 drinks/week in men, > 7 drinks/week in women
Defined as SBP ≥ 140 or DBP ≥ 90mm Hg or use of anti-hypertension medication
HDL < 40 mg/dL in men or < 50mg/dL in women
Defined as triglycerides ≥ 150mg/dL
Defined as fasting glucose ≥ 126 mg/dL or use of anti-diabetes medication
Metabolic Syndrome defined by modified ATP III criteria14
Fat volume and Incident CVD Risk Factors
Results of sex-specific linear and logistic regression models predicting changes in metabolic parameters from VAT and SAT volume are shown in Tables 2 and 3.
Table 2.
Sex-Specific Linear Regression Models predicting change (with 95% CI) in each outcome at follow-up per 500 cm3 increment in baseline fat volume†
| WOMEN | MEN | Sex Inter- action |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| MV‡ | p | MV + BMI | p | MV‡ | p | MV + BMI | p | p | |
| SBP | N=698 | N=848 | |||||||
| VAT | 1.77 (1.04, 2.50) | <0.0001 | 0.86 (−0.31, 2.03) | 0.15 | 1.02 (0.58, 1.47) | <0.0001 | 1.04 (0.40, 1.67) | 0.0013 | 0.65 |
| SAT | 0.96 (0.65,1.28) | <0.0001 | 1.22 (0.51, 1.92) | 0.0008 | 0.35 (0.05, 0.66) | 0.02 | −0.17 (−0.74, 0.39) | 0.55 | 0.13 |
| DBP | N=696 | N=848 | |||||||
| VAT | 1.04 (0.56, 1.51) | <0.0001 | 0.77 (>−0.01, 1.54) | 0.051 | 0.42 (0.10, 0.74) | 0.01 | 0.44 (−0.01, 0.88) | 0.06 | 0.06 |
| SAT | 0.55 (0.35, 0.76) | <0.0001 | 0.93 (0.46, 1.40) | 0.0001 | 0.12 (−0.09, 0.34) | 0.25 | −0.13 (−0.53, 0.27) | 0.53 | 0.02 |
| Glucose | N=761 | N=933 | |||||||
| VAT | 2.34 (1.71, 2.97) | <0.0001 | 2.81 (1.78, 3.84) | <0.0001 | 1.19 (0.56, 1.82) | 0.0002 | 0.87 (−0.02, 1.76) | 0.06 | 0.06 |
| SAT | 0.71 (0.43, 1.00) | <0.0001 | 0.36 (−0.28, 1.01) | 0.27 | 0.80 (0.38, 1.23) | 0.0002 | 0.67 (−0.16, 1.50) | 0.11 | 0.66 |
| HDL | N=732 | N=821 | |||||||
| VAT | −1.62 (−2.28, −0.97) | <0.0001 | −1.86 (−2.98, −0.74) | 0.001 | −0.44 (−0.85, −0.03) | 0.04 | 0.12 (−0.46, 0.70) | 0.69 | 0.01 |
| SAT | −0.60 (−0.89, −0.31) | <0.0001 | −0.67 (−1.37, 0.03) | 0.06 | −0.39 (−0.66, −0.13) | 0.004 | 0.01 (−0.51, 0.53) | 0.97 | 0.54 |
| Log Triglycerides | N=732 | N=821 | |||||||
| VAT | 0.05 (0.03, 0.07) | <0.0001 | 0.07 (0.03, 0.10) | 0.0002 | 0.01 (−0.00, 0.03) | 0.14 | 0.00 (−0.02, 0.03) | 0.86 | 0.08 |
| SAT | 0.02 (0.01, 0.02) | 0.0007 | 0.03 (< 0.01, 0.05) | 0.02 | 0.01 (−0.00, 0.02) | 0.28 | −0.01 (−0.03, 0.01) | 0.28 | 0.44 |
Estimates are change in the parameter (Exam 2 value − Exam 1 value) for a 500 cm3 increase in fat volume. Positive values indicate that larger values of SAT or VAT at Exam 1 are associated with increases in the parameter. Negative values indicate that larger values of SAT or VAT at Exam 1 are associated with decreases in the parameter at follow up.
MV model adjusted for age, physical activity, alcohol intake, smoking status, menopausal status (Women only), and Hormone Replacement Therapy (Women only). Additional covariates included Exam 1 values of the parameter. SBP and DBP models excluded participants on hypertensive medications at baseline (and imputed +10/+5 if patient was on hypertension medications at Exam 2). Other models excluded participants on diabetes medications or lipid lowering medications (as appropriate)
Table 3.
Sex-Specific Logistic Regression Models predicting incidence of each risk factor per 500cm3 increment in baseline fat volume†
| WOMEN | MEN | Sex Inter- action |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| MV‡ | p | MV + BMI | p | MV‡ | p | MV + BMI | p | p | |
| Hypertension | N=75/648 | N=121/728 | |||||||
| VAT | 1.24 (1.02, 1.51) | 0.03 | 1.13 (0.83, 1.53) | 0.44 | 1.28 (1.12, 1.47) | 0.0003 | 1.29 (1.07, 1.56) | 0.01 | 0.62 |
| SAT | 1.16 (1.06, 1.26) | 0.001 | 1.38 (1.11, 1.71) | 0.004 | 1.04 (0.95, 1.13) | 0.40 | 0.80 (0.67, 0.95) | 0.01 | 0.13 |
| Diabetes | N=20/753 | N=36/916 | |||||||
| VAT | 1.46 (1.05, 2.02) | 0.02 | 1.50 (0.88, 2.56) | 0.14 | 1.34 (1.08, 1.65) | 0.008 | 1.09 (0.83, 1.44) | 0.52 | 0.85 |
| SAT | 1.12 (0.97, 1.29) | 0.13 | 0.99 (0.73, 1.35) | 0.97 | 1.19 (1.05, 1.36) | 0.006 | 0.92 (0.72, 1.19) | 0.53 | 0.46 |
| Low HDL | N=19/590 | N=36/671 | |||||||
| VAT | 1.15 (0.85, 1.56) | 0.36 | 1.07 (0.63, 1.81) | 0.81 | 1.15 (0.93, 1.42) | 0.19 | 0.99 (0.74, 1.33) | 0.96 | 0.97 |
| SAT | 1.09 (0.95, 1.25) | 0.24 | 1.17 (0.80, 1.72) | 0.42 | 1.13 (0.99, 1.30) | 0.06 | 1.03 (0.77, 1.38) | 0.84 | 0.73 |
| Hyper-triglyceridemia | N=96/650 | N=125/570 | |||||||
| VAT | 1.19 (1.01, 1.40) | 0.04 | 1.36 (1.01, 1.84) | 0.045 | 1.13 (0.99, 1.29) | 0.06 | 1.07 (0.89, 1.29) | 0.46 | 0.64 |
| SAT | 1.02 (0.95, 1.11) | 0.59 | 0.92 (0.76, 1.10) | 0.35 | 1.04 (0.95, 1.13) | 0.41 | 0.87 (0.73, 1.03) | 0.12 | 0.79 |
| Metabolic Syndrome | N=78/639 | N=108/651 | |||||||
| VAT | 2.58 (2.05, 3.25) | <0.0001 | 2.50 (1.78, 3.52) | <0.0001 | 1.70 (1.45, 1.98) | <0.0001 | 1.31 (1.07, 1.60) | 0.01 | 0.007 |
| SAT | 1.36 (1.25, 1.49) | <0.0001 | 1.08 (0.87, 1.33) | 0.48 | 1.36 (1.23, 1.52) | <0.0001 | 0.91 (0.75, 1.11) | 0.36 | 0.87 |
Estimates are odds ratios (95% CI) of the condition for a 500 cm^3 increase in fat volume.
MV model adjusted for age, physical activity, alcohol intake, smoking status, menopausal status (Women only), and Hormone Replacement Therapy (Women only). Diabetes models were additionally adjusted for fasting glucose (at baseline). Hypertension model was additionally adjusted for baseline SBP and baseline DBP. Low HDL models and Hypertriglyceridemia models were additionally adjusted for baseline HDL and baseline log triglycerides, respectively.
Among women, baseline VAT volume was associated with incident increases in metabolic risk factors. For example, for each additional 500 cm3 in baseline VAT, we observed a 2.34 mg/dL increase in fasting glucose from baseline to follow up (95% CI 1.71–2.97), and a 1.62 mg/dL decrease in HDL-cholesterol (95% CI 0.97–2.28) (p<0.0001 for both) at follow-up. For each additional 500 cm3 in VAT at baseline, the odds of the metabolic syndrome were 2.58 times higher (95% CI 2.05–3.25, p<0.0001). In most cases, significance persisted after BMI adjustment.
In men, increased baseline VAT volume was also associated with higher odds of several incident risk factors. We observed higher odds of hypertension (OR 1.28, 95% CI 1.12–1.47) and metabolic syndrome (OR 1.70, 95% CI 1.45–1.98) (p<0.001 in both cases) for every 500 cm3 increment in VAT volume, and for these outcomes, the findings persisted after BMI adjustment. Baseline SAT volume was associated with changes in some risk factors over time among women. For example, each additional 500cm3 increment in baseline SAT volume was associated with a 0.96 mm Hg increase in SBP (95% CI 0.65–1.28, p<0.0001), and a 0.55 mm Hg increase in DBP (95% CI 0.35–0.76, p<0.0001). For each 500 cm3 increment in baseline SAT volume, the odds of hypertension at follow-up was 1.16 times higher (95% CI 1.06–1.26, p = 0.001). These blood pressure increases among women persisted after BMI adjustment. Among men, increments in baseline SAT volume were not associated with significant changes in risk factors over time.
Tests for sex interaction were significant for several outcomes, and uniformly the associations between each fat exposure and outcomes were more adverse among women (Tables 2 and 3). For example, a significant sex-interaction was detected for HDL-cholesterol predicted by baseline VAT volume (p = 0.01, Table 2), whereby the results were stronger in women (per 500 cm3 increment in VAT volume, HDL-cholesterol was 1.62 mg/dL lower among women at follow up versus 0.44 mg/dL lower among men at follow up).
Fat Attenuation and Incident Risk Factors
Results of sex-specific linear and logistic regression models for fat attenuation are shown in Tables 4 and 5. Among women, lower fat attenuation was associated with a more adverse metabolic profile at follow-up. For example, for a 5 unit decrease in VAT HU at baseline, we observed a 0.08 mg/dL increase in log triglycerides (95% CI 0.05–0.12, p=0.005) at follow up. For a 5 unit decrease in SAT HU at baseline, we observed a 2.19 mm Hg increase in SBP (95% 1.35–3.03, p<0.0001). These findings remained significant after adjustment for baseline fat volume. Among men, VAT HU and SAT HU were associated with changes in risk factors including SBP and glucose from baseline to follow up, however these changes were attenuated after adjusting for baseline fat volume. In secondary analyses adjusting the fat attenuation models for either VAT or BMI, the results were generally similar to the fat attenuation models adjusted for the corresponding fat volume only (Supplementary Tables 1 and 2).
Table 4.
Sex-Specific Linear Regression Models predicting change in each outcome between exams per 5 HU decrement baseline in fat attenuation†
| WOMEN | MEN | Sex Inter- action |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| MV‡ | p | MV + Fat Volume |
p | MV‡ | p | MV + Fat Volume |
p | p | |
| SBP | N=698 | N=848 | |||||||
| VAT HU | 1.49 (0.37, 2.60) | 0.01 | −0.61 (−2.11, 0.89) | 0.43 | 1.68 (0.88, 2.48) | <0.0001 | 0.65 (−0.61, 1.92) | 0.31 | 0.35 |
| SAT HU | 2.19 (1.35, 3.03) | <0.0001 | 1.23 (0.28, 2.19) | 0.01 | 0.80 (0.04, 1.56) | 0.04 | 0.43 (−0.51, 1.36) | 0.37 | 0.07 |
| DBP | N=696 | N=848 | |||||||
| VAT HU | 0.42 (−0.32, 1.16) | 0.27 | −1.15 (−2.13, −0.17) | 0.02 | 0.76 (0.19, 1.34) | 0.01 | 0.44 (−0.46, 1.33) | 0.34 | 0.54 |
| SAT HU | 1.35 (0.80, 1.89) | <0.0001 | 0.83 (0.20, 1.46) | 0.01 | 0.53 (−0.01, 1.07) | 0.053 | 0.52 (−0.13, 1.18) | 0.12 | 0.046 |
| Glucose | N=761 | N=933 | |||||||
| VAT HU | 2.91 (1.90, 3.91) | <0.0001 | 0.65 (−0.73, 2.04) | 0.36 | 2.23 (1.10, 3.37) | 0.0001 | 1.40 (−0.44, 3.24) | 0.14 | 0.44 |
| SAT HU | 1.34 (0.56, 2.12) | 0.0008 | 0.48 (−0.42, 1.37) | 0.30 | 1.69 (0.56, 2.82) | 0.004 | 0.66 (−0.75, 2.07) | 0.36 | 0.70 |
| HDL | N=732 | N=821 | |||||||
| VAT HU | −2.19 (−3.26, −1.13) | <0.0001 | −0.73 (−2.19, 0.74) | 0.33 | −0.44 (−1.23, 0.35) | 0.27 | 0.44 (−0.75, 1.64) | 0.47 | 0.04 |
| SAT HU | −2.68 (−3.46, −1.89) | <0.0001 | −2.46 (−3.37, −1.55) | <0.0001 | −0.66 (−1.39, 0.06) | 0.07 | −0.09 (−0.97, 0.79) | 0.84 | 0.0008 |
| Log Triglycerides | N=732 | N=821 | |||||||
| VAT HU | 0.08 (0.05, 0.12) | <0.0001 | 0.06 (0.02, 0.11) | 0.005 | 0.01 (−0.02, 0.04) | 0.64 | −0.02 (−0.07, 0.03) | 0.37 | 0.01 |
| SAT HU | 0.06 (0.03, 0.08) | <0.0001 | 0.05 (0.02, 0.07) | 0.002 | 0.01 (−0.02, 0.04) | 0.65 | > −0.01 (−0.04, 0.03) | 0.85 | 0.06 |
Estimates are change in the parameter (exam 2 value − exam 1 value) for a 5 unit decrease in HU (more negative).
MV model adjusted for age, physical activity, alcohol intake, smoking status, menopausal status (Women only), and Hormone Replacement Therapy (Women only). Additional covariates included Exam 1 values of the parameter. SBP and DBP models excluded participants on hypertensive medications at baseline (and imputed +10/+5 if patient was on hypertension medications at Exam 2). Other models excluded participants on diabetes medications or lipid lowering medications (as appropriate).
Table 5.
Sex-Specific Logistic Regression Models predicting incidence of each risk factor per 5 HU decrement in baseline fat attenuation†
| WOMEN | MEN | Sex Inter- action |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| MV‡ | p | MV + Fat Volume |
p | MV‡ | p | MV + Fat Volume |
p | p | |
| Hypertension | N=75/648 | N=121/728 | |||||||
| VAT HU | 1.48 (1.05, 2.08) | 0.02 | 1.31 (0.81, 2.10) | 0.27 | 1.34 (1.03, 1.74) | 0.03 | 0.81 (0.53, 1.23) | 0.31 | 0.77 |
| SAT HU | 1.29 (0.94, 1.77) | 0.12 | 1.06 (0.76, 1.50) | 0.72 | 1.02 (0.79, 1.31) | 0.91 | 0.94 (0.70, 1.27) | 0.69 | 0.40 |
| Diabetes | N=20/753 | N=36/916 | |||||||
| VAT HU | 1.40 (0.75, 2.64) | 0.29 | 0.59 (0.21, 1.64) | 0.31 | 1.54 (0.92, 2.59) | 0.10 | 0.92 (0.45, 1.87) | 0.81 | 0.80 |
| SAT HU | 1.38 (0.67, 2.85) | 0.38 | 1.19 (0.55, 2.58) | 0.66 | 1.24 (0.72, 2.13) | 0.44 | 0.84 (0.47, 1.51) | 0.57 | 0.84 |
| Low HDL | N=19/590 | N=36/671 | |||||||
| VAT HU | 1.26 (0.71, 2.21) | 0.43 | 1.08 (0.45, 2.60) | 0.86 | 0.88 (0.59, 1.32) | 0.54 | 0.42 (0.22, 0.81) | 0.01 | 0.34 |
| SAT HU | 1.24 (0.72, 2.14) | 0.44 | 1.10 (0.60, 2.00) | 0.75 | 0.83 (0.58, 1.19) | 0.31 | 0.57 (0.37, 0.89) | 0.01 | 0.16 |
| Hypertriglyceridemia | N=96/650 | N=125/570 | |||||||
| VAT HU | 1.38 (1.04, 1.85) | 0.03 | 1.27 (0.83, 1.94) | 0.27 | 1.05 (0.81, 1.35) | 0.72 | 0.74 (0.51, 1.09) | 0.13 | 0.22 |
| SAT HU | 1.42 (1.05, 1.93) | 0.02 | 1.48 (1.06, 2.07) | 0.02 | 0.91 (0.72, 1.15) | 0.42 | 0.80 (0.61, 1.06) | 0.13 | 0.03 |
| Metabolic Syndrome | N=78/639 | N=108/651 | |||||||
| VAT HU | 3.07 (2.20, 4.28) | <0.0001 | 1.20 (0.74, 1.95) | 0.47 | 1.87 (1.44, 2.44) | <0.0001 | 0.82 (0.54, 1.25) | 0.36 | 0.04 |
| SAT HU | 2.46 (1.72, 3.52) | <0.0001 | 1.68 (1.15, 2.47) | 0.01 | 1.25 (0.98, 1.59) | 0.07 | 0.76 (0.57, 1.01) | 0.06 | 0.003 |
Estimates are odds ratios (95% CI) for the condition for a 5 unit decrease in HU.
MV model adjusted for age, physical activity, alcohol intake, smoking status, menopausal status (Women only), and Hormone Replacement Therapy (Women only). Diabetes/IFG models were additionally adjusted for fasting glucose (at baseline). Hypertension model was additionally adjusted for baseline SBP and baseline DBP. Low HDL models and Hypertriglyceridemia models were additionally adjusted for baseline HDL and baseline triglycerides, respectively.
Several sex interactions were noted in the regression models predicting changes based on fat attenuation (Tables 4 and 5). The sex-specific models suggested that the changes in risk factors per decrement in fat attenuation were more pronounced among women. For example, significant sex interactions were detected between fat attenuation (both SAT HU and VAT HU) and HDL-cholesterol (p<0.001 and p<0.05, respectively); in the sex-specific models, HDL-cholesterol decreased more among women than among men per 5 HU decrement in fat attenuation.
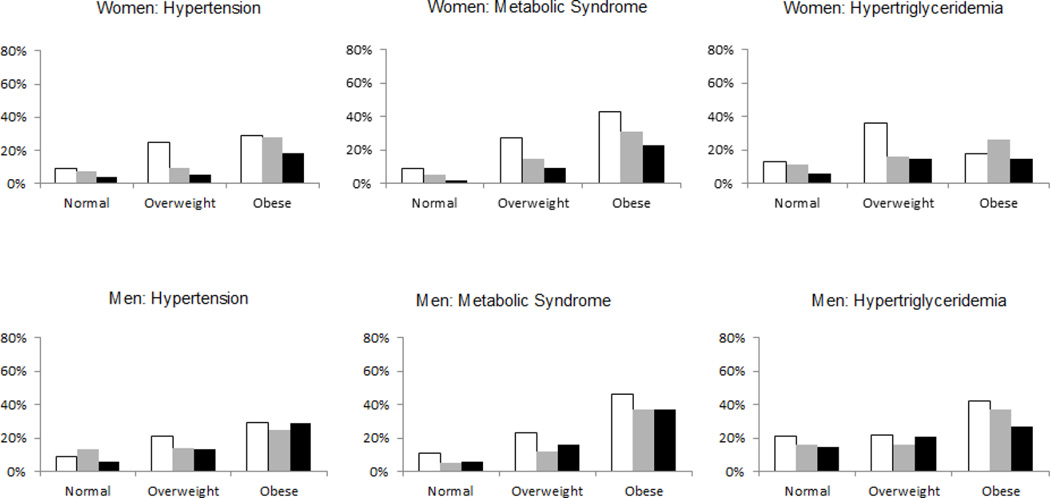
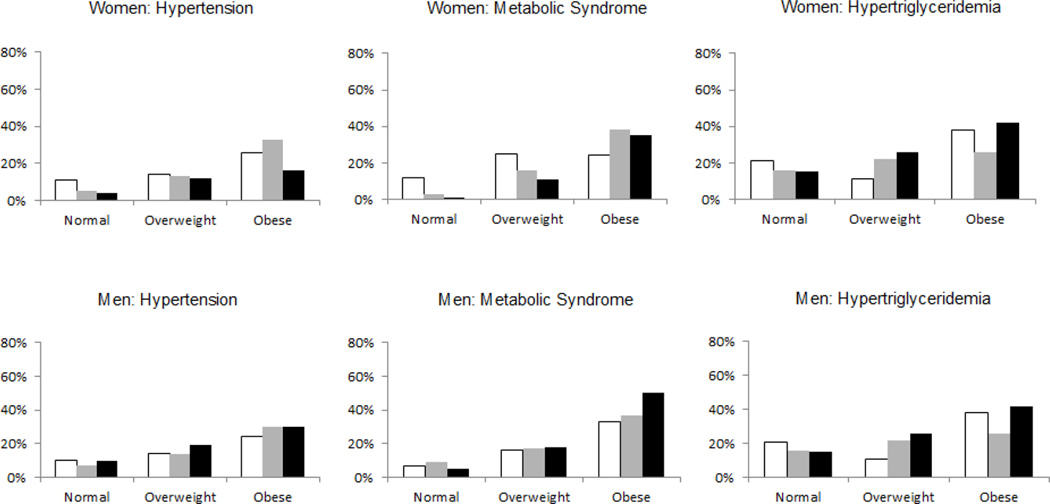
Figures 1 and 2 depict sex-specific baseline prevalence of selected risk factors stratified byVAT attenuation tertiles and SAT attenuation tertiles, respectively, within BMI categories (normal, overweight or obese). Figure 1 shows a general pattern of higher baseline prevalence of risk factors among the lowest VAT attenuation tertiles within each BMI category. This pattern is less pronounced for SAT attenuation.
Figure 1. Sex-specific incidence of risk factors stratified by VAT attenuation tertiles within BMI Categories.
Normal weight defined as BMI < 25 kg/m2, overweight defined as BMI 25 to < 30 kg/m2, obese defined as BMI ≥ 30 kg/m2. VAT attenuation tertiles depicted as white bars (lowest tertile, i.e. most negative attenuation), gray bars (middle tertile) or black bars (highest tertile, i.e. least negative attenuation). Due to exclusion of individuals with the outcome present at baseline, the study populations differed for each outcome. For hypertension, the study populations consisted of 361, 171 and 116 women and 205, 370 and 153 men in the normal, overweight and obese categories, respectively. For metabolic syndrome, the study populations were 389, 162 and 89 women and 222, 348 and 82 men in each successive BMI category. For hypertriglyceridemia, the study populations were 370, 165 and 115 women, and 187, 271 and 113 men in each successive BMI category.
Figure 2. Sex-specific incidence of risk factors stratified by SAT attenuation tertiles within BMI Categories.
Normal weight defined as BMI < 25 kg/m2, overweight defined as BMI 25 to < 30 kg/m2, obese defined as BMI ≥ 30 kg/m2. SAT attenuation tertiles depicted as white bars (lowest tertile, i.e. most negative attenuation), gray bars (middle tertile) or black bars (highest tertile, i.e. least negative attenuation). Due to exclusion of individuals with the outcome present at baseline, the study populations differed for each outcome. For hypertension, the study populations consisted of 361, 171 and 116 women and 205, 370 and 153 men in the normal, overweight and obese categories, respectively. For metabolic syndrome, the study populations were 389, 162 and 89 women and 222, 348 and 82 men in each successive BMI category. For hypertriglyceridemia, the study populations were 370, 165 and 115 women, and 187, 271 and 113 men in each successive BMI category.
Discussion
Principal Findings
Our principal findings are threefold. First, higher VAT volume is associated with the development of cardiovascular risk factors over time, and BMI did not fully account for these associations. Second, higher SAT volume is associated with incident risk factors, particularly among women, and these associations are not fully explained by BMI. Third, lower VAT and SAT attenuation, as indirect measures of fat quality, are associated with development of an adverse risk profile. Fat volume did not fully account for the associations of fat attenuation with incident risk factors.
In the context of the current literature
The present study prospectively examined the association between both fat volume and attenuation and incident metabolic risk factors. Our results expand upon prior work that has shown associations between VAT volume and incident metabolic risk. Published studies have shown a correlation between VAT volume and incidence of type 2 diabetes,6, 8 hypertension7 and dyslipidemia.16 Our findings were observed in both women and men. We robustly assessed and adjusted for a number of possible confounders. The association between VAT and metabolic risk factors persisted after BMI adjustment, further supporting the idea that VAT is a unique pathogenic depot that confers risk beyond its contribution to overall adiposity.
There is less published data about incident associations of SAT volume. A recent study of 732 obese adults divided participants into tertiles of SAT volume and found no trend for incident diabetes with increasing SAT.6 In the present study, we observed associations between SAT and several incident risk factors, particularly among women. These findings relate in part to our larger sample size, which lends better power to detect differences, and our robust assessment of an array of risk factors.
Fat quality in association with metabolic risk has been less extensively explored than fat volume. We have previously shown that lower VAT and SAT attenuation (i.e. more negative HU) was cross-sectionally associated with a more adverse metabolic profile even after adjustment for absolute fat volume.12 The results from the present study extend these findings to suggest that measurement of fat quality can predict future development of metabolic risk factors, and that for women, fat quality is predictive of selected incident CVD risk factors beyond fat volume alone.
Potential mechanisms
The unique pathogenic property of VAT, beyond its contributions to overall adiposity, may be due its role as an endocrine organ secreting adipokines that contribute to inflammation and insulin resistance. It is known that macrophages infiltrate VAT, leading to inflammation.17, 18 It is also known that VAT secretes a more pro-inflammatory cytokine profile than SAT, characterized by higher TNF-alpha and other pro-inflammatory molecules.19
The observation that SAT is associated with metabolic risk factors is partly explained by its contribution to overall adiposity. Our observation that, in women, SAT may have adverse metabolic effects beyond overall adiposity may be partly due to the fact that women have a wider baseline range of SAT volume compared to men (shown in Table 1). This wider range at baseline allows for better detection of differences based on SAT volume.20 Thus any unique pathogenic properties are likely to be more pronounced. For example serum leptin levels are more correlated with SAT than with VAT.19 High leptin may indicate leptin resistance, which is predictive of type 2 diabetes.21 Another possibility is that in women, SAT is less likely to proliferate new cells (hyperplasia) and instead expands primarily by increasing adipocyte size (hypertrophy).22 Increased adipocyte size is associated with insulin resistance.23, 24 This may represent failure of SAT to expand sufficiently to store excess fat, leading to accumulation into VAT or other ectopic depots. However, a secondary analysis found that the associations of SAT with CVD risk factors in women were not materially different after adjusting for VAT volume.
The observation that both VAT and SAT attenuation, as indirect measures of fat quality, are associated with metabolic risk factors may relate to the fact that more negative fat attenuation represents more lipid-dense fat tissue, which in turn correlates with adipocyte size.25 As noted above, larger adipocyte size may represent an inability to proliferate new adipocyte cells and is associated with insulin resistance.24 The observation that the associations of fat quality with metabolic risk are not fully explained by fat volume, again suggests that SAT in women may have limited capacity for hyperplasia, leading to ectopic fat deposition.26
An alternative hypothesis for the relevance of fat quality in predicting metabolic risk is that fat quality may be affected by vascularity of adipose tissue. Blood has higher HU attenuation than fat on CT.27 Thus, lower HU attenuation of a fat depot may indicate relative dearth of vascularity. It is plausible that lack of vascularity could lead to hypoxia and higher inflammation, both of which may mediate pathogenic effects of adipose tissue.3, 28
Implications
The results of the present study show that both VAT and SAT are associated with the development of metabolic risk independent of their contributions to overall adiposity. Our findings also demonstrate that fat attenuation is informative over and above fat volume alone in understanding metabolic risk associated with fat depots. Finally, our results suggest that there may be important differences between men and women in how fat depots contribute to metabolic risk. Future research is needed to better understand the cellular and molecular mechanisms by which these unique depots confer risk.
Strengths and limitations
The large sample size is a strength of our study. Fat depots were precisely assessed with validated tools and strong inter-reader reliability. The data is prospective and allowed us to assess change over time. Metabolic risk factors were directly measured on site, which is more reliable than self-report. Because this is an observational study, we cannot infer causality between the measures of VAT and SAT and the metabolic outcomes. The Framingham cohort is primarily white, thus results may not be generalizable to other ethnicities. Finally, the numbers of some of the incident clinical risk factors were low. Our continuous analysis reinforces the association between increasing fat quantity and lower attenuation and change in CVD risk factors over time.
Conclusion
Both VAT and SAT volume are associated with incident metabolic risk factors after BMI adjustment, particularly among women. Fat attenuation, as an indirect measure of fat quality, provides additional information beyond fat volume. These findings suggest that both volume and quality of distinct fat depots contribute to an individual’s metabolic risk, and that knowledge of the properties of fat depots has the potential to further our understanding of the heterogeneous metabolic risks conferred by obesity.
Supplementary Material
Acknowledgments
Funding Sources: The Framingham Heart Study of the National Heart, Lung and Blood Institute is supported by contract N01-HC-25195. T.M.A. is supported by an NIH-sponsored T32 training grant.
Footnotes
Disclosures: A.P. works for Merck.
References
- 1.Fox CS, Massaro JM, Hoffmann U, Pou KM, Maurovich-Horvat P, Liu CY, Vasan RS, Murabito JM, Meigs JB, Cupples LA, D'Agostino RB, Sr, O'Donnell CJ. Abdominal visceral and subcutaneous adipose tissue compartments: Association with metabolic risk factors in the framingham heart study. Circulation. 2007;116:39–48. doi: 10.1161/CIRCULATIONAHA.106.675355. [DOI] [PubMed] [Google Scholar]
- 2.Wagenknecht LE, Langefeld CD, Scherzinger AL, Norris JM, Haffner SM, Saad MF, Bergman RN. Insulin sensitivity, insulin secretion, and abdominal fat: The insulin resistance atherosclerosis study (iras) family study. Diabetes. 2003;52:2490–2496. doi: 10.2337/diabetes.52.10.2490. [DOI] [PubMed] [Google Scholar]
- 3.Pou KM, Massaro JM, Hoffmann U, Vasan RS, Maurovich-Horvat P, Larson MG, Keaney JF, Jr, Meigs JB, Lipinska I, Kathiresan S, Murabito JM, O'Donnell CJ, Benjamin EJ, Fox CS. Visceral and subcutaneous adipose tissue volumes are cross-sectionally related to markers of inflammation and oxidative stress: The framingham heart study. Circulation. 2007;116:1234–1241. doi: 10.1161/CIRCULATIONAHA.107.710509. [DOI] [PubMed] [Google Scholar]
- 4.Hamdy O, Porramatikul S, Al-Ozairi E. Metabolic obesity: The paradox between visceral and subcutaneous fat. Curr Diabetes Rev. 2006;2:367–373. doi: 10.2174/1573399810602040367. [DOI] [PubMed] [Google Scholar]
- 5.Bell JA, Kivimaki M, Hamer M. Metabolically healthy obesity and risk of incident type 2 diabetes: A meta-analysis of prospective cohort studies. Obes Rev. 2014;15:504–515. doi: 10.1111/obr.12157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Neeland IJ, Turer AT, Ayers CR, Powell-Wiley TM, Vega GL, Farzaneh-Far R, Grundy SM, Khera A, McGuire DK, de Lemos JA. Dysfunctional adiposity and the risk of prediabetes and type 2 diabetes in obese adults. JAMA. 2012;308:1150–1159. doi: 10.1001/2012.jama.11132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hayashi T, Boyko EJ, Leonetti DL, McNeely MJ, Newell-Morris L, Kahn SE, Fujimoto WY. Visceral adiposity is an independent predictor of incident hypertension in japanese americans. Ann Intern Med. 2004;140:992–1000. doi: 10.7326/0003-4819-140-12-200406150-00008. [DOI] [PubMed] [Google Scholar]
- 8.Boyko EJ, Fujimoto WY, Leonetti DL, Newell-Morris L. Visceral adiposity and risk of type 2 diabetes: A prospective study among japanese americans. Diabetes Care. 2000;23:465–471. doi: 10.2337/diacare.23.4.465. [DOI] [PubMed] [Google Scholar]
- 9.Nicklas BJ, Penninx BW, Cesari M, Kritchevsky SB, Newman AB, Kanaya AM, Pahor M, Jingzhong D, Harris TB. Association of visceral adipose tissue with incident myocardial infarction in older men and women: The health, aging and body composition study. Am J Epidemiol. 2004;160:741–749. doi: 10.1093/aje/kwh281. [DOI] [PubMed] [Google Scholar]
- 10.Dawber TR, Kannel WB, Lyell LP. An approach to longitudinal studies in a community: The framingham study. Ann N Y Acad Sci. 1963;107:539–556. doi: 10.1111/j.1749-6632.1963.tb13299.x. [DOI] [PubMed] [Google Scholar]
- 11.Splansky GL, Corey D, Yang Q, Atwood LD, Cupples LA, Benjamin EJ, D'Agostino RB, Sr, Fox CS, Larson MG, Murabito JM, O'Donnell CJ, Vasan RS, Wolf PA, Levy D. The third generation cohort of the national heart, lung, and blood institute's framingham heart study: Design, recruitment, and initial examination. Am J Epidemiol. 2007;165:1328–1335. doi: 10.1093/aje/kwm021. [DOI] [PubMed] [Google Scholar]
- 12.Rosenquist KJ, Pedley A, Massaro JM, Therkelsen KE, Murabito JM, Hoffmann U, Fox CS. Visceral and subcutaneous fat quality and cardiometabolic risk. JACC Cardiovasc Imaging. 2013;6:762–771. doi: 10.1016/j.jcmg.2012.11.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Maurovich-Horvat P, Massaro J, Fox CS, Moselewski F, O'Donnell CJ, Hoffmann U. Comparison of anthropometric, area- and volume-based assessment of abdominal subcutaneous and visceral adipose tissue volumes using multi-detector computed tomography. Int J Obes (Lond) 2007;31:500–506. doi: 10.1038/sj.ijo.0803454. [DOI] [PubMed] [Google Scholar]
- 14.Executive summary of the third report of the national cholesterol education program (ncep) expert panel on detection, evaluation, and treatment of high blood cholesterol in adults (adult treatment panel iii) JAMA. 2001;285:2486–2497. doi: 10.1001/jama.285.19.2486. [DOI] [PubMed] [Google Scholar]
- 15.Levy D, Ehret GB, Rice K, Verwoert GC, Launer LJ, Dehghan A, Glazer NL, Morrison AC, Johnson AD, Aspelund T, Aulchenko Y, Lumley T, Kottgen A, Vasan RS, Rivadeneira F, Eiriksdottir G, Guo X, Arking DE, Mitchell GF, Mattace-Raso FU, Smith AV, Taylor K, Scharpf RB, Hwang SJ, Sijbrands EJ, Bis J, Harris TB, Ganesh SK, O'Donnell CJ, Hofman A, Rotter JI, Coresh J, Benjamin EJ, Uitterlinden AG, Heiss G, Fox CS, Witteman JC, Boerwinkle E, Wang TJ, Gudnason V, Larson MG, Chakravarti A, Psaty BM, van Duijn CM. Genome-wide association study of blood pressure and hypertension. Nat Genet. 2009;41:677–687. doi: 10.1038/ng.384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nicklas BJ, Penninx BW, Ryan AS, Berman DM, Lynch NA, Dennis KE. Visceral adipose tissue cutoffs associated with metabolic risk factors for coronary heart disease in women. Diabetes Care. 2003;26:1413–1420. doi: 10.2337/diacare.26.5.1413. [DOI] [PubMed] [Google Scholar]
- 17.Britton KA, Fox CS. Ectopic fat depots and cardiovascular disease. Circulation. 2011;124:e837–841. doi: 10.1161/CIRCULATIONAHA.111.077602. [DOI] [PubMed] [Google Scholar]
- 18.Ouchi N, Parker JL, Lugus JJ, Walsh K. Adipokines in inflammation and metabolic disease. Nat Rev Immunol. 2011;11:85–97. doi: 10.1038/nri2921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Samaras K, Botelho NK, Chisholm DJ, Lord RV. Subcutaneous and visceral adipose tissue gene expression of serum adipokines that predict type 2 diabetes. Obesity (Silver Spring) 2010;18:884–889. doi: 10.1038/oby.2009.443. [DOI] [PubMed] [Google Scholar]
- 20.Cornier MA, Despres JP, Davis N, Grossniklaus DA, Klein S, Lamarche B, Lopez-Jimenez F, Rao G, St-Onge MP, Towfighi A, Poirier P. Assessing adiposity: A scientific statement from the american heart association. Circulation. 2011;124:1996–2019. doi: 10.1161/CIR.0b013e318233bc6a. [DOI] [PubMed] [Google Scholar]
- 21.Goossens L, Braet C, Decaluwe V. Loss of control over eating in obese youngsters. Behav Res Ther. 2007;45:1–9. doi: 10.1016/j.brat.2006.01.006. [DOI] [PubMed] [Google Scholar]
- 22.McLaughlin T, Lamendola C, Coghlan N, Liu TC, Lerner K, Sherman A, Cushman SW. Subcutaneous adipose cell size and distribution: Relationship to insulin resistance and body fat. Obesity (Silver Spring) 2014;22:673–680. doi: 10.1002/oby.20209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Heilbronn L, Smith SR, Ravussin E. Failure of fat cell proliferation, mitochondrial function and fat oxidation results in ectopic fat storage, insulin resistance and type ii diabetes mellitus. Int J Obes Relat Metab Disord. 2004;28(Suppl 4):S12–S21. doi: 10.1038/sj.ijo.0802853. [DOI] [PubMed] [Google Scholar]
- 24.Weyer C, Foley JE, Bogardus C, Tataranni PA, Pratley RE. Enlarged subcutaneous abdominal adipocyte size, but not obesity itself, predicts type ii diabetes independent of insulin resistance. Diabetologia. 2000;43:1498–1506. doi: 10.1007/s001250051560. [DOI] [PubMed] [Google Scholar]
- 25.Wronska A, Kmiec Z. Structural and biochemical characteristics of various white adipose tissue depots. Acta Physiol (Oxf) 2012;205:194–208. doi: 10.1111/j.1748-1716.2012.02409.x. [DOI] [PubMed] [Google Scholar]
- 26.McLaughlin T, Lamendola C, Coghlan N, Liu TC, Lerner K, Sherman A, Cushman SW. Subcutaneous adipose cell size and distribution: Relationship to insulin resistance and body fat. Obesity (Silver Spring) 2014;22:673–680. doi: 10.1002/oby.20209. Epub 2013 Dec 17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mettler FA. Essentials of radiology. Philadelphia: Elsevier/Saunders; 2014. [Google Scholar]
- 28.Pasarica M, Sereda OR, Redman LM, Albarado DC, Hymel DT, Roan LE, Rood JC, Burk DH, Smith SR. Reduced adipose tissue oxygenation in human obesity: Evidence for rarefaction, macrophage chemotaxis, and inflammation without an angiogenic response. Diabetes. 2009;58:718–725. doi: 10.2337/db08-1098. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.